ABSTRACT
Deinococcus radiodurans exhibits remarkable survival under extreme conditions, including ionizing radiation, desiccation, and various DNA-damaging agents. It employs unique repair mechanisms, such as single-strand annealing (SSA) and extended synthesis-dependent strand annealing (ESDSA), to efficiently restore damaged genome. In this study, we investigate the role of the natural transformation-specific protein DprA in DNA repair pathways following acute gamma radiation exposure. Our findings demonstrate that the absence of DprA leads to rapid repair of gamma radiation-induced DNA double-strand breaks primarily occur through SSA repair pathway. Additionally, our findings suggest that the DprA protein may hinder both the SSA and ESDSA repair pathways, albeit in distinct manners. Overall, our results highlight the crucial function of DprA in the selection between SSA and ESDSA pathways for DNA repair in heavily irradiated D. radiodurans.
IMPORTANCE
Deinococcus radiodurans exhibits an extraordinary ability to endure and thrive in extreme environments, including exposure to radiation, desiccation, and damaging chemicals, as well as intense UV radiation. The bacterium has evolved highly efficient repair mechanisms capable of rapidly mending hundreds of DNA fragments in its genome. Our research indicates that natural transformation (NT)-specific dprA genes play a pivotal role in regulating DNA repair in response to radiation. Remarkably, we found that DprA is instrumental in selecting DNA double-strand break repair pathways, a novel function that has not been reported before. This unique regulatory mechanism highlights the indispensable role of DprA beyond its native function in NT and underscores its ubiquitous presence across various bacterial species, regardless of their NT proficiency. These findings shed new light on the resilience and adaptability of Deinococcus radiodurans, opening avenues for further exploration into its exceptional survival strategies.
KEYWORDS: DprA, RecA, single strand annealing (SSA), extended synthesis-dependent strand annealing (ESDSA), DdrB
INTRODUCTION
Natural transformation (NT) is a regulated form of horizontal gene transfer in bacteria, serving various purposes, such as genome evolution, DNA repair, and fulfilling nutritional needs (1). The process involves interactions between extracellular DNA (eDNA) and pili structures, followed by the movement of eDNA into the cytoplasm with the assistance of specific proteins (2). Proteins like DprA and single-stranded DNA binding proteins (SSB) are indispensable for ensuring the integrity of translocated DNA against degradation by nucleases. DprA, as a member of the natural transformation-specific recombination mediator proteins (RMP) family, facilitates the loading of RecA on single-stranded DNA (ssDNA) (3, 4). The fate of the transformed DNA can be either chromosomal integration or reconstitution into an autonomous plasmid (5). The impact of natural transformation on bacterial evolution and genome stability remains debated (6–8). Studies on various bacterial species with natural competence have yielded mixed results on the effects of natural transformation on adaptive evolution, suggesting that its role in accelerating bacterial adaptation is not universal and may be beneficial (9, 10), neutral (11), and/or context dependent (10, 12–14). Despite its contribution to genome evolution, the immediate effects of DNA uptake and recombination on individual cells are not clear. Two non-mutually exclusive hypotheses have been proposed: one suggests that eDNA serves as a source of essential nutrients such as nitrogen, carbon, and nucleotides (6, 8, 14), while the other proposes that it can be used as a template for repair of genomic DNA damage (15, 16). Experimental evidence supports both hypotheses in different bacterial species, but competence induction may also be a general stress response (17–20).
DNA double-strand breaks (DSBs) are considered as the severest form of DNA damage and can lead to chromosomal abnormalities, genomic instability, and cell death (21). To counteract these effects, cells have developed several mechanisms for DSB repair, including non-homologous end joining, single-strand annealing (SSA), synthesis-dependent strand annealing (SDSA), break-induced replication, interstrand SSA, and copy choice (22, 23). Deinococcus radiodurans is a unique bacterium known for its exceptional ability to survive various DNA-damaging conditions (22). It employs distinct strategies and DNA repair mechanisms, including both RecA-independent and RecA-dependent repair phases (22, 24–35). However, DSB repair in D. radiodurans occurs through SSA and unique extended synthesis-dependent strand annealing (ESDSA) repair pathways, rather than the mechanisms mentioned above (23). The ESDSA pathway repairs the genome by joining together DNA fragments that were synthesized before irradiation with newly synthesized DNA blocks after irradiation, creating a patchwork-type DSB repair (22, 23, 36).
Moreover, D. radiodurans cells exhibit natural transformability, which persists throughout exponential growth phase (37). Key proteins, such as DprA, DdrB, and RecA/RecO/RecF, play pivotal roles in facilitating the natural transformation process of D. radiodurans (38, 39). While DprA and RecA are crucial for both plasmid and chromosomal DNA transformation, DdrB and RecO/RecF have distinct roles in this process (38, 40). Despite the early discovery of the natural transformation phenotype in D. radiodurans, there has been limited research on the underlying mechanism and its significance in DNA damage repair and radiation survival. Recent studies have highlighted the role of DprA in protecting and facilitating the integration of incoming DNA into the bacterial chromosome (38, 40). However, the exact contribution of DprA in DNA repair and its involvement in DSB repair mechanisms remain unclear.
In this study, we conducted a variety of experiments, including cell survival studies, DSB repair kinetics by pulse field gel electrophoresis (PFGE) analysis, BrdU-based ESDSA repair kinetics analysis, and microscopic studies, to learn more about DprA roles in DNA double-strand breaks repair and provide compelling evidence demonstrating that DprA helps in balancing the activities of SSA and ESDSA repair pathways in gamma-irradiated D. radiodurans.
RESULTS
dprA gene mutant showed differential response to DNA-damaging agents and follow fast DSBs repair
NT is a genetically regulated horizontal gene transfer mechanism in bacteria (1). The primary purpose of NT is to incorporate eDNA for various purposes, such as genome evolution, DNA repair, and nutritional needs (41). The NT process involves a series of steps, beginning with the interaction of eDNA with IV pili (Tfp) or a transformation-specific pilus/pseudo pilus, followed by the movement of eDNA from outside of the cells to the cytoplasm. This transfer is facilitated by Com proteins (ComEA, ComEC, and ComF) and an endonuclease A (2, 42, 43). The protection of the translocated ssDNA from cytoplasmic nuclease degradation is critical and is provided by DprA and SSB (44). DprA, a member of the natural transformation-specific RMP family, protects DNA from nuclease degradation and facilitates the loading of RecA on naked or SSB-coated ssDNA (3, 4). The fate of the transforming DNA can either be chromosomal integration or recirculation to reconstitute an autonomous plasmid or to use the DNA for cellular nutritional requirements. The chromosomal integration of eDNA is influenced by RecA recombinase in association with DprA protein, while reestablishment of plasmid DNA is accomplished through SSA activity by pairing complementary regions of plasmid DNA fragments (5, 45).
The NT-specific ΔdprA mutant of D. radiodurans was exposed to various DNA-damaging agents, including gamma radiation, mitomycin C (MMC), and UV radiation. The ΔdprA mutant exhibited distinct sensitivity patterns to these DNA-damaging agents. When treated with gamma radiation, the ΔdprA mutant displayed survival rates comparable to the wild type up to 10 kGy doses. However, at 18 kGy, the ΔdprA cells exhibited nearly a 1-log cycle drop in survival compared to the wild type (Fig. 1A). Upon treatment with MMC (20 µg/mL) for 30 min, the ΔdprA cells demonstrated a 1.3-log cycle drop in survival (Fig. 1B). UVC (Ultraviolet C) radiation resulted in a reduction of cell survival by approximately 0.5-log cycle at a dose of 1,000 J m⁻² (Fig. 1C). Furthermore, DSB repair kinetics was evaluated using PFGE. Interestingly, the ΔdprA mutant exhibited faster DSB repair compared to the wild type. Within 1 h of post-irradiation recovery (PIR), approximately 11 distinct bands appeared on the gel after genome digestion with NotI, while the corresponding pattern for the wild type emerged only after 2 h of PIR (Fig. 1D). The PFGE banding patterns of the ΔdprA mutant were nearly identical to those of the wild type, indicating the absence of large genomic rearrangements (Fig. 1D). These findings suggest that DprA is involved in DSB repair and that its absence leads to accelerated repair kinetics for gamma radiation-induced DSBs. Intriguingly, ΔdprA mutant cells showed sensitivity to the DNA-damaging agent MMC (Fig. 1B). However, the DSB repair profile of ΔdprA mutant cells revealed delayed repair of MMC-induced DSBs compared to wild-type cells, contrasting with the repair profile observed after exposure to gamma radiation-induced DSBs (see Fig. S1 at https://barc.gov.in/publications/aem01948-23r1/index.html). These findings suggest that DSBs induced by different agents may employ distinct repair strategies, highlighting the complexity of DSB repair mechanisms in D. radiodurans.
