Abstract
After central nervous system (CNS) trauma, axons have a low capacity for regeneration. Regeneration failure is associated with a muted regenerative response of the neuron itself, combined with a growth-inhibitory and cytotoxic post-injury environment. After spinal cord injury (SCI), resident and infiltrating immune cells (especially microglia/macrophages) contribute significantly to the growth-refractory milieu near the lesion. By targeting both the regenerative potential of the axon and the cytotoxic phenotype of microglia/macrophages, we may be able to improve CNS repair after SCI. In this review, we discuss molecules shown to impact CNS repair by affecting both immune cells and neurons. Specifically, we provide examples of pattern recognition receptors, integrins, cytokines/chemokines, nuclear receptors, and galectins that could improve CNS repair. In many cases, signaling by these molecules is complex and may have contradictory effects on recovery depending on the cell types involved or the model studied. Despite this caveat, deciphering convergent signaling pathways on immune cells (which affect axon growth indirectly) and neurons (direct effects on axon growth) could improve repair and recovery after SCI. Future studies must continue to consider how regenerative therapies targeting neurons impact other cells in the pathological CNS. By identifying molecules that simultaneously improve axon regenerative capacity and drive the protective, growth-promoting phenotype of immune cells, we may discover SCI therapies that act synergistically to improve CNS repair and functional recovery.
Keywords: inflammation, galectin-1, TLR, DAMPs, neurotrauma
Introduction
Injured mammalian central nervous system (CNS) axons regenerate poorly; however, recent data indicate that neuron intrinsic (e.g., Pten, low cAMP) and extrinsic barriers (e.g., myelin, CSPGs) to axon growth can be overcome (Park et al., 2010; Bradbury and Carter, 2011). In this review, we introduce the novel concept that successful CNS axon regeneration can be achieved by manipulating signaling pathways that are used by both the nervous and immune systems (Figure 1). Specifically, we discuss how pattern recognition receptors, integrins, cytokines, chemokines, nuclear receptors, and galectins can affect axon regeneration through dual activation of cells in the nervous and immune systems (Figure 2).
Fig. 1.
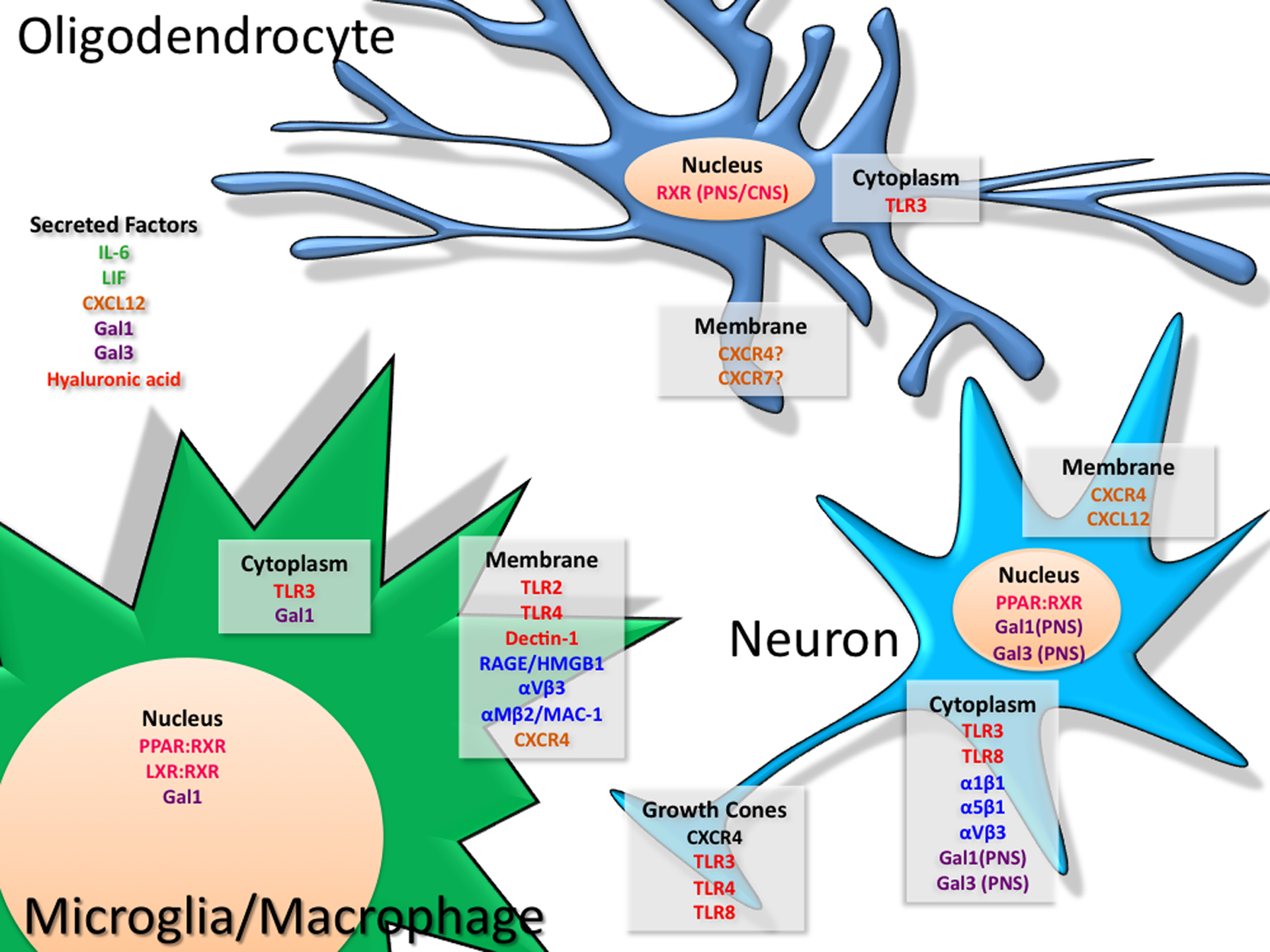
depicts the localization of factors and receptors that elicit convergent signaling in cells of the immune and nervous systems
Fig. 2.
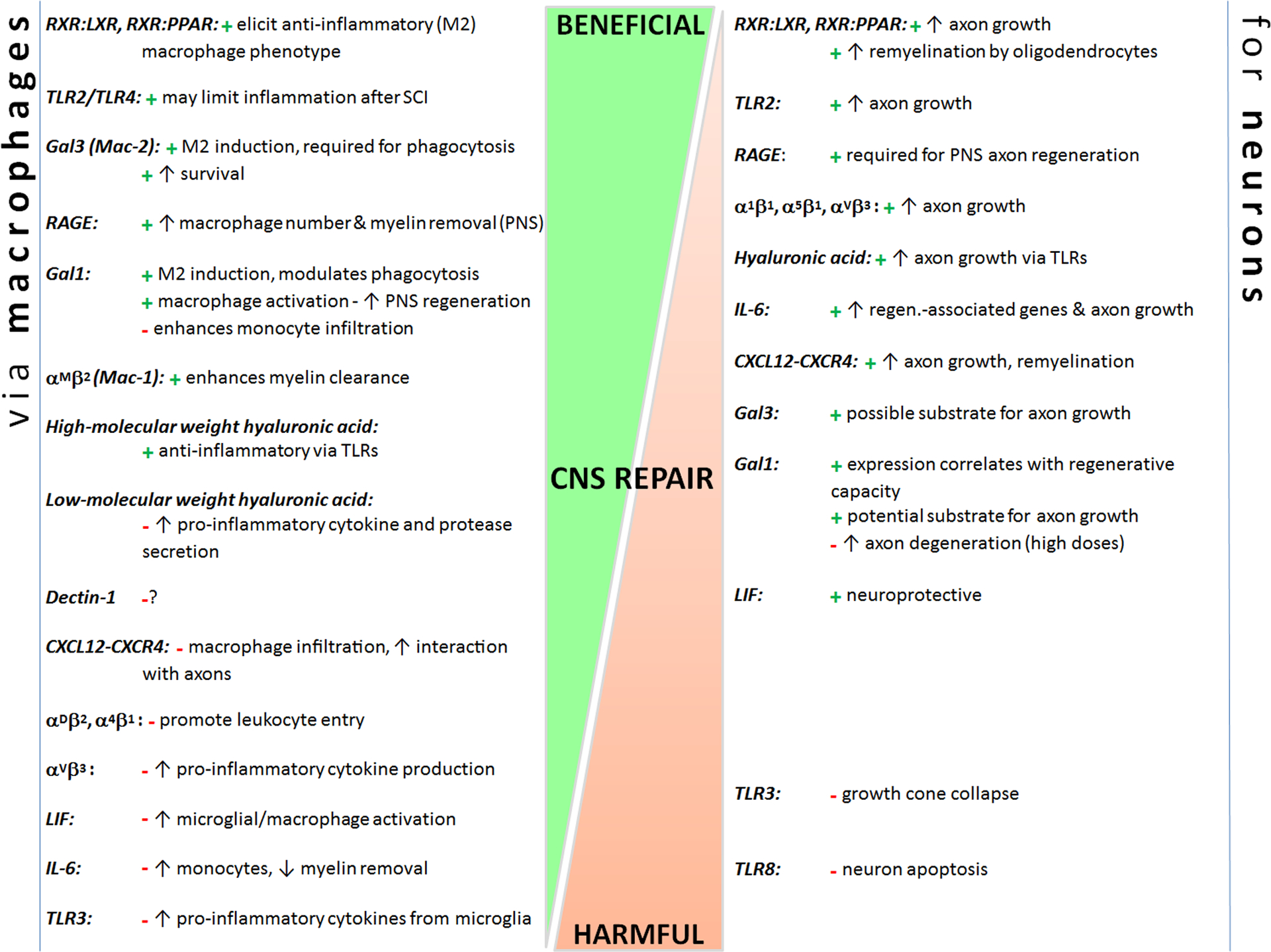
CNS repair-related processes can be initiated or prevented via similar pathways/receptors in both macrophages and neurons. These factors can alter neuron extrinsic (e.g., macrophages) and intrinsic responses to improve or hamper repair. Some factors have reparative effects on both macrophage phenotype and neuron growth/survival (e.g., TLR2, RXR heterodimers, Gal3), whereas others have contradictory effects on the two cell types (e.g., αVβ3 integrin, IL-6, CXCL12-CXCR4). Factors discussed are placed on a continuum of CNS repair, from beneficial (top) to harmful (bottom) (placements are estimates based on known effects). “+” represents a beneficial effect; “-” represents a harmful effect. See text for references.
