Abstract
Exposure of human fetuses to man-made estrogenic chemicals can occur through several sources. For example, fetal exposure to ethinylestradiol occurs because each year ≈3% of women taking oral contraceptives become pregnant. Exposure to the estrogenic chemical bisphenol A occurs through food and beverages because of significant leaching from polycarbonate plastic products and the lining of cans. We fed pregnant CD-1 mice ethinylestradiol (0.1 μg/kg per day) and bisphenol A (10 μg/kg per day), which are doses below the range of exposure by pregnant women. In male mouse fetuses, both ethinylestradiol and bisphenol A produced an increase in the number and size of dorsolateral prostate ducts and an overall increase in prostate duct volume. Histochemical staining of sections with antibodies to proliferating cell nuclear antigen and mouse keratin 5 indicated that these increases were due to a marked increase in proliferation of basal epithelial cells located in the primary ducts. The urethra was malformed in the colliculus region and was significantly constricted where it enters the bladder, which could contribute to urine flow disorders. These effects were identical to those caused by a similar dose (0.1 μg/kg per day) of the estrogenic drug diethylstilbestrol (DES), a known human developmental teratogen and carcinogen. In contrast, a 2,000-fold higher DES dose completely inhibited dorsolateral prostate duct formation, revealing opposite effects of high and low doses of estrogen. Acceleration in the rate of proliferation of prostate epithelium during fetal life by small amounts of estrogenic chemicals could permanently disrupt cellular control systems and predispose the prostate to disease in adulthood.
Keywords: bisphenol A, ethinylestradiol, urogenital sinus
More than 60 years ago, there was speculation that exposure of male fetuses to elevated estrogen levels during fetal life could predispose men to have an enlarged prostate in old age. This hypothesis was proposed because the prostate derives from a portion of the embryonic urogenital sinus (UGS) that differentiates into the estrogen-responsive vagina in females (1). In contrast to this prediction, numerous studies have shown that high doses of diethylstilbestrol (DES) and other estrogenic chemicals inhibit prostate development in mice and rats (2). These studies were conducted because pregnant women in the 1950s and 1960s were prescribed high doses of DES based on the mistaken assumption that DES would prevent spontaneous abortion. Maternal DES administration resulted in cancer and other abnormalities of the reproductive organs in offspring, which was not detected until subsequent adulthood and after millions of human fetuses had been exposed (3–5). It is now well known that hormones can have opposite effects at low vs. high doses. Studies that include only very high doses of drugs or chemicals can miss unique effects that are observed only within a physiologically relevant low dose range (6).
We examined whether very low doses of estrogenic chemicals in drugs and consumer products (7) could affect the development of prostate ducts in male mouse fetuses. Androgen is required for differentiation of the prostate, and our objective was to examine the modulating effect of estrogenic chemicals on the initial differentiation and growth of primary ducts in the fetal prostate. There are many possible opportunities for exposure of fetuses to estrogenic chemicals (7). An unexpected source of estrogen exposure by human fetuses is the drug ethinylestradiol, which is the estrogenic chemical used in oral contraceptives. It is estimated that each year almost 2 million of the more than 60 million women in the United States and Europe who use oral contraceptives become pregnant accidentally, primarily because of missed pills; the average is three missed pills per month and a 3% pregnancy rate per year for this population (8). Oral contraceptive pills often are taken for many months until the unplanned and unexpected pregnancy is discovered (9). Even though oral contraceptives have been used for decades, relatively little research has been conducted in experimental animals to assess effects on offspring of maternal administration of ethinylestradiol at or below the clinically relevant dose of 0.4–0.8 μg/kg per day, based on 30 μg of ethinylestradiol per pill and body weights ranging from 37 to 75 kg (10, 11).
Bisphenol A was shown to have full activity (efficacy similar to estradiol) as an estrogenic drug in 1936 (12). Since the 1950s, bisphenol A has been used as the monomer that is polymerized to manufacture polycarbonate plastic, certain dental sealants, and the resin lining of most food and beverage cans. Bisphenol A also is used as an additive in many other products, with global capacity at >6 billion pounds per year (13). Polycarbonate is less durable than commonly believed, because the ester bond linking polymerized bisphenol A molecules can be hydrolyzed, and hydrolysis increases dramatically at high or low pH and as temperature increases. Bisphenol A thus leaches into food and beverages under the normal conditions of use of tin cans and polycarbonate plastic containers (14–16), and when polycarbonate is scratched and discolored, the rate of leaching can be very high (16). There is significant exposure of pregnant women to bisphenol A, because mean blood levels of biologically active (unconjugated) bisphenol A in human fetuses at parturition are in the range of 2–3 ng/ml (≈10 nM) (17), and levels in human amniotic fluid during reproductive tract differentiation in fetuses are even higher at 8 ng/ml (18).
