Abstract
Several studies have characterized the upstream regulatory region of c-fos, and identified cis-acting elements termed the cyclic AMP (cAMP) response elements (CREs) that are critical for c-fos transcription in response to a variety of extracellular stimuli. Although several transcription factors can bind to CREs in vitro, the identity of the transcription factor(s) that activates the c-fos promoter via the CRE in vivo remains unclear. To help identify the trans-acting factors that regulate stimulus-dependent transcription of c-fos via the CREs, dominant-negative (D-N) inhibitor proteins that function by preventing DNA binding of B-ZIP proteins in a dimerization domain-dependent fashion were developed. A D-N inhibitor of CREB, termed A-CREB, was constructed by fusing a designed acidic amphipathic extension onto the N terminus of the CREB leucine zipper domain. The acidic extension of A-CREB interacts with the basic region of CREB forming a coiled-coil extension of the leucine zipper and thus prevents the basic region of wild-type CREB from binding to DNA. Other D-N inhibitors generated in a similar manner with the dimerization domains of Fos, Jun, C/EBP, ATF-2, or VBP did not block CREB DNA binding activity, nor did they inhibit transcriptional activation of a minimal promoter containing a single CRE in PC12 cells. A-CREB inhibited activation of CRE-mediated transcription evoked by three distinct stimuli: forskolin, which increases intracellular cAMP; membrane depolarization, which promotes Ca2+ influx; and nerve growth factor (NGF). A-CREB completely inhibited cAMP-mediated, but only partially inhibited Ca2+- and NGF-mediated, transcription of a reporter gene containing 750 bp of the native c-fos promoter. Moreover, glutamate induction of c-fos expression in primary cortical neurons was dependent on CREB. In contrast, induction of c-fos transcription by UV light was not inhibited by A-CREB. Lastly, A-CREB attenuated NGF induction of morphological differentiation in PC12 cells. These results suggest that CREB or its closely related family members are general mediators of stimulus-dependent transcription of c-fos and are required for at least some of the long-term actions of NGF.
Extracellular stimuli promote, at the transcriptional level, expression of a variety of immediate-early-response genes (IEGs). Many IEGs encode transcription factors which, in turn, influence the expression of secondary response genes (52). The products of secondary response genes contribute to the phenotypic response of the cell to extracellular stimuli. The prototypical IEG, c-fos (24), is activated by a variety of stimuli including activators of protein kinase C (8, 19), agents that increase intracellular cyclic AMP (cAMP) levels (3, 49), membrane depolarization or excitatory neurotransmitters, such as glutamate, that trigger an increase in intracellular levels of Ca2+ (1, 25, 38, 54), and peptide growth factors, such as nerve growth factor (NGF), that activate receptor tyrosine kinases (20, 23). In the upstream regulatory region of the c-fos gene, several cis-acting DNA sequences that mediate stimulus-dependent transcription of c-fos have been identified. These include at least three cAMP response elements (CREs) located 67, 293, and 343 nucleotides upstream of the transcriptional start site (3) and the serum response element (SRE) centered approximately 300 nucleotides upstream of the transcriptional start site (47, 59, 60). Transcription factors of the basic leucine zipper (B-ZIP) family such as CREB (CRE-binding protein) can bind to CRE-like elements (37). Likewise, the SRE can bind many factors (59); the best characterized is a ternary complex composed of a serum response factor (SRF) dimer and one p62TCF (ternary complex factor) molecule (reviewed in references 9 and 58). In transient transfection experiments, CREB, SRF, and p62TCF were found to be capable of mediating stimulus-dependent transcription of c-fos.
While the CREs within the promoters of c-fos and other IEGs are critical for stimulus-dependent transcription, it is unclear which trans-acting factors bind to these cis elements in vivo. The consensus CRE is 5′-TGAC:GTCA-3′, where the center of the dyad is marked by a colon. This DNA sequence may be bound by homodimer or heterodimer combinations of a variety of B-ZIP transcription factors including CREB homodimers (29), CREB-ATF heterodimers (37), and dimers consisting of other ATF family transcription factors (26). In addition, structurally related cis elements consisting of the same dyad half site (XXX:GTCA) exist. A TPA response element (TRE), or AP-1 site, is similar in sequence to the CRE with one of the central GC pairs of the CRE deleted, resulting in a pseudopalindromic site (consensus: TGA:GTCA). The TRE is thought to be bound by a B-ZIP heterodimer consisting of a Fos family member and a Jun family member (39).
Detailed experiments in vitro indicate that B-ZIP proteins are promiscuous in their DNA binding. For example, a Fra1-JunD heterodimer, a Jun–ATF-2 heterodimer, or a Jun–ATF-3 heterodimer can bind to a CRE better than to a TRE (27, 48). ATF-4 can heterodimerize with either Fos or Jun, and this complex preferentially binds to a CRE (28). A Jun–ATF-2 heterodimer has been reported to cooperate to form the enhanceosome on the human beta interferon gene (16, 57). Moreover, CREB can inhibit Jun-mediated transcription by competing for the same cis-acting elements (35), and c-Jun can repress gene expression by acting through a CRE (45). These results underscore the complexity of cis elements and the B-ZIP factors that bind to them.
We have developed dominant-negative (D-N) inhibitor proteins, termed A-ZIPs, which act by inhibiting the DNA binding of a B-ZIP protein in a dimerization-dependent fashion (33, 43). With these reagents in hand, we can establish in an intact cell or tissue the requirement for a particular B-ZIP transcription factor that acts via any cis element in response to extracellular stimuli to regulate gene expression. We have initiated the use of these A-ZIPs by examining the dimerization domains that are critical for mediating transcriptional activation, in response to several extracellular stimuli, of a minimal promoter with a single CRE and of the full-length native c-fos promoter. A-CREB, the D-N inhibitor of CREB used in this study, blocked transcription of a synthetic reporter gene containing a single CRE when activated by extracellular stimuli including forskolin, Ca2+ influx following membrane depolarization, and NGF. When the native c-fos promoter was examined, it was found that A-CREB completely inhibited cAMP-sensitive transcription but only partially inhibited Ca2+- and NGF-sensitive transcription. In contrast, A-CREB did not affect the induction of c-fos transcription by UV light. Moreover, A-CREB partially blocked NGF induction of morphological differentiation of PC12 cells. These results support the idea that CREB or its closely related family members play a general role in the regulation of IEG transcription in response to a wide variety of extracellular signals and that CREB-dependent gene expression contributes to the long-term actions of NGF.
MATERIALS AND METHODS
Proteins.
The amino acid sequence of A-CREB is as follows: DYKDDDDK MASMTGGQQMGRDPDLEQQLEELAQENEELEKEAEELEQELAE LENRVAVLENQNKTLIEELKALKDLYCHKSD. The first 8 amino acids encode the FLAG epitope tag, the next 13 amino acids are a φ10 protein sequence, the next 31 amino acids are the amphipathic acidic extension, and the final group of amino acids are the mouse CREB leucine zipper, which continues to the natural C terminus of the protein. The Leu residues of the leucine zipper domain are in boldface. The mouse CREB B-ZIP domain spans from Leu-274 to Asp-341, the natural C terminus. cDNAs encoding the different B-ZIP leucine zippers and a stop codon were generated by PCR and ligated to the C terminus of the acidic extension as XhoI-HindIII fragments in the prokaryotic expression vector pT5. The XhoI site is the Leu and Glu residues at the beginning of the leucine zipper. The recipient pT5 vector contained the φ10 sequence and the acidic extension. The entire open reading frame can be removed as an NdeI-HindIII fragment.
Protein purification.
Proteins for circular dichroism (CD) analysis and electrophoretic mobility shift assay (EMSA) were expressed in Escherichia coli. The proteins with DNA binding domains were purified over a heparin column and subsequently over a Rainin high-pressure liquid chromatography (HPLC) system, while the proteins without DNA binding domains were purified over a hydroxylapatite column and eluted with a buffer containing 200 mM phosphate, pH 7.4. The fractions enriched with the proteins were then purified on a Rainin HPLC system with a C18 column and eluted with a gradient consisting of 0 to 100% acetonitrile in 0.1% trifluoroacetic acid. Protein concentrations were calculated at 230 nm as described previously (33). The full-length CREB was expressed and purified from baculovirus (21).
