Abstract
Background
Pleural effusion (PE) is reported as a common complication in acute pancreatitis (AP), while the incidence of PE in AP varies widely among studies, and the association between PE and mortality is not clear. This study aimed to comprehensively analyze the pooled incidence of PE in patients with AP and to evaluate the influence of PE on mortality through a meta-analysis.
Method
Six databases (PubMed, Web of Science, EMBASE, Cochrane, Scopus, and OVID) were searched thoroughly for relevant studies. Data were extracted, and Stata SE 16.0 software was applied to compute the pooled incidence of PE and assess the association between PE and mortality, taking the risk ratio (RR) as the effect size.
Results
Thirty-five articles involving 7,675 patients with AP were eventually included in this meta-analysis. The pooled incidence of PE was 34% (95% CI: 28%-39%), with significant heterogeneity among studies (I2=96.7%). Further analysis revealed that the pooled incidence of unilateral and small PE occupied 49% (95% CI: 21%-77%) and 59% (95% CI: 38%-81%) of AP patients complicated by PE, respectively. The subgroup analysis revealed that “region” and “examination method” may contribute to heterogeneity. PE may be associated with increased mortality in AP patients (RR 3.99, 95% CI: 1.73-9.2).
Conclusion
This study suggested that PE is a common complication with high pooled incidence and that PE may be associated with increased mortality in AP patients. More studies should be performed to validate our findings.
Keywords: Acute pancreatitis, incidence, meta-analysis, mortality, pleural effusion
1. Introduction
Acute pancreatitis (AP) is a common inflammatory condition caused by varying etiologies and refers to the abnormal activation of pancreatic enzymes leading to digestion of pancreatic tissue itself as well as peripheral organs [1]. It has added medical and economic burden in countries to a large extent, with a rising incidence of approximately 34 cases per 100000 person-years throughout the world [2–4]. A majority of patients with AP had mild symptoms, which could recover after routine treatment according to the clinical guidelines during hospitalization. In addition, approximately 20% of patients develop severe acute pancreatitis (SAP) complicated by multiple organ failure (involved in the respiratory, cardiovascular and renal systems), which is associated with a mortality of up to 50% [5]. Hence, it is essential for physicians to identify and predict the severity of AP in a timely manner before formulating an adequate treatment strategy.
Currently, various scoring systems, including the Ranson score, Glasgow score and APACHE II, and some biochemical indicators, including C-reactive protein and serum calcium, are predominant assessment approaches to stratify AP, with nonnegligible limitations in the context of clinical practice [6–8]. Studies have reported that pleural effusion (PE) is a common complication of AP, has better accuracy in reflecting the severity of AP than scoring systems and is a reliable indicator for predicting SAP within 24 h of admission [9,10]. AP patients with concomitant PE were more likely to develop pancreatic pseudocysts, hasten pancreatic necrosis and increase mortality in comparison to non-PE AP patients [11]. A large number of studies have reported the incidence of PE in AP patients, but these studies were based on case reports and retrospective/prospective studies, so the results were variable. Owing to the inconsistent incidence of PE in AP patients reported in available studies and the relatively simple treatment of PE, some researchers have not paid enough attention to this complication.
Thus, in this study, we conducted a meta-analysis, systematically searching and reviewing available literature, to comprehensively analyze the pooled incidence of PE in patients with AP and to evaluate the influence of PE on the mortality of AP patients.
2. Methods
2.1. Literature search strategy
This meta-analysis was performed following the guidance of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [12,13].
Databases including PubMed, Web of Science, EMBASE, Cochrane, Scopus, and OVID were comprehensively searched for literature relevant to the incidence of PE in AP patients published from the date the database was established to February 2023. A combination of subject headings and random words was implemented to form most of the search strategy, and supplemental literature was added by manually tracing and searching the references of the relevant studies. The retrieval protocol was slightly adjusted according to the requirements of different databases and mainly determined as follows: ((“Pancreatitis” [Mesh]) OR (“Acute Pancreatitis” [Title/Abstract])) OR (“Pancreatic Parenchymal Edema” [Title/Abstract])) OR (“Acute Edematous Pancreatitis” [Title/Abstract])) AND ((“Pleural Effusion” [Mesh]) OR (“pleural Fluid” [Title/Abstract])).
2.2. Study selection procedure
Two reviewers (TTZ and JA) independently searched the databases above and screened the literature to select eligible studies with the aid of EndNote 20.0 software. A study was included if it met the following criteria (1): participants: patients with a confirmed diagnosis of AP; AP was diagnosed based on symptoms of abdominal pain, laboratory tests and imaging examinations (2); presenting clear diagnostic or assessment criteria for PE in AP patients (mainly based on Chest X-ray or computed tomography) (3); retrospective or prospective observational studies that reported incidence of PE in AP patients or provided sufficient information to calculate estimates of prognostic data; and (4) original studies published in English.
The exclusion criteria were as follows (1): conference abstracts, editors’ letters and comments, case reports, review articles and duplicate studies (2); study subjects were animals or children (3); studies did not provide accurate data or had a small scale (<20); and (4) full texts could not be obtained.