Fig 1.

The cell survival of D. radiodurans and its ΔdprA mutant. (A) Gamma radiation survival curves for wild-type (-●-) and ΔdprA (-■-) mutants in exponential growth phase. Cells were exposed to varying doses of gamma radiation, and the percentage of surviving cells was plotted as a function of radiation dose (kGy). Data represent the mean ± SEM of three independent experiments. (B) MMC survival curves for wild-type (-●-) and ΔdprA (-■-) mutants in exponential growth phase. Cells were treated with 20 µg/mL MMC for different time periods (0, 10, 20, and 30 min), and colony-forming unit counts were obtained after appropriate dilutions and plating on TGY plates. The percentage of surviving cells was plotted against treatment time (min). Data represent the mean ± SEM of two independent experiments. (C) UV survival curves for wild-type (-●-) and ΔdprA (-■-) mutants in exponential growth phase. Cells were subjected to varying UV doses, and after dilution, aliquots were spotted on TGY plates and incubated at 32°C for 48 h. The percentage of surviving cells was plotted against UV dose (J m−2). Data represent the mean ± SEM of two independent experiments. (D) The DSBs repair kinetics of wild type and ΔdprA of D. radiodurans. PFGE was utilized to evaluate the qualitative DSBs repair kinetics. The NotI-digested DNA from unirradiated cells (U) and irradiated cells at different PIR time (0, 1, 2, 4, and 8 h) after exposure to 6 kGy. Lambda PFG molecular mass standards (lane M).
ΔdprA mutant show a limited contribution of ESDSA repair
The observed faster kinetics of DSB repair in the ΔdprA mutant, in comparison to wild-type cells (Fig. 1D), prompted inquiries regarding the potential involvement of ESDSA repair in this mutant. The ESDSA repair process in D. radiodurans is a patchwork-like DSBs repair pathway that involves joining together DNA fragments synthesized before irradiation with newly synthesized double-stranded DNA (dsDNA) blocks after irradiation. Remarkably, both the pre-existing and newly formed dsDNA blocks in the repaired chromosomes exhibited a similar size, typically ranging from 20 to 30 kb, giving rise to what is known as ESDSA repair (22, 23, 36). It is worth emphasizing that BrdU, unlike natural DNA bases, is highly photosensitive. Consequently, if the repaired genome features segments of old-thymine DNA (dsDNA with both strands containing thymine) and new-BrdU DNA (dsDNA with both strands containing BrdU instead of thymine), intracellular UV-induced DNA photolysis will result in the generation of DSBs due to the photosensitive nature of new-BrdU DNA, and the repaired genome will appear as shattered genome when analyzed on a PFGE gel. Conversely, if only one DNA strand undergoes BrdU substitution via semi-conservative replication repair, leading to single-strand breaks, the repaired genome will not exhibit UV-induced DNA photolysis (22, 23, 36).
To investigate ESDSA repair in wild-type and ΔdprA mutants of D. radiodurans, we performed an ESDSA repair experiment by gamma irradiation of wild-type and ΔdprA mutant cells with 6 kGy doses followed by labeling cells with BrdU for 3 h and then using UV light to break the repaired DNA (Fig. 2). PFGE analysis revealed that the pattern of NotI-digested DNA bands in gamma-unirradiated cells of both wild-type and ΔdprA mutant strains was nearly identical (Fig. 2A, lanes 1–2). In contrast, 6kGy gamma-irradiated cells exhibited low-molecular-weight DNA fragments indicative of DSBs generation by radiation (Fig. 2A, lanes 3–4). Following 3 h of PIR in TGY medium, both wild-type and ΔdprA mutant cells exhibited repaired high-molecular-weight DNA bands when grown in TGY medium with and without BrdU supplementation, indicating DSB repair (Fig. 2A, lanes 5–6 and 9–10). Interestingly, the UV sensitivity of repaired DNA was similar for both wild-type and ΔdprA mutants after PIR in TGY medium (Fig. 2A, lanes 7–8). However, when cells were allowed to repair their genome in BrdU-supplemented TGY medium followed by UV photolysis (500 J m−2), a dramatic increase in the photosensitivity of repaired DNA (increased abundance of low-molecular-weight DNA) was observed for wild-type cells (Fig. 2A, lane 11) compared to ΔdprA mutant cells (Fig. 2A, lane 12). These results suggest that ΔdprA mutant exhibit a reduced contribution of ESDSA repair, as evidenced by the absence of renewed DSBs in the repaired genome after reconstitution in BrdU medium and subsequent UV photolysis. In contrast, wild type displayed the characteristic patchwork repair pattern associated with ESDSA repair and showed renewed DSBs formation due to increased photosensitivity in the BrdU substituted genome (Fig. 2A, compare lanes 11 and 12). To further investigate, we conducted a UV dose-dependent experiment. Both wild-type and ΔdprA mutant cells recovered in BrdU medium were exposed to incremental doses of UV radiation. The results demonstrated that wild-type repaired DNA exhibited increasing sensitivity to UV doses (100 to 3,000 J m−2), with incremental damage and generation of DSBs in the genome. In contrast, the repaired DNA of ΔdprA mutant cells did not display any UV photolysis sensitivity until exposed to a UV dose of 500 J m−2. However, further increases in UV doses led to the formation of DSBs and genome degradation (Fig. 2B).
Fig 2.
The UV photolysis of the genome of D. radiodurans repaired in the presence and absence of BrdU. (A) The NotI restriction pattern of wild-type D. radiodurans (W) and ΔdprA mutant (Δd) (lanes 1–12) is displayed, along with Lambda PFG molecular mass standards (lane 13). The DNA from cells that were unirradiated (lanes 1, 2), irradiated cells (6 kGy) immediately after exposure (0 h PIR) (lanes 3, 4), irradiated cells incubated for 3 h in TGY medium (lanes 5, 6), irradiated cells incubated for 3 h in TGY medium and exposed to 500 J m−2 of UV light (lanes 7, 8), and irradiated cells repaired in BrdU-supplemented TGY medium prior (lanes 9, 10) or after (lanes 11, 12) exposure to 500 J m−2 of UV are shown. (B) The UV photolysis of cells of wild type and ΔdprA recovered in BrdU-supplemented TGY medium post-irradiation is presented. The DNA from unirradiated cells and 6 kGy gamma-irradiated cells (I) repaired in BrdU-supplemented TGY medium for 3 h followed by UV exposure (0.1, 0.5, 1, 2, and 3 kJ m−2). Lambda PFG molecular mass standards (lane M).
These findings suggested that the ΔdprA mutant might have limited involvement of ESDSA repair pathway in the repair of DSBs and may alternatively be facilitated by the error-prone SSA pathway. This assertion gains further credence when examining the spontaneous rifampicin resistance frequency in wild-type and ΔdprA mutants. The objective was to explore whether the fast DSB repair might encourage error-prone SSA repair pathways, potentially resulting in a higher frequency of rifampicin resistance. To assess this, we exposed cells to varying doses of gamma radiation and performed 10−6 dilution plating in duplicate on both TGY and TGY rifampicin plates. This allowed us to count viable cells and rifampicin-resistant cells, respectively. Our results revealed a remarkable increase of over ~140-fold in normalized colony-forming units (CFU) of rifampicin-resistant colonies in the ΔdprA mutant compared to the wild type at 10 kGy (data not shown). This observation underscores the potential consequence of the fast DSB repair kinetics possibly driven by the SSA pathway in the ΔdprA mutant, leading to the error-prone DSB repair and gamma radiation-sensitive phenotype at higher doses. Additionally, these findings also suggest that the dprA gene might play a role in the selection of DSB repair pathways in heavily gamma-irradiated D. radiodurans.