CD.
All experiments were performed in 12.5 mM phosphate buffer (pH 7.4) containing 150 mM KCl, 0.25 mM EDTA, and 1 mM dithiothreitol. The concentration of each protein in the sample was 4 μM. Melting temperature (Tm) values were calculated as described before (32) and converted into values of Kd and ΔG at 25 and 37°C [Kd(25), ΔG(25), Kd(37), and ΔG(37)] by using a ΔCp of −1.25 kcal · mol−1 °C−1, which was calculated from a Tm-versus-ΔH plot of the five samples used in this study. Thermal denaturation was reversible in all cases.
DNA binding assay.
EMSA with recombinant proteins was performed as described previously (43). EMSA with nuclear extracts from HEK293-T cells was performed with a different reaction buffer (150 mM KCl, 12.5 mM potassium phosphate buffer [pH 7.4], 0.25 mM EDTA, 1 mg of bovine serum albumin per ml, 50 mg of dIdC per ml, 10 mM dithiothreitol, 2% glycerol). The sequences of the double-stranded DNA probes are as follows, with the DNA binding sites in boldface: CRE probe, 5-GTCAGTCAGTGAC:GTCAATCGGTCA-3; AP-1 probe, 5-GTCAGTCAGTGA:CTCAATCGGTCA-3; C/EBP probe, 5-GTCAGTCAGATTGC:GCAATATCGGTCAG-3. The α-ATF-1, α-CREM, and α-CREB antibodies for supershift experiments were obtained from Santa Cruz Biotechnology.
Eukaryotic plasmids.
The reporter constructs, which have been described previously, were pF4 and pAF42CRE (53), pAF42SRE (47), and pSVα-1 (51). The plasmid constructs for the generation of the riboprobes used in RNase protection assays, which have also been described previously, were pSP6-cfos (60) and pSP6α133 (10). pEGFP was obtained from Clontech.
A-ZIP coding sequences (A-CREB, A-C/EBP, A-Fos, A-Jun, A-ATF-2) were inserted as NdeI-HindIII fragments into a pRc/CMV vector (Invitrogen) which was modified to contain an N-terminal FLAG epitope (DYKDDDDK) and a new polylinker (pRc/CMV500). The NdeI-HindIII fragments were obtained from prokaryotic expression vector pT5, in which the A-ZIPs had been cloned previously (see Proteins). The A-C/EBP, A-VBP, A-Jun, and A-ATF-2 constructs contain an Asn in position a, while the A-CREB and A-Fos constructs contained a Leu in the same position.
Cell culture.
PC12 cells were grown on 100-mm-diameter tissue culture plates as described previously (6). Primary cultures of cortical neurons were prepared from cortical tissue of E19 rats as described previously (20).
Transient transfection.
Approximately 6 × 106 PC12 cells per 100-mm-diameter plate were used for transient transfection by a calcium phosphate precipitation technique (6). Twenty micrograms of reporter plasmid, the indicated amounts of A-CREB plasmid or an empty vector (pRc/CMV500), or 3 μg of acidic leucine zipper (A-ZIP) constructs and 4 μg of the internal-control α-globin expression vector (pSVα-1) were used. Primary rat cortical neurons were transfected by a modified calcium phosphate precipitation method as described previously (64). A total of 14 × 106 cortical neurons in 100-mm-diameter plates were transfected with 20 μg of reporter plasmid, 4 μg of pSVα-1, and 5 μg of A-CREB expression vector or empty vector. The expression of the transfected c-fos reporter gene was calculated as the ratio of the amount of protected 32P-labeled c-fos transcripts to that of α-globin transcripts as quantified by a PhosphorImager.
For morphological differentiation studies, 2 × 106 PC12 cells in 100-mm-diameter plates were transfected with 18 μg of Rous sarcoma virus lacZ and the indicated amounts of A-CREB plasmid. One day after the transfection, cells were stimulated with NGF (100 ng/ml) in low-serum-level media (Dulbecco modified Eagle medium plus 1% horse serum; Gibco-BRL). After an additional 3 days, cells were fixed with 2% formaldehyde–0.2% glutaraldehyde in phosphate-buffered saline for 5 min and then stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining solution overnight at room temperature. A differentiated cell was defined as one in which the length of processes was 1.5 times the cell body diameter. Morphological differentiation was calculated as the ratio of the differentiation of transfected cells to that of untransfected cells in the same plate.
Approximately 4.5 × 106 HEK293-T cells per 15-cm-diameter plate were transfected by the calcium phosphate precipitation method (4). Ten micrograms of pEGFP and 10 μg of either an empty vector or the A-CREB expression vector were used. Two days later, nuclear extracts were obtained as described previously (15).
RPA.
Two days after transfection, cells were either left unstimulated or were stimulated with NGF (100 or 200 ng/ml, 45 min), forskolin (10 μM, 1 h), KCl (50 mM, 45 min), glutamate (10 μM, 1 h), or UV light (400 J/m2, 45 min). Transfected cells were harvested, and RNA was isolated by the guanidinium isothiocyanate method (11). Thirty micrograms of RNA was used for the analysis by RNase protection assay (RPA) as described previously (51, 53). Quantification was done by PhosphorImager. Fold induction is the ratio of stimulated to unstimulated values which were calculated by dividing the signal of c-fosH by that of globin.
RESULTS
Design of A-CREB, a D-N inhibitor of CREB.
Previously, we developed D-N inhibitors that selectively inhibit the DNA binding activity of B-ZIP proteins C/EBP (33) and AP-1 (43) in equimolar competition assays. These D-N inhibitors, which we refer to as A-ZIPs, consist of an acidic amphipathic protein sequence that replaces the natural basic region fused to the N terminus of the dimerization domain (the leucine zipper). The rationale is that the acidic extension electrostatically mimics DNA and provides the B-ZIP basic region with an alternative interaction surface. We have demonstrated that the acidic amphipathic protein sequence forms a coiled-coil structure with the basic region of the endogenous B-ZIP protein and stabilizes the heterodimeric complex by 2.5 to 5.0 kcal/mol. This prevents the basic region of the endogenous B-ZIP protein from binding to DNA in a dimerization-dependent fashion.
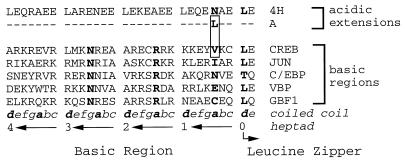
We used this strategy to construct a D-N inhibitor of CREB, termed A-CREB, by replacing the CREB basic region with an acidic amphipathic protein sequence. Figure 1 depicts the protein sequence of the CREB basic region, which is critical for sequence-specific DNA binding, and the designed amphipathic acidic residues that interact with the CREB basic region to form a coiled-coil structure. The basic regions of B-ZIP proteins are highly conserved (61), suggesting that the acidic extension may interact similarly with all B-ZIP basic regions (33). We have designed two acidic amphipathic extensions, differing by a single amino acid, that preferentially interact with different B-ZIP basic regions. The difference between these two acidic extensions is in the first a position N terminal of the first d position of the leucine zipper. The B-ZIP basic regions contain either a polar amino acid (e.g., C/EBP, VBP, and GBF1 contain Asn, Glu, and Cys, respectively) or a hydrophobic amino acid (Jun and CREB contain Ile and Val, respectively) at position a (Fig. 1). We have shown that an Asn in position a of the acidic extension can interact well with polar amino acids in the basic region (33). This type of interaction between polar amino acids is seen in the GCN4 leucine zipper, which contains an Asn in position a (44). To produce an interaction with the hydrophobic amino acids in positions a in CREB and Jun, we have placed a Leu in position a to create an acidic extension that will interact with B-ZIP basic regions containing hydrophobic amino acids in the corresponding a positions (Fig. 1).
FIG. 1.
Structure of A-CREB. Protein sequence of the acidic amphipathic extension that was fused with the CREB leucine zipper domain to produce A-CREB. The upper panel depicts the amino acid sequence of the acidic amphipathic extension (4H) and the single amino acid change in position a (N to L) needed to create the potent new acidic extension (A) that interacts with the CREB basic region. The lower panel shows the amino acid sequences of basic regions of CREB, Jun, C/EBP, VBP, and GBF1. The box encloses the potential interacting residues: Leu of the new acidic extension (A) and Val of the CREB basic region. The first Leu of the zipper, the invariant Asn and Arg of the basic region, and the position a amino acid which is critical for the efficacy of the new acidic extension, are in boldface. The coiled-coil nomenclature of the basic region extending from the leucine zipper is indicated below, with hydrophobic positions a and d in boldface. The numbering of the heptads in the acidic extension is indicated.
