SUMMARY
Local environmental factors influence CD8+ T cell priming in lymph nodes (LN). Here, we sought to understand how factors unique to the tumor-draining mediastinal LN (mLN) impact CD8+ T cell responses toward lung cancer. Type-1 conventional dendritic cells (DC1) showed a mLN-specific failure to induce robust cytotoxic T cells responses. Using regulatory T (Treg) cell depletion strategies, we found that Treg cells suppressed DC1 in a spatially coordinated manner within tissue-specific microniches within the mLN. Treg cell suppression required MHCII-dependent contact between DC1 and Treg cells. Elevated levels of IFNγ drove differentiation Treg cells into TH1-like effector Treg cells in the mLN. In patients with cancer, Treg cell TH1-polarization, but not CD8+/Treg cell ratios, correlated with poor responses to checkpoint blockade immunotherapy. Thus, IFNγ in the mLN skew Treg cells to be TH1-like effector Treg cells, driving their close interaction with DC1 and subsequent suppression of cytotoxic T cell responses.
Keywords: Regulatory T cells, cross-presenting dendritic cells, cytotoxic T cells, T cell dysfunction, T cell priming, anti-tumor immunity, non-small cell lung cancer, interferon-gamma, TH1-like effector regulatory T cells, tissue-specific immunity
INTRODUCTION
Cytotoxic CD8+ T cells are essential for anti-tumor immunity1,2. Naïve CD8+ T cells acquire cytotoxic function following priming by type-1 conventional dendritic cells (DC1) in the tumor-draining lymph nodes (tdLN)3–5. DC1 prime naïve T cells by providing three distinct stimulatory signals6. Signal 1 is cognate antigen, cross-presented on the major histocompatibility complex class I (MHCI) on DC14,5,7,8. Signal 2 comprises costimulatory ligands, such as CD80 and CD86, expressed on activated DC19–13, and signal 3 refers to cytokines, including DC-derived interleukin-12 (IL-12), that promote differentiation into cytotoxic T lymphocytes (CTL)14–17. The functional capacity of activated CD8+ T cells depends on the abundance, timing and context of these signals on DC118–22.
Distinct T cell activation trajectories established early after priming can have long-term effects on the quality of CD8+ T cell responses. For instance, elevated TOX expression four days post-chronic viral infection mediates commitment to the T cell exhaustion program23. In lung cancer, priming induces a T cell dysfunction program that prevents CTL differentiation and drives resistance to immune checkpoint blockade (ICB) therapy24. Hence, DC1-derived signals available during priming are instrumental for shaping the quality of the anti-tumor T cell response25.
The local environment regulates DC capacity to prime T cell responses25. At baseline, DC have low expression of signals 2 and 3 and are poorly stimulatory26–29. In the absence of signals 2 and 3, cognate antigen-presenting DC induce T cell tolerance, characterized by initial proliferation with failed accumulation and eventual T cell deletion30–32. Exposure to danger signals, such as tumor-derived dsDNA, promotes DC maturation and expression of signal 2 and 3 molecules27–29. DC stimulatory capacity can be regulated by other immune cells, most notably by regulatory T (Treg) cells. Treg cells inhibit DC maturation and cause decreased expression of signals 2 and 333–39. Treg cell-mediated suppression of DC stimulatory capacity in tumors can blunt protective anti-tumor CD8+ T cell responses38,40,41. Additionally, tissue-specific factors and microanatomical niches in the draining LN influence DC ability to prime distinct T cell subsets42. For instance, DC in lung and gut LN can imprint tissue-specific homing receptor expression on T cells43–45. Moreover, DC can skew T cell responses towards effector, tolerance, or memory programs in a tissue-specific manner46,47. Within a draining LN, the microanatomical location of DC1 and CD8+ T cells during priming dictates T cell exposure to stimulatory signals and influences effector differentiation48,49. Thus, several environmental factors can impact the T cell activation program induced during priming.
How tissue-specific immunoregulatory mechanisms influence anti-tumor T cell responses remains poorly understood. In this study, we sought to uncover lung cancer-specific mechanisms driving dysfunctional tumor-reactive CD8+ T cells during T cell priming24. By comparing T cell responses in the mediastinal (mLN) and inguinal (iLN) LN that drained lung and flank tumors, respectively, we determined that Treg cells in the mLN restrained DC1-mediated priming of CTL more effectively than their counterparts in the iLN. Treg cell-driven suppression of DC1 caused dysfunctional CD8+ T cell responses against lung tumors and required MHCII-dependent contact between Treg cells and DC1. Suppressive, clonally-expanded TH1-like Treg cells were preferentially induced in the mLN in response to the tissue-specific abundance of interferon-gamma (IFNγ). Blockade of IFNγ was sufficient to repolarize Treg cells and rescue CTL responses against lung tumors. Thus, tissue-specific induction of TH1-like Treg cells that suppress DC1-mediated CTL priming could represent a critical barrier to anti-tumor immunity.
RESULTS
DC1 in mLN prime dysfunctional CD8+ T cells against lung KP tumors
To study how priming of CTL is restrained in lung cancer24, we compared T cell responses against tumors growing orthotopically in the lungs or subcutaneously in the flanks. We used the KrasG12D Trp53−/− (KP) lung adenocarcinoma cell line, engineered to express the model antigen SIIINFEKL fused to ZsGreen (KP-zSIIN)24,50. We examined activation of SIIN-reactive CD8+ T cells in the mLN and iLN 7 days post-tumor implantation (Figure 1A). Despite comparable accumulation in both LN, SIIN-reactive T cells primed in the mLN failed to express effector differentiation markers CD25 and Granzyme B (GzmB) (Figure 1B), consistent with prior work24. This dysfunctional phenotype contrasted with the robust expression of effector molecules on SIIN-reactive T cells primed in the iLN (Figure 1B). Induction of CTL in the iLN was underscored by the increased expression of TIM3 relative to the mLN (Figure 1C).
Figure 1. DC1 in mLN prime dysfunctional CD8+ T cells against lung KP tumors.
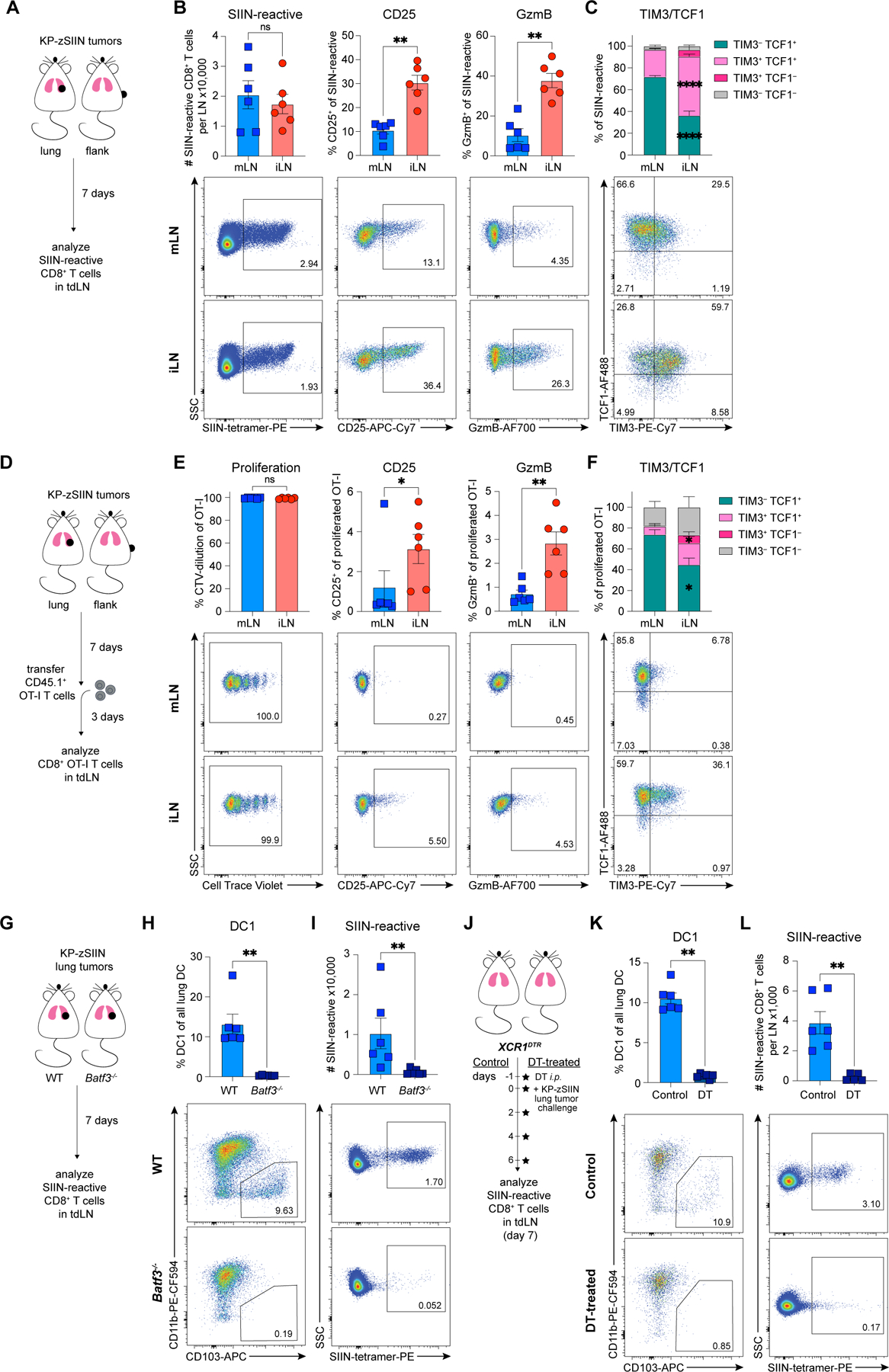
(A) Experimental design for (B-C).
(B-C) Representative flow plots and (B) numbers or CD25, GzmB, (C) TCF1 and TIM-3 expression of SIIN-reactive CD8+ T cells in tdLN, day 7 post-tumor implantation (n=3 mice/group; two independent experiments).
(D) Experimental design for (E-F).
(E-F) Representative flow plots and quantified (E) CellTrace Violet (CTV)-dilution or CD25, GzmB, (F) TCF1 and TIM-3 expression of adoptively-transferred CTV-labelled OT-I T cells primed in tdLN, day 10 post-tumor implantation (n=3 mice/group; two independent experiments).
(G) Experimental design for (H-I).
(H-I) Representative flow plots and quantified abundance of (H) DC1 in lungs and (I) SIIN-reactive CD8+ T cells in mLN of tumor-bearing WT or Batf3−/− mice, day 7 post-tumor implantation (n=3 mice/group; two independent experiments).
(J) Experimental design for (K-L).
(K-L) Representative flow plots and quantified abundance of (K) DC1 in lungs and (L) SIIN-reactive CD8+ T cells in mLN of control or DT-treated tumor-bearing XCR1DTR mice, day 7 post-tumor implantation (n=3 mice/group; two independent experiments).
*p<0.05, **p<0.01, ****p<0.0001, ns=not significant; MWU (B-C,E-F,H-I,K-L). Data shown as mean ± SEM.
We validated that the observed phenotypic and functional differences24 were independent of TCR signal strength using an in vivo priming assay. SIIN-reactive OT-I T cells activated in the mLN exhibited robust proliferation, yet reduced expression of CD25, GzmB, and TIM3 (Figures 1D-F;S1A-B). We affirmed that the dysfunction phenotype was not specific to the SIIN antigen using KP-SIY (SIYRYYGL) cells24 (Figures S1C-E).
Given the importance of cross-presenting DC1 for mounting anti-tumor immune responses4,51, we next tested whether DC1 were the dominant DC subset priming tumor-reactive T cells in both LN. SIIN-reactive T cell activation against both lung and flank tumors was severely impaired in Batf3−/− mice, lacking DC14 (Figures 1G-I;S1F-K). We further validated the requirement of DC1 for priming tumor-reactive T cells in the mLN using the XCR1DTR mouse model52 (Figures 1J-L) and KP-SIY tumor cells (Figures S1L-M). Since DC2 can also prime productive anti-tumor CD8+ T cell responses53, we compared the functional capacity of DC1 and DC2 for activating CD8+ T cells. Using ZsGreen (ZsG) fluorescence as a readout for tumor antigen uptake, we isolated ZsG+ and ZsG– DC1 and DC2 from both tdLN for ex vivo co-cultures (Figure S1N). ZsG+ DC but not ZsG– DC induced robust T cell proliferation, confirming that tumor-associated antigen was restricted to the ZsG+ fraction (Figure S1O-P). While both ZsG+ DC subsets could activate T cells, only ZsG+ DC1 induced productive priming, resulting in enhanced T cell proliferation and accumulation (Figure S1O-P). These data indicate that DC1 were the main DC subset activating tumor-reactive CD8+ T cells in both tdLN.
DC1 in tumor-draining mLN have high signal 1, but low signals 2 and 3
DC exist in distinct functional states with varied expression of signals 1 (antigen), 2 (costimulation) and 3 (cytokines), which shape the quality of anti-tumor immunity53,54. Since DC1 primed phenotypically distinct CD8+ T cell responses in the tumor-draining mLN and iLN (Figures 1A-L;S1A-M), we hypothesized that tissue-specific differences in DC1 states mediated the distinct priming outcomes. Therefore, we characterized signals 1, 2 and 3 on tumor-derived ZsG+ DC1 in both tdLN (Figures 2A-B).
Figure 2. DC1 in tumor-draining mLN have high signal 1, but low signals 2 and 3.
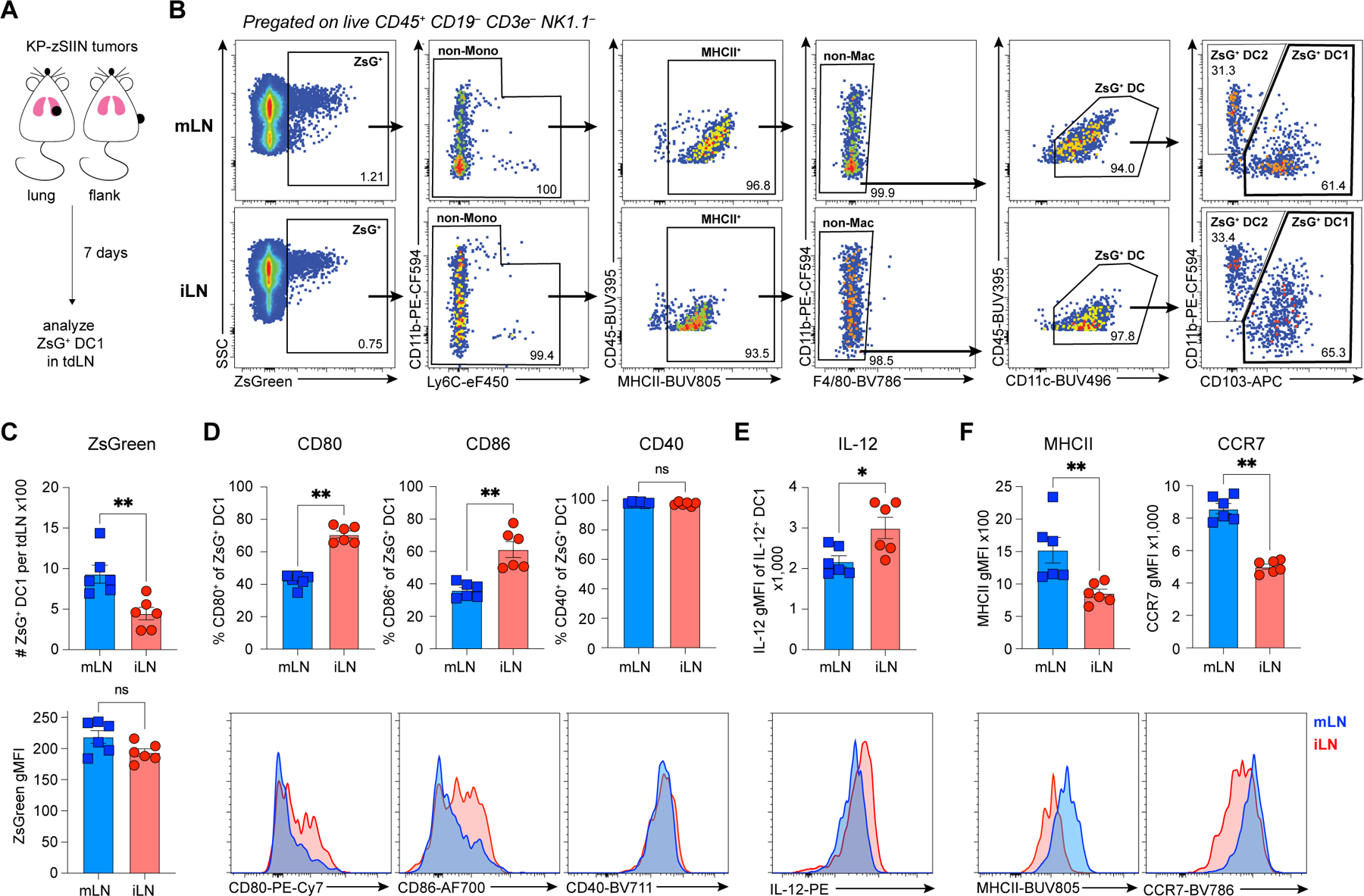
(A) Experimental design for (B-F).
