Abstract
FKS1 and FKS2 are alternative subunits of the glucan synthase complex, which is responsible for synthesizing 1,3-β-glucan chains, the major structural polymer of the Saccharomyces cerevisiae cell wall. Expression of FKS1 predominates during growth under optimal conditions. In contrast, FKS2 expression is induced by mating pheromone, high extracellular [Ca2+], growth on poor carbon sources, or in an fks1 mutant. Induction of FKS2 expression in response to pheromone, CaCl2, or loss of FKS1 function requires the Ca2+/calmodulin-dependent protein phosphatase calcineurin. Therefore, a double mutant in calcineurin (CNB1) and FKS1 is inviable due to a deficiency in FKS2 expression. To identify novel regulators of FKS2 expression, we isolated genes whose overexpression obviates the calcineurin requirement for viability of an fks1 mutant. Two components of the cell integrity signaling pathway controlled by the RHO1 G protein (MKK1 and RLM1) were identified through this screen. This signaling pathway is activated during growth at moderately high temperatures. We demonstrate that calcineurin and the cell integrity pathway function in parallel, through separable promoter elements, to induce FKS2 expression during growth at 39°C. Because RHO1 also serves as a regulatory subunit of the glucan synthase, our results define a regulatory circuit through which RHO1 controls both the activity of this enzyme complex and the expression of at least one of its components. We show also that FKS2 induction during growth on poor carbon sources is a response to glucose depletion and is under the control of the SNF1 protein kinase and the MIG1 transcriptional repressor. Finally, we show that FKS2 expression is induced as cells enter stationary phase through a SNF1-, calcineurin-, and cell integrity signaling-independent pathway.
The cell wall of the budding yeast Saccharomyces cerevisiae is required to maintain cell shape and integrity (4, 20). Vegetative proliferation requires that the cell remodels its wall to accommodate growth. The main structural components responsible for the rigidity of the yeast cell wall are 1,3-β-linked glucan polymers with some branches through 1,6-β linkages. The biochemistry of the yeast enzyme complex that catalyzes the synthesis of 1,3-β-glucan chains has been studied extensively (15, 29), and three genes that encode components of this complex have been identified. A pair of closely related genes, FKS1 and FKS2, encode alternative subunits of the 1,3-β-glucan synthase (GS) (8, 15, 28, 36). Either FKS1 or FKS2 function is sufficient for GS activity and cell viability. Additionally, the Rho1 GTPase is an essential regulatory subunit of the GS complex, serving to stimulate GS activity in a GTP-dependent manner (9, 35).
A second essential function of RHO1 is to regulate the cell integrity signaling pathway by binding and activating protein kinase C (19, 33), which is encoded by PKC1 (25). Loss of PKC1 function results in a cell lysis defect that is attributable to a deficiency in cell wall construction (23, 24, 34). Components on one branch of the RHO1-PKC1-regulated signaling pathway comprise a linear mitogen-activated protein kinase (MAPK) activation cascade. These include a MEK kinase (MEKK) homolog (BCK1 [5, 22]), a redundant pair of MEK homologs (MKK1 and MKK2 [16]), and a MAPK homolog (MPK1 [21], initially designated SLT2 [40]). Deletion of any of these components results in cell lysis when cells are cultivated under conditions of mild thermal stress (i.e., 37 to 39°C). Elevated growth temperature, thought to pose a challenge to the cells’ ability to construct adequate cell walls, also induces persistent activation of MPK1 (18). The regulatory output of the cell integrity signaling pathway is only now beginning to be explored.
The FKS1 and FKS2 genes differ primarily in the manner in which their expression is controlled. Under optimal growth conditions, FKS1 is the predominantly expressed gene, and its mRNA levels fluctuate periodically through the cell cycle (28, 36). Expression of FKS2 is low under optimal growth conditions, but expression is induced in response to treatment with mating pheromone, CaCl2, or growth on poor carbon sources or in the absence of FKS1 function (28). The pathway for induction of FKS2 expression by pheromone or CaCl2 or in fks1 mutants requires the Ca2+/calmodulin-dependent protein phosphatase calcineurin (PP2B [6, 39]). However, FKS2 induction by poor carbon sources is calcineurin independent.
A double mutant in calcineurin (CNB1) and FKS1 is inviable due to a deficiency in FKS2 expression (11). To identify novel regulators of FKS2 gene expression, we have isolated genes whose overexpression obviates the calcineurin requirement for viability of an fks1 mutant. In this study, we describe the isolation of MKK1, a component of the cell integrity signaling pathway (16), and RLM1, a putative transcription factor that has also been implicated in cell integrity signaling (7, 42, 43), as positive regulators of FKS2 expression. We demonstrate that calcineurin and the cell integrity pathway function in parallel, through separable promoter elements, to induce FKS2 expression under conditions of thermal stress and thereby provide the first clear evidence for a direct target of cell integrity signaling. We demonstrate also that FKS2 induction in response to glucose depletion is under the control of the SNF1-regulated MIG1 transcriptional repressor (31, 32). Finally, we show that FKS2 expression is induced strongly as cells enter stationary phase and that this induction is not mediated by any of the known regulatory inputs for FKS2 expression.
MATERIALS AND METHODS
Strains, growth conditions, and transformations.
