Abstract
Introduction:
Boerhaave’s syndrome, or the spontaneous transmural perforation of the esophagus, is typically thought to be due to an increase in esophageal pressure such as that which occurs during vomiting or retching. Another common etiology of esophageal perforation is esophageal instrumentation, such as during esophagogastroduodenoscopy or transesophageal echocardiography. This life-threatening condition requires prompt diagnosis and treatment to prevent patient demise. While a history of vomiting can aid in diagnosis, this history can be difficult to elicit in an unconscious patient or may be altogether absent. Additionally, Boerhaave’s syndrome can present similarly to more common upper gastrointestinal or cardiac conditions. Since mortality increases with delays in diagnosis and treatment, it is imperative that clinicians maintain a high level of suspicion for Boerhaave’s syndrome and initiate treatment urgently.
Case Description:
This report presents a 76-year-old man who presented to the emergency department after a history of several syncopal episodes and was found to be in complete heart block. Two days later, he acutely developed abdominal distention and coffee ground emesis. As the medical team was able to gather more history from the patient and his family, it was revealed that he had associated vomiting with his episodes of syncope. CT scan of the abdomen and pelvis demonstrated pneumomediastinum concerning for esophageal perforation. His clinical status subsequently deteriorated. He was intubated and a temporary transvenous pacer was placed before being transferred to our facility for emergent surgery.
Discussion:
Complete heart block in the setting of Boerhaave’s syndrome is exceptionally rare, with only 2 cases reported in the literature. The decision to place a pacemaker in the setting of esophageal perforation/sepsis is complicated and depends on the patient’s bacteremia status related to noncardiac comorbidities. Clearly this case represents the need for excellent multidisciplinary decision-making processes with excellent communication between hospital staff and all caretakers. Expeditious diagnosis and treatment of esophageal perforation is essential to prevent leaking of gastric contents into the mediastinum and worsening of cardiac complications and sepsis. Additionally, critical timing of various surgical procedures, especially the need for a permanent pacemaker implant with bacteremia is a complicated process not well described in the surgical literature.
Keywords: Bacteremia, Boerhaave’s syndrome, Heart block, Sepsis, Spontaneous esophageal rupture
INTRODUCTION
Boerhaave’s syndrome is the transmural rupture of the esophagus as a result of increased intraluminal pressure, such as with vomiting or retching. Incomplete relaxation of the cricopharyngeal muscle during episodes of vomiting, which impedes the escape of gastric contents, causes the increase in intraluminal pressure and subsequent perforation.4 Boerhaave’s syndrome is a rare surgical emergency with mortality rates as high as 20%, which can double with delays in treatment of more than 24 hours.5 The classic presentation of Boerhaave’s syndrome includes a history of alcohol use and ingestion of excess food with vomiting followed by chest pain and subcutaneous emphysema, also known as “Mackler’s Triad.” However, this trilogy is seen in only a minority of patients, causing the diagnosis to often be missed on initial presentation.18
Without prompt management, esophageal perforation can lead to infection such as mediastinitis, empyema, and pneumonia, which can quickly progress causing hemodynamic instability and sepsis. The chest pain associated with esophageal rupture can often mimic aortic dissection, pulmonary embolism, and acute coronary syndrome, especially if the patient has other comorbidities predisposing them to coronary artery disease.16 Cardiac complications of esophageal rupture are likely due to local inflammation secondary to leaking of gastric contents into the mediastinum. However, conduction abnormalities as a result of esophageal perforation have only been described twice in the literature and the mechanism of this complication is poorly understood.
CASE PRESENTATION
This patient is a 76-year-old man with a past medical history of hypertension, hyperlipidemia, diabetes mellitus type 2, and neuropathy who presented to the emergency department after reportedly having multiple episodes of syncope with associated fatigue and shortness of breath over the previous week. He was awake, alert, and hemodynamically stable at the time of interview. His only complaint was of moderate back and flank pain, which he attributed to a fall during one of the prior syncopal episodes. He denied recent dysphagia, abdominal pain, vomiting, diarrhea, or melena. Upon physical examination, he was found to be bradycardic with a heart rate of 33 beats/minute. Electrocardiogram (Figure 1) showed the patient was in third-degree heart block, and laboratory investigations revealed a troponin T of 27 ng/L. Cardiology was consulted and it was recommended that the patient be given an Isoproterenol infusion with plans for permanent pacemaker placement. He remained hemodynamically stable with SBP ranging 110–120 seconds.
