Summary
Type 1 diabetes is characterised by an autoimmune-mediated destruction of pancreatic beta-cell mass. With the implementation of insulin therapy a century ago, type 1 diabetes changed from a progressive, fatal disease to one that requires life-long complex self-management. Replacing the lost beta-cell mass through transplantation has proven successful, but limited donor supply and need for life-long immunosuppression restricts wide-spread use. In this review, we highlight recent advances and remaining challenges in regenerative medicine approaches to restore beta-cell mass and function in type 1 diabetes. We begin by summarising the role of endocrine islets in glucose homeostasis and how this is altered in disease. We then discuss the potential regenerative capacity of the remaining islet cells and the utility of stem-cell derived beta-like cells to restore beta-cell function. We conclude with tissue engineering approaches that may improve the engraftment, function and survival of beta-cell replacement therapies.
Keywords: Type 1 diabetes, insulin therapy, beta-cell, islet cell replacement, transplantation, tissue engineering, stem cell, regeneration
Limitations of conventional insulin replacement
The introduction of insulin therapy delivered a medical miracle in the early 20th Century rescuing those with type 1 diabetes mellitus (T1D) from an inexorably progressive and ultimately fatal wasting disease. Since then numbers of people with T1D have steadily increased1,2 in parallel with a global epidemic of type 2 diabetes (T2D) over the second half of the 20th Century and beyond3. In 2019, it was estimated that 463 million adults had been diagnosed diabetes world-wide. This includes up to 10% with T1D in addition to more than 1·1 million children and adolescents being dependent on insulin replacement therapy3. Although people with T2D and other rarer forms of diabetes are not absolutely dependent on insulin replacement, it is now acknowledged that all forms of diabetes are characterised by a sub-optimal pancreatic islet cell insulin response4.
As we celebrate the centenary of insulin treatment, diabetes remains a leading cause of blindness, renal failure and lower limb amputation5 with increased incidence of myocardial infarction and reduced life-expectancy6 despite incontrovertible evidence that chronic complications can be prevented by early correction of high blood glucose levels7. Achieving this with subcutaneous injected or infused insulin is unavoidably associated with risk of hypoglycaemia. Every year, nearly 50% of people with T1D of >15 years duration require assistance for severe hypoglycaemia, one of the greatest fears of dependence on exogenous insulin8. Great strides have been made in enhancing insulin delivery and glucose testing over the last century, including successful combination in integrated closed-loop systems9. In contrast to the physiological beta-cell which continuously senses glucose intravascularly with second-to-second control of insulin secretion directly into the portal vein, the wearable ‘bioartifical pancreas’ has to overcome delays in detecting blood glucose changes due to subcutaneous sensing and in effecting a change in circulating insulin levels due to subcutaneous insulin delivery. This necessitates frequent substantial changes in insulin infusion rate, typically associated with an average of at least 30 minutes each day with glucose less than 4 mmol/L even using the fastest marketed short-acting insulin analogue. This approach does not thus provide curative therapy, nor completely remove the burden of glucose uncertainty and unremitting daily self-management10.
Established beta-cell replacement therapy
Transplantation of vascularised whole pancreas from a deceased donor has confirmed that beta-cell replacement can immediately normalise glucose levels in T1D and in carefully selected recipients with insulin-treated T2D11. Despite established success, applicability to more than a minority is limited by the complexity of the surgery with unavoidable risk of mortality and significant morbidity12.
Mechanical and enzymatic dissociation of a donor pancreas has enabled separation of the endocrine cells within the Islets of Langerhans from their blood supply and from the digestive enzyme-producing majority of the organ13. Islet transplantation is a minimally invasive procedure that was initially undertaken as an autologous procedure following total pancreatectomy for painful pancreatitis13. Subsequently allogeneic islets were infused into the hepatic portal vein with systemic immunosuppression in T1D14.
Islet transplantation offers prevention of severe hypoglycaemia, stabilisation of glucose levels and attainment of optimal HbA1c (<53mmol/mol) and is established as standard of care for recurrent dangerous hypoglycaemia despite optimised conventional therapy in the integrated UK programme and in other countries around the world15–17. However, sustainable insulin independence cannot be reproducibly achieved from a single transplant procedure and the requirement for lifelong systemic immunosuppression currently limits recipients to those with life-threatening complications18.
Portal vein infusion via a percutaneous trans-hepatic or mini-laparotomy approach is minimally invasive, only very rarely complicated by haemorrhage or thrombosis in experienced centres15–17. Intravascularly transplanted cells are, however, subject to an Instant Blood-Mediated Inflammatory Reaction (IBMIR)19. PET scanning strongly suggests that this thrombotic and innate immune response leads to a substantial loss of transplanted islet mass immediately following infusion20. Moreover, beta-cells are uniquely sensitive to hypoxia, encountered during pancreas retrieval / preservation; islet isolation / culture / shipment for transplantation; and within the relatively low oxygen tension portal vein environment prior to revascularisation from the hepatic artery21. Transient ex vivo exposure of primary mouse islets to hypoxia has been shown to lead to upregulation of hypoxia-inducible genes and adaptation enabling beta-cell survival but with impaired glucose-stimulated insulin secretion persisting following transplantation22. Loss of fully mature end-differentiated phenotype (absence of beta-cell urocortin-3 expression) several years after transplantation despite full vascularisation has been reported following clinical islet transplantation23. Together these limitations have led to maximal engraftment of only approximately 25–40% of normal adult beta-cell functional mass despite often repeated transplants from sequential deceased donors24. Although this can deliver short term insulin independence, true physiological endocrine function has not yet been achieved25.
Human islets have been transplanted clinically without encapsulation into alternative sites including skeletal muscle and omentum26. Although islets may become well-vascularised, superior outcomes to portal transplantation have yet to be achieved, potentially due to irreversible adapatation during even short-term ischaemia and unpredictable inflammatory response.
Optimising beta-cell replacement through regenerative medicine
Here we consider how the remaining challenges eluding a T1D cure may be overcome through regenerative medicine approaches including stem cell-derived beta-cells, gene therapy and tissue engineering. The highly-vascularised endogenous islet niche and mechanisms for maintenance of beta-cell mass and function will be considered as the context for intelligent recapitulation through:
Generation of an unlimited source of physiologically functional islets
Vascularised engraftment of sufficient beta-cell mass without tissue ischaemia
Overcoming cell loss through innate and adaptive immune response
The endogenous beta-cell niche
In considering beta-cell replacement strategies, it it important to understand physiological beta-cell function in the context of its endogenous niche. Beta-cells do not exist in isolation in the normal human pancreas, but in islet ‘mini-organs’. Beta-cells respond to glucose stimulation above a threshold varying between species (⁓7·8–8·3 mmol/l in mice; ⁓4·4–5·0 mmol/l in humans27) with closure of ATP-sensitive potassium leak channels triggering opening of voltage-gated calcium channels and insulin secretory granule exocytosis. At higher glucose concentrations, beta-cells within the same islet will progressively synchronise glucose-stimulated insulin secretion via gap junction-mediated propagation of action potentials28.
Beta-cells are co-localised with alpha- and delta-cells, in addition to smaller numbers of pancreatic polypeptide and ghrelin-secreting cells. Human islets consist of 45–65% beta-cells and ⁓35% alpha-cells29–32. Alpha-cells secrete glucagon as part of a counter-regulatory response preventing significant hypoglycaemia by stimulating hepatic glucose production. Complex paracrine interactions between islet endocrine cells contributing to normal glucose homesostasis include positive as well as negative feedback loops33,34. For example, modest amounts of glucagon acting locally within the islet during the post-prandial phase paradoxically stimulate beta-cells in a paracrine amplification of glucose-stimulated insulin secretion35–37. Too much glucagon released during the post-prandial phase, however, counters the local amplifying actions of glucagon on insulin secretion aggravating hyperglycaemia; a scenario occurring in diabetes (see below). Delta-cells release the inhibitory hormone somatostatin, which exerts local modulatory feedback inhibition over insulin and glucagon release. Co-localisation of beta-cells with alpha- and delta-cells in the same islet affords local coordination of insulin and glucagon secretion, contributing to stable tightly regulated glycemic control38.
The paracrine mechanisms carefully balancing insulin and glucagon release in health become perturbed in diabetes due to loss of most of the functional beta-cell mass in T1D and loss of key paracrine factors in T2D33,39. Treatment with specific antagonists to the dominant somatostatin receptor expressed by alpha-cells can restore counter-regulation preventing hypoglycaemia40. This suggests that excessive inhibition of alpha-cells by somatostatin under hypoglycaemic conditions contributes to counter-regulatory failure in T1D. Conversely, in T2D models, hyperglycaemia is associated with reduced somatostatin release accompanied by elevated glucagon secretion post-prandially41,42, credited for as much as half of the hyperglycaemia in diabetes43,44. Collectively, these observations remind us of the elegant and coordinated role that islet endocrine cells play in glucose homeostasis. The gut-endocrine axis provides further refinement of physiological islet hormone secretion mediated by incretins including GLP1 and GIP45.
As we look towards a cure for T1D providing insulin independence and stable glycaemic control for decades, regenerative medicine approaches may ultimately require the recreation of an islet-like microenvironment containing a heterogeneous mixture of beta, alpha, and delta-cells that is appropriate vascularised.
Vascularisation and organisation of the islet niche
In addition to an organised inter-communicating network of endocrine cells, dense vascularisation is integral to the complex architecture of the endogenous islet. Despite comprising only 1–2% of total pancreatic mass, islets receive 10–20% of blood flow delivered through a more fenestrated endothelium with 1.5-fold higher oxygen tension than exocrine pancreas46. Larger islets have their own feeding arteriole and collecting venule with alpha- and beta-cells in direct contact with endothelial cells47. Overall islet structure is dependent on an extra-cellular matrix network including a specialised two-layered basement membrane providing structural integrity and cell-to-matrix connections that are important for coordinated endocrine cell function48. Additional cells, including fibroblasts, pericytes and tissue resident macrophages, also play significant roles in maintaining islet homeostasis through extra-cellular matrix synthesis, controlling blood flow and immune regulation49.
Endogenous islets are richly innervated50 providing bidirectional communication with central and enteric nervous systems (covered in detail elsewhere51,52). Recapitulating the important contributions of innervation using grafted islets including enhancement of insulin secretion in anticipation of food and inhibition following hypothalamic detection of low blood glucose – whether from adult donor or stem cell derived origins – may represent an inherent challenge to restoring fully functional beta-cell mass. Studies in mice and non-human primates have established proof-of-principle for re-innervation of islets grafted in the anterior chamber of the eye, leading to a Phase 1 clinical study53,54.
The impact of T1D on beta-cell mass
The specificity of T1D autoimmunity is illustrated by selective beta-cell ablation leaving behind islets consisting largely of alpha- and delta-cells. In most cases, sub-clinical loss of beta-cell function takes place over several years before onset of clinical symptoms55,56. Beta-cell mass loss is no longer considered absolute and few pancreases have been studied around the time of T1D diagnosis where on average 20% beta-cell mass may remain but with considerable heterogeneity57–59, potentially contributed to be beta-cell loss in a distinctly lobular fashion58,60–62. There is broad agreement that 85–95% of beta-cell mass is lost in more long-established T1D59,63 but many individuals retain modest numbers of residual insulin-positive cells for many years, including Joslin medalists (diabetes duration >50 years)61,62. Systematic evaluation within the Pancreatic Organ Donors with Diabetes (nPOD) biorepository of 47 T1D donors (age range 4–50 years; median diabetes duration 8 years) reported beta-cells in 64% with ⁓90% reductions in beta-cell area and mass compared to controls64. Another study reported insulin+ cells in all with recent T1D onset, but in only 8% with long-standing disease63. Striking differences according to age of diabetes presentation have been reported, with those diagnosed over the age of 13 years retaining insulin+ cells in up to 40% of islets65. Pancreatic imaging with radiolabelled GLP1 receptor agonist confirms significantly reduced islet mass in T1D66. Assessment of endocrine volume with the PET ligand 11C-5-HTP indicated ⁓50% reduction in T1D67, likely representing predominantly alpha- rather than beta-cells29–31.
