Abstract
Objectives
The presented study aimed to explore the presence and the self-identification of depressive symptoms among patients with rheumatic musculoskeletal diseases (RMDs) through the use of the Patient Health Questionnaire (PHQ-9).
Methods
Between June and October 2019, patients from the regional association for people with RMDs in Lombardy, Italy (ALOMAR), were invited to participate in a cross-sectional online survey. Participants completed PHQ-9 along with a survey about their perception of depressive symptoms. Patients were stratified according to PHQ-9 score as follows: not depressed (<4), subclinical or mild depression (5–9), moderate depression (10–14), moderately severe depression (10–14), and severe depression (20–27). Descriptive statistics and analyses of variance were used to explore data.
Results
Of the 192 RMD patients who completed PHQ-9, 35 (18.2%) were not depressed, 68 (35.4%) had subclinical or mild depression, 42 (21.9%) had moderate depression, 30 (15.6%) had moderately severe depression, and 17 (8.9%) had severe depression. Contrary to the above findings, only 16 respondents (8.3%) reported that they experienced depressive symptoms, and only 7 of the 16 were being followed by a psychiatrist. Respondents with higher PHQ-9 scores tended to have concomitant fibromyalgia, to be younger, and to be overweight.
Conclusions
The current results indicate the overall burden of depressive symptoms in RMD patients. While clinical depression (PHQ-9 >10) was detected in 41.2% of respondents, only 8.3% reported that they experience depressive symptoms. Routine screening of RMD patients for depression is therefore critical.
Key Words: depression, mood disorder, patient perspective, PHQ-9, research, rheumatic diseases
While major depression is one of the most prevalent and disabling disorders worldwide, it is often undiagnosed and therefore undertreated.1 Depressive symptoms play an important role in rheumatic musculoskeletal diseases (RMDs), representing one of the most common medical comorbidities and the most frequent psychiatric conditions associated with RMD.2–6 Rheumatic musculoskeletal diseases are a heterogeneous group of disorders with a high rate of morbidity and mortality.7–10 Thus, the concomitant presence of depressive symptoms among RMD patients implies a considerable economic and social burden.7–11
Depressive symptoms are associated with numerous deleterious outcomes, including increased mortality, work disability, disease activity, treatment noncompliance, physical dysfunction, pain, and fatigue.3,12,13 Moreover, depression impacts negatively on patient global assessment, thus diminishing clinical remission. While it is increasingly apparent that depressive symptoms are an intrinsic component of RMDs, they are still poorly recognized and managed in these patients.2,3,14–16
Although the co-occurrence of RMDs and depressive symptoms has been widely described, a common pathogenic pathway is currently not well understood. Recent evidence suggests shared pathways on the basis of circadian oscillations of inflammatory mediators, such as tumor necrosis factor α and interleukin 6 (IL-6), in patients with RMDs and depressive symptoms.17 Moreover, it has been demonstrated that IL-6 plays a crucial role in inducing and worsening mood disturbances by both increasing inflammation and directly affecting nociceptive neurons and the hypothalamic-pituitary-adrenal axis. These findings are critical to the optimization of treatment choices in patients with inflammatory arthritis.
A considerable proportion of patients with RMD respond poorly to therapy. However, the reason for this is currently not well understood. It is widely accepted that depressive symptoms may reduce the odds of full treatment efficacy and therefore diminish the probability of improvement over time.12
Despite a growing interest in the association between depressive symptoms and RMD, the self-identification of depressive symptoms among RMD patients is currently not known. Therefore, the current cross-sectional online study explored the perception of depressive symptoms among Italian patients with RMDs in contrast to the existence of depressive symptoms as measured by the Patient Health Questionnaire 9 (PHQ-9).
METHODS
Survey Design and Administration
The current nonprofit cross-sectional study was conducted online to screen for the presence and the self-identification of depressive symptoms in patients with RMDs. The ethical committee of the University of Milan granted approval for all study procedures (20.05.19-18/19). Between June and October 2019, the online questionnaire was disseminated to the patient association ALOMAR using a mailing list, website, and social networks (http://www.alomar.it/). Data protection was ensured by the information technology (IT) service of the Università degli Studi di Milano (Italy).
