Abstract
Objective:
Exposure-based therapy (EXP) and behavioral activation (BA) are empirically-supported behavioral intervention techniques that target avoidance and approach behavior to alleviate symptoms. Although EXP is an established treatment for generalized anxiety disorder (GAD), the effectiveness of BA for GAD has not been directly tested or compared with that of EXP. This study examined the efficacy of EXP and BA for adults with GAD.
Method:
In a randomized clinical trial (clinicaltrials.gov: NCT02807480) with partial blinding in Tulsa, OK, 102 adults with GAD were allocated to manualized, 10-session EXP or BA between April 2016–April 2021. Primary analyses were intention-to-treat and included the 94 (46 EXP, 48 BA) participants who started treatment. The GAD-7 self-report scale was the primary outcome measure.
Results:
Similar GAD-7 declines were observed at post-treatment for EXP (d=−0.97 [95% CI −1.40 to −0.53]) and BA (d=−1.14 [95% CI −1.57 to −0.70]), and were maintained through 6-month follow-up (EXP: d=−2.13, BA: d=−1.98). Compared to EXP, BA yielded more rapid declines in anxiety and depression scores during therapy (d=0.75–0.77), as well as lower anxiety and depression scores (d=0.13–0.14) and greater participant-rated improvement (d=0.64) at post-treatment. Bayesian analyses indicated 74–99% probability of greater change in BA than EXP at post-treatment.
Conclusions:
BA and EXP are both effective in treating GAD, and BA may confer greater benefit during treatment. Future research is warranted to inform personalized treatment approaches.
Keywords: Generalized anxiety disorder, Behavior therapy, Exposure therapy, Clinical trials
1. Introduction
Generalized anxiety disorder (GAD), a condition characterized by persistent, uncontrollable worry, has an estimated lifetime prevalence of 4% globally [1] and confers significant individual and socioeconomic burden [2,3]. Traditional first-line treatments for GAD, including medication and psychotherapy, are effective for many [4–6] but not all: only 40–60% of patients who engage in treatment for GAD experience meaningful symptom improvement, and 25% relapse within one year [4,7].
Clinicians and patients have little evidence-based guidance regarding how to tailor interventions to an individual case. A better understanding of the functional mechanisms of GAD and its treatment is therefore warranted to improve treatment outcomes. One such mechanism is persistent maladaptive avoidance of anxiogenic stimuli and situations: while avoidance is not a criterion for GAD in DSM-5 [8], GAD has been associated with overt behavioral avoidance, reassurance-seeking, and safety behaviors, similar to other anxiety disorders [9,10]. Worry, the cardinal symptom of GAD, is also thought to serve various avoidance functions, such as avoidance or prevention of negative outcomes through mental rehearsal [11,12], avoidance of unwanted internal experiences [13], and maintenance of a negative internal state to avoid unexpected emotional shifts [14]. Avoidance is a natural and often adaptive response to threat, but avoidance can be maladaptive when it involves relinquishing or withdrawing from valued goals, that is, when it occurs in the context of approach-avoidance conflict (AAC [15,16]). The maladaptive avoidance patterns seen in anxiety disorders, including GAD, can therefore be understood as stemming from an imbalance in the processing and resolution of AAC such that avoidance motivation is given undue priority. Treatment approaches would be expected to normalize this imbalance if they could reduce excessive avoidance motivation, bolster insufficient approach motivation, or both.
Exposure-based therapy (EXP) and behavioral activation (BA) are two behavioral intervention techniques frequently employed in GAD treatment, which putatively act on distinct systems with the shared aim of reducing avoidance. EXP is thought to normalize imbalanced AAC processing by reducing avoidance motivation. It centers on a series of exposure exercises in which the patient confronts a feared situation in the absence of the feared outcome and learns through repeated experience that the situation is in fact safe. Meta-analyses have demonstrated that EXP is highly effective for anxiety [6,17]. For GAD in particular, EXP has been shown to effectively reduce behavioral avoidance and worry [18,19]. BA, by contrast, is thought to normalize AAC processing by increasing positive affect and approach motivation, with a focus on scheduling rewarding and valued activities and monitoring their affective and functional consequences. BA has garnered substantial empirical support as a treatment for depression [20,21]. BA has also received increasing consideration as a potential treatment for anxiety: case studies have illustrated the promise of BA for anxiety [22,23], a meta-analysis of trials of BA clinical trials for depression found that BA reduced anxiety symptoms as well as depressive symptoms [24], a clinical trial of BA for social anxiety disorder showed favorable outcomes [25], and a recent meta-analysis has identified BA as an effective treatment for PTSD on par with cognitive processing therapy and exposure [26].
Given the theoretical relevance of BA to maladaptive avoidance, the growing support for BA as a treatment for anxiety, and the high comorbidity of GAD with depressive disorders, it is reasonable to consider the effectiveness of BA for GAD. Considering that most individuals with GAD present with depression and that BA is part of many CBT interventions, it is also likely that BA is frequently employed by providers when treating individuals with GAD. The existing evidence for the effectiveness of EXP and BA for GAD is encouraging; however, little is known about the relative effectiveness of these treatments, or the characteristics of patients who might benefit from one more than the other.
In the present study, we address this gap by investigating the efficacy of BA and EXP for GAD in a sample of community adults, implemented in a group format. This study represents a secondary set of analyses from a larger investigation of neural predictors of symptom change in behavioral therapies for GAD [52]. A control condition (e.g., waitlist or non-behavioral intervention) was not included given the known effectiveness of EXP for GAD [18,19]. Our first aim was to characterize symptom change over time, with the hypothesis that BA and EXP would both lead to linear declines in anxiety and depressive symptoms over the course of treatment, as well as symptom improvements from pre-to post-treatment and 3-and 6-month follow-ups; exploratory analyses were performed to investigate potential differences in effectiveness between treatments. Our second aim was to investigate the potential differential impact of BA and EXP on putative targeted psychological constructs, with the hypotheses that that BA would a greater impact on measures of behavioral activation and positive affect, and EXP would have a greater impact on negative affect measures.
2. Methods
The study method is described in detail in our published protocol paper [52] and summarized here. The study was registered in 2016 within 30 days of first participant enrollment in accordance with FDAAA 801 with ClinicalTrials.gov, identifier NCT02807480, which included pre-registration of primary outcome measures. Analysis plan for the present manuscript was not pre-registered.
