Abstract
Methods utilized for drug discovery and development within the kinome have rapidly evolved since the approval of imatinib, the first small molecule kinase inhibitor. Macrocycles have received increasing interest as a technique to improve kinase inhibitor drug properties evident by the FDA approvals of lorlatinib, pacritinib, and repotrectinib. Compared to their acyclic counterparts, macrocycles can possess improved pharmacodynamic and pharmacokinetic properties. This review highlights clinical success stories when implementing macrocycles in kinase-based drug discovery and showcases that macrocyclization is a clinically validated drug discovery strategy when targeting the kinome.
The development of macrocycles has emerged as an innovative approach to improve kinase inhibitor selectivity, as well as pharmacokinetic and pharmacodynamic properties.
Introduction
The integral activity of kinases in many diseases has led to the clinical development of numerous small-molecule inhibitors to shut down rogue kinase signaling.1–4 Since the approval of imatinib in 2001,5,6 kinases have become a prolific drug target in cancer therapy.7 One of the main challenges in the development of kinase inhibitors is non-selective, multi-kinase inhibition, because of the structural homology within kinase families.8,9 Beyond selectivity issues, the long-term efficacy of kinase inhibitors is hindered by on-target mutations that are especially difficult when targeting a heterogeneous disease like cancer.10–15 Hence, drug discovery approaches have been modified to more successfully target kinases.16–20 Macrocyclic kinase inhibitors have been widely investigated to overcome the aforementioned drawbacks of early kinase inhibitors. This review focuses on macrocycles as a method that has been utilized to increase druggability within the kinome (Fig. 1).
Fig. 1. Timeline highlighting clinical milestones in the development of macrocyclic kinase inhibitors.
Macrocycles have gained traction in kinase drug discovery as a rational approach of restricting the conformation of small molecules to improve potency and selectivity compared to their acyclic counterparts.19,21–24 The limited conformational freedom of macrocycles and their well-defined three-dimensional shape results in more energetically favored binding to the receptor, a function of reduced entropy and increased free energy of binding.25 Improvements in the pharmacokinetic profile such as improved cell permeability and oral bioavailability are also attainable due to the intrinsic chemical properties of macrocycles.22,26,27 While macrocyclization offers a space for chemical novelty, synthesis is challenging and often encountered with low yields posing a challenge for structure–activity relationship (SAR) exploration. In addition, there is a trade-off between increased molecular weight, metabolic susceptibility, and solubility. Despite these challenges, macrocyclic kinase inhibitors represent a promising approach for targeting kinases evident from their existence in various stages of the drug development process. Examples of successful macrocyclic inhibitors include lorlatinib,19 pacritinib,28,29 and repotrectinib30 that have all been recently FDA-approved. Focusing on these successful molecules, this review summarizes macrocyclization as a strategy to refine acyclic kinase inhibitors to better target the kinome.
Discovery of pacritinib (5, VONJO®)
JAKs (JAK1, JAK2, JAK3, and TYK2) are a family of intracellular nonreceptor tyrosine kinases that transduce signals via the JAK–STAT pathway.31,32 JAK2 mutations such as V617F are associated with hematological malignancies, particularly myeloproliferative neoplasms (MPNs) including thrombocythemia and myelofibrosis, as well as a wide range of leukemias and lymphomas.33–37 FLT3 (fms-like tyrosine kinase-3) is a receptor tyrosine kinase responsible for the maintenance, growth, and development of hematopoietic and nonhematopoietic cells. Mutations in the FLT3 receptor can lead to the development of acute myeloid leukemia (AML).38–40
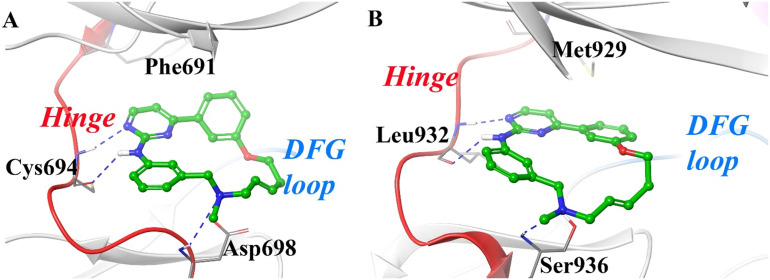
William and coworkers hypothesized that the dual inhibition of JAK2/FLT3 may improve anticancer efficacy compared to inhibiting either kinase independently.41 Screening of pyrimidine-based warheads uncovered a small low molecular weight compound (1) with a broad kinase inhibition profile. Small molecules can adopt numerous energetically favorable binding conformations in the active site that can lead to off-target toxicities through the inhibition of other targets. To circumvent this issue, the authors identified a low energy binding pose of compound 1 in the JAK2 active site and hypothesized that locking this energetically favorable conformation may improve kinome selectivity (Fig. 2).
Fig. 2. Docking poses and molecular interactions of compounds 1 and 2 with JAK2 (PDB 2B7A). A. Lowest energy pose of compound 1. Compound 1 was cyclized through rings A and C (Scheme 1) to generate a conformationally restricted macrocycle while retaining the structural attributes of the identified pose. B. Lowest energy pose of compound 2. Compound 2 forms two hydrogen bonds with the hinge region and a hydrogen bond with Ser936 in addition to hydrophobic interactions with Leu855, Val863, Ala880, and Leu983. The methoxy group serves as a solvent-accessible site to modify solubility of the macrocycle. Docking images were generated using AutoDock Vina and visualization was completed in Schrodinger Maestro Suite.
A series of constrained macrocycles were synthesized in an effort to improve selectivity profiles (Scheme 1).41 The SAR of these macrocycles was explored by modifying the conformationally defined arrangement of A, B, and C ring systems of compound 1. Ether-based macrocycles were found to be active against JAK2 with selectivity over JAK1, JAK3, and CDK2, as compared to their acyclic counterpart 1 that exhibited broad kinase inhibition profiles. Compound 2 achieved a JAK2 IC50 of 70 nM and was found to be 27-, 17-, and 12-fold selective against JAK1, JAK3, and CDK2, respectively. The hydrogen bond interaction between the benzylic ether oxygen and Ser936 (Fig. 2B) can explain the high JAK2 inhibitory activity of the compound. However, ether-based linkers also exhibited large clog P values that negatively impacted solubility and metabolic profiles and therefore introduction of hydrophilic groups were investigated to improve pharmacokinetics of the scaffold.
Scheme 1. Development of pacritinib (5, VONJO®), a macrocyclic JAK2/FLT3 dual inhibitor that received FDA approval on February 28, 2022, for the treatment of myelofibrosis.
JAK2 docking studies with compound 2 displayed that the methoxy group on ring C orients towards a solvent accessible pocket and this region was modified with functional groups capable of improving interactions with water. Substituting ring C with ortho/meta oxygen or nitrogen-based linkers resulted in compounds with sub-100 nM IC50 values against JAK2 and FLT3 (Table 1). While sterically larger substituents such as morpholine and piperazine in the ortho position were found to be potent and selective, solubility was not improved as predicted.
Biochemical activities of macrocyclic analogs against kinase targets involved in hematological malignancies41.
| Compound code | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| JAK1 | JAK2 | JAK3 | FLT3 | CDK2 | TYK2 | |
| 2 | 1.90 ± 0.02 | 0.070 ± 0.019 | 1.2 ± 0.3 | 0.190 ± 0.026 | 0.86 ± 1.20 | 0.21 ± 0.02 |
| 3 | 1.0 ± 0.0 | 0.007 ± 0.000 | 0.089 ± 0.004 | 0.019 ± 0.001 | >10 | 0.057 ± 0.004 |
| 4 | >10 | 0.019 ± 0.002 | 0.890 ± 0.042 | 0.092 ± 0.011 | >10 | 0.18 ± 0.00 |
| Pacritinib, 5 | 1.28 ± 0.37 | 0.023 ± 0.006 | 0.52 ± 0.11 | 0.022 ± 0.006 | 3.9 ± 1.1 | 0.050 ± 0.06 |
| 6 | 3.1 ± 0.3 | 0.084 ± 0.001 | 2.5 ± 0.3 | 0.070 ± 0.005 | 3.7 ± 0.2 | 0.16 ± 0.01 |
| 7 | 3.6 ± 0.6 | 0.077 ± 0.006 | ND | 0.031 ± 0.019 | 0.088 ± 0.000 | 0.071 ± 0.011 |
Hydroxyethyl piperazine (6) and open chain nitrogen substituents (7) were introduced at the solvent front to improve solubility. Although solubility improved, compounds displayed reduced JAK2 activity upon screening. Oxygen-linked compounds were explored and among this series aminoethyl ether substituents (5) displayed consistent potency and selectivity profiles. It is interesting to note that non-basic oxygen-linked analogs exhibited a reduction in FLT3 activity as well as solubility compared to the aminoethyl ether compounds, which can be explained partially by a lack of ionization at physiological pH.
