Abstract
The wide development in biomedical, regenerative medicine, and surgical techniques has ensured that new technologies are developed to improve patient-specific treatment and care. Tissue engineering is a special field in biomedical engineering that works toward cell development using scaffolds. Bone tissue engineering is a separate branch of tissue engineering, in which the construction of bone, functionalities of bone, and bone tissue regeneration are studied in detail to repair or regenerate new functional bone tissues. In India alone, people suffering from bone diseases are extensive in numbers. Almost 15% to 20% of the population suffers from osteoporosis. Bone scaffolds are proving to be an excellent solution for osseous abnormalities or defect treatment. Scaffolds are three dimensional (3D) and mostly porous structures created to enhance new tissue growth. Bone scaffolds are specially designed to promote osteoinductive cell growth, expansion, and migration on their surface. This review article aims to provide an overview of possible bone scaffolding materials in practice, different 3D techniques to fabricate these scaffolds, and effective bone scaffold characteristics targeted by researchers to fabricate tissue-engineered bone scaffolds.
Keywords: bone tissue engineering, bone disease, bone scaffold, osteoinductive cells, porous structure
Introduction
Tissue engineering is an integrative field that combines life sciences and engineering to generate or develop biological replacements to enhance tissue function. Tissue engineering aims to rejuvenate damaged or diseased tissue in the body attached to the extracellular matrix (ECM). ECM is a compound network of carbohydrates and proteins. Configuration of the ECM depends on tissue, and it also involves structural proteins, for example, elastin, collagen, adhesive protein of the types, laminin or fibronectin, and proteoglycans. Proteoglycans are the core proteins with sugar molecules attached in the form of protein–polysaccharide complexes.1–3 The two sugar units in a polysaccharide are called glycosaminoglycans (GAGs). GAGs are typically used as a lubricant and shock absorber in the body because of their ability to attract water.
There are five primary GAG groups: chondroitin sulfate, dermatan sulfate, heparin sulfate, keratin sulfate, and hyaluronic acid based on the sugar type.4 The ECM formation in cartilage comprises a component of collagen and hyaluronic acid together with GAG. The ECM of bone is mainly made up of collagen and hydroxyapatite. On the contrary, the skin's ECM is composed of collagen, elastin, and proteoglycans.5,6
Bone tissue engineering integrates the basic scientific principles with tissue engineering methods by only targeting bone cells, bone diseases, and bone regeneration. Bone tissue engineering offers adaptable solutions to widen clinical choices for the treatment of bone diseases and trauma. Bone is a matter that constructs the skeleton of the body. It is predominantly composed of calcium carbonate and calcium phosphate. Bone protects the internal organs of the body. It gives structural stability to body locomotion. Fully operative bone tissue engineering needs newly reinstated bone to be completely amalgamated with the host bone and also able to perform all the functions of bone mentioned above.7,8
Scaffolds are structures made up of specific materials that can be artificially made and implanted inside the body. Scaffolds promote cell interactions inside the body and help to form new native functional tissues. The design of scaffolds focuses on porosity having a predefined shape. The porous structure of the scaffold is responsible for cell proliferation, cell migration, and cell differentiation as it mimics the native ECM in the body.9,10 In the body, the cells constantly reabsorb the ECM and sediment more. For example, bone grows in response to load. Even healthy bone is being reabsorbed and sublimated. However, in normal bone, the rate of reabsorption and sedimentation is roundabout the same. However, in a bone disease such as osteoporosis, this balance is disturbed.
Tissue engineering scaffolds are conventionally designed to degrade in the body from the enzymes discharged by cells. Ideally, such scaffolds must be replaced by a natural ECM produced by cells in the body.11,12
Tissue engineering was instituted toward the end of the 1980s and rapidly became famous among researchers. The early part of the study focused on in vitro studies involving specific cells such as stem cells, which were mixed with other chemicals to observe cell growth in controlled conditions.13 Since ancient times, people have been using some form of implants to treat various human diseases. This includes dental implants, which were practiced in various civilizations all over the world. As early as 2500 BC, Egyptians used gold wires as a ligature to support the surrounding teeth structures. The French civilization mastered implants made from wrought iron on corpses around 200 AD. The Maya civilization practiced making nacre teeth using calcium carbonate derived from seashells around 600 AD; the present-day process is called osseointegration.11 Around 800 AD, the Honduran dynasty was able to make implants out of stones.10
These historical instances reveal that humans of almost every era have practiced tissue engineering and implants in one way or the other. This is a massive motivation for current researchers, and with the advancement of technology, tissue engineering is climbing an upward path.14
In vitro studies on scaffolds started around 25 years ago, where primarily bone tissue engineering was targeted. Since then, various materials have been used in bone scaffolding, and various technologies have been developed. Some methods used to fabricate the scaffolds include electrospinning, solvent casting, porogen leaching, gas foaming, phase separation, fiber mesh, melt molding, bonding, membrane lamination, and freeze-drying, along with rapid prototyping.15
Bone Tissue Engineering
Bone tissue engineering anchors on substitute options for the treatment that can discard the limitations such as donor-site morbidity, limited availability of cells, immune rejection, and pathogen transfer to name a few. Bone tissue engineering typically targets a few key aspects that include the development of a biocompatible scaffold that can closely mimic the natural ECM and osteogenic cells that lay down the bone tissue matrix. Bone tissue engineering demands the usage of porous three-dimensional (3D) scaffolds, which along with bioactive factors and cells can supply structural support for the cells to spread, differentiate, and migrate for new tissue development. The main components of bone tissue engineering involve cells, growth factors, and biomaterials.8
Functions of bone, treatment, and clinical problems
Bone is a naturally generated composite material that generates the skeleton of the human body consisting of an organic phase with collagen and an inorganic phase with hydroxyapatite. Viscoelastic behavior and toughness of bone are largely due to collagen. The major functions of bone are protecting the internal organs, providing load-bearing capacity to the skeleton, trapping dangerous materials such as lead, housing the biological elements required for hematopoiesis, and maintaining homeostasis of key electrolytes.
Treatment of bone defects that involve trauma or disease or disorder presents a major threat to all surgeons around the world. Massive bone loss due to damage is a very serious issue and needs to be addressed successfully. After a blood tissue transplant, bone tissue transplant is the largest transplanted tissue. Bone grafting is used to regenerate the bone and more than 2 million bone grafting surgeries are performed around the world annually. Bone grafting treatment involves autografting, in which tissues are grafted from the same individual. It offers good biocompatibility structurally and immunologically. The autografting procedure is very painful. It increases surgery time, bleeding, and donor-site morbidity. It can lead to nerve injuries at multiple harvest sites due to surgeries.
In allografting, tissues are grafted from different individuals but of the same species. Allografting is associated with pathogen transfer and immune rejection. All and all, a better treatment option is essential for enhancing bone regeneration and repair. Bone tissue engineering can provide synthetic bone substitutes/scaffolds, which will eliminate all the above limitations and enhance bone regeneration.16
Scaffolds in bone tissue engineering
A bone scaffold is a 3D web that energizes the attachment and proliferation of osteoinductive cells on its surface. Using bone scaffolds made with biocompatible biomaterials, cells can be seeded on top of it. While using scaffolds in bone tissue engineering, the general ideal revolves around removing the diseased or defected portion of bone and replacing it with the bone scaffold. Depending on the material of the bone scaffold and its structure, enhancement of osteoinductive cells is carried out and bone regeneration or remodeling is achieved.
Scaffold vascularization
Vascularization is the formation of new blood vessels. New bone formation rapidly happens in highly vascularized regions. Insufficiently vascularized sites are prone to decreased bone regeneration and repair. So vascularization plays a very vital role in bone tissue engineering and is identified as a major pitfall. There have been great efforts by researchers to come up with various methods to accelerate vascularization for the survival and integration of bone tissue engineering scaffolds with the inclusion of host tissue. Emphasis is given to enhancing scaffold design to incorporate vascularization, which can be best achieved by 3D printing (3DP) scaffolds. 3DP is used in both first- and second-generation scaffolds. Angiogenic growth factors are included to enhance endothelial cells, and scaffolds are 3D printed with the help of the fused deposition modeling (FDM) technique, which are also called biomimetic scaffolds.
In vitro prevascularization is used to check that the amount of vascularization is nothing but the coculture of endothelial cells and osteogenic cells. In vivo prevascularization is a two-step process. At first, the scaffold is vascularized in vivo at different locations, and then, this scaffold is removed and placed on the bone defect site. This method is very painful, time-consuming, and requires a minimum of two surgeries. It is very difficult to choose the best method that vascularizes scaffolds successfully. Maybe a typical combination of the two methods can prove more efficient in enhancing scaffold vascularization.17,18
There are a few challenges in increasing vascularization in bone tissue engineering. When the scaffold is placed in the desired location, the body immediately reacts in the form of an inflammatory response followed by tissue encapsulation. This encapsulation needs to be avoided and immunomodulatory strategies can come in very handy. To implement immunomodulatory strategies, the biggest challenge is the availability of animal models to test the bone tissue engineering scaffold approach preclinically. If bone tissue engineering needs to become clinically extensive, it should incorporate recent technologies such as 3DP, which can make use of all components, that is, cells, scaffolds, and growth factors to repair and regenerate the bone. Efforts should also be made in the direction to remove in vitro bone tissue engineering scaffold culture and increase in vivo bone tissue engineering scaffold for bone regeneration19 (Fig. 1).
FIG. 1.

Requirements for ideal bone scaffold (author's schematic).