A-CREB heterodimerizes selectively with CREB.
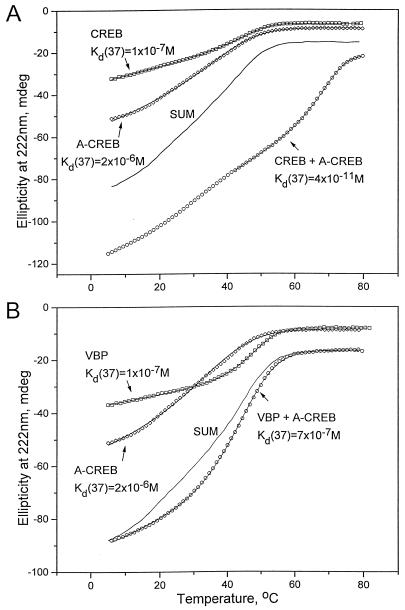
The α-helical content and thermal stability of the CREB B-ZIP domain and A-CREB, either alone or together, were measured by CD spectroscopy (Fig. 2A, Table 1) in which the amplitude of ellipticity at 222 nm is an indicator of α-helical structure. The CREB B-ZIP domain clearly showed a two-state thermal denaturation; it was more α-helical at low temperatures and had a Tm of 47°C, representing a Kd(37) of 1.3 × 10−7 M. Although A-CREB had a 50% greater ellipticity at 6°C, suggesting that the acidic extension was α-helical, A-CREB was less stable, as indicated by a Tm of 40°C or a Kd(37) of 2.3 × 10−6 M. The mixture of CREB and A-CREB also showed a two-state thermal transition and was more stable than a CREB homodimer, as shown by a Tm of 70°C or a Kd(37) of 3.9 × 10−11 M (Fig. 2A). This demonstrates that CREB and A-CREB formed a very stable heterodimer that was 3,300-fold more stable than a CREB B-ZIP homodimer. The mixture of CREB and 4H-CREB, which contains an Asn instead of Leu in position a (Fig. 1), is 4.5 kcal/mol less stable than the CREB–A-CREB mixture, demonstrating the importance of the Leu in the acidic extension for the heterodimerization with CREB. The mixture of CREB and A-CREB had more ellipticity at 6°C than was expected from the simple sum of the two homodimer samples, indicating that additional α-helical structure was formed in the heterodimer. We interpret this as a continuation of the “zippering” of the leucine zipper into the basic region to form a coiled-coil structure as represented in Fig. 2C.
FIG. 2.
Thermal stability of A-CREB mixed with CREB or VBP. (A) CD thermal denaturation curves recorded at 222 nm for CREB (squares), A-CREB (diamonds), and a mixture of CREB and A-CREB (circles). The solid line labeled SUM is expected if CREB and A-CREB do not interact. The line through each data set is a fitted curve that was used to calculate Tm as described previously (33), and the calculated Kd(37) is shown. (B) CD thermal denaturation curves at 222 nm of VBP (squares), A-CREB (diamonds), and a mixture of VBP and A-CREB (circles). The solid line labeled SUM is expected if VBP and A-CREB do not interact. (C) A schematic representation of an A-CREB–CREB dimer. The left panel shows a CREB homodimer with unhelical basic regions. The right panel shows a heterodimer of CREB and A-CREB resulting in an α-helical formation of the basic region.
TABLE 1.
Thermal stabilities of the CREB B-ZIP domain, two D-N inhibitors, and mixtures of CREB and D-N inhibitorsa
| Protein | Tm (°C) | ΔH (kcal/mol) | ΔG(37) (kcal/mol) | Kd(37) (M) | ΔG(25) (kcal/mol) | Kd(25) (M) |
|---|---|---|---|---|---|---|
| CREB | 47.2 | −73 | −9.8 | 1.3 × 10−7 | −11.5 | 4.1 × 10−9 |
| A-CREB | 40.0 | −39 | −8.1 | 2.3 × 10−6 | −8.8 | 3.7 × 10−7 |
| CREB + A-CREB | 69.9 | −96 | −14.8 | 3.9 × 10−11 | −16.1 | 1.8 × 10−12 |
| 4H-CREB | 22.1 | −37 | −5.4 | 1.8 × 10−4 | −7.0 | 7.6 × 10−6 |
| CREB + 4H-CREB | 53.9 | −61 | −10.3 | 5.9 × 10−8 | 11.2 | 7.1 × 10−9 |
The table presents the values for the indicated variables for CD thermal denaturation of proteins either as homodimers or as an equimolar mixture of two proteins. A higher Tm for the mixture compared to that for either homodimer alone indicates the formation of heterodimer. The root mean square errors in ΔG are 0.2 and 0.5 kcal/mol for homo- and heterodimers, respectively.
Next, the interaction between A-CREB and other B-ZIP domains was examined to address the specificity of the interaction between CREB and A-CREB. The B-ZIP domains of Vitellogenin binding protein (VBP) (31), the chicken homolog of mammalian TEF (18), and A-CREB were thermally denatured, either alone or together (Fig. 2B). VBP had a Tm of 50°C, and the mixture of VBP and A-CREB did not show an increase in Tm. The observed ellipticity value of the mixture at low temperatures was similar to the expected value assuming they do not interact, which indicates that a new α-helical structure was not produced. VBP and A-CREB presumably do not interact, which suggests that the acidic amphipathic extension is able to interact with the basic region and increase thermal stability only if the leucine zippers themselves can physically interact.
A-CREB inhibits CREB DNA binding activity.
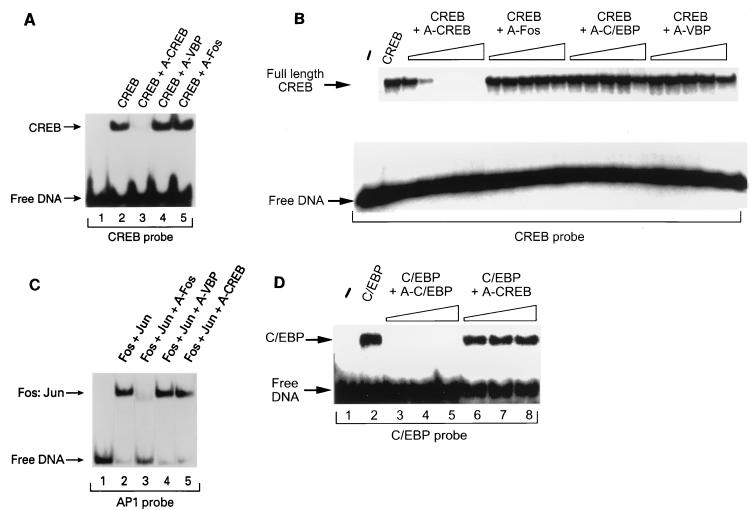
EMSAs were performed to determine whether A-CREB could inhibit the ability of the CREB B-ZIP domain to bind to its cognate DNA binding motif, the CRE. A CREB B-ZIP homodimer retarded the mobility of the radiolabeled double-stranded 25-bp oligonucleotide probe containing a single consensus CRE (Fig. 3A, lane 2), and an equimolar concentration of A-CREB (lane 3) effectively inhibited binding of the CREB B-ZIP domain to the CRE-containing oligonucleotide. In contrast, neither A-VBP (lane 4) nor A-Fos (lane 5), which contained leucine zipper domains derived from VBP or Fos, respectively, was able to modulate the DNA binding activity of CREB. These results demonstrate that A-CREB, but not A-VBP or A-Fos, prevents CREB from binding to the CRE.
FIG. 3.