Among all tested compounds, compound 5 (pacritinib, VONJO®) exhibited an optimal combination of potency (JAK2 IC50 = 0.023 ± 0.006 μM and FLT3 IC50 = 0.022 ± 0.006 μM) and selectivity over JAK1/JAK3/CDK2 (IC50 = 1.28/0.52/3.9 μM respectively). Modeling of compound 5 in JAK2 displayed two hydrogen bond interactions between the amino pyrimidine warhead and Leu932 and a hydrogen bond between the methoxy and Ser936 while the pyrrolidine basic amine orients towards the solvent. These interactions are retained within FLT3, suggesting that the macrocycle is geometrically constrained in a conformation that is optimal to bind to both JAK2 and FLT3 (Fig. 3). Based on the activity profiles and improved solubility and clog P values, compound 5 was further derivatized to evaluate SAR in the binding pocket.
Fig. 3. Molecular docking and interactions of compound 5 with JAK2 and FLT3. A. Docking pose of compound 5 (pacritinib) in JAK2 (PDB 2B7A). B. Docking pose of compound 5 in FLT3 (PDB 1RJB). C. Superimposed configurations of pacritinib in JAK2 (in green) and FLT3 (in pink) show that the overall conformation of the macrocycle is retained suggesting that compound 5 is geometrically constrained in a conformation that is optimal for binding to both JAK2 and FLT3.
To further investigate possible interactions with hydrophobic residues in the binding pocket, small lipophilic groups were incorporated into aryl rings A & B. Adding a methyl group on ring B (3) improved JAK2 potency but compromised selectivity against JAK3, whereas addition of a methoxy on ring A (4) improved potency and selectivity for JAK2 over JAK1. In addition, pacritinib and compound 4 displayed lower CYP3A4 inhibition (20% inhibition at 5 μM and IC50 > 10 μM, respectively) than compound 3. Although selectivity indices of compound 4 surpassed other compounds (Table 2), it was found to be less active against FLT3. Interestingly, the only difference between compounds 3 and 5 is a methyl group in ring B that results in distinct selectivity profiles (Table 2). Ultimately, pacritinib was progressed as the clinical candidate because of an overall balance between pharmacodynamic and pharmacokinetic properties.
In vitro activity and PK profiles of compounds 3, 4, and 5 (ref. 41).
| Compound code | IC50 selectivity index | Solubilitya (μg mL−1) | IC50 (μM) | GI50 (μM) | PAMPA Pappb (× 10−6 cm s−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| JAK1/JAK2 | JAK3/JAK2 | TYK2/JAK2 | CDK2/JAK2 | CYP3A4 | CYP2D6 | Ba/F3-JAK2 V617F | MV4-11 | |||
| 3 | 143 | 13 | 8.1 | >1000 | >150 | 2.5 | >10 | 0.18 | 0.051 | 16.8 |
| 4 | >500 | 47 | 9.5 | >500 | 60.83 | >10 | >10 | 0.10 | 0.085 | 10.1 |
| Pacritinib 5 | 56 | 23 | 2.2 | 170 | >150 | >5 | >5 | 0.16 | 0.047 | 9.3 |
High throughput solubility in PBS buffered at pH 7.0.
PAMPA: parallel artificial membrane permeability assay.
Pacritinib exhibited activity against JAK2 and FLT3 clinically relevant mutations (MV4-11 GI50 = 0.047 μM; Ba/F3 FLT3D835Y GI50 = 0.006 μM; Ba/F3 JAK2V617F GI50 = 0.16 μM), obtained selectivity within the kinome, and displayed a low drug–drug interaction potential (CYP3A4/2D6 IC50 > 5 μM). Pacritinib was found to be orally bioavailable with a favorable exposure profile and activity against FLT3-ITD and Ba/F3 JAK2V617F xenograft models. Following its entry into clinical trials and achieving favorable phase 3 results (NCT03165734), pacritinib was approved by the FDA for the treatment of myelofibrosis on February 28, 2022.
Pacritinib was one of the first FDA small-molecule kinase inhibitor macrocycles receiving approval in 2022. By predicting lowest energy binding conformations of an acyclic hit (compound 1) in JAK2 and FLT3, the pacritinib macrocycle was generated to affix the inhibitor into a desired conformation to optimize binding site interactions in both kinases. At the same time, locking conformation restricted the type of binding pose pacritinib could adopt in other targets, which improved selectivity and off target toxicity profiles of the macrocycle.
Discovery of zotiraciclib (8, SB1317/TG02)
The group that developed pacritinib also discovered a macrocyclic inhibitor of cyclin-dependent kinases (CDKs), JAK2, and FLT3 for the treatment of various advanced leukemias and multiple myeloma.42 CDKs play an essential role in cell cycle control (CDKs 1, 2, 4, and 6), transcription initiation (CDKs 7 and 9), and neuronal function (CDK5).43–45 Dysregulation of CDKs is a hallmark of cancer and researchers hypothesized that inhibition of CDK2, a core regulator of the cell cycle, concomitant with inhibition of JAK2 and FLT3, may provide a more robust profile for targeting hematological malignancies.42 Macrocycle linkages can dictate selectivity profiles and, accordingly, compound 1 served as a template to generate macrocycles that exhibit CDK2, JAK2, and FLT3 activity without compromising overall kinome selectivity (Scheme 2).
Scheme 2. Development of SB1317/TG02 (6), a macrocyclic JAK2/FLT3/CDK2 inhibitor.
A series of macrocyclic linking moieties with different substitutions (R1–R7) on rings A, B, or C were incorporated into compound 1 to enhance selectivity and potency and were tested for inhibition of CDK2, JAK2, and FLT3 (Fig. 4 and 5).42 Compounds with nitrogen- and amide-based linkers were synthesized to form additional interactions with residues at the solvent front of CDK2 (Asp86), FLT3 (Asp698), and JAK2 (Ser936) thereby improving selectivity as compared to their acyclic counterpart. Various amides, phenol ether amines, and benzylic ether amines were screened. Amides displayed weak CDK2 inhibition (IC50 > 10 μM) that was thought to be due to higher conformational energy and/or unfavorable electrostatic interactions. Benzylic ethers also exhibited low affinity for CDK2 due to steric clashes with the backbone carbonyl of Gln131. It is important to note that changing the phenolic linker to a benzylic ether decreased potency by one order of magnitude. Furthermore, replacing the benzylic ether with an N-methyl group restored activity of the compound against CDK2. This suggests that the orientation of the basic nitrogen in the allylic/benzylic position may dictate potency, which can be explained by a salt bridge interaction with Asp86 (Fig. 4B). More flexible and shorter phenol ether amine 5-carbon linkers were found to be active against CDK2 but not JAK2 or FLT3. Interestingly, phenolic ether amine linkers with an additional carbon were found to be active against all targeted kinases (IC50 < 0.100 μM, Table 3). The authors further optimized N-substitution of the linker by generating a series of compounds with different R4 groups (Scheme 2). While small lipophilic substituents displayed potency against all targeted kinases, bulky substituents lost activity likely due to a steric clash with Asp86. Furthermore, bulky substituents increased molecular weight and clog P of the compounds resulting in reduced solubility and a reduction in potency by an order of magnitude. When designing kinase-based macrocycles the choice of length of linker as well as the substitution in the linker can impact selectivity profiles.
Fig. 4. Docking poses and molecular interactions of compounds 1 and 8 into CDK2 (PDB 1AQ1). A. Docking pose of compound 1. B. Docking pose of compound 8 (zotiraciclib). A similar design strategy to pacritinib was employed to generate macrocycle 8 by using compound 1 as the template. The modification of linker in compound 1 helped retain the binding geometry and the activity of compound 8 against CDK2.
Fig. 5. Molecular docking of compound 8 into JAK2 and FLT3. A. Docking pose of compound 8 in JAK2 (PDB 2B7A). B. Compound 8 docked into FLT3 (PDB 6JQR). In both active sites, compound 8 forms conserved interactions at the hinge and solvent front amino acids, serine (JAK2) and aspartic acid (FLT3). These interactions are also shared with CDK2 suggesting that the constrained geometry of compound 8 locks the macrocycle in an orientation that is favorable to bind CDK2, FLT3, and JAK2.
Effect of linker length and composition on the activity of the compounds42.