Figure 1.

Electrocardiogram showing ventricular skip rhythm with right bundle-branch block. P waves at regular intervals consistent with third-degree heart block.
On admission day two, he developed acute abdominal distension and coffee ground emesis. Gastroenterology was consulted and further investigations into the patient’s history revealed vomiting with each episode of syncope. WBC at that time was elevated to 14.57 × 103 μ/L from his baseline of 7.21 × 103 μ/L on admission. Computed tomography with oral contrast revealed pneumomediastinum with a 3.6 × 6.9 cm air-fluid collection immediately adjacent to gastroesophageal junction on the left and several other foci of predominantly gas posterior to the gastric cardia which partially filled with small amount of contrast on postcontrast images (Figures 2–5). The diagnosis of esophageal perforation was made at that time. The patient was intubated, a nasogastric tube was inserted, and a temporary transvenous pacemaker was placed prior to transfer to our hospital for emergent repair of the esophagus.
Figure 2.

Coronal chest CT showing pneumomediastinum with left pleural effusion.
Figure 5.
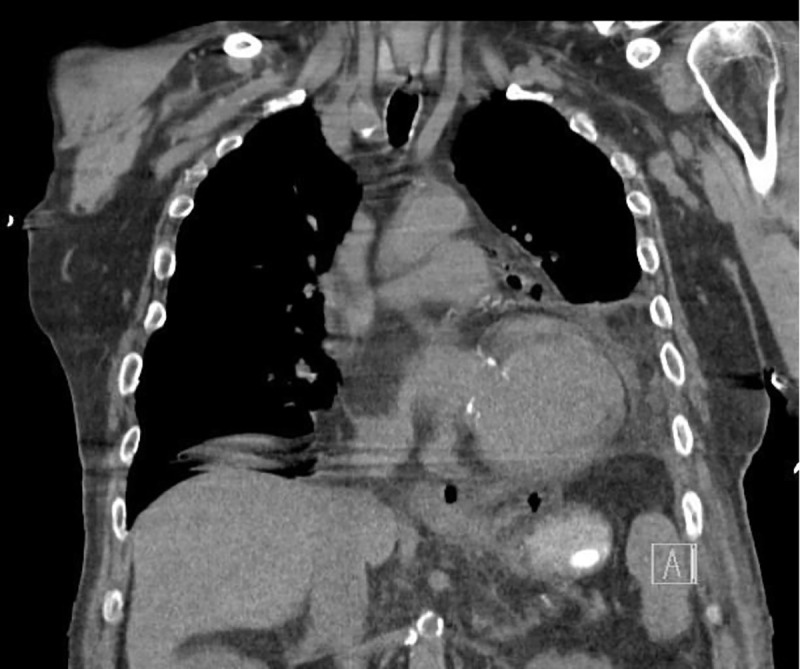
Coronal chest CT showing air adjacent to GE junction. These areas were filled with contrast on postcontrast images.
Figure 3.
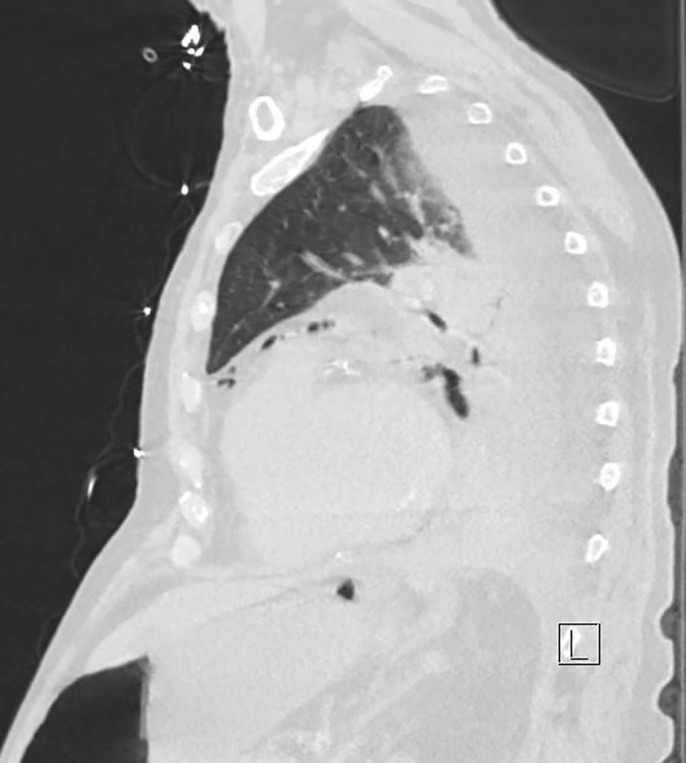
Sagittal view of pneumomediastinum and small focus of free air under the diaphragm from esophageal perforation.