2.3. Data abstraction and assessment of study quality
An Excel extraction form was used to collect the general characteristics of the included studies after carefully reading the included studies. The information included was as follows: first author, publication year, study design, country, sample size, incidence of PE, location and size of PE, examination method of PE and mortality.
In addition, the quality of all included studies was assessed utilizing the Newcastle–Ottawa Scale (NOS) recommended by the Cochrane Collaboration [14]. The NOS has eight score items and classifies studies into three quality levels—low (score 0-3), moderate (score 4-6) and high (score 7-9).
2.4. Data synthesis and statistical analysis
Stata SE 16.0 software was applied to perform statistical analysis. The incidence rate of PE in AP was computed on the basis of the number of patients with concurrent PE and the total number of AP patients and reported as a pooled estimate with 95% confidence intervals (CIs). Meanwhile, the prognostic effect of PE on the outcome of AP patients was also evaluated in this meta-analysis. The pooled estimates of the dichotomous variables were calculated using the risk ratio (RR) with 95% CI. Variations existed among studies, probably resulting in significant heterogeneity. A random-effects model was carried out to work with data when the inconsistency index (I2) was >50% or p < 0.05 (Cochran’s Q test) [15]. Then, subgroup analysis was conducted to explore the potential heterogeneity in different groups (study design, study scale, region and examination method), and the stability of the results was detected by sensitivity analysis (leave-one-out analysis). Finally, funnel plot was drawn to detect whether publication bias existed, and Begg’s and Egger’s tests were further used to analyze publication bias. p < 0.05 was considered statistically significant.
If any divergence emerged in the above process, a thorough negotiation between the two reviewers (TTZ and JA) was conducted, and a third reviewer (XH) was involved to reach an agreement.
3. Results
3.1. Literature selection results
According to the search strategy, 4,129 records from different databases were retrieved, in addition to seven records from the references of the relevant studies. After filtering duplicates, 2,193 records were retained for further selection. Then, by screening the titles and abstracts, we removed 2,002 records and retained 91 records to read details of the literature. The complete selection process is displayed in Figure 1. Ultimately, 35 articles that met the inclusion criteria were included in our study [16–50].
Figure 1.
The flow diagram of literature selection procedure.
The included studies covered a total of 7,675 AP patients, among which the number of patients with concurrent PE was 2,532. It is noteworthy that one article reported two different studies, which drove us to analyze the two studies separately [34]. There were 17 prospective [17,20,22–26,28,30,31,34,35,37,38,41,47,50] and 19 retrospective [16,18,19,21,27,29,32–34,36,39,40,42–46,48,49] studies conducted from 1983 to 2023. The sample sizes ranged from 25 to 909. Twenty-two studies [21,23,26–28,30,31,33,34,36–44,46–49] were from Asia, ten studies [16,17,20,22,25,29,32,35,45,50] were from Europe, and the rest [18,19,24,36] were from other regions. Among these studies, eleven studies [16–20,24,26,30,38,42,45] reported the location of PE, three studies [19,39,45] mentioned the size of PE, and five studies [20,23,32,35,42] provided data on mortality in AP patients with or without PE. Additionally, PE was detected by chest X-ray in eight studies [16,18,20,22–24,41,50], by CT in 14 studies [19,21,25–27,30,32,35,38,39,42,45–47], by transthoracic sonography in one study [17] and by combination of above methods in four studies [29,31,48,49]. Other studies did not clearly report the detecting method [28,33,34,36,37,40,43,44]. The general characteristics of the eligible studies are shown in Table 1. Additionally, the calculating scoring status of some studies are list in Supplement Table 1. Of the included studies, nine studies [16,18,24,31,32,40,45,48,49] classified the severity of AP and reported the incidence of PE in different severities of AP. The data were extracted and are displayed in Supplement Table 2.
Table 1.
The general information of included studies.