Deletion of dprA gene in ΔddrB mutant improves gamma radiation cell survival and recovery
The preceding results illustrate that in ΔdprA mutant cells, the SSA pathway may predominantly contribute to DSB repair, primarily due to the reduced involvement of ESDSA. These findings suggest that DprA may play a crucial role in determining the appropriate repair pathway selection between SSA and ESDSA. In light of these results, we sought to investigate further the role of DprA in regulating the differential use of SSA and ESDSA repair pathways in D. radiodurans cells subjected to acute doses of gamma radiation. Upon exposure to gamma radiation, Deinococcus cells upregulate a set of proteins, including novel SSA repair-specific DdrA and DdrB. Furthermore, DdrB has been shown to play multiple roles in cellular processes, including participating in the SSA repair, relieving blocked replication forks, and aiding in early genome repair after exposure to high doses of ionizing radiation (38, 46–51). Meanwhile, DdrA has been shown to assist DdrB in its repair functions. Another radiation-induced protein, PprA, is involved in DNA repair and cell division and is rapidly recruited to the site of damage where it forms a complex with other repair proteins to stabilize the broken ends of the DNA and facilitate efficient repair (52, 53).
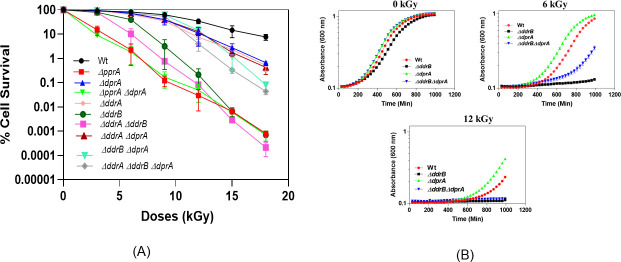
Our focus is on understanding the interplay between DprA and these proteins in modulating the balance between SSA and ESDSA repair pathways in response to gamma radiation exposure. To examine how DprA modulates the balance between SSA and ESDSA DSB repair pathways in response to gamma radiation, we deleted the dprA gene in the genetic background lacking pprA, ddrA, or ddrB genes, and monitored DSB repair and cell survival in the resulting double mutants. The results showed that the ΔdprA and ΔddrA single mutants were only sensitive to high doses of gamma radiation (>10 kGy), consistent with expectations, while the ΔpprA and ΔddrB mutants exhibited high sensitivity to gamma radiation (Fig. 3A and see Fig. S2 at https://barc.gov.in/publications/aem01948-23r1/index.html). The ΔddrA ΔddrB double mutant showed slightly lower survival rates compared to the ΔddrB single mutant (Fig. 3A and see Fig. S2A). However, the deletion of the dprA gene in the ΔddrB or ΔddrA ΔddrB background significantly increased cell survival (~1.5-log cycle) compared to the ΔddrB/ΔddrA ΔddrB mutant alone (Fig. 3A and see Fig. S2A). This finding suggests that dprA and ddrB genes do not exhibit epistasis, indicating that their functions are not mutually dependent. Moreover, this observation also suggests that DprA may also interfere with ESDSA repair, which is the sole active DSB repair pathway in the absence of the SSA repair (in ΔddrB mutant). The growth curve analysis further corroborated these findings where wild-type and different mutants displayed similar growth patterns in the absence of irradiation (Fig. 3B, 0kGy). However, gamma-irradiated ΔddrB cells required a considerable amount of time (>1,000 min PIR) to recover from the stress induced by gamma radiation, whereas the ΔddrB ΔdprA mutant cells initiated growth after 400 min of PIR (Fig. 3B, 6 and 12 kGy). Besides this, ΔdprA cells exhibited a shorter lag phase and resumed growth earlier compared to wild-type cells (Fig. 3B, 6 and 12 kGy). Together, these data suggest that SSA repair-specific ddrB gene deletion renders D. radiodurans cells sensitive to gamma radiation and the deletion of the dprA gene in the ΔddrB mutant positively impacts cell survival and recovery from gamma radiation-induced stress possibly by influencing ESDSA repair in ΔddrB cells. We also investigated the effect of deleting the dprA gene in the ΔpprA genetic background and found that it had no impact on gamma radiation cell survival, which remained comparable to that of the individual single mutants (Fig. 3A and see Fig. S2B), suggesting epistasis relation of these genes. However, the ΔpprA ΔddrA double mutant was more sensitive to MMC than the ΔpprA mutant alone and exhibited a DSB repair defect in PFGE gel analysis (see Fig. S1 at https://barc.gov.in/publications/aem01948-23r1/index.html).
Fig 3.
Gamma radiation survival of various mutants of D. radiodurans. (A) Exponential growth phase cells of various D. radiodurans R1 mutants and wild type were exposed to different doses of gamma radiation, and the percent cell survival fraction was plotted as a function of the radiation doses (0, 3, 6, 9, 12, 15, and 18 kGy). Data represented as the mean ± SEM of three independent experiments were shown. (B) Cell viability and growth curve of wild type and its mutants after exposure to gamma radiation. Optical density at 600 nm was continuously measured using a microtiter-based density reader. Panel 0 kGy shows the growth of normal, untreated cells, panel 6 kGy displays cells treated with a dose of 6 kGy of gamma radiation, and panel 12 kGy illustrates cells treated with a dose of 12 kGy of gamma radiation.
DprA has inhibitory role on ESDSA repair pathway in D. radiodurans
Nucleolytic processing of broken DNA ends is crucial for all recombination mechanisms, including SSA and ESDSA repair. In D. radiodurans, resection of DSBs directs repair toward ESDSA repair mediated by RecA or by SSA repair of repetitive sequences catalyzed by DdrB. ESDSA repair involves synthesis-dependent DNA end resection, while SSA repair does not require DNA synthesis following resection (23, 36, 46, 54). Therefore, in order to better understand the inhibitory role of the DprA protein on the ESDSA repair pathway, we examined the process of DNA end resection and synthesis involved in DSBs repair. [3H]-thymidine prelabeled genome analysis revealed minimal DNA degradation in the absence of gamma radiation exposure, with similar levels observed across the wild-type and various mutants (within a statistical variation of 10%) (Fig. 4A). However, upon exposure to a 6 kGy dose of gamma radiation, the wild type exhibited the characteristic DNA degradation pattern, initiating promptly after radiation treatment and persisting for 1.5 h post-irradiation time (PIR) (Fig. 4B). In contrast, the ΔdprA mutant displayed a brief DNA degradation phase of 0.5 h. Notably, the ΔddrB ΔdprA double mutant, which exhibited improved gamma survival compared to the ΔddrB alone mutant, showed a DNA degradation pattern similar to the wild type but for a reduced duration of approximately 1 h and with less DNA degradation than the ΔddrB alone mutant (Fig. 4B). The ΔpprA ΔdprA mutant demonstrated different results compared to the ΔpprA alone mutant, as DNA degradation was absent until 2 h PIR in both mutants, then ΔpprA showed DNA degradation but the degradation phase continued until 5 h PIR in the ΔpprA ΔdprA mutant (Fig. 4B).
Fig 4.

Measurement of DNA degradation and synthesis in wild-type and various mutants. (A and B) The DNA degradation was measured in [3H]- thymidine prelabeled unirradiated (A) and 6 kGy irradiated (B); wild-type and different mutants at different PIR or respective sham control and the data are represented as mean ± SEM of two independent experiments. (C to H) The global rate of DNA synthesis was measured by incorporating [3H]-thymidine during a 15-min pulse labeling in 6 kGy-irradiated wild-type and various mutants cells, and the results are presented as counts per minute (cpm).
ESDSA repair is characterized by DNA degradation followed by extensive DNA synthesis during repair following irradiation, while SSA repair does not involve significant DNA synthesis (23, 36, 46, 54). In our study, we evaluated DNA synthesis data in wild-type cells and different mutants using a [3H]-thymidine pulse for 15 min at various PIR time points (Fig. 4C through H). Concurrently, we examined the kinetics of DSB repair using PFGE (Fig. 1D and see Fig. S3 at https://barc.gov.in/publications/aem01948-23r1/index.html). Our results indicated that DNA synthesis in wild-type cells commenced after 1.5 h of PIR (Fig. 4C). Interestingly, the ΔdprA mutant exhibited an earlier onset of DNA synthesis, occurring after just 0.5 h PIR (Fig. 4D), while in the ΔddrB mutant, DNA synthesis began after 2 h PIR (Fig. 4E). Notably, the early repair of DSBs in the ΔdprA mutant coincided with the early onset of DNA synthesis in this mutant (Fig. 1D and 4D). Furthermore, the ΔddrB ΔdprA double mutant exhibited an earlier initiation of DNA synthesis (~1.5 h PIR) compared to the ΔddrB mutant (Fig. 4E and F). This observation suggests that the presence of DprA in the ΔddrB mutant may hinder ESDSA repair, as the ΔddrB mutant relies on the ESDSA pathway for DSB repair due to the absence of a functional SSA pathway.
