Abstract
Background
A range of strategies are used to communicate with parents, caregivers and communities regarding child vaccination in order to inform decisions and improve vaccination uptake. These strategies include interventions in which information is aimed at larger groups in the community, for instance at public meetings, through radio or through leaflets. This is one of two reviews on communication interventions for childhood vaccination. The companion review focuses on face‐to‐face interventions for informing or educating parents.
Objectives
To assess the effects of interventions aimed at communities to inform and/or educate people about vaccination in children six years and younger.
Search methods
We searched CENTRAL, MEDLINE, EMBASE and five other databases up to July 2012. We searched for grey literature in the Grey Literature Report and OpenGrey. We also contacted authors of included studies and experts in the field. There were no language, date or settings restrictions.
Selection criteria
Individual or cluster‐randomised and quasi‐randomised controlled trials, interrupted time series (ITS) and repeated measures studies, and controlled before‐and‐after (CBA) studies. We included interventions aimed at communities and intended to inform and/or educate about vaccination in children six years and younger, conducted in any setting. We defined interventions aimed at communities as those directed at a geographic area, and/or interventions directed to groups of people who share at least one common social or cultural characteristic. Primary outcomes were: knowledge among participants of vaccines or vaccine‐preventable diseases and of vaccine service delivery; child immunisation status; and unintended adverse effects. Secondary outcomes were: participants' attitudes towards vaccination; involvement in decision‐making regarding vaccination; confidence in the decision made; and resource use or cost of intervention.
Data collection and analysis
Two authors independently reviewed the references to identify studies for inclusion. We extracted data and assessed risk of bias in all included studies.
Main results
We included two cluster‐randomised trials that compared interventions aimed at communities to routine immunisation practices. In one study from India, families, teachers, children and village leaders were encouraged to attend information meetings where they received information about childhood vaccination and could ask questions. In the second study from Pakistan, people who were considered to be trusted in the community were invited to meetings to discuss vaccine coverage rates in their community and the costs and benefits of childhood vaccination. They were asked to develop local action plans and to share the information they had been given and continue the discussions in their communities.
The trials show low certainty evidence that interventions aimed at communities to inform and educate about childhood vaccination may improve knowledge of vaccines or vaccine‐preventable diseases among intervention participants (adjusted mean difference 0.121, 95% confidence interval (CI) 0.055 to 0.189). These interventions probably increase the number of children who are vaccinated. The study from India showed that the intervention probably increased the number of children who received vaccinations (risk ratio (RR) 1.67, 95% CI 1.21 to 2.31; moderate certainty evidence). The study from Pakistan showed that there is probably an increase in the uptake of both measles (RR 1.63, 95% CI 1.03 to 2.58) and DPT (diptheria, pertussis and tetanus) (RR 2.17, 95% CI 1.43 to 3.29) vaccines (both moderate certainty evidence), but there may be little or no difference in the number of children who received polio vaccine (RR 1.01, 95% CI 0.97 to 1.05; low certainty evidence). There is also low certainty evidence that these interventions may change attitudes in favour of vaccination among parents with young children (adjusted mean difference 0.054, 95% CI 0.013 to 0.105), but they may make little or no difference to the involvement of mothers in decision‐making regarding childhood vaccination (adjusted mean difference 0.043, 95% CI ‐0.009 to 0.097).
The studies did not assess knowledge among participants of vaccine service delivery; participant confidence in the vaccination decision; intervention costs; or any unintended harms as a consequence of the intervention. We did not identify any studies that compared interventions aimed at communities to inform and/or educate with interventions directed to individual parents or caregivers, or studies that compared two interventions aimed at communities to inform and/or educate about childhood vaccination.
Authors' conclusions
This review provides limited evidence that interventions aimed at communities to inform and educate about early childhood vaccination may improve attitudes towards vaccination and probably increase vaccination uptake under some circumstances. However, some of these interventions may be resource intensive when implemented on a large scale and further rigorous evaluations are needed. These interventions may achieve most benefit when targeted to areas or groups that have low childhood vaccination rates.’
Keywords: Child; Child, Preschool; Humans; Infant; Health Knowledge, Attitudes, Practice; Health Education; Health Education/methods; India; Information Dissemination; Information Dissemination/methods; Pakistan; Parents; Parents/education; Randomized Controlled Trials as Topic; Vaccination; Vaccination/statistics & numerical data
Plain language summary
Interventions aimed at communities for informing and/or educating about early childhood vaccination
Researchers in The Cochrane Collaboration conducted a review of the effect of informing or educating members of the community about early childhood vaccination. After searching for all relevant studies, they found two studies, published in 2007 and 2009. Their findings are summarised below.
What are interventions aimed at communities for childhood immunisation?
Childhood vaccinations can prevent illness and death, but many children do not get vaccinated. There are a number of reasons for this. One reason may be that families lack knowledge about the diseases that vaccines can prevent, how vaccinations work, or how, where or when to get their children vaccinated. People may also have concerns (or may be misinformed) about the benefits and harms of different vaccines.
Giving people information or education so that they can make informed decisions about their health is an important part of all health systems. Vaccine information and education aims to increase people's knowledge of and change their attitudes to vaccines and the diseases that these vaccines can prevent. Vaccine information or education is often given face‐to‐face to individual parents, for instance during home visits or at the clinic. Another Cochrane Review assessed the impact of this sort of information. But this information can also be given to larger groups in the community, for instance at public meetings and women's clubs, through television or radio programmes, or through posters and leaflets. In this review, we have looked at information or education that targeted whole communities rather than individual parents or caregivers.
The review found two studies. The first study took place in India. Here, families, teachers, children and village leaders were encouraged to attend information meetings where they were given information about childhood vaccination and could ask questions. Posters and leaflets were also distributed in the community. The second study was from Pakistan. Here, people who were considered to be trusted in the community were invited to meetings where they discussed the current rates of vaccine coverage in their community and the costs and benefits of childhood vaccination. They were also asked to develop local action plans, to share the information they had been given and continue the discussions with households in their communities.
What happens when members of the community are informed or educated about vaccines?
The studies showed that community‐based information or education:
‐ may improve knowledge of vaccines or vaccine‐preventable diseases;
‐ probably increases the number of children who get vaccinated (both the study in India and the study in Pakistan showed that there is probably an increase in the number of vaccinated children);
‐ may make little or no difference to the involvement of mothers in decision‐making about vaccination;
‐ may change attitudes in favour of vaccination among parents with young children;
We assessed all of this evidence to be of low or moderate certainty.
The studies did not assess whether this type of information or education led to better knowledge among participants about vaccine service delivery or increased their confidence in the decision made. Nor did the studies assess how much this information and education cost or whether it led to any unintended harms.
Summary of findings
for the main comparison.
| Interventions aimed at communities to inform and/or educate about early childhood vaccination versus routine immunisation practices in primary and community care | ||||||
|
People: community members Settings: primary and community care Intervention: interventions to inform and/or educate members of the community about early childhood vaccination Comparison: routine immunisation practices | ||||||
| Outcomes | Impact | Number of participants (studies) | Certainty of the evidence (GRADE)† | |||
| Absolute effect* | Estimated effects | Results in words | ||||
|
Without interventions aimed at communities |
With interventions aimed at communities |
|||||
| Knowledge among participants of vaccine or vaccine‐preventable disease (number of people whose vaccine knowledge had increased; follow‐up: mean = 2 years; assessed through household survey using a questionnaire) | 59 per 100 people | 71 per 100 people (from 65 to 78) | Adjusted mean difference 0.121 (95% CI 0.06 to 0.19) | The intervention may improve knowledge of vaccine‐preventable diseases among intervention participants | 55821 | ⊕⊕⊖⊖ Low2 |
| Knowledge among participants of vaccine service delivery | The included studies did not assess this outcome | |||||
| Immunisation status of child (follow‐up: mean = 2 years; assessed through household survey using a questionnaire) | Pooling of the data from these studies was not possible |
|
One study showed that the intervention probably increases the number of children who received one or more vaccinations. A second study showed that the intervention probably increases the uptake of both measles and DPT vaccines but makes little or no difference to the number of children who received polio vaccine |
3 |
⊕⊕⊕⊖ Moderate4 | |
| Participants' attitudes towards vaccination (number of parents who think it is worthwhile to vaccinate children; follow‐up: mean = 2 years; assessed through household survey using a questionnaire) | 86 per 100 parents | 91 per 100 parents (from 87 to 96) | Adjusted mean difference 0.054 (95% CI 0.01 to 0.11) | The intervention may improve attitudes towards vaccination among intervention participants | 56361 | ⊕⊕⊖⊖ Low5 |
| Participants' involvement in decision‐making regarding vaccination (number of mothers included in decisions about vaccination; follow‐up: mean = 2 years; assessed through household survey using a questionnaire) | 55 per 100 mothers | 60 per 100 mothers (from 54 to 65) | Adjusted mean difference 0.043 (95% CI ‐0.01 to 0.1) | The intervention may make little or no difference to the involvement of mothers in decision‐making regarding vaccination | 55651 | ⊕⊕⊖⊖ Low6 |
| Participant confidence in the decision made regarding vaccination | The included studies did not assess this outcome | |||||
| Unintended or adverse effects | The included studies did not assess this outcome | |||||
| Resource use or cost of the intervention | The included studies did not assess this outcome | |||||
| *The absolute effect WITHOUT the intervention is based on data from the trial control group. The corresponding absolute effect WITH the intervention is based on the estimated effect of the intervention relative to the control group. CI: confidence interval; RR: risk ratio; DPT: diphtheria, pertussis and tetanus vaccine | ||||||
|
†GRADE Working Group grades of evidence High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different^ is low. Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different^ is moderate. Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different^ is high. Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different^ is very high. ^Substantially different = a large enough difference that it might affect a decision | ||||||
1Andersson 2009. 2Downgraded due to risk of bias, as the outcome is based on self report; indirectness as the outcome assessed in the trial is not identical to that specified in the review; and sparse data drawn from a single study. 3Andersson 2009 (study 2), Pandey 2007 (study 1 ‐ unpublished data). 4Downgraded due to some imprecision (wide confidence intervals that include both little effect and a substantial effect) and some inconsistency across the findings from the two studies. 5Downgraded due to risk of bias, as the outcome is based on self report; indirectness as the outcome assessed in the trial is not identical to that specified in the review; and sparse data drawn from a single study. 6Downgraded due to risk of bias, as the outcome is based on self report; indirectness as the outcome assessed in the trial is not identical to that specified in the review; and sparse data drawn from a single study.
Background
This Cochrane Review was undertaken as part of a two‐year, multi‐stage research project called 'Communicate to Vaccinate 1' (COMMVAC 1) (Lewin 2011). The COMMVAC project focuses on building research knowledge and capacity to use evidence‐based strategies for improving communication about childhood vaccinations with parents and communities in low‐ and middle‐income countries (LMICs). This review is one of two reviews focusing on communication interventions to inform and/or educate about childhood vaccination. A series of deliberative forums identified the topics of both reviews as highly relevant to vaccine programme managers, policy‐makers and other key stakeholders in LMICs. The companion review focuses on face‐to‐face interventions for informing or educating parents (Kaufman 2013), while this review excludes interventions that target only individual parents or caregivers and focuses on interventions aimed at communities. The two reviews were developed in close collaboration, and our review uses some of the same text as Kaufman 2013 in the Background and Methods sections, with their permission.
Description of the condition
Vaccination has been described as one of the greatest public health achievements of the 20th century (CDC 1999), and is seen widely as a worthwhile and cost‐effective public health measure. Vaccination programmes have led to the global eradication of smallpox, and large reductions in disability and death from polio, measles, tetanus, rubella, diphtheria and Haemophilus influenzae type b (CDC 1999). However, over 24 million children are still without access to this important health intervention (Jheeta 2008; Wiysonge 2009), contributing to millions of preventable child deaths in LMICs (GAVI 2012). Efforts to improve vaccination coverage in LMICs are central to meeting the Millennium Development Goal (MDG) of reducing child mortality (United Nations 2011).
Routine vaccination is also an important issue in high‐income countries (HICs), many of which experience equity‐related challenges to achieving high vaccination coverage rates. A number of socio‐economic factors can affect coverage, including indigenous or ethnic status, poverty, large family size and low educational attainment. Consequently, certain population groups in HICs may have coverage rates below the national average, in some cases as low as those in some LMICs (Thomson 2012). For example, in Australia the 'fully immunised' vaccination coverage estimate in 2009 for Indigenous children aged 12 months was 85%, compared to 92.2% for non‐Indigenous children. However, this disparity was greatly reduced at 24 months, with 90.6% and 92.2% fully vaccinated for Indigenous and non‐Indigenous children respectively (Hull 2011). In the USA, there are still disparities in coverage rates based on socio‐economic status (Wooten 2010). A study from Austria has also shown that low educational attainment and high numbers of children in one family are associated with lower levels of vaccination coverage (Stronegger 2010).
Another cause of regional vaccination coverage variation in HICs is individuals who refuse some or all vaccination of their children. Vaccination objectors are probably more common in HICs and also tend to be grouped in particular regions. While they constitute a small proportion of the country's population overall, they may significantly lower coverage in certain areas (Diekema 2012; Hull 2010).
The term 'vaccine hesitancy' has been used in recent years to help understand behaviour in relation to vaccination. The WHO has defined vaccine hesitancy as "A behaviour, influenced by a number of factors including issues of confidence (do not trust vaccine or provider), complacency (do not perceive a need for a vaccine, do not value the vaccine), and convenience (access). Vaccine‐hesitant individuals are a heterogeneous group who hold varying degrees of indecision about specific vaccines or vaccination in general. Vaccine‐hesitant individuals may accept all vaccines but remain concerned about vaccines, some may refuse or delay some vaccines, but accept others; some individuals may refuse all vaccines" (WHO 2013). Determinants of vaccine hesitancy have been conceptualised as falling into three domains: contextual influences, including socio‐cultural and health systems factors; individual and group influences, including those rising from personal perceptions of a vaccine; and vaccine or vaccination‐specific issues, including individual assessments of risks and benefits and the effects of the mode of administration (WHO 2013).
Large numbers of qualitative and quantitative studies, as well as some reviews, have explored the reasons for vaccine hesitancy and the non‐vaccination of children (Dubé 2013; Larson 2014). Overall, the reviews highlight that vaccination decision‐making is a complex process, influenced by many factors. An important barrier in many settings is not being appropriately informed and, consequently, being in doubt about the trade‐offs between the benefits and harms of vaccination and having fears about side effects (Casiday 2006; Hadjikoumi 2006; Mills 2005; Pearce 2008; Taylor 2002). People may lack knowledge about how vaccinations and the process of immunisation 'works', but also about the diseases which vaccines may help prevent (Casiday 2006; Mills 2005; Woo 2004).
A key barrier to obtaining and using information on vaccination is people's poor understanding of medical and health‐related scientific concepts that are important for decision‐making, such as risk, uncertainty and causality (Casiday 2006; Tickner 2006; Woo 2004). Furthermore, people may not base their decision on evidence‐based information (Paulussen 2006; Tickner 2006). Studies have found that decision‐making about childhood vaccinations is often based on trust and personal experiences (Tickner 2006). For both vaccinators and non‐vaccinators, the decision is based on the intention to minimise the child's exposure to harm (Paulussen 2006; Tickner 2006). However, what people trust and who they trust have been found to differ somewhat between those deciding to vaccinate and those who decline. Vaccinators are generally more positive with regard to conventional sources of information, such as national health institutions and health personnel, while non‐vaccinators have been found to rely more on alternative sources of health information (Casiday 2006; Pearce 2008). The recent attacks on vaccination teams in Pakistan, with almost 30 vaccinators killed in the last two years, illustrate the extent to which suspicions about vaccination, linked to misinformation about the purpose and effects of vaccination, may have significant impacts on both vaccination programme staff and the communities in which they work (Boone 2014).
The perceived dangers of vaccination and the likelihood of vaccine side effects are also important influences on whether parents will allow their children to be vaccinated. Studies have shown that caregivers who do not vaccinate have very different views about the likelihood of a serious side effect than caregivers who take their children for vaccination (Meszaros 1996). Fear associated with vaccination has also been attributed to mass media reports about serious harms associated with vaccination (Woo 2004). Further, evidence suggests that there is more public controversy associated with new vaccines than with the more established vaccines, often based on a perception that new vaccines have not been tested adequately (Tickner 2006). A recent example of such a controversy is the debate about vaccination against swine flu (H1N1 influenza virus) (Teasdale 2011).
The extent to which socio‐demographic variables predict vaccination uptake varies somewhat across study populations. However, studies exploring uptake of, for example, the measles, mumps and rubella (MMR) vaccine have suggested that the number of children in a family, being a single parent and the mother's age may be related to vaccination status (Casiday 2006; Pearce 2008; Wright 2006). Vaccination coverage has also been found to be less optimal in areas with high population density and deprived populations (Wright 2006). Also, populations with higher education levels have been found to have a more rapid decline in coverage (Wright 2006) and to be more sceptical towards vaccination than populations with lower education levels. However, this may be explained by a differential response to health messages across these groups (Pearce 2008).
Other barriers to vaccination uptake include religious or philosophical beliefs (Mills 2005; Taylor 2002); practical issues such as costs, accessibility, forgetting appointments, lack of time and other commitments (Mills 2005; Tickner 2006); or medical issues including having allergies or an illness (Hadjikoumi 2006; Pearce 2008). Studies also indicate that communication between parents and health professionals may not be optimal as a consequence of time constraints during consultations; health professionals delivering information framed as recommendations instead of in ways that would facilitate a decision based on weighing the benefits and risks; and parents perceiving health professionals as biased or unwilling to discuss their concerns (Hobson‐West 2007; Mills 2005; Tickner 2006).
Description of the intervention
The exchange or delivery of information or education is a feature in all contexts of the health system, as people must decide whether or not to participate in health programmes or take particular actions. Such decisions are based on the information and knowledge that people have or acquire (Zyngier 2011). Successful vaccination programmes rely on people having sufficient knowledge to make an informed decision to participate (Shahrabani 2009).
Information provision and education may be undertaken in various ways. This review will focus on interventions aimed at communities to inform or educate about vaccination in children aged six years and younger. The interventions may include: printed materials such as brochures, pamphlets, posters or fact sheets; electronic media such as videos, slide shows, web‐based programmes or audio recordings; and large‐scale media such as billboards, newspaper, television and radio.
Information versus education
We have chosen to examine interventions whose purpose is to inform as well as those whose purpose is to educate. Most texts use the words 'inform' or 'educate' in tandem or interchangeably, and there is no consistent differentiation between these purposes. Kaufman 2012 conducted a content analysis to see how these terms were used, described and defined in practice. They found some consistencies in the ways that agencies and publications describe interventions to 'inform and educate'. Interventions to inform often: are utilised for the provision and dissemination of up‐to‐date, tailored and accurate information; involve limited interaction, as they are mainly targeted to many people at one time; and are recognised as being insufficient to lead to behaviour change (Hollands 2011). Interventions to educate were found to involve verbal communication and some level of interaction; can be supplemented with educational tools, educational materials and written information; and facilitate learning and greater comprehension.
In this review, we looked at interventions to inform and/or educate without distinguishing between them (see below for a definition of these interventions). The goal of these interventions is to achieve outcomes such as knowledge of vaccines, vaccine‐preventable diseases or service delivery; increased involvement in decision‐making; better informed decisions and more confidence in the decision made regarding vaccination; and improved vaccination coverage. Interventions to inform or educate may be tailored to address low literacy levels and can also serve to address misinformation. To better understand the content of these interventions, we recorded all information related to the nature of the intervention for each included study at the data extraction stage.
Delivery mechanisms
Interventions to inform and/or educate that are aimed at communities or groups of community members may be a cost‐effective method of reaching many people. The interventions can be delivered by a range of mechanisms, including face‐to‐face interactions (e.g. vaccination education sessions held at an immunisation carnival; vaccine information disseminated at public meetings or woman's clubs), mass‐media campaigns (e.g. vaccine information disseminated via television, radio, Internet, newspapers, billboards) or mail (e.g. postcards, letter or emails). The audience or target group for these interventions may include all people living in the communities in which the intervention takes place; groups with particular characteristics (e.g. young mothers); or virtual communities (e.g. online parents' forums).
Examples of interventions to inform and/or educate communities or community members about childhood vaccination include:
USA: a media‐based education and outreach campaign to improve knowledge and awareness of hepatitis B and children's receipt of hepatitis B vaccination (McPhee 2003a);
India: an information campaign focusing on services to which people were entitled, and consisting of meetings and the distribution of posters and leaflets directed towards resource‐poor rural populations; vaccinations received by infants was one of the outcomes assessed (Pandey 2007); and
Pakistan: evidence‐based, structured, group discussions in the community on the prevalence of measles among children and the importance of childhood immunisation (Andersson 2009).
This review aimed to assemble the global evidence on interventions to inform and/or educate communities or community members about childhood vaccination. We were interested in whether the effects of these interventions varied by type of delivery mechanism, and planned subgroup analyses to explore this (see Appendix 1).
How the intervention might work
The interventions aim to increase participants' levels of knowledge and/or change their attitudes regarding vaccination. Changes in knowledge and/or attitudes can be regarded as intermediate outcomes, and may lead to at least two more distal outcomes:
a change in the number of participants who make informed decisions regarding childhood vaccination (which may include the decision not to vaccinate); and
a change in childhood vaccination rates.
It is important to note that the pathway from improved knowledge and information to changes in attitudes towards vaccination and, finally, to improved uptake of vaccination is not necessarily linear or simple. Increased knowledge may, for example, result in more informed decision‐making among caregivers, but not in increased childhood vaccination uptake.
As noted above, interventions aimed at communities may be a cost‐effective method of reaching many people. In addition, the sharing and discussion of information within groups, such as women's groups, may enhance the uptake of this information, raise awareness of childhood vaccination issues and facilitate informed decision‐making regarding vaccination.
Why it is important to do this review
The COMMVAC project held a series of deliberative forums (both face‐to‐face and online) in June and July 2011 to discuss the project's taxonomy of communication interventions and to determine priority topics for systematic reviews of effects. Those invited to participate included vaccination programme managers, policy‐makers, researchers and other vaccination stakeholders. The forums included representatives from HICs and LMICs, but focused on the needs of LMICs in particular. The participants identified interventions aimed at communities or community members as a strategy that is used widely and is highly relevant (Willis 2013). However, the implementation of these interventions requires resources including money, time and trained personnel (UNICEF 2000). It is therefore critical to determine whether interventions aimed at communities to inform and/or educate are effective, so as to inform decisions about the use of resources on these strategies.
Overlap with other reviews
Our review is closely related to two other reviews: 'Interventions for improving coverage of child immunisation in low‐ and middle‐income countries' by Angela Oyo‐Ita and colleagues (Oyo‐Ita 2011) and 'Face‐to‐face interventions for informing or educating parents about early childhood vaccination' by Jessica Kaufman and colleagues (Kaufman 2013). It also has some overlap with other reviews. Table 2 describes the differences between our review and these other reviews. This review and the Kaufman review ('the COMMVAC reviews') consider specific sets of 'communicate to vaccinate' interventions, but with a global rather than a LMIC focus. We know from the COMMVAC mapping process that a significant proportion of these communication interventions have not been evaluated in LMICs. Although there is some overlap between Oyo‐Ita 2011 and the COMMVAC reviews, these global reviews will provide a more complete picture of the effects of these specific communication interventions (while the Oyo‐Ita review considers all interventions to increase vaccination coverage, but is restricted to studies conducted in LMICs). The review author teams for these three reviews were in close contact and addressed any overlap by:
1. Comparison between this review and other reviews on related topics.
| Review citation | Focus of cited review | Comparison with our review |
| Briss 2000 | This review considers the effects of population‐based interventions to improve vaccination coverage in children, adolescents and adults | Briss 2000 only includes studies from industrialised settings and studies published up to 1997, although it includes a wider scope of interventions than our review. Our review includes studies conducted in any setting and focusing on children, and updates findings from the Briss review for this range of interventions |
| Grilli 2002 | This review assesses the effect of mass media intervention on the utilisation of health services. 20 studies were included in the review; 2 were relevant to vaccination | Our review only includes mass media interventions focused on vaccination and not those focusing on other health services or issues Grilli 2002 has not been updated since 2001 |
| Stone 2002 | This review assesses the effects of interventions to increase the use of adult immunisation | Our review focuses on immunisation in children only |
| Maglione 2002 | This reviews assesses the effects of mass mailings designed to increase utilisation of influenza vaccine among Medicare beneficiaries in the USA | Our review focuses on immunisation in children only |
| Jacobson 2005 | This review assesses the effectiveness of patient reminder and recall systems to improve immunisation rates, and compares the effects of various types of reminders in different settings or patient populations | Our review excludes reminder and recall systems where there is no information and/or education component or purpose |
| Lewin 2010 | This review assesses the effects of lay health worker interventions on maternal and child health and the management of infectious diseases. 82 trials are included in the review; 8 are potentially relevant to vaccination. 7 of these deal with childhood vaccination | Our review is not limited to interventions delivered by lay health workers. Any childhood immunisation interventions included in the Lewin 2010 review and that were also aimed at communities were considered for inclusion in this review |
| Glenton 2011 | This review assesses the effects of lay health worker interventions on the uptake of childhood immunisation and develops a typology of intervention models | Our review is not limited to lay health worker interventions |
| Oyo‐Ita 2011 | This review evaluates the effectiveness of strategies to increase childhood vaccination rates in low‐ and middle‐income countries. 6 studies are included in the review. 4 involve some form of communication with parents or communities | This review only includes studies conducted in low‐ and middle‐income countries. Our review includes studies conducted in any location |
|
Williams 2011 |
This review assesses the effectiveness of strategies to improve childhood immunisation uptake in primary care settings in developed countries. Strategies may be directed to consumers or practitioners and include remind or recall interventions, education, parent‐held records and feedback | The studies included in this review were conducted in high‐income countries only and in primary care settings, while the scope of our review includes all locations and settings |
| Cairns 2012 | This review examined evidence on the effectiveness of European promotional communications for national immunisation schedule vaccinations. The review aimed to: describe the types of promotional communication that have been used; assess the quality of the evaluations these promotional communication interventions; and assess the applicability of this evidence to immunisation policy, strategy and practice priorities in Europe | Cairns 2012 considered immunisation in adults, adolescents and children and included studies conducted in a European country and evaluating a promotional communication intervention. Our review focuses on community‐directed interventions to inform or educate about childhood vaccination only and also includes studies from any setting. None of the studies in Cairns 2012 that focused on childhood vaccination and evaluated interventions aimed at communities were eligible for inclusion in our review |
| Kaufman 2013 | A companion COMMVAC review focused on face‐to‐face interventions directed at parents | Our review includes interventions which target community members (the general public), including, for example, parents and other caregivers and family members of young children, community leaders, teachers, health personnel (as part of a wider community intervention), and other influential community members We excluded interventions that targeted individuals directly and were not aimed at communities ‐ these interventions are considered in the Kaufman review |
| Sadaf 2013 | This review assessed the evidence on interventions to decrease parental vaccine refusal and hesitancy toward recommended childhood and adolescent vaccines | Sadaf 2013 includes a wider scope of interventions and study designs than our review and also included interventions focused on adolescents and young adults. The review does not specifically address the effects of interventions aimed at communities and does not include any studies that were eligible for inclusion in our review |
| Dubé 2013 | This review considers the possible causes of vaccine hesitancy in low‐ and middle‐income countries and the determinants of individual decision‐making about vaccination | This review did not focus on the effects of interventions to inform or educate about early childhood vaccination |
| Larson 2014 | This review aimed to: 1) identify research on vaccine hesitancy; 2) identify determinants of vaccine hesitancy in different settings; and 3) inform the development of a model for assessing determinants of vaccine hesitancy in different settings | This review did not focus on the effects of interventions to inform or educate about early childhood vaccination |
outlining clearly in each review the scope of the related reviews;
discussing studies identified from LMIC and HIC settings, and any differences and similarities between these;
discussing the effects of communication interventions in relation to other interventions to improve immunisation coverage, as identified in Oyo‐Ita 2011.