Participants were not remunerated and gave voluntary consent to complete the survey. All patient responses remained anonymous. The survey included questions about basic patient demographics and background information. Patients were asked to self-report disease characteristics and comorbidities. The results of the study were summarized by means and percentages and reported according to the Guidance for Reporting Involvement of Patients and the Public checklist.18,19
Patient Health Questionnaire 9
The PHQ-9, one of the most widely used depression scales in clinical practice,20 was administered to patients with RMDs.6,21,22 A recent manuscript confirmed the validity and reliability of PHQ-9 to assess depressive symptoms in patients affected by RMDs.23
The PHQ-916 rates frequency of symptoms over the past 2 weeks on a 0- to 3-point Likert-type scale (from “not at all” to “nearly every day”), with total scores ranging from 0 to 27.20 The items on PHQ-9 are derived from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition classification system and rate (1) anhedonia, (2) depressed mood, (3) trouble sleeping, (4) feeling tired, (5) change in appetite, (6) feelings of guilt or worthlessness, (7) trouble concentrating, (8) feeling slowed down or restless, and (9) suicidal thoughts. Patients were stratified according to PHQ-9 cutoff scores as follows: not depressed (<4), subclinical or mild depression (5–9), moderate depression (10–14), moderately severe depression (15–19), and severe depression (20–27).
Statistical Analysis
Descriptive statistics were used to summarize patient demographics, clinical data, and PHQ-9 results. Patients were grouped according to self-reported diagnosis as follows: group 1 (inflammatory arthritis), patients with rheumatoid arthritis (RA), psoriatic arthritis, and ankylosing spondylitis; group 2 (connective tissue diseases/vasculitis), patients with systemic lupus erythematous, undifferentiated or mixed connective tissue disease, systemic sclerosis, inflammatory myositis, Sjögren syndrome, and vasculitis; and group 3 (miscellaneous RMDs), all patients with RMDs other than those listed in groups 1 and 2 (osteoarthritis, polymyalgia rheumatica, fibromyalgia, and crystal arthropathies).
A 1-way analysis of variance was performed to compare PHQ-9 scores among the 3 groups. In addition, a multivariate linear regression analysis was conducted to explore the association between PHQ-9 score (dependent variable) and disease duration, age, age at diagnosis, sex, presence of obesity/overweight, and presence of fibromyalgia (independent variables). All analyses were performed using R software, version 3.5.2, with package Rcmdr (version 2.5–1).
RESULTS
A total of 192 RMD patients (124 with inflammatory arthritis, 49 with connective tissue diseases/vasculitis, and 19 with miscellaneous RMDs) responded to PHQ-9. One hundred seventy respondents were women (88.5%) with a median age of 50 years. All results were obtained from questionnaire responses; no medical records were reviewed. Gastroesophageal reflux disease was the most frequently reported comorbidity and was present in 37 of 192 patients (19.2%) (Table 1).
TABLE 1.