2.1. Procedure
This study received ethical approval and was carried out in accordance with the Declaration of Helsinki; participants provided written informed consent and were compensated for participation. The study was conducted as part of a randomized (two-condition), single-center (Tulsa, OK) clinical trial examining multilevel predictors of response to EXP versus BA for GAD. Fig. S1 illustrates the timeline of study events and collection of outcome measures. Interventions consisted of 10 weeks of manualized BA or EXP. Therapies were delivered in group format for 80% of participants: groups of 8–10 participants were assigned to closed treatment groups at time of enrollment, and each group was randomized to be either BA or EXP (randomization conducted in blocks of 4; sequence generated by RLA). Following the onset of the COVID-19 pandemic (March, 2020), interventions for the remaining 20% of participants were conducted in an individual virtual format. These participants were individually randomized into BA or EXP following enrollment. Participant counts for each therapy group and individual therapy are shown in Fig. S2. Participants were blinded to group assignment until after baseline assessments were completed; neither participants nor administrators of self-report scales were blinded during weekly and post-treatment assessments; the PI and clinicians were not blinded to intervention condition. The primary outcome measure was the GAD-7 [27], which has been used in previous clinical trials for GAD [28–31]. Secondary outcome measures included the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety and Depression scales [32] and the Sheehan Disability Scale (SDS [33]). For exploratory analyses of motivational and emotional processes impacted by the treatments, we administered a short form of the Behavioral Activation for Depression Scale [34] at all timepoints, and the BIS/BAS scale [35] and the Positive Affect, Affective Fear, and Somatic Fear scales from the Emotion Battery from the NIH Toolbox for Assessment of Neurological and Behavioral Function [36,37] at pre- and post-treatment timepoints. To assess treatment engagement and experience, the Homework Rating Scale [38] was administered weekly, and a scale assessing perceived therapy-related improvement adapted from previous measures [33,39] was administered at post-treatment.
Following initial screening assessments to confirm exclusion and inclusion criteria for the study, participants underwent a baseline assessment including self-report, behavioral, biological, and neuroimaging assessments. Participants were then randomized to EXP or BA treatment, during which weekly self-report symptom measures were obtained. After treatment, participants repeated the assessments that were conducted at baseline. Self-report symptom measures were then repeated at 3 and 6 months following treatment. Self-report data were collected electronically using Research Electronic Data Capture (REDCap [40]); interventions were conducted in-person at the Laureate Institute for Brain Research in Tulsa, OK or virtually over Zoom.
2.2. Participants
A total of 121 treatment-seeking individuals meeting criteria for GAD were consented to the study over a 5-year period (April 2016–April 2021); recruitment ended when the planned number of participants (n = 101) had been randomized to treatment. Of the 94 participants who started treatment, 69 participants completed treatment (31 EXP, 38 BA; 73.4%) with treatment completion defined as attending at least 7 therapy sessions (see Fig. S3 for participant flow diagram). Participants were recruited from local mental health clinics and the general population via electronic and print advertisements. Participants were between 18 and 55 years old, were fluent in English, and met criteria for GAD per the Mini International Neuropsychiatric Interview (MINI, version 6.0.0 for DSM-IV-TR or version 7.0.2 for DSM-5) [41], without severe depression or acute suicidal ideation with intent or plan. Participants reporting present use of selective serotonin reuptake inhibitors (SSRIs) were included if the dose had been stable for 6 weeks before enrollment. Inclusion/exclusion criteria were intended to minimize potential confounders while also promoting generalizability of results to GAD patient populations in the community. Full exclusion criteria are described in the supplement.
2.3. Interventions
EXP and BA treatments were both manualized, ten-session interventions. Participants in both treatment-arms were provided with a binder complementary to the intervention, including outlines of each session, basic descriptions of concepts, and homework worksheets. Both interventions centered on behavioral intervention techniques, and did not incorporate cognitive techniques such as cognitive restructuring or scheduled time for worry. Cognitive techniques were excluded in order to isolate the mechanisms of behavioral techniques, both for the purposes of the present analyses investigating positive and negative valence systems, and for the neuroimaging analyses not reported here. Groups met weekly for 90 min and were conducted by two co-therapists. Participants who received individual therapy following the onset of the COVID-19 pandemic had weekly 50-minute sessions with a single therapist. Detailed descriptions of the content of each session are published elsewhere [52]. Study therapists were licensed doctoral- or master’s-level clinicians, clinical psychology postdoctoral fellows, and clinical psychology graduate students, who were extensively trained in both treatment-arms. Fidelity ratings of select therapy session recordings were made by experts in each intervention and their trainees, and indicated acceptable fidelity to each treatment-arm (see Supplement for more detail). Therapists attended weekly consultation and supervision with RLA and/or consultants.
BA aims to help the patient re-engage with behaviors that increase opportunities for reward or reinforcement, especially those in line with the patient’s values. Avoidance patterns around these behaviors are specifically addressed. A ten-session, structured BA manual was developed by coauthors CM and RLA, with edits and amendments provided by AC, informed by existing BA treatment guides and altered to target general negative affect rather than only depression.
EXP involves a series of exposure exercises in which the patient safely confronts anxiogenic stimuli, enabling inhibitory learning or habituation. The manual accompanying the ten-session, structured EXP treatment was established using a previous group-based anxiety treatment manual developed by MGC [53] and adapted by MGC and RLA, with edits and amendments provided by KWT and AC, to isolate exposure techniques without cognitive restructuring and to target inhibitory learning.
Of note, BA and EXP share some similarities. Both interventions focus on identifying maladaptive behavior patterns, assigning weekly behavioral homework, and planning for long-term behavior change. Patients with similar presentations may receive similar homework in both treatments. However, even when this is the case, the treatments focus on different aspects of the activities (i.e., testing negative expectancies in EXP versus positive reinforcement in BA).
2.4. Sample size and power analysis
Sample size was originally determined for the purposes of supporting aims related to neuroimaging predictors of treatment response [52]. We aimed to recruit at least 100 participants, which with 20% attrition would allow for complete longitudinal data for 80 participants (i.e., ~ 40/intervention). The current analysis included the N = 94 individuals who started treatment; with this sample, we estimated having 80% power to detect large effects for changes in symptoms over time in each treatment-arm (d=0.70) and medium effects for differences between treatment-arms (d=0.47). Previous research suggests large effect sizes for changes over time within-treatment (d=1.62–2.41) [42,43], and small to medium effects when comparing active therapy interventions (e.g., d=0.17—0.54) [44–46].
2.5. Statistical analyses
Analysis scripts, data, and treatment manuals are publicly available in a data repository at https://osf.io/8cfwk/. R statistical software [47] was used to run all statistical analyses. Logistic regressions were conducted via the ‘glm’ function [47], linear mixed-effects regressions (LMEs) were conducted via the ‘lme’ function from the ‘nlme’ package [48], Bayesian analyses were conducted using the ‘rstanarm’ package [49]. Age, sex, and education were entered as covariates for all regression analyses.
2.5.1. Demographic and clinical characteristics across treatment-arms
To assess differences between treatment-arms in demographic and baseline clinical variables, we conducted chi-squared analyses for categorical variables (Fisher’s exact test when the number of observations in any cell < 5) and Welch’s t-tests for continuous variables. These demographic and clinical variables were also compared across groups of individuals who enrolled in the study but did not start treatment, those who started but did not complete treatment, and those who did complete treatment. Treatment completion and follow-up completion were compared across treatment-arms using logistic regression.
2.5.2. Treatment effects on symptom outcomes
LMEs, described in detail below, were used to assess treatment effects on the primary symptom outcome (GAD-7) and the four secondary outcome measures (i.e., PROMIS Depression and Anxiety, SDS, and BADS scores) between and within treatment-arms. We hypothesized that GAD-7, PROMIS Depression and Anxiety, and SDS scores would decrease and BADS scores would increase in both treatment-arms, and that BADS scores would increase more in BA than in EXP. An α threshold of p < .05 was used for analyses with GAD-7. To account for multiple comparisons across analyses of the four secondary outcome variables, a Bonferroni correction was used and only results for which p < .0125 were considered significant. These were intention-to-treat analyses and included data from all participants who began treatment; because the study involved a neuroimaging component before treatment, which may have affected participants’ decision to drop out before treatment started, the analyses excluded participants who dropped out before treatment. For participants who did not complete post-treatment ratings, scores from the last treatment session were used as the post-treatment data, i.e., “last one carried forward.” Those who withdrew from therapy were asked to complete the primary symptom outcome measures, and these scores were used as the post-treatment data.