(B) Representative flow gating strategy for ZsG+ DC1 from tdLN.
(C) (top) Abundance and (bottom) ZsG geometric mean fluorescence intensity (gMFI) of ZsG+ DC1 from tdLN, day 7 post-tumor implantation (LN from 3–4 mice pooled per datapoint; two independent experiments).
(D-F) Representative histograms and quantified expression of (D) CD80, CD86, CD40, (E) IL-12, (F) MHCII and CCR7 on ZsG+ DC1 from tdLN, day 7 post-tumor implantation (LN from 3–4 mice pooled per datapoint; two independent experiments).
*p<0.05, **p<0.01, ns=not significant; MWU (C-F). Data shown as mean ± SEM.
ZsG+ DC1 abundance was increased in the mLN compared to the iLN, with similar ZsG intensity, suggesting no defect in DC1 ability to engulf tumor debris and migrate to the tumor-draining mLN (Figure 2C). ZsG+ DC1 from both LN also had equal ability to prime naive CD8+ T cells ex vivo (Figures S1N-P), underscoring that DC1 from the mLN were not deficient in signal 1. Expression of CD80 and CD86 was reduced, while CD40 was unchanged on ZsG+ DC1 from mLN compared to those in the iLN (Figure 2D). Further, ZsG+ DC1 from the mLN produced less IL-12 relative to their counterparts in the iLN (Figure 2E). Since low expression of signals 2 (CD80 and CD86) and 3 (IL-12) is characteristic of immature DC55,56, we examined the maturation markers MHCII and CCR7. Both molecules were highly expressed on ZsG+ DC1 from the mLN (Figure 2F). We concluded that tumor-derived DC1 in the mLN were highly mature and provided sufficient signal 1, yet had reduced expression of CD80, CD86 and IL-12, required for priming of true CTL.
Since mature DC can acquire immunoregulatory molecules (mregDC) and suppress anti-tumor immunity following engulfment of tumor debris54, we examined mregDC markers (CD40, IL-12 and PD-L1)54. We observed similar expression of CD40 (Figure 2D) while IL-12 and PD-L1 were reduced on ZsG+ DC1 from mLN relative to iLN (Figures 2E;S1Q-R). Further, the mLN-specific decrease in CD80, CD86 and IL-12 expression (Figures 2D-E) was also detected on ZsG– DC1 (Figures S1S-U), suggesting a tissue-specific suppression distinct from the mregDC program.
Reduced expression of CD80, CD86 and IL-12 on DC1 from the tumor-draining mLN (Figures 2D-E;S1S-U) could indicate DC1-intrinsic tissue-specific suppression. However, the tissue-specific differences in signal 2 and 3 on DC1 (Figures 2D-E;S1S-U) were no longer detectable in naïve mice (Figures S1V-X), suggesting that the suppressed phenotype was not an intrinsic property of DC1 in the mLN. Additionally, in contrast to the mLN-specific induction of dysfunctional CD8+ T cells in vivo (Figures 1D-E), ZsG+ DC1 from both LN primed CTL with a similarly high expression of CD25 and GzmB ex vivo (Figures S1Y-Z). Thus, the dysfunctional CD8+ T cell responses in the tumor-draining mLN did not result from DC1-intrinsic defects, but instead a DC-extrinsic, mLN-specific and tumor-dependent factor restrained CTL activation in vivo.
Treg cells can induce CD8+ T cell dysfunction and DC1 suppression
Treg cells can suppress DC stimulatory capacity by depleting surface molecules CD80 and CD8634,35,37–39. Given the low expression of CD80 and CD86 on ZsG+ DC1 from tumor-draining mLN (Figure 2D), we hypothesized that Treg cells inhibited DC1 and thereby caused dysfunctional CD8+ T cell responses in the mLN.
We used FoxP3DTR mice57 to examine the impact of constitutive Treg cell depletion on SIIN-reactive CD8+ T cell responses in the tumor-draining mLN and iLN (Figure 3A). Expression of CD25 and GzmB on tumor-reactive T cells was markedly increased upon Treg cell depletion in both LN (Figure 3B). The rescue of both CD25 and GzmB expression on tumor-reactive T cells was greater in the mLN (Figure 3C), suggesting a more profound Treg cell suppression in the mLN compared to the iLN. While other tissue-specific suppressive factors cannot be fully excluded, these data provided a strong rationale to study the role of Treg cells in shaping the quality of tumor-specific T cell responses in the mLN.
Figure 3. Treg cells can induce CD8+ T cell dysfunction and DC1 suppression in the tumor-draining mLN.
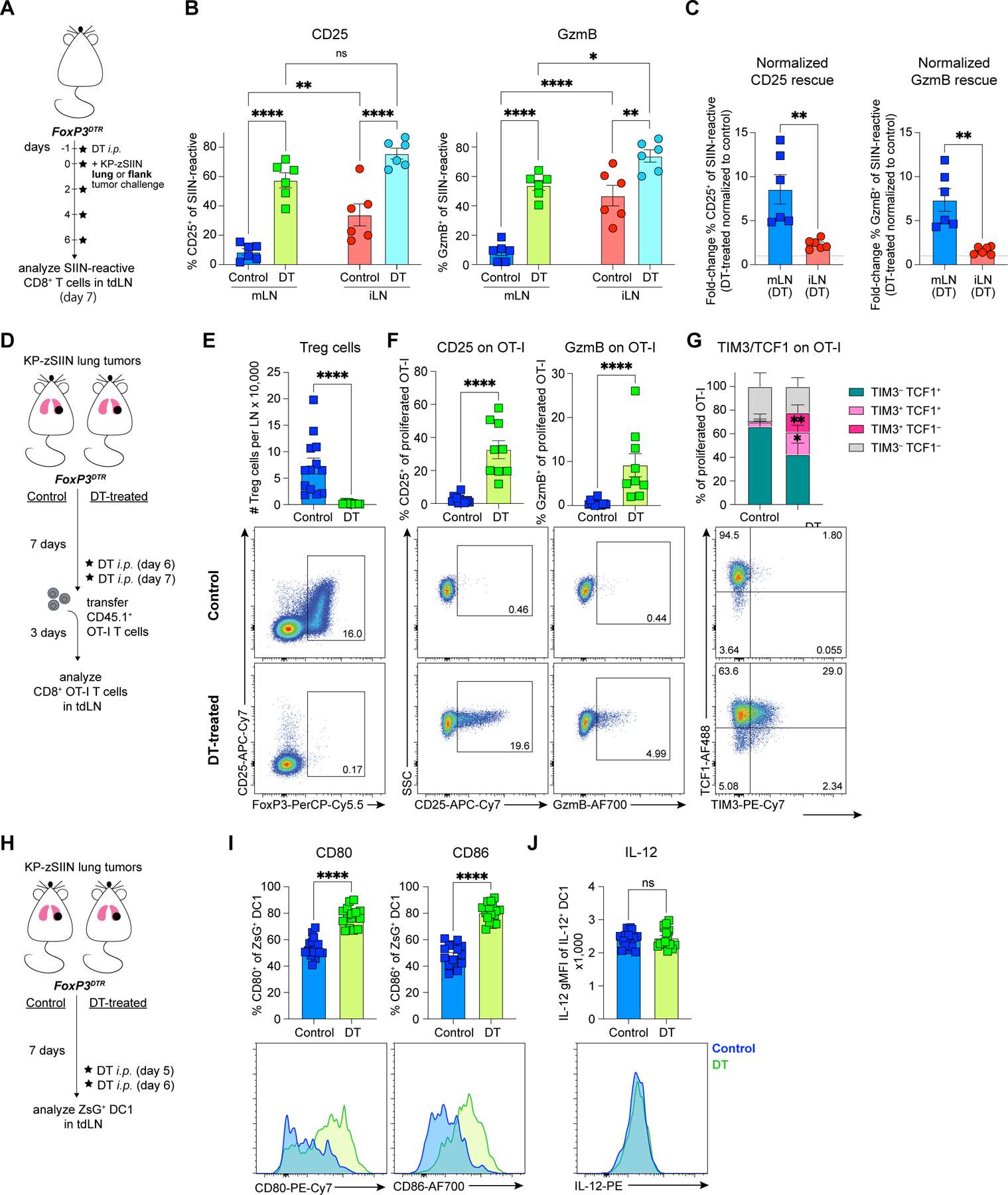
(A) Experimental design for (B-C).
(B-C) Quantification of (B) CD25 and GzmB expression, and (C) DT-treated/control ratios of CD25 and GzmB expression within each tdLN site for SIIN-reactive CD8+ T cells from control or DT-treated tumor-bearing FoxP3DTR mice, day 7 post-tumor implantation (n=3 mice/group; two independent experiments).
(D) Experimental design for (E-G).
(E-G) Representative flow plots and quantified (E) Treg cell number, (F) expression of CD25, GzmB, (G) TCF1 and TIM-3 on adoptively-transferred proliferated OT-I T cells from mLN of control or DT-treated tumor-bearing FoxP3DTR mice, day 10 post-tumor implantation (n=2–4 mice/group; four independent experiments).
(H) Experimental design for (I-J).
(I-J) Representative histograms and quantified expression of (I) CD80, CD86 and (J) IL-12 on ZsG+ DC1 from mLN of control or DT-treated tumor-bearing FoxP3DTR mice, day 7 post-tumor implantation (mLN from 3–5 mice pooled per datapoint; six independent experiments).
*p<0.05, **p<0.01, ****p<0.0001, ns=not significant; two-way ANOVA (B), MWU (C,E-G,I-J). Data shown as mean ± SEM.
As constitutive Treg cell ablation causes severe autoimmunity57, we further assessed the impact of transient Treg cell depletion. Transient depletion likewise restored CTL priming in the mLN (Figures 3D-G). Transient Treg cell depletion also led to an increased expression of CD80 and CD86, but not IL-12 on ZsG+ DC1 from the mLN (Figures 3H-J). Similar effects were observed in the iLN (Figures S1AA-AC). Therefore, Treg cells suppressed tumor-reactive T cell responses and DC1 stimulatory capacity in both LN, however, their inhibition of CTL priming was more potent in the mLN.
Treg cells from the tumor-draining mLN restrain cytotoxic T cell priming by suppressing DC-derived signals 2 and 3
As DC1 were required for T cell priming in the mLN (Figure 1) and Treg cells suppressed CTL differentiation (Figure 3), we hypothesized that mLN Treg cells restrained CD8+ T cell priming by inhibiting DC1. To delineate the effects of DC1 and Treg cells on CD8+ T cell priming, we utilized reductionist ex vivo co-cultures of naïve OT-I T cells with ZsG+ DC1 and Treg cells isolated from the tumor-draining mLN (Figure 4A; see methods for details). Treg cells caused a stark reduction in CD25 and GzmB expression on OT-I T cells at low Treg:OT-I T cell ratios (Figures 4B-D), while CD8+ T cell proliferation was only mildly reduced (Figures 4D;S2A-B). The resulting CD25low GzmBlow phenotype recapitulated the CD8+ T cell dysfunction phenotype observed in vivo (Figure 1E). To test whether Treg cells required DC1 to induce dysfunctional CD8+ T cells, we used plate-bound anti-(α)CD3 and αCD28 to stimulate OT-I T cells (Figure 4A). In the absence of DC1, the same number of Treg cells minimally impacted CD25 and GzmB expression on OT-I T cells (Figures 4C-D). Thus, Treg cells could redirect DC1-mediated priming of CTL towards dysfunction yet required the presence of DC1 for this suppression.
Figure 4. Treg cells from the tumor-draining mLN restrain cytotoxic T cell priming by suppressing DC-derived signals 2 and 3.
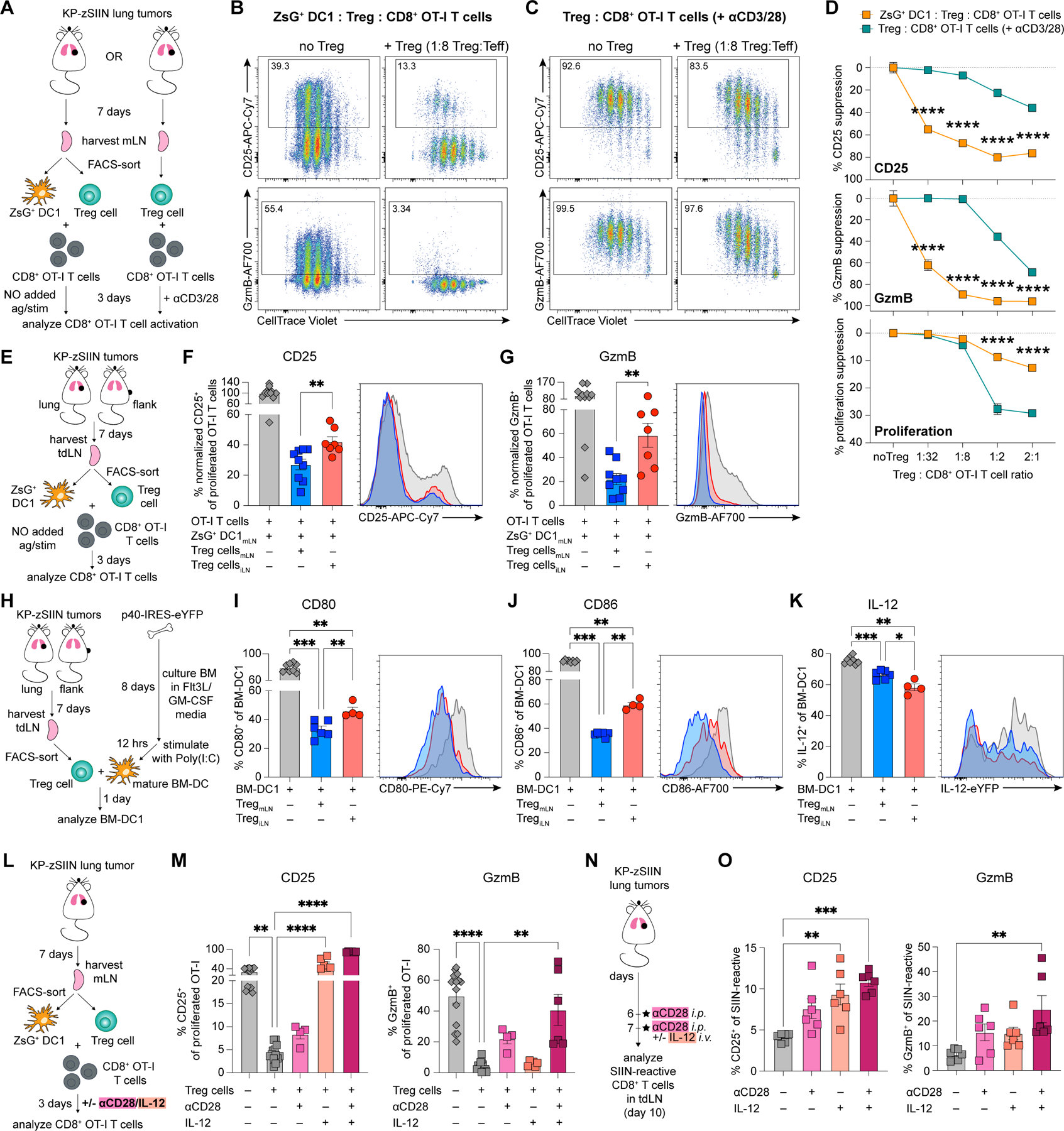
(A) Experimental design for (B-D).
(B-D) Representative flow plots of proliferation, CD25 and GzmB expression of CTV-labelled OT-I T cells after 3-day co-culture with (B) mLN-sorted ZsG+ DC1 and Treg cells or (C) mLN-sorted Treg cells with αCD3/αCD28-stimulation; representative example quantified in (D) (50 tdLN pooled for sorting, three independent experiments).
(E) Experimental design for (F-G).
(F-G) Representative histograms and quantified expression of (F) CD25 and (G) GzmB on proliferated OT-I T cells after 3-day co-culture with mLN-sorted ZsG+ DC1 and either mLN- or iLN-sorted Treg cells (50 tdLN pooled for sorting, three independent experiments).
(H) Experimental design for (I-K).
(I-K) Representative histograms and quantified expression of (I) CD80, (J) CD86 and (K) IL-12 on p40-IRES-eYFP BM-DC1 after 3-day co-culture with either mLN- or iLN-sorted Treg cells (15 tdLN pooled for sorting, two independent experiments).
(L) Experimental design for (M).
(M) CD25 and GzmB expression on proliferated OT-I T cells after 3-day co-culture with mLN-sorted DC1 and Treg cells at indicated culture conditions (50 mLN pooled for sorting, four independent experiments).
(N) Experimental design for (O).
