Abstract
Use of glyburide in gestational diabetes (GDM) has raised concerns about fetal and neonatal side effects, including increased birth weight. Placental nutrient transport is a key determinant of fetal growth, however the effect of glyburide on placental nutrient transporters is largely unknown. We hypothesized that glyburide treatment in GDM pregnancies is associated with increased expression of nutrient transporters in the syncytiotrophoblast plasma membranes.
We collected placentas from GDM pregnancies who delivered at term and were treated with either diet modification (n = 15) or glyburide (n = 8). Syncytiotrophoblast microvillous (MVM) and basal (BM) plasma membranes were isolated and expression of glucose (glucose transporter 1; GLUT1), amino acid (sodium-coupled neutral amino acid transporter 2; SNAT2 and L-type amino acid transporter 1; LAT1) and fatty acid (fatty acid translocase; FAT/CD36, fatty acid transporter 2 and 4; FATP2, FATP4) transporters was determined by Western blot. Additionally, we determined GLUT1 expression by confocal microscopy in cultured primary human trophoblasts (PHT) after exposure to glyburide.
Birth weight was higher in the glyburide-treated group as compared to diet-treated GDM women (3764 ± 126 g vs. 3386 ± 75 g; p < 0.05). GLUT1 expression was increased in both MVM (+50%; p < 0.01) and BM (+75%; p < 0.01). In contrast, MVM FAT/CD36 (−65%; p = 0.01) and FATP2 (−65%; p = 0.02) protein expression was reduced in mothers treated with glyburide. Glyburide increased membrane expression of GLUT1 in a dose-dependent manner in cultured PHT.
This data is the first to show that glyburide increases GLUT1 expression in syncytiotrophoblast MVM and BM in GDM pregnancies, and may promote transplacental glucose delivery contributing to fetal overgrowth.
Keywords: Glyburide, Placenta, Fetal growth, Pregnancy, Glucose transport, Syncytiotrophoblast
1. Introduction
Currently there is considerable controversy about the use of oral agents to treat hyperglycemia in women with gestational diabetes mellitus (GDM). Concomitant with the progressive increase in maternal pregnancy body mass index (BMI) [1], GDM has become a growing public health concern due to the rapidly rising rates (10–25%) [2,3] of women being diagnosed with GDM [4] in obstetric practices adopting the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria [5–8]. GDM is associated with poor infant outcomes, including fetal overgrowth, and increases the risk for the mother as well as the infant to develop metabolic and cardiovascular disease later in life [9,10]. GDM is currently treated by diet modification, oral insulin secretagogues or insulin. The use of oral insulin secretagogues as an adjunct therapy in GDM has been addressed by several guidelines [11,12], and are preferred over insulin by most obstetric providers [13].
Glyburide (a second-generation sulfonylurea; international nonproprietary name: glibenclamide) is the only sulfonylurea that has been studied in large randomized control trials in GDM women [3,14,15] and was approved as a possible alternative to insulin in GDM women [11]. However, recent retrospective studies and meta-analyses have challenged the efficacy and relative safety of glyburide. These reports suggest that macrosomia, neonatal hypoglycemia, and adverse neonatal outcomes are more common in women treated with glyburide compared to insulin and/or metformin [3,16]. Glyburide has a relatively short half-life in the circulation and early studies suggested that transplacental glyburide transport was negligible [15,17]. More recently, the use of methods with increased sensitivity has revealed that transplacental transfer of glyburide, albeit variable, does occur, with average neonatal glyburide levels below 10 ng/ml [18,19]. However, the effects of glyburide on placental function have received little attention [20].
Glyburide is considered primarily an insulin secretagogue and induces glucose-independent insulin release from pancreatic β-cells by inhibiting potassium flux through ATP-sensitive potassium channels (KATP) which are widely distributed throughout the body. However, sulfonylureas also have extra-pancreatic effects by stimulating glucose uptake, lipogenesis, glycogenesis, and stimulating the expression and translocation of glucose transporters (GLUT) [21,22]. Furthermore, in chondrocytes, KATP inhibition increases GLUT1 and GLUT3 expression [23].
KATP are expressed in whole placental homogenates and have been localized to the syncytiotrophoblast [20]. Thus, a mechanism completely unexplored at this time is the potential direct effect of glyburide on placental glucose transport, given KATP are inhibited by sulfonylureas and are localized to the syncytiotrophoblast which could increase the membrane abundance of glucose transporters, as in other organs. In term placenta, glucose transport is mainly mediated by GLUT1, a facilitative glucose transporter, expressed in the syncytiotrophoblast microvillous (MVM) and basal (BM) plasma membranes, the transporting epithelium of the human placenta [24,25].
Nutrient transport is a primary function of the syncytiotrophoblast and determines fetal nutrient availability, which in turn has a major impact on fetal growth. Changes in placental glucose, amino acid and fatty acid transporter expression and activity have been associated with pathological fetal growth [26]. Indeed, an increased placental capacity to transport amino acids has been shown in women with Type 1 Diabetes mellitus and fetal overgrowth [27,28]; and system A isoform sodium-coupled neutral amino acid transporter 2 (SNAT2) has been reported to be positively correlated to birth weight and upregulated in MVM isolated from placentas of obese women [29]. Furthermore, in trophoblast isolated from placentas from GDM mothers, L-type amino acid transporter 1 (LAT1) exhibited a greater contribution to the uptake of 14C-l-methionine [30]. Recently, expression of fatty acid transporter 2 (FATP2), a protein that mediates transplacental transport of fatty acids, was demonstrated to be increased in placentas of obese women [31].
Early studies on glyburide and other sulfonylureas focused on their ability to cross the placenta. However, almost nothing is known about a possible direct effect of glyburide on placental nutrient transporter expression. To address this question, we studied placentas from GDM pregnancies treated with either diet modification or glyburide. We isolated syncytiotrophoblast plasma membranes to test the hypothesis that the use of glyburide in GDM pregnancy is associated with increased expression of nutrient transporters in the syncytiotrophoblast plasma membranes. In addition, we used cultured primary human trophoblasts (PHT) to determine GLUT1 membrane transporter expression and localization after exposure to glyburide in vitro.