The S. cerevisiae strains used in this study are listed in Table 1. Yeast cultures were grown in YEP (1% Bacto-Yeast Extract, 2% Bacto-Peptone) supplemented with 2% glucose. Synthetic minimal medium (SD [37]) supplemented with the appropriate nutrients was used to select for plasmid maintenance. Yeast transformations were carried out by the lithium acetate method (17). Escherichia coli DH5α was used for the propagation of all plasmids. E. coli cells were cultured in Luria broth medium (1% Bacto-Tryptone, 0.5% Bacto-Yeast Extract, 1% NaCl) and transformed by standard methods (26).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| 1783 | MATaa | I. Herskowitz |
| 1788 | MATa/MATαa | I. Herskowitz |
| DL1009 | MATa/MATα mpk1Δ::TRP1/mpk1Δ::TRP1 (YEp351[mpk1 T190A, Y192F::HA])a | This study |
| DL251 | MATa/MATα bck1Δ::URA3/bck1Δ::URA3a | 22 |
| GMY63-5D | MATa rlm1Δ::LEU2a | K. Irie |
| PGY440 | MATα mpk1Δ::TRP1 fks1Δ::hisGa | This study |
| PGY482 | MATa rlm1Δ::LEU2 fks1Δ::hisGa | This study |
| YPH499 | MATab | P. Hieter |
| MCY3-1D | MATa cnb1Δ::LEU2b | 11 |
| PGY5 | MATa fks1Δ::URA3b | 11 |
| PGY220 | MATa fks1Δ::TRP1b | This study |
| PGY318 | MATa fks1Δ::HIS3 cnb1Δ::ADE2 (pGAL[CNB1])b | This study |
| MCY1093 | MATac | M. Carlson |
| MCY1846 | MATa snf1Δ10c | M. Carlson |
| YM4797 | MATa trp1-901 tyr1-501 met? can1?d | M. Johnston |
| YM4807 | MATa mig1Δ::ura3::LYS2 met− can1rd | M. Johnston |
EG123 background: leu2-3,112 trp1-1 ura3-52 his can1.
YPH499 background: ura3-52 lys2-801 ade2-101 trp-Δ63 his3-Δ200 leu 2-Δ1.
MCY1093 background: lys2-801 ura3-52 his4-539.
Congenic strains.
Isolation of high-copy-number suppressors of a cnb1Δ fks1Δ mutant.
PGY318 was transformed with one of two yeast genomic libraries constructed in high-copy-number LEU2-bearing plasmids, either YEp351 (13) or YEp13 (ATCC), and was grown at 25°C on solid synthetic medium lacking leucine and containing glucose. A portion of each transformation was grown on solid synthetic medium lacking leucine and containing galactose to estimate the transformation efficiency. Selection for plasmid loss was carried out by using the uracil biosynthesis antagonist 5-fluoro-orotic acid (5-FOA) (1). An FK506 sensitivity test was done by spotting the cells onto medium containing 2 mg of FK506/ml.
Plasmid construction.
Plasmids pLGΔ-178 and pLGΔ-312, which contain the CYC1-lacZ fusion genes were described previously (12). Plasmid FKS2(−928 to −1)-lacZ was constructed by two steps. First, pCZ-FKS2 was created by inserting a 955-bp PCR-amplified FKS2 fragment into the XbaI/BamHI sites of pCZ4 (2). PCR was carried out with the primer pair 5′-GGCCCGCTCTAGAATCTTCCGATCATCATCATCGGCGCGTTC-3′ (sense) and 5′-TCGGATCCGGTCATAACTATGACAGTTTAATAAT-3′ (antisense). (The XbaI and BamHI sites used for cloning are underlined.) Second, a SalI/BamHI fragment from pCZ-FKS2 was excised and cloned into the XhoI/BamHI sites of pLGΔ-178. To generate plasmid FKS2(−706 to −1)-lacZ, a 713-bp PCR-amplified FKS2 fragment was placed in frame into the XhoI/BamHI sites of pLGΔ-178, which had been made blunt with Klenow fragment. PCR was carried out with the primer pair 5′-TGGTGATGGGGTGTAGG-3′ (sense) and 5′-CGGACATAACTATGACAG-3′ (antisense). Plasmid FKS2(−706 to −361)-CYC1-lacZ was constructed by replacing the 134-bp SmaI/XhoI fragment of the CYC1 promoter of pLGΔ-312 with a 345-bp PCR-amplified FKS2 fragment, resulting in the removal of the CYC1 upstream activation sequence sites from the promoter region and the fusion of FKS2 upstream sequence from −706 to −361 to the CYC1 minimal promoter. PCR was carried out with the primer pair 5′-TGGTGATGGGGTGTAGG-3′ (sense) and 5′-AATACACTCGAGAAGCTTTTATTTTTGTA-3′ (antisense). (The XhoI site used for cloning is underlined.) All numbers are given with respect to the translational start codon.
RNA analysis.
Strains were grown to a density of 1.5 × 107 to 3 × 107 cells/ml (4.5 × 108 to 6 × 108 cells/ml for stationary-phase cells), collected by centrifugation at 1,500 × g for 5 min at 4°C, washed once with 1 ml of ice-cold diethylpyrocarbonate-H2O, frozen on dry ice, and stored at −70°C. Total RNA was prepared by resuspending the cell pellet in 400 μl of TES solution (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 0.5% sodium dodecyl sulfate), adding 400 μl of acidic phenol, vortexing vigorously for 10 s, and incubating for 1 h at 65°C with occasional, brief vortexing. The mixture was placed on ice for 5 min, and after centrifugation at 13,000 × g in a microcentrifuge for 5 min, the aqueous phase was removed. The RNA was purified by phenol and chloroform extractions and precipitated with 100% ethanol in the presence of 0.3 M sodium acetate (pH 5.3) at −20°C. RNA was separated on a 1% formaldehyde-agarose gel (1% agarose, 20 mM MOPS [morpholinepropanesulfonic acid], 1 mM EDTA, 5 mM sodium acetate, 2.2 M formaldehyde), transferred to a Hybond-N membrane (Amersham) as described in Maniatis et al. (26), and cross-linked by incubation in a vacuum incubator at 80°C for 2.5 h. Blots were probed with radiolabelled restriction fragments (ACT1 and RHO1) or PCR-amplified fragments (FKS1 and FKS2). The following primer pairs were used to synthesize FKS1 and FKS2 fragments, respectively: 5′-ATGAACACTGATCAAC-3′ (sense) and 5′-AATTACCGTAAATTGG-3′ (antisense) and 5′-ATGTCCTACAACGATCC-3′ (sense) and 5′-GAACCATCTTGATCAGG-3′ (antisense). The probes for FKS1 and FKS2 hybridize to a region encoding the divergent N termini of each protein product. RNA levels were quantitated by exposing the membrane to a Fuji phosphorimaging plate and the phosphorimaging data were analyzed by MacBAS 2.0 software. Autoradiographs of membranes were used for figures.
β-Galactosidase assays.