Residual beta-cells are long-term survivors
Perhaps the most likely explanation for residual beta-cells is that they are simply long-term survivors that have managed to escape ongoing autoimmunity for reasons that are not fully understood64. Beta-cells are long-lived evidenced by progressive accumulation with age of lipofuscin bodies in human beta-cells68, isotope labelling in rodents69,70, and very low rates of apoptosis – certainly in the absence of disease71.
It has been proposed that residual insulin+ cells reflect an altered or ‘dedifferentiated’ state characterized by impaired function and reduced expression of some mature beta-cell markers in rodent72 and human samples73. This includes autoantigens such as insulin potentially contribute to their ‘invisibility’ / adaptation / resilience to autoimmune attack and therefore long-term survival in T1D. Nevertheless, the notion of changing differentiation state of human beta-cells in response to T1D-related insults would certainly benefit from additional validation.
There are many potential ‘drivers’ of phenotypic switch towards a de-differentiated state. A combination of pro-inflammatory and cytotoxic T cell-mediated insults contribute to beta-cell stress, particularly in islets with active insulitis. Furthermore remaining beta-cells compensate for the diminishing total beta-cell mass by upregulating insulin secretion on a per-cell basis during the pre-clinical stages of T1D. However, this attempted compensatory increase in insulin release in the remaining beta-cells is in rodent models associated with accumulation of reactive oxygen species (ROS) and increased expression of the thioredoxin-interacting protein (TXNIP), which induces beta-cell apoptosis74. Furthermore, autophagy, ordinarily a protective mechanism against excess ROS, is impaired in beta-cells in NOD mice and in human T1D75 / T2D76, further compounding beta-cell loss. The increasing strain on a dwindling number of remaining beta-cells around T1D diagnosis is in rodents associated with increased ER stress and unfolded protein response. The relative importance of the detrimental contributions of ROS and ER stress to beta cell loss in human T1D is not fully established (Figure 1), although increased ER stress would be consistent with the defects in insulin processing observed in T1D through increased secreted proinsulin to insulin ratio77,78. Beta-cell islet amyloid polypeptide (IAPP) is also incompletely processed in T1D, being secreted as the pro-IAPP1–48 intermediate79. These partially processed peptides may act as neoepitopes further driving the autoimmune process80.
Figure 1: Residual beta-cells in type 1 diabetes are dysfunctional.
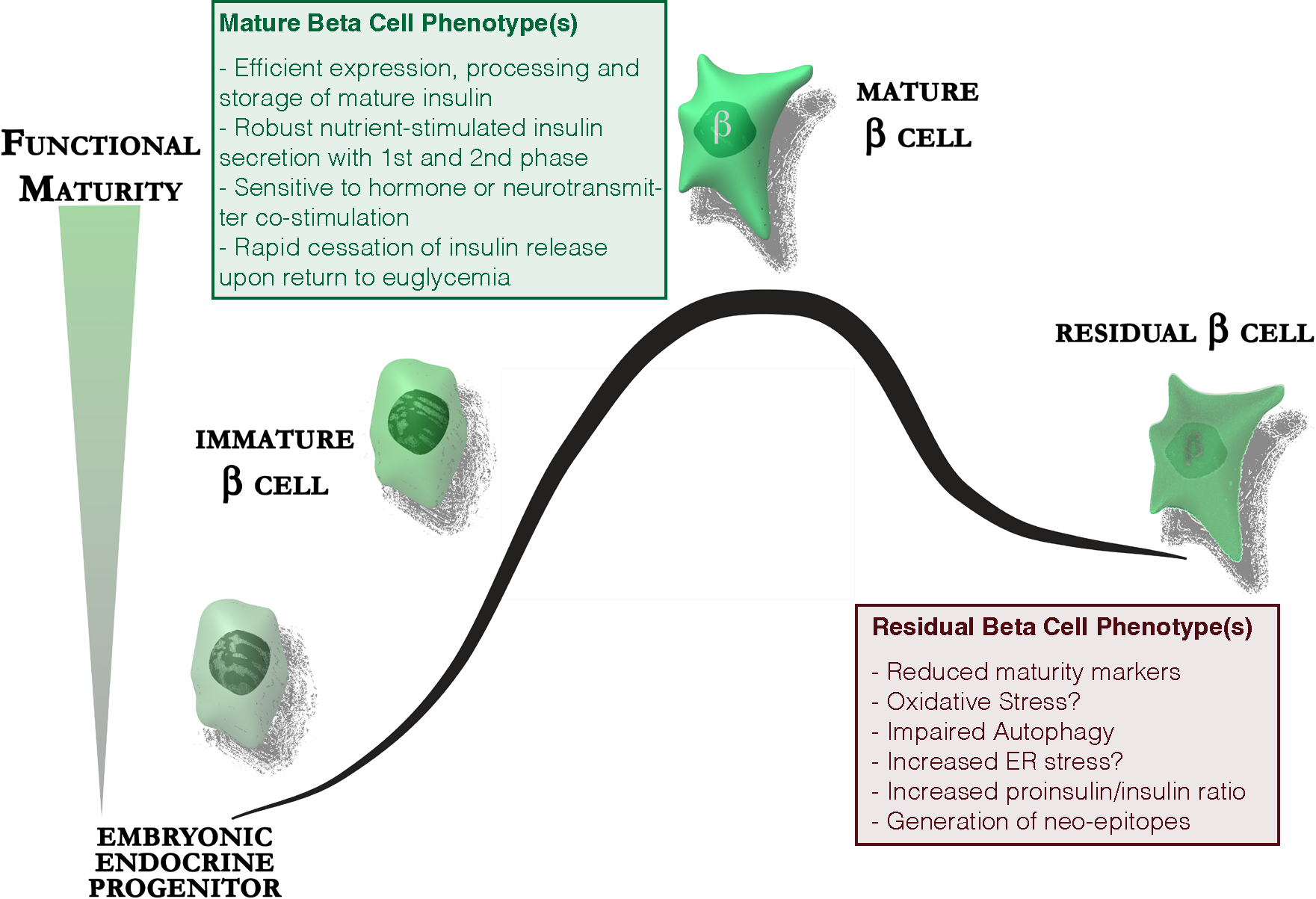
The majority of beta-cells in long-standing type 1 diabetes are lost. Residual beta-cells express lower levels of maturity markers, experience increased oxidative stress and have impaired autophagy. They demonstrate increased ER stress as a consequence of the increased demand for proinsulin biosynthesis placed upon a dwindling number of beta-cells. This is associated with increased release of incompletely processed proinsulin and could lead to the generation of neo-epitopes in the beta-cell specific autoimmune response.
Potential for endogenous beta-cell regeneration in T1D
There is no doubt that relatively modest residual insulin C-peptide secretion is associated with clinical benefits including reduced glucose variability, less hypoglycaemia and long-term microvascular complications81–84. Moreover, retention of residual beta-cell mass in even long-standing diabetes has raised hopes regarding potential for regeneration of sufficient insulin-secretory function to reverse type 1 diabetes. This goal has been further spurred by the demonstration of restored beta-cell function, previously thought irreversibly lost, in T2D through weight loss-induced remission85.
Reversal of functional impairment
A ‘honeymoon’ period following T1D diagnosis with transient reduction in requirement for exogenous insulin following initial glucose stabilisation supports the potential for beta-cell functional recovery. This ‘beta-cell rest’ is insufficient to prevent declining endogenous insulin levels as type 1 diabetes progresses, however, although a case series of three individuals aged 36–40 years presenting with T1D and confirmed underlying autoimmunity reported attainment of insulin independence with a low-carbohydrate high-fat diet in combination with regular exercise86. The case description of these patients is suggestive of Latent Autoimmune Diabetes in Adults (LADA), a particularly slowly progressing form of autoimmune diabetes.
Following demonstration of verapamil prevention of beta-cell apoptosis secondary to glucotoxicity-associated increase in TXNIP in a rodent model74, oral verapamil in a Phase 2 double-blind placebo-controlled clinical trial in recently diagnosed adult onset T1D was associated with improved mixed meal-stimulated C-peptide, fewer hypoglycemic events and better overall glycemic control87.
Evidence for prevention of diabetes progression through multifaceted immunotherapy approaches is rapidly accruing88, most recently an almost 3 year delay in diabetes onset with following 14 days’ teplizumab anti-CD3 antibody therapy89. In clinically established disease, the importance of combinatorial approaches to maintain functional beta-cell mass has been recognised (for example anti- IL21 / GLP1 receptor agonist90).
Proof of principle for successful cellular therapy holistically targeting immune and non-immune mechanisms towards regeneration of functional beta-cell mass within the endogenous pancreatic niche has been illustrated by a clinical trial in 23 participants in Brazil, 20 of whom achieved long-term insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantations91,92. Participants were 13–31 years’ old and received cell therapy within 6 weeks of diagnosis, in keeping with substantial residual beta-cell mass underlying their remarkable and sustained insulin independence. Longer term follow up revealed that participants who remained insulin-free for longer with higher C-peptide levels had fewer autoreactive memory cytoxic T lymphocytes and increased numbers of regulatory T- cells (T-regs) thought to be capable of controlling autoimmunity93.
The clinical benefit of intervention clearly varies greatly and is dependent on residual beta-cell mass, which is associated with age of diagnosis, and time since diagnosis. The tremendous variability among T1D patients with regards to their residual beta-cell mass as assessed by histopathalogical observations and C-peptide levels was recently formalised through designation of multiple T1D endotypes94. The endotype concept is relevant as it helps stratify the highly heterogeneous T1D patient population towards personalisation of most appropriate treatment, including strategies to preserve and restore functional beta-cell mass.
Beta-cell self-replication
It is generally accepted that self-replication of existing beta-cells is the principal mechanism to generate / maintain / expand mass95. Insulin-expressing cells in human pancreas first appear around 7–7.5 weeks post-conception (wpc) and beta-cell replication rates are highest perinatally96,97 before declining to 0.1% Ki67+ presumed proliferative beta-cells in adulthood98. By early adulthood, beta-cell mass is established at around 1 million islets collectively comprising ⁓1–2 grams of beta-cells68,99. Beta-cell mass during pregnancy increases by ⁓1.4-fold100,101 and is higher in obesity versus normal weight individuals. These observations suggest some capacity to increase beta-cell mass to compensate for increased metabolic demand102,103, although whether such compensation occurs by self-replication, islet neogenesis, or a combination of both, is difficult to resolve104.
Human beta-cell proliferation is notoriously difficult to stimulate even in vitro. Several groups have show that inhibition of the Dual Specificity Tyrosine-phosphorylarion-regulated kinase 1A (Dyrk1a) consistently stimulates beta-cell proliferation in dissociated islet cultures at daily rates of 0.1 – 0.4%105–107, although the effect is not beta-cell specific. More recently, the beta-cell proliferative actions of harmine were shown to synergize with clinically-approved GLP1 receptor agonists108. However, while these numbers are a clear improvement over our previous (in)ability to stimulate human beta-cell proliferation, significant challenges remain that preclude our ability to ‘proliferate our way out of’ T1D. Harmine is not a specific Dyrk1a agonist and does not selectively target beta-cells109,110. Daily proliferation rates stimulated to 0.4 % would require 1.5–3.5 years to increase 5% residual beta-cell mass to 25% of normal, considered the lower limit for insulin independence24 (Figure 2). This assumes that all beta-cells proliferate equally with a limited refractory period111,112, does not factor in any age-dependent decline in beta-cell replication rates, and assumes that proliferation rates reported in short-term in vitro assays are sustainable in situ in the face of ongoing autoimmunity. This suggests a best-case scenario of years of continuous stimulation with a systemically administered, non-beta-cell-specific mitogen to restore beta-cells to a lower threshold of clinically meaningful mass. Ongoing autoimmunity will need to be attenuated in parallel. Clinical success to date in meaningfully restoring beta-cell mass in T1D remains elusive despite years of ongoing effort towards this goal.
Figure 2: Requirements for restoring beta-cell mass by stimulating beta-cell proliferation in type 1 diabetes.