2. Materials and methods
2.1. Study participants
Informed written consent was obtained from all participants for placenta collection and access to their protected health information as approved by the Institutional Review Board at University of Texas Health Science Center San Antonio (HSC20100262H). GDM was diagnosed by the two step method of a 50 g glucose challenge which if abnormal (plasma glucose >130 mg/dL) was followed by a 100 g 3 h oral glucose tolerance test (3 h OGTT) with at least two abnormal plasma glucose measurements (≥95, 180, 155, and 140 mg/dL at fasting, 1, 2, and 3 h, respectively) at 24–28 weeks gestation per ACOG (American Congress of Obstetricians and Gynecologists) criteria [11,32]. Participants were stratified as GDM controlled by diet modification (Diet; A1GDM) or GDM treated with glyburide (Glyburide). The average glyburide dose was 6 mg per day (range 5.0–12.5 mg/day). Additional inclusion criteria included: pre-pregnancy/early pregnancy BMI range 18–45 kg/m2; ultrasound confirmation of gestational age at 14–18 weeks; singleton pregnancy; maternal age 18–45 years. Exclusion criteria were: pre-existing diabetes (Type 1 and Type 2 Diabetes mellitus), rheumatologic, hypertensive and cardiovascular disease, and assisted reproductive technology; current use of tobacco, street drugs, or medications (including corticosteroids); fetal malformations, or pre-term delivery (before 37 weeks). Table 1 summarizes the maternal and neonatal characteristics of the two groups. The mean maternal BMI was similar between groups. Z-scores for birth weight were calculated for each fetal sex and based on data for Mexican-American pregnancies [33] which was the predominant ethnicity of the participants. Z-scores for birth weight in White-Non-Hispanic pregnancies was calculated using the Fenton curve [34].
Table 1.
Clinical characteristics of study participants.
| Variable | Diet | Glyburide | p value |
|---|---|---|---|
| n | 15 | 8 | |
| Ethnicity (% Hispanic) | 93 | 100 | |
| Maternal BMI (kg/m2) | 30.3 ± 1.36 | 31.5 ± 2.71 | 0.219 |
| Gestational age (weeks) | 39.0 ± 0.32 | 39.1 ± 0.25 | 0.835 |
| Birth weight (g) | 3386 ± 74.77 | 3764 ± 126.40 | 0.012 |
| OGTT (mmol/L; 1 h/2 h/3 h) | 186.8/176.7/145.7 | 187.4/170.1/139.0 | 0.722/0.974/1.000 |
| Z-score (females) | 0.105 ± 0.271 | 0.578 ± 0.543 | 0.606 |
| Z-score (males) | −0.363 ± 0.235 | 0.520 ± 0.322 | 0.052 |
| Body length (cm) | 50.7 ± 0.28 | 51.9 ± 0.82 | 0.109 |
| Gender (%female) | 67 | 25 | |
| Delivery mode (C-section/vaginal) | 6/9 | 7/1 | |
| Placental weight (g) | 786.6 ± 44.24 | 826.3 ± 63.66 | 0.267 |
Selected clinical data. Values are mean ± SEM. OGTT: 3-h oral glucose tolerance test. Statistical significance was determined using Student's unpaired t-test.
BMI was calculated from self-reported pre-pregnancy body weight and length or obtained in early pregnancy.
2.2. Placenta collection and processing
Placentas from GDM pregnancies delivering at term (≥37 weeks gestation) were collected immediately after delivery and processed on ice. Approximately 100 g of villous tissue were rinsed in ice-cold physiological saline, transferred to ice-cold buffer D (250 mmol/L sucrose, 10 mmol/L HEPES; pH 7.4), containing protease and phosphatase inhibitors (Sigma-Aldrich; St Louis, MO) and homogenized on ice. Placental homogenates were frozen in liquid nitrogen and stored at −80 °C until further processing.
2.3. Isolation of microvillous and basal plasma membranes
Isolation of syncytiotrophoblast MVM and BM was performed according to established protocol [35]. Briefly, after initial separation of cellular debris, the MVM was purified by MgCl2-precipitation and BM was purified by sucrose gradient centrifugation. Isolated MVM and BM vesicles were snap-frozen in liquid nitrogen and stored at −80 °C in buffer D containing protease and phosphatase inhibitors. Alkaline phosphatase activity, a measurement of MVM enrichment, was 15.3 ± 0.8 fold enriched compared to placental homogenate; and ferroportin-1 protein expression, a BM marker, was 36.0 ± 8.0 fold enriched compared to homogenates. There was no difference in MVM or BM enrichments between the two groups.
2.4. Immunoblotting analysis
MVM or BM proteins (1–20 μg) were separated by SDS-PAGE on pre-cast gels (Bio-Rad, Hercules, CA) and transferred to poly-vinylidene fluoride membranes (at 30 V constant, overnight at 4 °C). Membranes were stained with Amido Black (Sigma-Aldrich) for loading normalization. Blocking was carried out for 1 h at room temperature in 5% nonfat milk in Tris-buffered saline (TBS)-Tween, and membranes were incubated in primary antibody diluted in 1–3% BSA in TBS-Tween. Protein expression of GLUT1 (Millipore; Billerica, MA), fatty acid translocase (FAT/CD36), FATP2 and FATP4 (AbCam, Cambridge, MA), SNAT2 (a generous gift from Dr. Prasad at University of Georgia, Augusta) and LAT1 (Cell Signaling, Beverly, MA) was determined in MVM and BM. Horseradish peroxidase-conjugated secondary anti-rabbit (Cell Signaling) was used and immunolabeling was visualized with enhanced chemiluminescent detection solution (Thermo Scientific, Waltham, MA) and a G:BOX Chemi XT4 gel imaging system (Syngene, Cambridge, United Kingdom). Densitometry was performed using ImageJ software (http://rsbweb.nih.gov/ij/; National Institutes of Health). For GLUT1, target protein expression in each individual lane was normalized to β-actin (Sigma-Aldrich) and for the remaining targets, total protein loaded was determined by Amido Black staining.