We examined the morphology (by using computer-assisted 3D reconstruction) and cytology (by using histochemical analysis) of the fetal mouse prostate after maternal exposure for 5 days to estrogenic chemicals during the initial period of development of the primary prostate ducts; this begins on gestation day (GD) 17 in mice and occurs during the 10th week of gestation in humans. The dose of bisphenol A used in this study results in average levels of parent (unconjugated) bisphenol A in mouse fetuses throughout the 24 h after administration (19) that are below average levels reported in blood from human fetuses at parturition (17). The dose of ethinylestradiol that we administered is ≈4- to 8-fold lower than the average dose in oral contraceptives taken by adult women (8). A low dose of DES also was examined as a positive control estrogen, and a high dose of DES was included for comparison to the large literature on developmental effects of high, pharmacological doses of estrogenic chemicals.
Materials and Methods
Animals. CD-1 mice (Mus musculus domesticus) were purchased from Charles River Laboratories. Pregnant and lactating females were housed in standard (11.5 × 7.5 × 5 in) polypropylene mouse cages on soy-based Purina 5008 chow. Drinking water was available ad libitum in glass bottles. The drinking water was purified by ion exchange, followed by passage through a series of carbon filters to remove chlorine as well as hydrophobic molecules, and flowed through copper pipes into our bottle-filling apparatus. Rooms were maintained at 25 ± 2°C under a 12-h/12-h light/dark cycle, with lights on at 1000 hours. All animal procedures were approved by the University of Missouri Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals.
Administration of Chemicals to Pregnant Females and Delivery of Pups. Adult virgin females were placed with a stud male for 4 h, beginning 2 h before the end of the dark phase of the light/dark cycle. Females with a vaginal plug were housed three per cage. We randomly assigned 21 pregnant mice to four different treatment groups, and on GD 14–18 (mating = GD 0), we fed pregnant mice oil vehicle (oil controls, n = 5); either ethinylestradiol (0.1 μg/kg per day) (n = 5) or DES (0.1 μg/kg per day) (n = 5); or bisphenol A (10 μg/kg per day) (n = 6). The chemicals (Sigma) were dissolved in tocopherol-stripped corn oil (ICN) and delivered through a pipette placed into the animal's mouth. Based on prior findings, DES (0.1 μg/kg per day) was included as a positive control (20, 21). Our dose level of bisphenol A was based on prior results suggesting that bisphenol A is ≈100-fold less potent than DES in terms of stimulating a permanent increase in prostate size in mice (20, 22, 23).
A separate study was conducted in which pregnant mice were fed either the oil vehicle (n = 4) or a high dose (200 μg/kg per day) of DES (n = 4) by using the same procedures described above. Our objective was to compare these results with those of other studies that examined effects on adult prostate size of exposure during development to high doses of DES, similar to doses that had been prescribed to pregnant women (3, 4, 20).
Just before normal parturition on GD 19, we removed the fetuses from the uterus by cesarean section. We recorded the intrauterine position of male fetuses relative to adjacent male and female fetuses as they were removed from the uterus. We reduced variation in background fetal blood levels of the sex hormones estradiol and testosterone due to the sex of the adjacent fetuses by examining only one male fetus per litter that developed in utero between a male and a female fetus, because intrauterine position influences serum hormone levels during fetal life, subsequent prostate size, and many other traits in litter-bearing species. The observation that male mouse fetuses with the highest serum levels of estradiol (due to developing between two female fetuses) had an enlarged prostate beginning in fetal life and persisting through adulthood (24–27) provided the basis for studying developmental effects of low doses of man-made estrogenic chemicals.
3D Reconstruction. Beginning on GD 17 in the mouse, the primary prostate ducts emerge from the UGS as solid cords of cells. The primary ducts elongate, and subsequent to the time examined in this study, branch, form lumens, and eventually become the functional glandular component of adult prostate. Prostate morphology on GD 19 was determined by a 3D computer reconstruction technique (26–28). Briefly, the UGS was removed, fixed in 4% paraformaldehyde, and sectioned. Digital images were used for 3D reconstruction and morphometric analysis of the developing prostate ducts, coupled with immunohistochemical analysis. We determined the number and volume of epithelial outgrowths (primary ducts) from the UGS. The individual primary ducts in each region (dorsal, lateral, and ventral) were counted. Prostate duct volume was determined as the sum of all of the individual cross-sectional areas for all of the ducts in a specific region.
Proliferating Cell Nuclear Antigen (PCNA) and Mouse Keratin 5 (MK5) Immunocytochemistry. Sections for PCNA staining were heated twice for 5 min in 50 ml of deionized water in a microwave oven at 600 W and then allowed to cool to room temperature before continuing. Immunocytochemical staining for PCNA was performed by using the avidin–biotin peroxidase method as follows. Endogenous peroxidase activity was blocked by using 3% hydrogen peroxide in methanol for 5 min, and slides were washed in PBS-TX [PBS containing 0.03% Triton X-100 (Fisher)]. Sections were then incubated for 30 min in a blocking serum (2% normal goat serum made in PBS-TX). All incubations were done in a humid chamber at room temperature. Excess serum was blotted from the slides, which were then incubated overnight with the primary antibody to PCNA (PC-10 clone; Santa Cruz Biotechnology) at a dilution of 1:600. After washing in PBS, sections were incubated for 30 min in the biotinylated secondary antibody from the Vectastain Elite ABC kit (Vector Laboratories) at a dilution of 1:200 in PBS-TX. Sections were washed in PBS and incubated in the avidin reagent as described in the ABC kit. Slides were washed again in PBS before immersion in the chromogen, diaminobenzidine, at a concentration of 0.025% made in PBS with 0.0025% hydrogen peroxide. Sections were washed in water and counterstained with hematoxylin. Slides were dehydrated, cleared, and mounted with Permount (Fisher Scientific). For MK5 staining with rabbit anti-MK5 (Covance Research Products, Berkeley, CA), antigen retrieval was performed by using procedures described by DAKO.