(O) CD25 and GzmB expression on SIIN-reactive CD8+ T cells in tumor-draining mLN, day 10 post-tumor implantation (n=3 mice/group; two independent experiments).
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; two-way ANOVA (D), MWU (F-G,I-K) or KW (M,O). Data shown as mean ± SEM.
To ascertain whether CD8+ T cell states from the ex vivo co-cultures (ZsG+ DC1:OT-I ± Treg cells, Figures 4A-D) and the in vivo priming assay (mLN versus iLN, Figures 1D-F) were comparable beyond phenotypic markers, we performed single-cell RNA sequencing (scRNA-seq) of ex vivo-primed T cells (Figure S2C). We examined the differentially expressed genes (DEG) between CD8+ T cells primed by DC1 in the presence or absence of Treg cells (Figure S2D; Table S1). Treg cell presence led to reduced expression of transcripts associated with effector function (GzmB, Il2ra, Il12rb1 and Il12rb2) and increased expression of transcripts associated with inhibition of effector T cell differentiation (Sell, Pecam1, Lef1, Tcf7I, S1pr1, S1pr4) and T cell fate decisions (Klf2, Klf3) on CD8+ T cells24,58–64 (Figure S2D). The transcriptional profiles of T cells primed ex vivo in the presence or absence of Treg cells strongly correlated with the published RNA-seq24 of T cells primed in vivo in the KP-tumor-draining mLN or iLN, respectively (Figures S2E-F). This result indicated that transcriptional differences between mLN- and iLN-primed T cells were largely conserved in T cells primed ex vivo.
Next, we directly compared the functional suppressive capacity of mLN and iLN Treg cells. Consistent with the in vivo Treg cell depletion data (Figure 3A-C), both mLN and iLN Treg cells inhibited CD25 and GzmB expression on primed CD8+ T cells (Figures 4E-G). However, mLN Treg cells induced more potent suppression than iLN Treg cells, regardless of whether ZsG+ DC1 were isolated from the mLN or iLN (Figures 4E-G;S2G-H). Tissue-specific differences in Treg cell-intrinsic suppressive capacity were only detectable in the DC1:Treg:OT-I T cell co-cultures, as the αCD3/αCD28-based co-cultures revealed comparable suppression (Figures S2I-J).
To compare DC suppression effects of mLN and iLN Treg cells in a controlled setting, we established co-cultures of mature bone marrow-derived-DC (BM-DC) derived from p40-IRES-eYFP IL-12 reporter mouse bone marrow and Treg cells sorted from the tumor-draining mLN and iLN (Figures 4H; see methods for details). Consistent with the in vivo data (Figures 2D;3I;S1AB), both mLN and iLN Treg cells suppressed CD80 and CD86 expression on BM-DC (Figures 4I-J). However, mLN Treg cells inhibited CD80 and CD86 expression more effectively (Figures 4I-J), mirroring in vivo differences in DC1 costimulatory molecule expression in the mLN and iLN (Figure 2D). Treg cells from the iLN induced increased IL-12 suppression compared to Treg cells from the mLN (Figure 4K), in contrast to the in vivo observation (Figure 2E). This discrepancy could be explained by a lack of effector T cells in this assay as T cell-derived IFNγ induces IL-12 expression65.
Insufficiency of DC-derived stimulatory signals can prevent differentiation of CTL18,19,31. If Treg cell-mediated inhibition of signals 2 and 3 on DC1 caused dysfunctional priming of CD8+ T cells in the mLN, then providing signals 2 and 3 exogenously should restore CTL priming. Using ZsG+ DC1:Treg:OT-I T cell co-cultures, we found that singular addition of αCD28 (signal 2) or IL-12 (signal 3) induced a partial increase of GzmB and high expression of CD25, respectively (Figures 4L-M). However, combining αCD28 and IL-12 led to a synergic effect, completely restoring both GzmB and CD25 expression (Figure 4M). We then examined the impact of αCD28 and IL-12 treatment on CD8+ T cell priming in the mLN of lung tumor-bearing mice (Figure 4N). Consistent with ex vivo results, we observed that the combination of αCD28 and IL-12 induced robust CD25 and GzmB expression on the tumor-reactive CD8+ T cells (Figure 4O). Thus, Treg cells in the tumor-draining mLN caused dysfunctional priming of CD8+ T cells due to an enhanced ability to suppress DC1 stimulatory signals.
Treg cells suppress CD8+ T cell priming in the mLN via direct interaction with DC1
Treg cells can suppress DC stimulatory molecules in a contact-dependent or independent manner34,35. To first evaluate the spatial arrangement of Treg cells and DC1 during CD8+ T cell priming, we performed immunofluorescence (IF) staining of tumor-draining mLN and iLN from XCR1DTR.Venus mice after adoptive transfer of CD45.1+ OT-I T cells (Figure 5A). We identified XCR1+ DC1, tumor-reactive OT-I T cells and Treg cells using the endogenous Venus signal, CD45.1 and FoxP3 markers, respectively (Figure 5B; see methods for details). We focused our analysis on the OT-I T cell microniches, defined as circular regions centered at OT-I T cell clusters that contain at least one DC1 (Figure 5B; see methods for details), and evaluated Treg:DC1 proximity by measuring the distance from each Treg cell to its closest DC1. The microniche-associated Treg cells and DC1 were closer to each other in the tumor-draining mLN compared to the iLN (Figures 5B-C;S2K). This spatial proximity of Treg cells and DC1 in the mLN was a microniche-specific effect, since outside of microniches the Treg:DC1 distance was increased (Figure 5C). Microniches in the mLN and iLN were similar in size, and the Treg:DC1 ratio within microniches was also not increased in the mLN (Figure 5D). Increased Treg cell suppression in the mLN was likely driven by the physical proximity of Treg cells and DC1 involved in tumor-specific CD8+ T cell activation.
Figure 5. Treg cells suppress CD8+ T cell priming in the mLN via direct interaction with DC1.
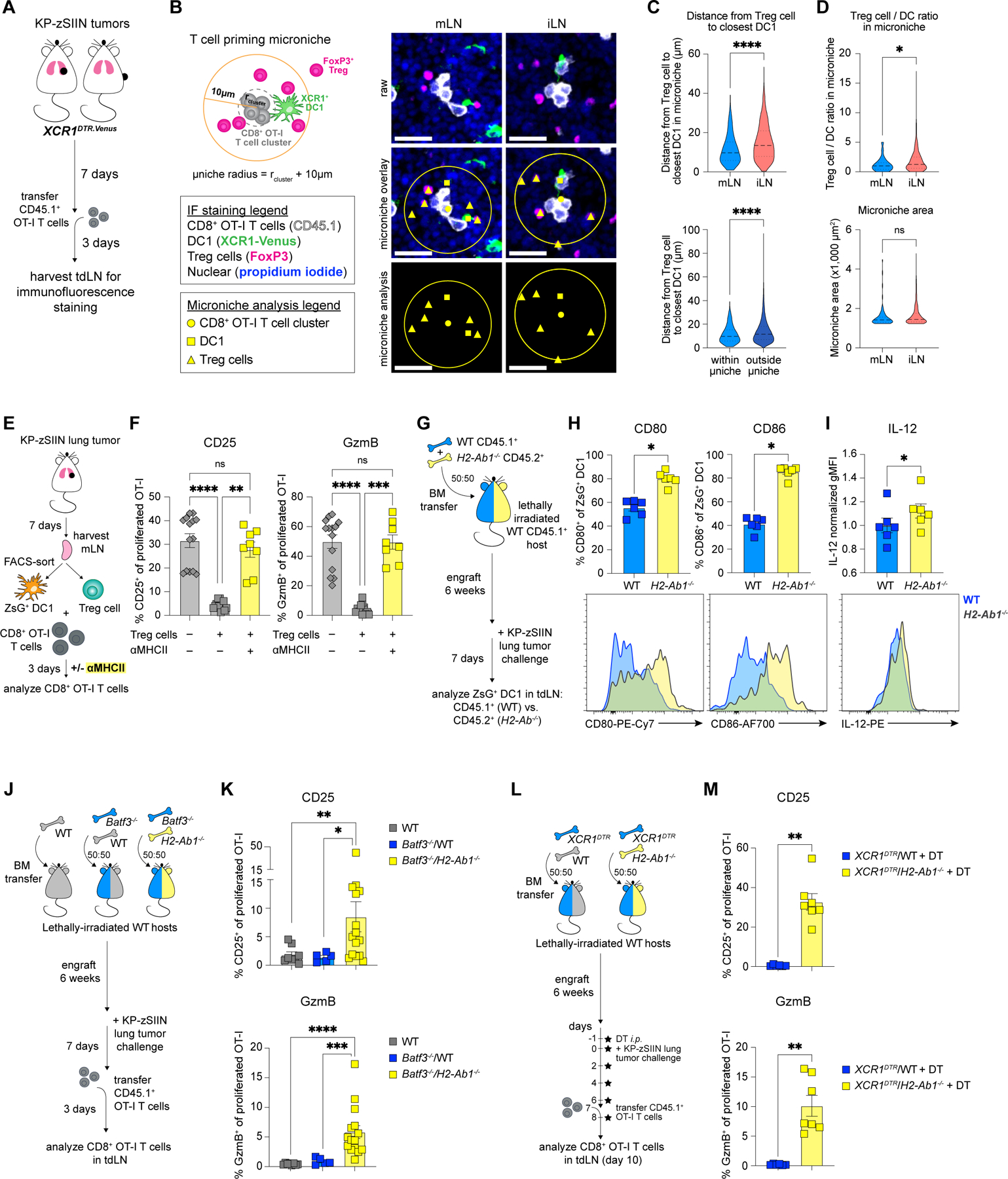
(A) Experimental design for (B-D) and Figure S2K.
(B) Schematic of microniche analysis for (C-D) and representative IF images of tdLN from tumor-bearing XCR1DTR.Venus mice, day 10 post-tumor implantation; scale bar, 20 μm.
(C-D) Distance from Treg cell to closest DC1 (C, top) within microniche in tumor-draining mLN and iLN or (C, bottom) in mLN within and outside of microniche, (D, top) Treg/DC1 ratio within microniche and (D, bottom) microniche area in tdLN; using microniche radius: rcluster + 10μm (n=4 mice/group including 399 mLN microniches and 74 iLN microniches; representative data from one of two independent experiments).
(E) Experimental design for (F).
(F) CD25 and GzmB expression on proliferated OT-I T cells after 3-day co-culture with mLN-sorted ZsG+ DC1 and Treg cells at indicated culture conditions (50 mLN pooled for sorting, four independent experiments); controls (gray bars) are the same as shown in Figure 4M.
(G) Experimental design for (H-I) and Figures S2L-M.
(H-I) Representative histograms and quantified expression of (H) CD80, CD86 and (I) IL-12 on WT and H2-Ab1−/− ZsG+ DC1 from mLN of tumor-bearing WT/H2-Ab1−/− BMCs, day 7 post-tumor implantation (mLN from 3–4 mice pooled per datapoint; two independent experiments).
(J) Experimental design for (K) and Figures S2N-O.
(K) CD25 and GzmB expression on adoptively-transferred proliferated OT-I T cells primed in mLN of tumor-bearing WT, Batf3−/−/WT or Batf3−/−/H2-Ab1−/− BMCs, day 10 post-tumor implantation (n=2–5 mice/group; 1–4 independent experiments).
(L) Experimental design for (M) and Figures S2P-Q.
(M) CD25 and GzmB expression on adoptively-transferred proliferated OT-I T cells primed in mLN of DT-treated tumor-bearing XCR1DTR/WT or XCR1DTR/H2-Ab1−/− BMCs, day 10 post-tumor implantation (n=3–4 mice/group; two independent experiments).
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant; MWU test (C-D,K,M), paired-MWU (H-I) or KW (F). Data shown as mean ± SEM.
To test whether Treg cell-mediated suppression of DC1 required direct cell:cell contact, we abrogated their MHCII-dependent interactions. Antibody-mediated MHCII blockade in ex vivo ZsG+ DC1:Treg:OT-I T cell co-cultures completely reversed the Treg cell suppression phenotype (Figures 5E-F), suggesting that MHCII accessibility on DC1 was critical for the Treg cell-mediated suppression of CD8+ T cell priming. To test the effect of MHCII ablation on DC1 stimulatory capacity in vivo, we generated WT:H2-Ab1−/− mixed bone marrow chimeras (BMCs) and compared the phenotypes of WT and H2-Ab1−/− ZsG+ DC1 within the tumor-draining mLN (Figures 5G;S2L-M). MHCII deletion resulted in increased expression of CD80, CD86 and IL12 on ZsG+ DC1 in vivo (Figures 5H-I), indicating that Treg cell-mediated suppression effects were conferred to DC1 through MHCII-dependent interactions.
We then examined the impact of DC1-specific MHCII deletion on CD8+ T cell priming in vivo. We generated WT, Batf3−/−:WT and Batf3−/−:H2-Ab1−/− mixed BMCs to compare the effect of MHCII+ DC1 (in WT and Batf3−/−:WT BMCs) and MHCII– DC1 (in Batf3−/−:H2-Ab1−/− BMCs) on CD8+ T cell priming (Figures 5J;S2N-O). DC1-specific MHCII deletion in Batf3−/−:H2-Ab1−/− BMCs restored the priming of CTL in the mLN compared to control BMCs (Figure 5K). We independently validated this result using XCRDTR:WT and XCRDTR:H2-Ab1−/− mixed BMCs (Figures 5L;S2P-Q). Consistently, the DC1-specific MHCII deletion led to effective priming of CTL in the tumor-draining mLN (Figure 5M). Therefore, MHCII-dependent Treg:DC1 interactions caused DC1 suppression and the associated CD8+ T cell dysfunction in the context of lung tumors.
TH1-like Treg cells expand in tumor-draining mLN
It remained unclear what tissue-specific factor(s) caused Treg cells to be more suppressive in the mLN compared to the iLN. Since increased suppression is often associated with a greater Treg cell abundance66,67, we enumerated Treg cells in both tdLN. However, Treg cells were equally abundant (Figures 6A-B) and SIIN-reactive CD8+/Treg cell ratios did not correlate with increased suppression observed in the tumor-draining mLN (Figure S3A).
Figure 6. TH1-like Treg cells expand in tumor-draining mLN.
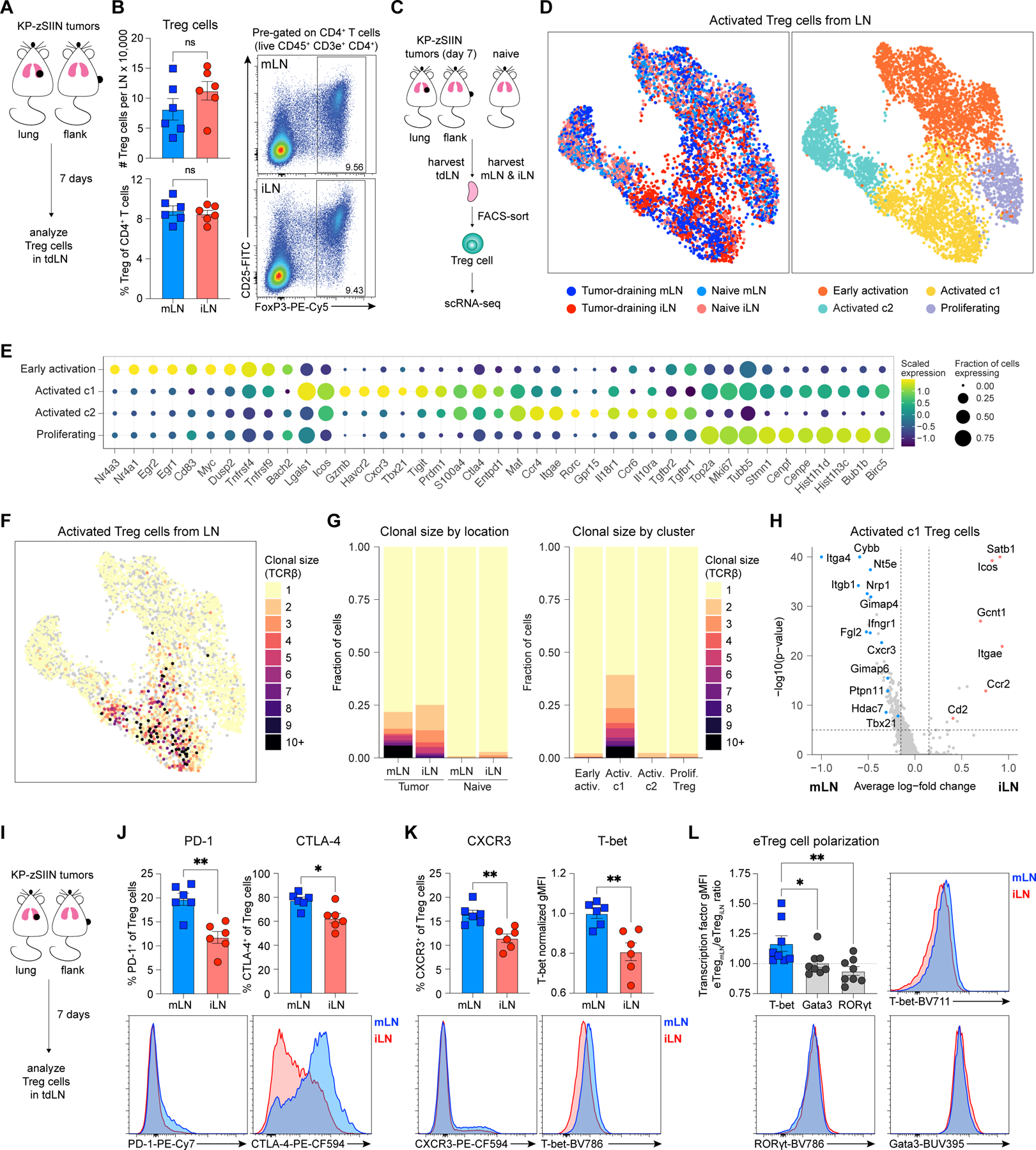
(A) Experimental design for (B) and Figure S3A.