Pattern Recognition Receptors and Danger Associated Molecular Patterns
Cells of the innate immune system play a pivotal role in defending organisms against infection. This is accomplished via a series of germ-line encoded receptors known as pattern recognition receptors (PRRs). PRRs can be membrane-bound or exist in the cytoplasm. Membrane-bound PRRs include most toll-like receptors (e.g., TLR2, TLR4, TLR5, TLR6), C-type lectin receptors (including dectin-1) and the receptor for advanced glycation end products (RAGE) (Kvarnhammar and Cardell, 2012). PRRs confined mainly to the cytoplasm include the nucleotide oligomerization domain (NOD) receptors, a subset of TLRs (e.g., TLR3, TLR7, TLR9) and RIG-I-like receptors.
PRRs bind pathogen-associated molecular patterns (PAMPs), which are conserved molecular sequences found on bacteria (e.g., endotoxin/LPS, peptidoglycans) and viruses. PRR ligation elicits inflammatory signaling resulting in the release of cytokines that augment inflammation culminating in pathogen removal from the host.
Although most cells of the CNS express PRRs, traumatic brain or spinal cord injuries (SCI) are considered to be “sterile” lesions, i.e., neither bacteria nor virus are found at sites of injury. However, endogenous PRR ligands do accumulate at sites of tissue damage. In contrast to PAMPs, these endogenous PRR ligands are referred to as damage-associated molecular patterns (DAMPs) or “alarmins” and include high mobility group box-1 (HMGB1), mRNA, fibronectin, oxidized lipids, hyaluronic acid and heat shock proteins (HSP) (Taylor et al., 2007).
The net effect of PRR signaling in the injured CNS is complex, with reported outcomes being either detrimental or beneficial to nerve repair or recovery of neurological function. For example, intraspinal inflammation is exacerbated and functional recovery is impaired in SCI mice lacking TLR2 or TLR4 (Kigerl et al., 2007). Conversely, TLR deficiency is neuroprotective in a mouse model of cerebral ischemia and remyelination is improved in TLR2 deficient mice (Sloane et al., 2010; Ziegler et al., 2011). Although the precise nature of the DAMP/PRR interactions that trigger these divergent effects is not clear, signaling via PRRs can simultaneously elicit tissue injury and repair in the CNS (Kigerl and Popovich, 2009). Data derived from experiments that apply exogenous zymosan to models of central or peripheral nervous system (PNS) injury, best illustrate the complexity of PRR signaling.
Zymosan is a complex of proteins and carbohydrates derived from yeast cell wall that potently activates inflammatory signaling after binding TLR2. Although technically a PAMP, zymosan elicits intracellular signaling pathways in microglia and macrophages that are identical to those activated by DAMPs. Thus, adding zymosan to intact or injured CNS/PNS elicits a threshold of cellular activation that is physiologically relevant and that can be compared to appropriate control groups. Zymosan-activated macrophages (ZAMs) can promote regeneration of injured axons ((Yin et al., 2003; Steinmetz et al., 2005; Gensel et al., 2009); however, ZAMs also cause axon degeneration, neurotoxicity and secondary demyelination (Fitch et al., 1999; Popovich et al., 2002; Schonberg et al., 2007; Gensel et al., 2009). Recently, we discovered that TLR2 is not solely responsible for the divergent effects of ZAMs on CNS repair (unpublished data); zymosan also affects macrophage function by binding the C-type lectin receptor, dectin-1 (Gantner et al., 2003). Although the importance of dectin-1 signaling in the injured CNS is an area of ongoing research, it is already known that activation of microglia and/or macrophages via TLR2 can promote growth of injured central (optic nerve) or peripheral (sciatic nerve) axons (Boivin et al., 2007; Hauk et al., 2010).
TLR3 is widely expressed by inflammatory cells, glia and neurons and is best known for its role in anti-viral CNS immunity. Less is known about the effects of TLR3 signaling in the context of CNS injury and nerve regeneration. In the injured PNS, necrotic cell debris signals Schwann cells via TLR3 (Lee et al., 2006). This is important for recruitment of macrophages and subsequent removal of myelin debris i.e., two necessary components of effective nerve regeneration (Lee et al., 2006).
Microglia, astrocytes and oligodendrocytes also express TLR3. When activated with synthetic TLR3 agonists, microglia and astrocytes upregulate inflammatory gene expression and release various inflammatory and anti-inflammatory cytokines with neurotoxic and neurotrophic effects. TLR3 stimulation of oligodendrocytes causes apoptosis (Lee et al., 2006; Bsibsi et al., 2012).
Extracellular mRNA released from dead or dying cells can bind TLR3 on axon growth cones in the CNS and PNS. This can cause growth cone collapse and reduce neurite outgrowth (Cameron et al., 2007). Intrathecal delivery of synthetic TLR3 agonists to neonatal mice reduces axon fiber outgrowth, decreases axon numbers and impairs motor coordination (Cameron et al., 2007) suggesting that TLR3 activation impairs developmental axon targeting in vivo. Whether this is through simultaneous activation of TLR3 on glia and neurons has not been determined. Like TLR3, TLR8 activation inhibits neurite outgrowth and causes neuron apoptosis (Ma et al., 2006; 2007).
Hyaluronic acid (HA or hyaluronan) is a prevalent long chain glucosaminoglycan and structural component of the CNS extracellular matrix (ECM) (Gladson, 1999). When injured, HA is degraded leading to a change in the ratio of high- to low-molecular weight HA. This will influence the magnitude of inflammation and also the ability of neurons and glia to respond to injury. High-molecular weight (HMW) HA has immune-regulatory properties. It can inhibit macrophage proliferation and cytokine release (Schimizzi et al., 2006; Wang et al., 2006; Taylor et al., 2007). However, hydrogels composed of HMW HA reduce synthesis of CSPGs by astrocytes and were shown to be anti-inflammatory in models of SCI (Brück and Friede, 1990; Reichert et al., 2001; Rotshenker, 2003; Yasuda, 2007; Khaing et al., 2011). HA also acts directly on neurons. For example, dorsal root ganglion neurons grown in HA-enriched hydrogel matrices extend neurites that are 50% longer than those grown in control matrices (Horn et al., 2007; Gardiner, 2011). These HA-mediated improvements in neurite extension are probably elicited by HA binding to TLRs and they could underlie the modest improvements in axon regeneration and functional recovery described in models of SCI (Condic, 2001; Gupta et al., 2006; Wakao et al., 2011). When degraded, the anti-inflammatory and growth-promoting effects of HA are lost. Low-molecular weight (LMW) HA binds TLR4 and TLR2 on immune cells, increasing their synthesis and release of inflammatory cytokines and proteases. LMW HA also binds TLR2 on oligodendrocyte progenitor cells preventing their differentiation and therefore their ability to remyelinate denuded axons (Bouhlel et al., 2007; Andrews et al., 2009; Sloane et al., 2010; Hawthorne and Popovich, 2011; Tan et al., 2011).