Characteristics of Patients With RMDs
| Inflammatory Arthritisa | Connective Tissue Diseaseb | Miscellaneousc | Total | |
|---|---|---|---|---|
| No. patients | 124 | 49 | 19 | 192 |
| Sex, male/female, n | 16/108 | 46/3 | 16/3 | 78/114 |
| Age, median (IQR), y | 49 (37.8–56) | 48 (38–60) | 52 (50–60) | 50 (38–57.3) |
| Disease duration, median (IQR), y | 9 (3–15) | 6 (1–13) | 6 (0.5–9.5) | 7 (3–14) |
| Age at diagnosis, median (IQR), y | 37.5 (22.8–47) | 38 (27–48) | 48 (41–51) | 38.5 (26.8–48) |
| Secondary fibromyalgia, n (%) | 25 (20.2%) | 6 (12.2%) | — | 31 (16.1%) |
| Concomitant OA, n (%) | 9 (7.3%) | 2 (4%) | — | 11 (5.7%) |
| Secondary sicca syndrome, n (%) | 6 (4.8%) | 5 (10.2%) | — | 11 (5.7%) |
| Comorbidities, n (%) | ||||
| Arterial hypertension | 22 (17.7%) | 5 (10.2%) | 4 (21%) | 31 (16.1%) |
| Diabetes | 5 (4%) | 1 (2%) | 1 (5.3%) | 7 (3.6%) |
| CV disease | 8 (6.5%) | 1 (2%) | 3 (15.8%) | 12 (6.2%) |
| Overweight/obesity | 16 (12.9%) | 3 (6.1%) | 4 (21%) | 23 (12%) |
| Depressive symptoms | 8 (6.5%) | 3 (6.1%) | 5 (26.3%) | 16 (8.3%) |
| Anxiety | 12 (9.7%) | 4 (8.1%) | 6 (31.6%) | 22 (11.5%) |
| Gastritis | 10 (8%) | 3 (6.1%) | 4 (21%) | 17 (8.9%) |
| GERD | 20 (16.1%) | 9 (18.4%) | 8 (42%) | 37 (19.3%) |
| Other GI disease | 20 (16.1%) | 2 (4%) | 5 (26.3%) | 27 (14%) |
| Thyroiditis | 16 (12.9%) | 11 (22.5%) | 4 (21%) | 31 (16.1%) |
| Ocular diseases | 13 (10.4%) | 5 (10.2%) | 3 (15.8%) | 21 (%) |
aRheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis.
bConnective tissue disease and vasculitis.
cOsteoarthritis, gout, chondrocalcinosis, polymyalgia, primary fibromyalgia.
CV, cardiovascular; GERD, gastroesophageal reflux disease; GI, gastrointestinal; IQR, interquartile range; OA, osteoarthritis.
According to PHQ-9 score, of the 192 respondents, 35 (18.2%) were not depressed. Depression was subclinical or mild in 68 (35.4%), moderate in 42 (21.9%), moderately severe in 30 (15.6%), and severe in 17 patients (8.9%). Among the participants with depression, only 16 (8.3%) perceived themselves to be depressed, and 7 of the 16 were followed by a psychiatrist.
There were no statistically significant differences in PHQ-9 scores between the 3 RMD groups (p = 0.2733) (Fig. 1). In group 1 with inflammatory arthritis (n = 124), 23 participants (18.5%) were not depressed. Depression was subclinical or mild in 41 (33%), moderate in 26 (21%), moderately severe in 21 (17%), and severe in 13 (10.5%). Among participants in group 1, only 8 (6.5%) self-identified as depressed, and only 3 of the 8 were followed by a psychiatrist.
FIGURE 1.
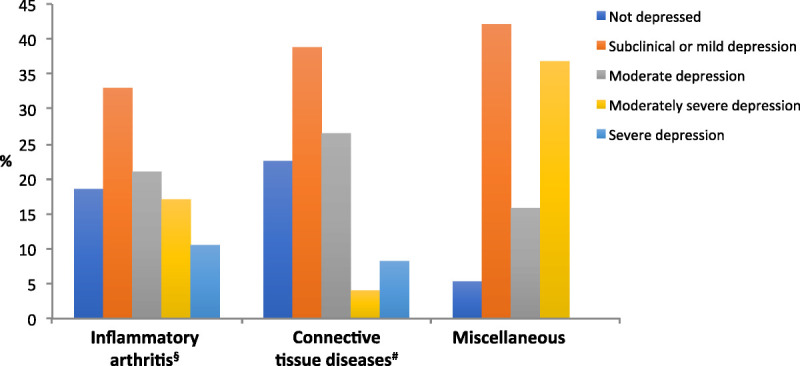
Depressive symptom severity according to PHQ-9 among groups of patients with inflammatory arthritis, connective tissue diseases, and miscellaneous RMDs. §Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis. #Connective tissue disease and vasculitis. *Osteoarthritis, gout, chondrocalcinosis, polymyalgia, primary fibromyalgia.
