Abstract
Background and purpose
microRNAs are small noncoding RNAs that play important roles in cancer regulation. In this study, we investigated the expression, functional effects and mechanisms of action of microRNA-29a (miR-29a) in glioblastoma (GBM).
Methods
miR-29a expression levels in GBM cells, stem cells (GSCs) and human tumors as well as normal astrocytes and normal brain were measured by quantitative PCR. miR-29a targets were uncovered by target prediction algorithms, and verified by immunoblotting and 3′ UTR reporter assays. The effects of miR-29a on cell proliferation, death, migration and invasion were assessed with cell counting, Annexin V-PE/7AAD flow cytometry, scratch assay and transwell assay, respectively. Orthotopic xenografts were used to determine the effects of miR-29a on tumor growth.
Results
Mir-29a was downregulated in human GBM specimens, GSCs and GBM cell lines. Exogenous expression of miR-29a inhibited GSC and GBM cell growth and induced apoptosis. miR-29a also inhibited GBM cell migration and invasion. PDGFC and PDGFA were uncovered and validated as direct targets of miR-29a in GBM. miR-29a downregulated PDGFC and PDGFA expressions at the transcriptional and translational levels. PDGFC and PDGFA expressions in GBM tumors, GSCs, and GBM established cell lines were higher than in normal brain and human astrocytes. Mir-29a expression inhibited orthotopic GBM xenograft growth.
Conclusions
miR-29a is a tumor suppressor miRNA in GBM, where it inhibits cancer stem cells and tumor growth by regulating the PDGF pathway.
Keywords: microRNA (miRNA), Glioblastoma (GBM), Glioblastoma stem cells (GSC), Platelet-derived growth factor (PDGF)
Introduction
microRNAs (miRNAs) are small noncoding RNAs that have strong regulatory roles in physiology and disease [1-3]. They modulate gene and protein expression by directly binding to the 3′ untranslated region (3′ UTR) of target mRNA and promoting RNA degradation and/or inhibiting mRNA translation. miRNAs are deregulated in a wide array of human cancers, where they can act as oncogenes or tumor suppressors by inhibiting tumor suppressor or oncogene mRNA expression, respectively [3-11].
Glioblastoma (GBM) is the most common and most deadly primary malignant brain tumor [12, 13]. Numerous studies and The Cancer Genome Atlas (TCGA) data have shown that aberrant expression or activation of receptor tyrosine kinases (RTKs) occurs in a majority of GBM and correlate with poor prognosis [14]. One of the main deregulated RTKs in GBM is platelet-derived growth factor receptor (PDGFR). The receptor exists in two forms: PDGFRA and PDGFRB, which are encoded by different genes [15]. They are activated by secreted PDGF ligands, which consist of five functional subunits that are disulfide-linked homo-or heterodimers of A-, B-, C-, and D-polypeptide chains (PDGFAA, PDGFBB, PDGFAB, PDGFCC and PDGFDD) [16, 17]. PDGFRA is activated by PDGFAA, PDGFBB, PDGFAB and PDGFCC, whereas PDGFRB is only activated by PDGFBB and PDGFDD [17-20]. Overexpression of PDGFs can activate PDGFRs and induce their oncogenic effects [21-25].
The failure of current treatments for GBM is arguably partly due to the presence in the tumors of GBM stem cells (GSCs) that are resistant to radio- and chemo-therapy and that are capable of maintaining and propagating the tumors [26-28]. PDGFs, PDGFRs and miRNAs have been implicated in the regulation of GSC functions [6, 29-34]. Mir-29a has been implicated in the regulation of GSC stemness by regulating CD133 and SOX4 and GBM cell growth and invasion via targeting TRAF4, CDC42, SCAP/SREBP, QKI-6, DNMT3A and 3B, MCL1 or HIC5 [35-42]. In this study, we show that miR-29a is downregulated in GBM cells, GSCs and tumors, leading to increased GBM and GSC cell proliferation, migration and invasion and in vivo orthotopic xenograft growth. We demonstrate, for the first time, that miR-29a downregulates PDGFC and PDGFA by directly binding to their 3′ untranslated region (3′ UTR). We therefore show that miR-29a is a tumor suppressor miRNA in GBM that acts via regulation of cancer stem cells and PDGF expression.
Materials and methods
Cells and tumor specimens
GBM cells lines U87, U373, A172, T98G and 293TN cells were from ATCC. GBM cell line U1242 is a kind gift of Dr. Isa Hussaini (University of Virginia). GSCs 11, 28, 240, 267, 295, 627 and 20 were isolated from surgical specimens of patient operated at MD Anderson Cancer Center and characterized for in vivo tumorigenesis, pluripotency, self-renewal, stem-cell markers, and neurosphere formation. CD133-positive GSCs (B4-GSC, NCH441-GSC, and NCH644-GSC) were isolated from GBM surgical specimens and CD133-positive neural stem cells (NSCs) were isolated from brain subventricular zone specimens at University Clinics Munich, Germany. GBM surgical specimens were obtained from the University of Virginia Brain Tumor Bank according to procedures that were reviewed and approved by the Review Board of the University of Virginia.