Objectives
To assess the effects of interventions aimed at communities to inform and/or educate people about vaccination in children six years and younger.
Methods
Criteria for considering studies for this review
Types of studies
Interventions to 'inform and educate' that aim to reach communities, or groups of community members, are likely to have been evaluated using a wide variety of approaches and designs. For some of these interventions, for example those delivered through mass media such as newspapers or radio, randomisation may not be feasible and other evaluation designs may be needed.
We therefore included the following types of studies.
Randomised controlled trials (RCTs), with randomisation at either individual or cluster level. For cluster‐RCTs, we only included those with at least two intervention and two control clusters.
Quasi‐randomised controlled trials, with allocation at either individual or cluster level. We included studies that allocated by alternation between groups, by the use of birth dates or weekdays or by other quasi‐random methods. For cluster trials, we only included those with at least two intervention and two control clusters.
Interrupted time series (ITS) and repeated measures studies with a clearly defined point in time when the intervention occurred and at least three data points both before and after the intervention.
Controlled before‐and‐after (CBA) studies with a minimum of two intervention and two control sites; comparable timing of the periods of study for the control and intervention groups; and comparability of the intervention and control groups on key characteristics.
Types of participants
We included interventions which targeted groups of people (the general public), including, for example, parents and other caregivers and family members of young children, community leaders, teachers, health personnel (as part of a wider community intervention) and other influential community members. Some of these groups are the 'end' target group for vaccination communication interventions (such as parents and other caregivers) while other groups are 'intermediaries' who are targeted because of their ability to convey information to or educate the end target group. Such intermediaries include community leaders, teachers and other influential community members, such as religious leaders.
We excluded interventions that targeted individuals directly and were not aimed at communities. Hence we excluded studies in which the interventions targeted individual parents or caregivers directly, except where these individuals functioned as the control group for an eligible intervention.
Some studies examined interventions aimed at a group (such as mothers of children attending a paediatric clinic) that comprised individual people attending a health facility for a health issue related to the intervention (i.e. they had no pre‐existing group relationship to one another, and were selected to participate as individuals). In such cases, we excluded the study if the group was constituted for the purposes of the trial, but included it if the group constituted a social or natural group prior to the trial.
Types of interventions
We included interventions aimed at communities, with a broad audience and purpose (see definition below) and that were intended to inform and/or educate about vaccination in children six years and younger. We defined 'inform and/or educate' interventions as those that enabled consumers to understand the meaning and relevance of vaccination to their health and the health of their family or community, and/or made them aware of the practical and logistical factors associated with vaccination. Interventions to inform and/or educate may be tailored to address issues such as low literacy levels or misinformation.
We defined interventions aimed at communities as those directed at a geographic area and/or interventions directed to groups of people who share at least one common social or cultural characteristic. This definition of interventions aimed at communities is based on definitions developed for other reviews, including Baker 2011. These interventions can be delivered by a broad range of people such as health personnel, lay people, governmental institutions, civil society organisations and other non‐governmental organisations. Delivery mechanisms may include: printed materials such as brochures, pamphlets, posters or fact sheets; electronic media such as videos, slide shows, web‐based programmes, virtual online communities or audio recordings; large‐scale media such as billboards, newspaper, television and radio; and face‐to‐face communication with groups of people.
Participants' involvement in these interventions may vary from passive to active. For example, people may be fairly passive recipients of mass media interventions, such as information provided on billboards, or more active participants in community meetings on child health.
We included interventions that aimed to inform and/or educate about vaccination in children six years and younger as well as in older children or others, provided that the main focus of the intervention was on children six years and younger, or that relevant outcomes for children aged six years and younger were reported separately.
We included multi‐faceted interventions if it was possible to separate out the effects of the communication component aimed at communities and that concerned childhood vaccination, i.e. the results for the interventions aimed at communities to inform and/or educate regarding vaccination needed to be reported separately.
We excluded interventions focused on reminding or recalling recipients regarding vaccination if they did not include an information and/or education component or purpose.
We included relevant studies conducted in any setting (including LMICs and HICs).
Comparisons:
-
Interventions aimed at communities to inform and/or educate versus:
routine immunisation practices in the study setting (i.e. the activities undertaken on a day‐to‐day basis in the study setting to promote immunisation uptake and deliver immunisation services, such as sending reminders to caregivers or writing the next immunisation date on the child's health card);
other interventions to promote immunisation uptake; or
no intervention.
Interventions aimed at communities to inform and/or educate versus interventions directed specifically to individual parents or caregivers of children.
One community‐aimed intervention to inform and/or educate versus another community‐aimed intervention to inform and/or educate.
Types of outcome measures
We only included studies if they assessed any of the following primary or secondary outcomes:
Primary outcomes
Knowledge among participants of vaccines or vaccine‐preventable diseases.
Knowledge among participants of vaccine service delivery.
Immunisation status of child (e.g. immunisation status up‐to‐date as defined by the author of the included study: receipt of one or more vaccines).
Any other measures of vaccination status in children (e.g. immunisation status for a specific vaccine, number of vaccine doses received).
Unintended adverse effects due to the intervention.
Immunisation status may be defined slightly differently across studies (e.g. receipt of a single or multiple vaccines; timeliness of vaccination). We have accepted the definition of immunisation status used by study authors.
Secondary outcomes
Participants' attitudes towards vaccination (the term 'attitudes' covers beliefs about vaccination, and may include intention to vaccinate).
Participant involvement in decision‐making regarding vaccination.
Participant confidence in the decision made regarding vaccination.
Resource use or cost of intervention.
We included the first three of the secondary outcomes listed above in this review as they relate to the pathway from improved knowledge and information to changes in attitudes towards vaccination and, finally, to improved uptake of vaccination (see Background).
The occurrence of vaccine‐preventable diseases is an outcome of immunisation, rather than of an intervention to inform and/or educate, and is affected by many other factors. We therefore have not reported this outcome.
To accommodate multiple or varying outcome assessment time points, we have recorded outcomes in the following categories:
Immediate: up to one month following completion of the intervention.
Short‐term: between one and six months following the completion of the intervention.
Long‐term: more than six months following the completion of the intervention.
Search methods for identification of studies
Electronic searches
We searched the following international and regional sources:
Cochrane Central Register of Controlled Trials (CENTRAL) (11 July 2012);
MEDLINE (OvidSP) MEDLINE In‐Process & Other Non‐Index Citations (OvidSP) (1946 to June 2012);
EMBASE (OvidSP) (1947 to July 2012);
CINAHL (EbscoHOST) (date of search: 19 July 2012);
PsycINFO (OvidSP) (1806 to July 2012);
ERIC (ProQuest) (1966 to July 2012);
Global Health (CAB) (1910 to June 2012);
WHO Global Health Library (including WHOLIS, LILACS and other regional databases) (date of search: 26 July 2012).
We also searched the following grey literature databases:
The Grey Literature Report: http://www.nyam.org/library/online‐resources/grey‐literature‐report/;
OpenGrey: http://www.opengrey.eu/.
All the strategies are presented in Appendix 2. Strategies have been tailored to the other databases and are reported in the Appendix. There were no date restrictions in the searches.
We conducted the searches in English. We assessed titles and abstracts published in any language, and obtained translation of potentially eligible studies where needed.
Searching other resources
We contacted the authors of included studies and the members of the COMMVAC project (www.commvac.com) advisory group for additional references.
Data collection and analysis
The data collection and analysis methods were described in the review protocol (Saeterdal 2012). We used Reference Manager software version 12 to store the records retrieved from the search (Reference Manager 12). We then used the Early Review Organizing Software version 2.0 to manage the records retrieved from the search following initial eligibility assessment and to facilitate full‐text screening of papers (EROS 2012).
Selection of studies
The review authors worked in pairs to independently screen all titles and abstracts identified in the search, and assess eligibility based on the Criteria for considering studies for this review. We retrieved all potentially eligible references in full text and the review authors then worked in pairs and independently assessed these references for inclusion against the Criteria for considering studies for this review. We resolved any disagreements through discussion and consulted one of the other review authors when required.
Data extraction and management
Two review authors working in pairs extracted data independently from all included studies. Data extraction was informed by lessons learned in the earlier evidence mapping stages of the COMMVAC project (Willis 2013). We used a data extraction form that combined features of the template developed by the Cochrane Consumers and Communication Review Group (CC & CRG 2011), the Cochrane equity checklist (Ueffing 2011), and the COMMVAC data extraction template. The form included questions to capture the following data:
Identification details of the study: authors, year of publication, the country and setting in which it was conducted, language and study design.
Participant characteristics: type of participant (type of community member), numbers, gender, age, ethnicity, religion, socio‐economic status, level of education, etc. We also recorded type and characteristics of the community (geographic area, social or cultural characteristics, local vaccination policy).
Intervention characteristics: type of intervention, intervention purpose, content of communication, intervention delivery mechanism. We also recorded information about the vaccine that was the focus of the intervention. We recorded similar characteristics for the comparator.
Outcomes: outcome data (results), methods for assessing/measuring the outcome data, length of follow‐up, loss to follow‐up data. We noted additional outcomes in the data extraction template.
One of the authors (IS) entered the data into the Review Manager software (RevMan 2012) and second author (AAD or SMB) then checked the data. We resolved disagreements regarding the data extracted by discussion and, when necessary, by consulting a third review author (CG or SL).
For studies published only as abstracts, we planned to contact the study authors for further information and to list these studies under 'Studies awaiting classification' until further information was obtained. For study reports that contained little information about methods and results, we contacted the authors to obtain further details on these elements.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the guidelines of the Cochrane Consumers and Communication Review Group (Ryan 2011), which recommends the explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting; and any other sources of bias such as contamination. For each domain we have described the relevant information provided by the authors and judged each item as being at high, low or unclear risk of bias as set out in the criteria provided by Higgins 2011.
For cluster‐RCTs we also assessed the risk of bias associated with an additional domain: selective recruitment of participants (Ryan 2011). Two authors assessed the risk of bias of included studies independently. We resolved disagreements between review authors regarding 'Risk of bias' assessment by discussion and, when necessary, by consulting a third review author (CG or SL).
We did not include any ITS or CBA studies. For a description of the methods we intended to use, see Appendix 1.
Overall risk of bias
We summarised the risk of bias on two levels: within studies (across domains) and across studies (for each primary outcome). Judgement on the overall risk of bias took into account the likely magnitude and direction of the bias and whether we considered the bias to impact on the findings.
We deemed studies to be at highest risk of bias if they scored 'high risk' in one or more of the following domains: sequence generation; allocation concealment; or selective outcome reporting (based on growing empirical evidence that these three factors are the most important in influencing risk of bias) (Higgins 2011). We judged the overall risk of bias as low if we assessed these key domains as low risk of bias, unclear if we assessed one or more key domains as unclear risk of bias, and high if we assessed one or more key domains as high risk of bias.
For the assessment across studies, the main findings of the review have been set out in 'Summary of findings' (SoF) tables prepared using GRADE profiler software (GRADEpro 2008). We have listed the primary review outcomes for each comparison with estimates of relative effects, along with the number of participants and studies contributing data for those outcomes. For each individual outcome, we have assessed the certainty of the evidence using the GRADE approach (Balshem 2011), which involves consideration of limitations in design, inconsistency, indirectness, imprecision, publication bias, magnitude of the effect, dose‐response effect and other plausible confounders. We have expressed the results as one of four levels of certainty (high, moderate, low or very low).
Measures of treatment effect
Dichotomous outcomes
For the included RCTs, we have recorded outcomes in each comparison group. Where possible we recorded or calculated risk ratios (RRs) for dichotomous outcomes. Where adjusted analyses were reported, we used estimates of effect from the primary analysis reported by the investigators and converted these to RRs, if possible.
Interrupted time series
We did not include any ITS studies. For a description of the methods we intended to use, see Appendix 1.
Continuous outcomes
For continuous outcomes, we have reported adjusted mean differences with 95% confidence intervals. We did not combine data from continuous outcomes. For a description of the methods we intended to use, see Appendix 1.
Studies reporting multiple measures of the same outcome
This issue did not arise for the studies included in this review. The methods we intended to use to manage this are described in Appendix 1.
Unit of analysis issues
Both included trials were cluster‐randomised. Although the results of these studies were adjusted for clustering, Andersson 2009 did not report risk ratios or intracluster correlation coefficients (ICCs) and further analysis was therefore required to calculate these. As this trial provided data on the cluster level, a formal re‐analysis was possible. For the outcome 'Proportion of children (12 to 23 months) reported to have received measles vaccine', we estimated the ICC as 0.25, and for the 'Proportion of children (12 to 23 months) reported to have received a full course of DPT (diphtheria, pertussis and tetanus) vaccine', we estimated the ICC as 0.14. We then used the more conservative ICC of 0.25 for re‐analysis of the polio data from Andersson 2009 (for which insufficient information was available to calculate an ICC).
Dealing with missing data
We contacted the study authors where outcome data were unclear or not reported fully.
For all outcomes, we planned to carry out analysis, as far as possible, on an intention‐to‐treat basis. That is, we planned to include all participants randomised to each group in the analyses, and to analyse data according to initial group allocation irrespective of whether or not participants received, or complied with, the planned intervention. For the two studies included in the review, intention‐to‐treat analysis was not applicable as these were cluster‐RCTs and the individual patient data were drawn from cross‐sectional surveys of children's immunisation status.
When assessing adverse events, adhering to the principle of 'intention‐to‐treat' may be misleading. We therefore planned to relate the results to the treatment received. This means that for side effects, we planned to base the analyses on the participants who actually received treatment and the number of adverse events that were reported in the studies. We did not undertake analysis of adverse events as neither of the included studies reported such data.
Assessment of heterogeneity
Since we did not combine data for any of the outcomes, assessment of heterogeneity is not possible. The method for how we intended to do this is described in Appendix 1.
Assessment of reporting biases
We had planned to generate funnel plots if more than 10 studies reported the same outcome of interest ‐ see Appendix 1.
Data synthesis
We have presented the results from the included studies in 'Summary of findings' tables (see Higgins 2011, chapter 11), prepared using the GRADE profiler software (GRADEpro 2008). Since the review included study results that could not be pooled because the settings and/or interventions were too heterogeneous, we have described the results in a narrative form. We have included the narrative information in the 'Summary of findings' table.
For a description of how we had planned to combine and present an overall estimate of treatment effect if more than one study had examined similar interventions, see Appendix 1.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were not possible due to the small number of studies included in this review. The planned methods for subgroup analysis are described in Appendix 1.
Sensitivity analysis
As there was a small number of included studies and because meta‐analysis was not conducted, it was not possible to carry out a sensitivity analysis to examine the effects of removing studies at overall high risk of bias across domains (based on 'Risk of bias' assessment within studies). As no individually randomised trials were included, we did not carry out a sensitivity analysis to examine the effects of removing data obtained from cluster‐randomised trials from meta‐analyses combining data from both individually and cluster‐randomised trials.
Consumer participation
Those with an interest in this review include vaccine programme managers, policy‐makers, practitioners and other community members (for example, parent interest groups, caregivers and family members of young children, teachers). The topic of this review was identified through a series of deliberative forums with stakeholders, as part of the 'Communicate to vaccinate 1' (COMMVAC 1) project (www.commvac.com). Reports of two of these deliberative forums are available on the COMMVAC website (see: http://www.commvac.com/publications.html#deliberative). In addition, in the peer review process we have liaised with the Cochrane Consumers and Communication Review Group to seek external referees reflecting the interests of relevant stakeholder groups.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified a total of 9130 records from the electronic databases (see flow chart of study selection in Figure 1). Of these, we excluded 8987 references based on titles and abstracts and retrieved 143 references in full text and assessed them for eligibility. We included two studies.
1.
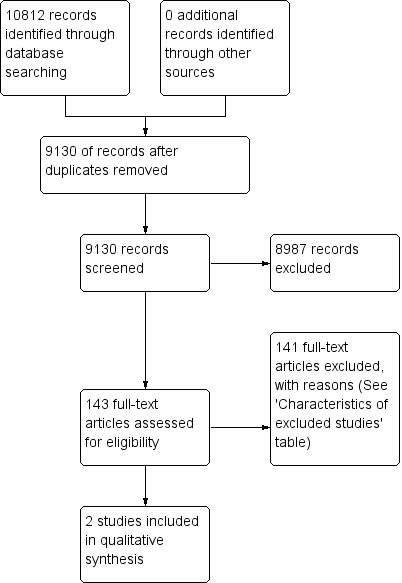
Study flow diagram.
Included studies
Two cluster‐randomised studies were included in this review (Andersson 2009; Pandey 2007): see Characteristics of included studies, which reports the numbers of participants who were assessed for the various outcomes and the details of the interventions delivered. Both studies compared interventions aimed at communities to inform and/or educate about early childhood vaccination with routine immunisation practices. We obtained additional data from the study authors for Pandey 2007.
Pandey 2007 involved 1050 households selected from 21 districts in the northern Indian state of Uttar Pradesh. At baseline, 46% of children in the control and intervention sites were immunised. Both low‐, middle‐ and high‐income households were included. Andersson 2009 involved 32 enumeration areas (including 5641 children) from a lower‐middle‐income district in Pakistan's Balochistan province. Each enumeration area included four or five villages. At baseline, measles vaccination rates among children (12 to 23 months) were 49% and 47% in the trial control and intervention clusters, respectively. For DPT (full schedule), they were 45% and 51%, respectively; and for polio vaccine in the last 12 months they were 100% and 99%, respectively.
In the Pandey 2007 study, the intervention consisted of information campaigns in each intervention cluster, conducted in two rounds separated by two weeks. Each round consisted of two to three meetings with each meeting lasting about one hour and consisting of a 15‐minute audiotape presentation that was played twice and opportunities to ask questions. Posters and leaflets were also distributed in the intervention villages (Pandey 2007). The intervention in the Andersson 2009 study comprised three phases of discussions in each community with small community groups of 8 to 10 people. In the first phase, the community groups considered information about child vaccination in their area and why vaccination might be important. In the second phase, the groups discussed the costs and benefits of vaccination, including the complications related to vaccine‐preventable illnesses and the adverse effects of vaccination. In the third phase, the groups considered the challenges to child vaccination in their settings and developed local action plans to address these, including ways to extend the discussions about vaccination to others in their community and ways to improve vaccination service access. Group participants were encouraged to share the information and continue the dialogue with households in their communities. The interventions were aimed at parents, other family members, village leaders, children and sometimes teachers (Pandey 2007) and, for Andersson 2009, at people selected because they were perceived to be trusted within their community and able to convince others.
We did not identify any studies that compared interventions aimed at communities to inform and/or educate and other interventions to promote immunisation uptake; or studies that compared these interventions with no intervention.
Excluded studies
We excluded 141 studies after screening the full texts (see Characteristics of excluded studies). The main reasons for exclusions were that the intervention or study design used did not meet our inclusion criteria.
Risk of bias in included studies
Assessments of risk of bias for the included studies are shown in the Characteristics of included studies table and are summarised in Figure 2 and Figure 3. We have reported risk of bias across all outcomes for each study as we assessed that the risk of bias did not differ significantly across outcomes within the studies. We judged both the included studies to be of unclear to low risk of bias, since they had low risk of bias for sequence generation; low risk of bias for allocation concealment; and low (Andersson 2009) or unclear (Pandey 2007) risk of bias for selective outcome reporting. These were the factors that we had determined a priori to be the most important in influencing overall risk of bias.
2.
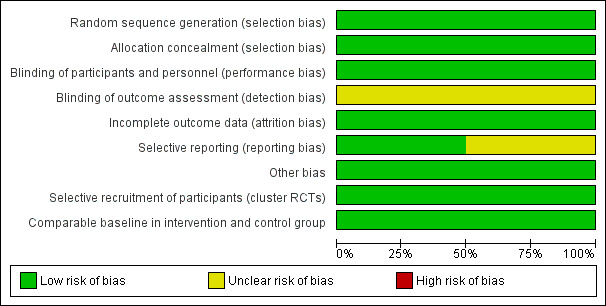
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
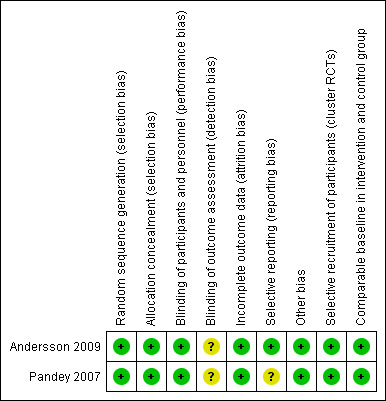
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both studies were at low risk of bias for random sequence generation. A random number generator was used to select communities for assignment to intervention and control groups. Allocation concealment was adequately described in one of the studies (Andersson 2009), but not mentioned in Pandey 2007.
Blinding
For these interventions, it was not possible to blind participants in the intervention clusters to receipt of the intervention. However, the clusters were spread geographically, so the risk of contamination between the clusters was probably low. In both of the included studies the field co‐ordinator for the surveys knew which clusters had received the intervention but the interviewers did not. The follow‐up interviews were performed by a research assistant who had no knowledge of the intervention. We assessed the studies to be at low risk of bias for performance bias, but unclear risk of bias for detection bias. We assess it as unlikely that it was possible to maintain the blinding of the people who performed the analysis.
Incomplete outcome data
We assessed the included studies to be at low risk for attrition bias. There was no loss of clusters in the Andersson 2009 trial and all loss of households in Pandey 2007 was accounted for by households having moved to another area prior to the final survey.
Selective reporting
We assessed Andersson 2009 as low risk as the published study protocol does not include any outcomes that were not assessed in the published trial report. For Pandey 2007, we were not able to identify a published protocol and were therefore not able to assess if all outcomes were reported. This domain was therefore assessed to be at unclear risk of bias.
Other potential sources of bias
We assessed that the trials were at low risk for other sources of bias.
Recall bias: information regarding the vaccination was obtained by interview. However, since any recall bias should have influenced both arms of the trial, we assessed the risk of bias to be low.
Selective recruitment of participants: as the study clusters were scattered geographically, it is unlikely that the participants knew which villages were control or intervention clusters. We therefore assessed this risk of bias to be low.
Groups comparable at baseline: there was a slightly uneven distribution of low‐caste versus mid‐to‐high‐caste households in one of the studies (Pandey 2007). However, we assessed the risk of bias to be low because the baseline differences were small. Willingness to travel to vaccinate was higher in intervention than control cluster (P value = 0.009) in the other study (Andersson 2009), but this was adjusted for in the analysis and we assessed the risk of bias to be low.
Effects of interventions
See: Table 1
Comparison 1: Interventions aimed at communities to inform and/or educate about early childhood vaccination versus routine immunisation practices in the study setting
Two studies assessed interventions aimed at communities to inform and/or educate about early childhood vaccination, versus routine immunisation practices in the study setting (Andersson 2009; Pandey 2007).
Primary outcomes
Knowledge among participants of vaccines or vaccine‐preventable diseases
One study reported results that we assessed as addressing knowledge among participants of vaccine or vaccine‐preventable diseases (Andersson 2009). The outcome was measured by surveying whether respondents were aware of an illness preventable by vaccination. The trial suggested that the intervention may improve knowledge of vaccine‐preventable diseases among intervention participants (adjusted mean difference 0.121, 95% confidence interval (CI) 0.055 to 0.189, low certainty evidence) at two years following the intervention. (Also see Table 3).
2. Data table for knowledge among participants of vaccine or vaccine‐preventable diseases, participants' attitudes towards vaccination and participant involvement in decision‐making regarding vaccination1.
| Outcome | Intervention clusters: number of parents (mean) | Control clusters: number of parents (mean) | Adjusted mean difference2 |
| Respondents were aware of an illness preventable by vaccination (knowledge) | 2368/3153 (mean 0.74) | 1437/2431 (mean 0.58) | 0.121 (95% CI 0.06 to 0.19) |
| Respondents thought it was worthwhile to vaccinate children (attitudes) | 3006/3161 (mean 0.95) | 2116/2475 (mean 0.84) | 0.054 (95% CI 0.01 to 0.11) |
| Mothers included in decisions about vaccination (involvement) | 1834/3131 (mean 0.59) | 1345/2434 (mean 0.54) | 0.043 (95% CI ‐0.01 to 0.1) |
Knowledge among participants of vaccine service delivery
The included studies did not assess this outcome.
Immunisation status of child
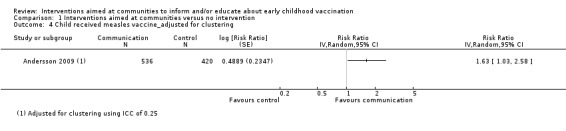
Children's immunisation status was measured in both of the included studies. However, it was not possible to pool these results as the interventions were too different. Pandey 2007 found that the intervention probably increases the number of children who received one or more vaccinations, compared to the control group (risk ratio (RR) 1.67, 95% CI 1.21 to 2.31, moderate certainty evidence, Analysis 1.2). For Andersson 2009, the results indicate that the intervention probably increase the uptake of both measles and the full course of diptheria, pertussis and tetanus (DPT) vaccines (RR 1.63, 95% CI 1.03 to 2.58 for measles, Analysis 1.4; RR 2.17, 95% CI 1.43 to 3.29 for DPT, Analysis 1.6). For both, the evidence was of moderate certainty. The intervention may make little or no difference to the number of children who received polio vaccination in the last 12 months (RR 1.01, 95% CI 0.97 to 1.05, low certainty evidence, Analysis 1.8). For polio, this may be because vaccination rates for both intervention and control sites were very high at baseline (see Analysis 1.7). Figure 4 summarises these findings.
1.2. Analysis.
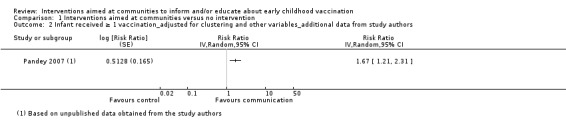
Comparison 1 Interventions aimed at communities versus no intervention, Outcome 2 Infant received ≥ 1 vaccination_adjusted for clustering and other variables_additional data from study authors.
1.4. Analysis.
Comparison 1 Interventions aimed at communities versus no intervention, Outcome 4 Child received measles vaccine_adjusted for clustering.
1.6. Analysis.
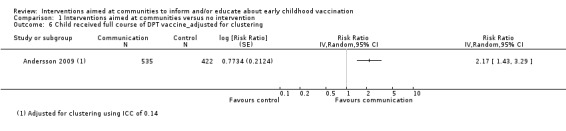
Comparison 1 Interventions aimed at communities versus no intervention, Outcome 6 Child received full course of DPT vaccine_adjusted for clustering.
