Abstract
We demonstrate, for the first time, catalysis by Escherichia coli ribonuclease P (RNase P) RNA with Zn2+ as the sole divalent metal ion cofactor in the presence of ammonium, but not sodium or potassium salts. Hill analysis suggests a role for two or more Zn2+ ions in catalysis. Whereas Zn2+ destabilizes substrate ground state binding to an extent that precludes reliable Kd determination, and Sr2+ in particular, both unable to support catalysis by themselves, promote high-substrate affinity. Zn2+ and substantially reduce the fraction of precursor tRNA molecules capable of binding to RNase P RNA. Stimulating and inhibitory effects of Sr2+ on the ribozyme reaction with Zn2+ as cofactor could be rationalized by a model involving two Sr2+ ions (or two classes of Sr2+ ions). Both ions improve substrate affinity in a cooperative manner, but one of the two inhibits substrate conversion in a non-competitive mode with respect to the substrate and the Zn2+. A single 2′-fluoro modification at nt −1 of the substrate substantially weakened the inhibitory effect of Sr2+. Our results demonstrate that the studies on RNase P RNA with metal cofactors other than Mg2+ entail complex effects on structural equilibria of ribozyme and substrate RNAs as well as E·S formation apart from the catalytic performance.
INTRODUCTION
The ribonucleoprotein enzyme ribonuclease P (RNase P) is an endonuclease that generates the mature 5′ ends of tRNAs in all three domains of life (Archaea, Bacteria and Eukarya) as well as in the mitochondria and the chloroplasts (1–3). Bacterial RNase P enzymes are composed of a catalytic RNA subunit, ∼400 nt in length, and a single small protein of typically 120 amino acids (4,5). Studies with RNase P RNA from Escherichia coli (structural RNase P RNA subtype A) and Bacillus subtilis (subtype B) have implied a specific role for two or more metal ions in substrate binding and cleavage chemistry (6–13). Mg2+ and Mn2+ efficiently support the precursor tRNA (ptRNA) processing reaction catalyzed by bacterial RNase P RNAs (14), which generates 3′-OH and 5′-phosphate termini. For E.coli RNase P RNA (referred to as M1 RNA further), processing under standard assay conditions was reported to be essentially abolished when Mg2+ or Mn2+ is replaced with earth alkaline metals such as Sr2+, transition metal ions, such as Zn2+, Co2+ and Ni2+, or as a potential mimic of hexaaquo Mg2+ (11,15). Ca2+, as an exception, supported the reaction, although inefficiently (11,14). Different results were obtained with a ptRNA carrying an Rp-phosphorothioate modification at the RNase P cleavage site. This substrate was cleaved quite efficiently by M1 RNA when Mg2+ was replaced with Cd2+, conditions under which cleavage of the unmodified ptRNA was hardly detectable (7). This result suggested that the failure of transition metal ions such as Cd2+ to support cleavage of unmodified ptRNA by M1 RNA is due to their inability to interact properly with the phosphate pro-Rp oxygen at the scissile phosphodiester in the transition state. The situation was found to be somewhat different in the reaction catalyzed by B.subtilis RNase P RNA. This ribozyme required a second metal ion, such as Ca2+, in addition to Cd2+ for processing a ptRNA with a single Rp-phosphorothioate modification at the cleavage site. Here, Ca2+ was inferred to be essential for productive enzyme–substrate complex formation (8), suggesting that there are differential roles for metal ions in RNase P RNA-catalyzed reactions. Synergistic effects of metal ion combinations were also observed for the reaction catalyzed by E.coli M1 RNA, although their molecular basis has been poorly understood. While the ribozyme failed to cleave the substrate in the presence of Ba2+, Sr2+, Zn2+ or alone, some cleavage activity was restored with the combinations Zn2+/Sr2+, Zn2+/Ba2+ or (11). Catalysis by M1 RNA with Sr2+ or Ba2+ alone has only been observed with low efficiency under very specialized conditions [at pH≫7 and in the presence of ethanol; (16)]. From the Pb2+-induced hydrolysis patterns of M1 RNA generated in the presence of different divalent metal ions and (17), it was concluded that the M1 RNA conformation is very similar in the presence of Mg2+, Mn2+, Ca2+, Sr2+, Ba2+ and apparently also , whereas transition metals, such as Zn2+ and particularly Cd2+, Co2+, Cu2+ and Ni2+, induce changes of the native M1 RNA conformation.
To obtain a deeper insight into how different metal ions modulate this ribozyme system, we have investigated the effects of Zn2+, Sr2+ and on different aspects of processing by M1 RNA: catalysis and its inhibition by Sr2+ in particular, enzyme–substrate affinity and changes in the fraction of substrate able to bind to the enzyme. Catalysis was analyzed under conditions of E≫S, and all assays included a relatively high monovalent salt concentration (1 M NH4OAc) to focus on the roles of metal ions which cannot be fulfilled by monovalent cations. To further characterize the metal ion interaction in vicinity of the 2′-OH group at nt −1 of the substrate, we have tested cleavage by M1 RNA in the presence of Zn2+/Sr2+ with ptRNA substrates carrying a single 2′-amino (2′-N), 2′-fluoro (2′-F) or 2′-deoxy (2′-H) substitution at nt −1. Such 2′-ribose modifications at the RNase P cleavage site were previously reported to affect the binding of catalytically important Mg2+ and to substantially reduce the rate of catalysis by M1 RNA (6,18–20). Recent NMR experiments have provided further evidence that the metal ion coordinated with the help of the 2′-OH group at nt −1 is actually ‘pre-bound’ to ptRNA before complexation with RNase P RNA (21).
MATERIALS AND METHODS
RNA synthesis and labeling
Chemical and enzymatic RNA synthesis, purification of RNA and assembly of ptRNA variants with single-site modifications have been described recently (20).
Kinetics
Processing assays were performed at 37°C under single turnover conditions (5 μM M1 RNA, <1 nM ptRNA, 1 M NH4OAc, 50 mM MES for the pH range of 5–6 and PIPES for pH 6–7; metal ion concentration and pH at 37°C as indicated) as described previously (22). Aliquots withdrawn from the enzyme–substrate mixtures were desalted by ethanol precipitation in the presence of 20 μg glycogen before analysis by 20% PAGE/8 M urea. Data analysis and calculation of single turnover rates of cleavage (kobs) were performed as described previously (22).