| Study/Year | Study Design | Country | AP, n | AP with PE, n | Total death / death with PE, n | Location of PE (Left/Right/Bilateral) | Size of PE (Small/Moderate/Large) | Assessment of PE |
|---|---|---|---|---|---|---|---|---|
| Millward 1983 [16] | Retrospective | U.K | 52 | 12 | NA | 8/4/0 | NA | Chest X-ray |
| Maringhini 1996 [17] | Prospective | Italy | 100 | 20 | NA | 12/4/4 | NA | ultrasound |
| Heller 1997 [18] | Retrospective | U.S.A. | 135 | 26 | NA | 7/1/18 | NA | Chest X-ray |
| Simmons 1997 [19] | Retrospective | U.S.A. | 50 | 10 | NA | 2/3/5 | 5/4/1 | CT |
| Tsushima 1999 [21] | Retrospective | Japan | 25 | 6 | NA | NA | NA | CT |
| Polyzogopoulou 2004 [22] | Prospective | Greece | 166 | 29 | NA | NA | NA | Chest X-ray |
| Maher 2010 [24] | Prospective | Egypt | 149 | 32 | NA | 8/2/22 | NA | Chest X-ray |
| Vasseur 2014 [25] | Prospective | France | 62 | 29 | NA | NA | NA | CT |
| Banday 2015 [26] | Prospective | India | 50 | 28 | NA | 16/0/12 | NA | CT |
| Qiu 2015 [27] | Retrospective | China | 909 | 206 | NA | NA | NA | CT |
| Vengadakrishnan 2015 [28] | Prospective | India | 110 | 15 | NA | NA | NA | NA |
| Dombernowsky 2016 [29] | Retrospective | Denmark | 359 | 59 | NA | NA | NA | Chest X-ray + CT |
| Raghuwanshi 2016 [30] | Prospective | India | 50 | 23 | NA | 10/0/13 | NA | CT |
| Rarthnakar 2017 [31] | Prospective | India | 82 | 27 | NA | NA | NA | Chest X-ray + ultrasound |
| Zhou 2018 [33] | Retrospective | China | 609 | 276 | NA | NA | NA | NA |
| Hong 2019 [34] | Retrospective | China | 700 | 135 | NA | NA | NA | NA |
| Hong 2019 [34] | Prospective | China | 194 | 83 | NA | NA | NA | NA |
| Milian 2020 [36] | Retrospective | Peru | 162 | 69 | NA | NA | NA | NA |
| Karim 2020 [37] | Prospective | India | 62 | 18 | NA | NA | NA | NA |
| Peng 2020 [38] | Prospective | China | 309 | 123 | NA | 37/6/80 | NA | CT |
| Gupta 2021 [39] | Retrospective | India | 103 | 47 | NA | NA | 36/11/0 | CT |
| He 2021 [40] | Retrospective | China | 198 | 46 | NA | NA | NA | NA |
| Shetty 2021 [41] | Prospective | India | 98 | 11 | NA | NA | NA | Chest X-ray |
| Zeng 2021 [43] | Retrospective | China | 222 | 25 | NA | NA | NA | NA |
| Huang 2022 [44] | Retrospective | China | 236 | 102 | NA | NA | NA | NA |
| Luiken 2022 [45] | Retrospective | Germany | 358 | 195 | NA | 36/9/150 | 94/59/42 | CT |
| Patidar 2022 [46] | Retrospective | India | 42 | 19 | NA | NA | NA | CT |
| Rajendran 2022 [47] | Prospective | India | 75 | 26 | NA | NA | NA | CT |
| Yao 2022 [48] | Retrospective | China | 309 | 214 | NA | NA | NA | Chest X-ray, CT, and ultrasound |
| Zhou 2022 [49] | Retrospective | China | 222 | 65 | NA | NA | NA | Chest X-ray, CT, and ultrasound |
| Selimovic 2023 [50] | Prospective | Bosnia and Herzegovina | 97 | 24 | NA | NA | NA | Chest X-ray |
| Talamini 1999 [20] | Prospective | Italy | 539 | 77 | 20/14 | 25/18/34 | NA | Chest X-ray |
| Raghu 2007 [23] | Prospective | India | 60 | 31 | 27/19 | NA | NA | Chest X-ray |
| Avanesov 2018 [32] | Retrospective | Germany | 167 | 106 | 25/21 | NA | NA | CT |
| Alberti 2020 [35] | Prospective | Spain | 149 | 76 | 13/12 | NA | NA | CT |
AP: Acute pancreatitis; PE: Pleural effusion; CT: Computed tomography.
3.2. Assessments of included studies
The quality of included studies was judged using the Newcastle–Ottawa Scale (NOS) (Supplement Table 3). Twenty-seven studies had a moderate level of quality, and nine studies had a high level of quality, which indicated that the overall risk of bias was moderate in 27 studies and low in nine studies.
3.3. Incidence of PE in AP
As shown in the forest plot (Figure 2A), we found significant heterogeneity (I2=96.7%) among studies, and the pooled incidence of PE in patients with AP was 34% (95% CI: 28%-39%), ranging from 11% (95% CI: 5%-17%) to 69% (95% CI: 64%-74%). Eleven and three studies reported the location and size of PE, respectively. The pooled incidence of unilateral and small PE was 49% (95% CI: 21%-77%) and 59% (95% CI: 38%-81%), respectively (Figure 2B, 2C). Nine studies reported the incidence of PE in severe acute pancreatitis (SAP), and the pooled estimate was 67% (95% CI: 53%-82%, I2=94.4%), as shown in Figure 2D. Of the nine studies, six studies also reported the incidence of PE in mild acute pancreatitis (MAP) [16,18,24,31,40,45], and the pooled estimate was 14% (95% CI: 7%-21%, I2=87.8%) (Figure 2E). We did not analyze the pooled incidence of PE in moderately severe acute pancreatitis (MSAP) due to the limited number of related studies.
Figure 2. .
Forest plot of incidence of PE in AP patients. (A) The incidence of PE (B) Incidence of unilateral PE. (C) Incidence of small size PE. (D) Incidence of PE in SAP patients. (E) Incidence of PE in MAP patients.
3.4. Subgroup analysis
The results are displayed in Table 2, which includes all studies and four subgroups. We found that “region” (e.g. Asia, Europe, America and Africa) (p = 0.033) and “examination method” (e.g. CT, chest X-ray and transthoracic sonography) (p = 0.0001) may play a role in the significant heterogeneity among studies, while study design and sample size did not affect the heterogeneity. Additionally, the subgroup analysis results provided more information. The pooled incidence of PE in AP patients in Asia and Europe were 35% (95%CI:28%-43%) and 33% (95%CI:21%-45%), respectively, which were higher than that in America (27%,95%CI:11%-43%) and Africa (21%,95%CI:15%-29%); the pooled incidence of PE detected by computed tomography (CT) was 43% (95%CI:34%-52%), while the incidence of PE detected by chest X-ray, ultrasonography and combination of above equipment were 21% (95%CI:16%-27%), 20% (95%CI:16%-27%) and 37% (95%CI: 11%-63%), respectively.
