Abstract
Although clinical use of the auditory brainstem response (ABR) to detect retrocochlear disorders has been largely replaced by imaging in recent years, the discovery of cochlear synaptopathy has thrown this foundational measure of auditory function back into the spotlight. While modern imaging now allows for non-invasive detection of vestibular schwannomas, imaging technology is not currently capable of detecting cochlear synaptopathy, the loss of the synaptic connections between inner hair cells and afferent auditory nerve fibers. However, animal models indicate that the amplitude of the first wave of the ABR, a far-field evoked potential generated by the synchronous firing of auditory nerve fibers, is highly correlated with synaptic integrity. This has led to many studies investigating the use of the ABR as a metric of synaptopathy in humans. However, these studies have yielded mixed results, leading to a lack of consensus about the utility of the ABR as an indicator of synaptopathy. This review summarizes the animal and human studies that have investigated the ABR as a measure of cochlear synaptic function, discusses factors that may have contributed to the mixed findings and the lessons learned, and provides recommendations for future use of this metric in the research and clinical setting.
Keywords: Auditory brainstem response, brainstem auditory evoked potential, cochlear synaptopathy, hidden hearing loss
I. INTRODUCTION
The discovery of cochlear synaptopathy in mice (Kujawa & Liberman 2009), the loss of the synapses between the inner hair cells (IHCs) and their afferent auditory nerve fiber targets, has motivated a search for non-invasive physiological measurements that can detect this condition in humans. Synaptopathy is of particular interest in the field because it has been suggested as a possible explanation for the paradoxical situation of auditory complaints that accompany a normal audiogram, such as tinnitus, hyperacusis, and difficulty with speech perception in noise (Kujawa & Liberman 2015). The finding that ABR wave 1 amplitude is correlated with cochlear synapse numbers in animal models (e.g., Kujawa & Liberman 2009; Sergeyenko et al. 2013) has led to renewed interest in this classic audiological test, particularly in ABR wave I amplitude, which was previously overlooked due to the large degree of variability associated with the measurement. Note that Arabic numerals (i.e., 1, 2, 3, 4, 5) are typically used when referring to ABR waves in animals, while Roman numerals (i.e., I, II, III, IV, V) are used when referring to ABR waves in humans. While several factors, such as sex, skull thickness, and tissue conductivity can impact ABR wave I amplitude (Trune et al. 1988; Irimia et al. 2013), much of the variability in wave I amplitude may result from differences in cochlear synaptic integrity. Although a large number of human studies have been conducted to investigate relationships between ABR measurements and risk factors or predicted perceptual consequences of synaptopathy, differences in study design, methodology, and statistical approach have resulted in mixed findings across studies. This has led to confusion about the feasibility of using the ABR as an indicator of synaptopathy. This review will provide an overview of the current state of knowledge concerning the use of the ABR to assess cochlear synaptic integrity in humans, including discussion of ABR measurement parameters, results from previous studies, limitations, and future directions.
II. OVERVIEW OF THE ABR WAVEFORM
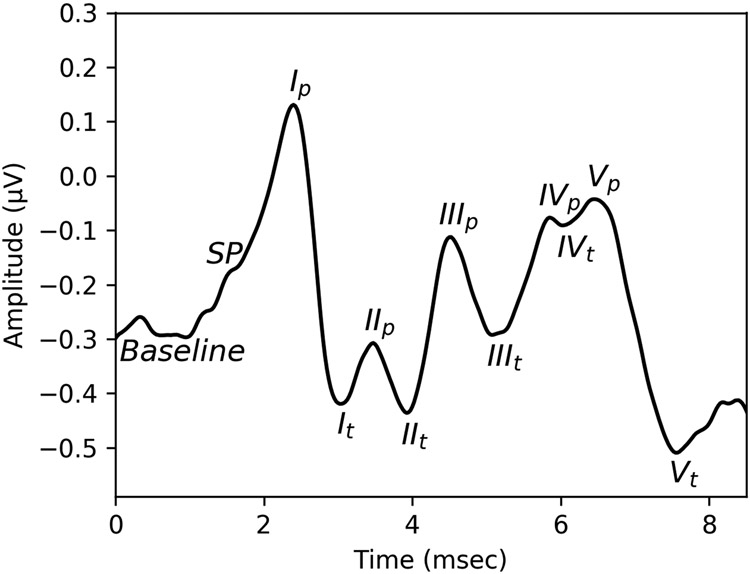
The human ABR waveform consists of five main peaks (or waves), illustrated in Figure 1. Intracranial recordings in humans suggest that waves I and II are generated by the distal and proximal ends of the auditory nerve, wave III by the cochlear nucleus, wave IV by the superior olivary complex, and wave V by the lateral lemniscus and inferior colliculus (Moller & Jannetta 1982). As stimulus intensity level increases, the amplitudes of these peaks increase and the latency decreases due to recruitment of higher frequency auditory nerve fibers and increased neural synchrony (Harris et al. 2018). Normative values exist for the latencies and amplitudes of the different peaks in response to suprathreshold clicks, collected from young adults with normal pure tone thresholds (e.g., Rosenhamer et al. 1978; Rowe 1978; Mochizuki et al. 1982), but these values have not been collected specifically from a population at low risk of synaptopathy (e.g., young people with minimal noise exposure history). Depending on the recording parameters, an additional peak is often visible sitting on the left-hand shoulder of wave I (see Figure 1). This is referred to as the summating potential (SP) and is thought to include contributions from the IHC and outer hair cell (OHC) receptor potentials as well as the auditory nerve (Pappa et al. 2019). The amplitude of wave I can be measured as the amplitude difference from the baseline to the wave I peak or from the wave I peak to the following trough. The trough is often easier to objectively identify than the baseline and the peak to trough measurement is the more common approach in human synaptopathy studies. Note however, that the trough appears to be impacted by contributions from the cochlear nucleus (Melcher et al. 1996) and the wave I peak can be contaminated by the SP. Therefore, regardless of how it is measured, wave I amplitude is not an entirely pure indicator of auditory nerve activity. Normative values are available for the ratio comparing the amplitudes of the SP and the action potential (AP, the wave I peak), defined relative to a prestimulus baseline (e.g., Margolis et al. 1995; Wuyts et al. 1997).
Figure 1. Example human ABR waveform.
ABR peaks I-V are labelled on an ABR waveform recorded in a human participant using a tiptrode. The subscript p indicates the wave peak and the subscript t indicates the following trough. SP indicates the summating potential.
III. HISTORICAL USE OF THE ABR IN THE AUDIOLOGY CLINIC
Use of the ABR as an indicator of peripheral auditory neuronal function is not new. In fact, the ABR has historically been used in audiology clinics as a non-invasive means for diagnosing retrocochlear disorders such as vestibular schwannomas, tumors that grow on the vestibular nerve and can impact auditory and balance function (Eggermont 1984). However, as imaging technology has improved and become more cost effective, use of the ABR for this purpose has declined (Fortnum et al. 2009). Despite this change in clinical practice, ABR testing remains an important part of an audiologist’s training and most audiology clinics have an ABR system available because several clinical applications of the ABR remain. The ABR can be used to assess auditory function in populations, such as infants, that are difficult to test behaviorally and the SP/AP amplitude ratio can be used to evaluate patients with suspected Meniere’s disease or other forms of endolymphatic hydrops. Additionally, the ABR can be used for diagnosis of auditory neuropathy, a broad term describing cases where OHC function, as indicated by otoacoustic emissions, is normal, but the ABR is absent or abnormal. One common cause of auditory neuropathy is a mutation in the gene that produces the otoferlin protein, which is important for the release of neurotransmitter at the IHC-auditory nerve synapse (Yasunaga et al. 1999). Functionally, a lack of otoferlin is very similar to cochlear synaptopathy, resulting in reduced transmission at the IHC-auditory nerve synapse and reduced or absent ABR wave I amplitude with normal OHC function (Santarelli et al. 2009; Santarelli et al. 2015). Although wave I amplitude is not typically assessed clinically, audiologists’ existing familiarity with the ABR make it an ideal metric for clinical diagnosis of cochlear synaptopathy.