2.5. Isolation, culture and treatment of primary human trophoblasts
Primary human trophoblasts (PHT) were isolated from term placentas using an adaptation of the Kliman method [36], as previously described [37]. Briefly, placental villous tissue was digested in trypsin (Invitrogen, Carlsbad, CA) and DNase (Sigma-Aldrich). The supernatant was layered onto newborn calf serum and spun for 10 min at 2200 rev/min (1000 × g) at 20 °C. Pellets were resuspended in DMEM (Sigma-Aldrich) and layered onto a discontinuous Percoll density gradient and centrifuged for 30 min at 2800 rev/min (1500 × g). The bands between 35 and 55% Percoll were collected and mixed with cell culture medium (DMEM: Ham’s F-12 Nutrient Mixture (Invitrogen) 1:1, 10% FBS (Atlanta Biologicals, Lawrenceville, GA), 1% gentamicin, 0.2% benzylpenicillin, 0.2% streptomycin, 0.6% glutamine) and centrifuged at 2200 rev/min for 10 min. The final pellet was resuspended in cell culture medium and plated at 37 °C, 95% air/5% CO2 onto chamber slides (Lab-Tek; Waltham, MA) at a cell density of 0.5 × 106. Following 18 h of culture, attached cytotrophoblasts were washed Dulbecco’s phosphate-buffered saline (PBS) and culture media was changed daily. At 66 h (total culture time), PHT were treated with 10, 20 or 50 μmol/L glyburide (glibenclamide; Sigma-Aldrich) or vehicle control (DMSO; max. concentration in culture medium 0.1%; untreated) for 24 h.
2.6. Measurement of lactate dehydrogenase
Lactate dehydrogenase (LDH) release into PHT-conditioned culture medium, an indicator of cell viability, was measured using a LDH Cytotoxicity Assay kit (Thermo Scientific) according to the instructions of the manufacturer. LDH release from PHT was compared to maximal LDH release using an internal positive control provided with the kit.
2.7. Immunofluorescence and confocal microscopy
At 90 h, PHT were fixed in 4% paraformaldehyde for 10 min at room temperature and blocked using 5% BSA in PBS for 1 h followed by anti-GLUT1 incubation for 1 h, and secondary goat anti-rabbit Alexa Fluor 633 (Invitrogen). An internal negative control was obtained by omission of the primary antibody. After washing with PBS-Tween, mounting was performed with ProLong-GOLD with DAPI (Thermo Scientific). Immunofluorescent images were captured using a Zeiss LSM 780 microscope at 63× magnification using oil immersion. Images were captured in the same laser settings with four Z-stack at 1 μm-step interval. Analysis of representative images was performed using Imaris software (Bitplane, Concord, MA). Channel-1 (GLUT1 staining), and the mean data intensity (maximun and minimun data intensity) was normalized to data volume (μm3).
2.8. Data presentation and statistical analysis
Data are presented as mean ± SEM or + SEM, n represents number of individual placentas studied. Statistical analysis and plotting was performed using GraphPad Prism version 6 (GraphPad Software, Inc. La Jolla, CA). Gaussian distribution of the samples was determined by D’Agostino and Pearson omnibus normality test and statistical significance between Diet and Glyburide groups was determined by Student’s unpaired t-test. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Increased birth weights in glyburide treated group
Maternal BMI and gestational age were comparable between glyburide versus diet groups (Table 1). However, birth weight was significantly increased in GDM mothers treated with glyburide compared to those treated with diet (Table 1; p = 0.012), in agreement with previous reports [38,39]. To adjust for gestational age and fetal sex, we calculated a birth weight Z-score. Z-score calculations revealed that one newborn male in the glyburide group (Z-score 1.403) and one female in the diet group (Z-score 1.530) were large for gestational age (LGA), and we found a strong trend towards a significant difference between Z-scores in the glyburide and the diet groups (p = 0.052) for male newborns. 3 h OGTT measurement results were comparable; and birth length and placental weights did not differ between groups.
3.2. Increased MVM and BM GLUT1 expression in glyburide treated group
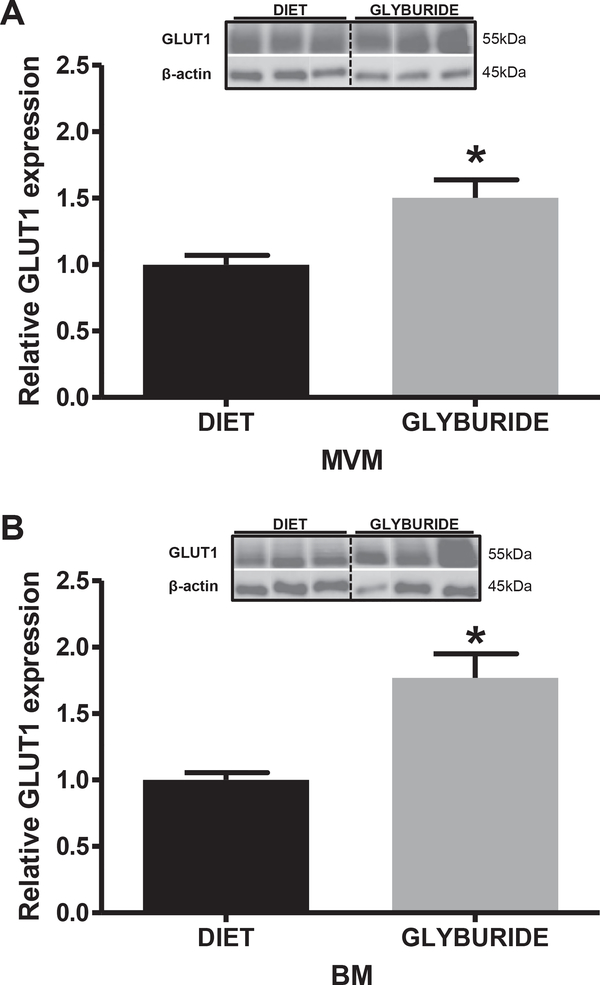
Protein expression of GLUT1 was assessed in isolated MVM and BM from syncytiotrophoblast of glyburide and diet-treated mothers. GLUT1 protein expression was significantly increased in placentas from glyburide treated GDM pregnancies in both MVM (+50%; p = 0.0015; Fig. 1A) and BM (+75%; p < 0.0001; Fig. 1B) compared to placentas from diet-treated GDM mothers.