Table 2.
Subgroup analysis of incidence of pleural effusion in acute pancreatitis.
| Subgroups | Studies | Pooled Estimated(95%CI) | I2 | P |
|---|---|---|---|---|
| Study design | ||||
| Prospective | 17 | 32%(25%-39%) | 93.52% | 0.539 |
| Retrospective | 19 | 35%(27%-43%) | 97.71% | |
| Study scale | ||||
| n < 100 | 13 | 34%(26%-42%) | 85.52% | 0.982 |
| n ≥ 100 | 23 | 34%(27%-41%) | 97.78% | |
| Region | ||||
| Asia | 22 | 35%(28%-43%) | 96.84% | 0.033 |
| Europe | 10 | 33%(21%-45%) | 97.51% | |
| America | 3 | 27%(11%-43%) | 91.2% | |
| Africa | 1 | 21%(15%-29%) | – | |
| Examination method | ||||
| CXR | 8 | 21% (16%-27%) | 83.23% | 0.0001 |
| CT | 14 | 43% (34%-52%) | 95.2% | |
| TS | 1 | 20% (16%-27%) | – | |
| combination | 4 | 37% (11%-63%) | 98.86% | |
| NA | 9 | 30% (20%-40%) | 96.72% |
Abbreviations: CXR: chest X-ray; TS: transthoracic sonography.
3.4. Association of PE with AP patients’ clinical outcome
We extracted useful data about mortality from a total of five studies [20,23,32,35,42]. The results indicated that the pooled mortality was higher in AP patients with PE (22%, 95% CI: 10%-34%) than in AP patients without PE (3%, 95% CI: 0.1%-5%), as shown in Table 3. Then, the random-effects model was carried out to calculate the pooled risk ratio (RR) of mortality, and the results demonstrated that PE may increase mortality in AP patients (RR 3.99, 95% CI: 1.73-9.2, p = 0.007) (Figure 3).
Table 3.
Mortality in acute pancreatitis patients with or without pleural effusion.
| Short time Mortality | Pooled Estimate(95%CI) | I² | P |
|---|---|---|---|
| AP with PE | 22%(10%-34%) | 93% | 0.0001 |
| AP without PE | 3% (0.1%-5%) | 71.6% | 0.007 |
Figure 3.
Forest plot of the association between PE and mortality.
3.5. Sensitivity analysis and publication bias evaluation
The sensitivity analysis (leave-one-out analysis) of the included studies on the incidence of PE in AP showed that the pooled results were stable, as the results changed few after any single study was deleted (Supplement Figure 1). Egger’s test indicated significant publication bias (p = 0.04).
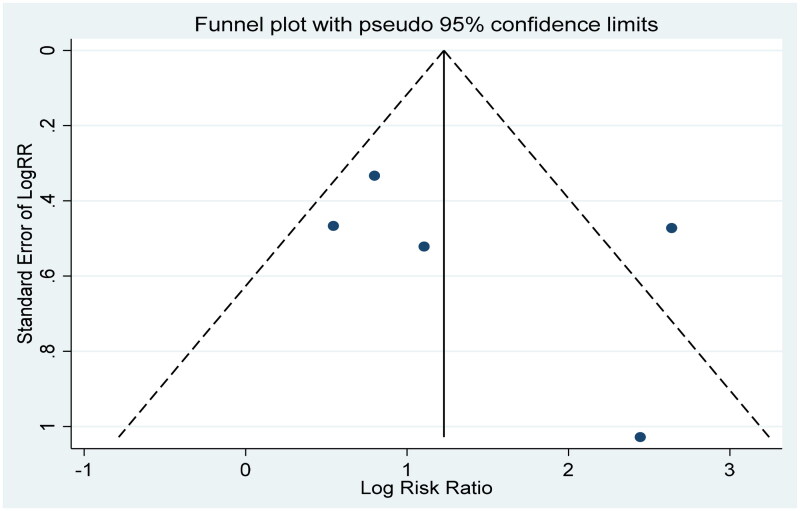
The results of the meta-analysis of the five studies associated with clinical outcomes showed significant heterogeneity (I2=71.9%). The sensitivity analysis was conducted to explore the source of heterogeneity, while the result suggested that our meta-analysis was stable (Figure 4). A funnel plot was used to examine publication bias, which revealed significant publication bias in our study (Figure 5). Apart from this, Begg’s test (p = 0.221) and Egger’s test (p = 0.437) showed no significant publication bias.
Figure 4.
Sensitivity analysis of five studies about PE and the mortality in AP.
Figure 5.
The funnel plot of five studies about PE and mortality in AP.