(B) Representative flow plots and quantified abundance of Treg cells in tdLN, day 7 post-tumor implantation (n=3 mice/group; two independent experiments).
(C) Experimental design for (D-H) and Figures S3B-K;S3N.
(D) UMAP plots of activated Treg cells from naïve and tumor-draining mLN and iLN colored (left) by tumor status and location or (right) by cluster (tdLN, n=5 mice/group; naïve LN, n=20 mice/group).
(E) Dot plot of select marker genes for each Treg cluster displaying average expression and frequency of expression for each gene.
(F-G) Clonal size of activated Treg cells (F) mapped onto UMAP plot and (G) graphed using stacked bar plots arranged (left) by tumor status and location or (right) by cluster.
(H) Volcano plot of DEGs between activated c1 Treg cells from mLN and iLN.
(I) Experimental design for (J-L).
(J-K) Representative histograms and quantified expression of (J) PD-1, CTLA-4, (K) CXCR3 and T-bet on Treg cells from tdLN, day 7 post-tumor implantation (n=3 mice/group; two independent experiments).
(L) Representative histograms and transcription factor gMFI ratios for eTreg cells from tumor-draining mLN and iLN, day 7 post-tumor implantation (n=4 mice/group; two independent experiments).
*p<0.05, **p<0.01, ns=not significant; MWU (B,J-L), MWU with Bonferroni correction (H). Data shown as mean ± SEM.
Treg cells are heterogeneous and have distinct tissue-specific functional specializations and TCR repertoires68, which could influence their suppressive capacity. To characterize their transcriptional states, we performed scRNA-seq paired with TCR-seq on Treg cells sorted from mLN and iLN of tumor-bearing and naïve mice (Figure 6C). We obtained high quality transcriptomes for 16,249 Treg cells (Figure S3B) and recovered TCRβ sequences from 55.4% of cells, TCRα sequences from 23.1% of cells, and paired TCRβ and TCRα sequences from 14.4% of cells (Figures S3C-D). Initial unsupervised analysis identified four clusters of Treg cells, and we focused subsequent analysis on three clusters of activated Treg cells that exhibited reduced expression of markers and gene sets associated with naïve T cells (Figures S3E-I)69–71. Among activated Treg cells, we identified four clusters, including Treg cells enriched for transcripts associated with early activation (Nr4a1, Egr1, Egr2, Myc, Dusp), proliferation (Mki67, Top2a, Stmn1, Cenpf, Birc5) and two activated Treg cell clusters (activated c1: Cxcr3, Icos, Tigit, Prdm1, Ctla4; activated c2: Rorc, Ccr6, Il10ra, Il18r1, Tgfbr2) (Figures 6D-E; Table S2)72,73. Activated c1 Treg cells were markedly enriched in both tdLN compared to the naïve LN, suggesting that this transcriptional program was induced in response to tumor (Figure S3J). As Treg cells can suppress in an antigen-specific manner74,75 and expand clonally in response to tumors76–78, we evaluated their clonal expansion. Treg cells expanded clonally in both tdLN, but not in either of the naïve LN (Figures 6F-G). The degree of overall clonal expansion was comparable between tumor-draining mLN and iLN and restricted to the activated c1 Treg cell cluster (Figures 6F-G).
To evaluate Treg cell specificity, we analyzed CDR3 sequences within activated c1 Treg cells. Four public TCRβ sequences were recovered independently from Treg cells in multiple mice and shared across mLN and iLN (Table S3). These sequences were often formed by distinct nucleotide rearrangements, demonstrating a convergence in the TCR repertoire (Table S3). These sequences were much more likely to exhibit clonal expansion than other CDR3β sequences (Figure S3K) and were entirely absent from the naïve iLN or mLN (Table S3), despite a comparable degree of sequence recovery (Figures S3C-D). These data suggested that the Treg cells analyzed here were responsive towards tumor-associated antigens present in both tdLN. While these data did not exclude the possibility that TCR repertoire differences could affect Treg cell suppressive capacity, they suggested that features other than the TCR might be dominant in mediating Treg cell suppressive functions.
The phenotype of activated c1 Treg cells correlated with increased functional suppression capacity (Figures S3L-M), which prompted us to interrogate transcriptional differences in activated c1 Treg cells from the mLN and iLN (Figure 6H; Table S4). Activated c1 Treg cells from the mLN differentially expressed transcripts associated with immunosuppression (Nrp1, Fgl2 and Nt5e)79–81, IFN response and TH1-polarization (Ifngr1, Cxcr3, Tbx21, Cybb and Ptpn11)82–84, while their iLN counterparts were enriched for transcripts associated with T cell activation (Icos and Gcnt1)85,86 and Treg cell survival and stability (Cd2 and Satb1)87,88 (Figure 6H). Additionally, since TH2-like Treg cells can effectively suppress anti-tumor immunity89,90, we examined expression of canonical TH1-, TH2- and TH17-polarizing transcription factors (Tbx21, Gata3 and Rorc, respectively) on activated c1 Treg cells. Consistently, Tbx21 expression was increased in the tumor-draining mLN of all analyzed mice, while Gata3 and Rorc were similarly expressed (Figure S3N). Thus, activated c1 Treg cells were TH1-polarized in the tumor-draining mLN.
Further flow characterization of Treg cells in the two tdLN revealed increased expression of natural Treg cell markers neuropilin 1 (NRP1) and Helios and effector activation markers PD-1 and CTLA-4 on Treg cells from the mLN (Figures 6I-J;S4A-B). Suppressive molecules NRP1, CTLA-4, CD39 and CD73 were differentially expressed on mLN Treg cells, while TGF-b1 and CD25 were highly expressed on iLN Treg cells (Figures 6J;S4A-D), suggesting qualitatively distinct modes of suppression. Ki67 and FoxP3 expression was similar (Figure S4E). Consistent with scRNA-seq results, TH1-like markers CXCR3 and T-bet were differentially expressed on mLN Treg cells (Figures 6K). Analysis of T-bet, Gata3 and RORγt expression confirmed the TH1-polarization, as T-bet was most highly expressed on both bulk and CD44+ CD62L– effector Treg (eTreg) cells in the mLN compared to the iLN (Figures 6L;S4F) and the frequency of T-bet+ CXCR3+ TH1-like eTreg cells was increased in the mLN (Figure S4G). Treg cells in the mLN acquired a TH1-like effector phenotype enriched in a distinct set of suppression molecules.
As Treg cells directly interacted with DC1 in the mLN (Figure 5), we tested whether DC1 regulated the Treg cell response. While Treg cells expressed PD-1 and CTLA-4 similarly in WT and Batf3−/− mice, T-bet was decreased and the frequency of T-bet+ CXCR3+ TH1-like eTreg cells was reduced in the absence of DC1 (Figure S4H-L). Thus, DC1 were required for the TH1-polarization of eTreg cells in the mLN.
Since IFN-regulated transcripts and proteins were preferentially expressed on Treg cells from the mLN compared to the iLN (Figures 6H;6K-L;S4F-G), we hypothesized that IFN-sensing induced TH1-like Treg cells in the mLN. We used WT:Ifnar1−/−, WT:Ifngr1−/− and WT:Ifngr1−/−Ifnar1−/− mixed BMCs91,92 to compare the phenotypes of WT and IFN receptor-deficient (KO) Treg cells within the tumor-draining mLN (Figures 7A;S5A-B). While Ifnar1 deletion induced a modest reduction in T-bet and had no impact on CXCR3 expression on Treg cells, Ifngr1 deletion caused a severe reduction of both T-bet and CXCR3 expression (Figures 7B;S5C), consistent with published data82. Although FoxP3, CD25, PD-1, CTLA-4 and CD73 expression was unaffected by IFN receptor deletion, CD39 was reduced on Ifngr1-deficient Treg cells in the mLN (Figures S5D-E). Expression of TH1 markers and CD39 on Treg cells was largely dependent on IFNγ-sensing in the tumor-draining mLN.
Figure 7. mLN-specific enrichment in IFNγ drives induction of TH1-like Treg cells and the associated dysfunctional T cell responses against lung cancer.
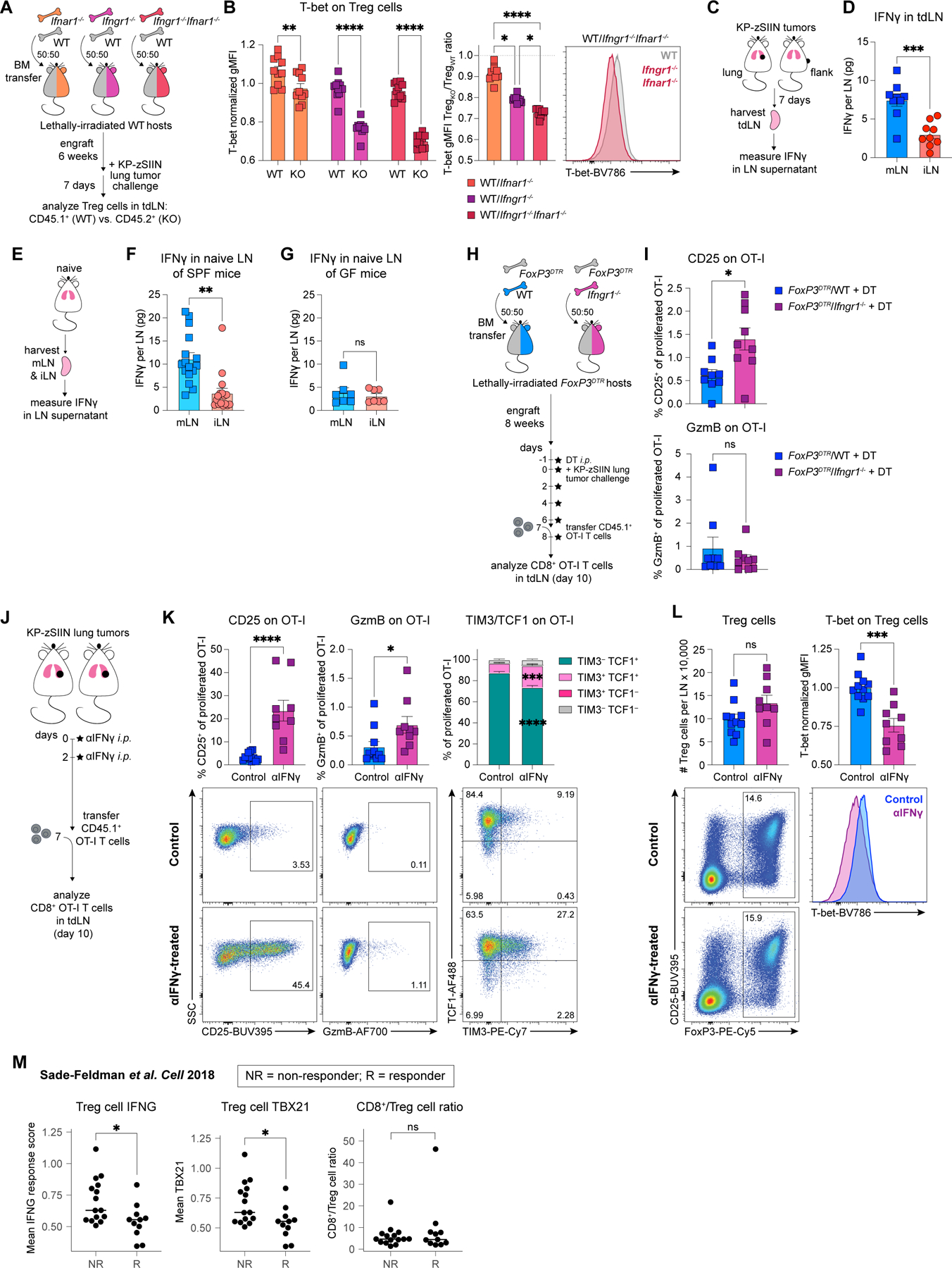
(A) Experimental design for (B).
(B) Representative histogram and quantified T-bet expression on WT and IFN receptor-deficient (KO) Treg cells from mLN of tumor-bearing WT/Ifnar1−/−, WT/Ifngr1−/− or WT/Ifngr1−/−Ifnar1−/− BMCs, day 7 post-tumor implantation (n=5 mice/group; two independent experiments).
(C) Experimental design for (D).
(D) IFNγ quantification in tdLN, day 7 post-tumor implantation (n=3 mice/group; three independent experiments).
(E) Experimental design for (F-G).
(F) IFNγ quantification in LN of naïve SPF mice (n=3–4 mice/group; five independent experiments).
(G) IFNγ quantification in LN of naïve GF mice (n=2–3 mice/group; three independent experiments).
(H) Experimental design for (I).
(I) CD25 and GzmB expression on adoptively-transferred proliferated OT-I T cells primed in mLN of DT-treated tumor-bearing FoxP3DTR/WT or FoxP3DTR/Ifngr1−/− BMCs, day 10 post-tumor implantation (n=3 mice/group; three independent experiments).
(J) Experimental design for (K-L).
(K-L) Representative flow plots and quantified expression of (K) CD25, GzmB, TIM3 and TCF1 on adoptively-transferred proliferated OT-I T cells and (L) abundance and T-bet expression for Treg cells from mLN of control and αIFNγ-treated tumor-bearing mice, day 10 post-tumor implantation (n=3–4 mice/group; three independent experiments).
(M) IFNG response hallmark signature scores and TBX21 expression on intratumoral Treg cells, along with CD8+/Treg cell ratios in melanoma patients, including ICB-responders (R) and ICB-non-responders (NR)103.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns=not significant; two-way ANOVA (B, left) or KW (B, middle), paired-MWU (F-G), MWU (D,I,K-L,M). Data shown as mean ± SEM, except in (M) where the median is shown.
We then tested whether induction of TH1-like eTreg cells similarly depended on IFN-sensing in both tdLN. We generated FoxP3DTR.eGFP:Ifnar1−/−, FoxP3DTR.eGFP:Ifngr1−/− and FoxP3DTR.eGFP:Ifngr1−/−Ifnar1−/− mixed BMCs using congenically-labeled hosts and compared phenotypes of donor-derived WT and IFN receptor-deficient (KO) eTreg cells within the tumor-draining mLN and iLN (Figures S5F-G). These BMCs enabled us to exclude host-derived Treg cells and verify that these radio-resistant cells did not confound our analysis. Consistent with earlier analysis of bulk Treg cells (Figures 7B;S5C), eTreg cell expression of T-bet and CXCR3 in the mLN depended on IFNγ-sensing (Figures S5H-I). Similarly, Ifngr1 but not Ifnar1 ablation blunted T-bet and CXCR3 expression on eTreg cells in the tumor-draining iLN (Figures S5H-I). Although T-bet+ CXCR3+ TH1-like eTreg cells were preferentially enriched in the WT immune fraction of the mLN relative to the iLN (Figure S5J), as shown before (Figure S4G), their frequency was comparable between mLN and iLN when Ifngr was ablated (Figure S5J). Hence, the TH1-like eTreg cell program was IFNγ-dependent in both LN, but was predominantly induced in Treg cells of the mLN.
Since induction of TH1-like eTreg cells relied on IFNγ-sensing, we hypothesized that the mLN-specific enrichment in TH1-like eTreg cells could result from a tissue-specific difference in IFNγ abundance. Indeed, IFNγ was 3.78-fold more enriched in the tumor-draining mLN compared to the iLN (Figures 7C-D). In contrast, IL-2 abundance was comparable between the two tdLN (Figures S6A-B). Further, IFNγ measurements from naïve LN mirrored those from tdLN, as IFNγ was more abundant in the mLN compared to matched iLN of naïve mice (Figures 7E-F). The mLN-specific enrichment in IFNγ was independent of tumor presence and was determined by the anatomical location of the LN. As lung microbiome-dependent IFN signals can impact lung-specific immunity93, we tested whether the tissue-specific differences in IFNγ would be maintained in germ-free (GF) mice. Unlike in specific-pathogen free (SPF) mice, the amount of IFNγ in the mLN and matched iLN of naïve GF mice was equalized (Figure 7G). Therefore, observed mLN-specific abundance of IFNγ was caused by the presence of commensal bacteria.
mLN-specific enrichment in IFNγ drives induction of TH1-like Treg cells and the associated dysfunctional T cell responses against lung cancer
Treg cells in the mLN expressed the chemokine receptor CXCR3 (Figure 6K), which guides intranodal positioning of effector T cells and facilitates interactions with DC94–96. To test whether CXCR3 was functionally required for Treg cells to suppress CTL responses against lung tumors, we generated FoxP3DTR:WT and FoxP3DTR:Cxcr3−/− mixed BMCs. We treated both groups with DT to compare the suppressive effects of CXCR3-deficient Treg cells (in FoxP3DTR:Cxcr3−/− BMCs) and WT Treg cells (in FoxP3DTR:WT BMCs) on CD8+ T cell priming (Figure S6C). Treg cell-specific deletion of CXCR3 has no impact on the CD8+ T cell phenotype in the tumor-draining mLN (Figures S6D-E), suggesting that several redundant chemokine receptor/ligand pathways might promote Treg:DC communication97–99.