In group 2 with connective tissue diseases and vasculitis (n = 49), 11 (22.5%) were not depressed. Depression was subclinical or mild in 19 (38.8%), moderate in 13 (26.5%), moderately severe in 2 (4%), and severe in 4 (8.2%). Among participants in group 2, only 3 (6%) self-identified as depressed, and only 1 of the 6 was followed by a psychiatrist.
In group 3 with miscellaneous RMDs (n = 19), 1 participant (5.3%) was not depressed, whereas 8 (42.1%) had subclinical or mild depression, 3 (15.8%) had moderate depression, and 7 (36.8%) had moderately severe depression. Among participants in group 3, 5 (26.3%) self-identified as depressed, and 3 of the 5 were being followed by a psychiatrist.
Finally, factors that have known comorbidities with depressive symptoms, such as fibromyalgia, being overweight and age were analyzed (Table 2 and Fig. 2). Of the 192 respondents, 46 had fibromyalgia, and 23 were overweight/obese. A multivariate linear regression analysis found a significant association between depressive symptom severity and age (estimate: −0.059; p = 0.046), fibromyalgia (estimate: 4.364; p < 0.001), and weight (estimate: 4.380; p < 0.001).
TABLE 2.
Multivariate Linear Regression Analysis for the Association Between Depressive Symptom Severity as Measured by PHQ-9 and Known Risk Factors
| Independent Variable Description | Estimate | SE | p value |
|---|---|---|---|
| Disease duration | 0.029 | 0.040 | 0.469 |
| Age at visit | −0.059 | 0.029 | 0.046 |
| Fibromylagia | 4.364 | 0.953 | <0.001 |
| Overweight/obesity | 4.380 | 4.380 | <0.001 |
| Sex | −2.302 | 1.262 | 0.069 |
FIGURE 2.
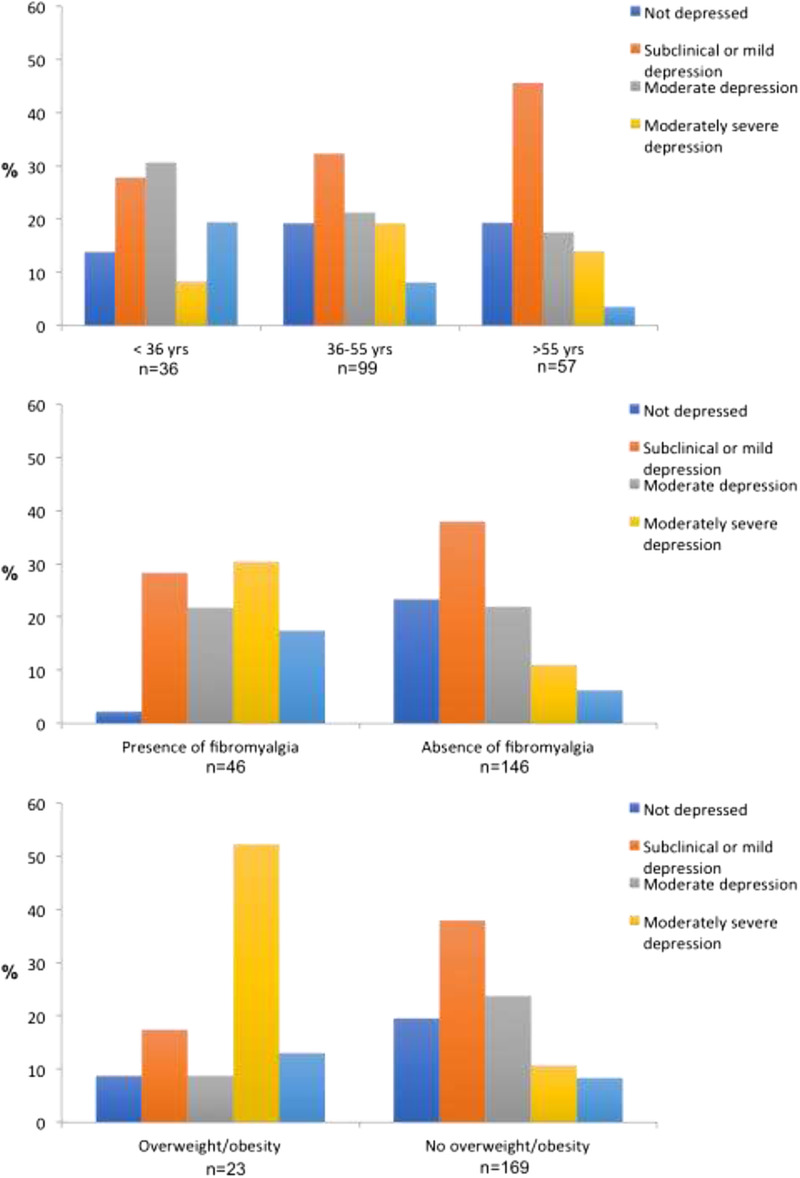
Depressive symptom severity according to PHQ-9 scores among patients with RMDs stratified by age, fibromyalgia, and weight disorder.