Another example of a PRR that if manipulated, would simultaneously affect cells of the immune and nervous systems is the receptor for advanced glycation end products (RAGE). RAGE binds diverse ligands including advanced glycation end products, amyloid fibrils, HMGB1 and S100/calgranulins (Alexiou et al., 2010; Hawthorne and Popovich, 2011; 2011). Existing data indicate that in the presence of these ligands, RAGE signaling leads to nerve repair. In a model of peripheral nerve injury, overexpression of a dominant negative (DN-) RAGE in monocytes or neurons reduces nerve conduction velocity, myelin debris clearance and density of myelinated fibers (decrease ~40% vs. control)(Rong et al., 2004). However, if DN-RAGE was expressed in both monocytes and neurons, impairment was significantly greater (decrease ~70% vs. control)(Rong et al., 2004). These elegant studies illustrate that PRR activation can affect repair processes via convergent signaling in immune cells and neurons. These data also illustrate the importance of considering convergent signaling mechanisms when developing therapeutics for repairing the pathological CNS or PNS, where inflammatory cells coexist with neurons and glia.
Integrins
Integrins are transmembrane heterodimeric receptors that facilitate bidirectional signaling between cells and the extracellular environment, most notably proteins found in the ECM or on other cells (e.g., laminin, fibronectin). Integrin binding activates intracellular signaling cascades that affect cell differentiation, survival, growth and division (Luo et al., 2007).
Integrins also are essential for regulating the tightly coordinated process of leukocyte extravasation into sites of inflammation (Rose et al., 2007). In response to cytokines and chemotactic gradients that develop at inflammatory foci, circulating leukocytes increase their surface expression of β2 integrins. This allows them to adhere to the vascular lumen and subsequently to enter sites of inflammation. It is possible to reduce leukocyte entry and subsequent inflammation by blocking integrin binding/signaling. For example, in models of SCI where acute inflammation has neurotoxic effects, anti-integrin antibodies (e.g., αDβ2 or α4β1) confer neuroprotection and improve recovery of function (Yip et al., 1998; Yip and Siu, 2001; Bao et al., 2004; Gris et al., 2004; Pittier et al., 2005; Fleming et al., 2008; 2009; Bao et al., 2011). Activation of other integrins (αMβ2/Mac-1) expressed on the surface of microglia and macrophages could improve axon regeneration indirectly by enhancing phagocytic clearance of myelin debris (Brück and Friede, 1990; Reichert et al., 2001; Rotshenker, 2003; Mukaino et al., 2010).
Integrins also can directly regulate axon outgrowth (Gris et al., 2007; Sofroniew, 2009; Gardiner, 2011). Axonal integrins are developmentally regulated with reduced expression in the mature CNS. This could explain why regeneration of injured adult CNS axons is inefficient. Indeed, transfection of adult DRGs with α1β1 or α5β1 integrin receptors improves axon elongation on laminin or fibronectin, respectively (Condic, 2001; Okada et al., 2006; Herrmann et al., 2008). Similarly, overexpression of integrins also can improve axonal outgrowth on inhibitory substrates found at sites of CNS injury (e.g., CSPGs and tenascin-C) (Hakkoum et al., 2007; Andrews et al., 2009; Tan et al., 2011). Serotonergic axons, known for their ability to undergo robust regenerative sprouting, even in the context of a growth inhibitory environment, express high levels of β1 integrins (Cafferty et al., 2004; Cao et al., 2006; Hawthorne et al., 2011). Conversely, cortical neurons, with limited regenerative sprouting, express low levels of β1 integrins (Richardson and Issa, 1984; Neumann and Woolf, 1999; Cameron et al., 2007; Hong and Tontonoz, 2008; Hawthorne et al., 2011).
After CNS injury, activation of αVβ3 integrins is likely to elicit convergent signaling in neurons, glia and inflammatory cells. For example, overexpression of the cell adhesion molecule L1 can augment neurite outgrowth via activation of αVβ3 integrins (Yip et al., 1998; Yip and Siu, 2001; Pittier et al., 2005; Cao et al., 2006). Vitronectin, a glycoprotein ligand of αVβ3 integrins found in plasma and the ECM, elicits NFĸB activation and downstream inflammatory signaling in microglia and macrophages (e.g., synthesis of MMPs, TNFα, IL-1β, IL-6).
Cytokines
Although originally defined as the soluble effector molecules of inflammatory cells, cytokines are now known to be pivotal mediators of intercellular communication between most cells in the body. Cytokines are pleotropic, i.e., the same cytokine exerts multiple effects on different cell types. This makes it difficult to understand how cytokines affect CNS repair. This complexity and the sheer numbers of cytokines that have been characterized in the injured CNS (Nakamura et al., 2003), preclude a comprehensive overview of this topic. In this review, the potential for convergent signaling of IL-6 and leukemia inhibitory factor (LIF) in cells of the neuro-immune axis will be discussed.
Within the first few hours after injury to the brain or spinal cord, concentrations of IL-6 increase in the CNS (Nakamura et al., 2005). Neurons, glia and infiltrating leukocytes all produce and respond to IL-6 signifying that it is a pivotal mediator of the acute CNS injury response. However, the net effect of IL-6-mediated signaling is difficult to determine. For example, injecting anti-IL-6 antibodies early after SCI confers neuroprotection – a response that is associated with reduced monocyte infiltration and increased efficiency of microglia phagocytosis (Cafferty et al., 2004; Cao et al., 2006; Mukaino et al., 2010). IL-6 signaling also elicits reactive astrogliosis, a stereotypical response to CNS injury that restricts acute post-traumatic inflammation and inhibits axon regeneration in part through the transcriptional activation of genes associated with CSPG formation (Kerr and Patterson, 2004; Gris et al., 2007; Sofroniew, 2009). The effects of IL-6 (and LIF; see below) are likely mediated via STAT-3 signaling (Kerr and Patterson, 2004; Okada et al., 2006; Herrmann et al., 2008). Collectively, the above examples illustrate neuron extrinsic effects of IL-6 that could influence axon regeneration in vivo.
IL-6 can also affect neuron-intrinsic growth. Transection of axons in an organotypic hippocampal slice culture increases IL-6 leading to spontaneous recovery of synaptic activity with evidence of regenerative sprouting. In the presence of an anti-IL-6 antibody, the expression of regeneration-associated genes (e.g., GAP43) and recovery of synaptic activity were abolished (Zang and Cheema, 2003; Kerr and Patterson, 2005; Hakkoum et al., 2007). IL-6 also plays a role in the “conditioning lesion” phenomenon (Azari et al., 2003; Cafferty et al., 2004; Butzkueven et al., 2006; Cao et al., 2006). Conditioning lesions improve the intrinsic growth capacity of adult neurons. For example, sciatic nerve crush augments the capacity of adult dorsal root ganglia (DRGs) neurons to mount a regenerative response after a subsequent injury to the central projections of those axons (e.g., spinal cord dorsal column axons) (Richardson and Issa, 1984; Neumann and Woolf, 1999; Ni et al., 2009). Conditioning lesions normally increase IL-6 in DRG neurons and intrathecal delivery of IL-6 can mimic the effects of a conditioning lesion (Gonzalez et al., 2003; Cao et al., 2006; Kohler et al., 2008). IL-6 may be sufficient but not necessary to induce the effects of conditioning. Indeed, conflicting data exist from two different studies using IL-6 knockout mice. In one report, the effects of conditioning were lost in the absence of IL-6 whereas the other report showed a normal effect of conditioning in IL-6 deficient mice (Cafferty et al., 2004; Cao et al., 2006; Chalasani et al., 2007).