Cells were harvested by centrifugation at 3,000 × g for 5 min and resuspended in 300 μl of breaking buffer (100 mM Tris-HCl [pH 8.0], 1 mM dithiothreitol, and 20% glycerol). The cells were not to be used immediately, and they were quickly frozen at −70°C and stored at −20°C. An equal volume of glass beads, 2 μl of phenylmethylsulfonyl fluoride (10 mg/ml), and 1 μl of leupeptin (10 mg/ml) were added to this suspension, and cells were broken by vigorous vortexing for 4 min. After removal of the beads and cell debris by centrifugation at 13,000 × g for 1 min, the supernatant was further clarified by additional centrifugation for 10 min. All steps were carried out at 4°C. Protein concentrations of cell extracts were measured by the Bradford method (Bio-Rad). β-Galactosidase assays were carried out as previously described (37). Specific activities were given in nanomoles of ONPG (o-nitro-phenyl-β-d-galactopyranoside) converted per minute per milligram of protein. Data presented are mean values from two or three experiments.
GS assays.
Crude extracts were prepared as previously described (18) and stored at −80°C in lysis buffer supplemented with 33% glycerol. GS activity was measured as previously described (29) with the following modifications: uridine diphosphate-[3H]glucose was used as the substrate and α-amylase (1 U/40 μl) was added to reaction mixtures to eliminate [3H]glucose incorporation into glycogen.
RESULTS
Isolation of MKK1 and RLM1 as high-copy-number suppressors of a cnb1Δ fks1Δ mutant.
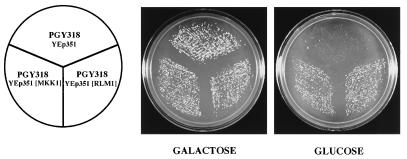
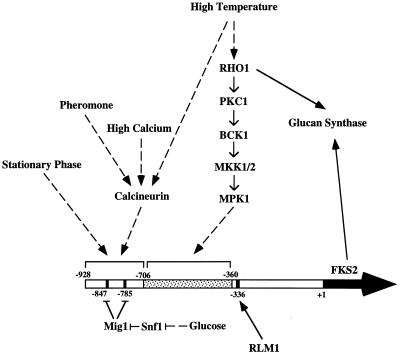
To identify novel regulators of FKS2 expression, we screened a high-copy-number genomic yeast library for clones that could suppress the synthetic lethality of a cnb1Δ fks1Δ mutant, presumably by restoring FKS2 expression. We used a cnb1Δ fks1Δ mutant that carries a plasmid with CNB1 under the inducible control of the GAL1-10 promoter (PGY318). This strain grows normally on galactose-containing medium but is inviable on glucose-containing medium. PGY318 was transformed with either of two high-copy-number S. cerevisiae genomic libraries, and transformants were screened for the ability to grow on glucose-containing medium. To eliminate galactose regulatory mutants, the CNB1-containing plasmid was evicted with 5-FOA (1). A total of 32 5-FOA-resistant transformants were isolated and tested for sensitivity to the calcineurin inhibitor FK506. FK506-sensitive isolates were assumed to carry a CNB1 gene and were not characterized further. Plasmids from 12 transformants were rescued and back-transformed into PGY318 to confirm that the plasmids allowed this strain to grow on glucose-containing medium. Among 10 plasmids isolated, three subjected to DNA sequence analysis are described here. One of these contained a region of chromosome XV with two complete open reading frames, MKK1 and MGE1. A subclone containing only MKK1, a MEK homolog of the cell integrity signaling pathway (16), effectively suppressed the growth defect of PGY318 (Fig. 1). The other two plasmids contained an overlapping region of chromosome XVI that included RLM1, which encodes a member of the serum response factor family of transcription factors (38) that has also been implicated in cell integrity signaling (7, 42, 43). A subclone containing only RLM1 was competent for suppression activity (Fig. 1). The finding that overproduction of either MKK1 or RLM1 suppresses the synthetic lethality of a fks1Δ cnb1Δ mutant suggests that the cell integrity pathway contributes to the expression of FKS2.
FIG. 1.
Suppression of a cnb1Δ fks1Δ mutant by high-copy-number MKK1 or RLM1. Yeast strain PGY318, transformed with YEp351(MKK1), YEp351(RLM1), or YEp351, was streaked onto SD-Leu medium supplemented with 2% glucose or 2% galactose and incubated at 30°C.
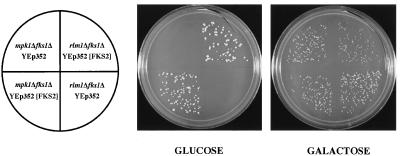
Additional genetic evidence supports the notion that the cell integrity pathway positively regulates FKS2 expression. First, as reported previously, an fks1Δ mpk1Δ double mutant is inviable on glucose-containing medium (11). That result may now be explained by a deficiency in FKS2 expression, similar to the synthetic lethality of an fks1Δ cnb1Δ double mutant. Consistent with this interpretation, we found that expression of FKS2 from a high-copy-number plasmid suppressed the growth defect of an fks1Δ mpk1Δ double mutant (Fig. 2). Second, we found that an fks1Δ rlm1Δ double mutant is inviable on glucose-containing medium and that this defect is suppressed by overexpression of FKS2 (Fig. 2). Interestingly, both double mutants are viable on galactose-containing medium (see section on glucose starvation below).
FIG. 2.
Suppression of an mpk1Δ fks1Δ mutant and an rlm1Δ fks1Δ mutant by high-copy-number FKS2. Yeast strains PGY440 and PGY48, transformed with YEp352(FKS2) or YEp352, were streaked onto SD-Ura medium supplemented with either 2% glucose or 2% galactose and incubated at 30°C.
Ca2+ induction of FKS2 is not dependent on the cell integrity pathway.