A-CREB inhibits DNA binding activity of CREB. The inhibition of CREB DNA binding activity is leucine zipper specific. (A) A-CREB, but not A-VBP or A-Fos, inhibits the DNA binding activity of the DNA binding domain of CREB. An EMSA was performed with a 25-bp double-stranded DNA oligonucleotide containing a consensus CRE and the DNA binding domain of CREB (5 × 10−6 M; lane 2). The binding reaction mixture contained 1.0 molar equivalent of A-CREB (lane 3), A-VBP (lane 4), or A-Fos (lane 5). (B) A-CREB, but not A-Fos, A-C/EBP, or A-VBP, inhibits the DNA binding activity of full-length CREB. Increasing concentrations (10−8 M, 3 × 10−7 M, 10−7 M, 3 × 10−6 M, and 10−6 M) of A-CREB, A-Fos, A-C/EBP, or A-VBP were added to the reaction mixture containing CREB (2 × 10−7 M), and an EMSA was performed as for panel A. (C) A-CREB does not inhibit a Fos-Jun complex from binding to the AP-1 site. An EMSA was performed with a probe containing an AP-1 site (lane 1). A Fos-Jun complex (5 × 10−7 M) binds DNA (lane 2), and 1 molar equivalent of A-Fos completely inhibits Fos-Jun DNA binding (lane 3). Neither A-VBP (lane 4) nor A-CREB (lane 5) inhibits AP-1 DNA binding activity. (D) A-CREB does not inhibit DNA binding activity of C/EBP. An EMSA was performed with a probe containing a C/EBP binding site (lane 1). C/EBP (10−8 M) was challenged with A-C/EBP or A-CREB at 1, 3, and 10 molar excesses. A-C/EBP completely inhibits C/EBP binding (lanes 3 to 5), while A-CREB has no effect on C/EBP DNA binding activity (lanes 6 to 8).
The ability of A-CREB to specifically inhibit DNA binding of full-length CREB was also examined (Fig. 3B). The full-length CREB protein of 342 amino acids was produced in baculovirus and showed CRE-specific DNA binding. Five different concentrations of A-CREB, at half-log-unit concentration intervals, were used to compete the binding of CREB (2 × 10−7 M) to the CRE probe. A-CREB, at a concentration of 10−7 M (lane 6), effectively blocked CREB from binding to the CRE. The ability of A-CREB to completely inhibit CREB DNA binding at a molar ratio of 1:2 suggests that only 50% of the baculovirus-expressed CREB protein was able to bind DNA. A-Fos, A-C/EBP, and A-VBP proteins, which did inhibit the DNA binding of their respective B-ZIP dimerization partners, did not inhibit CREB DNA binding even at a 30-fold molar excess (Fig. 3D and data not shown).
We further addressed the specificity of A-CREB inhibition of CREB DNA binding activity by determining whether A-CREB could nonspecifically inhibit DNA binding of the AP-1 complex, which is a heterodimer of Fos and Jun (Fig. 3C). For these experiments, a probe containing a single AP-1 binding site was used (lane 1) and the migration of this probe was retarded by the Fos-Jun complex (lane 2). A-Fos completely inhibited DNA binding of the Fos-Jun complex in an equimolar challenge (lane 3). However, neither A-VBP (lane 4) nor A-CREB (lane 5) had an effect on the DNA binding activity of the Fos-Jun complex. The ability of A-CREB to inhibit C/EBP DNA binding was also examined. A-C/EBP was able to block the B-ZIP domain of C/EBP from binding to its cognate DNA binding element (Fig. 3D, lanes 3 to 5), while A-CREB did not inhibit the DNA binding activity of C/EBP (lanes 6 to 8). These results suggest that A-CREB selectively inhibited the DNA binding activity of B-ZIP proteins containing the CREB leucine zipper domain. Together, these data demonstrate a remarkable degree of specificity of the A-ZIP D-N molecules via their respective leucine zipper domains.
A-CREB inhibits the DNA binding activity of CREB in extracts of transfected cells.
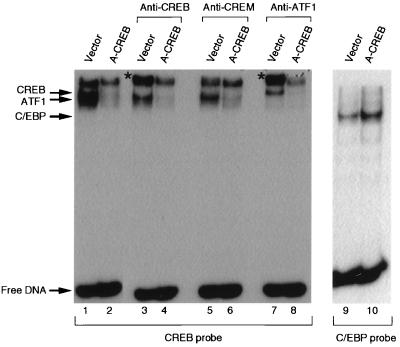
To further demonstrate that A-CREB inhibits the DNA binding activity of nuclear CREB in intact cells, nuclear extracts prepared from HEK293-T cells that were transfected with either an empty vector or an A-CREB expression vector were used for EMSA (Fig. 4). Cotransfection of HEK293-T cells with pEGFP allowed us to estimate a transfection efficiency of 60% by fluorescence microscopy. When the probe containing a consensus CRE was used for EMSA, three bands were observed with the nuclear extracts from the empty-vector-transfected cells (lane 1), while only the upper band was observed with nuclear extracts from A-CREB-expressing cells (lane 2). The disappearance of the two lower bands indicates that A-CREB heterodimerized with CREB-like leucine zipper proteins in the nuclear extracts and prevented them from binding to the CRE probe.
FIG. 4.
A-CREB inhibits CREB DNA binding activity in extracts of transfected cells. CREB DNA binding activity is inhibited in nuclear extracts prepared from HEK293-T cells that were transfected with an expression vector encoding A-CREB (lane 2) or the control vector (lane 1). For supershift analysis, antibodies against CREB (lanes 3 and 4), CREM (lanes 5 and 6), and ATF-1 (lanes 7 and 8) were added to the DNA binding reaction mixtures. A-CREB inhibited CREB and ATF-1 binding activity; the supershifted products are indicated by a star. C/EBP DNA binding activity is not inhibited in nuclear extracts from cells transfected with A-CREB (lanes 9 and 10).
The identities of protein-DNA complexes in the two lower bands were determined by supershift experiments using antibodies against CREB, ATF-1, and CREM. The middle band was supershifted by the α-CREB antibody (lane 3), while the lower band was supershifted by the α-ATF-1 antibody (lane 7). An antibody against CREM was without effect, suggesting that CREM was not a component of any of the CRE-protein complexes (lane 5). The identity of the protein(s) that resulted in the upper band was not determined, and the protein is thought to be a factor without a CREB-like leucine zipper domain. In the presence of A-CREB, no supershift was observed with any of the antibodies (lanes 4, 6, and 8), indicating that CREB-like proteins did not bind to CRE-containing probes in extracts of A-CREB-transfected cells. In contrast, C/EBP DNA binding activities present in cell extracts prepared from vector- and A-CREB-transfected cells were the same (lanes 9 and 10).
A-CREB inhibits cAMP-dependent, CRE-mediated transcrip- tion.
Since CREB and A-CREB heterodimerize with an affinity 3.3 orders of magnitude greater than that of CREB homodimers (Fig. 2A) and since A-CREB effectively and selectively inhibits CREB DNA binding activity in vitro (Fig. 3) and in vivo (Fig. 4), we next asked whether A-CREB can potently and specifically block CRE-mediated transcription when expressed in intact cells. For these experiments, the ability of A-CREB to influence the expression of a transiently transfected reporter gene, termed pAF42CRE, which contains a single consensus CRE upstream of the transcribed region of the human c-fos gene, was determined (Fig. 5A). The pAF42CRE reporter gene was cotransfected into pheochromocytoma PC12 cells along with expression vectors encoding A-CREB and α-globin (pSVα-1). The expression of α-globin served as an internal control of transfection efficiency since it is constitutively transcribed regardless of PC12 cell treatment (Fig. 5). Transcription of the reporter genes was assessed by an RPA. The protected RNA product of the pAF42CRE reporter gene, c-fosH (296 nucleotides), is larger than the protected RNA product of the endogenous rat c-fos gene (65 nucleotides), which allowed us to distinguish between them when they were resolved on denaturing gels. The presence of endogenous c-fosR transcripts served as a positive indication that the cells responded to the stimuli examined. Any effect of A-CREB on expression of endogenous c-fosR was not detectable because the transfection efficiency of PC12 cells is less than 5% by our protocol.
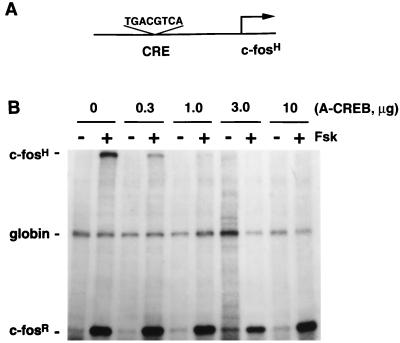
FIG. 5.