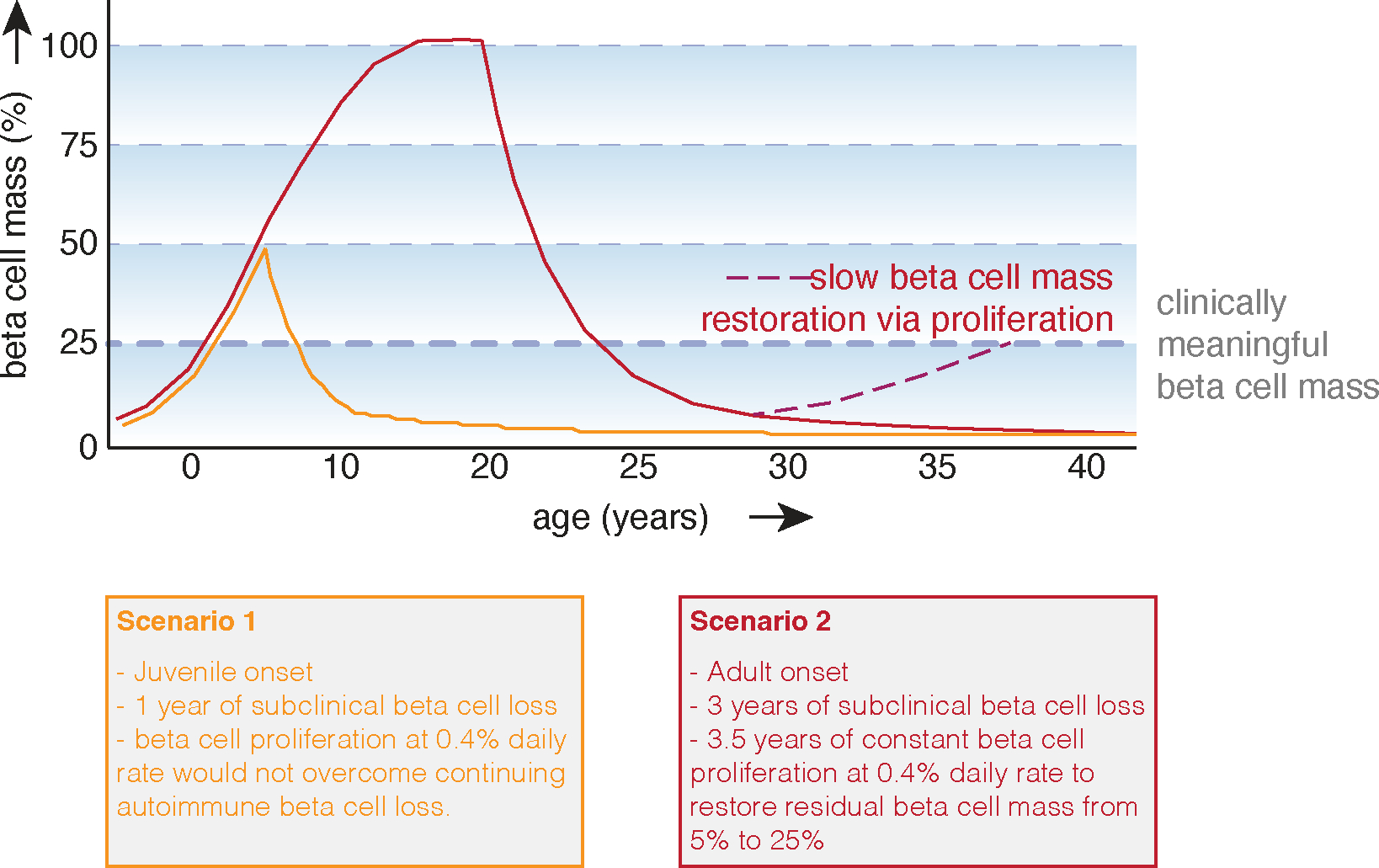
To determine the prospects of restoring beta-cell mass via self-replication one needs to make several assumptions. Here we assumed that beta-cell mass increases with somatic growth during the first two decades of life, that residual beta-cell mass in long-standing diabetes is 5% and that the rate of beta-cell loss is lower in adult-onset type 1 diabetes. Although human beta-cell proliferation rates likely decline with age, we did not factor this into these scenarios. We further assume that beta-cell proliferation rate are similar across the pool of residual beta-cells in type 1 diabetes and did not account for a beta-cell refractory period, even though each beta-cell will have to complete the cell cycle multiple times in order to achieve restoration to even 25% of original beta cells mass starting from 5% of residual beta-cell mass. We further assumed a basal daily proliferation rate of 0.4%, based on published studies of primary human beta-cell basal proliferation in vitro. Such a daily proliferation rate is unlikely to offset the ongoing loss of beta-cell mass in early onset type 1 diabetes in a child who has not yet achieved their full beta-cell mass (Scenario 1). In adult onset type 1 diabetes with a slower rate of autoimmune-mediated beta cell loss (Scenario 2), a daily proliferation rate of 0.4% would gradually restore some functional beta-cell mass over the course of several years. Whether such a proliferation rate could be achieved in situ in the pancreas affected by type 1 diabetes is unknown and would depend on dosing with beta-cell mitogens that are weak and relatively non-specific over the course of many years, possible life-long, with any benefit to the individual not emerging until after years of medication adherence.
Evidence for plasticity in the pancreas
An alternative explanation for continued presence of insulin+ cells in established T1D is attempted beta-cell regeneration from other cell types. Both endocrine and exocrine pancreas share a common developmental origin from endoderm-derived progenitors. This shared lineage of beta-cells with most of the rest of the pancreas continues to fuel a search for therapeutically targetable endogenous progenitors, largely focused on finding either a local stem cell-like progenitor, or a mechanism by which to promote transdifferentiation of non-beta endocrine cells or acinar cells into insulin-expressing cells. While it is established that self-replication of existing beta-cells constitutes the predominant path to form and maintain endogenous mass95, this does not rule out alternative pathways. Most of the evidence in favour of endogenous stem cells in the pancreas is derived from the setting of severe experimentally-induced pancreatic injury models in mice that are limited in their resemblance to human T1D113. Nevertheless, the possibility of beta-cell replacement therapy via neogenesis from a pancreatic progenitor cell remains an active research area.
The possibility of generating beta-cells from other terminally differentiated cells including via direct transdifferentiation has in recent years generated considerable interest, but is largely based on observations in mouse models114,115. Promotion of lineage conversion between alpha and beta-cells in mice has been reported through small molecule treatment with Gamma Aminobutyric Acid (GABA) or with antimalarial compounds purported to act through GABA signalling116,117. However, this approach has proven difficult to reproduce in rodents118–120 and – perhaps more importantly – without effect in non-human primates121. Definitive demonstration of transdifferentiation normally relies on genetic lineage tracing experiments that are relatively straightforward in rodent models, but incredibly challenging to achieve rigorously in human islets. This further complicates determination of the role transdifferentiation could play in attempted restoration of functional beta-cell mass in clinical T1D. The detection of insulin/glucagon double positive cells is often held up as an alternative method to detect and quantify alpha-to-beta transdifferentiation, but is vulnerable to several caveats. It has not been established that such dual hormone+ cells do indeed represent transdifferentiation. If they do, directionality of the transdifferentiation is hard to establish and sub-optimal microscopy approaches can generate the mistaken appearance of overlap within the same cell where none exists.
Restoration of lost endogenous beta-cell mass in the face of continuing autoimmune-mediated beta-cell loss has proven to be a formidable challenge that, despite continuing advances in our understanding accrued over decades-long effort among hundreds of labs, have not yet resulted in clinically meaningful restoration of functional beta-cell mass. Nevertheless, these efforts continue to generate strong foundational knowledge that informs collective efforts to restore functional beta-mass from endogenous or exogenous sources.
hPSC-derived pancreatic progenitor cells for the treatment of diabetes
Human pluripotent stem cells (hPSCs) are capable of generating all cell types in vitro, if provided with the right environment. One source of hPSCs is human embryonic stem cells which are derived from the blastocyst of a pre-implantation embryo. Protocols to culture pluripotent human embryonic stem cells in the lab became available in 1998122, but was limited by the need for embryonic donor tissue. In a series of seminal studies leading to award of the Nobel Prize in Physiology or Medicine jointly with Sir John Gurdon in 2012, Shinya Yamanaka showed that forced expression of four key pluripotency transcription factors can reprogram mature cells to become pluripotent123. These cells, known as induced pluripotent stem cells, can be generated from widely available somatic cells including a patient’s own cells in culture.
Generating functional beta-cells for the treatment of diabetes from hPSCs has been a major goal of regenerative medicine. Success requires in-depth understanding of how islet endocrine cells form during embryonic development in order to replicate this complicated process in vitro. Building on mouse development studies that described the signalling pathways involved in germ layer specification and organogenesis, the first major advance towards the goal of generating insulin-producing cells was making pancreatic progenitor cells in vitro124. The protocol used recombinant proteins and small molecules to activate and inhibit key signalling pathways to recapitulate key stages in embryological pancreas development (definitive endoderm / posterior foregut / pancreatic endoderm)125. The resulting pancreatic progenitor cells express the transcription factors PDX1 and NKX6·1, both of which are necessary for endocrine cell differentiation126,127. When pancreatic progenitors were implanted into immunocompromised mice, the in vivo environment provided unknown cues leading to further differentiation. After three months, grafts comprised >50% insulin-producing cells secreting glucose-stimulated serum C-peptide levels similar to that of ~3000 human islets after 3 months128. Iterative empirical refinement of the differentiation protocol including PKC activator addition129 generated pancreatic progenitor cells producing endocrine cells which were single hormone-expressing (a sign of maturity) and capable of reversing chemically-induced (streptozotocin) diabetes in immunodeficient mice following in vivo maturation130. These proof-of-concept studies provided evidence that hPSC-derived pancreatic progenitor cells could be a therapeutic source of insulin-producing cells.
One concern with transplanting a progenitor population derived from embryonic or adult somatic cells is the potential for teratoma formation seen in seven out of 46 (15%) of grafts in these pioneering studies128. To reduce risk, cellular products can be encapsulated in a device allowing easy retrieval in the event of teratoma formation and protecting the cells against immune attack. hPSC-derived pancreatic progenitor cells implanted into mice within the TheraCyte macroencapsulation device were able to generate glucose-responsive insulin-producing cells that reversed diabetes131. This enabled the first clinical evaluation of hPSC-derived cellular replacement therapy for the treatment of type 1 diabetes, within a Phase 1/2 clinical trial (VC-01; ClinicalTrials.gov Identifier: NCT04678557) sponsored by ViaCyte, in which hPSC-derived pancreatic progenitor cells termed PEC-01™ were encapsulated in the semipermeable Encaptra® device and implanted subcutaneously132 (Figure 3). Nineteen participants each received six to eight implants - two large units and additional sentinel units to provide insight into engraftment and maturation of cells133. While the trial provided some evidence of cell survival at two years post-implantation, most grafts showed minimal cell survival due to hypoxia exacerbated by a foreign body response to the encapsulation device133. However, the trial did provide evidence that the Encaptra® device was sufficient to protect against both allo- and auto-immunity and, in partnership with the materials science company Gore, efforts are underway to improve the device to prevent hypoxia133. Building upon these results, ViaCyte, Inc. has initiated a second Phase 1/2 clinical trial, VC-02 (ClinicalTrials.gov Identifier: NCT03163511), encapsulating PEC-01 cells in an open device, allowing vascularisation, in combination with systemic anti-inflammatory and immunosuppression treatment133.
Figure 3: Current status of stem-cell derived insulin-producing cells.