Reagents
Oligofectamine transfection reagent and Lipofectamine RNAimax were from Invitrogen (Grand Island, NY, USA). The Dual Luciferase kit was from Promega (Fitchburg, WI). The Xtream HD transfection reagent was from Roche (Pleasanton, CA). MiR-29a, anti-miR-29a and scrambled controls were from Fisher Scientific (Hampton, NH). The miScriptRNA Extraction kit, miScript Reverse Transcriptase kit, and miR-29a Primer Assay were from Qiagen (Chatsworth, CA). All primers for quantitative PCR were from Integrated DNA Technology (San Jose, CA). Annexin V-PE, and 7 AAD were from BD Pharmingen (San Diego, CA).
Vectors
The 3′ UTR reporter plasmids with firefly/Renilla Dual-Luciferase reporter vector are from Genecopoeia (Rockville, MD). Site-directed mutagenesis of predicted miR-29a target sites was performed to generate mutant-control vectors using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Lenti-vectors containing miR- 29a or scrambled control sequences and package vectors are from System Biosciences (Palo Alto, CA).
Quantitative RT-PCR
Total RNA was extracted from GBM cells, GSCs, and tissues using the miScript RNA extraction kit (Qiagen, Valencia, CA), according to the instructions of the manufacturer. Each RNA sample was reverse transcribed. cDNA was synthesized using the miScript II RT kit (Qiagen, Valencia, CA) and 800 ng RNA. Quantitative PCR analysis was performed using miScript SYBR Green PCR kit (Qiagen, Valencia, CA). For miR-29a precursor expression analysis, miR-29a primer assay from Qiagen (Valencia, CA) was used. A human U6B primer assay from Qiagen (Valencia, CA) was used as an endogenous control. For mRNA measurements, total RNA was extracted with RNeasy Mini Kit (Qiagen, Valencia, CA) and cDNA was synthesized using random hexamers with iScript cDNA Synthesis kit (BioRad, Hercules, CA). For human PDGFC and PDGFA expression analysis, PDGFC primers (forward, 5′-GCCTCTTCGGGCTTCTCC-3′; reverse, 5′-TGAGGATCTTGTACTCCGTTCTGTT-3′), PDGFA primers (forward, 5′-CACACCTCCTCGCTGTAGTATTTA-3′; reverse, 5′-GTTATCGGTGTAAATGTCATCCAA-3′) were used and 18S rRNA primers (forward, 5′-AACTTTCGATGGTAGTCGCCG-3′; reverse, 5′-CCTTGGATGTGGTAGCCGTTT-3′) were used as endogenous control. All primers are from Thermo Fisher (Tewksbury, MA). Quantitative real-time PCR analysis was performed using the CFX Connect Real-time PCR System (BioRad, Hercules, CA).
Cell transfections
Cells were cultured to 30–80% confluence and transiently transfected with 20 nM pre-miRNA or anti-miRNA using Oligofectamine or Lipofectamine RNAimax according to the manufacturer’s instructions. Lenti-vector transfections were performed with Xtrem HD reagent (Roche, San Francisco, CA) according to the instructions of the manufacturer.
3′UTR reporter assays
To determine whether miR-29a directly binds to the PDGFC and PDGFA 3′UTR, cells were transfected with pre-miR-29a or pre-miR-con for 24 h. The cells were then transfected with 3′ UTR control, 3′ UTR-PDGFC, mutant 3′ UTR-PDGFC, 3′UTR-PDGFA, or mutant 3′ UTR-PDGFA for 24 h. Luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) and luminescence was measured. Firefly luciferase activity was double normalized by dividing each well first by Renilla activity and then by average luciferase/Renilla value in a parallel set done with a constitutive luciferase plasmid.
Immunoblotting and immunocytochemistry
Immunoblotting and immunocytochemistry were performed as previously described [43] using antibodies specific for PDGFC (Abcam, Cambridge, UK), PDGFA (Santa Cruz Biotechnologies, Dallas, Texas), Ki67, CD31 and cleaved Caspase 3 (Cell Signaling, Danvers, MA). All blots were stripped and re-probed with GAPDH or β-actin (Santa Cruz Biotechnologies, Dallas, Texas) as a loading control.
Cell proliferation assays
Cells (20,000 cells/well) were transfected with pre-miR-29a, or anti-miR-29a or pre-miR-con as described above. After 72 h, the cells were collected every other day for 7 days, counted with a hemocytometer, and growth curves were established.
Annexin V-PE and 7AAD flow cytometry
Cell death and apoptosis were assessed by Annexin V-PE and 7AAD flow cytometry as previously described [43]. Briefly, cells were transfected with pre-miR-29a or pre-miR-con for 96 h. The cells were harvested and stained with Annexin V-PE and 7AAD according to the instructions of the manufacturer. Cell samples were analyzed on a FACscan and apoptotic and dead cell fractions were determined.
Migration and invasion assays
The effects of miR-29a expression on cell migration and invasion were assessed using the wound healing and trans-well assays as previously described [44]. Briefly, cells were transfected with pre-miR-29a or pre-miR-con for 72 h. The same number of transfected cells (1 × 105) were resuspended in 300 μl low serum medium (0.1% FBS) and placed in the upper chamber of the wells (Corning Life Sciences, Lowell, MA) that are pre-coated with Collagen IV matrix and 600 μl of 10% FBS medium were placed in the lower chamber. After incubation for 6 h at 37 °C in 5% CO2, the cells on the upper membrane surface were mechanically removed. Cells that had migrated to the lower side of the collagen IV-coated membrane were fixed and stained for 5 min with 0.1% crystal violet. Photographs were taken and migrated cells were counted under a microscope in five randomly chosen fields. Migration was assessed by wound healing assay: The same number of transfected cells (4 × 105) were resuspended in 2 ml of low serum medium (1% FBS) and seeded in 6 well plate and put it into incubator for overnight allowing cells to adhere and spread on the plate. Next day, the cell monolayer was scraped to create a “wound”. After 8–24 h, pictures were under a microscope and migration was quantified by measuring the width of the wound.