IV. ABR WAVE 1 AMPLITUDE IN ANIMAL MODELS OF SYNAPTOPATHY
Cochlear synaptopathy was initially identified in mice following a two hour exposure to a 100 dB SPL 8-16 kHz band of noise (Kujawa & Liberman 2009). After noise exposure, the amplitude of the first wave of the ABR (wave 1) was permanently reduced in mice with histologically confirmed synapse loss compared with control mice for suprathreshold tone pips at frequencies above the noise exposure band. In contrast, there was no long-term change in ABR threshold and OHCs were preserved. Later studies revealed similar findings of noise-induced synapse loss in multiple other rodent species (see review by Hickox et al. 2017 for more details) and in non-human primates (Valero et al. 2017), many of them also showing associated reductions in ABR wave 1 amplitude. Animal studies also indicate that synaptopathy is associated with age (Sergeyenko et al. 2013) and can occur in response to some ototoxic drugs (Ruan et al. 2014).
Auditory nerve fibers with low spontaneous rates (SRs) appear to be the most vulnerable to synaptopathy, whereas high SR fibers are generally preserved (Schmiedt et al. 1996; Furman et al. 2013). Low SR fibers have higher response thresholds, larger dynamic ranges, and are more resistant to background noise than high SR fibers (reviewed in Bharadwaj et al. 2014). Preservation of high SR fibers, which have low response thresholds, provides an explanation for why ABR thresholds are not impacted by synaptopathy.
V. OVERVIEW OF THE USE OF THE ABR IN HUMAN STUDIES OF SYNAPTOPATHY
A. Study design
Participant selection is an important source of variability in human studies of synaptopathy. Many previous synaptopathy studies were observational studies that compared ABR measurements from an experimental group with measurements from a control group. Participants in the experimental group were selected based on synaptopathy risk factors (noise exposure history or age) or predicted consequences of synaptopathy (tinnitus or speech perception difficulty). However, the criteria for participant selection varied across studies. For instance, in some noise exposure studies, the experimental group was composed of individuals who reported significant recreational noise exposure (e.g., Grose et al. 2017; Prendergast et al. 2018; Skoe & Tufts 2018), while other studies focused on music students (Liberman et al. 2016) or military Veterans (Bramhall et al. 2017). Given the differences in the intensity of the noise exposures experienced by these different populations and the crude nature of evaluating noise exposure history using self-report questionnaires, it may not be realistic to expect similar results across studies. Equally important is the participant selection for the control group, which should be composed of individuals at low risk of synaptopathy based on their noise exposure history and age. Ideally, anyone who has ever used a firearm should be excluded from the control group because a history of firearm use is associated with reduced ABR wave I amplitudes (Bramhall et al. 2017). Additionally, individuals reporting any auditory complaints such as tinnitus, hyperacusis, or speech perception difficulty should not be included in a control group as these complaints suggest the presence of auditory damage and potentially synaptopathy.
It is also important to remember that humans will likely exhibit much more variability in their genetic susceptibility to synaptopathy than congenic mice. This will make it more difficult to detect associations between ABR measures and synaptopathy risk factors such as noise exposure or age.
Finally, auditory complaints such as tinnitus and speech perception difficulty are likely multifactorial, with synaptopathy as only a single possible etiology. This makes it difficult to detect associations between these complaints and the ABR unless a subpopulation of individuals with these complaints is chosen that is at particularly high risk for synaptopathy, such as firearm users who report tinnitus.
B. ABR measures
A number of different ABR measures have been used in human studies of synaptopathy. This is due in part to differing approaches to accounting for discrepancies in ABRs measured in animals versus humans. For instance, in animals, the ABR is typically measured with needle electrodes while the animal is anesthetized. Additionally, the animals have considerably smaller heads than humans. In contrast, in humans, the ABR is usually measured using surface electrodes without anesthesia and the electrodes are further away from the source generators due to the larger head size. This can lead to greater degrees of variability across human subjects due to differences in anatomy, head size, and muscle artifact. In mice, ABR wave 1 amplitudes can easily be identified across a broad range of frequencies and intensity levels, whereas in humans, where the noise floor is higher and the electrodes are further from the source, it can be difficult to reliably identify ABR wave I amplitude in all subjects at levels of 80-90 dB peSPL, even if pure tone thresholds are clinically normal. In addition, the length of the cochlea differs between mice and humans (Greenwood 1961) and between males and females (Don et al. 1994), resulting in differences in cochlear traveling wave delay that will impact the synchrony of the response across auditory nerve fibers and the amplitude of wave I.
One approach to dealing with these sources of variability is to use a differential measure of ABR wave I amplitude, where a ratio is calculated between ABR wave I amplitude and another ABR peak, such as the SP or wave V amplitude, or by calculating wave I amplitude growth across multiple stimulus levels. The rationale is that by calculating ratios or growth functions, subject-specific factors that impact all peaks will cancel out, reducing measurement variability. Test re-test reliability of ABR wave I amplitude (measured peak to following trough) and the wave I/V ratio appears to be quite good, while the reliability of the SP/AP ratio and wave I amplitude growth functions is poorer (Prendergast et al. 2018; Guest et al. 2019b). However, Kamerer et al. (2020) showed that a summed-Gaussian model performs better at identifying the SP than visual determination, particularly for noisy waveforms and cases where the SP and wave I were overlapping in time. This suggests that the reliability of the SP/AP ratio might be improved by using a model-based peak picking approach. The majority of human synaptopathy studies have focused on ABR wave I amplitude and have measured this as the difference in voltage between the peak of wave I and the following trough (Le Prell 2019), although a number of studies have also presented wave I/V amplitude ratios, particularly those investigating the relationship between synaptopathy and tinnitus (e.g., Schaette & McAlpine 2011; Guest et al. 2017; Bramhall et al. 2018).
Other ABR measures that have been proposed for their ability to serve as a biomarker of synaptopathy include masked wave I amplitude and wave V latency. Giraudet et al. (2021) found that measuring ABR wave 1 amplitude in the presence of a 60 dB SPL broadband ipsilateral masker was better at separating mice with histologically confirmed noise-induced synaptopathy from controls than unmasked ABR wave 1 amplitude and suggested that use of the masker saturates the response of high SR fibers, allowing for improved specificity to loss of low SR fibers. Mehraei et al. (2016) measured the shift in wave 4 latency (analogous to wave V latency in humans) in mice when a 60 or 80 dB SPL broadband masker was added to the tone pip stimulus. They found that mice with histologically-confirmed noise-induced synaptopathy exhibited smaller latency changes upon adding the masker than control mice, particularly for the 60 dB SPL masker. They suggest that this smaller change in latency indicates a loss of low SR fibers because low SR fibers are more resistant to background noise and have a delayed onset response compared with high SR fibers.
C. ABR parameters
Differences in ABR parameters such as electrode placement, stimuli, repetition rate, and response filtering can also contribute to variability across studies.
(1). Electrode placement
Resolution of wave I and the SP increases with closer proximity of the active electrode to the cochlea, with the greatest resolution obtained with a transtympanic electrode and the poorest resolution with a mastoid electrode (see review by Margolis 1999 for a detailed comparison of the data obtained with different electrode placements). However, placement of a transtympanic electrode is an invasive procedure that requires anesthetizing the tympanic membrane and is typically performed by a surgeon. The resolution of wave I with a TM electrode is poorer than with a transtympanic electrode, but better than with an ear canal electrode (tiptrode) or a mastoid electrode. However, placement of a TM electrode requires more skill than placement of a tiptrode or mastoid electrode and may cause some patient discomfort. One early human synaptopathy study used a TM electrode (Stamper & Johnson 2015), but most human synaptopathy studies have used either a mastoid electrode or an ear canal electrode (tiptrode). While use of a tiptrode increases the size of ABR wave I amplitude compared to the use of a mastoid electrode, it only slightly increases the reliability of ABR wave I amplitude measurements (Prendergast et al. 2018). The SP, however, cannot be identified using a mastoid electrode. Therefore, the choice of the type of electrode may depend on the population being tested and the precise measurement being obtained. For example, a tiptrode may be preferred when testing older populations or populations with overt hearing loss where wave I amplitude may be more difficult to identify or when collecting SP measurements.