As IFNγ cytokine and IFNγ-dependent TH1-like Treg cells were enriched in the mLN (Figures 6K-L;7A-F;S5C;S5H-J), we tested whether IFNγ-sensing could regulate Treg cell ability to suppress CTL priming. We generated FoxP3DTR:WT and FoxP3DTR:Ifngr1−/− mixed BMCs and treated the mice with DT to evaluate the impact of the Ifngr1-deficient Treg cells (in FoxP3DTR:Ifngr1−/− BMCs) or WT Treg cells (in FoxP3DTR:WT BMCs) on CD8+ T cell priming in the tumor-draining mLN (Figures S6F-G). Compared to the control, Ifngr1 ablation on Treg cells led to increased CD25 expression on CD8+ T cells primed in the mLN (Figures 7H-I). This 2.24-fold increase (±0.374 SEM) (Figures 7H-I) was comparable to the 3.32-fold difference in CD25 expression (±0.947 SEM) between iLN and mLN-primed T cells (Figure 1E). Although GzmB and TIM3/TCF1 expression was unchanged (Figures 7I;S6H), the partial rescue was encouraging, as roughly half of all immune cells in FoxP3DTR:Ifngr1−/− BMCs were Ifngr1-deficient, including DC1, which depend on IFNγ for IL-12 production54,65. In the absence of DT treatment, CD8+ T cells primed in the mLN of FoxP3DTR:WT and FoxP3DTR:Ifngr1−/− BMCs exhibited unchanged CD25 expression (Figures S6I-K), indicating that the observed rescue effect (Figure 7I) was driven by Treg cell-specific ablation of IFNγ-sensing.
We then evaluated whether antibody-mediated IFNγ blockade could counter the IFNγ-rich environment of the mLN and rewire Treg cell polarization and CD8+ T cell priming. Since IFNγ plays an important role in CD8+ T cell effector differentiation100–102, we administered IFNγ blockade early post-tumor implantation and evaluated priming of adoptively-transferred T cells (Figure 7J). IFNγ blockade caused increased CD25, GzmB and TIM-3 expression on the OT-I T cells in the tumor-draining mLN (Figure 7K), but had no impact in the tumor-draining iLN (Figures S7A-B), underscoring that this immunoregulation was tissue-specific. Additionally, IFNγ blockade caused decreased T-bet expression in both mLN and iLN Treg cells, while leaving the total number of Treg cells unchanged (Figures 7L;S7C). Transient IFNγ blockade was sufficient to rescue CTL priming and alter the Treg cell phenotype in the IFNγ-rich environment of the tumor-draining mLN.
To examine whether the IFN-dependent TH1-like Treg cells correlated with blunted anti-tumor immunity in humans, we reanalyzed a scRNA-seq dataset of tumor-infiltrating T cells from human melanoma103. Treg cell expression of the IFN response program and either TBX21 or CXCR3 transcripts strongly correlated with ICB resistance in these patients (Figures 7M;S7D). The CD8+/Treg cell ratio did not associate with ICB response (Figure 7M), suggesting that the Treg cell quality, rather than quantity, determined anti-tumor immunity outcomes. Combined with our published finding that lung cancer-specific CD8+ T cell dysfunction drives ICB resistance24, these data suggested that IFNγ-dependent TH1-like Treg cells correlated with ICB resistance in humans and mice.
DISCUSSION
We established that TH1-like eTreg cells in the lung tumor-draining mLN restrained activation of anti-tumor CTL and induced a dysfunctional CD8+ T cell state. Mechanistically, Treg cells in the tumor-draining mLN suppressed DC1 stimulatory signals needed to drive successful CTL differentiation. The suppression was mediated via MHCII-dependent contact between Treg cells and DC1 and was associated with the mLN-specific TH1-like eTreg cell program characterized by increased T-bet and CXCR3 expression. TH1-like eTreg cells were induced by IFNγ, which was preferentially enriched in the tumor-draining mLN. Antibody-mediated IFNγ blockade could counter the naturally increased IFNγ abundance in the mLN, resulting in Treg cell repolarization and enhanced CTL responses against lung tumors. Additionally, IFNγ-induced TH1-like Treg cells correlated with ICB resistance in patients with cancer. Collectively, our data suggested that TH1-like eTreg cells acted on DC1 in the tdLN to prevent priming of CTL responses against lung tumors.
Mounting evidence suggests that the tissue site of tumor growth can influence anti-tumor immunity104,105. By directly comparing T cell responses mounted against KP tumor cells implanted into the lungs or flanks of mice, we identified a Treg cell-driven lung-specific mechanism of immunosuppression. Lung tumor growth led to IFNγ-dependent TH1-like eTreg responses associated with highly potent suppression capacity and enhanced spatial proximity to DC1. These findings are consistent with prior reports describing the importance of IFNγ-sensing TH1-like Treg cells for suppressing autoimmunity in type-1-diabetes and graft-versus-host disease106–108. In addition to restraining CTL priming locally in the lung tdLN, we predict that this Treg cell state could systemically suppress anti-tumor immunity and potentially blunt secondary tumor control, as shown in prior studies109.
We found that TH1-like eTreg cell responses in the lung setting were driven by the mLN-specific abundance in IFNγ, which was induced by commensal bacteria. Consistent with our findings, the lung microbiome can directly impact lung-specific immunoregulatory mechanisms110 as well as anti-tumor immunity111. The microbiome has further been reported to regulate the suppression capacity and frequency of Treg cells112,113. Local immune populations that respond to commensal bacteria114, such as unconventional T cells, have been described to migrate to the draining LN and shape conventional T cell responses in a tissue-specific manner115. It remains to be determined which cells mediate the mLN-specific abundance in IFNγ observed in this study.
Our observations do not exclude the possibility that Treg cell-independent tissue-specific factors might also contribute to the immunosuppressive microenvironment of the tumor-draining mLN. For instance, tumors from different anatomic sites differ in local nutrient availability116, and the local metabolic environment directly regulates DC and T cell functions in cancer117–121. It is plausible that differential nutrient availability in the tumor-draining iLN and mLN could further promote the qualitatively distinct T cell responses observed in our study.
Establishment of protective anti-tumor immunity requires a breach of immune tolerance122, however, the mechanisms that maintain peripheral tolerance in cancer remain poorly understood. We uncovered a mechanism of MHCII-dependent Treg cell-mediated DC1 suppression that restrained the activation of a protective anti-tumor T cell response in the tdLN. TH1-like eTreg cells were the critical mediators of this CD8+ T cell tolerance. Although we described this mechanism in the context of lung tumors, MHCII ablation on DC1 can similarly disrupt peripheral tolerance of CD8+ T cells specific to a keratinocyte-derived self-antigen in the skin123. Thus, this peripheral tolerance mechanism is likely generalizable and not restricted to the lung setting. The preferential induction of peripheral tolerance in response to KP lung tumors but not flank tumors suggested that distinct tissue sites might have different tolerance setpoints. Since differential IFNγ abundance regulated Treg cell phenotypes and suppression capacity, tissue-specific availability of cytokines might influence the tolerance setpoint in different contexts. Further research is needed to discern additional stimuli that regulate peripheral tolerance at different anatomic sites. An improved mechanistic understanding of peripheral tolerance regulation could inspire new therapeutic approaches to disrupt immune tolerance against cancer and boost protective anti-tumor immunity.
LIMITATIONS OF STUDY
Our results strongly support the conclusion that IFNγ-sensing by Treg cells leads to enhanced suppression in the mLN. Refined studies utilizing mouse models that harbor Treg cell-specific Ifngr1−/− are needed to fully elucidate the molecular mediators of this suppression. Additionally, it is important to further validate this immunoregulatory mechanism in the context of human biology using immune cell co-cultures and spatial analyses of Treg cells, DC1 and CD8+ T cells in tdLN of patients.
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Stefani Spranger (spranger@mit.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The scRNA-seq data generated in this study has been deposited to the GEO database (GSE216086), and code used to process and analyze scRNA-seq data was made available at https://github.com/duncanmorgan/kptumors_immunity and is archived at https://doi.org/10.5281/zenodo.7331914. Code used for the spatial analysis of IF images was made available at https://github.com/awedwards/DC-Treg-CD8T-interactions-project. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6 mice were purchased from Taconic Biosciences or Jackson Laboratories. H2-Ab1−/− mice were purchased from Taconic Biosciences. Batf3−/−, FoxP3DTR.eGFP, p40-IRES-eYFP, Ifnar1−/−, Ifngr1−/−, Ifngr1−/−Ifnar1−/−, Cxcr3−/−, Thy1.1+ and CD45.1+ mice were purchased from Jackson Laboratories and bred in-house. TCR-transgenic OT-I Rag2−/− and XCR1DTR.Venus mice were bred and maintained in-house. All mice were housed under specific pathogen free conditions at the Koch Institute animal facility. Mice were gender-matched and age-matched to be 6–12 weeks old at the time of experimentation. All experimental animal procedures were approved by the Committee on Animal Care at MIT.
Cell lines and tumor injections
KP-zSIIN and KP-SIY were cultured at 37°C and 5% CO2 in DMEM (GIBCO) supplemented with 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO), and 1X non-essential amino acids (GIBCO). For tumor implantation, tumor cells were harvested by trypsinization (GIBCO), washed twice with 1X PBS (GIBCO), resuspended in PBS and 2.5x105 tumor cells were injected subcutaneously or intravenously.
METHOD DETAILS
Generation of expression vectors and modified tumor cell lines
The pLV-EF1α-IRES-puro vector (Addgene no. 85132) was linearized by digestion with BamHI and EcoRI restriction enzymes (NEB). The ZsGreen-SIINFEKL insert was generated using the pCAGGS_ZsGreen_minOVA construct (a gift from Max Krummel at UCSF), then cloned into the linearized pLV-EF1α-IRES-puro vector using the In-Fusion cloning kit (Takara Bio). The resulting pLV-EF1α-ZsGreen-SIINFEKL-IRES-puro construct was amplified and sequenced for accuracy. The KP-zSIIN cell line was genetically engineered to stably express ZsGreen-SIINFEKL using lentiviral transduction of the KP parental tumor line (a gift from Tyler Jacks) with the pLV-EF1a-cerulean-SIIN-SIY-IRES-puro construct. KP-zSIIN cell line was puromycin (GIBCO) selected and further FACS-sorted to enrich for cells with maximal expression of ZsGreen. Generation of the KP-SIY cell line was previously described24.
Adoptive transfer of T cells
The in vivo priming assay was adapted from Horton et al.24. Briefly, OT-I T cells were isolated from spleens and LN of OT-I Rag2−/− CD45.1+ mice, labeled with CTV dye (Invitrogen) following manufacturer’s instructions and at least 106 (for flow analysis) or precisely 2.5x104 cells (for immunostaining) were transferred retro-orbitally (r.o.) to mice with KP-zSIIN tumors at day 7 post-tumor implantation. Transferred OT-I T cells were analyzed in tdLN of recipient mice 3 days later. Frequency of proliferated T cells was quantified as the percentage of cells that had undergone one or more rounds of division, gated using an unstimulated, CTV-labeled sample as the undivided reference control. The T cell proliferation index was quantified as the total number of divisions divided by the number of cells that went into division using the proliferation modeling function in FlowJo v10.5.3 software (TreeStar).
Tissue processing for flow cytometry, cell sorting and ELISpot
LN were either directly mashed through a 70 μm filter into RPMI (GIBCO) for T cell analysis, or processed using a method adapted from Ruhland et al.124 for DC analysis and sorting. For the latter, LN were pierced with sharp forceps, incubated for 15 min at 37°C in digestion buffer (250 μg/ml Liberase (Sigma-Aldrich) and 50 μg/ml DNase (Sigma-Aldrich) in RPMI (GIBCO)), then pipetted up and down vigorously, followed by a second 15 min incubation at 37°C. The LN cell mixture was filtered through a 70 μm filter to generate a single cell suspension. For sorting, LN cells were next incubated with αCD16/CD32 (clone 93, BioLegend) in FACS buffer (PBS (GIBCO) with 1% FBS (Atlanta Biologicals) and 2 mM EDTA (Invitrogen)) for 15 min at 4°C to prevent non-specific antibody binding, then washed with FACS buffer. The cells were then incubated with PE-labeled antibodies (αCD19-PE clone 1D3 and αCD3e-PE clone 17A2 if only DC were sorted, or αCD19-PE alone if Treg cells were also sorted, Biolegend) in FACS buffer for 10 min at 4°C, followed by negative selection using anti-PE microbeads (Miltenyi Biotec) according to manufacturer’s instructions, prior to staining for FACS. Lungs and flank tumors were dissected, minced, then incubated in digestion buffer for 40 min at 37°C and mashed through a 70 μm filter to generate a single cell suspension. Spleens were dissected and directly mashed through a 70 μm filter to generate a single cell suspension. ACK Lysing Buffer (GIBCO) was used to lyse red blood cells in lungs and spleens (lysis performed for 2 min on ice, followed by two washes with PBS), prior to staining for flow or plating for ELISpot.
Flow cytometry and FACS staining
Cells were resuspended in FACS buffer (PBS (GIBCO) with 1% FBS (Atlanta Biologicals) and 2 mM EDTA (Invitrogen)) containing Fixable Viability Dye eFluor 780, eFluor 506 or eFluor 405 (eBioscience) to distinguish live and dead cells and αCD16/CD32 (clone 93, BioLegend) to prevent non-specific antibody binding, and incubated for 15 min at 4°C. Cells were then washed with FACS buffer and stained for surface proteins using fluorophore-conjugated antibodies resuspended in FACS buffer at the specified dilutions (Table S5) for 20 min at 4°C. Following surface staining, cells were washed twice with FACS buffer and analyzed directly or fixed for downstream intracellular staining and/or analysis the next day. Cell fixation was achieved using the Foxp3 Transcription Factor Fixation/Permeabilization buffer (eBioscience) when staining for transcription factors, or the Cytofix/Cytoperm Fixation/Permeabilization buffer (BD Biosciences) when staining for cytokines, following manufacturer’s instructions. Brefeldin A (BioLegend) was added to all buffers prior to fixation when staining for cytokines. After fixation, cells were washed twice with FACS buffer, stained for intracellular proteins (antibodies and dilutions specified in Table S5) in FACS buffer overnight at 4°C and then washed twice with FACS buffer prior to flow analysis. To obtain absolute counts of cells, Precision Count Beads (BioLegend) were added to samples following manufacturer’s instructions. Flow cytometry sample acquisition was performed on a LSR Fortessa cytometer (BD), and the collected data was analyzed using FlowJo v10.5.3 software (TreeStar). For cell sorting, the surface staining was performed as described above under sterile conditions, and cells were acquired and sorted into co-culture media (RPMI (GIBCO) containing 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO), 1X non-essential amino acids (GIBCO) and 1X β-mercaptoethanol (GIBCO)) using a FACSAria III sorter (BD). For CD8+ T cell analysis, cells were pre-gated on live, singlets, CD45+, CD3e+, CD4–, CD8+ (and CD45.1+ if congenically labeled) markers. For DC analysis and cell sorting, cells were pre-gated on live, singlets, CD45+, CD19–, CD3e–, NK1.1–, Ly6C–, MHCII+, F4/80–, CD11c+ and ZsG+ if appropriate (also autofluorescence– in lungs to exclude autofluorescent CD11c+ alveolar macrophages). For Treg cell analysis, cells were pre-gated on live, singlets, CD45+, CD3e+, CD8–, CD4+ and FoxP3+ markers (and eTreg cells were further defined as CD44+ CD62L– Treg cells). For cell sorting, Treg cells were gated as live, singlets, CD45+, CD3e+, CD8–, CD4+ and CD25high and an example gating strategy is shown in Extended Data S1A.
SIIN-tetramer staining
To identify SIIN-reactive CD8+ T cells, PE-conjugated SIIN tetramer was added during the surface staining step in the flow cytometry methods described above. PE-conjugated SIIN tetramer was assembled in-house using biotinylated peptide-MHC monomer (NIH Tetramer Core Facility) and PE-streptavidin (Invitrogen) following the NIH tetramerization protocol, and titrated to empirically determine the optimal staining concentration.
