Abstract
Health-related quality of life (HRQoL) assesses the perceived impact of health status across life domains. Although research has explored the relationship between specific conditions, including HIV, and HRQoL in low-resource settings, less attention has been paid to the association between multimorbidity and HRQoL. In a secondary analysis of cross-sectional data from the Vukuzazi (“Wake up and know ourselves” in isiZulu) study, which identified the prevalence and overlap of non-communicable and infectious diseases in the uMkhanyakunde district of KwaZulu-Natal, we (1) evaluated the impact of multimorbidity on HRQoL; (2) determined the relative associations among infectious diseases, non-communicable diseases (NCDs), and HRQoL; and (3) examined the effects of controlled versus non-controlled disease on HRQoL. HRQoL was measured using the EQ-5D-3L, which assesses overall perceived health, five specific domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), and three levels of problems (no problems, some problems, and extreme problems). Six diseases and disease states were included in this analysis: HIV, diabetes, stroke, heart attack, high blood pressure, and TB. After examining the degree to which number of conditions affects HRQoL, we estimated the effect of joint associations among combinations of diseases, each HRQoL domain, and overall health. Then, in one set of ridge regression models, we assessed the relative impact of HIV, diabetes, stroke, heart attack, high blood pressure, and tuberculosis on the HRQoL domains; in a second set of models, the contribution of treatment (controlled vs. uncontrolled disease) was added. A total of 14,008 individuals were included in this analysis. Having more conditions adversely affected perceived health (r = -0.060, p<0.001, 95% CI: -0.073 to -0.046) and all HRQoL domains. Infectious conditions were related to better perceived health (r = 0.051, p<0.001, 95% CI: 0.037 to 0.064) and better HRQoL, whereas non-communicable diseases (NCDs) were associated with worse perceived health (r = -0.124, p<0.001, -95% CI: 0.137 to -0.110) and lower HRQoL. Particular combinations of NCDs were detrimental to perceived health, whereas HIV, which was characterized by access to care and suppressed viral load in the large majority of those affected, was counterintuitively associated with better perceived health. With respect to disease control, unique combinations of uncontrolled NCDs were significantly related to worse perceived health, and controlled HIV was associated with better perceived health. The presence of controlled and uncontrolled NCDs was associated with poor perceived health and worse HRQoL, whereas the presence of controlled HIV was associated with improved HRQoL. HIV disease control may be critical for HRQoL among people with HIV, and incorporating NCD prevention and attention to multimorbidity into healthcare strategies may improve HRQoL.
Introduction
Chronic diseases relapse and remit over time, with substantial impact on quality of life and daily functioning. Health-related quality of life (HRQoL) is a multidimensional construct that captures the perceived effects of illness and chronic disease, as well as associated treatments, on physical, emotional, and social wellbeing [1]. Among those living with health conditions for which efficacious treatments are available, long-term management and quality of life, rather than survival, are the ultimate goals. Yet, in sub-Saharan Africa and other low resource regions, people living with chronic illness—including HIV [2], tuberculosis (TB) [3], and diabetes [4]—report poor HRQoL, which has been associated with low adherence to medications [5] and other behaviors that negatively affect both physical and mental health [6, 7].
Although research has assessed the degree to which individual diseases and disease states impact HRQoL, less is known about the effects of multimorbidity—defined as multiple medical conditions occurring simultaneously in a single individual [8]—on HRQoL in sub-Saharan Africa (SSA). Data from high-income settings has isolated specific co-occurring disease groups or patterns of multimorbidity (e.g., cardiovascular and metabolic disease, mental health conditions, musculoskeletal disorders [9]) and demonstrated that patients with multimorbidity have increased utilization of healthcare services, reduced quality of life, and poorer health outcomes [10]. However, patterns of multimorbidity in high-income countries differ from those in low- and middle-income countries (LMICs), many of which are located in SSA. Countries in SSA have under-resourced health systems and similar disease profiles that characterized by emerging and re-emerging infectious and communicable diseases, included HIV, TB, and malaria. A recent epidemiological shift from infectious diseases to multiple non-communicable diseases (NCDs) signals a higher risk for mortality and disease-related disability in SSA than in other areas over the coming decades [11–13]. This shift has taken place in the context of large declines in HIV-related deaths and corresponding increases in life expectancy [14], with the HIV epidemic now concentrated in middle-aged groups that have high NCD risk. For example, in Uganda, the HIV prevalence rate had reduced to 6% by 2017, but rates among men and women aged 45–49 were much higher (14% and 11%, respectively) [15]. In South Africa, HIV prevalence rates are also highest among middle-aged men and women (approximately 26% and 38%, respectively) [16].
South Africa (SA), rapid demographic shifts to urban centers have contributed to changes in diet, decreases in physical activity, and increases in NCDs alongside established HIV and TB epidemics [17]. Moreover, whereas there has been tremendous investment and relative success in confronting the HIV, malaria, and TB epidemics in the region, less attention has been paid to NCD prevention and care [18]. Though a few recent studies have examined multimorbidity in SA [19–22], with a review estimating the prevalence of multimorbidity to be 3–23% in studies that included younger people and 30–87% in studies of older adults [23], none have addressed the impact of multimorbidity on HRQoL across a large cohort of community-dwelling adults with extensive phenotyping for both infectious diseases and NCDs. In addition, the effects of disease treatment (i.e., disease control) on HRQoL have yet to be examined in the context of multimorbidity.
The objectives of this secondary analysis of data collected for the Vukuzazi (“Wake up and know ourselves” in isiZulu) study [24] were to (1) evaluate the impact of multimorbidity on HRQoL; (2) determine the relative associations among infectious diseases, NCDs, and HRQoL; and (3) examine the effects of controlled versus non-controlled disease on HRQoL in a large South African cohort. This study may inform public health priorities and the design of interventions or services that target the needs of patients with multimorbidity, both in SA and in other countries that face a convergence of infectious and non-communicable disease epidemics.
Methods
Study setting and recruitment for the parent study
Adolescents and adults, aged 15 years and older, living in the Africa Health Research Institute (AHRI) Demographic Surveillance Area in the uMkhanyakunde district of KwaZulu-Natal were invited to participate in the parent study. Typical of rural SA, the uMkhanyakunde district has a high unemployment rate (58%), and one third of homes within the district do not have access to piped water.
Participants were recruited over an 18-month period, from May 2018 to November 2019, through home visits of all households in the catchment area. If no household members were home at the first visit, return visits were conducted. The recruitment team also issued proxy invitations to allow eligible household members who were away from the home at the time of the visit to participate in data collection. Approximately 50% of all adults in the area participated in the study. Please see Gunda et al. for a detailed cohort profile [25].
Procedures of the parent study
All participants provided written informed consent. For non-emancipated participants under 18 years of age, we also obtained written parental consent for participation.
This was a secondary analysis of data collected for the Vukuzazi study [24], which leveraged an existing demographic and health surveillance cohort to determine the distribution and overlap of four common infectious and non-communicable diseases (HIV, TB, elevated blood glucose, and high blood pressure) in KwaZulu-Natal. The Vukuzazi data collection protocol is described briefly below (for a more detailed description, please see Wong et al., 2021) [24]. We used the STROBE cross-sectional reporting guidelines to complete this report [26].
Between May 2018 and November 2019, data collection was conducted via mobile health camp, a mobile facility where data is gathered on-site, in real-time, throughout the study area. At the mobile health camps, research nurses assessed history of tuberculosis (TB), HIV, hypertension, and diabetes. Participants who were not pregnant were screened for TB via digital chest x-ray, which were read in real-time by computer-assisted image analysis or by an experienced central radiologist [27]. From participants reporting current TB symptoms and participants with abnormal lung fields, sputum was collected and tested for Mycobacterium tuberculosis (Mtb) by Xpert MTP/RIF Ultra test (Cepheid, Sunnyvale, USA) and liquid mycobacterial culture (BACTEC MGIT 960 System, Becton Dickinson, Berkshire, UK). Blood was collected to measure glycosylated hemoglobin (HbA1c, VARIANT II TURBO Haemoglobin testing system; Bio-Rad, Marnes-la-Coquette, France) and to assess for HIV. Participants with a positive HIV immunoassay completed HIV-1 RNA viral load testing (Abbott RealTime HIV-1 Viral Load, Abbott, Illinois, USA).
After data collection, specimens were processed in the central laboratory, and results were reported back to participants. Normal results were communicated via text message. Abnormal results, including a new HIV diagnosis or a positive TB Xpert test, were reported by study nurses through home visits. These results were not available on the day that the EQ-5D-3L was administered; therefore, EQ-5D-3L results pertain to participants’ perceived HRQoL prior to receiving any information about new diagnoses.
Data measurement for the current analyses
The predictor variables: Disease and disease control
Six diseases and disease states were included as predictor variables in this analysis: HIV, diabetes, stroke, heart attack, high blood pressure, and TB. Medical history and study assessments were used to (1) define the presence of the diseases and disease states included in the analysis and to (2) determine whether the disease was “controlled” (i.e., optimally managed via medical intervention).
