Abstract
Over the past decade, interest on multitarget anticancer drugs -including heterometallic compounds- has increased considerably. Heterometallic species display improved efficacy and physicochemical properties compared to the individual metallic fragments for a variety of metal pair combinations. By 2018, several compounds had emerged as promising candidates against cisplatin resistant cancers. Here, we summarize research contributions to this topic over the past four years (July 2018-July 2022). In particular, we highlight five articles reporting on the in vivo activity and preliminary mechanisms of action for five groups of compounds. From this selection, we further feature two families of compounds based on Pt(IV)-Gd(III) and Ti(IV)-Au(I) metal combinations, given their potential for clinical translation.
Keywords: anticancer agents, multitarget therapy, bimetallic molecules, mice models, mechanisms
Introduction
One-drug-single-target has long been the paradigm in drug discovery. However, the use and design of multitarget drugs has recently become a very active area of research [1]. This is especially true for most challenging diseases like cancer, where multiple pathways may operate concomitantly, or where resistance to chemotherapy develops [2]. Starting in the 1960s, combination chemotherapy has been the standard to treat cancer and has most recently involved the use of both chemotherapeutics and targeted therapies and/or immunotherapies [2]. A large number of cancers respond better to combination therapies and these treatments are beneficial to override acquired resistance [2]. This realization has paved the way for the development of strategies combining multitarget fragments in a single molecule. For instance, the incorporation of bioactive or targeting ligands [3] or a second different metallic fragment [4] to metal-based drugs.
We previously reviewed heterometallic compounds as anticancer agents in a book chapter published in 2019 [4]. At that time there were ca. 81 publications and two patents on this topic. The compounds described were extremely diverse not only because of the variety of metals and metal-combinations involved, but also due to the ligands employed. We classified them into families of compounds with similar biologically active motifs. We concluded that in vitro studies strongly supported that synergistic and/or cooperative effects from the different metals were indeed able to improve the pharmacological profile of the heterometallic complexes described. While broad generalizations could not be made at the time due to scarcity of mechanistic/comparative studies, we indicated that in some cases one of the metals is responsible for the anticancer activity, while the second metal acts as modulator of chemicophysical properties (cooperative effect). However, in the majority of cases anticancer activity arises from the distinct action of each metallic center (synergistic effect). We identified two families of compounds as very promising potential candidates against metastatic cancers: a) ruthenium(II)-arene compounds containing platinum or gold, and titanocene(IV) containing gold compounds. We also pinpointed rhenium(I)-based derivatives for their potential as theranostic agents [4].
For the past four years (until July 2022) a few reviews in this topic have been published, either more general [5], or focused on heterometallic compounds as theranostics [6-9], heterometallic platinum compounds [10], ferrocene multimetallic complexes [11], multinuclear rhodium compounds [12], platinum-ruthenium [13], or titanocene-gold [14] complexes. In addition, thirty-five experimental articles describing studies in vitro (in cells) [15-41] or in vitro and in vivo [42-49] have been published. We list these in chronological order in the reference section, including six highly relevant articles and reviews that we have annotated [9,10,13,24,25,42]. In this review, we highlight the findings of five articles [44-48] that report on detailed studies in mice models and propose some plausible mechanisms of action. We consider that some of the compounds featured have high potential for clinical translation. We focus solely on bimetallic compounds containing motifs with known biological activity.
Results
Heterometallic Compounds Containing Platinum-Based Motifs
We describe findings of three articles based on platinum(II) [48] or on Pt(IV) fragments based on the FDA-approved cisplatin core [45], or the oxaliplatin core [46]. Cytotoxic cisplatin and oxaliplatin bind to genomic and mitochondrial DNA preventing DNA replication and transcription causing apoptosis. Additional potential apoptotic pathways involve inactivation of RNA polymerases by platinum DNA adducts causing transcription cessation and cell death through p53 dependent and independent pathways [50].
Platinum(II)-Ferrocene Hybrids
Anticancer ferrocene-based hybrids have been reviewed recently [51]. In 2022, Patra and co-authors have reported on Pt(II)-Fc (Fc= ferrocene) hybrids (Fig. 1) with high efficacy against ovarian A2780 cell lines [48]. Their design improves on the activity of Pt(II)-based compounds causing DNA damage (like cisplatin and oxaliplatin, Fig. 1) by the incorporation of Fc units which are thought to produce reactive oxygen species (ROS) in cancer cells (from unregulated endogenous H2O2) inducing oxidative stress or modify cellular redox homeostasis. The resulting cationic and highly lipophilic compounds were expected to have improved accumulation in mitochondria.
Figure 1.
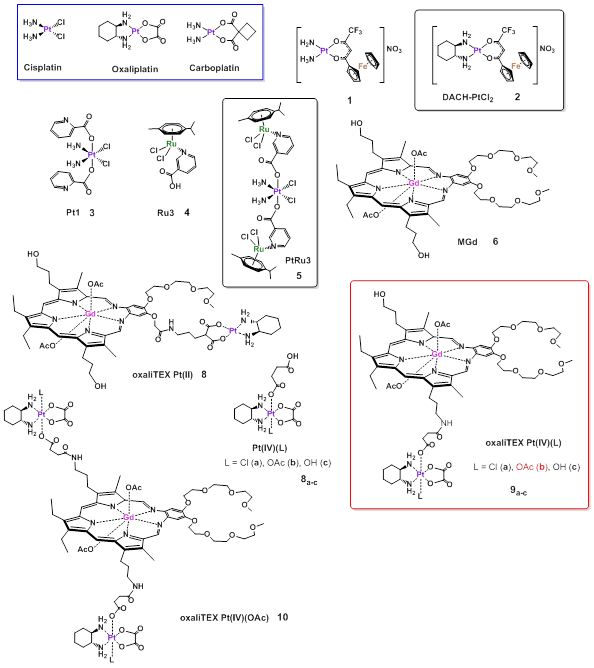
FDA Pt(II) approved FDA anticancer agents (in blue box). Selected heterometallic Pt(II) and Pt(IV) compounds (2, 5, 7, 9, and 10) with high in vivo efficacy against ovarian cancer and monometallic fragments (compounds 3, 4, 6, 8) [45,46,48].