(2). ABR stimuli
Both broadband (e.g., a click) and frequency specific toneburst ABR stimuli have been used in human studies of synaptopathy. There is no clear difference in the results of studies that have used high intensity broadband versus frequency specific stimuli. Bramhall et al. plotted raw data from several studies that measured ABR wave I amplitude in a group with tinnitus (a predicted perceptual consequence of synaptopathy) and a control group without tinnitus (see Figure 3 from Bramhall et al. 2019). Across the plotted studies, broadband stimuli resulted in considerably larger contrasts in mean wave I amplitude between the tinnitus and control groups than toneburst stimuli. This could be interpreted as indicating that broadband stimuli are more sensitive to differences in synapse numbers between individuals with and without tinnitus. However, this comparison should be interpreted cautiously because the sample characteristics differed across studies and it’s possible that the broadband stimuli only perform well in populations likely to have widespread cochlear synaptic loss rather than focal lesions.
A broad range of stimulus levels have also been employed in human studies of synaptopathy. Animal studies suggest that higher intensity levels (e.g., 90 dB SPL) are more sensitive to synaptopathy than lower levels (e.g., Kujawa & Liberman 2009). In humans, the range of intensity levels over which wave I amplitudes can be reliably identified is more limited due to higher noise floors. For this reason, human studies have tended to use higher stimulus levels (up to 99 dB nHL) than the animal studies, particularly if one objective of the study was to measure the SP. However, raw data from multiple studies of the relationship between tinnitus and ABR wave I amplitude indicate that contrasts between the tinnitus and control groups tend to grow larger as stimulus level increases up to 100 dB peSPL, but then decline for higher stimulus levels (see Figure 3 from Bramhall et al. 2019). In addition, in a statistical model that adjusted for sex and average distortion product otoacoustic emission (DPOAE) level, Bramhall et al. (2021a) showed the biggest contrast in wave I amplitude for Veterans with high levels of noise exposure compared with non-Veteran controls at 90 dB peSPL, with progressively smaller contrasts as the stimulus level increased to 100 and 110 dB pe SPL. These results suggest that ABR stimulus levels of 90-100 dB peSPL may represent a “sweet spot” for detecting synaptopathy in humans. This sweet spot may be reflect a balance between greater sensitivity to synaptopathy at higher stimulus intensity levels and OHC dysfunction resulting in small increases in ABR wave I amplitude at very high stimulus levels (above 100 dB peSPL), as suggested by computational modeling from Verhulst et al. (2018). Given that OHC dysfunction is likely to be greater in the tinnitus and high noise exposure groups, this may result in inflated wave I amplitudes in these groups at very high stimulus levels, narrowing the contrasts with the control groups.
(3). Other parameters
Several additional parameters need to be considered when using the ABR to assess synaptopathy, including stimulus repetition rate, number of sweeps, stimulus polarity, and filtering of the response. For the early waves, the latency increases and the amplitude decreases at stimulus rates greater than ~30/sec and comparison of responses across low and high stimulus rates are sometimes used for detection of neural pathologies (Hood 1998). Human studies that have assessed relationships between ABR measurements and either noise exposure history or speech-in-noise perception have used stimulus rates ranging from 10-30/sec, with the majority using a rate from 10-20/sec (reviewed in Le Prell 2019). However, some studies (e.g., Konrad-Martin et al. 2012) have also included higher rates, up to 71/sec, for comparison with the lower rates.
Because background noise is expected to be random and should not be time-locked to the stimulus, averaging over a large number of sweeps will reduce background noise and improve the clarity of the ABR response. However, there is a trade-off in that collecting more sweeps requires greater test time. The mean amplitude of the background noise approaches zero as a function of the square root of the number of sweeps. Therefore, increasing the number of sweeps past ~1000 for suprathreshold stimuli and ~4000 for stimuli near threshold results in limited improvement in the signal-to-noise ratio (Hall 1992). Synaptopathy studies have typically obtained 1024-2048 sweeps, although sweep numbers vary from extremes of 500 to 12,500 (reviewed in Le Prell 2019). To reduce variability due to measurement error, two replicate waveforms are often obtained and peak amplitudes are then averaged across the replicates.
Stimulus polarity is an additional consideration for ABR measurement. Animal and human studies of synaptopathy typically use alternating polarity stimuli for measuring the amplitude of the first wave of the ABR. The advantage of using an alternating polarity stimulus is that it gets rid of the cochlear microphonic, which can bleed into wave I, making it harder to identify and contributing to variability in the measurement. Wave amplitudes and latencies will differ somewhat depending on the polarity used, so it is important to use a consistent polarity when making comparisons across measurements.
Finally, bandpass filtering of the ABR waveform helps remove background noise in the response. Typically a low frequency cut-off of 10-30 Hz and a high frequency cut-off of 1500 or 3000 Hz is used. The filter cut-offs can be adjusted to maximize resolution of the SP, wave I, and wave V as desired.
VI. LIMITATIONS OF ABR WAVE I AMPLITUDE AS AN INDICATOR OF SYNAPOTOPATHY
A. OHC dysfunction may impact ABR wave I amplitude
Given that the risk factors for synaptopathy (age and exposure to noise or ototoxins) are also risk factors for OHC damage, it is important to consider the possibility that ABR wave I amplitude may be impacted by OHC damage, even in individuals with clinically normal audiograms. This could complicate the interpretation of wave I amplitude as an indicator of synaptopathy. Don and Eggermont (1978) showed that more basal off-frequency auditory nerve fibers contribute to wave I amplitude at high stimulus levels. This suggests that basal OHC dysfunction could result in lower wave I amplitudes, making it difficult to separate OHC dysfunction from synaptopathy. Computational modeling suggests that the ABR wave I amplitude growth function is steepened by loss of cochlear gain, such that at lower stimulus levels, wave I amplitude is decreased by loss of cochlear gain, while at higher stimulus levels, it is increased (Verhulst et al. 2018). Model simulations by Verhulst et al. indicate that the cross-over point between the wave I amplitude growth function for normal cochlear gain versus sloping cochlear gain loss occurs at 90 dB peSPL for a click stimulus. This suggests that the impact of OHC dysfunction is minimal for a 90 dB peSPL stimulus, which is consistent with previous human studies suggesting that contrasts between groups with tinnitus or high noise exposure and controls are greatest for stimulus levels of 90-100 dB peSPL (Bramhall et al. 2019; Bramhall et al. 2021a).
Most previous human studies of human synaptopathy have investigated populations with clinically normal audiograms to limit the impacts of OHC dysfunction. However, subclinical OHC dysfunction can still occur in these populations and could theoretically impact ABR wave I amplitude. Previous studies of synaptopathy have dealt with the possible effects of subclinical OHC dysfunction in various ways. One approach has been to take no further action if mean DPOAEs measured in the experimental and control group are not significantly different. The problem with this approach is that just because there is no significant difference in the mean DPOAEs between the groups does not indicate that the DPOAEs (and OHC function) in the two groups are equivalent. A second approach has been to match experimental and control groups on pure tone thresholds. The problem with this approach is that analysis of a matched dataset requires that the statistical analysis account for the matched structure of the data, typically through conditional logistic regression (Collett 1991). Matched case-control designs are usually used for cases where the disease is so rare that the case count in a cross sectional sample would be exceptionally small, which is not true when investigating tinnitus or difficulty with speech perception. Ignoring the matched structure of the data could lead to overly conservative estimates of the effects of ABR wave I amplitude on the outcome variable (Breslow & Day 1980, Section 7.6). A more common approach has been to include average pure tone thresholds or DPOAE levels as predictors in a regression analysis. There are a couple of potential problems to consider with this approach. Use of pure tone thresholds as an indicator of OHC function is problematic because pure tone thresholds are behavioral responses that can theoretically be impacted by dysfunction at any point in the auditory system. Although cochlear deafferentation of up to 80% (due to loss of cochlear synapses, IHCs, or spiral ganglion cells) is thought to have little impact on pure tone thresholds (Schuknecht & Woellner 1955; Lobarinas et al. 2013), data from chinchillas with selective IHC loss indicates that threshold shifts of up to 10 dB can occur even with only 35% IHC loss (a form of cochlear deafferentation) (Lobarinas et al. 2013). This is supported by a statistical modeling study from Bramhall et al. (2021b) that showed that pure tone thresholds predicted from measured DPOAE levels were lower (indicating better hearing) than actual measured pure tone thresholds in Veterans, individuals with tinnitus, and subjects of older age, but not in younger control subjects. This could be explained by synaptopathy-related pure tone threshold shifts on the order of 1-8 dB in the groups at higher risk of synaptopathy due to noise exposure history, perception of tinnitus, and older age. Therefore, it’s possible that small changes in pure tone thresholds will result from synapse loss and that statistically adjusting ABR wave I amplitude for pure tone thresholds will then partially adjust out the synaptopathy effect (Figure 2). Otoacoustic emissions also appear to be more sensitive to noise exposure than pure tone thresholds (Engdahl & Kemp 1996; Seixas et al. 2005; Marshall et al. 2009). For these reasons, statistical adjustment for otoacoustic emissions, such as DPOAEs, as a more specific measure of OHC function is preferable. However, many labs do not have the necessary equipment to measure DPOAEs in the extended high frequencies, which may limit the utility of this approach. In addition, an important consideration when statistically adjusting for OHC function is the possibility of post-treatment bias (Montgomery et al. 2018). Post-treatment bias occurs when the treatment (or exposure in the case of synaptopathy) impacts both a predictor variable and the outcome variable. For example, noise exposure can result in damage to both OHCs and cochlear synapses. If we are evaluating the impact of noise exposure on ABR wave I amplitude (as an indicator of synaptopathy) and we include DPOAEs as a predictor, we may be not only adjusting out the impact of OHC dysfunction on wave I amplitude, but also the effect of noise exposure on wave I amplitude (Figure 3). Post-treatment bias will be a problem when trying to adjust for OHC function in any analysis of the effects of age, noise exposure, or ototoxin exposure on ABR wave I amplitude because these are “treatments”. Post-treatment bias is not a problem when evaluating relationships between wave I amplitude and potential perceptual consequences of synaptopathy, such as tinnitus or speech perception difficulty because no “treatment” is being evaluated in these cases. It is also important to remember that statistically adjusting for OHC function also depends on the assumption that the relationship between OHC function and ABR wave I amplitude is linear. The statistical adjustment is only helpful if the true relationship is actually linear.