Biomaterials
A biomaterial is a natural or synthetic substance that is engineered to perform a specific biological function with a medical purpose either diagnostic or therapeutic. Biomaterials are classified based on their ease of usage, their type, biocompatibility, biodegradability, and biological responses, as given in Table 1.
Table 1.
Classification of Biomaterials
| Biomaterials | ||
|---|---|---|
| First-generation biomaterials | Second-generation biomaterials | Third-generation biomaterials |
| These are bioinert with minimum interaction with the surrounding tissues and normally osteoinductive materials | These are bioactive and biodegradable hybrid materials with a combination of two or more biomaterials with enhanced functions in the form of copolymers, polymer–polymer blends, or polymer–ceramic composites | These are specifically designed based on second-generation biomaterials to increase biological response with bioresorbable properties |
| Mainly include metals, that is, titanium alloys, synthetic polymers (polymethyl methacrylate, polyether ether ketone), and ceramics (alumina and zirconia) | Mainly include synthetic and natural polymers (collagen, calcium carbonates, calcium phosphates, sulfates, bioactive glasses, etc.) | Mainly include polymers, polymer composites, hydroxyapatite, etc. |
Essential properties required in biomaterials
Pore size
Pore size ranging from 20 to 1500 μm is used mainly in tissue engineering applications to mimic natural bone. It is recommended to have a pore size of 80 to 120 μm for considerable bone growth. If the pore size is tiny, that is, less than 80 μm, then migration of cells is affected, and if the pore size is huge, that is, more than 500 μm, then cell attachment is affected because of decreased specific surface area. Optimum pore size provides a good number of cell sites and increases bone ingrowth.20 However, achieving a uniform pore size is very difficult; therefore, gradient porosity is preferred. A gradient porous structure has a nonuniform pore size, and it is considered more efficient than a uniform porous structure as it enhances cell migration, cell attachment, and intracellular signaling.21
Biocompatibility
Biocompatibility is a material property to react with a suitable host response under a particular situation. It refers to the appropriateness of the biomaterial to the body and bodily fluids. The bone scaffold should be biocompatible to increase bone tissue interaction with the scaffold material. A biocompatible polymer can improve normal body functions without affecting its operations and activating allergies or other side effects.22
Biodegradability
It is defined as a chemically reactive process that produces a sharp division of covalent bonds. Biomaterials such as polymers degrade by the hydrolysis mechanism, which typically means chemical breakage in the presence of water molecules. Biodegradable polymers can be defined concerning the time frame they degrade once they are implanted inside the patient. When the new bone tissue in the body starts recreating itself along with the pores of scaffolds, the scaffold material should conveniently decompose inside the body. This is a significant property when selecting a type of biomaterial for scaffolds.23
Nontoxicity
Cytotoxicity is defined as a quality that makes cells toxic due to the addition of foreign elements in the body. When foreign materials are implanted in the body utilizing the scaffold, there is a chance that the scaffold material generates toxicity among the surrounding cells. As an implant material, it should not possess or exhibit cytotoxicity. In other words, the bone scaffold should be nontoxic.24
Chemical bonding at surface
The bone scaffold material should promote cell attachment, cell proliferation, and cell migration. To achieve this, there should be a chemical bond established with the scaffold surface and surrounding tissues. It is also termed cytocompatibility. It is a property confirmed by in vitro studies showcasing enough cell activity and tissue-specific functions.25,26
Mechanical strength
The bone scaffold material should possess adequate mechanical strength. To place the scaffold at an appropriate location in the patient's body, the scaffold should withstand a certain amount of compressive stress. Also, when implanted, depending upon its specific location, it should possess enough strength to support adjacent bones if necessary.27
Printability
Biomaterials should exhibit excellent printability. In various 3DP techniques, the definition of printability is different. In stereolithography, it is the ease with which liquid resin solidifies and cures with the help of ultraviolet (UV) light. In selective laser sintering, powder material can fuse and bind with a subsequent sintered layer at a controlled temperature. In binder jet printing, it is the property of powder to bind with subsequent layers depending upon the properties of the binder substance. Finally, in FDM, it is considered the melt flow index of the filament, which is the amount of material flow rate when the melted material flows out of the nozzle. It is determined using glass transition temperature along with the flowability of thermoplastics24–26,28 (Fig. 2).
FIG. 2.

Essential properties of biomaterials (author's schematic).
Polymers as biomaterials
Polymers and polymer composites are used as biomaterials in tissue engineering applications to fabricate scaffolds. The selection of polymer material depends on various properties such as molecular weight, shape, lubricity, chemistry, hydrophilicity, hydrophobicity, solubility, and biodegradability. Scaffolds made with polymers possess good mechanical strength, biodegradation, and porous structure when 3D printed with various 3DP technologies. Polymers are classified into two broad categories, natural polymers and synthetic polymers. Natural polymers are further classified into proteins, polysaccharides, and polynucleotides. Synthetic polymers are classified as copolymers, microbial polymers, and bioactive ceramics (Figs. 3 and 5).28
FIG. 3.
Classification of polymers (author's schematic).
FIG. 5.
Fused deposition modeling process (author's schematic).
Natural polymers
Natural polymers are obtained from animals, plants, and microorganisms, which are called renewable resources. They possess complex structures with different physiological functionalities depending on the source from which they are derived. They are generally formed by polymerization of the type addition or condensation. Regular polymers exhibit exceptional properties. These properties often arise from the structural organization of the material and not from molecular formation.29 Emphasis is given to studying and mimicking natural polymer structure and functionality to develop composite polymers with enhanced properties. Proteins are widely used for developing natural polymers. Many proteins, for example, silk, gelatin, soy protein, casein, and keratin, have demonstrated excellent attributes. When these proteins are blended with polymers, they exhibit better shear and flexural strength, toughness, elasticity, and tensile modulus.30
Polysaccharides are extended shackles of polymeric carbohydrate molecules consisting of monosaccharides connected by o-glycosidic chains. Polysaccharides manifest various physiological functions. Some examples of polysaccharides include cellulose, starch, alginate, chitosan, GAGs, hyaluronic acid, pullulan, and dextran.31
Polynucleotides are long polymer chains of nucleotide monomers that are covalently bonded together. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are examples of polynucleotides. Research is going on in gene therapy using bionanocomposites of DNA and RNA.32
Collagen
It is the most abundant protein by weight in the human body (50% of total protein in the human body). Collagen provides structural support for the body. Studies have identified 46 different collagen genes in the human genome, which can generate 28 different types of collagen fibrils, for example, type I, type II, and type III. Type I collagen is seen in skin, tendon, vascular ligature, and bone. It is the main component of bone. Collagen monomers self-associate into a triple helical structure called collagen fibrils. These collagen fibrils are 10 to 300 monomers thick and aggregate to form collagen fiber. This collagen then forms the ECM.18
Silk fibroin
It is a fiber-rich protein that is mainly produced by insects such as silkworms, spiders, and flies. Silk produced from spiders and silkworms possesses excellent biological properties. Silk obtained from insects varies in its physical and chemical properties vastly because of different geophysical properties. Thus, they possess different biological properties as well. Silk is made from two different proteins, that is, fibrous protein (silk fibroin) and globular protein (sericin). The silk-producing ability of various insects has greatly evolved over the years. Thus, silk is produced with different molecular structures and mechanical properties.
In general, silk obtained from Bombyx mori silkworm has Young's modulus of 12.4–17.9 GPa and ultimate tensile strength of 360–530 MPa at an elongation of 18–21%. Silk obtained from cocoon yields using degumming and sericin protein coat has Young's modulus in a similar range, but a slightly more ultimate tensile strength of 450–700 MPa and an elongation of the range of 12–24%. The excellent mechanical properties of silk fibroin make it a suitable candidate material for bone scaffolds. Whereas the processing technique to make bone scaffolds from silk fibroin gradually alters its properties and should be controlled and monitored closely.33,34
Gelatin
Gelatin is a counterpart of collagen and possesses good biocompatibility, mechanical strength, and chemical characteristics. These properties can be further enhanced in controlled conditions. Permanent hydrolysis of collagen produces gelatin by lowering the protein fibers into small peptide groups with varying molecular weights. Gelatin can sustain matrix metalloproteinase recognition flow allowing degradation enzymatically. The great tuning capabilities of gelatin in terms of its chemical and physical properties make it a highly suitable candidate for making bone scaffolds. Young's modulus of gelatin can be enhanced up to 152 MPa making it stronger than collagen by crosslinking it with glutaraldehyde.18,35
Hyaluronic acid
Hyaluronic acid is a natural polymer and an essential part of human ECM and thus found in literally every tissue of the vertebrates. It is highly hydrophilic with high water solubility. Hyaluronic acid has got a tendency to trap water molecules, which allows it to obtain 3D complex shapes by forming gels. Hyaluronic acid is used to repair tissues because it can readily promote stem cell and epithelial cell migration and differentiation. This unique property of hyaluronic acid makes it a highly suitable candidate for tissue engineering applications. It is mixed with natural and synthetic polymers to make hydrogels by the crosslinking process. It improves the enzymatic degradation of hyaluronic acid. Using an aqueous hyaluronic acid solution, three porous structures can be created.18,36
Chitosan
Chitosan is a naturally occurring GAG in the human body. Compared with the other polysaccharides, chitosan is easy to obtain. Chitosan is a linear polysaccharide of (1-4)-linked d-glucosamine and N-acetyl-d-glucosamine. It is a natural polymer obtained from chitin, which is the main substance of the crustacean exoskeleton. Chitosan is extracted from the marine crustacean shells by chemical hydrolysis. It can also be obtained from typical fungi by enzymatic digestion of its cell walls. Since chitosan has a very similar structure to GAG, it is highly useful to create porous scaffolds. It promotes bone ingrowth and can be easily shaped into different forms that include fibers, sponges, beads, films, and complicated structures for orthopedic applications. It is also used as an injectable biopolymer in bone tissue engineering applications.18,37
Starch
Starch is a carbohydrate reserve molecule of higher plants. It is one of the cheapest biopolymers that are biodegradable in water and carbon dioxide. Starch contains alpha-d-glucose units, which can be organized as amylose and amylopectin. Amylose is a linear polymer that is sparsely branched and linked by 1–4 glycosidic bonds. Amylopectin is also a heavily branched polymer that contains 1–4 bonds and 1–6 branching points appearing for every 25–30 glucose units. Starch is semicrystalline in structure with crystallinity ranging between 15% and 50%. Starch possesses biological renewability, but is brittle and very difficult to process as well. Starch also has a low surface area and its affinity with water limits its usage as a bone scaffold material in bone tissue engineering.38
Synthetic polymers
Synthetic polymers have shown a lot of advantages over natural polymers. Synthetic polymers possess desired mechanical properties, process control capabilities, and reliability. Synthetic polymers can be engineered to achieve required chemical bonding, cell interaction, porosity, surface roughness, and so on. Synthetic polymers can also demonstrate controlled resorption and biocompatibility, and beneficial properties while designing bone scaffolds.39 They are more uniform and have predictable responses toward chemical as well as mechanical properties. Synthetic polymers can be made to exhibit nontoxic behavior toward the surrounding tissues, and this property makes them a more preferred material choice than natural polymers. Synthetic polymers are broadly classified according to their ability to degrade biologically without causing any harm or damage to the surrounding tissues and their inability to degrade biologically.