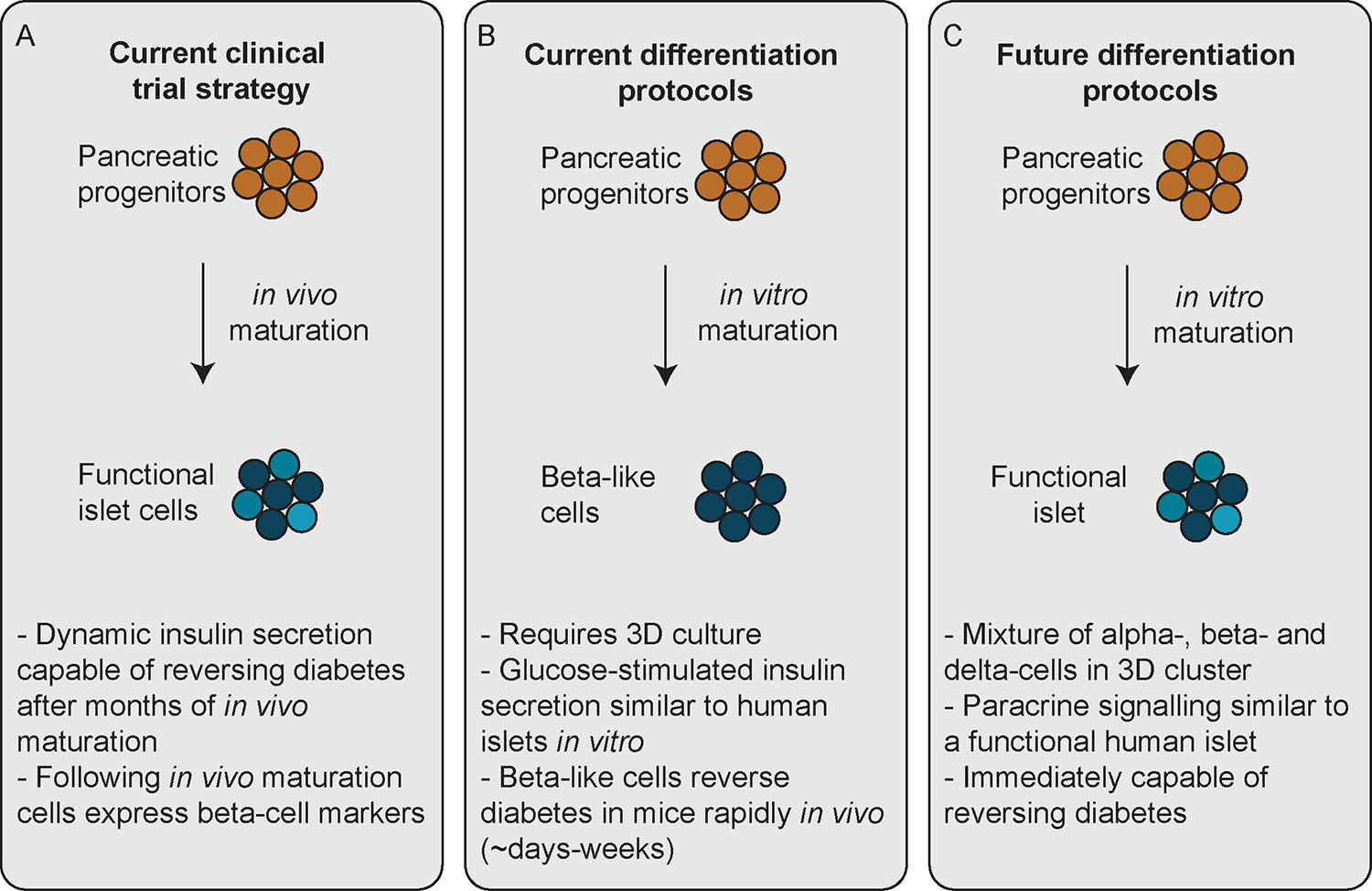
A. The first clinical trial using stem-cell derived insulin-producing cells for diabetes treatment (ClinicalTrials.gov Identifier: NCT04678557) uses pancreatic progenitors that will mature in vivo into islet-like structures. B. Current differentiation protocols are focused on improving the differentiation of pancreatic progenitors into beta-like cells in vitro. C. We believe that the goal of future differentiation protocols will be to generate islet-like organoids with alpha-, beta- and delta-cells that are highly vascularised pre- or rapidly post-transplantation.
Generation of insulin-producing cells from pluripotent stem cells
In parallel with these first clinical trials, research efforts continued to generate fully functional insulin-producing cells from hPSCs in vitro. While hormone-positive cells are seen as early as 8 weeks during human fetal development, it is not until 13 weeks that the hormone-expressing cells are located in islet-like structures which are vascularised and express maturity markers including PC1/3, Chromogranin A134, and Ucn3135. Building upon decades of work by developmental biologists and stem cell scientists, two landmark papers in 2014 outlined differentiation protocols capable of generating insulin-producing ‘beta-like cells’ in vitro129,136. These multi-stage protocols attempted to recapitulate 3 months of in vivo development over 27–43 days in culture and included TGF-beta inhibition and thyroid hormone stimulation during the final stages (Figure 3). Using static glucose-stimulated insulin secretion assays, beta-like cells generated in vitro secreted C-peptide in response to 16.7 mM glucose with a fold increase from basal more closely resembling primary human islets136. Careful analysis of dynamic cytosolic calcium signalling in in vitro assays revealed differences between hPSC-derived beta-like cells and human islets, suggesting that they do not exactly replicate adult human beta-cells136. However, the beta-like cells were able to rapidly reverse diabetes in streptozotocin-induced136 and Akita129 mouse hyperglycaemia models. Further refinement of the differentiation protocols identified the key factors required for formation of glucose-responsive beta-like cells in vitro137. FDA approval has recently been granted for a Phase 1/2 trial of an investigational stem cell-derived, fully differentiated pancreatic islet cell therapy (VX-880) in combination with systemic immunosuppression in T1D complicated by severe hypoglycaemia (ClinicalTrials.gov Identifier: NCT04786262).
Ongoing efforts continue to focus on understanding the ‘black box’ of the in vivo maturation period that allows stem cell-derived pancreatic progenitors or beta-like cells to mature into fully functional islet cells. Two areas of particularly active research include understanding the role of the microenvironment in beta-like cell maturation and careful characterization of developmental trajectories using single-cell RNA-sequencing.
Mimicking the microenvironment during in vitro stem cell differentiation
One of the major advances to differentiation protocols that generate insulin-producing beta-like cells is the introduction of 3D cell culture, either with an intermediate step that transfers monolayer cultures to an air-liquid interface136 or using suspension-based culture of cell clusters throughout the differentiation protocol129. The suspension-based differentiation protocol was further refined, both by controlling TGF-beta signaling and by adding a cellular cluster resizing step138. More recently, Nair and colleagues established a differentiation protocol using the INSGFP/W reporter hPSC line including fluorescence-activated cell sorting (FACS) enrichment for GFP+ beta-like cells before reaggregation into clusters with similar cell density to that of a human islet equivalent (150 μm diameter)139. These enriched beta-clusters (eBCs) had functional properties similar to human islet cells, including rapid first-phase insulin secretion in response to glucose in a perifusion assay139. The functional improvement provided by enriching and clustering hPSC-derived beta-like cells is consistent with pseudo-islet studies in the MIN6 mouse beta-cell line, highlighting the importance of cell-cell contact in insulin secretion140,141. Based on the heterogeneity of previous differentiation protocols and the lack of beta-like cell-to-cell contact in vitro, the in vivo maturation period may, in part, allow for restructuring and clustering of beta-like cells that is necessary for functional maturation.
Researchers next set out to mimic cell-cell contact using small molecules in vitro. By manipulating the actin cytoskeleton, Hogrebe and colleagues142 were able to create a fully planar differentiation protocol that produced hPSC-derived beta-like cells with similar properties to those cells generated in suspension, circumventing the need for 3D culture. Interestingly, altering the actin cytoskeleton dynamics improved the differentiation of other cell types derived from the endoderm lineage, suggesting a common mechanism for cell-cell contact in the maturation and development of terminally-differentiated cell types142.
Using single-cell RNA-sequencing to improve stem cell differentiation protocols
While differentiation protocols continue to improve, variation and heterogeneity remain challenging125,143. Deeper comparison at the single cell level enables identification of key differences between hPSC-derived beta-like cells and adult human beta-cells, providing information that can be used to develop differentiation protocols generating homogenous cell populations at high efficiency. Several studies have characterised the developing fetal mouse144–148 and human149,150 pancreas at a single cell level. Together, these studies have begun to map more deeply the developmental trajectories through which mature beta-cells are formed.
In summary, the past two decades have seen rapid progress towards the ultimate goal of generating an unlimited source of insulin-producing cells for diabetes treatment with or without an in vivo maturation step (Figure 3). This work has culminated in clinical trials that, while promising, highlight areas that still need to be addressed, including preventing hypoxia, promoting vascularisation, and protecting the cells from immune attack.
Harnessing tissue engineering in beta-cell replacement therapy
Systems capable of recreating a ‘pancreatic niche’ for transplanted islets hold great promise for enhancing survival, engraftment, and long-term function. The following sections address some of the major challenges associated with transplantation, with a particular focus on access to nutrients and a supportive environment for survival and function, as well as modulating inflammation and adaptive cell responses to limit immune-mediated cell destruction.
Combination of cell transplants with biomaterial encapsulation / scaffolds
Transplantation to extrahepatic sites has largely been attempted using an encapsulation approach that creates a semi-permeable physical barrier between therapeutic cells and the host immune system151. Clinical trials with porcine islets have relied on encapsulation usually using alginate to prevent hyperacute rejection through direct exposure of xenogeneic cells. Intraperitoneal delivery of these capsules has been associated with a giant cell foreign body reaction significantly limiting function152.
Innovation with encapsulation technologies continues including novel materials to limit fibrosis153, or thinner coatings154 to reduce graft volume and minimize the diffusion barrier. Nevertheless, this approach restricts access to vascular ingrowth limiting graft oxygenation necessary for optimal beta-cell survival and function. Furthermore, the sensing of blood glucose can be delayed by diffusion times between the vasculature and islets, also impairing systemic insulin secretion pharmacokinetics.
In proof-of-concept clinical studies, oxygen has been directly delivered to the transplantation site using an implantable device developed by Beta O2 called βAir, which has a refillable oxygen chamber designed to provide oxygen to encapsulated islets155,156. Oxygen-generating compounds can be incorporated with the graft to provide oxygen to the transplanted cells for days / weeks, bridging the critical time prior to integration with host vasculature (Figure 4)157–159. Once the oxygen-generating compound is depleted, hypoxia will return unless sufficient host vasularisation has occurred. Loading and release must be balanced to meet oxygen demands without excessive production of harmful reactive oxygen species159.
Figure 4: Integration of transplanted cells.
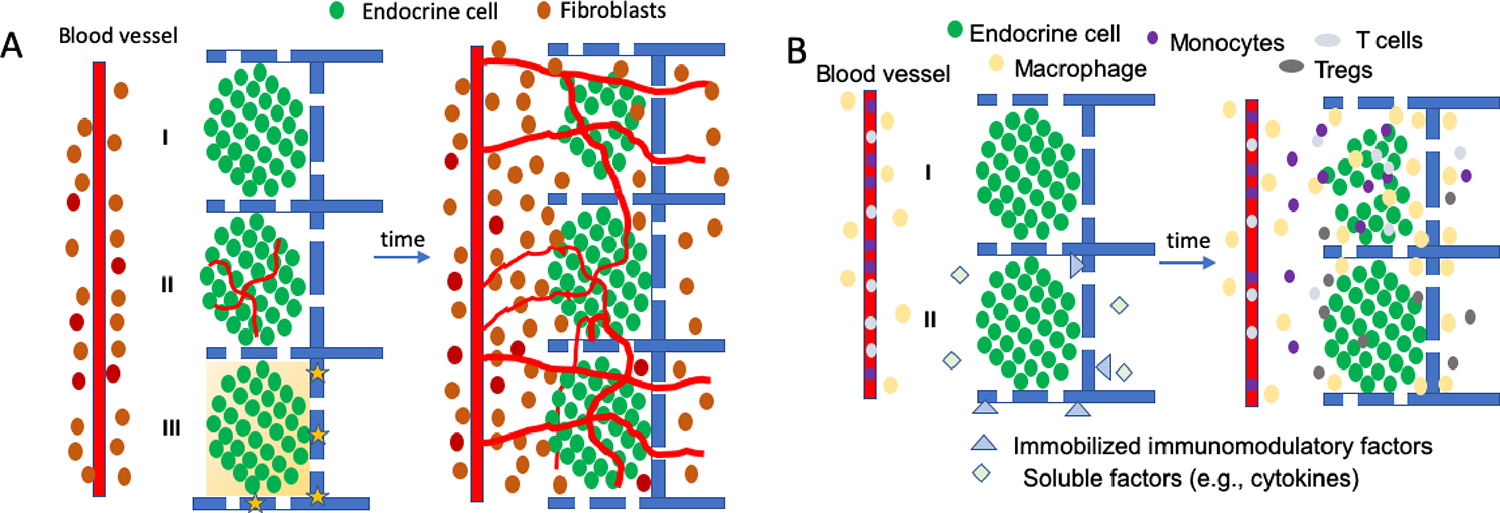
A. Survival and vascularization of transplanted cells. Endocrine cells are transplanted on biomaterial scaffolds near to vasculature, which can be modified with pre-vascularized structures (II) or oxygen-releasing materials (yellow stars) that can transiently create local oxygen gradients (yellow gradient) (III). Over time, these cells become integrated with host cells such as fibroblasts and vascular ingrowth occurs. Transplantation can lead to hypoxic states for days that limit cell survival (I). Pre-vascularized structures (II) and oxygen release (III) can decrease the time to vascularization and potentially enhance survival post transplantation, with vessels providing nutrients to the transplanted endocrine cells, and allowing for glucose sensing and the distribution of insulin. B. Immunomodulation to enhance integration. The transplantation process and the graft will also stimulate an inflammatory response that recruits immune cells (e.g., neutrophils, monocytes, macrophages, T cells) from the adjacent tissue and the host vasculature. Immunomodulatory factors can be released locally from the implant or the transplanted cells (II), or immobilized to the material or cells, with these factors influencing the extent to which immune cells infiltrate the graft, and can also influence the phenotype such as reducing inflammatory cytokine production than can harm beta cells or inducing expression of anti-inflammatory cytokines that support vessel ingrowth and graft integration. Furthermore, effector T cells entering graft can be directed toward anergy or induced to become regulatory T cells that can promote long-term graft acceptance.