Participants with a positive HIV immunoassay were defined as having HIV, and HIV disease was considered to be controlled if the following conditions were met: (1) HIV-1 RNA viral load < 40 copies/mL and (2) currently on antiretroviral therapy. Patients who were currently on antiretroviral therapy but did not have suppressed HIV-1 RNA viral loads were categorized as in treatment, with uncontrolled HIV. Diabetes was defined as hemoglobin A1c > 6.5% or self-reported use of diabetes medications in the past two weeks, irrespective of hemoglobin A1c. Controlled diabetes was defined per WHO’s STEPwise approach to NCD risk factor surveillance as self-reported use of diabetes management medications in the past two weeks with a hemoglobin A1c < 6.5% [28]. A lifetime history of stroke or heart attack was determined by self-report. High blood pressure was defined as a mean systolic BP ≥ 140 mmHg and diastolic BP ≥ 90 mmHg or a reported diagnosis of hypertension with use of anti-hypertensive medicines in the past two weeks. Finally, participants who were categorized as having active TB were either in the active phase of TB treatment at the time of the survey or had sputum that was positive for Mtb by Xpert MTB/RIF Ultra or liquid mycobacterial culture. Controlled TB was defined as currently receiving treatment for TB; participants who were categorized as having controlled TB were diagnosed with TB prior to completing the survey and were currently receiving treatment—at any phase—outside of the context of the study.
The outcome variable: HRQoL
HRQoLwas measured using the EQ-5D-3L, a well-known and widely used health status tool that was developed by the EuroQol Group [29]. The EQ-5D-3L includes a short descriptive system questionnaire that assesses five dimensions—mobility, self-care, usual activities, pain/discomfort, and anxiety/depression—and three levels of perceived problems (no problems, some problems, and extreme problems. The three levels are measured on a 3-point scale, which was recoded for ease of interpretation (with 3 indicating no problems, 2 indicating some problems, and 1 indicating extreme problems), creating a descriptive profile of a respondent’s health state. This descriptive system is linked to a value set (i.e., a set of weights for each health state description that varies by country/region), but value sets were not used in the current study. The measure also includes a visual analog scale (EQ VAS) that assesses overall health (“We would like to know how good or bad your health is TODAY”) on a scale from 0 to 100, with 0 defined as “the worst health you can imagine” and 100 defined as “the best health you can imagine”. The EQ-5D-3L was chosen over other widely-used tools (e.g., SF-36 [30], SF-12 [31–33]) first and foremost for its brevity, which was essential for use in a large sample, but also for its validation across languages (over 150, including multiple South African languages) and reproducibility.
Statistical analyses
Two broad analytic strategies were used to understand multimorbidity’s impact on HRQoL. First, Pearson correlational analyses were conducted to understand the general relationships among participants’ number of diagnosed conditions (noninfectious, infectious, and total), overall perceived health, and the five HRQoL domains. Second, to understand the complex inter-relationships among all disease states, level of disease control, and their cumulative impact on HRQoL, we employed a regularized regression strategy known as “elastic net”, a process of selecting predictor variables for multiple regression through a sophisticated penalization strategy or shrinking algorithm. The elastic net process is flexible and data-driven, such that the statistics determine the precise parameters of the shrinking algorithm that is applied. A standard ridge regression approach, which implicitly accounts for the high degree of intercorrelations among the variables, ultimately emerged from the data [34]. This approach statistically manages and overcomes issues of multicollinearity, which may have compromised the results if left unaddressed (variance inflation factors are reported in S1 Table). Ridge regression is not only recommended for its ability to handle intercorrelations among predictors but, critically, is valuable for its ability to minimize overfitting [35]. Importantly, the ridge regressions enabled us to prioritize our goal of understanding of the roles that all conditions play in HRQoL rather than simpler predictor combinations–specifically, in a more accurate manner than afforded by other traditional regression approaches [36, 37].
We employed k-fold cross-validation procedures during the modeling pipeline at several stages, including the parameter optimization and model evaluation processes, to prevent arbitrary overfitting of the data and to compare models against each other. The shrinkage parameter was tuned via the cva.glmnet function of the glmnetUtils package in R [38]. This function is designed to tune the shrinkage parameter via cross-validation. Specifically, we used cross-validation to identify optimal elastic net parameters for our initial modeling of multimorbidity’s impact on HRQoL domains [39]. Then, we performed additional 10-fold cross-validation of our ridge regressions to derive conservative, realistic estimates of our account of variance so as to not report R-squared values from overfit models.
We controlled for age and gender because they are generally considered to be confounding variables [40, 41]; between-group comparisons (see Table 1) and Pearson correlations (see Supporting Information) also suggested that age and gender were potential confounds in our sample, which provided further justification for residualizing age and gender out of the models described below. Even though HRQoL was measured on an ordinal scale, we did not use ordinal regressions because modeling out the confounds of age and gender does not produce ordinal residuals; therefore, per established guidelines [42, 43], we treated the residuals as continuous variables for the primary analyses.
Table 1. Demographics, disease states, total number of disease conditions, and HRQoL domains by gender.
| Women | Men | t-value/z-value† | |
|---|---|---|---|
| Age (M, SD) | 41.72 (18.88) | 35.88 (18.52) | 17.15*** |
| Disease states (n, %)–Predictor variables for subsequent models | |||
| HIV | 3850 (40.20) | 1139 (25.70) | 16.57*** |
| Diabetes | 1130 (11.80) | 239 (5.39) | 11.60*** |
| Stroke | 224 (2.33) | 46 (1.04) | 5.076*** |
| Heart attack | 115 (1.20) | 23 (0.52) | 3.696*** |
| High blood pressure | 2565 (26.80) | 709 (16.00) | 13.91*** |
| Active TB | 163 (0.02) | 191 (0.04) | -8.823*** |
| Number of conditions (M, SD) | 0.84 (0.79) | 0.52 (0.72) | 22.27*** |
| Number of infectious conditions (M, SD) | 0.42 (0.30) | 0.51 (0.50) | 12.90*** |
| Number of non-infectious conditions (M, SD) | 0.42 (0.68) | 0.23 (0.50) | 16.87*** |
| Quality of life domains (M, SD)a –Outcome variables for subsequent models | |||
| Overall health (EQ VAS) | 87.43 (15.17) | 90.70 (13.31) | -12.23*** |
| Mobility | 2.86 (0.34) | 2.92 (0.27) | -9.542*** |
| Pain/discomfort | 2.76 (0.46) | 2.86 (0.36) | -12.84*** |
| Self-Care | 2.88 (0.33) | 2.93 (0.34) | -8.771*** |
| Usual activity | 2.82 (0.39) | 2.89 (0.32) | -10.37*** |
| Anxiety/Depression | 2.85 (0.37) | 2.91 (0.30) | -8.368*** |
*** p < 0.001
† Continuous measures (age, numbers of conditions, and quality of life domains) were compared via an independent-samples t-test (t-values are reported). Categorical measures (i.e., positive diagnosis of disease states) compared via binomial regression (z-values are reported).
aNote that the quality of life domains were assessed on a three-point scale (1–3), which were recoded for ease of interpretation so that 1 indicated extreme problems and 3 indicated no problems.
We ran two sets of regression models. In the first set of models, we examined each disease and disease state as predictors of overall health and each of the five HRQoL domains. In the second set, we included variables reflecting disease control to assess the degree to which treatment influenced the relationships among comorbid disease and the HRQoL domains. All analyses were conducted in the R software environment using the glmnet package [44, 45], with significance test estimates generated using Cule et al.’s method [46]. We note, however, that such estimates for penalized regressions are problematic and, while heuristically useful, we strongly caution against overinterpretation (see [47, 48]). Finally, we ran follow-up relative weights analyses to test for whether some conditions played significantly stronger roles in HRQoL than others within any given model.
Patient and public involvement
The AHRI Community Advisory Board contributed to the study design and the selection of the measures. The Community Advisory Board also reviewed and approved the study protocol. Throughout each phase of the project, the AHRI Public Engagement Department routinely shared study results via public communications and road shows.
Results
Study participation and demographics
Out of the 18,053 participants who enrolled in the Vukuzazi study, 14,008 individuals were included in this analysis (4,045 participants were excluded because they enrolled prior to inclusion of the EQ5D HRQoL questionnaire). Women comprised the majority of the final sub-sample (N = 9,573; 68.34%), and the average age of all participants was 39.87 years (SD = 18.96). See Table 1 for information on demographics, the prevalence of each of the six diseases and disease states, and the HRQoL domains by gender.
With respect to HRQoL, most participants (80–90%) reported no problems with mobility, pain/discomfort, self-care, usual activity, and anxiety/depression, 10–20% of participants reported some problems across the five domains, and less than 2% of participants reported extreme problems across the five domains (Table 2).