Various methods are used to create synthetic polymer scaffolds. Some include gas leaching, salt leaching, electrospinning, solvent casting, gas foaming, and 3DP. Out of these, 3DP is a more suitable technique for bone scaffolds because it can produce the desired shape and achieve adequate porosity.40 The 3D-printed bone scaffold can mimic the bone shape and also provide cell sites for attachment. When this scaffold is implanted in the patient, it can be resorbed easily and promote bone ingrowth. In this section, highly suitable synthetic polymers for four-dimensional printing using the FDM technique are discussed. The FDM technique is opted for because of its cost-effectiveness and ability to use biodegradable materials.
Polylactic acid
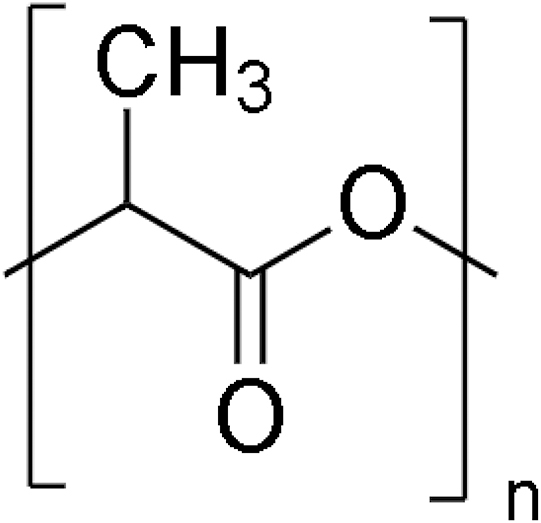
Polylactic acid (PLA) is a ubiquitous biodegradable material used in tissue engineering applications to fabricate bone scaffolds. See Table 2 for the chemical structure of PLA. PLA comes in different forms, for example, amorphous and crystalline, based on the levels of isomers present in its molecules. PLA is a copolymer of polyglycolic acid (PGA) specifically developed to increase its hydrophobicity. PLA is a chiral molecule in a couple of stereoregular polymers, that is, d-PLA and l-PLA, and a racemic type D, l-PLA. l-PLA is largely used in tissue engineering and bone scaffolding applications because it is a semicrystalline material.
Table 2.
Chemical Structures of Synthetic Polymers (All Images Are Author's Schematic)
| Sr. no. | Name | Chemical structures of synthetic polymers |
|---|---|---|
| Fig. 1 | PLA (author's schematic) |
|
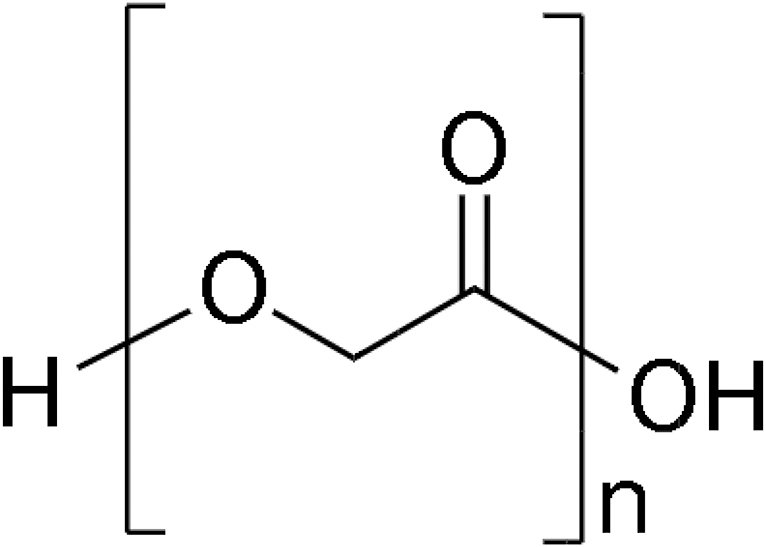
| Fig. 2 | PGA (author's schematic) |
|
| Fig. 3 | PLGA (author's schematic) |
|
| Fig. 4 | PCL (author's schematic) |
|
| Fig. 5 | PEG (author's schematic) |
|
| Fig. 6 | PPF (author's schematic) |

|
| Fig. 7 | PVA (author's schematic) |
|
| Fig. 8 | PU (author's schematic) |
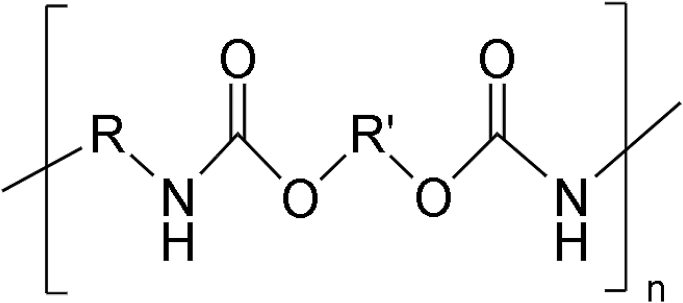
|
PCL, polycaprolactone; PEG, polyethylene glycol; PGA, polyglycolic acid; PLA, polylactic acid; PLGA, polylactide-co-glycolide; PPF, polypropylene fumarate; PU, polyurethane; PVA, polyvinyl alcohol.
Its hydrolysis produces L lactic acid, a naturally available stereoisomer of lactic acid. It degrades inside the human body producing CO2 and water, which are not harmful. Also, because of its semicrystallinity, it possesses high mechanical strength and toughness, which is a desirable property of a bone scaffold. To achieve the desired porosity for bone ingrowth and adequate mechanical strength, PLA is highly suitable.24–26,41,42
Polyglycolic acid
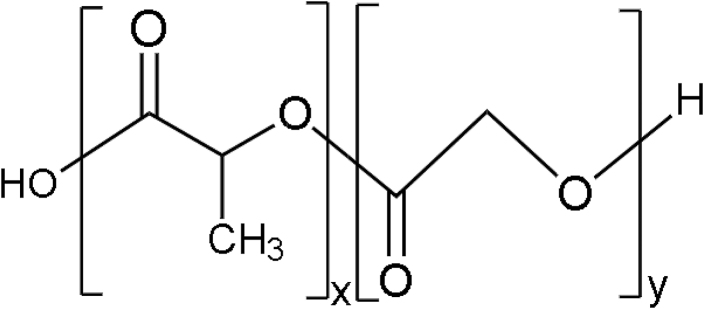
PGA is simple linear aliphatic polyester. It is crystalline along with a high melting point as well as low solubility in organic solvents. See Table 2 for its chemical structure. It is biodegradable and promotes cell migration, proliferation, and bone ingrowth. PGA also exhibits excellent mechanical properties when 3D printed with the FDM technique. When PLA and PGA are mixed with an equal percentage by weight (50% PLA and 50% PGA), it forms a copolymer called polylactide-co-glycolide. See Table 2 for its chemical structure. It demonstrates faster degradation rates compared with both PLA and PGA. It is nontoxic and has recorded no side effects when used as bone scaffold material.43
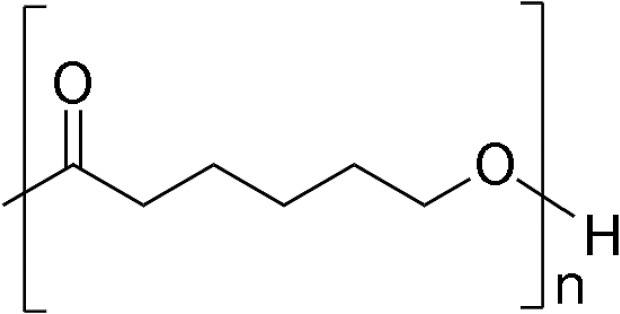
Polycaprolactone
Polycaprolactone (PCL) is an aliphatic semicrystalline polymer with adequate biocompatibility and mechanical strength. See Table 2 for its chemical structure. PCL is highly hydrophobic and created by ring-opening polymerization. It then forms degradable ester linkages. The rate of degradation of PCL is prolonged, taking place by bulk or surface hydrolysis of ester linkages. Slow degradation ensures that PCL remains in the patient's body for a maximum period of 2 years. PCL is used in bone tissue engineering, adheres to osteoblasts, and produces alkaline phosphatase, a biomineralization marker. The degradation rate of PCL can be increased by copolymerization with polyethylene glycol (PEG) or polyvinyl alcohol (PVA).44 PCL is also available in filament form for material extrusion-type 3DP. Its low melting point is advantageous compared with other filament materials.