DNA synthesis was considerably delayed (>3 h) in the ΔpprA and ΔpprA ΔdprA double mutants (Fig. 4G and H). The delay in DNA synthesis observed in the ΔpprA and ΔddrB mutants correlated well with their gamma radiation-sensitive phenotype and DSB repair kinetics, as evidenced by the NotI pattern on the PFGE gel and consistent with previous studies (see Fig. S3 at https://barc.gov.in/publications/aem01948-23r1/index.html). To provide further support for these findings, cell survival and growth analysis data of both the wild type and ΔdprA mutant strains overexpressing DprA from the pRAD plasmid was done (Fig. 5). Notably, a dominant negative effect on cell survival and growth curve was observed in wild-type cells overexpressing DprA, particularly at gamma radiation doses tested (6 and 12 kGy) (Fig. 5A and B). A similar effect was also observed in the ΔdprA mutant strain overexpressing DprA, albeit with less severity due to the absence of chromosomal doses of DprA (Fig. 5A). The dominant negative effect of the dprA gene was further substantiated by growth curve analysis. The results revealed that ectopic expression of DprA significantly extended the lag phase of growth in both wild-type and ΔdprA mutant cells, leading to an approximate 200-min delay (Fig. 5C). In conclusion, data suggest that DprA protein plays an inhibitory role in the ESDSA DSB repair pathway along with SSA pathway of D. radiodurans and overexpression of DprA negatively affects cell survival and growth.
Fig 5.

The cell survival of wild-type and ΔdprA mutant of D. radiodurans was assessed under gamma radiation by overexpressing DprA from the pRAD shuttle vector. Both the wild-type and ΔdprA mutant strains were transformed with either the pRAD control plasmid or the pRADdprA plasmid for ectopic expression of DprA. (A) These transformed cells were exposed to various gamma radiation doses, and after dilution, aliquots were spotted on TGY plates and incubated at 32°C for 48 h. (B) The percentage of cell survival fraction was plotted as a function of gamma radiation doses (kGy). (C) Additionally, the optical density at 600 nm was measured for the wild-type and ΔdprA mutant strains overexpressing DprA under untreated conditions, as well as after exposure to 6 and 12 kGy of gamma radiation. The data suggested that ectopic overexpression of DprA negatively affects the cell survival and growth of D. radiodurans.
DprA has potential to sequester RecA focus in vivo
RecA protein plays a crucial role in the ESDSA repair pathway in D. radiodurans. RecA loading onto ssDNA facilitated by RecFOR complex as RecBC complex is absent in D. radiodurans (55). DprA is proposed to bind to ssDNA and protect it from degradation by nucleases during natural transformation (56) and facilitate RecA loading during DNA recombination between different bacterial species (39). The cost and benefit of transforming DNA during natural transformation are subjective and context-specific. However, under genotoxic stress, transforming DNA may introduce additional DNA strand breaks, potentially negating the beneficial effects such as the use of transforming DNA as a nutritional source (57). Therefore, the observed inhibitory effect of DprA in ESDSA repair could be explained by the hypothesis that during early ESDSA repair, the RecFOR complex facilitates RecA loading onto ssDNA. However, due to its recombination mediator-like activities, DprA may compete with RecFOR to load RecA protein onto ssDNA generated through radiation-induced ssDNA ends or on transforming DNA, thereby reducing the availability of ssDNA-bound RecA foci capable of catalyzing ESDSA repair.
To test this hypothesis, we evaluated the percentage frequency of RecARFP foci formation in wild-type, ΔddrB, and ΔdprA mutants. The data showed that RecA foci formation increased immediately after irradiation but gradually returned to unirradiated levels by 4 h PIR. In ΔddrB mutant, RecA foci formation elevated at 4 h PIR. However, this dynamic pattern was not observed in the ΔdprA mutants until 4 h PIR (Fig. 6 and see Fig. S4 at https://barc.gov.in/publications/aem01948-23r1/index.html). Furthermore, one-way analysis of variance (ANOVA) of the 0-h PIR samples from the wild type, ΔdprA, and ΔddrB showed a significant difference in RecA foci formation (P = 0.006) (Fig. 6 and see Table S1 at https://barc.gov.in/publications/aem01948-23r1/index.html). These findings suggest that DprA may actively facilitate the formation of the RecA nucleoprotein complex with ssDNA, potentially due to its recombination mediator-like activities. However, it remains unclear whether RecA foci formed with DprA-loaded ssDNA are proficient in recombination repair or defective. Nevertheless, the microscopy data, in conjunction with the previously presented findings on cell survival and DSB repair kinetics, suggest that DprA may exerts its function by diminishing the effective availability of RecA-ssDNA complex molecules for the RecFOR-mediated ESDSA recombination repair pathway. This reduction ultimately leads to a deceleration of the ESDSA repair process.
Fig 6.

Frequency of RecA foci formation in wild-type and different mutants. Fluorescence microscopy images of D. radiodurans cells expressing DrRecARFP in logarithmic growing wild-type and mutant strains (ΔdprA and ΔddrB). The expression of DrRecA was visualized in the RFP channel (561 nm) and under differential interference contrast (DIC). Error bars show the SEM, and asterisks indicate statistically significant differences when 0-hr PIR was compared to unirradiated cells (one-way ANOVA, P = 0.006).
DISCUSSION
D. radiodurans, known for its exceptional resistance to extreme conditions, possesses distinct repair mechanisms that efficiently mend DNA fragments in to functional genome. The bacterium employs two primary pathways for repairing DSBs: the RecA-dependent ESDSA pathway and the RecA-independent SSA pathway. The SSA pathway plays a crucial role in the early repair of fragmented genomes, while the ESDSA pathway involves DNA degradation and extensive synthesis to accomplish patch-type repair (22, 23, 36, 46, 47, 54). SSA repair reduces the number of small DNA fragments by transforming them into larger fragments suitable for ESDSA-mediated homologous recombination repair (46, 54). The SSA pathway in D. radiodurans relies on DdrB and DdrA proteins. DdrB aids in DNA annealing during SSA repair, while DdrA helps to limit DNA degradation by nucleases (46, 47, 51). The presented data in this study emphasize the crucial role of the NT-specific protein DprA in governing the selection between DSB repair pathways (ESDSA and SSA) in D. radiodurans. Cell survival experiments revealed distinct susceptibility of ΔdprA mutant to gamma radiation, UV radiation, and MMC, indicating the vital functions of dprA gene in cell survival particularly against MMC and higher doses of gamma radiation (Fig. 1 and and see Fig. S1 at https://barc.gov.in/publications/aem01948-23r1/index.html). The differential responses of the ΔdprA mutants to MMC and gamma radiation may be attributed to distinct mechanisms of DSB repair for each agent, as supported by previous studies (35, 58).
Interestingly, data presented in Fig. 1D and 2 reveal that in wild-type cells, both the SSA and ESDSA pathways contribute to the repair of severely damaged DNA. However, in the absence of DprA, the SSA pathway becomes the primary mechanism for repairing DSBs, resulting in diminished efficiency in error-free patchwork ESDSA repair (Fig. 2). During DSB repair, the resected ssDNA can serve as a template for either SSA or ESDSA, depending on factors such as the length of ssDNA tails or the presence of homology/DNA damage in the resected region. The selection of one pathway over the other is influenced by various factors, leading to an interdependent relationship between the two pathways. In D. radiodurans, maintaining a balance between the SSA and ESDSA repair pathways is critical for survival during recovery from genotoxic stress (22). SSA repair plays a pivotal role in initiating ESDSA repair by providing a suitable substrate for the formation of RecA nucleoprotein filaments (46). However, key repair proteins from both pathways may compete for repair, favoring SSA repair in the early stages of DSB repair (46, 47). DdrB promotes rapid annealing of complementary strands using short homology, providing a suitable DNA substrate for ESDSA repair (50). RecA, a crucial protein for ESDSA repair, efficiently searches for homology and facilitates error-free repair (36, 59). In heavily irradiated D. radiodurans cells, small DNA fragments may not serve as optimal substrates for RecA-catalyzed ESDSA repair. Hence, prioritizing the RecA-independent phase becomes necessary for efficient DSB repair.