To determine the percentage of cells labeled for PCNA within the ducts of each region of the developing prostate, cell counts were performed on dorsal, lateral, and ventral ducts as well as the urethra. A total of 500–1,000 cells were counted in each region. Care was taken to ensure that sections selected for analysis included the entire length (both proximal and distal portions) of a duct. Sections were viewed with a BX60 microscope (Olympus, Melville, NY), and digital photographs of alternate sections throughout the entire developing gland were captured by using a DVC1301C camera (Digital Video Camera, Austin, TX). Images were analyzed by using image-pro plus (Media Cybernetics, Silver Spring, MD). To determine cell counts in an area of interest, nonconvolution filters were used to enhance the outline of individual cells and differentiate between stained and unstained cells. We counted the number of cells within each area and calculated the percent of stained vs. unstained cells within each area.
Statistical Procedures. ANOVA was conducted by using the Statistical Analysis System general linear model procedure (SAS Institute, Cary, NC). Planned comparisons of differences between controls and treatment groups were made by using the Fisher's least-squares means test in SAS if the overall analysis was statistically significant. The confidence level for rejecting the null hypothesis was P < 0.05. All procedures were conducted blind to ensure the absence of bias.
Results
A High Dose of DES Inhibits Prostate Morphogenesis. As predicted, administration of the high, 200-μg/kg per day, dose of DES to pregnant mice completely inhibited the formation of ducts in the dorsal and lateral prostate in male fetuses, including the coagulating glands, which form from dorsal ducts that develop in the most cranial region of the UGS. The Müllerian ducts also were clearly evident in the DES-treated animals, suggesting that this treatment interfered with the action of Müllerian-inhibiting hormone. Relative to the oil-treated controls, this high dose of DES caused a very different pattern of outgrowths in the ventral UGS. Numerous abnormally short outgrowths were apparent throughout the entire length of the prostatic urethra, compared with 7–10 elongated ducts that normally develop in the ventral region of the prostatic urethra. This high dose of DES also reduced the size of the seminal vesicles relative to controls (Fig. 1). Because of the gross abnormalities caused by this dose of DES, no attempt was made to quantify differences relative to controls.
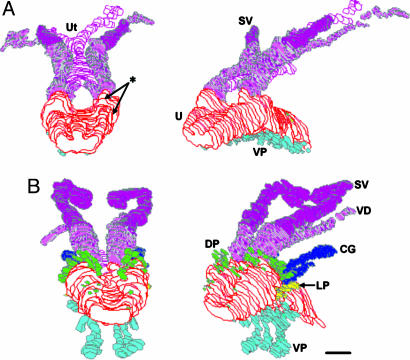
Fig. 1.
3D serial section reconstruction of the UGS from GD-19 mice exposed to a high dose (200 μg/kg per day) of DES from GD 14–18 (A), compared with vehicle-treated controls (B). Reconstructions are shown from the caudal–dorsal perspective in the left images and caudal–dorsolateral on the right. Exposure of pregnant females to this DES dose resulted in the inhibition (*) of dorsal (DP, green) and lateral (LP, yellow) prostate ducts and coagulating glands (CG, dark blue). There is an abnormal pattern of development in ventral duct formation (VP, light blue). The utricle (Ut, pink) was present in oil controls only as a small remnant of the Müllerian ducts, whereas these ducts did not regress in the DES-exposed males. DES also resulted in a decrease in the size of the seminal vesicles (SV, purple). VD, vas deferens, U, urethra. (Scale bar, 100 μm.)
Low Doses of Estrogenic Chemicals Stimulate Prostate Duct Development. In sharp contrast with the high dose of DES, the low doses of DES, ethinylestradiol, and bisphenol A significantly increased the total number of ducts in the dorsal, lateral, and ventral prostate relative to controls (ethinylestradiol, 24%; DES, 26%; and bisphenol A, 41%) and also increased total volume of these ducts (ethinylestradiol, 67%; DES, 68%; and bisphenol A, 81%). A significant increase in volume of the paired coagulating glands (that consist of two to three ducts per side) also was caused by the low dose of each chemical (ethinylestradiol, 40%; DES, 47%; and bisphenol A, 56%). These effects were thus apparent within the first 48 h after the initiation of prostate duct development (Fig. 2A and Table 1).
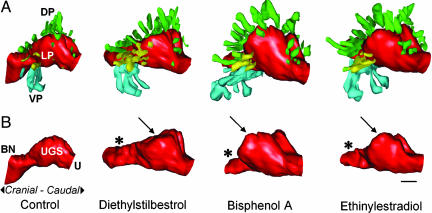
Fig. 2.