4. Discussion
Pulmonary complications are an inevitable topic in AP patients, as AP is an inflammatory condition involving not only the pancreas but also extrapancreatic organs, especially the lung [51]. Currently, there is still a lack of reliable parameters to predict AP outcome. In accordance with the revised and updated Atlanta Classification of AP, severity stratification is based on organ failure, local or systemic complications and effusions rather than the degree of pancreatic necrosis [48,52]. PE is regarded as one of indicators of classifying the severity of AP, and its presence is related to increasing mortality in SAP [51]. Early identification of PE on admission, awareness of the incidence and significance of PE and intervention if necessary are helpful to prevent deterioration of AP. However, when PE in AP patients was detected by imaging examination, it is crucial to exclude patients comorbid with heart failure, cancer, underlying respiratory or other diseases that may cause PE. In the included studies, part of them excluded PE preceding the development of AP, the presence of severe debilitating illness, and lung infection or pulmonary embolism, while some studies only reported PE was detected at or after admission and was a complication of AP. Thus, Comprehensive consideration of the occurrence and development of PE in the clinical course of AP is necessary.
This meta-analysis focused on 35 eligible articles involving 7,675 participants that reported the incidence of PE, and among them, five studies additionally reported the mortality of AP patients with or without PE. Our results suggested that the overall pooled incidence of PE in AP was 34% (95% CI: 28%-39%), with significant heterogeneity (I2=96.7%). The subgroup analysis identified that regions of studies and examination methods affected the heterogeneity. The reason for this result may be the upgrades and updates of guidelines for AP management, development of clinicians’ deeper understanding of pulmonary complications in AP patients, and the advancement of examination equipment contributing to timely and correct detection of PE.
In previous studies, more than half of PE cases were detected on the bilateral side [38,42,45]. In other studies, PEs were mainly unilateral [16,17,53]. These two completely discordant findings may be explained by the different proportions of SAP and diverse examination methods [45]. Furthermore, the volume and presence of PE could be affected by the detection time points as they changed according to the process of disease. In general, a group of studies suggested that the appearance of bilateral and moderate to large amounts of PE in the early phase of disease were predictors of SAP, even more accurate than the BISAP and APACHE II scores [38,45]. Our results suggested that the pooled incidence of unilateral PE was 49% (95% CI: 21%-77%), and the pooled incidence of small PE was 59% (95% CI: 38%-81%). Our meta-analysis also calculated the pooled incidence of PE in SAP and MAP, which were 67% (95% CI: 53%-82%) and 14% (95% CI: 7%-21%), respectively. It is obvious that the incidence of PE in SAP was much higher than that in MAP. However, the results need further rigorous investigation, especially when examination methods are different and the characteristics of patients vary largely in the clinical context.
Based on published studies, the range of the incidence of PE in AP had a wide distribution. Among the included studies, Yao et al. and Avanesov et al. reported the two highest incidences of PE in AP, 69% and 63%, respectively [32,48]. This is not surprising, as their subjects were confined to the SAP population alone. A Chinese study even reported an up to 72.82% incidence of PE in SAP [54]. However, our study showed an incidence of 34%, which was consistent with previous studies involving patients with AP [38,43]. Detection by CT had the highest incidence of PE in AP (43% 95% CI: 34%-52%). Contrast-enhanced abdominal CT is generally regarded as the gold standard to diagnose AP and evaluate the degree of pancreatic necrosis, while its application within 48 h of onset is limited. In the setting of AP, chest CT could be applied to detect the minimal volume of PE with brilliant sensitivity, while chest X-ray performed beside the bed may ignore some effusion due to the positions [51]. Furthermore, ultrasonography is considered a convenient and economical method to detect PE, but relevant studies on its utilization in AP patients are few. Moreover, ultrasonography is unsuitable for obese AP patients.
The correlation between PE and the clinical severity of AP (including pseudocysts, necrosis, organ failure and mortality) was reported in previous studies, suggesting that PE had a significant influence on death, as per available studies [18,55]. Our meta-analysis covering five studies justified that PE may be associated with the increased mortality in AP. However, PE is rarely an early, independent predictor of severity AP. Combining PE with other indicators in the evaluation of severity of AP is necessary. Simple tests such as age, ascites, blood glucose, BMI along with some prognostic criteria suggested by scores system provided significant predictive power for clinical decision-making [17,42]. The meta-analysis has inspirations for clinicians when they need to assess disease at an early stage in the absence of CT or organ dysfunction indicator.
The underlying mechanisms of PE accumulation in AP are various and could be explained by the following reasons: the permeability change of capillaries caused by inflammation reactions, the blockage of transdiaphragmatic lymphatic, pleuro-pancreatic fistula formation and sinus formation between the pleural cavity and the pancreatic pseudocyst [11,56,57]. Previous studies reported that patients with PE but not atelectasis and consolidation were more likely to develop respiratory failure. PE was also associated with pancreatic necrosis, renal dysfunction and other organ failure [43]. These life-threatening complications are closely related to the prognosis of AP and may prolong the hospital stay and increase mortality. PE could exacerbate impairment in the respiratory system. Studies have reported that proteolytic enzymes secreted by the pancreas may be present in PE and thus impair the lung directly [11,58]. However, limited by the number of available studies, the mechanism of PE and adverse outcomes of AP has not been interpreted clearly and requires further investigation. Finally, of great importance is the timely treatment of PE. Yao et al. reported that indwelling pleural catheters in the early stage could reduce the complication rate and mortality in SAP [48].