In vivo mouse treatments
For DT-mediated cell depletion, DT (Sigma-Aldrich) was diluted in PBS (GIBCO) and administered intraperitoneally (i.p.) to deplete FoxP3+ Treg cells in FoxP3DTR mice, FoxP3DTR/WT and FoxP3DTR/Ifngr1−/− BMCs (1μg DT/mouse) or XCR1+ DC1 in XCR1DTR mice, XCR1DTR/WT and XCR1DTR/H2-Ab1−/− BMCs (0.5μg DT/mouse) at the indicated timepoints. For in vivo treatments, mice were injected with 100 μg αCD28 antibody i.p. (clone 37.51, Bio X Cell), 1.42x10−11 mol IL-12 protein fused to murine serum albumin r.o. (IL-12-MSA, manufactured in-house) or 200 μg IFNγ antibody i.p. (clone XMG1.2, Bio X Cell) at the indicated timepoints.
IFNγ ELISpot
ELISpot plates (EMD Millipore) were coated overnight at 4°C with αIFNγ capture antibody (BD Biosciences). Plates were washed and blocked with complete media (DMEM (GIBCO) supplemented with 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO), and 1X non-essential amino acids (GIBCO)) for 2 hr at room temperature (RT). Splenocytes were plated in complete media at 106 cells/well in the presence or absence of 160 nM SIINFEKL peptide (Invivogen). As a positive control, splenocytes were plated in complete media containing 100 ng/ml PMA (Sigma-Aldrich) and 1 μg/ml ionomycin (Sigma-Aldrich). Plates were incubated overnight at 37°C and 5% CO2, then developed using the mouse IFNγ ELISpot kit (BD Biosciences), following manufacturer’s protocol.
Treg cell transcription factor analysis
To accurately compare differences in Treg cell transcription factor expression, lung and flank KP-zSIIN tumors were implanted into Thy1.2+ and Thy1.1+ mice, respectively, and tdLN from congenically-labeled mice were combined for downstream processing and flow analysis. Relative expression of transcription factor expression were quantified as the ratio of gMFI values for matched Thy1.2+ mLN-derived and Thy1.1+ iLN-derived fractions of bulk or eTreg cells, as indicated. The experiment was repeated with reversed congenic marker assignments, i.e. using tdLN samples from Thy1.1+ lung- and Thy1.2+ flank-tumor-bearing mice, to ensure reproducibility, and the data were pooled. The same experimental approach was used to evaluate transcription factor expression in Treg cells from WT and Batf3−/− lung tumor-bearing mice.
Generation of BMC mice
Host mice were irradiated with 500 rad, allowed to recover for 3 hr, and subsequently irradiated again with 550 rad. The next day, BM was harvested from the femur and tibia of donor mice, depleted of T cells using CD90.2 microbeads (Miltenyi Biotec), washed twice with PBS and 107 cells were injected r.o. into the irradiated host mice. For mixed BM chimeras, 107 total cells of a 50:50 mixture of BM from donor mice was transferred. A period of at least 6 weeks was allowed for engraftment prior to the start of experiments.
Ex vivo T cell priming co-cultures
DC and Treg cells were FACS-sorted from LN as described above (LN from many mice were pooled to reach appropriate cell yield). SIIN-reactive CD8+ T cells were isolated from spleens and LN of naïve OT-I TCR-transgenic mice using the untouched CD8+ T cell isolation kit (Miltenyi Biotec), following manufacturer’s instructions. Isolated CD8+ OT-I T cells were washed twice with PBS and stained with CTV dye (Life Technologies) following manufacturer’s instructions. For DC:OT-I co-cultures, 5x103 sorted ZsG+ or ZsG– DC were cultured with 5x104 dye-labeled CD8+ OT-I T cells, respectively (1:10 DC:T cell ratio). For ZsG+ DC1:Treg:OT-I T cell suppression co-cultures, 5x103 sorted ZsG+ DC1 were cultured with 5x104 dye-labeled CD8+ OT-I T cells, in the presence or absence of 6.25x103 sorted Treg cells (1:8 Treg:OT-I ratio), unless indicated otherwise. For Treg:OT-I suppression co-cultures performed without DC1, various numbers of Treg cells were cultured with 5x104 dye-labeled CD8+ OT-I T cells (at the indicated Treg:OT-I ratios) on αCD3/αCD28-coated plates. Antibody-coated plates were coated overnight at 4°C with 0.2 μg/ml αCD3 (clone 145–2C11, BD Biosciences) and 0.5 μg/ml αCD28 (clone 37.51, BD Biosciences) in PBS, washed with PBS, then blocked with co-culture media (RPMI (GIBCO) containing 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO) and 1X β-mercaptoethanol (GIBCO)) for at least 30 min at RT, prior to adding cells. For all ex vivo T cell priming co-cultures, cells were resuspended in co-culture medium, added to V-bottom tissue culture-treated 96-well plates and cultured at 37°C and 5% CO2 for 3 days. Following co-culture, cells were stained and analyzed by flow cytometry. Frequency of proliferated T cells was quantified as the percentage of cells that had undergone one or more rounds of division, gated using an unstimulated, CTV-labeled sample as the undivided reference control. The T cell proliferation index was quantified as the total number of divisions divided by the the number of cells that went into division using the proliferation modeling function in FlowJo v10.5.3 software (TreeStar). Expression of T cell activation markers was also assessed by flow staining as described. For co-culture experiments performed in the presence of additional biomolecules, 5 μg/ml αCD28 (clone 37.51, BD Biosciences), 10 nM IL-12 (using IL-12-MSA fusion protein generated in-house) or 10 μg/ml αMHCII (clone M5/114, Bio X Cell) were added at the beginning of co-culture where indicated.
Generation of BM-DC
BM-DC generation protocol was adapted from de Mingo Pulido et al.125. BM was harvested from the femur and tibia of p40-IRES-eYFP mice, passed through a 70 μm filter, ACK-lysed and cultured at a density of 2x106 cells/ml in RPMI (GIBCO) supplemented with 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO), 25 mM HEPES (GIBCO), 1X non-essential amino acids (GIBCO), 10 mM sodium pyruvate (GIBCO), 1X β-mercaptoethanol (GIBCO), 100 ng/ml recombinant human FLT3-L (Bio X Cell), and 5 ng/ml recombinant mouse GM-CSF (BioLegend) for 6 days at 37°C and 5% CO2. On day 6, floating and semi-adherent cells were collected as BM-DC, frozen in 10% DMSO (Sigma-Aldrich) in FBS (Atlanta Biologicals) and stored in liquid nitrogen.
BM-DC:Treg cell co-cultures
Treg cells were FACS-sorted from LN as described above. Frozen p40-IRES-eYFP BM-DC were thawed and cultured in RPMI (GIBCO) supplemented with 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO), 25 mM HEPES (GIBCO), 1X non-essential amino acids (GIBCO), 10 mM sodium pyruvate (GIBCO), 1X β-mercaptoethanol (GIBCO), 100 ng/ml recombinant human FLT3-L (Bio X Cell), and 5 ng/ml recombinant mouse GM-CSF (BioLegend) for 2 days at 37°C and 5% CO2. BM-DC were then washed in PBS and cultured overnight in 1 μg/ml Poly(I:C) (Invivogen) in co-culture media (RPMI (GIBCO) containing 10% FBS (Atlanta Biologicals), 1% penicillin/streptomycin (GIBCO) and 1X β-mercaptoethanol (GIBCO)) at 37°C and 5% CO2 to generate mature BM-DC. On the next day mature BM-DC were collected and washed twice with PBS, prior to addition to assays. For BM-DC:Treg cell co-cultures, 2x104 mature BM-DC and 2.5x104 sorted Treg cells were resuspended in co-culture medium with 10 nM IL-2 (using IL-2-MSA fusion protein generated in-house), added to V-bottom tissue culture-treated 96-well plates and cultured at 37°C and 5% CO2 for 24 hr. Following co-culture, cells were stained and analyzed by flow cytometry. A representative gating strategy of BM-DC1 is shown in Extended Data S1B.
Tissue section preparation and immunostaining
Adapted from Gerner et al.126. LN were harvested, fixed in periodate-lysine-paraformaldehyde buffer (0.05 M phosphate buffer containing 0.1 M lysine, 2 mg/ml NaIO4, and 1% of paraformaldehyde (Electron Microscopy Grade), pH 7.4) overnight at 4°C, followed by cryoprotection in 30% sucrose in PBS for 24 hr at 4°C. LN were then carefully cleaned of fat, embedded in 100% OCT in cryomolds, snap-frozen by floating the cryomolds on a solution of 2-methylbutane cooled by dry ice and stored at −80°C until processing. Frozen LN were completely sectioned (10 μm thickness) using Cryostar NX70 (Thermo Scientific) to make sure that imaged tissue cross-sections contained all representative LN regions. Tissue sections were left to dry for 30 min at RT, post-fixed in ice-cold acetone for 10 min at −20°C, then left to dry again for 30 min at RT and stored at −20°C. For immunostaining, sections were first warmed to RT, washed twice with PBS and incubated with blocking buffer (PBS containing 1% bovine serum albumin, 1% normal mouse serum, 10% normal goat serum, and 0.3% Triton X-100) for 2 hr at RT. Tissue sections were then stained with primary antibody (chicken polyclonal αGFP, 1:1000; abcam) diluted in blocking buffer for 2 hr at RT, washed three times with wash buffer (PBS with 0.3% Triton X-100), followed by staining with secondary antibody (goat anti-chicken AF488 1:500, Jackson ImmunoResearch) diluted in blocking buffer for 1 hr at RT. Next, samples were washed three times with wash buffer and stained with fluorophore-conjugated antibodies (FoxP3-AF750 clone 1054C, 1:100, R&D Systems; CD45.1-APC clone A20, 1:100, Biolegend) diluted in blocking buffer for 1 hr at RT. Then, nuclei were counterstained with 1.5 μm propidium iodide (1:1000, Sigma-Aldrich) diluted in wash buffer for 10 min at RT, followed by a wash in PBS. Samples were mounted using ProLong Diamond Antifade Mountant (Thermo Fisher Scientific).
Imaging
Tissue sections were imaged using the TissueFAXS Plus automated slide scanning system (TissueGnostics, USA) combining a Zeiss Axio Imager 2 upright microscope with a Märzhäuser motorized stage (Märzhäuser Wetzlar). We utilized the Zeiss 20x Plan-Neofluor 0.5 N.A. air objective, in combination with filters sets (from Chroma Technology, USA) with the following specifications: AF488 (470/24) ET470/30x T495lpxr ET515/30m, AF750 (740/20) ET740/40x T770lpxr ET780lp, multiband dichroic qTexasRed/qCy5 (550/15, 640/30). A Lumencor Spectra 3 LED light engine (500 mW per channel) provided excitation. Fluorescence images were captured using a Hamamatsu Orca Flash 4.0 V2 cooled digital CMOS camera C11440–22CU. Exposure times were 94 ms for AF488, 146 ms for AF750, 10 ms for qTexasRed and 48 ms for qCy5 channels. Tissue sections were acquired as z-stacks in a process called extended focus, with a 3 μm step size, including one step above and one step below the focal plane. Extended focus takes each in-focus area of each image within the z-stack and combines those regions into a single image. Image processing included stitching performed using the TissueFAXS capture/control software.
Image processing and spatial analysis
Images were exported as single channel TIFF files using the TissueFAXS Viewer software (TissueGnostics USA) and downstream image analysis was completed in Imaris software v9.7.2 (Bitplane) and using custom scripts in Python. Imaris Surface objects were created for FoxP3+ Treg cells (smoothing sigma = 0.5μm, intensity threshold = 17.2, seed point diameter = 3.00μm, seed point quality filter = 1.0), XCR1+ DC1 (smoothing sigma = 0.20μm, background subtraction rolling ball radius = 5.00μm, background subtraction threshold = 8.69, seed point diameter = 5.00μm, seed point quality threshold = 2.07, size threshold >= 50.0 voxels), CD45.1+ CD8+ OT-I T cells (smoothing sigma = 0.5μm, intensity threshold = 16.7, seed point diameter = 6.00μm, seed point quality threshold = 4.87, area threshold >= 11.5μm2) and CD45.1+ CD8+ OT-I T cell clusters (smoothing sigma = 0.5μm, intensity threshold = 9.12, area threshold > 300μm2). Surfaces for FoxP3+ Treg cells, XCR1+ DC1 cells and CD45.1+ CD8+ OT-I T cells were further segmented using the “Split touching objects” function. A surface was also made for the entire lymph node using the nuclear channel (smoothing sigma = 5.00μm, intensity threshold = 10.0). For each surface set, the surface area and centroid coordinates were exported and collated across images. For each CD8+ OT-I T cell cluster surface, the area of the cluster was used to calculate the radius. The microniche area for each CD8+ OT-I T cell cluster was defined using the radius of the CD8+ OT-I T cell cluster plus 5μm, 10μm, 15μm and 20μm. Distance matrices encoding the distance between the centroid of the CD8+ OT-I T cell cluster and centroids all DC1 and Treg cells in the image were generated. These distance matrices were thresholded using the microniche radius to find the DC1 and Treg cells that were positioned within each microniche. Finally, a new distance matrix was calculated between the DC1 and Treg cells within each microniche to find the minimum distance from each Treg cell to a DC1. Code made available: https://github.com/awedwards/DC-Treg-CD8T-interactions-project. Example images of the segmentation and the microniche analysis workflow are provided in Extended Data S1C-F.
Generation of IL-2 and IL-12 fusion proteins
Extended half-life cytokine-MSA fusions were generated as previously described127,128. HEK293 cells (at 1 million cells/ml) were transfected with sterile-filtered plasmid DNA (1 mg/L cell culture) using polyethylenimine (2 mg per liter cell culture) in OptiPro serum-free medium (40 ml/L cell culture) (Thermo Fisher). His-tagged proteins were isolated from HEK293 supernatant using TALON Metal Affinity Resin (Takara Bio Inc.). Cytokine-fusion proteins were then further purified by size exclusion chromatography using a HiLoad 16/600 Superdex 200 pg column on an ÄKTA FPLC system (GE Healthcare) that had been pretreated for 4 hours with 1 M NaOH to remove endotoxin and subsequently equilibrated in sterile PBS (Corning). After purification, all proteins were buffer exchanged into sterile PBS (Corning), 0.2 micron sterile-filtered (Pall Corporation), and confirmed to contain minimal endotoxin (<0.1 EU per injection) usinga chromogenic LAL assay (Lonza). To confirm their molecular weights, proteins were run alongside a Novex Prestaind Sharp Protein Ladder on a 4–12% NuPAGE Bis-Tris protein gel (Life Technologies) with 1% MES running buffer. Proteins were flash-frozen and stored at −80°C, but before therapeutic injection, cytokine fusion proteins were warmed to room temperature.
Cytokine measurements in LN supernatant
LN were homogenized in T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific) supplemented with cOmplete™ Protease Inhibitor Cocktail (Sigma-Aldrich) and centrifuged for 5 min at 500 g. LN supernatant was collected, flash-frozen and stored at −80°C. IFNγ and IL-2 cytokines were quantified via ELISA (Mouse IFNγ DuoSet ELISA, R&D Systems; Mouse IL-2 DuoSet ELISA) following manufacturer’s instructions.
Seq-Well scRNA-seq
For scRNA-seq of ex vivo-primed T cells, CD8+ OT-I T cells and ZsG+ DC1 sorted from tumor-draining mLN were cultured alone or in the presence of Treg cells sorted from tumor-draining mLN (added at the 1:8 Treg:OT-I ratio) as described above. After the 3-day co-culture, cells were collected for sequencing. For scRNA-seq of Treg cells, LN were mashed through a 70 μm filter into RPMI (GIBCO), then incubated with αCD16/CD32 (clone 93, BioLegend) in FACS buffer (PBS (GIBCO) with 1% FBS (Atlanta Biologicals) and 2 mM EDTA (Invitrogen)) for 15 min at 4°C, washed with FACS buffer and stained with a mix of αCD45-APC (1:200, clone 30-F11, Biolegend) and Total-seq A mouse hashtag antibody (1:100, clone 30-F11, Biolegend) in FACS buffer to label individual mice (hashtag labeling was only performed for tdLN, not naïve LN). Cells were then washed twice in FACS buffer and tdLN cells from multiple mice were pooled together for subsequent CD4+ T cell enrichment sing the mouse CD4+ T cell isolation kit (Miltenyi Biotec), following manufacturer’s instructions. Next, cells were stained for FACS and Treg cells were sorted as previously described. Cells from ex vivo co-cultures and sorted Treg cells were processed for scRNA-seq using the Seq-Well platform with second strand chemistry, as previously described129,130. Whole transcriptome libraries were barcoded and amplified using the Nextera XT kit (Illumina) and were sequenced on a Novaseq 6000 (Illumina). Hashtag oligo libraries were amplified as described previously and were sequenced on a Nextseq 550131.