LIF is a member of the IL-6 cytokine family and like IL-6, LIF exerts divergent effects axonal regeneration. After injury to the nervous system, LIF acts as a pro-inflammatory cytokine and can exacerbate tissue damage and functional impairment via its effects on microglia/macrophages. Indeed, intraspinal activation of microglia and macrophages after SCI is reduced and spontaneous recovery of function is improved in LIF knockout mice (Reichert and Rotshenker, 1999; Kerr and Patterson, 2004; Park et al., 2008; De Giusti et al., 2011). Moreover, viral-mediated over-expression of LIF in the naive spinal cord causes mild neurological impairment with enhanced microgliosis, a response that can be reversed using the anti-inflammatory drug minocycline (Kerr and Patterson, 2004; Bouhlel et al., 2007; Odegaard et al., 2008; Rotshenker, 2009). However, LIF also may be essential for coordinating CNS repair. Data obtained from several reports using different models of neurological disease indicate that systemic delivery of LIF is neuroprotective (Pesheva et al., 1998; Mahoney et al., 2000; Zang and Cheema, 2003; Kerr and Patterson, 2005; Diez-Revuelta et al., 2010) (Azari et al., 2003; Butzkueven et al., 2006; Boivin et al., 2007; Diez-Revuelta et al., 2010; 2010). These protective effects are likely to be mediated indirectly via an increase in the synthesis of insulin-like growth factor 1 (IGF-1), a potent trophic factor for neurons and oligodendrocytes (Kerr and Patterson, 2005).
Chemokines
Chemokines are chemotactic cytokines that act on cognate G-protein coupled transmembrane receptors (Luster, 1998). In response to tissue injury or infection, chemokines induce the directed migration of leukocytes along a chemokine gradient. Genetic or pharmacologic manipulation of chemokines or their cognate receptors can attenuate neurodestructive inflammation in the injured brain or spinal cord (Gonzalez et al., 2003; Kohler et al., 2008; Ni et al., 2009; Donnelly et al., 2011). However, chemokines also are important during development and emerging data show that chemokines and their cognate receptors are constitutively expressed in the CNS. For example, CXCL12, also known as stromal cell-derived factor (SDF1), binds CXCR4 found on axons and is important for axonal pathfinding during CNS development (Bouhlel et al., 2007; Chalasani et al., 2007; Odegaard et al., 2007; 2008).
The functional significance of CXCL12 in the adult spinal cord is not clear; however, it is normally expressed in corticospinal tract (CST) axons and in the meninges (Tysseling et al., 2011) while its cognate receptors, CXCR4 and CXCR7 are found on adult DRG neurons, CST axons, and ependyma. Both trauma and demyelination cause CXCR4+ macrophages and oligodendrocyte precursor cells to infiltrate sites of pathology (Patel et al., 2010; Tysseling et al., 2011; Zhang et al., 2011). Endogenous signaling via CXCR4 or CXCR7 is pivotal for promoting differentiation and maturation of new oligodendrocytes, a prerequisite for successful remyelination (Göttle et al., 2010; Patel et al., 2010).
These same chemokine-receptor interactions may also regulate regenerative sprouting and/or die-back of injured axons. CXCR4 is localized to axon growth cones and intrathecal infusion of CXCL12 was able to increase axonal growth after SCI in vivo (Opatz et al., 2009; Sloane et al., 2010). Because CXCL12 is a potent chemoattractant for monocytes and microglia (Tanabe et al., 1997; Ransohoff, 2009), it is perhaps not surprising that CXCR4+ macrophages are found in close proximity to injured CXCL12+ axons (Tysseling et al., 2011). The endogenous macrophage response after SCI is known to exacerbate acute tissue damage (Popovich et al., 1999)(Blight; Popovich), in part through augmenting die-back of injured axons (Horn et al., 2008). A direct role for CXCL12/CXCR4 in regulating macrophage-mediated die-back has not been determined.
Nuclear Receptors
Nuclear receptors are a superfamily of ~50 ligand activated transcription factors. These receptors are structurally conserved and regulate gene transcription by directly binding DNA response elements in the promoters of target genes. As such, they play a critical role during development and in maintaining homeostasis throughout the body (Olefsky, 2001).
Type II nuclear receptors form obligate heterodimers with the Retinoid X Receptors (RXRs) and regardless of their ligand binding status are localized to the nucleus (Klinge et al., 1997). In the absence of ligand, this heterodimeric complex is associated with a nuclear corepressor complex. Upon ligand binding, a conformational change occurs in the receptor causing the corepressor complex to detach. A coactivator complex with intrinsic histone acetyltransferase activity is subsequently recruited that allows chromatin decondensation and gene transcription (Desvergne et al., 2006; Schimizzi et al., 2006; Wang et al., 2006; Taylor et al., 2007).
Peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs) form obligate heterodimers with RXRs to form functional transcription factors. These RXR containing heterodimers are termed “permissive” because their transcriptional activity can be induced by ligation of either member of the receptor pair. Thus, RXR activation may be an optimal mechanism to activate both PPAR and LXR pathways in vivo.
Little is known about the role of RXRs after injury to the brain or spinal cord. One report describes constitutive RXR expression in neurons and glia in intact spinal cord (Schrage et al., 2006). After injury, RXR-expressing leukocytes appeared in the lesion site with increased translocation of RXRs to the nucleus of leukocytes and neurons evident within the first two weeks post-injury (Schrage et al., 2006). Although the functional implications of these changes are unknown, activating type II nuclear receptors in macrophages can profoundly change their phenotype and function. For example, activation of LXRs or PPARs suppresses inflammatory macrophage functions and promotes the development of an “alternative” M2 macrophage phenotype (Hong and Tontonoz, 2008; Chawla, 2010). Genetic disruption of either PPAR γ or PPARδ blocks the acquisition of the M2 phenotype (Bouhlel et al., 2007; Odegaard et al., 2007; 2008; Chinetti-Gbaguidi et al., 2011). M2 macrophages might promote CNS repair. Indeed, recent data indicate that M2 macrophages increase the intrinsic growth potential of neurons, even on inhibitory substrates found at site of CNS injury (Kigerl et al., 2009).
In addition to their role in regulating microglia/macrophage function, LXR and PPAR agonists were shown to confer neuroprotection with improved spontaneous recovery of function in experimental models of SCI (Mctigue et al., 2007; Park et al., 2007; Paterniti et al., 2010; Meng et al., 2011). Activation of PPARγ also promotes axonal outgrowth in neuronal cell lines and primary DRG neurons (Miglio et al., 2009; Geeven et al., 2011), an effect that involves RhoA inhibition (Dill et al., 2010). In the peripheral nervous system, myelin thickness is reduced in LXR knockout mice suggesting that LXRs may regulate myelin gene expression (Makoukji et al., 2011). A similar role for LXRs has not been proven but in the injured CNS, RXR activation after SCI enhances oligodendrocyte differentiation and remyelination (Meng et al., 2011). RXRγ expression was shown to increase in myelinating schwann cells again suggesting a role for type II nuclear receptors in myelination (Latasa and Cosgaya, 2011). Due to its central role in stimulation both LXRs and PPARs, selective activation of RXR could represent a powerful therapeutic target for CNS repair.
Galectins
Galectins are carbohydrate-binding proteins found in the ECM, on cell surfaces and inside the cytoplasm and nucleus of cells (Norling et al., 2009). This diverse distribution, combined with their affinity for binding ubiquitous β-galactosides, enables galectins to bind various ligands including ECM molecules, glycoproteins, integrins, and immune receptors (Camby et al., 2006). Through protein-carbohydrate and protein-protein interactions, galectins affect cell adhesion, cell proliferation, cell survival, inflammation, mRNA splicing and axon growth (Perillo et al., 1998). While 15 mammalian galectins have been identified, studies thus far support roles for Gal1 and Gal3 in enhancing axon growth through activation of the both the nervous and immune systems.
Gal1 is a 14.5-kDa protein that is expressed throughout the body and has a variety of cellular functions (Camby et al., 2006). Gal1 is expressed in the developing peripheral nervous system (PNS) and CNS; in adults, Gal1 expression is confined mainly to the more growth-permissive PNS. The structure and behavior of Gal1 depend on its oxidation state. In the extracellular space, Gal1 exists as a homodimer with two active carbohydrate recognition domains (Gladson, 1999; López-Lucendo et al., 2004). Under oxidizing conditions (e.g., early after injury to CNS/PNS), Gal1 assumes an oxidized, monomeric form that lacks lectin activity (Inagaki et al., 2000).