Table 2. EQ-5D-3L scores across the five domains.
| Mobility | Pain/Discomfort | Self-Care | Usual Activity | Anxiety/ Depression | |
|---|---|---|---|---|---|
| No Problems | 12369 (88.30%) | 11297 (80.65%) | 12532 (89.46%) | 11873 (84.76%) | 12298 (87.79%) |
| Some Problems | 1630 (11.64%) | 2539 (18.13%) | 1448 (10.34%) | 2078 (14.83%) | 1644 (11.74%) |
| Extreme problems | 9 (00.06%) | 172 (01.23%) | 28 (00.20%) | 57 (00.41%) | 66 (00.47%) |
The prevalence rates of multimorbidity were as follows: 14% of participants had two conditions, 2% of participants had three conditions, 0.24% had four conditions, and 0.007% had five conditions (Table 3). We also provide a breakdown of perceived overall health by number of conditions, number of non-infectious, and number of infectious conditions in Table 3.
Table 3. Prevalence of multimorbidity and perceived overall health by number of conditions, number of non-infectious conditions, and number of infectious conditions, controlling for age and gender.
| N (%) | Mean of Overall Health | Standard deviation | Median of Overall Health | |
|---|---|---|---|---|
| Number of conditions (Fig 1) | ||||
| 0 | 6600 (47.12) | 74.73 | 9.54 | 77.02 |
| 1 | 6011 (42.91) | 75.30** | 10.69 | 77.52 |
| 2 | 1965 (14.03) | 73.39*** | 12.43 | 74.89 |
| 3 | 329 (2.35) | 70.69*** | 14.27 | 72.73 |
| 4 | 33 (0.24) | 68.23 | 11.76 | 67.22 |
| 5 | 1 (0.007) | 69.70 | NA | 69.70 |
| Number of non-infectious conditions (Fig 2) | ||||
| 0 | 10660 (76.1) | 75.20 | 9.60 | 77.20 |
| 1 | 3160 (22.56) | 74.04*** | 12.08 | 75.33 |
| 2 | 1021 (7.29) | 71.81*** | 13.82 | 73.47 |
| 3 | 92 (0.66) | 66.50*** | 13.38 | 67.12 |
| 4 | 6 (0.04) | 71.32 | 5.17 | 69.20 |
| Number of infectious conditions (Fig 3) | ||||
| 0 | 9568 (68.3) | 74.26 | 10.70 | 76.82 |
| 1 | 5179 (36.97) | 75.41*** | 10.20 | 78.01 |
| 2 | 192 (1.37) | 74.84 | 11.68 | 77.52 |
** p < 0.01
*** p < 0.001
These p-values correspond to statistically differences between X number of conditions and the preceding number of conditions (X-1). For example, the difference between the mean value of perceived overall health among participants with 3 total conditions (70.69) is significantly lower than the mean value of perceived overall health among participants with 2 total conditions at the p <0.001 level.
Multimorbidity and health-related quality of life
Correlational analyses revealed that more conditions, regardless of condition type (non-communicable vs. infectious), was associated with poor perceived overall health (measured via the EQ VAS) and with decreased HRQoL across the five domains (see Table 3 for Pearson correlation coefficients). Similarly, correlational analyses demonstrated that higher numbers of comorbid NCDs were associated with worse overall health and decreased HRQoL across the five domains (see Table 4). The strongest negative correlations between number of NCDs and the HRQoL domains were for usual activity (r = -0.136) and mobility (r = -0.134). However, unlike the relationships among number of NCDs and the HRQoL domains, higher numbers of infectious diseases were positively associated with overall health and HRQoL. The strongest positive correlation was between number of infectious diseases and mobility (r = 0.097). With a sample size of 14,008, differences between coefficients (i.e., differences in the strengths of the relationships) of a magnitude ≥ 0.017 were associated with a p-value < 0.05 (see Table 4 note). For example, the correlation between overall perceived health and number of conditions (-0.060) is a significantly stronger association than the correlation between mobility and number of conditions (-0.032). Differences in the strength of these relationships were assessed using the cocor package in R [49].
Table 4. Pearson correlation coefficients among HRQoL domains, total number of conditions, number of infectious conditions, and number of non-infectious conditions, after controlling for age and gender.
| Domain | Number of Conditions | Number of Infectious Conditions | Number of Non-Infectious Conditions |
|---|---|---|---|
| Overall Health (EQ VAS) | -0.060 [-0.073, -0.046] | 0.051 [0.037, 0.064] | -0.124 [-0.137, -0.110] |
| Mobility | -0.032 [-0.045, -0.018] | 0.097 [0.084, 0.110] | -0.134 [-0.147, -0.121] |
| Pain/Discomfort | -0.042 [-0.055, -0.028] | 0.073 [0.060, 0.086] | -0.125 [-0.138, -0.112] |
| Self-Care | -0.025* [-0.038, -0.011] | 0.082 [0.069, 0.095] | -0.111 [-0.125, -0.098] |
| Usual Activity | -0.045 [-0.059, -0.032] | 0.080 [0.066, 0.093] | -0.136 [-0.149, -0.123] |
| Anxiety/ Depression | -0.048 [-0.062, -0.035] | 0.041 [0.028, 0.054] | -0.102 [-0.115, -0.089] |
* p = 0.002; all other ps < 0.001.
Note. Given our sample size and the generally high intercorrelations between HRQoL measures (Cronbach’s α = 0.86) and number of conditions (α = 0.69), differences between coefficients of a magnitude ≥ 0.017 were associated with a p-value < 0.05.
In the first set of regression models, combinations of NCDs were associated with lower overall health and lower HRQoL for each of the domains. Table 5 presents the relative contribution of each disease to each HRQoL domain. For any given disease/predictor, the beta values reflect the change in each HRQoL domain, measured by a tool that has been rescaled and residualized (per the description in the Methods), as a function of the presence of a given condition or disease state.
Table 5. Beta weights for diseases/disease states that were significantly associated with overall health and/or the five HRQoL domains in the first set of regression models (Model 1).
| Predictor | Overall Health | Mobility | Pain/ Discomfort |
Self-Care | Usual Activity | Anxiety/ Depression |
|---|---|---|---|---|---|---|
| Intercept | 74.984 | 4.135 | 3.818 | 4.177 | 3.965 | 4.126 |
| Diabetes | -0.156* | -0.006† | -0.015*** | -0.008* | -0.011** | -0.016*** |
| History of stroke | -0.932*** | -0.039*** | -0.045*** | -0.031*** | -0.038*** | -0.040*** |
| History of heart attack | -0.339*** | -0.008* | -0.023*** | -0.005 | -0.013*** | -0.013** |
| HIV | 0.354*** | 0.036*** | 0.032*** | 0.034*** | 0.031*** | 0.019*** |
| Hypertension | -0.918*** | -0.040*** | -0.036*** | -0.033*** | -0.045*** | -0.023*** |
| Active TB | -0.020*** | 0.001 | 0.001 | -0.006† | -0.001 | -0.005 |
| R 2 | 0.023 | 0.029 | 0.024 | 0.021 | 0.027 | 0.016 |
| 10-fold cross-validation R2 | 0.023 | 0.029 | 0.024 | 0.020 | 0.027 | 0.016 |
† p < 0.10
* p < 0.05
** p < 0.01
*** p < 0.001
The majority of diagnosed conditions contributed negatively to perceived overall health. Notably, a history of stroke and current hypertension were most strongly associated with poorer overall health (β = -0.932 and β = -0.918, respectively). HIV was most strongly associated with better overall health (β = 0.354). It is important to note that this result is counterintuitive; we address factors that may be driving this association, including the role of treatment and disease control, in the results section below and in the Discussion. These relationships were fairly consistent across the five HRQoL domains (see Table 5). Combining the beta weights for individual diseases or disease states provides cumulative estimates of the strength of the association between a set of comorbid or multimorbid conditions and each of the HRQoL domains (i.e., beta weights are additive). For example, while stroke and hypertension each detract from HRQoL, perceived overall health is negatively impacted if a person is diagnosed with both relative to either condition individually. For a quantification of multicollinearity in this model, please see the Supporting Information, which include a variance inflation factor (VIF) table. Any VIF above 1 indicates a modest degree of collinearity.