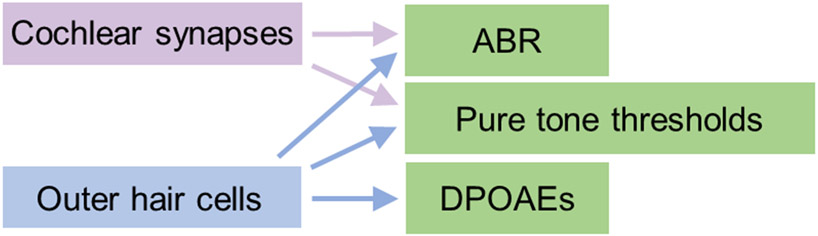
Figure 2. Schematic illustrating relationships between elements of the peripheral auditory system and audiological measurements.
Cochlear synaptic function impacts the ABR, but can also have a small effect on pure tone thresholds. OHC function influences pure tone thresholds, DPOAEs, and potentially the ABR. If a regression model includes pure tone thresholds as a predictor, this may adjust out both OHC function and cochlear synaptic function.
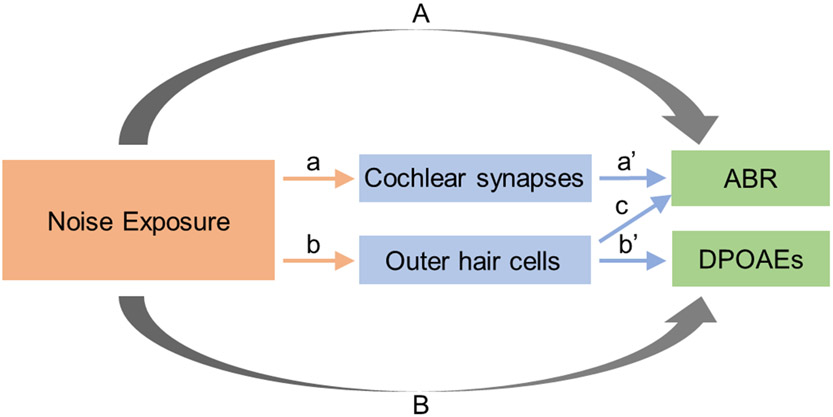
Figure 3. Schematic demonstrating the concept of post-treatment bias.
Noise exposure is assumed to impact both cochlear synapses (a) and OHCs (b). Cochlear synaptic function is measured using the ABR (a’) and OHC function using DPOAEs (b’). To assess the impact of noise exposure on cochlear synapses, we evaluate the effect of noise exposure on the ABR (A). OHC function may alter the ABR (c), but if we try to adjust out the impact of OHC function on the ABR by including DPOAEs as a predictor in our regression model, we may also be adjusting out the noise exposure effect on the ABR (A) because noise exposure also impacts DPOAEs (B). This problem is referred to as post-treatment bias.
Masking can be used to eliminate the impacts of OHC dysfunction in the extended high frequencies, but this does not solve the problem of accounting for possible OHC dysfunction in the standard frequency range.
Ideally, an ABR stimulus (or stimulus level) will be identified that is relatively insensitive to OHC dysfunction so that we can avoid the complexities of trying to separate out the impacts of OHC and synapse function statistically.
B. ABR wave I amplitude may be insensitive to loss of low spontaneous rate auditory nerve fibers
Animal studies suggest that low SR auditory nerve fibers may be particularly vulnerable to age- and noise exposure-related cochlear synaptopathy (Schmiedt et al. 1996; Furman et al. 2013). However, the results of Bourien et al. (2014) suggest that the contribution of the low SR fibers to ABR wave 1 amplitude is limited because the ABR is an onset response and the low SR fibers have a long first spike latency. Wave 1 amplitude is dominated by medium and high SR fibers, which have shorter first spike latencies. A reanalysis of the data from Furman et al. (2013) suggests that medium and high SR fibers are also impacted to some degree by noise-induced synaptopathy (Marmel et al. 2015). This could explain why clear reductions in ABR wave 1 amplitude can be observed in animal models of synaptopathy (e.g., Kujawa & Liberman 2009; Lin et al. 2011; Sergeyenko et al. 2013), even if wave 1 amplitude is not a good indicator of the integrity of low SR fibers.
The relative distribution of auditory nerve fibers with various SRs is unknown in humans and it is unclear how well the relative vulnerability of different subtypes of auditory nerve fibers to synaptopathy will parallel animal models. Therefore, it is presently unclear how accurately ABR wave I amplitude measurements in humans reflect underlying synaptopathy.
C. ABR wave I amplitude appears to be affected by sex
Sex differences in ABR wave I amplitude, due at least in part to head size, have been reported previously, with smaller wave I amplitudes in males than in females (Trune et al. 1988; Mitchell et al. 1989). This is likely due to sex-related differences in the length of the cochlea (shorter for females than for males), which lead to differences in cochlear dispersion (reviewed in Bharadwaj et al. 2019). The shorter time it takes for the traveling wave to go from the base to the apex in females, on average, would be expected to result in greater neural synchrony among different regions of the cochlea, leading to larger ABR wave I amplitudes for females compared with males. This is an important factor to consider when using wave I amplitude as a physiological indicator of synaptopathy. Previous human synaptopathy studies have addressed this issue by testing only a single sex, performing analyses separately for males and females, matching participants in the experimental and control groups by sex, or including sex as a predictor in regression analyses.
D. ABR wave I amplitude is altered by other types of cochlear deafferentation
As an indicator of auditory nerve activity, ABR wave I amplitude will be similarly impacted by any form of cochlear deafferentation (cochlear synapse, IHC, and spiral ganglion loss). This means that ABR wave I amplitude is not a specific indicator of synaptopathy because it cannot differentiate synaptopathy from other forms of cochlear deafferentation. However, the functional impacts of these different types of deafferentation should be similar, so from a treatment standpoint, it may not be necessary to differentiate synaptopathy from other forms of deafferentation unless the goal is to repair or regenerate synapses. In addition, data from animal models and human temporal bone studies suggest that age- and noise exposure-related synapse loss exceeds IHC loss and that synapse loss occurs prior to loss of spiral ganglion cells (Kujawa & Liberman 2009; Sergeyenko et al. 2013; Viana et al. 2015; Wu et al. 2019; Fernandez et al. 2020; Wu et al. 2021). For example, based on human temporal bone data, Wu et al. (2021) estimated a 6.3% loss of synapses per decade of life as compared with a 3.4% loss of IHCs per decade. This suggests that after accounting for possible OHC dysfunction, synapse loss will be the primary factor leading to abnormal ABR wave I amplitude measurements.