3D serial section reconstruction of the UGS from GD-19 mice exposed to low doses of estrogenic chemicals, DES, bisphenol A, and ethinylestradiol, during fetal development. The UGS depicted for each treatment was closest to the group mean for prostate duct number and size. All images are viewed from a left-lateral perspective. (A) Shown are the differences in patterns of prostate ductal development after fetal exposure to these chemicals, compared with oil-treated controls. There is a significant increase in the total number of ducts in estrogen-treated animals and a corresponding increase in overall prostate volume. (B) Shown is the marked alteration in the shape of the urethra (U, red) in the region of the bladder neck (BN), which is markedly constricted (*) inthe mice exposed to the estrogenic chemicals, compared with controls. In addition, the region of the UGS (the prostatic sulcus or colliculus, arrow) associated with the development of the dorsal (DP, green) and lateral (LP, yellow) prostate ducts is enlarged, particularly by bisphenol A, compared with controls. Ventral prostate (VP, light blue). (Scale bar, 100 μm.)
Table 1. Data from reconstruction of the prostate and urethra and immunochemistry in control and estrogenic chemical-treated male fetuses.
| No. of prostate ducts
|
Prostate duct volume, μm2
|
PCNA staining, %
|
Cranial urethra volume, μm3
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | DL | V | DLV | DL | V | DLV | DL | V | |
| Control | 45.4 ± 6.3 | 7.2 ± 1.3 | 53.0 ± 6.7 | 25,616 ± 4,773 | 26,531 ± 5130 | 25,921 ± 3561 | 36.3 ± 3.2 | 35.0 ± 7.8 | 35,033 ± 8126 |
| DES (0.1 μg) | 57.2 ± 2.5* | 7.8 ± 0.9 | 65.0 ± 2.8 | 48,321 ± 5,156*** | 56,576 ± 2692*** | 51,073 ± 3589*** | 64.2 ± 2.7** | 42.1 ± 7.2 | 25,556 ± 2911 |
| BPA (10 μg) | 64.2 ± 4.6* | 10.0 ± 1.4 | 74.1 ± 5.2** | 50,886 ± 6,921*** | 47,112 ± 4726* | 49,592 ± 4790*** | 52.4 ± 2.5* | 34.1 ± 7.5 | 22,767 ± 2875* |
| EE (0.1 μg) | 56.4 ± 3.9* | 7.6 ± 1.2 | 64.0 ± 5.1 | 45,508 ± 5,215*** | 49,369 ± 3947* | 46,795 ± 3688*** | 69.2 ± 3.0** | 40.9 ± 9.0 | 21,748 ± 2047* |
All results are presented as mean ± SEM. Shown are the number of developing epithelial ducts in the entire prostate (DLV) and for the individual dorsolateral (DL) and ventral (V) regions of the prostate on GD 19. For control, DES, and ethinylestradiol (EE) treatments, n = 5 fetuses per group; for bisphenol A (BPA) n = 6 fetuses. Prostate volume on GD 19 was calculated as the sum of all the individual cross-sectional areas based on all of the ducts in a specific region in histological sections as described in ref. 27. Urethral volume is of the initial 200 μm of the urethra beginning at the bladder neck, cranial to the region of prostate duct formation. *, P < 0.05; **, P < 0.01; ***, P < 0.005 vs. controls.
To determine whether an increase in cell proliferation accounted for differences in the size of prostate ducts in fetuses exposed to estrogenic chemicals, tissue sections were labeled with an antibody to PCNA, a marker of proliferative activity (29). All of the estrogenic chemicals resulted in a significant increase in the number of proliferating epithelial cells (Table 1 and Fig. 3 A–D). A significant increase in proliferative activity was observed only in the dorsolateral ducts but not in the ventral ducts of the mice exposed to the estrogenic chemicals. The pattern of PCNA staining was similar to the pattern of staining in cells that expressed a marker, MK5, of basal cells (Fig. 3E).
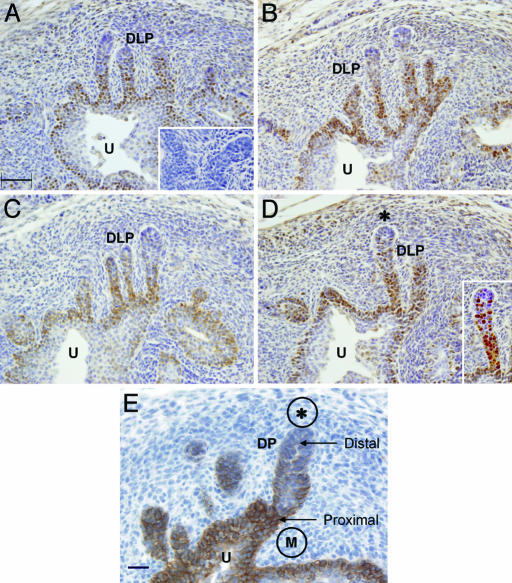
Fig. 3.