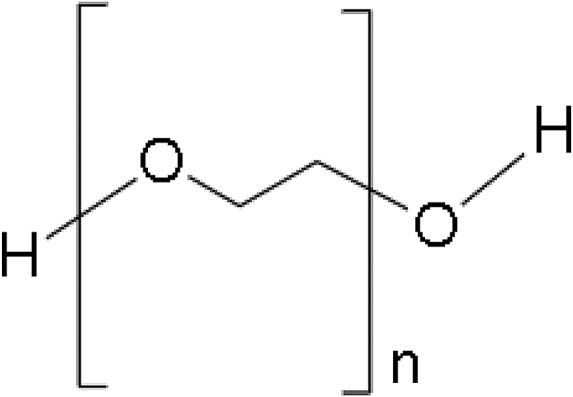
Polyethylene glycol
PEG is a direct chained polyether comprising repeating units of ethylene oxide. See Table 2 for its chemical structure. PEG is highly soluble in water and few organic solutions, namely ethanol, acetonitrile, and benzene.45 PEG is nontoxic and therefore often used in tissue engineering applications. The swelling capacity of PEG makes it highly suitable to produce hydrogels. However, the linear chain-type structure of PEG ushers in low mechanical stability and quick diffusion. PEG is nonbiodegradable, but copolymerization makes it biodegradable. It has a degradation time of ∼16 to 18 weeks and produces a minimal erythrogenic response when examined in vivo.46 The photopolymerization potential of PEG allows it to structure in any complex shape, retaining its mechanical, chemical, and structural characteristics, which makes it a promising candidate for 3DP scaffold material in a resin-based technology.42
PEG is used as an additive while making ultrahigh-molecular-weight polyethylene (UHMWPE). UHMWPE filament can be 3D printed using the FDM 3D printer.47
Polypropylene fumarate
Polypropylene fumarate (PPF) is a biodegradable, crosslinkable linear polyester having repeating units of ester bonds. See Table 2 for its chemical structure. The high mechanical strength, controllable degradation rate, and biocompatibility of PPF make it suitable for making tissue engineering bone scaffolds. Crosslinked PPF exhibits good thermal stability in situ.48 PPF degrades into fumaric acid and propylene glycol in the existence of water. These byproducts are rapidly thrown out of the human body through a metabolic biochemical process. PPF demonstrates controllable degradation capability; this feature improves the hydrophobicity of materials such as PLA, PGA, and PCL.42 PPF is seldom used in liquid form with resin-based 3D printers.
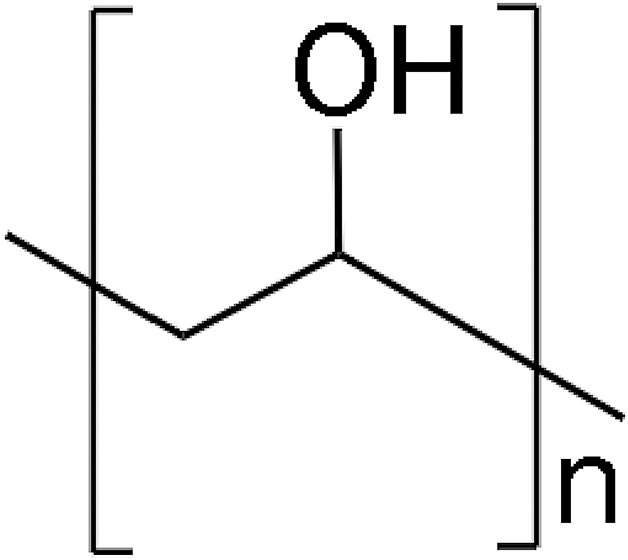
Polyvinyl alcohol
PVA demonstrates excellent biocompatibility and water solubility. See Table 2 for its chemical structure. It is a biodegradable polymer with an associated matrix that permits easy resorption.49 Since vinyl alcohol is unstable, it is synthesized in a two-step manner. Initially, vinyl acetate has undergone radical polymerization to form polyvinyl acetate. Then, the hydrolysis is carried out on polyvinyl acetate to form PVA. The degree of hydrolysis can be effectively controlled, resulting in different physical and mechanical properties for PVA.50 PVA is typically used as a support material in FDM 3D printers. It can also be mixed with other materials to increase the biodegradability index.
Polyurethane
Polyurethane (PU) exhibits excellent biocompatibility, mechanical properties, and fatigue resistance. PU is considered to be the best suitable material for making bone scaffolds. See Table 2 for its chemical structure. PU is made using the catalytic-polymerization process in which repeating urethane groups are synthesized by reacting hard segments of polyisocyanates with soft segments of polyols.51 PU is a biodegradable material and undergoes physical degradation because of water absorption.52 PU is used to create devices that can contact blood and blood veins and shows no adverse effects. PU is mixed with other polymers to enhance their biocompatibility. PU is also used to make solid filaments used in FDM 3D printers for biomedical applications and showcase good printability and structural properties.53
Table 3 explains the suitability of the above-discussed synthetic polymers and their parameters. The parameters include Fourier transform infrared (FTIR) spectroscopy, contact angle, glass transition temperature, tensile modulus, and resorption period of these materials (Table 3).
Table 3.
Polymer Properties
| Polymer | Fourier transform infrared spectroscopy (cm−1) | Contact angle (°) | Glass transition temperature (°C) | Tensile modulus (GPa) | Resorption period (weeks/months/years) | Refs. |
|---|---|---|---|---|---|---|
| PLA | CH3 group resonance 2929 cm−1 peak C = O group 1756 cm−1 carboxyl group 1090 cm−1 |
74.3° ± 11° | 45–60 | 0.35–3.5 | 1–2 years | 24,54 |
| PGA | 1751 cm−1 because of carboxyl stretch | 66.1° ± 8° | 35–45 | 6–7 | 6–12 months | 24,54–56 |
| PCL | Peak at 3500 cm−1, sharp signal at 1750 cm−1 corresponding to hydroxyl and ester group | 140° ± 5° | 60 | 0.2–0.4 | Up to 2 years | 54,57,58 |
| PEG | Peak at 3446 cm−1 corresponding to terminal hydroxyl group | 63° ± 5° | (-56)–(-52) | 0.12–0.266 | Nonbiodegradable in pure form | 59,60 |
| PPF | Peak at 3540 cm−1 corresponding to CH bonds | 60.6° ± 8.2° | 30–32 | 0.21–0.24 | 6–8 weeks | 61–64 |
| PVA | The main peak at 3280 cm−1 corresponds to the off-stretching vibration of the hydroxyl group | 60.6° ± 5° | 85 | Up to 2 months | 49,65,66 | |
| PU | The main peak at 3352 cm−1 due to strong absorption of N-H stretching | 66° ± 5° | −35 | 0.091 | Up to 3 months | 67–69 |
According to Table 2, PLA shows a favorable chemical composition for bone scaffold application to its FTIR spectroscopy results. The contact angle of PLA is <90° making it hydrophilic, which can provide enough cell sites for bone tissues to grow. PLA has a moderately high glass transition temperature of 45°C to 60°C compared with the average human body temperature. When PLA is used as a bone scaffold, it can retain its solid form and provide adequate mechanical strength to the adjacent bones. The tensile modulus of PLA is between 0.35 and 3.5 GPa, which ensures enough load-bearing capacity when used as a bone scaffold implant in the lower limb.
Hydrogels
Hydrogels are matter that can absorb water and retain moisture. Hydrogels are associated and connected nexus of hydrophilic macroscopic monomers that are initially soluble in water, but, as the crosslinking is created to a higher degree, become insoluble in water. Hydrogels can absorb and retain a large amount of water. Crosslinking plays a vital role while creating hydrogels and there are many types of crosslinking, that is, chemical crosslinking and physical crosslinking. Chemical crosslinking is inter- or intramolecular joining of two or more molecules by a covalent bond with the help of crosslinking reagents. Physical crosslinking is a process of bond formation between polymer chains through weak interactions, that is, ionic bonds. Crosslinking alters the physical properties and gives more rigidity to the material.
Hydrogels are normally used above the glass transition temperature so that they have a rubber-like flexible structure. Hydrogels can absorb a high amount of water because they possess hydrophilic functional groups on the polymer with which it is made. Hydrogels have compositional and structural similarities when compared with the ECM. They are used as moisture-retaining agents in the process of wound healing. They have a substantial framework that accelerates cell proliferation and cell survival in tissue engineering applications. Hydrogels can provide efficient solute transport through vascularization while making 3D scaffolds.