1.8. Analysis.
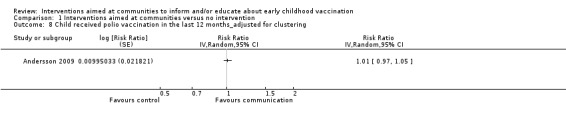
Comparison 1 Interventions aimed at communities versus no intervention, Outcome 8 Child received polio vaccination in the last 12 months_adjusted for clustering.
1.7. Analysis.

Comparison 1 Interventions aimed at communities versus no intervention, Outcome 7 Child received polio vaccination in the last 12 months_unadjusted.
4.
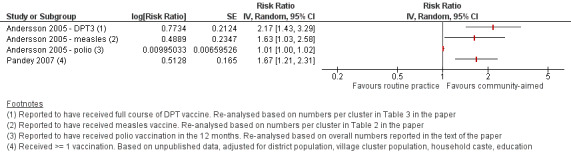
Forest plot of comparison: Interventions aimed at communities versus routine immunisation practices, outcome: Immunisation status of child.
Any other measures of vaccination status in children
The included studies did not assess any other measures of vaccination status in children.
Unintended or adverse effects due to the intervention
None of the included studies reported any unintended or adverse effects due to the intervention.
Secondary outcomes
Participants' attitudes towards vaccination
One study reported results that we assessed to address participants' attitudes towards vaccination (Andersson 2009). The outcome was measured by surveying whether parents of children aged 9 to 60 months thought it was worthwhile to vaccinate children. The trial suggested that the intervention may change attitudes ‐ in this group in favour of vaccination (adjusted mean difference 0.054, 95% CI 0.013 to 0.105, low certainty evidence), at two years following the intervention. (Also see Table 3).
Participant involvement in decision‐making regarding vaccination
One study reported results that we assessed to address participant involvement in decision‐making regarding vaccination (Andersson 2009). The outcome was measured by surveying whether mothers in the study sites in Pakistan were included in household decisions about childhood vaccination. The trial suggested that the intervention may make little or no difference to mothers' involvement in decision‐making regarding vaccination (adjusted mean difference: 0.043 (95% CI ‐0.009 to 0.097), low certainty evidence), at two years following the intervention. (Also see Table 3).
Participant confidence in the decision made regarding vaccination
The included studies did not assess this outcome.
Resource use or cost of the intervention
The included studies did not assess this outcome. Some uncontrolled data on the costs of the interventions are reported in the Characteristics of included studies table.
Comparison 2: Interventions aimed at communities to inform and/or educate about early childhood vaccination versus interventions directed specifically to individual parents or caregivers of children
We did not identify any eligible studies that compared interventions aimed at communities to inform and/or educate about early childhood vaccination with interventions directed specifically to individual parents or caregivers of children.
Comparison 3: One community‐aimed intervention to inform and/or educate about early childhood vaccination versus another community‐aimed intervention to inform and/or educate
We did not identify any eligible studies that compared one community‐aimed intervention to inform and/or educate about early childhood vaccination with another community‐aimed intervention to inform and/or educate.
Discussion
Summary of main results
The two randomised controlled trials (RCTs) identified by this review show low certainty evidence that interventions aimed at communities to inform and educate about childhood vaccination may improve knowledge of vaccine‐preventable diseases and probably improve the immunisation status of children. The study in India showed that these interventions probably increase the number of children who received one or more vaccinations. The study in Pakistan showed that there is probably an increase in the uptake of both measles and diptheria, pertussis and tetanus (DPT) vaccines, and that there may be little or no difference in the number of children who received polio vaccine. There is also low certainty evidence that these interventions may change attitudes in favour of vaccination among parents with young children, but may make little or no difference to the involvement of mothers in household decision‐making regarding childhood vaccination. The included studies did not assess the other review outcomes including knowledge among participants of vaccine service delivery; participant confidence in the decision made regarding vaccination; or any unintended adverse effects as a consequence of the intervention (see Table 1). The sparse data available for several outcomes indicate that further trials are needed.
Overall completeness and applicability of evidence
This review considered studies with a wide range of designs and is based on comprehensive searches, following the methods recommended by The Cochrane Collaboration and the Cochrane Consumers and Communication Review Group and without language or publication status restrictions. However, searches in this area are challenging for several reasons: firstly, there is no single term used in the literature to describe interventions aimed at communities to inform and educate about early childhood vaccination. These interventions encompass a wide range of different approaches and strategies, each of which may be indexed differently within the major medical databases. Secondly, studies are generally not indexed on the basis of the purpose of the intervention, i.e. to inform and educate. Thirdly, although filters have been developed to identify the range of study designs included in this review, these filters may not identify all relevant published studies. It is therefore possible that eligible studies were missed despite our efforts. Finally, it is also possible that we missed eligible non‐randomised studies, published in the grey literature, of interventions aimed at communities. However, we undertook searches of the Grey Literature Report and Open Grey to attempt to identify such studies.
The review searched for RCTs, quasi‐RCTs, controlled before‐and‐after (CBA) studies and interrupted time series (ITS) studies. We included this range of designs as randomisation may not be feasible for some interventions aimed at communities, such as those delivered through mass media. Such interventions may be best assessed using ITS studies. For interventions aimed at specific small groups in communities, such as women's groups, randomised approaches may be both feasible and desirable. Given the focus of this review on interventions aimed at communities, it is perhaps surprising that the two included studies were RCTs. Methodological work on the extent to which reviews on health systems questions identify non‐randomised studies when their selection criteria include these suggests that this is variable, and may depend on whether the intervention addresses governance, financial or delivery arrangements (Glenton 2013). As non‐randomised studies are believed to be at higher risk of bias and their inclusion entails a considerable effort, further work is needed on the types of questions for which these designs are likely to be used, including within the field of vaccination communication, and where they may add value to the evidence obtained from RCTs.
The two included studies evaluated quite similar interventions in which community meetings were used to disseminate vaccination information and build awareness of vaccination, although the Andersson 2009 intervention was more participatory in its design. We identified no eligible studies that used large‐scale media such as billboards, newspaper, television, and radio to inform or educate or that used electronic media such as videos, slide shows, web‐based programmes or virtual online communities. These delivery mechanisms are widely used in low‐, middle‐ and high‐income countries to inform and educate regarding childhood vaccination, and the absence of rigorous evaluations of these mechanisms is therefore an important gap. We also did not identify any studies addressing the following comparisons:
interventions aimed at communities to inform and/or educate versus interventions directed specifically to individual parents or caregivers of children;
one community‐aimed intervention to inform and/or educate versus another community‐aimed intervention to inform and/or educate.
It is therefore not possible to draw reliable conclusions regarding the relative effectiveness of these interventions compared to interventions using other strategies to inform and educate about childhood vaccination, or using other types of strategies (such as interventions to remind and recall) to increase knowledge of vaccination and childhood vaccination uptake. A companion review identified low certainty evidence that face‐to‐face interventions to inform or educate parents about childhood vaccination may have little to no impact on immunisation status, or knowledge or understanding of vaccination (Kaufman 2013). These review findings are discussed in more detail below. A recently published taxonomy of interventions to communicate about childhood vaccination provides a framework that can inform the development of both future trials and reviews in this field (Willis 2013).
The included studies did not assess several review outcomes: knowledge among participants of vaccine service delivery; participant confidence in the decision made regarding vaccination; any unintended adverse effects or harms as a consequence the intervention; and resource use or cost of the intervention. Only uncontrolled data on intervention costs were reported. Intermediate outcomes, such as participant confidence in the decision made regarding vaccination, are important to understanding the pathways between exposure to a communication intervention and a decision regarding childhood vaccination. Others have noted that inadequate assessment of intermediate outcomes is a common limitation of trials in the area of behaviour change (Kaufman 2013; Ryan 2011), making it difficult to understand how an intervention works or does not work. A focus on outcomes such as vaccination uptake may also act to undermine the importance of informed choice in relation to childhood vaccination. Outcomes that assess informed choice, such as confidence in the decision made, are considered important endpoints by many parents, caregivers and members of the public and are central to the principles of evidence‐based practice (Austvoll‐Dahlgren 2010; Coulter 2006; Dawes 2005; Stacey 2011).
The included trials also differed in the ways in which they assessed childhood immunisation status, making it challenging to pool these data. Greater standardisation of these assessments across trials of vaccination interventions would be helpful for future reviews, and the World Health Organization (WHO) monitoring standards may be useful in this regard (WHO 2014). Work on an outcome framework for vaccination communication interventions is also underway as part of the COMMVAC 2 project (http://www.commvac.com/).
Both included studies were conducted in middle‐income countries in South Asia, and the findings may therefore be transferable to similar settings in South Asia or to other middle‐income countries. As the study participants were also relatively poor, the findings may also be transferable to low‐income countries. We outline below some of the factors that decision‐makers need to consider in assessing whether the effects of interventions aimed at communities to inform and educate about childhood vaccination are likely to be transferable to other settings with different systems of health care (see Implications for practice).
Quality of the evidence
The Table 1 summarises the certainty of the evidence for the following key outcomes:
knowledge among participants of vaccine or vaccine‐preventable disease;
knowledge among participants of vaccine service delivery;
immunisation status of the child;
participants' attitudes towards vaccination;
participants' involvement in decision‐making regarding vaccination;
participant confidence in the decision made regarding vaccination;
unintended or adverse effects;
resource use or cost of the intervention.
Using GRADE, we assessed the certainty of the evidence to be moderate to low for outcomes for which data were available. The reasons for these judgements are outlined in the Table 1. We assessed the two included trials as being at low risk of bias. However, there were sparse data for several outcomes indicating that further trials are needed.
Potential biases in the review process
Communication interventions are poorly indexed in electronic databases. To attempt to address this, we searched a large number of databases, including grey literature sources, using a broad range of search terms. There is also no widely accepted definition of 'intervention aimed at communities' or of the intervention purpose: to inform and educate about childhood vaccination. Our definition of 'community‐aimed' was based on that developed for other reviews (Baker 2011). We also used a definition of 'inform and educate' included in a recently published taxonomy of vaccination communication interventions (Willis 2013). However, judgements were required in applying these definitions to identified studies. This is compounded by the fact that many studies do not describe adequately the interventions used. Together these issues may have made it difficult to identify all eligible studies.
A new taxonomy of communication interventions for vaccination, in which these interventions are organised by purpose, may help to provide standard language and terms both for describing these interventions in primary studies and for indexing these interventions in electronic databases (Willis 2013). This could facilitate searching in future review updates.
Agreements and disagreements with other studies or reviews
Our results differ somewhat from those of a companion Cochrane Review assessing the effects of face‐to‐face interventions for informing or educating parents about early childhood vaccination (Kaufman 2013). This companion review found low certainty evidence suggesting that face‐to‐face interventions to inform or educate parents about childhood vaccination may have little to no impact on immunisation status, or knowledge or understanding of vaccination. In contrast, our review suggests that interventions aimed at communities for informing or educating about early childhood vaccination have mixed results: they may improve knowledge of vaccine‐preventable diseases and probably lead to increased vaccination uptake. There is also low certainty evidence that these interventions may improve attitudes towards vaccination among intervention participants but may make to little or no difference to the involvement of mothers in decision‐making regarding vaccination. However, this review identified only two studies and the evidence from both reviews is of moderate to low certainty, suggesting that there is a moderate to high likelihood that the effect will be substantially different from that found in the research (EPOC 2013). A third Cochrane Review of interventions to improve coverage of child immunisation in low‐ and middle‐income countries is currently being updated (Oyo‐Ita 2011). Discussion of the relationship to the results of that review will be addressed in a future update of this review.
Two recently published systematic reviews have explored vaccine hesitancy. One review examined interventions for reducing parental vaccine refusal and vaccine hesitancy and identified 17 studies on the impact of written educational information. Neither of the studies included in our review were included in this review. Many of the included interventions focused on human papillomavirus (HPV), and it is not clear how many of the interventions were aimed at communities to inform or educate regarding childhood vaccination. Overall, the effects of these interventions were mixed (Sadaf 2013).
A second systematic review focused on understanding the determinants of vaccine hesitancy in different settings and did not address the effects of interventions aimed at communities to inform and educate about early childhood vaccination (Larson 2014).
Authors' conclusions
Implications for practice.
This review provides limited evidence to inform decisions regarding the implementation of interventions aimed at communities to inform and educate about early childhood vaccination. Some of these interventions, such as community meetings or some forms of mass media, may be resource intensive when implemented at scale and caution may therefore be needed. Such interventions may need to be targeted to areas or groups that have low childhood vaccination rates and therefore have the potential to benefit most. Other interventions, such as the use of electronic media directed to communities, may be less costly and possibly more feasible at scale. However, it is also important to consider that interventions aimed at communities may be cost‐effective in some settings even if these interventions result in small increases in vaccination uptake, as the costs of non‐vaccination are likely to be very high. The participatory approach used in the Andersson 2009 trial has parallels with that used in a series of trials of participatory women's groups to mobilise communities around maternal and newborn health (Azad 2010; Colbourn 2013; Manandhar 2004; Tripathy 2010). These trials, in which lay health workers facilitated the formation of the women's groups, showed important impacts on neonatal mortality (Azad 2010; Colbourn 2013; Lewin 2010; Manandhar 2004; Tripathy 2010), and perhaps reinforce the value of participatory approaches. Where such interventions directed by communities or aimed at communities are implemented, this should be in the context of rigorous evaluation so as to build the evidence base in this area (see Implications for research).
The two studies identified were conducted in quite similar settings, therefore it is difficult to assess the transferability of the review findings. Factors that need to be considered in assessing whether the effects of interventions aimed at communities to inform and educate about childhood vaccination are likely to be transferable to other settings with different systems of health care delivery include the following (Lavis 2009):
Whether the studies from which the evidence was drawn were conducted in similar settings to that in which the implementation decision is being taken.
Whether there are important differences in on‐the‐ground realities and constraints that might substantially alter the feasibility and acceptability of the intervention, compared to the sites in which the studies were done. For example, whether there are already high levels of knowledge regarding vaccination, its benefits and adverse effects and how to access vaccination services. Also whether there are sufficient resources to support the delivery of interventions aimed at communities (such as community meetings), including for transport and materials and to facilitate access to locations at which vaccination is available.
Whether there are important differences in health system arrangements that may mean that these interventions could not work in the same way as in the sites in which the studies were conducted. For example, if there are no comparable community‐based cadres to deliver these interventions; if there are important direct and indirect financial barriers to vaccination access; or if the organisation of primary care services does not facilitate the scaling up of communication interventions.
Whether there are important differences in the baseline conditions between where the studies were done and the implementation setting. For example, if childhood vaccination rates (for some or all vaccines) are much higher than in the study settings, perhaps making it less cost‐effective to use interventions aimed at communities, which may be fairly resource intensive.
The availability of routine data on who might benefit from the intervention (for example, areas in which there are large numbers of children whose immunisation is not up to date). These data are needed to target these programmes towards the areas of greatest need.
The findings of this review must be considered alongside other relevant reviews in order to understand the best mix of interventions in different settings (see Table 2). A recently published taxonomy of communication interventions for childhood vaccination may assist decision‐makers in considering the range of interventions that might be used to improve vaccination uptake (Willis 2013).
Implications for research.
The implications for research are organised into key messages for trialists, systematic review authors and other researchers.
Trialists
Sparse or no data were available for the outcomes assessed in this review. Given the extensive use across many settings of interventions aimed at communities to inform and educate about early childhood vaccination, it is surprising that so few rigorous evaluations have been conducted. Trials, or other rigorous evaluations, are needed of the following:
interventions aimed at communities and delivered by different groups of people, such as health personnel, lay people, governmental institutions, civil society organisations and other non‐governmental organisations;
interventions aimed at communities and delivered via different mechanisms, including: printed materials such as brochures, pamphlets, posters or fact sheets; electronic media such as videos, slide shows, web‐based programmes, virtual online communities or audio recordings; large‐scale media such as billboards, newspaper, television and radio; and face‐to‐face communication with groups of people;
community‐aimed interventions delivered in different geographical settings.
In planning these trials, investigators should consult the recently published taxonomy of communication interventions for childhood vaccination (Willis 2013); consider how to assess vaccination status; include a logic model outlining the hypothesised pathway/s of the intervention effects (Anderson 2011; Burford 2013; Petticrew 2013), and also relevant intermediate outcomes, such as attitudes towards vaccination and participant confidence in the decision made regarding vaccination; carefully describe the different components of the intervention (and any co‐interventions), their implementation (Burford 2013; Harper 2013; Shepperd 2009) and key elements of the implementation context; and assess possible harms or unintended consequences of the intervention.
Economic studies and process evaluations (Craig 2008; Lewin 2009; Oakley 2006) should also accompany trials to establish the cost‐effectiveness of different interventions aimed at communities to inform and educate, and to better understand the factors affecting their successful implementation.
Systematic review authors
Further systematic reviews, including of studies of effects, process evaluations and economic evaluations, are needed on:
people's views regarding the information they receive on childhood vaccination and on the types of information they would like to receive;
the cost‐effectiveness of interventions aimed at communities to inform and educate about early childhood vaccination;
the effects of other types of communication interventions for childhood vaccination (Willis 2013).
Acknowledgements
We thank John Eyers and John Kis‐Rigo for their help in devising the search strategy and valuable input regarding bibliographic databases. Thanks also to Marit Johansen for agreeing to run the search in the ERIC database, to Jan Odgaard‐Jensen for his valuable comments and help on the statistical issues, and to Kjetil Olsen for administrative support. We also thank the authors of the companion review, Kaufman 2013, for useful discussions while drafting the protocol and review. Anthony Morgan, Chris Morgan and Ruth Stewart provided valuable comments on an earlier version of this review and the Review Group base provided support throughout the review process.
Appendices
Appendix 1. Methods that could not be implemented for this version of the review
This appendix includes text from the methods section of the original protocol for this review (Saeterdal 2012). Due to the small number of included studies and their characteristics, these parts of the methods section were not relevant for this review. However, these methods are described below as they may be relevant when updating the review.
Assessment of risk of bias in included studies: CBAs and ITS studies
For CBAs we will also assess whether the intervention and control groups were comparable at baseline, as part of the assessment of other sources of bias (Ryan 2011).
For ITS studies we will assess the following risk of bias domains (Ryan 2011): the extent to which the intervention was independent of other changes; whether the shape of the intervention effect was pre‐specified; whether the intervention was likely to affect data collection; blinding (participants and personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting; and any other sources of bias.
Measures of treatment effect
Interrupted time series
For ITS studies we will record changes in level and in slope. If papers with ITS design do not provide an appropriate analysis or reporting of results, but present the data points in a graph or in a table that can be scanned, we will re‐analyse the data using methods described in Ramsay 2003.
Continuous outcomes
For continuous outcomes, the effect size will be expressed as mean differences (MDs) with standard deviations if outcomes are measured in the same way between studies. If some studies have reported endpoint data and others have reported change from baseline data (with errors), we will combine these in the meta‐analysis if the outcomes are reported using the same scale (Higgins 2011). We will use standardised mean difference (SMD) with 95% confidence intervals (CIs) to combine data from trials that measure the same outcome but use different scales. We will standardise the data to their effect size by dividing the estimated mean difference by its standard deviation.
For CBA studies, we will use difference in differences between pre‐ and post‐observation in intervention and control group.
Studies reporting multiple measures of the same outcome
When a single study uses two separate methods to measure the same outcome (e.g. two measures of knowledge among participants), or measures two different outcomes that could be considered part of the same outcome category (e.g. beliefs about vaccination and views regarding vaccination, which both fall under 'attitudes of participants towards vaccination'), we will adopt the approach to measures of treatment effect as outlined by Brennan and colleagues (Brennan 2009):
Select the primary outcome identified by the study authors that correlates to our stated outcomes of interest.
If no primary outcome is specified, select the one specified in the sample size calculation.
If no sample size calculations are reported, we will rank the reported effect estimates and select the outcome with the median effect estimate. When there is an even number of outcomes, we will include the outcome whose effect estimate is ranked n/2, where n is the number of outcomes.
Assessment of heterogeneity
We will consider whether there is substantive heterogeneity of settings, interventions or outcomes among the included studies. We will examine the forest plots from the meta‐analyses to visually assess the levels of heterogeneity (in terms of the size or direction of treatment effect and by looking at the overlap between confidence intervals around the treatment effect estimate for each included study). We will employ the Chi2 test to assess whether observed differences in results across studies are compatible with chance alone. When the observed intervention effects are more different from each other than one would expect due to chance alone, we will assume that the studies have 'clinical' and/or statistical heterogeneity. We will use the I2 statistic to quantify the level of statistical heterogeneity among the trials in each analysis. If we identify substantial or considerable heterogeneity (approximately I2 = 50% to 100%) we will note this in the text and explore this heterogeneity through the prespecified subgroup analyses (see 'Subgroup analysis and investigation of heterogeneity').
We will use caution in the interpretation of those meta‐analysis results with high levels of unexplained heterogeneity.
Assessment of reporting biases
We will generate funnel plots using the Review Manager software (RevMan 2012) and visually examine them for asymmetry if more than 10 studies reporting the same outcome of interest are available.
Data synthesis
We will carry out meta‐analysis to provide an overall estimate of treatment effect when more than one study examines similar interventions, provided that: studies use similar methods; studies are homogeneous when it comes to setting; and studies measure the same outcome in similar ways in comparable populations. We will carry out the statistical analysis using Review Manager software (RevMan 2012). We will not combine results from randomised and quasi‐randomised trials together in meta‐analysis, nor will we present pooled estimates for quasi‐randomised studies with different types of study designs.
Evidence on different interventions may be available from different types of studies (for example, it is likely that data from interventions delivered through mass media, such as newspapers or radio, will be reported in quasi‐randomised studies). Where there is evidence on a particular outcome from both randomised trials and quasi‐randomised studies, we will use the evidence from trials which are at lower risk of bias to estimate treatment effect.
We will use a random‐effects meta‐analysis for combining data, as we anticipate that there may be natural heterogeneity between studies attributable to the different interventions, populations and implementation strategies.
For continuous variables we will use the inverse variance method while for dichotomous variables we will use the method proposed by Mantel‐Haenszel. If cluster‐randomised trials are included we will use the generic inverse variance method in RevMan 2012 for meta‐analysis. For quasi‐randomised studies, where results have been adjusted to take account of possible confounding factors, we will use the generic inverse variance method in RevMan 2012 to carry out any meta‐analysis. If both adjusted and non‐adjusted figures are provided we will carry out a sensitivity analysis using the unadjusted figures to examine any possible impact on the estimate of treatment effect.
For ITS and repeated measures studies, the preferred analysis method is either a regression analysis with time trends before and after the intervention, adjusted for autocorrelation and any periodic changes, or auto regressive integrated moving average (ARIMA) analysis. We will attempt to present the results for outcomes as changes along two dimensions: change in level and change in slope. Change in level is the immediate effect of the policy and is measured as the difference between the fitted value for the first post intervention data point (one month after the intervention) minus the predicted outcome one month after the intervention, based on the pre‐intervention slope only. Change in slope is the change in the trend from pre to post intervention, reflecting the 'long‐term' effect of the intervention. Since the interpretation of change in slope can be difficult, we will present the long‐term effects similarly to the way we propose to calculate and present the immediate effects. We will present the effects after half a year as the difference between the fitted value for the sixth month post intervention data point (half a year after the intervention) minus the predicted outcome six months after the intervention based on the pre‐intervention slope only. The effects after one year and two years, if available, will be measured similarly. Where studies report a transition phase, we will exclude these data.
If papers with CBA design do not provide an appropriate analysis or reporting of results, but present the data for each district/region in the intervention and control groups respectively, for dichotomous outcomes we will re‐analyse the data using a generalised linear model to calculate adjusted RR.
We will use the generic inverse variance method for combining the data in a meta‐analysis for ITS and CBA studies.
We will present the results from the meta‐analysis in 'Summary of findings' tables (see Higgins 2011, chapter 11) prepared using GRADE profiler software (GRADEpro 2008). If the review includes study results that cannot be pooled because the settings and/or interventions are too heterogeneous, we will describe the results in a narrative form. We will include the narrative information in the 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
Where data are available we will carry out the following subgroup analyses:
By baseline vaccination status (0% to 50%; 51% to 80%; and 81% to 100% vaccination coverage).
By types of delivery mechanisms (mass media, electronic media, face‐to‐face, single versus multi‐component interventions).
By the target group/s of the intervention (community leaders, teachers, health personnel, the general public).
This review is global in scope but arose from a project with a LMIC focus, so in preparing the review we considered whether the country in which the intervention was delivered would change the effect of the intervention. Consultation indicated that there is insufficient empirical evidence at present to suggest a setting‐based variation in effect. Given that the knowledge and beliefs of parents and other key stakeholders affect vaccination coverage around the world, and that the delivery of information is easily adapted to local settings, we believe that there are not likely to be differences in effect that would justify subgroup analysis based on country income level.
We will use only the primary outcomes in subgroup analyses and limit these analyses to those outcomes for which three or more trials contributed data. We will examine differences between subgroups by visual inspection of the subgroups' CIs; with non‐overlapping CIs suggesting a statistically significant difference in treatment effect between the subgroups. We will test for subgroup differences as described by Borenstein 2008 and implemented in RevMan 2012.
Appendix 2. Search strategies
OVID databases
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) 1946 to present (search result for 3 June 2012).
EMBASE Classic+EMBASE 1947 to 3July 2012 (search result for 7 July 2012).
Global Health 1910 to June 2012 (search result for 7 July 2012).
PsycINFO 1806 to July Week 1 2012 (search result for 7 July 2012).
MEDLINE
1. Immunization/
2. Immunization Schedule/
3. Immunization, Secondary/
4. Immunotherapy, Active/
5. Mass Immunization/
6. Immunization Programs/
7. Vaccination/
8. (vaccinat$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
9. or/1‐8
10. Tetanus Toxoid/
11. Diphtheria Toxoid/
12. Diphtheria‐Tetanus‐Acellular Pertussis Vaccines/
13. Diphtheria‐Tetanus‐Pertussis Vaccine/
14. Diphtheria‐Tetanus Vaccine/
15. Pertussis Vaccine/
16. Measles‐Mumps‐Rubella Vaccine/
17. Measles Vaccine/
18. Mumps Vaccine/
19. Rubella Vaccine/
20. Poliovirus Vaccines/
21. Poliovirus Vaccine, Inactivated/
22. Poliovirus Vaccine, Oral/
23. Tuberculosis Vaccines/
24. BCG Vaccine/
25. Viral Hepatitis Vaccines/
26. Hepatitis B Vaccines/
27. Haemophilus Vaccines/
28. Pneumococcal Vaccines/
29. Meningococcal Vaccines/
30. Rotavirus Vaccines/
31. Japanese Encephalitis Vaccine/
32. Yellow Fever Vaccine/
33. ((tetanus or diphtheria) adj toxoid).tw.
34. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
35. or/10‐34
36. Tetanus/
37. Diphtheria/
38. Measles/
39. Mumps/
40. Rubella/
41. Whooping Cough/
42. Poliomyelitis/
43. Poliomyelitis, Bulbar/
44. Tuberculosis/
45. Tuberculosis, Pulmonary/
46. Mycobacterium Tuberculosis/
47. Hepatitis B/
48. Hepatitis B, Chronic/
49. Haemophilus Influenzae/
50. Haemophilus Influenzae Type B/
51. Pneumococcal Infections/
52. Meningitis, Pneumococcal/
53. Meningitis, Meningococcal/
54. Pneumonia, Pneumococcal/
55. Rotavirus Infections/
56. Japanese Encephalitis/
57. Yellow Fever/
58. Encephalitis, Tick‐borne/
59. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
60. or/36‐59
61. Child/
62. Child, Preschool/
63. Infant/
64. exp Infant, Newborn/
65. Child Care/
66. Infant Care/
67. exp Perinatal Care/
68. exp Parents/
69. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
70. or/61‐69
71. Mothers/
72. Women/
73. Pregnant Women/
74. Female/
75. (woman or women or mother? or female?).tw.
76. or/71‐75
77. exp Communication/
78. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
79. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
80. (readability or intelligibility or credibility).mp.
81. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
82. exp Interpersonal Relations/
83. hospital patient relations/
84. community institutional relations/
85. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
86. ((health or patient or client) adj (education or knowledge or promotion)).mp.
87. exp health promotion/
88. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
89. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
90. (self adj (teaching or education or instruction)).mp.
91. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
92. marketing.mp.
93. ((family or office or work* or school or faith or church) adj based).tw.
94. (educational status or literacy).mp.
95. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
96. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
97. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
98. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
99. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
100. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
101. ((social or community) adj2 network*).mp.
102. (self‐care or self‐management).mp.
103. (motivat* or incentive* or goal*).mp.
104. exp Communications Media/
105. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
106. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
107. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
108. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
109. exp computer systems/
110. software/
111. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
112. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
113. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
114. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
115. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
116. (participation or advocacy or consumer* or empower*).mp.
117. exp decision making/
118. (decision adj (making or support or aid*)).mp.
119. exp informed consent/
120. (informed adj (consent or choice* or decision*)).tw.
121. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
122. (therapeutic adj (relation* or alliance*)).mp.
123. or/77‐122
124. 70 or 76
125. 9 and 60
126. 35 or 125
127. randomised controlled trial.pt.
128. controlled clinical trial.pt.
129. multicenter study.pt.
130. (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab.
131. groups.ab.
132. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
133. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
134. or/127‐133
135. exp Animals/
136. Humans/
137. 135 not (135 and 136)
138. review.pt.
139. meta analysis.pt.
140. news.pt.
141. comment.pt.
142. editorial.pt.
143. cochrane database of systematic reviews.jn.
144. comment on.cm.
145. (systematic review or literature review).ti.
146. 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145
147. 134 not 146
148. 9 and 60
149. 35 or 148
150. 123 and 124 and 147 and 149 (Result 3897)
EMBASE
Searched 3 July 2012
1. immunization/
2. active immunization/
3. mass immunization/
4. vaccination/
5. revaccination/
6. exp vaccine/
7. (vaccin$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
8. or/1‐7
9. tetanus toxoid/
10. diphtheria toxoid/
11. diphtheria pertussis tetanus vaccine/
12. pertussis vaccine/
13. measles mumps rubella vaccine/
14. measles vaccine/
15. mumps vaccine/
16. rubella vaccine/
17. poliomyelitis vaccine/
18. oral poliomyelitis vaccine/
19. BCG vaccine/
20. hepatitis vaccine/
21. hepatitis B vaccine/
22. Haemophilus vaccine/
23. Meningococcus vaccine/
24. Pneumococcus vaccine/
25. Rotavirus vaccine/
26. Japanese encephalitis vaccine/
27. yellow fever vaccine/
28. ((tetanus or diphtheria) adj toxoid).tw.
29. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
30. or/9‐29
31. tetanus/
32. diphtheria/
33. measles/
34. mumps/
35. rubella/
36. pertussis/
37. poliomyelitis/
38. tuberculosis/
39. lung tuberculosis/
40. Mycobacterium tuberculosis/
41. hepatitis B/
42. haemophilus influenzae/ or haemophilus influenzae type b/
43. pneumococcal infection/
44. pneumococcal meningitis/
45. epidemic meningitis/
46. pneumonia/
47. Rotavirus infection/
48. Japanese encephalitis/
49. yellow fever/
50. tick borne encephalitis/
51. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
52. or/31‐51
53. child/
54. preschool child/
55. infant/
56. newborn/
57. child care/
58. perinatal care/
59. exp parent/
60. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
61. or/53‐60
62. mother/ or adolescent mother/ or expectant mother/
63. female/
64. pregnant woman/
65. (woman or women or mother? or female?).tw.
66. or/62‐65
67. exp interpersonal communication/
68. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
69. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
70. (readability or intelligibility or credibility).mp.
71. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
72. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
73. ((health or patient or client) adj (education or knowledge or promotion)).mp.
74. health promotion/
75. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
76. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
77. (self adj (teaching or education or instruction)).mp.
78. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
79. marketing.mp.
80. ((family or office or work* or school or faith or church) adj based).tw.
81. (educational status or literacy).mp.
82. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
83. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
84. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
85. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
86. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
87. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
88. ((social or community) adj2 network*).mp.
89. (self‐care or self‐management).mp.
90. (motivat* or incentive* or goal*).mp.
91. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
92. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
93. social media/
94. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
95. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
96. computer system/
97. computer program/
98. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
99. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
100. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
101. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
102. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
103. (participation or advocacy or consumer* or empower*).mp.
104. decision making/
105. patient decision making/
106. decision support system/
107. (decision adj (making or support or aid*)).mp.
108. parental consent/ or informed consent/
109. (informed adj (consent or choice* or decision*)).tw.
110. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
111. (therapeutic adj (relation* or alliance*)).mp.
112. or/67‐111
113. 61 or 66
114. 8 and 52
115. 114 or 30
116. randomised controlled trial/
117. controlled clinical trial/
118. quasi experimental study/
119. pretest posttest control group design/
120. time series analysis/
121. experimental design/
122. multicenter study/
123. (randomis* or randomiz* or randomly or random allocat*).ti,ab.
124. groups.ab.
125. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
126. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
127. or/116‐126
128. (systematic review or literature review).ti.
129. "cochrane database of systematic reviews".jn.
130. nonhuman/
131. or/128‐130
132. 127 not 131
133. 112 and 113 and 115 and 132 (Result 4936)
Global Health
Searched 6 July 2012
1. immunization/
2. exp vaccination/
3. immunization programmes/
4. immunotherapy/
5. (vaccinat$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
6. or/1‐5
7. tetanus toxoid/
8. diphtheria toxoid/
9. diphtheria pertussis tetanus vaccines/
10. measles mumps rubella vaccines/
11. poliomyelitis/
12. bcg vaccine/
13. ((tetanus or diphtheria) adj toxoid).tw.
14. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
15. or/7‐14
16. exp tetanus/
17. diphtheria/
18. measles/
19. mumps/
20. exp rubella/
21. pertussis/
22. poliomyelitis/
23. exp tuberculosis/
24. mycobacterium tuberculosis/
25. hepatitis b/
26. exp haemophilus influenzae/
27. meningitis/ or bacterial meningitis/ or cryptococcal meningitis/ or neonatal meningitis/
28. rotavirus/
29. japanese encephalitis/
30. yellow fever/
31. tickborne encephalitis/
32. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
33. or/16‐32
34. children/
35. preschool children/
36. infants/ or neonates/
37. child care/
38. exp parents/
39. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
40. mothers/
41. fathers/
42. women/ or housewives/ or lactating women/ or rural women/
43. females/
44. (woman or women or mother? or female?).tw.
45. or/34‐44
46. communication/ or interpretation/ or nonverbal communication/ or verbal communication/
47. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
48. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
49. (readability or intelligibility or credibility).mp.
50. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
51. exp interpersonal relations/
52. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
53. ((health or patient or client) adj (education or knowledge or promotion)).mp.
54. health promotion/
55. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
56. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
57. (self adj (teaching or education or instruction)).mp.
58. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
59. marketing.mp.
60. ((family or office or work* or school or faith or church) adj based).tw.
61. (educational status or literacy).mp.
62. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
63. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
64. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
65. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
66. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
67. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
68. ((social or community) adj2 network*).mp.
69. (self‐care or self‐management).mp.
70. (motivat* or incentive* or goal*).mp.
71. exp mass media/
72. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
73. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
74. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
75. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
76. exp computer techniques/
77. computer software/
78. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
79. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
80. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
81. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
82. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
83. (participation or advocacy or consumer* or empower*).mp.
84. decision making/
85. (decision adj (making or support or aid*)).mp.
86. consent/
87. (informed adj (consent or choice* or decision*)).tw.
88. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
89. (therapeutic adj (relation* or alliance*)).mp.
90. or/46‐89
91. randomised controlled trials/
92. (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab.
93. groups.ab.
94. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
95. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
96. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
97. or/91‐96
98. exp animals/
99. man/
100. 98 not (98 and 99)
101. systematic reviews/
102. meta‐analysis/
103. (systematic review or literature review).ti.
104. literature reviews/
105. or/100‐104
106. 97 not 105
107. 6 and 33
108. 107 or 15
109. 45 and 90 and 106 and 108 (Result 2679)
PsycINFO
Searched 7 July 2012
1. immunization/
2. (vaccinat$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
3. 1 or 2
4. ((tetanus or diphtheria) adj toxoid).tw.
5. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
6. 4 or 5
7. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
8. exp child care/
9. parents/ or adoptive parents/ or fathers/ or foster parents/ or mothers/ or single parents/ or "surrogate parents (humans)"/
10. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
11. mothers/ or parents/ or adolescent mothers/ or single mothers/ or unwed mothers/
12. human females/ or sisters/ or wives/ or working women/
13. (woman or women or mother? or female?).tw.
14. or/8‐13
15. exp Communication/
16. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
17. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
18. (readability or intelligibility or credibility).mp.
19. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
20. family relations/ or interpersonal relationships/ or childrearing practices/ or parental role/
21. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
22. ((health or patient or client) adj (education or knowledge or promotion)).mp.
23. health promotion/
24. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
25. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
26. (self adj (teaching or education or instruction)).mp.
27. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
28. marketing.mp.
29. ((family or office or work* or school or faith or church) adj based).tw.
30. (educational status or literacy).mp.
31. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
32. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
33. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
34. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
35. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
36. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
37. ((social or community) adj2 network*).mp.
38. (self‐care or self‐management).mp.
39. (motivat* or incentive* or goal*).mp.
40. exp communications media/
41. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
42. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
43. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
44. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
45. computer applications/
46. computer software/ or decision support systems/
47. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
48. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
49. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
50. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
51. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
52. (participation or advocacy or consumer* or empower*).mp.
53. decision making/ or choice behavior/ or group decision making/
54. (decision adj (making or support or aid*)).mp.
55. informed consent/
56. (informed adj (consent or choice* or decision*)).tw.
57. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
58. (therapeutic adj (relation* or alliance*)).mp.
59. or/15‐58
60. (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab.
61. groups.ab.
62. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
63. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
64. or/60‐63
65. 3 and 7
66. 6 or 65
67. 14 and 59 and 64 and 66
68. 14 and 59 and 66 (Result 187)
COMMVAC Multifile Community‐wide –(files pmoz emczd cagz psyh)
Duplicates removed & MEDLINE excluded 7 July 2012 – Result 7802
1. Immunization/
2. Immunization Schedule/
3. Immunization, Secondary/
4. Immunotherapy, Active/
5. Mass Immunization/
6. Immunization Programs/
7. Vaccination/
8. (vaccinat$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
9. or/1‐8
10. Tetanus Toxoid/
11. Diphtheria Toxoid/
12. Diphtheria‐Tetanus‐Acellular Pertussis Vaccines/
13. Diphtheria‐Tetanus‐Pertussis Vaccine/
14. Diphtheria‐Tetanus Vaccine/
15. Pertussis Vaccine/
16. Measles‐Mumps‐Rubella Vaccine/
17. Measles Vaccine/
18. Mumps Vaccine/
19. Rubella Vaccine/
20. Poliovirus Vaccines/
21. Poliovirus Vaccine, Inactivated/
22. Poliovirus Vaccine, Oral/
23. Tuberculosis Vaccines/
24. BCG Vaccine/
25. Viral Hepatitis Vaccines/
26. Hepatitis B Vaccines/
27. Haemophilus Vaccines/
28. Pneumococcal Vaccines/
29. Meningococcal Vaccines/
30. Rotavirus Vaccines/
31. Japanese Encephalitis Vaccine/
32. Yellow Fever Vaccine/
33. ((tetanus or diphtheria) adj toxoid).tw.
34. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
35. or/10‐34
36. Tetanus/
37. Diphtheria/
38. Measles/
39. Mumps/
40. Rubella/
41. Whooping Cough/
42. Poliomyelitis/
43. Poliomyelitis, Bulbar/
44. Tuberculosis/
45. Tuberculosis, Pulmonary/
46. Mycobacterium Tuberculosis/
47. Hepatitis B/
48. Hepatitis B, Chronic/
49. Haemophilus Influenzae/
50. Haemophilus Influenzae Type B/
51. Pneumococcal Infections/
52. Meningitis, Pneumococcal/
53. Meningitis, Meningococcal/
54. Pneumonia, Pneumococcal/
55. Rotavirus Infections/
56. Japanese Encephalitis/
57. Yellow Fever/
58. Encephalitis, Tick‐borne/
59. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
60. or/36‐59
61. Child/
62. Child, Preschool/
63. Infant/
64. exp Infant, Newborn/
65. Child Care/
66. Infant Care/
67. exp Perinatal Care/
68. exp Parents/
69. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
70. or/61‐69
71. Mothers/
72. Women/
73. Pregnant Women/
74. Female/
75. (woman or women or mother? or female?).tw.
76. or/71‐75
77. exp Communication/
78. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
79. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
80. (readability or intelligibility or credibility).mp.
81. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
82. exp Interpersonal Relations/
83. hospital patient relations/
84. community institutional relations/
85. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
86. ((health or patient or client) adj (education or knowledge or promotion)).mp.
87. exp health promotion/
88. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
89. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
90. (self adj (teaching or education or instruction)).mp.
91. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
92. marketing.mp.
93. ((family or office or work* or school or faith or church) adj based).tw.
94. (educational status or literacy).mp.
95. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
96. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
97. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
98. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
99. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
100. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
101. ((social or community) adj2 network*).mp.
102. (self‐care or self‐management).mp.
103. (motivat* or incentive* or goal*).mp.
104. exp Communications Media/
105. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
106. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
107. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
108. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
109. exp computer systems/
110. software/
111. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
112. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
113. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
114. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
115. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
116. (participation or advocacy or consumer* or empower*).mp.
117. exp decision making/
118. (decision adj (making or support or aid*)).mp.
119. exp informed consent/
120. (informed adj (consent or choice* or decision*)).tw.
121. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
122. (therapeutic adj (relation* or alliance*)).mp.
123. or/77‐122
124. 70 or 76
125. 9 and 60
126. 35 or 125
127. randomised controlled trial.pt.
128. controlled clinical trial.pt.
129. multicenter study.pt.
130. (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab.
131. groups.ab.
132. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
133. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
134. or/127‐133
135. exp Animals/
136. Humans/
137. 135 not (135 and 136)
138. review.pt.
139. meta analysis.pt.
140. news.pt.
141. comment.pt.
142. editorial.pt.
143. cochrane database of systematic reviews.jn.
144. comment on.cm.
145. (systematic review or literature review).ti.
146. 137 or 138 or 139 or 140 or 141 or 142 or 143 or 144 or 145
147. 134 not 146
148. 9 and 60
149. 35 or 148
150. 123 and 124 and 147 and 149
151. 150 use pmoz
152. immunization/
153. active immunization/
154. mass immunization/
155. vaccination/
156. revaccination/
157. exp vaccine/
158. (vaccin$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
159. or/152‐158
160. tetanus toxoid/
161. diphtheria toxoid/
162. diphtheria pertussis tetanus vaccine/
163. pertussis vaccine/
164. measles mumps rubella vaccine/
165. measles vaccine/
166. mumps vaccine/
167. rubella vaccine/
168. poliomyelitis vaccine/
169. oral poliomyelitis vaccine/
170. BCG vaccine/
171. hepatitis vaccine/
172. hepatitis B vaccine/
173. Haemophilus vaccine/
174. Meningococcus vaccine/
175. Pneumococcus vaccine/
176. Rotavirus vaccine/
177. Japanese encephalitis vaccine/
178. yellow fever vaccine/
179. ((tetanus or diphtheria) adj toxoid).tw.
180. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
181. or/160‐180
182. tetanus/
183. diphtheria/
184. measles/
185. mumps/
186. rubella/
187. pertussis/
188. poliomyelitis/
189. tuberculosis/
190. lung tuberculosis/
191. Mycobacterium tuberculosis/
192. hepatitis B/
193. haemophilus influenzae/ or haemophilus influenzae type b/
194. pneumococcal infection/
195. pneumococcal meningitis/
196. epidemic meningitis/
197. pneumonia/
198. Rotavirus infection/
199. Japanese encephalitis/
200. yellow fever/
201. tick borne encephalitis/
202. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
203. or/182‐202
204. child/
205. preschool child/
206. infant/
207. newborn/
208. child care/
209. perinatal care/
210. exp parent/
211. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
212. or/204‐211
213. mother/ or adolescent mother/ or expectant mother/
214. female/
215. pregnant woman/
216. (woman or women or mother? or female?).tw.
217. or/213‐216
218. exp interpersonal communication/
219. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
220. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
221. (readability or intelligibility or credibility).mp.
222. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
223. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
224. ((health or patient or client) adj (education or knowledge or promotion)).mp.
225. health promotion/
226. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
227. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
228. (self adj (teaching or education or instruction)).mp.
229. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
230. marketing.mp.
231. ((family or office or work* or school or faith or church) adj based).tw.
232. (educational status or literacy).mp.
233. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
234. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
235. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
236. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
237. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
238. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
239. ((social or community) adj2 network*).mp.
240. (self‐care or self‐management).mp.
241. (motivat* or incentive* or goal*).mp.
242. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
243. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
244. social media/
245. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
246. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
247. computer system/
248. computer program/
249. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
250. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
251. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
252. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
253. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
254. (participation or advocacy or consumer* or empower*).mp.
255. decision making/
256. patient decision making/
257. decision support system/
258. (decision adj (making or support or aid*)).mp.
259. parental consent/ or informed consent/
260. (informed adj (consent or choice* or decision*)).tw.
261. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
262. (therapeutic adj (relation* or alliance*)).mp.
263. or/218‐262
264. 212 or 217
265. 159 and 203
266. 265 or 181
267. randomized controlled trial/
268. controlled clinical trial/
269. quasi experimental study/
270. pretest posttest control group design/
271. time series analysis/
272. experimental design/
273. multicenter study/
274. (randomis* or randomiz* or randomly or random allocat*).ti,ab.
275. groups.ab.
276. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
277. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
278. or/267‐277
279. (systematic review or literature review).ti.
280. "cochrane database of systematic reviews".jn.
281. nonhuman/
282. or/279‐281
283. 278 not 282
284. 263 and 264 and 266 and 283
285. 284 use emczd
286. immunization/
287. exp vaccination/
288. immunization programmes/
289. immunotherapy/
290. (vaccinat$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
291. or/286‐290
292. tetanus toxoid/
293. diphtheria toxoid/
294. diphtheria pertussis tetanus vaccines/
295. measles mumps rubella vaccines/
296. poliomyelitis/
297. bcg vaccine/
298. ((tetanus or diphtheria) adj toxoid).tw.
299. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
300. or/292‐299
301. exp tetanus/
302. diphtheria/
303. measles/
304. mumps/
305. exp rubella/
306. pertussis/
307. poliomyelitis/
308. exp tuberculosis/
309. mycobacterium tuberculosis/
310. hepatitis b/
311. exp haemophilus influenzae/
312. meningitis/ or bacterial meningitis/ or cryptococcal meningitis/ or neonatal meningitis/
313. rotavirus/
314. japanese encephalitis/
315. yellow fever/
316. tickborne encephalitis/
317. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
318. or/301‐317
319. children/
320. preschool children/
321. infants/ or neonates/
322. child care/
323. exp parents/
324. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
325. mothers/
326. fathers/
327. women/ or housewives/ or lactating women/ or rural women/
328. females/
329. (woman or women or mother? or female?).tw.
330. or/319‐329
331. communication/ or interpretation/ or nonverbal communication/ or verbal communication/
332. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
333. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
334. (readability or intelligibility or credibility).mp.
335. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
336. exp interpersonal relations/
337. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
338. ((health or patient or client) adj (education or knowledge or promotion)).mp.
339. health promotion/
340. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
341. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
342. (self adj (teaching or education or instruction)).mp.
343. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
344. marketing.mp.
345. ((family or office or work* or school or faith or church) adj based).tw.
346. (educational status or literacy).mp.
347. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
348. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
349. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
350. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
351. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
352. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
353. ((social or community) adj2 network*).mp.
354. (self‐care or self‐management).mp.
355. (motivat* or incentive* or goal*).mp.
356. exp mass media/
357. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
358. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
359. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
360. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
361. exp computer techniques/
362. computer software/
363. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
364. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
365. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
366. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
367. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
368. (participation or advocacy or consumer* or empower*).mp.
369. decision making/
370. (decision adj (making or support or aid*)).mp.
371. consent/
372. (informed adj (consent or choice* or decision*)).tw.
373. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
374. (therapeutic adj (relation* or alliance*)).mp.
375. or/331‐374
376. randomized controlled trials/
377. (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab.
378. groups.ab.
379. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
380. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
381. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
382. or/376‐381
383. exp animals/
384. man/
385. 383 not (383 and 384)
386. systematic reviews/
387. meta‐analysis/
388. (systematic review or literature review).ti.
389. literature reviews/
390. or/385‐389
391. 382 not 390
392. 291 and 318
393. 392 or 300
394. 330 and 375 and 391 and 393
395. 394 use cagz
396. immunization/
397. (vaccinat$ or revaccinat$ or immuniz$ or immunis$ or immunother$ or inoculat$ or innoculat* or prophyla*).tw.
398. 396 or 397
399. ((tetanus or diphtheria) adj toxoid).tw.
400. ((tetanus or diphtheria? or pertussis or whooping cough or measles or mumps or rubella? or rubeola or mmr or polio$ or tuberculosis or tuberculoses or bcg or calmette$ or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$) adj vaccin$).tw.
401. 399 or 400
402. (tetanus or diphtheria? or measles or rubella? or rubeola or mumps or epidemic parotit$ or pertussis or whooping cough or polio$ or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza? or hemophilus influenza? or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus$ or MMR).tw.
403. exp child care/
404. parents/ or adoptive parents/ or fathers/ or foster parents/ or mothers/ or single parents/ or "surrogate parents (humans)"/
405. (child$ or infant$ or newborn? or neonat$ or baby or babies or toddler? or parent* or father*).tw.
406. mothers/ or parents/ or adolescent mothers/ or single mothers/ or unwed mothers/
407. human females/ or sisters/ or wives/ or working women/
408. (woman or women or mother? or female?).tw.
409. or/403‐408
410. exp Communication/
411. ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) adj1 communication).mp.
412. (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating).hw,ti.
413. (readability or intelligibility or credibility).mp.
414. (disclos* or trust* or truth* or deceiv* or deception or misinform*).hw,ti.
415. family relations/ or interpersonal relationships/ or childrearing practices/ or parental role/
416. ((professional or physician or doctor or clinician or nurse or provider) adj1 (patient or client or family)).tw.
417. ((health or patient or client) adj (education or knowledge or promotion)).mp.
418. health promotion/
419. ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) adj1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)).mp.
420. (((medical or continuing or residency or distance) adj2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning).mp.
421. (self adj (teaching or education or instruction)).mp.
422. ((media adj3 campaign*) or (promotion adj1 program*) or (community based adj3 intervention*) or (home adj3 visit*) or (awareness adj3 (rais* or increas*))).tw.
423. marketing.mp.
424. ((family or office or work* or school or faith or church) adj based).tw.
425. (educational status or literacy).mp.
426. ((improv* or increas* or enhanc* or patient) adj3 (understanding or comprehension)).tw.
427. (information* adj (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)).mp.
428. ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) adj2 inform*).mp.
429. (((inform* or message* or communicat* or effect* or gain or positive or negative) adj2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) adj (format* or presentation* or display*))).mp.
430. (counsel* or ((social or carer* or caregiver* or care giver* or patient*) adj1 support*) or psychosocial or ((social or pastoral or spiritual) adj care) or religio* or chaplaincy or behavior modification or behaviour modification).mp.
431. (counsel*ing session* or ((support or peer or self‐help or self‐care) adj2 (intervention* or group* or program*))).mp.
432. ((social or community) adj2 network*).mp.
433. (self‐care or self‐management).mp.
434. (motivat* or incentive* or goal*).mp.
435. exp communications media/
436. ((mass or communication* or electronic or digital or multi or print* or social or new) adj media).tw.
437. ((print* adj (material* or based)) or paper‐based or written material* or (paper adj1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*).mp.
438. (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual adj (reality or world or environment*))).mp.
439. ((electronic or e‐) adj (mail or prescri* or health or game*)).mp.
440. computer applications/
441. computer software/ or decision support systems/
442. (computer* adj1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)).mp.
443. (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*).mp.
444. (((automat* or interactive*) adj3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) adj (response or recognition or messag* or system* or technolog*))).mp.
445. (cultural* adj (competen* or sensitiv* or appropriate)).mp.
446. ((cultural* or linguistic* or language) adj3 (service* or care or intervention* or message*)).mp.
447. (participation or advocacy or consumer* or empower*).mp.
448. decision making/ or choice behavior/ or group decision making/
449. (decision adj (making or support or aid*)).mp.
450. informed consent/
451. (informed adj (consent or choice* or decision*)).tw.
452. ((patient or person or family or client) adj (cent*red or focus*ed or oriented)).mp.
453. (therapeutic adj (relation* or alliance*)).mp.
454. or/410‐453
455. (randomis* or randomiz* or randomly allocat* or random allocat*).ti,ab.
456. groups.ab.
457. (trial or multicenter or multi center or multicentre or multi centre).ti,ab.
458. (intervention* or controlled or control group or compare or compared or (before adj5 after) or (pre adj5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point? or repeated measur*).ti,ab.