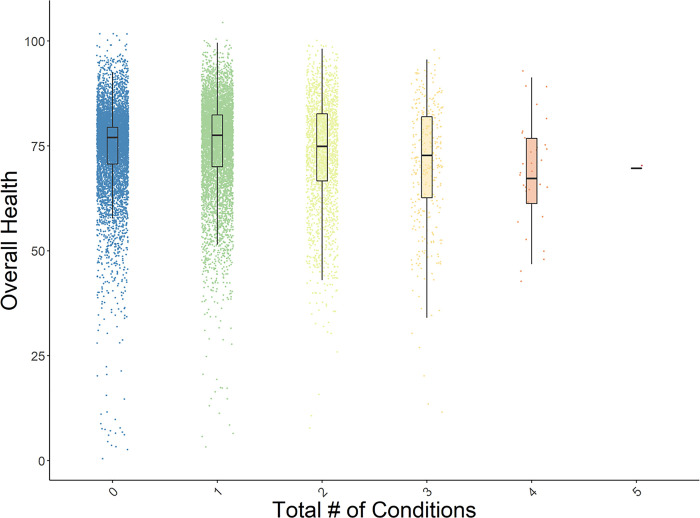
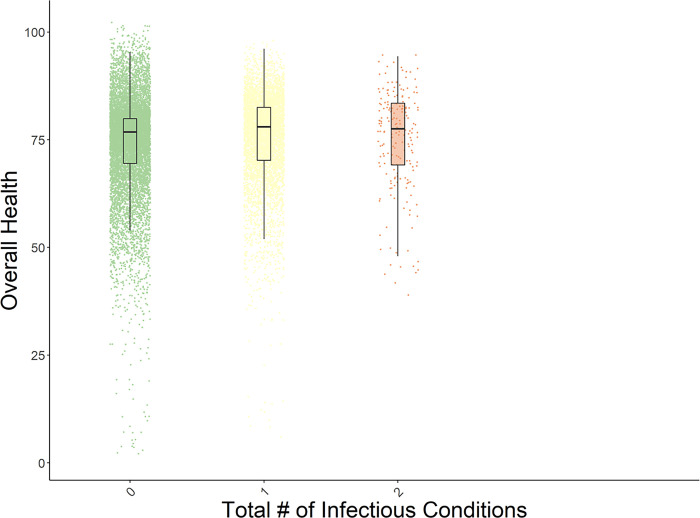
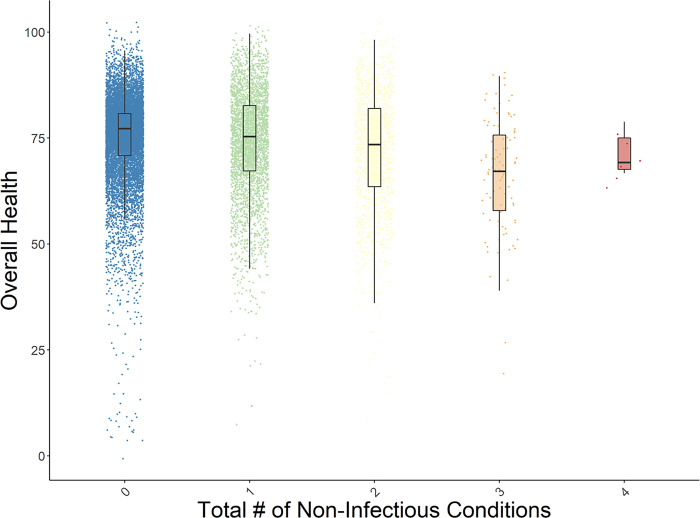
See Figs 1–3 for box plots of the associations between number of conditions and perceived health, number of NCDs and perceived health, and number of infectious diseases and perceived health. Each figure indicates the medians for overall health ratings per number of conditions, controlling for age and gender. Similar relationships were evident across all five HRQoL domains. These box plots depict the relative decreases in perceived health as multimorbidity increases. Table 3 presents the descriptive data (means, standard deviations, and medians) that are associated with the Figures.
Fig 1. Box plot of the distribution of perceived overall health by number of conditions, with age and gender residualized.
The plots present the medians of the data.
Fig 3. Box plot of the distribution of perceived overall health by number of infectious conditions, with age and gender residualized.
The plots present the medians of the data.
Fig 2. Box plot of the distribution of perceived overall health by number of non-infectious conditions, with age and gender residualized.
The plots present the medians of the data.
The role of treatment in the relationship between multimorbidity and health-related quality of life
To understand the impact of disease treatment on HRQoL, a second set of regression models were performed parallel to those described above, albeit with more nuanced measures of diseases ranging from diagnosed but uncontrolled to diagnosed and medically controlled; Table 5 presents results from these analyses. Much like the first set of models, uncontrolled hypertension was most strongly associated with poor health (β = -0.918), as was history of stroke (β = -0.913), and; similarly, most diagnosed disease and disease states, whether controlled or uncontrolled, negatively impacted HRQoL. The strength of the associations among HIV, overall health, and the HRQoL domains differed based on level of disease control; that is, the relationship between controlled HIV and good overall health was stronger than the relationship between uncontrolled HIV and good health (β = 0.383 vs. β = 0.145, respectively). In a follow-up bootstrapped relative weights analysis, the difference between these two beta weights had a p-value < .05, CI = [-0.0032, -0.0003] (see [50]). Again, combining the beta weights for individual diseases or disease states provides an estimate of the strength of the association between a set of comorbid/multimorbid conditions and each of the HRQoL domains. For a quantification of multicollinearity in this model, please see the VIF table in the Supporting Information.
Finally, using a five-by-two-fold cross validation procedure as described in the Methods, we compared the set of models that accounts for treatment/disease control with the set of models that do not; R-squared values were compared to each other using a paired samples t-test (see Table 6) [51]. In the models estimating overall perceived health, the model that included disease control variables accounted for significantly more variance than the model that did not include those variables (5×2 paired-t(9)ΔR2 = 11.116, p < 0.001). With respect to the anxiety/depression domain of HRQoL, the model with disease control variables accounted for significantly less variance than the model without those variables (5×2 paired-t(9)ΔR2 = -3.084, p < 0.01). However, the differences in the expected R-squared values derived from cross-validation suggest that, in general, the models are functionally comparable in their ability to account for variance. Therefore, in the case of the anxiety/depression models, one should not necessarily be seen as “better” or “worse” from a predictive standpoint, as these differences in variance accounted for are so small as to be arbitrary.
Table 6. Beta weights for diseases/disease states that were significantly associated with overall health and/or the five HRQoL domains in the second set of regression models (Model 2), which included variables that indicate disease control.
| Predictor | Overall Health | Mobility | Pain/ Discomfort |
Self-Care | Usual Activity | Anxiety/ Depression |
|---|---|---|---|---|---|---|
| Intercept | 74.984 | 4.135 | 3.818 | 4.177 | 3.965 | 4.126 |
| Current smoker | -0.504*** | 0.003 | -0.003 | 0.009 | 0.002 | -0.002 |
| Controlled diabetes | -0.076 | -0.010** | -0.008† | -0.010** | -0.013*** | -0.009* |
| Uncontrolled diabetes | -0.161** | -0.004 | -0.014** | -0.006 | -0.008* | -0.014*** |
| History of stroke | -0.913*** | -0.039*** | -0.044*** | -0.030*** | -0.037*** | -0.039*** |
| History of heart attack | -0.326*** | -0.008* | -0.022*** | -0.005 | -0.013*** | -0.012** |
| Controlled HIV | 0.383*** | 0.035*** | 0.029*** | 0.032*** | 0.029*** | 0.020*** |
| On treatment, but uncontrolled HIV | 0.058 | 0.009* | 0.012** | 0.008* | 0.010** | 0.005 |
| Uncontrolled HIV | 0.145 | 0.014*** | 0.015*** | 0.013*** | 0.013*** | 0.003 |
| Hypertension, uncontrolled | -0.918*** | -0.040*** | -0.036*** | -0.032*** | -0.044*** | -0.022*** |
| Active TB | 0.024 | 0.001 | 0.001 | -0.005* | 0.000 | -0.004 |
| Controlled TB | -0.314*** | -0.002 | -0.007† | -0.007* | -0.006 | -0.007* |
| R 2 | 0.027 | 0.029 | 0.025 | 0.022 | 0.027 | .017 |
| 10-fold cross-validation R2 | 0.026 | 0.028 | 0.024 | 0.020 | 0.027 | .015 |
| 5×2 paired-t(9)ΔR2 | 11.116*** | -0.683 | -1.606 | 0.717 | -1.011 | -3.084** |
† p < 0.10
* p < 0.05
** p < 0.01
*** p < 0.001
Discussion
In a large sample of adults living in rural KwaZulu-Natal, multimorbidity was associated with poor HRQoL; as number of conditions increased, HRQoL decreased across all domains. Notably, the impact of multimorbidity on HRQoL differed by type and management of disease. The presence of both controlled and uncontrolled NCDs was associated with poorer HRQoL, whereas the presence of controlled infectious diseases, driven by high rates of disease control among people with HIV, was associated with higher HRQoL. Unlike most previous studies, which either determined the prevalence of multimorbidity in certain regions or assessed the impact of specific diseases on HRQoL, this analysis was the first to demonstrate the association of treatment on the relationship between multimorbidity and HRQoL. Understanding the degree to which disease control is related to overall functioning has important implications for health policy and health service delivery in low-resource settings, as it may enable treatment prioritization based on individual patient values and risk for poor HRQoL.
Data from high-resource settings consistently demonstrate that HRQoL decreases as number of conditions increases. A recent meta-analysis indicated that the mean decrease in HRQoL per each added disease ranged from 1.55% to 4.37%, depending on the HRQoL scale that was used [52]. The majority of the studies included in the meta-analysis were performed in high-income countries, with only one SA-based study representing the entire African continent. Although the relationship between increasing number of diseases and poor HRQoL may seem evident, the nuances of this relationship likely differ between high-income settings, which have lower prevalence rates of infectious diseases, and low-income or middle-income settings, where NCD and infectious disease epidemics co-occur.