Hydrogels are produced by the crosslinking process. The subunits then form a network of macroscopic dimensions. Initially, these subunits grow and branch out, and at the same time remain soluble and disperse. Then, clusters form and grow in size and become very large. Gel point is accomplished where all subunits are connected with each other at numerous points. The crosslinks also confer mechanical and structural integrity and prevent dissolution in an aqueous environment. Hydrogels are heterogeneous, when put in water their solid-rich regions get distributed within the liquid and swell to a great extent. Hydrogels have very high viscosity but a defined shape as well, but they can have different porosities.54
Hydrogels for bone tissue engineering
Being a type of polymer scaffold, hydrogels have various prospective supremacies in bone tissue engineering and bone repair. Hydrogels can provide a suitable environment for the growth of endogenous cells. Hydrogels possess an excellent network structure to trap the proteins or cells and release them at the required cell site very efficiently. Receptivity of hydrogels is very large and showcases good integration with the surrounding tissues, helping to avoid complexity in surgery and reducing the inflammatory response. Hydrogels greatly promote bone regeneration through their cell and drug delivery mechanism. It creates a natural hydrophilic 3D environment favorable for the survival of cells and helps to form new bones.54
Even though hydrogels are best when it comes to bone tissue engineering applications and bone scaffold material, there are few challenges. The biocompatibility of hydrogels is a bit of concern. When hydrogels are used for bone tissue regeneration, many times an inflammatory response is recorded. To stop this, biocompatible materials such as collagen, chitosan, and alginate can be used to prepare hydrogels. However, hydrogels created using these natural polymers exhibit poor mechanical strength and rigidity. The use of suitable synthetic polymers can overcome this problem.55
Hydrogels should exhibit good interaction with the surrounding tissues to promote osteointegration. Various studies have showcased that blood vessels are not present at the defect site, to improve this condition, angiogenic factors can be incorporated into hydrogels for quick bone regeneration.56
Role of 3DP in Biomedical Applications
3DP involves unique ways of creating parts. The process begins by creating a 3D model using a CAD package. This 3D solid model is then converted into a valid surface format, either .stl or .obj. This surface file is then accessed using slicing software. The slicing software uses this surface file and creates all inner details, including infill pattern, infill density, layer height, and wall count, to convert a surface file into a layer file. A 3D printer uses this layer definition to fabricate the part layer by layer.5 The ability of all 3DP technologies to create intricate shape geometries makes it highly suitable to use in biomedical and tissue engineering applications. Precisely speaking, scaffolds with porous structures can be 3D printed using various technologies.6
In 3DP, the scaffold is printed with polymer material or ink and then cells are seeded on top of it. There are almost 40 different 3DP technologies available today. They are based on different approaches.9,10
Stereolithography
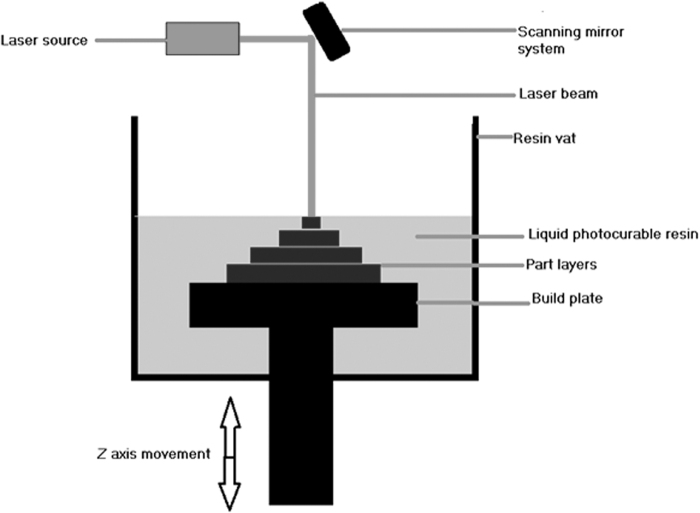
The first 3DP technique was developed way back in 1986 by Charles Hull. It uses liquid resin material and UV light. It works on the principle of photopolymerization in which the liquid resin solidifies when a particular wavelength of an UV light source is made to fall upon it. It finds applications in tissue engineering to 3D print bone scaffolds. Biocompatible and biodegradable liquid resin is a mandatory need to fabricate bone scaffolds using this technique. Examples of liquid composite resins used in stereolithography are ceramic suspension mixed with hydroxyapatite, di-trimethylolpropane tetraacrylate, and hexanediol diacrylate in a paste or gel form. This composite resin in gel form was 3D printed using stereolithography and cured under UV light to obtain a ceramic scaffold. It underwent sintering and debinding to provide adequate strength to the scaffold, and the toxic polymer was also removed from it.12
A scaffold made with this resin showcased good compressive strength of the order 5.6 to 18.4 MPa, and an elastic modulus value was recorded between 2.4 and 5.9 GPa. These values even matched with actual bone values.13 However, in the above example, a 3D-printed scaffold goes through various fabrication stages, including solidification using UV light, washing to remove uncured resin, heating in the oven, and then again curing in UV light. The resin itself is very costly, and at each stage, the cost associated is considerable, making stereolithography a costly technique to 3D print scaffolds. Commercially available resins are very limited in numbers, and most of them are not biocompatible, which is a limitation. To make resin respond to UV light, photoinitiators are required.14,57 Most of the photoinitiators are toxic and must be removed after the print.
So, stereolithography offers excellent quality prints with detailed internal geometry and porosity, but at the expense of higher costs (Fig. 4).
FIG. 4.
Stereolithography process (author's schematic).
Fused deposition modeling
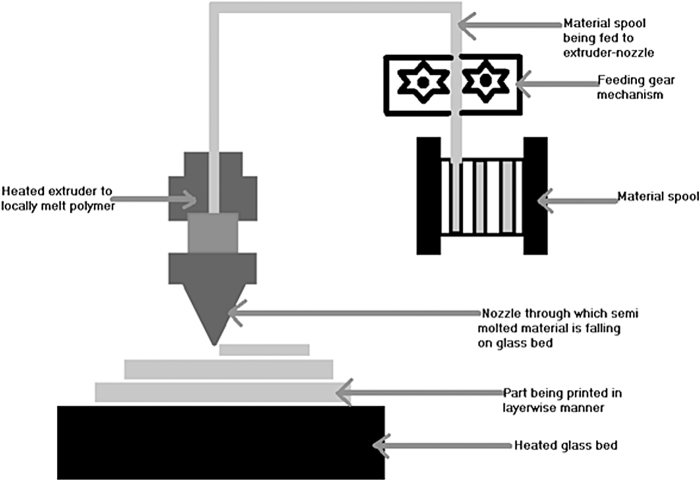
FDM is a technique in which the material is in solid filament form. It was developed around 1988 by Scott and Lisa Crump. It is also called fused filament fabrication and material extrusion technique because it uses polymer filament. These polymer filaments come in standard sizes of 1.75 mm diameter and 2.85 mm diameter. The polymer filament is heated in the extruder, which consists of heating coils. The temperature of the heating coil is controlled very precisely such that the polymer melts locally. A feeding gear mechanism continuously pushes the filament into this extruder. Once the filament locally melts because of the pressure from feeding gear, this semimolten polymer is made to come out through a nozzle onto the heated glass bed to manufacture parts layer by layer.
Usually, two extruders and nozzles are used: primary extruder and nozzle to deposit base polymer material and secondary extruder and nozzle to deposit support material for overhang geometries. This support material can be easily removed, and a clean part is obtained. The selection of material for FDM precisely depends upon the flowability of polymer close to its melting point and thermal characteristics.13
Because of the ability of a material to reshape itself upon heating up to a melting point, thermoplastics are used in FDM. Some of the common materials used include PLA, acrylonitrile butadiene styrene, polycarbonate, nylon, and copolyester for general applications. Important material properties to 3D print bone scaffolds using FDM include biodegradability, biocompatibility, and nontoxicity.15,24–26 Polymers are available as natural and synthetic polymers. Among natural polymers, collagen, hydroxyapatite, and chitosan are some of the materials that are used for the 3DP of scaffolds. Synthetic polymers exhibit better mechanical properties compared with natural polymers. It is found from the study that synthetic polymers mixed with natural polymers such as collagen, hydroxyapatite, chitosan, alginate, fibroin, and hyaluronic acid tend to increase bone ingrowth and cell attachment. Compared with stereolithography, selective laser sintering (SLS), and inkjet printing, FDM is cost-effective and easy to use17 (Fig. 5).
Binder jet/inkjet printing
There are two printing strategies in inkjet printing: direct printing and indirect printing. In direct printing, the synthetic powder is evenly spread over a printing bed with the help of a roller. The print head deposits the binder material on the bed in a layer-wise manner according to the geometry. The binder binds the ink. Again the bed is lowered, the powder is spread over the bed again, and the print head spreads the binder material. This cycle continues till the whole part is fabricated. This technique is also called three-dimensional printing. The part is retrieved, and unbounded powder can be reused for printing purposes. The synthetic powder used in this technique includes PCL and polylactide-co-glycolide alongside organic solvents used as binder material.
Also, some natural polymers can be used in powder form, which includes materials such as starch, gelatin, and dextran. For natural polymers, water can be used as a binder material.13,58,59 Inkjet printing allows fabricating complex geometries because the powder present on the bed readily acts as a support structure. Complex internal features and highly porous structures are very easily printed using this technology. Resolution as low as 100 μm can be easily achieved with this. Using a direct printing approach, cells/tissues can be effectively printed, and desired porosity ensures cell proliferation and growth.