459. or/455‐458
460. 398 and 402
461. 401 or 460
462. 409 and 454 and 459 and 461
463. 409 and 454 and 461
464. 463 use psyh
465. 151 or 285 or 395 or 464
466. limit 465 to yr="2005 ‐Current"
467. 465 not 466
468. remove duplicates from 467
469. remove duplicates from 466
470. 468 or 469
471. 470 not 151 (Result 7802)
CENTRAL (Cochrane Central Register of Controlled Trials)
Searched 11 July 2012
#1 MeSH descriptor Immunization, this term only
#2 MeSH descriptor Immunization Schedule, this term only
#3 MeSH descriptor Immunization, Secondary, this term only
#4 MeSH descriptor Immunotherapy, Active explode all trees
#5 MeSH descriptor Mass Vaccination, this term only
#6 MeSH descriptor Immunization Programs, this term only
#7 MeSH descriptor Vaccination, this term only
#8 (vaccinat* or revaccinat* or immuniz* or immunis* or immunother* or inoculat* or innoculat* or prophyla*):ti,ab in Trials
#9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
#10 MeSH descriptor Tetanus Toxoid, this term only
#11 MeSH descriptor Diphtheria Toxoid, this term only
#12 MeSH descriptor Diphtheria‐Tetanus‐acellular Pertussis Vaccines, this term only
#13 MeSH descriptor Diphtheria‐Tetanus‐Pertussis Vaccine, this term only
#14 MeSH descriptor Diphtheria‐Tetanus Vaccine, this term only
#15 MeSH descriptor Pertussis Vaccine, this term only
#16 MeSH descriptor Measles‐Mumps‐Rubella Vaccine, this term only
#17 MeSH descriptor Measles Vaccine, this term only
#18 MeSH descriptor Mumps Vaccine, this term only
#19 MeSH descriptor Rubella Vaccine, this term only
#20 MeSH descriptor Poliovirus Vaccines, this term only
#21 MeSH descriptor Poliovirus Vaccine, Inactivated, this term only
#22 MeSH descriptor Poliovirus Vaccine, Oral, this term only
#23 MeSH descriptor Tuberculosis Vaccines, this term only
#24 MeSH descriptor BCG Vaccine, this term only
#25 MeSH descriptor Viral Hepatitis Vaccines, this term only
#26 MeSH descriptor Hepatitis B Vaccines, this term only
#27 MeSH descriptor Haemophilus Vaccines, this term only
#28 MeSH descriptor Pneumococcal Vaccines, this term only
#29 MeSH descriptor Meningococcal Vaccines, this term only
#30 MeSH descriptor Rotavirus Vaccines, this term only
#31 MeSH descriptor Japanese Encephalitis Vaccines, this term only
#32 MeSH descriptor Yellow Fever Vaccine, this term only
#33 ((tetanus or diphtheria) near toxoid):ti,ab in Trials
#34 ((tetanus or diphtheria* or pertussis or whooping cough or measles or mumps or rubella* or rubeola or mmr or polio* or tuberculosis or tuberculoses or bcg or calmette* or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus*) near vaccin*):ti,ab in Trials
#35 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34)
#36 MeSH descriptor Tetanus, this term only
#37 MeSH descriptor Diphtheria explode all trees
#38 MeSH descriptor Measles, this term only
#39 MeSH descriptor Mumps, this term only
#40 MeSH descriptor Rubella, this term only
#41 MeSH descriptor Whooping Cough, this term only
#42 MeSH descriptor Poliomyelitis, this term only
#43 MeSH descriptor Poliomyelitis, Bulbar, this term only
#44 MeSH descriptor Tuberculosis, this term only
#45 MeSH descriptor Tuberculosis, Pulmonary, this term only
#46 MeSH descriptor Mycobacterium tuberculosis, this term only
#47 MeSH descriptor Hepatitis B, this term only
#48 MeSH descriptor Hepatitis B, Chronic, this term only
#49 MeSH descriptor Haemophilus influenzae, this term only
#50 MeSH descriptor Haemophilus influenzae type b, this term only
#51 MeSH descriptor Pneumococcal Infections, this term only
#52 MeSH descriptor Meningitis, Pneumococcal, this term only
#53 MeSH descriptor Meningitis, Meningococcal, this term only
#54 MeSH descriptor Pneumonia, Pneumococcal, this term only
#55 MeSH descriptor Rotavirus Infections, this term only
#56 MeSH descriptor Encephalitis, Japanese, this term only
#57 MeSH descriptor Yellow Fever, this term only
#58 MeSH descriptor Encephalitis, Tick‐Borne, this term only
#59 (tetanus or diphtheria* or measles or rubella* or rubeola or mumps or epidemic parotit* or pertussis or whooping cough or polio* or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza* or hemophilus influenza* or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus* or MMR):ti,ab in Trials
#60 (#36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59)
#61 MeSH descriptor Child, this term only
#62 MeSH descriptor Child, Preschool, this term only
#63 MeSH descriptor Infant, this term only
#64 MeSH descriptor Infant, Newborn explode all trees
#65 MeSH descriptor Child Care, this term only
#66 MeSH descriptor Infant Care, this term only
#67 MeSH descriptor Perinatal Care explode all trees
#68 MeSH descriptor Parents explode all trees
#69 (child* or infant* or newborn* or neonat* or baby or babies or toddler* or parent* or father*):ti,ab in Trials
#70 MeSH descriptor Women, this term only
#71 MeSH descriptor Pregnant Women, this term only
#72 (woman or women or mother* or female*):ti,ab in Trials
#73 (#61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72)
#74 MeSH descriptor Communication explode all trees
#75 ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) near1 communication):ti,ab in Trials
#76 (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or discuss* or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or interview* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating):ti,ab in Trials
#77 (readability or intelligibility or credibility):ti,ab in Trials
#78 (disclos* or trust* or truth* or deceiv* or deception or misinform*):ti,ab in Trials
#79 MeSH descriptor Interpersonal Relations explode all trees
#80 MeSH descriptor Hospital‐Patient Relations, this term only
#81 MeSH descriptor Community‐Institutional Relations, this term only
#82 ((professional or physician or doctor or clinician or nurse or provider) near1 (patient or client or family)):ti,ab in Trials
#83 ((health or patient or client) near (education or knowledge or promotion)):ti,ab in Trials
#84 MeSH descriptor Health Promotion explode all trees
#85 ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) near1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)):ti,ab in Trials
#86 (((medical or continuing or residency or distance) near2 education) or internship or inservice or in‐service or staff development or professional development or mentor* or lifelong learning):ti,ab in Trials
#87 (self near (teaching or education or instruction)):ti,ab in Trials
#88 ((media near3 campaign*) or (promotion near1 program*) or (community based near3 intervention*) or (home near3 visit*) or (awareness near3 (rais* or increas*))):ti,ab in Trials
#89 marketing:ti,ab in Trials
#90 ((family or office or work* or school or faith or church) near based):ti,ab in Trials
#91 (educational status or literacy):ti,ab in Trials
#92 ((improv* or increas* or enhanc* or patient) near3 (understanding or comprehension)):ti,ab in Trials
#93 (information* near (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)):ti,ab in Trials
#94 ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) near2 inform*):ti,ab in Trials
#95 (((inform* or message* or communicat* or effect* or gain or positive or negative) near2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) near (format* or presentation* or display*))):ti,ab in Trials
#96 (counsel* or ((social or carer* or caregiver* or care giver* or patient*) near1 support*) or psychosocial or ((social or pastoral or spiritual) near care) or religio* or chaplaincy or behavior modification or behaviour modification):ti,ab in Trials
#97 (counsel*ing session* or ((support or peer or self‐help or self‐care) near2 (intervention* or group* or program*))):ti,ab in Trials
#98 ((social or community) near2 network*):ti,ab in Trials
#99 (self‐care or self‐management):ti,ab in Trials
#100 (motivat* or incentive* or goal*):ti,ab in Trials
#101 MeSH descriptor Communications Media explode all trees
#102 ((mass or communication* or electronic or digital or multi or print* or social or new) near media):ti,ab in Trials
#103 ((print* near (material* or based)) or paper‐based or written material* or (paper near1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or card or cards or picture* or pictogram*):ti,ab in Trials
#104 (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual near (reality or world or environment*))):ti,ab in Trials
#105 ((electronic or e‐) near (mail or prescri* or health or game*)):ti,ab in Trials
#106 MeSH descriptor Computer Systems explode all trees
#107 MeSH descriptor Software, this term only
#108 (computer* near1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)):ti,ab in Trials
#109 (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*):ti,ab in Trials
#110 (((automat* or interactive*) near3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) near (response or recognition or messag* or system* or technolog*))):ti,ab in Trials
#111 (cultural* near (competen* or sensitiv* or appropriate)):ti,ab in Trials
#112 ((cultural* or linguistic* or language) near3 (service* or care or intervention* or message*)):ti,ab in Trials
#113 (participation or advocacy or consumer* or empower*):ti,ab in Trials
#114 MeSH descriptor Decision Making explode all trees
#115 (decision near (making or support or aid*)):ti,ab in Trials
#116 MeSH descriptor Informed Consent explode all trees
#117 (informed near (consent or choice* or decision*)):ti,ab in Trials
#118 ((patient or person or family or client) near (cent*red or focus*ed or oriented)):ti,ab in Trials
#119 (therapeutic near (relation* or alliance*)):ti,ab in Trials
#120 (#74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112 OR #113 OR #114 OR #115 OR #116 OR #117 OR #118 OR #119)
#121 (#9 AND #60)
#122 (#35 OR #121)
#123 (#122 AND #73 AND #120) Result 307
CINAHL
Searched 19 July 2012
| # | Query |
| S123 | S122 NOT S119 |
| S122 | S53 and S102 and S110 and S121 |
| S121 | S24 or S120 |
| S120 | S7 and S43 |
| S119 | S114 or S115 or S116 or S117 or S118 |
| S118 | TI (systematic review or literature review) |
| S117 | CR (Comment on) |
| S116 | JN (cochrane database of systematic reviews) |
| S115 | PT (review or meta analysis or news or comment or editorial) |
| S114 | S111 NOT S113 |
| S113 | S111 and S112 |
| S112 | (MH "Human") |
| S111 | (MH "Animals+") |
| S110 | S103 or S104 or S105 or S106 or S107 or S108 or S109 |
| S109 | TI (intervention* or controlled or control group or compare or compared or (before N5 after) or (pre N5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point* or repeated measur*) OR AB (intervention* or controlled or control group or compare or compared or (before N5 after) or (pre N5 post) or pretest or pre test or posttest or post test or quasiexperiment* or quasi experiment* or evaluat* or effect or impact or time series or time point* or repeated measur*) |
| S108 | TI (trial or multicenter or multi center or multicentre or multi centre) OR AB (trial or multicenter or multi center or multicentre or multi centre) |
| S107 | AB Groups |
| S106 | TI (randomis* or randomiz* or randomly allocat* or random allocat*) OR AB (randomis* or randomiz* or randomly allocat* or random allocat*) |
| S105 | (MH "Nonrandomized Trials") |
| S104 | (MH "Multicenter Studies") |
| S103 | (MH "Randomized Controlled Trials") |
| S102 | S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 or S73 or S74 or S75 or S76 or S77 or S78 or S79 or S80 or S81 or S82 or S83 or S84 or S85 or S86 or S87 or S88 or S89 or S90 or S91 or S92 or S93 or S94 or S95 or S96 or S97 or S99 or S100 or S101 |
| S101 | TI (therapeutic N1 (relation* or alliance*)) OR AB (therapeutic N1 (relation* or alliance*)) |
| S100 | TI ((patient or person or family or client) N1 (cent*red or focus*ed or oriented)) OR AB ((patient or person or family or client) N1 (cent*red or focus*ed or oriented)) |
| S99 | TI (informed N1 (consent or choice* or decision*)) OR AB (informed N1 (consent or choice* or decision*)) |
| S98 | (MH "Consent (Research)") |
| S97 | TI (decision N1 (making or support or aid*)) OR AB (decision N1 (making or support or aid*)) |
| S96 | (MH "Decision Making+") |
| S95 | TI (participation or advocacy or consumer* or empower*) OR AB (participation or advocacy or consumer* or empower*) |
| S94 | TI ((cultural* or linguistic* or language) N3 (service* or care or intervention* or message*)) OR AB ((cultural* or linguistic* or language) N3 (service* or care or intervention* or message*)) |
| S93 | TI (cultural* N1 (competen* or sensitiv* or appropriate)) OR AB (cultural* N1 (competen* or sensitiv* or appropriate)) |
| S92 | TI (((automat* or interactive*) N3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) N1 (response or recognition or messag* or system* or technolog*))) OR AB (((automat* or interactive*) N3 (telephon* or phone or phones or voice or hotline* or hot line*)) or ((voice or speech) N1 (response or recognition or messag* or system* or technolog*))) |
| S91 | TI (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*) OR AB (touch screen or digital assistant* or pda or blackberry or mobile‐device* or laptop* or notebook computer* or computer* or netbook*) |
| S90 | TI (computer* N1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)) OR AB (computer* N1 (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or assisted instruction)) |
| S89 | (MH "Software") |
| S88 | (MH "Computer Systems+") |
| S87 | TI ((electronic or e‐) N1 (mail or prescri* or health or game*)) OR AB ((electronic or e‐) N1 (mail or prescri* or health or game*)) |
| S86 | TI (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual N1 (reality or world or environment*))) OR AB (radio or television or audiovisual or video* or tape recording* or cassette* or cd‐rom* or dvd* or motion picture* or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or short message* or text message* or i‐pod* or ipod* or i‐pad* or ipad* or mp3 player* or hotline* or answering service* or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare or (virtual N1 (reality or world or environment*))) |
| S85 | TI ((print* N1 (material* or based)) or paper‐based or written material* or (paper N1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or picture* or pictogram*) OR AB ((print* N1 (material* or based)) or paper‐based or written material* or (paper N1 pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or picture* or pictogram*) |
| S84 | TI ((mass or communication* or electronic or digital or multi or print* or social or new) N1 media) OR AB ((mass or communication* or electronic or digital or multi or print* or social or new) N1 media) |
| S83 | TI (motivat* or incentive* or goal*) OR AB (motivat* or incentive* or goal*) |
| S82 | TI (self‐care or self‐management) OR AB (self‐care or self‐management) |
| S81 | TI ((social or community) N2 network*) OR AB ((social or community) N2 network*) |
| S80 | TI (counsel*ing session* or ((support or peer or self‐help or self‐care) N2 (intervention* or group* or program*))) OR AB (counsel*ing session* or ((support or peer or self‐help or self‐care) N2 (intervention* or group* or program*))) |
| S79 | TI (counsel* or ((social or carer* or caregiver* or care giver* or patient*) N1 support*) or psychosocial or ((social or pastoral or spiritual) N1 care) or religio* or chaplaincy or behavior modification or behaviour modification) OR AB (counsel* or ((social or carer* or caregiver* or care giver* or patient*) N1 support*) or psychosocial or ((social or pastoral or spiritual) N1 care) or religio* or chaplaincy or behavior modification or behaviour modification) |
| S78 | TI (((inform* or message* or communicat* or effect* or gain or positive or negative) N2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) N1 (format* or presentation* or display*))) OR AB (((inform* or message* or communicat* or effect* or gain or positive or negative) N2 fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) N1 (format* or presentation* or display*))) |
| S77 | TI ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) N2 inform*) OR AB ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) N2 inform*) |
| S76 | TI (information* N3 (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)) OR AB (information* N3 (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)) |
| S75 | TI ((improv* or increas* or enhanc* or patient) N3 (understanding or comprehension)) OR AB ((improv* or increas* or enhanc* or patient) N3 (understanding or comprehension)) |
| S74 | TI (educational status or literacy) OR AB (educational status or literacy) |
| S73 | TI ((family or office or work* or school or faith or church) N3 based) OR AB ((family or office or work* or school or faith or church) N3 based) |
| S72 | TI marketing OR AB marketing |
| S71 | TI ((media N3 campaign*) or (promotion N1 program*) or (community based N3 intervention*) or (awareness N3 (rais* or increas*))) OR AB ((media N3 campaign*) or (promotion N1 program*) or (community based N3 intervention*) or (awareness N3 (rais* or increas*))) |
| S70 | TI (self N1 (teaching or education or instruction)) OR AB (self N1 (teaching or education or instruction)) |
| S69 | TI ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) N1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)) OR AB ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) N1 (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)) |
| S68 | (MH "Health Promotion+") |
| S67 | TI ((health or patient or client) N3 (education or knowledge or promotion)) OR AB ((health or patient or client) N3 (education or knowledge or promotion)) |
| S66 | TI ((health or patient or client) N1 (education or knowledge or promotion)) OR AB ((health or patient or client) N1 (education or knowledge or promotion)) |
| S65 | TI ((professional or physician or doctor or clinician or nurse or provider) N1 (patient or client or family)) OR AB ((professional or physician or doctor or clinician or nurse or provider) N1 (patient or client or family)) |
| S64 | (MH "Physician‐Patient Relations") OR (MH "Professional‐Patient Relations+") OR (MH "Nurse‐Patient Relations") OR (MH "Community‐Institutional Relations") |
| S63 | (MH "Interpersonal Relations+") |
| S62 | TI (disclos* or trust* or truth* or deceiv* or deception or misinform*) OR AB (disclos* or trust* or truth* or deceiv* or deception or misinform*) |
| S61 | TI (readability or intelligibility or credibility) OR AB (readability or intelligibility or credibility) |
| S60 | TI (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating) OR AB (communicat* or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating) |
| S59 | TI ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) N1 communication) OR AB ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) N1 communication) |
| S58 | (MH "Communications Media+") |
| S57 | (MH "Telephone") OR (MH "Wireless Communications") |
| S56 | (MH "Reminder Systems") |
| S55 | (MH "Communication+") |
| S54 | (MH "Parental Attitudes+") |
| S53 | S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S54 |
| S52 | TI (child* or infant* or newborn* or neonat* or baby or babies or toddler or parent* or father* or woman or women or mother* or female*) OR AB (child* or infant* or newborn* or neonat* or baby or babies or toddler or parent* or father* or woman or women or mother* or female*) |
| S51 | (MH "Female") |
| S50 | (MH "Expectant Mothers") |
| S49 | (MH "Women") |
| S48 | (MH "Parents+") |
| S47 | (MH "Perinatal Care") |
| S46 | (MH "Infant Care") |
| S45 | (MH "Child Care") |
| S44 | (MH "Child") OR (MH "Child, Preschool") OR (MH "Infant") OR (MH "Infant, Newborn+") |
| S43 | S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 |
| S42 | TI (tetanus or diphtheria* or measles or rubella* or rubeola or mumps or epidemic parotit* or pertussis or whooping cough or polio* or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza* or hemophilus influenza* or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus* or MMR) OR AB (tetanus or diphtheria* or measles or rubella* or rubeola or mumps or epidemic parotit* or pertussis or whooping cough or polio* or infantile paralysis or tuberculosis or tuberculoses or hepatitis b or haemophilus influenza* or hemophilus influenza* or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus* or MMR) |
| S41 | (MH "Tick‐Borne Diseases") |
| S40 | (MH "Yellow Fever") |
| S39 | (MH "Encephalitis, Arbovirus") |
| S38 | (MH "Rotavirus Infections") |
| S37 | (MH "Community‐Acquired Pneumonia") OR (MH "Pneumonia") |
| S36 | (MH "Meningitis, Meningococcal") OR (MH "Meningitis, Pneumococcal") |
| S35 | (MH "Pneumococcal Infections") |
| S34 | (MH "Haemophilus Influenzae") |
| S33 | (MH "Hepatitis B, Chronic") OR (MH "Hepatitis B") |
| S32 | (MH "Tuberculosis") OR (MH "Tuberculosis, Pulmonary") OR (MH "Mycobacterium Tuberculosis") |
| S31 | (MH "Poliomyelitis") |
| S30 | (MH "Whooping Cough") |
| S29 | (MH "Rubella") |
| S28 | (MH "Mumps") |
| S27 | (MH "Measles") |
| S26 | (MH "Diphtheria") |
| S25 | (MH "Tetanus") |
| S24 | S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 |
| S23 | TI ((tetanus or diphtheria* or pertussis or whooping cough or measles or mumps or rubella* or rubeola or mmr or polio* or tuberculosis or tuberculoses or bcg or calmette* or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus*) N1 vaccin*) OR AB ((tetanus or diphtheria* or pertussis or whooping cough or measles or mumps or rubella* or rubeola or mmr or polio* or tuberculosis or tuberculoses or bcg or calmette* or hepatitis b or haemophilus or triple or pneumococcal or meningococcal or yellow fever or japanese encephalitis or tick‐borne encephalitis or rotavirus*) N1 vaccin*) |
| S22 | TI ((tetanus or diphtheria) N1 toxoid) OR AB ((tetanus or diphtheria) N1 toxoid) |
| S21 | (MH "Rotavirus Vaccines") |
| S20 | (MH "Meningococcal Vaccines") |
| S19 | (MH "Pneumococcal Vaccine") |
| S18 | (MH "HIB Vaccine") |
| S17 | (MH "Viral Hepatitis Vaccines") OR (MH "Hepatitis B Vaccines") |
| S16 | (MH "BCG Vaccine") |
| S15 | (MH "Poliovirus Vaccine, Oral") OR (MH "Poliovirus Vaccine, Inactivated") OR (MH "Poliovirus Vaccine") |
| S14 | (MH "Rubella Vaccine") |
| S13 | (MH "Mumps Vaccine") |
| S12 | (MH "Measles Vaccine") OR (MH "Measles‐Mumps‐Rubella Vaccine") |
| S11 | (MH "Pertussis Vaccine") |
| S10 | (MH "Diphtheria‐Tetanus‐acellular Pertussis Vaccines") OR (MH "Diphtheria‐Tetanus‐Pertussis Vaccine") OR (MH "Diphtheria‐Tetanus Vaccine") |
| S9 | (MH "Diphtheria Toxoid") |
| S8 | (MH "Tetanus Toxoid") |
| S7 | S1 or S2 or S3 or S4 or S5 or S6 |
| S6 | TI (vaccinat* or revaccinat* or immuniz* or immunis* or immunother* or inoculat* or innoculat* or prophyla*) OR AB (vaccinat* or revaccinat* or immuniz* or immunis* or immunother* or inoculat* or innoculat* or prophyla*) |
| S5 | (MH "Immunization Programs") |
| S4 | (MH "Immunotherapy") |
| S3 | (MH "Immunization, Secondary") |
| S2 | (MH "Immunization Schedule") |
| S1 | (MH "Immunization") |
WHO Global Health Library – COMMVAC Search
Date searched: 26 July 2012
(immuniz* or immunis* or vaccin* or immunotherap* or inoculat* or innoculat* or prophyla*) and (child* or infant* or newborn* or neonat* or preschool* or "primary school*" or baby or babies or toddler* or parent* or mother* or father*) and (communicati* or face‐to‐face or messag* or verbal* or nonverbal* or written or writing or reading or language or speech or speak* or spoken or talk* or conversation* or voice or visual‐perception or feedback or listen* or negotiat* or notify* or notification or remind* or narrat* or music* or humor or humour or humorous or adverti* or persua* or interpreting or interpreters or interpret*‐service or translat* service* or translating or disclos* or trust* or truth* or deceiv* or deception or misinform* or participation or advocacy or consumer* or empower* or (decision and (making or support or aid*)) or (informed and (consent or choice* or decision*)) or (radio or television or audiovisual or video* or "tape recording*" or cassette* or cd‐rom* or dvd* or "motion picture*" or movie* or cinema* or multimedia or hypermedia or telephon* or phone or phones or sms or "short message*" or "text message*" or i‐pod* or ipod* or i‐pad* or ipad* or "mp3 player*" or hotline* or "answering service*" or internet or web* or online or on‐line or blog* or telemedicine or telehealth or telecare) or ((health or patient or client) and (education or knowledge or promotion)) or ((education* or teaching or learning or instruction* or training or skills or online or web* or internet or video* or multimedia or multi‐media) and (intervention* or session* or course* or program* or material* or package* or module* or demonstration or method* or process*)) or (((inform* or message* or communicat* or effect* or gain or positive or negative) and fram*) or ((verbal or oral or written or text or data or numerical or statistical or visual or graphic* or pictorial or audio* or video* or multimedia or multi‐media or narrative) and (format* or presentation* or display*))) or (counsel* or ((social or carer* or caregiver* or "care giver*" or patient*) and support*) or psychosocial or ((social or pastoral or spiritual) and care) or religio* or chaplaincy or "behavior modification" or "behaviour modification") or ("counsel*ing session*" or ((support or peer or self‐help or self‐care) and (intervention* or group* or program*))) or motivat* or incentive* or goal* or ((mass or communication* or electronic or digital or multi or print* or social or new) and media) or ((media and campaign*) or (promotion and program*) or ("community based" and intervention*) or (awareness and (rais* or increas*))) or ("educational status" or literacy) or ((improv* or increas* or enhanc* or patient) and (understanding or comprehension)) or ((patient or client or health or medical or drug or written or print* or visual* or provid* or present*) and inform*) or ((print* and (material* or based)) or paper‐based or "written material*" or (paper and pen*) or publication* or newsletter* or brochure* or booklet* or pamphlet* or leaflet* or flyer* or handout* or poster* or illustrat* or picture* or pictogram*) or ((social or community) and network*) or marketing or ((mass or communication* or electronic or digital or multi or print* or social or new) and media) or ((cultural* or linguistic* or language) and (service* or care or intervention* or message*)) or (cultural* and (competen* or sensitiv* or appropriate)) or ((patient or person or family or client) and (cent*red or focus*ed or oriented)) or (therapeutic and (relation* or alliance*)) or (information* and (service* or center* or centre* or system* or dissemination or seeking or retrieval or transfer* or campaign* or provision or aid or material* or sheet* or pack*)) or (computer* and (system* or network* or program* or terminal* or interfac* or interact* or handheld or intervention* or therapy or graphic* or simulation* or searching or mediated or based or tailored or communication or "assisted instruction")) or ("touch screen" or "digital assistant*" or pda or blackberry or mobile‐device* or laptop* or "notebook computer*" or computer* or netbook*) or (((automat* or interactive*) and (telephon* or phone or phones or voice or hotline* or "hot line*")) or ((voice or speech) and (response or recognition or messag* or system* or technolog*))) or ((health or patient* or mediated or facilitated or augmentative or alternative or total or simultaneous or manual or mass or face‐to‐face or one‐to‐one or one‐on‐one or oral or cultural or risk or intervention* or interaction* or program* or skill* or aid* or tool* or board* or device* or system* or barrier*) and communication) or (readability or intelligibility or credibility) or ((professional or physician or doctor or clinician or nurse or provider) and (patient or client or family)) or (self and (teaching or education or instruction)) or ((family or office or work* or school or faith or church) and based) or ((electronic or e‐) and (mail or prescri* or health or game*)) or (self‐care or self‐management))
ERIC (ProQuest)
Searched: 12 July 2012
ALL(vaccinat* or revaccinat* or immuniz* or immunis* or immunother* or inoculat* or innoculat* or prophyla*) AND ALL(randomis* or randomiz* or randomly or intervention* or control* or compar* or evaluat* or "time series" or pretest or posttest or "pre test" or "post test" or impact or chang* or effect* or experiment* or "repeated measure" or "repeated measures")
Grey Literature Searches
ICTRP
Using the 'Advanced search' function, the following was used in each field (truncation symbol * is not necessary). You may want to tick box to restrict to clinical trials in children
Title: vaccin OR immuniz OR immunis OR immunother OR inoculat OR innoculat
Intervention: educat OR inform OR promot OR knowledge OR attitude OR teach OR instruct OR train OR session OR face to face OR communicat OR discuss OR negotiate OR remind OR counsel OR persuad
Result: 111 records altogether (or 47 records when records were restricted to children in clinical trials)
ClinicalTrials.gov
(vaccine OR vaccination OR vaccinated OR vaccinate OR immunise OR immunised OR immunising OR immunisation OR immunize OR immunizing OR immunized OR immunization AND (educate OR education OR teach OR teaching OR train OR training OR instruction OR inform OR information OR promotion OR promote OR communication OR knowledge OR attitude OR session OR face to face OR negotiation OR persuasion OR persuade OR discuss OR discussion)
Result: 89 trials
Open Grey
(vaccin* OR immunis* OR immuniz* OR immunotherap* OR inoculat*) AND (child* OR infant* OR parent* OR mother* or father* OR famil*) AND (educat* OR teach* OR train* OR instruct* OR inform* OR promot* OR persua* OR influenc* OR explain* OR advis* OR counsel* OR communicat* OR knowledge OR understand* OR attitude* OR session* OR campaign* OR messag* OR adverti* OR visit* OR "face to face" OR verbal* OR personal* OR individual* OR meeting* OR peer* OR "health* aide*" OR "health*‐worker*" OR "community‐based" OR "family‐based" OR "school‐based" OR "work*‐based" OR "church‐based" OR intervention* OR remind* OR negotiat*)
Result: 16 records
Grey Literature Report
The Grey Literature Report was searched on 29 August 2012 using different search terms:
“Vaccination communication” = 37 hits, 1 excluded at FT
“Vaccine communication” = 37 hits, 2 excluded at FT
“Immunization communication” = 19 hits, 1 excluded at FT
“Immunization education” = 14 hits, 2 excluded at FT
“Vaccination education” = 16 hits, 3 excluded at FT
“Vaccine education” = 16 hits
“Face to face vaccination” = 8 hits
“Face to face vaccine communication” = 3 hits
“Face to face vaccine education” = 1 hit
“Verbal vaccine information” = 0 hits
“Verbal vaccine education” = 0 hits
“Verbal immunization communication” = 0 hits
“Verbal immunization education” = 0 hits
“Parental vaccination education” = 1 hit, 1 excluded at FT
“Parental vaccination communication” = 1 hit
“Vaccination education session” = 0 hits
“Vaccine education session” = 0 hits
“Immunization education session” = 0 hits
“Vaccination information session” = 0 hits
“Vaccine information session” = 0 hits
“Interpersonal vaccine communication” = 0 hits
“Interpersonal vaccine information” = 0 hits
“Interpersonal immunization education” = 0 hits
Data and analyses
Comparison 1. Interventions aimed at communities versus no intervention.