When the contribution of multimorbidity to HRQoL was examined by disease type, the impact of NCDs was negative, as was the impact of TB. An HIV diagnosis, however, was associated with better HRQoL, which was counterintuitive, given that the negative influence of HIV-related symptoms on both physical and mental HRQoL domains has been well-documented [53, 54]. However, the role of HIV treatment is meaningful here. Successful engagement in care, adherence to therapy and virologic suppression is often associated with a return to health, sometimes after grave illness, and significant improvements in HRQoL [55]. HIV care is also associated with increased social support and other pro-health behaviors, thereby potentially increasing HRQoL. Indeed, when disease control was factored into the models, controlled HIV was associated with greater HRQoL than uncontrolled HIV, suggesting a possible positive impact of HIV treatment on HRQoL. Similar effects of antiretroviral therapy (ART) on HRQoL among people with HIV have been previously reported in both South Africa and Zambia [56]. Notably, this population-based cohort included individuals with relatively well controlled HIV; 83% of participants living with HIV were virologically suppressed, with a median CD4 count of 693 cells/uL (SD = 340.40). These data add to growing evidence for positive secondary effects of HIV care that are not always available those without HIV infection [57].
With the possibility of these secondary effects in mind, it is important to consider the societal and health systems implications of the association between well-controlled HIV and better perceived health across domains, as well as the implications of the stronger association between controlled HIV and better perceived health. A potential hypothesis that would explain this counterintuitive phenomenon is that HIV care provides greater frequency and intensity of contact between individuals and their health care providers, counselors, pharmacists, and the health care system than what is typically received for those without a chronic disease (or those with one, such as hypertension and diabetes, which is not as well addressed by the South African healthcare system). This increased interface with healthcare providers may in turn increase opportunities for health education as well as improve health literacy, health screening and maintenance, and other such services, which may ultimately have downstream effects on different domains of HRQoL. For example, numerous studies have demonstrated that people with HIV in sub-Saharan Africa have improved health indicators for other conditions, such as diabetes, hypertension, and atherosclerosis, and that HIV care may lead to increases in smoking cessation [58–63]. Though cost effectiveness will need to be considered, the integrated ART program model may be harnessed to increase touchpoints for care in populations with NCDs or comorbid HIV/NCDs [64], especially if longitudinal studies support the association between controlled HIV and better perceived HRQoL and if mediational analyses suggest that strong relationships with providers and associated social support may be driving these relationships.
Other studies have also found that ART is associated with improved HRQoL in sub-Saharan contexts [65, 66], but few have done so in a sample of this size, and none have examined these relationships in the context of comorbidities. Although treatment engagement lessened the negative effects of some of these disease states on HRQoL, controlled NCDs either had no significant impact on HRQoL or were associated with poor HRQoL. This suggests that, in SA, public health efforts that aim to prevent NCDs may have a meaningful impact on HRQoL. These efforts could be integrated into or independent of HIV treatment.
It is important to address the implications of the size of the correlation coefficients and the beta weights that we report in this analysis. Although the coefficients and beta weights that we report are small, they are typical of studies that use self-report measures to assess psychological constructs. In fact, the beta weights that we report are actually larger than others documented in the multimorbidity literature [67]. Even models that include variables like age and sex may at best account for 14% of the total variance, for example [68], corresponding to an overall correlation of ~.37. There is also a substantial established literature on the interpretation of effect sizes, particularly those reported in real-world contexts (i.e., outside of the lab). These papers typically stress the importance of small effects, especially when they occur in non-trivial contexts, challenge existing theory and assumptions, and may have large cumulative consequences if replicated [69, 70]. Many of our findings fit all three criteria and therefore warrant documentation and further exploration in future work. Furthermore, the statistically significant differences in correlation strengths provide important clinical insights; this nuanced information about which disease states and associated treatments may impact HRQoL could help inform treatment priorities and decision-making, especially in resource-limited settings, where some treatments may need to be prioritized over others.
With NCDs projected to account for nearly half of the burden of disease in low-income countries by 2030 [71], awareness of the relationships between these diseases and HRQoL reinforces the importance of early identification of modifiable risk factors in maintaining population health, functioning, and overall quality of life. In settings with high HIV-burden like SA, multimorbidity is occurring at younger ages, as younger individuals living with HIV are at higher risk for heart-related conditions [72] and have increased levels of cholesterol and triglycerides compared to uninfected adolescents [73]. Missed opportunities to address modifiable NCD risk factors, as early as adolescence [74], may strain under-resourced health systems that are not yet equipped to manage multimorbidity and have long-term negative effects, given that NCD disease treatment does not appear to be associated with improved HRQoL.
Several limitations of these data should be acknowledged. First, two thirds of the sample were women, which may have resulted from bias during data collection. Given this imbalance, we chose to residualize gender out of all variables. In addition, though the study used a full population and recruited individuals directly from the community to minimize healthcare seeking bias, approximately 50% of the eligible population enrolled. As such, there may be residual bias related to participation. As described by Gunda and colleagues [25], contact rates were low among participants who were unavailable due to work or other commitments, which made it difficult to enroll specific subpopulations (e.g., working men). Non-participation could therefore be non-random, which should be considered when interpreting disease prevalences and HRQoL and should be investigated in future studies. Second, the blood pressure and blood glucose measurements that were used to define hypertension and diabetes were captured on a single day. To confirm these diagnoses, additional measurements need to be captured over time. Moreover, some disease state measurements were self-reported, whereas others were directly measured. For example, self-reported histories of disease conditions (e.g., lifetime history of stroke, heart attack) were not confirmed by medical providers or medical records, which may have introduced bias. Third, several key categories of NCDs were not assessed in this study, including cancer and chronic respiratory disease, which may have unique associations with HRQoL or interactions with other comorbid conditions that may impact HRQoL. Logistical barriers and ethical considerations informed the selection of diseases that were assessed. Investigators considered the burden that expanded health screenings might place on rural primary health care providers and clinics, and in choosing to measure certain diseases, interagency and interdisciplinary collaboration was required to establish appropriate referral and treatment pathways. In addition, given the extensive nature of the project, other important and nuanced factors that may impact HRQoL—including but not limited to socioeconomic status, structural issues, social circumstances, interpersonal relationships, discrimination and other forms of stigma—are difficult to measure and were not assessed in this study, and therefore could not be included in the analyses. Similarly, other measurement instruments—for example, HRQoL tools that were developed specifically for people with HIV—would have been valuable, but as one might expect, the logistics of a study of this magnitude (total N > 18,000) required the incorporation of brief, reproducible, validated scales (such as the EQ-5D-3L), with a view to characterizing the entire population, independent of any single comorbidity (e.g., HIV). Fourth, we could not account for the timing or severity of diagnoses and associated treatments, which may impact HRQoL, nor did we account for the interpersonal, community, and structural factors that may have protected against the negative effects of diseases and their treatments on HRQoL. Notably, the EQ-5D-3L assessment was administered to participants before they received any information about new diagnoses. It is possible that knowledge of a new diagnosis or a change in health status may have influenced responding had the assessment been administered after participants received all of their final results. The relationship between knowledge of health status and HRQoL should be explored in future research. Fifth, the current data and analytic methods precluded the broadband inclusion of all possible interaction terms within our dataset, limiting the degree to which our model recognizes the ways in which one disease may amplify or suppress the impact of other diseases on HRQoL. That is, two conditions or disease states may interact to produce a worse outcome than the additive effect of both conditions combined. While we acknowledge that there are likely interactive effects between conditions that extend beyond a summative process, modeling all interaction terms would be neither feasible nor interpretable with the current sample. Finally, as with all cross-sectional data, these results do not speak to causal associations. To gain insight on causal relationships in the context of multimorbidity, future studies should conduct longitudinal examinations of HRQoL that start from prevention programming and continue throughout treatment trajectories. Future work should also thoroughly assess the structural and social determinants of HRQoL and include these variables in models, alongside those indicating different levels of disease control. The inclusion of those data would enable a holistic representation of the multidimensional factors that may be associated with HRQoL. Until these additional studies are conducted, these findings should be interpreted with caution.
Under ideal circumstances, multimorbidity would be addressed by providers who are trained to consider quality of life in the management of complex co-occurring conditions, and prevention programs would specifically target diseases and disease states that most negatively impact HRQoL. Emerging programs that address mental health in the context of comorbid HIV disease through stepped care [75] or task shifted approaches [76] may be an important model to support improved HRQoL among people living with HIV in Southern Africa. In SA, an integrated chronic disease model has been proposed and variably implemented [77], and pilot programs that facilitate integrated treatment for infectious diseases and NCDs are underway [78]. Some preliminary feedback on these programs from both providers and patients has highlighted structural and process-oriented implementation challenges [79], whereas other studies have found high levels of fidelity in implementing the integrated model [80] and high satisfaction with integrated services [81]. Although integration of services for comorbid conditions is critical, more attention must be paid to the development and implementation of interventions that aim to prevent stroke, heart attack, and other NCDs, particularly in populations that have elevated risk for NCDs, such as women living with HIV [82].
