Abstract
Titanium aluminide (TiAl)-based intermetallics, especially Ti-48Al-2Cr-2Nb, are a well-established class of materials for producing bulky components using the electron beam powder bed fusion (EB-PBF) process. The biological properties of Ti-48Al-2Cr-2Nb alloy have been rarely investigated, specifically using complex cellular structures. This work investigates the viability and proliferation of NIH-3T3 fibroblasts on Ti-48Al-2Cr-2Nb dodecahedral open scaffolds manufactured by the EB-PBF process. A process parameter optimization is carried out to produce a fully dense part. Then scaffolds are produced and characterized using different techniques, including scanning electron microscopy and X-ray tomography. In vitro viability tests are performed with NIH-3T3 cells after incubation for 1, 4, and 7 days. The results show that Ti-48Al-2Cr-2Nb represents a promising new entry in the biomaterial field.
Keywords: additive manufacturing, titanium aluminide, biocompatibility, X-ray analysis, 3D printing
Introduction
The electron beam powder bed fusion (EB-PBF) process is an additive manufacturing (AM) process used to produce metallic components following a layer-wise approach. In this process, metallic powder particles are melted selectively using a high-energy electron beam. Compared with the most common laser-based AM processes, EB-PBF is a hot process because of its high working temperatures achieved through a preheating step during the building process.1,2 The industrial evolution of this process lies mainly in two fields of application: medical and aerospace.3 The medical sector, essentially based on the production of prostheses, explored the following advantages of the process: no oxygen contamination thanks to the use of a vacuum environment, building complex structures, such as trabecular lattices, without the use of support structures, and the nesting production of the parts thanks to the strength of the powder layers sintered during the preheating step.4
In aerospace, EB-PBF is the most suitable process for producing structural components made of intermetallics (e.g., titanium aluminide [TiAl]), mainly owing to the EB-PBF process effectiveness in reducing hot cracking and overcoming the difficulties related to the conventional or laser-based methods.5 Thanks to its mechanical performance and high specific strength, TiAl parts fabricated by the EB-PBF process are used today to replace heavyweight Ni-based superalloys components, such as turbine blades, at a working temperature of up to 900°C.6 TiAl alloys consist of titanium, aluminum between 44 at.% and 48 at.%, and small quantities of other elements. The additional elements aim to improve specific alloy properties, such as oxidation and corrosion resistance in the case of niobium. These properties are the most attractive for applications that deal with aggressive and harsh environments.7
TiAl intermetallic materials have the potential for being applied to the biomedical field, especially if coupled with the benefits of EB-PBF technology. Titanium alloys processed by the EB-PBF technology are extensively used for biomedical applications.4,8 However, the most used alloy is Ti6Al4V.9 This alloy has shown some drawbacks associated with the presence of vanadium and its oxide, which may cause allergic and inflammatory reactions, leading to implant rejection.10 The use of TiAl intermetallic materials could overcome these issues.11 It is interesting to notice that the predominant oxide of TiAl alloys is aluminum oxide, which exhibited a better structural and oxidation resistance than titanium oxide.11 For instance, Escudero et al12 showed an excellent corrosion behavior of Ti-45Al-2W-0.6Si-0.7B (at.%) bars under body fluid action. In particular, TiAl intermetallic pre-oxidized at 700°C had a much lower concentration of Al and Ti ions, which were two orders of magnitude lower than those obtained from the Ti6Al4V counterpart.
On the contrary, Kyzio et al13 and Santiago-Medina et al14 reported that surface treatment could enhance the material biocompatibility compared with the as-built conditions. Kyzio et al13 suggested a plasma-chemical process to obtain specific surface roughness properties. However, using the EB-PBF process, the surface topography can be controlled locally without requiring additional surface modifications. For instance, Som et al15 assessed the influence of thermal oxidation in the air on the biocompatibility properties of the EB-PBF processed Ti-48Al-2Cr–2Nb (at.%) bulk samples. The results revealed that the oxidation induced no harmful consequences and significantly improved cell proliferation. However, the roughness and porosity of the samples were not suitable for wear resistance.15 All the previous tests have been conducted using bulk material. However, bulk material induces stress shielding for biomedical applications, and therefore, porous structures have been developed to be used for the production of implants.16
However, only a couple of studies17,18 on porous structures made with TiAl were found in the literature. Nevertheless, none of these previous works has investigated the biological properties of the TiAl porous structure. Hernandez et al17 investigated the effect of geometrical changes on the microstructure of the TiAl foams. Mohammad et al18 optimized the EB-PBF process to build a simple 2D ordered lattice structure made by horizontal and vertical beams ranging from 0.5 to 1.5 mm in thickness. In their work, the scanning strategy included only hatching mode without contour, which results in the build of the struts larger than the nominal one.
In addition, internal pores with comparable dimensions of the strut thickness were detected by computed tomography. It is interesting to highlight that their compression tests carried out on the structure with struts of 0.5 mm revealed that Young's modulus of their lattice structures was close to the stiffness of the bone, which could solve the issue related to the stress shielding phenomena.