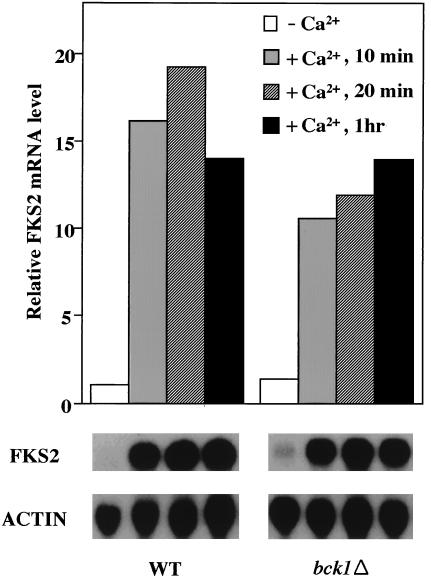
Exogenous Ca2+ stimulates FKS2 expression through a calcineurin-dependent pathway (28). We tested the possibility that the cell integrity signaling pathway is also required for Ca2+-dependent FKS2 expression. A bck1Δ mutant, which is defective in the MEKK of the cell integrity pathway and grows normally at room temperature, was used for this experiment. Figure 3 shows that FKS2 mRNA accumulation was induced rapidly in both wild-type and bck1Δ cells grown at 23°C after addition of CaCl2 (30 mM) to the growth medium, indicating that the MAPK branch of the cell integrity pathway is not required for this calcineurin-dependent response.
FIG. 3.
Calcium induction of FKS2 gene is independent of cell integrity pathway signaling. Log-phase cultures of wild-type (1788) and bck1Δ (DL251) cells were grown at 23°C in the presence or absence of 30mM CaCl2 for the indicated times. Total RNA, isolated after the indicated times, was probed for FKS2 and ACT1 mRNAs. FKS2 mRNA was normalized to ACT1 mRNA. The ACT1-normalized FKS2 mRNA level from wild-type cells grown in the absence of CaCl2 was arbitrarily designated a value of 1. WT, wild type; ACTIN, ACT1.
FKS2 mRNA accumulates in response to growth at high temperature.
We showed previously that the cell integrity signaling pathway is strongly activated in response to growth at moderately high temperatures (i.e., 37 to 39°C [18]). Mutants in cell integrity signaling lyse when cultivated at elevated temperatures due to a deficiency in cell wall construction (18). We have interpreted these findings to indicate that growth under thermal stress poses a challenge to the cells’ ability to construct adequate cell walls, to which the cell responds by activation of cell integrity signaling (18). However, the cellular targets that are induced by this signaling have yet to be identified. The observations described above suggested that FKS2 might be one such target.
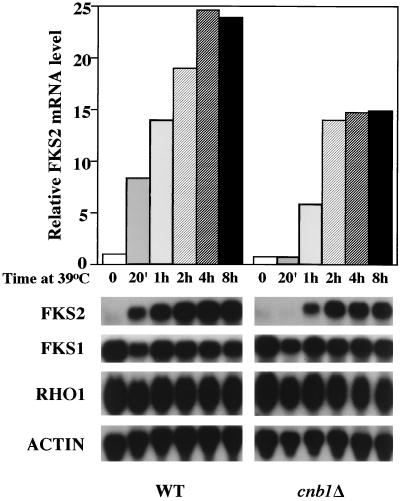
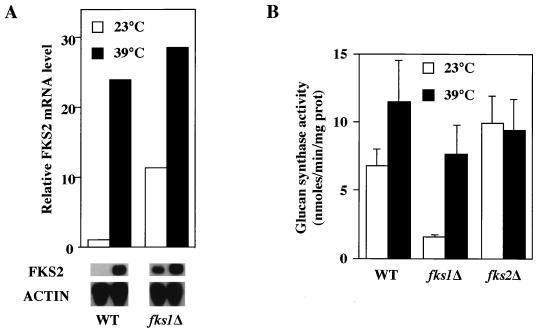
As a first step to determine if the expression of FKS2 is regulated in response to cell integrity signaling, we examined the effect of growth temperature on the steady-state levels of mRNA from FKS2. Figure 4 shows that the level of FKS2 mRNA, which is barely detectable in cells growing at 23°C, is strongly induced by a shift to growth at 39°C, peaking 2 to 4 h after shift, and persists at the elevated level as long as the cells continue to grow at high temperature (not shown). In contrast, accumulation of neither FKS1 nor RHO1 mRNA, which encode the other known components of the GS complex, was induced by growth at high temperature (Fig. 4).
FIG. 4.
Temperature-induced accumulation of FKS2 mRNA. Accumulation of FKS1, FKS2, and RHO1 mRNA in response to temperature upshift was examined in wild-type (YPH499) and cnb1Δ (MCY3-1D) cells. Cells were grown to an A600 of 0.5 to 1 in YEP containing 2% glucose at 23°C. An immediate temperature shift to 39°C was achieved by adding an equal volume of fresh medium prewarmed to 55°C, followed by incubation at 39°C with agitation for the indicated times. Total RNA was probed for FKS2, FKS1, RHO1, and ACT1 mRNAs. WT, wild type; ACTIN, ACT1.
Because FKS1 and FKS2 are catalytic subunits of the GS complex, we examined the effect of growth temperature on GS activity. Wild-type cells shifted from growth at 23°C to growth at 39°C for 6 h strongly induced FKS2 expression (Fig. 5A), but this induction was accompanied by an increase in GS activity of less than twofold (Fig. 5B). This may be explained by the relatively large contribution of FKS1 to GS activity (28). In an fks1Δ mutant, FKS2 expression was elevated (relative to wild type) during growth at low temperature, but was further induced (approximately threefold) in response to elevated temperature (Fig. 5A). This induction was accompanied by a fivefold increase in GS activity (Fig. 5B), presumably because all of the GS activity in this mutant is derived from FKS2. GS activity in an FKS2 mutant was unaffected by growth temperature, consistent with the observation that FKS1 mRNA levels are not influenced by thermal stress (Fig. 4).
FIG. 5.
Effect of growth temperature on GS activity in fks1Δ and fks2Δ mutants. (A) Cultures of wild-type (YPH499) and fks1Δ (PGY5) cells were shifted from log-phase growth at 23 to growth at 39°C. Total RNA, isolated 0 and 6 h after shift, was probed for FKS2 and ACT1 mRNA. (B) Log-phase cultures of wild-type (YPH499), fks1Δ (PGY5), and fks2Δ (PGY220) cells were split and incubated at 23 or 39°C for 6 h. Crude extracts were prepared, and GS activities were determined as described in Materials and Methods. prot, protein; WT, wild type; ACTIN, ACT1.