E. The test time required to measure ABR wave I amplitude may be prohibitive
One limitation of ABR testing is the time involved in setting up the test. Prior to testing, the skin must be prepared, electrodes attached, impedances checked, and the subject must be situated in a comfortable position that they can maintain throughout the test and that will encourage sleep. This set-up process typically takes 10-15 minutes. The length of the data collection process itself depends on the number of stimuli collected and the number of stimulus presentations. However, the best quality data is collected when the subject is asleep and this typically only occurs after several minutes of data collection. The time required to complete ABR testing makes it difficult to tack the ABR onto the end of the standard audiological evaluation. Instead, ABR testing to diagnose synaptopathy/deafferentation would likely need to be completed at a separate clinic visit.
VII. OBSERVED RELATIONSHIPS BETWEEN ABR MEASURES AND SYNAPTOPATHY RISK FACTORS
A. ABR wave I amplitude and age
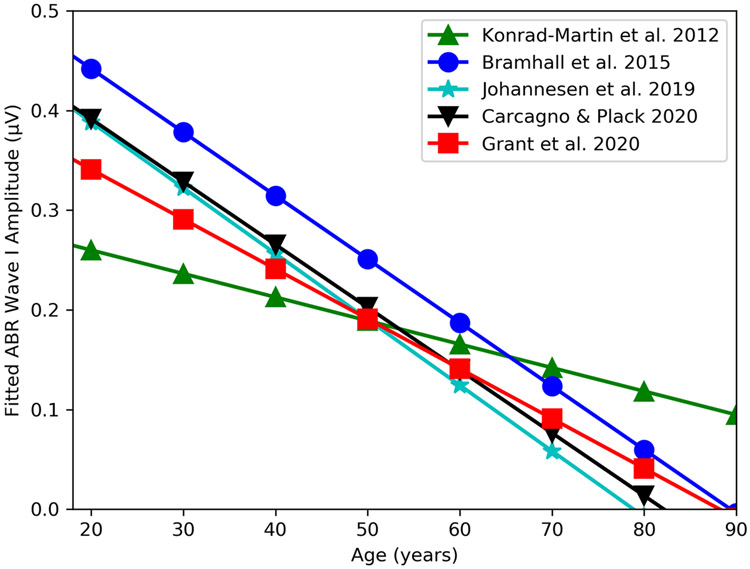
In mice, an age-related decrease in suprathreshold ABR wave I amplitude is highly correlated with degree of synapse loss (Sergeyenko et al. 2013). Several human studies have shown similar age-related decreases in suprathreshold ABR wave I amplitude (Konrad-Martin et al. 2012; Bramhall et al. 2015; Johannesen et al. 2019; Carcagno & Plack 2020; Grant et al. 2020). Regression analyses were completed using data from each of these studies and included an adjustment for average pure tone thresholds. The results of these analyses are summarized in Figure 4. Despite methodological differences, data from all five studies suggest a decrease in wave I amplitude with advancing age, varying from 0.024 to 0.066 μV per decade of life. This translates to a 56%, 38%, 63%, 61%, and 63% loss of wave I amplitude at 50 years of age for the Bramhall et al., Konrad-Martin et al., Johannesen et al., Carcagno & Plack, and Grant et al. studies, respectively. These ABR findings are consistent with human temporal bone data that shows, for the 1.4-8 kHz cochlear frequency range, an average decline in auditory nerve fibers per IHC position of 1.2 per decade of life (Wu et al. 2019). This corresponds to a 42% decrease in auditory nerve fibers at age 50. This suggests that ABR wave I amplitude is sensitive to age-related cochlear deafferentation in humans.
Figure 4. Comparison of relationship between age and ABR wave I amplitude across human studies.
Results of multivariate regression models relating age and ABR wave I amplitude are plotted for five studies. Note that there are some differences between these studies/analyses that may impact the intercept and slope for each study. The Konrad-Martin et al. and Johannesen et al. studies measured wave I amplitude using a mastoid electrode, while the other studies used a tiptrode. Konrad-Martin et al., Johannesen et al., Carcagno & Plack, and Grant et al. used click stimuli, while Bramhall et al. used a 4 kHz toneburst and stimulus presentation level varied across study (Konrad-Martin et al: 110 dB peSPL, Bramhall et al.: 80 dB nHL, Johannesen et al.: 105 dB peSPL, Carcagno & Plack: 105 dB peSPL, Grant et al.: 125 dB peSPL). Pure tone thresholds were averaged over 2, 3, and 4 kHz for the Konrad-Martin et al. study, over .5, 1, 2, and 4 kHz for the Bramhall et al., Johannesen et al., and Carcagno & Plack studies, and over 0.25-8 kHz for the Grant et al. study. In addition to including the predictors age and pure tone average in the regression model, the Bramhall et al. analysis included an interaction effect between age and average pure tone threshold, while the Konrad-Martin et al. study included stimulus rate and an interaction between stimulus rate and age. The data from Johannesen et al, Carcagno & Plack, and Grant et al. were reanalyzed so that the regression models were similar to the models from the other two studies. To be consistent with the other studies, Carcagno & Plack’s unmasked ABR wave I amplitude data recorded using a tiptrode and Grant et al.’s unmasked ABR data measured from peak to the following trough was used for the modeling. The revised regression models included age and average pure tone thresholds as predictors of wave I amplitude. Sex was also included as a predictor for the Carcagno & Plack and Johannesen et al. datasets. As in Bramhall et al. and Konrad-Martin et al., subject-specific random effects were used to account for variation in wave I amplitude due to sex/head size and the correlation between the two ears of a subject for the Grant et al. dataset, which included data for the right and left ears of each subject. For the purpose of plotting, average pure tone thresholds were set to 15 dB HL, sex was set to male, and rate was set at 11/sec. Equations are as follows, Bramhall et al.: y= 0.72597 -(0.00892 * age) – (0.01044 * 15) + (0.00017 * age *15), Konrad-Martin et al.: y=0.39448 - (0.00395 *15) – (0.00255 * 11) – (0.00269 * age) + (0.00003 * 11 * age), Johannesen et al.: y = 0.5264 – (0.0066 * age) - (0.0004 * 15), Carcagno & Plack: y = 0.5181 – (0.0063 * age) - (0.00003854 *15), Grant et al.: y = 0.537 - (0.006 * age) – (0.004 *15).
B. ABR wave I amplitude and noise exposure history
Mouse models of age- and noise exposure-related synaptopathy indicate different patterns of damage. Age-related synaptopathy is widespread across cochlear frequency (Sergeyenko et al. 2013). In the acute stage, noise-induced synaptopathy is more frequency-specific, resulting in a focal reduction of synapses and ABR wave I amplitude in the frequency regions above the noise exposure band (e.g., Kujawa & Liberman 2009). However over time, noise-induced synaptopathy in mice expands from a focal lesion to a broader synapse loss across a large frequency range (Fernandez et al. 2015). Although it’s unclear how the time course of this frequency spreading might translate to humans, it’s possible that unless the noise exposure occurred very recently, individuals with a history of noise exposure may have broad rather than focal synaptic loss. This view is supported by human temporal bone data that demonstrates a consistent noise exposure-related reduction in cochlear synapse numbers across frequency (Wu et al. 2021) and the results of Bramhall et al. (2017), where reductions in ABR wave I amplitude were noted in young military Veterans reporting high levels of noise exposure and non-Veterans with a history of firearm use compared to non-Veteran controls at frequencies of 1, 3, 4, and 6 kHz.