1.1. Analysis.
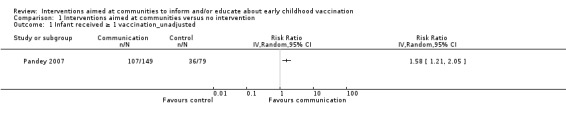
Comparison 1 Interventions aimed at communities versus no intervention, Outcome 1 Infant received ≥ 1 vaccination_unadjusted.
1.3. Analysis.

Comparison 1 Interventions aimed at communities versus no intervention, Outcome 3 Child received measles vaccine_unadjusted.
1.5. Analysis.
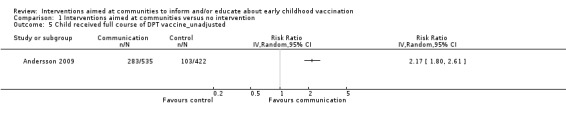
Comparison 1 Interventions aimed at communities versus no intervention, Outcome 5 Child received full course of DPT vaccine_unadjusted.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersson 2009.
| Methods | Study design: cluster‐randomised controlled trial Duration of study: baseline 2005, intervention delivered August 2006 to March 2007 Study arms: intervention in 18 enumerated areas, each with 4 or 5 villages, and including a total of 3166 children under 5 years. 14 control enumeration areas, each with 4/5 villages, and including a total 2475 children under 5 years. Total number of clusters randomised = 32. None of the clusters were lost to follow‐up or withdrew from the trial Methods of recruitment of participants: a random number generator was used to allocate baseline communities to 18 intervention and 14 controls. Local men and women were recruited to lead and record the discussions in the intervention communities. The teams organised male and female groups in the 94 intervention villages (a total of 180 community groups, each of 8 to 10 people) to participate in the intervention Informed consent obtained: yes ‐ field teams visited the community leaders to explain the purpose of the intervention and seek permission to work in the community Ethical approval: yes Funding: grant from the International Development Research Centre, Canada as part of the Canadian International Immunisation Initiative, Phase 2 |
|
| Participants | Setting: Lasbela, one of the poorest districts in the Balochistan province of Pakistan, with limited access to health services. The language spoken was not indicated. In the trial control and intervention clusters, the baseline measles vaccination rates among children (12 to 23 months) were 49% and 47% respectively. For DPT (full schedule), they were 45% and 51% respectively; and for polio vaccine in the last 12 months they were 100% and 99% respectively Description of participants:
Number of participants:
|
|
| Interventions | Aim of intervention: to facilitate informed discussion of childhood vaccination costs and benefits Deliverer: community members (women and men) from the study area Format or delivery mode: meetings of small (8 to 10 people) community groups. The groups consisted of people selected because they were perceived to be trusted within their community and able to convince others Content of communication: the intervention comprised 3 phases of discussions in each community with small community groups. In the first phase, the community groups considered information about child vaccination in their area and why vaccination might be important. In the second phase, the groups discussed the costs and benefits of vaccination, including the complications related to vaccine‐preventable illnesses and the adverse effects of vaccination. In the third phase, the groups considered the challenges to child vaccination in their settings and developed local action plans to address these, including ways to extend the discussions about vaccination to others in their community and ways to improve vaccination service access. Group participants were encouraged to share the information and continue the dialogue with households in their communities. Vaccines delivered or described: the intervention focused on measles, but also measured DPT (diptheria, pertussis and tetanus vaccine) and polio vaccine uptake Direction of communication: small group discussions led by trained community members. Those in the group discussions then took the information to the wider community Where the intervention took place: in communities ‐ further details not provided Frequency or timing of communication: 3 phases of discussion (see above), sometimes with several meetings for each phase Training required for the intervention: those leading and recording the discussions received 2 to 3 days of training for each phase of the intervention. The training included both classroom sessions and field practice Theoretical basis for the intervention: utilised a behaviour change model or cascade (cascada) based on that developed to measure youth responses to risk. "[The] 'cascada' refers to conscious knowledge about immunisation and its side effects, attitudes to childhood immunisation, social norms (what neighbours do) and positive or negative deviation from those norms, intentions to change or to vaccinate in the future, agency (expectancy of self‐efficacy or collective efficacy) and discussion about immunisation, its benefits and side effects. The outcome of this 'cascada' is the action, immunisation." Cost of the intervention: the direct cost of implementation of the intervention was estimated to be USD 63,600. This included the cost of 6 field teams undertaking the 3 phased discussions in 18 communities (94 villages), with a total of 180 community groups. Costs of the baseline and follow‐up surveys were not included Intervention quality: "Local supervisors supported and monitored the work of the field teams and documented the outcome of the three phase discussions, using structured checklists and reporting formats. They visited the teams in the field, provided feedback, and assisted them to remedy any problems encountered with the intervention implementation." (Andersson 2009 pg 3) Fidelity/integrity of the intervention: not described Details of control/usual or routine care: usual care not described Details of co‐interventions in all groups: "During the period of the intervention, the Lasbela government health department implemented a health education programme in the district, aiming to reach all communities, with messages particularly about household hygiene and prevention of diarrhoea in children. Both intervention and control communities received this health education programme, implemented mainly through lady health workers (LHW) and other local officers, who received specific training for this activity" (Andersson 2009 pg 3) |
|
| Outcomes | Primary trial outcome:
Secondary trial outcomes specified were the theory‐based "cascada" of intermediate outcomes leading to vaccination uptake:
The baseline and follow‐up questionnaire included questions about the vulnerability of the household and questions to mothers concerning their education, childcare knowledge, attitudes and practices Outcomes included in the review: uptake of measles, full DPT and polio vaccination of 12‐ to 23‐month old children, as reported by the main caregiver, conscious knowledge, attitudes about vaccination and agency/self efficacy |
|
| Notes | Contact with the authors: we contacted the author to ask for further information regarding the secondary outcomes; if they were able to provide the data for these outcomes by cluster, for all intervention and control clusters. No additional data were received from the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | [All outcomes] Randomly selected 32 enumeration areas from the district population census. A random number generator allocated communities to 18 intervention and 14 control enumeration areas |
| Allocation concealment (selection bias) | Low risk | [All outcomes] The sequence was concealed and intervention assigned centrally |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | [All outcomes] Blinding not mentioned but this is a cluster trial so scored low risk. However, participants in the intervention district would know they were receiving intervention. The field co‐ordinator for the surveys knew which clusters received the intervention but interviewers did not |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | [All outcomes] The field co‐ordinator for the household surveys knew which clusters had received the intervention but interviewers did not. However, they did not evaluate the success of this blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | [All outcomes] No loss of clusters during this trial. Outcomes were assessed on cross‐sections of the population so difficult to assess how many of those available at baseline were lost at follow‐up |
| Selective reporting (reporting bias) | Low risk | [All outcomes] The published study protocol (Andersson 2005) does not include any outcomes that were not assessed in the published trial report |
| Other bias | Low risk | [All outcomes] Recall bias: used maternal report to assess measles and DPT vaccination status and these were not verified by vaccination card. Scored low as this may influence both arms of the trial |
| Selective recruitment of participants (cluster RCTs) | Low risk | [All outcomes] The district population is scattered so it unlikely the participants knew which villages were control or intervention clusters |
| Comparable baseline in intervention and control group | Low risk | [All outcomes] Willingness to travel to vaccinate was higher in intervention than control cluster (P value = 0.009) but this was adjusted for in the analysis |
Pandey 2007.
| Methods | Study design: cluster‐randomised controlled trial Duration of study: May 2004 to May 2005 Study arms: intervention arm (public meetings) + control arm (no intervention). 21 of 70 districts were the focus of the intervention. 1 district consist of proximately 14 blocks and each block consists of 65 village clusters Methods of recruitment of participants: using a random number generator, 1 block was randomly selected within each of the 21 districts, and then 5 villages were randomly selected from each block. No blocks were adjacent to each other. The 21 districts, and the selected blocks and villages, were then randomly assigned to either intervention or control arm. 10 households were sampled in each village of which 5 were low‐caste and five were mid‐ to high‐caste. In total 548 intervention households and 497 control households were included Informed consent obtained: yes, verbal consent of individual participants was obtained Ethical approval: yes Funding: financial support from Sahbhagi Shikshan Kendra (a non‐governmental organisation based in Uttar Pradesh) and the World Bank |
|
| Participants | Setting: 21 central, central‐eastern and southern districts out of the 70 districts in Uttar Pradesh, a State in Northern India with a population of 170 million. Uttar Pradesh is ranked 23 of 32 states in India in terms of the proportion of people living below the poverty line, with one living under the poverty line. Infant mortality rate is 89 per 1000. Literacy is 56% while 46% of children are immunised. The main language spoken is Hindi Description of participants:
Number of participants: The number of households with infants < 1 year was as follows:
|
|
| Interventions | Aim of the intervention: to support resource‐poor populations through providing information about entitled health services, educational services and governance requirements Deliverer: project research assistants. No further information provided Format or delivery mode: information campaigns were conducted in 2 rounds for each cluster, separated by 2 weeks. Each round consisted of 2 to 3 meetings and also included the distribution of leaflets and posters in the intervention villages. Residents were informed in advance about the dates and locations of meetings and separate meetings were held in low and mid‐ to high‐caste neighbourhoods. Each meeting lasted about an hour and consisted of the following: a 15‐minute audiotape presentation that was played twice; opportunities to ask questions; and distribution of leaflets. People were informed that the information was obtained from the government and distributed in the public interest by the research team and a nongovernmental organisation based in Uttar Pradesh, Sahbhagi Shikstan Kendra Content of communication: information included health services information; availability of midwives; obligations of the midwife to provide free prenatal and postnatal care including tetanus vaccines and prenatal supplements for the mother and health care and vaccines for infants; the health centres available for more specialised care; and where to complain about quality or quantity of services. Information was also included on school fees; sources and oversight of education funds; obligations of oversight committees; requirements for semi‐annual village government meetings; organisation and funding of village government and development work; rights to obtain copies of village records; and where to complain about education or village governance problems. The information in the presentation and leaflets was obtained from Uttar Pradesh health, education and village governance departments Vaccines delivered or described: infant vaccinations (not specified) and tetanus vaccination for pregnant women Direction of communication: from research assistants to community members. Community members had opportunities to ask questions Where the intervention took place: intervention communities. Separate meetings were held in low‐ and mid‐ to high‐caste areas Frequency or timing of communication: 2 rounds of 2 to 3 meetings, separated by a period of 2 weeks Training required for the intervention: not described Theoretical basis for the intervention: not described Cost of the intervention: the total cost of the intervention was USD 4000 (approximately USD 0.22 per household in a village cluster) Intervention quality: to help ensure that the intervention was delivered in a uniform way, those delivering the information read a scripted introduction and were allowed to answer questions only to which the answers were already written on the information leaflets provided Fidelity/integrity of the intervention: "According to headcounts of everyone attending our informational meetings, 14% of the residents of the entire village cluster attended in round 1 and 11% attended in round 2. If there was some overlap in attendance, we estimate that 14% to 25% of the residents of each intervention village cluster attended a presentation. We reached our target level of attendance, which was 250 people in each round (about 11% of the average village population)" (Pandey 2007 pg 1870) Details of control/usual or routine care: not described Details of co‐interventions in all groups: not described |
|
| Outcomes |
Outcomes included in the review: vaccinations received by infants |
|
| Notes | The total cost of the intervention was approximately USD 4000. This included the cost of designing the intervention (for example, a professional radio journalist was hired for the audio‐recording of information played in the villages); printing materials; the tools and equipment (tape‐recorders); and the cost of hiring the research team to carry out the campaign (personal communication) Contact with the authors: we contacted the author to ask for additional information regarding: (1) The intervention. This included further details related to vaccination and in what way vaccines were a part of the intervention and more information about how communities participated in the meetings. (2) The relative risk for the vaccination outcome, adjusted for clustering and other variables. Additional information on the intervention and this outcome was received from the study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | [All outcomes] From a comprehensive list of blocks and village clusters, they used a random number generator to randomly select 1 block within each district and then randomly select 5 village clusters within each block. They then randomly assigned districts to intervention and control arms. The method of randomisation was not described |
| Allocation concealment (selection bias) | Low risk | [All outcomes] The unit of allocation was by cluster and allocation was performed on all units at the start of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | [All outcomes] 5 village clusters of about 1000 in each district were selected. The total selection of 105 village clusters was spread over the 21 districts to minimise any potential for contamination between intervention and control villages. However, participants in the intervention district would have known they were receiving intervention. The field co‐ordinator for the surveys knew which clusters received the intervention but interviewers did not. Participants in the intervention clusters would have known if they were receiving intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | [All outcomes] The follow‐up interviews were performed by a research assistant who had no knowledge of the intervention. To maintain this blinding, intervention group subjects were not asked whether they attended an informational meeting. We assessed it as unlikely that it was possible to maintain the blinding, and we are not able to find information on blinding of the people that performed the analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | [All outcomes] 12 households in the intervention group and 8 in the control group moved before the final survey |
| Selective reporting (reporting bias) | Unclear risk | [All outcomes] We were not able to locate a published protocol to assess if all of the outcomes listed in the original protocol were reported. Results are reported for all of the main outcomes mentioned in the trial report |
| Other bias | Low risk | [All outcomes] Recall bias ‐ information regarding vaccinations was obtained through interviews. We scored this as low risk of bias as it may influence both arms of the trial |
| Selective recruitment of participants (cluster RCTs) | Low risk | [All outcomes] The districts population were scattered (105 village clusters were spread over the 21 districts), so it is unlikely that the participants knew which villages were control or intervention clusters |
| Comparable baseline in intervention and control group | Low risk | [All outcomes] There was a slightly uneven distribution of low‐caste versus mid‐ to high‐caste households. Of 548 households in intervention village clusters, 252 (46%) were low‐caste. Of 497 households in control village clusters, 245 (49%) were low‐caste |
DPT: diptheria, pertussis and tetanus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abhyankar 2008 | Intervention |
| Agboatwalla 1997 | Population |
| Andrianarivelo 2001 | Design |
| Anjum 2002 | Intervention |
| Ansari 2007 | Design/intervention |
| Aylward 2011 | Design/intervention |
| Balraj 1986 | Design |
| Barzgar 1997 | Design |
| Belmaker 2006 | Intervention |
| Berhane 1993 | Intervention |
| Bhargava 1972 | Intervention |
| Black 1981 | Intervention/population |
| Bolivia study 1985 | Intervention |
| Bollag 1979 | Population |
| Bonu 2003 | Design |
| Browngoehl 1997 | Intervention |
| Bruneau 2001 | Population |
| Budroni 1991 | Population |
| Casas 2001 | Intervention/population/design |
| Chandra 1992 | Intervention |
| Chen 2012 | Population |
| Chongsuvivatwong 1993 | Intervention |
| Cliff 1984 | Design |
| Cockman 2011 | Intervention |
| Cui 2009 | Population |
| Dammann 1990 | Intervention |
| Davis 1998 | Population |
| de Nuncio 1999 | Design |
| Devadas 1983 | Design |
| Devedas 1994 | Design |
| Dini 2000 | Intervention |
| Donovan 2000 | Intervention |
| Dutta 1989 | Design |
| Dyer 1996 | Design |
| Ekerete 1997 | Design |
| Elkharrat 1999 | Population |
| Estrella 2001 | Full text not in file |
| Euler 2003 | Intervention |
| Fairhead 2006 | Design |
| Ferson 1995 | Intervention |
| Fischer 1995 | Design |
| Fu 2012 | Design |
| Gateff 1968 | Full text not in file |
| Gilca 2006 | Intervention |
| Glogowska‐Ligus 2011 | Population |
| Goel 2012 | Design |
| Gold 1994 | Design |
| Guimaraes 2009 | Design |
| Gutierrez 1996 | Intervention |
| Haelterman 1996 | Design |
| Hamkar 2006 | Design |
| Holzman 2005 | Population |
| Hong 2005 | Design |
| Igarashi 2010 | Intervention |
| Jackson 2010 | Design /intervention |
| Jackson 2011 | Intervention |
| Johnson 2000 | Intervention |
| Jones 1980 | Population |
| Joo 2001 | Design |
| Kar 1968 | Design |
| Kavanagh 2011 | Design |
| Khanom 1983 | Design |
| Koehlmoos 2011 | Design |
| Kowli 1990 | Unable to assess article |
| Lahariya 2007 | Design |
| Leiner 2004 | Intervention |
| Lin 1971 | Design |
| Lindegger 2007 | Design |
| Linkins 1994 | Intervention |
| Linkins 1995 | Design |
| Luo 2009 | Design |
| Macdonald 1985 | Design |
| Maher 1993 | Design |
| Main 2001 | Design |
| Majdzadeh 2008 | Design |
| Mann 1972 | Design |
| Marchand 1992 | Design |
| Martinez‐Campillo 2003 | Design |
| Mason 2000 | Design |
| Mason 2000b | Intervention |
| Massoudi 1999 | Intervention |
| Mathur 1979 | Intervention |
| Mayer 1999 | Intervention |
| McBean 1976 | Design |
| McPhee 2003b | Design |
| Meegan 2001 | Intervention |
| Mexico study 1994 | Design |
| Morgan 1998 | Intervention |
| Movahedi 2008 | Design |
| Muhuri 1995 | Design/study aim |
| Nanyunja 2003 | Design |
| Niederhauser 1999 | Full text not in file |
| Nuwaha 2000 | Intervention |
| Nwogu 2008 | Intervention |
| O'Mahony 1986 | Population |
| Oche 2011 | Design |
| Odusanya 2003 | Design |
| Oeffinger 1992 | Intervention |
| Oliphant 2010 | Intervention |
| Painvin 2011 | Population |
| Pallasch 2005a | Unable to assess article |
| Pallasch 2005b | Duplicate of Pallasch 2005a |
| Paunio 1991 | Intervention |
| Peng 2012 | Design |
| Perdue 1955 | Design |
| Phommathansy 2002 | Full text not in file |
| Pietsch 2002 | Full text not in file |
| Porter 2000 | Design |
| Porter‐Jones 2009 | Intervention |
| Przewlocka 2000 | Design |
| Quaiyum 1997 | Design |
| Rahman 1988 | Design/intervention |
| Rajakumar 2010 | Design/intervention |
| Rao 1974 | Design |
| Reichler 1997 | Design |
| Ryman 2011 | Intervention |
| Salmond 1994 | Intervention |
| Schwarz 2008 | Population |
| Shaikh 2003 | Design |
| Shamma 1970 | Intervention |
| Sheikh 2009 | Population |
| Shenson 2001 | Population |
| Shevlin 2002 | Intervention |
| Smith 1999 | Intervention |
| Sokhey 1985 | Design |
| Sudan study 1995 | Full text not in file |
| Sultana 2008 | Design |
| Swift 1969 | Design |
| Tandon 1992 | Intervention |
| Teberg 1970 | Design/intervention |
| Thomas 2008 | Intervention |
| Thompson 2009 | Design |
| Tung 2005 | Intervention/population |
| Usman 2011 | Population |
| Vijayaraghavan 1983 | Design |
| Waisbord 2010 | Design |
| Wallace 2006 | Intervention |
| Wang 2007 | Intervention |
| Waterman 1996 | Design |
| White 1976 | Design |
| WHO 1986 | Design |
| Zajicek‐Farber 2010 | Intervention |
| Zimicki 1994 | Design |
| Zuber 2001 | Intervention |
Differences between protocol and review
In the protocol (Saeterdal 2012), unintended adverse effects due to the intervention were listed as a secondary outcome. In the review, these are listed as a primary outcome, as per Cochrane recommendations.
Contributions of authors
Ingvil Sæterdal (IS) and Simon Lewin (SL) drafted the protocol for the review with methodological and content‐specific input from the rest of the authors. IS and SL and Susan Munabi‐Babigumira (Background) drafted the review with input from the other authors. Claire Glenton drafted the plain language summary. All authors participated in the inclusion and assessment of the literature and in drafting the summary of findings.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Norwegian Research Council, Norway.
This review was supported by a grant from the Norwegian Research Council for the 'Communicate to vaccinate' (COMMVAC) project (www.commvac.com).
Declarations of interest
The authors of this review are employed by the Norwegian Knowledge Centre for the Health Services. We receive in‐kind support from the Knowledge Centre such as time, office materials, library access and support from colleagues. The Knowledge Centre has no inappropriate interest in the findings of this review.
The funder of this work, the Norwegian Research Council's GLOBVAC programme, does not have any inappropriate vested interest in the findings of this review.
Simon Lewin and Claire Glenton are editors for the Cochrane Consumers and Communication Review Group. They did not have any influence over the Review Group's editorial process or decision to publish this review.