Results suggested that in the ΔdprA mutant, SSA takes precedence as the primary mechanism for repair and ramps up DSB repair (Fig. 1D and 2). Furthermore, the diminished role of ESDSA repair in the ΔdprA mutant indicates the apparent role of DprA in regulating the selection between SSA and ESDSA repair pathways in D. radiodurans exposed to gamma radiation (Fig. 2 and 4). This observation is further supported by cell biology data showing improved survival of the ΔddrB ΔdprA double mutant and ΔddrA ΔddrB ΔdprA triple mutant compared to the ΔddrB and ΔddrA ΔddrB mutants (Fig. 3 and see Fig. S2A at https://barc.gov.in/publications/aem01948-23r1/index.html). Based on our findings, the decrease in ESDSA repair observed in the ΔdprA mutant may be attributed to a concurrent increase in SSA repair. Consequently, we anticipate that the deletion of the dprA gene in the ΔddrB or ΔddrA ΔddrB mutant (which lacks SSA repair) would enhance ESDSA repair, resulting in improved cell survival of these cells (Fig. 3A and see Fig. S2A). This notion is further supported by the relatively faster repair of DSBs in ΔddrB ΔdprA mutants compared to ΔddrB alone (see Fig. S3 at https://barc.gov.in/publications/aem01948-23r1/index.html), cellular growth (Fig. 3B), and the rapid DNA synthesis kinetics with a short DNA degradation phase observed in the ΔdprA and ΔddrB ΔdprA mutants (Fig. 4). These observations were further supported by the dominant negative effect of DprA overexpression in wild-type cells (Fig. 5). Thus, data suggested that DprA may inhibit RecA-mediated ESDSA recombination repair, and its deletion in the ΔddrB mutant can mitigate this inhibition, ultimately enhancing the survival of ΔddrB ΔdprA mutant cells. The deletion of the dprA gene in the ΔpprA genetic background had no effect on gamma radiation cell survival, indicating that DprA is less likely to affect PprA functions during DSB repair in D. radiodurans (52, 53).
The right selection of DSB repair pathway is crucial to preserve genomic stability. For eukaryotes, cell growth phase and availability of undamaged homologous DNA for repair are crucial parameters for the selection of DSB repair pathway. Bloom syndrome helicase (BLM helicase), along with 53BP1 and RIF1, has been shown to be key players in the initial stages of DSB repair pathway determination in mammalian cells (60). In bacteria, RecA proteins act as molecular search engines during recombination repair, facilitating DNA strand exchange between homologous DNA strands for error-free repair (61, 62). RecA activity is regulated by various RMPs that interact with it, aiding in its loading onto ssDNA or its removal from it. These RMPs include RecF, RecO, RecR, DprA, PprA, RecU, and RecX. The RecFOR and DprA assist in assembling RecA onto ssDNA to form RecA-nucleoprotein filament, while PprA, RecU, and RecX negatively regulate RecA activity to prevent hyper-recombination by dislodging RecA molecules from ssDNA (3, 39, 59, 63–69). The formation of RecA foci on ssDNA is significantly reduced in the ΔdprA mutant compared to the wild type, and there are no dynamic changes in RecA foci formation during PIR (Fig. 6 and see Fig. S4 at https://barc.gov.in/publications/aem01948-23r1/index.html). These findings suggest that DprA, through its RMP-like activity, may be able to effectively compete with repair-specific RMPs like RecFOR for the loading of RecA onto SSB-coated ssDNA ends, thereby slowing down the ESDSA repair process. This may indirectly enhance the preference for SSA repair. For D. radiodurans, the level of RecA increases immediately after cells receive acute doses of gamma radiation (70). However, the role of RecA in ESDSA and homologous recombination (HR)-mediated repair begins at a somewhat later time during post-irradiation recovery (~1.5 h) (23, 36). Therefore, it is crucial to control RecA function until SSA repair is active in the early hours of post-irradiation recovery, and the role of DprA in interfering with RecA recombination activity appears to be an effective and attractive mechanism for regulating RecA activity and efficiently utilizing various DSB repair pathways. Thus, DprA protein RMP-like activities justify its inhibitory effect on ESDSA repair. The intriguing aspect of DprA involvement in inhibiting SSA repair calls for a deeper exploration of its potential direct or indirect contributions to the SSA repair process. One plausible mechanism could involve direct competition with key DdrA/DdrB repair proteins for access to ssDNA, as DprA has previously demonstrated the capacity to effectively replace SSBs (67). Another plausible mechanism by which DprA may hinder both SSA and ESDSA repair, albeit operating at distinct levels, is its potential to induce the generation of additional DSBs. This proposition draws from observations made during the recovery process after UV stress in Acinetobacter baylyi, which implies a potential role for DprA in such DSB generation (57). Together, the findings of this study are summarized in a proposed scheme for efficient DSB repair in D. radiodurans that involves DprA-mediated selection of SSA and ESDSA repair pathways (Fig. 7). It is suggested that in the ΔdprA mutant, SSA repair takes on a more significant role in the repair process with modest increase in ESDSA function as well. In other genetic backgrounds, varying levels of SSA and ESDSA contribution are suggested (Fig. 7).
Fig 7.

Model explaining the DprA role in DSB repair of D. radiodurans. The DSB repair process in D. radiodurans proceeds through a RecA-independent phase (A), which is supported by the SSA repair pathway, with crucial involvement of the DdrB and DdrA proteins. During this RecA-independent phase, the DNA fragments size reached up to 25–30 kb fragments. These smaller DNA fragments are then joined together with the assistance of ESDSA and HR repair mechanisms, resulting in the complete repair of the genome (B). In the lower panel, the relative status of SSA and ESDSA repair is depicted. In the wild-type D. radiodurans, SSA repair takes place first, followed by ESDSA repair. However, in the ΔddrB mutant, the contribution of SSA repair is diminished, and repair primarily occurs through the ESDSA repair pathway. In the ΔdprA mutant, the data from the present study suggest that SSA repair contributes more significantly while ESDSA role may also be alleviated. In the ΔddrB ΔddrA mutant, SSA repair is significantly reduced, and the status of ESDSA repair remains unclear. Conversely, in the ΔddrB ΔdprA mutant, SSA repair is diminished, while ESDSA repair appears to be enhanced. For the ΔpprA mutant, the exact proportion of SSA and ESDSA repair contributions in the DSB repair process remains uncertain.
Collectively, our findings highlight the role of DprA in selecting appropriate DSB repair pathways in heavily irradiated D. radiodurans cells and demonstrate that DprA function extends beyond natural transformation in bacteria. This expanded role may explain the widespread presence of DprA even in bacteria lacking natural transformation capabilities (39).
MATERIALS AND METHODS
Bacterial strains, plasmids, chemicals, and growth medium
Bacterial strains were employed, including wild-type D. radiodurans R1 sourced from the ATCC (ATCC 13939), a ΔpprA mutant obtained from Prof. I. Narumi, Japan, and ΔddrA, ΔddrB, and ΔddrA ΔddrB double mutants obtained from Prof. Pascale Servant, France. The construction of ΔdprA mutant, as well as the deletion of the dprA gene in different genetic backgrounds, was carried out in the laboratory using previously described methods. To isolate the thymine-requiring (thy -) derivatives of the wild-type and various mutant strains, they were grown on a solid minimal medium containing thymine (50 µg/mL) and trimethoprim (100 µg/mL) as described elsewhere (23). All strains were grown in TGY medium (1% Bacto-tryptone, 0.1% glucose, 0.5% yeast extract) supplemented with appropriate antibiotics, as previously described (27). The media were supplemented with a variety of antibiotics as and when required, including kanamycin (8 µg/mL), spectinomycin (75 µg/mL), and chloramphenicol (3 µg/mL) for D. radiodurans. Ampicillin (100 mg/mL) for Escherichia coli. The shuttle vector pRADgro and its derivatives were maintained in E. coli strain HB101, as previously described (71). Standard molecular biology techniques were utilized, as outlined in the manual (Molecular Cloning: A Laboratory Manual. 4th Edition, Vol. II, Cold Spring Harbor Laboratory Press, New York. Green et al., 2012). Molecular biology-grade chemicals, enzymes, and salts were obtained from various sources, including Sigma Chemicals Company, USA, Roche Biochemicals, New England Biolabs (USA), and Merck India Pvt. Ltd., India. Radiolabeled nucleotides were obtained from the Board of Radiation and Isotope Technology (BRIT), Department of Atomic Energy, India. Please refer to Table S1 at https://barc.gov.in/publications/aem01948-23r1/index.html for a complete list of the bacterial strains, plasmids, and PCR primers employed in this study.