| ||||
|---|---|---|---|---|
| –Z– | CDK2 IC50 (μM) | JAK2 IC50 (μM) | FLT3 IC50 (μM) | clog P |

|
0.15 ± 0.015 | 3.3 ± 0.92 | 2.9 ± 1.2 | 3.8 |

|
0.66 ± 0.19 | 1.7 ± 0.14 | 0.21 ± 0.022 | 3.7 |

|
0.021 ± 0.012 | 0.15 ± 0.083 | 0.11 ± 0.038 | 3.8 |

|
0.013 ± 0.004 | 0.073 ± 0.017 | 0.056 ± 0.026 | 4.1 |
The authors also investigated the effect of substitution at the aromatic region (R5 substitution, Scheme 5). Lipophilic groups that contribute to a higher clog P resulted in reduced activity against all enzymes. Various polar substituents were tested to decrease clog P and increase solubility while maintaining potency. However, polar groups in the aromatic region caused a reduction in activity against either CDK2 or FLT3. This was justified by a hypothesized twist in the biaryl ring system following substitution, which likely reduced the interaction between the aromatic region and the enzyme.
Scheme 5. Development of the macrocyclic inhibitor lorlatinib (15).
Since substitution at the R5 position did not improve potency, compound 8 was progressed as the lead candidate. Modeling studies of compound 8 (SB1317/TG02) in CDK2, FLT3, and JAK2 displayed energetically favorable conformations within all active sites. This illustrates how the linker region of macrocycles can be fine-tuned to target specific kinases by modifying composition.
Compound 8 displayed in vitro efficacy in various cancer cell lines including HL-60, HCT-116, COLO205, and DU145. Advanced ADME profiling of compound 8 demonstrated favorable pharmacokinetic properties.42 Importantly, compound 8 displayed in vivo efficacy in xenograft mouse models of colon (HCT-116) and lymphoma (Ramos) cancer models. Compound 8 caused tumor regression and extended the survival of mice with AML xenografts.46 Favorable pharmacodynamic and pharmacokinetic properties of compound 8 (zotiraciclib) have led to its clinical development against various cancers as a monotherapy or in combination with other anticancer agents (NCT01699152 and NCT02942264).47,48
Discovery of SB1578 (11)
Due to the plasticity of macrocyclic variations of the hit compound 1, another iteration of small molecule macrocycles were generated to selectively inhibit JAK2, FLT3, and TYK2 kinases for the treatment of rheumatoid arthritis (RA).49 Interruption of cytokine signaling networks has emerged as a new treatment paradigm for RA,50 which was brought to fruition through the clinical development of the JAK1/3 inhibitor tofacitinib (XELJANZ®).51 The FDA has since required tofacitinib to include a boxed warning of injury or death due to drug-induced problems such as opportunistic infections, lymphoma, and other malignancies.52 Since JAK3 inhibition can cause severe combined immunodeficiency syndrome (SCID), selective blockade of other JAK members has been investigated as an alternative. In addition to the upregulation of the FLT3 ligand in RA patients, JAK2 and TYK2 contribute to inflammation through activation of the JAK–STAT pathway.53–55 Hence, the development of a selective JAK2/TYK2/FLT3 macrocycle inhibitor may have therapeutic efficacy in the treatment of RA without the side effect profile of first generation JAK1/3 inhibitors.49 Importantly, deucravacitinib (Sotyktu®) a selective TYK2 inhibitor was approved for plaque psoriasis without requiring a black box warning supporting this hypothesis.56,57
Both pacritinib (JAK2/FLT3 inhibitor) and zotiraciclib (JAK2/CDK2/FLT3 inhibitor) have been indicated in cancer therapy with modest safety profiles. However, for autoimmune diseases such as RA, a higher safety profile is typically required. Therefore, new analogs were investigated to improve the safety profile of macrocyclic inhibitors (Scheme 3). Initially, the biaryl ring system was modified with saturated, heteroaryl, and/or non-aromatic ring systems to lower clog P and improve solubility as well as selectivity profiles. However, the saturated compounds completely lacked activity against all enzymes. The macrocycle ring size was reduced to 18 or 17 atoms, which was found to be optimal for JAK2 activity.41 Among the various tested heterocyclic macrocycles, the pyridine substituted analogue 9 displayed modest improvement in JAK2 and FLT3 activity with improved CDK2/JAK2 selectivity but reduced JAK3/JAK2 selectivity. The pyrrolidinyl-ethyloxy side chain in compound 9 was introduced as a solubilizing group and displayed JAK2 and FLT3 inhibition. In addition, compound 9 was active against TYK2 with improved CDK2/JAK1/JAK3 selectivity. It is interesting to note that replacing the pyrrolidinyl-ethyloxy side chain with a methoxyethoxy side chain decreased JAK2/FLT3 potency positioning pyrrolidine as an important heterocycle for JAK2/FLT3 potency.
Scheme 3. Development strategy of the JAK2/TYK2/FLT3 macrocycle inhibitor SB1578 (9).
Various 5-membered heterocycles were also investigated with pyrrolidine fixed as a solvent interacting group. Furan and thiophene analogues with an N-methyl linker were less active against all enzymes as compared to the diether linker. Since thiophene analogues had higher clog P values compared to furan, the furan analogue 11 (SB1578) exhibited preferred solubility properties. The furan analogue 11 exhibited sub-micromolar JAK2, TYK2, and FLT3 potency, as well as improved JAK1/3 selectivity.49 Docking studies of macrocycle 11 in JAK2 and FLT3 demonstrated that the pyrimidine warhead interacts at the hinge in a manner similar to that of compound 1 (Fig. 6).
Fig. 6. Molecular docking and interactions of compound 9 with JAK2 (PDB 2B7A) and FLT3 (PDB 6JQR) A. Compound 11 docked into JAK2 (PDB 2B7A). B. Compound 11 docked into FLT3 (PDB 6JQR). Binding interactions of compound 11 in JAK2 and FLT3 are retained. The warhead region is shared between pacritinib and compound 8, but the linker region utilized to generate the macrocycle is varied. This suggests that the same warhead can be utilized for multiple kinases, and the linker region utilized to generate the macrocycle will dictate selectivity.
Compound 11 exhibited a JAK2 IC50 of 0.046 μM and selectivity of 152-, 59-, and 93-fold against CDK2, JAK1, and JAK3, respectively. While placing the furan oxygen at the regioisomeric position at the exterior of the macrocycle (10) increases solubility, it displays a comparable overall potency profile suggesting that the macrocyclic geometry of the five membered ring system dictates receptor affinity with little influence on heteroatom arrangement. Furthermore, compound 11 lacked hERG activity whereas compound 9 had an IC50 of 2.4 μM against hERG.
Next, compound 11 and its regioisomer 10 were further profiled against Ba/F3-JAK2V617F murine B-cells and FLT3-driven HL-60 cells. Although growth inhibition values were similar for both compounds, the 2,5-disubstituted furans were more stable in vivo suggesting that compound 11 would have a better overall pharmacokinetic profile. To test this, a pharmacokinetic analysis of compound 11 was completed, and the compound exhibited rapid to moderate absorption (Tmax ranged between 0.5 to ∼2.3 h) in mice, rats, and monkeys but was relatively slower in dogs. The terminal t1/2 ranged between 0.8 and 3.6 h. Exposures achieved in mice at 50 mg kg−1 exceeded the pharmacological concentrations necessary to inhibit the desired kinase targets. Compound 11 also exhibited dose-dependent inhibition of JAK2 signaling in four different cell lines including cells that express constitutively activated mutant JAK2 (SET2), express constitutively activated wild type (WT) JAK2 (MDA-MB-231), require exogenous ligand for WT JAK2 (HEK293) activation, and primary human T cells. Taken together, this suggests that compound 11 is a robust inhibitor of inducible inflammation common in RA.49
In mammalian efficacy studies, a murine collagen-induced arthritis model was utilized to evaluate the efficacy of compound 11 for human rheumatoid arthritis. After the onset of collagen-induced arthritis, mice were administered doses of 105 mg kg−1 or 210 mg kg−1 twice daily for 10 days. A dose-dependent reduction in collagen-induced arthritis was observed and was related back to a reduction in JAK2 signaling. A phase 1 clinical trial for compound 11 was then initiated in healthy volunteers to evaluate pharmacokinetics of the macrocycle (NCT01235871).
The development of pacritinib (5), zotiraciclib (8), and SB1578 (11) all shared compound 1 as a common starting point (Scheme 4). By predicting the lowest energy binding conformations of compound 1 in kinase targets, unique macrocycles were generated to lock the inhibitor in a desired conformation for optimal binding. Constraining conformation and restricting the binding pose a macrocycle can adopt improves selectivity. This is evident by selective inhibitory profiles of pacritinib and zotiraciclib against CDK2. The only difference between pacritinib and zotiraciclib is the linker region utilized to generate the macrocycle. Similarly, to develop a macrocycle with improved safety profiles to be a candidate to treat RA, SB1758 was generated. The difference between pacritinib and SB1578 is the heterocycle to which the linker is attached. The slight change in geometry between six and five membered ring systems can lead to macrocycles with augmented safety profiles. Therefore, the choice of linker but also geometry linking the macrocycle can influence pharmacodynamic as well as pharmacokinetic profiles (see Table 4), which can be modified depending on intended therapeutic use.