The main analyses were LMEs comparing the effects on primary and secondary symptom outcomes of each treatment-arm linearly from pretreatment through weekly and post-treatment timepoints. A treatment-arm by timepoint (ordered factor; i.e., pre-treatment, weeks 1–10, and post-treatment) effect was the predictor of interest; random effects were included for participant and treatment group number (i.e., group 1, 2, 3, etc.; participants assigned to individual therapy due to COVID were coded as group 11). Treatment group number was included as a random effect, given that the intervention was conducted in groups and to control for the possibility that some groups may have had better or worse outcomes (e.g., due to being earlier or later in the study). An auto-correlation function was used to account for within-group residuals in the models. Given the high comorbidity of GAD with MDD, and BA’s history as an evidence-based treatment for depression, we next assessed whether the efficacy of BA is specific to depressed patients, by repeating these analyses with major depressive episode status (current MDE vs. no current MDE at baseline) entered as a binary predictor variable, and the three-way interaction of time, treatment and MDE status as the predictor of interest.
Additional analyses were conducted to examine the differences in treatment effects on primary and secondary symptom outcomes for each time-point as a categorical factor (i.e., to obtain effect sizes for each time point separately). LMEs were run with symptom outcomes as the DV and a treatment-arm by timepoint (non-ordered factor; i.e., pre-treatment, post-treatment, the 10 therapy sessions, 3-month follow-up, and 6-month follow-up) interaction effect as the predictor of interest, with random effects for participant and treatment group. The treatment-arm by timepoint interaction is reported for post-treatment, 3-month follow-up and 6-month follow up timepoints, respectively. These secondary analyses were then repeated with a Bayesian approach, to obtain the posterior probability of a treatment-arm difference in symptom change from baseline at each timepoint.
2.5.3. Positive and negative valence systems
Changes in BIS/BAS scores and positive affect, behavioral approach and inhibition tendencies, and affective and somatic fear were compared pre- and post-treatment across treatment-arms, with LMEs in which treatment-arm, timepoint, and the treatment-arm by timepoint interaction were entered as predictors and treatment group and participant were entered as random effects. A Bonferroni correction was applied and only results for which p < .007 were considered significant. We hypothesized that BA would increase positive affect and behavioral approach, and EXP would decrease behavioral inhibition and fear.
2.5.4. Treatment engagement and experience
Measures of treatment engagement and experience (number of sessions attended, homework completion, working alliance, perceived therapy-related improvement) were compared across treatment-arms using LMEs with random effects for treatment group. Homework completion and working alliance were assessed at multiple timepoints and thus also included timepoint as a predictor of interest and participant as a random effect.
3. Results
3.1. Demographic and clinical characteristics
All 94 (46 EXP, 48 BA) participants who started treatment were included in analyses. The BA treatment-arm included more male participants and lower GAD-7 scores at a trend level; treatment-arms were otherwise comparable on demographic and baseline clinical characteristics (Table 1). Differences among those who completed, did not complete, and did not start treatment are described in the supplement. As shown in Table S2, treatment completion and follow-up completion did not significantly differ across treatment-arms.
Table 1.
Demographics and Comparisons across Treatment-Arms.
| BA | EXP | P | |
|---|---|---|---|
| n | 48 | 46 | |
| Age (mean (SD)) | 34 (11) | 35 (11) | 0.754 |
| Sex = Male (%) | 10 (20.8) | 2 (4.3) | 0.028† |
| Income (%) | 0.445† | ||
| Not provided | 0 (0.0) | 2 (4.3) | |
| Less than 50,000 | 25 (52.1) | 19 (41.3) | |
| 50,000 to 100,000 | 9 (18.8) | 13 (28.3) | |
| 100,000 to 150,000 | 11 (22.9) | 8 (17.4) | |
| over 150,000 | 3 (6.2) | 4 (8.7) | |
| Race (%) | 0.232† | ||
| American Indian | 11 (22.9) | 9 (19.6) | |
| Asian | 0 (0.0) | 2 (4.3) | |
| Black | 6 (12.5) | 1 (2.2) | |
| Hispanic | 2 (4.2) | 2 (4.3) | |
| Multiracial | 1 (2.1) | 0 (0.0) | |
| White | 27 (56.2) | 30 (65.2) | |
| Race = White (%) | 27 (56.2) | 30 (65.2) | 0.498 |
| Education (%) | 0.734† | ||
| Less than high school | 1 (2.1) | 1 (2.2) | |
| High school or GED | 4 (8.3) | 4 (8.7) | |
| Some college, no degree | 14 (29.2) | 12 (26.1) | |
| Bachelor’s degree | 16 (33.3) | 10 (21.7) | |
| Associate’s degree | 3 (6.2) | 4 (8.7) | |
| Master’s degree | 10 (20.8) | 13 (28.3) | |
| Professional/doctoral degree | 0 (0.0) | 2 (4.3) | |
| GAD-7 (Mean (SD)) | 11.3 (3.7) | 12.9 (4.6) | 0.066 |
| PROMIS Anxiety (Mean (SD)) | 64.7 (4.7) | 65.7 (5.1) | 0.328 |
| PROMIS Depression (Mean (SD)) | 58.5 (6.6) | 57.9 (8.0) | 0.719 |
| SDS (Mean (SD)) | 12.3 (5.6) | 12.9 (6.5) | 0.609 |
| BADS (Mean (SD)) | 23.6 (7.1) | 25.6 (9.2) | 0.256 |
| MDD Current Episode (%) | 18 (37.5) | 13 (28.3) | 0.464 |
| MDD Lifetime (%) | 44 (91.7) | 38 (82.6) | 0.227† |
| Social Anxiety Disorder (%) | 16 (33.3) | 15 (32.6) | > 0.99 |
| Agoraphobia (%) | 1 (2.1) | 5 (10.9) | 0.107† |
| Panic Disorder (%) | 2 (4.2) | 3 (6.5) | 0.674† |
| Suicidality Current (%) | 19 (39.6) | 12 (26.1) | 0.241 |
| PTSD (%) | 1 (2.1) | 2 (4.3) | 0.613† |
| Binge Eating Disorder (%) | 1 (2.1) | 1 (2.2) | > 0.99† |
Note. BA: behavioral activation; EXP: exposure; GAD-7: Generalized Anxiety Disorder Scale – 7-item, PROMIS: Patient-Reported Outcomes Measurement Information System; SDS: Sheehan Disability Scale; MDD: major depressive disorder; PTSD: post-traumatic stress disorder.
Fisher’s exact test.