Fig. 1.
GLUT1 expression in purified syncytiotrophoblast membranes isolated from placentas of GDM women treated with diet or glyburide. Representative immunoblots with corresponding histograms illustrating relative protein expression of GLUT1 in syncytiotrophoblast MVM (A) and BM (B) isolated from placentas from diet and glyburide treated groups. Data are mean + SEM; n = 15/diet; n = 8/glyburide. Statistical significance was determined using Student’s unpaired t-test, *p < 0.01.
3.3. Unaltered placental expression of amino acid transporters between diet and glyburide treated group
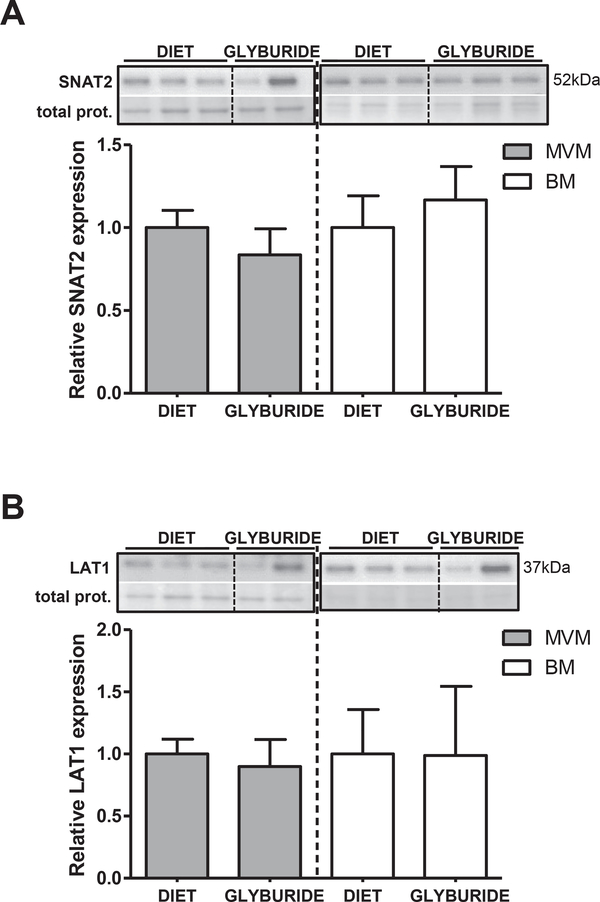
Protein expression of amino acid transporters SNAT2 and LAT1 was assessed in MVM and BM from syncytiotrophoblast isolated from placentas of glyburide or diet-treated mothers. SNAT2 (Fig. 2A) and LAT1 (Fig. 2B) protein expression levels were comparable between groups (Fig. 2A–B).
Fig. 2.
Amino acid transporter expression in purified syncytiotrophoblast membranes isolated from placentas of GDM women treated with diet or glyburide. Representative immunoblots with corresponding histograms illustrating relative protein expression of SNAT2 (A) and LAT1 (B) in syncytiotrophoblast MVM and BM isolated from placentas from diet and glyburide groups. Data are mean + SEM; n = 15/diet; n = 8/glyburide. Statistical significance was determined using Student’s unpaired t-test.
3.4. Reduced MVM fatty acid transporter expression in GDM mothers treated with glyburide
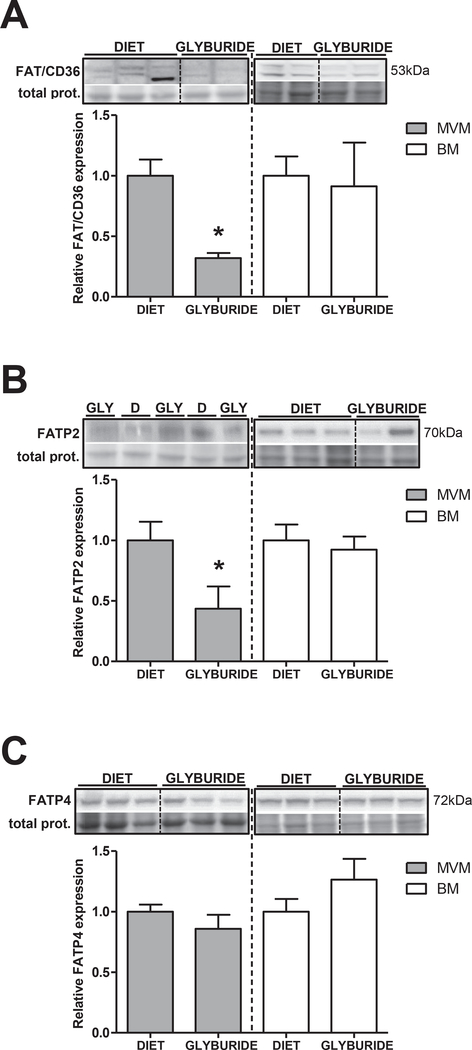
Expression of fatty acid transport proteins FAT/CD36, FATP2 and FATP4 was assessed in purified syncytiotrophoblast MVM and BM isolated from placentas of glyburide and diet-treated mothers. FAT/ CD36 and FATP2 protein expression were significantly reduced (−65%; p = 0.01 and −65%; p = 0.02 respectively) in MVM from the glyburide group (Fig. 3A and B respectively), compared to the diet group. In contrast, protein expression of FAT/CD36 and FATP2 was unaltered in syncytiotrophoblast BM. FATP4 protein was expressed in both MVM and BM and levels were comparable between placentas from glyburide and diet-treated mothers (Fig. 3C).