Processing of cell hashing data
Cell hashing data was aligned to HTO barcodes using CITE-seq-Count v1.4.2132. To establish thresholds for positivity for each HTO barcode, we first performed centered log-ratio normalization of the HTO matrix and then performed k-medoids clustering with k=5 (one for each HTO). This produced consistently five clusters, each dominated by one of the 5 barcodes. For each cluster, we first identified the HTO barcode that was dominant in that cluster. We then considered the threshold to be the lowest value for that HTO barcode among the cells classified in that cluster. To account for the scenario in which this value was substantially lower than the rest of the values in the cluster, we used Grubbs’ test to determine whether this threshold was statistically an outlier relative to the rest of the cluster. If the lower bound was determined to be an outlier at p=0.05, it was removed from the cluster, and the next lowest value was used as the new threshold. This procedure was iteratively applied until the lowest value in the cluster was no longer considered an outlier at p=0.05. Cells were then determined to be “positive” or “negative” for each HTO barcode based on these thresholds. Cells that were positive for multiple HTOs or were negative for all HTOs were excluded from downstream analysis. To account for differences in sequencing depth between samples, these steps were performed separately for each Seq-Well array that was processed.
scRNA-seq data processing and visualization
Raw read processing of scRNA-seq reads was performed as previously described133. Briefly, reads were aligned to the mm10 reference genome and collapsed by cell barcode and unique molecular identifier (UMI). Then, cells with less than 1000 (for the co-culture dataset) or 700 (for the Treg cell dataset) unique genes detected and genes detected in fewer than 5 cells were filtered out, and the data for each cell was log-normalized to account for library size. Genes with log-mean expression values greater than 0.1 and a dispersion of greater than 1 were selected as variable genes, and the ScaleData function in Seurat v2.3.4134 was used to regress out the number of UMI and percentage of mitochondrial genes in each cell. Principal components analysis was performed. The number of principal components used for visualization was determined by examination of the elbow plot, and two-dimensional embeddings were generated using uniform manifold approximation and projection (UMAP). Clusters were determined using Louvain clustering, as implemented in the FindClusters function in Seurat. In the ex vivo co-culture dataset, CD8+ T cells were specifically identified based on the expression of canonical CD8+ T cell transcripts including CD3e, CD8a, Lck and Thy1, and these CD8+ T cell clusters were selected further analysis. The CD8+ T cells were reprocessed with the same processing and clustering steps described above. DEG analysis was performed for the CD8+ T cells between the two co-culture conditions using the FindMarkers function. Gene signature scores defined as the top 50 DEGs of the in vivo-primed CD8+ T cells from tumor-draining mLN and iLN24 were calculated using Seurat’s AddModuleScore function135 and compared between the two co-culture conditions. In the Treg cell dataset, Treg cells were identified based on the expression of canonical Treg cell transcripts including CD3e, CD4, FoxP3 and Il2ra, and the contaminating cell clusters were removed. Naïve T cell gene set signature scores69–71 were obtained from MSigDB136 and calculated using Seurat’s AddModuleScore function135 and compared across Treg cell clusters. Clusters that contained activated Treg cells were selected for further analysis. These cells were reprocessed with the same processing and clustering steps described above. DEG analysis was performed for each cluster and between indicated cell populations using the FindMarkers function.
Paired single-cell TCR-seq and analysis
Paired TCR-seq and read alignment was performed as previously described137. Briefly, whole transcriptome amplification product from each single-cell library was enriched for TCR transcripts using biotinylated Tcrb and Tcra probes and magnetic streptavidin beads. The enrichment product was further amplified using V-region primers and Nextera sequencing handles, and the resulting libraries were sequenced on an Illumina Novaseq 6000. Processing of reads was performed using the Immcantation software suite138,139. Briefly, reads were aggregated by cell barcode and UMI, and UMI with under 10 reads were discarded. ClusterSets.py was used to divide sequences for each UMI into sets of similar sequences. Only sets of sequences that comprised greater than 90% of the sequences obtained for that UMI were considered further. Consensus sequences for each UMI were determined using the BuildConsensus.py function. Consensus sequences were then mapped against TCRV and TCRJ IMGT references sequences with IgBlast. Sequences for which a CDR3 sequence could not be unambiguously determined were discarded. UMI for consensus sequences were corrected using a directional UMI collapse, as implemented in UMI-tools140. TCR sequences were then mapped to single cell transcriptomes by matching cell barcodes. If multiple Tcra or Tcrb sequences were detected for a single cell barcode, then the corresponding sequence with the highest number of UMI and raw reads was retained. Expanded clones were defined as multiple cells from the same mouse (i.e. with same hashtag signal where hashing antibodies were used) containing identical CDR3β junction nucleotide sequences. Clonal size was defined as the number of cells from a given mouse (identified based on the hashtag signal where hashing antibodies were used) that share the same CDR3β junction nucleotide sequence.
Re-analysis of dataset from Sade-Feldman et al. (Cell 2018)
Patient characteristics and scRNA-seq of tumor-infiltrating immune cells from metastatic melanoma patients published by Sade-Feldman et al.103 were downloaded from the Gene Expression Omnibus (GEO, accession GSE120575). This dataset was processed as previously described141. Briefly, a Seurat v3.0.0134 object was created such that the counts slot and data slot were populated with TPM counts and log(TPM+1) counts, respectively. Highly variable genes were identified (FindVariableFeatures) and the data was scaled (ScaleData, while regressing out percentage mitochondrial content per cell and the number of detected reads per cell) followed by dimensionality reduction with PCA using Seurat’s standard preprocessing procedure. Clusters were determined using FindNeighbors and FindClusters Seurat functions with 21 principal components (clustering resolution 0.6). Subsequently, hallmark IFN response gene set signature scores136,142 for cells within the FoxP3+ Treg cell cluster were calculated using Seurat’s AddModuleScore function135 and compared between ICB-responders and non-responders.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analyses were performed using GraphPad Prism (GraphPad) and R. All data are shown as mean ± SEM, unless otherwise indicated. For flow cytometry and immunofluorescence data, statistical analyses were performed with Mann-Whitney U (MWU) test for comparison of two groups, Kruskal-Wallis (KW) test for comparison of more than two groups, or two-way ANOVA for multiple comparisons with *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns=not significant. Nonparametric tests were used as the small sample sizes in our study did not allow us to assume a normal distribution143. For scRNA-seq differential gene expression and pathway analyses, p-values were calculated using a two-sided MWU test and adjusted with Bonferroni correction.
Supplementary Material
Table S3. Public TCRβ sequences recovered from Treg cell TCR-seq.
Table S2. DEG markers for the four activated Treg cell clusters (0: early activation, 1: activated c1, 2: activated c2, 3: proliferating Treg cells) identified in the scRNA-seq dataset.
Table S1. DEG between activated CD8+ OT-I T cells from DC1:OT-I T cell and DC1:Treg:OT-I T cell ex vivo co-cultures.
Table S5. Flow cytometry and immunofluorescence antibodies.
Table S4. DEG between mLN and iLN Treg cells sorted from mice bearing KP-zSIIN tumors.
ACKNOWLEDGEMENTS
We thank Ömer Yilmaz, Heaji Shin and Andrea Vargas for providing GF mice; Arlene Sharpe (Harvard) and Max Krummel (UCSF) for advice; Ellen Duong, Yash Agarwal, Megan Ruhland (UCSF), Thomas Diefenbach (Ragon) and Peter Savage (UChicago) for experimental guidance; Arjun Bhutkar and Sarah Blatt for assistance with initial processing of the human dataset; Melissa Duquette for mouse colony maintenance and laboratory support; Paul Thompson for administrative support; Koch Institute Swanson Biotechnology Center and the Ragon Institute Microscopy Core for technical support. This work was supported by the Pew Stewart scholarship, the Koch Institute Frontier Research program, the Ludwig Center at MIT and in part by the MIT School of Science Fellowship in Cancer Research.
Footnotes
DECLARATION OF INTERESTS
S.S. is a SAB member for Related Sciences, Arcus Biosciences, Ankyra Therapeutics and Venn Therapeutics. S.S. is a co-founder of Danger Bio. S.S. is a consultant for TAKEDA, Merck, Tango Therapeutics, and Ribon Therapeutics and receives funding for unrelated projects from Leap Therapeutics. J.C.L. has interests in Sunflower Therapeutics PBC, Pfizer, Honeycomb Biotechnologies, OneCyte Biotechnologies, SQZ Biotechnologies, Alloy Therapeutics, QuantumCyte, Amgen, and Repligen. S.S. and J.C.L.’s interests are reviewed and managed under MIT’s policies for potential conflicts of interest. J.C.L. receives sponsored research support at MIT from Amgen, the Bill & Melinda Gates Foundation, Biogen, Pfizer, Roche, Takeda, and Sanofi.
REFERENCES
- 1.Fridman WH, Pages F, Sautes-Fridman C, and Galon J (2012). The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12, 298–306. 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 2.Weigelin B, den Boer AT, Wagena E, Broen K, Dolstra H, de Boer RJ, Figdor CG, Textor J, and Friedl P (2021). Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat Commun 12, 5217. 10.1038/s41467-021-25282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DS, and Mellman I (2013). Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10. 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. (2008). Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100. 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. (2014). Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell 26, 938. 10.1016/j.ccell.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, and Sallusto F (2001). Regulation of T cell immunity by dendritic cells. Cell 106, 263–266. 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 7.den Haan JM, Lehar SM, and Bevan MJ (2000). CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med 192, 1685–1696. 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Plaen E, Lurquin C, Van Pel A, Mariame B, Szikora JP, Wolfel T, Sibille C, Chomez P, and Boon T (1988). Immunogenic (tum-) variants of mouse tumor P815: cloning of the gene of tum- antigen P91A and identification of the tum- mutation. Proc Natl Acad Sci U S A 85, 2274–2278. 10.1073/pnas.85.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley PS, Ikehara S, et al. (1994). The tissue distribution of the B7–2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med 180, 1849–1860. 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caux C, Vanbervliet B, Massacrier C, Azuma M, Okumura K, Lanier LL, and Banchereau J (1994). B70/B7–2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J Exp Med 180, 1841–1847. 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeths MJ, and Mescher MF (1997). B7–1-dependent co-stimulation results in qualitatively and quantitatively different responses by CD4+ and CD8+ T cells. Eur J Immunol 27, 598–608. 10.1002/eji.1830270305. [DOI] [PubMed] [Google Scholar]
- 12.Harding FA, McArthur JG, Gross JA, Raulet DH, and Allison JP (1992). CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356, 607–609. 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins MK, Ashwell JD, and Schwartz RH (1988). Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol 140, 3324–3330. [PubMed] [Google Scholar]
- 14.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, and Alber G (1996). Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 184, 747–752. 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, and Tough DF (2003). Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 4, 1009–1015. 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 16.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, and Mescher MF (1999). Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 162, 3256–3262. [PubMed] [Google Scholar]
- 17.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, and Gajewski TF (2011). Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 208, 2005–2016. 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtsinger JM, Lins DC, and Mescher MF (2003). Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med 197, 1141–1151. 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez J, Aung S, Marquardt K, and Sherman LA (2002). Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med 196, 323–333. 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gett AV, Sallusto F, Lanzavecchia A, and Geginat J (2003). T cell fitness determined by signal strength. Nat Immunol 4, 355–360. 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 21.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, and Schoenberger SP (2003). Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol 4, 361–365. 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 22.D'Souza WN, and Hedrick SM (2006). Cutting edge: latecomer CD8 T cells are imprinted with a unique differentiation program. J Immunol 177, 777–781. 10.4049/jimmunol.177.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. (2019). TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 571, 211–218. 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton BL, Morgan DM, Momin N, Zagorulya M, Torres-Mejia E, Bhandarkar V, Wittrup KD, Love JC, and Spranger S (2021). Lack of CD8(+) T cell effector differentiation during priming mediates checkpoint blockade resistance in non-small cell lung cancer. Sci Immunol 6, eabi8800. 10.1126/sciimmunol.abi8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zagorulya M, and Spranger S (2022). Once upon a prime: DCs shape cancer immunity. Trends Cancer 10.1016/j.trecan.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone AM, Fathman CG, Inaba K, and Steinman RM (1989). Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med 169, 1169–1178. 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, and Moser M (1996). Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med 184, 1413–1424. 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, and Biron CA (2002). Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med 195, 517–528. 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, and Sher A (1997). In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med 186, 1819–1829. 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, and Nussenzweig MC (2001). Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194, 769–779. 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, and Steinman RM (2002). Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 196, 1627–1638. 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, and Steinman RM (2002). Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med 196, 1091–1097. 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onishi Y, Fehervari Z, Yamaguchi T, and Sakaguchi S (2008). Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A 105, 10113–10118. 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu P, Gao JF, D'Souza CA, Kowalczyk A, Chou KY, and Zhang L (2012). Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell Mol Immunol 9, 136–146. 10.1038/cmi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. (2011). Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600–603. 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mavin E, Nicholson L, Rafez Ahmed S, Gao F, Dickinson A, and Wang XN (2017). Human Regulatory T Cells Mediate Transcriptional Modulation of Dendritic Cell Function. J Immunol 198, 138–146. 10.4049/jimmunol.1502487. [DOI] [PubMed] [Google Scholar]
- 37.Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, Tsui J, Ruhland MK, Kersten K, Abushawish MA, et al. (2019). Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4(+) T Cell Immunity. Cell 177, 556–571 e516. 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, and Mempel TR (2014). Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest 124, 2425–2440. 10.1172/JCI66375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marangoni F, Zhakyp A, Corsini M, Geels SN, Carrizosa E, Thelen M, Mani V, Prussmann JN, Warner RD, Ozga AJ, et al. (2021). Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell 184, 3998–4015 e3919. 10.1016/j.cell.2021.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, and Bar-Sagi D (2017). Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep 20, 558–571. 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, et al. (2015). Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity 43, 579–590. 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poholek AC (2021). Tissue-Specific Contributions to Control of T Cell Immunity. Immunohorizons 5, 410–423. 10.4049/immunohorizons.2000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikhak Z, Strassner JP, and Luster AD (2013). Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med 210, 1855–1869. 10.1084/jem.20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim TS, Gorski SA, Hahn S, Murphy KM, and Braciale TJ (2014). Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8(+) T cell differentiation by a CD24-dependent mechanism. Immunity 40, 400–413. 10.1016/j.immuni.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa LM, Blomhoff R, and Agace WW (2008). Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol 1, 38–48. 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esterhazy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, and Mucida D (2019). Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130. 10.1038/s41586-019-1125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mani V, Bromley SK, Aijo T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. (2019). Migratory DCs activate TGF-beta to precondition naive CD8(+) T cells for tissue-resident memory fate. Science 366. 10.1126/science.aav5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leal JM, Huang JY, Kohli K, Stoltzfus C, Lyons-Cohen MR, Olin BE, Gale M Jr., and Gerner MY (2021). Innate cell microenvironments in lymph nodes shape the generation of T cell responses during type I inflammation. Sci Immunol 6. 10.1126/sciimmunol.abb9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duckworth BC, Lafouresse F, Wimmer VC, Broomfield BJ, Dalit L, Alexandre YO, Sheikh AA, Qin RZ, Alvarado C, Mielke LA, et al. (2021). Effector and stem-like memory cell fates are imprinted in distinct lymph node niches directed by CXCR3 ligands. Nat Immunol 22, 434–448. 10.1038/s41590-021-00878-5. [DOI] [PubMed] [Google Scholar]
- 50.LaFave LM, Kartha VK, Ma S, Meli K, Del Priore I, Lareau C, Naranjo S, Westcott PMK, Duarte FM, Sankar V, et al. (2020). Epigenomic State Transitions Characterize Tumor Progression in Mouse Lung Adenocarcinoma. Cancer Cell 38, 212–228 e213. 10.1016/j.ccell.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, and Krummel MF (2016). Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 30, 324–336. 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, Fukuda Y, Yano T, Nobuoka M, Hirashima T, et al. (2013). Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J Immunol 190, 6071–6082. 10.4049/jimmunol.1202798. [DOI] [PubMed] [Google Scholar]
- 53.Duong E, Fessenden TB, Lutz E, Dinter T, Yim L, Blatt S, Bhutkar A, Wittrup KD, and Spranger S (2022). Type I interferon activates MHC class I-dressed CD11b(+) conventional dendritic cells to promote protective anti-tumor CD8(+) T cell immunity. Immunity 55, 308–323 e309. 10.1016/j.immuni.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J, Chudnovskiy A, Maskey S, Walker L, Finnigan JP, et al. (2020). A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262. 10.1038/s41586-020-2134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, and Lanzavecchia A (1998). Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 28, 2760–2769. . [DOI] [PubMed] [Google Scholar]