Next we examined the mechanism by which FKS2 expression is induced in response to temperature upshift. Accumulation of FKS2 mRNA in response to extracellular Ca2+ or treatment with mating pheromone is strictly dependent on a calcineurin-mediated pathway (28). Therefore, we examined the effect of increased growth temperature on FKS2 expression in a calcineurin mutant (cnb1Δ [6]). Thermal induction of FKS2 mRNA accumulation was nearly as strongly induced in this mutant as in the wild type (Fig. 4) but with somewhat delayed kinetics. Most notably, the response observed in the wild-type strain 20 min after shift was absent in the cnb1Δ mutant, suggesting that calcineurin may play a role in the early stages of this response to thermal stress.
The cell integrity pathway and calcineurin collaborate to induce FKS2 expression at high temperature.
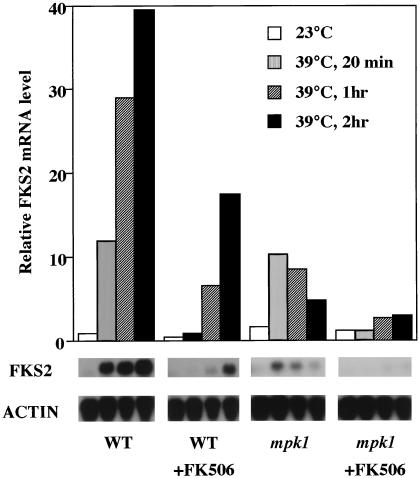
To determine if the cell integrity signaling pathway is required for thermal induction of FKS2 mRNA accumulation, we examined a mutant in MPK1, the gene encoding the MAPK of this pathway. Because mpk1 mutants lyse when cultivated at high temperature, we suppressed the growth defect of an mpk1Δ mutant by overexpression of a nonactivatable allele of MPK1 (mpk1-TAYF [18]). The basal kinase activity provided by this allele allows survival at otherwise lethal temperatures, but because the mutant kinase cannot be phosphorylated by its activating kinase, it is not subject to regulation (18). Figure 6 shows that FKS2 mRNA accumulation was induced briefly in the mpk1 mutant by a shift from growth at 23°C to growth at 39°C, but this induction was transient, with FKS2 mRNA levels diminishing to nearly the preinduction level by 2 h after shift. Therefore, persistent expression of FKS2 at elevated temperature requires the MAPK branch of the cell integrity signaling pathway.
FIG. 6.
Effect of an mpk1 mutation on FKS2 mRNA accumulation in response to elevated growth temperature. Cell growth and temperature shift were conducted as described in the legend for Fig. 4. Total RNA, isolated at the indicated times from wild type (1788) or an mpk1 mutant (DL1009), was probed for FKS2 and ACT1 mRNA. Cells were pretreated with FK506 by addition of drug to the cultures to a final concentration of 0.2 mg/ml 30 min before temperature shift. WT, wild type; ACTIN, ACT1.
To determine if the transient induction of FKS2 mRNA accumulation observed in the mpk1 mutant is calcineurin dependent, we pretreated cultures with the calcineurin inhibitor FK506. Wild-type and mpk1 mutant strains, growing at 23°C, were treated with FK506 (200 ng/ml) for 30 min prior to increasing the temperature to 39°C. As expected, the kinetics of FKS2 mRNA accumulation in the wild-type strain treated with FK506 (Fig. 6) approximated those observed in the cnb1Δ mutant (Fig. 4). Pretreatment of the mpk1 mutant with FK506 completely blocked FKS2 mRNA accumulation, indicating that the transient induction observed in this mutant is calcineurin dependent. Therefore, calcineurin collaborates in parallel with the cell integrity signaling pathway to induce the accumulation of FKS2 mRNA in response to elevated growth temperature. The calcineurin-dependent pathway induces a rapid but transient increase in FKS2 mRNA, whereas the cell integrity pathway (acting through MPK1) induces a delayed but sustained increase. These pathways appear to function independently of each other because their effects on FKS2 expression were roughly additive and because both pathways must be blocked to prevent FKS2 mRNA accumulation completely in response to temperature upshift. This conclusion is supported by the observation that the cell integrity pathway is not required for the calcineurin-mediated induction of FKS2 by exogenous Ca2+. Moreover, the calcineurin- and PKC1-responsive sequences in the FKS2 promoter are separable (see below).
Dissecting the FKS2 promoter.
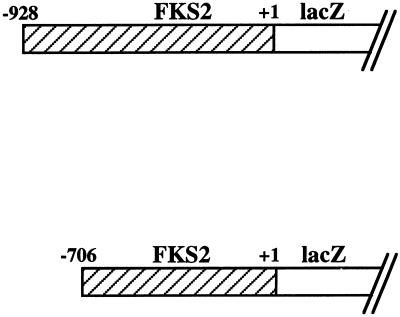
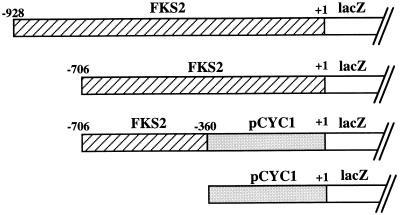
To further define the molecular mechanisms of calcineurin- and cell integrity-dependent control of FKS2 expression, we created several FKS2 reporter plasmids. Sequences 5′ to the FKS2 translational start site, spanning from either nucleotide −706 to −1 or from −928 to −1, were fused to the E. coli lacZ gene (encoding β-galactosidase). Table 2 shows that a wild-type strain bearing either of these plasmids induced β-galactosidase activity 10- or 20-fold, respectively, in response to a 24-h shift from growth at 23°C to growth at 39°C. A third reporter plasmid that contains FKS2 sequences from nucleotide −706 to −360 fused to the basal CYC1 promoter was also strongly activated by growth at high temperature (Table 2), indicating that the region from −359 to −1 is dispensable for thermal induction of FKS2 transcription. A strain bearing a control plasmid with no FKS2 sequences and only the basal CYC1 promoter expressed similar levels of β-galactosidase activity at both temperatures. We conclude that increased transcription of FKS2 mRNA, mediated by promoter sequences between nucleotides −928 and −360, accounts for part or all of the observed increase in mRNA accumulation in response to temperature upshift.