3.2. Treatment Effects on Symptom Outcomes
Linear declines were observed in GAD-7 scores across both treatment-arms (Table 2, Fig. 1). A main effect of treatment-arm was also found, indicating overall higher GAD-7 scores across these timepoints in EXP (M=10.7, SD= 4.7) than BA (M=8.3, SD= 4.7). The timepoint by treatment-arm interaction was not significant, indicating comparable symptom improvement across treatment-arms.
Table 2.
Linear Effects of Timepoint and Interactions with Treatment-Arm, from Pre-Treatment through Weekly and Post-Treatment Timepoints.
| Coef. | d | 95% CI of Coef. | ||
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| GAD-7 | ||||
| Timepoint | −5.37 | −0.54 | −6.74 | −4.00 |
| Treatment-Arm | 2.34 ** | 0.70 | 0.84 | 3.84 |
| Treatment-Arm by Timepoint | 1.79 | 0.40 | −0.17 | 3.75 |
| PROMIS Anxiety | ||||
| Timepoint | −8.22 *** | −0.65 | −9.95 | −6.49 |
| Treatment-Arm | 2.48 * | 0.57 | 0.53 | 4.43 |
| Treatment-Arm by Timepoint | 4.21 *** | 0.75 | 1.72 | 6.70 |
| PROMIS Depression | ||||
| Timepoint | −6.75 *** | −0.53 | −8.50 | −5.00 |
| Treatment-Arm | 0.32 | 0.05 | −2.32 | 2.95 |
| Treatment-Arm by Timepoint Sheehan | 4.41 *** | 0.77 | 1.89 | 6.94 |
| Timepoint | −8.75 *** | −0.71 | −10.45 | −7.05 |
| Treatment-Arm | 2.62 | 0.43 | −0.09 | 5.32 |
| Treatment-Arm by Timepoint | 3.34 ** | 0.59 | 0.83 | 5.85 |
| BADS | ||||
| Timepoint | 11.53 *** | 0.64 | 9.02 | 14.04 |
| Treatment-Arm | −1.53 | −0.21 | −4.73 | 1.67 |
| Treatment-Arm by Timepoint | −4.64 * | −0.55 | −8.35 | −0.94 |
Note. Models take the form symptom ~ timepoint × treatment + age + sex + education, with participant ID and treatment group entered as random effects. BA: behavioral activation; EXP: exposure; GAD-7: Generalized Anxiety Disorder Scale – 7-item, PROMIS: Patient-Reported Outcomes Measurement Information System; SDS: Sheehan Disability Scale; BADS: Behavioral Activation for Depression Scale.
p < .001,
p < .01,
p < .05.
Fig. 1.
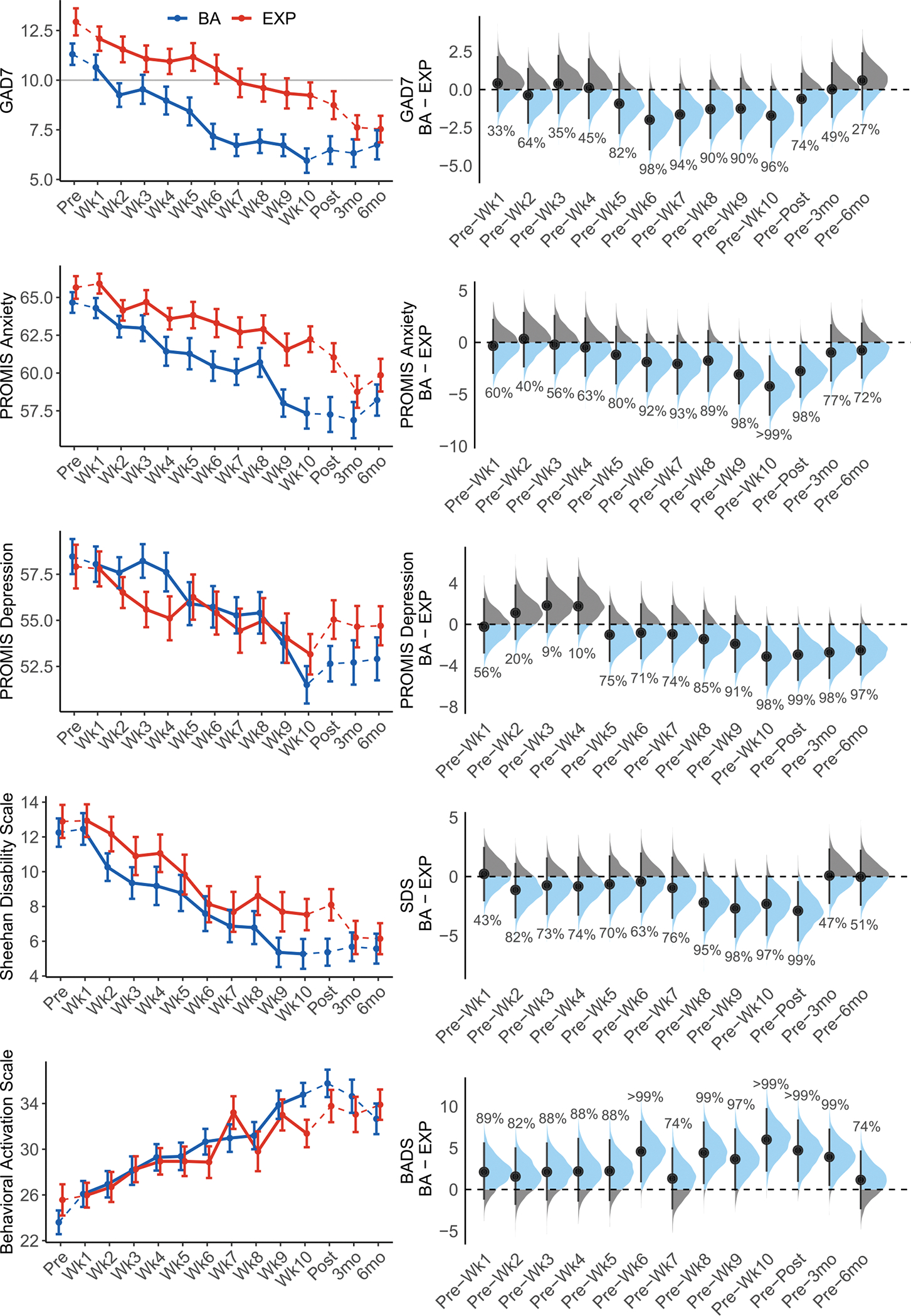
Symptom Change, Line plots on the left reflect raw means and standard errors for self-reported symptoms across time in each treatment-type. Dotted lines reflect assessment timepoints outside of the weekly therapy. The horizontal line on the plot of GAD-7 scores represents a clinical cutoff score of 10 [27]. Forest plots on the right reflect results from Bayesian analyses. Points and vertical lines show posterior medians for the EXP − BA difference of change from pre-treatment scores with 95% credibility intervals (see Table S8), blue areas reflect iterations in which change scores were greater in BA than EXP (i.e., a greater decrease for BA than for EXP on symptom measures, or a greater increase for BA than for EXP on the BADS), and gray areas reflect iterations in which change scores were greater EXP than BA. Percentages reflect the posterior probabilities that the EXP − BA difference of change score is greater than 0 for each timepoint. BA: behavioral activation treatment; EXP: exposure-based treatment; GAD-7: Generalized Anxiety Disorder Scale – 7-item, PROMIS: Patient-Reported Outcomes Measurement Information System; SDS: Sheehan Disability Scale; BADS: Behavioral Activation for Depression Scale; Wk: week of treatment; 3mo: 3-month post-treatment follow-up; 6mo: 6-month post-treatment follow-up.