Non-encapsulating scaffolds, in contrast to encapsulating hydrogels, allow for integration of transplanted cells with host tissue, which can support long term survival and function151,160. Endogenous pancreatic endocrine function is supported by a dense intra-islet capillary network which can potentially be recapitulated by strategies that integrate transplanted cells with the host vasculature161. Insulin independence has been reported following islet transplantation onto the omentum within a biodegradable thrombin scaffold, although functional decline was observed at 12 months160. Beyond serving as a support for the transplanted cells, a number of materials are being developed to control the microenvironment around transplanted islets or stem cell-derived beta-cells at extrahepatic sites151.
Optimised graft vascularisation
Enhanced vascularisation of the site prior to transplantation or along with transplantation is also being developed as a means to optimse engraftment, glucose sensing and insulin distribution promoting long-term survival (Figure 4). Adjuvant mesenchymal stromal cells (MSCs) with transplantation may enhance graft function and longevity162 and allogeneic human umbilical cord perivascular MSCs (HUCPVCs) co-transplantation with islets led to enhanced vascularization of the graft and improved glycemic control in mice relative to islet transplant alone163. There is now considerable clinical experience with MSC therapy and a clinical study in islet transplantation led by Uppsala University has been proposed (ClinicalTrials.gov Identifier: NCT01967186). Other approaches involve delivery of angiogenic factors, or genes encoding these factors, to induce blood vessel ingrowth into the graft. Hydrogels functionalized with angiogenic factors delivered locally, or islets transfected to express VEGF-A improved survival, engraftment, and function of pancreatic donor islets in preclinical studies164–166. Vascularization can also be enhanced by directly delivering endothelial cells or endothelial progenitor cells with the graft167 or following ex vivo seeding on endothelialised scaffolds168,169.
Attenuating inflammation and innate immune response
A number of pharmaceutical-based anti-inflammatory approaches are being applied systemically at the time of transplantation to limit beta cell loss and promote engraftment. TNF-α inhibition in tandem with T-cell depleting induction has been associated with improved alloislet outcomes in an international registry analysis170. The combination of etanercept (anti-TNF-alpha) and anakinra (anti-IL1-beta) is well-tolerated in total pancreatectomy / islet autotransplant recipients with reduced circulating IL-6, IL-8 and MCP-1 and corresponding modest improvements in basal C-peptide and HbA1c compared with historical controls171. Protection against inflammatory stress and hypoxia is being attempted through a range of small molecule approaches172–174, Agents targeting reactive oxygen species172, the NLRP3 inflammasome173, or providing essential phospholipids174 can protect islets against inflammatory stress and hypoxia.
Diabetes has long been considered a candidate disease for gene therapy175 with an initial focus on transducing non-islet tissues including muscle to secrete insulin. Increasingly effective clinical gene transfer holds considerable potential for generic engineering of islets to modulate their response to inflammatory signals176,177. Overexpression of the ubiquitin-editing enzyme A20 in human islets reduced expression of inflammatory mediators, without negatively impacting glucose-stimulated insulin secretion178. This may promote islet allogeneic survival post-transplant by raising inflammatory signalling threshold. Pax4 overexpression in islets enhanced islet transplant efficacy in mice with mechanisms thought to include increased resilience to metabolic and proinflammatory stress177. Despite this promise, we are unaware of any clinical beta-cell gene therapy trials to date.
Conventional anti-inflammatory agents can be delivered locally, limiting systemic exposure and adverse events (Figure 4). The local delivery of dexamethasone from porous scaffolds179 or presentation of tannic acid on encapsulation materials180 enhanced islet engraftment and improved glucose control in the early transplant period. Treatment with dexamethasone correlated with a greater polarization of macrophages toward an M2 immunomodulatory phenotype179. More recently, biologic therapies, such as local delivery of TGF-beta1181, CXCL12182, or CCL22183, are being developed to inhibit immune cell recruitment or alter immunophenotypes in order to create a niche with decreased inflammation that can enhance graft survival and function.
Promoting in vivo function
Stem cell-derived β-cells may not be fully differentiated following in vitro manufacture, and transplanted islets may need support due to the harsh environment post-transplantation. Trophic factors delivered at the transplantation site can enhance proliferation and maturation in addition to survival, ultimately leading to optimal function in vivo. In pre-clinical studies, normoglycaemia was achieved more effectively following transplantation of a marginal mass of human islets cultured on a scaffold eluting exendin-4, a glucagon-like peptide-1 (GLP-1) agonist that augments glucose-dependent insulin secretion in β-cells, promotes β-cell proliferation and protects against apoptosis, in comparison to an inert scaffold 184. Scaffolds similarly releasing exendin-4 were used to support hPSC-derived pancreatic progenitors, enhancing maturation and C-peptide secretion185. Incorporation of additional trophic factors, such as prolactin, growth hormone, IGF-1, betacellulin and activin-A into the engineered transplant niche may further enhance in vivo maturation and function of the stem cell derived beta-cells.
Eliminating immunosuppression without immunobarrier encapsulation
The major impediment to much wider inclusion criteria for beta-cell replacement therapy is need for lifelong systemic immunosuppression with associated risk of malignancy and severe infection in addition to potential for toxicity directly impairing engraftment and beta-cell function. Immunosuppression is required, even for recipient-derived cells to prevent recurrent autoimmune graft destruction, previously reported after pancreas transplantation from an identical twin live donor without diabetes186. Alternative strategies towards operational tolerance, where the graft is effectively ignored by the immune system, are being explored. Transient treatment with the blocking antibody anti-CD154/CD40L in the peri-transplant period improved outcomes of intraocular islet allografts, enabling immunosuppression-free survival in mice and in a baboon54.
In addition to considering transplantation into naturally immunoprivileged sites such as the eye53,54, generation of novel immunoprivileged sites has been explored through genetic engineering of the graft to express immunomodulatory factors or co-transplantation with cells from immunoprivileged sites, such as testicular Sertoli cells 187. Expression of PDL1-CTLA4Ig or an agonistic membrane-bound single-chain anti-CTLA-4 Fv led to protection of allo-islets from acute rejection in mice with established autoimmune diabetes188,189. Immunoprivileged cells constitutively express FasL to control the entry of lymphoid cells expressing Fas. FasL modification of donor islets has been able to promote long-term function of donor islets following experimental allotransplantation190. FasL initially reduced frequency of alloreactive T cells in draining lymph nodes with maintenance requiring CD4+ CD25+ Foxp3+ regulatory T-cells (T-regs) and donor antigen persistence. More recent studies have engineered localised delivery of FasL191,192, PD-L1193, or CTLA4/Fc fusion proteins194 (Figure 4). This strategy has delivered long-term allogeneic islet survival and function, associated with expansion of Foxp3+ T-regs.
In addition to their pro-angiogenic and anti-inflammatory properties that can enhance engraftment, MSCs have been co-transplanted with islets to capitalise on potential immunomodulatory properties195. MSC treatment significantly increased T-reg numbers in murine allografts and draining lymph nodes196, in parallel with enhanced islet engraftment and function. A positive impact on regulatory T-cells is a common goal for many islet immunomodulatory approaches and T-reg cell therapy has effectively reduced ongoing immunosuppression requirements in solid organ transplantation197. T-regs have been delivered systemically along with islet transplantation, and also directly co-transplanted with islets198. Locally delivered T-regs protecting islet grafts198 were ultimately replaced by recipient-derived T-regs over time, suggesting that host-derived T-regs may enable long-term immunotolerisation. The recent exciting advances in chimeric antigen receptor modified T-cells have been applied to T-regs, which are long-lived and can effectively suppress antigen-specific effector T-cell function in vivo199–201.
Systemic approaches for antigen-specific immune modulation are also being investigated as they offer the opportunity to prevent rejection of the graft while leaving the remainder of the immune system intact. Possibilities are reviewed elsewhere202, yet one regenerative medicine approach that has made its way to patients has been intravenous infusion of nanoparticles modified with disease-associated antigens, which recently completed a Phase II clinical study in autoimmunity and successfully induced tolerance to those antigens203. These nanoparticles were developed based on has treatment of donor splenocytes with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (ECDI) and subsequent intravenous infusion204, which was recently applied to allotransplantation. In non-human primates, infusion of ECDI treated apoptotic donor leukocytes combined with short-term immunotherapy using anti-CD40, rapamycin, TNF receptor, and anti-IL-6 receptor205 promoted stable islet allograft tolerance (>356 days by suppressing effector cell expansion and expanding regulatory networks involving antigen-specific Tr1 cells exhibiting indirect specificity for matched MHC class II and mismatched class I peptides. Preclinical studies have demonstrated the potential for nanoparticles based on this ECDI approach to immunotolerise to prevent islet allograft rejection206.
Strategies for reducing destructive inflammatory response and requirement for ongoing immunosuppression necessitate meaningful biomarkers given the inability to easily biopsy the graft site. Elevated levels of circulating beta-cell-derived unmethylated insulin DNA have been reported in rapid progressors towards clinical T1D207. Persistent elevation after autologous islet transplantation was associated with worse graft function208. This has led to proposal of whole genome sequencing of cell-free DNA as a ‘liquid biopsy’ of cell death in all phenotypes undergoing cell death following beta-cell replacement therapy209. Withdrawal of immunosuupression following immunomodulatory interventions will be very challenging without biopsy access to graft tissue in situ. Subcutaneously implanted microscaffolds engineered to express beta-cell antigens and allowing immune cell infiltration may provide an accessible ‘immunological niche’ to better guide these decisions210.
Conclusions
Whether the approach is to maintain / restore beta-cell function in situ or replace beta-cell mass through transplantation, truly transformative regenerative medicine for T1D is dependent on fundamental understanding of human islets within the pancreas from which we draw a number of conclusions:
Despite the presence of considerable functional reserve in those without diabetes for increasing insulin secretion several fold in response to increasing demand, for example during pregnancy and with obesity; evidence for induction of significant expansion of beta-cell mass from adult human pancreatic cells in vitro or in vivo in T1D remains extremely limited.
Recent strategies to consider identification of individuals prior to clinical T1D onset and to ‘endotype’ according to underlying aetiopathogenesis, level of residual beta-cell function and rate of functional decline offer the potential for better-informed trials combining immunotherapy with regenerative medicine approaches. Personalised medicine approaches may enable intervention at a stage where harnessing beta-cell regenerative potential, despite likely being fairly modest, may enable restoration of sustainable insulin independence. We envisage these trials moving beyond those with new-onset T1D to include those with longer standing disease but more stable C-peptide levels.
Recovery from the substantial loss of beta-cell mass in many with T1D is likely to necessitate cell replacement strategies, with the initial challenge being the manufacture of physiologically functioning islets at sufficient scale for all potential recipients. Stem cell-derived beta-cells have the potential to satisfy the supply, with efficient platforms yielding a reproducibly consistent product under development. Functional specifications include sufficient insulin storage, full processing and appropriate secretion in response to nutrient stimulation in combination with effective counter-regulation avoiding biochemical hypoglycaemia through cell-to-cell communication within a 3-dimensional unit with or without other pancreatic endocrine cells and absence of teratogenicity / tumorigenicity.