Gal1 interacts with various ECM glycoproteins (e.g., laminin, fibronectin, integrins) and their associated receptors. Gal1 binding partially activates β1 integrin (Moiseeva et al., 2003) and modulates α7β1 integrin interaction with laminin during skeletal muscle differentiation (Gu et al., 1994). Gal1 also co-localizes with αMβ2 integrin (complement receptor 3) on mouse macrophages (Avni et al., 1998). Together, these data suggest that extracellular Gal1 could modulate neuron and macrophage phenotype. In fact, Gal1 may even act as a PRR (Vasta, 2012).
Gal1 has both pro- and anti-inflammatory effects (Liu and Rabinovich, 2010). Neutrophil extravasation is reduced upon exposure to Gal1 (La et al., 2003; Cooper et al., 2008), but these cells also secrete more reactive oxygen species (Almkvist et al., 2002). Gal1-treated macrophages release less arachadonic acid and nitric oxide, while increasing arginase-1 activity (Rabinovich et al., 2000; Correa et al., 2003). Enhanced arginase-1 activity is characteristic of anti-inflammatory M2 macrophages (see above). By binding to saccharides on the surface of monocytes, Gal1 upregulates constitutive expression of FcgRI; however, in the presence of inflammatory stimuli (e.g., IFNγ), Gal1 blocks the induction of FcgRI (Barrionuevo et al., 2007). This complex regulation of Fc receptors can positively and negatively affect macrophage phagocytosis. Finally, Gal1 has mainly anti-inflammatory effects on T cells: it limits T cell transmigration across endothelia (Rabinovich et al., 1999) and promotes Th2 cytokine expression (Motran et al., 2008). Although Gal1 regulation of pro- and anti-inflammatory responses is complicated, the balance of data suggest that Gal1 suppresses immune cell transmigration and promotes an anti-inflammatory response that could help to support CNS repair and axon regeneration.
In the nervous system, Gal1 is associated with developmental and regenerative axon growth (Gaudet et al., 2005). Gal1 is required for the proper developmental targeting of axonal subsets projecting to the olfactory bulb (Puche et al., 1996) and the spinal cord dorsal horn (McGraw et al., 2005; Horn et al., 2007). In Gal1 knockout mice, defective targeting of central branches of DRG neurons manifests as decreased thermal nociceptive sensitivity. After nerve trauma, Gal1 expression correlates with regenerative capacity, both intrinisic and extrinsic to the injured neuron. Injured facial motoneurons (PNS projections), but not injured rubrospinal neurons (CNS projections) upregulate Gal1 during the regenerative period (McGraw et al., 2004) Similarly, DRG neurons upregulate Gal1 after injury to their peripheral but not central branch (McGraw et al., 2005). Therefore, Gal1 may contribute to neuron intrinsic regenerative programs.
The role of Gal1 in the environment extrinsic to the injured neuron has been explored more extensively. Horie et al., (Horie et al., 1999) showed that exogenous delivery of Gal1 augments regeneration of peripheral axons. More recent studies have implicated the oxidized form of Gal1 in improving regeneration: oxidized Gal1 binds macrophages, causing them to secrete an unidentified factor that elicits Schwann cell migration and axon regeneration (Horie et al., 2004). In the CNS, intravitreal injection of oxidized Gal1 improves axon regeneration after optic nerve injury (Okada et al., 2005; Gupta et al., 2006; Yasuda, 2007; Khaing et al., 2011; Wakao et al., 2011), possibly by activating macrophages in the retina. Gal1 also regulates macrophage accumulation after peripheral axotomy (Gaudet et al., 2009). Oxidized Gal1 injection into the sciatic nerve facilitates macrophage accumulation in the nerve comparable to that elicited by zymosan; conversely, macrophage accumulation is delayed and diminished after peripheral nerve injury in Gal1 knockout mice. Since Gal1 increases monocyte chemotaxis (Malik et al., 2009), Gal1 may act in concert with chemokines to augment macrophage accumulation at sites of injury. Thus, selective manipulation of Gal1 could elicit a reparative macrophage phenotype (e.g., M2) with concurrent activation of neuron intrinsic growth programs.
Gal3 (also known as Mac-2) is a ~30 kDa protein that is expressed throughout the body. Upon ligand binding, Gal3 oligomerization induces conformational changes that enhance its affinity for binding carbohydrate ligands. This allows Gal3 to create lattices that can cross-link and activate glycoconjugate-containing receptors. In the CNS, GAL3 is mainly expressed by astrocytes, endothelial cells, and microglia.
In the immune system, Gal3 is secreted mostly by macrophages (Liu et al., 1995; Henderson and Sethi, 2009). At sites of inflammation, Gal3 is upregulated and binds neutrophils and macrophages. Gal3 induces neutrophil activation and adhesion (Kuwabara and Liu, 1996; Liu et al., 1996) and facilitates opsonization and macrophage phagocytosis of apoptotic neutrophils (Farnworth et al., 2008; Karlsson et al., 2009). Gal3 expression in macrophages predicts their phenotype: Gal3 is upregulated in both M1 and M2 macrophages but it is expressed more highly in M2 macrophages (Novak et al., 2011). Gal3 also promotes monocyte and macrophage chemotaxis (Sano et al., 2000). Studies using Gal3 null mutant macrophages have defined important roles for Gal3 in protecting macrophages against apoptosis (Hsu et al., 2000; Alexiou et al., 2010). Moreover, whereas Gal3 null mutant macrophages respond normally to classical M1 activators and to IL-10-mediated deactivation, they cannot mount anti-inflammatory M2 responses (MacKinnon et al., 2008). Gal3 may modulate macrophage phenotype by binding the glycoprotein CD98 and enhancing its association with B1 integrin, in a pathway that seems to converge on the intracellular signaling pathway initiated by M2 factors IL-4 and IL-13. Therefore, Gal3 has key roles in modulating neutrophil activation and survival, and is a switch that drives a more protective M2 macrophage phenotype.
In the CNS, Gal3 is upregulated by astrocytes and microglia/macrophages at sites of pathology (Reichert and Rotshenker, 1999; Cameron et al., 2007; Park et al., 2008; De Giusti et al., 2011) including SCI (Redensek et al., 2011). Gal3 is expressed by macrophages and microglia that phagocytose degenerated myelin. In fact, cells that do not express Gal3 do not phagocytose myelin (Rotshenker, 2009).
Gal3 is also a permissive substrate for axon growth. Neurons grown on Gal3 extend longer, more complex axons compared to neurons cultured on control substrates (Pesheva et al., 1998; Mahoney et al., 2000; Ma et al., 2006; 2007; Diez-Revuelta et al., 2010). These effects may require Gal3 binding to the neuronal adhesion molecule L1; dominant negative-L1 impairs Gal3-mediated axon branching (Diez-Revuelta et al., 2010).
In summary, galectins (especially Gal1 and Gal3) represent unique targets for concomitant modulation of the immune and nervous systems to enhance CNS repair. Gal1 and Gal3 are promiscuous through binding their ubiquitous carbohydrate ligands. Also, they are highly expressed in tissues that repair efficiently including the PNS, but are not expressed significantly in the adult CNS. Finally, these galectins generally promote a protective immune cell phenotype, while simultaneously improving axon growth. These characteristics point to Gal1 and Gal3 as important potential therapeutic targets for improving recovery after CNS trauma.
Conclusions:
The use of common ligands, receptors, and signaling systems between diverse cell types is striking. In this review, we have highlighted the effects of specific molecules that impact both the nervous and immune systems, thereby promoting CNS axon regeneration. These multi-functional factors – which include PRRs, integrins, cytokines/chemokines, nuclear receptors, and galectins – act on various cell types and can affect pathophysiology in both reparative and detrimental manners. Given that the nervous system does not act in isolation, it is critical to consider the potential effects that a novel therapy could have on other cells, including immune cells (Gensel et al., 2011). An ideal treatment for improving CNS repair might drive an anti-inflammatory M2 macrophage phenotype, while simultaneously enhancing axon extension or neuron survival. Therefore, future studies should continue to explore potential treatments that act synergistically on the nervous and immune systems to promote recovery after CNS injury.
Acknowledgments:
This work was supported by the National Institute of Neurological Disorders and Stroke (NS047175 and NS072304), the Craig H. Neilsen Foundation (JCG & KAK), Canadian Institutes of Health Research (ADG), and the Ray W. Poppleton Endowment (PGP).