A-CREB blocks cAMP-induced gene expression in a dose-dependent manner. (A) A schematic representation of the pAF42CRE reporter gene construct used in these experiments. (B) PC12 cells were transfected with the reporter plasmid pAF42CRE, an α-globin internal control expression vector (pSVα-1), and indicated amounts of A-CREB expression vector as described in Materials and Methods. The total DNA concentration was kept constant by including empty expression vector DNA. After 48 h of transfection, cells were stimulated with forskolin (Fsk) (10 μM, 1 h), and RNA was analyzed by an RPA. c-fosH, protected RNA fragment from the transfected human c-fos reporter gene; globin, fragment protected from the α-globin internal control plasmid; c-fosR, fragment protected by the endogenous rat c-fos mRNA.
cAMP-dependent transcription of pAF42CRE induced by forskolin was blocked by A-CREB in a dose-dependent manner; transfection of 3 μg of A-CREB plasmid per 6 × 106 cells completely inhibited cAMP induction of c-fosH transcription (Fig. 5). This result demonstrates that A-CREB potently blocks CRE-mediated gene transcription. Three micrograms of A-CREB plasmid was used for all subsequent transfection experiments.
A-CREB inhibition of CRE-mediated transcription is leucine zipper dependent.
In vitro, A-CREB abolished the DNA binding activity of CREB, but not that of other B-ZIP proteins (Fig. 3). In order to establish the specificity of A-CREB for inhibition of CREB DNA binding and transactivation in intact cells, the ability of other acidic leucine zippers (A-ZIPs) to influence CRE-mediated transcription was assessed (Fig. 6). The A-ZIP proteins have leucine zipper dimerization domains derived from different B-ZIP proteins (C/EBP, Fos, Jun, ATF-2, and VBP). Three-microgram amounts of the eukaryotic expression vectors encoding different A-ZIPs were transfected into PC12 cells, and cAMP-sensitive transcription of pAF42CRE was assessed by RPA. As before, A-CREB completely blocked cAMP-sensitive expression of the reporter gene. In contrast, A-C/EBP, A-Fos, A-Jun, A-ATF-2, and A-VBP had no inhibitory effect. Yet these inhibitors were expressed (data not shown), and these A-ZIPs blocked the DNA binding activities of the partners of their respective B-ZIP domains (Fig. 3 and data not shown) (43). These results demonstrate that A-CREB inhibition of cAMP-sensitive gene expression was via the inhibition of the DNA binding activity of CREB and not via the inhibition of any dimerization partner of the other tested B-ZIP proteins.
FIG. 6.
A-CREB, but not other acidic leucine-zippers (A-ZIPs), inhibits cAMP activation of CRE-mediated gene expression. Transcriptional activation of pAF42CRE was evoked by forskolin. For this experiment, PC12 cells were transfected with pAF42CRE, α-globin expression vector, and expression vectors encoding the indicated A-ZIP proteins (3 μg each). Two days later, cells were treated with forskolin (Fsk; 10 μM, 1 h) or were left untreated and an RPA was performed. This experiment was performed two times with similar results.
To examine the cis element dependence of A-CREB inhibition of CRE-mediated transcription, we employed an alternative reporter gene in which the single CRE was replaced with a single copy of the SRE (pAF42SRE) (Fig. 7). Previous studies have shown that the SRE can mediate growth factor induction of c-fos transcription (47, 60). However, A-CREB did not block NGF induction of transcription of pAF42SRE (Fig. 6). Taken together, these results demonstrate that A-CREB is a specific inhibitor of CREB-mediated gene transcription.
FIG. 7.
A-CREB does not inhibit SRE-mediated gene expression. (A) A schematic representation of the pAF42SRE construct used in this experiment. (B) PC12 cells were transfected with the pAF42SRE reporter gene, an α-globin expression vector, and an empty vector or the A-CREB expression vector. Two days later, cells were treated with NGF (100 ng/ml, 45 min) or were left untreated and an RPA was performed. This experiment was performed three times with similar results.
CREB mediates CRE-dependent transcriptional activation of c-fos in response to three distinct extracellular stimuli.
After it was established that A-CREB specifically and potently inhibits CREB function, the potential role of CREB in mediating the expression of pAF42CRE evoked by multiple distinct extracellular stimuli was analyzed. For these experiments, PC12 cells were cotransfected with pAF42CRE, pSVα-1, and an expression vector encoding A-CREB. Subsequently, cells were exposed to NGF, forskolin, or KCl, and the expression of the pAF42CRE reporter gene was assessed by RPA (Fig. 8). As seen previously, forskolin and KCl, which increase intracellular levels of cAMP and Ca2+, respectively, were effective in triggering expression of pAF42CRE (lanes 3 and 4), while NGF only weakly induced expression of pAF42CRE (lane 2) (6). Nevertheless, A-CREB completely blocked expression of the reporter gene in response to all three stimuli (lanes 6 to 8). While these results implicate CREB in CRE-mediated transcription, they were obtained with a reporter gene containing a perfect consensus CRE (TGAC:GTCA), whereas the CREs within the upstream regulatory region of c-fos are imperfect, nonpalindromic CRE-like elements. Identical results were obtained with a reporter gene that contained the −67 human c-fos CRE (TGAC:GTTT) placed in front of a c-fos minimal promoter (data not shown). These results demonstrate that CREB is essential for CRE-mediated transcription of c-fos in PC12 cells.
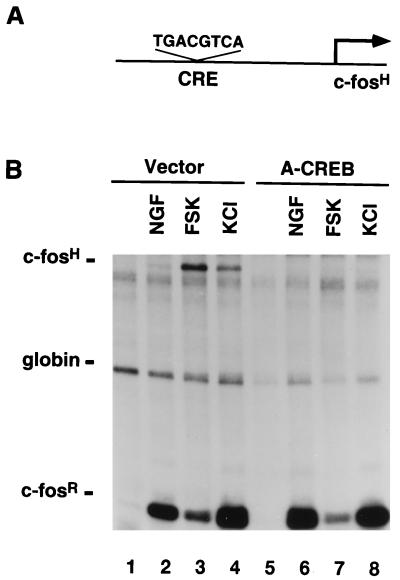
FIG. 8.
CREB is critical for cAMP, Ca2+, and NGF induction of CRE-mediated gene expression. (A) A schematic representation of the pAF42CRE reporter construct. (B) Transcriptional activation of pAF42CRE by NGF, cAMP, or Ca2+. PC12 cells were transfected with the pAF42CRE reporter gene, an α-globin expression vector, and either an empty vector or the A-CREB expression vector (3 μg). Two days later, cells were either left unstimulated (lanes 1 and 5) or were stimulated with NGF (100 ng/ml, 45 min; lanes 2 and 6) or forskolin (FSK) (10 μM, 1 h; lanes 3 and 7) or were subjected to membrane depolarization with KCl (50 mM, 1 h; lanes 4 and 8) and an RPA was performed. A-CREB blocked NGF, forskolin, and KCl induction of pAF42CRE by 95, 100, and 98%, respectively. The quantification was done as described in Materials and Methods. This experiment was performed two times with similar results.
CREB is critical for c-fos expression in response to many, but not all, extracellular stimuli.
While CREB is critical for stimulus-dependent transcription via the CRE, the full upstream regulatory region of the c-fos gene contains binding sites for CREB-ATFs, C/EBP, AP-1, SRF, p62TCF, STATs, and other stimulus-sensitive transcription factors (reviewed in reference 17). Therefore, we sought to determine the contribution of CREB to stimulus-dependent transcription of the intact, full-length c-fos gene. For these experiments, we employed a reporter gene, pF4, which contains 750 bp of the human c-fos promoter with all cis elements known to be important for inducible transcription of c-fos (Fig. 9A). A-CREB completely inhibited cAMP-sensitive expression of pF4 (Fig. 9B, lane 8). However, CREB is only partly responsible for Ca2+ and NGF induction of c-fos in PC12 cells. Upon its expression in PC12 cells, A-CREB inhibited Ca2+ and NGF induction of transcription of pF4 by 50 to 75% (lanes 7 and 9, respectively). In contrast, c-fos expression induced by UV light, which acts via the stress pathway through stress-induced mitogen-activated protein kinases (SAPK/JNKs and p38MAPK) and involves SRE-p62TCF-dependent transcription (46), was not inhibited by A-CREB (lane 10).