To date, several studies have investigated the production and application of TiAl parts for aerospace applications, but far too little attention has been paid to the strong potential of TiAl components produced via the EB-PBF process for biomedical applications. Hence, this current study aims to produce porous complex 3D structures made with Ti-48Al-2Cr-2Nb (Ti-48-2-2) alloy and evaluate their performance for biomedical applications. For this reason, a dodecahedral design with nominal porosity and strut dimension equal to 96% and 0.29 mm, respectively, was considered the main strut type. Before producing the final samples, the scanning strategy was optimized to obtain dense specimens without internal defects for the biological evaluations. After that, a theme consisting of the selected process parameters was used to produce the final samples for biological tests. In vitro viability tests with NIH-3T3 cells were performed after incubation for 1, 4, and 7 days.
Materials and Methods
Scaffold design and production
The elementary cell selected for the study was a dodecahedral structure (Fig. 1).
FIG. 1.

Dodecahedral elementary cell.
In this study, sample production was performed in two steps. At first, a set of samples was produced to calibrate the process parameters. Thereafter, the second set of samples for the biological tests, called scaffolds, was produced using the selected parameters of the first samples.
The process calibration aimed to obtain a dense structure with no defect that may jeopardize the structural strength of the strut. According to the standard melting strategy for an EB-PBF process, the section to be melted is split into perimetral and inner areas. The perimetral area is melted using one or more contours to ensure dimensional accuracy, whereas the inner area is usually melted using the hatching strategy.1 However, this melting strategy is suitable for the bulk part, whereas it can generate heat accumulation and distortions for thinner structures, such as the lattice structures. For this reason, the melting strategy consisted only of a series of contours. Therefore, the machine set of process parameters related to the hatching strategy was deactivated.
For the contour, the following parameters varied (Table 1): speed on the outer contour (VOUT) and inner contour (VIN), beam focus offset (FOOUT), and beam current (IOUT) for the outer contour. The focus offset and the beam current for the inner perimeter values were set at 0 and 2.4 mA, respectively. The preheating temperature and the layer thickness were set at 1050°C and 90 μm, respectively. Two replicas were manufactured for each process parameter set.
Table 1.
Combination of Different Sets of Process Parameters Used to Find the Optimum Process Parameters
| VOUT (mm/s) | IOUT (mA) | FOOUT (mA) | VIN (mm/s) | |
|---|---|---|---|---|
| NET1 | 380 | 2.4 | 0 | 400 |
| NET2 | 380 | 2.4 | 5 | 400 |
| NET3 | 380 | 2.0 | 5 | 400 |
| NET4 | 400 | 2.4 | 5 | 420 |
| NET5 | 400 | 2.4 | 0 | 420 |
For the first set, the size of the elementary unit cell selected was equal to 5 mm, whereas the strut size was 0.3 mm. The so designed unit cell was used to fill a cubic volume of overall dimensions equal to 20 × 20 × 20 mm. To correctly fit the experimental equipment for biological tests, the second set of samples was smaller cubes with the overall dimension corresponding to a cubic volume with an edge of 10 mm. Owing to the reduced size of the samples, the elementary cell was reduced to 3 mm to fit a higher number of cells in the predefined volume. The nominal porosity of the structure is 96%. A total of 40 replicas were produced. Ten replicas were used for scaffold characterization (defect and mechanical characteristics), whereas 30 replicas were used for the biological tests.
All samples were produced using an Arcam A2X, an EB-PBF machine, and standard Arcam Ti48-2-2 powder with a particle size range of 45–150 μm. Each replica was built tilted 45° with respect to the build platform to avoid using support structures and preserve the structural integrity. After the production, the samples were cooled inside the machine down to room temperature. Then the samples were blasted using the same powder used during the EB-PBF to clean the lattice from the sintered powder. Moreover, clean compressed air at 4.5 bar was used for the final cleaning of the scaffolds from the loose powder.
Powder and scaffold analysis
The morphology and the chemical composition of the Ti-48-2-2 powder were analyzed using a scanning electron microscope (SEM) Tescan VEGA 3, equipped with an EDAX energy dispersive X-ray spectroscopy (EDS) system and by a Zeiss Supra 40 field emission scanning electron microscope (FESEM), equipped with Bruker Z200 EDS. The EDS was also performed on the scaffold by averaging six different areas at the same magnification (500 × ). Results were reported as average value and standard deviation (SD). X-ray diffraction (XRD) analysis was implemented on representative samples to study their phase compositions. The XRD analysis was carried out using a Bruker D8 Advance diffractometer operating at V = 40 kV and I = 40 mA, with Cu-Kα radiation in the angular range 2θ = 20°–90°.