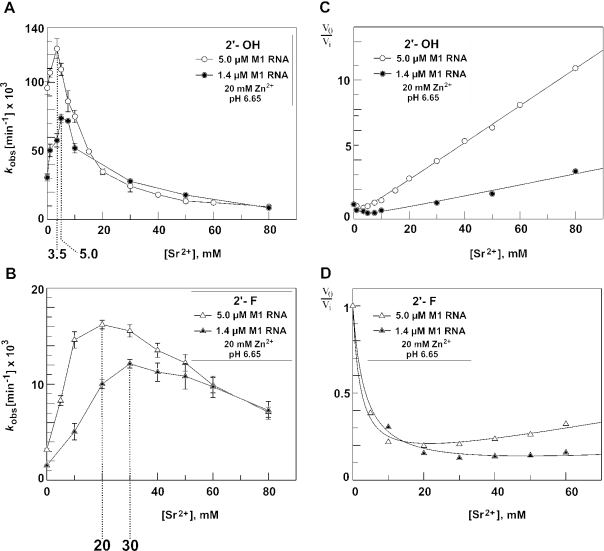
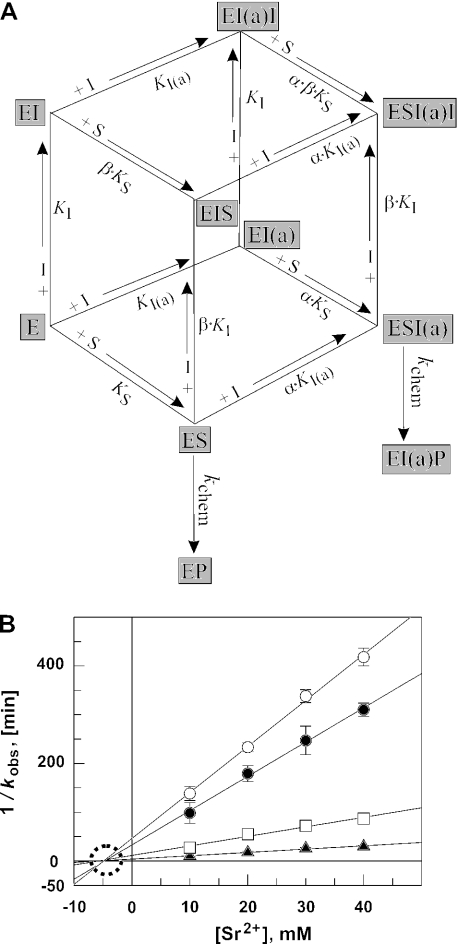
The dependence of kobs on [Sr2+] in Figure 7A and B showed a stimulatory and inhibitory phase. These primary data were replotted as kobs in the absence of Sr2+ divided by kobs at individual Sr2+ concentrations (termed v0/vi) over [Sr2+] (Figure 7C and D). These secondary plots were fit to the equation v0/vi = (KS + [E](1 + (1/αKI(a) + 1/βKI)[I] + [I]2/αβKI(a) KI))/((KS + [E])(1 + [I]/αKI(a))), derived from a model (Figure 8A) involving two Sr2+ ions (or two classes of Sr2+ ions): both improve substrate affinity in a cooperative manner, but one of the two inhibits substrate conversion in a non-competitive mode with respect to the substrate. The equation was derived as follows [E, M1 RNA; S, ptRNA substrate; I(a), Sr2+ with ‘activating’ effect owing to a reduction in KS, expressed as α · KS; I, inhibitory Sr2+ which also reduces KS, expressed as β · KS].
Figure 7.
Sensitivity of processing rates as a function of [Sr2+] to differences in [E]: (A) all-ribose ptRNAGly (2′-OH); (B) 2′-F-ptRNAGly. (C and D) Secondary v0/vi plots of the data (v0 = kobs in the absence of Sr2+; vi = kobs at the respective Sr2+ concentration) from (A) and (B). Data fitting was best with the model depicted in Figure 8A, using Equation 8 (see Materials and Methods and Figure 8): v0/vi = (KS + [E] (1 + (1/αKI(a) + 1/βKI) [I] + [I]2/αβKI(a) KI))/((KS + [E]) (1 + [I]/αKI(a))). Curve fits yielded the following values for αKI(a) and βKI: 1.46 ± 0.46 mM and 0.72 ± 0.025 mM [(C), 1.4 μM M1 RNA, 2′-OH], 1.49 ± 0.45 mM and 0.72 ± 0.005 mM [(C) 5 μM M1 RNA, 2′-OH], 3.5 ± 0.4 and 9.1 ± 2.1 mM [(D) 1.4 μM M1 RNA, 2′-F] and 2.2 ± 0.2 and 11 ± 0.8 mM [(D) 5 μM M1 RNA, 2′-F]; estimates for KS were 50 and 120 μM in (C) and (D), respectively.
Figure 8.
(A) Equilibria for the M1 RNA-catalyzed processing reaction in the presence of constant [Zn2+] as the catalytic cofactor and varying concentrations of [Sr2+] that activate the reaction at low but inhibit at higher concentrations. The model involves two Sr2+ ions (or two classes of Sr2+ ions) that both improve substrate affinity in a cooperative manner (see Figure 3B), but one of the two inhibiting substrate conversion non-competitively with respect to the substrate. Other models, for example, assuming the involvement of two ‘activating’ and one inhibitory Sr2+ ion or predicting that E·S·I and E·S·I(a)·I complexes retain residual reactivity, failed to give satisfactory curve fits of the data in Figure 7C and D. I(a) = Sr2+ ion that activates processing by decreasing KS to αKS; I = inhibitory Sr2+ ion that inhibits cleavage chemistry but also decreases KS to βKS. (B) Dixon plot of the M1 RNA-catalyzed ptRNA cleavage rate at 37°C as a function of [Sr2+] in the presence of four different fixed Zn2+ concentrations and 5 μM M1 RNA, <1 nM ptRNA, 50 mM PIPES, pH 6.65 and 1 M NH4OAc; open circles, 8.1 mM Zn2+; filled circles, 10 mM Zn2+; open squares, 15 mM Zn2+; and filled triangles, 20 mM Zn2+. All four datasets fit to straight lines intersecting on the x-axis, indicating that Sr2+ acts as a non-competitive inhibitor with respect to Zn2+. The point of intersection on the x-axis yields a KI-value of ∼5 mM.
The velocity dependence equation is:
| 1 |
Dividing both sides of the velocity dependence Equation 1 by [S]t:
| 2 |
| 3 |
Replacing [S]t in the right-hand side of Equation 2:
| 4 |
Expressing the concentration of each species in terms of [E]:
| 5 |
Multiplying the numerator and the denominator with KS/[S] and simplifying:
| 6 |
When [I] = 0, vi equals v0, and Equation 6 simplifies to:
| 7 |
[S]t · kchem is Vmax under conditions [E] > [S].
Dividing Equation 7 by Equation 6 and simplifying:
| 8 |
Spin column assays
Spin column assays for the determination of equilibrium dissociation constants (Kd) of enzyme–substrate complexes were performed as described previously (8,23) in a buffer containing 50 mM MES, pH 6.0, 1 M NH4OAc, 0.1% (w/v) SDS, 0.05% (w/v) Nonidet P-40, and indicated concentrations of SrCl2, Zn(OAc)2 and/or Co(NH3)6Cl3. For the Hill plot analysis shown in Figure 3, see (9).
Figure 3.
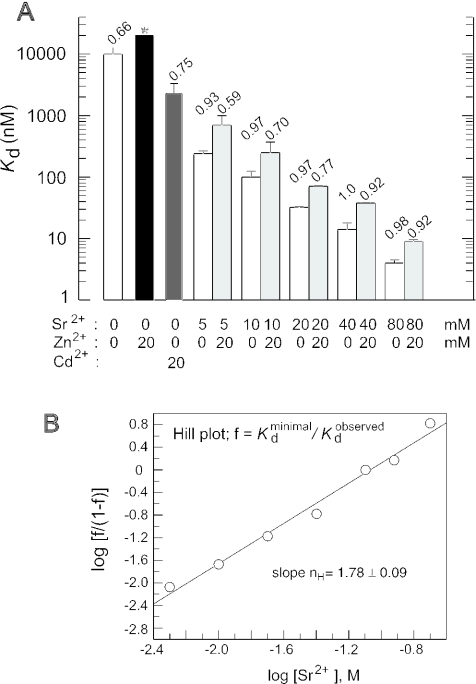
Kd values for ptRNAGly binding to M1 RNA determined by the spin column assay (8,23). Assay buffers contained 50 mM MES, pH 6.0, 1 M NH4OAc and indicated concentrations of Sr2+ and/or Zn2+. (A) Bar diagram of log Kd dependence on Sr2+ and/or Zn2+ (or Cd2+) concentration as indicated below the diagram. Values above bars represent the proportion of ptRNAGly that was able to form a complex with M1 RNA at the theoretical endpoint (M1 RNA saturation), normalized to conditions of 40 mM Sr2+. Asterisk above the black bar for conditions of 20 mM Zn2+ alone: the experimental endpoint could not be reached due to very weak complex formation, which resulted in high errors for Kd determinations; the Kd of ≥20 μM is therefore only an estimate. Individual values are based on average on four independent experiments; errors are indicated by error bars. (B) Hill plot analysis of Kd dependence on Sr2+ concentration. f = (minimal Kd)/(observed Kd); the minimal Kd at saturating Sr2+ concentrations was determined as 2 nM.