Fig. 3.
Fatty acid transporter expression in purified syncytiotrophoblast membranes isolated from placentas from diet (D) and glyburide treated (GLY) mothers. Representative immunoblots with corresponding histograms illustrating relative protein expression of FAT/CD36 (A), FATP2 (B) and FATP4 (C) in syncytiotrophoblast MVM and BM isolated from placentas from diet and glyburide groups. Data are mean + SEM; n = 15/diet; n = 8/glyburide. Statistical significance was determined using Student’s unpaired t-test, *p < 0.05.
3.5. Glyburide promotes GLUT1 translocation to the plasma membrane in PHT
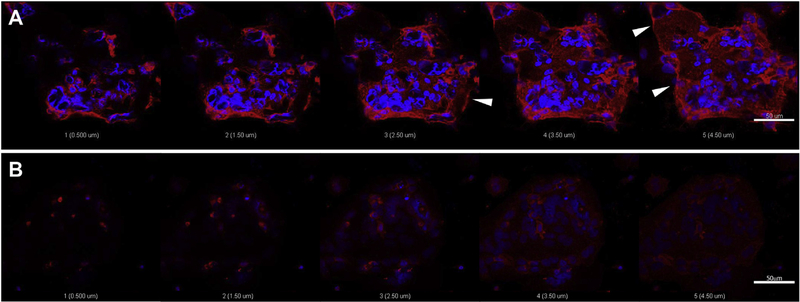
PHT were isolated from normal term healthy placentas and cultured for 90 h. GLUT1 protein expression was confirmed at 90 h using immunofluorescent staining with an antibody which detects the C-terminus of human GLUT1. Pixel intensity mean per volume (μm3) analysis, calculated for each of the concentrations of glyburide used demonstrated that treatment with 10, 20 and 50 μmol/L glyburide in vitro for 24 h resulted in progressively increased immunofluorescent staining of GLUT1, compared to untreated control cells (Suppl. Fig.1). Specifically, 50 μmol/L glyburide treatment for 24 h resulted in maximun GLUT1 immunostaining intensity without affecting cell viability (Suppl. Fig. 2), and exhibited increased GLUT1 localization to the plasma membrane of PHT (Fig. 4A) compared to vehicle treated cells (Fig. 4B). Z-stack images at 1 μm step interval revealed localized GLUT1 staining at the PHT plasma membrane, arrows indicate representative staining associated with the plasma membrane/cell surface (Fig. 4A). GLUT1 staining intensity was markedly lower in untreated PHT (Fig. 4B).
Fig. 4.
Representative confocal laser scanning microscopy images showing dual immunofluorescent staining for GLUT1 protein (red) and nuclear counterstain (blue) in PHT at 90 h. PHT were treated for 24 h with 50 μmol/L glyburide (A) or were untreated (B). 1 μm step interval Z-stack images were captured at 63× (A–B); scale bar 50 μm. Arrows indicate GLUT1 staining associated with PHT cell surface (A).
4. Discussion
Glyburide treatment for GDM in this small cohort was associated with increased birth weight as previously described [38,39]. We report, for the first time, that glyburide treatment of GDM pregnancies was associated with increased GLUT1 expression in syncytiotrophoblast MVM and BM. Additionally, women receiving glyburide treatment demonstrated a significant reduction of fatty acid transporter expression in MVM with no change in the BM. Furthermore, 24 h glyburide treatment in cultured PHT increased GLUT1 expression and localization to the plasma membrane. We propose that the increase in GLUT1 expression in MVM and BM in GDM pregnancies treated with glyburide directly contributes to transplacental glucose delivery, and provides a novel explanation for accelerated fetal growth in these pregnancies.
Fetal growth is dependent on fetal nutrient availability, which is determined by the capacity of the placenta to transport nutrients. The syncytiotrophoblast is a highly specialized multinucleated epithelial cell layer covering the surface of the chorionic villi that mediates nutrient transport; it is strategically positioned at the maternal-fetal interface, therefore regulating nutrient supply to the fetus [26]. Glucose transfer across the human placenta occurs by facilitated diffusion, which at term is primarily mediated by GLUT1. GLUT1 is asymmetrically distributed in the syncytiotrophoblast, expressed at several-fold higher levels in the MVM (maternal facing) as compared to than BM (fetal-facing) [24,25]. The BM is therefore believed to be the rate-limiting step for transplacental glucose transport [40], and changes in BM glucose transporter expression will have significant consequences for the maternal-fetal transfer of glucose and fetal growth. There is evidence that GLUT1 expression is increased in the BM of pregnancies complicated by Type 1 Diabetes mellitus and fetal overgrowth [41,42], favoring glucose transfer to the fetus.
We report, for the first time, that glyburide treatment of GDM mothers significantly increased expression of GLUT1 compared to GDM women treated with diet modification in both MVM and BM of the syncytiotrophoblast. Factors determining net glucose transfer include the maternal-fetal concentration gradient and the transport capacity of the exchange area determined by the density of GLUT1 transporters. The increased expression of GLUT1 in the syncytiotrophoblast MVM and BM in the glyburide treated group reported in this study is therefore expected to increase the functional capacity of the exchange barrier to transport glucose and the net flux of glucose according to the prevailing maternal-fetal gradient. This suggests that, glyburide may directly enhance net placental transfer of glucose to the fetus contributing to increased birth weight.