Animal experiments
The effects of miR-29a on in vivo tumor growth were tested in an intracranial xenograft model. GSCs (267) or GBM (U87) cells were infected with lentiviral vectors encoding miR-29a or miR-con. The cells (2 × 105) were stereotactically implanted into the striata of immunodeficient mice (n = 6). Two to four weeks after tumor implantation, the animals were subjected to MRI scan and tumor volumes were quantified according to established and validated protocols [6].
Statistics
All experiments were repeated at least three times. When appropriate, two group comparisons were analyzed with a t-test, and P-values were calculated.
Results
miR-29a is downregulated in GBM tumors, GSCs and GBM cell lines
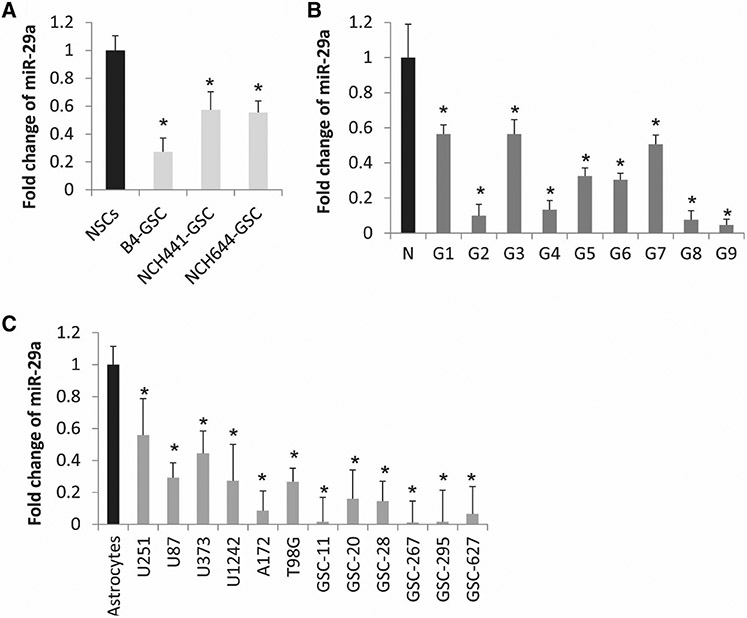
We analyzed global miRNA expression in GBM stem cells that were FACS-sorted for CD133 (B4, NCH441 and NCH644) and compared them to miRNA expressions in normal human neural stem cells (NSCs) [45]. We found that miR-29a was strongly decreased in all three CD133 positive GSC samples compared to NSCs (Fig. 1a). We speculated that miR-29a might be generally downregulated in GBM cells and tumors. To determine whether that is true, we measured miR-29a levels in GBM cell lines, GSCs, and human GBM specimens, as well as in normal human astrocytes (NHAs), and normal brain using quantitative RT-PCR. We found that miR-29a was significantly lower in GBM cell lines (n = 6) and GSCs (n = 6) than in NHAs (P < 0.05) (Fig. 1b) and in GBM tumors (n = 9) as compared to normal brain (P < 0.05) (Fig. 1c). These results show that miR-29a is downregulated in GBM cells and tumors and GSC and suggest that miR-29a might be an important regulator of GBM malignancy.
Fig. 1.
miR-29a expression is downregulated in GSCs, GBM cells, and human tumors. a Quantitative RT-PCR of miR-29a in GSCs and GBM cells showing lower expression levels in GSCs isolated from GBM tumors by CD133+ as compared to normal neural stem cells (NSCs) (CD133+ ) isolated from normal brain. b Quantitative RT-PCR of miR-29a in GBM surgical specimens (G) showing lower levels of miR-29a than in normal brain (N). c Quantitative RT-PCR of miR-29a in GBM cell lines (U251, U87, U373, U1242 and T98G) and GSCs (GSC-11, GSC-20, GSC-28, GSC-267, GSC-295 and GSC-627) showing lower levels of expression as compared to normal human astrocytes. *P < 0.05
miR-29a inhibits GBM cell proliferation and induces apoptosis
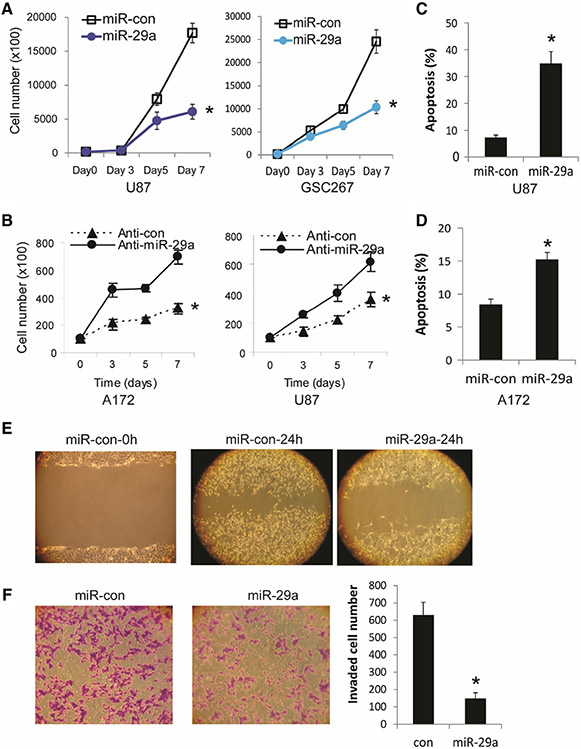
We then assessed the effects of exogenous expression of miR-29a or control on cell growth and survival in GBM cells and GSCs. Pre-miR-29a, anti-miR-29a or control were transfected into GBM cells and GSCs for 72 h. For cell proliferation assay, cells were collected and counted every other day for 5 days. Overexpression of miR-29a significantly inhibited the proliferation of GBM (U87) and GSC cells (GSC267) (Fig. 2a), while inhibition of miR-29a by transfection of anti-miR-29a significantly increased the cell counts in GMB cells (U87 and A172) supporting the miR-29a effects on inhibition of GBM cell proliferation (P < 0.05) (Fig. 2b). To assess the effects of miR-29a on apoptosis, pre-miR-29a or control was transfected into GBM U87 and A172 cells for 96 h and floating and adherent cells were collected and double stained with Annexin V and 7-AAD. The flow cytometry assays with AnnexinV-7AAD revealed that miR-29a transfection significantly induced apoptosis and cell death in GBM cells (P < 0.05) (Fig. 2c, d).
