Abstract
Steroid receptor complexes are assembled through an ordered, multistep pathway involving multiple components of the cytoplasmic chaperone machinery. Two of these components are Hsp70-binding proteins, Hip and Hop, that have some limited homology in their C-terminal regions, outside the sequences mapped for Hsp70 binding. Within this region of Hip is a DPEV sequence that occurs twice; in Hop, one DPEV sequence plus a partial second sequence occurs. In an effort to better understand Hip function as it relates to assembly of progesterone receptor complexes, the DPEV region of Hip was targeted for mutations. Each DPEV sequence was mutated to an APAV sequence, singly or in combination. The combined mutation, APAV2, was further combined with a deletion of Hip’s tetratricopeptide repeat region that is required for Hsp70 binding or with a deletion of Hip’s GGMP repeat. An additional mutant was prepared by truncation of Hip’s DPEV-containing C terminus. By comparing interactions of various Hip forms with Hsp70, it was determined that mutation of the DPEV sequences created a dominant inhibitory form of Hip. The mutant Hip-Hsp70 complex was not prevented from interacting with progesterone receptor, but the mutant caused a dose-dependent inhibition of receptor assembly with Hsp90. The behavior of the Hip mutant is consistent with a model in which Hip and Hop are required to facilitate the transition from an early receptor complex with Hsp70 into later complexes containing Hsp90.
Prior to binding hormone, steroid receptor monomers are typically found in a multiprotein complex containing Hsp90 and Hsp90-associated proteins such as p23 and large immunophilins (for an extensive review, see reference 21). Receptors for progesterone (PR) and glucocorticoids (GR) must be assembled in these complexes in order to bind hormone with high affinity and efficiency. Studies of the receptor assembly process, relying primarily on cell-free reactions in rabbit reticulocyte lysate (RL), have revealed that assembly is an ordered process and involves at least eight proteins that are components of the molecular chaperone machinery. Several of these chaperone components appear transiently prior to formation of mature receptor complexes. For example, Hsp70 is the first protein observed to bind PR in a cell-free assembly, but Hsp70 is probably not a component of mature complexes (1, 25). Two Hsp70-associated proteins, Hip and Hop, are recovered in PR complexes shortly after the first appearance of Hsp70, but these proteins are also absent from mature complexes. A model in which Hip and Hop function in a coordinated manner to facilitate Hsp70-mediated folding of proteins has been proposed (8), but it is not clear how this relates to steroid receptor assembly.
Hop (alternate names in the literature are p60, IEF SSP 3521, mSti1, and RF-hsp70) is required for formation of mature PR (4) and GR (5, 6) complexes. In a yeast model system, deletion of the gene for Sti1, a Saccharomyces cerevisiae homolog for Hop, resulted in decreased function of heterologously expressed vertebrate GR (3). Hop binds Hsp70 through an N-terminal tetratricopeptide repeat (TPR) region, but it can simultaneously bind Hsp90 through an internal TPR (4, 17). Hop appears to be quantitatively associated with Hsp90, but there is typically a substoichiometric amount of Hsp70 in immunoaffinity-purified Hop complexes (24). In one report, evidence that Hop catalyzes the dissociation of ADP from Hsp70 in exchange for ATP was presented (11), but the absence of contaminating DnaJ or other activities in the protein preparations was not demonstrated.
Less is known about the functional requirement for Hip in the receptor assembly pathway. Hip was first noted as a transient component during the cell-free assembly of PR complexes (25) and was subsequently found to be associated with Hsp70 (19). In a screen for proteins interacting with the ATPase domain of Hsp70, Hip was identified and shown to stabilize binding of Hsp70 to a misfolded protein substrate (12). Hip binds Hsp70 in an ADP-dependent manner (12) through a central TPR motif (15, 20). There is no apparent homolog for Hip in the S. cerevisiae genomic database. Since vertebrate steroid receptors function in yeast, this would argue against an absolute need for Hip, but it is possible that Hip’s function is fulfilled by some other factor in yeast.
In designing Hip mutants, we chose to target the C-terminal region of Hip that shows some limited homology with the C-terminal region of Hop. In this report, mutations in this region of Hip are shown to generate dominant inhibitory forms of Hip that block in vitro assembly of Hop and Hsp90 with PR complexes.
MATERIALS AND METHODS
In vitro expression plasmids.
A QuikChange site-directed mutagenesis kit (Stratagene) was used to substitute alanines for the aspartic and glutamic acid residues in the DPEV sequences, beginning at codons 318 and 327, contained in Hip’s C-terminal region. A 1.4-kb cDNA containing the open reading frame and 3′ untranslated region of the human Hip gene (19) was subcloned into pSPUTK plasmid (Stratagene) for construction of APAV mutants. Sequences for the mutagenic oligonucleotides targeting the first and second DPEV sequences, respectively, were 5′-GAAATTCTTAGTGCTCCAGCGGTTCTTGCAGCCATG and 5′-CATGGCTGCAAGAACCGCTGGAGCACTAAGAATTTC (the mutated bases are underlined). Mutants were created for each DPEV alone (APAV-a and APAV-b) and for the two DPEV sites in combination (APAV2).
The generation of plasmids encoding ΔGGMP, ΔTPR, and N-303 was described previously (20). To create combinations of APAV2 with ΔTPR (ΔTAP) and ΔGGMP (ΔGAP), the mutated region of the APAV2 cDNA was substituted into the corresponding position of the deletion mutants by using a unique SphI restriction enzyme site.
The preparation of a plasmid encoding the ATPase domain of rat Hsc70 (Hsc70-AD) was described previously (20). A plasmid encoding rat Hsc70’s peptide-binding domain (Hsc70-PD) was prepared by first using site-directed mutagenesis to introduce an NcoI site at codon 379. The resulting cDNA, containing an EcoRI site at the 3′ cloning site, was digested with NcoI and EcoRI and subcloned into pSPUTK. The point mutant K71E was prepared by site-directed mutagenesis of codon 71 of rat Hsc70 cDNA cloned into pSPUTK. The sequences of all mutated plasmids were confirmed by automated sequencing.
All plasmids were expressed in vitro in a combined transcription-translation system (TnT Lysate; Promega) according to the guidelines suggested by the manufacturer. Protein products were radiolabeled by inclusion of [35S]methionine (DuPont/NEN; specific activity, 1,200 Ci/mmol) in the synthesis mixture. Two microliters of each synthesis mixture was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the dried gel was autoradiographed. Bands were quantitated by laser scanning densitometry (Molecular Dynamics). After adjustment for the number of methionines in each product, equivalent molar amounts of radiolabeled products could be determined.
Bacterial expression and purification of recombinant Hip forms.
Expression and purification of Hip and mutants involved generating a fusion protein (Hip-intein/CBD) in which Hip’s C terminus is contiguous with a self-cutting intein and chitin-binding domain (CBD). First, a PCR product was generated by using Hip/pSPUTK as a template, a forward oligonucleotide primer complementary to the 5′ pSPUTK cloning site (5′-GCAGAAGCTCAGAATAAACGC), and a reverse primer complementary to the final seven codons of the open reading frame for Hip (5′-CGCTTGACCTCCAAATTTGGC). A high-fidelity polymerase (Deep Vent Exo+; New England Biolabs) was used for 25 cycles of amplification. The PCR product was digested with NcoI and subcloned into pCYB4 (Impact I kit; New England Biolabs) that had been digested with NcoI and SmaI. Automated sequencing was performed to verify the correct sequence of the entire cloned PCR product.