RESULTS
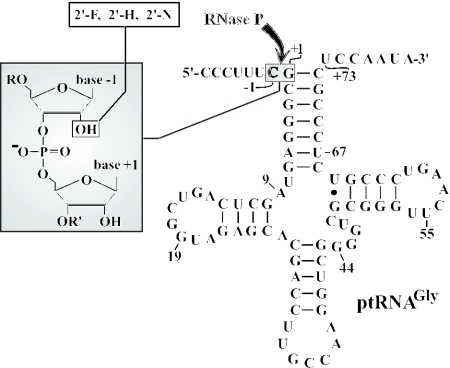
The well-characterized bacterial ptRNAGly used as the substrate for processing by M1 RNA is illustrated in Figure 1, including the variants carrying single-site 2′-ribose modifications at nt −1.
Figure 1.
Secondary structure of the ptRNAGly substrate. 2′-Ribose modifications introduced at the canonical RNase P cleavage site (nt −1) are illustrated in the gray-shaded box on the left. The arrow marks the canonical RNase P cleavage site between nucleotides −1 and +1. For further details, see (20).
Cleavage in the presence of Zn2+ alone or combinations of Zn2+/Sr2+ and
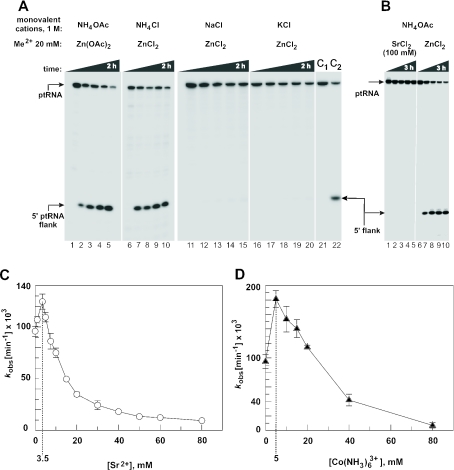
Surprisingly, Zn2+ as the only divalent metal ion was able to support ptRNA processing by M1 RNA, but only in the presence of ammonium salts (Figure 2A). The nature of the counter anion (chloride or acetate) was not critical (Figure 2A), although some RNA degradation was observed with chloride salts. Neither significant degradation nor precipitation of ptRNA or M1 RNA occurred under our standard assay conditions (1 M NH4OAc, 20–80 mM Zn[OAc]2, pH 6.65; data not shown). Sr2+ (Figure 2B) or (data not shown) alone did not support M1 RNA-catalyzed cleavage, in line with the previous observations (11).
Figure 2.
Processing of the all-ribose ptRNAGly by E.coli M1 RNA under single turnover conditions at 37°C in the presence of 20 mM Zn2+. (A) Dependence on the type of monovalent cation; processing occurred in the presence of ammonium, but not sodium or potassium salts; reaction conditions: 5 μM M1 RNA, <1 nM ptRNA, 50 mM PIPES, pH 6.65, 1 M monovalent salt as indicated, and 20 mM ZnCl2 or Zn(OAc)2; control lanes 1, 6, 11, 16 and 21: incubation for 2 h in the absence of M1 RNA; time points were 5 min (lanes 2, 7, 12, 17 and 22), 20 min (lanes 3, 8, 13 and 18), 1 h (lanes 4, 9, 14 and 19) and 2 h (lanes 5, 10, 15 and 20); lanes 21 and 22 (C1, C2) are equal to lanes 1 and 2. (B) Assay documenting that no processing occurs in the presence of Sr2+ as the sole metal ion; reaction conditions: 5 μM M1 RNA, <1 nM ptRNA, 50 mM PIPES, pH 6.65, 1 M NH4OAc and 100 mM SrCl2 (left) or 20 mM ZnCl2 (right). Control lanes 1 and 6: incubation for 3 h in the absence of M1 RNA; time points were 5 min (lane 7), 20 min (lanes 2 and 8), 40 min (lane 3), 1 h (lanes 4 and 9) and 3 h (lanes 5 and 10). (C and D) Processing rates as a function of increasing concentrations of SrCl2 (C) or Co(NH3)6Cl3 (D); reaction conditions: 5 μM M1 RNA, <1 nM ptRNA, 50 mM PIPES, pH 6.65, 1 M NH4OAc and 20 mM Zn(OAc)2; transition points between the stimulatory and the inhibitory phases of the curves are marked by dashed lines.
We then analyzed single turnover cleavage by M1 RNA (see Materials and Methods) in the presence of Zn2+ and increasing concentrations of Sr2+ or (Figure 2C and D). The pH 6.65 was chosen to combine substantial substrate turnover with conditions where cleavage chemistry mainly determines the rate of cleavage at saturating enzyme concentrations (7). At constant 20 mM Zn2+, both Sr2+ and started to stimulate substrate turnover at lower concentrations, followed by an inhibitory phase at higher concentrations (Figure 2C and D). This suggested that the two different Sr2+ or ions (or classes of ions) affected the cleavage reaction under these experimental conditions.
dependence of substrate binding to M1 RNA
As a next step, we analyzed the binding of ptRNAGly to M1 RNA as a function of Zn2+, Sr2+ and/or . The following results were obtained by a spin column assay (see Materials and Methods) and are summarized in Table 1. In the absence of any metal ion, a Kd of ∼10 μM was measured under our assay conditions. The Kd increased to ≥20 μM in the presence of 20 mM Zn2+, demonstrating that the Zn2+ destabilizes the substrate ground state binding. Another transition metal ion, Cd2+, supported E·S formation poorly as well (Kd of 2.1 μM), although more efficiently than the Zn2+. In contrast to the transition metal ions, Sr2+ or support substrate binding, Sr2+ more efficiently than . Kd values in the presence of 20 mM Zn2+ progressively decreased with increasing Sr2+ concentrations (Figure 3A). However, at all tested Sr2+ concentrations (5, 10, 20, 40 and 80 mM), the addition of 20 mM Zn2+ resulted in a constant 2–3-fold increase in Kd (Figure 3A). Moreover, the proportion of substrate that is able to form a stable complex with the enzyme at the endpoint (i.e. point of enzyme saturation; Figure 3A, numbers above bars) decreased with increasing ratios of [Zn2+] to [Sr2+], for example, the binding-proficient substrate fraction decreased from 0.92 at 20 mM Zn2+/80 mM Sr2+ to 0.59 at 20 mM Zn2+/5 mM Sr2+ (normalized to 1.0 measured at 40 mM Sr2+ alone, Figure 3A). Hill analysis of Kd in the presence of varying concentrations of Sr2+ and in the absence of Zn2+ gave a slope of nH = 1.8 (Figure 3B), suggesting that at least two Sr2+ ions are additionally taken up into the enzyme–substrate complex under the applied conditions.
Table 1.
Influence of Zn2+, Sr2+ and/or on the binding of ptRNAGly to E.coli M1 RNA
| Metal ion(s) | Kd (nM) | Average endpoint | |
|---|---|---|---|
| Transition Me2+ | Other | ||
| — | — | 10 000 ± 3000 | 0.66 |
| 20 mM Zn2+ | — | ≥20 000a | n.d.a |
| 20 mM Cd2+ | — | 2100 ± 400 | 0.75 |
| — | 5 mM Sr2+ | 240 ± 15 | 0.93 |
| 20 mM Zn2+ | 5 mM Sr2+ | 700 ± 300 | 0.59 |
| — | 80 mM Sr2+ | 4 ± 0.5 | 0.98 |
| 20 mM Zn2+ | 80 mM Sr2+ | 9 ± 0.5 | 0.92 |
| 20 mM Zn2+ | 5 mM | 2486 ± 400 | 0.55 |
| — | 20 mM | 751 ± 200 | 0.75 |
| 20 mM Zn2+ | 20 mM | 350 ± 120 | 0.65 |
| — | 80 mM | 79 ± 35 | 0.26 |
| 20 mM Zn2+ | 80 mM | 53 ± 6 | 0.28 |
Spin column assay for the determination of Kd values were performed at pH 6.0 and 1 M NH4OAc using trace amounts of 5′-endlabeled ptRNAGly; individual Kd values are based on three to six independent experiments and were calculated by non-linear regression analysis (program Grafit, Erithacus Software) using the equation: fc = ft · [P RNA]free/(Kd + [P RNA]free), where fc = fraction of ptRNA in the complex, and ft = maximum fraction of ptRNA that is able to bind to P RNA (endpoint). Endpoints in the right column are the theoretical ones obtained by the fitting procedure; however, theoretical and experimentally measured endpoints were generally in good agreement; average endpoints were normalized to that for 40 mM Sr2+ (see Figure 3A).