References:
- Alexiou P, Chatzopoulou M, Pegklidou K, Demopoulos VJ (2010) RAGE: a multi-ligand receptor unveiling novel insights in health and disease. Curr Med Chem 17:2232–2252. [DOI] [PubMed] [Google Scholar]
- Almkvist J, Dahlgren C, Leffler H, Karlsson A (2002) Activation of the neutrophil nicotinamide adenine dinucleotide phosphate oxidase by galectin-1. J Immunol 168:4034–4041. [DOI] [PubMed] [Google Scholar]
- Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, Gage FH, Ffrench-Constant C, Fawcett JW (2009) Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. Journal of Neuroscience 29:5546–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni O, Pur Z, Yefenof E, Baniyash M (1998) Complement receptor 3 of macrophages is associated with galectin-1-like protein. J Immunol 160:6151–6158. [PubMed] [Google Scholar]
- Azari MF, Lopes EC, Stubna C, Turner BJ, Zang D, Nicola NA, Kurek JB, Cheema SS (2003) Behavioural and anatomical effects of systemically administered leukemia inhibitory factor in the SOD1(G93A G1H) mouse model of familial amyotrophic lateral sclerosis. Brain Res 982:92–97. [DOI] [PubMed] [Google Scholar]
- Bao F, Brown A, Dekaban GA, Omana V, Weaver LC (2011) CD11d integrin blockade reduces the systemic inflammatory response syndrome after spinal cord injury. Exp Neurol 231:272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC (2004) Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem 88:1335–1344. [DOI] [PubMed] [Google Scholar]
- Barrionuevo P, Beigier-Bompadre M, Ilarregui JM, Toscano MA, Bianco GA, Isturiz MA, Rabinovich GA (2007) A novel function for galectin-1 at the crossroad of innate and adaptive immunity: galectin-1 regulates monocyte/macrophage physiology through a nonapoptotic ERK-dependent pathway. J Immunol 178:436–445. [DOI] [PubMed] [Google Scholar]
- Boivin A, Pineau I, Barrette B, Filali M, Vallières N, Rivest S, Lacroix S (2007) Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci 27:12565–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6:137–143. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM (2011) Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull 84:306–316. [DOI] [PubMed] [Google Scholar]
- Brück W, Friede RL (1990) Anti-macrophage CR3 antibody blocks myelin phagocytosis by macrophages in vitro. Acta Neuropathol 80:415–418. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Nomden A, van Noort JM, Baron W (2012) Toll-like receptors 2 and 3 agonists differentially affect oligodendrocyte survival, differentiation, and myelin membrane formation. J Neurosci Res 90:388–398. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Emery B, Cipriani T, Marriott MP, Kilpatrick TJ (2006) Endogenous leukemia inhibitory factor production limits autoimmune demyelination and oligodendrocyte loss. Glia 53:696–703. [DOI] [PubMed] [Google Scholar]
- Cafferty WBJ, Gardiner NJ, Das P, Qiu J, Mcmahon SB, Thompson SWN (2004) Conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. Journal of Neuroscience 24:4432–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camby I, Le Mercier M, Lefranc F, Kiss R (2006) Galectin-1: a small protein with major functions. Glycobiology 16:137R–157R. [DOI] [PubMed] [Google Scholar]
- Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, Flavell R, Strittmatter SM, Volpe J, Sidman R, Vartanian T (2007) Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. Journal of Neuroscience 27:13033–13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT (2006) The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. Journal of Neuroscience 26:5565–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Sabol A, Xu H, Gyda MA, Rasband K, Granato M, Chien C-B, Raper JA (2007) Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo. Journal of Neuroscience 27:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A (2010) Control of macrophage activation and function by PPARs. Circ Res 106:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A, Haulon S, Zawadzki C, Jude B, Staels B (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ Res 108:985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic ML (2001) Adult neuronal regeneration induced by transgenic integrin expression. Journal of Neuroscience 21:4782–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D, Norling LV, Perretti M (2008) Novel insights into the inhibitory effects of Galectin-1 on neutrophil recruitment under flow. J Leukoc Biol 83:1459–1466. [DOI] [PubMed] [Google Scholar]
- Correa SG, Sotomayor CE, Aoki MP, Maldonado CA, Rabinovich GA (2003) Opposite effects of galectin-1 on alternative metabolic pathways of L-arginine in resident, inflammatory, and activated macrophages. Glycobiology 13:119–128. [DOI] [PubMed] [Google Scholar]
- De Giusti CJ, Alberdi L, Frik J, Ferrer MF, Scharrig E, Schattner M, Gomez RM (2011) Galectin-3 is upregulated in activated glia during Junin virus-induced murine encephalitis. Neurosci Lett 501:163–166. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Michalik L, Wahli W (2006) Transcriptional regulation of metabolism. Physiol Rev 86:465–514. [DOI] [PubMed] [Google Scholar]
- Diez-Revuelta N, Velasco S, Andre S, Kaltner H, Kubler D, Gabius HJ, Abad-Rodriguez J (2010) Phosphorylation of adhesion- and growth-regulatory human galectin-3 leads to the induction of axonal branching by local membrane L1 and ERM redistribution. Journal of Cell Science 123:671–681. [DOI] [PubMed] [Google Scholar]
- Dill J, Patel AR, Yang X-L, Bachoo R, Powell CM, Li S (2010) A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. Journal of Neuroscience 30:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG (2011) Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. Journal of Neuroscience 31:9910–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnworth SL, Henderson NC, MacKinnon AC, Atkinson KM, Wilkinson T, Dhaliwal K, Hayashi K, Simpson AJ, Rossi AG, Haslett C, Sethi T (2008) Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol 172:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J (1999) Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 19:8182–8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Gonzalez-Lara LE, Foster PJ, Weaver LC (2009) Timing and duration of anti-alpha4beta1 integrin treatment after spinal cord injury: effect on therapeutic efficacy. J Neurosurg Spine 11:575–587. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC (2008) Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp Neurol 214:147–159. [DOI] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197:1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner NJ (2011) Integrins and the extracellular matrix: key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol 71:1054–1072. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Leung M, Poirier F, Kadoya T, Horie H, Ramer MS (2009) A role for galectin-1 in the immune response to peripheral nerve injury. Exp Neurol 220:320–327. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Steeves JD, Tetzlaff W, Ramer MS (2005) Expression and functions of galectin-1 in sensory and motoneurons. Curr Drug Targets 6:419–425. [DOI] [PubMed] [Google Scholar]
- Geeven G, Macgillavry HD, Eggers R, Sassen MM, Verhaagen J, Smit AB, de Gunst MCM, van Kesteren RE (2011) LLM3D: a log-linear modeling-based method to predict functional gene regulatory interactions from genome-wide expression data. Nucleic Acids Res 39:5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Donnelly DJ, Popovich PG (2011) Spinal cord injury therapies in humans: an overview of current clinical trials and their potential effects on intrinsic CNS macrophages. Expert Opin Ther Targets 15:505–518. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, Van Rooijen N, Ankeny DP, Popovich PG (2009) Macrophages promote axon regeneration with concurrent neurotoxicity. Journal of Neuroscience 29:3956–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson CL (1999) The extracellular matrix of gliomas: modulation of cell function. J Neuropathol Exp Neurol 58:1029–1040. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS (2003) Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol 184:456–463. [DOI] [PubMed] [Google Scholar]
- Göttle P, Kremer D, Jander S, Odemis V, Engele J, Hartung H-P, Küry P (2010) Activation of CXCR7 receptor promotes oligodendroglial cell maturation. Ann Neurol 68:915–924. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC (2004) Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci 24:4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris P, Tighe A, Levin D, Sharma R, Brown A (2007) Transcriptional regulation of scar gene expression in primary astrocytes. Glia 55:1145–1155. [DOI] [PubMed] [Google Scholar]
- Gu M, Wang W, Song WK, Cooper DN, Kaufman SJ (1994) Selective modulation of the interaction of alpha 7 beta 1 integrin with fibronectin and laminin by L-14 lectin during skeletal muscle differentiation. Journal of Cell Science 107 (Pt 1):175–181. [DOI] [PubMed] [Google Scholar]
- Gupta D, Tator CH, Shoichet MS (2006) Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 27:2370–2379. [DOI] [PubMed] [Google Scholar]
- Hakkoum D, Stoppini L, Muller D (2007) Interleukin-6 promotes sprouting and functional recovery in lesioned organotypic hippocampal slice cultures. J Neurochem 100:747–757. [DOI] [PubMed] [Google Scholar]