Immunohistochemical localization of PCNA in developing dorsolateral prostate ducts (DLP) of the UGS of GD-19 fetal mice. (A–D) Representative sections of comparable regions are shown for oil-treated control mice (A) and animals exposed to the estrogenic compounds DES (0.1 μg/kg per day) (B), bisphenol A (10 μg/kg per day) (C), and ethinylestradiol (0.1 μg/kg per day) (D). In each of the groups studied, the predominant site of PCNA staining was associated with the proximal region of the longest ducts. In D, the Inset is a digitally enhanced image of developing prostate duct [PCNA-stained cells (brown) vs. nonstained cells (blue)]. These proliferating cells were continuous with the basal layer of urothelial epithelial cells in the urethra (U). PCNA was not detected at the distal tips of the longest ducts that are juxtaposed to a condensation of mesenchyme (D; area with *). This region of mesenchyme is associated with induction of postnatal branching morphogenesis, after which formation of a ductal lumen and development of differentiated, secretory epithelial cells occurs. A negative immunohistochemistry control section is shown in A Inset. A comparison of cellular proliferation in these experimental groups is presented in Table 1. (Scale bar, 50 μm.) (E) Immunohistochemical localization of MK5, a marker for basal epithelial cells, in the developing prostate ducts of an oil-treated control GD-19 mouse fetus. The majority of labeled cells are found in the proximal region of the developing ducts (DP, dorsal prostate) and the basal epithelial cells of the urethra (U). The initial duct formation is associated with periurethral mesenchyme (M). Cells in the distal portion of the duct are infrequently stained. The latter region is juxtaposed to a condensation of mesenchyme (*). The pattern of staining seen with this antibody closely resembles the pattern seen for PCNA staining. (Scale bar, 20 μm.)
Low Doses of Estrogenic Chemicals Cause Urethral Malformations. Fetal exposure to a low dose of DES, ethinylestradiol, and bisphenol A also caused a malformation (an abnormal narrowing or urethral stricture) in the portion of the urethra associated with the neck of the bladder (Table 1 and Fig. 2B). The internal sphincter is associated with this region and is involved in the control of the retention of urine in the bladder. Our findings show that this malformation is observed in a region cranial to the region of the UGS where these chemicals result in prostate duct epithelial proliferation. Fig. 2B also shows a distinct malformation (enlargement) caused by low doses of all of the estrogenic chemicals in the region of the paired prostatic sulci that form the lateral walls of the colliculus (a protuberance on the posterior wall of the prostatic urethra).
Discussion
The findings of this study demonstrate that the differentiating UGS in male mouse fetuses is exquisitely sensitive to low-dose bisphenol A and ethinylestradiol exposure via the pregnant female, which can result in male fetuses beginning life with a greater number of primary ducts in the dorsolateral prostate, increased epithelial proliferation in these ducts, an overall increase in prostate volume, constriction of the urethra at the bladder neck, and malformation of the prostatic sulci that form the colliculus. These effects are virtually identical to effects of the low dose (0.1 μg/kg per day) of DES (Table 1).
Studies have demonstrated that there is a permanent “imprinted” increase (up to 7-fold) in prostate androgen receptors, associated with an increase in prostate size, in adult male mice due to fetal exposure to low doses of estradiol, ethinylestradiol, DES, and bisphenol A, whereas fetal exposure to much higher doses reduces adult prostate androgen receptors and prostate size (10, 20, 23, 30). Bisphenol A over a 1,000-fold dose range (between 100 pM and 100 nM) also showed an inverted-U dose–response curve for the stimulation of proliferation of human LnCAP prostate cancer cells (31). Bisphenol A stimulates a wide range of effects through binding to estrogen receptors in the nucleus (6, 32). However, extremely low doses (1 pM or 0.23 pg/ml) of bisphenol A also stimulate a rapid response (calcium influx within 1 min) in rat pituitary tumor cells resulting in prolactin secretion. This response occurs through binding of bisphenol A or estradiol to receptors associated with the cell membrane and the rapid activation of kinases, and the dose–response curve also forms an inverted U over a 10,000-fold dose range (33).
Taken together, these findings suggest that effects of fetal exposure to low doses of bisphenol A and ethinylestradiol occur through multiple mechanisms. In addition, the low doses of these estrogenic chemicals to which human fetuses are exposed may have opposite effects on the developing human prostate (and other tissues) relative to the high doses of DES experienced by human males whose mothers took DES during pregnancy (5). The cohort of “DES sons” may thus not be an appropriate reference population for predicting effects of exposure to low doses of estrogenic chemicals. Based on these and other findings, we propose that the use of high, pharmacological doses of estrogenic chemicals in research with experimental animals does not provide information that is relevant to understanding the role of endogenous estradiol in development (20, 32), and that the results are not relevant for predicting the effects of exposure to low levels of man-made estrogenic chemicals present in the environment (6).
An increase in prostate androgen receptors as a result of fetal exposure to very low doses of estrogenic chemicals is likely only one of many potential bases for the altered rate of epithelial proliferation. Extensive research involving experimental recombination of mesenchyme and epithelial tissues has shown that mesenchyme adjacent to specific regions of epithelium “instructs” epithelial differentiation under the control of androgen (34, 35). There have been numerous studies seeking to determine the androgen-mediated factors secreted by adjacent mesenchyme that regulate epithelial growth and differentiation. Results of these studies suggest that multiple paracrine factors, both stimulatory, such as FGF-10 (36), and inhibitory, such as TGF-β, and bone morphogenetic protein 4 (37), are involved (2, 35). A permanent derangement by estrogenic chemicals in the activity of genes that regulate cell proliferation could be a basis for the reawakening of prostate growth (both benign and malignant) during aging in men (38). There is evidence that disruption of the temporal organization of developmental events is associated with permanent functional outcomes in other organs that form ducts, such as the lungs (39), breast (40), and salivary glands (41).