From these hybrids, compound 2 (containing DACH = 1R,2R-diaminocyclohexane, Fig. 1) was identified as the candidate for further mechanistic and in vivo studies. The two metallic fragments imparted complementary physicochemical properties as their solubility in water was improved with respect to the Fc compound, while the lipophilicity was increased with respect to cisplatin. 2 is very stable in polar organic media, water, PBS and cell media (RPMI) and more stable in human plasma than oxaliplatin (half-life of 118 minutes versus 70 minutes) displaying lower platinum-cross resistance similar to oxaliplatin and certain selectivity in cancer cells. Compound 2 works in vitro via synergism and cooperative effects of both metallic fragments, which would not occur separately, such as nuclear and mitochondrial DNA damage (platination), ROS production in mitochondria and endoplasmic reticulum (ER), disruption of mitochondrial oxygen consumption rate, induction of ER dilation and stress leading to cell death by necroptosis and paraptosis [48].
A pilot efficacy study in an ovarian A2780 xenograft model (n= 5/group) in NOD/SCID 100 mice (IP injection, 32 days, 9 doses) with doses 5 mg/kg of 2 or oxaliplatin showed growth inhibition at 25 days of 63% and 40% for compound 2 and oxaliplatin respectively. Mice treated with 2 did not show signs of toxicity or bodyweight loss. Histopathological analysis showed reduced tumor vascularization in treated mice and regulation of the NLRP3 inflammasome innate pathway. However, the histological analysis of organ tissues was not discussed. Biodistribution studies showed highest accumulation of Pt in excretory organs (spleen, kidney and liver) followed by tumor, heart, lungs and blood [48].
Platinum(IV)-Ruthenium(II) Compounds
Exploiting the use of Pt(IV) pro-drugs to improve the therapeutic index of more toxic Pt(II) compounds [52], Chen, Wang and co-workers have developed chimeric complexes containing Pt(IV) and Ru(II) centers bonded by a niacin linker (Fig. 1) [45]. These compounds are based on Pt(IV) species containing a cytotoxic cisplatin core (Pt1, 3) and antimetastatic arene-Ru(II)-chloride fragments (like Ru3, 4) and were expected to have a synergistic effect by combination of the two metallic fragments. In in vitro studies, PtRu1 (5) emerged as the best candidate to move forward with (IC50s 1-6.5 μM range in different cell lines including ovarian A2780). PtRu1 (5) is stable for 24 h in DMSO and under incubation with ascorbic acid releases Ru3 4. PtRu1 (5) induced apoptosis (late apoptosis in A2780 cells) and arrested the cells in the G2 phase (differing from cisplatin which induces S phase arrest). PtRu1 (like Ru3 4 and unlike cisplatin) displays antimetastatic and antiangiogenic properties (invasion and tube-forming assay). The in vivo efficacy of 5 was tested using a transcoelomic metastatic A2780 ovarian cancer model in Balb/c nude mouse. PtRu1 5 at a dosage of 3 mg/kg reduced tumor growth completely and prevented metastasis to other organs better than Ru3 4 or cisplatin [45]. Histological analysis showed that organs excised from mice treated with 5 presented comparable histopathological features to saline-treated mice.
Platinum(IV)-Gadolinium-Texaphyrin Prodrugs
Pt(IV)-based prodrugs were also exploited by Sessler and co-workers in the preparation of gadolinium(III) texaphyrin platinum(IV) conjugates (Fig. 1) [46] based on a a metallotexaphyrin core (MGd 6) and Pt(IV) compounds containing an oxaliplatin core (7a-d).Platinum(II) compound oxaliTEX Pt(II) 8 and analogues of oxaliTEX Pt(IV)(L) 9 and 10 containing Cl had already been reported [53] and it was demonstrated that Pt(IV) compounds (under reducing conditions) displayed potent anti-proliferative activity in ovarian cancer cell lines. Compound 9a was photo-inductively reduced to a Pt(II) species upon exposure to glass-filtered daylight binding DNA in a controlled manner [57]. In this excellent report, the authors identified compound 9b (with an acetate group) as the best candidate to move forward with. 9b contains a number of key components including the metallotexaphyrin core (motexafin gadolinium MGd 6) shown to accumulate in primary and metastatic tumors in rodents and humans, and for which the redox activity on the macrocyclic ligand imparts anticancer activity. Other components include a Pt(IV) core (for reduced toxicity with respect to Pt(II)), an oxalate group coordinated to Pt(IV) and to texaphyrin through a linker, and a diaminiocyclohexyl (DACH) ligand around the platinum center (that as demonstrated for compound 2 [48] in this review, imparts higher selectivity). These structural elements provide several advantages: 1) reduced toxicity when compared to FDA-approved drugs, 2) ability to overcome p53-based platinum resistance, 3) good efficacy in vitro and in vivo, and 4) ease of formulation. Compound 9b does not platinate DNA unless in presence of excess ascorbate (via reduction of Pt(IV) to Pt(II)) [46].
In ovarian cancer cell lines (A2780 and 2780CP/CI-16 platinum resistant) as well as other cell lines, 9b is highly cytotoxic and apoptotic, with a higher cellular uptake compared to oxaliplatin (due to MGd). 9b also displays high water solubility and strong binding to albumin (90%) which does not decrease activity [46]. A synergistic/cooperative effect on the basis of MGd activation the Pt(IV) species through redox cycling (e.g. improvement of antiproliferative activity, Fig. 2) was established [46].
Figure 2.