- Hauk TG, Leibinger M, Müller A, Andreadaki A, Knippschild U, Fischer D (2010) Stimulation of axon regeneration in the mature optic nerve by intravitreal application of the toll-like receptor 2 agonist Pam3Cys. Invest Ophthalmol Vis Sci 51:459–464. [DOI] [PubMed] [Google Scholar]
- Hawthorne AL, Hu H, Kundu B, Steinmetz MP, Wylie CJ, Deneris ES, Silver J (2011) The unusual response of serotonergic neurons after CNS Injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. Journal of Neuroscience 31:5605–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne AL, Popovich PG (2011) Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics 8:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Sethi T (2009) The regulation of inflammation by galectin-3. Immunol Rev 230:160–171. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. Journal of Neuroscience 28:7231–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Tontonoz P (2008) Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev 18:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie H, Inagaki Y, Sohma Y, Nozawa R, Okawa K, Hasegawa M, Muramatsu N, Kawano H, Horie M, Koyama H, Sakai I, Takeshita K, Kowada Y, Takano M, Kadoya T (1999) Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. Journal of Neuroscience 19:9964–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie H, Kadoya T, Hikawa N, Sango K, Inoue H, Takeshita K, Asawa R, Hiroi T, Sato M, Yoshioka T, Ishikawa Y (2004) Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. Journal of Neuroscience 24:1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn EM, Beaumont M, Shu XZ, Harvey A, Prestwich GD, Horn KM, Gibson AR, Preul MC, Panitch A (2007) Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J Neurosurg Spine 6:133–140. [DOI] [PubMed] [Google Scholar]
- Horn KP, Busch SA, Hawthorne AL, Van Rooijen N, Silver J (2008) Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci 28:9330–9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, Liu FT (2000) Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol 156:1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y, Sohma Y, Horie H, Nozawa R, Kadoya T (2000) Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur J Biochem 267:2955–2964. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Christenson K, Matlak M, Björstad A, Brown KL, Telemo E, Salomonsson E, Leffler H, Bylund J (2009) Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology 19:16–20. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Patterson PH (2004) Potent pro-inflammatory actions of leukemia inhibitory factor in the spinal cord of the adult mouse. Exp Neurol 188:391–407. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Patterson PH (2005) Leukemia inhibitory factor promotes oligodendrocyte survival after spinal cord injury. Glia 51:73–79. [DOI] [PubMed] [Google Scholar]
- Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE (2011) High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng 8:046033. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG (2007) Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem 102:37–50. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Popovich PG (2009) Toll-like receptors in spinal cord injury. Curr Top Microbiol Immunol 336:121–136. [DOI] [PubMed] [Google Scholar]
- Klinge CM, Bodenner DL, Desai D, Niles RM, Traish AM (1997) Binding of type II nuclear receptors and estrogen receptor to full and half-site estrogen response elements in vitro. Nucleic Acids Res 25:1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler RE, Comerford I, Townley S, Haylock-Jacobs S, Clark-Lewis I, McColl SR (2008) Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain pathology (Zurich, Switzerland) 18:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara I, Liu FT (1996) Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol 156:3939–3944. [PubMed] [Google Scholar]
- Kvarnhammar AM, Cardell LO (2012) Pattern-recognition receptors in human eosinophils. Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La M, Cao TV, Cerchiaro G, Chilton K, Hirabayashi J, Kasai K-I, Oliani SM, Chernajovsky Y, Perretti M (2003) A novel biological activity for galectin-1: inhibition of leukocyte-endothelial cell interactions in experimental inflammation. Am J Pathol 163:1505–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latasa M-J, Cosgaya JM (2011) Regulation of retinoid receptors by retinoic acid and axonal contact in Schwann cells. PLoS ONE 6:e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Jo E-K, Choi S-Y, Oh SB, Park K, Kim JS, Lee SJ (2006) Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochemical and Biophysical Research Communications 350:742–747. [DOI] [PubMed] [Google Scholar]
- Liu F-T, Rabinovich GA (2010) Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci 1183:158–182. [DOI] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Hill PN, Shenhav A, Kuwabara I, Chen SS (1996) Modulation of functional properties of galectin-3 by monoclonal antibodies binding to the non-lectin domains. Biochemistry 35:6073–6079. [DOI] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR (1995) Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol 147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- López-Lucendo MF, Solís D, André S, Hirabayashi J, Kasai K-I, Kaltner H, Gabius H-J, Romero A (2004) Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol 343:957–970. [DOI] [PubMed] [Google Scholar]
- Luo B-H, Carman CV, Springer TA (2007) Structural basis of integrin regulation and signaling. Annu Rev Immunol 25:619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD (1998) Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med 338:436–445. [DOI] [PubMed] [Google Scholar]
- Ma Y, Haynes RL, Sidman RL, Vartanian T (2007) TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle 6:2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lü J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T (2006) Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol 175:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T (2008) Regulation of alternative macrophage activation by galectin-3. J Immunol 180:2650–2658. [DOI] [PubMed] [Google Scholar]
- Mahoney SA, Wilkinson M, Smith S, Haynes LW (2000) Stabilization of neurites in cerebellar granule cells by transglutaminase activity: identification of midkine and galectin-3 as substrates. Neuroscience 101:141–155. [DOI] [PubMed] [Google Scholar]
- Makoukji J, Shackleford G, Meffre D, Grenier J, Liere P, Lobaccaro J-MA, Schumacher M, Massaad C (2011) Interplay between LXR and Wnt/β-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. Journal of Neuroscience 31:9620–9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik RKJ, Ghurye RR, Lawrence-Watt DJ, Stewart HJS (2009) Galectin-1 stimulates monocyte chemotaxis via the p44/42 MAP kinase pathway and a pertussis toxin-sensitive pathway. Glycobiology 19:1402–1407. [DOI] [PubMed] [Google Scholar]
- McGraw J, Gaudet AD, Oschipok LW, Steeves JD, Poirier F, Tetzlaff W, Ramer MS (2005) Altered primary afferent anatomy and reduced thermal sensitivity in mice lacking galectin-1. Pain 114:7–18. [DOI] [PubMed] [Google Scholar]
- McGraw J, McPhail LT, Oschipok LW, Horie H, Poirier F, Steeves JD, Ramer MS, Tetzlaff W (2004) Galectin-1 in regenerating motoneurons. Eur J Neurosci 20:2872–2880. [DOI] [PubMed] [Google Scholar]
- Mctigue DM, Tripathi R, Wei P, Lash AT (2007) The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol 205:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng B, Zhang Q, Huang C, Zhang HT, Tang T, Yang HL (2011) Effects of a single dose of methylprednisolone versus three doses of rosiglitazone on nerve growth factor levels after spinal cord injury. J Int Med Res 39:805–814. [DOI] [PubMed] [Google Scholar]
- Miglio G, Rattazzi L, Rosa AC, Fantozzi R (2009) PPARgamma stimulation promotes neurite outgrowth in SH-SY5Y human neuroblastoma cells. Neurosci Lett 454:134–138. [DOI] [PubMed] [Google Scholar]
- Moiseeva EP, Williams B, Goodall AH, Samani NJ (2003) Galectin-1 interacts with beta-1 subunit of integrin. Biochemical and Biophysical Research Communications 310:1010–1016. [DOI] [PubMed] [Google Scholar]
- Motran CC, Molinder KM, Liu SD, Poirier F, Miceli MC (2008) Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur J Immunol 38:3015–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaino M, Nakamura M, Yamada O, Okada S, Morikawa S, Renault-Mihara F, Iwanami A, Ikegami T, Ohsugi Y, Tsuji O, Katoh H, Matsuzaki Y, Toyama Y, Liu M, Okano H (2010) Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp Neurol 224:403–414. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS (2003) Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol 184:313–325. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Okada S, Toyama Y, Okano H (2005) Role of IL-6 in spinal cord injury in a mouse model. Clin Rev Allergy Immunol 28:197–204. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ (1999) Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23:83–91. [DOI] [PubMed] [Google Scholar]