A second study of military Veterans also found a reduction in ABR wave I amplitude (for a 90 dB peSPL stimulus) for Veterans reporting high levels of noise exposure compared with controls (Bramhall et al. 2021a). However, a number of studies of young adults with a history of recreational noise exposure from sources such as music concerts, night clubs, playing in a band, and listening to music with headphones have either showed no clear correlation between noise exposure score and wave I amplitude (e.g., Fulbright et al. 2017; Grinn et al. 2017; Prendergast et al. 2017) or very similar mean wave I amplitudes for low and high noise exposure groups (Prendergast et al. 2018). Grose et al. (2017) found mean wave I amplitude was smaller for a noise exposed group (71% whom identified as musicians) compared with a control group, but the difference was not statistically significant. Liberman et al. (2016) showed an increase in the SP/AP ratio and a mean decrease in wave I amplitude among college music students compared with controls, but only the SP/AP contrast was statistically significant. One interpretation of these results is that due to the higher intensity levels, military noise exposure and firearm use pose a higher risk for noise-induced synaptopathy than common sources of recreational noise exposure, such as exposure to loud music, even for musicians. Other factors that may contribute to the differing results across studies include: 1) asking participants to retroactively assess their noise exposure history and use of hearing protection is imprecise, 2) humans vary in their genetic susceptibility to noise damage, and 3) many studies have not included firearm use as part of their assessment of noise exposure history or have not excluded individuals with a history of firearm use from their control group. Given that Bramhall et al. (2017) showed lower ABR wave I amplitudes among non-Veterans reporting any history of firearm use, suggesting synaptopathy, failure to account for firearm use could make it difficult to detect relationships between noise exposure history and ABR wave I amplitude because noise exposure history will be underestimated in firearm users. See Le Prell (2019) for a comprehensive review of human studies that have assessed the relationship between ABR wave I amplitude and noise exposure history.
VIII. OBSERVED RELATIONSHIPS BETWEEN ABR MEASURES AND PREDICTED PERCEPTUAL CONSEQUENCES OF SYNAPTOPATHY
Predicted perceptual consequences of cochlear synaptopathy include tinnitus, hyperacusis, and difficulty understanding speech in background noise (Kujawa & Liberman 2015). All three are common complaints among Veterans, who are at high risk for synaptopathy due to their history of military noise exposure. Henry et al. (2019) reported a 44% prevalence of constant tinnitus among Veterans who had separated from military service in the past 2.5 years, even though mean audiometric thresholds for this sample were clinically normal. Theodoroff et al. (2019) found that 48% of Veterans with a history of blast exposure and normal audiograms had some degree of decreased tolerance to the loudness of everyday sounds. Finally, despite average hearing thresholds within the normal range, 76% of a sample of 100 Service members and recently separated Veterans reported difficulty hearing speech or other sounds (Gordon et al. 2017).
A. ABR wave I amplitude and tinnitus
Several studies have shown reductions in ABR wave I amplitude associated with the report of tinnitus (Schaette & McAlpine 2011; Gu et al. 2012; Bramhall et al. 2018; Bramhall et al. 2019), suggesting that some types of tinnitus are related to synaptopathy. The observed reductions in wave I amplitude have not been accompanied by reductions in wave V amplitude, resulting in a decreased wave I/V amplitude ratio. Decreased wave I/V ratios have generally been interpreted as indicating compensatory gain occurring in the central auditory system, which may lead to the percept of tinnitus. However, because OHC damage in the basal end of the cochlea will impact the amplitude of wave I more than the amplitude of wave V (Don & Eggermont 1978), poorer OHC function in the tinnitus group compared with the control group could also explain the observed reduction in the wave I/V amplitude ratio for the tinnitus group. In contrast, Guest et al. (2017) found very similar mean wave I amplitudes for a tinnitus and a control group. One possible explanation for the differing results include the multifactorial nature of tinnitus. The tinnitus sample studied by Guest et al. may have tended towards tinnitus etiologies other than synaptopathy because it was not drawn specifically from a noise exposed population, making it more difficult to detect group differences in wave I amplitude.
B. ABR wave I amplitude and hyperacusis
Mice with noise-induced cochlear synaptopathy have elevated acoustic startle responses to moderate level sounds compared to control mice, which is suggestive of hyperacusis (Hickox & Liberman 2014). However, the relationship between synaptopathy and hyperacusis has not been well studied in humans. In a sample of young Veterans and non-Veterans with normal audiograms, Bramhall et al. (2018) did not find a clear relationship between loudness discomfort level (LDL) and ABR wave I amplitude, but cautioned against the use of LDL as a measure of hyperacusis. In contrast, the results of Bramhall et al. (2020) suggest that Veterans who report decreased sound tolerance (DST) exhibit steeper ABR wave I amplitude growth functions compared with non-Veteran controls or Veterans without DST, resulting in a reduction in raw mean wave I amplitude for Veterans with DST compared with controls at 90 dB peSPL, but not at 100 and 110 dB peSPL.
C. ABR wave I amplitude and complex speech perception
Several studies have shown poorer performance on temporal processing and signal-in-noise detection tasks in animals with synaptopathy, IHC loss, or spiral ganglion cell loss compared with controls (Lobarinas et al. 2017; Lobarinas et al. 2020; Monaghan et al. 2020; Resnik & Polley 2021). All three of these types of deafferentation will result in reduced peripheral input to the central auditory system and should have similar functional impacts. The results of these studies suggest that synaptopathy would be expected to negatively impact complex speech perception.
However, despite these predictions, previous studies investigating the relationship between ABR wave I amplitude and speech perception have resulted in mixed findings. Several studies have shown a relationship between ABR measurements and performance on complex speech perception or signal-in-noise detection tasks (Bramhall et al. 2015; Liberman et al. 2016; Ridley et al. 2018; Buran et al. 2020; Grant et al. 2020; Mepani et al. 2020), while others have failed to detect a relationship (Fulbright et al. 2017; Bramhall et al. 2018; Guest et al. 2018; Guest et al. 2019a). Possible explanations for the mixed findings include: 1) differences across studies in the ABR measure that was used (e.g., ABR wave I amplitude, ABR wave I/V amplitude ratio, ABR SP/AP ratio), 2) differences in the perceptual task – some tasks may be more sensitive to deafferentation impacts than others, 3) differences in the study population – for example the results of Bramhall et al. (2015) suggest that relationships between physiological indicators of deafferentation and speech perception measures may be more apparent when there is some OHC dysfunction present (i.e., deafferentation has a greater impact on speech perception in people with poorer OHC function), 4) differences in cognitive ability across participants (e.g., working memory) may obscure differences in speech perception performance due to synaptopathy if increased listening effort can be used to compensate for the degraded speech signal so that task performance is relatively unaffected.
VIII. FUTURE DIRECTIONS FOR ABR MEASURES IN DIAGNOSIS OF SYNAPTOPATHY/DEAFFERENTATION
A. Clinical diagnosis of deafferentation to guide treatment
A physiological test for deafferentation is meaningless without a method for assessing individual patients using that test. Many clinical audiological tests are interpreted based on a normative reference range established in young people expected to have normal auditory function (e.g., pure tone thresholds, tympanometry, and ABR peak latencies). Normative ranges for ABR wave I amplitude do not currently exist for a sample at low risk of synaptopathy, but once the optimal ABR stimulus parameters are determined, sex-specific normative ranges for these stimuli could be obtained by measuring ABR wave I amplitudes in a large sample of young adults with minimal noise exposure history.
Another option for individualized assessment of deafferentation is to combine physiological measurements with computational modeling to generate predictions of the degree of deafferentation for each patient based on their ABR wave I amplitude measurements. Buran et al. (2020) illustrate how multiple ABR measurements (in response to different stimuli/levels) can be combined with a computational model of the human auditory periphery to predict synapse numbers in individual patients, presumably enhancing the precision of the estimate of deafferentation over what can be accomplished with a single ABR measurement.
B. Diagnosis of synaptopathy for clinical trials of pharmaceutical treatments
Most treatments for deafferentation-related perceptual deficits, such as specialized hearing aid algorithms or use of low gain hearing aids, would not require identification of the exact site of lesion. However, the site of lesion is important for pharmaceutical treatments aimed at synapse regeneration or repair. Studies in animals and human temporal bones indicate that synapse loss is the most common type of age- or noise exposure-related deafferentation (e.g., Kujawa & Liberman 2009; Sergeyenko et al. 2013; Wu et al. 2019). Accordingly, individuals with physiological measurements that indicate deafferentation, such as low ABR wave I amplitudes, who are also at high risk for synaptopathy due to age, noise exposure, or use of ototoxic drugs would be good candidates for these pharmaceutical treatments.
The treatment efficacy of a candidate pharmaceutical could be determined in a clinical trial by measuring ABR wave I amplitude before and after treatment to determine if the drug led to greater increases in ABR wave I amplitude, suggesting decreased synaptopathy, compared to treatment with a placebo. Treatment-related improvements in auditory perception could also be evaluated, such as decreased tinnitus severity or improved speech perception performance.