Linear declines in anxiety symptoms (PROMIS Anxiety), depressive symptoms (PROMIS Depression), and functional impairment (SDS), and linear increases in self-reported behavioral activation (BADS), were also observed (Table 2, Fig. 1). Significant timepoint by treatment-arm interactions were observed for PROMIS Anxiety, PROMIS Depression, and SDS scores; the timepoint by treatment-arm interaction for BADS scores was not significant after correcting for multiple comparisons (p = .014). Stronger linear trends reflecting steeper declines in symptoms were observed in BA (GAD-7: β = −5.12, p < .001; PROMIS Anxiety: β = −8.19, p < .001; PROMIS Depression: β = −6.82, p < .001; SDS: β = −8.78, p < .001) compared to EXP (GAD-7: β = −3.71, p < .001; PROMIS Anxiety: β = −4.02, p < .001, PROMIS Depression: β = −2.23, p = .019; SDS: β = −5.36, p < .001). When these analyses were repeated with MDD status included as a predictor, the MDD × treatment-arm, MDD × timepoint, and MDD × treatment-arm × timepoint interactions were not significant for any outcome measure, indicating comparable treatment effectiveness for those with vs. without active MDD (Table S3, Fig. S4).
The secondary analyses examining pairwise contrasts of pre-treatment scores with post-treatment, 3-month follow-up and 6-month follow-up scores revealed significant timepoint effects but no significant timepoint by treatment-arm interactions for GAD-7. When examining secondary outcome measures, the timepoint by treatment-arm interactions were not significant after correcting for multiple comparisons, though the timepoint by treatment-arm interaction for pre- to post-treatment on BADS scores approached the Bonferroni-corrected significance threshold (p = .018; Table 3). A Bayesian approach to these secondary analyses indicated the probabilities of symptom change from pre-treatment to each timepoint being greater in BA than in EXP (i.e., the probability of the [EXP – BA] difference between [timepoint – pre-treatment] difference scores being less than 0 for symptom scores). The probabilities of pre- to post-treatment symptom change being greater in BA than in EXP range from 74.20% (GAD-7) to 99% (SDS); the probabilities of pre-treatment to 6-month follow up symptom change being greater in BA than in EXP ranged from 26.7% (GAD-7) to 97.10% (PROMIS Depression) (Fig. 1). Clinically significant change in PROMIS Depression scores was more likely in BA (60%) than EXP (39%), and participant-rated perceived improvement was higher in BA (M=5.5, SD=1.0) compared to EXP (M=4.9, SD=0.7), as described in the supplement.
Table 3.
Effects of Time and Treatment-Arm on Symptoms: Paired Contrasts.
| Coef. | d | 95% CI of Coef. | ||
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| GAD | ||||
| Timepoint (Pre vs. Post) | −4.82 *** | −1.54 | −6.19 | −3.44 |
| Timepoint (Pre vs. 3mo) | −4.94 *** | −1.59 | −6.32 | −3.57 |
| Timepoint (Pre vs. 6mo) | −4.43 *** | −0.40 | −5.81 | −3.06 |
| Treatment × Timepoint (Pre vs. Post) | 0.61 | 0.04 | −1.34 | 2.57 |
| Treatment × Timepoint (Pre vs. 3mo) | 0.01 | < 0.01 | −1.96 | 1.98 |
| Treatment × Timepoint (Pre vs. 6mo) | −0.56 | −0.12 | −2.54 | 1.43 |
| PROMIS Anxiety | ||||
| Timepoint (Pre vs. Post) | −7.40 *** | −1.70 | −9.32 | −5.48 |
| Timepoint (Pre vs. 3mo) | −7.59 *** | −1.75 | −9.51 | −5.67 |
| Timepoint (Pre vs. 6mo) | −6.26 *** | −0.40 | −8.19 | −4.33 |
| Treatment × Timepoint (Pre vs. Post) | 2.89* | 0.13 | 0.15 | 5.63 |
| Treatment × Timepoint (Pre vs. 3mo) | 1.08 | 0.05 | −1.68 | 3.85 |
| Treatment × Timepoint (Pre vs. 6mo) | 0.93 | 0.15 | −1.86 | 3.73 |
| PROMIS Depression | ||||
| Timepoint (Pre vs. Post) | −5.78 *** | −1.37 | −7.65 | −3.91 |
| Timepoint (Pre vs. 3mo) | −5.64 *** | −1.33 | −7.51 | −3.77 |
| Timepoint (Pre vs. 6mo) | −5.16*** | −0.34 | −7.04 | −3.28 |
| Treatment × Timepoint (Pre vs. Post) | 2.97* | 0.14 | 0.30 | 5.64 |
| Treatment × Timepoint (Pre vs. 3mo) | 2.72 * | 0.13 | 0.02 | 5.42 |
| Treatment × Timepoint (Pre vs. 6mo) | 2.52 | 0.41 | −0.20 | 5.24 |
| SDS | ||||
| Timepoint (Pre vs. Post) | −6.93 *** | −1.66 | −8.77 | −5.08 |
| Timepoint (Pre vs. 3mo) | −6.53 *** | −1.67 | −8.26 | −4.80 |
| Timepoint (Pre vs. 6mo) | −6.51 *** | −0.47 | −8.25 | −4.77 |
| Treatment × Timepoint (Pre vs. Post) | 2.53 | 0.12 | −0.17 | 5.23 |
| Treatment × Timepoint (Pre vs. 3mo) | −0.16 | −0.01 | −2.66 | 2.35 |
| Treatment × Timepoint (Pre vs. 6mo) | −0.01 | < 0.01 | −2.53 | 2.52 |
| BADS | ||||
| Timepoint (Pre vs. Post) | 11.71 *** | 1.94 | 9.05 | 14.36 |
| Timepoint (Pre vs. 3mo) | 11.07 *** | 1.96 | 8.57 | 13.56 |
| Timepoint (Pre vs. 6mo) | 8.80 *** | 0.44 | 6.29 | 11.30 |
| Treatment × Timepoint (Pre vs. Post) | −4.68 * | −0.15 | −8.56 | −0.79 |
| Treatment × Timepoint (Pre vs. 3mo) | −4.10 * | −0.14 | −7.70 | −0.49 |
| Treatment × Timepoint (Pre vs. 6mo) | −1.10 | −0.13 | −4.74 | 2.53 |
Note. Models take the form symptom ~ timepoint × treatment-arm + age + sex + education, with participant ID and treatment group entered as random effects. BA: behavioral activation; EXP: exposure; GAD-7: Generalized Anxiety Disorder Scale - 7-item, PROMIS: Patient-Reported Outcomes Measurement Information System; SDS: Sheehan Disability Scale; BADS: Behavioral Activation for Depression Scale.
p < .001,
p < .01,
p < .05.