In indirect printing, commercially available materials such as calcium sulfate and hemihydrate plaster powder can be used as powder material, and water can be used as a binder material. Mold is printed first, and then it is cast with biodegradable material such as hydroxyapatite and polylactide-co-glycolide.60–62 Highly customized and patient-specific scaffolds can be printed with this technology. Any particular damaged bone/diseased bone can be recreated using computed tomography (CT)/magnetic resonance imaging (MRI) scan data. Using an indirect printing approach, proper size molds can be made in which biodegradable material can be cast and patient-specific, customized scaffolds are fabricated.63–65 It is even possible to combine inkjet printing with other techniques such as electrospinning to achieve an even lower resolution of the order of 70 μm.66 A recent study also showcases examples of scaffold fabrication using inkjet printing, which provides sites for cell attachment and even improves drug-delivering abilities67 (Fig. 6).
FIG. 6.

Binder jet/inkjet process (author's schematic).
Selective laser sintering
In this process, fine polymer powder is scanned with the help of a high-power laser to create a fine layer of the part. The powder is evenly spread over a print bed with the help of a roller. The powder, along with the bed, is preheated to make it ready for sintering. When the laser beam falls on a thin layer of powder, it sinters the powder, and the first layer is formed. The print bed is lowered, and the roller again fetches the powder from the stock bed and spreads it over on the print bed. The laser, with the help of a scanner, scans the next layer and sinters it with the previous layer, and this process continues.61,62,68,69
The reported resolution of SLS can be as low as 40 μm, and it can go up to 200 μm.70–72 SLS is capable of using polymers that include nylon, ceramics, PCL, hydroxyapatite, and so on. The grain size of powder should be small and uniform for an improved sintering process.73
The fact that SLS can work with even biodegradable materials makes it useful in tissue engineering applications. SLS is capable of creating highly porous geometries with ease. Because the powder is sintered on a print bed, the surrounding powder acts as a readymade support structure, and no additional support structure needs to be modeled. In SLS, primarily, polymer powders are used with significant effects. PCL is a common choice because of its low melting point of 55°C to 60°C. It is biocompatible and biodegradable, and it can also be used as an implant material. It degrades slowly when implanted in the human body and, in the meantime, does not harm other tissues. Food and Drug Administration (FDA) also clears it for clinical use.
Along with PCL, there are several other materials such as hydroxyapatite (HAp), tricalcium phosphate (TCP), and poly (d, l-lactide acid co-glycolic acid), a few examples of materials that possess excellent sintering properties and are also approved by the FDA for its clinical usage. Few other materials such as polyether ether ketone and PVA possess excellent mechanical properties and are bioinert at the same time. Even though SLS has a considerable material base, the overall printing and postprocessing are still very costly. In vitro studies have showcased cell attachment and cell proliferation with scaffolds printed with SLS, but more intuitive research will confirm prolonged use of SLS along with all its material base72–75 (Fig. 7).
FIG. 7.
Selective laser sintering process (author's schematic).
Polymer composites in bone tissue engineering
Ranjan et al demonstrated through in vitro studies that the PLA-HAp-chitosan composite in proportions of 91-8-1 (by % of weight) was used to 3D print bone scaffold using the FDM technique. This composite bone scaffold exhibited good biocompatibility and bioactivity from the Ra profile and serum stability test.25,26 Ales Gregor et al 3D printed PLA scaffolds and performed in vitro studies to showcase a porosity of 30% to 60% promotes cell attachment and proliferation. It provides more cell sites for a natural ECM to grow.24 Donate et al suggested the use of additives that include HAp, β-TCP, and so on to increase the mechanical properties of PLA and enhance its osteoconductivity, the use of surface treatments such as alkali and plasma treatments to increase the hydrophilicity of PLA, and the use of bioactive substances such as chitosan, calcium phosphate, collagen, and alginate to enhance cell bioactivity in PLA.76
Bruna et al suggested that the FDM technique is suitable to 3D print PLA scaffolds and exhibit structural properties comparable with cancellous bone.77 Zhang et al created optimized poly-l-lactic acid (l-PLA)/nano-hydroxyapatite (nHA) composites with cost-effective FDM technology to 3D print PLLA/nHA porous bone scaffolds to showcase good compressive strength. They also exhibited good osteogenic properties when compared with HA ceramic scaffold and cancellous bone.78 Valentina et al studied the mechanical properties of polymers such as polyolefins when mixed with natural fillers and showcased significant improvement.70
FDM is the most widely used 3DP technology across the globe. However, still, the cost of FDM 3D printers is comparatively higher. To overcome this barrier, open-source 3D printers have been manufactured in several parts of the world and getting more popular. These open-source FDM 3D printers work on open-source software that includes slicing software, for example, Ultimaker Cura and slice 3r, and open source community setup provides free access to users for different 3D models and designs, for example, Thingiverse and Backster.79 FDM is specifically useful in tissue engineering to print porous scaffolds that can be used for bones. FDM can produce scaffolds with good mechanical properties and structural integrity, a critical factor in making bone scaffolds. FDM helps to make patient-specific defect designs using patients' MRI or CT scan data. Also, the infill structures can be set to match the defect sites and promote cell attachment and cell migration.
3D bioprinting and bone tissue engineering
3D bioprinting is a relatively new technology in the field of tissue engineering. It is used for regenerating the tissues as well. Bone regeneration is greatly realized through 3D bioprinting of bone scaffolds. Various steps to achieve this include material selection, scaffold printing, tissue culture, cell seeding, and finally, experimenting on an animal under the controlled environment.80 It is different from 3DP technology where live cells are used along with the ink. These two approaches are to design scaffolds and tissue engineering constructs. First is a top-down approach in which the materials that look similar to the shape of tissue are prepared and then cells are seeded on top of it for their growth, differentiation, and proliferation. This is then implanted into the body. The main problem with this approach is the lack of control over cell distribution and the underdevelopment of the ECM microenvironment.
The second approach is the bottom-up approach in which biomaterials along with micro-/nano-sized blocks of cells are used to make the tissue. In this approach, it is possible to construct very small size structures containing cells that can be combined to create the ECM that can act as a scaffold.
There are three types of 3D bioprinting techniques: extrusion-based/pressure-assisted/pneumatic bioprinting, ink-based bioprinting, and laser-assisted bioprinting (Fig. 8).
FIG. 8.

Techniques of three-dimensional bioprinting (author's schematic).
Extrusion based/pressure-assisted/pneumatic bioprinting
Extrusion-based bioprinting technique is very efficient and is currently in practice. Compressed air is used to force the bioink out of a nozzle. The pressure of the pneumatic system is kept at such a value at which live cells can survive and sustain. In this pneumatic system, some delay is expected because of the time taken to build the pressure. Biomaterials used for printing are generally pastes, solutions that are extracted in a controlled manner by applying pneumatic pressure through a plunger or screw-type piston. The nozzle assembly is made to move in X and Y directions to form the first layer of the scaffold. Then the bed moves down and subsequent layers are printed. This bioprinting technique is carried out at normal room temperature and is applied to those cells that can showcase activity retention capability81 (Fig. 9).
FIG. 9.
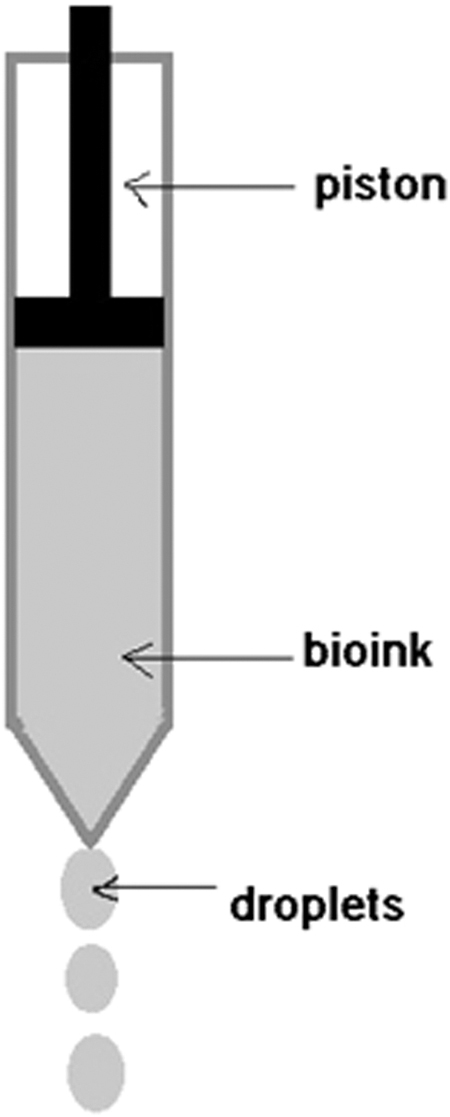
Extrusion-based method (author's schematic).
Inkjet bioprinting
It resembles a normal inkjet desktop printing process. Precisely measured droplets of bioink are made to fall on a substrate of hydrogel controlled by a computer. It is further classified based on a mechanism of droplet actuation as the thermal actuator method and the piezoelectric actuator method.
In the thermal actuator method, the localized temperature can be increased very rapidly to generate the desired pulse pressure. However, the ink droplets are normally disordered, disoriented, and of variable sizes. Clogging of the nozzle is also a major issue in this printing process.
In the piezoelectric actuator method, the required pressure is generated by the piezoelectric actuator, which creates the droplets, as shown in Figure 10. Since this method does not use any heating in its process, nozzle clogging is prevented from making ordered droplet delivery (Fig. 10).
FIG. 10.

Inkjet bioprinting methods (author's schematic).