Density analysis and structure characterization
The structural integrity of the samples was investigated following the image analysis procedure. For this purpose, a Leica stereomicroscope was used for image acquisition. Density evaluation of the samples was performed using the image analysis approach. In this approach, the samples were prepared following the standard procedure for metallography of the TiAl samples reported elsewhere. This procedure to achieve a mirror-like surface consists of several grinding steps using abrasive paper with different grain dimensions (600, 800, and 1200 grit), followed by final polishing steps using diamond pastes of 3 and 1 μm. After that, the as-polished surfaces were analyzed by a Leica S9i microscope at a constant magnification (50 × ). For the porosity evaluations, 6 random images per sample were acquired, and the outcomes were reported as an average of 6 analyses together with the SD. The images were assessed using ImageJ, an open-source software, to investigate the presence of pores and determine the density of the as-built specimens.
The porosity of scaffolds, p, was calculated according to the following Equation:

where Vnom is the volume of the cube, W is the scaffold weight, ρmat is the material density. W is equal to 0.63 ± 0.03 g, and ρmat was set equal to 4040 kg/m3.
On the contrary, an X-ray microcomputed tomography (XμCT) analysis was carried out on a replica of the scaffolds using a Bruker Skyscan 1174 tomographic system. The 2D projections were obtained using a voltage and beam current equal to 50 kV and 800 μA, respectively, with a corresponding resolution equal to 11.5 μm. The total rotation was equal to 180°, stepped with 0.2°. The exposure time was set to 10.5 s, and an aluminum filter with 1 mm thickness was used. The projections were processed by SkyScan reconstruction program NRecon (version 1.10.6.2), using smoothing, ring artifacts reduction, and beam hardening factors equal to 5%, 3%, and 30%, respectively. Scaffold morphometric parameters for average strut thickness, average macro-pore size, and total porosity were measured using SkyScan CT-analyzer software (version 1.17.7.2). XμCT analysis was also used to evaluate the bulk density of the samples. Furthermore, the morphology of the scaffold surfaces in the as-built state was observed using an FESEM.
Mechanical properties
The mechanical behavior of Ti-48-2-2 lattice samples was investigated under a uniaxial compressive load. The tests were performed using an Instron 5567 system (30 kN load cell, 0.5 mm/min speed). Young's modulus (E) of the lattice structures was calculated as an average of the two replicas in the case of the first set of samples and five replicas from the second set.
Cell culture and seeding
Murine fibroblast cell line, NIH-3T3 (CRL1658), was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). NIH-3T3 cells were cultured in Dulbecco modified Eagle medium with 4.5 g/L glucose (Invitrogen), supplemented with 10% bovine calf serum (Sigma-Aldrich) and 1% p/v l-glutamine (Lonza). Cells were incubated at 37°C with 5% CO2, routinely trypsinized after confluence, and then counted and seeded into wells. Before cell seeding, scaffolds were sterilized in an autoclave at 120°C for 20 min, washed several times with sterile distilled water followed by phosphate-buffered saline (PBS 1 × ), and finally soaked overnight in the culture medium. Afterward, on day 0, a drop of cell suspension containing 5 × 105 cells was seeded onto the scaffold, which was previously placed in a 24-well ultralow cell attachment plate (Corning, Inc., Corning, NY, USA). After 30 min, 1.5 mL of cell culture medium was added to each well, changing the medium every 2 days. Cells seeded on a tissue culture polystyrene plate (TCPS) were inserted as control.
Cell viability
Resazurin-based assay (TOX8; Sigma-Aldrich) was performed to assess cell viability onto scaffold after 1, 4, and 7 days from cell seeding as previously described.19 According to manufacturer instructions, resazurin solution was added in a 1:10 ratio with respect to culture volume to each well plate and incubated for 4 h at 37°C in 5% CO2. At the end of incubation time, the absorbance of the samples was measured at a wavelength of 600 nm with a reference wavelength of 690 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). A standard cell viability curve was used to obtain the cell number per sample.
DNA content
The released DNA content of the samples was assessed after 1, 4, and 7 days of culture. For this reason, the samples were processed through the three freeze/thaw cycles method in sterile deionized distilled water. Between each freeze/thaw cycle, scaffolds were roughly vortexed. The released DNA content was measured with the fluorometric DNA quantification kit (PicoGreen; Molecular Probes, Eugene, OR, USA), following the manufacturing protocols.20 The amount of DNA was expressed as weight of DNA per sample (μg/sample).
Cell morphology
On day 7 of culture, the samples were fixed with 2.5% (v/v) glutaraldehyde solution in 0.1 M Na-cacodylate buffer (pH 7.2) for 1 h at 4°C, washed with Na-cacodylate buffer, dehydrated at room temperature in an ethanol gradient series up to 100%, and then lyophilized for 4 h up to complete dehydration. Subsequently, scaffolds were sputter coated with gold and observed with a FESEM at 350 × , 650 × , and 2000 × magnifications. Cells seeded on plastic cell culture coverslip disks (Thermanox Plastic; Nalge Nunc International, Rochester, NY, USA) were inserted as control.