Fig. 2.
miR-29a inhibits GBM cell and GSC proliferation, induces GBM cell apoptosis and inhibits GBM cell migration and invasion. a Proliferation assay showing the inhibition of GBM cell U87 and GSC-267 proliferation by pre-miR-29a. b miR-29a inhibitor (anti-miR-29a) transfection enhances GBM cell A172 and U87 proliferation. c, d AnnexinV-PE and 7-AAD flow-cytometric analysis of GBM cells U87 (c) and A172 (d) showing induction of apoptosis after pre-miR-29a transfection. e GBM cells A172 were transfected with either Pre-miR-29a or controls and assessed for migration with the wound healing assay. f Pre-miR-29a or control-transfected A172 cells were used in a transwell invasion assay. The left panel shows a representative invasion assay, the right panel shows the quantification of invading cells. The data show that miR-29a overexpression inhibits GBM cell migration and invasion. *P < 0.05
miR-29a decreases migration and invasion of GBM cells
Tumor cell invasion is one of the hallmarks of GBM. We therefore assessed the effects of miR-29a on GBM cell migration and invasion. Pre-miR-29a or control were transfected into A172 cells for 72 h followed by a wound healing assay to assess migration. Overexpression of miR-29a inhibited GBM cells migration (Fig. 2e). To assess the effects of miR-29a on GBM cell invasion, pre-miR-29a or control was transfected into A172 cells followed by a transwell invasion assay. After incubation for 6 h, the migrated cells to the lower side of the collagen IV-coated membrane were fixed, stained with 0.1% crystal violet. Photographs were taken and invading cells in 5 representative areas were counted under a microscope and the average invading cell number was quantified. Transfection of miR-29a inhibited GBM cell invasion through collagen IV-coated transwells (Fig. 2f). These data show that miR-29a might suppress tumor growth by inhibiting tumor cell migration and invasion.
miR-29a directly targets and regulates PDGFC and PDGFA in GBM
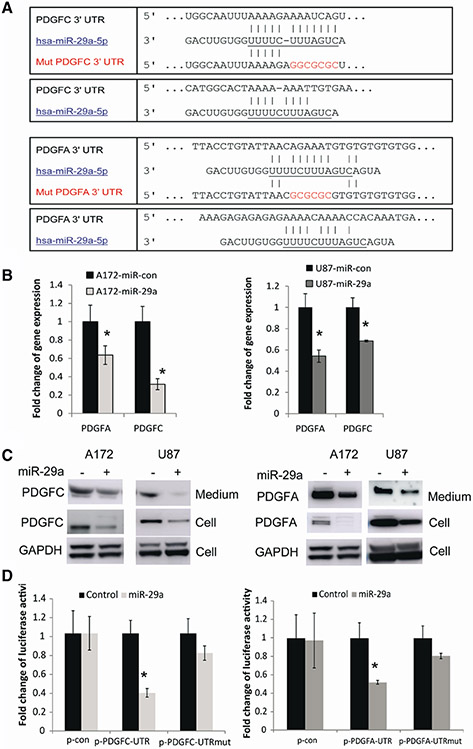
To identify targets of miR-29a in GBM, we used a bioinformatic database (Targetscan) and our unpublished PAR-CLIP data and identified PDGFC and PDGFA as potential targets of miR-29a (Fig. 3a). To verify whether miR-29a downregulates PDGFC and PDGFA, we tested the effects of miR-29a overexpression on transcription and protein synthesis of PDGFC and PDGFA, as microRNAs regulate gene expression by degrading mRNA or/and inhibiting protein translation. We transfected GBM cells (U87 and A172) with pre-miR-29a or control and measured the effects on PDGFC and PDGFA mRNA with quantitative RT-PCR. We found that overexpression of miR-29a significantly reduced PDGFC mRNA level (31–68%) and PDGFA mRNA levels (36–45%) (Fig. 3b). We then determined their expression level in cells and growth medium, respectively, after transfection with miR-29a, since PDGFC and PDGFA are both secreted proteins. Overexpression of miR-29a significantly decreased protein level in GBM cells and secreted proteins in the medium compared with control (Fig. 3c). These data suggest that miR-29a reduces the levels of cellular and secreted PDGFC and PDGFA through both mRNA degradation and inhibition of protein synthesis.