Figure 4.

Axial view of contrast extravasation and pneumomediastinum into left pleural effusion due to esophageal perforation.
TREATMENT
The patient was emergently brought into the operating room for a left posterolateral thoracotomy with incision made around the temporary pacing electrodes. Approximately 5 liters of infected pleural fluid was removed from the thoracic cavity with visualization of the esophageal perforation at the level of the diaphragmatic hiatus. Due to the severe contamination of the left chest and poor quality of the esophageal tissue in the area of the perforation, primary repair was not an option for treatment. An 18 French Malecot catheter was placed, and a diaphragmatic patch placed over the esophageal perforation. The Malecot drain was tacked to the diaphragm and brought out on the lateral chest wall through a separate stab incision. During the surgery, the patient became bradycardic again and hemodynamically unstable. There was poor electrical and mechanical capture from the temporary pacemaker. The pericardium was opened and epicardial pacemaker leads were placed and pacing resumed with improved hemodynamics. Two 28 French chest tubes were placed and the epicardial pacemaker wire was sutured to the skin. Additionally, a gastrostomy and a jejunostomy were performed. The patient was transferred back to the intensive care unit.
FOLLOW-UP
The patient recovered after several weeks in the intensive care unit and 2 wash out thoracotomies and maintained a sinus rhythm with the epicardial pacer. After stabilization, a tracheostomy tube was placed for prolonged ventilatory support. After the patient was stabilized and his infection controlled, discussion was held in relation to “what time is it safe to place a permanent pacemaker in the setting of bacteremia after an esophageal perforation.” After two sets of negative blood cultures collected 48 hours apart and procalcitonin levels were trending towards normal, the patient underwent permanent internal cardiac pacemaker placement and removal of the temporary pacemaker leads 2 weeks after his esophageal perforation. Shortly after this, he was able to be weaned from the ventilator. The chest tubes were removed in the intensive care unit and the patient was transferred to an acute rehabilitation care unit. He returned in 6 weeks for the removal of his Malecot drain and placement of an esophageal stent. His tracheostomy was downsized at that time. He was recovering well and improving with physical therapy.
DISCUSSION
This case of a patient with Boerhaave’s syndrome presenting primarily in third-degree heart block is an extremely rare complication with a poorly understood mechanism (primary synchronous cardiac conductions abnormality versus cardiac irritation from the inflammatory process), possibly unrelated to the esophageal perforation. There have been several cases of esophageal perforation presenting as an acute myocardial infarction or cardiac arrest, but cardiac arrhythmia has only been noted in 2 cases upon literature review.9,16 This patient with third degree heart block, presenting with symptomatic bradyarrhythmia, would be a candidate for implanted permanent pacemaker (class I indication). However, the decision of when to place the pacemaker was complicated by his comorbidities and the presentation with sepsis.8,12 While cardiac stability and resolution of sepsis are preferred prior to any surgical procedure, this is not always possible in an emergency setting. A retrospective cohort study of patients undergoing Cardiac Implantable Electronic Device (CIED) implantation found that there was no significant difference in the incidence of CIED infection between patients with and without recent infection (local or systemic) after adequate duration of afebrile status and antibiotic therapy, defined here as 14 days of treatment for bacteremia, 7–10 days for nonbloodstream-related infections, and 5–7 days for local wound infections.7 Patients with recent infection did, however, have a higher incidence of in-hospital all-cause mortality rate and 1-year all-cause mortality when compared to patients without recent infection.7 Other important factors associated with a high incidence of postoperative device infection include the presence of indwelling catheters, chronic skin conditions, corticosteroid treatment, and chronic kidney disease requiring hemodialysis.13 Most notably, presence of fever less than 24 hours prior to surgery was associated with a 5.8-fold higher risk of infection.11