Statistical analysis
Statistical analysis was generated using GraphPad Prism 6.0 (GraphPad, Inc., San Diego, CA, USA) software package. Each experiment reported in the Results and Discussion section was run in triplicate, at least in three separate experiments. Results were expressed as mean ± SD. Student's unpaired t-test was performed to determine statistical significance, with a significance level (p-value) at 0.05.
Results and Discussion
Powder analysis
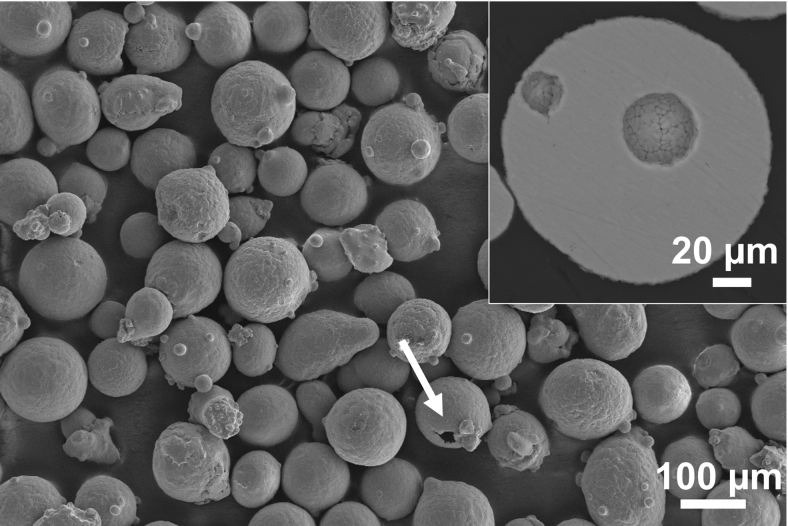
Micrographs of Ti-48-2-2 powder are given in Figure 2. Powder particles are generally spherical, with diameters ranging from 50 to 140 μm (Fig. 2). Some satellite particles attached to the main particles can also be observed (Fig. 2). In some powder particles, holes are visible as indicated by an arrow in Figure 2. In cross-section, holes appear as spherical pores (inset in Fig. 2) and can be explained by the gas-atomization method used to produce the powder. In fact, Argon entrapment during the gas-atomization process commonly leads to the formation of internal gas porosity, typically spherical in morphology.21
FIG. 2.
SEM micrograph of Ti-48-2-2 powder with satellite particles and surface holes, indicated by arrow. Inset of cross-sectional particles reveals holes as spherical pores. SEM, scanning electron microscope.
The chemical composition analysis of Ti-48-2-2 powder, obtained by EDS, is listed in Table 2.
Table 2.
Chemical Composition (at.%) of Ti-48-2-2 Powder and Scaffold, Obtained by Energy Dispersive X-Ray Spectroscopy Analysis
| |
Ti-48-2-2 powder |
Ti-48-2-2 scaffold |
||
|---|---|---|---|---|
| Elements (at.%) | AV | SD | AV | SD |
| Ti | 53.30 | 0.40 | 52.30 | 1.30 |
| Al | 43.20 | 0.50 | 44.00 | 1.30 |
| Cr | 1.92 | 0.07 | 1.82 | 0.09 |
| Nb | 1.83 | 0.05 | 1.90 | 0.10 |
AV, average value; SD, standard deviation.
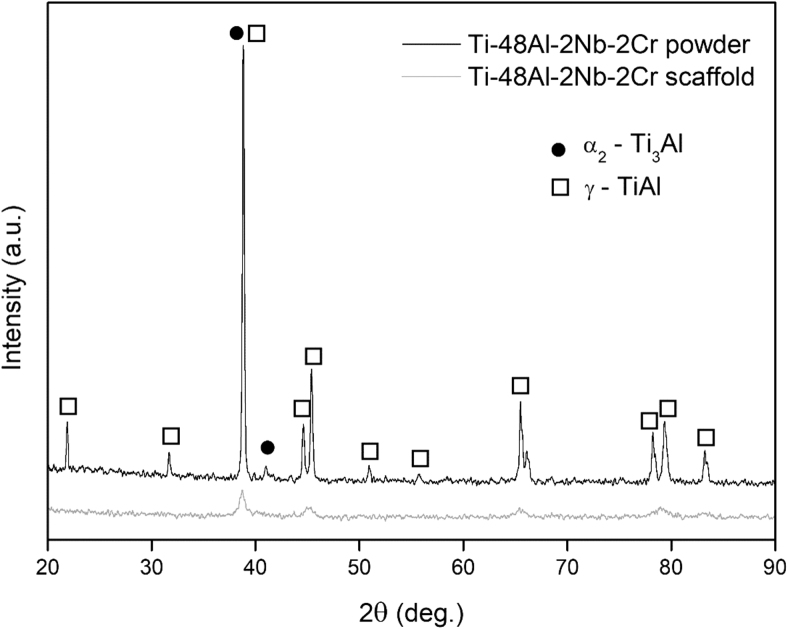
XRD analysis (Fig. 3) highlights the presence of the γ-TiAl phase (ICDD File No. 65-8565) and the α2-Ti3Al phase (ICDD File No. 65-7534).17 Sharp and intense peaks were identified for the main phase (γ-TiAl), whereas fewer peaks related to the α2-Ti3Al phase were detected.