To enhance expression of the Hip-intein/CBD fusion protein in bacteria, the region coding for the fusion protein was removed from pCYB4 and subcloned into pET28 (Novagen). With the Hip-intein/CBD/pET28 plasmid as the host, mutant Hip-intein/CBD constructs were prepared by replacing the wild-type Hip sequence with mutant Hip cDNA sequences. Transformation of BL21 bacterial cells, expression of protein, and cell extract preparation were performed as described previously (20).
Proteins were purified from bacterial extracts by using reagents and instructions supplied with the Impact I kit. Briefly, fusion proteins were bound to a chitin affinity resin. After being washed, the resin was incubated overnight with reducing agent to promote intein-mediated cleavage of Hip proteins from the fusion product. The cleaved Hip products were collected in 20 mM Tris-HCl–50 mM NaCl–0.1 mM EDTA and concentrated by using Centriprep 30 devices (Amicon). The C terminus of each product differed from that of native Hip by the addition of a single glycine residue.
Interaction of Hip forms with Hsp70.
The abilities of Hip forms to bind Hsp70 immobilized on an immunoaffinity resin were assessed essentially as described previously (20). Briefly, anti-Hsp70 monoclonal antibody BB70 was adsorbed to protein G-Sepharose (Pharmacia) and used to immunoprecipitate Hsp70 from rabbit RL (1:1; from Green Hectares, Oregon, Wis.) that was supplemented with a radiolabeled Hip form. Each sample contained the same molar equivalent of radiolabeled Hip or Hip mutant in 100 μl of RL with 15 μg of BB70 on a 10-μl resin pellet. All samples were incubated at 30°C for 30 min. The resin pellets were divided and washed three times with wash buffer (WB) (20 mM Tris [pH 7.4], 50 mM NaCl, 10 mM monothioglycerol, 0.5% Tween 20) or WB plus 0.5 M NaCl. Proteins adsorbed to resin were separated by SDS-PAGE and visualized by Coomassie blue staining and autoradiography of the dried gel.
In an alternate set of experiments to compare interactions of wild-type Hip (wtHip) and APAV2 with Hsp70, purified recombinant Hip forms were first adsorbed to an immunoaffinity resin. The resin was prepared by binding the anti-Hip monoclonal antibody 2G6 (19) to protein A-Sepharose (Pharmacia). To avoid later contamination by antibody heavy chain in the gel migration region for Hip, 2G6 was covalently coupled to resin according to a published procedure (23). Radiolabeled rat Hsc70 or mutant K71E was prepared by transcription-translation (TnT kit; Promega) of in vitro expression plasmids in the presence of [35S]methionine. A 10-μl pellet of immunoaffinity resin containing 10 μg of wtHip or APAV2 was added to 100 μl of rabbit RL (Green Hectares) supplemented with radiolabeled Hsc70 or K71E. Resin complexes were incubated at 30°C for 30 min, washed with WB, and separated by SDS-PAGE for Coomassie blue staining and autoradiography.
Radiolabeled Hsc70-AD and Hsp70-PD were prepared by in vitro transcription-translation of the appropriate plasmids. To distinguish interactions of Hip forms with the separate Hsp70 domains, an aliquot of each radiolabeled synthesis mixture was added to 100 μl of incubation buffer (20 mM Tris-HCl, 10 mM monothioglycerol, 50 mM NaCl, 5 mM ADP, 5 mM MgCl2, and 0.5% Tween 20) containing wtHip or APAV2 resin complexes. Samples were incubated at 30°C for 30 min, divided equally, and washed with either WB alone or WB plus 0.5 M NaCl. Protein components on resins were separated by SDS-PAGE and visualized by Coomassie blue staining and autoradiography.
PR reconstitution reactions.
With some minor modifications, PR assembly reactions were performed as described previously (27). Briefly, individual reactions were performed in 100 to 200 μl of RL supplemented with an ATP-regenerating system and containing approximately 1 μg of recombinant chicken PR-A bound to 15 μg of monoclonal antibody PR22 on a 10-μl pellet of protein G- or protein A-Sepharose. The resins were incubated for 30 to 45 min at 30°C, washed four times with WB, and extracted with SDS sample buffer.
In one set of reactions, RL was immunodepleted of endogenous Hip in the following manner. In the first depletion step, 100 μg of monoclonal antibody 3A4, which recognizes only free Hip (19), was adsorbed to a 15-μl pellet of protein G-Sepharose and gently rocked with 1 ml of RL at 4°C for 8 h. The supernatant from this step was retreated with a combination of anti-Hip antibodies (3A4, 2G6, and 10D1; 100 μg total) for an additional 8 h at 4°C. Mock-depleted RL was prepared by two treatments with PR22. The efficiency of Hip depletion was estimated by quantifying Western-immunostained bands from 2 μl of mock-depleted or Hip-depleted lysates. PR reconstitutions were performed with either mock-depleted or Hip-depleted lysates, and the relative recovery of Hip in PR complexes was quantitated by densitometry of Western-stained bands.
In another set of experiments, purified recombinant Hip forms were added to RL for use in PR assembly reactions. Either wtHip or mutant Hip forms were added to RL at final concentrations as high as 12 μM (approximately a 12-fold molar excess over endogenous Hip levels). In control experiments, up to 32 μM α-casein or reduced carboxymethylated lactalbumin (RCMLA) (Sigma) was added in place of Hip forms. PR assembly reactions were performed as usual.
Dynamics of Hip and Hsp70 interactions.
Pulse-chase-type experiments were performed to monitor the exchange of Hsp70 in PR complexes or in Hip-Hsp70 complexes. In the PR experiment, complexes were assembled in two phases. In both phases, 800 μl of RL which contained an ATP-regenerating system and 20 μg of geldanamycin (GA) per ml to enhance formation of early and intermediate PR complexes containing Hsp70 and Hip was used (27). Also in both phases, the RL was supplemented with a 10-fold molar excess of purified wtHip, APAV2, or N-303. In the first assembly phase only, samples contained radiolabeled Hsp70. After steady-state assembly conditions were established in the first phase (30 min at 30°C), PR-resin complexes were transferred to fresh RL prewarmed to 30°C for the second assembly phase. At 0, 1, 2, 3, 5, 10, 15, and 25 min, 100-μl aliquots were removed from the second-assembly mixtures and immediately quenched with 1 ml of cold WB to inhibit further assembly reactions. As a negative control, a parallel set of samples with PR22 resin lacking PR-A was used. All samples were separated by SDS-PAGE, Coomassie blue stained, and autoradiographed. Stained protein bands and bands on X-ray film were quantitated by densitometry.