DISCUSSION
The current study highlights the burden of depressive symptoms among patients with RMDs, along with a lack of patient insight into this problem. Of note, 46.4% of the respondents scored more than 10 on PHQ-9, indicating clinical depression. However, only 8.3% of these patients self-identified as depressed, and even fewer (4%) were regularly followed up by a psychiatrist.
The percentage of RMD patients with depressive symptoms in the current study was higher than that previously reported.24–26 The prevalence for depression among individuals with RA has been reported as ranging from 15% to 39%,26 whereas the percentage of patients with systemic lupus erythematosus who experience depression is between 24% and 39%.25 Furthermore, the overall risk of depression among RA patients has been reported to be 2 to 3 times higher than that in the general population.24
In agreement with previous studies, the current study found significant associations between depressive symptoms and age, fibromyalgia, and being overweight. Interestingly, prior studies have postulated that the association between depression and being overweight/obese is bidirectional.6,27 One explanation for this finding is based on the inflammatory theory of depression in which proinflammatory cytokines, such as IL-6 and tumor necrosis factor α, have been reportedly observed in patients with depression, and concurrently, inflammation is correlated with metabolic abnormalities (e.g., leptin).28,29
Despite the high prevalence of depressive symptoms, few participants self-identified as depressed. One explanation for this finding is that depressive symptoms, including pain and fatigue, are also associated with RMDs,30 and therefore, patients who experience these symptoms ascribe them to their disease and not to depression. In addition, patients may be apprehensive of the stigma associated with psychiatric disorders and be reluctant to speak about depressive symptoms with doctors and relatives.31 Alternatively, patients may not have the opportunity to discuss psychiatric issues with their rheumatologists or general practitioners.31 In fact, prior studies have reported that rheumatologists seldom inquire about their patients’ mental health.31,32 However, considering the negative consequences of neglecting depressive symptoms, such as declining physical disability, poor quality of life, and increased suicidal risk, rheumatologists should monitor their patients’ mental health.33 Furthermore, RMD patients with depressive symptoms tend to be less compliant with medical treatment than patients without depression.34 These findings further support the importance of early recognition and treatment of depression in patients with RMDs.
The high prevalence of depression in individuals affected by RMDs suggests the possibility of a common biological pathway. Pain and physical disability associated with RMDs have been shown to increase inflammation and cause abnormalities in circadian rhythm, which in turn worsen physical functioning and can cause persistence of depressive symptoms.35 This deleterious loop can be disrupted by early diagnosis and appropriate treatment of mood disorders, leading to better mental health in patients affected by RMDs.35
Taken together, the findings from the current and prior studies underline the importance of the establishment of guidelines for the management of depressive symptoms in patients affected by RMDs. Specifically, a collaboration between rheumatologists and psychiatrists is vital for the management of the most severe patients. The presented study provides important data on the mental health of patients living with RMDs. There were many strengths to the study design. For example, the participants were assured anonymity, thus avoiding the potential confounding factor of fear about social stigmas. In addition, participants were able to respond to questionnaires online, avoiding confounding factors associated with the type of clinical setting where they were being treated (e.g., university clinics vs. general hospitals). However, limitations of the study include the cross-sectional design, the small sample size, and the potential biased selection of patients who are more confident with IT and therefore more prone to respond to online surveys.