Our findings show a marked increase in PCNA staining in the epithelium of the primary dorsolateral prostate ducts. Basal cells are a subset of epithelial cells found in the undifferentiated UGS that contribute to the proliferative pool of epithelial cells in the developing prostate ducts (35). Based on the known instructive mechanisms that involve mesenchymal induction of epithelial proliferation, our finding that PCNA had a similar distribution to cells that stained for MK5 suggests that estrogenic chemicals influence mesenchymal growth factors, which results in stimulation of the epithelial basal cell population. The recent identification of specific markers, such as p63 (42), which distinguishes progenitor cells in developing epithelial tissues, will permit further elucidation of the subpopulations of these cells that exhibit the greatest proliferative response to estrogenic stimulation.
In assessing whether organs in different species are homologous, functional as well as structural similarities should be assessed. With regard to functional similarities, mesenchyme from the mouse prostate has been shown to produce the appropriate regulatory factors that induce differentiation of human bladder epithelium into epithelium characteristic of the human prostate (43). The dorsolateral prostate in both human and mouse male fetuses also shows structural similarities during early fetal development, namely, the same pattern of epithelial duct formation. Based on these findings, we have proposed that the dorsolateral region of the human prostate is homologous to the dorsolateral region of the mouse prostate (2, 28). In contrast, for the ventral region of the prostate, there are differences, because unlike the mouse prostate, the human adult prostate does not contain ducts that emerge from the ventral region of the urethra. We, and others, also have reported differences between the rodent dorsolateral and ventral prostate in regulatory factors and the response to other chemicals (34, 44). The rodent dorsolateral prostate thus may be a valuable model for predicting the effects of estrogenic chemicals on human prostate development.
The changes in the structure of the urethra induced by estrogenic chemicals may have implications for human disease. Low doses of bisphenol A, ethinylestradiol and DES caused similar morphological changes relative to oil-treated controls in the region of the paired prostatic sulci that form the lateral walls of the colliculus. This region is of particular interest with regard to estrogen-induced malformations, because the primary secretory ducts in the human prostate, which are long ducts that project into the peripheral zone, develop from these urethral ridges, and it is within these ducts that the great majority of malignant prostate tumors form in men as they age (45). The unexpected observation of a malformation of the urethra at the bladder neck (specifically, a narrowing of the urethral lumen by approximately one-third) could affect bladder function and have implications for bladder outlet obstruction disease (46).
There are now >90 published studies showing adverse effects of low doses of bisphenol A in a wide variety of experimental animals (32). For example, during postnatal life very low doses of bisphenol A have been shown to disrupt chromosomes during meiosis in mouse oocytes (47) and decrease sperm production in male rats (48). Fetal exposure to very low doses of bisphenol A accelerates postnatal growth and advances puberty (49) and also stimulates mammary gland epithelium in mice (50). A recent case-control study has shown a correlation between blood levels of bisphenol A and both obesity and polycystic ovarian disease in Japanese women (51).
With regard to ethinylestradiol, the focus of the relatively few studies of exposure by human fetuses during the critical period of reproductive organ development due to continued use of oral contraceptives during an undetected pregnancy has been on externally visible malformations at birth (reviewed in ref. 52). Our findings concerning ethinylestradiol exposure in fetal mice should be viewed from the perspective that the long-term effects of fetal DES exposure in mice and humans have been documented to be highly concordant (3–5), and we show here that the effects of the same low doses of ethinylestradiol and DES on the developing prostate and urethra are virtually identical.
Based on the general absence of grossly observable external malformations at birth, DES was considered safe for administration to millions of women during pregnancy for more than two decades. DES was subsequently found to result in serious harm to offspring that became apparent in adulthood (3–5). Similar conclusions about the safety of ethinylestradiol exposure for human fetuses based on the absence of consistent findings from studies focusing on grossly observable external malformations should be reevaluated based on several lines of evidence. Ethinylestradiol has been shown to readily pass from the maternal to fetal circulation across the primate placenta (52). Fetal exposure to DES caused uterine cancer in humans and mice (3, 4), and similar to our prostate and urethra findings here, DES and ethinylestradiol have virtually identical effects on the developing uterus in female rats (11, 53). The dose of ethinylestradiol in oral contraceptives is typically ≈4- to 8-fold higher than the dose used in our experiment with mice that caused malformations of the urethra and altered differentiation and growth of the prostate. In summary, we propose that the data from this and other published animal studies, and the similarity to effects of low doses of DES, warrant a thorough reevaluation of the risks posed by doses of both ethinylestradiol and bisphenol A below those to which human fetuses are exposed.
Acknowledgments
We thank Drs. Leland Chung and Joseph Monte for comments on an earlier draft of the manuscript. This work was supported by National Institute of Environmental Health Sciences Grants ES11283 (to F.S.v.S.) and ES-11549 (to C.A.R.) and Environmental Protection Agency Grant R-827403 (to B.G.T.).