A major further challenge is for this cell product to be delivered into an environment which can support long-term islet function, while also modulating the host immune response to prevent rejection. Tissue engineering strategies are required to overcome hypoxia and lack of vascularisation early post-transplantation to prevent loss of mass / function and to rebuild the complex architecture of the normal islet niche providing an inter-communicating network of endocrine cells, vasculature, and non-endocrine / non-vascular intra-islet cells enabling truly normal islet homeostasis.
Modulation of inflammation post-transplantation and adaptive immune responses in the long term is critical for graft survival and avoidance of sustained immunosuppression. Overcoming this major remaining challenge will be essential for conversion of beta-cell replacement therapy from a life-saving procedure for those with dangerous hypoglycaemia to a curative option for all with T1D.
Incremental steps have continued since the seminal success achieved through islet transplantation at the dawn of the current millennium. Twenty years later as regenerative medicine comes of age, islet transplantation is embedded around the world as standard-of-care for those with highest need; multidisciplinary collaborative working bringing together patients, molecular biologists, immunologists, bioengineers, clinical scientists, industrial partners and regulators has delivered first-in-man trials; and an exciting pathway towards reproducible sustained insulin independence after a single transplant procedure and ultimately immunosuppression-free beta-cell replacement has emerged.
Search strategy and selection criteria.
References for this review were identified through searches of PubMed for articles published since 2015, by use of the terms “beta-cell differentiation” AND “stem cells”, “beta-cell transdifferentiation”, “beta-cell neogenesis”, “beta-cell dedifferentiation”, “human beta-cell proliferation”, “beta-cell heterogeneity”, “beta cell transplantation”, “islet transplantation” AND “inflammation”, and “islet transplantation” AND “immunomodulation”. Articles published in English resulting from these searches and relevant references cited in those articles were reviewed. The authors generally focused on studies conducted on human participants, human biospecimens, and human cells / cell lines supplemented by pertinent observations from rodent and non-human primate pre-clinical models of human physiology and pathology.
Acknowledgements
NAJK is funded by the Wellcome Trust (200837 [Grant to A. Gloyn]). Research in the lab of LDS is supported by NIH DK121462, JDRF (2-SRA-2020-775-S-B) and the JDRF Center of Excellence at the University of Michigan. Research in the lab of MOH is supported by grants from the NIH (NIDDK-110276), JDRF (2-SRA-2021-1054-M-N), and ADA (1-19-IBS-078). Research in the lab of JAMS is supported by grants from the MRC (Quality and Safety in Organ Donation Tissue Bank - Expansion to include Pancreas/Islets, Heart and Lungs); Innovate UK (Northern Alliance Advanced Therapies Treatment Centre), MRC/BBSRC/EPSRC UK Regenerative Medicine Platform (Defining the role of tissue-resident immune cells in alveolar epithelial cell regeneration), Diabetes UK (Establishment of optimised biomedical and psychosocial measures to determine overall outcomes in islet transplant recipients) and a Cystic Fibrosis Trust Strategic Research Centre (Mechanisms and measures of the pathways through which cystic fibrosis exocrine pancreatic disease leads to beta-cell dysfunction and diabetes).
Footnotes
Conflict of interest statement
NAJK has no conflicts of interest to declare. LDS holds a licensed patent on nanoparticles for autoimmune tolerance, with a patent applications pending on scaffolds for islet transplantation and a local immune monitoring. MOH has received funding and honoraria from Crinetics Inc. to investigate somatostatin analogs that are not discussed in this review. JAMS has received hororaria for chairing academic meetings organised by Novo Nordisk and for participating in a Medtronic Scientific Advisory Board.
Contributor Information
Nicole A. J. Krentz, Division of Endocrinology, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA, USA.
Lonnie D. Shea, Departments of Biomedical Engineering, Chemical Engineering, and Surgery, College of Engineering and School of Medicine, University of Michigan, Ann Arbor, MI USA.
Mark O. Huising, Department of Neurobiology, Physiology & Behavior, College of Biological Sciences, University of California, Davis, California and Department of Physiology and Membrane Biology, School of Medicine, University of California, Davis. CA, USA.
James Shaw, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, NE2 4HH, UK; and Institute of Transplantation, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, NE7 7DN, UK.
References
- 1.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39(3): 481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med 2017; 376(15): 1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium; 2019. Available at: https://www.diabetesatlas.org [Google Scholar]
- 4.Cersosimo E, Triplitt C, Solis-Herrera C, et al. Pathogenesis of Type 2 Diabetes Mellitus. [Updated 2018 Feb 27]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [Google Scholar]
- 5.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018; 391(10138): 2449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA 2015; 313(1): 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014; 37(1): 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50(6): 1140–7. [DOI] [PubMed] [Google Scholar]
- 9.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018; 361: k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnard KD, Wysocki T, Thabit H, et al. Psychosocial aspects of closed- and open-loop insulin delivery: closing the loop in adults with Type 1 diabetes in the home setting. Diabet Med 2015; 32(5): 601–8. [DOI] [PubMed] [Google Scholar]
- 11.White SA, Shaw JA, Sutherland DER. Pancreas Transplantation. Lancet 2009; 373: 1808–17. [DOI] [PubMed] [Google Scholar]
- 12.Flatt AJS, Bennett D, Counter C, Brown AL, White SA, Shaw JAM. Beta-cell and renal transplantation options for diabetes. Diabet Med 2020; 37(4): 580–92. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro AMJ. A Historical Perspective on Experimental and Clinical Islet Transplantation. In: Shapiro AJM, Shaw JA, ed. Islet Transplantation and Beta Cell Replacement Therapy. Boca Raton: CRC Press, 2007:1–28. [Google Scholar]
- 14.Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4): 230–8. [DOI] [PubMed] [Google Scholar]
- 15.Vantyghem MC, Kerr-Conte J, Arnalsteen L, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 2009; 32(8): 1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks AM, Walker N, Aldibbiat A, et al. Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transplant 2013; 13(12): 3236–43. [DOI] [PubMed] [Google Scholar]
- 17.Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016; 39(7): 1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012; 35(7): 1436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory response in islet transplantation. Int J Endocrinol 2014; 2014: 451035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant 2009; 9(12): 2816–24. [DOI] [PubMed] [Google Scholar]
- 21.Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol 2013; 2013: 352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantley J, Walters SN, Jung MH, et al. A preexistent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis. Cell Transplant 2013; 22(11): 2147–59. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SJ, White MG, Armour SL, et al. Loss of end-differentiated beta-cell phenotype following pancreatic islet transplantation. Am J Transplant 2018; 18(3): 750–5. [DOI] [PubMed] [Google Scholar]
- 24.Flatt AJS, Greenbaum CJ, Shaw JAM, Rickels MR. Pancreatic islet reserve in type 1 diabetes. Ann N Y Acad Sci 2021; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. Improvement in beta-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013; 62(8): 2890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Addison P, Fatakhova K, Rodriguez Rilo HL. Considerations for an Alternative Site of Islet Cell Transplantation. J Diabetes Sci Technol 2020; 14(2): 338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Diaz R, Molano RD, Weitz JR, et al. Paracrine Interactions within the Pancreatic Islet Determine the Glycemic Set Point. Cell Metab 2018; 27(3): 549–58 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benninger RK, Piston DW. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol Metab 2014; 25(8): 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005; 53(9): 1087–97. [DOI] [PubMed] [Google Scholar]
- 30.Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2010; 2(3): 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006; 103(7): 2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisania A, Weir GC, O’Neil JJ, et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest 2010; 90(11): 1661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noguchi GM, Huising MO. Integrating the inputs that shape pancreatic islet hormone release. Nat Metab 2019; 1(12): 1189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartig SM, Cox AR. Paracrine signaling in islet function and survival. J Mol Med (Berl) 2020; 98(4): 451–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svendsen B, Larsen O, Gabe MBN, et al. Insulin Secretion Depends on Intra-islet Glucagon Signaling. Cell Rep 2018; 25(5): 1127–34 e2. [DOI] [PubMed] [Google Scholar]
- 36.Capozzi ME, Svendsen B, Encisco SE, et al. Beta cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019; 4(5): e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu L, Dattaroy D, Pham J, et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019; 5(10): e127994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huising MO, Lee S, van der Meulen T. Evidence for a Neogenic Niche at the Periphery of Pancreatic Islets. Bioessays 2018; 40(11): e1800119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Meulen T, Donaldson CJ, Caceres E, et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med 2015; 21(7): 769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue JT, Riddell MC, Burdett E, Coy DH, Efendic S, Vranic M. Amelioration of hypoglycemia via somatostatin receptor type 2 antagonism in recurrently hypoglycemic diabetic rats. Diabetes 2013; 62(7): 2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellard JA, Rorsman NJG, Hill TG, et al. Reduced somatostatin signalling leads to hypersecretion of glucagon in mice fed a high-fat diet. Mol Metab 2020; 40: 101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao R, Yang T, Zhang Q. Delta-Cells: The Neighborhood Watch in the Islet Community. Biology (Basel) 2021; 10(2): 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A 2010; 107(37): 16009–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherr JL, Palau Collazo M, Cengiz E, et al. Safety of nighttime 2-hour suspension of Basal insulin in pump-treated type 1 diabetes even in the absence of low glucose. Diabetes Care 2014; 37(3): 773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garber AJ. Incretin effects on beta-cell function, replication, and mass: the human perspective. Diabetes Care 2011; 34(2): S258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansson L, Carlsson P-O. Pancreatic Blood Flow with Special Emphasis on Blood Perfusion of the Islets of Langerhans. Compr Physiol 2019; 9(2): 799–837. [DOI] [PubMed] [Google Scholar]
- 47.Bosco D, Armanet M, Morel P, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 2010; 59(5): 1202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia 2018; 61(6): 1261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almaca J, Caicedo A, Landsman L. Beta cell dysfunction in diabetes: the islet microenvironment as an unusual suspect. Diabetologia 2020; 63(10): 2076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang SC, Baeyens L, Shen CN, et al. Human pancreatic neuro-insular network in health and fatty infiltration. Diabetologia 2018; 61(1): 168–81. [DOI] [PubMed] [Google Scholar]
- 51.Makhmutova M, Weitz J, Tamayo A, et al. Pancreatic Beta-Cells Communicate With Vagal Sensory Neurons. Gastroenterology 2021; 160(3): 875–88.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Diaz R, Caicedo A. Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 2014; 28(5): 745–56. [DOI] [PubMed] [Google Scholar]
- 53.Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008; 14(5): 574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdulreda MH, Berman DM, Shishido A, et al. Operational immune tolerance towards transplanted allogeneic pancreatic islets in mice and a non-human primate. Diabetologia 2019; 62(5): 811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisenbarth GS. Type 1 diabetes mellitus. N Engl J Med 1986; 314(21): 1360–8. [DOI] [PubMed] [Google Scholar]
- 56.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015; 38(10): 1964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damond N, Engler S, Zanotelli VRT, et al. A Map of Human Type 1 Diabetes Progression by Imaging Mass Cytometry. Cell Metab 2019; 29(3): 755–68 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 2007; 50(11): 2323–31. [DOI] [PubMed] [Google Scholar]