Scheme 4. Selective JAK2/CDK2/FLT3 inhibitors.
In vitro ADME of pacritinib, zotiraciclib and SB1578 (ref. 58).
| Compound | Solubility (μg mL−1) | CYP3A4 IC50 (μM) | CYP2D6 IC50 (μM) | HLM t1/2 (min) | MLMat1/2 (min) | PPBb (%) |
|---|---|---|---|---|---|---|
| Pacritinib | >150 | >5 | >5 | >60 | 22 | 99.4 |
| Zotiraciclib | 71.8 | >25 | 0.95 | 45 | 12 | 99.4 |
| SB1578 | >250 | >10 | >10 | >60 | 18 | 87.3 |
MLM: mouse liver enzyme.
Plasma protein binding in mice.
Development of lorlatinib (17, LORBRENA®)
Anaplastic lymphoma kinase (ALK) is a member of the insulin receptor (IR) subfamily of tyrosine kinase receptors.59,60 ALK-fusions are oncogenic drivers in diffuse large-B-cell lymphoma (DLBCL),61 inflammatory myofibroblastic tumors (IMTs),62 and in 3–7% of non-small-cell lung carcinomas (NSCLC).63–68 Crizotinib (PF-02341066), a MET, ALK, and ROS1 kinase inhibitor received FDA approval in 2011 for the treatment of ALK-positive NSCLC.69,70 Unfortunately patients receiving crizotinib eventually developed resistance from on-target ALK mutations and CNS issues.71 Poor blood–brain barrier permeability of crizotinib does not permit targeting ALK-positive NSCLC brain metastasis. In addition, conformational flexibility of crizotinib reduces its affinity for secondary ALK mutations.72 This is particularly apparent with the L1196M gatekeeper mutation that confers resistance to crizotinib. It was hypothesized that simultaneous improvement in pharmacodynamics and pharmacokinetics of crizotinib could be achieved by restricting adoptable conformations using structure-based and lipophilic-efficiency-based (LipE) drug design approaches.
To determine how compound 15 bound to ALK, a co-crystal structure was solved, which depicted an acyclic U-shaped binding pose with the methoxy and triazole moieties in proximity (Fig. 7). Adoption of this U-shaped binding pose in ALK point mutations causes a negative impact on binding energies because of sterics. Therefore, it was hypothesized that compound 15 could be linked through the methoxy and triazole regions to restrict its conformation to improve binding energies against secondary ALK mutations. This linkage would also cause a restriction of rotation in rotatable bonds that could result in improved blood brain barrier penetration to target CNS metastasis.19,73
Fig. 7. Crystal structure of ALK with compound 15 (PDB 4CNH). A. Cocrystal structure of compound 15 in the ALK kinase domain highlighting the proximity of the methoxy fluorophenyl head group and triazole tail group. Linking these two regions was hypothesized to reduce steric interactions with the gatekeeper mutant ALKL1196M. B. L1196M mutation resulting in a steric clash with compound 15.
To reduce adoptable conformations, two macrocyclic design templates were followed as shown in Scheme 5 (as A and B). The first template linked the pyrazole carbon and amide nitrogen to form analogue 16a, whereas the second template linked the methoxy carbon to the triazole 4-carbon (15) to form analogue 16b. A series of 12 to 14-membered ether linked macrocycles were generated to evaluated. It is notable that smaller ring sizes had higher lipophilic efficiencies in addition to lower binding strains. These compact structural derivatives of crizotinib with restriction in rotation of rotatable bonds were found to provide improved CNS penetration/efficacy.19 Increased efflux of the analogues was seen in cases of higher molecular weight and/or hydrogen bond donor count whereas decreased efflux was seen in compounds with higher lipophilic efficiencies.
The cyclization and SAR studies of crizotinib led to the discovery of macrocycle 16a. The crystal structure of 16a in the ALK active site depicts that the N-methyl group is conformationally restricted and pulled tightly towards the G-loop. This conformational restriction also provides closer contact with the carbonyl group of Leu1122 (3.4 Å) and nearby side chains of Leu1122 (4.1 Å), Gly1123 (4.7 Å), and Val1130 (4.7 Å). The amide carbonyl of macrocycle 16a engages in a water bridge with Lys1150 and His1124, which effectively lowers binding desolvation penalties and improves binding energies. Compound 16a exhibited an improvement in ALK potency and common secondary ALK mutations that are resistant to crizotinib (ALK Ki = 0.0002 μM, ALKL1196MKi = 0.00029 μM). Permeability of 16a, measured by Pgp efflux potential, also improved compared to the acyclic analogue suggesting an enhancement in drug absorption and penetration into the CNS (Table 5). While both 16a and 16b showed similar cellular potencies against both wildtype and mutated ALK kinases, the use of an amide linker reduced lipophilicity of 16a by one unit and increased lipophilic efficiency by almost two units. Furthermore, amide-linked macrocycles exhibited the most desirable CNS ADME properties with low MDR BA/AB efflux ratios without a compromise in efficacy.
Comparison of inhibition values and physicochemical properties of crizotinib and lorlatinib19.
| Compound | K i (nM) | log D | LipE | HLMa Cl | MDRb BA/AB (ratio) | |
|---|---|---|---|---|---|---|
| ALK | ALK-L1196M | |||||
| Crizotinib (12, acyclic) | 0.74 | 8.2 | 2.0 | 4.1 | 44 | 12.5/0.28 (44.5) |
| 13 | 0.85 | 8.2 | 3.0 | 3.5 | 76 | 33.8/7.7 (1.4) |
| 14 | 2.8 | 35 | 3.7 | 3.7 | 28 | 12.6/0.74 (17.0) |
| 15 | 4.0 | 38 | 2.4 | 3.1 | 25 | 16.3/20.0 (0.82) |
| 16a | <0.2 | 0.29 | 2.2 | 5.7 | 8.6 | 28.3/8.1 (4.2) |
| 16b | <0.1 | 0.62 | 3.8 | 3.9 | — | — |
| Lorlatinib (17, macrocycle) | <0.07 | 0.70 | 2.3 | 5.4 | <8 | 28.0/19.3 (1.5) |
Human liver microsomes.
Multiple drug resistance.
Compound 16a was further optimized for activity against ALK secondary mutations and CNS penetration, leading to the discovery of compound 17 (lorlatinib, LORBRENA® [US], LORVIQUA® [EU]). To generate lorlatinib, the methyl group of 16a was replaced with a cyano group, which was found active against the ALKL1196M mutation that exhibits resistance to crizotinib. The ALKL1196M mutation occurs at the gatekeeper region of ALK, where leucine is replaced by a bulky methionine residue. The conformational restriction of lorlatinib prevents the macrocycle from engaging in a steric clash with methionine and highlights the ability of a macrocycle to retain inhibition against binding site mutations (Fig. 8).
Fig. 8. Crystal structure of the ALK-kinase domain bound to 16a, lorlatinib (17) and crizotinib (12). A. Cocrystal structure of compound 16a in the ALK-kinase domain (PDB 4CMU). B. Lorlatinib (17) cocrystal structure in the ALK-kinase domain (PDB 4CLI). C. Lorlatinib (17) cocrystal structure in the ALK-kinase domain with an L1196M mutation (PDB 4CLI with L1196M mutation). D. Crizotinib (12) cocrystal structure in the ALK-kinase domain with an L1196M mutation (PDB 2YFX). The conformational restriction of the lorlatinib macrocycle circumvents a steric clash with methionine, which leads to retention of activity against the ALKL1196M mutation.
The macrocyclization of crizotinib to lorlatinib resulted in increased kinase activity against wildtype and various secondary mutations (Table 6), increased lipophilic efficiency (LipE 4.1 vs. 5.4), improved membrane permeability (MDR BA/AB ratio 44.5 vs. 1.5), and improved metabolic stability (44 vs. <8). In addition, lorlatinib exhibited enhanced CNS penetration, making it suitable to target brain metastases. In November 2018, lorlatinib received accelerated approval for second- and third-line treatment of ALK positive NSCLC followed by its regular approval on March 3, 2021, for ALK positive NSCLC.