Glibenclamide (glyburide) has been an attractive alternative to insulin treatment in GDM given it is the only sulfonylurea that has been studied in large randomized controlled trials in women with GDM, and transplacental transfer is less than metformin. However, a retrospective cohort study in 9173 women treated with glyburide or insulin demonstrated that newborns treated with glyburide were more likely to experience adverse outcomes than those treated with insulin [3], and a recent meta-analysis suggested that glyburide was inferior to insulin and treatment failures were higher with metformin alone than glyburide alone [16]. It has been postulated that this could be due to transplacental transfer of glyburide which could result in fetal hyperinsulinemia and excess growth [13,18]. Glyburide is transported across the placenta mediated by drug efflux systems expressed in the syncytiotrophoblast, in particular by breast cancer resistance proteins [43], multidrug resistance proteins and, to a lesser extent, through P-glycoprotein [44]. However, the relationship between placental glyburide transport and effects of glyburide on placental function is unclear. Moreover, there are no previous studies of the direct effect of glyburide on placental nutrient transport. Our data supports a direct effect of glyburide on the placenta by altering the delivery of nutrients to the fetus, but the mechanism by which glyburide is exerting these effects remains unexplored.
To test causality, we performed in vitro experiments using cultured placental trophoblasts treated with different doses of glyburide to determine the translocation of GLUT1 to the plasma membrane. We used a range of glyburide doses (10, 20 or 50 μmol/ L), previously tested in vitro in placental models [20,45], which did not induce cellular toxicity (as measured by PHT LDH release). Our results demonstrated that in vitro, glyburide treatment induced a dose-dependent increase in GLUT1 immunostaining in PHT. Indeed, evaluation of GLUT1 staining by confocal microscopy using 1 μm Z-stack step intervals confirmed specific plasma membrane localization, compared to untreated cells. The redistribution of GLUT1 to the plasma membrane was dose-dependent with the lower glyburide doses tested (10 and 20 μmol/L) exhibiting less intense immunostaining (data not shown). These experiments provide indirect support for the concept that glyburide, rather than more severe hyperglycemia, causes the up-regulation in MVM and BM GLUT1 expression observed in GDM women treated with glyburide. Additionally, previous studies from Illsley and coworkers [46] show that glucose transport activity and, to a lesser extent, glucose transporter expression are down-regulated in choriocarcinoma cells and placental villus explants in response to hyperglycemia supporting that the increased translocation of GLUT1 to the plasma membrane reported in this study was due to glyburide and not hyperglycemia. Furthermore, the 3 h OGTT values were comparable between clinical groups, providing further support that hyperglycemia is not the cause of the increased GLUT1 expression in the glyburide treated group. Collectively, these data suggest that the increased translocation of GLUT1 to the syncytiotrophoblast plasma membrane in the glyburide treated group is an effect of glyburide rather than metabolic differences between the two groups.
It is well established that glyburide inhibits KATP [47]. In the placenta, KATP has been previously shown to be localized to the syncytiotrophoblast [20]. While KATP have been functionally linked to pancreatic β-cell insulin secretion [48], KATP appears to regulate the abundance of GLUT1 and GLUT3 in chondrocytes [23] and GLUT1 in adipocytes [49]. Whether glyburide has a similar effect in the placenta is unknown. We hypothesized that glyburide modulates expression and localization of GLUT1 to the MVM and BM and found increased expression in the placental plasma membranes of GDM women treated with glyburide compared to diet alone. Our in vitro data is consistent with a cause-and-effect relationship between glyburide and the observed increase in expression of GLUT1 in the MVM and BM of glyburide treated GDM mothers. We speculate that glyburide, through its pharmacological inhibition of syncytiotrophoblast KATP, mediates an increase in GLUT1 abundance to the microvillous and basal plasma membrane. However, the underlying mechanism by which KATP interacts with GLUT1 (directly or indirectly) remains to be explored.
We also determined the effect of glyburide treatment on the expression of placental amino acid and fatty acid transporters in GDM women. MVM and BM SNAT2 and LAT1 expression were not significantly different between the glyburide and diet treated groups. At present, there are no literature reports demonstrating regulation of system A or L amino acid transporters by glyburide in any tissue. On the other hand, glyburide treatment of GDM mothers was associated with reduced expression of FAT/CD36 and FATP2 in MVM of the syncytiotrophoblast, with no effect on the expression of fatty acid transporters in the BM. To what extent these changes impact transport of fatty acids across the placenta remains elusive, given the lack of understanding of the mechanisms mediating transplacental fatty acid transport and that the changes occurred only in the MVM.
Our study, although limited to small numbers, has provided the first evidence that glyburide promotes expression and localization of GLUT1 to the MVM and BM of the syncytiotrophoblast. Some placental functions show sexual dimorphism [50], however the relatively small sample size in the glyburide group precludes a robust assessment of sex differences in placental responses to glyburide treatment in our study. However, the similar birth weight Z-scores in male and females in the glyburide group suggest that the overrepresentation of males in this group does not drive the difference in birth weights. In addition, our studies in cultured PHT isolated from both male and female placentas, showing a robust effect of glyburide on GLUT1 plasma membrane translocation, does not support a sex difference in the effects of glyburide on GLUT1 membrane trafficking.
In conclusion, we propose that increased GLUT1 translocation to the plasma membrane could directly lead to increased transplacental glucose transfer, fetal hyperglycemia, fetal hyperinsulinemia, and contribute to increased birth weight. This may therefore provide a novel mechanism for the association between glyburide treatment and increased birth weight in GDM.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the help of Vanessa I. Ramirez for excellent technical assistance and to Evelyn Miller for patient recruitment.
Funding
This work was supported by NIH grant DK89989. P. Díaz was also supported by the National Fund for Scientific and Technological Development (FONDECYT)-Chile postdoctoral grant 3170140.