- 59.Oram RA, Sims EK, Evans-Molina C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia 2019; 62(4): 567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Calvo T, Suwandi JS, Amirian N, et al. Heterogeneity and Lobularity of Pancreatic Pathology in Type 1 Diabetes during the Prediabetic Phase. J Histochem Cytochem 2015; 63(8): 626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu MG, Keenan HA, Shah HS, et al. Residual beta cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest 2019; 129(8): 3252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010; 59(11): 2846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and Beta-Cell Mass in the Natural History of Type 1 Diabetes. Diabetes 2016; 65(3): 719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lam CJ, Jacobson DR, Rankin MM, Cox AR, Kushner JA. Beta Cells Persist in T1D Pancreata Without Evidence of Ongoing Beta-Cell Turnover or Neogenesis. J Clin Endocrinol Metab 2017; 102(8): 2647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leete P, Willcox A, Krogvold L, et al. Differential Insulitic Profiles Determine the Extent of beta-Cell Destruction and the Age at Onset of Type 1 Diabetes. Diabetes 2016; 65(5): 1362–9. [DOI] [PubMed] [Google Scholar]
- 66.Brom M, Woliner-van der Weg W, Joosten L, et al. Non-invasive quantification of the beta cell mass by SPECT with (1)(1)(1)In-labelled exendin. Diabetologia 2014; 57(5): 950–9. [DOI] [PubMed] [Google Scholar]
- 67.Espes D, Carlsson P-O, Selvaraju RK, et al. Longitudinal Assessment of 11 C-5-Hydroxytryptophan Uptake in Pancreas After Debut of Type 1 Diabetes. Diabetes 2021; 70(4):966–75. [DOI] [PubMed] [Google Scholar]
- 68.Cnop M, Hughes SJ, Igoillo-Esteve M, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 2010; 53(2): 321–30. [DOI] [PubMed] [Google Scholar]
- 69.Arrojo EDR, Lev-Ram V, Tyagi S, et al. Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metab 2019; 30(2): 343–51 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sender R, Milo R. The distribution of cellular turnover in the human body. Nat Med 2021; 27(1): 45–8. [DOI] [PubMed] [Google Scholar]
- 71.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52(1): 102–10. [DOI] [PubMed] [Google Scholar]
- 72.Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. Beta Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice. Cell Metab 2017; 25(3): 727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Calvo T, Richardson SJ, Pugliese A. Pancreas Pathology During the Natural History of Type 1 Diabetes. Curr Diab Rep 2018; 18(11): 124. [DOI] [PubMed] [Google Scholar]
- 74.Xu G, Chen J, Jing G, Shalev A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes 2012; 61(4): 848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muralidharan C, Conteh AM, Marasco MR, et al. Pancreatic beta cell autophagy is impaired in type 1 diabetes. Diabetologia 2021; 64: 865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JAM, Lovat PE, Arden C. Glucagon-Like Peptide 1 Protects Pancreatic beta-Cells From Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes 2017; 66(5): 1272–85. [DOI] [PubMed] [Google Scholar]
- 77.Sims EK, Chaudhry Z, Watkins R, et al. Elevations in the Fasting Serum Proinsulin-to-C-Peptide Ratio Precede the Onset of Type 1 Diabetes. Diabetes Care 2016; 39(9): 1519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watkins RA, Evans-Molina C, Terrell JK, et al. Proinsulin and heat shock protein 90 as biomarkers of beta-cell stress in the early period after onset of type 1 diabetes. Transl Res 2016; 168: 96–106 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Courtade JA, Klimek-Abercrombie AM, Chen YC, et al. Measurement of Pro-Islet Amyloid Polypeptide (1–48) in Diabetes and Islet Transplants. J Clin Endocrinol Metab 2017; 102(7): 2595–603. [DOI] [PubMed] [Google Scholar]
- 80.Marrack P, Kappler JW. Do MHCII-presented neoantigens drive type 1 diabetes and other autoimmune diseases? Cold Spring Harb Perspect Med 2012; 2(9): a007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lachin JM, McGee P, Palmer JP, Group DER. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014; 63(2): 739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brooks AM, Oram R, Home P, Steen N, Shaw JA. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care 2015; 38(1): 105–12. [DOI] [PubMed] [Google Scholar]
- 83.Rickels MR, Evans-Molina C, Bahnson HT, et al. High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest 2020; 130(4): 1850–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor GS, Smith K, Capper TE, et al. Postexercise Glycemic Control in Type 1 Diabetes Is Associated With Residual beta-Cell Function. Diabetes Care 2020; 43(10): 2362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. The Lancet 2018; 391(10120): 541–51. [DOI] [PubMed] [Google Scholar]
- 86.Bouillet B, Rouland A, Petit JM, Verges B. A low-carbohydrate high-fat diet initiated promptly after diagnosis provides clinical remission in three patients with type 1 diabetes. Diabetes Metab 2020; 46(6): 511–3. [DOI] [PubMed] [Google Scholar]
- 87.Ovalle F, Grimes T, Xu G, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med 2018; 24(8): 1108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jacobsen LM, Bundy BN, Greco MN, et al. Comparing Beta Cell Preservation Across Clinical Trials in Recent-Onset Type 1 Diabetes. Diabetes Technol Ther 2020; 22(12): 948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sims EK, Bundy BN, Stier K, et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med 2021; 13(583): eabc8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Herrath M, Bain SC, Bode B, et al. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Diabetes & Endocrinology 2021; 9(4): 212–24. [DOI] [PubMed] [Google Scholar]
- 91.Voltarelli JC, Couri CEB, Stracieri ABPL, et al. Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in Newly Diagnosed Type 1 Diabetes Mellitus. JAMA 2007; 297(14): 1568–76. [DOI] [PubMed] [Google Scholar]
- 92.Couri CEB, Oliveira MCB, Stracieri ABPL, et al. C-Peptide Levels and Insulin Independence Following Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in Newly Diagnosed Type 1 Diabetes Mellitus. JAMA 2009; 301(15): 1573–9. [DOI] [PubMed] [Google Scholar]
- 93.Malmegrim KC, de Azevedo JT, Arruda LC, et al. Immunological Balance Is Associated with Clinical Outcome after Autologous Hematopoietic Stem Cell Transplantation in Type 1 Diabetes. Front Immunol 2017; 8: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Battaglia M, Ahmed S, Anderson MS, et al. Introducing the Endotype Concept to Address the Challenge of Disease Heterogeneity in Type 1 Diabetes. Diabetes Care 2020; 43(1): 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dor Y, Brown J, Martinez OI, Melton D. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004; 429: 41–6. [DOI] [PubMed] [Google Scholar]
- 96.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008; 57(6): 1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meier JJ, Kohler CU, Alkhatib B, et al. Beta-cell development and turnover during prenatal life in humans. Eur J Endocrinol 2010; 162(3): 559–68. [DOI] [PubMed] [Google Scholar]
- 98.Perl S, Kushner JA, Buchholz BA, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab 2010; 95(10): E234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weir GC, Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci 2013; 1281: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butler AE, Cao-Minh L, Galasso R, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010; 53(10): 2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Assche FA, Aerts L, Prins FD. A morphological study of the endocrine pancreas in human pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology 1978; 85(11): 818–20. [DOI] [PubMed] [Google Scholar]
- 102.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. Beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 2013; 36(1): 111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008; 10(4): 32–42. [DOI] [PubMed] [Google Scholar]
- 104.Baeyens L, Lemper M, Staels W, et al. (Re)generating Human Beta Cells: Status, Pitfalls, and Perspectives. Physiol Rev 2018; 98(3): 1143–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang P, Alvarez-Perez JC, Felsenfeld DP, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 2015; 21(4): 383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dirice E, Walpita D, Vetere A, et al. Inhibition of DYRK1A Stimulates Human beta-Cell Proliferation. Diabetes 2016; 65(6): 1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen W, Taylor B, Jin Q, et al. Inhibition of DYRK1A and GSK3B induces human beta-cell proliferation. Nat Commun 2015; 6: 8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ackeifi C, Wang P, Karakose E, et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human beta cell regeneration. Sci Transl Med 2020; 12(530): eaaw9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ackeifi C, Swartz E, Kumar K, et al. Pharmacologic and genetic approaches define human pancreatic beta cell mitogenic targets of DYRK1A inhibitors. JCI Insight 2020; 5(1): e132594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Allegretti PA, Horton TM, Abdolazimi Y, et al. Generation of highly potent DYRK1A-dependent inducers of human beta-Cell replication via Multi-Dimensional compound optimization. Bioorg Med Chem 2020; 28(1): 115193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol 2007; 5(7): e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cox AR, Lam CJ, Rankin MM, et al. Extreme obesity induces massive beta cell expansion in mice through self-renewal and does not alter the beta cell lineage. Diabetologia 2016; 59(6): 1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic beta Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab 2018; 27(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thorel F, Nepote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010; 464(7292): 1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chera S, Baronnier D, Ghila L, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature 2014; 514(7523): 503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ben-Othman N, Vieira A, Courtney M, et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 2017; 168(1–2): 73–85 e11. [DOI] [PubMed] [Google Scholar]
- 117.Li J, Casteels T, Frogne T, et al. Artemisinins Target GABAA Receptor Signaling and Impair alpha Cell Identity. Cell 2017; 168(1–2): 86–100 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van der Meulen T, Lee S, Noordeloos E, et al. Artemether Does Not Turn alpha Cells into beta Cells. Cell Metab 2018; 27(1): 218–25 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.von Herrath M, Pagni PP, Grove K, et al. Case Reports of Pre-clinical Replication Studies in Metabolism and Diabetes. Cell Metab 2019; 29(4): 795–802. [DOI] [PubMed] [Google Scholar]
- 120.Ackermann AM, Moss NG, Kaestner KH. GABA and Artesunate Do Not Induce Pancreatic alpha-to-beta Cell Transdifferentiation In Vivo. Cell Metab 2018; 28(5): 787–92 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shin JS, Kim JM, Min BH, Chung H, Park CG. Absence of spontaneous regeneration of endogenous pancreatic beta-cells after chemical-induced diabetes and no effect of GABA on alpha-to-beta cell transdifferentiation in rhesus monkeys. Biochem Biophys Res Commun 2019; 508(4): 1056–61. [DOI] [PubMed] [Google Scholar]
- 122.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998; 282: 1145–7. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131(5): 861–72. [DOI] [PubMed] [Google Scholar]
- 124.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006; 24(11): 1392–401. [DOI] [PubMed] [Google Scholar]
- 125.Migliorini A, Nostro MC, Sneddon JB. Human pluripotent stem cell-derived insulin-producing cells: A regenerative medicine perspective. Cell Metab 2021; 33(4): 721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002; 129(10): 2447–57. [DOI] [PubMed] [Google Scholar]
- 127.Sander M, Sussel L, Conners J, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 2000; 127(24): 5533–40. [DOI] [PubMed] [Google Scholar]
- 128.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008; 26(4): 443–52. [DOI] [PubMed] [Google Scholar]
- 129.Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2): 428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012; 61(8): 2016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bruin JE, Rezania A, Xu J, et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 2013; 56(9): 1987–98. [DOI] [PubMed] [Google Scholar]
- 132.Henry R, Pettus J, Wilensky J, et al. Initial Clinical Evaluation of VC-01TM Combination Product—A Stem Cell–Derived Islet Replacement for Type 1 Diabetes (T1D). Diabetes 2018; 67(1): 138–OR. [Google Scholar]
- 133.Pullen LC. Stem cell-derived pancreatic progneitor cells have now been transplanted into patients: report from IPITA 2018. Am J Transplant 2018; 18: 1581–2. [DOI] [PubMed] [Google Scholar]
- 134.Piper K, Brickwood S, Turnpenny LW, et al. Beta cell differentiation during early human pancreas development. J Endocrinol 2004; 181(1): 11–23. [DOI] [PubMed] [Google Scholar]
- 135.van der Meulen T, Huising MO. Maturation of stem cell-derived beta-cells guided by the expression of urocortin 3. Rev Diabet Stud 2014; 11(1): 115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11): 1121–33. [DOI] [PubMed] [Google Scholar]
- 137.Russ HA, Parent AV, Ringler JJ, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 2015; 34(13): 1759–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Velazco-Cruz L, Song J, Maxwell KG, et al. Acquisition of Dynamic Function in Human Stem Cell-Derived beta Cells. Stem Cell Reports 2019; 12(2): 351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nair GG, Liu JS, Russ HA, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat Cell Biol 2019; 21(2): 263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luther MJ, Hauge-Evans A, Souza KL, et al. MIN6 beta-cell-beta-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem Biophys Res Commun 2006; 343(1): 99–104. [DOI] [PubMed] [Google Scholar]
- 141.Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 1999; 48(7): 1402–8. [DOI] [PubMed] [Google Scholar]