TABLE 2.
Temperature-induced expression of β-galactosidase from FKS2-lacZ reporter plasmidsa
| FKS2-lacZ reporter plasmid | β-Galactosidase sp act (nmol/min/mg of protein)
|
||
|---|---|---|---|
| 23°C | 39°C | 23°C + Ca2+ | |
 |
3.3 | 67.2 | 51.8 |
| 1.8 | 17.7 | 1.9 | |
| 1.8 | 17.1 | 2.0 | |
| 1.5 | 2.1 | 1.9 | |
Wild-type (1788) cells were transformed with the indicated reporter plasmids. Transformants were grown to saturation at 23°C in SD-Ura medium. These cultures were diluted into 20 ml of YEP containing 2% glucose (YEPD) so that subsequent incubation at either 23 or 39°C for 24 h resulted in mid-log-phase cultures (densities of 1.5 × 107 to 3 × 107 cells/ml). For the Ca2+ experiment, CaCl2 was added to YEPD cultures to a final concentration of 30 mM, and the mixture was incubated for 24 h at 23°C. Crude extracts were prepared and enzyme activities were determined as described in Materials and Methods.
We also examined the response of various FKS2-lacZ reporter plasmids to exogenous CaCl2. Although the FKS2 reporter plasmid containing 928 bp of 5′ sequence was induced strongly in response to exogenous Ca2+, which stimulates FKS2 expression through a calcineurin-dependent pathway (28), the reporter plasmid containing only 706 bp of FKS2 was unresponsive to this stimulus (Table 2). This indicates that the calcineurin-responsive sequence(s) resides within the 222 bp between nucleotides −928 and −706. Because the reporter that carries FKS2 sequences from −706 to −360 was stimulated by growth at high temperature, the PKC1-responsive sequences must reside between −360 and −706 and are, therefore, separable from the calcineurin-responsive sequences. These results further support the conclusion that the mechanisms by which calcineurin and cell integrity signaling regulate FKS2 expression are completely independent.
We next examined the effect of overproducing MKK1 or RLM1 from high-copy-number plasmids on FKS2 expression using the reporter plasmids described above. Overexpression of MKK1 or RLM1 increased the basal level (at 25°C) of β-galactosidase expression from the reporter that contains FKS2 sequences from −706 to −1 by approximately three- or eightfold, respectively (data not shown). As expected for a signaling component that controls FKS2 expression through the cell integrity pathway, the region from nucleotide −359 to −1 was dispensable for the effect of MKK1 overproduction on β-galactosidase expression. Surprisingly, the effect of RLM1 overproduction was eliminated in the reporter lacking this region (not shown), suggesting that RLM1 affects FKS2 expression through a mechanism other than cell integrity signaling. No perfect matches to the consensus DNA binding site for RLM1 (CTA[T/A]4TAG [7]) exist within the 1 kb of sequence 5′ to the FKS2 translational initiation site, but a lone sequence with a single mismatch to this consensus exists at position −336 (CTAAAGATAG).
The RLM1 transcription factor is not involved in thermal induction of FKS2 expression.
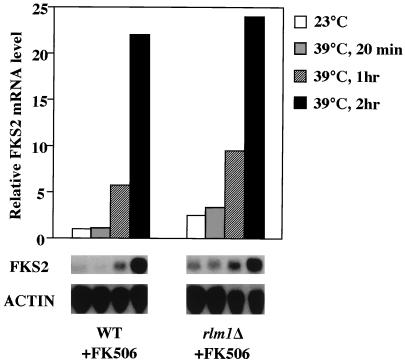
RLM1 has been proposed to mediate some functions of MPK1 (7, 42, 43). Therefore, even though the cell integrity pathway-responsive sequences in the FKS2 promoter were separable from the RLM1-responsive sequences, we examined an rlm1Δ mutant for the ability to accumulate FKS2 mRNA in response to elevated growth temperature. To eliminate the contribution of the calcineurin-dependent pathway, the rlm1Δ strain was pretreated with FK506. Figure 7 shows that this mutant was not impaired for thermal induction of FKS2, suggesting that the putative transcription factor does not mediate this MPK1-dependent function. Additionally, the fks1Δ rlm1Δ double mutant, which fails to grow at 25 to 30°C, is rescued by growth at 38°C (data not shown), consistent with thermal induction of FKS2 being independent of RLM1.
FIG. 7.
Transcription factor RLM1 is not required for the temperature-induced expression of FKS2. Log-phase cultures of wild-type (1783) and rlm1Δ (GMY63-5D) cells, grown at 23°C, were subjected to temperature shift and FK506 treatment as described in the legends for Fig. 4 and 6. Total RNA, isolated at the indicated times after temperature shift, was probed for FKS2 and ACT1 mRNAs. WT, wild type; ACTIN, ACT1.
Induction of FKS2 expression by glucose starvation and entry to stationary phase.
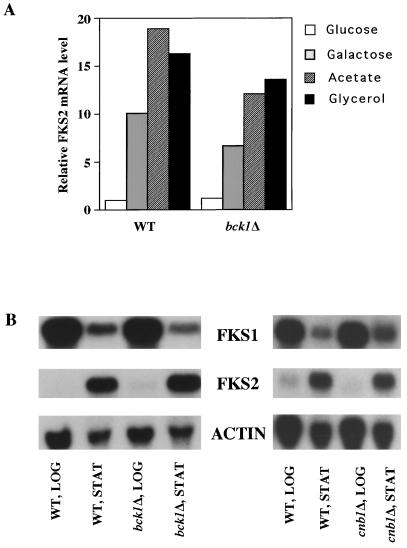
Accumulation of FKS2 mRNA has been reported to increase during growth on nonglucose carbon sources in a calcineurin-independent manner (28). Figure 8A shows that a bck1Δ mutant responds similarly to wild type in its accumulation of FKS2 mRNA in cells growing on galactose, acetate, or glycerol as carbon sources, indicating that this response does not depend on the MAPK branch of the cell integrity pathway. Similarly, an rlm1Δ mutant induces FKS2 expression normally in response to growth on galactose-containing medium (not shown). These results are consistent with the observation that fks1Δ mpk1Δ and fks1Δ rlm1Δ double mutants can grow on galactose but not glucose as a carbon source (Fig. 2). To determine if the accumulation of FKS2 mRNA in response to growth on nonglucose carbon sources might reflect a general response to glucose starvation, we examined FKS2 mRNA levels in response to a shift from high glucose (2%) to low glucose (0.05%) growth conditions. Within 2 h after shift to low glucose, FKS2 mRNA levels were induced sevenfold (data not shown), indicating that the absence of glucose rather than the presence of alternate carbon sources is responsible for FKS2 induction.