Laser-based bioprinting
In this method, a laser is used as the source of energy to sublimate the biomaterial onto the substrate. It consists of three main parts: a source of the laser, a ribbon, and a receiving substrate. The laser illuminates the ribbon, which in turn evaporates the biomaterial and it is made to reach up to the receiving substrate in the droplet form. It uses a near UV wavelength laser as a source to print cells, proteins, hydrogels, and so on. It offers a very good resolution of pico- to microlevel. Researchers prefer this technique because of its unique advantages such as noncontact-type printing, nozzle-free printing, and higher control on droplet delivery (Fig. 11).
FIG. 11.

Laser-based method (author's schematic).
Conclusion
Profound knowledge regarding the vibrance of bone tissue engineering is required for encountering the challenges that limit the use of polymers and their composites for bone tissue engineering clinically. Current strategies in 3D modeling including 3D imaging using CT/MRI scan data are having limited design flexibilities. Getting the exact microarchitecture is very critical but very difficult at the same time. 3DP using the FDM, stereolithography apparatus (SLA), SLS, and inkjet binder techniques, along with 3D bioprinting, is capable of providing the exact microarchitecture required to fabricate bone scaffold. Further analysis techniques such as finite element analysis and computational fluid dynamics can evaluate the mechanical and fluid flow properties of the 3D-printed scaffolds before actually printing them. Even optimization of scaffold design is also evident for the enhanced properties of bone scaffolds.
To summarize, the material of scaffold and the fabrication technique play a very vital role and the aspects discussed in this review will help to decide suitable parameters for the fabrication of bone scaffolds.
Future Scope
Research in tissue engineering is growing at a rapid rate with advancements in additive manufacturing processes. New bioprinters are getting developed with increased efficiency, an extensive material library, and improved accuracy. Despite all this advancement, there are a few challenges ahead. The high cost of these bioprinters and the high cost of materials and technologies such as SLA, SLS, and binder jet printing are important and somewhat limiting aspects of research. Also, most studies are in vitro studies, more emphasis should be given to in vivo studies, and clinical trials should be followed. Multimaterial printing is still a very nascent stage; accelerating research toward this is the need of the future to enable multipolymer material printing, multimetal printing, and so on. Surface structures, layer adhesion, and cell interactions should improve and allow for customized implants used in surgeries.
Acknowledgments
The authors would like to acknowledge the Assam Don Bosco University, School of Mechanical Engineering, for their guidance and approval to carry out this research, Tezpur Medical College and Hospital, Assam, for their guidance in the field of medical data, and the Don Bosco Institute of Technology, Mumbai, for their continuous support toward this research.
Authors' Contributions
S.S.M. was the main author of the article and along with C.D. and V.K. jointly reviewed, corrected, and edited the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this review article.
References
- 1. Kusindarta DL, Wihadmadyatami H.. The role of extracellular matrix in tissue regeneration. In: Kaoud HA, ed. Tissue Regeneration [Internet]. London: IntechOpen; 2018. [cited 2022 Oct 30]; doi: 10.5772/intechopen.75728 Available from: https://www.intechopen.com/chapters/60312 [DOI]
- 2. Zheng XQ, Huang JF, Lin JL, et al. 3D bioprinting in orthopedics translational research. J Biomater Sci Polym Ed 2019;30(13):1172–1187; doi: 10.1080/09205063.2019.1623989 [DOI] [PubMed] [Google Scholar]
- 3. Biazar E, Najafi SM, Heidari KS, et al. 3D bio-printing technology for body tissues and organs regeneration. J Med Eng Technol 2018;42(3):187–202; doi: 10.1080/03091902.2018.1457094 [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Yang G, Johnson BN, et al. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater 2019;84:16–33; doi: 10.1016/j.actbio.2018.11.039 [DOI] [PubMed] [Google Scholar]
- 5. Chocholata P, Kulda V, Babuska V. Fabrication of scaffolds for bone-tissue regeneration. Materials (Basel) 2019;12(4):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hospodiuk M, Dey M, Sosnoski D, et al. The bioink: A comprehensive review on bioprintable materials. Biotechnol Adv 2017;35(2):217–239; doi: 10.1016/j.biotechadv.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 7. Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nat Rev Mater 2020;5(8):584–603; doi: 10.1038/s41578-020-0204-2 [DOI] [Google Scholar]
- 8. Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: Recent advances and challenges. Crit Rev Biomed Eng 2012;40(5):363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. An J, Teoh JEM, Suntornnond R, et al. Design and 3D printing of scaffolds and tissues. Engineering 2015;1(2):261–268; doi: 10.15302/J-ENG-2015061 [DOI] [Google Scholar]
- 10. Wang C, Huang W, Zhou Y, et al. 3D printing of bone tissue engineering scaffolds. Bioact Mater 2020;5(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abraham CM. A brief historical perspective on dental implants, their surface coatings and treatments. Open Dent J 2014;8(1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z, Huang C, Wang J, et al. Design and characterization of hydroxyapatite scaffolds fabricated by stereolithography for bone tissue engineering application. Procedia CIRP 2020;89:170–175; doi: 10.1016/j.procir.2020.05.138 [DOI] [Google Scholar]
- 13. Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng 2015;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qi L, Kash JC, Dugan VG, et al. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013;34(2):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Velasco MA, Narváez-Tovar CA, Garzón-Alvarado DA. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Biomed Res Int 2015;2015; doi: 10.1155/2015/729076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guerado E, Caso E. Challenges of bone tissue engineering in orthopaedic patients. World J Orthop 2017;8(2):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qu H, Fu H, Han Z, et al. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv 2019;9(45):26252–26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Filippi M, Born G, Chaaban M, et al. Natural polymeric scaffolds in bone regeneration. Front Bioeng Biotechnol 2020;8:474; doi: 10.3389/fbioe.2020.00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simunovic F, Finkenzeller G. Vascularization strategies in bone tissue engineering. Cells 2021;10:1749; doi: 10.3390/cells10071749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy CM, O'Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes Migr 2010;4(3):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng Part B Rev 2013;19(6):485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arif U, Haider S, Haider A, et al. Biocompatible polymers and their potential biomedical applications: A review. Curr Pharm Des 2019;25(34):3608–3619; doi: 10.2174/1381612825999191011105148 [DOI] [PubMed] [Google Scholar]
- 23. Ratner BD, Hoffman AS, Schoen FJ, et al. Biomaterials Science: An Introduction to Materials in Medicine. Chemical Engineering. San Diego, California, USA: Academic Press: Elsevier, Inc.; 2013. [Google Scholar]
- 24. Gregor A, Filová E, Novák M, et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng 2017;11(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ranjan N, Singh R, Ahuja IPS, et al. On 3D printed scaffolds for orthopedic tissue engineering applications. SN Appl Sci 2020;2(2):1–8; doi: 10.1007/s42452-020-1936-8 [DOI] [Google Scholar]
- 26. Ranjan N, Singh R, Ahuja IPS, et al. Fabrication of pla-hap-cs based biocompatible and biodegradable feedstock filament using twin screw extrusion. Addit Manuf Emerging Mater 2018;325–345. [Google Scholar]
- 27. Subia B, Kundu J, Kundu SC. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. In: Eberli D, ed. Tissue Engineering [Internet]. London: IntechOpen; 2010. [cited 2022 Oct 30]; doi: 10.5772/8581 Available from: https://www.intechopen.com/chapters/9798 [DOI]
- 28. Dhandayuthapani B, Yoshida Y, Maekawa T, et al. Polymeric scaffolds in tissue engineering application: A review. Int J Polym Sci 2011:290602; doi: 10.1155/2011/290602 [DOI] [Google Scholar]
- 29. Bassas-Galia M, Follonier S, Pusnik M, et al. , Chapter 2—Natural polymers: A source of inspiration. In: Perale G, Hilborn J, eds. Bioresorbable Polymers for Biomedical Applications, Woodhead Publishing, 2017, pp. 31–64. Available from: 10.1016/B978-0-08-100262-9.00002-1 [DOI] [Google Scholar]
- 30. Gupta P, Nayak KK. Characteristics of protein-based biopolymer and its application. Polym Eng Sci 2015;55(3):485–498; doi: 10.1002/pen.23928 [DOI] [Google Scholar]
- 31. Aravamudhan A, Ramos DM, Nada AA, et al. Chapter 4—Natural polymers: Polysaccharides and their derivatives for biomedical applications. In: Kumbar SG, Laurencin CT, Deng M, eds. Natural and Synthetic Biomedical Polymers, Elsevier, 2014, pp. 67–89. Available from: 10.1016/B978-0-12-396983-5.00004-1 [DOI] [Google Scholar]
- 32. Noreen A, Sultana S, Sultana T, et al. Chapter 3—Natural polymers as constituents of bionanocomposites. In: Zia KM, Jabeen F, Anjum MN, Ikram S, eds. In Micro and Nano Technologies, Bionanocomposites, Elsevier, 2020, pp. 55–85. Available from: 10.1016/B978-0-12-816751-9.00003-9 [DOI] [Google Scholar]