To measure exchange of Hsp70 on immobilized Hip forms, recombinant intein-CBD fusion proteins (wtHip, APAV2, or ΔTAP) were preadsorbed to chitin resin. In a similar experiment, His-tagged wtHip or mutant N-303 was adsorbed to Ni2+-resin. Hip forms on resin (42 μg of protein on a 70-μl resin pellet) or Ni2+-resin alone was separately added to 700 μl of RL supplemented with an ATP-regenerating system and 5 to 8 μl of radiolabeled Hsp70 synthesis mixture. After a 30-min incubation at 30°C, the resins were transferred to fresh, prewarmed RL lacking radiolabeled Hsp70. As with PR complexes, aliquots were removed at various times and quenched in cold WB. Samples were separated by SDS-PAGE, Coomassie blue stained, and autoradiographed.
Hop binding to purified Hsp70.
Hop was immunoaffinity purified from RL that was adjusted to 0.4 M NaCl to dissociate Hop complexes containing Hsp70 and Hsp90. Each sample contained 10 μg of monoclonal antibody F5 adsorbed to a 10-μl pellet of protein A-Sepharose (Pharmacia) and approximately 1 μg of immunoadsorbed Hop. Reaction mixtures (100 μl, final volume) were prepared by combining 70 nM purified bovine Hsc70 (StressGen) alone or with 700 nM α-casein, RCMLA, or bovine serum albumin (BSA) in buffer (20 mM Tris [pH 7.4], 50 mM KCl, 5 mM ADP, 5 mM MgCl2, 10 mM monothioglycerol, and 0.5% Tween 20) and preincubating the mixture at 30°C for 15 min. Each reaction mixture was then added to Hop or control resin pellets and incubated for an additional 20 min at 30°C. The resins were washed four times in cold WB, and bound proteins were extracted into SDS sample buffer for gel separations.
RESULTS
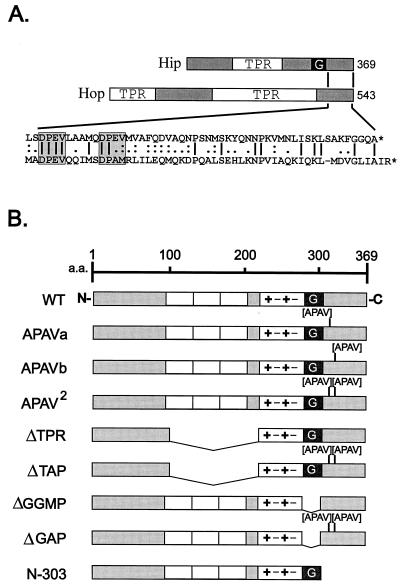
It was noted previously that the C-terminal region of Hip shows some limited homology with Hop (12), although this region does not appear to be required for known protein interactions of either Hip (15, 20) or Hop (4, 17). In Fig. 1A, the sequences of Hip and Hop are diagramed with the amino acid sequences of their C termini aligned. The two DPEV sequences in the C terminus of Hip were selected as target sites for mutagenesis.
FIG. 1.
(A) Comparison of Hip and Hop sequences. Hsp70-binding proteins Hip and Hop both contain TPR domains. In Hip, the TPR domain plus surrounding highly charged sequences is required for Hsp70 binding. Also contained in Hip is a GGMP repeat domain (G) whose function is unknown. Hop contains two TPR regions; the N-terminal one is required for Hsp70 binding, and the central TPR binds to Hsp90. Little homology is shared between the highly degenerate TPR regions of Hip and Hop. The region of greatest similarity is near the C terminus of each protein (amino acid sequence alignment in exploded view at bottom). Most notable in this region are two DPEV sequences in Hip that align with a DPEV and DPAM in Hop (highlighted by shaded boxes). (B) Hip mutants. Each of the DPEV sequences in Hip was mutated to APAV, as illustrated. The mutation of both DPEV sequences (APAV2) was also combined with previously developed mutants in which the TPR or GGMP domains had been deleted. Another mutant from previous studies is the truncation mutant N-303, which lacks the DPEV-containing C terminus of Hip. TPR domains (open boxes), highly charged regions (+−+−), and a region of Hop homology (shaded box) at the C terminus are shown. a.a., amino acids; WT, wild type.
Expression plasmids encoding each of the Hip mutants diagramed in Fig. 1B were generated. The two DPEV sequences were mutated to APAV, either singly (APAV-a and APAV-b) or in combination (APAV2). ΔTAP is a combination of APAV2 with ΔTPR, a mutant shown to be deficient in Hsp70 binding (20), and ΔGAP is an APAV2 combination with ΔGGMP, a mutant in which Hip’s GGMP repeat region is deleted. N-303 is a previously described truncation mutant lacking the C-terminal 66 amino acids of Hip (20).
The ability of Hip mutants to enter PR complexes was tested in the following manner. Plasmids encoding wtHip and mutant Hip proteins were expressed in vitro in the presence of [35S]methionine to produce radiolabeled products. An aliquot of each synthesis mixture was individually added to normal rabbit RL containing an ATP-regenerating system and the Hsp90-binding drug GA (28). GA competes for ATP binding to Hsp90 (10, 22) and blocks formation of mature PR complexes while enhancing recovery of early and intermediate complexes containing Hsp70, Hsp90, Hop, and Hip (27). The RL mixtures were then used to support the cell-free assembly of PR complexes with each sample containing an equimolar amount of radiolabeled wtHip or mutant.
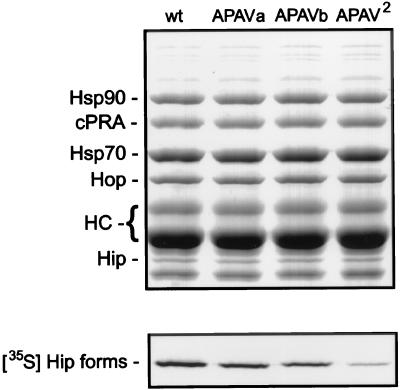
PR complexes were separated by SDS-PAGE and Coomassie blue stained (Fig. 2, upper panel), and then the dried gel was subjected to autoradiography (lower panel). Stained bands representing recombinant chicken PR-A, nonreceptor components of the complexes, and antibody heavy chains are indicated. The Hip bands seen in the upper panel are endogenous rabbit Hip from RL; its abundance in the assembly mixtures far exceeds the amount of radiolabeled human Hip forms added to the mixtures. The unmarked band below rabbit Hip and other minor bands are RL proteins that bind nonspecifically to antibody resins. As measured by densitometry of bands on the autoradiograph, APAV-a and APAV-b were recovered in PR complexes at approximately one-half the level of wtHip, but recovery of the combination APAV2 mutant was only 10% of the level of wtHip.
FIG. 2.
Association of radiolabeled APAV mutants with PR complexes. PR complexes were assembled in vitro by using RL supplemented with radiolabeled wtHip (wt) or the three APAV mutants. After assembly, components were separated by SDS-PAGE and visualized by Coomassie blue staining (upper panel). Proteins associated with recombinant chicken PR-A (cPRA) are indicated on the left. Also labeled are the heavy-chain bands (HC) from antibody used to immobilize PR-A for assembly reactions. The gel was dried and autoradiographed (lower panel) to reveal binding by radiolabeled Hip forms.
Since the amount of radiolabeled Hip forms present in the RL assembly mixtures is much less than the amount of endogenous Hip, no conclusions as to whether Hip mutants disrupt PR assembly can be drawn from this experiment. However, there is a clear deficit of DPEV mutants relative to wtHip recovered in PR complexes.
Interactions of Hip mutants with Hsp70.