FIG. 8.
Induction of FKS2 expression by poor carbon sources and entry to stationary phase are independent of the cell integrity pathway and calcineurin. (A) Wild-type (1788) and bck1Δ (DL251) cells were grown to mid-log phase at 23°C in YEP supplemented with 2% glucose, 2% galactose, 2% glycerol, or 2% sodium acetate. Total RNA was probed for FKS2 and ACT1 mRNA. Levels of FKS2 mRNA are presented relative to that of the wild type grown in YEP supplemented with 2% glucose. (B) Cultures of bck1Δ (DL251) and cnb1Δ (MCY3-1D) and their isogenic wild-type strains (1788 and YPH499, respectively) were grown in YEP containing 2% glucose. Total RNA, extracted from cultures in log phase (LOG) (1.5 × 107 to 3 × 107 cells/ml) or stationary phase (STAT) (4.5 × 108 to 6 × 108 cells/ml), was probed for FKS1, FKS2, and ACT1 mRNAs. WT, wild type; ACTIN, ACT1.
Next we examined the effect of nutrient depletion on FKS2 expression as cells entered stationary phase. Figure 8B shows that although FKS2 expression is low in cells growing in log phase at 23°C, its mRNA accumulates in cells that have exhausted their glucose supply and entered stationary phase at this temperature. A concomitant decrease in FKS1 mRNA was observed in stationary-phase cells. Both a calcineurin mutant(cnb1Δ) and a cell integrity pathway mutant (bck1Δ) behaved similarly to wild-type cells in this response (Fig. 8B), indicating that, like the response to glucose starvation, stationary phase-induced expression of FKS2 is independent of both signaling pathways.
Because neither calcineurin nor the cell integrity signaling pathway are responsible for starvation-induced expression of FKS2, we hypothesized that the glucose derepression pathway controlled by SNF1 might regulate FKS2 under these conditions. The protein kinase encoded by the SNF1 gene is required for derepression of many glucose-repressible genes (3). To determine if induction of FKS2 mRNA accumulation in response to glucose starvation and entry to stationary phase is dependent on the SNF1 gene, we tested a snf1Δ10 mutant for transcriptional activation of the FKS2 reporter plasmids described above. Table 3 shows that the FKS2 reporter plasmid bearing 928 bp of 5′ sequence was responsive to glucose starvation in SNF1 cells, but not in an otherwise isogenic snf1Δ10 mutant, indicating that SNF1-dependent derepression is responsible for FKS1 transcriptional induction in response to glucose starvation. In contrast, the reporter with only 706 bp of FKS2 sequence failed to respond to glucose starvation even in SNF1 cells, indicating that SNF1-dependent sequences reside within the 222-bp region between the endpoints of these reporters.
TABLE 3.
Roles of SNF1 and MIG1 in the induction of FKS2 expression by glucose starvation and entry to stationary phasea
Log-phase cultures (A600 = 0.5 to 1) of SNF1 (MCY1093), snf1Δ10 (MCY1846), MIG1 (YM4797), and mig1Δ (YM4807) cells, harboring the indicated reporter plasmids, were grown in YEP containing 2% glucose (YEPD) at 23°C. For glucose starvation, cultures were washed with YEP twice, resuspended in YEP supplemented with 0.05% glucose, and incubated for 3 h at 23°C. Stationary-phase samples were obtained by incubating YEPD cultures at 23°C to an A600 of 8 to 11. Samples designated 2% glucose and Log phase were treated identically but were harvested from cultures prior to low glucose and stationary-phase treatments, respectively. Cell extracts were made and enzyme activities measured as described in Materials and Methods.
ND, not determined.
Mazur et al. (28) identified two putative “carbon source-regulatory sequences” within the FKS2 promoter region at positions −583 and −489. Our promoter analysis indicates that these sequences are not sufficient for induction of FKS2 in response to glucose starvation and are probably not involved in this regulation. Rather, the SNF1 protein kinase mediates derepression of glucose-repressible genes by inhibition of the MIG1 transcriptional repressor (41). Examination of the FKS2 promoter region revealed two consensus MIG1 binding sites (31, 32) at positions −785 and −847. Both of these sites are within the 222-bp region that distinguishes the starvation-responsive FKS2 reporter from the nonresponsive reporter, strongly suggesting the importance of the MIG1 repressor in starvation-induced expression of FKS2. This was supported by the observation that a mig1Δ mutant displayed a ninefold greater basal level of expression (on 2% glucose) from the starvation-responsive reporter compared with a congenic MIG1 strain (Table 3). As anticipated, the mig1Δ mutant displayed a less than twofold increase in basal expression from the nonresponsive reporter relative to wild type.
Similar to the effect of glucose starvation, only the reporter plasmid with the longer FKS2 regulatory sequence was transcriptionally activated upon entry to stationary phase (Table 3). However, unlike the response to glucose starvation, this activation was not SNF1 dependent. The ability of a snf1Δ10 mutant to derepress FKS2 expression upon entry to stationary phase reveals the existence of yet another regulatory sequence within the 222-bp region of the FKS2 promoter between nucleotides −928 and −706.
DISCUSSION
The cell integrity signaling pathway and calcineurin act in parallel to stimulate FKS2 expression in response to thermal stress.
FKS1 and FKS2 are alternative subunits of the GS complex (8, 15, 28, 36). Calcineurin is an important regulator of FKS2 expression, and thus a double mutant in calcineurin (CNB1) and FKS1 is inviable due to a deficiency in FKS2 expression (11). To identify additional regulators of FKS2 expression, dosage-dependent suppressors of a strain bearing deletions in CNB1 and FKS1 were isolated. Two of the suppressors isolated through this screen were MKK1 and RLM1, which encode a MEK kinase and a member of the serum response factor family of transcription factors, respectively (16, 42). Both of these genes have been implicated previously in the cell integrity signaling pathway mediated by RHO1 and PKC1. These results suggested that FKS2 expression is also under the control of cell integrity signaling.