IX. CONCLUSIONS
Given that the ABR has been used in audiology clinics for decades and a clear relationship between ABR wave 1 amplitude and cochlear synaptic integrity has been demonstrated in multiple animal models, measurement of ABR wave I amplitude has potential for future clinical assessment of cochlear synaptopathy/deafferentation. However, further work is necessary to determine normative values in a population at low risk for synaptopathy and the optimal ABR parameters for this purpose. In addition, because cochlear synaptopathy can only be confirmed though post-mortem temporal bone analysis and we do not currently have any ABR wave I amplitude measurements that can be linked to human temporal bone data, our understanding of the relationship between synaptopathy and wave I amplitude is based on animal data. Use of ABR wave I amplitude as an indicator of synaptopathy requires recognition of the limitations associated with using an indirect measure of auditory nerve function and our uncertainty about how well human auditory nerve anatomy parallels what has been observed in rodent models.
Acknowledgements
The author thanks Dr. Stéphane Maison for sharing the ABR wave I amplitude data presented in Grant et al. (2020), Drs. Peter Johannesen and Enrique Lopez-Poveda for sharing the ABR data presented in Johannesen et al. (2019), and Dr. Samuele Carcagno for making the ABR data presented in Carcagno & Plack (2020) publicly available. Dr. Bramhall is supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service - Award #C2104-W. The opinions and assertions presented are private views of the author and are not to be construed as official or as necessarily reflecting the views of the Department of Veterans Affairs.
References
- Bharadwaj HM, Mai AR, Simpson JM, Choi I, Heinz MG, Shinn-Cunningham BG (2019). Non-Invasive Assays of Cochlear Synaptopathy - Candidates and Considerations. Neuroscience, 407, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG (2014). Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Desmadryl G, Nouvian R, Puel JL, Wang J (2014). Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol, 112, 1025–1039. [DOI] [PubMed] [Google Scholar]
- Bramhall N, Ong B, Ko J, Parker M (2015). Speech Perception Ability in Noise is Correlated with Auditory Brainstem Response Wave I Amplitude. J Am Acad Audiol, 26, 509–517. [DOI] [PubMed] [Google Scholar]
- Bramhall NB, McMillan GP, Kampel SD (2021a). Envelope following response measurements in young veterans are consistent with noise-induced cochlear synaptopathy. Hear Res, 108310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP (2018). Tinnitus and Auditory Perception After a History of Noise Exposure: Relationship to Auditory Brainstem Response Measures. Ear Hear, 39, 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP, Griest SE (2017). Auditory Brainstem Response Altered in Humans With Noise Exposure Despite Normal Outer Hair Cell Function. Ear Hear, 38, e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, McMillan GP, Gallun FJ, Konrad-Martin D (2019). Auditory brainstem response demonstrates that reduced peripheral auditory input is associated with self-report of tinnitus. J Acoust Soc Am, 146, 3849. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, McMillan GP, Mashburn AN (2021b). Subclinical Auditory Dysfunction: Relationship Between Distortion Product Otoacoustic Emissions and the Audiogram. Am J Audiol, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Theodoroff SM, Kampel SD (2020). Measures of Synaptopathy Linked with Tinnitus and Hyperacusis. In Association for Research in Otolaryngology MidWinter Meeting. San Jose, CA. [Google Scholar]
- Breslow NE, Day NE (1980). Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ, 32, 5–338. [PubMed] [Google Scholar]
- Buran BN, McMillan G, Keshishzadeh S, Verhulst S, Bramhall N (2020). Predicting synapse counts in living humans by combining computational models with auditory physiology. OSF Preprints, Available from 10.31219/osf.io/f8bx6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcagno S, Plack CJ (2020). Effects of age on electrophysiological measures of cochlear synaptopathy in humans. Hear Res, 396, 108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett D. (1991). Modelling Binary Data. London: Chapman and Hall. [Google Scholar]
- Don M, Eggermont JJ (1978). Analysis of the click-evoked brainstem potentials in man unsing high-pass noise masking. J Acoust Soc Am, 63, 1084–1092. [DOI] [PubMed] [Google Scholar]
- Don M, Ponton CW, Eggermont JJ, Masuda A (1994). Auditory brainstem response (ABR) peak amplitude variability reflects individual differences in cochlear response times. J Acoust Soc Am, 96, 3476–3491. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ (1984). Use of electrocochleography and brain stem auditory evoked potentials in the diagnosis of cerebellopontine angle pathology. Adv Otorhinolaryngol, 34, 47–56. [DOI] [PubMed] [Google Scholar]
- Engdahl B, Kemp DT (1996). The effect of noise exposure on the details of distortion product otoacoustic emissions in humans. The Journal of the Acoustical Society of America, 99, 1573–1587. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Guo D, Micucci S, De Gruttola V, Liberman MC, Kujawa SG (2020). Noise-induced Cochlear Synaptopathy with and Without Sensory Cell Loss. Neuroscience, 427, 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG (2015). Aging after noise exposure: acceleration of cochlear synaptopathy in "recovered" ears. J Neurosci, 35, 7509–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnum H, O'Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, O'Donoghue G, Mason S, Baguley D, Jones H, Mulvaney C (2009). The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess, 13, iii–iv, ix-xi, 1-154. [DOI] [PubMed] [Google Scholar]
- Fulbright ANC, Le Prell CG, Griffiths SK, Lobarinas E (2017). Effects of Recreational Noise on Threshold and Suprathreshold Measures of Auditory Function. Semin Hear, 38, 298–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC (2013). Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol, 110, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudet F, Labanca L, Souchal M, Avan P (2021). Decreased Reemerging Auditory Brainstem Responses Under Ipsilateral Broadband Masking as a Marker of Noise-Induced Cochlear Synaptopathy. Ear Hear. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Griest SE, Thielman EJ, Carlson KF, Helt WJ, Lewis MS, Blankenship C, Austin D, Theodoroff SM, Henry JA (2017). Audiologic characteristics in a sample of recently-separated military Veterans: The Noise Outcomes in Servicemembers Epidemiology Study (NOISE Study). Hear Res, 349, 21–30. [DOI] [PubMed] [Google Scholar]
- Grant KJ, Mepani AM, Wu P, Hancock KE, de Gruttola V, Liberman MC, Maison SF (2020). Electrophysiological markers of cochlear function correlate with hearing-in-noise performance among audiometrically normal subjects. J Neurophysiol, 124, 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD (1961). Critical bandwidth and the frequency coordinates of the basilar membrane. J Acoust Soc Am, 33, 1344–1356. [Google Scholar]
- Grinn SK, Wiseman KB, Baker JA, Le Prell CG (2017). Hidden Hearing Loss? No Effect of Common Recreational Noise Exposure on Cochlear Nerve Response Amplitude in Humans. Front Neurosci, 11, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Buss E, Hall JW 3rd. (2017). Loud Music Exposure and Cochlear Synaptopathy in Young Adults: Isolated Auditory Brainstem Response Effects but No Perceptual Consequences. Trends Hear, 21, 2331216517737417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, Melcher JR (2012). Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol, 13, 819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Plack CJ (2019a). Acoustic Middle-Ear-Muscle-Reflex Thresholds in Humans with Normal Audiograms: No Relations to Tinnitus, Speech Perception in Noise, or Noise Exposure. Neuroscience, 407, 75–82. [DOI] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ (2017). Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear Res, 344, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Millman RE, Plack CJ (2018). Impaired speech perception in noise with a normal audiogram: No evidence for cochlear synaptopathy and no relation to lifetime noise exposure. Hear Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Plack CJ (2019b). Reliability and interrelations of seven proxy measures of cochlear synaptopathy. Hear Res, 375, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JWI (1992). Handbook of Auditory Evoked Responses. Boston: Allyn and Bacon. [Google Scholar]
- Harris KC, Vaden KI Jr., McClaskey CM, Dias JW, Dubno JR (2018). Complementary metrics of human auditory nerve function derived from compound action potentials. J Neurophysiol, 119, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JA, Griest SE, Blankenship C, Thielman EJ, Theodoroff SM, Hammill T, Carlson KF (2019). Impact of Tinnitus on Military Service Members. Mil Med, 184, 604–614. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Larsen E, Heinz MG, Shinobu L, Whitton JP (2017). Translational issues in cochlear synaptopathy. Hear Res, 349, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC (2014). Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol, 111, 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood LJ (1998). Clinical Applications of the Auditory Brainstem Response. United States: Singular Publishing Group. [Google Scholar]