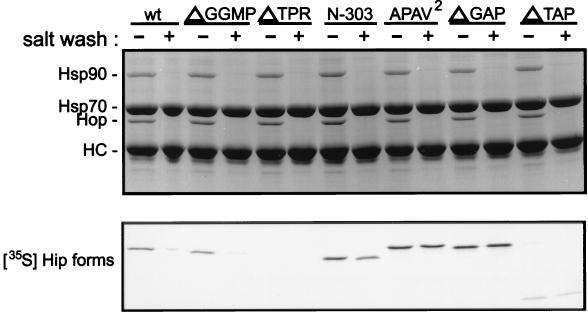
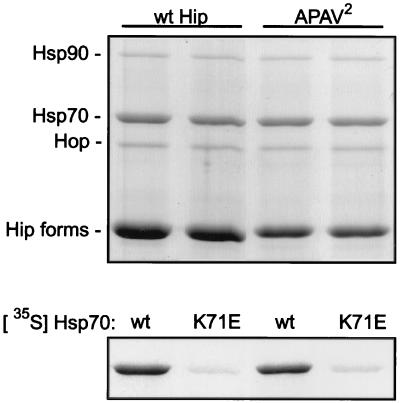
Hip transiently associates with PR complexes (19, 25, 27) indirectly through its binding to the ATPase domain of Hsp70 (12). Mutants were tested for their association with Hsp70, as shown in Fig. 3. Radiolabeled Hip forms were added to RL, and Hsp70 complexes were immunoprecipitated with anti-Hsp70 monoclonal antibody BB70. Hip binding to Hsp70 is normally salt sensitive, but mutant N-303 was earlier observed to bind Hsp70 in a salt-resistant manner (20). The immunoprecipitates were divided and washed in buffer either lacking NaCl or containing 0.5 M NaCl. Samples were separated by SDS-PAGE and Coomassie blue stained (Fig. 3, upper panel), and the dried gel was subjected to autoradiography (lower panel).
FIG. 3.
Coprecipitation of Hip forms with Hsp70. Hsp70 complexes were immunoprecipitated from RL supplemented with the radiolabeled Hip forms indicated above the lanes (wt, wtHip). An equimolar amount of each radiolabeled Hip form was added to separate mixtures for immunoprecipitation reactions. Immunoprecipitates were washed in buffer containing either no additional salt (−) or 0.5 M NaCl (+). Proteins in resin complexes were separated by SDS-PAGE and visualized by Coomassie blue staining (upper panel). Hsp70 and the coprecipitating proteins Hop and Hsp90 are indicated on the left. Hip is not evident in the stained gel due to its comigration with anti-Hsp70 BB70 heavy chain (HC). Coprecipitating radiolabeled Hip forms were detected by autoradiography of the dried gel (lower panel).
In the stained gel, the coprecipitation of Hsp90 and Hop with Hsp70 is evident, but endogenous rabbit Hip in Hsp70 complexes is not apparent, since Hip comigrates with the heavy chain of BB70. Previous studies have shown that Hsp90 does not directly interact with Hsp70 but is recovered due to the mutual binding of Hsp70 and Hsp90 with Hop (4). Hip and Hop bind separate sites on Hsp70, since either protein binds Hsp70 alone and the two proteins can bind Hsp70 concomitantly (19).
As observed previously (20), wtHip and ΔGGMP coprecipitate with Hsp70 in a salt-sensitive manner, ΔTPR shows no binding to Hsp70, and N-303 binds to Hsp70 in a salt-resistant manner. It has been shown that Hip’s TPR and adjacent highly charged region are required for Hsp70 binding (15, 20). To explain the salt-resistant binding of N-303 to Hsp70, it was previously proposed that the mutant is capable of binding both the ATPase and the peptide-binding domains of Hsp70, with the exposed GGMP in the truncated protein being a substrate for Hsp70’s peptide-binding domain. Supporting this view was the observed return to salt-sensitive binding in the truncation mutant N-276, which lacks the GGMP repeat (20). If true, then mutant N-303 would be expected to compete with misfolded protein substrates for binding to Hsp70. However, the proposal for dual binding to Hsp70 domains by N-303 must be revised in light of the results with APAV2, ΔGAP, and ΔTAP. As seen in Fig. 3, APAV2 and ΔGAP, which lacks the GGMP repeat, both bind Hsp70 in a salt-resistant manner. On the other hand, little binding to Hsp70 is observed with ΔTAP, which retains the GGMP repeat. These results suggest that binding of Hip mutants to Hsp70 may be strictly dependent on Hip TPR interactions with Hsp70’s ATPase domain, although GGMP and C-terminal Hip sequences can influence the biochemical nature of Hip interactions, perhaps by altering the conformation of Hip’s TPR domain.
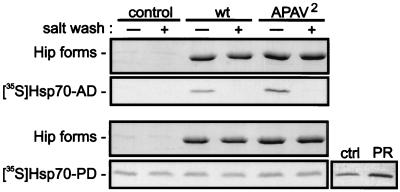
Binding of wtHip and APAV2 to the separate Hsp70 domains was assessed directly (Fig. 4). Purified recombinant Hip forms were bound to a covalently coupled immunoaffinity resin and suspended in buffer supplemented with the radiolabeled ATPase domain (Hsc70-AD) or peptide-binding domain (Hsc70-PD). After incubation, the resins were washed in either low- or high-salt buffer, and proteins were separated by SDS-PAGE. Coomassie blue staining was used to demonstrate the quantity of wtHip or APAV2 in each sample. Autoradiographs of the stained gels revealed the salt-sensitive binding of either Hip form to Hsc70-AD. However, no specific interaction was detected between Hsc70-PD and either wtHip or APAV2. Recovery of radiolabeled Hsc70-PD in the Hip samples was no greater than its recovery on immunoaffinity resin lacking Hip. Although Hsc70-PD binding in this assay is somewhat promiscuous, specific binding of Hsc70-PD to PR, a natural substrate for Hsp70, was detected, indicating that the isolated peptide-binding domain retains its ability to bind substrates. As judged by densitometry of bands on the autoradiograph, the association of Hsc70-PD with PR was 2.5-fold greater than its association with control resin lacking PR. As expected for Hsp70-substrate interactions, the enhanced recovery of Hsc70-PD with PR was maintained following washing with 0.5 M NaCl (not shown).
FIG. 4.
Association of wtHip and APAV2 with separate Hsp70 domains. For the main panels, recombinant wtHip (wt) or APAV2 was adsorbed to a covalently coupled immunoaffinity resin. The Hip resins or resin lacking bound Hip (control) were incubated in WB plus 5 mM ADP and 5 mM MgCl2. The buffer was further supplemented with the radiolabeled synthesis mixtures for the ATPase domain of Hsc70 (Hsc70-AD) or the peptide-binding domain (Hsc70-PD). Hip immunoprecipitates were divided and washed in buffer lacking (−) or containing (+) 0.5 M NaCl. As a positive control for Hsc70-PD binding to substrate, radiolabeled Hsc70-PD was incubated with immunoadsorbed PR or with immunoaffinity resin lacking PR (ctrl). After SDS-PAGE separation of components, the gels were Coomassie blue stained (Hip forms) and autoradiographed ([35S]Hsp70AD and [35S]Hsp70-PD).