New
References
References to studies included in this review
Andersson 2009 {published data only (unpublished sought but not used)}
- Andersson N, Cockcroft A, Ansari NM, Omer K, Baloch M, Ho Foster A, et al. Evidence‐based discussion increases childhood vaccination uptake: a randomised cluster controlled trial of knowledge translation in Pakistan. BMC International Health and Human Rights 2009;9 Suppl 1:S8. [PUBMED: 19828066] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson N, Cockcroft A, Ansari NM, Omer K, Losos J, Ledogar RJ, et al. Household cost‐benefit equations and sustainable universal childhood immunisation: a randomised cluster controlled trial in south Pakistan [ISRCTN12421731]. BMC Public Health 2005 2005;5:72. [PUBMED: 15985160] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandey 2007 {published and unpublished data}
- Pandey P, Sehgal AR, Riboud M, Levine D, Goyal M. Informing resource‐poor populations and the delivery of entitled health and social services in rural India: a cluster randomized controlled trial. JAMA 2007;298(16):1867‐75. [PUBMED: 17954538] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abhyankar 2008 {published data only}
- Abhyankar P, O'Connor DB, Lawton R. The role of message framing in promoting MMR vaccination: evidence of a loss‐frame advantage. Psychology, Health & Medicine 2008;13(1):1‐16. [DOI] [PubMed] [Google Scholar]
Agboatwalla 1997 {published data only}
- Agboatwalla M, Akram DS. Impact of health education on mothers' knowledge of preventive health practices. Tropical Doctor 1997;27(4):199‐202. [DOI] [PubMed] [Google Scholar]
Andrianarivelo 2001 {published data only}
- Andrianarivelo MR, Boisier P, Rabarijaona L, Ratsitorahina M, Migliani R, Zeller H. Mass vaccination campaigns to eradicate poliomyelitis in Madagascar: oral poliovirus vaccine increased immunity of children who missed routine programme. Tropical Medicine & International Health 2001;6(12):1032‐9. [DOI] [PubMed] [Google Scholar]
Anjum 2002 {published data only}
- Anjum Q, Omair A, Inam SNB, Ahmed Y, Usman Y, Shaikh S. Improving vaccination status of children under five through health education. Journal of the Pakistan Medical Association 2002;54(12):610‐3. [PubMed] [Google Scholar]
Ansari 2007 {published data only}
- Ansari MA, Khan Z, Khan IM. Reducing resistance against polio drops. Journal of the Royal Society for the Promotion of Health 2007;127(6):276‐9. [DOI] [PubMed] [Google Scholar]
Aylward 2011 {published data only}
- Aylward B, Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine 2011;29 Suppl 4:D80‐5. [DOI] [PubMed] [Google Scholar]
Balraj 1986 {published data only}
- Balraj V, John TJ. Evaluation of a poliomyelitis immunization campaign in Madras city. Bulletin of the World Health Organization 1986;64(6):861‐5. [PMC free article] [PubMed] [Google Scholar]
Barzgar 1997 {published data only}
- Barzgar MA, Sheikh MR, Bile MK. Female health workers boost primary care. World Health Forum 1997;18(2):202‐10. [PubMed] [Google Scholar]
Belmaker 2006 {published data only}
- Belmaker I, Dukhan L, Elgrici M, Yosef Y, Shahar‐Rotberg L. Reduction of vaccine‐preventable communicable diseases in a Bedouin population: summary of a community‐based intervention programme. Lancet 2006;367(9515):987‐91. [DOI] [PubMed] [Google Scholar]
Berhane 1993 {published data only}
- Berhane Y, Pickering J. Are reminder stickers effective in reducing immunization dropout rates in Addis Ababa, Ethiopia?. Journal of Tropical Medicine and Hygiene 1993;96(3):139‐45. [PubMed] [Google Scholar]
Bhargava 1972 {published data only}
- Bhargava SK. Evaluation of methods for mass immunizations in children. Indian Pediatrics 1972;9(7):378‐83. [PubMed] [Google Scholar]
Black 1981 {published data only}
- Black NA. Response to rubella immunisation education in health authority family planning clinics: a controlled clinical trial. Health Education Journal 1981;40(4):111‐8. [DOI] [PubMed] [Google Scholar]
Bolivia study 1985 {published data only}
- Higher coverage attained through public participation: National Vaccination Days in Bolivia. EPI newsletter / c Expanded Program on Immunization in the Americas 1985;7(1):4‐6. [PubMed] [Google Scholar]
Bollag 1979 {published data only}
- Bollag U. Practical evaluation of a pilot immunization campaign against typhoid fever in a Cambodian refugee camp. Disasters 1979;3(4):413‐5. [DOI] [PubMed] [Google Scholar]
Bonu 2003 {published data only}
- Bonu S, Rani M, Baker TD. The impact of the national polio immunization campaign on levels and equity in immunization coverage: evidence from rural North India. Social Science & Medicine (1982) 2003;57(10):1807‐19. [DOI] [PubMed] [Google Scholar]
Browngoehl 1997 {published data only}
- Browngoehl K, Kennedy K, Krotki K, Mainzer H. Increasing immunization: a Medicaid managed care model. Pediatrics 1997;99(1):E4. [DOI] [PubMed] [Google Scholar]
Bruneau 2001 {published data only}
- Bruneau A, Haley N, Valiquette L. Evaluation of a program to promote hepatitis B vaccination among adolescents in a school environment of Central Montreal. Canadian Journal of Public Health 2001;92(5):348‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Budroni 1991 {published data only}
- Budroni M, Delogu S, Dettori S, Rodella D, Sechi O, Angius M. Results and evaluation of an immunization campaign against measles at local level. Igiene Moderna 1991;96(3):341‐8. [Google Scholar]
Casas 2001 {published data only}
- Casas GI, Ruiz MJ, Carreras MA, Segura EA, Esteve PM, Casas GX. Prevalence of tuberculosis in a population from Kosovo sheltered in Catalonia, Spain. Medicina Clinica 2001;116(20):770‐1. [PubMed] [Google Scholar]
Chandra 1992 {published data only}
- Chandra R, Srivastava VK, Nirupam S. Impact of Urban Basic Services on immunization coverage in a slum area of northern India. Asia‐Pacific Journal of Public Health/Asia‐Pacific Academic Consortium for Public Health 1992;6(3):153‐5. [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen JJ, Chang ET, Chen YR, Bailey MB, So SKS. A model program for hepatitis B vaccination and education of schoolchildren in rural China. International Journal of Public Health 2012;57(3):581‐8. [DOI] [PubMed] [Google Scholar]
Chongsuvivatwong 1993 {published data only}
- Chongsuvivatwong V, Bujakorn L, Kanpoy V, Treetrong R. Control of neonatal tetanus in southern Thailand. International Journal of Epidemiology 1993;22(5):931‐5. [DOI] [PubMed] [Google Scholar]
Cliff 1984 {published data only}
- Cliff J, Lemos L. Prevention of neonatal tetanus in Maputo, Mozambique. Revista Medica de Mocambique 1984;2(2):65‐8. [Google Scholar]
Cockman 2011 {published data only}
- Cockman P, Dawson L, Mathur R, Hull S. Improving MMR vaccination rates: herd immunity is a realistic goal. BMJ 2011;343:d5703. [DOI] [PubMed] [Google Scholar]
Cui 2009 {published data only}
- Cui FQ, Miao N, Hu YS. Effect of hepatitis health promotion project in schools of Beijing and Gansu. Zhongguo Yi Miao He Mian Yi 2009;15(5):409‐16. [PubMed] [Google Scholar]
Dammann 1990 {published data only}
- Dammann DF, Solarsh GC, Patrick ME, Ijsselmuiden CB. Vaccination‐‐coverage of under‐fives, validity of records, and the impact of mass campaigns in the Edendale/Vulindlela district of KwaZulu. South African Medical Journal 1990;78(12):729‐33. [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis TC, Fredrickson DD, Arnold C, Murphy PW, Herbst M, Bocchini JA. A polio immunization pamphlet with increased appeal and simplified language does not improve comprehension to an acceptable level. Patient Education and Counseling 1998;33(1):25‐37. [DOI] [PubMed] [Google Scholar]
de Nuncio 1999 {published data only}
- Nuncio MLZ, Price SA, Tjoa T, Lashuay N, Jones MC, Elder JP. Pretesting Spanish‐language educational radio messages to promote timely and complete infant immunization in California. Journal of Community Health 1999;24:269‐84. [DOI] [PubMed] [Google Scholar]
Devadas 1983 {published data only}
- Devadas RP, Premakumari S. Potentials of the multipurpose health workers (MPHW) for improving the nutritional status of rural communities. Indian Journal of Nutrition and Dietetics 1983;20(10):310‐5. [Google Scholar]
Devedas 1994 {published data only}
- Devedas RP, Parvathi E, Ruckmani P. Impact of nutrition education of the Tamil Nadu Integrated Nutrition Project on the nutritional status of selected preschool children. Indian Journal of Nutrition and Dietetics 1994;31(7):193‐8. [Google Scholar]
Dini 2000 {published data only}
- Dini EF, Linkins RW, Sigafoos J. Erratum: The impact of computer‐generated messages on childhood immunization coverage (American Journal of Preventive Medicine). American Journal of Preventive Medicine 2000;19(1):68‐70. [DOI] [PubMed] [Google Scholar]
Donovan 2000 {published data only}
- Donovan RJ, Jalleh G. Positive versus negative framing of a hypothetical infant immunization: the influence of involvement. Health Education & Behavior 2000;27(1):82‐95. [DOI] [PubMed] [Google Scholar]
Dutta 1989 {published data only}
- Dutta PK, Vaz LS, Singh H. Knowledge, attitude and beliefs about measles and vaccination coverage in a rural area. Journal of Communicable Diseases 1989;21(4):285‐9. [PubMed] [Google Scholar]
Dyer 1996 {published data only}
- Dyer JJ, Naidoo KN, Knight S, Mjekevu T, Munro G, Robinson A. Effect of an immunisation campaign in Natal and KwaZulu on vaccination coverage rates, 1990‐1991. South African Medical Journal 1996;86(2):158‐61. [PubMed] [Google Scholar]
Ekerete 1997 {published data only}
- Ekerete PP. Motivating consumers for National Programme on Immunization (NPI) and Oral Rehydration Therapy (ORT) in Nigeria. Journal of Hospital Marketing 1997;12(1):33‐60. [DOI] [PubMed] [Google Scholar]
Elkharrat 1999 {published data only}
- Elkharrat D, Boyer Chammard A, Raskine L, Durand‐Zaleski I, Zerbani A, Caulin C, et al. Impact of guidelines to alter antitetanus prophylaxis practices and reduce costs in the emergency department. American Journal of Therapeutics 1999;6(4):203‐9. [DOI] [PubMed] [Google Scholar]
Estrella 2001 {published data only}
- Estrella Silva FF, Mejia R, Osorio F. Cual es el efecto que produce un programa de educacion en vacunacion sobre el aumento en la demanda de vacunacion dado en los meses de enero a junio de 1980 a las personas responsables de las familias con ninos de 0‐5 anos de edad 2001:152.
Euler 2003 {published data only}
- Euler GL, Copeland J, Williams WW. Impact of four urban perinatal hepatitis B prevention programs on screening and vaccination of infants and household members. American Journal of Epidemiology 2003;157(8):747‐53. [DOI] [PubMed] [Google Scholar]
Fairhead 2006 {published data only}
- Fairhead J, Leach M, Small M. Public engagement with science? Local understandings of a vaccine trial in the Gambia. Journal of Biosocial Science 2006;38(1):103‐16. [DOI] [PubMed] [Google Scholar]
Ferson 1995 {published data only}
- Ferson MJ, Fitzsimmons G, Christie D, Woollett H. School‐health nurse interventions to increase immunization uptake in school entrants. Public Health 1995;109:25‐9. [DOI] [PubMed] [Google Scholar]
Fischer 1995 {published data only}
- Fischer S, Baron D. Testing strategies to raise immunization rates. Report of the Joyce Foundation's Special Project on Immunization. Joyce Foundation, Chicago, Illinois, USA 1995.
Fu 2012 {published data only}
- Fu LY, Weissman M, McLaren R, Thomas C, Campbell J, Mbafor J, et al. Improving the quality of immunization delivery to an at‐risk population: a comprehensive approach. Pediatrics 2012;129(2):e496‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gateff 1968 {published data only}
- Gateff C, Lefevre M. Health education contribution to BCG vaccination: a pilot project in Africa. International Journal of Health Education 1968;11(1):38‐46. [Google Scholar]
Gilca 2006 {published data only}
- Gilca V, Duval B, Boulianne N, Dion R, Serres G. Impact of the Quebec school‐based hepatitis B immunization program and potential benefit of the addition of an infant immunization program. Pediatric Infectious Disease Journal 2006;25(4):372‐4. [DOI] [PubMed] [Google Scholar]
Glogowska‐Ligus 2011 {published data only}
- Glogowska‐Ligus J, Dabek J, Koj J, Bonek‐Wytrych G, Lepich T, Bajor G. B hepatitis vaccination evaluated in population of non‐medical staff members. Polski Merkuriusz Lekarski 2011;31(183):154‐8. [PubMed] [Google Scholar]
Goel 2012 {published data only}
- Goel S, Dogra V, Gupta SK, Lakshmi PV, Varkey S, Pradhan N, et al. Effectiveness of Muskaan Ek Abhiyan (the smile campaign) for strengthening routine immunization in Bihar, India. Indian Pediatrics 2012;49(2):103‐8. [DOI] [PubMed] [Google Scholar]
Gold 1994 {published data only}
- Gold R, Bjornson GL. What do parents learn by reading a DPT vaccine information form?. Canadian Journal of Infectious Diseases 1994;5(2):67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guimaraes 2009 {published data only}
- Guimaraes TMR, Alves JGB, Tavares MMF. Impact of immunization measures by the Family Health Program on infant mortality from preventable diseases in Olinda, Pernambuco State, Brazil. Cadernos de Saude Publica/Ministerio da Saude, Fundacao Oswaldo Cruz, Escola Nacional de Saude Publica 2009;25(4):868‐76. [DOI] [PubMed] [Google Scholar]
Gutierrez 1996 {published data only}
- Gutierrez G, Tapia‐Conyer R, Guiscafre H, Reyes H, Martinez H, Kumate J. Impact of oral rehydration and selected public health interventions on reduction of mortality from childhood diarrhoeal diseases in Mexico. Bulletin of the World Health Organization 1996;74(2):189‐97. [PMC free article] [PubMed] [Google Scholar]
Haelterman 1996 {published data only}
- Haelterman E, Boelaert M, Suetens C, Blok L, Henkens M, Toole MJ. Impact of a mass vaccination campaign against a meningitis epidemic in a refugee camp. Tropical Medicine & International Health 1996;1(3):385‐92. [DOI] [PubMed] [Google Scholar]
Hamkar 2006 {published data only}
- Hamkar R, Jalilvand S, Mokhtari‐Azad T, Jelyani KN, Nategh R. Evaluation of immunity against rubella in Iranian after mass campaign for measles‐rubella vaccination on December 2003. American Journal of Infection Control 2006;34(9):588‐92. [DOI] [PubMed] [Google Scholar]
Holzman 2005 {published data only}
- Holzman GS, Harwell TS, Johnson EA, Goldbaum G, Helgerson SD. A media campaign to promote pneumococcal vaccinations: is a telephone survey an effective evaluation strategy?. Journal of Public Health Management and Practice 2005;11(3):228‐34. [DOI] [PubMed] [Google Scholar]
Hong 2005 {published data only}
- Hong R, Banta JE. Effects of extra immunization efforts on routine immunization at district level in Pakistan. Eastern Mediterranean Health Journal 2005;11(4):745‐52. [PubMed] [Google Scholar]
Igarashi 2010 {published data only}
- Igarashi K, Sasaki S, Fujino Y, Tanabe N, Muleya CM, Tambatamba B, et al. The impact of an immunization programme administered through the Growth Monitoring Programme Plus as an alternative way of implementing Integrated Management of Childhood Illnesses in urban‐slum areas of Lusaka, Zambia. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010;104(9):577‐82. [DOI] [PubMed] [Google Scholar]
Jackson 2010 {published data only}
- Jackson C, Cheater FM, Rose P, Julie L, Lyndal T. Evaluating a web‐based MMR decision aid to support informed decision making by UK parents: a before‐and‐after feasibility study. Health Education Journal 2010;73(4):74‐83. [DOI: 10.1177/0017896910363146] [DOI] [Google Scholar]
Jackson 2011 {published data only}
- Jackson C, Cheater FM, Harrison W, Peacock R, Bekker H, West R, et al. Randomised cluster trial to support informed parental decision‐making for the MMR vaccine. BMC Public Health 2011;11:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2000 {published data only}
- Johnson Z, Molloy B, Scallan E, Fitzpatrick P, Rooney B, Keegan T, et al. Community Mothers Programme‐‐seven year follow‐up of a randomized controlled trial of non‐professional intervention in parenting. Journal of Public Health Medicine 2000;22(3):337‐42. [DOI] [PubMed] [Google Scholar]
Jones 1980 {published data only}
- Jones SA. Health education to improve rubella immunisation in schools. British Medical Journal 1980;281(6241):649‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joo 2001 {published data only}
- Joo CB. A study on delivery environments and immunization practice of the maternal & child health services in a rural area. Journal of Korean Academy of Nursing 2001;5(2):92‐103. [Google Scholar]
Kar 1968 {published data only}
- Kar SB, Srivastava VP. Impact of mass communication in a smallpox vaccination campaign. Health Education Journal 1968;27(4):205‐14. [Google Scholar]
Kavanagh 2011 {published data only}
- Kavanagh J, Oakley A, Harden A, Trouton A, Powell C. Are incentive schemes effective in changing young people's behaviour? A systematic review. Health Education Journal 2011;70(2):192‐205. [Google Scholar]
Khanom 1983 {published data only}
- Khanom K, Salahuddin AK. A study on impact of an educational programme on immunization behaviour of parents. Bangladesh Medical Research Council Bulletin 1983;9(1):18‐24. [PubMed] [Google Scholar]
Koehlmoos 2011 {published data only}
- Koehlmoos TP, Uddin J, Sarma H. Impact of measles eradication activities on routine immunization services and health systems in Bangladesh. Journal of Infectious Diseases 2011;204 Suppl 1:S90‐7. [DOI] [PubMed] [Google Scholar]
Kowli 1990 {published data only}
- Kowli SS, Bhalerao VR, Jagtap AS, Shrivastav R. Community participation improves vaccination coverage. Forum Mondial de la Sante 1990;11(2):181‐4. [PubMed] [Google Scholar]
Lahariya 2007 {published data only}
- Lahariya C, Khandekar J, Ray TK, Meenakshi, Pradhan SK. Role of an area specific approach to increase community participation in pulse polio program in a locality of South Delhi. Journal of Communicable Diseases 2007;39(4):245‐8. [PubMed] [Google Scholar]
Leiner 2004 {published data only}
- Leiner M, Handal G, Williams D. Patient communication: a multidisciplinary approach using animated cartoons. Health Education Research 2004;19(5):591‐5. [DOI] [PubMed] [Google Scholar]
Lin 1971 {published data only}
- Lin N, Hingson R, Allwood‐Paredes J. Mass immunization campaign in El Salvador, 1969. Evaluation of receptivity and recommendation for future campaigns. HSMHA Health Reports 1971;86(12):1112‐21. [PMC free article] [PubMed] [Google Scholar]
Lindegger 2007 {published data only}
- Lindegger G, Quayle M, Ndlovu M. Local knowledge and experiences of vaccination: implications for HIV‐preventive vaccine trials in South Africa. Health Education & Behavior 2007;34(1):108‐23. [DOI] [PubMed] [Google Scholar]
Linkins 1994 {published data only}
- Linkins RW, Dini EF, Watson G, Patriarca PA. A randomized trial of the effectiveness of computer‐generated telephone messages in increasing immunization visits among preschool children. Archives of Pediatrics & Adolescent Medicine 1994;148(9):908‐14. [DOI] [PubMed] [Google Scholar]
Linkins 1995 {published data only}
- Linkins RW, Mansour E, Wassif O, Hassan MH, Patriarca PA. Evaluation of house‐to‐house versus fixed‐site oral poliovirus vaccine delivery strategies in a mass immunization campaign in Egypt. Bulletin of the World Health Organization 1995;73(5):589‐95. [PMC free article] [PubMed] [Google Scholar]
Luo 2009 {published data only}
- Luo FJ, Liu F, Zhang J. The effective evaluation on the supplementary immunization activities among the migrant pre‐school children in Chaoyang District of Beijing. Zhongguo Yi Miao He Mian Yi 2009;15(3):276‐8. [PubMed] [Google Scholar]
Macdonald 1985 {published data only}
- Macdonald H, Roder D. The planning, implementation and evaluation of an immunization promotion campaign in South Australia. Hygie 1985;4(2):13‐7. [PubMed] [Google Scholar]
Maher 1993 {published data only}
- Maher CP, Hall JJ, Yakam W, Naupa M, Leonard D. Improving vaccination coverage: the experience of the Expanded Programme on Immunization in Vanuatu. Papua and New Guinea Medical Journal 1993;36(3):228‐33. [PubMed] [Google Scholar]
Main 2001 {published data only}
- Main B, Lower T, James R, Rouse I. Changes in Expanded Program for Immunization coverage for mother and child in Krakor, Cambodia 1996‐‐1998. Tropical Medicine & International Health 2001;6(7):526‐8. [DOI] [PubMed] [Google Scholar]
Majdzadeh 2008 {published data only}
- Majdzadeh R, Moradi A, Zeraati H, Sepanlou SG, Zamani G, Zonobi V. Evaluation of the measles‐rubella mass vaccination campaign in the population covered by Tehran University of Medical Sciences. Eastern Mediterranean Health Journal 2008;14(4):810‐7. [PubMed] [Google Scholar]
Mann 1972 {published data only}
- Mann CK. Meeting the polio threat through health education. International Health Education 1972;15(3):149‐58. [Google Scholar]
Marchand 1992 {published data only}
- Marchand RS, Poulin C. Strategies to Increase and Maintain High Immunization Levels in Children. Canadian Journal of Public Health 1992;83:113‐5. [PubMed] [Google Scholar]
Martinez‐Campillo 2003 {published data only}
- Martinez‐Campillo Garcia F, Maura da Fonseca A, Santiago Oliva J, Verdu Perez M, Serramia del Prisco A, Ibanez Molina M, et al. Vaccine coverage study and intervention with health community agents in a marginal gypsy community of Alicante. Atencion Primaria/Sociedad Espanola de Medicina de Familia y Comunitaria 2003;31(4):234‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2000 {published data only}
- Mason BW, Donnelly PD. Impact of a local newspaper campaign on the uptake of the measles mumps and rubella vaccine. Journal of Epidemiology and Community Health 2000;54(6):473‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2000b {published data only}
- Mason BW, Donnelly PD. Targeted mailing of information to improve uptake of measles, mumps, and rubella vaccine: a randomised controlled trial. Communicable Disease and Public Health 2000;3(1):67‐8. [PUBMED: 10743326] [PubMed] [Google Scholar]
Massoudi 1999 {published data only}
- Massoudi MS, Walsh J, Stokley S, Rosenthal J, Stevenson J, Miljanovic B, et al. Assessing immunization performance of private practitioners in Maine: impact of the assessment, feedback, incentives, and exchange strategy. Pediatrics 1999;103(6 Pt 1):1218‐23. [DOI] [PubMed] [Google Scholar]
Mathur 1979 {published data only}
- Mathur HN, Damodar, Sharma PN, Jain TP. The impact of training traditional birth attendants on the utilisation of maternal health services. Journal of Epidemiology and Community Health 1979;33(2):142‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayer 1999 {published data only}
- Mayer JP, Housemann R, Piepenbrok B. Evaluation of a campaign to improve immunization in a rural Headstart program. Journal of Community Health 1999;24(1):13‐27. [DOI] [PubMed] [Google Scholar]
McBean 1976 {published data only}
- McBean AM, Foster SO, Herrmann KL, Gateff C. Evaluation of a mass measles immunization campaign in Yaounde, Cameroun. Transactions of the Royal Society of Tropical Medicine and Hygiene 1976;70(3):206‐12. [DOI] [PubMed] [Google Scholar]
McPhee 2003b {published data only}
- McPhee SJ, Nguyen T, Euler GL, Mock J, Wong C, Lam T, et al. Successful promotion of hepatitis B vaccinations among Vietnamese‐American children ages 3 to 18: results of a controlled trial. Pediatrics 2003;111(6 Pt 1):1278‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meegan 2001 {published data only}
- Meegan ME, Conroy RM, Lengeny SO, Renhault K, Nyangole J. Effect on neonatal tetanus mortality after a culturally‐based health promotion programme. Lancet 2001;358(9282):640‐1. [DOI] [PubMed] [Google Scholar]
Mexico study 1994 {published data only}
- Mexico's immunization programme gets results. CVI Forum 1994;6:4‐5. [PubMed] [Google Scholar]
Morgan 1998 {published data only}
- Morgan MZ, Evans MR. Initiatives to improve childhood immunisation uptake: a randomised controlled trial. BMJ 1998;316(7144):1569‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Movahedi 2008 {published data only}
- Movahedi M, Haghdoost AA, Pournik O, Hajarizadeh B, Fallah MS. Temporal variations of health indicators in Iran comparing with other Eastern Mediterranean Region countries in the last two decades. Journal of Public Health 2008;30(4):499‐504. [DOI] [PubMed] [Google Scholar]
Muhuri 1995 {published data only}
- Muhuri PK. Health programs, maternal education, and differential child mortality in Matlab, Bangladesh. Population and Development Review 1995;21:813. [Google Scholar]
Nanyunja 2003 {published data only}
- Nanyunja M, Lewis RF, Makumbi I, Seruyange R, Kabwongera E, Mugyenyi P, et al. Impact of mass measles campaigns among children less than 5 years old in Uganda. Journal of Infectious Diseases 2003;187 Suppl 1:S63‐8. [DOI] [PubMed] [Google Scholar]
Niederhauser 1999 {published data only}
- Niederhauser VP. Parental Decision‐making for the Varicella and Measles, Mump and Rubella Vaccines [PhD dissertation]. University of Hawaii at Manoa, 1999. [Google Scholar]
Nuwaha 2000 {published data only}
- Nuwaha F, Kabwongyera E, Mulindwa G, Barenzi E. National immunisation days for polio eradication in Uganda: did immunisation cards increase coverage?. East African Medical Journal 2000;77(2):66‐70. [DOI] [PubMed] [Google Scholar]
Nwogu 2008 {published data only}
- Nwogu R, Larson JS, Kim MS. Reducing child mortality in Nigeria: a case study of immunization and systemic factors. Social Science & Medicine (1982) 2008;67(1):161‐4. [DOI] [PubMed] [Google Scholar]
O'Mahony 1986 {published data only}
- O'Mahony MC, Begg N. Rubella vaccination: the effect of health education and administrative systems on vaccination rates in two health authorities. Public Health 1986;100(2):84‐90. [DOI] [PubMed] [Google Scholar]
Oche 2011 {published data only}
- Oche MO, Umar AS, Ibrahim MTO, Sabitu K. An assessment of the impact of health education on maternal knowledge and practice of childhood immunization in Kware, Sokoto State. Journal of Public Health and Epidemiology 2011;3(10):440‐7. [Google Scholar]
Odusanya 2003 {published data only}
- Odusanya OO, Alufohai JE, Meurice FP, Clemens R, Ahonkhai VI. Short term evaluation of a rural immunization program in Nigeria. Journal of the National Medical Association 2003;95(2):175‐9. [PMC free article] [PubMed] [Google Scholar]
Oeffinger 1992 {published data only}
- Oeffinger KC, Roaten SP, Hitchcock MA, Oeffinger PK. The effect of patient education on pediatric immunization rates. Journal of Family Practice 1992;35(3):288‐93. [PubMed] [Google Scholar]
Oliphant 2010 {published data only}
- Oliphant NP, Mason JB, Doherty T, Chopra M, Mann P, Tomlinson M, et al. The contribution of child health days to improving coverage of periodic interventions in six African countries. Food and Nutrition Bulletin 2010;31(3 Suppl):S248‐63. [DOI] [PubMed] [Google Scholar]
Painvin 2011 {published data only}
- Painvin C, Schlumberger M, Chhem DB, Savannarom D, Phong P, Gilberg S. Positive impact of a video and TV documentary on attendance of women to catch‐up collective vaccinations and reasons for non‐attendance. Bulletin de la Societe de Pathologie Exotique 2011;104(1):29‐37. [DOI] [PubMed] [Google Scholar]
Pallasch 2005a {published data only}
- Pallasch G, Salman R, Hartwig C. Improvement of protection given by vaccination for socially underprivileged groups on the basis of "key persons approach" ‐ Results of an intervention based on cultural and language aspects for children of immigrants in Altlander Viertel provided by the health department of Stade. Gesundheitswesen 2005;67:33‐8. [DOI] [PubMed] [Google Scholar]
Pallasch 2005b {published data only}
- Pallasch G, Salman R, Hartwig C. Improvement of protection given by vaccination for socially underprivileged groups on the basis of "key persons approach" ‐‐ results of an intervention based on cultural and language aspects for children of immigrants in Altlander Viertel provided by the Health Department of Stade. Gesundheitswesen 2005;67(1):33‐8. [DOI] [PubMed] [Google Scholar]
Paunio 1991 {published data only}
- Paunio M, Virtanen M, Peltola H, Cantell K, Paunio P, Valle M, et al. Increase of vaccination coverage by mass media and individual approach: intensified measles, mumps, and rubella prevention program in Finland. American Journal of Epidemiology 1991;133(11):1152‐60. [DOI] [PubMed] [Google Scholar]
Peng 2012 {published data only}