Tools such as the American Society of Anesthesiologists Physical Status Classification System (ASA), the Acute Physiology and Chronic Health Evaluation (APACHE), the Physiological and Severity Score for the Enumeration of Mortality and Morbidity (POSSUM), and the quick Sepsis Related Organ Failure Assessment (qSOFA) have all been classically used for risk stratification in the preoperative evaluation of patients.6 In addition to these tools, the presence of a transvenous temporary pacemaker, low estimated Glomeruli Filtration Rate (eGFR), and a history of diabetes mellitus, coronary artery disease, or other comorbidities have proven to be effective in estimating the likelihood of negative outcomes in patients requiring implantation of cardiac devices.2,7,11 The decision to implant a cardiac device in a patient with current sepsis is dependent on the patient’s 1-year life-expectancy, their current comorbidities (most significantly the presence of end-stage renal disease), their clinical manifestations of infection (i.e., fever), and whether they have had adequate antibiotic therapy.2 Additional pertinent laboratory evaluations include serum erythrocyte sedimentation rate, serum C-reactive protein, serum interleukin-6,17 platelet count, serum bilirubin levels, serum creatinine, procalcitonin, and urine output.3 Consideration should also be taken if the patient is taking steroids or immunosuppressant drugs. Colonization of the nares or skin with Methaacillin Sensitive Staph Aureus (MSSA) and/or Methacillin Resistant Staph Aureus (MRSA) is a risk factor for infection of an implanted device, therefore nasal swabbing and treatment prior to surgery in addition to surgical site preparation with chlorhexidine gluconate (CHG) has been shown to reduce the risk of MRSA-related surgical site infections.1 Guidelines for Enhanced Recovery After Surgery (ERAS) for high-risk surgical patients recommend assessment with a validated sepsis score (such as the qSOFA) and monitoring of serum lactate as a risk marker even in the absence of sepsis. Imagining with computed tomography is also recommended as soon as possible as indicated in patients requiring emergency laparotomy, so long as it does not cause a time-delay.14
CONCLUSION
Esophageal perforation is an uncommon condition that may be missed in critically ill patients. Few present with the classical Mackler’s Triad of a history of vomiting, chest pain, and subcutaneous emphysema.18 The chest pain associated with esophageal perforation presents similarly to cardiac-related causes of chest pain like aortic dissection or myocardial infarction.10 Iatrogenic perforation from upper endoscopy or instrumentation should be considered in patients who present with chest pain after these procedures. Providers should maintain a high index of suspicion for esophageal perforation, as delays in treatment greatly contribute to the high mortality rate. While it is crucial to rule out a cardiac cause for a patient presenting with chest pain, esophageal perforation should remain in the differential diagnosis, even if a cardiac cause is found. This case highlights an extremely rare presentation of combined third-degree heart block and esophageal perforation. The mechanism of esophageal perforation leading to any degree of heart-block is not yet understood and requires more investigations into similar cases. The close proximity of the heart and esophagus suggests that disease or trauma of one organ can significantly impact the other. Therefore, physicians should be vigilant in assessing the integrity of both the cardiac and upper gastrointestinal systems in a patient presenting with vague symptoms that could be related to either cardiac or gastrointestinal etiologies.
The decision to implant a permanent pacemaker into this patient with sepsis was crucial for his survival due to the degree of heart block he was experiencing.15 Tools like the qSOFA, which considers the Glasgow Coma Scale (GCS), PaO2/FiO2, and mean arterial pressure, can be particularly useful when deciding whether to proceed with device implantation in a septic or bacteremic patient as this is a predictor of mortality based on laboratory and clinical data.3 While there are several risk stratification tools available to determine the benefit of proceeding with surgical intervention in a patient experiencing current or recent infection, there is no single tool that can accurately calculate risk. A combination of assessment scores, relevant laboratory data based on the patient’s status and comorbidities as mentioned previously, as well as the intuition and experience of the surgeon(s) and anesthesiologist(s) all play a valuable role in the decision-making process. This process must be highly individualized and should involve several members of the care team to assure the most beneficial outcome for the patient while minimizing risk. To review, the following algorithm should be considered when implanting a device into the bloodstream after sepsis based on our reviews of the literature:
Temperature > 100.4 within the last 24 hours
qSOFA score ≥ 2
eGFR ≤ 59
Serum creatinine ≥ 1.3
Lactate ≥ 2 mmol/L
Presence of a transvenous temporary pacemaker
Duration of appropriate antibiotic therapy
Comorbidities (ESRD, DM, CAD)
ESR, CRP, IL-6
Negative blood cultures 48 hours apart
Procalcitonin level
Each of these factors increases the risk for intraoperative and postoperative complications, most notably postoperative infection of the implanted device.