Conclusion
In conclusion, we found that presence of NCDs, whether controlled or not, was associated with poor HRQoL. However, the presence of HIV was associated with higher HRQoL, and the strength of that relationship increased when HIV was controlled. These findings suggest that investing in programs that prevent NCDs will likely have a meaningful impact on HRQoL in SA and other contexts where NCD and infectious disease epidemics converge.
Supporting information
(PDF)
(DOCX)
(DOCX)
Acknowledgments
We thank the residents of the AHRI demographic surveillance area and their leaders for many years of continuous engagement in population health research. We are particularly grateful to those who engaged with Vukuzazi either through participation or through considering participation. We appreciate members of the Community Advisory Board for their critical input throughout the lifecycle of the project. We additionally acknowledge the partnership of the local and provincial Department of Health in their support of this project. We thank all the members of the Vukuzazi Study Team for their valuable contributions to this study. The members of the Vukuzazi Population Science Programme include Deenan Pillay, Willem Hanekom, Emily Wong, Mark Siedner, Olivier Koole, Thumbi Ndung’u, Thandeka Khoza, Kobus Herbst, Kathy Baisley, Janet Seeley, Alison Grant, Resign Gunda, Ashmika Surujdeen, Theresa Smit, Dickman Gareta, Day Munatsi, Ngcebo Mhlongo, Tshwaraganang Modise, Jaco Dreyer, Siyabonga Nxumalo, Stephen Olivier, and Gregory Ording- Jespersen, Innocentia Mpofana, Khadija Khan, Zizile Sikhosana, Sashen Moodley, Hollis Shen, Philippa Mathews, Nompilo Buthelezi, Hlolisile Khumalo, Sanah Bucibo, Nozipho Mbonambi, Hloniphile Ngubane, Thokozani Simelane, Khanyisani Buthelezi, Sphiwe Ntuli, Nombuyiselo Zondi, Siboniso Nene, Bongumenzi Ndlovu, Talente Ntimbane, Mbali Mbuyisa, Xolani Mkhize, Melusi Sibiya, Ntombiyenkosi Ntombela, Mandisi Dlamini, Hlobisile Chonco, Hlengiwe Dlamini, Doctar Mlambo, Nonhlanhla Mzimela, Zinhle Buthelezi, Zinhle Mthembu, Thokozani Bhengu, Sandile Mthembu, Phumelele Mthethwa, Zamashandu Mbatha, Welcome Petros Mthembu, Anele Mkhwanazi, Mandlakayise Zikhali, Phakamani Mkhwanazi, Ntombiyenhlanhla Mkhwanazi, Rose Myeni, Fezeka Mfeka, Hlobisile Gumede, Nonceba Mfeka, Ayanda Zungu, Nonhlanhla Mfekayi, Smangaliso Zulu, Mzamo Buthelezi, Senzeni Mkhwanazi, Mlungisi Dube, Hosea Kambonde, Lindani Mthembu, Seneme Mchunu, Sibahle Gumbi, Tumi Madolo, Thengokwakhe Nkosi, Sibusiso Mkhwanazi, Sibusiso Nsibande, Mpumelelo Steto, Sibusiso Mhlongo, Velile Vellem, Pfarelo Tshivase, Jabu Kwinda, Bongani Magwaza, Siyabonga Nsibande, Skhumbuzo Mthombeni, Sphiwe Clement Mthembu, Antony Rapulana, Jade Cousins, Thabile Zondi, Nagavelli Padayachi, Freddy Mabetlela, Simphiwe Ntshangase, Nomfundo Luthuli, Sithembile Ngcobo, Kayleen Brien, Sizwe Ndlela, Nomfundo Ngema, Nokukhanya Ntshakala, Anupa Singh, Rochelle Singh, Logan Pillay, Kandaseelan Chetty, Ashentha Govender, Pamela Ramkalawon, Nondumiso Mabaso, Kimeshree Perumal, Senamile Makhari, Nondumiso Khuluse, Nondumiso Zitha, Hlengiwe Khathi, Mbuti Mofokeng, Nomathamsanqa Majozi, Nceba Gqaleni, Hannah Keal, Phumla Ngcobo, Costa Criticos, Raynold Zondo, Dilip Kalyan, Clive Mavimbela, Anand Ramnanan, and Sashin Harilall of African Health Research Institute; Kennedy Nyamande of the University of KwaZulu-Natal; Jaikrishna Kalideen of Perumal and Partners Radiologist Inc.; Ramesh Jackpersad of Jacpersad and Partners Inc.; and Kgaugelo Moropane, Boitsholo Mfolo, Khabonina Malomane of Aurum Innova (Pty) Ltd. The lead authors for this group are Deenan Pillay (d.pillay@ucl.ac.uk) and Willem Hanekom (willem.hanekom@ahri.org) of the Africa Health Research Institute.
Data Availability
De-identified participant data and a data dictionary, as well as other study documents (e.g., study protocol, informed consent forms) are available and accessible upon electronic request via the AHRI Data Repository at https://data.ahri.org/index.php/home. Requests can be made at: RDMServiceDesk@ahri.org. Data that requires additional levels of secure treatment to protect confidentiality will be made available upon approval of proposed analyses by the Vukuzazi Scientific Steering Committee and completion of a data access agreement.
Funding Statement
This project was supported by the Africa Health Research Institute and funding from the Wellcome Trust (201433/Z/16/Z), awarded to WH, Bill and Melinda Gates Foundation (OPP1175182), awarded to WH, the South African Department of Science and Innovation, South African Medical Research Council and South African Population Research Infrastructure Network (SAPRIN), awarded to KH. AMS receives funding from NIMH, National Institutes of Health (K23MH131438). EBW receives funding from the NIAID, National Institutes of Health (K08AI118538). MJS receives funding from the NHLBI, National Institutes of Health (K24HL166024). TN was partially funded through the South African Research Chairs Initiative, the Victor Daitz Foundation and the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency), with funding from the Wellcome Trust (107752/Z/15/Z) and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. The funders played no role in the study design, collection and analysis of the data, writing of the report, or decision to submit the paper for publication.