Comparison of GI50 of crizotinib and lorlatinib among various mutated cell lines19.
| Compound | pALK GI50 (nM) in 3T3-EML4-ALK cell lines | |||||||
|---|---|---|---|---|---|---|---|---|
| ALK | F1174L | C1156Y | G1269A | S1206Y | L1196M | L1152R | G1202R | |
| Crizotinib (12, acyclic) | 80 | 165 | 478 | 605 | 626 | 843 | 1026 | 1148 |
| Lorlatinib (17, macrocycle) | 1.3 | 0.2 | 1.6 | 15 | 4.2 | 21 | 9 | 77 |
Development of Selitrectinib (19, LOXO-195) and Repotrectinib (20, TPX-0005, AUGTYRO™)
Tropomyosin receptor kinases (Trks) are activated by the neurotrophin family of growth factors and have been recognized as a useful therapeutic target for various cancers.74,75 Trk fusions or rearrangements occur in 3.3% of lung cancers, 2.2% of colorectal cancers, 16.7% of thyroid cancers, 2.5% of glioblastomas, and 7.1% of pediatric gliomas.76,77 Several Trk inhibitors have been developed including larotrectinib (VITRAKVI®) that was FDA-approved in 2018 for the treatment of cancers containing neurotrophic tropomyosin receptor kinase (NTRK) fusions.78–80 Unfortunately, due to the heterogeneity within Trk-driven cancers, larotrectinib exhibited issues from acquired drug resistance due to secondary mutations in the Trk kinase domain.
Across the most common Trk types (A and C), frequent Trk secondary mutations that confer resistance to larotrectinib are located at the solvent-front (TrkAG595R and TrkCG623R) and the DFG motif (TrkAG667C) of the active site.20,76,81 Molecular modeling of both TrkA and TrkC solvent front mutations suggests that mutation from glycine to arginine introduces steric bulk that clashes with the hydroxypyrrolidine group of larotrectinib (18, Fig. 9). Similarly, the mutation from glycine to cysteine at the DFG motif of TrkA increases steric hindrance with the difluorophenyl group of larotrectinib. The flexibility of larotrectinib causes the molecule to clash with these mutations resulting in unfavorable binding energies against Trk mutants.
Fig. 9. Docking of larotrectinib in TrkA and TrkAG595R kinase domains. A. Larotrectinib in the TrkA kinase domain (PDB 4AOJ). B. Larotrectinib in TrkAG595R kinase domain (PDB 7VKN). The hydroxypyrrolidine group in larotrectinib clashes with the bulky arginine residue at the solvent front in TrkG595R. C. A G667C mutation resulting in a steric clash with the diflurophenyl moiety.
To improve activity against Trk solvent front and DFG mutations, constrained macrocyclic derivatives of larotrectinib were synthesized (Scheme 6), which uncovered 19 (selitrectinib, LOXO-195) and 20 (repotrectinib, TPX-0005).20,82,83 These macrocycles were designed with conformational rigidity to avoid steric clashes with TrkAG595R, TrkAG667C, and TrkCG623R secondary kinase mutations. Molecular modeling indicated that selitrectinib and repotrectinib both bind to the Trk active site without steric clashes with solvent front or gatekeeper mutations (Fig. 10). In support of these modeling studies, both selitrectinib and repotrectinib achieved low nanomolar inhibitory activity against secondary Trk mutations (Tables 7 and 8).
Scheme 6. Development of macrocyclic selitrectinib (19) and repotrectinib (20).
Fig. 10. Docking studies of selitrectinib and repotrectinib in TrkA (PDB 7VKN). A. Molecular docking of selitrectinib in TrkA (G595R, PDB 7VKN) with a G667C mutation. B. Co-crystal structure of repotrectinib in TrkA (G595R, PDB 7VKN) with a G667C mutation. Both inhibitors are designed to restrict steric hindrance with TrkAG595R and TrkAG667C mutants by limiting conformational freedom. This reduction in conformational freedom lowers steric interactions with the TrkA secondary mutations to overcome acquired drug resistance seen with larotrectinib.
Comparison of IC50 and GI50 of larotrectinib and selitrectinib against various Trk mutations20.
| Compound | IC50 (nM) | GI50 (nM) in NIH3T3 cell lines | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TrkA WT | TrkA G595R | TrkA G667C | TrkC WT | TrkC G623R | TrkC G696A | TrkA WT | TrkA G595R | TrkA G667C | TrkA V573M | TrkA F589L | TrkA G667S | TrkC WT | TrkC G623R | TrkC G696A | |
| Larotrectinib (18, acyclic) | 0.9 | 69.0 | 45.5 | 2.8 | 48 | 4.5 | 5 | 819 | 315 | 1018 | 47 | 70 | 18.8 | >10 000 | 172 |
| Selitrectinib (19, macrocycle) | 0.6 | 2.0 | 9.8 | <2.5 | 2.3 | <2.5 | 1.6 | 7 | 64 | 29 | 9 | 25 | 2 | 45.5 | 17.5 |
Comparison of IC50 and GI50 of larotrectinib and repotrectinib against various Trk mutations82.
| Compound | IC50 (nM) | GI50 (nM) in BA/F3 cell | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TrkA WT | TrkA G595R | TrkC WT | TrkC G623R | TrkA WT | TrkA G595R | TrkC WT | TrkC G623R | TrkC G623E | |
| Larotrectinib (18, acyclic) | 0.9 | 69.0 | 2.8 | 48 | 4 | 1024 | 10.2 | 3293 | 742.3 |
| Repotrectinib (20, macrocycle) | 0.533 | 2.67 | 0.211 | 4.46 | <0.2 | 0.2 | <0.2 | 0.39 | 1.4 |
Repotrectinib demonstrated in vivo efficacy in xenograft tumor models bearing TrkA WT and TrkA mutants, as well as ALK and ROS1. ALK and ROS1 also exhibit secondary solvent-front mutations and repotrectinib exhibited affinity for these mutations as well. Favorable results from the TRIDENT-1 trial (NCT03093116) have led to breakthrough therapy designation of repotrectinib granted by the FDA in May 2022 for the treatment of ROS1-positive NSCLC and full FDA approval on November 15, 2023.30
Conclusions and future perspectives
Methods for drug development in the kinome is a rapidly evolving field. Successful clinical implementation of a kinase inhibitor relies on both a balance between pharmacokinetic and pharmacodynamic properties. Initial FDA approvals for first-generation kinase inhibitors such as imatinib, sorafenib, and erlotinib were groundbreaking, however, the use of these agents introduced clinical challenges when targeting the kinome. Circumvention of these issues followed suit by using strategies such as irreversible inhibition to overcome drug resistance and the introduction of chirality to improve selectivity and efficacy profiles.84–87 Clinical limitations to these methods were quickly realized88–90 and the development of small-molecule kinase macrocycles has emerged as an innovative approach to supplement drug development efforts in the kinome (Table 9).
Macrocyclic small molecule kinase inhibitors under clinical investigation or with FDA approval.
| Compound name | Indication | Targets | NCT identifier | Clinical trial |
|---|---|---|---|---|
| Pacritinib (5, VONJO®) | Primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis | JAK2, FLT3, and IRAK1 | — | FDA approval in 2022 |
| Zotiraciclib (8, SB1317/TG02) | Glioma | CDK2, JAK2, and FLT3 | NCT01699152 | Phase 1 completed |
| SB1578 (11) | Rheumatoid arthritis | JAK2, FLT3, and TYK2 | NCT01235871 | Phase 1 completed |
| Lorlatinib (17, LORBRENA®) | ALK positive non-small cell lung cancer | ALK | — | FDA approval in 2018 |
| Selitrectinib (19, LOXO-195) | Trk fusion solid tumors | Trk | NCT03215511, NCT03206931 | Phase 1/2 |
| Repotrectinib (20, Augtyro™) | ROS1-positive metastatic non-small cell lung cancer | Trk, ROS1, ALK | — | FDA approval in 2023 |
The FDA approval of lorlatinib in 2018 was a milestone that marked the first small-molecule kinase inhibitor macrocycle approved for use in human disease. From the success stories of lorlatinib, pacritinib, and repotrectinib, generating macrocycles from acyclic kinase inhibitors can provide a substantial clinical advantage. Promiscuous inhibitors can be tuned for precision, broad-spectrum mutations can be effectively targeted, and pharmacokinetic properties of acyclic drugs can be improved. Macrocyclic kinase inhibitors compared to their acyclic counterparts are rigid in conformation with reduced number of rotatable bonds thereby improving selectivity by avoiding steric clashes with mutations. For instance, selitrectinib and repotrectinib can better accommodate TrkA mutations while their acyclic counterpart (larotrectinib) engages in steric clashes with these mutations. It is also important to note that the rigid macrocycles developed from acyclic counterparts are not always selective. This was observed in the development of lorlatinib, as intermediate 16a lacked selectivity for ALKL1196M and exhibited higher affinity for TrkB.19 This suggests that selectivity profiles of macrocycles must be determined on a case-by-case basis. To improve selectivity of 16a, substitution on the pyrazole ring system was modified from methyl to cyano showcasing that selectivity profiles can be fine-tuned by exploring substitutions within the macrocycle rather than focusing solely on linker length and composition.