- Ni J, Zhu Y-N, Zhong X-G, Ding Y, Hou L-F, Tong X-K, Tang W, Ono S, Yang Y-F, Zuo J-P (2009) The chemokine receptor antagonist, TAK-779, decreased experimental autoimmune encephalomyelitis by reducing inflammatory cell migration into the central nervous system, without affecting T cell function. Br J Pharmacol 158:2046–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Perretti M, Cooper D (2009) Endogenous galectins and the control of the host inflammatory response. J Endocrinol 201:169–184. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447:1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A (2008) Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 7:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834. [DOI] [PubMed] [Google Scholar]
- Okada T, Ichikawa M, Tokita Y, Horie H, Saito K, Yoshida J, Watanabe M (2005) Intravitreal macrophage activation enables cat retinal ganglion cells to regenerate injured axons into the mature optic nerve. Exp Neurol 196:153–163. [DOI] [PubMed] [Google Scholar]
- Olefsky JM (2001) Nuclear receptor minireview series. J Biol Chem 276:36863–36864. [DOI] [PubMed] [Google Scholar]
- Opatz J, Küry P, Schiwy N, Järve A, Estrada V, Brazda N, Bosse F, Müller HW (2009) SDF-1 stimulates neurite growth on inhibitory CNS myelin. Mol Cell Neurosci 40:293–300. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z (2010) PTEN/mTOR and axon regeneration. Exp Neurol 223:45–50. [DOI] [PubMed] [Google Scholar]
- Park S-H, Min HS, Kim B, Myung J, Paek SH (2008) Galectin-3: a useful biomarker for differential diagnosis of brain tumors. Neuropathology 28:497–506. [DOI] [PubMed] [Google Scholar]
- Park S-W, Yi J-H, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R (2007) Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 320:1002–1012. [DOI] [PubMed] [Google Scholar]
- Patel JR, McCandless EE, Dorsey D, Klein RS (2010) CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci USA 107:11062–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti I, Esposito E, Mazzon E, Galuppo M, Di Paola R, Bramanti P, Kapoor A, Thiemermann C, Cuzzocrea S (2010) Evidence for the role of peroxisome proliferator-activated receptor-beta/delta in the development of spinal cord injury. J Pharmacol Exp Ther 333:465–477. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Marcus ME, Baum LG (1998) Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med 76:402–412. [DOI] [PubMed] [Google Scholar]
- Pesheva P, Kuklinski S, Schmitz B, Probstmeier R (1998) Galectin-3 promotes neural cell adhesion and neurite growth. J Neurosci Res 54:639–654. [DOI] [PubMed] [Google Scholar]
- Pittier R, Sauthier F, Hubbell JA, Hall H (2005) Neurite extension and in vitro myelination within three-dimensional modified fibrin matrices. J Neurobiol 63:1–14. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM (2002) The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol 61:623–633. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT (1999) Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158:351–365. [DOI] [PubMed] [Google Scholar]
- Puche AC, Poirier F, Hair M, Bartlett PF, Key B (1996) Role of galectin-1 in the developing mouse olfactory system. Dev Biol 179:274–287. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Ariel A, Hershkoviz R, Hirabayashi J, Kasai KI, Lider O (1999) Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology 97:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG (2000) Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol 30:1331–1339. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM (2009) Chemokines and Chemokine Receptors: Standing at the Crossroads of Immunobiology and Neurobiology. Immunity 31:711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redensek A, Rathore KI, Berard JL, López-Vales R, Swayne LA, Bennett SAL, Mohri I, Taniike M, Urade Y, David S (2011) Expression and detrimental role of hematopoietic prostaglandin D synthase in spinal cord contusion injury. Glia 59:603–614. [DOI] [PubMed] [Google Scholar]
- Reichert F, Rotshenker S (1999) Galectin-3/MAC-2 in experimental allergic encephalomyelitis. Exp Neurol 160:508–514. [DOI] [PubMed] [Google Scholar]
- Reichert F, Slobodov U, Makranz C, Rotshenker S (2001) Modulation (inhibition and augmentation) of complement receptor-3-mediated myelin phagocytosis. Neurobiol Dis 8:504–512. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM (1984) Peripheral injury enhances central regeneration of primary sensory neurones. Nature 309:791–793. [DOI] [PubMed] [Google Scholar]
- Rong LL, Yan S-F, Wendt T, Hans D, Pachydaki S, Bucciarelli LG, Adebayo A, Qu W, Lu Y, Kostov K, Lalla E, Yan SD, Gooch C, Szabolcs M, Trojaborg W, Hays AP, Schmidt AM (2004) RAGE modulates peripheral nerve regeneration via recruitment of both inflammatory and axonal outgrowth pathways. The FASEB Journal 18:1818–1825. [DOI] [PubMed] [Google Scholar]
- Rose DM, Alon R, Ginsberg MH (2007) Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev 218:126–134. [DOI] [PubMed] [Google Scholar]
- Rotshenker S (2003) Microglia and macrophage activation and the regulation of complement-receptor-3 (CR3/MAC-1)-mediated myelin phagocytosis in injury and disease. J Mol Neurosci 21:65–72. [DOI] [PubMed] [Google Scholar]
- Rotshenker S (2009) The role of Galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J Mol Neurosci 39:99–103. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT (2000) Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol 165:2156–2164. [DOI] [PubMed] [Google Scholar]
- Schimizzi AL, Massie JB, Murphy M, Perry A, Kim CW, Garfin SR, Akeson WH (2006) High-molecular-weight hyaluronan inhibits macrophage proliferation and cytokine release in the early wound of a preclinical postlaminectomy rat model. Spine J 6:550–556. [DOI] [PubMed] [Google Scholar]
- Schonberg DL, Popovich PG, Mctigue DM (2007) Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J Neuropathol Exp Neurol 66:1124–1135. [DOI] [PubMed] [Google Scholar]
- Schrage K, Koopmans G, Joosten EAJ, Mey J (2006) Macrophages and neurons are targets of retinoic acid signaling after spinal cord contusion injury. Eur J Neurosci 23:285–295. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T (2010) Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci USA 107:11555–11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J (2005) Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci 25:8066–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Kwok JCF, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW (2011) Integrin Activation Promotes Axon Growth on Inhibitory Chondroitin Sulfate Proteoglycans by Enhancing Integrin Signaling. J Neurosci 31:6289–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Heesen M, Yoshizawa I, Berman MA, Luo Y, Bleul CC, Springer TA, Okuda K, Gerard N, Dorf ME (1997) Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J Immunol 159:905–911. [PubMed] [Google Scholar]
- Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL (2007) Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem 282:18265–18275. [DOI] [PubMed] [Google Scholar]
- Tysseling VM, Mithal D, Sahni V, Birch D, Jung H, Belmadani A, Miller RJ, Kessler JA (2011) SDF1 in the dorsal corticospinal tract promotes CXCR4+ cell migration after spinal cord injury. J Neuroinflammation 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR (2012) Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol 946:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao N, Imagama S, Zhang H, Tauchi R, Muramoto A, Natori T, Takeshita S, Ishiguro N, Matsuyama Y, Kadomatsu K (2011) Hyaluronan oligosaccharides promote functional recovery after spinal cord injury in rats. Neurosci Lett 488:299–304. [DOI] [PubMed] [Google Scholar]
- Wang M-J, Kuo J-S, Lee W-W, Huang H-Y, Chen W-F, Lin S-Z (2006) Translational event mediates differential production of tumor necrosis factor-alpha in hyaluronan-stimulated microglia and macrophages. J Neurochem 97:857–871. [DOI] [PubMed] [Google Scholar]
- Yasuda T (2007) Hyaluronan inhibits cytokine production by lipopolysaccharide-stimulated U937 macrophages through down-regulation of NF-kappaB via ICAM-1. Inflamm Res 56:246–253. [DOI] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI (2003) Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci 23:2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip PM, Siu CH (2001) PC12 cells utilize the homophilic binding site of L1 for cell-cell adhesion but L1-alphavbeta3 interaction for neurite outgrowth. J Neurochem 76:1552–1564. [DOI] [PubMed] [Google Scholar]
- Yip PM, Zhao X, Montgomery AM, Siu CH (1998) The Arg-Gly-Asp motif in the cell adhesion molecule L1 promotes neurite outgrowth via interaction with the alphavbeta3 integrin. Mol Biol Cell 9:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang DW, Cheema SS (2003) Leukemia inhibitory factor promotes recovery of locomotor function following spinal cord injury in the mouse. J Neurotrauma 20:1215–1222. [DOI] [PubMed] [Google Scholar]
- Zhang H, Trivedi A, Lee J-U, Lohela M, Lee SM, Fandel TM, Werb Z, Noble-Haeusslein LJ (2011) Matrix metalloproteinase-9 and stromal cell-derived factor-1 act synergistically to support migration of blood-borne monocytes into the injured spinal cord. Journal of Neuroscience 31:15894–15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Freyer D, Harhausen D, Khojasteh U, Nietfeld W, Trendelenburg G (2011) Blocking TLR2 in vivo protects against accumulation of inflammatory cells and neuronal injury in experimental stroke. J Cereb Blood Flow Metab 31:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]