FIG. 3.
XRD pattern of Ti-48-2-2 powder and scaffold. α2-Ti3Al—full dot, γ-TiAl—empty square. TiAl, titanium aluminide; XRD, X-ray diffraction;.
Process parameter optimization
Image analysis of the samples reveals that using all the tested process parameters resulted in the formation of complete and defect-free struts in all the cells. Figure 4 provides an example of analysis carried out on a sample produced with the parameters set named NET1. In particular, Figure 4a–c demonstrates the produced lattice at different magnifications, in which the integrity of the struts and the elementary cells can be denoted. Figure 4c and d shows the representative images from the cross-section of a sample produced using the parameters set Named NET1. As can be seen in these images, the porosity content and size in various cross-sections are different. The pores were categorized according to their dimensions, and the largest pores were nearly spherical with diameters <100 μm. The presence of this kind of gas-induced porosities can be associated with the presence of internal pores in the feedstock materials (Fig. 2). It is interesting to note that no lack of fusion was detected in all the samples.
FIG. 4.
Cross-sections at different magnifications of a sample produced using the process parameters set named NET1. Pores are grouped according to their dimension using colored circles. Blue circles identify a pore dimension smaller than 0.025 mm. Green circle highlights pores with dimension between 0.026 and 0.050 mm. Yellow circles were used for pores with a size between 0.050 and 0.099 mm, whereas orange for pores larger than 0.100 mm.
Table 3 provides the relative density values obtained from the image analysis for each set of process parameters and the corresponding Young's modulus. As can be seen, for all process parameters, the measured relative density of the samples was >98.5%, with the highest values equal to 99.5%, corresponding to NET4. The Young's modulus varied in a wide range, from 97 to 177 MPa. However, the lowest value of Young's modulus (NET5) can presumably be explained by a combined effect of lower density and superficial defects on the strut,20 which also explains the high deviation. Notwithstanding the deviations, all Young's moduli are comparable with the counterparts measured on the bulk sample.22 The outcomes density evaluations and the results of the mechanical strength of the samples, imply that the best set of process parameters that results in a high density and mechanical strength is NET4. Hence, this set of process parameters was chosen to produce the second set of samples for the biological tests.
Table 3.
Relative Density of the Samples Measured by Images Analysis
| Process setting | Relative density (%) | Young modulus (MPa) |
|---|---|---|
| NET1 | 98.90 | 155.04 (50.00) |
| NET2 | 98.52 | 177.20 (47.20) |
| NET3 | 99.33 | 150.81 (52.00) |
| NET4 | 99.50 | 141.28 (53.00) |
| NET5 | 98.77 | 97.12 (70.00) |
Scaffold analysis
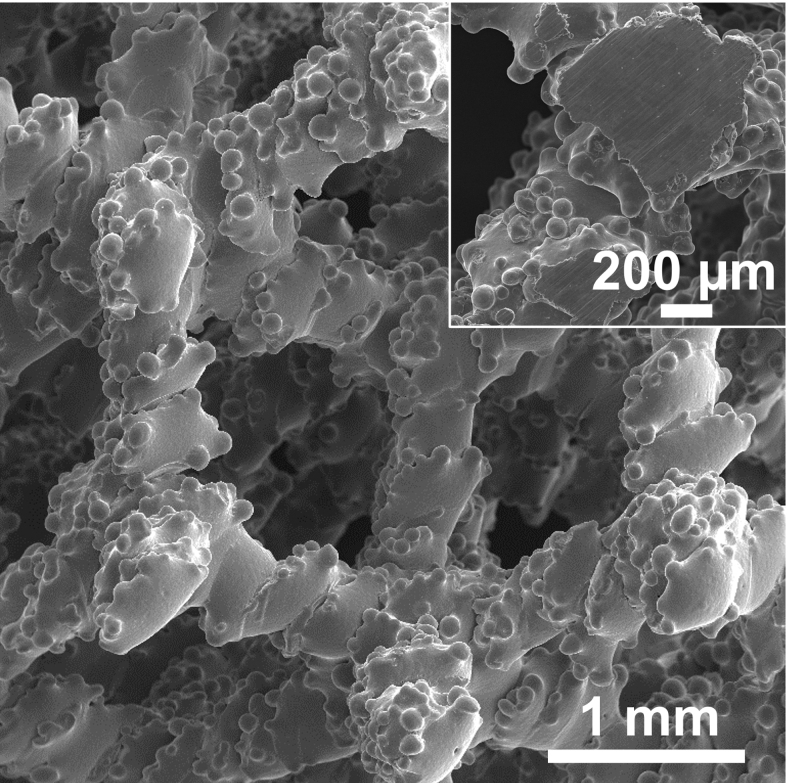
Figure 5 provides the SEM micrograph of the scaffolds produced using NET4 process parameters, whereas the inset in Figure 5 is the cross-section of the strut. This analysis reveals the typical as-built surface obtained by an EB-PBF in which unmelted powders stick to the external surface of the components.23
FIG. 5.