- Irimia A, Goh SY, Torgerson CM, Chambers MC, Kikinis R, Van Horn JD (2013). Forward and inverse electroencephalographic modeling in health and in acute traumatic brain injury. Clin Neurophysiol, 124, 2129–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen PT, Buzo BC, Lopez-Poveda EA (2019). Evidence for age-related cochlear synaptopathy in humans unconnected to speech-in-noise intelligibility deficits. Hearing Research. [DOI] [PubMed] [Google Scholar]
- Kamerer AM, Neely ST, Rasetshwane DM (2020). A model of auditory brainstem response wave I morphology. J Acoust Soc Am, 147, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, Austin DF (2012). Age-related changes in the auditory brainstem response. J Am Acad Audiol, 23, 18–35; quiz 74-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2009). Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci, 29, 14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2015). Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res, 330, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG (2019). Effects of noise exposure on auditory brainstem response and speech-in-noise tasks: a review of the literature. Int J Audiol, 58, S3–S32. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF (2016). Toward a Differential Diagnosis of Hidden Hearing Loss in Humans. PLoS One, 11, e0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC (2011). Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol, 12, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D (2013). Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res, 302, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D (2020). Gap Detection Deficits in Chinchillas with Selective Carboplatin-Induced Inner Hair Cell Loss. J Assoc Res Otolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Spankovich C, Le Prell CG (2017). Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear Res, 349, 155–163. [DOI] [PubMed] [Google Scholar]
- Margolis RH (1999). Electrocochleography. Semin Hear, 20, 45–60. [Google Scholar]
- Margolis RH, Rieks D, Fournier EM, Levine SE (1995). Tympanic electrocochleography for diagnosis of Meniere's disease. Arch Otolaryngol Head Neck Surg, 121, 44–55. [DOI] [PubMed] [Google Scholar]
- Marmel F, Rodríguez-Mendoza MA, Lopez-Poveda EA (2015). Stochastic undersampling steepens auditory threshold/duration functions: implications for understanding auditory deafferentation and aging. Front Aging Neurosci, 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Lapsley Miller JA, Heller LM, Wolgemuth KS, Hughes LM, Smith SD, Kopke RD (2009). Detecting incipient inner-ear damage from impulse noise with otoacoustic emissions. J Acoust Soc Am, 125, 995–1013. [DOI] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG (2016). Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci, 36, 3755–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Guinan JJ Jr., Knudson IM, Kiang NY (1996). Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hear Res, 93, 28–51. [DOI] [PubMed] [Google Scholar]
- Mepani AM, Kirk SA, Hancock KE, Bennett K, de Gruttola V, Liberman MC, Maison SF (2020). Middle Ear Muscle Reflex and Word Recognition in "Normal-Hearing" Adults: Evidence for Cochlear Synaptopathy? Ear Hear, 41, 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Phillips DS, Trune DR (1989). Variables affecting the auditory brainstem response: audiogram, age, gender and head size. Hear Res, 40, 75–85. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Go T, Ohkubo H, Tatara T, Motomura T (1982). Developmental changes of brainstem auditory evoked potentials (BAEPs) in normal human subjects from infants to young adults. Brain Dev, 4, 127–136. [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ (1982). Comparison between intracranially recorded potentials from the human auditory nerve and scalp recorded auditory brainstem responses (ABR). Scand Audiol, 11, 33–40. [DOI] [PubMed] [Google Scholar]
- Monaghan JJM, Garcia-Lazaro JA, McAlpine D, Schaette R (2020). Hidden Hearing Loss Impacts the Neural Representation of Speech in Background Noise. Curr Biol, 30, 4710–4721 e4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JM, Nyhan B, Torres M (2018). How conditioning on posttreatment variables can ruin your experiment and what to do about it. Am J Pol Sci, 62, 760–775. [Google Scholar]
- Pappa AK, Hutson KA, Scott WC, Wilson JD, Fox KE, Masood MM, Giardina CK, Pulver SH, Grana GD, Askew C, Fitzpatrick DC (2019). Hair cell and neural contributions to the cochlear summating potential. J Neurophysiol, 121, 2163–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Guest H, Munro KJ, Kluk K, Leger A, Hall DA, Heinz MG, Plack CJ (2017). Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hear Res, 344, 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Tu W, Guest H, Millman RE, Kluk K, Couth S, Munro KJ, Plack CJ (2018). Supra-threshold auditory brainstem response amplitudes in humans: Test-retest reliability, electrode montage and noise exposure. Hear Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik J, Polley DB (2021). Cochlear neural degeneration disrupts hearing in background noise by increasing auditory cortex internal noise. Neuron, 109, 984–996 e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley CL, Kopun JG, Neely ST, Gorga MP, Rasetshwane DM (2018). Using Thresholds in Noise to Identify Hidden Hearing Loss in Humans. Ear Hear, 39, 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhamer HJ, Lindstrom B, Lundborg T (1978). On the use of click-evoked electric brainstem responses in audiological diagnosis. I. The variability of the normal response. Scand Audiol, 7, 193–205. [DOI] [PubMed] [Google Scholar]
- Rowe MJ 3rd. (1978). Normal variability of the brain-stem auditory evoked response in young and old adult subjects. Electroencephalogr Clin Neurophysiol, 44, 459–470. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Ao H, He J, Chen Z, Yu Z, Zhang R, Wang J, Yin S (2014). Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology, 40, 86–96. [DOI] [PubMed] [Google Scholar]
- Santarelli R, del Castillo I, Cama E, Scimemi P, Starr A (2015). Audibility, speech perception and processing of temporal cues in ribbon synaptic disorders due to OTOF mutations. Hear Res, 330, 200–212. [DOI] [PubMed] [Google Scholar]
- Santarelli R, Del Castillo I, Rodriguez-Ballesteros M, Scimemi P, Cama E, Arslan E, Starr A (2009). Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene. J Assoc Res Otolaryngol, 10, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, McAlpine D (2011). Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci, 31, 13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA (1996). Age-related loss of activity of auditory-nerve fibers. J Neurophysiol, 76, 2799–2803. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC (1955). An experimental and clinical study of deafness from lesions of the cochlear nerve. J Laryngol Otol, 69, 75–97. [DOI] [PubMed] [Google Scholar]
- Seixas N, Goldman B, Sheppard L, Neitzel R, Norton S, Kujawa S (2005). Prospective noise induced changes to hearing among construction industry apprentices. Occupational and environmental medicine, 62, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013). Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci, 33, 13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E, Tufts J (2018). Evidence of noise-induced subclinical hearing loss using auditory brainstem responses and objective measures of noise exposure in humans. Hearing research, 361, 80–91. [DOI] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA (2015). Auditory function in normal-hearing, noise-exposed human ears. Ear Hear, 36, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoroff SM, Reavis KM, Griest SE, Carlson KF, Hammill TL, Henry JA (2019). Decreased sound tolerance associated with blast exposure. Sci Rep, 9, 10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Mitchell C, Phillips DS (1988). The relative importance of head size, gender and age on the auditory brainstem response. Hear Res, 32, 165–174. [DOI] [PubMed] [Google Scholar]
- Valero MD, Burton JA, Hauser SN, Hackett TA, Ramachandran R, Liberman MC (2017). Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hear Res, 353, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Altoe A, Vasilkov V (2018). Computational modeling of the human auditory periphery: Auditory-nerve responses, evoked potentials and hearing loss. Hear Res, 360, 55–75. [DOI] [PubMed] [Google Scholar]
- Viana LM, O'Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, Merchant SN, Liberman LD, Liberman MC (2015). Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res, 327, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O'Malley JT, Liberman MC (2019). Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience, 407, 8–2s0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, O'Malley JT, de Gruttola V, Liberman MC (2021). Primary Neural Degeneration in Noise-Exposed Human Cochleas: Correlations with Outer Hair Cell Loss and Word-Discrimination Scores. J Neurosci, 41, 4439–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuyts FL, Van de Heyning PH, Van Spaendonck MP, Molenberghs G (1997). A review of electrocochleography: instrumentation settings and meta-analysis of criteria for diagnosis of endolymphatic hydrops. Acta Otolaryngol Suppl, 526, 14–20. [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C (1999). A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet, 21, 363–369. [DOI] [PubMed] [Google Scholar]