3.3. Positive and negative valence systems
Positive affect and BAS Fun-Seeking scores increased across both treatments, while BIS scores decreased across both treatments. Contrary to expectations, a timepoint by treatment-arm interaction was observed for somatic fear (p = .014; not significant with a Bonferroni-corrected threshold of α = 0.007). A post hoc Welch’s t-test comparison indicated marginally greater declines in somatic fear in BA (t(79.95) = −2.78, p = .007) than in EXP (t(57.79) = −0.24, p = .812). The timepoint by treatment-arm interaction was observed at a trend level for BAS Drive (p = .062) and positive affect (p = .181), and not for the other measures (ps > 0.290). (Table S7, Fig. S7).
3.4. Treatment engagement and experience
Patient- and therapist-rated working alliance, as well as homework completion, increased across sessions; no main effects of treatment-arm on these measures were found. A timepoint by treatment-arm interaction was found for therapist-rated working alliance, reflecting greater therapist-rated working alliance in EXP than BA at Week 9 (t (33.64) = −2.31, p = .027), but not at Week 3 or Week 6 (ps > 0.280). A timepoint by treatment-arm interaction was also found for homework completion; examining timepoint effects in each treatment-arm separately revealed an overall steeper linear increase in EXP (β = 6.21, p < .001) than in BA (β = 3.02, p < .001). A main effect of treatment-arm on participant-rated perceived improvement reflected higher improvement ratings in BA compared to EXP (t(64.09) = 2.59, p = .012) (Table S6, Fig. S6).
4. Discussion
4.1. Behavioral activation and exposure were both effective for GAD
Our findings provide evidence for BA and EXP as effective behavioral interventions for GAD. The effect sizes corresponding to changes in GAD-7 scores observed in the current study (absolute values of Cohen’s d for post-treatment, 3-month and 6-month follow-ups: BA: 1.14, 2.23, 1.98; EXP: .97, 2.27, 2.13) are consistent with the large effects previously reported, including in a large meta-analysis examining various forms of psychotherapy for GAD, across treatment types and outcome measures [4]; seminal trials of cognitive-behavioral therapy for GAD delivered in group [42]; and individual [43] formats; and trials of GAD treatment using GAD-7 as an outcome measure, all of which involved internet-based cognitive-behavioral therapy [28–31]. Regarding the secondary outcome measures, large effects on PROMIS Anxiety, SDS, and BADS scores were observed in both treatment-arms at post-treatment and through follow-up timepoints. Of note, only a medium effect was observed on PROMIS Depression scores in EXP at post-treatment, whereas a large effect was observed in BA; however, effects were large at 3-month and 6-month follow-up for both treatments, suggesting continued improvement of depressive symptoms in the months following treatment for those who received EXP.
The hypothesis that BADS scores would increase in BA more than in EXP was not supported. Pre- to post-treatment increases in BADS scores were marginally greater in BA than in EXP, but this difference was not significant after correction for multiple comparisons, and was greatly reduced by 6-month follow-up. It is noteworthy that the BADS includes dimensions of avoidance and engagement. Accordingly, changes in avoidance can be attributed to the fact that both interventions explicitly target avoidance. While only BA has an explicit focus on activation, it is possible that EXP may have also led to increased activation through its focus on reducing avoidance.
It is noteworthy that the effect sizes reported in the current study reflect the effects of behavioral intervention techniques alone; treatment manuals instructed against the use of any of the cognitive techniques commonly employed in GAD treatment, such as cognitive restructuring or scheduling limited time for worry. While we cannot directly compare these interventions to other protocols, the large treatment effects reported here underscore the importance of behavioral techniques such as EXP and BA in GAD treatment.
By some measures, patients who received BA experienced greater symptom reductions than patients who received EXP, particularly in the later sessions of treatment. Compared to participants in EXP, those in BA demonstrated steeper declines in self-reported anxiety, depression, functional impairment, and behavioral activation tendencies; greater pre- to post-treatment differences in anxiety, depression, and behavioral activation tendencies; and a greater likelihood of clinically significant change in depression from pre- to post-treatment; as well as greater participant-rated perceived improvement across domains of functioning at post-treatment. At the 3-month follow-up timepoint, BA was associated with greater declines in PROMIS Depression and greater increases in BADS compared to EXP; by the 6-month follow-up timepoint, no differences between treatment-arms were observed. Of note, these findings did not appear to be driven by greater working alliance or homework completion in BA. Participants in EXP appeared to make continued gains following treatment, in line with learning theories of EXP, which postulate that post-treatment approach behavior can lead to continued symptom improvement [34,50]. In the present study, participants in EXP may have gleaned particular benefit from post-treatment approach behavior if it served to consolidate and enhance the corrective safety learning they acquired in treatment.
The finding that BA is at least as effective as EXP in treating GAD challenges the prevailing wisdom that exposure-based treatments and behavioral activation are separately indicated for anxiety and depressive disorders. Given the well-established link between anxiety and avoidance, we hypothesized that BA and EXP, which both aim to increase behavioral approach and reduce avoidance, would both be effective treatments for anxiety; in line with this, self-reported anxiety and avoidance declined in both BA and EXP.
To test the possibility that BA’s efficacy was limited to cases with comorbid depression, we conducted an exploratory analysis of symptom change in patients with and without depression. Symptom improvement was similar for those with and without depression in both treatment-arms. We are cautious to over-interpret these findings, given that they are likely underpowered due to the limited number of participants with active MDD in each treatment-arm (n = 13 in EXP, n = 18 in BA). However, these results provide initial evidence that BA may be effective in the treatment of GAD, even without comorbid depression.
4.2. Treatments exert broadly similar effects on positive and negative valence systems
Our predictions that BA would uniquely influence positive-valence systems and EXP would uniquely influence negative-valence systems were not fully supported. Both treatments increased positive affect and the fun-seeking aspect of behavioral approach, and both treatments decreased behavioral inhibition and the affective experience of fear. BA was associated with greater declines at a trend level in the somatic experience of fear compared to EXP, contrary to prediction. Of note, these ratings were only collected at pre- and post-treatment. Given that participants in EXP demonstrated continued reductions in anxiety symptoms following the termination of treatment, it is possible that patients in EXP also experienced continuing declines in somatic fear and increases in positive affect and drive following treatment that were not assessed at follow-up timepoints. In general, our findings demonstrate a striking similarity between the psychological effects of these two treatments. These findings also echo the results from a separate clinical trial, which demonstrated that negative- and positive-affect focused therapies were both effective in increasing positive affect and decreasing negative affect for individuals with depression and anxiety [51]. Positive affect is implicated as an important treatment target in interventions for anxiety disorders.