SEM micrograph of Ti-48-2-2 scaffold top surface. Inset shows a scaffold node, cut in correspondence of half scaffold height (∼5 mm).
Table 2 provides the chemical composition of the scaffold that was evaluated through the EDS. As can be seen in this table, the chemical composition of the scaffold is comparable with that of powder.
Figure 3 provides the XRD pattern of the scaffold (red pattern) compared with the powder counterpart (black pattern). The γ-TiAl phase is also, in this case, the predominant phase. Moreover, the width of the main peak in the scaffold pattern is wider than the one in the powder pattern. This discrepancy in the width of the main peaks in both cases can be related to the cooling rate during their processing. As a matter of fact, a wider peak in the case of scaffold implies that the cooling rate during the EB-PBF process was higher than the gas-atomizing process and, consequently, results in the formation of finer crystallite size in the scaffold.
The porosity evaluations using Equation (1) show that the scaffold's porosity was equal to 84%, which agrees with the value (82%) exploited using XμCT analysis (Table 4). As given in Table 4, the average strut width of the scaffold was larger than the nominal one (0.300 mm). This result is explained by the actual dimensions of the melt pool generated during the process, which depend on a combined effect of beam current, focus offset, and beam speed.24 The thicker struts explain the lower actual porosity with respect to the nominal counterpart (96%). The thicker struts reduce the size of the scaffold structure open volumes.
Table 4.
Lattice Scaffold Morphometric Parameters Obtained from XμCT Analysis
| Average strut thickness (mm) | 0.37 ± 0.03 |
| Average macropore size (mm) | 1.50 ± 0.11 |
| Total porosity (%) | 82 |
The compressive test showed an extremely high Young's modulus equal to 270 ± 60 MPa. This higher value with respect to the previous measure can be explained by the smaller cell size and consequently higher relative density.
Biocompatibility results
To evaluate the feasibility of using the Ti-48-2-2 scaffolds for potential applications in tissue engineering, the in vitro biocompatibility tests were performed using the NIH-3T3 fibroblast murine cell line. This cell line is one of the most frequently used cell lines for studying material biocompatibility25 and has been previously used for cytotoxicity testing of titanium surfaces.26,27 To evaluate the overall biocompatibility of Ti-48-2-2, cell proliferation, DNA content, and morphology analyses were carried out. Cells plated on a standard tissue culture plate (TCPS) were used as a positive control. As given in Figure 6a, at day 7, a significant difference in cell growth was observed in Ti-48-2-2 compared with day 1 (**p < 0.01). Remarkably, DNA measurement corroborated the above data. DNA content significantly increased in Ti-48-2-2 from day 1 to 7 days of incubation (*p < 0.05) (Fig. 6b). Overall, these data demonstrated that the metabolic activity significantly increased over time, despite the low initial cell adhesion (≈40%) ascribed to scaffold morphology.
FIG. 6.
Ti-48-2-2 scaffold biocompatibility was analyzed after 1, 4, and 7 from NIH-3T3 cell seeding by cell viability assay (a) and DNA content (b). For viability assay (a), the cell proliferation was plotted as the ratio between viable cell number and the adherent cell number at day 1 set as 100%. For DNA quantification (b), results were expressed as the total μg of DNA per sample. For both (a) and (b), cells seeded on TCPS were used as control. Bars represent the mean ± SD (****p < 0.0001, **p < 0.01, and *p < 0.05). SD, standard deviation; TCPS, tissue culture polystyrene plate.
The biocompatibility of Ti-48-2-2 surfaces was further investigated by evaluating the morphology of NIH-3T3 cells using FESEM microscopy (Fig. 7). After 7 days of cultures, SEM images showed cellular colonization and a robust spreading of healthy and flattened-shape cells covering Ti-48-2-2 surfaces (Fig. 7b). In addition, at high magnification micrographs (Fig. 7d), spread and adherent cells with noticeable filopodia extensions and cellular protrusions across the scaffold pores can be observed. This result was clear evidence of the binding between NIH-3T3 and Ti-48-2-2 (inset in Fig. 7d). Figure 7 compares the cell proliferation of Ti-48-2-2 with a commercial cell culture plate made in TCPS. Overall, cell proliferation was found lower in Ti-48-2-2 compared with the TCPS. However, this result is easily explained by the different surface properties, such as stiffness, porosity, chemistry, and topography.28
FIG. 7.