Hip binds Hsp70, and Hsp70 can simultaneously bind Hop-Hsp90 (19). To test whether APAV2 alters Hsp70 interactions with Hop-Hsp90, recombinant Hip forms were bound to immunoaffinity resin and incubated with RL. As determined from gel analysis of immunoprecipitates (Fig. 5, upper panel), Hop and Hsp90 coprecipitate equally with wtHip and APAV2.
FIG. 5.
Bulk interactions of Hip forms and their association with an Hsp70 mutant lacking ATPase activity. Recombinant wtHip or APAV2 was preadsorbed to a covalently coupled immunoaffinity resin. The resins were subsequently incubated in RL containing an ATP-regenerating system. Proteins on the resin complexes were separated by SDS-PAGE and Coomassie blue stained (upper panel). Stained bands representing coprecipitating Hsp70, Hop, and Hsp90 are indicated on the left. Radiolabeled Hsp70 forms were included in the bulk reaction mixtures, and the association of these forms with wtHip and APAV2 was resolved by autoradiography of the stained gel (lower panel). Either radiolabeled wild-type Hsp70 (wt) or an ATPase-deficient point mutant (K71E) was included in the reaction mixtures.
Another characteristic of Hip is that it binds Hsp70 in an ADP-dependent manner (12, 19). To test whether APAV2 differs in this regard, we compared it with wtHip for binding to two forms of Hsp70: wild-type Hsp70, which hydrolyzes bound ATP to ADP, and a K71E point mutant that binds ATP but lacks any ATPase activity (18). Radiolabeled Hsp70 forms were included in samples, and autoradiography of the gel (Fig. 5, lower panel) revealed that neither wtHip nor APAV2 bound K71E.
Inhibition of PR assembly by C-terminal Hip mutants.
The transient participation of Hip in PR assembly in vitro argues for a functional role of Hip in this process. Evidence that Hip stabilizes Hsp70 interactions with misfolded substrates has been presented elsewhere (12), but we have had difficulty in demonstrating a requirement for Hip in PR assembly. Having multiple specific monoclonal antibodies which recognize Hip (19), we have added them to RL in various combinations in hopes of inhibiting Hip-mediated steps in PR assembly but have observed no effects (20a). Another approach has been to immunodeplete Hip from RL prior to assembly reactions. By this method, we have estimated that Hip’s concentration in RL is approximately 1 μM (35 to 40 μg/ml), about the same as Hsp70’s molar concentration.
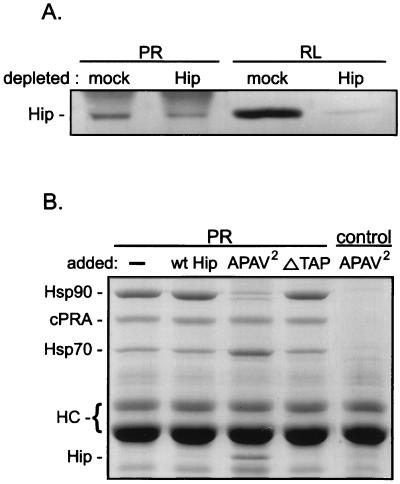
Immunodepletion of Hip from RL was attempted to demonstrate a requirement for Hip in PR assembly. However, the results were inconclusive but informative. The image in Fig. 6A is a Western-immunostained blot illustrating relative Hip levels in mock-depleted and depleted RL and in PR complexes assembled from these lysates. Densitometry of the RL bands indicated that greater than 90% of the total Hip was removed, but Hip recovery from PR complexes was reduced only by 40%. The compositions of the PR complexes, whether assembled in mock- or Hip-depleted lysate, were identical (Coomassie blue-stained lanes not shown). The discrepancy between Hip removal from RL and its recovery from PR complexes demonstrates a selective enrichment of Hip in PR complexes. Possibly, the small fraction of Hip remaining in the depleted RL is sufficient to maintain any requirement for Hip in the assembly process.
FIG. 6.
Hip involvement in PR assembly. (A) RL that was mock depleted or immunodepleted of endogenous Hip was used for assembly of PR complexes in vitro. The resulting PR complexes (PR lanes) and the total RL used for assembly reactions (RL lanes) were immunostained for the presence of Hip. (B) Purified recombinant Hip forms were added to RL at a 10-fold molar excess over endogenous Hip levels. The RL mixtures were used for PR assembly reactions, and the resulting PR complexes were separated by SDS-PAGE and visualized by Coomassie blue staining. The sample in the first lane contained no added protein, and the sample in the final lane (control) lacked PR in the assembly reaction mixture. Proteins associated with PR (recombinant chicken PR-A [cPRA]), along with the PR22 heavy chains (HC), are indicated on the left.
As an alternate functional test of Hip, PR complexes were assembled in RL supplemented with purified, recombinant Hip forms (Fig. 6B). The assembly mixtures contained a 10-fold molar excess (relative to endogenous Hip levels in RL) of wtHip, APAV2, or ΔTAP. Several changes in PR complexes are observed exclusively for APAV2. First, Hsp90 is greatly reduced in PR complexes, essentially down to the background level of nonspecific binding; at the same time, Hsp70 recovery increases. Note also that APAV2 is readily evident in PR complexes but not on a control resin lacking PR. Evidently, APAV2 arrests PR assembly at an early stage at which Hsp70 and APAV2 bind but formation of the intermediate complex containing Hsp90 and Hop is prevented. Since this effect is not seen with an equal amount of ΔTAP that lacks Hsp70 binding, the inhibition of Hsp90 binding to PR by APAV2 probably relates to APAV2 interactions with Hsp70.
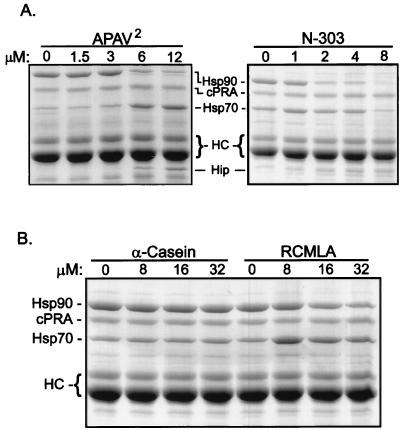
As shown in Fig. 7A, APAV2 and the truncation mutant N-303, which lacks the DPEV-containing C terminus, both inhibit PR assembly in a dose-dependent manner. Again, APAV2 inhibited Hsp90 association, with concomitant increases in Hsp70 and APAV2 in the receptor complex. N-303 also efficiently blocks Hsp90 binding. Half-maximal inhibition of Hsp90 binding occurs when Hip mutants are present in three- to sixfold molar excess over endogenous Hip. Unlike APAV2, N-303 does not cause a dose-dependent increase in Hsp70, and recovery of N-303 on PR complexes is somewhat less than for APAV2. N-303, which is 66 amino acids shorter than wtHip or APAV2, comigrates with an RL protein that binds nonspecifically to resins; however, a modest dose-dependent increase in N-303’s association with PR complexes can be detected. It is not understood why APAV2 and N-303 differ in their effects on Hsp70 accumulation in PR complexes, but it might relate to C-terminal sequences other than DPEV that are absent from N-303 and retained in APAV2.
FIG. 7.