Fig. 3.
miR-29a regulates PDGFC and PDGFA expressions by directly binding to their mRNA 3′ UTRs. a Alignment of PDGFC, PDGFA 3′ UTRs and miR-29a sequences showing the predicted binding sites. The sites of targeted mutagenesis for the generation of mutant controls are shown in red. b Quantitative RT-PCR showing the inhibitory effects of miR-29a on the mRNA levels of PDGFC and PDGFA in GBM cells A172 and U87. c Immunoblots (left panel) showing the effects of miR-29a overexpression on the protein levels of secreted and intracellular PDGFC in GBM cells. The right panel shows that miR-29a downregulates the protein levels of PDGFA in growth medium and cell lystaes. d 3′ UTR luciferase assays for PDGFC (left panel) and PDGFA (right panel) showing the inhibition of luciferase activity by miR-29a in GBM cells. No significant alteration in luciferase activity was observed when 3′UTR binding sites were mutated. *P < 0.05
To determine if miR-29a can directly bind the 3′ UTRs of PDGFC and PDGFA to inhibit their expression, PDGFC and PDGFA 3′ UTR reporters, mutant or control vectors were generated and co-transfected with pre-miR-29a or pre-miR-control into GBM cells and luciferase assays were performed. Expression of miR-29a (confirmed by q-PCR; data not shown) significantly decreased PDGFC luciferase activity by approximately 60% in experimental but not in mutant or control-transfected cells (Fig. 3d). miR-29a also inhibited normalized activity of the PDGFA 3′ UTR reporter by ~ 50% in A172 cells (n = 3; P < 0.05 for all). Altogether, the data show that miR-29a inhibits PDGFC and PDGFA mRNA and protein expressions by directly binding to their 3′ UTRs.
PDGFA and PDGFC are upregulated in GBM cells, GSCs and GBM tumors
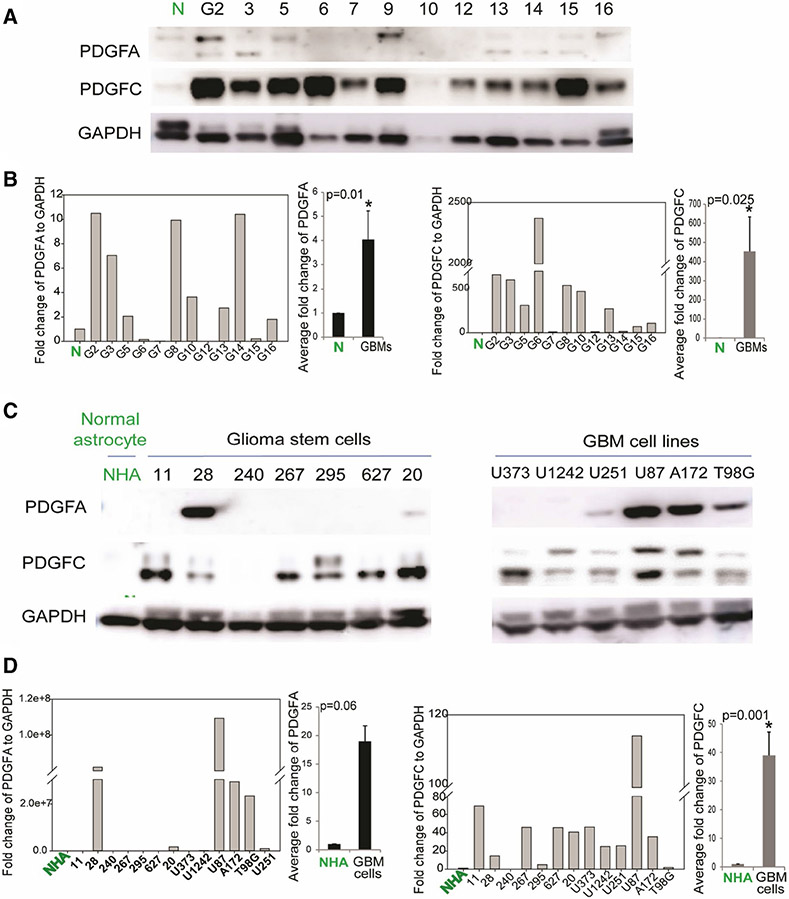
Receptor tyrosine kinases are frequently activated in GBM. Growth factors that function as RTK ligands are able to activate RTKs and downstream signaling pathways. Since miR-29a is downregulated in GBM and it inhibits PDGFC and PDGFA expression, we speculated that PDGFC and/or PDGFA are upregulated in GBM tumors and cells. To determine whether that is true, we measured PDGFC and PDGFA protein levels in GBM cells, GSCs, and human tumor specimens as well as in normal human astrocytes and normal brain using immunoblotting. We found that PDGFC was significantly higher in all GBM tumors as compared with normal brain (Fig. 4a) and significantly higher in most GSCs and GBM cell lines as compared to normal human astrocytes (NHA) (Fig. 4c). PDGFC signal was quantified using ImageJ software (Fig. 4b (left panel) and Fig. 4d (left panel)), and average signal was calculated (P > 0.05). We also found that PDGFA was relatively high in a subset of GBM tumors (Fig. 4a) and highly expressed in most of the established GBM cell lines, and two GSCs (Fig. 4b). PDGFA signal was quantified and average signal was calculated (Fig. 4b (right panel, P > 0.05) and Fig. 4d (right panel, P = 0.06).