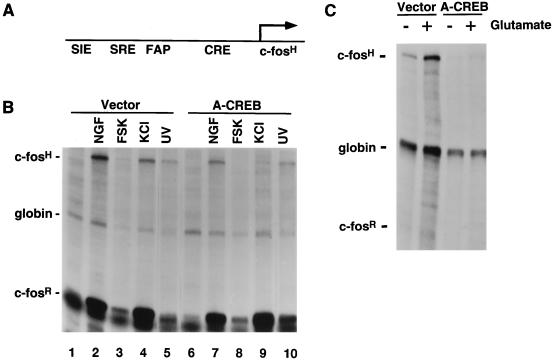
FIG. 9.
A-CREB inhibits NGF, cAMP, and Ca2+ induction of c-fos expression but not induction by UV light. (A) A schematic representation of the pF4 reporter construct. (B) Transcriptional activation of pF4 by various stimuli. PC12 cells were transfected with reporter gene pF4, an α-globin expression vector, and either an empty vector or the A-CREB expression vector. After 2 days, cells were either left unstimulated (lanes 1 and 6) or were stimulated with NGF (100 ng/ml, 45 min; lanes 2 and 7), forskolin (FSK) (10 μM, 1 h; lanes 3 and 8), KCl (50 mM, 1 h; lanes 4 and 9), or UV light (400 J/m2; lanes 5 and 10) and an RPA was performed. (C) Primary E19 rat cortical neurons were transfected as for panel B. Two days later, cells were stimulated with glutamate (10 μM, 1 h) and an RPA was performed. A-CREB blocked NGF, forskolin, KCl, and UV light induction of pF4 by 64, 99, 84, and 2%, respectively. The quantification was done as described in Materials and Methods. These experiments were performed three times with similar results.
Another extracellular stimulus, glutamate, induces Ca2+-dependent activation of c-fos expression in hippocampal and cortical neurons (1). Dissociated cultures of E19 rat cortical neurons were transfected with the pF4 reporter gene, the A-CREB expression vector, and pSVα-1. A-CREB, but not the control vector, completely blocked glutamate-induced c-fos transcription (Fig. 9C). These results demonstrate that CREB is critical for the activation of transcription of c-fos in response to many, but not all, stimuli and that the contribution of CREB to c-fos transcription depends on the nature of the extracellular stimulus.
Several studies have suggested that additional B-ZIP family members, including C/EBP (36, 50), may contribute to c-fos expression. Therefore, we next sought to determine the involvement of other B-ZIPs by transiently transfecting different A-ZIP expression vectors into PC12 cells and subsequently assessing the expression of the pF4 reporter gene after NGF treatment (Fig. 10). As seen previously, A-CREB attenuated NGF induction of the pF4 reporter gene. In contrast, A-C/EBP, A-Fos, A-Jun, A-ATF-2, and A-VBP had no effect on NGF-sensitive c-fos expression. Therefore, CREB is the only B-ZIP transcription factor thus far tested that contributes to NGF induction of c-fos expression.
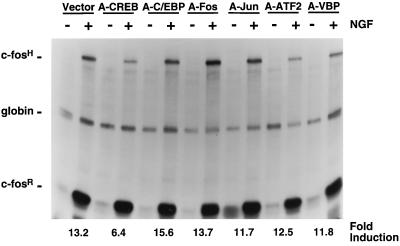
FIG. 10.
A-CREB, but not other A-ZIPs, attenuates NGF induction of c-fos expression. For this experiment, PC12 cells were transfected with the pF4 reporter gene, α-globin, and either an empty vector or expression vectors encoding A-CREB, A-C/EBP, A-Fos, A-Jun, A-ATF-2, or A-VBP. After 2 days, cells were either left untreated or were treated with NGF (200 ng/ml, 45 min) and an RPA was performed. The quantification was done as described in Materials and Methods. This experiment was performed two times with similar results.
CREB is required for NGF induction of the morphological differentiation of PC12 cells.
Since CREB contributes to NGF-induced transcription of c-fos and since many other NGF-induced genes contain putative CREB binding sites, we investigated the role of CREB in NGF induction of the morphological differentiation of PC12 cells (Fig. 11). For these experiments, PC12 cells were cotransfected with a β-galactosidase-containing reporter gene and either a control expression vector or an expression vector encoding A-CREB. Cells were subsequently grown in the presence or absence of NGF. Three days after NGF treatment, cells were fixed and stained for β-galactosidase activity to identify transfected cells, and cells with processes greater than 1.5 times the cell body diameter were scored as differentiated cells. The extent of morphological differentiation of untransfected cells from the same plate served as an internal control. Results shown in Fig. 11A demonstrate that A-CREB, but not an empty vector, attenuated NGF-mediated morphological differentiation of PC12 cells. Moreover, this effect was dose dependent (Fig. 11B). In fact, the 50% inhibitory concentrations for A-CREB inhibition of differentiation and for inhibition of CRE-mediated gene transcription were identical (approximately 0.2 μg of A-CREB expression vector), suggesting that A-CREB blocked differentiation via inhibition of CREB-dependent gene transcription and not via some other nonspecific effect. Therefore, in addition to its role in NGF induction of c-fos expression, CREB contributes to NGF induction of the morphological differentiation of PC12 cells.
FIG. 11.
CREB contributes to NGF induction of morphological differentiation in PC12 cells. (A) PC12 cells were transfected with a β-galactosidase (β-Gal) plasmid and either an empty expression vector or the A-CREB expression vector. Then, cells were stimulated with NGF (100 ng/ml). After 3 days, cells were fixed and stained for β-Gal activity and the morphological differentiation of transfected cells was calculated as described in the Materials and Methods (the results shown are means ± the standard errors of the means; n = 8). (B) A-CREB inhibition of morphological differentiation is dose dependent, and the 50% inhibitory concentration is equivalent to that seen for A-CREB inhibition of CRE-mediated transcription (quantification of the results shown in Fig. 5B).
DISCUSSION
In many cases, the identities of the DNA sequences that are critical for the function of a promoter have been determined by a combination of cis element mutagenesis and the expression of D-N inhibitors of transcription factors that bind to these cis elements. Many D-N inhibitors are created by a simple deletion or point mutation of the transactivation domain; they function by binding to a cis element, thereby occluding the binding of endogenous trans-acting factors. This approach is valuable for the identification of a particular cis element that is critical for gene expression. However, this combination of methodologies reveals nothing about the oligomerization properties of the actual transcription factors that mediate transactivation events in vivo. The fact that multiple proteins can bind to identical cis elements makes it difficult to identify the protein(s) which mediates stimulus-dependent transcription in vivo by these approaches. We have developed and characterized D-N inhibitors that act via a novel mechanism; they inhibit the activity of a transcription factor, not by occluding the DNA cis element itself, but by heterodimerizing with endogenous transcription factors, preventing them from binding to their cognate DNA elements. These D-N inhibitors allow us to identify the B-ZIP transcription factors that are critical for influencing transcription via a particular cis element.
In the present study, we have shown that A-CREB, a novel D-N inhibitor of the B-ZIP family member CREB, potently and selectively inhibits DNA binding of CREB and CREB-mediated gene transcription. Using this reagent, we show that CREB or its closely related family members are critical for cAMP, Ca2+, and NGF induction of transcription of a synthetic reporter gene containing a single consensus canonical CRE (5′-TGAC:GTCA-3′). Moreover, our results demonstrate that CREB contributes to the activation of the native c-fos gene promoter in response to many, but not all, extracellular stimuli.
Another established approach to assess the function of a particular gene is to use mice with deletions or mutations in that gene. Targeted disruption of the CREB gene has been performed, and CREB hypomorphs exist; these mice express a CREB protein that is competent to promote transcription (5, 30). Moreover, in these mice, there are elevated levels of expression of other members of the CREB/ATF family, ATF-1 and CREM (CRE modulator), and these transcription factors may compensate for CREB deficiency (30). However, a requirement for CREB in stimulus-dependent gene transcription has not been demonstrated in cells from mice with a targeted CREB mutation. Other attempts to perturb CREB function include the use of an antisense oligonucleotide for CREB (40), a CREB protein with a mutation in the basic DNA binding domain (63), and a CREB protein with a mutation at Ser-133 (2, 56). We propose that A-CREB is a potent and specific tool that will be generally useful to block CREB-dependent transcription.