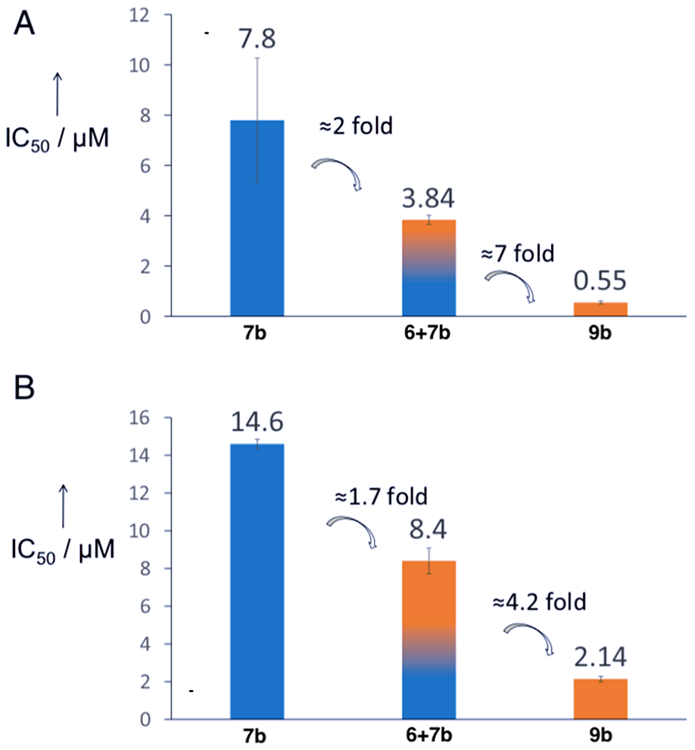
Synergistic antiproliferative effect of 9b in (A) ovarian A2780 and (B) lung A549 compared to 7b and a mixture of 6 and 7b. Reprinted and adapted with permission from Ref. [46]. Copyright © 2020, PNAS
In a number of in vivo studies on athymic mice bearing established subcutaneous A549 xenografts, compound 9b displayed a higher tumor growth inhibition (50-60%) and easiness to be formulated in 5% dextrose/deionized water compared to compound 7 and oxaliplatin. In mice bearing cell-derived subcutaneous (s.c) xenografts (A2780 ovarian and HCT116 colon) and syngeneic tumors (CT26 colon and EMT6 breast), compound 9b displayed statistically significant higher anticancer activity compared to oxaliplatin. In an orthotopic model of A549 lung cancer, 9b led to tumor growth inhibition but not significantly different than that of oxaliplatin. Efficacy studies in patient-derived xenografts (PDX, n = 10) with poor responses to traditional platinum treatments (0253 ovarian, 0069 colorectal) compared 9b to the platinum standard of care for particular tumor types. Remarkably, mice bearing 0253 ovarian PDX tumors did not respond to treatment with carboplatin but treatment with 9b led to 95% tumor growth inhibition. Similar results were obtained for 0069 colon PDX models (80% tumor growth inhibition) in comparison to oxaliplatin (50%) [46].
Mice can tolerate a higher repeated dose of platinum when administered as 9b than as oxaliplatin. Intratumoral Pt concentration in mice treated with 9b was 5.5-fold higher than in animals treated with oxaliplatin. These studies demonstrated that the gadolinium(III) texaphyrin core (MGd) in 9b led to improved tumor localization. In addition, MGd alters the biodistribution away from the kidney and towards the liver (advantageous to fight renal toxicity). In terms of hematology, 9b produces pancytopenia (leukopenia, anemia, and thrombocytopenia) although this was more pronounced in animals treated with oxaliplatin and could potentially be managed in the clinic [46].
Heterometallic Compounds Containing Gold-Based Motifs
Since 2011, our laboratory has developed gold-based compounds containing a second metal (titanium or ruthenium) [4]. Gold(I) compounds such as antirheumatic FDA-approved Auranofin (Fig. 3) and related derivatives are becoming attractive not only due to their antitumor effects that involve apoptosis, antimigratory and antiangiogenic properties, but also because they may produce immunogenic cell death [54]. Gold(I)-thiolate complexes containing phosphanes or N-Heterocyclic carbenes are known to exert cytotoxicity by inhibiting the function of the mitochondrial thioredoxin system and that of enzymes that are involved in DNA replication [55]. Bimetallic compounds containing titanium and ruthenium displayed high efficacy against different cancers (ovarian, prostate and colon) [4]. A number of titanium-gold [4,58] and ruthenium-gold [4,59,60] complexes containing organometallic titanocene and arene-Ru(II) scaffolds and gold-phosphane or gold N-heterocyclic carbenes (Fig. 3) are very efficacious against renal cancer both in vitro and in vivo. By 2018, we had identified three compounds (two Ti-Au Titanocref 11 and Titanofin 12 [4,16,47,58], and one Ru-Au [4,59,60], RANCE-1, 13) as leading candidates for treatment of clear cell renal cell carcinoma (ccRCC). Here we describe more recent and detailed in vivo studies [44,47].
Figure 3.
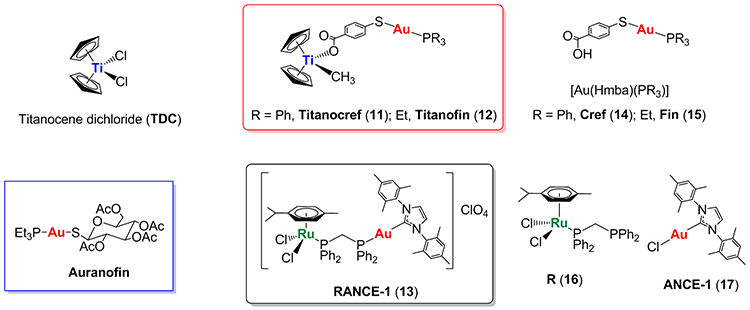
FDA-approved gold-based compound Auranofin (in blue box). Selected heterometallic compounds developed in our laboratory display high efficacy against ccRCC in vitro and in vivo (a) titanium-gold (Titanocref 11, and Titanofin 12) based on the moieties of titanocene dichloride and gold(I) compounds Cref (14) and Fin (15) [16,23,44,58]; (b) ruthenium-gold compound (RANCE-1, 13) based on ruthenium(II) compound R (16) and gold(I) compound ANCE-1 (17) [47,59,60].