- 33. Melke J, Midha S, Ghosh S, et al. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater 2016;31:1–16; doi: 10.1016/j.actbio.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 34. Li G, Sun S. Silk fibroin-based biomaterials for tissue engineering applications. Molecules 2022;27:2757; doi: 10.3390/molecules27092757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lantigua D, Wu X, Suvarnapathaki S, et al. Composite scaffolds from gelatin and bone meal powder for tissue engineering. Bioengineering 2021;8:169; doi: 10.3390/bioengineering8110169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sionkowska A, Gadomska M, Musiał K, et al. Hyaluronic acid as a component of natural polymer blends for biomedical applications: A review. Molecules 2020;25:4035; doi: 10.3390/molecules25184035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sukpaita T, Chirachanchai S, Pimkhaokham A, et al. Chitosan-based scaffold for mineralized tissues regeneration. Mar Drugs 2021;19(10):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo L, Liang Z, Yang L, et al. The role of natural polymers in bone tissue engineering. J Control Release 2021;338:571–582; doi: 10.1016/j.jconrel.2021.08.055 [DOI] [PubMed] [Google Scholar]
- 39. Gunatillake PA, Adhikari R, Gadegaard N. Biodegradable synthetic polymers for tissue engineering. Eur Cells Mater 2003;5:1–16. [DOI] [PubMed] [Google Scholar]
- 40. Bolívar-Monsalve EJ, Alvarez MM, Hosseini S, et al. Engineering bioactive synthetic polymers for biomedical applications: A review with emphasis on tissue engineering and controlled release. Mater Adv 2021;2:4447–4478; doi: 10.1039/D1MA00092F [DOI] [Google Scholar]
- 41. Serra T, Mateos-Timoneda MA, Planell JA, et al. 3D printed PLA-based scaffolds: A versatile tool in regenerative medicine. Organogenesis 2013;9(4):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reddy MSB, Ponnamma D, Choudhary R, et al. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers (Basel) 2021;13(7):1105; doi: 10.3390/polym13071105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Savioli Lopes M, Jardini AL, Maciel Filho R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng 2012;42:1402–1413; doi: 10.1016/j.proeng.2012.07.534 [DOI] [Google Scholar]
- 44. Chen D, Bei J, Wang S. Polycaprolactone microparticles and their biodegradation. Polym Degrad Stab 2000;67(3):455–459. [Google Scholar]
- 45. Zarrintaj P, Saeb MR, Jafari SH, et al. Chapter 18—Application of compatibilized polymer blends in biomedical fields. In: Ajitha AR, Thomas S, eds. Compatibilization of Polymer Blends, Elsevier, 2020, pp. 511–537. Available from: 10.1016/B978-0-12-816006-0.00018-9 [DOI] [Google Scholar]
- 46. Chae MP, Hunter-Smith DJ, Murphy SV, et al. 15—3D bioprinting adipose tissue for breast reconstruction. In: Thomas DJ, Jessop ZM, Whitaker IS, eds. 3D Bioprinting for Reconstructive Surgery, Woodhead Publishing, 2018, pp. 305–353. Available from: 10.1016/B978-0-08-101103-4.00028-4 [DOI] [Google Scholar]
- 47. Ramli MS, Wahab MS, Ahmad M, et al. FDM preparation of bio-compatible UHMWPE polymer for artificial implant. ARPN J Eng Appl Sci 2016;11(8):5474–5480. [Google Scholar]
- 48. Lee K-W, Wang S, Lu L, et al. Fabrication and characterization of poly(propylene fumarate) scaffolds with controlled pore structures using 3-dimensional printing and injection molding. Tissue Eng 2006;12(10):2801–2811; doi: 10.1089/ten.2006.12.2801 [DOI] [PubMed] [Google Scholar]
- 49. Liu P, Chen W, Liu C, et al. A novel poly (vinyl alcohol)/poly (ethylene glycol) scaffold for tissue engineering with a unique bimodal open-celled structure fabricated using supercritical fluid foaming. Sci Rep 2019;9(1):9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arakawa CK, DeForest CA, Chapter 19—Polymer Design and Development. In: Vishwakarma A, Karp JM, eds. Biology and Engineering of Stem Cell Niches, Academic Press, 2017, pp. 295–314. Available from: 10.1016/B978-0-12-802734-9.00019-6 [DOI] [Google Scholar]
- 51. Ahmad S. Polyurethane: A versatile scaffold for biomedical applications. Significances Bioeng Biosci 2018;2(3); doi: 10.31031/SBB.2018.02.000536 [DOI] [Google Scholar]
- 52. Wendels S, Avérous L. Biobased polyurethanes for biomedical applications. Bioact Mater 2021;6(4):1083–1106; doi: 10.1016/j.bioactmat.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haryńska A, Kucinska-Lipka J, Sulowska A, et al. Medical-grade PCL based polyurethane system for FDM 3D printing—Characterization and fabrication. Materials (Basel) 2019;12(6):887.. https://www.mdpi.com/1996-1944/12/6/887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bai X, Gao M, Syed S, et al. Bioactive hydrogels for bone regeneration. Bioact Mater 2018;3(4):401–417; doi: 10.1016/j.bioactmat.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Short AR, Koralla D, Deshmukh A, et al. Hydrogels that allow and facilitate bone repair, remodeling, and regeneration. J Mater Chem B 2015;3(40):7818–7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. He B, Ou Y, Zhou A, et al. Functionalized D-form self-assembling peptide hydrogels for bone regeneration. Drug Des Devel Ther 2016;10:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Elomaa L, Teixeira S, Hakala R, et al. Preparation of poly(ɛ-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater 2011;7(11):3850–3856; doi: 10.1016/j.actbio.2011.06.039 [DOI] [PubMed] [Google Scholar]
- 58. Billiet T, Vandenhaute M, Schelfhout J, et al. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012;33(26):6020–6041; doi: 10.1016/j.biomaterials.2012.04.050 [DOI] [PubMed] [Google Scholar]
- 59. Lam CXF, Mo XM, Teoh SH, et al. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C 2002;20(1–2):49–56. [Google Scholar]
- 60. Seitz H, Rieder W, Irsen S, et al. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater 2005;74(2):782–788. [DOI] [PubMed] [Google Scholar]
- 61. Zhao Y, Hou Y, Li Z, et al. Powder-based 3D printed porous structure and its application as bone scaffold. Front Mater 2020;7:1–5. [Google Scholar]
- 62. Chin SY, Dikshit V, Priyadarshini BM, et al. Powder-based 3D printing for the fabrication of device with micro and mesoscale features. Micromachines 2020;11(7):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee M, Dunn JCY, Wu BM. Scaffold fabrication by indirect three-dimensional printing. Biomaterials 2005;26(20):4281–4289. [DOI] [PubMed] [Google Scholar]
- 64. Lee M, Wu BM, Dunn JCY. Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A 2008;87(4):1010–1016. [DOI] [PubMed] [Google Scholar]
- 65. Sherwood JK, Riley SL, Palazzolo R, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002;23(24):4739–4751. [DOI] [PubMed] [Google Scholar]
- 66. Yang Z, Song Z, Nie X, et al. A smart scaffold composed of three-dimensional printing and electrospinning techniques and its application in rat abdominal wall defects. Stem Cell Res Ther 2020;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today 2013;16(12):496–504; doi: 10.1016/j.mattod.2013.11.017 [DOI] [Google Scholar]
- 68. Lakshmi KS, Arumaikkannu G. Influence of process parameters on surface finish in customized bone implant using selective laser sintering. Adv Mater Res 2014;845:862–867. [Google Scholar]
- 69. Bahraminasab M. Challenges on optimization of 3D-printed bone scaffolds. Biomed Eng Online 2020;19(1):1–33; doi: 10.1186/s12938-020-00810-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mazzanti V, Malagutti L, Mollica F. FDM 3D printing of polymers containing natural fillers: A review of their mechanical properties. Polymers (Basel) 2019;11(7):1094; doi: 10.3390/polym11071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roy NK, Behera D, Dibua OG, et al. A novel microscale selective laser sintering (μ-SLS) process for the fabrication of microelectronic parts. Microsystems Nanoeng 2019;5(1):64; doi: 10.1038/s41378-019-0116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yeong WY, Sudarmadji N, Yu HY, et al. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater 2010;6(6):2028–2034; doi: 10.1016/j.actbio.2009.12.033 [DOI] [PubMed] [Google Scholar]
- 73. Munir KS, Li Y, Wen C. Metallic scaffolds manufactured by selective laser melting for biomedical applications. Metallic Foam Bone Process Modification Charact Prop 2017;1–23. [Google Scholar]
- 74. Tamay DG, Usal TD, Alagoz AS, et al. 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol 2019;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buyuksungur S, Endogan Tanir T, Buyuksungur A, et al. 3D printed poly(ϵ-caprolactone) scaffolds modified with hydroxyapatite and poly(propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater Sci 2017;5(10):2144–2158. [DOI] [PubMed] [Google Scholar]
- 76. Donate R, Monzón M, Alemán-Domínguez ME. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020;20(1):571–599. [Google Scholar]
- 77. Marianna C, Bruna T, Daniel K, et al. Structural evaluation of PLA scaffolds obtained by 3D printing via fused deposition modeling (FDM) technique for applications in tissue engineering. Front Bioeng Biotechnol 2016;4:995–997. [Google Scholar]
- 78. Zhang B, Wang L, Song P, et al. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater Des 2021;201:109490; doi: 10.1016/j.matdes.2021.109490 [DOI] [Google Scholar]
- 79. Alagoz AS, Hasirci V. 3D printing of polymeric tissue engineering scaffolds using open-source fused deposition modeling. Emergent Mater 2020;3(4):429–439. [Google Scholar]
- 80. Vanaei S, Parizi MS, Salemizadehparizi F, et al. An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng Regener 2021;2:1–18; doi: 10.1016/j.engreg.2020.12.001 [DOI] [Google Scholar]
- 81. Salah M, Tayebi L, Moharamzadeh K, et al. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac Plast Reconstr Surg 2020;42:18; doi: 10.1186/s40902-020-00263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]