The present study describes a useful alternative approach to gene targeting to assess the roles of B-ZIP transcription factors in stimulus-dependent gene expression. Since the CREB leucine zipper domain has a high degree of homology with those of ATF-1 and CREM and since these proteins can form heterodimers with each other (26), we expect that A-CREB has the capacity to dimerize with endogenous ATF-1 and CREM, thereby blocking their ability to bind to DNA. Therefore, our approach most likely does not distinguish between the role of CREB and that of highly related ATF-1 and CREM-τ as positive mediators of stimulus-dependent transcription. The gel shift results shown in Fig. 4 underscore this point. However, our results demonstrate that CREB or one of these very closely related family members mediate stimulus-dependent transcription of c-fos. A similar conclusion, however, may not be drawn from model systems in which upregulation of other ATF/CREB family members may compensate for CREB deficiency.
In addition to providing insight into CRE-mediated transcription, the B-ZIP D-N inhibitors described in this study have allowed us to distinguish between the importance of different B-ZIP transcription factors in the context of a promoter consisting of multiple distinct cis-acting elements. Several B-ZIP-containing transcription factors have been implicated in the regulation of the expression of c-fos and other IEGs. For example, it has been reported that C/EBPβ contributes to cAMP-dependent activation of c-fos transcription by directly binding to an element within the 3′ end of the SRE (36). Moreover, Sealy et al. reported that C/EBPβ may contribute to the serum activation of c-fos through the SRE (50). Our data do not support a role for C/EBP in cAMP or NGF induction of c-fos expression in PC12 cells (Fig. 6 and 10). While it is possible that B-ZIP transcription factors not tested in the present study contribute to c-fos transcription, our results demonstrate that CREB plays a major role in the stimulus-dependent transcription of c-fos.
In addition to its role in cAMP regulation of gene expression, CREB also contributes to Ca2+-induced gene expression. In the nervous system, the excitatory neurotransmitter glutamate modulates the postsynaptic intracellular level of calcium through the ionotropic glutamate receptor, the NMDA (N-methyl d-aspartate) receptor. Ca2+ influx via the NMDA receptor triggers the phosphorylation of CREB on Ser-133 (14, 21) and promotes the transcription of IEGs that contain potential CREB binding sites, including c-fos (1). Furthermore, the expression of IEGs may be critical for long-term neuronal responses such as the expression of the late phase of long-term potentiation and long-term depression (13, 34, 42, 55). Thus, glutamate- and Ca2+-sensitive genes may be critical substrates for complex neurobiological phenomena including learning and memory. Despite the observations that glutamatergic stimuli that induce neuronal adaptive responses also trigger the phosphorylation of CREB on Ser-133 and that the CREB hypomorph displays a disrupted late phase of long-term potentiation and impaired spatial learning and memory (7), a requirement for CREB during trans-synaptic activation of gene expression in vivo has not been demonstrated. Our results in the present study clearly show that CREB or a closely related family member is indeed required for glutamate-sensitive c-fos expression in cortical neurons. A-CREB may be useful in future studies to determine the role of CREB in trans-synaptic activation of gene expression and activity-dependent adaptive responses in vivo.
Our data also support a central role for CREB in growth factor-dependent gene expression. Like agents that increase intracellular levels of cAMP and Ca2+, NGF induces robust phosphorylation of CREB on its transcriptional regulatory site, Ser-133 (20). In addition, many NGF-sensitive genes contain putative CREs in their upstream regulatory regions; these include IEGs as well as late-response genes such as those encoding vgf, tyrosine hydroxylase, neurotensin, and CGRP (calcitonin gene-related peptide) (6, 62). Lastly, A-CREB inhibits NGF induction of c-fos. Although the specific genes responsible for NGF induction of the biochemical and morphological differentiation of PC12 cells are not well characterized, CREB-dependent gene expression contributes to this process since A-CREB was effective at inhibiting NGF induction of morphological differentiation. However, a maximum dose of A-CREB only partially blocked NGF induction of morphological differentiation. This result suggests that there are CREB-dependent and CREB-independent mechanisms responsible for NGF-mediated differentiation. Likewise, our data demonstrate that CREB-dependent and CREB-independent signaling pathways contribute to NGF induction of c-fos expression.
The signal transduction pathways that influence CREB-mediated gene activation in response to extracellular stimuli have been extensively characterized. Several types of stimuli, including growth factors that activate receptor tyrosine kinases and agents that increase intracellular levels of cAMP and Ca2+, induce phosphorylation of CREB on its transcriptional regulatory site, Ser-133 (20–22, 53). This phosphorylation event is critical for stimulus-dependent, CREB-mediated transcription. Phosphorylation of this residue promotes the association of CREB with coactivator protein CBP (CREB-binding protein) or p300 (12). Interestingly, although NGF and agents that trigger an increase in intracellular levels of cAMP or Ca2+ induce the phosphorylation of CREB Ser-133 to a similar extent, these stimuli influence the transcription of genes containing CREs in distinct ways. For example, agents that increase intracellular levels of cAMP or Ca2+ effectively stimulate the transcription of pAF42CRE, a reporter gene that contains only a single CRE in its upstream regulatory region (Fig. 8), while NGF is ineffective in stimulating transcription of pAF42CRE (Fig. 8). Rather, NGF induction of the transcription of c-fos requires the CREs as well as other elements including the SRE (Fig. 7 and 9) (6). Likewise, GAL4CREB can confer cAMP and Ca2+, but not NGF, activation of a reporter gene containing GAL4 binding sites (6). Thus, the phosphorylation of CREB on Ser-133 alone is not sufficient to promote the transcription of genes containing CREs. One possible explanation for these observations is that, depending on the nature of the stimulus, distinct B-ZIP transcription factors are responsible for the activation of c-fos transcription. Our results presented in this study do not support this possibility. Rather, our data support the idea that CREB is the B-ZIP transcription factor responsible for cAMP as well as NGF induction of c-fos expression. The mechanism by which cAMP, Ca2+, and NGF influence CREB-mediated transcription in distinct ways remains to be determined. One possibility is that CBP or proteins that associate with CBP are differentially regulated depending on the nature of the stimulus, as suggested by Nakajima et al. (41).
Taken together with results of previous studies, our data demonstrate that CREB is a general mediator of stimulus-dependent gene transcription. First, many distinct extracellular stimuli that employ different signal transduction pathways trigger the phosphorylation of CREB on its transcriptional regulatory site, Ser-133. Second, many IEGs and delayed response genes that are sensitive to these extracellular stimuli contain CREs or CRE-like elements in their upstream regulatory regions. Third, the present study demonstrates that A-CREB, a potent inhibitor of the DNA binding activity of CREB, inhibits cAMP, Ca2+, and NGF induction of the transcription of the prototypical IEG, c-fos. Therefore, CREB and/or its closely related family members are important for stimulus-dependent gene expression and are likely to be critical mediators of the long-term changes that underlie the growth, differentiation, and adaptive responses of various cell types.
ACKNOWLEDGMENTS
We thank L. Szilak for making the DNA probes, Susan Rutberg in Stuart Yuspa’s laboratory for the CREB and ATF-1 antibodies, and J. Moitra and B. A. Pierchala for comments on the manuscript.
This work was supported by NIH grant N534814, March of Dimes Birth Defects Foundation research grant 5-FY95-1114, a grant from The Esther A. and Joseph Klingenstein Fund, and a Pew Scholars Award (D.D.G.).