Fig. 4.
PDGFC and PDGFA are upregulated in GBM cells, GSCs and GBM tumors. a Immunoblots showing higher levels of PDGFA and PDGFC protein in a subset of GBM specimens (G) as compared to normal brain (N). b Quantification of PDGFA (left panel) and PDGFC level (middle panel) in GBM specimens as compared to normal brain (P = 0.01 and P = 0.025, respectively). c Immunoblots showing PDGFA and PDGFC levels in GSCs (left panel) and GBM cell lines (right panel) and normal human astrocytes (NHA). d Quantification of PDGFA (left panel) and PDGFC levels (right panel) in GBM cell lines, GSCs as compared to NHA (P = 0.06 and P < 0.001, respectively)
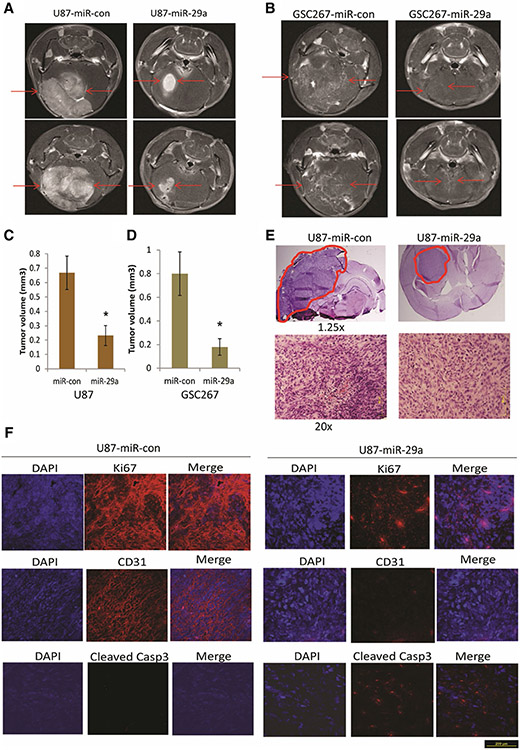
miR-29a inhibits in vivo GBM cell and GSC-derived xenograft growth
To determine whether miR-29a suppresses in vivo tumor growth, the GBM cell line U87 and GSC-267 were infected with lentiviruses encoding pre-miR-29a or scrambled control for 24 h and then implanted into the brains of immunodeficient mice (n = 6). The tumors were visualized by MRI 3 weeks post tumor implantation and tumor volumes were quantified. Both GBM and GSC-derived xenografts that overexpressed miR-29a were significantly smaller than control xenografts (Fig. 5a, b). The quantified tumor volumes are shown in Fig. 5c and d for U87 and GSC-267 xenografts, respectively (P < 0.05). H&E staining (Fig. 5e) also showed reduced tumor growth after overexpression of miR-29a. Moreover, miR-29a decreased the levels of proliferation marker Ki67 and endothelial cell marker CD31 level and increased the levels of apoptotic marker cleaved Caspase 3 in GBM xenografts (Fig. 5f). These data indicate that miR-29a exerts tumor suppressive effects in GBM.
Fig. 5.
miR-29a inhibits GBM cell and GSC derived xenograft growth. a, b GBM cells U87 (a) and GSC-267 cells (b) were infected with lentivirus encoding pre-miR-29a or pre-miR-control for 24 h and implanted into the brains of immunodeficient mice (n = 6). After 3 weeks, the mice were subjected to MRI scan and tumor volumes were calculated (c, d). e H&E staining of U87 xenografts generated from Lenti-control or pre-miR-29a U87 cells (red lines indicate the tumors). f Immunohistochemistry staining of U87 xenograft sections with antibodies against Ki67, CD31 and Cleaved Caspase 3. The data show that miR-29a inhibits in vivo GBM xenograft growth, reduces Ki67 and CD31 levels and enhances Cleaved Caspase 3 levels. *P < 0.05
Discussion
Our study demonstrates that miR-29a is a tumor suppressive miRNA in GBM. We found that miR-29a is downregulated in GBM cells, GSCs and human tumors. We show that miR-29a inhibits malignancy parameters in GBM cells and stem cells, including cell proliferation, apoptosis, migration and invasion. Importantly our data demonstrate inhibitory effects of miR-29a on vivo tumor growth in both GBM cell line and human derived GSC-based orthotopic GBM models. We uncover the PDGF pathway as direct target for miR-29a in GBM by showing for the first time, that miR-29a regulates PDGFC and PDGFA. miR-29a has been investigated in brain tumors and some other cancers [35-42]. However, no previous study has described its targeting of PDGF and no previous study has shown its effects on orthotropic and GSC-derived GBM tumors.