The success stories of macrocyclic inhibitors suggest this approach can be effectively utilized by groups with active drug discovery programs targeting the kinome. The design of macrocyclic inhibitors can be completed by predicting the binding mode of an inhibitor and identifying regions in proximity for cyclization via a chemically suitable linker. Key attributes of the macrocycle such as linker length and composition can be adjusted to optimize for a specific inhibition profile. For instance, use of amide linkers to generate zotiraciclib analogs displayed weaker CDK2 activity (IC50 > 10 μM) as compared to benzylic amine linkers. Among benzylic ethers and benzylic ether amines, selectivity was enhanced by ether amines. This suggests that linkers can also be involved in interaction/clashes with amino acid residues in the active site. The strategic development of different linker compositions is necessary to achieve desired selectivity and potency profiles. Furthermore, the macrocycle architecture as a whole can be modified to augment selectivity of the scaffold. This is seen with pacritinib and SB1578 as the only difference is the heterocycle to which the linker is attached – changing from a six-membered ring to five-membered ring can alter selectivity of the compound.
The linker also affects physicochemical properties of the compounds thereby altering blood brain barrier penetration properties. This is mainly due to a restriction in rotatable bonds from the macrocyclization process as CNS available drugs have significantly fewer rotatable bonds than non-CNS available drugs. This phenomenon is observed in the development of lorlatinib. Crizotinib, the acyclic counterpart of lorlatinib, has poor CNS penetration and is not able to effectively target CNS metastases. The macrocycle lorlatinib with restriction in rotation of rotatable bonds exhibited reduced efflux potential and achieved greater CNS penetration compared to crizotinib. The metabolic stability of lorlatinib was also found to be higher compared to crizotinib.
Despite the potential for significant improvements in drug properties using macrocycles, the implementation of macrocyclization also comes with distinct disadvantages. These challenges include difficulties in chemical synthesis, a molecular weight increase, and a potential negative effect on solubility and metabolic profiles. Issues dealing with chemical synthesis can delay macrocycle optimization, which is commonly observed during ring-closing reactions that are frequently low yielding. The choice of linker is also important since the linker can negatively impact metabolic and solubility profiles. In addition, macrocyclization leads to an increase in molecular weight and often clog P, which can negatively impact metabolic and solubility profiles.
Wider adoption of macrocyclic-based drug development will create new chemical space, which will allow the expansion of intellectual property in an otherwise crowded area within the kinome. Other medicinal chemistry practices such as in silico approaches and X-ray crystallography are necessary to improve the design of macrocyclic templates. Synthetic advances in the generation of functionalized macrocycles are also required to make macrocyclic drug discovery routine and robust.91,92 Enhancing druggability by implementing macrocycle-focused drug discovery will expand structural diversity, which in turn will uncover new macrocyclic templates with augmented inhibitory profiles. Medicinal chemists should now consider macrocyclization as a clinically validated drug discovery strategy when targeting the kinome and implement this method to fine-tune inhibitors for specific kinase targets.
Author contributions
All the authors contributed to the conceptualization, analysis, and discussion of the article.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of General Medical Sciences (P20GM109005), a UAMS College of Pharmacy Seed grant, and a 2020 UAMS College of Pharmacy Summer Research Fellowship.
References
- Maurer G. Tarkowski B. Baccarini M. Oncogene. 2011;30:3477–3488. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- Paul M. K. Mukhopadhyay A. K. Int. J. Med. Sci. 2004;1:101–115. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardelli A. Parsons D. W. Silliman N. Ptak J. Szabo S. Saha S. Markowitz S. Willson J. K. V. Parmigiani G. Kinzler K. W. Vogelstein B. Velculescu V. E. Science. 2003;300:949–949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- Kittler H. Tschandl P. Br. J. Dermatol. 2018;178:26–27. doi: 10.1111/bjd.16119. [DOI] [PubMed] [Google Scholar]
- Cohen M. H. Williams G. Johnson J. R. Duan J. Gobburu J. Rahman A. Benson K. Leighton J. Kim S. K. Wood R. Rothmann M. Chen G. U K. M. Staten A. M. Pazdur R. Clin. Cancer Res. 2002;8:935–942. [PubMed] [Google Scholar]
- Dagher R. Cohen M. Williams G. Rothmann M. Gobburu J. Robbie G. Rahman A. Chen G. Staten A. Griebel D. Pazdur R. Clin. Cancer Res. 2002;8:3034–3038. [PubMed] [Google Scholar]
- Bhullar K. S. Lagarón N. O. McGowan E. M. Parmar I. Jha A. Hubbard B. P. Rupasinghe H. P. V. Mol. Cancer. 2018;17:48. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight Z. A. Shokat K. M. Chem. Biol. 2005;12:621–637. doi: 10.1016/j.chembiol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Duong-Ly K. C. Peterson J. R. Curr. Protoc. Pharmacol. 2013;60:2.9.1–2.9.14. doi: 10.1002/0471141755.ph0209s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Cross D. Jänne P. A. Nat. Rev. Drug Discovery. 2021;20:551–569. doi: 10.1038/s41573-021-00195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovly C. M. Shaw A. T. Clin. Cancer Res. 2014;20:2249–2256. doi: 10.1158/1078-0432.CCR-13-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram A. M. Chang M. T. Jonsson P. Drilon A. Nat. Rev. Clin. Oncol. 2017;14:735–748. doi: 10.1038/nrclinonc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C. Fresnais M. Gilon M. Jérusalem G. Longuespée R. Sounni N. E. Cancers. 2020;12:731. doi: 10.3390/cancers12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Yang P. L. Gray N. S. Nat. Rev. Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski Jr R. Pharmacol. Res. 2022;175:106037. doi: 10.1016/j.phrs.2021.106037. [DOI] [PubMed] [Google Scholar]
- Newton R. Waszkowycz B. Seewooruthun C. Burschowsky D. Richards M. Hitchin S. Begum H. Watson A. French E. Hamilton N. Jones S. Lin L.-Y. Waddell I. Echalier A. Bayliss R. Jordan A. M. Ogilvie D. ACS Med. Chem. Lett. 2020;11:497–505. doi: 10.1021/acsmedchemlett.9b00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah V. Velcheti V. Tuch B. B. Ebata K. Busaidy N. L. Cabanillas M. E. Wirth L. J. Stock S. Smith S. Lauriault V. Corsi-Travali S. Henry D. Burkard M. Hamor R. Bouhana K. Winski S. Wallace R. D. Hartley D. Rhodes S. Reddy M. Brandhuber B. J. Andrews S. Rothenberg S. M. Drilon A. Ann. Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah V. Gainor J. F. Rahal R. Brubaker J. D. Kim J. L. Maynard M. Hu W. Cao Q. Sheets M. P. Wilson D. Wilson K. J. DiPietro L. Fleming P. Palmer M. Hu M. I. Wirth L. Brose M. S. Ou S.-H. I. Taylor M. Garralda E. Miller S. Wolf B. Lengauer C. Guzi T. Evans E. K. Cancer Discovery. 2018;8:836–849. doi: 10.1158/2159-8290.CD-18-0338. [DOI] [PubMed] [Google Scholar]