Cell survival studies
The experimental treatments for D. radiodurans cells survival studies included exposure to different doses of UV and gamma radiation as previously described (72), as well as treatment with MMC (20 µg/mL) following the protocol described elsewhere (27). Briefly, bacterial cultures grown in TGY medium at 32°C were washed and suspended in sterile phosphate buffered saline (PBS) and exposed to varying doses of gamma radiation at a dose rate of 5.86 kGy per hour (GC-5000, 60CO, Board of Radiation and Isotopes Technology, DAE, India). The treated cells were then plated on TGY agar plates supplemented with appropriate antibiotics, if necessary, and CFU were counted after a 48-h incubation period at 32°C. For UVC treatment, different dilutions of cells were plated and exposed to various doses of UV (254 nm) radiation and CFU measured after a 48-h incubation period at 32°C. For MMC treatment, cells were treated with 20 µg/mL MMC for different time periods (0, 10, 20, and 30 min), and CFU counts were obtained after appropriate dilutions and plating on TGY plates.
Measurement of DNA repair kinetics using pulsed-field gel electrophoresis
Irradiated cultures were diluted in TGY to an optical density at 600 nm (OD600)= 0.2 and incubated at 32°C. At indicated intervals, 5 mL samples were taken to prepare DNA plugs as described by Mattimore and Battista (73). The DNA contained in the plugs was digested with 60 units of NotI restriction enzyme for 16 h at 37°C. After digestion, the plugs were subjected to pulsed-field gel electrophoresis in 0.5 × Tris-borate-EDTA (TBE) buffer using a CHEF-DR III electrophoresis system (Bio-Rad) at 6 V/cm2 for 20 h at 14°C, with a linear pulse ramp of 50–90 s and a switching angle of 120o.
Rate of DNA degradation and DNA synthesis measured by [3H]-thymidine labeling
DNA degradation was estimated as mentioned elsewhere (22, 23, 36). In brief, pre-label D. radiodurans cultures, 10 mCi/mL [3H]-thymidine (BRIT, DAE, India, specific activity 18 Ci/mmol) was added and the cultures were grown for 18 h. To eliminate the radioactive thymidine present in the intracellular pools, the cultures were incubated for an additional hour in fresh TGY. Both irradiated and unirradiated cultures were diluted in TGY to an OD600 of 0.2 and agitated at 32°C. Samples of 50 µL were withdrawn at different time points and filtered using Whatman GF/C filters. The filters were then dried and washed twice with 10% TCA, once with 5% TCA, and briefly with 96% ethanol. The nondegraded DNA content was determined by scintillation counting of the dried filters using TRI-CARB 4910TR 110 V Liquid Scintillation Counter, Perkin Elmer.
For the DNA synthesis studies, unirradiated and irradiated exponentially growing cultures were incubated and 0.5 mL samples were taken and mixed with a 0.1 mL pre-warmed TGY medium containing 10 µCi [3H]-thymidine (BRIT, DAE, India, specific activity 18 Ci/mmol). Radioactive pulses were terminated after 15 min by addition of 2 mL ice-cold 10% TCA. Samples were kept on ice for at least 1 h, and then collected by suction onto Whatman GF/C filters followed by washing with 5% TCA and 96% ethanol. Filters were dried overnight at room temperature, and placed in 5 mL scintillation liquid. The precipitated counts were measured in a liquid scintillation counter.
BrdU incorporation and UV-induced photolysis of BrdU-substituted DNA
The D. radiodurans and its various mutants thy- culture were subjected to 6 kGy gamma radiation as described above. The irradiated culture was then diluted to an OD600 of 0.2 and grown in TGY supplemented with 5-BrdU for 3 h. After collecting the cells by centrifugation, they were resuspended in phosphate buffer and starved in the buffer for 1 h at 32°C. The cell suspension was then cooled on ice and exposed to various doses of 254 nm UV light in a thin layer. Both UV-irradiated and unirradiated cells were embedded in agarose plugs for subsequent analysis of DNA using PFGE (as described above).
RecARFP foci evaluation by confocal microscopy
Confocal microscopy was performed on an Olympus FV3000 confocal microscope with a 100×, 1.45 NA oil-immersion apochromatic objective lens. The laser beams were focused on the back focal plane, and the Fluoview software was used to control the intensity and time sequence of laser illumination. Fluorescence emission was collected through a DM-405/488 dichroic mirror and single-band emission filters (561 nm). For fixed-cell imaging, bacterial cells in TYG broth were grown to the mid-log phase. They were then fixed with 4% paraformaldehyde on ice for 10 min, followed by two washes with PBS. The cells were mounted on glass slides with a 1% agarose bed and observed under the microscope. Image analysis was performed using the cellSens software, analyzing 400–500 cells from at least two separate microscopic fields captured in two independent experiments. The data obtained were analyzed by one-way ANOVA in GraphPad Prism.
Statistical methods
The figures display the average values of at least three separate experiments with the standard error of the mean. For Fig. 6, a one-way ANOVA was conducted to determine whether the difference is statistically significant between unirradiated and 0-h irradiated samples (P = 0.006).
ACKNOWLEDGMENTS
The authors are also thankful to Shri Ranjit Sharma and Shri Lokesh Kumar from BARC for their valuable assistance in scintillation counting.
The authors would like to express their gratitude to Prof. Fabrice Confalonieri and Prof. Pascale Servant from Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell, France, for kindly providing various D. radiodurans mutant strains (ΔddrA, ΔddrB, and ΔddrA ΔddrB). Authors further would also like to extend their appreciation to Prof. Issay Narumi from Toyo University, Japan, for sharing the ΔpprA mutant.
Contributor Information
Yogendra Singh Rajpurohit, Email: ysraj@barc.gov.in.
Gladys Alexandre, University of Tennessee at Knoxville, Knoxville, Tennessee, USA.