GBM cell invasive growth contributes to tumor aggressiveness and therapeutic failure. Clinically GBMs show highly infiltrative growth patterns that disperse tumor cells throughout the brain [46]. The invasive tumor cells contribute to incomplete surgical removal and inevitable tumor recurrence primarily along the resection margin. Our data showed that miR-29a inhibits glioma cell migration and invasion. Because GBM stem cells (GSCs) are not adherent cells that grow as spheres in the growth medium, invasion assays using GSCs could not be performed. GBM is also a fast growing tumor that resists cell death and apoptosis. Our data show that miR-29a downregulation in GBM contributes to increased cell proliferation and resistance to apoptosis. Increased GBM cell invasion, proliferation and survival together lead to increased tumor growth as evidenced by our in vivo findings. Future studies will examine the effects of miR-29a on animal survival.
RTKs are frequently over activated in GBM. Our findings indicate that downregulation of miR-29a is partly responsible for PDGFR activation in GBM. According to TCGA data, 88% of GBM harbor one or more functional mutations in RTK pathways. Transcriptional and autocrine activation of RTKs are even more frequent. Activation of PDGFR in GBM enhances tumorigenicity, tumor growth, and tumor-associated angiogenesis [47]. PDGFs are critical regulators of gliomagenesis and neurogenesis and regulate tumor cell proliferation, migration, and angiogenesis [47, 48]. PDGFA and PDGFC are frequently upregulated in GBM [25, 49] where they induce autocrine signaling. PDGFC and PDGFA activate the PDGFR pathway, a major cell survival pathway in cancers and GBM. Our study suggests that miR-29a downregulation is an important mechanism of PDGFC and PDGFA overexpression.
GSCs have been implicated in GBM recurrence and resistance to therapy [26, 27]. We show that miR-29a regulates PDGFC and PDGFA and GSC proliferation and inhibits GSC-derived tumor formation in an orthotopic xenograft mouse model. miR-29a has been implicated in the regulation of cancer stemness by targeting targets other than PDGF, including CD133 and SOX4, TRAF4, CDC42, SCAP, QKI-6, DNMT3, MCL1 or HIC5 [35-42]. Therefore, miR-29a is a regulator and cancer stem cells in GBM.
Altogether, our study demonstrates that miR-29a is a tumor suppressor that regulates GBM cell and stem cell pathobiology by controlling PDGFC and PDGFA.
Acknowledgements
This study was supported by NIH grant UO1 CA CA220841 (Roger Abounader).
References
- 1.Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9(10):1274–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YS, Dutta A (2009) MicroRNAs in cancer. Annu Rev Pathol 4:199–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldas C, Brenton JD (2005) Sizing up miRNAs as cancer genes. Nat Med 11(7):712–714 [DOI] [PubMed] [Google Scholar]
- 5.Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11):857–866 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Kim J, Mueller AC, Dey B, Yang Y, Lee DH, Hachmann J, Finderle S, Park DM, Christensen J, Schiff D, Purow B, Dutta A, Abounader R (2014) Multiple receptor tyrosine kinases converge on microRNA-134 to control KRAS, STAT5B, and glioblastoma. Cell Death Differ 21:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Dutta A, Abounader R (2012) The role of microRNAs in glioma initiation and progression. Front Biosci 17:700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez MA, Nicoloso MS, Shimizu M, Rossi S, Gopisetty G, Molina JR, Carlotti C, Tirapelli D, Neder L, Brassesco MS, Scrideli CA, Tone LG, Georgescu MM, Zhang W, Puduvalli V, Calin GA (2010) miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes Chromosom Cancer 49(11):981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabriely G, Yi M, Narayan RS, Niers JM, Wurdinger T, Imitola J, Ligon KL, Kesari S, Esau C, Stephens RM, Tannous BA, Krichevsky AM (2011) Human glioma growth is controlled by microRNA-10b. Cancer Res 71(10):3563–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guessous F, Alvarado-Velez M, Marcinkiewicz L, Zhang Y, Kim J, Heister S, Kefas B, Godlewski J, Schiff D, Purow B, Abounader R (2013) Oncogenic effects of miR-10b in glioblastoma stem cells. J Neurooncol 112:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE (2010) MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell 37(5):620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CBTRUS (1998) Central brain tumor registry of the United States. CBTRUS, Chicago [Google Scholar]
- 13.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15(11):1311–1333 [DOI] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455 (7216), 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldin CH, Westermark B (1989) Platelet-derived growth factors: a family of isoforms that bind to two distinct receptors. Br Med Bull 45(2):453–464 [DOI] [PubMed] [Google Scholar]
- 16.Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316 [DOI] [PubMed] [Google Scholar]
- 17.Kazlauskas A (2017) PDGFs and their receptors. Gene 614:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidaran MA, Pierce JH, Yu JC, Lombardi D, Artrip JE, Fleming TP, Thomason A, Aaronson SA (1991) Role of alpha beta receptor heterodimer formation in beta platelet-derived growth factor (PDGF) receptor activation by PDGF-AB. J Biol Chem 266(30):20232–20237 [PubMed] [Google Scholar]
- 19.Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22(10):1276–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen PH, Chen X, He X (2013) Platelet-derived growth factors and their receptors: structural and functional perspectives. Biochim Biophys Acta 1834(10):2176–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nister M, Libermann TA, Betsholtz C, Pettersson M, Claesson-Welsh L, Heldin CH, Schlessinger J, Westermark B (1988) Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res 48(14):3910–3918 [PubMed] [Google Scholar]