- Johnson T. W. Richardson P. F. Bailey S. Brooun A. Burke B. J. Collins M. R. Cui J. J. Deal J. G. Deng Y.-L. Dinh D. Engstrom L. D. He M. Hoffman J. Hoffman R. L. Huang Q. Kania R. S. Kath J. C. Lam H. Lam J. L. Le P. T. Lingardo L. Liu W. McTigue M. Palmer C. L. Sach N. W. Smeal T. Smith G. L. Stewart A. E. Timofeevski S. Zhu H. Zhu J. Zou H. Y. Edwards M. P. J. Med. Chem. 2014;57:4720–4744. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- Drilon A. Nagasubramanian R. Blake J. F. Ku N. Tuch B. B. Ebata K. Smith S. Lauriault V. Kolakowski G. R. Brandhuber B. J. Larsen P. D. Bouhana K. S. Winski S. L. Hamor R. Wu W.-I. Parker A. Morales T. H. Sullivan F. X. DeWolf W. E. Wollenberg L. A. Gordon P. R. Douglas-Lindsay D. N. Scaltriti M. Benayed R. Raj S. Hanusch B. Schram A. M. Jonsson P. Berger M. F. Hechtman J. F. Taylor B. S. Andrews S. Rothenberg S. M. Hyman D. M. Cancer Discovery. 2017;7:963–972. doi: 10.1158/2159-8290.CD-17-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers E. M. Hale S. P. Lee J. Terrett N. K. Nat. Rev. Drug Discovery. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- Mallinson J. Collins I. Future Med. Chem. 2012;4:1409–1438. doi: 10.4155/fmc.12.93. [DOI] [PubMed] [Google Scholar]
- Liang Y. Fang R. Rao Q. Molecules. 2022;27:2837. doi: 10.3390/molecules27092837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M. D. Sekharan S. J. Med. Chem. 2019;62:6843–6853. doi: 10.1021/acs.jmedchem.8b01985. [DOI] [PubMed] [Google Scholar]
- Marsault E. Peterson M. L. J. Med. Chem. 2011;54:1961–2004. doi: 10.1021/jm1012374. [DOI] [PubMed] [Google Scholar]
- Garcia Jimenez D. Poongavanam V. Kihlberg J. J. Med. Chem. 2023;66:5377–5396. doi: 10.1021/acs.jmedchem.3c00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordanetto F. Kihlberg J. J. Med. Chem. 2014;57:278–295. doi: 10.1021/jm400887j. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J. Hoffman R. Talpaz M. Gerds A. T. Stein B. Gupta V. Szoke A. Drummond M. Pristupa A. Granston T. Daly R. Al-Fayoumi S. Callahan J. A. Singer J. W. Gotlib J. Jamieson C. Harrison C. Mesa R. Verstovsek S. JAMA Oncol. 2018;4:652–659. doi: 10.1001/jamaoncol.2017.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa R. A. Vannucchi A. M. Mead A. Egyed M. Szoke A. Suvorov A. Jakucs J. Perkins A. Prasad R. Mayer J. Demeter J. Ganly P. Singer J. W. Zhou H. Dean J. P. te Boekhorst P. A. Nangalia J. Kiladjian J.-J. Harrison C. N. Lancet Haematol. 2017;4:e225–e236. doi: 10.1016/S2352-3026(17)30027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration Approves Augtyro™ (repotrectinib), a Next-Generation Tyrosine Kinase Inhibitor (TKI), for the Treatment of Locally Advanced or Metastatic ROS1-Positive Non-Small Cell Lung Cancer (NSCLC), https://news.bms.com/news/corporate-financial/2023/US-Food-and-Drug-Administration-Approves-Augtyro/default.aspx
- Rane S. G. Reddy E. P. Oncogene. 2000;19:5662–5679. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- Yamaoka K. Saharinen P. Pesu M. Holt V. E. T. Silvennoinen O. O'Shea J. J. Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C. Ugo V. Le Couédic J.-P. Staerk J. Delhommeau F. Lacout C. Garçon L. Raslova H. Berger R. Bennaceur-Griscelli A. Villeval J. L. Constantinescu S. N. Casadevall N. Vainchenker W. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Baxter E. J. Scott L. M. Campbell P. J. East C. Fourouclas N. Swanton S. Vassiliou G. S. Bench A. J. Boyd E. M. Curtin N. Scott M. A. Erber W. N. Green A. R. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Levine R. L. Wadleigh M. Cools J. Ebert B. L. Wernig G. Huntly B. J. P. Boggon T. J. Wlodarska I. Clark J. J. Moore S. Adelsperger J. Koo S. Lee J. C. Gabriel S. Mercher T. D'Andrea A. Fröhling S. Döhner K. Marynen P. Vandenberghe P. Mesa R. A. Tefferi A. Griffin J. D. Eck M. J. Sellers W. R. Meyerson M. Golub T. R. Lee S. J. Gilliland D. G. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Levine R. L. Best Pract. Res., Clin. Haematol. 2009;22:489–494. doi: 10.1016/j.beha.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Tefferi A. Leuk. Lymphoma. 2008;49:388–397. doi: 10.1080/10428190801895360. [DOI] [PubMed] [Google Scholar]
- Gilliland D. G. Best Pract. Res., Clin. Haematol. 2003;16:409–417. doi: 10.1016/S1521-6926(03)00063-X. [DOI] [PubMed] [Google Scholar]
- Quentmeier H. Reinhardt J. Zaborski M. Drexler H. G. Leukemia. 2003;17:120–124. doi: 10.1038/sj.leu.2402740. [DOI] [PubMed] [Google Scholar]
- Kindler T. Lipka D. B. Fischer T. Blood. 2010;116:5089–5102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- William A. D. Lee A. C. H. Blanchard S. Poulsen A. Teo E. L. Nagaraj H. Tan E. Chen D. Williams M. Sun E. T. Goh K. C. Ong W. C. Goh S. K. Hart S. Jayaraman R. Pasha M. K. Ethirajulu K. Wood J. M. Dymock B. W. J. Med. Chem. 2011;54:4638–4658. doi: 10.1021/jm200326p. [DOI] [PubMed] [Google Scholar]
- William A. D. Lee A. C. H. Goh K. C. Blanchard S. Poulsen A. Teo E. L. Nagaraj H. Lee C. P. Wang H. Williams M. Sun E. T. Hu C. Jayaraman R. Pasha M. K. Ethirajulu K. Wood J. M. Dymock B. W. J. Med. Chem. 2012;55:169–196. doi: 10.1021/jm201112g. [DOI] [PubMed] [Google Scholar]
- Cai D. Latham, Jr. V. M. Zhang X. Shapiro G. I. Cancer Res. 2006;66:9270–9280. doi: 10.1158/0008-5472.CAN-06-1758. [DOI] [PubMed] [Google Scholar]
- Shapiro G. I. J. Clin. Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- Knockaert M. Greengard P. Meijer L. Trends Pharmacol. Sci. 2002;23:417–425. doi: 10.1016/S0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- Goh K. C. Novotny-Diermayr V. Hart S. Ong L. C. Loh Y. K. Cheong A. Tan Y. C. Hu C. Jayaraman R. William A. D. Sun E. T. Dymock B. W. Ong K. H. Ethirajulu K. Burrows F. Wood J. M. Leukemia. 2012;26:236–243. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- Pasha R. J. M. K. Reddy V. P. Yeo P. Goh E. Williams A. Goh K. C. Kantharaj E. Drug Metab. Lett. 2012;6:33–42. doi: 10.2174/187231212800229336. [DOI] [PubMed] [Google Scholar]
- Wu J. Yuan Y. Long Priel D. A. Fink D. Peer C. J. Sissung T. M. Su Y.-T. Pang Y. Yu G. Butler M. K. Mendoza T. R. Vera E. Ahmad S. Bryla C. Lindsley M. Grajkowska E. Mentges K. Boris L. Antony R. Garren N. Siegel C. Lollo N. Cordova C. Aboud O. Theeler B. J. Burton E. M. Penas-Prado M. Leeper H. Gonzales J. Armstrong T. S. Calvo K. R. Figg W. D. Kuhns D. B. Gallin J. I. Gilbert M. R. Clin. Cancer Res. 2021;27:3298–3306. doi: 10.1158/1078-0432.CCR-20-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William A. D. Lee A. C. H. Poulsen A. Goh K. C. Madan B. Hart S. Tan E. Wang H. Nagaraj H. Chen D. Lee C. P. Sun E. T. Jayaraman R. Pasha M. K. Ethirajulu K. Wood J. M. Dymock B. W. J. Med. Chem. 2012;55:2623–2640. doi: 10.1021/jm201454n. [DOI] [PubMed] [Google Scholar]
- Buch M. H. Emery P. Curr. Opin. Rheumatol. 2011;23:245–251. doi: 10.1097/BOR.0b013e3283454124. [DOI] [PubMed] [Google Scholar]
- Dhillon S. Drugs. 2017;77:1987–2001. doi: 10.1007/s40265-017-0835-9. [DOI] [PubMed] [Google Scholar]