4.3. Limitations and future directions
Because the present study reports exploratory analyses from a larger study aimed at identifying neural predictors of treatment outcomes [52], some caution is warranted in interpreting the results. The present study did not include a control group (e.g., waitlist), given that the effectiveness of EXP for GAD has already been demonstrated [18,19]; however, this limits our ability to identify treatment-specific effects. In addition, the present study did not include a non-behavioral treatment-arm (e.g., cognitive, interpersonal, or supportive therapies), limiting our ability to draw conclusions as to the relative effects of behavioral interventions compared to other types of therapeutic approaches. The ability to stratify demographic and clinical characteristics across treatment-arms was limited due to therapies being conducted in groups for most of the study; participants were assigned to the next available group to minimize wait times. A significantly greater number of male participants received BA than EXP, and participants who received EXP reported marginally greater baseline symptom severity than those who received BA. In addition, the current study had a predominantly female (87%) and White (61%) participant sample, with Black, Hispanic, and Asian populations being significantly underrepresented and American Indian participants overrepresented; the generalizability of these findings to more diverse populations is unknown. Our decision to exclude participants with severe depression and/or suicidal intent or plan also limits the generalizability of findings. Finally, the present study was under-powered to detect small differences between treatment-arms. Replication of current findings in larger clinical trials that address the above limitations would strengthen conclusions about the relative efficacy of EXP and BA.
5. Conclusion
These findings represent a promising step toward improving treatment outcomes for GAD, by identifying two highly effective psychotherapeutic interventions. These behavioral interventions, which did not include any cognitive intervention techniques, were observed to have large effects on symptom outcomes. BA was effective regardless of the presence of comorbid depression. BA yielded some benefit over EXP through the later sessions of treatment and the three months following treatment, though the treatment effects were equivalent six months following treatment. Behavioral interventions that increase approach behavior, including BA and EXP, should be considered as important tools in the treatment of GAD.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Mental Health (grant number F31MH122090). The authors would like to acknowledge the recruitment and assessment team at the Laureate Institute for Brain Research; the therapists who were involved in implementing study interventions, particularly Jessica Santiago, M.Ed., LPC, and Sydnee Nelson Hunt, LCSW, LMFT; and those who provided fidelity ratings for the interventions, including Ruth Hermann-Dunn, PhD, Christine Hough, Allison Metts, Averi Gaines, Clara DeFontes, and Parker Longwell. We also acknowledge Jerzy Bodurka for his contribution as a consultant on the training grant that funded this study and for contributing to the fMRI study design. Lastly, we acknowledge the participants who volunteered their time to this study.
Abbreviations:
- BA
behavioral activation
- EXP
exposure-based therapy
- AAC
approach-avoidance conflict
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests, Robin Aupperle reports financial support was provided by National Institute of Mental Health. Christopher Martell reports receiving royalties for four books on the topic of behavioral activation. Martin Paulus has received royalties for an article about methamphetamine published in UpToDate. The other authors have no conflicts of interest to declare.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.xjmad.2023.100004.
References
- [1].Ruscio AM, Hallion LS, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry 2017;74:465–75. 10.1001/jamapsychiatry.2017.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andlin-Sobocki P, Wittchen H-U. Cost of anxiety disorders in Europe. Eur J Neurol 2005;12(Suppl 1):39–44. 10.1111/j.1468-1331.2005.01196.x [DOI] [PubMed] [Google Scholar]
- [3].Hoffman DL, Dukes EM, Wittchen H-U. Human and economic burden of generalized anxiety disorder. Depress Anxiety 2008;25:72–90. 10.1002/da.20257 [DOI] [PubMed] [Google Scholar]
- [4].Cuijpers P, Sijbrandij M, Koole S, Huibers M, Berking M, Andersson G. Psychological treatment of generalized anxiety disorder: A meta-analysis. Clin Psychol Rev 2014;34:130–40. 10.1016/j.cpr.2014.01.002 [DOI] [PubMed] [Google Scholar]
- [5].Hidalgo RB, Sheehan DV. Chapter 19 - Generalized anxiety disorder. In: Aminoff MJ, Boller F, Swaab DF, editors. Handb. Clin. Neurol. vol. 106. Elsevier; 2012. p. 343–62. 10.1016/B978-0-444-52002-9.00019-X [DOI] [PubMed] [Google Scholar]
- [6].Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry 2008;69:3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hollon SD, Stewart MO, Strunk D. Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annu Rev Psychol 2006;57:285–315. 10.1146/annurev.psych.57.102904.190044 [DOI] [PubMed] [Google Scholar]
- [8].American Psychiatric Association. (Author). Arlington VA, editor. Diagnostic and Statistical Manual of Mental Disorders. 5th ed.,2013. [Google Scholar]
- [9].Beesdo-Baum K, Jenjahn E, Höfler M, Lueken U, Becker ES, Hoyer J. Avoidance, safety behavior, and reassurance seeking in generalized anxiety disorder. Depress Anxiety 2012;29:948–57. 10.1002/da.21955 [DOI] [PubMed] [Google Scholar]
- [10].Mahoney AEJ, Hobbs MJ, Newby JM, Williams AD, Sunderland M, Andrews G. The Worry Behaviors Inventory: Assessing the behavioral avoidance associated with generalized anxiety disorder. J Affect Disord 2016;203:256–64. 10.1016/j.jad.2016.06.020 [DOI] [PubMed] [Google Scholar]
- [11].Borkovec TD, Alcaine OM, Behar E. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, editors. Gen. Anxiety Disord. Adv. Res. Pract. Guilford Press; 2004. p. 77–108. [Google Scholar]
- [12].Sibrava NJ, Borkovec TD. The cognitive avoidance theory of worry. In: Davey G, Wells A, editors. Worry Its Psychol. Disord. Theory Assess. Treat. Chichester, England; Hoboken, NJ: Wiley; 2006. p. 239–56. [Google Scholar]
- [13].Roemer L, Salters K, Raffa SD, Orsillo SM. Fear and avoidance of internal experiences in GAD: Preliminary tests of a conceptual model. Cogn Ther Res 2005;29:71–88. 10.1007/s10608-005-1650-2 [DOI] [Google Scholar]
- [14].Newman MG, Llera SJ. A novel theory of experiential avoidance in generalized anxiety disorder: A review and synthesis of research supporting a contrast avoidance model of worry. Clin Psychol Rev 2011;31:371–82. 10.1016/j.cpr.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB. Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp 2015;36:449–62. 10.1002/hbm.22639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kirlic N, Young J, Aupperle RL. Animal to human translational paradigms relevant for approach avoidance conflict decision making. Behav Res Ther 2017;96:14–29. 10.1016/j.brat.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Norton PJ, Price EC. A meta-analytic review of adult cognitive-behavioral treatment outcome across the anxiety disorders. J Nerv Ment Dis 2007;195:521–31. 10.1097/01.nmd.0000253843.70149.9a [DOI] [PubMed] [Google Scholar]