References
- 1.Hays RD, Reeve BB. Measurement and modeling of health-related quality of life. In: Epidemiology and Demography in Public Health [Internet]. San Diego: Academic Press; 2008. [cited 2021 Apr 1]. p. 195–205. Available from: https://escholarship.org/uc/item/70x7m955 [Google Scholar]
- 2.Pozniak A. Quality of life in chronic HIV infection. The Lancet HIV. 2014. Oct 1;1(1):e6–7. doi: 10.1016/S2352-3018(14)70003-7 [DOI] [PubMed] [Google Scholar]
- 3.Kastien-Hilka T, Abulfathi A, Rosenkranz B, Bennett B, Schwenkglenks M, Sinanovic E. Health-related quality of life and its association with medication adherence in active pulmonary tuberculosis- a systematic review of global literature with focus on South Africa. Health Qual Life Outcomes. 2016. Mar 11;14:42. doi: 10.1186/s12955-016-0442-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rwegerera GM, Moshomo T, Gaenamong M, Oyewo TA, Gollakota S, Rivera YP, et al. Health-related quality of life and associated factors among patients with diabetes mellitus in Botswana. Alexandria Journal of Medicine. 2018. Jun 1;54(2):111–8. [Google Scholar]
- 5.Vagiri RV, Meyer JC, Godman B, Gous AGS. Relationship between adherence and health-related quality of life among HIV-patients in South Africa: findings and implications. Journal of AIDS and HIV Research. 2018. Dec 31;10(8):121–32. [Google Scholar]
- 6.Bayliss M, Rendas-Baum R, White MK, Maruish M, Bjorner J, Tunis SL. Health-related quality of life (HRQL) for individuals with self-reported chronic physical and/or mental health conditions: panel survey of an adult sample in the United States. Health Qual Life Outcomes. 2012. Dec 19;10:154. doi: 10.1186/1477-7525-10-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strine TW, Kroenke K, Dhingra S, Balluz LS, Gonzalez O, Berry JT, et al. The associations between depression, health-related quality of life, social support, life satisfaction, and disability in community-dwelling US adults. J Nerv Ment Dis. 2009. Jan;197(1):61–4. doi: 10.1097/NMD.0b013e3181924ad8 [DOI] [PubMed] [Google Scholar]
- 8.Multiple Long-Term Conditions (Multimorbidity): a priority for global health research [Internet]. [cited 2022 Nov 22]. Available from: https://acmedsci.ac.uk/policy/policy-projects/multimorbidity
- 9.Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014. Mar;67(3):254–66. doi: 10.1016/j.jclinepi.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 10.Smith SM O ’Dowd T Chronic diseases: what happens when they come in multiples? Br J Gen Pract. 2007. Apr;57(537):268–70. [PMC free article] [PubMed] [Google Scholar]
- 11.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012. Dec 15;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 12.Hall V, Thomsen RW, Henriksen O, Lohse N. Diabetes in Sub Saharan Africa 1999–2011: Epidemiology and public health implications. a systematic review. BMC Public Health. 2011. Jul 14;11(1):564. doi: 10.1186/1471-2458-11-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhatib A, Nnyanzi L, Mujuni B, Amanya G, Ibingira C. Preventing Multimorbidity with Lifestyle Interventions in Sub-Saharan Africa: A New Challenge for Public Health in Low and Middle-Income Countries. International journal of environmental research and public health [Internet]. 2021. Nov 26 [cited 2022 Nov 22];18(23). Available from: http://pubmed.ncbi.nlm.nih.gov/34886172/ doi: 10.3390/ijerph182312449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016. Sep;11(5):492–500. doi: 10.1097/COH.0000000000000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health-Uganda. Uganda Population-Based HIV Impact Assessment [Internet]. 2018. Apr. Available from: https://phia.icap.columbia.edu/wp-content/uploads/2018/07/3430%E2%80%A2PHIA-Uganda-SS_NEW.v14.pdf [Google Scholar]
- 16.Zuma K, Simbayi L, Zungu N, Moyo S, Marinda E, Jooste S, et al. The HIV Epidemic in South Africa: Key Findings from 2017 National Population-Based Survey. Int J Environ Res Public Health. 2022. Jul 1;19(13):8125. doi: 10.3390/ijerph19138125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009. Sep 12;374(9693):934–47. doi: 10.1016/S0140-6736(09)61087-4 [DOI] [PubMed] [Google Scholar]
- 18.Gouda HN, Charlson F, Sorsdahl K, Ahmadzada S, Ferrari AJ, Erskine H, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. The Lancet Global Health. 2019. Oct 1;7(10):e1375–87. doi: 10.1016/S2214-109X(19)30374-2 [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Pu R, Li Z, Ji L, Li X, Ghose B, et al. Subjective health and quality of life among elderly people living with chronic multimorbidity and difficulty in activities of daily living in rural South Africa. Clin Interv Aging. 2019;14:1285–96. doi: 10.2147/CIA.S205734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peltzer K. Tuberculosis non-communicable disease comorbidity and multimorbidity in public primary care patients in South Africa. Afr J Prim Health Care Fam Med. 2018. Apr 11;10(1):e1–6. doi: 10.4102/phcfm.v10i1.1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang AY, Gómez-Olivé FX, Payne C, Rohr JK, Manne-Goehler J, Wade AN, et al. Chronic multimorbidity among older adults in rural South Africa. BMJ Global Health. 2019. Aug 1;4(4):e001386. doi: 10.1136/bmjgh-2018-001386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odland ML, Payne C, Witham MD, Siedner MJ, Bärnighausen T, Bountogo M, et al. Epidemiology of multimorbidity in conditions of extreme poverty: a population-based study of older adults in rural Burkina Faso. BMJ Glob Health. 2020;5(3):e002096. doi: 10.1136/bmjgh-2019-002096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roomaney RA, Wyk B van, Turawa EB, van Wyk VP. Multimorbidity in South Africa: a systematic review of prevalence studies. BMJ Open. 2021. Oct 1;11(10):e048676. doi: 10.1136/bmjopen-2021-048676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong EB, Olivier S, Gunda R, Koole O, Surujdeen A, Gareta D, et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: a cross-sectional, population-based multimorbidity study. The Lancet Global Health. 2021;9(7):e967–76. doi: 10.1016/S2214-109X(21)00176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunda R, Koole O, Gareta D, Olivier S, Surujdeen A, Smit T, et al. Cohort Profile: The Vukuzazi (’Wake Up and Know Yourself’in isiZulu) population science programme. International journal of epidemiology. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elm E von Altman DG, Egger M Pocock SJ, Gøtzsche PC Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007. Oct 18;335(7624):806–8. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breuninger M, van Ginneken B, Philipsen RHHM, Mhimbira F, Hella JJ, Lwilla F, et al. Diagnostic accuracy of computer-aided detection of pulmonary tuberculosis in chest radiographs: a validation study from sub-Saharan Africa. PLoS One. 2014;9(9):e106381. doi: 10.1371/journal.pone.0106381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.STEPwise Approach to NCD Risk Factor Surveillance (STEPS). World Health Organization. https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps/manuals. Accessed October 2021.
- 29.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990. Dec;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992. Jun;30(6):473–83. [PubMed] [Google Scholar]
- 31.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996. Mar;34(3):220–33. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 32.Ware J, Kosinski M, Keller S. How to score the SF-12 physical and mental health summary scales. 2nd ed. Boston, MA; 1995. [Google Scholar]
- 33.Ware J. E. SF-12: an even shorter health survey. Medical Outcomes Trust Bulletin. 1996;4(2). [Google Scholar]
- 34.Schreiber-Gregory DN. Ridge Regression and multicollinearity: An in-depth review. Model Assisted Statistics and Applications. 2018;13(4):359–65. [Google Scholar]
- 35.Firinguetti L, Kibria G, Araya R. Study of partial least squares and ridge regression methods. Communications in Statistics-Simulation and Computation. 2017;46(8):6631–44. [Google Scholar]
- 36.McNeish DM. Using Lasso for Predictor Selection and to Assuage Overfitting: A Method Long Overlooked in Behavioral Sciences. Multivariate Behavioral Research. 2015. Sep 3;50(5):471–84. doi: 10.1080/00273171.2015.1036965 [DOI] [PubMed] [Google Scholar]
- 37.Hawkins DM. The Problem of Overfitting. J Chem Inf Comput Sci. 2004. Jan 1;44(1):1–12. doi: 10.1021/ci0342472 [DOI] [PubMed] [Google Scholar]
- 38.Microsoft, & Ooi H. (2020). glmnetUtils: Utilities for “Glmnet.” Accessed January 11, 2022. https://CRAN.R-project.org/package=glmnetUtils. [Google Scholar]
- 39.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. Journal of statistical software. 2010;33(1):1. doi: 10.1109/TPAMI.2005.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen MP. Partial regression and residualized variables. Understanding Regression Analysis. 1997;86–90. [Google Scholar]
- 41.Pearl J. Simpson’s paradox, confounding, and collapibility. Causality: models, reasoning and inference. 2000;(7):173–200. [Google Scholar]
- 42.Li C, Shepherd BE. A new residual for ordinal outcomes. Biometrika. 2012. Jun 1;99(2):473–80. doi: 10.1093/biomet/asr073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu D, Zhang H. Residuals and Diagnostics for Ordinal Regression Models: A Surrogate Approach. Journal of the American Statistical Association. 2018. Apr 3;113(522):845–54. doi: 10.1080/01621459.2017.1292915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman J, Hastie T, Tibshirani R, Narasimhan B, Tay K, Simon N, et al. glmnet: Lasso and Elastic-Net Regularized Generalized Linear Models [Internet]. 2021. [cited 2021 Apr 1]. Available from: https://CRAN.R-project.org/package=glmnet [Google Scholar]
- 45.Team RC. R: A language and environment for statistical computing. 2013. [Google Scholar]
- 46.Cule E, Vineis P, De Iorio M. Significance testing in ridge regression for genetic data. BMC Bioinformatics. 2011. Sep 19;12(1):372. doi: 10.1186/1471-2105-12-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goeman J, Meijer R, Chaturvedi N, Lueder M. penalized: L1 (Lasso and Fused Lasso) and L2 (Ridge) Penalized Estimation in GLMs and in the Cox Model [Internet]. 2018. [cited 2021 Apr 1]. Available from: https://CRAN.R-project.org/package=penalized [Google Scholar]