Our meta-analysis is the first to study the pooled incidence and mortality of PE in patients with AP to date, with some inevitable limitations. First of all, the study is a meta-analysis of individual rates, with significant heterogeneity among the included studies. Although subgroup analysis and sensitivity analysis were performed, heterogeneity still existed. Therefore, we should interpret the data with caution. Moreover, Clinical heterogeneity between studies should also be noted. As the detailed baseline characteristics of patients, for instance, the disease severity and other comorbidities in PE patients, in the included studies were lacked, the stratified analysis according to the characteristics of the patients were confined. Second, our study is mainly a single-arm meta-analysis, most of the included studies were cross-sectional and did not set up a control group, so there was a lack of reasonable comparison. Third, there are only a small number of observational studies reporting mortality in AP patients complicated with or without PE. Thus, we merely demonstrated an association between PE and mortality in AP patients, and the causality still needs to be confirmed by a large number of clinical trials or population-based studies of high quality in future. Our results of the study just provided hypotheses for further risk studies. In other words, well-designed clinical trials are needed to evaluate the prognostic role of PE in AP in the future. Future studies could also explore the effects of PE on systemic function in patients with AP as well as investigating the production mechanisms of PE in AP patients Finally, all included studies were published in English, and conference abstracts were excluded, which may result in selection bias.
Despite these limitations, to the best of our knowledge, this is the first meta-analysis that summarized a large number of existing studies about the incidence PE in AP patients. The findings of our study also shed light on mortality in AP with or without PE and has inspirations for clinicians when they need to assess disease at an early stage in the absence of CT or organ dysfunction indicator.
5. Conclusion
Above all, current evidence suggests a high pooled incidence of PE in AP patients (34%, 95% CI: 28%-39%), and PE may be associated with the increased mortality in AP patients. Clinicians should pay more attention to pulmonary complications, especially PE, in AP patients and take measures in a timely manner when necessary.
Supplementary Material
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This study was supported by the 1•3•5 Project for Disciplines of Excellence at West China Hospital of Sichuan University (2019HXFH042), the Sichuan Key Research and Development Program (2020YFS0147), the National Natural Science Foundation of China(82100047)and China Postdoctoral Science Foundation (2021M702350). These funding agencies were not involved in designing the study, collecting or analyzing the data, writing the manuscript, or making decisions related to publication.
Authors contributions
T.Z and J.A performed literature search, wrote the draft and contributed equally to the study. Study design and manuscript reviewer: Y.S. and L.L; Statistic analysis: X.H, Y.W and N. A; Software: L.G and C.W. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors declare no conflict of interest.
Ethical approval
Because all the analyses were based on previously published studies, ethical approval and informed consent were not required.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Petrov MS, Yadav D.. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(3):1–13. doi: 10.1038/s41575-018-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts SE, Morrison-Rees S, John A, et al. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17(2):155–165. doi: 10.1016/j.pan.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1(1):45–55. doi: 10.1016/S2468-1253(16)30004-8. [DOI] [PubMed] [Google Scholar]
- 4.Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):122–134. doi: 10.1053/j.gastro.2021.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Gliem N, Ammer-Herrmenau C, Ellenrieder V, et al. Management of severe acute pancreatitis: an update. Digestion. 2021;102(4):503–507. doi: 10.1159/000506830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Cho CM.. Predicting severity of acute pancreatitis. Medicina-Lithuania. 2022;58(6):787. doi: 10.3390/medicina58060787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda K, Yokoe M, Takada T, et al. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J Hepatobiliary Pancreat Sci. 2010;17(1):37–44. doi: 10.1007/s00534-009-0213-4. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Zhang W, Xiang X.. Role of CT signs of thoracoabdominal complications, serum calcium, and serum C-reactive protein in evaluating disease severity in patients with acute pancreatitis. J Clin Hepatol. 2019;35(8):1766–1769. [Google Scholar]
- 9.Ocampo C, Silva W, Zandalazini H, et al. [Pleural effusion is superior to multiple factor scoring system in predicting acute pancreatitis outcome]. Acta Gastroenterol Latinoam. 2008;38(1):34–42. [PubMed] [Google Scholar]