- [18].Butler G, Fennell M, Robson P, Gelder M. Comparison of behavior therapy and cognitive behavior therapy in the treatment of generalized anxiety disorder. J Consult Clin Psychol 1991;59:167–75. 10.1037//0022-006x.59.1.167 [DOI] [PubMed] [Google Scholar]
- [19].Hoyer J, Beesdo K, Gloster AT, Runge J, Höfler M, Becker ES. Worry exposure versus applied relaxation in the treatment of generalized anxiety disorder. Psychother Psychosom 2009;78:106–15. 10.1159/000201936 [DOI] [PubMed] [Google Scholar]
- [20].Cuijpers P, van Straten A, Warmerdam EH. Behavioral treatment of depression: A meta-analysis of activity scheduling. Clin Psychol Rev 2007;27:318–26. 10.1016/j.cpr.2006.11.001 [DOI] [PubMed] [Google Scholar]
- [21].Mazzucchelli T, Kane R, Rees C. Behavioral activation treatments for depression in adults: A meta-analysis and review. Clin Psychol Sci Pr 2009;16:383–411. 10.1111/j.1468-2850.2009.01178.x [DOI] [Google Scholar]
- [22].Boswell JF, Iles BR, Gallagher MW, Farchione TJ. Behavioral activation strategies in cognitive-behavioral therapy for anxiety disorders. Psychotherapy 2017;54:231–6. 10.1037/pst0000119 [DOI] [PubMed] [Google Scholar]
- [23].Hopko DR, Lejuez CW, Hopko SD. Behavioral activation as an intervention for coexistent depressive and anxiety symptoms. Clin Case Stud 2004;3:37–48. 10.1177/1534650103258969 [DOI] [Google Scholar]
- [24].Stein AT, Carl E, Cuijpers P, Karyotaki E, Smits JAJ. Looking beyond depression: a meta-analysis of the effect of behavioral activation on depression, anxiety, and activation. Psychol Med 2021;51:1491–504. 10.1017/S0033291720000239 [DOI] [PubMed] [Google Scholar]
- [25].Taheri H, Taheri E, Amiri M. Efficacy of group behavioral activation on social anxiety, avoidance and negative evaluations among individuals whit social anxiety. J Fundam Ment Health 2017;19:361–5. 10.22038/jfmh.2017.9057 [DOI] [Google Scholar]
- [26].Etherton JL, Farley R. Behavioral activation for PTSD: A meta-analysis. Psychol Trauma Theory Res Pr Policy 2022;14:894–901. 10.1037/tra0000566 [DOI] [PubMed] [Google Scholar]
- [27].Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- [28].Dear BF, Titov N, Sunderland M, McMillan D, Anderson T, Lorian C, et al. Psychometric comparison of the Generalized Anxiety Disorder Scale-7 and the Penn State Worry Questionnaire for measuring response during treatment of generalised anxiety disorder. Cogn Behav Ther 2011;40:216–27. 10.1080/16506073.2011.582138 [DOI] [PubMed] [Google Scholar]
- [29].Dear BF, Staples LG, Terides MD, Karin E, Zou J, Johnston L, et al. Transdiagnostic versus disorder-specific and clinician-guided versus self-guided internet-delivered treatment for generalized anxiety disorder and comorbid disorders: A randomized controlled trial. J Anxiety Disord 2015;36:63–77. 10.1016/j.janxdis.2015.09.003 [DOI] [PubMed] [Google Scholar]
- [30].Johnston L, Titov N, Andrews G, Spence J, Dear BF. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: Examination of support roles and disorder-specific outcomes. PLOS ONE 2011;6:e28079 10.1371/journal.pone.0028079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mewton L, Wong N, Andrews G. The effectiveness of internet cognitive behavioural therapy for generalized anxiety disorder in clinical practice. Depress Anxiety 2012;29:843–9. 10.1002/da.21995 [DOI] [PubMed] [Google Scholar]
- [32].Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, et al. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J Clin Epidemiol 2016;73:119–27. 10.1016/j.jclinepi.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol 1996;11:89–95. 10.1097/00004850-199606003-00015 [DOI] [PubMed] [Google Scholar]
- [34].Manos RC, Kanter JW, Luo W. The Behavioral Activation for Depression Scale–Short Form: Development and validation. Behav Ther 2011;42:726–39. 10.1016/j.beth.2011.04.004 [DOI] [PubMed] [Google Scholar]
- [35].Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol 1994;67:319–33. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- [36].Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology 2013;80:S2–6. 10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, et al. Emotion assessment using the NIH Toolbox. Neurology 2013;80:S76–86. 10.1212/WNL.0b013e3182872e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kazantzis N, Deane FP, Ronan KR. Assessing compliance with homework assignments: Review and recommendations for clinical practice. J Clin Psychol 2004;60:627–41. 10.1002/jclp.10239 [DOI] [PubMed] [Google Scholar]
- [39].Miller SD, Duncan BL, Brown J, Sparks JA, Claud DA. The Outcome Rating Scale: A preliminary study of the reliability, validity, and feasibility of a brief visual analog measure. J Brief Ther 2003;2:91–100. [Google Scholar]
- [40].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:11980. [PubMed] [Google Scholar]
- [42].Dugas MJ, Ladouceur R, Léger E, Freeston MH, Langolis F, Provencher MD, et al. Group cognitive-behavioral therapy for generalized anxiety disorder: Treatment outcome and long-term follow-up. J Consult Clin Psychol 2003;71:821–5. 10.1037/0022-006X.71.4.821 [DOI] [PubMed] [Google Scholar]
- [43].Ladouceur R, Dugas MJ, Freeston MH, Léger E, Gagnon F, Thibodeau N. Efficacy of a cognitive-behavioral treatment for generalized anxiety disorder: evaluation in a controlled clinical trial. J Consult Clin Psychol 2000;68:957–64. [PubMed] [Google Scholar]
- [44].Arch JJ, Eifert GH, Davies C, Vilardaga JCP, Rose RD, Craske MG. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol 2012;80:750–65. 10.1037/a0028310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hayes-Skelton SA, Roemer L, Orsillo SM. A randomized clinical trial comparing an acceptance-based behavior therapy to applied relaxation for generalized anxiety disorder. J Consult Clin Psychol 2013;81:761–73. 10.1037/a0032871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stefan S, Cristea IA, Szentagotai Tatar A, David D. Cognitive-behavioral therapy (CBT) for generalized anxiety disorder: Contrasting various CBT approaches in a randomized clinical trial. J Clin Psychol 2019;75:1188–202. 10.1002/jclp.22779 [DOI] [PubMed] [Google Scholar]
- [47].Core Team RR: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing,; 2021. [Google Scholar]
- [48].Pinheiro J, Bates D. R Core Team. nlme. Linear Nonlinear Mixed Eff Models 2022. [Google Scholar]
- [49].Goodrich B, Gabry J rstanarm. Bayesian Appl Regres Model via Stan 2022. [Google Scholar]
- [50].Wolitzky-Taylor KB, Horowitz JD, Powers MB, Telch MJ. Psychological approaches in the treatment of specific phobias: A meta-analysis. Clin Psychol Rev 2008;28:1021–37. 10.1016/j.cpr.2008.02.007 [DOI] [PubMed] [Google Scholar]
- [51].Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, Rosenfield D. Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol 2019;87:457–71. 10.1037/ccp0000396 [DOI] [PubMed] [Google Scholar]
- [52].Santiago J, Akeman E, Kirlic N, Clausen AN, Cosgrove KT, McDermott TJ, et al. Protocol for a randomized controlled trial examining multilevel prediction of response to behavioral activation and exposure-based therapy for generalized anxiety disorder. Trials 2020;21:17. 10.1186/s13063-019-3802-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Arch JJ, Ayers CR, Baker A, Almklov E, Dean DJ, Craske MG. Randomized clinical trial of adapted mindfulness-based stress reduction versus group cognitive behavioral therapy for heterogeneous anxiety disorders. Behav Res Ther 2013;51:185–96. 10.1016/j.brat.2013.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