aKd and endpoint values (n.d. = not determined) could not be determined with reasonably low errors owing to very low ribozyme–substrate affinity; the Kd of 20 000 nM in the presence of 20 mM Zn2+ alone is a lower limit estimate.
also decreased the Kd values, but the substrate affinity was lower relative to the equal concentrations of Sr2+ (Table 1), suggesting that is a rather inefficient substitute for hexahydrated Mg2+ in this system. Whereas Kd values in the presence of Sr2+ were generally increased by the addition of 20 mM Zn2+ (Figure 3A), addition of 20 mM Zn2+ to 20 or 80 mM tended to lower Kd to some extent compared with the corresponding alone conditions (Table 1). This suggests that Zn2+ and weakly complement each other in promoting E·S complex formation. Remarkably, higher concentrations (e.g. 80 mM) of substantially lowered the proportion of ptRNA capable of complex formation under saturating enzyme concentrations [0.26 for 80 mM versus 0.98 for 80 mM Sr2+; Table 1].
Zn2+ cooperativity in catalysis by M1 RNA
We observed that M1 RNA is able to catalyze ptRNA processing in the presence of Zn2+ as the sole divalent metal ion (Figure 2A). On the other hand, Zn2+ failed to promote thermodynamically stable E·S complex formation (Table 1 and Figure 3A), in contrast to other divalent metal ions, such as Mg2+ or Mn2+, which support both catalysis and E·S formation (14). Thus, processing analyses with Zn2+ suggested the potential to use Zn2+ as a specific tool to study catalysis apart from E·S complex formation.
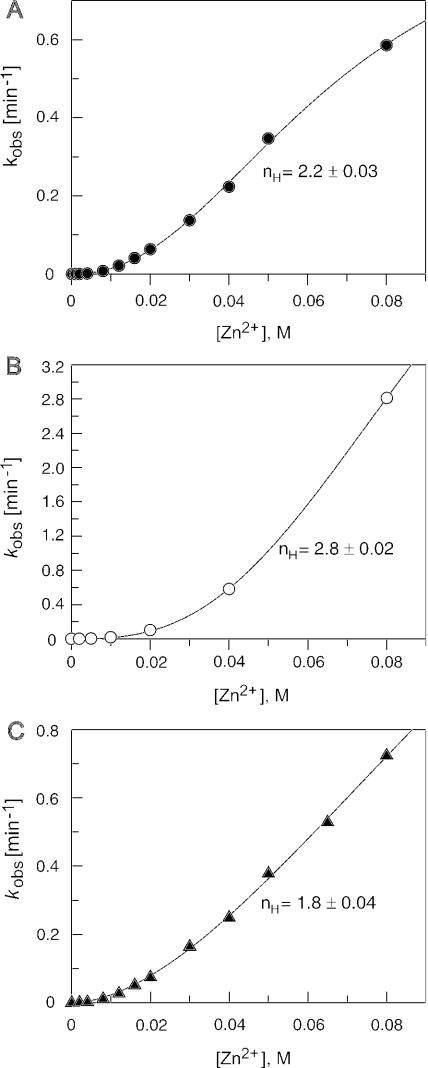
To assess the number of catalytic Zn2+ ions involved, we analyzed single turnover cleavage of ptRNAGly in the presence of increasing Zn2+ concentrations, either in the presence of constant 12 mM Sr2+ or 20 mM , or without any second metal ion (Figure 4). For the conditions including Sr2+ and , enzyme concentration (5 μM) was saturated based on our Kd measurements (Table 1 and Figure 3A). In all cases, reasonable fits to the Hill equation resulted in coefficients of nH = 2.2 (Figure 4A, at constant 12 mM Sr2+), nH = 2.8 (Figure 4B, Zn2+ alone) and nH = 1.8 [Figure 4C, at constant 20 mM ]. These nH-values in the range 1.8–2.8 support the cooperative involvement of two or more Zn2+ ions in catalysis by E.coli M1 RNA. Results from the Zn2+-alone reaction, although supporting this Zn2+ cooperativity, should yet be interpreted with some caution as the enzyme concentration was subsaturating under these conditions (Table 1); thus, we cannot exclude that, in addition to the rate of the catalytic step, changes in the E·S binding equilibrium upon variation of [Zn2+] may also have affected the observed cleavage rates.
Figure 4.
Zn2+-dependence of M1 RNA-catalyzed cleavage of all-ribose ptRNAGly (Hill analysis). The Zn2+-concentration was varied in the range of 0–80 mM: (A) at constant [Sr2+] (12 mM) and pH 6.0; (B) in the absence of a second metal ion at pH 6.5; (C) at constant [] (20 mM) and pH 6.0. Data were analyzed by non-linear regression analysis using the Hill equation v = Vmax·[Zn2+]n/(K′Zn + [Zn2+]n) as described previously (8). The Hill coefficient (nH) was determined as 2.2 ± 0.03 in (A), 2.8 ± 0.02 in (B) and 1.80 ± 0.04 in (C). For further details, see Materials and Methods.
Processing of substrates with 2′-ribose modifications at nt −1 in the presence of Zn2+/Sr2+
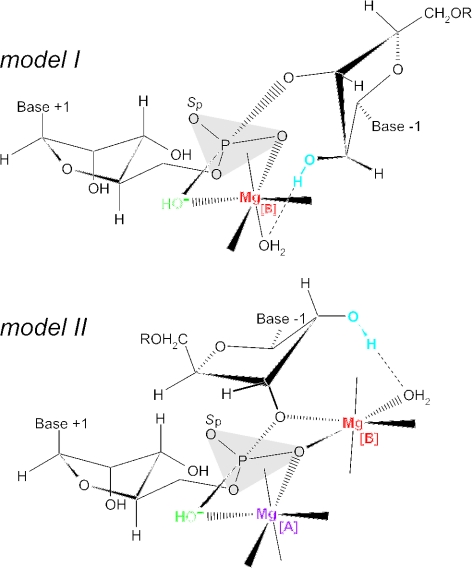
A model of the RNase P RNA cleavage mechanism [(24); Figure 5, model I] proposes that one Mg2+ ion (termed here Mg[B]) simultaneously interacts with the OH− nucleophile (inner-sphere) and the 2′-OH at position −1 of the substrate via an inner-sphere water molecule, and directly coordinates to the pro-Rp phosphate oxygen at the cleavage site. In an alternative model (Figure 5, model II), two metal ions (Mg[A] and Mg[B]) directly coordinate to the pro-Rp oxygen, but Mg[A] instead of Mg[B] interacts with the OH− nucleophile via inner-sphere coordination (7,25). In both models, Mg[B] interacts with the 2′-OH function at nt −1 of the substrate via an inner-sphere water molecule.
Figure 5.
Transition state models for phosphodiester hydrolysis by E.coli M1 RNA. The single 2′-OH at nt −1 (in blue) was replaced with a 2′-deoxy, 2′-amino or 2′-fluoro group in the modified substrates analyzed in Figure 6. Putative, catalytically important Mg2+ ions are shown in magenta (Mg [A]) or red (Mg [B]); metal ion site [B] was in the focus of the present study. The first transition state model (model I) for hydrolysis of the scissile phosphodiester connecting nt +1 and −1 is derived from that proposed in (24), according to which the Mg2+ ion at the site termed [B] here directly coordinates to the pro-Rp phosphate oxygen and OH− nucleophile (in green), and simultaneously interacts with the 2′-OH at position −1 via an inner-sphere water molecule. According to model II, two Mg2+ ions, Mg[A] and Mg[B], directly coordinate to the pro-Rp oxygen (7,25), but Mg[A] instead of Mg[B] interacts with the OH− nucleophile via inner-sphere coordination. Additional metal ion interactions at the pro-Sp oxygen (marked Sp; models I and II) and the 3′-bridging oxygen (model I) are conceivable based on strong inhibition effects caused by sulfur substitutions at these positions (7,24,35). The ribose at nt +1 are drawn in the A-helical C3′-endo and the ribose at position −1 in the C2′-endo conformation based on the results of NMR investigations (36,37).