- 10.de Oliveira C, Khatua B, Bag A, et al. Multimodal transgastric local pancreatic hypothermia reduces severity of acute pancreatitis in rats and increases survival. Gastroenterology. 2019;156(3):735–747.e10. doi: 10.1053/j.gastro.2018.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne GW, Pitchumoni CS.. Pathophysiology of pulmonary complications of acute pancreatitis. World J Gastroenterol. 2006;12(44):7087–7096. doi: 10.3748/wjg.v12.i44.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Ades AE, Lu G, Higgins JP.. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 16.Millward SF, Breatnach E, Simpkins KC, et al. Do plain films of the chest and abdomen have a role in the diagnosis of acute pancreatitis? Clin Radiol. 1983;34(2):133–137. doi: 10.1016/s0009-9260(83)80290-6. [DOI] [PubMed] [Google Scholar]
- 17.Maringhini A, Ciambra M, Patti R, et al. Ascites, pleural, and pericardial effusions in acute pancreatitis. A prospective study of incidence, natural history, and prognostic role. Dig Dis Sci. 1996;41(5):848–852. doi: 10.1007/BF02091521. [DOI] [PubMed] [Google Scholar]
- 18.Heller SJ, Noordhoek E, Tenner SM, et al. Pleural effusion as a predictor of severity in acute pancreatitis. Pancreas. 1997;15(3):222–225. doi: 10.1097/00006676-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Simmons MZ, Miller JA, Zurlo JV, et al. Pleural effusions associated with acute pancreatitis: incidence and appearance based on computed tomography. Emergency Radiology. 1997;4(5):287–289. doi: 10.1007/BF01461734. [DOI] [Google Scholar]
- 20.Talamini G, Uomo G, Pezzilli R, et al. Serum creatinine and chest radiographs in the early assessment of acute pancreatitis. Am J Surg. 1999;177(1):7–14. doi: 10.1016/s0002-9610(98)00296-7. [DOI] [PubMed] [Google Scholar]
- 21.Tsushima Y, Tamura T, Tomioka K, et al. Transient splenomegaly in acute pancreatitis. Br J Radiol. 1999;72(859):637–643. doi: 10.1259/bjr.72.859.10624319. [DOI] [PubMed] [Google Scholar]
- 22.Polyzogopoulou E, Bikas C, Danikas D, et al. Baseline hypoxemia as a prognostic marker for pulmonary complications and outcome in patients with acute pancreatitis. Dig Dis Sci. 2004;49(1):150–154. doi: 10.1023/b:ddas.0000011617.00308.e3. [DOI] [PubMed] [Google Scholar]
- 23.Raghu MG, Wig JD, Kochhar R, et al. Lung complications in acute pancreatitis. JOP: journal of the Pancreas. 2007;8(2):177–185. [PubMed] [Google Scholar]
- 24.Maher MM, Dessouky BA.. Simplified early predictors of severe acute pancreatitis: a prospective study. Gastroenterology Res. 2010;3(1):25–31. doi: 10.4021/gr2010.02.172w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasseur P, Devaure I, Sellier J, et al. High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology. 2014;14(6):465–469. doi: 10.1016/j.pan.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Banday IA, Gattoo I, Khan AM, et al. Modified computed tomography severity index for evaluation of acute pancreatitis and its correlation with clinical outcome: a tertiary care hospital based observational study. J Clin Diagn Res. 2015;9(8):Tc01–5. doi: 10.7860/JCDR/2015/14824.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu L, Sun RQ, Jia RR, et al. Comparison of existing clinical scoring systems in predicting severity and prognoses of hyperlipidemic acute pancreatitis in Chinese patients a retrospective study. Medicine (Baltimore). 2015;94(23):e957. doi: 10.1097/MD.0000000000000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vengadakrishnan K, Koushik AK.. A study of the clinical profile of acute pancreatitis and its correlation with severity indices. Int J Health Sci (Qassim). 2015;9(4):410–417. [PMC free article] [PubMed] [Google Scholar]
- 29.Dombernowsky T, Kristensen M, Rysgaard S, et al. Risk factors for and impact of respiratory failure on mortality in the early phase of acute pancreatitis. Pancreatology. 2016;16(5):756–760. doi: 10.1016/j.pan.2016.06.664. [DOI] [PubMed] [Google Scholar]
- 30.Raghuwanshi S, Gupta R, Vyas MM, et al. CT evaluation of acute pancreatitis and its prognostic correlation with CT severity index. J Clin Diagn Res. 2016;10(6):Tc06–11. doi: 10.7860/JCDR/2016/19849.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rathnakar SK, Vishnu VH, Muniyappa S, et al. Accuracy and predictability of PANC-3 scoring system over APACHE II in acute pancreatitis: a prospective study. J Clin Diagn Res. 2017;11(2):Pc10–PC13. doi: 10.7860/JCDR/2017/23168.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avanesov M, Löser A, Smagarynska A, et al. Clinico-radiological comparison and short-term prognosis of single acute pancreatitis and recurrent acute pancreatitis including pancreatic volumetry. PLoS One. 2018;13(10):e0206062. doi: 10.1371/journal.pone.0206062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Xiao B.. Analysis of the pathogenesis and clinical features of acute pancreatitis: an observation from 609 cases. Int J Clin Exp Med. 2018;11(9):9988–9994. [Google Scholar]
- 34.Hong W, Lillemoe KD, Pan S, et al. Development and validation of a risk prediction score for severe acute pancreatitis. J Transl Med. 2019;17(1):146. doi: 10.1186/s12967-019-1903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberti P, Pando E, Mata R, et al. Evaluation of the modified CT severity index (MCTSI) and CT severity index (CTSI) in predicting severity and clinical outcomes in acute pancreatitis. J Dig Dis. 2020;22(1):41–48. doi: 10.1111/1751-2980.12961. [DOI] [PubMed] [Google Scholar]