REFERENCES
- 1.Bading H, Ginty D D, Greenberg M E. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathway. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 2.Barton H, Muthusamy M, Chanyangam C, Fischer C, Cledenin C, Leiden J M. Defective thymocyte proliferation and IL-2 production in transgenic mice expressing a dominant-negative form of CREB. Nature. 1996;379:81–85. doi: 10.1038/379081a0. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz L A, Riabowal K T, Gilman M Z. Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Mol Cell Biol. 1989;9:4272–4281. doi: 10.1128/mcb.9.10.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackstone C D, Moss S J, Martin L J, Price D L, Huganir R L. Biochemical characterization and localization of a non-NMDA receptor in rat brain. J Neurochem. 1992;58:1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- 5.Blendy J A, Kaestner K H, Schmid W, Gass P, Schutz G. Targeting of the CREB gene leads to up-regulation of a novel CREB mRNA isoform. EMBO J. 1996;15:1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 6.Bonni A, Ginty D D, Dudek H, Greenberg M E. Serine 133-phosphorylated CREB induces transcription via a cooperative mechanism that may confer specificity to neurotrophin signals. Mol Cell Neurosci. 1995;6:168–183. doi: 10.1006/mcne.1995.1015. [DOI] [PubMed] [Google Scholar]
- 7.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 8.Buscher M, Rahmsdorf H J, Litfin M, Karin M, Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal pathways converge to the same enhancer element. Oncogene. 1988;3:301–311. [PubMed] [Google Scholar]
- 9.Cahill M A, Janknecht R, Nordheim A. Signalling pathways: jack of all cascades. Curr Biol. 1996;6:16–19. doi: 10.1016/s0960-9822(02)00410-4. [DOI] [PubMed] [Google Scholar]
- 10.Charnay P, Treisman R, Mellon M, Chao R, Axel R, Maniatis T. Differences in human alpha- and beta-globin gene expression in mouse erythroleukemia cells: the role of intragenic sequences. Cell. 1984;38:251–263. doi: 10.1016/0092-8674(84)90547-6. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 13.Dash P K, Hochner B, Kandel E R. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 14.Deisseroth K, Bito H, Tsien R W. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 15.Dignam J, Lebovitz R, Roeder R. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du W, Thanos D, Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993;74:887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- 17.Edwards D R. Cell signaling and the control of gene transcription. Trends Pharmacol Sci. 1994;15:239–244. doi: 10.1016/0165-6147(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 18.Fonjallaz P, Ossipow V, Wanner G, Schibler U. The two PAR leucine zipper proteins, TEF and DBP, display similar circadian and tissue-specific expression, but have different target promoter preferences. EMBO J. 1996;15:351–362. [PMC free article] [PubMed] [Google Scholar]
- 19.Gilman M Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988;2:394–399. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- 20.Ginty D D, Bonni A, Greenberg M E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 21.Ginty D D, Kornhauser J M, Thompson M A, Bading H, Mayo K E, Takahashi J S, Greenberg M E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg M E, Greene L A, Ziff E B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985;260:14101–14110. [PubMed] [Google Scholar]
- 24.Greenberg M E, Ziff E B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg M E, Ziff E B, Greene L A. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986;234:80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- 26.Habener J F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990;4:1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- 27.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hai T, Liu F, Coukos W, Green M. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 29.Hoeffler J P, Meyer T E, Yun Y, Jameson J L, Habener J F. Cyclic-AMP responsive DNA-binding protein: structure based on a cloned placental DNA. Science. 1988;242:1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- 30.Hummler E, Cole T J, Blendy J A, Ganss R, Aguzzi A, Schmid W, Beermann F, Schutz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci USA. 1994;91:5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyer S V, Davis D L, Seal S N, Burch J B E. Chicken vitellogenin gene-binding protein, a leucine zipper transcription factor that binds to an important control element in the chicken vitellogenin II promoter, is related to rat DBP. Mol Cell Biol. 1991;11:4863–4875. doi: 10.1128/mcb.11.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krylov D, Mikhailenko I, Vinson C. A thermodynamic scale for leucine zipper stability and dimerization specificity: e and g interhelical interactions. EMBO J. 1994;13:1849–1861. doi: 10.1002/j.1460-2075.1994.tb06579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krylov D, Olive M, Vinson C. Extending dimerization interfaces: the bZIP basic region can form a coiled coil. EMBO J. 1995;14:5329–5337. doi: 10.1002/j.1460-2075.1995.tb00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linden D J. A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron. 1996;17:483–490. doi: 10.1016/s0896-6273(00)80180-4. [DOI] [PubMed] [Google Scholar]
- 35.Masquilier D, Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J Biol Chem. 1992;267:22460–22466. [PubMed] [Google Scholar]
- 36.Metz R, Ziff E. cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to trans-locate to the nucleus and induce c-fos transcription. Genes Dev. 1991;5:1754–1766. doi: 10.1101/gad.5.10.1754. [DOI] [PubMed] [Google Scholar]
- 37.Meyer T E, Habener J F. Cyclic AMP response element binding protein (CREB) and related transcription-activating DNA-binding proteins. Endocr Rev. 1993;14:269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- 38.Morgan J I, Curran T. Role of ion fluxes in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- 39.Morgan J I, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 40.Nagamoto-Combs K, Piech K, Best J, Sun B, Tank A. Tyrosine hydroxylase gene promoter activity is regulated by both cAMP-responsive element and AP1 sites following calcium influx: evidence for CREB-independent regulation. J Biol Chem. 1997;272:6051–6058. doi: 10.1074/jbc.272.9.6051. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima T, Fukamizu A, Takahashi J, Gage F H, Fisher T, Blenis J, Montminy M R. The signal-dependent coactivator CBP is a nuclear target for pp90RSK. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen P V, Abel T, Kandel E R. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 43.Olive M, Krylov D, Echlin D R, Gardner K, Taparowsky E, Vinson C. A dominant-negative to AP1 that abolishes DNA binding and inhibits oncogenesis. J Biol Chem. 1997;272:18586–18594. doi: 10.1074/jbc.272.30.18586. [DOI] [PubMed] [Google Scholar]
- 44.O’Shea E K, Klemm J D, Kim P S, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 45.Pestell R, Hollenberg A, Albanese C, Jameson J. c-Jun represses transcription of the human chorionic gonadotropin alpha and beta genes through distinct types of CREs. J Biol Chem. 1994;269:31090–31096. [PubMed] [Google Scholar]
- 46.Price M A, Cruzalegui F H, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 47.Rivera V M, Sheng M, Greenberg M E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990;4:255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- 48.Ryseck R, Bravo R. c-Jun, Jun B, and Jun D differ in their binding affinities to AP-1 and CRE consensus sequences: effects of FOS proteins. Oncogene. 1991;6:533–542. [PubMed] [Google Scholar]
- 49.Sassone-Corsi P, Visvader J, Ferland L, Mellon P, Verma I M. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-response element. Genes Dev. 1988;2:1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- 50.Sealy L, Malone D, Pawlak M. Regulation of the cfos serum response element by C/EBPβ. Mol Cell Biol. 1997;17:1744–1755. doi: 10.1128/mcb.17.3.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheng M, Dougan S T, McFadden G, Greenberg M E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988;8:2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheng M, Greenberg M E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 53.Sheng M, McFadden G, Greenberg M E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 54.Sheng M E, Thompson M A, Greenberg M E. CREB: a Ca2+-regulated transcription factor phosphorylated by CaM kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 55.Stanton P K, Sarvey J M. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Struthers R S, Vale W W, Arias C, Sawchenko P E, Montminy M R. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature. 1991;350:622–624. doi: 10.1038/350622a0. [DOI] [PubMed] [Google Scholar]
- 57.Thanos D, Maniatis T. Virus induction of human IFNβ gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 58.Treisman R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 1995;14:4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treisman R. The serum response element. Trends Biochem Sci. 1992;17:423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- 60.Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5′ element and c-fos 3′ sequences. Cell. 1985;42:567–574. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- 61.Vinson C R, Sigler P B, McKnight S L. A scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989;246:911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- 62.Watson A, Latchman D. The cyclic AMP response element in the calcitonin/calcitonin gene-related peptide gene promoter is necessary but not sufficient for its activation by nerve growth factor. J Biol Chem. 1995;270:9655–9660. doi: 10.1074/jbc.270.16.9655. [DOI] [PubMed] [Google Scholar]
- 63.Woloshin P, Walton K, Rehfuss R, Goodman R, Cone R. CREB activity is required for normal growth and differentiated phenotype in the FRTL5 thyroid follicular cell line. Mol Endocrinol. 1992;5:1725–1733. doi: 10.1210/mend.6.10.1333055. [DOI] [PubMed] [Google Scholar]
- 64.Xia Z, Dudek H, Miranti C K, Greenberg M E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]