Dose dependence and specificity of PR assembly inhibition. (A) PR complexes were assembled in RL containing the concentrations of added APAV2 or N-303 indicated above the lanes. Protein components on the resulting resin complexes were separated by SDS-PAGE and Coomassie stained. (B) Similar PR reconstitutions were performed with Hip mutants replaced by the concentrations of the Hsp70 substrate α-casein or RCMLA indicated above the lanes.
Since Hsp70 is required at an early stage for assembly of receptor complexes (14, 26), anything that inhibits Hsp70 function would be expected to inhibit PR assembly at some level. If APAV2 is misfolded, it could competitively inhibit Hsp70 binding to PR. Inconsistently with this interpretation, APAV2 is recovered in PR complexes and Hsp70 recovery increases. Also, APAV2 does not appear to bind Hsp70’s peptide-binding domain (Fig. 3 and 4). To further minimize the likelihood that APAV2 is competitively inhibiting Hsp70 by binding its peptide-binding site, we compared two frequently studied Hsp70 substrates for their abilities to inhibit PR assembly (Fig. 7B). Since Hsp70 is required for PR assembly and recognizes PR as a substrate, other Hsp70 substrates should inhibit PR assembly to some degree. However, little inhibition of PR assembly was observed with a 32 μM concentration of α-casein, although inhibition by this substrate was observed at much higher concentrations (results not shown). Partial inhibition of PR assembly was observed with RCMLA, but RCMLA is less effective as an inhibitor than either APAV2 or N-303.
Hip is able to oligomerize (12), and this activity maps within the first 14 amino acids at Hip’s N terminus (20). The physiological significance of Hip oligomerization is unknown, but oligomerization-deficient N-terminal truncation mutants were observed to bind Hsp70 and assemble in PR complexes, similarly to wtHip (20). To test if oligomerization is necessary for the inhibitory property of APAV2, this mutant was combined with a truncation of Hip’s first 14 amino acids. However, the monomeric combined mutant inhibited PR assembly, similarly to APAV2 (results not shown).
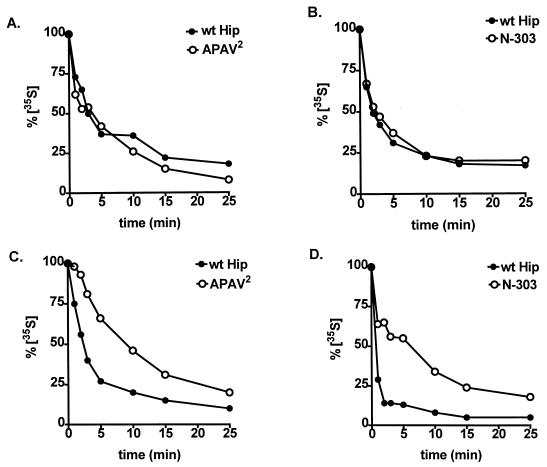
Effects of Hip forms on Hsp70 binding dynamics.
Since Hip can stabilize Hsp70 binding to some substrates (12), we tested whether APAV2 or N-303 inhibits PR assembly by altering the dynamics of Hsp70-PR interactions (Fig. 8A and B). PR assembly proceeded through an initial pulse phase during which radiolabeled Hsp70 was present and steady-state assembly dynamics were established. In a subsequent chase phase, radiolabeled Hsp70 was excluded, and assembly was allowed to proceed for different times. During both phases, a 10-fold molar excess of recombinant wtHip, APAV2, or N-303 was present. Neither APAV2 (Fig. 8A) nor N-303 (Fig. 8B) altered the exchange of Hsp70 on PR complexes compared with wtHip. In all cases, the half-time to reach new steady-state levels of radiolabeled Hsp70 in PR complexes was approximately 2 min.
FIG. 8.
Effects of mutant Hip forms on the dynamics of Hsp70 interactions. The rates of exchange of radiolabeled Hsp70 on PR complexes (A and B) and on Hip complexes (C and D) were compared in the presence of wtHip (A to D), APAV2 (A and C), or N-303 (B and D). As detailed in Materials and Methods, PR or Hip complexes were assembled in RL containing radiolabeled Hsp70 and then transferred to fresh RL lacking radioactivity. At the times indicated, aliquots were removed from the secondary assemblies. Each sample was separated by SDS-PAGE, Coomassie blue stained to monitor total protein levels, and autoradiographed to measure the binding of radioactive Hsp70. Bands were quantitated by densitometry, and radioactive bands were normalized to the amount of Coomassie blue-stained Hsp70 in each sample. The resulting values are plotted as a percentage of radiolabeled Hsp70 present at initiation of the secondary assembly reactions (0 min).
In a similar set of reactions, the exchange of Hsp70 on Hip forms was measured (Fig. 8C and D). APAV2 and wtHip, as intein/CBD fusion proteins, were adsorbed to chitin resin, and N-terminally His-tagged N-303 and wtHip were adsorbed to Ni2+-resin. Negative-control samples, with ΔTAP-intein/CBD bound to chitin resin or Ni2+-resin lacking any His-tagged protein, failed to show any binding by radiolabeled Hsp70. Contrasting with the similarity of Hsp70-PR exchange rates, the rate of Hsp70 exchange on wtHip was markedly lowered by mutations in Hip’s C-terminal region. The half-time to reach new steady-state levels of radiolabeled Hsp70 on wtHip was approximately 1 to 2 min but was closer to 5 min with either APAV2 (Fig. 8C) or N-303 (Fig. 8D). Briefly, Hip mutants differ from wtHip in their interactions with Hsp70, but the more stable binding of mutant Hip to Hsp70 does not alter the normal binding and release of Hsp70 from PR complexes.
Hsp70 binding to Hop is inhibited by misfolded substrates.
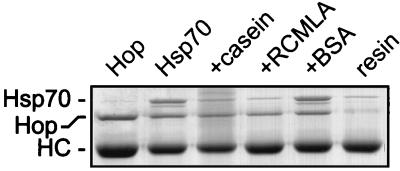
Hip mutants APAV2 and N-303 do not prevent binding of Hsp70 and the mutant to PR, but PR assembly is arrested immediately prior to incorporation of Hop and Hsp90 into intermediate complexes. Hop-Hsp90 is able to bind Hsp70-APAV2 in the absence of PR (Fig. 5) but not, apparently, in the presence of PR. Perhaps, then, there is a difference in Hop binding to Hsp70 that is dependent on whether Hsp70 is associated with a substrate. This was confirmed when the binding of Hop to Hsp70 in the presence and absence of misfolded substrates was compared (Fig. 9). Hsp70 alone or in the presence of native BSA will readily bind immunoaffinity-purified Hop. On the other hand, if Hsp70 is preincubated with the substrate α-casein or RCMLA, binding of Hsp70 to Hop is reduced to the background level observed with immunoaffinity resin alone.
FIG. 9.
Inhibition of Hop binding to Hsp70 in the presence of misfolded substrates. Hop was immunoadsorbed from RL in the presence of 0.5 M NaCl to dissociate Hsp70 and Hsp90. Hop-resin (lane Hop) was then incubated with purified Hsp70 (lane Hsp70) or with Hsp70 preincubated with a 10-fold molar excess of α-casein, RCMLA, or BSA as indicated above the lanes. In the final lane, immunoaffinity resin lacking Hop was incubated with Hsp70. Migration positions for Hsp70, Hop, and the anti-Hop immunoglobulin heavy chain (HC) are indicated on the left.