- Peng ZQ, Chen WS, He Q, Peng GW, Wu CG, Xu N, et al. Evaluation of the mass measles vaccination campaign in Guangdong Province, China. International Journal of Infectious Diseases 2012;16(2):e99‐103. [DOI] [PubMed] [Google Scholar]
Perdue 1955 {published data only}
- Perdue TR, Malcolm JC. Poliomyelitis vaccine; a report on field trials in Southern Alameda County in 1954. California Medicine 1955;83(3):233‐6. [PMC free article] [PubMed] [Google Scholar]
Phommathansy 2002 {published data only}
- Phommathansy K, Wichiencharoen K, Nookong A. The effects of planned instruction on mother's knowledge, health beliefs and number of children receiving immunization in Sikhothtaboung district, Vientiane, Lao P.D.R. Journal of Nursing Science 2010;28(3):22‐9. [Google Scholar]
Pietsch 2002 {published data only}
- Pietsch M, Michels H, Diwo J, Martens H, Jacob R, Lossen‐Geissler E, et al. Influence of information campaigns on the vaccination immunity among the population of a small town area ‐ seroepidemiological results of the 'Wittlich Vaccination Study'. Gesundheitswesen 2002;64(1):60‐4. [DOI] [PubMed] [Google Scholar]
Porter 2000 {published data only}
- Porter RW, Steinglass R, Kaiser J, Olkhovsky P, Rasmuson M, Dzhatdoeva FA, et al. Role of health communications in Russia's diphtheria immunization program. Journal of Infectious Diseases 2000;181 Suppl 1:S220‐7. [DOI] [PubMed] [Google Scholar]
Porter‐Jones 2009 {published data only}
- Porter‐Jones G, Williams S, Powell C, Pusey L, Roberts RJ. Impact of a novel way to communicate information about MMR on uptake of MMR vaccine: a randomized controlled trial. Public Health 2009;123(1):78‐80. [DOI] [PubMed] [Google Scholar]
Przewlocka 2000 {published data only}
- Przewlocka T. Evaluation of the hepatitis B prevention education programme in Poland. Vaccine 2000;18 Suppl 1:S46‐8. [DOI] [PubMed] [Google Scholar]
Quaiyum 1997 {published data only}
- Quaiyum MA, Tunon C, Hel Baqui A, Quayyum Z, Khatun J. Impact of national immunization days on polio‐related knowledge and practice of urban women in Bangladesh. Health Policy and Planning 1997;12(4):363‐71. [DOI] [PubMed] [Google Scholar]
Rahman 1988 {published data only}
- Rahman S, Nessa F. Improvement of Maternity and Child Health Services in rural Bangladesh: an experimental project. Public Health 1988;102(4):355‐8. [DOI] [PubMed] [Google Scholar]
Rajakumar 2010 {published data only}
- Rajakumar D, Lemstra M, Thompson A. The effectiveness of an intervention to improve MMR immunization coverage rates in children. Canadian Journal of Infectious Diseases and Medical Microbiology 2010;21(4):196. [Google Scholar]
Rao 1974 {published data only}
- Rao NM. Health education in immunization of children. Nursing Journal of India 1974;65(11):291‐4. [PubMed] [Google Scholar]
Reichler 1997 {published data only}
- Reichler MR, Aslanian R, Lodhi ZH, Latif I, Khan MA, Chaudhry R, et al. Evaluation of oral poliovirus vaccine delivery during the 1994 national immunization days in Pakistan. Journal of Infectious Diseases 1997;175 Suppl 1:S205‐9. [DOI] [PubMed] [Google Scholar]
Ryman 2011 {published data only}
- Ryman TK, Trakroo A, Wallace A, Gupta SK, Wilkins K, Mehta P, et al. Implementation and evaluation of the Reaching Every District (RED) strategy in Assam, India, 2005‐2008. Vaccine 2011;29(14):2555‐60. [DOI] [PubMed] [Google Scholar]
Salmond 1994 {published data only}
- Salmond CE, Soljak MA, Bandaranayake DR, Stehr‐Green P. Impact of a promotion program for hepatitis B immunisation. Australian Journal of Public Health 1994;18(3):253‐7. [DOI] [PubMed] [Google Scholar]
Schwarz 2008 {published data only}
- Schwarz K, Garrett B, Lee J, Thompson D, Thiel T, Alter MJ, et al. Positive impact of a shelter‐based hepatitis B vaccine program in homeless Baltimore children and adolescents. Journal of Urban Health 2008;85(2):228‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaikh 2003 {published data only}
- Shaikh I, Omair A, Inam SNB, Safdar S, Kazmi T, Anjum Q. National Polio Day campaign in a squatter settlement through medical students. Journal of the Pakistan Medical Association 2003;53(3):98‐101. [PubMed] [Google Scholar]
Shamma 1970 {published data only}
- Shamma AC. Health education project in Beirut. Health Education Journal 1970;29(2):40‐6. [Google Scholar]
Sheikh 2009 {published data only}
- Sheikh M, MacIntyre CR. The impact of intensive health promotion to a targeted refugee population on utilisation of a new refugee paediatric clinic at the Children's Hospital at Westmead. Ethnicity & Health 2009;14(4):393‐405. [DOI] [PubMed] [Google Scholar]
Shenson 2001 {published data only}
- Shenson D, Quinley J, DiMartino D, Stumpf P, Caldwell M, Lee T. Pneumococcal immunizations at flu clinics: the impact of community‐wide outreach. Journal of Community Health 2001;26(3):191‐201. [DOI] [PubMed] [Google Scholar]
Shevlin 2002 {published data only}
- Shevlin JD, Summers‐Bean C, Thomas D, Whitney CG, Todd D, Ray SM. A systematic approach for increasing pneumococcal vaccination rates at an inner‐city public hospital. American Journal of Preventive Medicine 2002;22(2):92‐7. [DOI] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith SW, Connery P, Knudsen K, Scott KL, Frintner MP, Outlaw G, et al. A preschool immunization project to enhance immunization levels, the public‐private relationship, and continuity of care. Journal of Community Health 1999;24(5):347‐58. [DOI] [PubMed] [Google Scholar]
Sokhey 1985 {published data only}
- Sokhey J. National programme for the control of poliomyelitis. Indian Journal of Public Health 1985;29(3):168‐74. [PubMed] [Google Scholar]
Sudan study 1995 {published data only}
- CDC. Implementation of health initiatives during a cease‐fire‐Sudan, 1995. Morbidity and Mortality Weekly Report 1995;44(23):433‐6. [PubMed] [Google Scholar]
Sultana 2008 {published data only}
- Sultana H. Impact of media health campaign for Primary Health Care among mothers in Karachi. Journal of the Dow University of Health Sciences 2008;2(1):11‐5. [Google Scholar]
Swift 1969 {published data only}
- Swift DW. Interracial effectiveness of subprofessional aides. Phylon 1969;30(4):394‐7. [Google Scholar]
Tandon 1992 {published data only}
- Tandon BN, Gandhi N. Immunization coverage in India for areas served by the Integrated Child Development Services programme. The Integrated Child Development Services Consultants. Bulletin of the World Health Organization 1992;70(4):461‐5. [PMC free article] [PubMed] [Google Scholar]
Teberg 1970 {published data only}
- Teberg A, Friedman DB. Success and failure in a family‐centered comprehensive care teaching program for pediatric residents. Journal of Medical Education 1970;45(10):777‐83. [DOI] [PubMed] [Google Scholar]
Thomas 2008 {published data only}
- Thomas P, Joseph TL, Menzies RI. Evaluation of a targeted immunisation program for Aboriginal and Torres Strait Islander infants in an urban setting. New South Wales Public Health Bulletin 2008;19(5‐6):96‐9. [DOI] [PubMed] [Google Scholar]
Thompson 2009 {published data only}
- Thompson ME, Harutyunyan TL. Impact of a community‐based integrated management of childhood illnesses (IMCI) programme in Gegharkunik, Armenia. Health Policy and Planning 2009;24(2):101‐7. [PUBMED: 19147699] [DOI] [PubMed] [Google Scholar]
Tung 2005 {published data only}
- Tung CS, Middleman AB. An evaluation of school‐level factors used in a successful school‐based hepatitis B immunization initiative. Journal of Adolescent Health 2005;37(1):61‐8. [DOI] [PubMed] [Google Scholar]
Usman 2011 {published data only}
- Usman HR, Rahbar MH, Kristensen S, Vermund SH, Kirby RS, Habib F, et al. Randomized controlled trial to improve childhood immunization adherence in rural Pakistan: redesigned immunization card and maternal education. Tropical Medicine & International Health 2011;16(3):334‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vijayaraghavan 1983 {published data only}
- Vijayaraghavan K, Hanumantha Rao D, Swaminathan MC. India Population Project, Karnataka: evaluation of nutrition education activities. Hygie 1983;1(3/4):9‐14. [PubMed] [Google Scholar]
Waisbord 2010 {published data only}
- Waisbord S, Shimp L, Ogden EW, Morry C. Communication for polio eradication: improving the quality of communication programming through real‐time monitoring and evaluation. Journal of Health Communication 2010;15 Suppl 1:9‐24. [DOI] [PubMed] [Google Scholar]
Wallace 2006 {published data only}
- Wallace C, Leask J, Trevena LJ. Effects of a web based decision aid on parental attitudes to MMR vaccination: a before and after study. BMJ 2006;332(7534):146‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, et al. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village‐based, out‐of‐cold‐chain delivery strategy. Bulletin of the World Health Organization 2007;85(9):688‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterman 1996 {published data only}
- Waterman SH, Maes EF, Hill LL, Stevenson JM, Robyn B, Anderson KN, et al. A model immunization demonstration for preschoolers in an inner‐city barrio, San Diego, California, 1992‐1994. American Journal of Preventive Medicine 1996;12(4 Suppl):8‐13. [PubMed] [Google Scholar]
White 1976 {published data only}
- White J. Because we care. American Education 1976;12(10):26‐8. [PubMed] [Google Scholar]
WHO 1986 {published data only}
- WHO. Maternal care for the reduction of perinatal and neonatal mortality. A Joint WHO/UNICEF Statement. Geneva: World Health Organization 1986.
Zajicek‐Farber 2010 {published data only}
- Zajicek‐Farber ML. Building practice evidence for parent mentoring home visiting in early childhood. Research on Social Work Practice 2010;20(1):46‐64. [Google Scholar]
Zimicki 1994 {published data only}
- Zimicki S, Hornik RC, Verzosa CC, Hernandez JR, Guzman E, Dayrit M, et al. Improving vaccination coverage in urban areas through a health communication campaign: the 1990 Philippine experience. Bulletin of the World Health Organization 1994;72(3):409‐22. [PMC free article] [PubMed] [Google Scholar]
Zuber 2001 {published data only}
- Zuber PL, Conombo KS, Traore AD, Millogo JD, Ouattara A, Ouedraogo IB, et al. Mass measles vaccination in urban Burkina Faso, 1998. Bulletin of the World Health Organization 2001;79(4):296‐300. [PMC free article] [PubMed] [Google Scholar]
Additional references
Anderson 2011
- Anderson LM, Petticrew M, Rehfuess E, Armstrong R, Ueffing E, Baker P, et al. Using logic models to capture complexity in systematic reviews. Research Synthesis Methods 2011;2(1):33e42. [DOI: 10.1002/jrsm.32] [DOI] [PubMed] [Google Scholar]
Austvoll‐Dahlgren 2010
- Austvoll‐Dahlgren A, Helseth S. What informs parents' decision‐making about childhood vaccinations?. Journal of Advanced Nursing 2010;66(11):2421‐30. [DOI: 10.1111/j.1365-2648.2010.05403.x; PUBMED: 20722796] [DOI] [PubMed] [Google Scholar]
Azad 2010
- Azad K, Barnett S, Banerjee B, Shaha S, Khan K, Rego AR, et al. Effect of scaling up women's groups on birth outcomes in three rural districts in Bangladesh: a cluster‐randomised controlled trial. Lancet 2010;375(9721):1193‐202. [PUBMED: 20207412] [DOI] [PubMed] [Google Scholar]
Baker 2011
- Baker PR, Francis DP, Soares J, Weightman AL, Foster C. Community wide interventions for increasing physical activity. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD008366.pub2] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Boone 2014
- Boone J. Pakistan polio vaccinator's murder by militants raises health workers' fears. The Guardian 25 March 2014. Available at: http://www.theguardian.com/society/2014/mar/25/pakistan‐polo‐vaccinators‐murder‐militants‐salma‐farooqi.
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐analysis. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Brennan 2009
- Brennan S, McKenzie J, Whitty P, Buchan H, Green S. Continuous quality improvement: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003319.pub2] [DOI] [Google Scholar]
Briss 2000
- Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernier RR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The Task Force on Community Preventive Services. American Journal of Preventive Medicine 2000;18(1 Suppl):97‐140. [DOI] [PubMed] [Google Scholar]
Burford 2013
- Burford B, Lewin S, Welch V, Rehfuess E, Waters E. Assessing the applicability of findings in systematic reviews of complex interventions can enhance the utility of reviews for decision making. Journal of Clinical Epidemiology 2013;66(11):1251‐61. [PUBMED: 23953081] [DOI] [PubMed] [Google Scholar]
Cairns 2012
- Cairns G, MacDonald L, Angus K, Walker L, Cairns‐Haylor T, Bowdler T. Systematic Literature Review of the Evidence for Effective National Immunisation Schedule Promotional Communications. European Centre for Disease Prevention and Control (ECDC), 2012. [DOI: 10.2900/63521] [DOI] [Google Scholar]
Casiday 2006
- Casiday R, Cresswell T, Wilson D, Panter‐Brick C. A survey of UK parental attitudes to the MMR vaccine and trust in medical authority. Vaccine 2006;24(2):177‐84. [DOI] [PubMed] [Google Scholar]
CC & CRG 2011
- Cochrane Consumers and Communication Review Group. Data Extraction Template for Cochrane Reviews. Version 1.5.0. http://www.latrobe.edu.au/chcp/assets/downloads/DET_2011.doc (accessed 2 July 2012).
CDC 1999
- Centers for Disease Control and Prevention. Ten great public health achievements: United States, 1990‐1999. Morbidity and Mortality Weekly Report 1999;48(12):241‐3. [PubMed] [Google Scholar]
Colbourn 2013
- Colbourn T, Nambiar B, Bondo A, Makwenda C, Tsetekani E, Makonda‐Ridley A, et al. Effects of quality improvement in health facilities and community mobilization through women's groups on maternal, neonatal and perinatal mortality in three districts of Malawi: MaiKhanda, a cluster randomized controlled effectiveness trial. International Health 2013;5(3):180‐95. [PUBMED: 24030269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulter 2006
- Coulter A, Ellins J. Patient‐focused Interventions: A Review of the Evidence. London: Health Foundation, 2006. [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [PUBMED: 18824488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawes 2005
- Dawes M, Summerskill W, Glasziou P, Cartabellotta A, Martin J, Hopayian K, et al. Second International Conference of Evidence‐Based Health Care Teachers and Developers. Sicily statement on evidence‐based practice. BMC Medical Education 2005;5(1):1. [DOI: 10.1186/1472-6920-5-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diekema 2012
- Diekema DS. Improving childhood vaccination rates. New England Journal of Medicine 2012;366(5):391‐3. [DOI] [PubMed] [Google Scholar]
Dubé 2013
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Human Vaccines and Immunotherapeutics 2013;9(8):1763‐73. [PUBMED: 23584253] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2013
- Effective Practice, Organisation of Care (EPOC). Worksheets for preparing Summary of Findings tables using GRADE (Resource 23). EPOC Resources for Review Authors. Available at: http://epocoslo.cochrane.org/epoc‐specific‐resources‐review‐authors. Oslo: Norwegian Knowledge Centre for the Health Services, 2013. [Google Scholar]
EROS 2012
- EROS. Early Review Organization Software version 2.0. Institute for Clinical Effectiveness and Health Policy, Argentina 2012.
GAVI 2012
- GAVI Alliance. Investing in immunisation through the GAVi Alliance: the evidence base. (accessed 24 May 2012) 2012.
Glenton 2011
- Glenton C, Scheel IB, Lewin S, Swingler GH. Can lay health workers increase the uptake of childhood immunisation? Systematic review and typology. Tropical Medicine & International Health 2011;16(9):1044‐53. [PUBMED: 21707877] [DOI] [PubMed] [Google Scholar]
Glenton 2013
- Glenton C, Lewin S, Mayhew A, Scheel I, Odgaard‐Jensen J. Nonrandomized studies are not always found even when selection criteria for health systems intervention reviews include them: a methodological study. Journal of Clinical Epidemiology 2013;66(4):367‐70. [PUBMED: 23380445] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Grade Working Group. Grade Profiler. Version 3.2 for Windows. Brozek J, Oxman A, Schünemann H, 2008.
Grilli 2002
- Grilli R, Ramsay C, Minozzi S. Mass media interventions: effects on health services utilisation. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000389] [DOI] [PubMed] [Google Scholar]
Hadjikoumi 2006
- Hadjikoumi I, Niekerk KV, Scott C. MMR Catch up Campaign: reasons for refusal to consent. Archives of Disease in Childhood 2006;91(7):621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harper 2013
- Harper R, Lewin S, Glenton C, Peña‐Rosas JP. Completeness of reporting of setting and health worker cadre among trials on antenatal iron and folic acid supplementation in pregnancy: an assessment based on two Cochrane reviews. Systematic Reviews 2013;2:42. [DOI: 10.1186/2046-4053-2-42; PUBMED: 23773404] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hobson‐West 2007
- Hobson‐West P. 'Trusting blindly can be the biggest risk of all': organised resistance to childhood vaccination in the UK. Sociology of Health and Illness 2007;29(2):198‐215. [DOI] [PubMed] [Google Scholar]
Hollands 2011
- Hollands G, Cameron L, Crockett R, Marteau T. Presentation of aversive visual images in health communication for changing health behaviour. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD009086] [DOI] [Google Scholar]
Hull 2010
- Hull BP, Mahajan D, Dey A, Menzies RI, McIntyre PB. Immunisation coverage annual report, 2008. Communicable Diseases Intelligence 2010;34(3):241‐58. [DOI] [PubMed] [Google Scholar]
Hull 2011
- Hull B, Dey A, Mahajan D, Menzies R, McIntyre PB. Immunisation coverage annual report, 2009. Communicable Diseases Intelligence 2011;35(2):132‐48. [DOI] [PubMed] [Google Scholar]
Jacobson 2005
- Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jheeta 2008
- Jheeta M, Newell J. Childhood vaccination in Africa and Asia: the effects of parents' knowledge and attitudes. Bulletin of the World Health Organization 2008;86(6):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 2012
- Kaufman J, Synnot A, Hill S, Willis N, Horey D, Lin V, et al. Face to face interventions for informing or educating parents about early childhood vaccination. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD010038] [DOI] [PubMed] [Google Scholar]
Kaufman 2013
- Kaufman J, Synnot A, Ryan R, Hill S, Horey D, Willis N, et al. Face to face interventions for informing or educating parents about early childhood vaccination. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD010038.pub2] [DOI] [PubMed] [Google Scholar]
Larson 2014
- Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine 2014;32(19):2150‐9. [PUBMED: 24598724] [DOI] [PubMed] [Google Scholar]
Lewin 2009
- Lewin S, Glenton C, Oxman A. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339:b3496. [PUBMED: 19744976] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewin 2010
- Lewin S, Munabi‐Babigumira S, Glenton C, Daniels K, Bosch‐Capblanch X, Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004015.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewin 2011
- Lewin S, Hill S, Abdullahi LH, Castro Freire SB, Bosch‐Capblanch X, Glenton C, et al. 'Communicate to vaccinate' (COMMVAC). Building evidence for improving communication about childhood vaccinations in low‐ and middle‐income countries: protocol for a programme of research. Implementation Science 2011;6:125. [DOI: 10.1186/1748-5908-6-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maglione 2002
- Maglione MA, Stone EG, Shekelle PG. Mass mailings have little effect on utilization of influenza vaccine among Medicare beneficiaries. American Journal of Preventive Medicine 2002;23(1):43‐6. [DOI] [PubMed] [Google Scholar]
Manandhar 2004
- Manandhar DS, Osrin D, Shrestha BP, Mesko N, Morrison J, Tumbahangphe KM, et al. Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster‐randomised controlled trial. Lancet 2004;364(9438):970‐9. [PUBMED: 15364188] [DOI] [PubMed] [Google Scholar]
McPhee 2003a
- McPhee SJ, Nguyen T, Euler GL, Mock J, Wong C, Lam T, et al. Successful promotion of hepatitis B vaccinations among Vietnamese‐American children ages 3 to 18: results of a controlled trial. Pediatrics 2003;111(6 Pt 1):1278‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meszaros 1996
- Meszaros JR, Asch DA, Baron J, Hershey JC, Kunreuther H, Schwartz‐Buzaglo J. Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. Journal of Clinical Epidemiology 1996;49(6):697‐703. [DOI] [PubMed] [Google Scholar]
Mills 2005
- Mills E, Jadad AR, Ross C, Wilson K. Systematic review of qualitative studies exploring parental beliefs and attitudes toward childhood vaccination identifies common barriers to vaccination. Journal of Clinical Epidemiology 2005;58(11):1081‐8. [DOI] [PubMed] [Google Scholar]
Oakley 2006
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J, RIPPLE Study Team. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413‐6. [PUBMED: 16484270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oyo‐Ita 2011
- Oyo‐Ita A, Nwachukwu CE, Oringanje C, Meremikwu MM. Interventions for improving coverage of child immunization in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008145.pub2] [DOI] [PubMed] [Google Scholar]
Paulussen 2006
- Paulussen TG, Hoekstra F, Lanting CI, Buijs GB, Hirasing RA. Determinants of Dutch parents' decisions to vaccinate their child. Vaccine 2006;24(5):644‐51. [DOI] [PubMed] [Google Scholar]
Pearce 2008
- Pearce A, Law C, Elliman D, Cole TJ, Bedford H. Factors associated with uptake of measles, mumps, and rubella vaccine (MMR) and use of single antigen vaccines in a contemporary UK cohort: prospective cohort study. BMJ 2008;336(7647):754‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petticrew 2013
- Petticrew M, Rehfuess E, Noyes J, Higgins JP, Mayhew A, Pantoja T, et al. Synthesizing evidence on complex interventions: how meta‐analytical, qualitative, and mixed‐method approaches can contribute. Journal of Clinical Epidemiology 2013;66(11):1230‐43. [PUBMED: 23953082] [DOI] [PubMed] [Google Scholar]
Ramsay 2003
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. International Journal of Technology Assessment in Health Care 2003;19(4):613‐23. [DOI] [PubMed] [Google Scholar]
Reference Manager 12 [Computer program]
- Thomson Reuters. Reference Manager. Version 12. New York: Thomson Reuters, 2012.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ryan 2011
- Ryan R, Hill S, Prictor M, McKenzie J. Cochrane Consumers and Communication Review Group. Study Quality Guide. Available from: http://cccrg.cochrane.org/author‐resources May 2011.
Sadaf 2013
- Sadaf A, Richards JL, Glanz J, Salmon DA, Omer SB. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine 2013;31(40):4293‐304. [PUBMED: 23859839] [DOI] [PubMed] [Google Scholar]
Shahrabani 2009
- Shahrabani S, Benzion U, Yom Din G. Factors affecting nurses' decision to get the flu vaccine. European Journal of Health Economics 2009;10(2):227‐31. [DOI] [PubMed] [Google Scholar]
Shepperd 2009
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions?. PLoS Medicine 2009;68(8):e1000086. [PUBMED: 19668360] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2011
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes‐Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD001431.pub3] [DOI] [PubMed] [Google Scholar]
Stone 2002
- Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, et al. Interventions that increase use of adult immunization and cancer screening services: a meta‐analysis. Annals of Internal Medicine 2002;136(9):641‐51. [DOI] [PubMed] [Google Scholar]
Stronegger 2010
- Stronegger WJ, Freidl W. A hierarchical analysis of social determinants of measles vaccination coverage in Austrian schoolchildren. European Journal of Public Health 2010;20(3):354‐9. [DOI] [PubMed] [Google Scholar]
Taylor 2002
- Taylor JA, Darden PM, Brooks DA, Hendricks JW, Wasserman RC, Bocian AB. Association between parents' preferences and perceptions of barriers to vaccination and the immunization status of their children: a study from Pediatric Research in Office Settings and the National Medical Association. Pediatrics 2002;110(6):1110‐6. [DOI] [PubMed] [Google Scholar]
Teasdale 2011
- Teasdale E, Yardley L. Understanding responses to government health recommendations: public perceptions of government advice for managing the H1N1 (swine flu) influenza pandemic. Patient Education and Counseling 2011;85(3):413‐8. [DOI] [PubMed] [Google Scholar]
Thomson 2012
- Thomson N, MacRae A, Brankovich J, Burns J, Catto M, Gray C, et al. Overview of Australian Indigenous health status 2011. http://www.healthinfonet.ecu.edu.au/overview_2012 (accessed 23 May 2012) 2012.
Tickner 2006
- Tickner S, Leman PJ, Woodcock A. Factors underlying suboptimal childhood immunisation. Vaccine 2006;24(49‐50):7030‐6. [DOI] [PubMed] [Google Scholar]
Tripathy 2010
- Tripathy P, Nair N, Barnett S, Mahapatra R, Borghi J, Rath S, et al. Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster‐randomised controlled trial. Lancet 2010;375(9721):1182‐92. [PUBMED: 20207411] [DOI] [PubMed] [Google Scholar]
Ueffing 2011
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E. Equity Checklist for Systematic Review Authors. Version 2011‐11‐08. Campbell and Cochrane Equity Methods Group. http://equity.cochrane.org/our‐publications (accessed 2 July 2012).
UNICEF 2000
- UNICEF and World Health Organization. Communication Handbook for Polio Eradication and Routine EPI. http://www.unicef.org/cbsc/files/polio.pdf (accessed 13 December 2013). New York: UNICEF and WHO, 2000. [Google Scholar]
United Nations 2011
- United Nations. The Millennium Development Goals Report. http://www.un.org/en/additions/documents/MDG_Report_2011.pdf (accessed 13 December 2013). New York: United Nations, 2011. [Google Scholar]
WHO 2013
- WHO. What influences vaccine acceptance: a model of determinants of vaccine hesitancy. The SAGE Vaccine Hesitancy Working Group. Available at: http://www.who.int/immunization/sage/meetings/2013/april/1_Model_analyze_driversofvaccineConfidence_22_March.pdf 2013.
WHO 2014
- WHO/UNICEF. Global Immunization Data.Available from: http://www.who.int/entity/immunization/monitoring_surveillance/Global_Immunization_Data.pdf?ua=1. Geneva: World Health Organization, 2014. [Google Scholar]
Williams 2011
- Williams N, Woodward H, Majeed A, Saxena S. Primary care strategies to improve childhood immunisation uptake in developed countries: systematic review. JRSM Short Reports 2011;2(10):81. [PUBMED: 22046500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willis 2013
- Willis N, Hill S, Kaufman J, Lewin S, Kis‐Rigo J, Bensaude De Castro Freire S, et al. 'Communicate to vaccinate': the development of a taxonomy to organise the evidence of communication interventions to improve vaccination in low‐ and middle‐income countries. BMC International Health and Human Rights 2013;13:23. [DOI: 10.1186/1472-698X-13-23] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiysonge 2009
- Wiysonge CS, Waggie Z, Rhoda L, Hussey G. Vaccines for Africa (VACFA) website: an innovative immunisation advocacy tool. South African Medical Journal 2009;99(5):275. [PubMed] [Google Scholar]
Woo 2004
- Woo EJ, Ball R, Bostrom A, Shadomy SV, Ball LK, Evans G, et al. Vaccine risk perception among reporters of autism after vaccination: vaccine adverse event reporting system 1990‐2001. American Journal of Public Health 2004;94(6):990‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wooten 2010
- Wooten K, Kolasa M, Singleton J, Shefer A. National, state, and local area vaccination coverage among children aged 19‐35 months: United States. Morbidity and Mortality Weekly Report 2010;59(36):1170‐7. [PubMed] [Google Scholar]
Wright 2006
- Wright JA, Polack C. Understanding variation in measles‐mumps‐rubella immunization coverage: a population‐based study. European Journal of Public Health 2006;16(2):137‐42. [DOI] [PubMed] [Google Scholar]
Zyngier 2011
- Zyngier S, D'Souza C, Robinson P, Schlotterlein M. Knowledge transfer: examining a public vaccination initiative in a digital age. Proceedings of the 44th Hawaii International Conference on System Science (HICSS‐44), 4‐7 Jan 2011. IEEE Computer Society, 2011. [DOI: 10.1109/HICSS.2011.277] [DOI]
References to other published versions of this review
Saeterdal 2012
- Saeterdal I, Glenton C, Austvoll‐Dahlgren A, Munabi‐Babigumira S, Lewin S. Community‐directed interventions for informing and/or educating about early childhood vaccination. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010232] [DOI] [PMC free article] [PubMed] [Google Scholar]