Author contributions: B.G.T. and F.S.v.S. designed research; B.G.T., K.L.H., L.B., S.B., C.A.R., and F.S.v.S. performed research; B.G.T., L.B., S.B., C.A.R., and F.S.v.S. analyzed data; and B.G.T., K.L.H., C.A.R., and F.S.v.S. wrote the paper.
Abbreviations: DES, diethylstilbestrol; UGS, urogenital sinus; GD, gestation day; PCNA, proliferating cell nuclear antigen; MK5, mouse keratin 5.
References
- 1.Zuckerman, S. (1936) Proc. R. Soc. Med. 29, 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter, C. A., Timms, B. G. & vom Saal, F. S. (2004) in Endocrine Disruptors: Effects on Male and Female Reproductive Systems, ed. Naz, R. K. (CRC, Boca Raton, FL), 2nd Ed., pp. 379–410.
- 3.Bern, H. A. (1992) in Chemically Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection, Advances in Modern Environmental Toxicology, eds. Colborn, T. & Clement, C. (Princeton Scientific, Princeton), Vol. 21, pp. 9–15. [Google Scholar]
- 4.Newbold, R. (1995) Environ. Health Perspect. 103, 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swan, S. H. & vom Saal, F. S. (2001) in Endocrine Disruptors in the Environment, ed. Metzler, M. (Springer, Heidelberg), Vol. 3, pp. 131–170. [Google Scholar]
- 6.Welshons, W. V., Thayer, K. S., Taylor, J., Judy, B. & vom Saal, F. S. (2003) Environ. Health Perspect. 111, 994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colborn, T., vom Saal, F. S. & Soto, A. M. (1993) Environ. Health Perspect. 101, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickey, R. P. (1998) Managing Contraceptive Pill Patients (EMIS Publishers, Durant, OK).
- 9.Li, D., Daling, J. R., Mueller, B. A., Hickok, D. E., Fantel, A. G. & Weiss, N. S. (1995) Teratology 51, 30–36. [DOI] [PubMed] [Google Scholar]
- 10.Thayer, K. A., Ruhlen, R. L., Howdeshell, K. L., Buchanan, D., Cooke, P. S., Welshons, W. V. & vom Saal, F. S. (2001) Hum. Reprod. 16, 988–996. [DOI] [PubMed] [Google Scholar]
- 11.Branham, W. S., Zehr, D. R. & Sheehan, D. M. (1993) Proc. Soc. Exp. Biol. Med. 230, 297–303. [DOI] [PubMed] [Google Scholar]
- 12.Dodds, E. C. & Lawson, W. (1936) Nature 137, 996. [Google Scholar]
- 13.Burridge, E. (2003) Eur. Chem. News, April 14–20, p. 17.
- 14.Brotons, J. A., Olea-Serrano, M. F., Villalobos, M., Pedraza, V. & Olea, N. (1995) Environ. Health Perspect. 103, 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brede, C., Fjeldal, P., Skjevrak, I. & Herikstad, H. (2003) Food Addit. Contam. 20, 684–689. [DOI] [PubMed] [Google Scholar]
- 16.Howdeshell, K. L., Peterman, P. H., Judy, B. M., Taylor, J. A., Orazio, C. E., Ruhlen, R. L., vom Saal, F. S. & Welshons, W. V. (2003) Environ. Health Perspect. 111, 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schonfelder, G., Wittfoht, W., Hopp, H., Talsness, C. E., Paul, M. & Chahoud, I. (2002) Environ. Health Perspect. 110, A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikezuki, Y., Tsutsumi, O., Takai, Y., Kamei, Y. & Taketani, Y. (2002) Hum. Reprod. 17, 2839–2841. [DOI] [PubMed] [Google Scholar]
- 19.Zalko, D., Soto, A. M., Dolo, L., Dorio, C., Rathahao, E., Debrauwer, L., Faure, R. & Cravedi, J. P. (2002) Environ. Health Perspect. 111, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.vom Saal, F. S., Timms, B. G., Montano, M. M., Palanza, P., Thayer, K. A., Nagel, S. C., Dhar, M. D., Ganjam, V. K., Parmigiani, S. & Welshons, W. V. (1997) Proc. Natl. Acad. Sci. USA 94, 2056–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welshons, W. V., Nagel, S. C., Thayer, K. A., Judy, B. M. & vom Saal, F. S. (1999) Toxicol. Ind. Health 15, 12–25. [DOI] [PubMed] [Google Scholar]
- 22.Nagel, S. C., vom Saal, F. S., Thayer, K. A., Dhar, M. G., Boechler, M. & Welshons, W. V. (1997) Environ. Health Perspect. 105, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta, C. (2000) Proc. Soc. Exp. Biol. Med. 224, 61–68. [DOI] [PubMed] [Google Scholar]
- 24.vom Saal, F. S. (1989) J. Anim. Sci. 67, 1824–1840. [DOI] [PubMed] [Google Scholar]
- 25.Nonneman, D. J., Ganjam, V. K., Welshons, W. V. & vom Saal, F. S. (1992) Biol. Reprod. 47, 723–729. [DOI] [PubMed] [Google Scholar]
- 26.Timms, B. G., Petersen, S. L. & vom Saal, F. S. (1999) J. Urol. 161, 1694–1701. [PubMed] [Google Scholar]
- 27.Timms, B. G., Peterson, R. E. & vom Saal, F. S. (2002) Toxicol. Sci. 67, 264–274. [DOI] [PubMed] [Google Scholar]
- 28.Timms, B. G., Mohs, T. J. & Didio, L. J. A. (1994) J. Urol. 151, 1427–1432. [DOI] [PubMed] [Google Scholar]
- 29.Iatropoulos, M. J. & Williams, G. M. (1996) Exp. Toxicol. Pathol. 48, 175–181. [DOI] [PubMed] [Google Scholar]
- 30.Prins, G. S. (1997) in Prostate: Basic and Clinical Aspects, ed. Naz, R. K. (CRC, Boca Raton, FL), pp. 247–265.