DISCUSSION
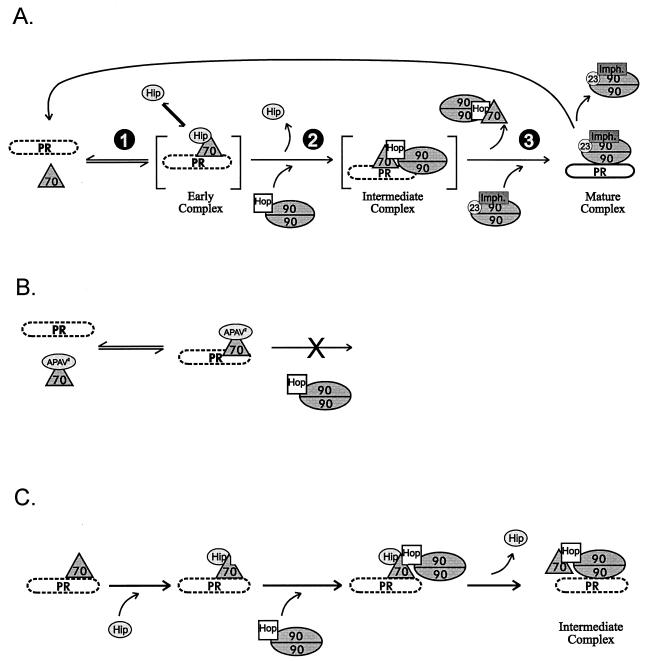
The normal assembly pathway for PR complexes (25, 27) is illustrated in Fig. 10A. PR must reach the mature complex in order to bind hormone with efficiency and high affinity, and since the mature complex is not inherently stable, complexes must be constantly reassembled to maintain a PR pool competent for hormone-dependent activation.
FIG. 10.
(A) Normal assembly cycle. During cell-free assembly of PR complexes, an ordered pathway is followed. (1) Hsp70 and Hip bind at an early stage. (2) An intermediate complex containing Hsp70, Hop, and Hsp90 is formed. (3) The functionally mature complex contains Hsp90, p23, and any one of three immunophilins (Imph.). Hormone binding by PR is deficient (dashed outline of PR) until the mature complex is formed. The mature complex is not stable; if PR dissociates without binding hormone, it reenters the assembly cycle. (B) APAV2-arrested assembly. Hip mutant APAV2, or N-303, is permissive for Hsp70 binding and dissociation but prevents the coassociation of Hop-Hsp90 and Hsp70 on PR. (C) Hip-mediated transition in PR complexes. It is proposed that Hip normally facilitates binding of Hop to Hsp70 when Hsp70 is associated with PR, thus promoting progression of PR assembly to the intermediate complex containing Hsp90. The indirect association of Hsp90 with PR complexes may favor displacement of Hsp70 and direct binding of Hsp90 to PR.
Whereas the compositions of receptor complexes at different assembly stages are fairly well defined, the mechanisms for transition from one complex to the next are not. It is known that blocking of p23 function prevents the transition from intermediate to mature complexes (13, 16, 27), inhibition of Hop function blocks formation of intermediate complexes (4–6), and inhibition of Hsp70 prevents all assembly steps (14, 26). What we’ve found here is that certain Hip mutants, while allowing Hsp70 to transiently associate with PR, prevent formation of intermediate complexes containing Hop and Hsp90 (Fig. 10B).
The initial characterization of APAV2 showed that the radiolabeled protein was underrepresented in PR complexes (Fig. 2), yet the experiments whose results are presented in Fig. 6B and 7A show enhanced recovery of APAV2 in PR complexes. These seemingly paradoxical results can be explained in the following manner. When APAV2 is present in RL at low levels relative to endogenous Hip, as in Fig. 2, assembly can proceed via the actions of endogenous Hip. The small pool of PR complexes containing APAV2 do not progress to transitional stages and quickly dissociate. In contrast, when APAV2 is the dominant Hip form (as in Fig. 6B and 7A), assembly progression is efficiently blocked, and the pool of PR-Hsp70-APAV2 complexes increases even though the complexes remain dynamic.
Mechanism for inhibition of PR assembly by Hip mutants.
Inhibition of PR assembly by C-terminal Hip mutants requires binding of the Hip mutants to Hsp70 (Fig. 6B, lane ΔTAP), and the mutants retain several of wtHip’s Hsp70-binding characteristics. For example, the mutants still bind the ATPase domain of Hsp70 in an ADP-dependent manner (Fig. 3 to 5). Also, the Hip mutants do not block Hsp70 binding to PR (Fig. 6B and 7), nor do they alter the dynamics of Hsp70-PR interactions (Fig. 8A and B). One distinguishing characteristic of the Hip mutants is their more stable binding to Hsp70. Unlike wtHip, association of APAV2 and N-303 with full-length Hsp70 is resistant to increased ionic strength (Fig. 3), and the mutants exchange on Hsp70 more slowly than wtHip (Fig. 8C and D). More stable binding of Hip mutants to Hsp70 competitively favors their occupation of Hsp70, thus accounting for the dominance over wtHip of mutant Hip interactions with Hsp70. However, more stable Hsp70 binding alone probably does not account for inhibition of PR assembly.
The sequences mutated in APAV2 and N-303 are similar to sequences in the C-terminal region of Hop. Also, Hip and Hop both bind Hsp70 and can do so concomitantly. Hop is required for the entry of Hsp90 into steroid receptor complexes (4–6), and the Hip mutants tested in this study arrest PR assembly prior to incorporation of Hop into PR complexes. Hence, there is a potential connection between Hip function and Hop’s ability to enter PR complexes.
While Hop can bind substrate-free Hsp70 alone (Fig. 9) or in the presence of APAV2 (Fig. 5), Hop is unable to associate with Hsp70 when misfolded substrates are present (Fig. 9). This suggests either that Hop is sterically excluded from binding Hsp70 by misfolded substrates or that the conformation of Hsp70 recognized by Hop is altered by substrate binding. We have been unsuccessful in mapping Hop’s binding site on Hsp70 using Hsc70-AD, Hsc70-PD, or a number of additional Hsp70 truncation mutants (4b), indicating that Hop’s binding is sensitive to Hsp70’s overall conformation. This is further suggested by Hop’s failure to recognize the ATP-bound form of Hsp70. Finally, Hop can clearly associate with Hsp70 in PR complexes—indeed, Hop is required for progression of PR assembly—arguing against a strict steric exclusion of Hop from this particular Hsp70-substrate complex. It seems most likely that the occupancy status of Hsp70’s peptide-binding domain conformationally alters the binding site for Hop, requiring some mechanism to facilitate Hop binding to PR-associated Hsp70, Hip may be serving this role.
A model (Fig. 10C) in which Hip and Hop direct the transition from early PR complexes (containing Hsp70 and Hip) to intermediate complexes (containing Hsp70, Hop, and Hsp90) is proposed. In the scheme shown, Hsp70 binds to PR, apparently recognizing PR as a misfolded protein; Hip then binds Hsp70 and induces a conformational change that facilitates Hop-Hsp90 binding. Hop binding may promote release of Hip from Hsp70, or Hip dissociation may occur spontaneously. The Hop-mediated localization of Hsp90 to intermediate complexes might favor direct binding of Hsp90 to PR with concomitant displacement of Hsp70 as a precedent to formation of mature complexes.