- 36.Jamanca-Milian H, Cano-Cardenas L.. Severity prognostic factors in acute pancreatitis in the national hospitao sergio E. RFMH. 2020;20(1):14–19. doi: 10.25176/RFMH.v20i1.2543. [DOI] [Google Scholar]
- 37.Karim T, Jain A, Kumar V, et al. Clinical and severity profile of acute pancreatitis in a hospital for low socioeconomic strata. Indian J Endocrinol Metab. 2020;24(5):416–421. doi: 10.4103/ijem.IJEM_447_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng R, Zhang L, Zhang Z-M, et al. Chest computed tomography semi-quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity. Quant Imaging Med Surg. 2020;10(2):451–463. doi: 10.21037/qims.2019.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta P, Kumar MP, Verma M, et al. Development and validation of a computed tomography index for assessing outcomes in patients with acute pancreatitis: "SMART-CT" index. Abdom Radiol (NY). 2021;46(4):1618–1628. doi: 10.1007/s00261-020-02740-y. [DOI] [PubMed] [Google Scholar]
- 40.He F, Zhu H-M, Li B-y, et al. Factors predicting the severity of acute pancreatitis in elderly patients. Aging Clin Exp Res. 2021;33(1):183–192. doi: 10.1007/s40520-020-01523-1. [DOI] [PubMed] [Google Scholar]
- 41.Shetty RV, Shetty AS, Shetty RN.. Comparative study of PANC3 score and Ranson’s score for predictability of the severity of pancreatitis. J Cardiovas Dis Res. 2021;12(6):1430–1434. [Google Scholar]
- 42.Yan G, Li H, Bhetuwal A, et al. Pleural effusion volume in patients with acute pancreatitis: a retrospective study from three acute pancreatitis centers. Ann Med. 2021;53(1):2003–2018. doi: 10.1080/07853890.2021.1998594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng Q-X, Jiang K-L, Wu Z-H, et al. Pleural effusion is associated with severe renal dysfunction in patients with acute pancreatitis. Med Sci Monit. 2021;27:e928118. doi: 10.12659/MSM.928118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang D-N, Zhong H-J, Cai Y-L, et al. Serum lactate dehydrogenase is a sensitive predictor of systemic complications of acute pancreatitis. Gastroenterol Res Pract. 2022;2022:1131235–1131236. doi: 10.1155/2022/1131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luiken I, Eisenmann S, Garbe J, et al. Pleuropulmonary pathologies in the early phase of acute pancreatitis correlate with disease severity. PLoS One. 2022;17(2):e0263739. (2 February). doi: 10.1371/journal.pone.0263739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patidar A, Gupta PK, Mishra RK, et al. Retrospective study: evaluation of CT imaging spectrum in acute pancreatitis, its severity and complications in tertiary care center. European Journal of Molecular and Clinical Medicine. 2022;9(3):5070–5081. [Google Scholar]
- 47.Rajendran S, Soundararajan S, Jayaraman GV.. Radiological study to assess the severity of acute pancreatitis using revised Atlanta classification and its correlation with clinical outcomes. International Journal of Academic Medicine and Pharmacy. 2022;4(5):74–78. [Google Scholar]
- 48.Yao Q, Peng S, Wu Y, et al. Effects of indwelling pleural catheter on severe acute pancreatitis: a retrospective study. Gastroenterol Res Pract. 2022;2022:1919729–1919727. doi: 10.1155/2022/1919729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou B-M, Qiu Z-L, Niu K-X, et al. Construction of a nomogram model for predicting pleural effusion secondary to severe acute pancreatitis. Emerg Med Int. 2022;2022:4199209–4199205. doi: 10.1155/2022/4199209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Husic-Selimovic A, Bijedic N, Sofic A, et al. Prediction of surgical treatment in acute pancreatitis using biochemical and clinical parameters. Med Arch. 2023;77(1):29–33. doi: 10.5455/medarh.2023.77.29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar P, Gupta P, Rana S.. Thoracic complications of pancreatitis. JGH Open. 2019;3(1):71–79. doi: 10.1002/jgh3.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 53.Saleem H, Arshad F, Shaikh MW, et al. Frequency of left plueral effusion in acute necrotizing pancreatitis. PJMHS. 2021;15(10):3349–3351. doi: 10.53350/pjmhs2115103349. [DOI] [Google Scholar]
- 54.Zuo L, Zhang H, Chen J, et al. Factors influencing acute pancreatitis complicated with pleural effusion. Chinese General Practice. 2019;22(18):2194–2199. [Google Scholar]
- 55.Gumaste V, Singh V, Dave P.. Significance of pleural effusion in patients with acute pancreatitis. Am J Gastroenterol. 1992;87(7):871–874. [PubMed] [Google Scholar]
- 56.Karki A, Riley L, Mehta HJ, et al. Abdominal etiologies of pleural effusion. Dis Mon. 2019;65(4):95–103. doi: 10.1016/j.disamonth.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Suna N, Öztaş E, Kuzu UB, et al. Pleural effusion in acute pancreatitis, not always related. Acta Gastroenterol Belg. 2017;80(3):434–435. [PubMed] [Google Scholar]
- 58.Chelliah T, Werge M, Merc AI, et al. Pulmonary dysfunction due to combination of extra-pulmonary causes and alveolar damage is present from first the day of hospital admission in the early phase of acute pancreatitis. Pancreatology. 2019;19(4):519–523. doi: 10.1016/j.pan.2019.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.