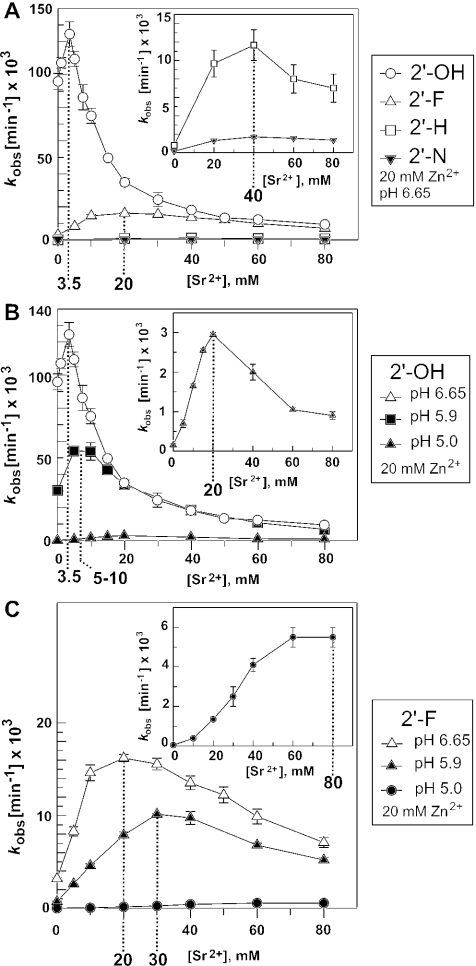
We addressed the possibility that Sr2+ may displace catalytically important Zn2+ at the aforementioned metal ion binding site [B], assuming that the binding sites for the two different metal ions overlap to such an extent that their binding is mutually exclusive. We have recently shown that 2′-substitutions at nt −1 of ptRNA decreased cleavage efficiency by M1 RNA in the order 2′-H ≤ 2′-N < 2′-F < 2′-OH under conditions of rate-limiting chemistry (20). Assuming that Sr2+ indeed displaces a Zn2+ ion that is bound to site [B] involving the 2′-OH at nt −1 of ptRNA (Figure 5), one would expect that these 2′-modifications change the affinities of Zn2+ and Sr2+ (which largely differ in their electronic properties and the details of their coordination spheres; see Discussion) to different extents. In contrast, the competition profile should be much less affected if Sr2+ displaces catalytic Zn2+ at a site other than [B], where metal ion coordination is not directly dependent on the 2′-OH at nt −1. In fact, the concentration of Sr2+ at the transition point between the stimulatory and the inhibitory phases of the curve was substantially shifted toward higher Sr2+ concentrations (20–40 mM versus 3.5 mM, Figure 6A) when the 2′-OH at nt −1 was replaced with a 2′-F, 2′-N or 2′-H substituent. We then tested if this shift in the inflection point of the curve is a specific feature associated with the 2′-ribose modifications at nt −1 rather than a general effect, for example, related to a reduction in the rate of the chemical step (kchem). We, therefore, analyzed the Sr2+ dependence of cleavage rate for the all-ribose substrate at two lower pH values to reduce the rate of kchem. The inflection point of the curve indeed increased with decreasing pH (Figure 6B), which also holds for cleavage of the 2′-F-ptRNAmeasured at the same three pH values (Figure 6C). These findings suggested that it may be difficult to extract metal ion-specific information based on changes of the inflection point between the stimulatory and the inhibitory phases.
Figure 6.
Processing by M1 RNA of the all-ribose ptRNAGly (2′-OH) and variants thereof with a single 2′-fluoro (2′-F), 2′-deoxy (2′-H) or 2′-amino (2′-N) modification at nt −1. (A) Processing rates in the presence of 20 mM Zn2+ and varying concentrations of Sr2+ at pH 6.65. Inflection points between stimulatory and inhibitory phases of curves are marked by dashed lines. The curve for the all-ribose ptRNAGly is identical to that shown in Figure 2C. The inset shows the curves for the 2′-H- and 2′-N-modified substrates at higher resolution. (B) Processing rates for all-ribose ptRNAGly at three different pH values. (C) As in (B), but using the 2′-F-modified ptRNAGly. For further details, see Materials and Methods.
Our affinity measurements (Figure 3A and Table 1) showed that an enzyme concentration of 5 μM was subsaturating at 20 mM Zn2+ and ≪5 mM Sr2+. Since we attributed the stimulatory effect of Sr2+ at low concentrations to its stabilization of E·S complexes, we suspected that changes in [E] may also affect the inflection point between the stimulatory and the inhibitory phases. Indeed, the Sr2+-dependence of processing rate displayed changes in the inflection point between the two phases for the all-ribose as well as the 2′-F-ptRNA when monitored at enzyme concentrations of 5 versus 1.4 μM (Figure 7A and B). We then replotted the data of Figure 7A and B as v0/vi (v0 and vi correspond to kobs in the absence and the presence of Sr2+, respectively; Figure 7C and D). The best fit of the data was obtained utilizing Equation 8 (see Materials and Methods) based on a model outlined in Figure 8A, which involves two Sr2+ ions (or two classes of Sr2+ ions); both ions improve substrate affinity in a cooperative manner, but one of the two inhibits substrate conversion in a non-competitive mode with respect to the substrate. The two Sr2+ ions may well be those suggested by the Hill coefficient of the binding data in Figure 3B. The fact that both Sr2+ ions contribute to the formation of high-affinity E·S complexes is accounted for by introducing the interaction factors α and β in the scheme of Figure 8A. Other models, for example, assuming the involvement of two ‘activating’ and one inhibitory Sr2+ ion or predicting that E·S·I and E·S·I(a)·I complexes retain residual reactivity, failed to give satisfactory curve fits of the data.
Mode of inhibition by Sr2+
We finally investigated the inhibition mode of Sr2+ with respect to the Zn2+ by varying the Sr2+ concentration between 10 and 40 mM at four different Zn2+ concentrations. Under these conditions, the enzyme concentration (5 μM) was assumed to be saturating at all variations of Sr2+ and Zn2+, even at the combination of 10 mM Sr2+ and 20 mM Zn2+, where a Kd of ∼250 nM was determined (Figure 3A). Thus, cleavage chemistry was expected to limit the rate of substrate turnover. The Dixon plot of the data (Figure 8B) gave straight lines that intersect on the [I] axis, which is a specific feature of non-competitive inhibition. The point of intersection yields a KI value of ∼5 mM. The result argues against a direct displacement of catalytic Zn2+ by a Sr2+ ion at the aforementioned metal ion site [B] (Figure 5).
DISCUSSION
Catalysis in the presence of Zn2+
M1 RNA-catalyzed processing in the presence of Zn2+ as the only divalent metal ion present in the cleavage assay (termed Zn2+ alone conditions in the following) is in contrast to the previous studies (11,15). This discrepancy can be explained by the fact that our study was performed under conditions of E≫S and in the presence of high concentrations of NH4OAc. With potassium or sodium instead of ammonium salts, we were unable to detect M1 RNA-catalyzed cleavage under Zn2+-alone conditions (Figure 2A). One possibility is that Zn2+ ions partly replace water ligands with ammonia (26) as a requirement to be able to sustain catalysis by M1 RNA. Although proficient in catalysis, Zn2+ is unable to support thermodynamically stable E·S complex formation (Table 1 and Figure 3A). Yet selection of the canonical cleavage site (between nt −1 and +1) was not changed in the presence of Zn2+ alone (relative to Mg2+ alone; data not shown), despite the very low substrate affinity under Zn2+ alone conditions. This shows that low affinity substrate ground state binding not necessarily favors aberrant cleavage (between nt −2 and −1) relative to cleavage at the canonical site. One interpretation is that the high activation barrier difference for aberrant cleavage (at −2/−1) relative to canonical cleavage (−1/+1) is maintained under these conditions. In conclusion, RNase P RNA catalysis with Zn2+ as the metal cofactor represents a ribozyme case where the specific transition state is achieved despite a dramatic destabilization of the substrate ground state binding.