It is clear that the two domains of Hsp70 are conformationally linked and mutually influenced by the presence of ATP or ADP in the ATPase domain and by the presence or absence of a substrate in the peptide-binding domain (2, 7, 9). The influence of the peptide domain on ATPase domain interactions with other proteins is evident in two examples presented here. First, mutant APAV2 binds Hsp70’s isolated ATPase domain in a salt-sensitive manner, but APAV2 binding is salt resistant with full-length Hsp70 (Fig. 3 and 4). We found no direct interaction between APAV2 and Hsp70’s peptide-binding domain to account for salt-resistant binding. Second, Hop readily binds free Hsp70 but fails to bind Hsp70 in the presence of misfolded substrates (Fig. 9). However, under the right conditions (namely, the presence of wtHip), Hop can bind Hsp70 while its peptide-binding domain is occupied with PR.
Recently, we have generated mutations in the DPEV region of Hop and found that the mutants fail to bind Hsp70 and fail to support PR assembly (4a). This observation strengthens the potential importance of Hip’s and Hop’s DPEV sequences in directing assembly of functionally mature PR complexes. In conclusion, Hip may have evolved as a coadapter with Hop to enhance and coordinate recognition of steroid receptors, and perhaps other Hsp70 substrates, by Hsp90.
ACKNOWLEDGMENT
This work was supported by NIH grant R01-44923.
REFERENCES
- 1.Barent, R. L., S. C. Nair, D. C. Carr, R. A. Rimerman, Y. Ruan, Y. Zhang, J. Brennan, and D. F. Smith. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol., in press. [DOI] [PubMed]
- 2.Buchberger A, Theyssen H, Schroder H, McCarty J S, Virgallita G, Milkereit P, Reinstein J, Bukau B. Nucleotide-induced conformational changes in the ATPase and substrate binding domains of the DnaK chaperone provide evidence for interdomain communication. J Biol Chem. 1995;270:16903–16910. doi: 10.1074/jbc.270.28.16903. [DOI] [PubMed] [Google Scholar]
- 3.Chang H C, Nathan D F, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Prapapanich V, Rimerman R, Honore B, Smith D. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Mol Endocrinol. 1996;10:682–693. doi: 10.1210/mend.10.6.8776728. [DOI] [PubMed] [Google Scholar]
- 4a.Chen, S., V. Prapapanich, and D. F. Smith. Unpublished data.
- 4b.Chen, S., and D. F. Smith. Unpublished results.
- 5.Dittmar K D, Hutchison K A, Owens-Grillo J K, Pratt W B. Reconstitution of the steroid receptor-hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271:12833–12839. doi: 10.1074/jbc.271.22.12833. [DOI] [PubMed] [Google Scholar]
- 6.Dittmar K D, Pratt W B. Folding of the glucocorticoid receptor by the reconstituted hsp90-based chaperone machinery. J Biol Chem. 1997;272:13047–13054. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- 7.Freeman B, Myers M, Schumacher R, Morimoto R. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frydman J, Hohfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- 9.Fung K L, Hilgenberg L, Wang N M, Chirico W J. Conformations of the nucleotide and polypeptide binding domains of a cytosolic Hsp70 molecular chaperone are coupled. J Biol Chem. 1996;271:21559–21565. doi: 10.1074/jbc.271.35.21559. [DOI] [PubMed] [Google Scholar]
- 10.Grenert J P, Sullivan W P, Fadden P, Haystead T A J, Clark J, Mimnaugh E, Krutzsch H, Ochel J, Schulte T W, Sausville E, Neckers L M, Toft D O. The amino terminal domain of heat shock proteins 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates Hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 11.Gross M, Hessefort S. Purification and characterization of a 66-kDa protein from rabbit reticulocyte lysate which promotes the recycling of hsp 70. J Biol Chem. 1996;271:16833–16841. doi: 10.1074/jbc.271.28.16833. [DOI] [PubMed] [Google Scholar]
- 12.Hoehfeld J, Minami Y, Hartl F U. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 13.Hutchison K, Stancato L, Owens-Grillo J, Johnson J, Krishna P, Toft D, Pratt W. The 23-kDa acidic protein in reticulocyte lysate is the weakly bound component of the hsp foldosome that is required for assembly of the glucocorticoid receptor into a functional heterocomplex with hsp90. J Biol Chem. 1995;270:18841–18847. doi: 10.1074/jbc.270.32.18841. [DOI] [PubMed] [Google Scholar]
- 14.Hutchison K A, Dittmar K D, Czar M J, Pratt W B. Proof that hsp70 is required for assembly of the glucocorticoid receptor into a heterocomplex with hsp90. J Biol Chem. 1993;269:5043–5049. [PubMed] [Google Scholar]
- 15.Irmer H, Hoehfeld J. Characterization of functional domains of the eukaryotic co-chaperone Hip. J Biol Chem. 1997;272:2230–2235. doi: 10.1074/jbc.272.4.2230. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J, Beito T, Krco C, Toft D. Characterization of a novel 23-kilodalton protein of unactive progesterone receptor complexes. Mol Cell Biol. 1994;14:1956–1963. doi: 10.1128/mcb.14.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassle M, Blatch G L, Kundra V, Takatori T, Zetter B R. Stress-inducible, murine protein mSTI1. Characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien M C, Flaherty K M, McKay D B. Lysine 71 of the chaperone protein Hsc70 is essential for ATP hydrolysis. J Biol Chem. 1996;271:15874–15878. doi: 10.1074/jbc.271.27.15874. [DOI] [PubMed] [Google Scholar]
- 19.Prapapanich V, Chen S, Nair S C, Rimerman R A, Smith D F. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an Hsp70-binding protein. Mol Endocrinol. 1996;10:420–431. doi: 10.1210/mend.10.4.8721986. [DOI] [PubMed] [Google Scholar]
- 20.Prapapanich V, Chen S, Toran E J, Rimerman R A, Smith D F. Mutational analysis of the Hsp70-interacting protein Hip. Mol Cell Biol. 1996;16:6200–6207. doi: 10.1128/mcb.16.11.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Prapapanich, V., and D. F. Smith. Unpublished results.
- 21.Pratt W B, Toft D O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrine Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 22.Prodromou C, Roe S M, O’Brien R, Ladbury J E, Piper P W, Pearl L H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 23.Schneider C, Newman R A, Sutherland D R, Asser U, Greaves M F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
- 24.Smith D, Sullivan W, Marion T, Zaitsu K, Madden B, McCormick D, Toft D. Identification of a 60-kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol Cell Biol. 1993;13:869–876. doi: 10.1128/mcb.13.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith D F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- 26.Smith D F, Stensgard B A, Welch W J, Toft D O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992;267:1350–1356. [PubMed] [Google Scholar]
- 27.Smith D F, Whitesell L, Nair S C, Chen S, Prapapanich V, Rimerman R A. Progesterone receptor structure and function altered by geldanamycin, an Hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Inhibition of heat shock protein Hsp90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]