Gold(I)-Titanium(IV) Hybrids
The mode of action of titanocene dichloride (figure 3) is based on the formation of titanocene-DNA adducts which inhibit DNA and RNA synthesis, as well as the accumulation of titanium in nucleic acid-rich regions of tumor cells [56]. In ccRCC cell lines, Ti(IV)-Au(I) Titanocref 11 and Titanofin 12 are more cytotoxic (nanomolar range) and apoptotic than gold monometallic derivatives (14 and 15, and auranofin) and significantly more cytotoxic than titanocene dichloride while displaying high selectivity [16,47]. In Caki-1 clear cell renal cell carcinoma cells, Titanocref 11 and Titanofin 12 inhibit migration, invasion, and angiogenic assembly along with molecular markers associated with these processes such as prometastatic IL(s), MMP(s), TNF-α, and proangiogenic VEGF, FGF-basic [16]. The Ti-Au compounds also strongly inhibit the mitochondrial protein TrxR often overexpressed in cancer cells able to evade apoptosis. These compounds also inhibit FOXC2, PECAM-1, and HIF-1α whose overexpression is linked to resistance to genotoxic chemotherapy [16]. This comparative in vitro evaluation indicates that the mode of action is primarily due to the gold center and that these compounds, although similar to Auranofin, have improved properties such as selectivity towards tumorigenic cells. The titanocene fragment has an appreciable role in some of the chemicophysical properties of 11 and 12 with remarkable improvement of their pharmacological profile [4,16,23]. Microarray and qRT-PCR analysis of ccRCC Caki-1 cells treated with Titanocref 11 revealed that the compound alters apoptosis, JNK MAP kinase, and ROS pathways within 3 hours of treatment [47].
Evaluation of Titanocref 11 and Titanofin 12 in NOD.CB17-Prkdc SCID/J mice bearing xenograft ccRCC Caki-1 tumors to assess their in vivo activity accompanied by detailed pharmacokinetic and pathology studies confirmed most in vitro results [47]. Doses of 5 mg/kg/72h for 11 and 10 mg/kg/72h for 12 (21-day trial) yield a significant reduction of 51% and 60% respectively (p<0.01) in tumor size, while vehicle-treated mice exhibited a tumor size increase of 138% (p<0.01). Importantly, we find no signs of pathological complication as a result of treatment. Additionally, treatment with 11 and 12 reduced angiogenesis by 38% and 54% respectively. We further demonstrated activation of apoptosis by 11 and 12 in vivo via caspase 3 assays.
Gold(I)-Ruthenium(II) Hybrids
The design of anticancer gold-ruthenium heterometallic complexes is based on the combination of the potential antimetastatic/cytotoxic properties of [Ru(p-cymene)Cl2(η1-dppm)] 16 and the antimitochondrial/thioredoxin reductase inhibition effects of [(NHC)Au]+ and [(NHC)Au(PR3)]+ species [59]. The leading ruthenium-gold compound RANCE-1 (13) had been studied in vitro in ccRCC Caki-1 cell lines including assessment of synergistic effects and comparisons with Auranofin [59,60]. In addition to being cytotoxic and apoptotic, RANCE-1 (13) displays major inhibition of migration (82%), invasion (66%) and angiogenesis, while also inhibiting molecular pathways associated with these processes, and thioredoxin reductase [60]. It is noteworthy that the inhibition observed is generally better than that of the individual monometallic fragments present in 13. In some cases, this inhibition can be correlated with either ruthenium R (16) or gold ANCE-1 (17) which supports the notion of synergistic effects improving efficacy. The treatment of NOD.CB17-Prkdc SCID/J mice bearing xenograft ccRCC Caki-1 tumors (21 days) with 13 (10 mg/kg/72h) afforded complete tumor inhibition. As for the Ti-Au derivatives, there were no signs of pathological complications as a result of treatment [44]. Furthermore, significant reductions in the expression of VEGF, PDGF, FGF, EGFR, and HGRF, all key to the proliferation of tumor cells and stromal cells serving protumorigenic purposes, were observed [44].
Conclusions
The research highlighted in this review demonstrates the high efficacy of various heterometallic compounds against cancers resistant to FDA-approved platinum drugs. The results also support the idea that synergistic/cooperative effects from two different metallic fragments lead to higher effectiveness of the heterometallic hybrids and improved pharmacological profile. From the compounds described (all of them efficacious in vivo) we select Pt(IV)-Gd(II): Pt(IV)oxalitex(OAc) 9b and Ti-Au: Titanocref (11)/Titanofin (12) (red boxes in Figs. 1 and 3) as candidates for further pharmacological studies due to their potential clinical significance. Pt(IV)-Gd(II) 9b is superior to current standard of care for certain platinum-resistant cancers in several mice models (including PDX models), in terms of efficacy and tolerability. Ti-Au 11 and 12 afforded an impressive tumor size reduction (not only tumor growth inhibition) and angiogenesis inhibition in renal cancer xenograft mice models with minimal pathological complications. We anticipate more advanced pre-clinical studies with these types of multitarget heterometallic therapies.
Figure 4.