Footnotes
Disclosure: none.
Conflict of interest: none.
Contributor Information
Jay A. Redan, Department of Surgery, AdventHealth Celebration, Florida, Kissimmee, Florida, USA. (Drs Redan and Gaughan).
Taylor Croteau, St. Matthew’s University School of Medicine, Orlando, Florida, USA. (Dr Croteau).
Colleen Gaughan, Department of Surgery, AdventHealth Celebration, Florida, Kissimmee, Florida, USA. (Drs Redan and Gaughan).
References:
- 1.Antonelli B, Chen AF. Reducing the risk of infection after total joint arthroplasty: preoperative optimization. Arthroplasty. 2019;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomstrom-Lundqvist C, Ostrowska B. Prevention of cardiac implantable electronic device infections: guidelines and conventional prophylaxis. Europace. 2021;23(Suppl 4):iv11–iv19. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsetti A, Vitali E, Pesaresi L, et al. Anesthetic management of patients with sepsis/septic shock. Front Med (Lausanne). 2023;10:1150124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrozza F, Dragean C. Spontaneous esophageal rupture or Boerhaave’s syndrome. J Belg Soc Radiol. 2020;104(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR, Kucharczuk JC. Evolving options in the management of esophageal perforation. Ann Thorac Surg. 2004;77(4):1475–1483. [DOI] [PubMed] [Google Scholar]
- 6.Chand M, Armstrong T, Britton G, Nash GF. How and why do we measure surgical risk? J R Soc Med. 2007;100(11):508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Chen Y, Lee W, et al. Clinical outcomes of patients undergoing a cardiac implantable electronic device implantation following a recent non-device-related infection. J Hosp Infect. 2020;105(2):272–279. [DOI] [PubMed] [Google Scholar]
- 8.Dalia T, Amr BS. Pacemaker indications. [Updated August 22, 2022]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2022, January. Available at: https://www.ncbi.nlm.nih.gov/books/NBK507823/. [PubMed] [Google Scholar]
- 9.Davies J, Spitzer D, Phylactou M, Glasser M. Cardiac arrest due to a missed diagnosis of Boerhaave’s syndrome. BMJ Case Rep. 2016. Available at: https://casereports.bmj.com/content/2016/bcr-2014-208659.full (Accessed: March 7, 2023). [DOI] [PMC free article] [PubMed]
- 10.Janjua KJ. Eponyms medicine revisited Boerhaave’s syndrome. bmj.com. 1997. Available at: https://pmj.bmj.com/content/postgradmedj/73/859/265.full.pdf (Accessed: March 6, 2023).
- 11.Klug D, Balde M, Pavin D, et al. PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116(12):1349–1355. [DOI] [PubMed] [Google Scholar]
- 12.Link MS. Permanent cardiac pacing: overview of devices and indications. UpToDate. February 15, 2023. Retrieved March 22, 2023, from https://www.uptodate.com/contents/permanent-cardiac-pacing-overview-of-devices-and-indications?source=bookmarks.
- 13.Nielsen JC, Gerdes JC, Varma N. Infected cardiac-implantable electronic devices: prevention, diagnosis, and treatment. Eur Heart J. 2015;36(37):2484–2490. [DOI] [PubMed] [Google Scholar]
- 14.Peden CJ, Aggarwal G, Aitken RJ, et al. Guidelines for perioperative care for emergency laparotomy enhanced recovery after surgery (ERAS) society recommendations: part 1-preoperative: diagnosis, rapid assessment and optimization. World J Surg. 2021;45(5):1272–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccini J. Cardiac implantable electronic devices: periprocedural complications. UpToDate. January 12, 2021. Retrieved March 22, 2023, from https://www.uptodate.com/contents/cardiac-implantable-electronic-devices-periprocedural-complications?source=bookmarks.
- 16.Rigger W, Mai R, Maddux PT, Cavalieri S, Calkins J. Esophageal rupture presenting with ST segment elevation and junctional rhythm mimicking acute myocardial infarction. Case Rep Crit Care. 2021;2021:8843477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tubb CC, Polkowksi GG, Krause B. Diagnosis and prevention of periprosthetic joint infections. J Am Acad Orthop Surg. 2020;28(8):e340–e348. [DOI] [PubMed] [Google Scholar]
- 18.Turner AR, Turner SD. Boerhaave syndrome. [Updated 2023 June 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. January, 2023. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430808/. [Google Scholar]