- 48.Wasserman L. Assumption-Free High-Dimensional Inference [Internet]. Normal Deviate. 2013. [cited 2021 Apr 1]. Available from: https://normaldeviate.wordpress.com/2013/10/03/assumption-free-high-dimensional-inference/ [Google Scholar]
- 49.Diedenhofen B, Musch J. cocor: A comprehensive solution for the statistical comparison of correlations. PloS one. 2015;10(4):e0121945. doi: 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tonidandel S, LeBreton JM. RWA Web: A Free, Comprehensive, Web-Based, and User-Friendly Tool for Relative Weight Analyses. J Bus Psychol. 2015. Jun 1;30(2):207–16. [Google Scholar]
- 51.Dietterich TG. Approximate Statistical Tests for Comparing Supervised Classification Learning Algorithms. Neural Computation. 1998. Oct;10(7):1895–923. doi: 10.1162/089976698300017197 [DOI] [PubMed] [Google Scholar]
- 52.Makovski TT, Schmitz S, Zeegers MP, Stranges S, van den Akker M. Multimorbidity and quality of life: Systematic literature review and meta-analysis. Ageing Res Rev. 2019. Aug;53:100903. doi: 10.1016/j.arr.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 53.Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV Cost and Services Utilization Study. Am J Med. 2000. Jun 15;108(9):714–22. doi: 10.1016/s0002-9343(00)00387-9 [DOI] [PubMed] [Google Scholar]
- 54.Rueda S, Raboud J, Mustard C, Bayoumi A, Lavis JN, Rourke SB. Employment status is associated with both physical and mental health quality of life in people living with HIV. AIDS Care. 2011. Apr;23(4):435–43. doi: 10.1080/09540121.2010.507952 [DOI] [PubMed] [Google Scholar]
- 55.Beard J, Feeley F, Rosen S. Economic and quality of life outcomes of antiretroviral therapy for HIV/AIDS in developing countries: a systematic literature review. AIDS care. 2009;21(11):1343–56. doi: 10.1080/09540120902889926 [DOI] [PubMed] [Google Scholar]
- 56.Thomas R, Burger R, Harper A, Kanema S, Mwenge L, Vanqa N, et al. Differences in health-related quality of life between HIV-positive and HIV-negative people in Zambia and South Africa: a cross-sectional baseline survey of the HPTN 071 (PopART) trial. The Lancet Global Health. 2017;5(11):e1133–41. doi: 10.1016/S2214-109X(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manne-Goehler J, Siedner MJ, Montana L, Harling G, Geldsetzer P, Rohr J, et al. Hypertension and diabetes control along the HIV care cascade in rural South Africa. J Int AIDS Soc. 2019. Mar;22(3):e25213. doi: 10.1002/jia2.25213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manne-Goehler J, Montana L, Gómez-Olivé FX, Rohr J, Harling G, Wagner RG, et al. The ART advantage: Healthcare utilization for diabetes and hypertension in rural South Africa. Journal of acquired immune deficiency syndromes (1999). 2017;75(5):561. doi: 10.1097/QAI.0000000000001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwarisiima D, Atukunda M, Owaraganise A, Chamie G, Clark T, Kabami J, et al. Hypertension control in integrated HIV and chronic disease clinics in Uganda in the SEARCH study. BMC public health. 2019;19(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phalane E, Fourie CM, Mels CM, Schutte AE. A comparative analysis of blood pressure in HIV-infected patients versus uninfected controls residing in sub-Saharan Africa: A narrative review. Journal of Human Hypertension. 2020;34(10):692–708. doi: 10.1038/s41371-020-0385-6 [DOI] [PubMed] [Google Scholar]
- 61.Nonterah EA, Boua PR, Klipstein‐Grobusch K, Asiki G, Micklesfield LK, Agongo G, et al. Classical cardiovascular risk factors and HIV are associated with carotid intima‐media thickness in adults from Sub‐Saharan Africa: findings from H3Africa AWI‐Gen study. Journal of the American Heart Association. 2019;8(14):e011506. doi: 10.1161/JAHA.118.011506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feinstein MJ, Kim JH, Bibangambah P, Sentongo R, Martin JN, Tsai AC, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV-infected and uninfected Ugandans. AIDS research and human retroviruses. 2017;33(1):49–56. doi: 10.1089/AID.2016.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitton JA, North CM, Muyanja D, Okello S, Vořechovská D, Kakuhikire B, et al. Smoking cessation after engagement in HIV care in rural Uganda. AIDS care. 2018;30(12):1622–9. doi: 10.1080/09540121.2018.1484070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kintu A, Sando D, Okello S, Mutungi G, Guwatudde D, Menzies NA, et al. Integrating care for non‐communicable diseases into routine HIV services: key considerations for policy design in sub‐Saharan Africa. Journal of the International AIDS Society. 2020;23:e25508. doi: 10.1002/jia2.25508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomita A, Garrett N, Werner L, Burns JK, Ngcobo N, Zuma N, et al. Impact of antiretroviral therapy on health-related quality of life among South African women in the CAPRISA 002 acute infection study. AIDS Behav. 2014. Sep;18(9):1801–7. doi: 10.1007/s10461-014-0800-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nglazi MD, West SJ, Dave JA, Levitt NS, Lambert EV. Quality of life in individuals living with HIV/AIDS attending a public sector antiretroviral service in Cape Town, South Africa. BMC Public Health. 2014. Jul 3;14:676. doi: 10.1186/1471-2458-14-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao XY, Xie YX, Zhang XX, Peng X, Huang JX, Du QF, et al. The association between multimorbidity and health-related quality of life: a cross-sectional survey among community middle-aged and elderly residents in southern China. Health and quality of life outcomes. 2019;17(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brettschneider C, Leicht H, Bickel H, Dahlhaus A, Fuchs A, Gensichen J, et al. Relative impact of multimorbid chronic conditions on health-related quality of life–results from the MultiCare Cohort Study. PloS one. 2013;8(6):e66742. doi: 10.1371/journal.pone.0066742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cortina JM, Landis RS. When small effect sizes tell a big story, and when large effect sizes don’t. Statistical and methodological myths and urban legends: Doctrine, verity and fable in the organizational and social sciences. 2009;287–308. [Google Scholar]
- 70.Matz SC, Gladstone JJ, Stillwell D. In a world of big data, small effects can still matter: A reply to Boyce, Daly, Hounkpatin, and Wood (2017). Psychological science. 2017;28(4):547–50. doi: 10.1177/0956797617697445 [DOI] [PubMed] [Google Scholar]
- 71.Non communicable diseases [Internet]. Geneva, Switzerland: World Health Organization; 2018. Jun [cited 2021 Apr 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases [Google Scholar]
- 72.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–85. doi: 10.1146/annurev.med.050108.151127 [DOI] [PubMed] [Google Scholar]
- 73.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013. Nov 2;382(9903):1525–33. doi: 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamkuemah M, Gausi B, Oni T. Missed opportunities for NCD multimorbidity prevention in adolescents and youth living with HIV in urban South Africa. BMC Public Health. 2020. Jun 1;20(1):821. doi: 10.1186/s12889-020-08921-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abas M, Nyamayaro P, Bere T, Saruchera E, Mothobi N, Simms V, et al. Feasibility and Acceptability of a Task-Shifted Intervention to Enhance Adherence to HIV Medication and Improve Depression in People Living with HIV in Zimbabwe, a Low Income Country in Sub-Saharan Africa. AIDS Behav. 2018;22(1):86–101. doi: 10.1007/s10461-016-1659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andersen LS, Magidson JF, O’Cleirigh C, Remmert JE, Kagee A, Leaver M, et al. A pilot study of a nurse-delivered cognitive behavioral therapy intervention (Ziphamandla) for adherence and depression in HIV in South Africa. J Health Psychol. 2018;23(6):776–87. doi: 10.1177/1359105316643375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahomed OH, Asmall S. Development and implementation of an integrated chronic disease model in South Africa: lessons in the management of change through improving the quality of clinical practice. Int J Integr Care. 2015. Dec;15:e038. doi: 10.5334/ijic.1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ameh S, Klipstein-Grobusch K, Musenge E, Kahn K, Tollman S, Gómez-Olivé FX. Effectiveness of an Integrated Approach to HIV and Hypertension Care in Rural South Africa: Controlled Interrupted Time-Series Analysis. J Acquir Immune Defic Syndr. 2017. Aug 1;75(4):472–9. doi: 10.1097/QAI.0000000000001437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ameh S, Klipstein-Grobusch K, D’ambruoso L, Kahn K, Tollman SM, Gómez-Olivé FX. Quality of integrated chronic disease care in rural South Africa: user and provider perspectives. Health Policy Plan. 2017. Mar;32(2):257–66. doi: 10.1093/heapol/czw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lebina L, Alaba O, Ringane A, Hlongwane K, Pule P, Oni T, et al. Process evaluation of implementation fidelity of the integrated chronic disease management model in two districts, South Africa. BMC Health Services Research. 2019. Dec 16;19(1):965. doi: 10.1186/s12913-019-4785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins KL, Hlongwane KE, Otwombe K, Dietrich J, Cheyip M, Khanyile N, et al. Level of adult client satisfaction with clinic flow time and services of an integrated non-communicable disease-HIV testing services clinic in Soweto, South Africa: a cross-sectional study. BMC Health Serv Res. 2020. May 11;20(1):404. doi: 10.1186/s12913-020-05256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chow FC, Regan S, Zanni MV, Looby SE, Bushnell CD, Meigs JB, et al. Elevated ischemic stroke risk among women living with HIV infection. AIDS. 2018. Jan 2;32(1):59–67. doi: 10.1097/QAD.0000000000001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
Data Availability Statement
De-identified participant data and a data dictionary, as well as other study documents (e.g., study protocol, informed consent forms) are available and accessible upon electronic request via the AHRI Data Repository at https://data.ahri.org/index.php/home. Requests can be made at: RDMServiceDesk@ahri.org. Data that requires additional levels of secure treatment to protect confidentiality will be made available upon approval of proposed analyses by the Vukuzazi Scientific Steering Committee and completion of a data access agreement.