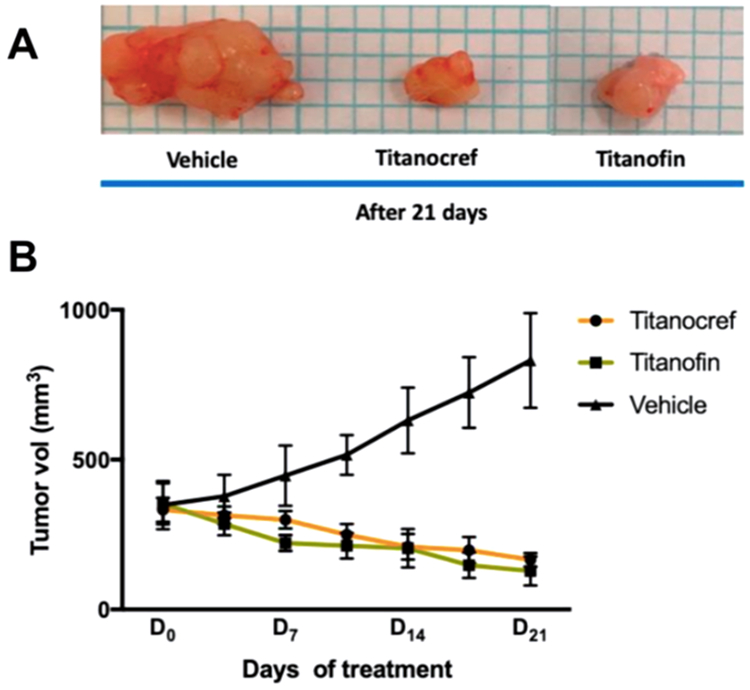
High tumor suppression of xenograft renal cancer mouse model over 21 days upon the treatment with Titanocref 11 and Titanofin 12. (A) Resected tumors and (B) Average tumor volume after treatment of Titanocref 11 and Titanofin 12. Reprinted with permission from Ref. [47]. Copyright © 2020, American Chemical Society
Acknowledgements
This research is supported by the National Institute of Health for General Medical Sciences (NIGMS) grant 2SC1GM127278 to M.C. We thank PNAS journal for granting us permission to reuse and modify Figure 2 from reference [46]. We thank the American Chemical Society Publications’ ACS Pharmacology & Translational Science journal for granting us permission to reprint Figure 3 from reference [47]. We are grateful to Prof. Mariana Torrente (Brooklyn College) for proofreading this article.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: M.C. has patent #US Patent 9,315,531 issued to Maria Contel, Jacob Fernández-Gallardo, Benelita T. Elie, and Joe W. Ramos.
References
* of special interest
** of outstanding interest
- [1].Proschak E, Stark H, Merk D: Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J Med Chem 2019, 62:420–444. [DOI] [PubMed] [Google Scholar]
- [2].Makhoba XH, Viegas C Jr, Mosa RA, Viegas FPD, Pooe OJ: Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des Devel Ther 2020, 14:3235–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen ZF, Orvig C, Liang H: Multi-Target Metal-Based Anticancer Agents. Curr Top Med Chem 2017, 17:3131–3145. [DOI] [PubMed] [Google Scholar]
- [4].Curado N; Contel M: Heterometallic Complexes as Anticancer Agents – Metallobiology. R. Soc. Chem. Cambridge 2019, Chapter 6:143–168 and refs. therein. [Google Scholar]
- [5].van Niekerk A, Chellan P, Mapolie SF: Heterometallic Multinuclear Complexes as Anti-Cancer Agents-An Overview of Recent Developments. Eur J Inorg Chem 2019, 30:3432–3455. [Google Scholar]
- [6].Redrado M, Fernández-Moreira V, Gimeno MC: Theranostics Through the Synergistic Cooperation of Heterometallic Complexes. ChemMedChem 2021, 16:932–941. [DOI] [PubMed] [Google Scholar]
- [7].Tan C-P, Zhong Y-M, Ji L-N, Mao Z-W: Phosphorescent Metal Complexes as Theranostic Anticancer Agents: Combining Imaging and Therapy in a Single Molecule. Chem Sci 2021, 12:2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vaidya SP, Gadre S, Kamisetti RT, Patra M: Challenges and Opportunities in the Development of Metal-Based Anticancer Theranostic Agents. Biosci Rep 2022, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[9]. Huang Z, Wilson JJ: Therapeutic and Diagnostic Applications of Multimetallic Rhenium(I) Tricarbonyl Complexes. Eur J Inorg Chem 2021, 14:1312–1324. An overview of multimetallic Re(CO)3 compounds developed specifically for biomedical applications. The authors collect reports on Re-containing homonuclear and heteronuclear complexes and draw comparisons based on their cytotoxic profiles, biological activity, and imaging properties.
- *[10]. Ma L, Li L, Zhu G: Platinum-Containing Heterometallic Complexes in Cancer Therapy: Advances and Perspectives. Inorg Chem Front 2022, 9:2424–2453. This review details numerous approaches and synthetic routes leading to Pt-containing heterometallic anticancer agents in the past ten years. The authors elaborate on the mechanistic contribution and anticancer properties attributable to the non-platinum metal center in these complexes. They also enumerate the limitations yet to be overcome for potential clinical translation.
- [11].Basu U, Roy M, Chakravarty AR: Recent Advances in the Chemistry of Iron-Based Chemotherapeutic Agents. Coord Chem Rev 2020, 417:1–30. [Google Scholar]
- [12].Máliková K, Masaryk L, Štarha P: Anticancer Half-Sandwich Rhodium(III) Complexes. Inorganics 2021, 9:1–24. [Google Scholar]
- *[13]. Jain A: Multifunctional, Heterometallic Ruthenium-Platinum Complexes with Medicinal Applications. Coord Chem Rev 2019, 401:1–10. This review focuses on ruthenium-platinum multimetallic complexes containing the two-metal centers combination Ru(II)/Ru(III) with Pt(II)/Pt(IV). The authors highlight the synthetic design, the activation upon reduction, and photoactive versus non-photoactive activation of the resulting heterometallic complexes. The authors describe in vitro and in vivo activity as well as plausible mechanisms of action (covalent and non-covalent DNA binding, and RNA and protein interactions).