- 142.Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol 2020; 38(4): 460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Melton D The promise of stem cell-derived islet replacement therapy. Diabetologia 2021; 64(5): 1030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Byrnes LE, Wong DM, Subramaniam M, et al. Lineage dynamics of murine pancreatic development at single-cell resolution. Nat Commun 2018; 9(1): 3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scavuzzo MA, Hill MC, Chmielowiec J, et al. Endocrine lineage biases arise in temporally distinct endocrine progenitors during pancreatic morphogenesis. Nat Commun 2018; 9(1): 3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krentz NAJ, Lee MYY, Xu EE, et al. Single-Cell Transcriptome Profiling of Mouse and hESC-Derived Pancreatic Progenitors. Stem Cell Reports 2018; 11(6): 1551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stanescu DE, Yu R, Won KJ, Stoffers DA. Single cell transcriptomic profiling of mouse pancreatic progenitors. Physiol Genomics 2017; 49(2): 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bastidas-Ponce A, Tritschler S, Dony L, et al. Comprehensive single cell mRNA profiling reveals a detailed roadmap for pancreatic endocrinogenesis. Development 2019; 146(12): dev173849. [DOI] [PubMed] [Google Scholar]
- 149.Ramond C, Beydag-Tasoz BS, Azad A, et al. Understanding human fetal pancreas development using subpopulation sorting, RNA sequencing and single-cell profiling. Development 2018; 145(16): dev165480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cao J, O’Day DR, Pliner HA, et al. A human cell atlas of fetal gene expression. Science 2020; 370(6518): eaba7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov 2017; 16(5): 338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cooper DK, Matsumoto S, Abalovich A, et al. Progress in Clinical Encapsulated Islet Xenotransplantation. Transplantation 2016; 100(11): 2301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vegas AJ, Veiseh O, Doloff JC, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nature Biotechnology 2016; 34: 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stock AA, Manzoli V, De Toni T, et al. Conformal Coating of Stem Cell-Derived Islets for beta Cell Replacement in Type 1 Diabetes. Stem Cell Reports 2020; 14(1): 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barkai U, Weir GC, Colton CK, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplantation 2013; 22: 1463–76. [DOI] [PubMed] [Google Scholar]
- 156.Carlsson PO, Espes D, Sedigh A, et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas betaAir to patients with type 1 diabetes mellitus. Am J Transplant 2018; 18(7): 1735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Montazeri L, Hojjati-Emami S, Bonakdar S, et al. Improvement of islet engrafts by enhanced angiogenesis and microparticle-mediated oxygenation. Biomaterials 2016; 89: 157–65. [DOI] [PubMed] [Google Scholar]
- 158.Coronel MM, Liang JP, Li Y, Stabler CL. Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device. Biomaterials 2019; 210: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Razavi M, Primavera R, Kevadiya BD, Wang J, Buchwald P, Thakor AS. A Collagen Based Cryogel Bioscaffold that Generates Oxygen for Islet Transplantation. Advanced Functional Materials 2020; 30(15): 1902463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baidal DA, Ricordi C, Berman DM, et al. Bioengineering of an Intraabdominal Endocrine Pancreas. N Engl J Med 2017; 376(19): 1887–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg 2007; 31(4): 705–14. [DOI] [PubMed] [Google Scholar]
- 162.Hubber EL, Rackham CL, Jones PM. Protecting islet functional viability using mesenchymal stromal cells. Stem Cells Transl Med 2021; 10(5): 674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Forbes S, Bond AR, Thirlwell KL, et al. Human umbilical cord perivascular cells improve human pancreatic islet transplant function by increasing vascularization. Sci Transl Med 2020; 12: eaan5907. [DOI] [PubMed] [Google Scholar]
- 164.Weaver JD, Headen DM, Aquart J, et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci Adv 2017; 3(6): e1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Najjar M, Manzoli V, Abreu M, et al. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol Bioeng 2015; 112(9): 1916–26. [DOI] [PubMed] [Google Scholar]
- 166.Staels W, Verdonck Y, Heremans Y, et al. Vegf-A mRNA transfection as a novel approach to improve mouse and human islet graft revascularisation. Diabetologia 2018; 61(8): 1804–10. [DOI] [PubMed] [Google Scholar]
- 167.Grapensparr L, Christoffersson G, Carlsson PO. Bioengineering with Endothelial Progenitor Cells Improves the Vascular Engraftment of Transplanted Human Islets. Cell Transplant 2018; 27(6): 948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vlahos AE, Kinney SM, Kingston BR, et al. Endothelialized collagen based pseudo-islets enables tuneable subcutaneous diabetes therapy. Biomaterials 2020; 232: 119710. [DOI] [PubMed] [Google Scholar]
- 169.Citro A, Moser PT, Dugnani E, et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials 2019; 199: 40–51. [DOI] [PubMed] [Google Scholar]
- 170.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant 2012; 12(6): 1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Naziruddin B, Kanak MA, Chang CA, et al. Improved outcomes of islet autotransplant after total pancreatectomy by combined blockade of IL-1beta and TNFalpha. Am J Transplant 2018; 18(9): 2322–9. [DOI] [PubMed] [Google Scholar]
- 172.Kim G, Lee HS, Oh BJ, et al. Protective effect of a novel clinical-grade small molecule necrosis inhibitor against oxidative stress and inflammation during islet transplantation. Am J Transplant 2021; 21(4): 1440–52. [DOI] [PubMed] [Google Scholar]
- 173.Matsuoka T, Yoshimatsu G, Sakata N, et al. Inhibition of NLRP3 inflammasome by MCC950 improves the metabolic outcome of islet transplantation by suppressing IL-1beta and islet cellular death. Sci Rep 2020; 10(1): 17920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shahbazov R, Kanak MA, Takita M, et al. Essential phospholipids prevent islet damage induced by proinflammatory cytokines and hypoxic conditions. Diabetes Metab Res Rev 2016; 32(3): 268–77. [DOI] [PubMed] [Google Scholar]
- 175.Shaw JA, Docherty K. Gene therapy in diabetes mellitus. In: Wass JAH, Stewart PM, Armiel SA, Davies MJ, eds. Oxford Textbook of Endocrinology and Diabetes (2 edn): Oxford University Press; 2011. [Google Scholar]
- 176.Noguchi H, Miyagi-Shiohira C, Nakashima Y, Saitoh I, Watanabe M. Novel cell-permeable p38-MAPK inhibitor efficiently prevents porcine islet apoptosis and improves islet graft function. Am J Transplant 2020; 20(5): 1296–308. [DOI] [PubMed] [Google Scholar]
- 177.Parajuli KR, Zhang Y, Cao AM, Wang H, Fonseca VA, Wu H. Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting beta Cell Survival and alpha-to-beta Cell Transdifferentiation. Cell Transplant 2020; 29: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zammit NW, Walters SN, Seeberger KL, O’Connell PJ, Korbutt GS, Grey ST. A20 as an immune tolerance factor can determine islet transplant outcomes. JCI Insight 2019; 4(21): e131028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang K, Weaver JD, Li Y, Chen X, Liang J, Stabler CL. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials 2017; 114: 71–81. [DOI] [PubMed] [Google Scholar]
- 180.Pham-Hua D, Padgett LE, Xue B, et al. Islet encapsulation with polyphenol coatings decreases pro-inflammatory chemokine synthesis and T cell trafficking. Biomaterials 2017; 128: 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu JMH, Zhang J, Zhang X, et al. Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials 2016; 80: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alagpulinsa DA, Cao JJL, Driscoll RK, et al. Alginate-microencapsulation of human stem cell–derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. American Journal of Transplantation 2019; 19: 1930–40. [DOI] [PubMed] [Google Scholar]
- 183.Bischoff L, Alvarez S, Dai DL, Soukhatcheva G, Orban PC, Verchere CB. Cellular mechanisms of CCL22-mediated attenuation of autoimmune diabetes. J Immunol 2015; 194(7): 3054–64. [DOI] [PubMed] [Google Scholar]
- 184.Hlavaty KA, Gibly RF, Zhang X, et al. Enhancing human islet transplantation by localized release of trophic factors from PLG scaffolds. Am J Transplant 2014; 14(7): 1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kasputis T, Clough D, Noto F, Rychel K, Dye B, Shea LD. Microporous Polymer Scaffolds for the Transplantation of Embryonic Stem Cell Derived Pancreatic Progenitors to a Clinically Translatable Site for the Treatment of Type I Diabetes. ACS Biomater Sci Eng 2018; 4(5): 1770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tyden G, Reinholt FP, Sundkvist G, Bolinder J. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. N Engl J Med 1996; 335(12): 860–3. [DOI] [PubMed] [Google Scholar]
- 187.Kin T, Rajotte RV, Dufour JM, Korbutt GS. Development of an immunoprivileged site to prolong islet allograft survival. Cell Transplant 2002; 11(6): 547–52. [PubMed] [Google Scholar]
- 188.El Khatib MM, Sakuma T, Tonne JM, et al. beta-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection. Gene Ther 2015; 22(5): 430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shieh SJ, Chou FC, Yu PN, et al. Transgenic expression of single-chain anti-CTLA-4 Fv on beta cells protects nonobese diabetic mice from autoimmune diabetes. J Immunol 2009; 183(4): 2277–85. [DOI] [PubMed] [Google Scholar]
- 190.Woodward KB, Zhao H, Shrestha P, et al. Pancreatic islets engineered with a FasL protein induce systemic tolerance at the induction phase that evolves into long-term graft-localized immune privilege. Am J Transplant 2020; 20(5): 1285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Headen DM, Woodward KB, Coronel MM, et al. Local immunomodulation Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nat Mater 2018; 17(8): 732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Skoumal M, Woodward KB, Zhao H, et al. Localized immune tolerance from FasL-functionalized PLG scaffolds. Biomaterials 2019; 192: 271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Coronel MM, Martin KE, Hunckler MD, et al. Immunotherapy via PD-L1-presenting biomaterials leads to long-term islet graft survival. Sci Adv 2020; 6(35): eaba5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang W, Gorantla VS, Campbell PG, et al. Biopatterned CTLA4/Fc Matrices Facilitate Local Immunomodulation, Engraftment, and Glucose Homeostasis After Pancreatic Islet Transplantation. Diabetes 2016; 65(12): 3660–6. [DOI] [PubMed] [Google Scholar]
- 195.Gamble A, Pawlick R, Pepper AR, et al. Improved islet recovery and efficacy through co-culture and co-transplantation of islets with human adipose-derived mesenchymal stem cells. PLOS ONE 2018; 13: e0206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Duan W, Yu X, Ma D, et al. Mesenchymal stem cells in combination with low-dose rapamycin significantly prolong islet allograft survival through induction of regulatory T cells. Biochem Biophys Res Commun 2018; 506(3): 619–25. [DOI] [PubMed] [Google Scholar]
- 197.Sawitzki B, Harden PN, Reinke P, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. The Lancet 2020; 395(10237): 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Graham JG, Zhang X, Goodman A, et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A 2013; 19(11–12): 1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tenspolde M, Zimmermann K, Weber LC, et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J Autoimmun 2019; 103: 102289. [DOI] [PubMed] [Google Scholar]
- 200.Serr I, Furst RW, Achenbach P, et al. Type 1 diabetes vaccine candidates promote human Foxp3(+)Treg induction in humanized mice. Nat Commun 2016; 7: 10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dawson NAJ, Rosado-Sanchez I, Novakovsky GE, et al. Functional effects of chimeric antigen receptor co-receptor signaling domains in human regulatory T cells. Sci Transl Med 2020; 12(557): eaaz3866. [DOI] [PubMed] [Google Scholar]
- 202.Serra P, Santamaria P. Antigen-specific therapeutic approaches for autoimmunity. Nat Biotech 2019; 37(3): 238–51. [DOI] [PubMed] [Google Scholar]
- 203.Kelly CP, Murray JA, Leffler DA, et al. TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Gastroenterology 2021; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lutterotti A, Yousef S, Sputtek A, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med 2013; 5(188): 188ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Singh A, Ramachandran S, Graham ML, et al. Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes. Nat Commun 2019; 10(1): 3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bryant J, Hlavaty KA, Zhang X, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials 2014; 35(31): 8887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Herold KC, Usmani-Brown S, Ghazi T, et al. Beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest 2015; 125(3): 1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bellin MD, Clark P, Usmani-Brown S, et al. Unmethylated Insulin DNA Is Elevated After Total Pancreatectomy With Islet Autotransplantation: Assessment of a Novel Beta Cell Marker. Am J Transplant 2017; 17(4): 1112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Neiman D, Gillis D, Piyanzin S, et al. Multiplexing DNA methylation markers to detect circulating cell-free DNA derived from human pancreatic beta cells. JCI Insight 2020; 5(14): e136579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Morris AH, Hughes KR, Oakes RS, et al. Engineered immunological niches to monitor disease activity and treatment efficacy in relapsing multiple sclerosis. Nat Commun 2020; 11(1): 3871. [DOI] [PMC free article] [PubMed] [Google Scholar]