- 22.Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M (1992) Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 52(11):3213–3219 [PubMed] [Google Scholar]
- 23.Di Rocco F, Carroll RS, Zhang J, Black PM (1998) Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery 42(2):341–346 [DOI] [PubMed] [Google Scholar]
- 24.Martinho O, Longatto-Filho A, Lambros MB, Martins A, Pinheiro C, Silva A, Pardal F, Amorim J, Mackay A, Milanezi F, Tamber N, Fenwick K, Ashworth A, Reis-Filho JS, Lopes JM, Reis RM (2009) Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br J Cancer 101(6):973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA (2002) Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res 62(13):3729–3735 [PubMed] [Google Scholar]
- 26.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A (2004) Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64(19):7011–7021 [DOI] [PubMed] [Google Scholar]
- 27.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401 [DOI] [PubMed] [Google Scholar]
- 28.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120):756–760 [DOI] [PubMed] [Google Scholar]
- 29.Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH (2006) Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci USA 103(20):7853–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P (2006) Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci 26(25):6781–6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guessous F, Zhang Y, Kofman A, Catania A, Li Y, Schiff D, Purow B, Abounader R (2010) microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle 9(6):1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen TD, Lopes B, Schiff D, Purow B, Abounader R (2009) MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res 69(19):7569–7576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ernst A, Campos B, Meier J, Devens F, Liesenberg F, Wolter M, Reifenberger G, Herold-Mende C, Lichter P, Radlwimmer B (2010) De-repression of CTGF via the miR-17-92 cluster upon differentiation of human glioblastoma spheroid cultures. Oncogene 29(23):3411–3422. [DOI] [PubMed] [Google Scholar]
- 34.Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S (2008) Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res 68(22):9125–9130 [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Huang W, Kim TM, Jung Y, Menon LG, Xing H, Li H, Carroll RS, Park PJ, Yang HW, Johnson MD (2019) MicroRNA-29a activates a multi-component growth and invasion program in glioblastoma. J Exp Clin Cancer Res 38(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Li N, Yan Z, Li C, Zhao Z (2018) MiR-29a-mediated CD133 expression contributes to cisplatin resistance in CD133(+) glioblastoma stem cells. J Mol Neurosci 66(3):369–377 [DOI] [PubMed] [Google Scholar]
- 37.Shi C, Ren L, Sun C, Yu L, Bian X, Zhou X, Wen Y, Hua D, Zhao S, Luo W, Wang R, Rao C, Wang Q, Yu S (2017) miR-29a/b/c function as invasion suppressors for gliomas by targeting CDC42 and predict the prognosis of patients. Br J Cancer 117(7):1036–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Duan N, Duan S (2018) MiR-29a inhibits glioma tumorigenesis through a negative feedback loop of TRAF4/Akt signaling. Biomed Res Int 2018:2461363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ru P, Guo D (2017) microRNA-29 mediates a novel negative feedback loop to regulate SCAP/SREBP-1 and lipid metabolism. RNA Dis 4(1):e1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xi Z, Wang P, Xue Y, Shang C, Liu X, Ma J, Li Z, Li Z, Bao M, Liu Y (2017) Overexpression of miR-29a reduces the oncogenic properties of glioblastoma stem cells by downregulating Quaking gene isoform 6. Oncotarget 8(15):24949–24963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Sun J, Shi C, Sun C, Yu L, Wen Y, Zhao S, Liu J, Xu J, Li H, An T, Zhou X, Ren L, Wang Q, Yu S (2015) miR-29s inhibit the malignant behavior of U87MG glioblastoma cell line by targeting DNMT3A and 3B. Neurosci Lett 590:40–46 [DOI] [PubMed] [Google Scholar]
- 42.Aldaz B, Sagardoy A, Nogueira L, Guruceaga E, Grande L, Huse JT, Aznar MA, Diez-Valle R, Tejada-Solis S, Alonso MM, Fernandez-Luna JL, Martinez-Climent JA, Malumbres R (2013) Involvement of miRNAs in the differentiation of human glioblastoma multiforme stem-like cells. PLoS ONE 8(10):e77098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Guessous F, Kwon S, Kumar M, Ibidapo O, Fuller L, Johnson E, Lal B, Hussaini I, Bao Y, Laterra J, Schiff D, Abounader R (2008) PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res 68(6):1723–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Guessous F, Johnson EB, Eberhart CG, Li XN, Shu Q, Fan S, Lal B, Laterra J, Schiff D, Abounader R (2008) Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab Invest 88(2):98–111 [DOI] [PubMed] [Google Scholar]
- 45.Floyd DH, Zhang Y, Dey BK, Kefas B, Breit H, Marks K, Dutta A, Herold-Mende C, Synowitz M, Glass R, Abounader R, Purow BW (2014) Novel anti-apoptotic microRNAs 582–5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS ONE 9(5):e96239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21(8):1624–1636 [DOI] [PubMed] [Google Scholar]
- 47.Shih AH, Holland EC (2006) Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett 232(2):139–147 [DOI] [PubMed] [Google Scholar]
- 48.Yeh HJ, Silos-Santiago I, Wang YX, George RJ, Snider WD, Deuel TF (1993) Developmental expression of the platelet-derived growth factor alpha-receptor gene in mammalian central nervous system. Proc Natl Acad Sci USA 90(5):1952–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sil S, Periyasamy P, Thangaraj A, Chivero ET, Buch S (2018) PDGF/PDGFR axis in the neural systems. Mol Aspects Med 62:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]