We had speculated that the inability of Zn2+ to mediate high-affinity substrate binding might enable us to dissect the metal ions that mediate substrate ground state binding from those specifically involved in transition state stabilization in order to define the subset of catalytic metal ions. This, however, turned out to be difficult because saturating enzyme concentrations could not be reached in the absence of a second non-catalytic metal ion, such as Sr2+, but Sr2+ in turn [as ] inhibited catalysis. This resulted in complex velocity versus [Sr2+] curves with ascending and descending sections (Figure 2C, 6, 7A and B). Secondary plots of these data gave reasonable fits to a model involving two Sr2+ ions (or two classes of Sr2+ ions). Both Sr2+ ions support substrate binding in a cooperative manner, but one of the two inhibits substrate conversion. In a non-competitive mode with respect to the substrate. As a result, Sr2+ stimulated processing at low concentrations by shifting the E·S equilibrium toward complex formation, whereas the inhibitory effect dominated at higher concentrations. Curves of kobs versus [Sr2+] turned out to be highly sensitive to changes in [E] or kchem (Figures 6 and 7). By analyzing Sr2+ inhibition of M1 RNA cleavage with Zn2+ as catalytic cofactor in the context of different substrates with 2′-ribose modifications, we had hoped to extract specific information regarding the catalytic metal ion binding to site [B] (Figure 5). Indeed, the βKI values for Sr2+ inhibition derived from the secondary plots in Figure 7C and D are ∼15-fold higher for the 2′-F versus all-ribose ptRNA substrate, while αKI(a) values are equal within a factor of 2. This finding is consistent with the binding of an Sr2+ ion at or near metal ion site [B] with the help of the 2′-OH at nt −1. However, inhibition kinetics (Figure 8B) with the all-ribose substrate favored a model of non-competitive inhibition of Sr2+ with respect to Zn2+, thus arguing against a mechanism in which the inhibitory Sr2+ ion directly displaces a catalytic Zn2+ at metal ion site [B]. It, therefore, remains to be defined how Sr2+ inhibits the catalytic process with Zn2+ as catalytic cofactor.
Our kinetic analyses (Figure 4) have revealed an involvement of two or more Zn2+ ions, or classes of Zn2+ binding sites, in M1 RNA catalysis. This result is similar to those obtained with Mg2+ as the metal ion cofactor (6). These authors concluded that at least three Mg2+ ions take part in the catalytic step.
Zn2+ has also been explored as a catalytic cofactor in the reaction catalyzed by the B.subtilis holoenzyme (9) with an RNA subunit of the structural type B. Single turnover activity (with 1 μM holoenzyme and 100 mM KCl, pH 8.0) was very low in the presence of 10 mM Zn2+ (2.4 × 10−3 min−1). However, the addition of 2 mM in combination with only 0.2 mM Zn2+ resulted in a processing rate of ∼11 min−1, which was only ∼5-fold lower than the cleavage rate under saturating Mg2+ conditions, with Zn2+ activating cleavage at lower concentrations than Mg2+ (9). The latter is attributable to the fact that Zn2+ (as Mn2+) is a better Lewis acid than Mg2+ [pKa for the formation of Me[H2O]5[OH]+ of ∼9.0 versus 11.4 for Mg2+; (27)]. These results combined with ours obtained for the E.coli system document that Zn2+ is a proficient cofactor of bacterial RNase P (RNA) catalysis.
Comparison with results from the previous studies of M1 RNA
The global conformation of M1 RNA has previously been probed by lead ion-induced hydrolysis in the absence of substrate (17). Cd2+ substantially altered the lead hydrolysis pattern of M1 RNA relative to Mg2+, while changes were more moderate in the presence of Zn2+, which suggested substantial changes of M1 conformation induced by Cd2+ but to a lower extent by Zn2+ (17). These findings are entirely different from the relative effects of Zn2+ and Cd2+ on the substrate affinity observed in the work presented here, indicating that Zn2+ is much more detrimental to E·S formation than Cd2+ at the same concentrations (Table 1). Yet, despite the better performance of Cd2+ in E·S formation, Zn2+ supports cleavage of the all-ribose ptRNA at a 16-fold higher rate than Cd2+ (data not shown) under our standard conditions (Figure 2A, in the presence of 1 M NH4OAc and 20 mM Me2+). This may be related to the fact that the formation of an Me[H2O]5[OH]+ species required for RNase P catalysis (see below) is favored with Zn2+ over Cd2+ [pKa of ∼9.0 for Zn2+ versus >10 for Cd2+; (27)].
The lead-induced hydrolysis patterns of M1 RNA also suggested that the M1 RNA conformation is rather similar in the presence of Mg2+, Mn2+, Ca2+, Sr2+, Ba2+ and (17). However, we found that at higher concentrations substantially reduced the fraction of ptRNA substrates capable of binding to saturating concentrations of M1 RNA, which was also observed to some extent for Zn2+ (Table 1 and Figure 3A). Likewise, Zn2+ and are expected to affect the proportion of catalytically competent M1 RNA, which will be of particular importance when cleavage assays are performed in the presence of limited amounts of ribozyme (E ≪ S). In conclusion, future studies will have to incorporate the differential effects that metal ions (or metal ion mimics) other than Mg2+ have on structural equilibria of ribozyme and substrate RNAs as well as E·S complex formation in addition to the catalytic performance.
Failure of Sr2+ to support catalysis
Little is known on the binding of Sr2+ ions to RNA, but a coordination geometry different from the canonical octahedral Mg2+ geometry may be the cause for the failure of Sr2+ to activate catalysis by RNase P RNA under standard conditions and its inhibitory mode in the reaction with Zn2+ as the metal cofactor. Indeed, a coordination geometry resembling a slightly distorted trigonal prism and involving nine oxygen atoms (four ribose hydroxyl groups and five waters) were observed for a Sr2+ ion in the crystal structure of the tRNAAla acceptor stem (28). Taking into account that four hydroxyl groups were inner-sphere ligands of this Sr2+ ion, whereas inner-sphere coordination of 2′-OH ligands to Mg2+ seems to be rare (29), it is an intriguing possibility that Sr2+ fails to support M1 RNA catalysis owing to inner-sphere coordination to the 2′-OH at nt −1 of the substrate. A role for this substituent in Sr2+ binding is indeed indicated by a weaker inhibitory effect of Sr2+ in the context of the ptRNA substrate with a 2′-F modification at nt −1 (Figure 7, see Discussion).
Further information on binding of Sr2+ to RNA stems from high-resolution structures of the leadzyme in the presence of Mg2+ versus Mg2+ plus Sr2+ (30). Three Mg2+ and three Sr2+ ions were identified, the Sr2+ ions occupying different sites on the RNA than the Mg2+ ions. All three Mg2+ ions contacted the RNA duplex via their canonical octahedral hexa-hydration sphere, while ligand spheres of the three Sr2+ ions varied in number and did not uniformly consist of inner-shell water molecules. One Sr2+ ion ([Sr]3,(30)) had three water molecules, three inner-sphere base or phosphate oxygen ligands, and was 3.8 Å from the oxygen of the 2′-OH at C23 that serves as the nucleophile in the leadzyme reaction after proton abstraction by catalytic Pb2+. Sr2+ coordination next to the 2′-OH of C23 offered an explanation why Sr2+ inhibits catalysis by Pb2+ (30). The same Sr2+ ion also caused modest but significant local changes in the immediate vicinity of the cleavage site, thereby favoring a ‘pre-catalytic’ over the ‘ground-state’ conformation of the leadzyme. Such local, Sr2+-induced changes in the active site of RNase P RNA–substrate complexes may well have contributed to the inhibition effects seen in the RNase P system.