- FDA, Janus Kinase (JAK) inhibitors: Drug Safety Communication - FDA Requires Warnings about Increased Risk of Serious Heart-related Events, Cancer, Blood Clots, and Death, https://www.fda.gov/safety/medical-product-safety-information/janus-kinase-jak-inhibitors-drug-safety-communication-fda-requires-warnings-about-increased-risk
- Paunović V. Carroll H. P. Vandenbroeck K. Gadina M. Rheumatology. 2008;47:771–776. doi: 10.1093/rheumatology/kem352. [DOI] [PubMed] [Google Scholar]
- Ishizaki M. Muromoto R. Akimoto T. Ohshiro Y. Takahashi M. Sekine Y. Maeda H. Shimoda K. Oritani K. Matsuda T. Int. Immunol. 2011;23:575–582. doi: 10.1093/intimm/dxr057. [DOI] [PubMed] [Google Scholar]
- Dehlin M. Bokarewa M. Rottapel R. Foster S. J. Magnusson M. Dahlberg L. E. Tarkowski A. PLoS One. 2008;3:e3633. doi: 10.1371/journal.pone.0003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalac T., BMS Wins Landmark Approval in Plaque Psoriasis, Avoids Black Box Label, https://www.biospace.com/article/bms-wins-clean-label-approval-for-first-plaque-psoriasis-oral-innovation-in-a-decade/, (accessed 03/09/2023)
- Mease P. J. Deodhar A. A. van der Heijde D. Behrens F. Kivitz A. J. Neal J. Kim J. Singhal S. Nowak M. Banerjee S. Ann. Rheum. Dis. 2022;81:815. doi: 10.1136/annrheumdis-2021-221664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen A., William A. D. and Dymock B. W., in Macrocycles in Drug Discovery, ed. J. Levin, The Royal Society of Chemistry, 2014, 10.1039/9781782623113-00141 [DOI] [Google Scholar]
- Morris S. W. Naeve C. Mathew P. James P. L. Kirstein M. N. Cui X. Witte D. P. Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- Bilsland J. G. Wheeldon A. Mead A. Znamenskiy P. Almond S. Waters K. A. Thakur M. Beaumont V. Bonnert T. P. Heavens R. Whiting P. McAllister G. Munoz-Sanjuan I. Neuropsychopharmacology. 2008;33:685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- Lee H. W. Kim K. Kim W. Ko Y. H. Hematol. Oncol. 2008;26:108–113. doi: 10.1002/hon.841. [DOI] [PubMed] [Google Scholar]
- Griffin C. A. Hawkins A. L. Dvorak C. Henkle C. Ellingham T. Perlman E. J. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- Mano H. Cancer Sci. 2008;99:2349–2355. doi: 10.1111/j.1349-7006.2008.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. T. Solomon B. Clin. Cancer Res. 2011;17:2081–2086. doi: 10.1158/1078-0432.CCR-10-1591. [DOI] [PubMed] [Google Scholar]
- Soda M. Choi Y. L. Enomoto M. Takada S. Yamashita Y. Ishikawa S. Fujiwara S.-i. Watanabe H. Kurashina K. Hatanaka H. Bando M. Ohno S. Ishikawa Y. Aburatani H. Niki T. Sohara Y. Sugiyama Y. Mano H. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- Roskoski R. Pharmacol. Res. 2017;117:343–356. doi: 10.1016/j.phrs.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Lei Y. Lei Y. Shi X. Wang J. Oncol. Lett. 2022;24:277. doi: 10.3892/ol.2022.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X. Shao Y. Qin H.-F. Tai Y.-H. Gao H.-J. Thorac. Cancer. 2018;9:423–430. doi: 10.1111/1759-7714.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J. J. Tran-Dubé M. Shen H. Nambu M. Kung P.-P. Pairish M. Jia L. Meng J. Funk L. Botrous I. McTigue M. Grodsky N. Ryan K. Padrique E. Alton G. Timofeevski S. Yamazaki S. Li Q. Zou H. Christensen J. Mroczkowski B. Bender S. Kania R. S. Edwards M. P. J. Med. Chem. 2011;54:6342–6363. doi: 10.1021/jm2007613. [DOI] [PubMed] [Google Scholar]
- Ou S.-H. I. Kwak E. L. Siwak-Tapp C. Dy J. Bergethon K. Clark J. W. Camidge D. R. Solomon B. J. Maki R. G. Bang Y.-J. Kim D.-W. Christensen J. Tan W. Wilner K. D. Salgia R. Iafrate A. J. J. Thorac. Oncol. 2011;6:942–946. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- Pao W. Chmielecki J. Nat. Rev. Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. Oya Y. Tanaka K. Shimizu J. Horio Y. Kuroda H. Sakao Y. Hida T. Yatabe Y. Lung Cancer. 2016;97:43–47. doi: 10.1016/j.lungcan.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Mikitsh J. L. Chacko A.-M. Perspect. Med. Chem. 2014;6:11–24. doi: 10.4137/PMC.S13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. J. Reichardt L. F. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Vaishnavi A. Le A. T. Doebele R. C. Cancer Discovery. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco E. Scaltriti M. Drilon A. Nat. Rev. Clin. Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman J. F. Mod. Pathol. 2022;35:298–305. doi: 10.1038/s41379-021-00913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W. Lakkaniga N. R. Carlomagno F. Santoro M. McDonald N. Q. Lv F. Gunaganti N. Frett B. Li H.-y. J. Med. Chem. 2019;62:1731–1760. doi: 10.1021/acs.jmedchem.8b01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardini E. Menichincheri M. Banfi P. Bosotti R. De Ponti C. Pulci R. Ballinari D. Ciomei M. Texido G. Degrassi A. Avanzi N. Amboldi N. Saccardo M. B. Casero D. Orsini P. Bandiera T. Mologni L. Anderson D. Wei G. Harris J. Vernier J.-M. Li G. Felder E. Donati D. Isacchi A. Pesenti E. Magnaghi P. Galvani A. Mol. Cancer Ther. 2016;15:628–639. doi: 10.1158/1535-7163.MCT-15-0758. [DOI] [PubMed] [Google Scholar]
- Al-Salama Z. T. Keam S. J. Drugs. 2019;79:1477–1483. doi: 10.1007/s40265-019-01177-y. [DOI] [PubMed] [Google Scholar]
- Russo M. Misale S. Wei G. Siravegna G. Crisafulli G. Lazzari L. Corti G. Rospo G. Novara L. Mussolin B. Bartolini A. Cam N. Patel R. Yan S. Shoemaker R. Wild R. Di Nicolantonio F. Bianchi A. S. Li G. Siena S. Bardelli A. Cancer Discovery. 2016;6:36–44. doi: 10.1158/2159-8290.CD-15-0940. [DOI] [PubMed] [Google Scholar]
- Drilon A. Ou S.-H. I. Cho B. C. Kim D.-W. Lee J. Lin J. J. Zhu V. W. Ahn M.-J. Camidge D. R. Nguyen J. Zhai D. Deng W. Huang Z. Rogers E. Liu J. Whitten J. Lim J. K. Stopatschinskaja S. Hyman D. M. Doebele R. C. Cui J. J. Shaw A. T. Cancer Discovery. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- El-Nassan H. B. Al-Qadhi M. A. Eur. J. Med. Chem. 2023;258:115618. doi: 10.1016/j.ejmech.2023.115618. [DOI] [PubMed] [Google Scholar]
- Dungo R. T. Keating G. M. Drugs. 2013;73:1503–1515. doi: 10.1007/s40265-013-0111-6. [DOI] [PubMed] [Google Scholar]
- Koch A. L. Vellanki P. J. Drezner N. Li X. Mishra-Kalyani P. S. Shen Y. L. Xia H. Li Y. Liu J. Zirkelbach J. F. Palazov E. Gamarian A. Choo Q. Girčys A. Rohr U.-P. Fesenko N. Spillman D. Pazdur R. Beaver J. A. Singh H. Clin. Cancer Res. 2021;27:6638–6643. doi: 10.1158/1078-0432.CCR-21-1034. [DOI] [PubMed] [Google Scholar]
- Saha D. Kharbanda A. Yan W. Lakkaniga N. R. Frett B. Li H.-Y. J. Med. Chem. 2020;63:441–469. doi: 10.1021/acs.jmedchem.9b00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F. Sanford M. Drugs. 2014;74:263–271. doi: 10.1007/s40265-014-0178-8. [DOI] [PubMed] [Google Scholar]
- Scott L. J. Drugs. 2019;79:201–206. doi: 10.1007/s40265-018-1044-x. [DOI] [PubMed] [Google Scholar]
- Qin H. Patel M. R. Int. J. Mol. Sci. 2022;23:2916. doi: 10.3390/ijms23062916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. Bourne P. E. Drug Discovery Today. 2018;23:727–735. doi: 10.1016/j.drudis.2018.01.035. [DOI] [PubMed] [Google Scholar]
- Amrhein J. A. Knapp S. Hanke T. J. Med. Chem. 2021;64:7991–8009. doi: 10.1021/acs.jmedchem.1c00217. [DOI] [PubMed] [Google Scholar]
- Mortensen K. T. Osberger T. J. King T. A. Sore H. F. Spring D. R. Chem. Rev. 2019;119:10288–10317. doi: 10.1021/acs.chemrev.9b00084. [DOI] [PubMed] [Google Scholar]