Representative FESEM images of NIH-3T3 cells after 7 days of incubation on control plastic dish (a, c) and Ti-48-2-2 scaffold (b, d) at different magnification (350 × and 650 × , respectively). Insert 2000 × magnification. Red arrows indicate cell distribution onto the scaffold. After 7 days, cells formed a widespread cell monolayer throughout the TCPS surfaces owing to the higher initial cell adhesion on TCPS (≈ 100%) than on Ti-48-2-2 (≈ 40%). FESEM, field emission scanning electron microscope.
Conclusions
This study investigated the application of Ti48-2-2 for biomedical applications. First, an optimized EB-PBF process set was identified to produce a dense part. Then, viability tests in vitro were performed with NIH-3T3 fibroblast murine cells after 1, 4, and 7 days of incubation on a set of open porous scaffolds with an actual porosity equal to 82%. The use of Ti-48-2-2 produced by EB-PBF has been shown to be extremely promising. Healthy and flattened-shaped cells were observed on the scaffold surfaces with a uniform spreading, and complete cellular colonization of 3D structure was achieved. These preliminary results encourage further efforts and investigations on Ti-48Al-2Cr-2Nb as a promising new entry in the biomaterial field.
Acknowledgments
The authors acknowledge the Integrated Additive Manufacturing centre at Politecnico di Torino (IAM@PoliTo), where the specimens were produced. Nora Bloise and Livia Visai acknowledge the grant of the Italian Ministry of Education, University and Research (MIUR) to the Department of Molecular Medicine of the University of Pavia under the initiative “Dipartimenti di Eccellenza (2018–2022).”
Authors' Contributions
M.G. Conceptualization, methodology, investigation of material production and process optimization, formal analysis of the process performance, writing—original draft; M.L.G. Investigation of material characterization and 3D analysis, formal analysis of characterization results, writing—original draft; N.B. Investigation of in vitro biocompatibility, formal analysis of data, writing—original draft; L.F. Writing—original draft—review and editing; A.S. Conceptualization, writing—review and editing; L.V. Supervision of in vitro biocompatibility experiments, resources; P.M. Supervision of material study; Luca Iuliano Supervision of process optimization, Resources (powder and EB-PBF system).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Galati M, Iuliano L. A literature review of powder-based electron beam melting focusing on numerical simulations. Addit Manuf 2018;19:1–20; doi: 10.1016/j.addma.2017.11.001 [DOI] [Google Scholar]
- 2. Saboori A, Abdi A, Fatemi SA, et al. Hot deformation behavior and flow stress modeling of Ti–6Al–4V alloy produced via electron beam melting additive manufacturing technology in single β-phase field. Mater Sci Eng A 2020;792:139822; doi: 10.1016/j.msea.2020.139822 [DOI] [Google Scholar]
- 3. Del Guercio G, Galati M, Saboori A, et al. Microstructure and mechanical performance of Ti-6Al-4V lattice structures manufactured via electron beam melting (EBM): A review. Acta Metall Sin (English Lett) 2020;33(2):183–203; doi: 10.1007/s40195-020-00998-1 [DOI] [Google Scholar]
- 4. Calignano F, Galati M, Iuliano L, et al. Design of additively manufactured structures for biomedical applications: A review of the additive manufacturing processes applied to the biomedical sector. J Healthc Eng 2019;2019; doi: 10.1155/2019/9748212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loeber L, Biamino S, Ackelid U, et al. Comparison of selective laser and electron beam melted titanium aluminides. In: 22nd Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, SFF. Austin, Texas, USA, 2011. [Google Scholar]
- 6. Wartbichler R, Clemens H, Mayer S, et al. On the Formation mechanism of banded microstructures in electron beam melted Ti–48Al–2Cr–2Nb and the design of heat treatments as remedial action. Adv Eng Mater 2021;23(12); doi: 10.1002/adem.202101199 [DOI] [Google Scholar]
- 7. Dzogbewu TC. Additive manufacturing of TiAl-based alloys. Manuf Rev 2020;7(35):8; doi: 10.1051/mfreview/2020032 [DOI] [Google Scholar]
- 8. Del Guercio G, Galati M, Saboori A. Innovative approach to evaluate the mechanical performance of Ti–6Al–4V lattice structures produced by electron beam melting process. Met Mater Int 2020;27:55–67; doi: 10.1007/s12540-020-00745-2 [DOI] [Google Scholar]