Effects of Sr2+ and Zn2+ on substrate binding and structure
Kd measurements (Figure 3A and Table 1) were performed with trace amounts of 32P-labeled ptRNA and varying excess amounts of enzyme using a gel filtration spin column assay (8,23). Zn2+ increased the proportion of binding-deficient ptRNA molecules at saturating enzyme concentration (i.e. at the endpoint), a feature that is attributable to Zn2+ ions bound to ptRNA. Increasing Sr2+ concentrations at constant 20 mM Zn2+ largely reduced this binding-deficient ptRNA fraction, suggesting that Sr2+ can displace many of the deleterious Zn2+ ions from the substrate. The presence of 20 mM Zn2+ also caused a constant 2–3-fold increase in Kd over the entire range of tested Sr2+ concentrations (5–80 mM, Figure 3A). This indicates that Sr2+ is unable to displace Zn2+ (or to compensate its deleterious effects) at some sites where Zn2+ directly or indirectly impairs high-affinity substrate binding. Since Kd reflects structural properties of enzyme and substrate, the Zn2+ binding sites responsible for this Kd increase may be on the substrate and/or enzyme. To understand the structural effects of Zn2+ observed in the present study, it is instructive to inspect the Zn2+ binding sites detected in yeast tRNAPhe crystals (31). Five bound Zn2+ ions were identified, two of which, Zn(1) and Zn(2), replaced tightly bound Mg2+ ions in the U8–U12 region and in the D loop [corresponding to the Mg2+ binding sites 1 and 3 in Jovine et al. (32)], one [Zn(3)] overlapping with the weak Mg2+ binding site 7 in (32), and the remaining two [Zn(4,5)] being Zn2+-specific or transition metal ion-specific sites in base-paired regions. All five Zn2+ ions were coordinated tetrahedrally, and four of them were bound by direct coordination to a guanine N7 at positions where the G residue is flanked by a purine residue on its 5′ side. Zn(1) is shifted ∼2 Å relative to Mg2+ at this site. Based on these observations, Zn2+ may well cause specific changes of tRNA conformation or may occupy novel Zn2+-specific sites that disturb ptRNA interaction with M1 RNA. The preference of Zn2+ for purine–guanine dinucleotides also in paired regions implies that (i) some Zn2+ binding sites may directly perturb E·S contacts involving acceptor and T stems regions and (ii) that effects of Zn2+ will be to some extent sequence-specific and thus specific for every individual RNA under investigation.
Effects of on M1 RNA-catalyzed cleavage
The addition of 5 mM to 20 mM Zn2+ stimulated ptRNA turnover ∼2-fold, but higher concentrations were inhibitory as observed for Sr2+ (Figure 2C and D). Recently, inhibition of the B.subtilis RNase P holoenzyme by with Mg2+ as the catalytic cofactor (9) was discussed to indicate that displaces a metal ion for which the ionization or the displacement of a water molecule from the metal hydration shell is required. Here, it is instructive to compare the properties of Sr2+ and , which showed similar inhibition effects (Figure 2C and D). Since water ligands can dissociate from the hydration shell of Sr2+ (28) but ammine ligands do not dissociate from the inert octahedral complex cation [] (33), the remaining common feature of the two is the low degree of ionization of water ligands at pH ≤ 7 [pKa of Sr2+ aqua ion = 13.2; (27)] in the case of Sr2+ and the complete absence of ionizable water ligands in the case of . This would be consistent with the involvement of a Me[H2O]5[OH]+ species in the catalytic process (9,15,24).
Effects of on substrate structure and binding
Soaking of yeast tRNAGly crystals with Co(NH3)6Cl3 identified three [Co(NH3)6]3+ complexes which, however, did not replace strongly bound Mg2+ ions (34). Two bound to double-helical guanylguanosine sequences (G3/G4 [Co(2)] and G42/G43 [Co(1)]) and the third [Co(3)] to the purine–purine sequence A44/G45, in all cases in the major groove via hydrogen bonding of cis-ammine ligands to the N7 and O6 functions of adjacent purine bases. Additional hydrogen bonding occurred to O4 of U residues and to phosphate oxygens, but no direct metal–nucleotide bonds were observed (34). Interestingly, the binding site for Zn(5) [see above; (31)] overlapped with the site for Co(1), both contacting the N7 and O6 functions of G42 and G43. However, coordination of the tetrahedral Zn(5) involved an innersphere contact to the N7 of G43, and the octahedral Co(1) formed two additional contacts to non-bridging phosphate oxygens at positions 24 and 42 (34).
We observed that only about one-fourth of the ptRNA molecules were capable of binding to M1 RNA at saturating enzyme concentrations in the presence of 80 mM compared with the conditions of 80 mM Sr2+ (Table 1). One explanation may be related to the preference of for GG dinucleotides in paired regions (see above) and the multiple presence of such potential binding sites in our ptRNAGly (Figure 1). Binding of to some sites in the acceptor stem and T arm may prevent crucial contacts to M1 RNA either directly or may perturb the tRNA tertiary fold.
Acknowledgments
We thank Dagmar K. Willkomm for critical reading of the manuscript, Sybille Siedler and Dominik Helmecke for excellent technical assistance, and Michael Weber for the design of Figure 5. Financial support for these studies from the Deutsche Forschungsgemeinschaft (HA 1672/7-3/7-4) is acknowledged. Funding to pay the Open Access publication charges for this article was provided by the Fonds der Chemischen Industrie.
Conflict of interest statement. None declared.
REFERENCES
- 1.Frank D.N., Pace N.R. Ribonuclease P: unity and diversity in a tRNA processing ribozyme. Annu. Rev. Biochem. 1998;67:153–180. doi: 10.1146/annurev.biochem.67.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Altman S., Kirsebom L.A. Ribonuclease P. In: Gesteland R.F., Cech T., Atkins J.F., editors. The RNA World, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. pp. 351–380. [Google Scholar]
- 3.Schön A. Ribonuclease P: the diversity of a ubiquitous RNA processing enzyme. FEMS Microbiol. Rev. 1999;23:391–406. doi: 10.1111/j.1574-6976.1999.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 4.Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 5.Brown J.W. The ribonuclease P database. Nucleic Acids Res. 1998;26:351–352. doi: 10.1093/nar/26.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith D., Pace N.R. Multiple magnesium ions in the ribonuclease P reaction mechanism. Biochemistry. 1993;32:5273–5281. doi: 10.1021/bi00071a001. [DOI] [PubMed] [Google Scholar]
- 7.Warnecke J.M., Fürste J.P., Hardt W.-D., Erdmann V.A., Hartmann R.K. Ribonuclease P (RNase P) RNA is converted to a Cd2+-ribozyme by a single Rp-phosphorothioate modification in the precursor tRNA at the RNase P cleavage site. Proc. Natl Acad. Sci. USA. 1996;93:8924–8928. doi: 10.1073/pnas.93.17.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warnecke J.M., Held R., Busch S., Hartmann R.K. Role of metal ions in the hydrolysis reaction catalyzed by RNase P RNA from Bacillus subtilis. J. Mol. Biol. 1999;290:433–445. doi: 10.1006/jmbi.1999.2890. [DOI] [PubMed] [Google Scholar]