- 56.Cella M, Engering A, Pinet V, Pieters J, and Lanzavecchia A (1997). Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388, 782–787. 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 57.Fontenot JD, Gavin MA, and Rudensky AY (2003). Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4, 330–336. 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, Johnson J, Staupe RP, Bengsch B, Xu C, et al. (2019). TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision. Immunity 51, 840–855 e845. 10.1016/j.immuni.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, and Rao A (2010). Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32, 79–90. 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, and Ahmed R (2010). Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103. 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Marelli-Berg FM, Clement M, Mauro C, and Caligiuri G (2013). An immunologisťs guide to CD31 function in T-cells. J Cell Sci 126, 2343–2352. 10.1242/jcs.124099. [DOI] [PubMed] [Google Scholar]
- 62.Xing S, Li F, Zeng Z, Zhao Y, Yu S, Shan Q, Li Y, Phillips FC, Maina PK, Qi HH, et al. (2016). Tcf1 and Lef1 transcription factors establish CD8(+) T cell identity through intrinsic HDAC activity. Nat Immunol 17, 695–703. 10.1038/ni.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorsam G, Graeler MH, Seroogy C, Kong Y, Voice JK, and Goetzl EJ (2003). Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J Immunol 171, 3500–3507. 10.4049/jimmunol.171.7.3500. [DOI] [PubMed] [Google Scholar]
- 64.Hart GT, Hogquist KA, and Jameson SC (2012). Kruppel-like factors in lymphocyte biology. J Immunol 188, 521–526. 10.4049/jimmunol.1101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M, Gungabeesoon J, et al. (2018). Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-gamma and IL-12. Immunity 49, 1148–1161 e1147. 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saleh R, and Elkord E (2020). FoxP3(+) T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett 490, 174–185. 10.1016/j.canlet.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 67.Gubser C, Schmaler M, Rossi SW, and Palmer E (2016). Monoclonal regulatory T cells provide insights into T cell suppression. Sci Rep 6, 25758. 10.1038/srep25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shevyrev D, and Tereshchenko V (2019). Treg Heterogeneity, Function, and Homeostasis. Front Immunol 10, 3100. 10.3389/fimmu.2019.03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. (2009). Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911. 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. (2009). Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167. 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbas AR, Wolslegel K, Seshasayee D, Modrusan Z, and Clark HF (2009). Deconvolution of blood microarray data identifies cellular activation patterns in systemic lupus erythematosus. PLoS One 4, e6098. 10.1371/journal.pone.0006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zemmour D, Zilionis R, Kiner E, Klein AM, Mathis D, and Benoist C (2018). Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat Immunol 19, 291–301. 10.1038/s41590-018-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miragaia RJ, Gomes T, Chomka A, Jardine L, Riedel A, Hegazy AN, Whibley N, Tucci A, Chen X, Lindeman I, et al. (2019). Single-Cell Transcriptomics of Regulatory T Cells Reveals Trajectories of Tissue Adaptation. Immunity 50, 493–504 e497. 10.1016/j.immuni.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hori S, Haury M, Coutinho A, and Demengeot J (2002). Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci U S A 99, 8213–8218. 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tarbell KV, Yamazaki S, Olson K, Toy P, and Steinman RM (2004). CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 199, 1467–1477. 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, and Rudensky AY (2016). Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 45, 1122–1134. 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. (2013). Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339, 1219–1224. 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, et al. (2017). Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 169, 1342–1356 e1316. 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 79.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, et al. (2013). Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256. 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, and Smyth MJ (2011). CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 71, 2892–2900. 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 81.Shalev I, Liu H, Koscik C, Bartczak A, Javadi M, Wong KM, Maknojia A, He W, Liu MF, Diao J, et al. (2008). Targeted deletion of fgl2 leads to impaired regulatory T cell activity and development of autoimmune glomerulonephritis. J Immunol 180, 249–260. 10.4049/jimmunol.180.1.249. [DOI] [PubMed] [Google Scholar]
- 82.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, and Campbell DJ (2009). The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 10, 595–602. 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Condino-Neto A, and Newburger PE (2000). Interferon-gamma improves splicing efficiency of CYBB gene transcripts in an interferon-responsive variant of chronic granulomatous disease due to a splice site consensus region mutation. Blood 95, 3548–3554. [PubMed] [Google Scholar]
- 84.You M, Yu DH, and Feng GS (1999). Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol 19, 2416–2424. 10.1128/MCB.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perkey E, Maurice De Sousa D, Carrington L, Chung J, Dils A, Granadier D, Koch U, Radtke F, Ludewig B, Blazar BR, et al. (2020). GCNT1-Mediated O-Glycosylation of the Sialomucin CD43 Is a Sensitive Indicator of Notch Signaling in Activated T Cells. J Immunol 204, 1674–1688. 10.4049/jimmunol.1901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, and Kroczek RA (1999). ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266. 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 87.Kakugawa K, Kojo S, Tanaka H, Seo W, Endo TA, Kitagawa Y, Muroi S, Tenno M, Yasmin N, Kohwi Y, et al. (2017). Essential Roles of SATB1 in Specifying T Lymphocyte Subsets. Cell Rep 19, 1176–1188. 10.1016/j.celrep.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 88.Kashiwakura Y, Sakurai D, Kanno Y, Hashiguchi M, Kobayashi A, Kurosu A, Tokudome S, Kobata T, and Kojima H (2013). CD2-mediated regulation of peripheral CD4(+) CD25(+) regulatory T-cell apoptosis accompanied by down-regulation of Bim. Immunology 139, 48–60. 10.1111/imm.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li A, Herbst RH, Canner D, Schenkel JM, Smith OC, Kim JY, Hillman M, Bhutkar A, Cuoco MS, Rappazzo CG, et al. (2019). IL-33 Signaling Alters Regulatory T Cell Diversity in Support of Tumor Development. Cell Rep 29, 2998–3008 e2998. 10.1016/j.celrep.2019.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halim L, Romano M, McGregor R, Correa I, Pavlidis P, Grageda N, Hoong SJ, Yuksel M, Jassem W, Hannen RF, et al. (2017). An Atlas of Human Regulatory T Helper-like Cells Reveals Features of Th2-like Tregs that Support a Tumorigenic Environment. Cell Rep 20, 757–770. 10.1016/j.celrep.2017.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, and Aguet M (1993). Immune response in mice that lack the interferon-gamma receptor. Science 259, 1742–1745. 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 92.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, and Aguet M (1994). Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921. 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 93.Bradley KC, Finsterbusch K, Schnepf D, Crotta S, Llorian M, Davidson S, Fuchs SY, Staeheli P, and Wack A (2019). Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection. Cell Rep 28, 245–256 e244. 10.1016/j.celrep.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 94.Maurice NJ, McElrath MJ, Andersen-Nissen E, Frahm N, and Prlic M (2019). CXCR3 enables recruitment and site-specific bystander activation of memory CD8(+) T cells. Nat Commun 10, 4987. 10.1038/s41467-019-12980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bangs DJ, Tsitsiklis A, Steier Z, Chan SW, Kaminski J, Streets A, Yosef N, and Robey EA (2022). CXCR3 regulates stem and proliferative CD8+ T cells during chronic infection by promoting interactions with DCs in splenic bridging channels. Cell Rep 38, 110266. 10.1016/j.celrep.2021.110266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, and Luster AD (2012). CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 37, 1091–1103. 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rapp M, Wintergerst MWM, Kunz WG, Vetter VK, Knott MML, Lisowski D, Haubner S, Moder S, Thaler R, Eiber S, et al. (2019). CCL22 controls immunity by promoting regulatory T cell communication with dendritic cells in lymph nodes. J Exp Med 216, 1170–1181. 10.1084/jem.20170277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coghill JM, Fowler KA, West ML, Fulton LM, van Deventer H, McKinnon KP, Vincent BG, Lin K, Panoskaltsis-Mortari A, Cook DN, et al. (2013). CC chemokine receptor 8 potentiates donor Treg survival and is critical for the prevention of murine graft-versus-host disease. Blood 122, 825–836. 10.1182/blood-2012-06-435735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, and Kalinski P (2008). Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res 68, 5972–5978. 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Curtsinger JM, Agarwal P, Lins DC, and Mescher MF (2012). Autocrine IFN-gamma promotes naive CD8 T cell differentiation and synergizes with IFN-alpha to stimulate strong function. J Immunol 189, 659–668. 10.4049/jimmunol.1102727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tau GZ, Cowan SN, Weisburg J, Braunstein NS, and Rothman PB (2001). Regulation of IFN-gamma signaling is essential for the cytotoxic activity of CD8(+) T cells. J Immunol 167, 5574–5582. 10.4049/jimmunol.167.10.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhat P, Leggatt G, Waterhouse N, and Frazer IH (2017). Interferon-gamma derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis 8, e2836. 10.1038/cddis.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, et al. (2018). Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013 e1020. 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horton BL, Fessenden TB, and Spranger S (2019). Tissue Site and the Cancer Immunity Cycle. Trends Cancer 5, 593–603. 10.1016/j.trecan.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zagorulya M, Duong E, and Spranger S (2020). Impact of anatomic site on antigen-presenting cells in cancer. J Immunother Cancer 8. 10.1136/jitc-2020-001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan TG, Mathis D, and Benoist C (2016). Singular role for T-BET+CXCR3+ regulatory T cells in protection from autoimmune diabetes. Proc Natl Acad Sci U S A 113, 14103–14108. 10.1073/pnas.1616710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi J, Ziga ED, Ritchey J, Collins L, Prior JL, Cooper ML, Piwnica-Worms D, and DiPersio JF (2012). IFNgammaR signaling mediates alloreactive T-cell trafficking and GVHD. Blood 120, 4093–4103. 10.1182/blood-2012-01-403196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, Hoyos BE, Putintseva EV, Chaudhry A, Dikiy S, et al. (2017). Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 546, 421–425. 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, Daud A, and Bluestone JA (2020). Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol 5. 10.1126/sciimmunol.aba0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huffnagle GB, Dickson RP, and Lukacs NW (2017). The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol 10, 299–306. 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramirez-Labrada AG, Isla D, Artal A, Arias M, Rezusta A, Pardo J, and Galvez EM (2020). The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends Cancer 6, 86–97. 10.1016/j.trecan.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 112.Lee N, and Kim WU (2017). Microbiota in T-cell homeostasis and inflammatory diseases. Exp Mol Med 49, e340. 10.1038/emm.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ansaldo E, Farley TK, and Belkaid Y (2021). Control of Immunity by the Microbiota. Annu Rev Immunol 39, 449–479. 10.1146/annurev-immunol-093019-112348. [DOI] [PubMed] [Google Scholar]
- 114.Tastan C, Karhan E, Zhou W, Fleming E, Voigt AY, Yao X, Wang L, Horne M, Placek L, Kozhaya L, et al. (2018). Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol 11, 1591–1605. 10.1038/s41385-018-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ataide MA, Knopper K, Cruz de Casas P, Ugur M, Eickhoff S, Zou M, Shaikh H, Trivedi A, Grafen A, Yang T, et al. (2022). Lymphatic migration of unconventional T cells promotes site-specific immunity in distinct lymph nodes. Immunity. 10.1016/j.immuni.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 116.Sullivan MR, Danai LV, Lewis CA, Chan SH, Gui DY, Kunchok T, Dennstedt EA, Vander Heiden MG, and Muir A (2019). Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife 8. 10.7554/eLife.44235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, Wilhelm J, Li S, Song J, Li W, et al. (2022). Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun 13, 4981. 10.1038/s41467-022-32521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Best SA, Gubser PM, Sethumadhavan S, Kersbergen A, Negron Abril YL, Goldford J, Sellers K, Abeysekera W, Garnham AL, McDonald JA, et al. (2022). Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab 34, 874–887 e876. 10.1016/j.cmet.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Zhao S, Peralta RM, Avina-Ochoa N, Delgoffe GM, and Kaech SM (2021). Metabolic regulation of T cells in the tumor microenvironment by nutrient availability and diet. Semin Immunol 52, 101485. 10.1016/j.smim.2021.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng X, He Y, Huang J, Tao Y, and Liu S (2021). Metabolism of Dendritic Cells in Tumor Microenvironment: For Immunotherapy. Front Immunol 12, 613492. 10.3389/fimmu.2021.613492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, Menk AV, Rittenhouse NL, DePeaux K, Whetstone RD, et al. (2021). Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651. 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nussing S, Trapani JA, and Parish IA (2020). Revisiting T Cell Tolerance as a Checkpoint Target for Cancer Immunotherapy. Front Immunol 11, 589641. 10.3389/fimmu.2020.589641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wohn C, Le Guen V, Voluzan O, Fiore F, Henri S, and Malissen B (2020). Absence of MHC class II on cDC1 dendritic cells triggers fatal autoimmunity to a cross-presented self-antigen. Sci Immunol 5. 10.1126/sciimmunol.aba1896. [DOI] [PubMed] [Google Scholar]
- 124.Ruhland MK, Roberts EW, Cai E, Mujal AM, Marchuk K, Beppler C, Nam D, Serwas NK, Binnewies M, and Krummel MF (2020). Visualizing Synaptic Transfer of Tumor Antigens among Dendritic Cells. Cancer Cell 37, 786–799 e785. 10.1016/j.ccell.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Mingo Pulido A, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, Coussens LM, and Ruffell B (2018). TIM-3 Regulates CD103(+) Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 33, 60–74 e66. 10.1016/j.ccell.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, and Germain RN (2012). Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37, 364–376. 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Momin N, Mehta NK, Bennett NR, Ma L, Palmeri JR, Chinn MM, Lutz EA, Kang B, Irvine DJ, Spranger S, and Wittrup KD (2019). Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci Transl Med 11. 10.1126/scitranslmed.aaw2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu EF, Gai SA, Opel CF, Kwan BH, Surana R, Mihm MC, Kauke MJ, Moynihan KD, Angelini A, Williams RT, et al. (2015). Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell 27, 489–501. 10.1016/j.ccell.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hughes TK, Wadsworth MH 2nd, Gierahn TM, Do T, Weiss D, Andrade PR, Ma F, de Andrade Silva BJ, Shao S, Tsoi LC, et al. (2020). Second-Strand Synthesis-Based Massively Parallel scRNA-Seq Reveals Cellular States and Molecular Features of Human Inflammatory Skin Pathologies. Immunity 53, 878–894 e877. 10.1016/j.immuni.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gierahn TM, Wadsworth MH 2nd, Hughes TK, Bryson BD, Butler A, Satija R, Fortune S, Love JC, and Shalek AK (2017). Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat Methods 14, 395–398. 10.1038/nmeth.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stoeckius M, Zheng S, Houck-Loomis B, Hao S, Yeung BZ, Mauck WM 3rd, Smibert P, and Satija R (2018). Cell Hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol 19, 224. 10.1186/s13059-018-1603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roelli P, Flynn B, and Gui G (2019). Hoohm/CITE-seq-Count: 1.4.2. (Version 1.4.2). Zenodo 10.5281/zenodo.2590196. [DOI] [Google Scholar]
- 133.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, et al. (2015). Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214. 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hafemeister C, and Satija R (2019). Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 20, 296. 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Butler A, Hoffman P, Smibert P, Papalexi E, and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36, 411–420. 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, and Mesirov JP (2011). Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740. 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tu AA, Gierahn TM, Monian B, Morgan DM, Mehta NK, Ruiter B, Shreffler WG, Shalek AK, and Love JC (2019). TCR sequencing paired with massively parallel 3' RNA-seq reveals clonotypic T cell signatures. Nat Immunol 20, 1692–1699. 10.1038/s41590-019-0544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vander Heiden JA, Yaari G, Uduman M, Stern JN, O'Connor KC, Hafler DA, Vigneault F, and Kleinstein SH (2014). pRESTO: a toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics 30, 1930–1932. 10.1093/bioinformatics/btu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gupta NT, Vander Heiden JA, Uduman M, Gadala-Maria D, Yaari G, and Kleinstein SH (2015). Change-O: a toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics 31, 3356–3358. 10.1093/bioinformatics/btv359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith TS, Heger A, and Sudbery I (2017). UMI-tools: Modelling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Research 27, 491–499. 10.1101/gr.209601.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burger ML, Cruz AM, Crossland GE, Gaglia G, Ritch CC, Blatt SE, Bhutkar A, Canner D, Kienka T, Tavana SZ, et al. (2021). Antigen dominance hierarchies shape TCF1(+) progenitor CD8 T cell phenotypes in tumors. Cell 184, 4996–5014 e4926. 10.1016/j.cell.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, and Tamayo P (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425. 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Skinner J (2018). Statistics for Immunologists. Curr Protoc Immunol 122, 54. 10.1002/cpim.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S3. Public TCRβ sequences recovered from Treg cell TCR-seq.
Table S2. DEG markers for the four activated Treg cell clusters (0: early activation, 1: activated c1, 2: activated c2, 3: proliferating Treg cells) identified in the scRNA-seq dataset.
Table S1. DEG between activated CD8+ OT-I T cells from DC1:OT-I T cell and DC1:Treg:OT-I T cell ex vivo co-cultures.
Table S5. Flow cytometry and immunofluorescence antibodies.
Table S4. DEG between mLN and iLN Treg cells sorted from mice bearing KP-zSIIN tumors.
Data Availability Statement
The scRNA-seq data generated in this study has been deposited to the GEO database (GSE216086), and code used to process and analyze scRNA-seq data was made available at https://github.com/duncanmorgan/kptumors_immunity and is archived at https://doi.org/10.5281/zenodo.7331914. Code used for the spatial analysis of IF images was made available at https://github.com/awedwards/DC-Treg-CD8T-interactions-project. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