- [14].Fernández-Vega L, Ruiz Silva VA, Domínguez-González TM, Claudio-Betancourt S, Toro-Maldonado RE, Capre Maso LC, Sanabria Ortiz K, Pérez-Verdejo JA, Román González J, Rosado-Fraticelli GT, Pagán Meléndez F, Betancourt Santiago FM, Rivera-Rivera DA, Martínez Navarro C, Bruno Chardón AC, Vera AO, Tinoco AD: Evaluating Ligand Modifications of the Titanocene and Auranofin Moieties for the Development of More Potent Anticancer Drugs. Inorganics 2020, 8:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luengo A, Fernández-Moreira V, Marzo I, Gimeno MC: Bioactive Heterobimetallic Re(I)/Au(I) Complexes Containing Bidentate N-Heterocyclic Carbenes. Organometallics 2018, 37:3993–4001. [Google Scholar]
- [16].Elie BT, Fernández-Gallardo J, Curado N, Cornejo MA, Ramos JW, Contel M: Bimetallic Titanocene-Gold Phosphane Complexes Inhibit Invasion, Metastasis, and Angiogenesis-Associated Signaling Molecules in Renal Cancer. Eur J Med Chem 2019, 161:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shahsavari HR, Giménez N, Lalinde E, Moreno MT, Fereidoonnezhad M, Babadi Aghakhanpour R, Khatami M, Kalantari F, Jamshidi Z, Mohammadpour M: Heterobimetallic PtII -AuI Complexes Comprising Unsymmetrical 1,1-Bis(Diphenylphosphanyl)Methane Bridges: Synthesis, Photophysical, and Cytotoxic Studies. Eur J Inorg Chem 2019, 10:1360–1373. [Google Scholar]
- [18].Curado N, Giménez N, Miachin K, Aliaga-Lavrijsen M, Cornejo MA, Jarzecki AA, Contel M: Preparation of Titanocene–Gold Compounds Based on Highly Active Gold(I)- N -Heterocyclic Carbene Anticancer Agents: Preliminary in Vitro Studies in Renal and Prostate Cancer Cell Lines. ChemMedChem 2019, 14:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ohui K, Babak MV, Darvasiova D, Roller A, Vegh D, Rapta P, Guan GRS, Ou YH, Pastorin G, Arion VB: Redox-Active Organoruthenium(II)– and Organoosmium(II)–Copper(II) Complexes, with an Amidrazone–Morpholine Hybrid and [CuICl2]− as Counteranion and Their Antiproliferative Activity. Organometallics 2019, 38:2307–2318. [Google Scholar]
- [20].Thiabaud G, Harden-Bull L, Ghang Y-J, Sen S, Chi X, Bachman JL, Lynch VM, Siddik ZH, Sessler JL: Platinum(IV)-Ferrocene Conjugates and Their Cyclodextrin Host–Guest Complexes. Inorg Chem 2019, 58:7886–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu SD, Wu XH: Bimetallic DppfM(II) (M = Pt and Pd) Dithiocarbamate Complexes: Synthesis, Characterization, and Anticancer Activity. J Chem Res 2019, 43:437–442. [Google Scholar]
- [22].Tabrizi L, Olasunkanmi LO, Fadare OA: De Novo Design of Thioredoxin Reductase-Targeted Heterometallic Titanocene–Gold Compounds of Chlorambucil for Mechanistic Insights into Renal Cancer. Chem Commun 2020, 56:297–300. [DOI] [PubMed] [Google Scholar]
- [23].Sarpong-Kumankomah S, Contel M, Gailer J: SEC Hyphenated to a Multielement-Specific Detector Unravels the Degradation Pathway of a Bimetallic Anticancer Complex in Human Plasma. J Chromatogr B 2020, 1145:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[24]. Karges J, Yempala T, Tharaud M, Gibson D, Gasser G: A Multi-action and Multi-target RuII –PtIV Conjugate Combining Cancer-Activated Chemotherapy and Photodynamic Therapy to Overcome Drug Resistant Cancers. Angew Chem In Ed 2020, 59:7069–7075. The authors report on the synthesis of a Ru(II) polypyridine-Pt(IV) heterobimetallic complex which combines cancer activated chemotherapy with photodynamic therapy. Upon entering the cancer cell, the Pt(IV) center is reduced to Pt(II) and the axial ligands (Ru(II) complex and phenylbutyrate) are released. The conjugate exerts a multi-target and multi-action effect due to the individual metallic components with (photo-)cytotoxicity values upon irradiation up to 595 nm in the low nanomolar range in various 2D monolayer cancer cells and 3D multicellular tumor spheroids.
- **[25]. Saeed HK, Sreedharan S, Jarman PJ, Archer SA, Fairbanks SD, Foxon SP, Auty AJ, Chekulaev D, Keane T, Meijer AJHM, Weinstein JA, Smythe CGW, Bernardino de la Serna J, Thomas JA: Making the Right Link to Theranostics: The Photophysical and Biological Properties of Dinuclear RuII-ReI Dppz Complexes Depend on Their Tether. J Am Chem Soc 2020, 142:1101–1111. This report on ruthenium-rhenium heterometallic theranostics linked through N,N’-bis(4-pyridylmethyl)-1,6-hexanediaminea compares them with an analogue with a different linker. Steady-state and time-resolved photophysical studies revealed that the nature of the linker affects the excited state dynamics of the complexes and their DNA photocleavage properties, as well as their intracellular localization with one compound being able to be tracked by super resolution (SIM and STED) nanoscopy.