- 9.Kurz J.C., Fierke C.A. The affinity of magnesium binding sites in the Bacillus subtilis RNase P × pre-tRNA complex is enhanced by the protein subunit. Biochemistry. 2002;41:9545–9558. doi: 10.1021/bi025553w. [DOI] [PubMed] [Google Scholar]
- 10.Kufel J., Kirsebom L.A. The P15-loop of Escherichia coli RNase P RNA is an autonomous divalent metal ion binding domain. RNA. 1998;4:777–788. doi: 10.1017/s1355838298970923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brännvall M., Kirsebom L.A. Metal ion cooperativity in ribozyme cleavage of RNA. Proc. Natl Acad. Sci. USA. 2001;98:12943–12947. doi: 10.1073/pnas.221456598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brännvall M., Pettersson F., Kirsebom L.A. The residue immediately upstream of the RNase P cleavage site is a positive determinant. Biochimie. 2002;84:693–703. doi: 10.1016/s0300-9084(02)01462-1. [DOI] [PubMed] [Google Scholar]
- 13.Christian E.L., Kaye N.M., Harris M.E. Evidence for a polynuclear metal ion binding site in the catalytic domain of ribonuclease P RNA. EMBO J. 2002;21:2253–2262. doi: 10.1093/emboj/21.9.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith D., Burgin A.B., Haas E.S., Pace N.R. Influence of metal ions on the ribonuclease P reaction. Distinguishing substrate binding from catalysis. J. Biol. Chem. 1992;267:2429–2436. [PubMed] [Google Scholar]
- 15.Guerrier-Takada C., Haydock K., Allen L., Altman S. Metal ion requirements and other aspects of the reaction catalyzed by M1 RNA, the RNA subunit of ribonuclease P from Escherichia coli. Biochemistry. 1986;25:1509–1515. doi: 10.1021/bi00355a006. [DOI] [PubMed] [Google Scholar]
- 16.Kazakov S., Altman S. Site-specific cleavage by metal ion cofactors and inhibitors of M1 RNA, the catalytic subunit of RNase P from Escherichia coli. Proc. Natl Acad. Sci. USA. 1991;88:9193–9197. doi: 10.1073/pnas.88.20.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brännvall M., Mikkelsen N.E., Kirsebom L.A. Monitoring the structure of Escherichia coli RNase P RNA in the presence of various divalent metal ions. Nucleic Acids Res. 2001;29:1426–1432. doi: 10.1093/nar/29.7.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perreault J.P., Altman S. Important 2′-hydroxyl groups in model substrates for M1 RNA, the catalytic RNA subunit of RNase P from Escherichia coli. J. Mol. Biol. 1992;226:399–409. doi: 10.1016/0022-2836(92)90955-j. [DOI] [PubMed] [Google Scholar]
- 19.Perreault J.P., Altman S. Pathway of activation by magnesium ions of substrates for the catalytic subunit of RNase P from Escherichia coli. J. Mol. Biol. 1993;230:750–756. doi: 10.1006/jmbi.1993.1197. [DOI] [PubMed] [Google Scholar]
- 20.Persson T., Cuzic S., Hartmann R.K. Catalysis by RNase P RNA: unique features and unprecedented active site plasticity. J. Biol. Chem. 2003;278:43394–43401. doi: 10.1074/jbc.M305939200. [DOI] [PubMed] [Google Scholar]
- 21.Zuleeg T., Hartmann R.K., Kreutzer R., Limmer S. NMR spectroscopic evidence for Mn2+(Mg2+) binding to a precursor-tRNA microhelix near the potential RNase P cleavage site. J. Mol. Biol. 2001;305:181–189. doi: 10.1006/jmbi.2000.4299. [DOI] [PubMed] [Google Scholar]
- 22.Busch S., Kirsebom L.A., Notbohm H., Hartmann R.K. Differential role of the intermolecular base-pairs G292-C75 and G293-C74 in the reaction catalyzed by Escherichia coli RNase P RNA. J. Mol. Biol. 2000;299:941–951. doi: 10.1006/jmbi.2000.3789. [DOI] [PubMed] [Google Scholar]
- 23.Beebe J.A., Fierke C.A. A kinetic mechanism for cleavage of precursor tRNAAsp catalyzed by the RNA component of Bacillus subtilis ribonuclease P. Biochemistry. 1994;33:10294–10304. doi: 10.1021/bi00200a009. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y., Li X., Gegenheimer P. Ribonuclease P catalysis requires Mg2+ coordinated to the pro-RP oxygen of the scissile bond. Biochemistry. 1997;36:2425–2438. doi: 10.1021/bi9620464. [DOI] [PubMed] [Google Scholar]
- 25.Kuimelis R.G., McLaughlin L.W. Mechanisms of ribozyme-mediated RNA cleavage. Chem. Rev. 1998;98:1027–1044. doi: 10.1021/cr960426p. [DOI] [PubMed] [Google Scholar]
- 26.Cotton F.A., Wilkinson G. Advanced Inorganic Chemistry, 5th edn. NY: John Wiley & Sons; 1988. Chichester, Brisbane, Toronto, Singapore. [Google Scholar]
- 27.Feig A.L., Uhlenbeck O.C. The role of metal ions in RNA biochemistry. In: Gesteland R.F., Cech T., Atkins J.F., editors. The RNA World, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. pp. 287–319. John Wiley & Sons, NY, Chichester, Brisbane, Toronto, Singapore. [Google Scholar]
- 28.Mueller U., Schübel H., Sprinzl M., Heinemann U. Crystal structure of acceptor stem of tRNAAla from Escherichia coli shows unique G·U wobble base pair at 1.16 Å resolution. RNA. 1999;5:670–677. doi: 10.1017/s1355838299982304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juneau K., Podell E., Harrington D.J., Cech T. Structural basis of the enhanced stability of a mutant ribozyme domain and a detailed view of RNA–solvent interactions. Structure. 2001;9:221–231. doi: 10.1016/s0969-2126(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 30.Wedekind J.E., McKay D.B. Crystal structure of the leadzyme at 1.8 Å resolution: metal ion binding and the implications for catalytic mechanism and allo site ion regulation. Biochemistry. 2003;42:9554–9563. doi: 10.1021/bi0300783. [DOI] [PubMed] [Google Scholar]
- 31.Rubin J.R., Wang J., Sundaralingam M. X-ray diffraction study of the zinc(II) binding sites in yeast phenylalanine transfer RNA. Preferential binding of zinc to guanines in purine–purine sequences. Biochim. Biophys. Acta. 1983;756:111–118. doi: 10.1016/0304-4165(83)90030-2. [DOI] [PubMed] [Google Scholar]
- 32.Jovine L., Djordjevic S., Rhodes D. The crystal structure of yeast phenylalanine tRNA at 2.0 Å resolution: cleavage by Mg2+ in 15-year old crystals. J. Mol. Biol. 2000;301:401–414. doi: 10.1006/jmbi.2000.3950. [DOI] [PubMed] [Google Scholar]
- 33.Cowan J.A. Metallobiochemistry of RNA. as a probe for Mg2 (aq) binding sites. J. Inorg. Biochem. 1993;49:171–175. doi: 10.1016/0162-0134(93)80002-q. [DOI] [PubMed] [Google Scholar]
- 34.Hingerty B.E., Brown R.S., Klug A. Stabilization of the tertiary structure of yeast phenylalanine tRNA by [Co(NH3)6]3+. X-ray evidence for hydrogen bonding to pairs of guanine bases in the major groove. Biochim. Biophys. Acta. 1982;697:78–82. doi: 10.1016/0167-4781(82)90047-1. [DOI] [PubMed] [Google Scholar]
- 35.Warnecke J.M., Sontheimer E.J., Piccirilli J.A., Hartmann R.K. Active site constraints in the hydrolysis reaction catalyzed by bacterial RNase P: analysis of precursor tRNAs with a single 3′-S-phosphorothiolate internucleotide linkage. Nucleic Acids Res. 2000;28:720–727. doi: 10.1093/nar/28.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuleeg T., Hansen A., Pfeiffer T., Schubel H., Kreutzer R., Hartmann R.K., Limmer S. Correlation between processing efficiency for ribonuclease P minimal substrates and conformation of the nucleotide −1 at the cleavage position. Biochemistry. 2001;40:3363–3369. doi: 10.1021/bi0016974. [DOI] [PubMed] [Google Scholar]
- 37.Zuleeg T., Hartmann R.K., Kreutzer R., Limmer S. NMR spectroscopic evidence for Mn2+ (Mg2+) binding to a precursor-tRNA microhelix near the potential RNase P cleavage site. J. Mol. Biol. 2001;305:181–189. doi: 10.1006/jmbi.2000.4299. [DOI] [PubMed] [Google Scholar]