Future research should better clarify shared underlying biological mechanisms of depression and RMDs and explore whether antidepressants can modify inflammatory parameters and, conversely, if inflammatory modulators, such as immunosuppressant therapies, can improve mood symptoms.
In conclusion, considering the current and previously reported findings, the importance of screening psychiatric comorbidities, particularly mood disorders, in patients with RMDs is critical. Treatment of comorbid psychiatric conditions is likely to improve the global course of RMDs.
KEY POINTS
Depressive symptoms are common among rheumatic patients, but are often not identified.
Depressive symptoms in rheumatic patients are related to age, fibromyalgia, and weight disorders.
ACKNOWLEDGMENTS
The authors thank the IT department of the University of Milan for their contributions to the survey and maintaining database integrity, the Lombard Association of Rheumatic Diseases (ALOMAR) for invaluable contributions to the planning and dissemination of the survey, and Gruppo Italiano per la Lotta alla Sclerodermia, for disseminating the survey through social media. The authors are grateful to all patients who volunteered their perspectives.
Footnotes
No specific funding was received from the general public, commercial, or not-for-profit sectors to carry out the work described in this article.
The authors declare no conflict of interest.
Contributor Information
Tommaso Schioppo, Email: t.schioppo@gmail.com.
Tania Ubiali, Email: tania.ubiali@gmail.com.
Silvia Ostuzzi, Email: silviaostuzzi.alomar@gmail.com.
Valentina Bollati, Email: valentina.bollati@unimi.it.
Massimiliano Buoli, Email: massimiliano.buoli@unimi.it.
Roberto Caporali, Email: roberto.caporali@unimi.it.
REFERENCES
- 1.Moussavi S Chatterji S Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. 2007;370:851–858. [DOI] [PubMed] [Google Scholar]
- 2.Sturgeon JA, Finan PH, Zautra AJ. Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nat Rev Rheumatol. 2016;12:532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moustafa AT Moazzami M Engel L, et al. Prevalence and metric of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. Semin Arthritis Rheum. 2020;50:84–94. [DOI] [PubMed] [Google Scholar]
- 4.Anyfanti P Gavriilaki E Pyrpasopoulou A, et al. Depression, anxiety, and quality of life in a large cohort of patients with rheumatic diseases: common, yet undertreated. Clin Rheumatol. 2016;35:733–739. [DOI] [PubMed] [Google Scholar]
- 5.Faezi ST Paragomi P Shahali A, et al. Prevalence and severity of depression and anxiety in patients with systemic sclerosis: an epidemiologic survey and investigation of clinical correlates. J Clin Rheumatol. 2017;23:80–86. [DOI] [PubMed] [Google Scholar]
- 6.Gota CE, Kaouk S, Wilke WS. Fibromyalgia and obesity: the association between body mass index and disability, depression, history of abuse, medications, and comorbidities. J Clin Rheumatol. 2015;21:289–295. [DOI] [PubMed] [Google Scholar]
- 7.Moradi-Lakeh M Forouzanfar MH Vollset SE, et al. Burden of musculoskeletal disorders in the eastern Mediterranean region, 1990–2013: findings from the Global Burden of Disease Study 2013. Ann Rheum Dis. 2017;76:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross M Smith E Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 Study. Ann Rheum Dis. 2014;73:1323–1330. [DOI] [PubMed] [Google Scholar]
- 9.Smith E Hoy DG Cross M, et al. The global burden of other musculoskeletal disorders: estimates from the Global Burden of Disease 2010 Study. Ann Rheum Dis. 2014;73:1462–1469. [DOI] [PubMed] [Google Scholar]
- 10.Safiri S Kolahi AA Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2019;78:1463–1471. [DOI] [PubMed] [Google Scholar]
- 11.Fiest KM Hitchon CA Bernstein CN, et al. Systematic review and meta-analysis of interventions for depression and anxiety in persons with rheumatoid arthritis. J Clin Rheumatol. 2017;23:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Studenic P, Smolen JS, Aletaha D. Near misses of ACR/EULAR criteria for remission: effects of patient global assessment in Boolean and index-based definitions. Ann Rheum Dis. 2012;71:1702–1705. [DOI] [PubMed] [Google Scholar]