Abbreviations:
- GDM
gestational diabetes mellitus
- BMI
body mass index
- KATP
ATP-sensitive potassium channel
- MVM
microvillous plasma membrane
- BM
basal plasma membrane
- GLUT
glucose transporter
- SNAT
sodium-coupled neutral amino acid transporter
- LAT
L-type amino acid transporter
- FAT/CD36
fatty acid translocase
- FATP
fatty acid transporter
- PHT
primary human trophoblasts
- LDH
lactate dehydrogenase
Footnotes
Conflict of interest statement
The author(s) report(s) no conflict of interest.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.placenta.2017.05.016.
Contributor Information
Paula Díaz, Email: pauladiaz@med.uchile.cl.
Theresa L. Powell, Email: theresa.powell@ucdenver.edu.
References
- [1].Stuebe AM, Landon MB, Lai Y, Spong CY, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Sciscione A, Catalano P, Harper M, Saade G, Sorokin Y, Peaceman AM, Tolosa JE, Maternal BMI, glucose tolerance, and adverse pregnancy outcomes, Am. J. obstetrics Gynecol 207(1) (62) (2012) e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].DeSisto CL, Kim SY, Sharma AJ, Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (PRAMS), 2007–2010, Prev. chronic Dis 11 (2014) E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Camelo Castillo W, Boggess K, Sturmer T, Brookhart MA, Benjamin DK Jr., Jonsson Funk M, Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes, JAMA Pediatr. 169 (5) (2015) 452–458. [DOI] [PubMed] [Google Scholar]
- [4].McIntyre HD, Colagiuri S, Roglic G, Hod M, Diagnosis of GDM: a suggested consensus, Best. Pract. Res. Clin. Obstet. Gynaecol 29 (2) (2015) 194–205. [DOI] [PubMed] [Google Scholar]
- [5].Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI, International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy, Diabetes care 33 (3) (2010) 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Standards of medical care in diabetese2014, Diabetes care 37 (Suppl 1) (2014) S14–S80. [DOI] [PubMed] [Google Scholar]
- [7].Blumer I, Hadar E, Hadden DR, Jovanovic L, Mestman JH, Murad MH, Yogev Y, Diabetes and pregnancy: an endocrine society clinical practice guideline, J. Clin. Endocrinol. Metab 98 (11) (2013) 4227–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A world health organization guideline, Diabetes Res. Clin. Pract 103 (3) (2014) 341–363. [DOI] [PubMed] [Google Scholar]
- [9].Yessoufou A, Moutairou K, Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory”, Exp. diabetes Res 2011 (2011) 218598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marshall NE, Spong CY, Obesity, pregnancy complications, and birth outcomes, Seminars reproductive Med 30 (6) (2012) 465–471. [DOI] [PubMed] [Google Scholar]
- [11].Bulletins–obstetrics CoP. Practice bulletin No. 137: gestational diabetes mellitus, Obstet. Gynecol 122 (2 Pt 1) (2013) 406–416. [DOI] [PubMed] [Google Scholar]
- [12].Practice bulletin No. 137: gestational diabetes mellitus, Obstetrics Gynecol 122 (2, PART 1) (2013) 406–416. [DOI] [PubMed] [Google Scholar]
- [13].Barbour LA, Unresolved controversies in gestational diabetes: implications on maternal and infant health, Curr. Opin. Endocrinol. diabetes, Obes 21 (4) (2014) 264–270. [DOI] [PubMed] [Google Scholar]
- [14].Camelo Castillo W, Boggess K, Sturmer T, Brookhart MA, Benjamin DK Jr., Jonsson Funk M, Trends in glyburide compared with insulin use for gestational diabetes treatment in the United States, 2000–2011, Obstet. Gynecol 123 (6) (2014) 1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O, A comparison of glyburide and insulin in women with gestational diabetes mellitus, N. Engl. J. Med 343 (16) (2000) 1134–1138. [DOI] [PubMed] [Google Scholar]
- [16].Balsells M, Garcia-Patterson A, Sola I, Roque M, Gich I, Corcoy R, Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis, Bmj 350 (2015) h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Elliott BD, Langer O, Schenker S, Johnson RF, Insignificant transfer of glyburide occurs across the human placenta, Am. J. obstetrics Gynecol 165 (4 Pt 1) (1991) 807–812. [DOI] [PubMed] [Google Scholar]
- [18].Schwartz RA, Rosenn B, Aleksa K, Koren G, Glyburide transport across the human placenta, Obstet. Gynecol 125 (3) (2015) 583–588. [DOI] [PubMed] [Google Scholar]
- [19].Naraharisetti SB, Kirby BJ, Hebert MF, Easterling TR, Unadkat JD, Validation of a sensitive LC-MS assay for quantification of glyburide and its metabolite 4-transhydroxy glyburide in plasma and urine: an OPRU Network study, J. Chromatography B, Anal. Technol. Biomed. life Sci 860 (1) (2007) 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lybaert P, Hoofd C, Guldner D, Vegh G, Delporte C, Meuris S, Lebrun P, Detection of KATP channels subunits in human term placental explants and evaluation of their implication in human placental lactogen (hPL) and human chorionic gonadotropin (hCG) release, Placenta 34 (6) (2013) 467–473. [DOI] [PubMed] [Google Scholar]
- [21].Tsiani E, Ramlal T, Leiter LA, Klip A, Fantus IG, Stimulation of glucose uptake and increased plasma membrane content of glucose transporters in L6 skeletal muscle cells by the sulfonylureas gliclazide and glyburide, Endocrinology 136 (6) (1995) 2505–2512. [DOI] [PubMed] [Google Scholar]
- [22].Schmitz O, Lund S, Bak JF, Orskov L, Andersen PH, Moller N, Rasmussen O, Christiansen JS, Pedersen O, Effects of glipizide on glucose metabolism and muscle content of the insulin-regulatable glucose transporter (GLUT 4) and glycogen synthase activity during hyperglycaemia in type 2 diabetic patients, Acta diabetol. 31 (1) (1994) 31–36. [DOI] [PubMed] [Google Scholar]