- 31.Wetherill, Y. B., Petra, C. E., Monk, K. R., Puga, A. & Knudsen, K. E. (2002) Mol. Cancer Ther. 7, 515–524. [PubMed] [Google Scholar]
- 32.vom Saal, F. S. & Hughes, C. (April 13, 2005) Environ. Health Perspect., 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed]
- 33.Wozniak, A. L., Bulayeva, N. N. & Watson, C. S. (2005) Environ. Health Perspect. 113, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timms, B. G., Lee, C. W., Aumuller, G. & Seitz, J. (1995) Microsc. Res. Tech. 30, 319–332. [DOI] [PubMed] [Google Scholar]
- 35.Marker, P. C., Donjacour, A. A., Dahiya, R. & Cunha, G. R. (2003) Dev. Biol. 253, 165–174. [DOI] [PubMed] [Google Scholar]
- 36.Donjacour, A. A., Thomson, A. A. & Cunha, G. R. (2003) Dev. Biol. 261, 39–54. [DOI] [PubMed] [Google Scholar]
- 37.Lamm, M. L. G., Podlasek, C. A., Barnett, D. H., Lee, J., Clemens, J. Q., Hebner, C. M. & Bushman, W. (2001) Dev. Biol. 232, 301–314. [DOI] [PubMed] [Google Scholar]
- 38.McNeal, J. E. (1978) Invest. Urol. 15, 340–345. [PubMed] [Google Scholar]
- 39.Cardoso, W. V. (2000) Dev. Dyn. 219, 121–130. [DOI] [PubMed] [Google Scholar]
- 40.Wiesen, J. F., Young, P., Werb, Z. & Cunha, G. R. (1999) Development (Cambridge, U.K.) 126, 335–344. [DOI] [PubMed] [Google Scholar]
- 41.Melnick, M. & Jaskoll, T. (2000) Crit. Rev. Oral Biol. Med. 11, 199–215. [DOI] [PubMed] [Google Scholar]
- 42.Westfall, M. D. & Pietenpol, J. A. (2004) Carcinogenesis 25, 857–864. [DOI] [PubMed] [Google Scholar]
- 43.Aboseif, S., El-Sakka, A., Young, P. & Cunha, G. R. (1999) Differentiation (Berlin) 65, 113–118. [DOI] [PubMed] [Google Scholar]
- 44.Ko, K., Theobald, H. M., Moore, R. W. & Peterson, R. E. (2004) 79, 360–369. [DOI] [PubMed]
- 45.McNeal, J. E. (1983) Monogr. Urol. 4, 1–33. [Google Scholar]
- 46.Streng, T., Launonen, A., Salmi, S., Saarinen, N., Talo, A., Makela, S. & Santti, R. (2001) J. Urol. 165, 1305–1309. [PubMed] [Google Scholar]
- 47.Hunt, P. A., Koehler, K. E., Susiarjo, M., Hodges, C. A., Hagan, A., Voigt, R. C., Thomas, S., Thomas, B. F. & Hassold, T. J. (2003) Curr. Biol. 13, 546–553. [DOI] [PubMed] [Google Scholar]
- 48.Sakaue, M., Ohsako, S., Ishimura, R., Kurosawa, S., Kurohmaru, M., Hayashi, Y., Aoki, Y., Yonemoto, J. & Tohyama, C. (2001) J. Occup. Health 43, 185–190. [Google Scholar]
- 49.Howdeshell, K. L., Hotchkiss, A. K., Thayer, K. A., Vandenbergh, J. G. & vom Saal, F. S. (1999) Nature 401, 763–764. [DOI] [PubMed] [Google Scholar]
- 50.Markey, C. M., Luque, E. H., Munoz De Toro, M., Sonnenschein, C. & Soto, A. M. (2001) Biol. Reprod. 65, 1215–1223. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi, T., Tsutsumi, O., Ikezuki, Y., Takai, Y. & Taketani, Y. (2004) Endocr. J. 51, 165–169. [DOI] [PubMed] [Google Scholar]
- 52.Slikker, W., Bailey, J. R., Newport, G. D., Lipe, G. W. & Hill, D. E. (1982) J. Pharmacol. Exp. Ther. 223, 483–489. [PubMed] [Google Scholar]
- 53.Sheehan, D. M. & Branham, W. S. (1987) Teratog. Carcinog. Mutagen. 7, 411–422. [DOI] [PubMed] [Google Scholar]