- 13.Krug HE, Woods SR, Mahowald ML. The importance of identifying depression in patients with rheumatoid arthritis: evaluation of the Beck Depression Inventory. J Clin Rheumatol. 1997;3:248–257. [DOI] [PubMed] [Google Scholar]
- 14.Heiman E Lim SS Bao G, et al. Depressive symptoms are associated with low treatment adherence in African American individuals with systemic lupus erythematosus. J Clin Rheumatol. 2018;24:368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juarez-Rojop IE Nolasco-Rosales GA Perez-Mandujano A, et al. Prevalence for and factors associated with depression and anxiety symptoms in Mexican patients with rheumatoid arthritis [published online June 11, 2019]. J Clin Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- 16.Cui C, Li Y, Wang L. The association of illness uncertainty and hope with depression and anxiety symptoms in women with systemic lupus erythematosus: a cross-sectional study of psychological distress in systemic lupus erythematosus women [published online February 19, 2020]. J Clin Rheumatol. 2020. [DOI] [PubMed] [Google Scholar]
- 17.Nerurkar L Siebert S McInnes IB, et al. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry. 2019;6:164–173. [DOI] [PubMed] [Google Scholar]
- 18.Staniszewska S Brett J Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staniszewska S Brett J Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals. 2002;32:509–515. [Google Scholar]
- 21.Hyphantis T Kotsis K Kroenke K, et al. Lower PHQ-9 cutpoint accurately diagnosed depression in people with long-term conditions attending the accident and emergency department. J Affect Disord. 2015;176:155–163. [DOI] [PubMed] [Google Scholar]
- 22.Gonzales JA Chou A Rose-Nussbaumer JR, et al. How are ocular signs and symptoms of dry eye associated with depression in women with and without Sjogren syndrome? Am J Ophthalmol. 2018;191:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hitchon CA Zhang L Peschken CA, et al. The validity and reliability of screening measures for depression and anxiety disorders in rheumatoid arthritis. Arthritis Care Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheehy C, Murphy E, Barry M. Depression in rheumatoid arthritis—underscoring the problem. Rheumatology. 2006;45:1325–1327. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L Fu T Yin R, et al. Prevalence of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. BMC Psychiatry. 2017;17:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matcham F Rayner L Steer S, et al. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology. 2013;52:2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luppino FS de Wit LM Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. [DOI] [PubMed] [Google Scholar]
- 28.Lamers F Vogelzangs N Merikangas KR, et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18:692–699. [DOI] [PubMed] [Google Scholar]
- 29.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;7:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicassio PM. The problem of detecting and managing depression in the rheumatology clinic. Arthritis Rheum. 2008;59:155–158. [DOI] [PubMed] [Google Scholar]
- 31.Sleath B Chewning B de Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum. 2008;59:186–191. [DOI] [PubMed] [Google Scholar]
- 32.Heiman E, Kravitz RL, Wise BL. Rheumatologists' approaches to diagnosis and treatment of depression. J Clin Rheumatol. 2016;22:307–311. [DOI] [PubMed] [Google Scholar]
- 33.Edwards RR Cahalan C Mensing G, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7:216–224. [DOI] [PubMed] [Google Scholar]
- 34.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. [DOI] [PubMed] [Google Scholar]
- 35.Pryce CR, Fontana A. Depression in autoimmune diseases. Curr Top Behav Neurosci. 2017;31:139–154. [DOI] [PubMed] [Google Scholar]