- 9. Gatto ML, Groppo R, Bloise N, et al. Topological, mechanical and biological properties of Ti6Al4V scaffolds for bone tissue regeneration fabricated with reused powders via electron beam melting. Materials (Basel) 2021;14; doi: 10.3390/ma14010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siemers C, Bäker M, Brunke F, et al. Aluminum- and Vanadium-Free Titanium Alloys for Application in Medical Engineering. In: Froes FH, Qian M, eds. Titanium in Medical and Dental Applications. Elsevier, 2018. [Google Scholar]
- 11. Rivera-Denizard O, Diffoot-Carlo N, Navas V, et al. Biocompatibility studies of human fetal osteoblast cells cultured on gamma titanium aluminide. J Mater Sci Mater Med 2008;19(1):8; doi: 10.1007/s10856-006-0039-4 [DOI] [PubMed] [Google Scholar]
- 12. Escudero ML, Muñoz-Morris MA, García-Alonso MC, et al. In vitro evaluation of a γ-TiAl intermetallic for potential endoprothesic applications. Intermetallics 2004;12(3):253–260; doi: 10.1016/j.intermet.2003.10.004 [DOI] [Google Scholar]
- 13. Kyzioł K, Kaczmarek Ł, Brzezinka G, et al. Structure, characterization and cytotoxicity study on plasma surface modified Ti-6Al-4V and γ-TiAl alloys. Chem Eng J 2014;240:516–526; doi: 10.1016/j.cej.2013.10.091 [DOI] [Google Scholar]
- 14. Santiago-Medina P, Sundaram PA, Diffoot-Carlo N. The effects of micro arc oxidation of gamma titanium aluminide surfaces on osteoblast adhesion and differentiation. J Mater Sci Mater Med 2014;25:1577–1587; doi: 10.1007/s10856-014-5179-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Som I, Balla VK, Das M, et al. Thermally oxidized electron beam melted γ-TiAl: In vitro wear, corrosion, and biocompatibility properties. J Mater Res 2018;33:2096–2105; doi: 10.1557/jmr.2018.175 [DOI] [Google Scholar]
- 16. Ikeo N, Ishimoto T, Fukuda H, et al. Fabrication and Characterization of Porous Implant Products with Aligned Pores by EBM Method for Biomedical Application. In: Chandra T, Ionescu M, Mantovani D, et al., eds. Advanced Materials Research. Germany: Trans Tech Publications. Vol. 409; 2012. [Google Scholar]
- 17. Hernandez J, Murr LE, Gaytan SM, et al. Microstructures for two-phase gamma titanium aluminide fabricated by electron beam melting. Metallogr Microstruct Anal 2012;1:14–27; doi: 10.1007/s13632-011-0001-9 [DOI] [Google Scholar]
- 18. Mohammad A, Alahmari AM, Moiduddin K, et al. Porous γ-TiAl structures fabricated by electron beam melting process. Metals (Basel) 2016;6(1):25; doi: 10.3390/met6010025 [DOI] [Google Scholar]
- 19. Gatto ML, Furlani M, Giuliani A, et al. Biomechanical performances of PCL/HA micro- and macro-porous lattice scaffolds fabricated via laser powder bed fusion for bone tissue engineering. Mater Sci Eng C 2021;128; doi: 10.1016/j.msec.2021.112300 [DOI] [PubMed] [Google Scholar]
- 20. Bloise N, Patrucco A, Bruni G, et al. In vitro production of calcified bone matrix onto wool keratin scaffolds via osteogenic factors and electromagnetic stimulus. Materials (Basel) 2020;13; doi: 10.3390/ma13143052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biamino S, Penna A, Ackelid U, et al. Electron beam melting of Ti–48Al–2Cr–2Nb alloy: Microstructure and mechanical properties investigation. Intermetallics 2011;19:776–781; doi: 10.1016/J.INTERMET.2010.11.017 [DOI] [Google Scholar]
- 22. Teschke M, Moritz J, Telgheder L, et al. Characterization of the high-temperature behavior of PBF-EB/M manufactured γ titanium aluminides. Prog Addit Manuf 2022;7:471–480; doi: 10.1007/s40964-022-00274-x [DOI] [Google Scholar]
- 23. Galati M, Rizza G, Defanti S, et al. Surface roughness prediction model for Electron Beam Melting (EBM) processing Ti6Al4V. Precis Eng 2021;69:19–28; doi: 10.1016/j.precisioneng.2021.01.002 [DOI] [Google Scholar]
- 24. Galati M, Snis A, Iuliano L. Powder bed properties modelling and 3D thermo-mechanical simulation of the additive manufacturing Electron Beam Melting process. Addit Manuf 2019;30; doi: 10.1016/j.addma.2019.100897 [DOI] [Google Scholar]
- 25. Rejmontová P, Capáková Z, Mikušová N, et al. Adhesion, proliferation and migration of NIH/3T3 cells on modified polyaniline surfaces. Int J Mol Sci 2016;17:82016; doi: 10.3390/ijms17091439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tavangar A, Tan B, Venkatakrishnan K. The influence of laser-induced 3-D titania nanofibrous platforms on cell behavior. J Biomed Nanotechnol 2013;9(11); doi: 10.1166/jbn.2013.1679 [DOI] [PubMed] [Google Scholar]
- 27. Pandiyaraj KN, Kumar AA, Ramkumar MC, et al. Influence of non-thermal TiCl4/Ar + O2 plasma-assisted TiOx based coatings on the surface of polypropylene (PP) films for the tailoring of surface properties and cytocompatibility. Mater Sci Eng C 2016;62; doi: 10.1016/j.msec.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Wang C, Wu S, et al. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater Sci 2020;8(10); doi: 10.1039/d0bm00269k [DOI] [PubMed] [Google Scholar]