REFERENCES
- 1. Chen I, Dubnau D. 2004. DNA uptake during bacterial transformation. Nat Rev Microbiol 2:241–249. doi: 10.1038/nrmicro844 [DOI] [PubMed] [Google Scholar]
- 2. Claverys J-P, Martin B, Polard P. 2009. The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiol Rev 33:643–656. doi: 10.1111/j.1574-6976.2009.00164.x [DOI] [PubMed] [Google Scholar]
- 3. Mortier-Barrière I, Velten M, Dupaigne P, Mirouze N, Piétrement O, McGovern S, Fichant G, Martin B, Noirot P, Le Cam E, Polard P, Claverys J-P. 2007. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell 130:824–836. doi: 10.1016/j.cell.2007.07.038 [DOI] [PubMed] [Google Scholar]
- 4. Quevillon-Cheruel S, Campo N, Mirouze N, Mortier-Barrière I, Brooks MA, Boudes M, Durand D, Soulet A-L, Lisboa J, Noirot P, Martin B, van Tilbeurgh H, Noirot-Gros M-F, Claverys J-P, Polard P. 2012. Structure–function analysis of pneumococcal DprA protein reveals that dimerization is crucial for loading RecA recombinase onto DNA during transformation. Proc Natl Acad Sci U S A 109:E2466–E2475. doi: 10.1073/pnas.1205638109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saunders CW, Guild WR. 1981. Pathway of plasmid transformation in Pneumococcus: open circular and linear molecules are active. J Bacteriol 146:517–526. doi: 10.1128/jb.146.2.517-526.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston C, Martin B, Fichant G, Polard P, Claverys J-P. 2014. Bacterial transformation: distribution, shared mechanisms and divergent control. Nat Rev Microbiol 12:181–196. doi: 10.1038/nrmicro3199 [DOI] [PubMed] [Google Scholar]
- 7. Moradigaravand D, Engelstädter J. 2013. The evolution of natural competence: disentangling costs and benefits of sex in bacteria. Am Nat 182:E112–E126. doi: 10.1086/671909 [DOI] [PubMed] [Google Scholar]
- 8. Stewart GJ, Carlson CA. 1986. The biology of natural transformation. Annu Rev Microbiol 40:211–235. doi: 10.1146/annurev.mi.40.100186.001235 [DOI] [PubMed] [Google Scholar]
- 9. Baltrus DA, Guillemin K, Phillips PC. 2008. Natural transformation increases the rate of adaptation in the human pathogen Helicobacter pylori. Evolution 62:39–49. doi: 10.1111/j.1558-5646.2007.00271.x [DOI] [PubMed] [Google Scholar]
- 10. Perron GG, Lee AEG, Wang Y, Huang WE, Barraclough TG. 2012. Bacterial recombination promotes the evolution of multi-drug-resistance in functionally diverse populations. Proc Biol Sci 279:1477–1484. doi: 10.1098/rspb.2011.1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bacher JM, Metzgar D, de Crécy-Lagard V. 2006. Rapid evolution of diminished transformability in Acinetobacter baylyi. J Bacteriol 188:8534–8542. doi: 10.1128/JB.00846-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engelmoer DJP, Donaldson I, Rozen DE. 2013. Conservative sex and the benefits of transformation in Streptococcus pneumoniae. PLoS Pathog 9:e1003758. doi: 10.1371/journal.ppat.1003758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Utnes ALG, Sørum V, Hülter N, Primicerio R, Hegstad J, Kloos J, Nielsen KM, Johnsen PJ. 2015. Growth phase-specific evolutionary benefits of natural transformation in Acinetobacter baylyi. ISME J 9:2221–2231. doi: 10.1038/ismej.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinha S, Mell J, Redfield R. 2013. The availability of purine nucleotides regulates natural competence by controlling translation of the competence activator Sxy. Mol Microbiol 88:1106–1119. doi: 10.1111/mmi.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoelzer MA, Michod RE. 1991. DNA repair and the evolution of transformation in Bacillus subtilis. III. Sex with damaged DNA. Genetics 128:215–223. doi: 10.1093/genetics/128.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michod RE, Wojciechowski MF, Hoelzer MA. 1988. DNA repair and the evolution of transformation in the bacterium Bacillus subtilis. Genetics 118:31–39. doi: 10.1093/genetics/118.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mongold JA. 1992. DNA repair and the evolution of transformation in Haemophilus influenzae. Genetics 132:893–898. doi: 10.1093/genetics/132.4.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Redfield RJ. 1993. Evolution of natural transformation: testing the DNA repair hypothesis in Bacillus subtilis and Haemophilus influenzae. Genetics 133:755–761. doi: 10.1093/genetics/133.4.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Claverys JP, Prudhomme M, Martin B. 2006. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu Rev Microbiol 60:451–475. doi: 10.1146/annurev.micro.60.080805.142139 [DOI] [PubMed] [Google Scholar]
- 20. Engelmoer DJP, Rozen DE. 2011. Competence increases survival during stress in Streptococcus pneumoniae. Evolution 65:3475–3485. doi: 10.1111/j.1558-5646.2011.01402.x [DOI] [PubMed] [Google Scholar]
- 21. Kavanagh JN, Redmond KM, Schettino G, Prise KM. 2013. DNA double strand break repair: a radiation perspective. Antioxid Redox Signal 18:2458–2472. doi: 10.1089/ars.2012.5151 [DOI] [PubMed] [Google Scholar]
- 22. Slade D, Radman M. 2011. Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev 75:133–191. doi: 10.1128/MMBR.00015-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zahradka K, Slade D, Bailone A, Sommer S, Averbeck D, Petranovic M, Lindner AB, Radman M. 2006. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature 443:569–573. doi: 10.1038/nature05160 [DOI] [PubMed] [Google Scholar]
- 24. Misra H, Rajpurohit Y, Kota S. 2013. Physiological and molecular basis of extreme radioresistance in Deinococcus radiodurans. Curr Sci 104:194–205. [Google Scholar]
- 25. Misra HS, Khairnar NP, Barik A, Indira Priyadarsini K, Mohan H, Apte SK. 2004. Pyrroloquinoline-quinone: a reactive oxygen species scavenger in bacteria. FEBS Lett 578:26–30. doi: 10.1016/j.febslet.2004.10.061 [DOI] [PubMed] [Google Scholar]
- 26. Rajpurohit YS, Gopalakrishnan R, Misra HS. 2008. Involvement of a protein kinase activity inducer in DNA double strand break repair and radioresistance of Deinococcus radiodurans. J Bacteriol 190:3948–3954. doi: 10.1128/JB.00026-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajpurohit YS, Misra HS. 2010. Characterization of a DNA damage-inducible membrane protein kinase from Deinococcus radiodurans and its role in bacterial radioresistance and DNA strand break repair. Mol Microbiol 77:1470–1482. doi: 10.1111/j.1365-2958.2010.07301.x [DOI] [PubMed] [Google Scholar]
- 28. Sharma DK, Bihani SC, Siddiqui MQ, Misra HS, Rajpurohit YS. 2022. WD40 domain of RqkA regulates its kinase activity and role in extraordinary radioresistance of D. radiodurans. J Biomol Struct Dyn 40:1246–1259. doi: 10.1080/07391102.2020.1824810 [DOI] [PubMed] [Google Scholar]
- 29. Cox MM, Battista JR. 2005. Deinococcus radiodurans - the consummate survivor. Nat Rev Microbiol 3:882–892. doi: 10.1038/nrmicro1264 [DOI] [PubMed] [Google Scholar]
- 30. Timmins J, Moe E. 2016. A decade of biochemical and structural studies of the DNA repair machinery of Deinococcus radiodurans: major findings, functional and mechanistic insight and challenges. Comput Struct Biotechnol J 14:168–176. doi: 10.1016/j.csbj.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minton KW. 1994. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol Microbiol 13:9–15. doi: 10.1111/j.1365-2958.1994.tb00397.x [DOI] [PubMed] [Google Scholar]
- 32. Daly MJ, Gaidamakova EK, Matrosova VY, Kiang JG, Fukumoto R, Lee D-Y, Wehr NB, Viteri GA, Berlett BS, Levine RL. 2010. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS One 5:e12570. doi: 10.1371/journal.pone.0012570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daly MJ. 2009. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol 7:237–245. doi: 10.1038/nrmicro2073 [DOI] [PubMed] [Google Scholar]
- 34. Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Leapman RD, Lai B, Ravel B, Li S-M, Kemner KM, Fredrickson JK. 2007. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol 5:e92. doi: 10.1371/journal.pbio.0050092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim S, Jung J-H, Blanchard L, de Groot A. 2019. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol Rev 43:19–52. doi: 10.1093/femsre/fuy037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slade D, Lindner AB, Paul G, Radman M. 2009. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell 136:1044–1055. doi: 10.1016/j.cell.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 37. Moseley BE, Setlow JK. 1968. Transformation in Micrococcus radiodurans and the ultraviolet sensitivity of its transforming DNA. Proc Natl Acad Sci U S A 61:176–183. doi: 10.1073/pnas.61.1.