- [23].Rufino AT, Rosa SC, Judas F, Mobasheri A, Lopes MC, Mendes AF, Expression and function of K(ATP) channels in normal and osteoarthritic human chondrocytes: possible role in glucose sensing, J. Cell Biochem 114 (8) (2013) 1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jansson T, Wennergren M, Illsley NP, Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation, J. Clin. Endocrinol. Metab 77 (6) (1993) 1554–1562. [DOI] [PubMed] [Google Scholar]
- [25].Barros LF, Yudilevich DL, Jarvis SM, Beaumont N, Baldwin SA, Quantitation and immunolocalization of glucose transporters in the human placenta, Placenta 16 (7) (1995) 623–633. [DOI] [PubMed] [Google Scholar]
- [26].Díaz P, Powell TL, Jansson T, The role of placental nutrient sensing in maternal-fetal resource allocation, Biol. reproduction 91 (4) (2014) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jansson T, Wennergren M, Powell TL, Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes, Am. J. obstetrics Gynecol 180 (1 Pt 1) (1999) 163–168. [DOI] [PubMed] [Google Scholar]
- [28].Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL, Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes, Diabetes 51 (7) (2002) 2214–2219. [DOI] [PubMed] [Google Scholar]
- [29].Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL, Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies, J. Clin. Endocrinol. Metab 98 (1) (2013) 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Araujo JR, Correia-Branco A, Ramalho C, Goncalves P, Pinho MJ, Keating E, Martel F, L-methionine placental uptake: characterization and modulation in gestational diabetes mellitus, Reprod. Sci 20 (12) (2013) 1492–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL, Protein expression of Fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women, Placenta 40 (2016) 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ramirez VI, Miller E, Meireles CL, Gelfond J, Krummel DA, Powell TL, Adiponectin and IGFBP-1 in the development of gestational diabetes in obese mothers, BMJ open diabetes Res. care 2 (1) (2014) e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Overpeck MD, Hediger ML, Zhang J, Trumble AC, Klebanoff MA, Birth weight for gestational age of Mexican American infants born in the United States, Obstet. Gynecol 93 (6) (1999) 943–947. [DOI] [PubMed] [Google Scholar]
- [34].Fenton TR, Kim JH, A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants, BMC Pediatr. 13 (2013) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM, Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta, Biochimica biophysica acta 1029 (2) (1990) 218–226. [DOI] [PubMed] [Google Scholar]
- [36].Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3rd, Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae, Endocrinology 118 (4) (1986) 1567–1582. [DOI] [PubMed] [Google Scholar]
- [37].Aye IL, Jansson T, Powell TL, TNF-alpha stimulates System A amino acid transport in primary human trophoblast cells mediated by p38 MAPK signaling, Physiol. Rep 3 (10) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Amin M, Suksomboon N, Poolsup N, Malik O, Comparison of glyburide with metformin in treating gestational diabetes mellitus: a systematic review and meta-analysis, Clin. drug Investig 35 (6) (2015) 343–351. [DOI] [PubMed] [Google Scholar]
- [39].Zeng YC, Li MJ, Chen Y, Jiang L, Wang SM, Mo XL, Li BY, The use of glyburide in the management of gestational diabetes mellitus: a meta-analysis, Adv. Med. Sci 59 (1) (2014) 95–101. [DOI] [PubMed] [Google Scholar]
- [40].Vardhana PA, Illsley NP, Transepithelial glucose transport and metabolism in BeWo choriocarcinoma cells, Placenta 23 (8e9) (2002) 653–660. [DOI] [PubMed] [Google Scholar]
- [41].Jansson T, Ekstrand Y, Wennergren M, Powell TL, Placental glucose transport in gestational diabetes mellitus, Am. J. obstetrics Gynecol 184 (2) (2001) 111–116. [DOI] [PubMed] [Google Scholar]
- [42].Gaither K, Quraishi AN, Illsley NP, Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter, J. Clin. Endocrinol. Metab 84 (2) (1999) 695–701. [DOI] [PubMed] [Google Scholar]
- [43].Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG, Placental drug transporters and their role in fetal protection, Placenta 33 (3) (2012) 137–142. [DOI] [PubMed] [Google Scholar]
- [44].Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS, Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin, Am. J. obstetrics Gynecol 202 (4) (2010), 383 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Marino GI, Assef YA, Kotsias BA, An outwardly rectifying chloride channel in BeWo choriocarcinoma cell line, Placenta 31 (12) (2010) 1093–1100. [DOI] [PubMed] [Google Scholar]
- [46].Illsley NP, Sellers MC, Wright RL, Glycaemic regulation of glucose transporter expression and activity in the human placenta, Placenta 19 (7) (1998) 517–524. [DOI] [PubMed] [Google Scholar]
- [47].Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, Lazdunski M, Nichols CG, Seino S, Vandenberg CA, International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels, Pharmacol. Rev 57 (4) (2005) 509–526. [DOI] [PubMed] [Google Scholar]
- [48].Ashcroft FM, The Walter B, Cannon Physiology in Perspective Lecture, 2007. ATP-sensitive K+ channels and disease: from molecule to malady, Am. J. Physiol. Endocrinol. Metab 293 (4) (2007) E880–E889. [DOI] [PubMed] [Google Scholar]
- [49].Muller G, Wied S, The sulfonylurea drug, glimepiride, stimulates glucose transport, glucose transporter translocation, and dephosphorylation in insulin-resistant rat adipocytes in vitro, Diabetes 42 (12) (1993) 1852–1867. [DOI] [PubMed] [Google Scholar]
- [50].Evans L, Myatt L, Sexual dimorphism in the effect of maternal obesity on antioxidant defense mechanisms in the human placenta, Placenta 51 (2017) 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.