- [26].Adams CJ, Meade TJ: Gd(III)-Pt(IV) Theranostic Contrast Agents for Tandem MR Imaging and Chemotherapy. Chem Sci 2020, 11:2524–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johnson A, Marzo I, Gimeno MC: Heterobimetallic Propargyl Gold Complexes with π-Bound Copper or Silver with Enhanced Anticancer Activity. Dalton Trans 2020, 49:11736–11742. [DOI] [PubMed] [Google Scholar]
- [28].Luengo A, Marzo I, Reback M, Daubit IM, Fernández-Moreira V, Metzler-Nolte N, Gimeno MC: Luminescent Bimetallic IrIII /AuI Peptide Bioconjugates as Potential Theranostic Agents. Chem Eur J 2020, 26:12158–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Luengo A, Redrado M, Marzo I, Fernández-Moreira V, Gimeno MC: Luminescent Re(I)/Au(I) Species As Selective Anticancer Agents for HeLa Cells. Inorg. Chem 2020, 59:8960–8970. [DOI] [PubMed] [Google Scholar]
- [30].Guedes APM, Mello-Andrade F, Pires WC, de Sousa MAM, da Silva PFF, de Camargo MS, Gemeiner H, Amauri MA, Gomes Cardoso C, de Melo Reis PR, Silveira-Lacerda E de P, Batista AA: Heterobimetallic Ru(II)/Fe(II) Complexes as Potent Anticancer Agents against Breast Cancer Cells, Inducing Apoptosis through Multiple Targets. Metallomics 2020, 12:547–561. [DOI] [PubMed] [Google Scholar]
- [31].BenYosef D, Romano D, Hadiji M, Dyson PJ, Blom B: Facile Synthesis of Heterobimetallic [FeII(μ-Diphosphine)RuII] and Homobimetallic [FeII(μ-Diphosphine)FeII] Complexes and Their in Vitro Cytotoxic Activity on Cisplatin-Resistant Cancer Cells. Inorg. Chim Acta 2020, 510:119731. [Google Scholar]
- [32].Reigosa-Chamorro F, Raposo LR, Munín-Cruz P, Pereira MT, Roma-Rodrigues C, Baptista PV, Fernandes AR, Vila JM: In Vitro and In Vivo Effect of Palladacycles: Targeting A2780 Ovarian Carcinoma Cells and Modulation of Angiogenesis. Inorg. Chem 2021, 60:3939–3951. [DOI] [PubMed] [Google Scholar]
- [33].Yao K, Karunanithy G, Howarth A, Holdship P, Thompson AL, Christensen KE, Baldwin AJ, Faulkner S, Farrer NJ: Cell-Permeable Lanthanide–Platinum(iv) Anti-Cancer Prodrugs. Dalton Trans 2021, 50:8761–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Redrado M, Benedi A, Marzo I, García-Otín AL, Fernández-Moreira V, Concepción Gimeno M: Multifunctional Heterometallic IrIII–AuI Probes as Promising Anticancer and Antiangiogenic Agents. Chem Eur J 2021, 27:9885–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang Z, King AP, Lovett J, Lai B, Woods JJ, Harris HH, Wilson JJ: Photochemistry and in Vitro Anticancer Activity of Pt(IV)Re(I) Conjugates. Chem Commun 2021, 57:11189–11192. [DOI] [PubMed] [Google Scholar]
- [36].Luengo A, Marzo I, Fernández-Moreira V, Gimeno MC: Synthesis and Antiproliferative Study of Phosphorescent Multimetallic Re(I)/Au(I) Complexes Containing Fused Imidazo[4,5-f]-1,10-phenanthroline Core. Appl. Organomet. Chem 2022, 1–11. [Google Scholar]
- [37].Mohamed AS, Jourdain I, Knorr M, Elmi A, Chtita S, Scheel R, Strohmann C, Hussien MA: Design of Hydroxyl- and Thioether-Functionalized Iron-Platinum Dimetallacyclopentenone Complexes. Crystal and Electronic Structures, Hirshfeld and Docking Analyses and Anticancer Activity Evaluated by in Silico Simulation. J. Mol. Struct 2022, 1251:1–19. [Google Scholar]
- [38].Ma X, Lu J, Yang P, Huang B, Li R, Ye R: Synthesis, Characterization and Antitumor Mechanism Investigation of Heterometallic Ru(II)-Re(I) Complexes. Front. Chem 2022, 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gascón E, Otal I, Maisanaba S, Llana-Ruiz-Cabello M, Valero E, Repetto G, Jones PG, Oriol L, Jiménez J: Gold(I) Metallocyclophosphazenes with Antibacterial Potency and Antitumor Efficacy. Synergistic Antibacterial Action of a Heterometallic Gold and Silver-Cyclophosphazene. Dalton Trans 2022, 51:13657–13674. [DOI] [PubMed] [Google Scholar]
- [40].Xiong Z, Jiang M, Zhang M, Qiu Y, Zhang D, Lin X, Lamu Z, Zhuoga G, Zhen J, Li H, Lu X, Wu Z: A Novel Heterometallic Ruthenium-Silver Complex as Potential Antitumor Agent: Studies on Its Synthesis, in Vitro Assays and Interactions with Biomolecular Targets. Eur J Pharm Sci 2022, 106276. [DOI] [PubMed] [Google Scholar]
- [41].Lu J-J, Ma X-R, Xie K, Yang P-X, Li R-T, Ye R-R: Novel Heterobimetallic Ir(III)–Re(I) Complexes: Design, Synthesis and Antitumor Mechanism Investigation. Dalton Trans. 2022, 51:7907–7917. [DOI] [PubMed] [Google Scholar]
- **[42]. Zhou Z, Liu J, Rees TW, Wang H, Li X, Chao H, Stang PJ: Heterometallic Ru–Pt Metallacycle for Two-Photon Photodynamic Therapy. Proc. Natl. Acad. Sci 2018, 115:5664–5669. The authors describe a supramolecular heterometallic Ru–Pt metallacycle via coordination-driven self-assembly, a potential PDT candidate, which combines excellent photostability and two-photon absorption (Ru) with red-shifted luminescence to the near-infrared region, a larger two-photon absorption cross-section and higher singlet oxygen generation efficiency. The compound accumulates in mitochondria and nuclei leading to cell death via DNA damage and mitochondrial function impairment. In vivo studies showed efficient tumor growth reduction (low light dose) with negligible side effects.