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ithurbide S, Coste G, Lisboa J, Eugénie N, Bentchikou E, Bouthier de la Tour C, Liger D, Confalonieri F, Sommer S, Quevillon-Cheruel S, Servant P. 2020. Natural transformation in Deinococcus radiodurans: a genetic analysis reveals the major roles of DprA, DdrB, RecA, RecF, and RecO proteins. Front Microbiol 11:1253. doi: 10.3389/fmicb.2020.01253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharma DK, Misra HS, Bihani SC, Rajpurohit YS. 2023. Biochemical properties and roles of DprA protein in bacterial natural transformation, virulence, and pilin variation. J Bacteriol 205:e0046522. doi: 10.1128/jb.00465-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma DK, Misra HS, Soni I, Rajpurohit YS. 2022. Characterization of DNA processing protein A (DprA) of the radiation-resistant bacterium Deinococcus radiodurans. Microbiol Spectr 10:e0347022. doi: 10.1128/spectrum.03470-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arber W. 2014. Horizontal gene transfer among bacteria and its role in biological evolution. Life (Basel) 4:217–224. doi: 10.3390/life4020217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seitz P, Blokesch M. 2014. DNA transport across the outer and inner membranes of naturally transformable Vibrio cholerae is spatially but not temporally coupled. mBio 5:e01409-14. doi: 10.1128/mBio.01409-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pimentel ZT, Zhang Y. 2018. Evolution of the natural transformation protein, ComEC, in bacteria. Front Microbiol 9:2980. doi: 10.3389/fmicb.2018.02980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Attaiech L, Olivier A, Mortier-Barrière I, Soulet A-L, Granadel C, Martin B, Polard P, Claverys J-P. 2011. Role of the single-stranded DNA-binding protein SsbB in pneumococcal transformation: maintenance of a reservoir for genetic plasticity. PLoS Genet 7:e1002156. doi: 10.1371/journal.pgen.1002156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kidane D, Carrasco B, Manfredi C, Rothmaier K, Ayora S, Tadesse S, Alonso JC, Graumann PL. 2009. Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells. PLoS Genet 5:e1000630. doi: 10.1371/journal.pgen.1000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu G, Lu H, Wang L, Chen H, Xu Z, Hu Y, Tian B, Hua Y. 2010. DdrB stimulates single-stranded DNA annealing and facilitates RecA-independent DNA repair in Deinococcus radiodurans. DNA Repair (Amst) 9:805–812. doi: 10.1016/j.dnarep.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 47. Ithurbide S, Bentchikou E, Coste G, Bost B, Servant P, Sommer S. 2015. Single strand annealing plays a major role in RecA-independent recombination between repeated sequences in the radioresistant Deinococcus radiodurans bacterium. PLoS Genet 11:e1005636. doi: 10.1371/journal.pgen.1005636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Norais CA, Chitteni-Pattu S, Wood EA, Inman RB, Cox MM. 2009. DdrB protein, an alternative Deinococcus radiodurans SSB induced by ionizing radiation. J Biol Chem 284:21402–21411. doi: 10.1074/jbc.M109.010454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sugiman-Marangos S, Junop M. 2012. Crystallization of the DdrB-DNA complex from Deinococcus radiodurans. Acta Crystallogr Sect F Struct Biol Cryst Commun 68:1534–1537. doi: 10.1107/S1744309112044041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sugiman-Marangos SN, Weiss YM, Junop MS. 2016. Mechanism for accurate, protein-assisted DNA annealing by Deinococcus radiodurans DdrB. Proc Natl Acad Sci U S A 113:4308–4313. doi: 10.1073/pnas.1520847113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harris DR, Tanaka M, Saveliev SV, Jolivet E, Earl AM, Cox MM, Battista JR. 2004. Preserving genome integrity: the DdrA protein of Deinococcus radiodurans R1. PLoS Biol 2:e304. doi: 10.1371/journal.pbio.0020304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rajpurohit YS, Misra HS. 2013. Structure-function study of deinococcal serine/threonine protein kinase implicates its kinase activity and DNA repair protein phosphorylation roles in radioresistance of Deinococcus radiodurans. Int J Biochem Cell Biol 45:2541–2552. doi: 10.1016/j.biocel.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 53. Narumi I, Satoh K, Cui S, Funayama T, Kitayama S, Watanabe H. 2004. PprA: a novel protein from Deinococcus radiodurans that stimulates DNA ligation. Mol Microbiol 54:278–285. doi: 10.1111/j.1365-2958.2004.04272.x [DOI] [PubMed] [Google Scholar]
- 54. Daly MJ, Minton KW. 1996. An alternative pathway of recombination of chromosomal fragments precedes recA-dependent recombination in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol 178:4461–4471. doi: 10.1128/jb.178.15.4461-4471.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Makarova KS, Aravind L, Wolf YI, Tatusov RL, Minton KW, Koonin EV, Daly MJ. 2001. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol Mol Biol Rev 65:44–79. doi: 10.1128/MMBR.65.1.44-79.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang L, Tian X, Liu M, Wang M, Biville F, Cheng A, Zhu D, Jia R, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Huang J, Tian B, Yu Y, Liu Y, Zhang L, Pan L, Rehman MU, Chen X. 2019. DprA is essential for natural competence in Riemerella anatipestifer and has a conserved evolutionary mechanism. Front Genet 10:429. doi: 10.3389/fgene.2019.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hülter N, Sørum V, Borch-Pedersen K, Liljegren MM, Utnes ALG, Primicerio R, Harms K, Johnsen PJ. 2017. Costs and benefits of natural transformation in Acinetobacter baylyi. BMC Microbiol 17:34. doi: 10.1186/s12866-017-0953-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weng M, Zheng Y, Jasti VP, Champeil E, Tomasz M, Wang Y, Basu AK, Tang M. 2010. Repair of mitomycin C mono- and interstrand cross-linked DNA adducts by UvrABC: a new model. Nucleic Acids Res 38:6976–6984. doi: 10.1093/nar/gkq576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bentchikou E, Servant P, Coste G, Sommer S. 2010. A major role of the RecFOR pathway in DNA double-strand-break repair through ESDSA in Deinococcus radiodurans. PLoS Genet 6:e1000774. doi: 10.1371/journal.pgen.1000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grabarz A, Guirouilh-Barbat J, Barascu A, Pennarun G, Genet D, Rass E, Germann SM, Bertrand P, Hickson ID, Lopez BS. 2013. A role for BLM in double-strand break repair pathway choice: prevention of CtIP/Mre11-mediated alternative nonhomologous end-joining. Cell Rep 5:21–28. doi: 10.1016/j.celrep.2013.08.034 [DOI] [PubMed] [Google Scholar]
- 61. Lusetti SL, Cox MM. 2002. The bacterial RecA protein and the recombinational DNA repair of stalled replication forks. Annu Rev Biochem 71:71–100. doi: 10.1146/annurev.biochem.71.083101.133940 [DOI] [PubMed] [Google Scholar]
- 62. Bell JC, Kowalczykowski SC. 2016. RecA: regulation and mechanism of a molecular search engine. Trends Biochem Sci 41:491–507. doi: 10.1016/j.tibs.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carrasco B, Manfredi C, Ayora S, Alonso JC. 2008. Bacillus subtilis SsbA and dATP regulate RecA nucleation onto single-stranded DNA. DNA Repair (Amst) 7:990–996. doi: 10.1016/j.dnarep.2008.03.019 [DOI] [PubMed] [Google Scholar]
- 64. Carrasco B, Yadav T, Serrano E, Alonso JC. 2015. Bacillus subtilis RecO and SsbA are crucial for RecA-mediated recombinational DNA repair. Nucleic Acids Res 43:5984–5997. doi: 10.1093/nar/gkv545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lenhart JS, Brandes ER, Schroeder JW, Sorenson RJ, Showalter HD, Simmons LA. 2014. RecO and RecR are necessary for RecA loading in response to DNA damage and replication fork stress. J Bacteriol 196:2851–2860. doi: 10.1128/JB.01494-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Serrano E, Carrasco B, Gilmore JL, Takeyasu K, Alonso JC. 2018. RecA regulation by RecU and DprA during Bacillus subtilis natural plasmid transformation. Front Microbiol 9:1514. doi: 10.3389/fmicb.2018.01514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yadav T, Carrasco B, Serrano E, Alonso JC. 2014. Roles of Bacillus subtilis DprA and SsbA in RecA-mediated genetic recombination. J Biol Chem 289:27640–27652. doi: 10.1074/jbc.M114.577924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Y, Xu G, Wang L, Hua Y. 2019. Distinct roles of Deinococcus radiodurans RecFOR and RecA in DNA transformation. Biochem Biophys Res Commun 513:740–745. doi: 10.1016/j.bbrc.2019.04.042 [DOI] [PubMed] [Google Scholar]
- 69. Rajpurohit YS, Sharma DK, Misra HS. 2021. PprA protein inhibits DNA strand exchange and ATP hydrolysis of Deinococcus RecA and regulates the recombination in gamma-irradiated cells. Front Cell Dev Biol 9:636178. doi: 10.3389/fcell.2021.636178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tanaka M, Earl AM, Howell HA, Park M-J, Eisen JA, Peterson SN, Battista JR. 2004. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics 168:21–33. doi: 10.1534/genetics.104.029249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Misra HS, Khairnar NP, Kota S, Shrivastava S, Joshi VP, Apte SK. 2006. An exonuclease I-sensitive DNA repair pathway in Deinococcus radiodurans: a major determinant of radiation resistance. Mol Microbiol 59:1308–1316. doi: 10.1111/j.1365-2958.2005.05005.x [DOI] [PubMed] [Google Scholar]
- 72. Rajpurohit YS, Bihani SC, Waldor MK, Misra HS. 2016. Phosphorylation of Deinococcus radiodurans RecA regulates its activity and may contribute to radioresistance. J Biol Chem 291:16672–16685. doi: 10.1074/jbc.M116.736389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mattimore V, Battista JR. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol 178:633–637. doi: 10.1128/jb.178.3.633-637.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]