- [43].Milutinović MM, Čanović PP, Stevanović D, Masnikosa R, Vraneš M, Tot A, Zarić MM, Simović Marković B, Misirkić Marjanović M, Vučićević L, Savić M, Jakovljević V, Trajković V, Volarević V, Kanjevac T, Rilak Simović A: Newly Synthesized Heteronuclear Ruthenium(II)/Ferrocene Complexes Suppress the Growth of Mammary Carcinoma in 4T1-Treated BALB/c Mice by Promoting Activation of Antitumor Immunity. Organometallics 2018, 37:4250–4266. [Google Scholar]
- [44].Elie BT, Hubbard K, Pechenyy Y, Layek B, Prabha S, Contel M: Preclinical Evaluation of an Unconventional Ruthenium-gold-based Chemotherapeutic: RANCE-1, in Clear Cell Renal Cell Carcinoma. Cancer Med. 2019, 8:4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shu L, Ren L, Wang Y, Fang T, Ye Z, Han W, Chen C, Wang H: Niacin-Ligated Platinum(IV)–Ruthenium(II) Chimeric Complexes Synergistically Suppress Tumor Metastasis and Growth with Potentially Reduced Toxicity in Vivo. Chem Commun 2020, 56:3069–3072. [DOI] [PubMed] [Google Scholar]
- [46].Thiabaud G, He G, Sen S, Shelton KA, Baze WB, Segura L, Alaniz J, Munoz Macias R, Lyness G, Watts AB, Kim HM, Lee H, Cho MY, Hong KS, Finch R, Siddik ZH, Arambula JF, Sessler JL: Oxaliplatin Pt(IV) Prodrugs Conjugated to Gadolinium-Texaphyrin as Potential Antitumor Agents. Proc Natl Acad Sci 2020, 117:7021–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Elie BT, Hubbard K, Layek B, Yang WS, Prabha S, Ramos JW, Contel M: Auranofin-Based Analogues Are Effective Against Clear Cell Renal Carcinoma In Vivo and Display No Significant Systemic Toxicity. ACS Pharmacol Transl Sci 2020, 3:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gadre S, Manikandan M, Duari P, Chhatar S, Sharma A, Khatri S, Kode J, Barkume M, Kasinathan NK, Nagare M, Patkar M, Ingle A, Kumar M, Kolthur-Seetharam U, Patra MA: Rationally Designed Bimetallic Platinum (II)-Ferrocene Antitumor Agent Induces Non-Apoptotic Cell Death and Exerts in Vivo Efficacy. Chem Eur J 2022, 28:1–13. [DOI] [PubMed] [Google Scholar]
- [49].Bellam R, Jaganyi D, Robinson RS: Heterodinuclear Ru–Pt Complexes Bridged with 2,3-Bis(Pyridyl)Pyrazinyl Ligands: Studies on Kinetics, Deoxyribonucleic Acid/Bovine Serum Albumin Binding and Cleavage, In Vitro Cytotoxicity, and In Vivo Toxicity on Zebrafish Embryo Activities. ACS Omega 2022, 7:26226–26245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mehmood RK: Review of Cisplatin and Oxaliplatin in Current Immunogenic and Monoclonal Antibody Treatments. Oncol Rev 2014, 8:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang R, Chen H, Yan W, Zheng M, Zhang T, Zhang Y: Ferrocene-Containing Hybrids as Potential Anticancer Agents: Current Developments, Mechanisms of Action and Structure-Activity Relationships. Eur J Med Chem 2020, 190:1–21. [DOI] [PubMed] [Google Scholar]
- [52].Gibson D: Platinum(IV) anticancer agents; are we en route to the holy grail or to a dead end? J Inor Biochem 2021, 217:1–10. [DOI] [PubMed] [Google Scholar]
- [53].Arambula JF, Sessler JL, Siddik ZH: A Texaphyrin–Oxaliplatin Conjugate That Overcomes Both Pharmacologic and Molecular Mechanisms of Cisplatin Resistance in Cancer Cells. MedChemComm 2012, 3:1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yue S, Luo M, Liu H, Wei S: Recent Advances of Gold Compounds in Anticancer Immunity. Front Chem 2020, 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yeo CI, Ooi KK, Tiekink ERT: Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Olszewski U, Claffey J, Hogan M, Tacke M, Zeillinger R, Bednarski PJ, Hamilton G: Anticancer Activity and Mode of Action of Titanocene C. Invest New Drugs 2011, 29:607–614. [DOI] [PubMed] [Google Scholar]
- [57].Thaibaud G, Arambula JF, Siddik ZH, Sessler JL: Photoinduced Reduction of Pt(IV) within an Anti-Proliferative Pt(IV)-Texaphyrin Conjugate. Chem Eur J 2014, 20:8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].(a) Fernández-Gallardo J, Elie BT, Sadhukha T, Prabha S, Sanaú M, Rotenberg SA, Ramos JW, Contel M: Heterometallic Titanium–gold Complexes Inhibit Renal Cancer Cells in Vitro and in Vivo. Chem Sci 2015, 6:5269–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Contel M, Fernández-Gallardo J, Elie BT, Ramos JW: US Patent 9,315,531 (April/19/2016).
- [59].Fernández-Gallardo J, Elie BT, Sanaú M, Contel M: Versatile Synthesis of Cationic N-Heterocyclic Carbene–gold(I) Complexes Containing a Second Ancillary Ligand. Design of Heterobimetallic Ruthenium–gold Anticancer Agents. Chem Commun 2016, 52:3155–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Elie BT, Pechenyy Y, Uddin F, Contel M: A heterometallic ruthenium-gold complex displays antiproliferative, antimigratory and antiangiogenic properties and inhibits metastasis and angiogenesis-associated proteases in renal cancer. JBIC 2018, 23:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]