Cell integrity signaling is stimulated strongly by growth at elevated temperatures (e.g., 37 to 39°C) but does not regulate either the well-characterized heat shock response or the expression of stress element (STRE)-controlled stress response genes (18). We found that expression of FKS2 was stimulated by shift from growth at 23°C to 39°C, though this gene possesses no heat shock elements (30) or STREs (27) within the 1 kb of sequence 5′ to its translational start site. Similar to the maintenance of cell integrity signaling at high-growth temperature (18), increased expression of FKS2 was sustained indefinitely while cells continued to grow at elevated temperature. Three lines of evidence indicate that the cell integrity pathway and calcineurin act in parallel to stimulate FKS2 expression. First, a calcineurin mutant displayed a delayed but sustained induction of FKS2 expression in response to temperature upshift, whereas a cell integrity signaling mutant (mpk1) displayed a rapid but transient induction of FKS2 in response to this challenge. The contributions of these pathways to FKS2 expression was roughly additive to that of the wild type. These results indicate that calcineurin mediates the early response to temperature shift and that the cell integrity signaling pathway mediates the persistent maintenance of FKS2 expression at elevated temperature. Simultaneous inhibition of both pathways completely prevented thermal induction of FKS2. Second, induction of FKS2 expression by exogenous Ca2+, a calcineurin-dependent process, was not impaired in mutants defective in cell integrity signaling. Third, the calcineurin-responsive region of the FKS2 promoter was separable from the cell integrity signaling-responsive region. The calcineurin-responsive sequences reside in the region between −928 and −706, whereas the cell integrity-responsive sequences reside in the region between −706 and −360. In this regard, it is interesting to note that in mammalian T cells, calcineurin and protein kinase C act synergistically to activate transcription of the interleukin-2 gene (10). Thus, cooperative activity of these signaling molecules may be a common theme.
The involvement of cell integrity signaling in FKS2 expression indicates that a regulatory circuit for cell wall construction exists in which the RHO1 G protein controls both the activity of GS and the expression of at least one of its components. However, temperature upshift during growth had only a modest effect on GS activity in wild-type cells. This can be explained by the large contribution to GS activity of FKS1, whose expression was not influenced by temperature upshift. Only in an fks1 mutant was an appreciable increase in GS activity observed in response to temperature upshift. These results suggest that FKS2 is of minor importance as an effector of cell integrity signaling. This conclusion is supported by the absence of a growth defect associated with loss of FKS2 function (28).
Our results provide the first clear evidence for a direct target of cell integrity signaling. Igual et al. (14) proposed that the cell integrity pathway positively regulates FKS1. However, this was based on the observation that FKS1 mRNA levels were modestly reduced (25 to 80%) in pkc1 and mpk1 mutants. Moreover, the most severely impaired signaling mutant tested in that study, a pkc1Δ strain maintained in the presence of osmotic support, displayed only a 25 to 35% decrease in the steady-state level of FKS1 mRNA compared with wild type. Finally, the failure of Igual et al. to observe an increase in FKS1 mRNA in response to stimulation of cell integrity signaling by temperature upshift of wild-type cells was confirmed in the present study and suggests to us that FKS1 is not under the control of this signaling pathway. The reported diminution of FKS1 mRNA levels in cell integrity pathway mutants might be explained by differences in growth rates of these mutants or by other secondary factors.
The RLM1 transcription factor is not required for thermal induction of FKS2 expression.
RLM1 encodes a member of the serum response factor family of transcription factors that has been implicated in cell integrity signaling (7, 42, 43). A recessive mutation in RLM1 was isolated as a suppressor of the growth defect associated with expressing a hyperactive MKK1 allele (42). Recent reports suggest that transcriptional activity of RLM1 is regulated positively in response to phosphorylation by MPK1 (7, 43). However, an rlm1Δ mutant does not display a cell integrity defect, suggesting that any role it may have in cell integrity signaling is minor. Additionally, mutation of the only RLM1-related yeast gene, designated SMP1, also failed to display cell integrity defects alone or in combination with rlm1 (7, 43).
We found that overexpression of RLM1 suppressed the synthetic lethality of a cnb1Δ fks1Δ double mutant, presumably by mediating increased expression of FKS2. This conclusion was supported by the observation that RLM1 overproduction increased the basal level of FKS2 expression at room temperature by eightfold. Additionally, an rlm1Δ fks1Δ double mutant displayed synthetic lethality that was suppressed by overexpression of FKS2, further suggesting that RLM1 normally contributes to FKS2 expression. However, an rlm1Δ mutant was not deficient for induction of FKS2 expression in response to temperature upshift. Moreover, the only potential RLM1 binding site (7) within the FKS2 promoter resides within a region that is dispensable for thermal induction of this gene. These results support the conclusion that although RLM1 contributes to FKS2 expression, it does not mediate the thermal induction of this gene stimulated by cell integrity signaling. Because the inputs to FKS2 expression from calcineurin, RLM1, and cell integrity signaling appear to be independent, the synthetic lethality of each of these inputs with fks1Δ suggests that they all contribute significantly to FKS2 expression in the absence of FKS1.
Expression of FKS2 in response to glucose starvation and entry to stationary phase.
Regulation of FKS2 expression is under the independent control of a variety of signaling pathways. In addition to the roles of the cell integrity pathway, calcineurin, and RLM1 in the regulation of this gene, FKS2 induction in response to glucose starvation was found to be under the control of the SNF1 protein kinase. Glucose repression of FKS2 is apparently mediated by the MIG1 transcriptional repressor at two consensus MIG1 binding sites in the region between residues −706 and −928 of the FKS2 promoter. Additionally, FKS2 expression was strongly induced as cells entered stationary phase. However, though the regulatory site for this induction was mapped to the same region as the SNF1- and calcineurin-responsive sequences (−928 to −706), neither a snf1Δ mutant nor a cnb1Δ mutant were impaired for the stationary-phase response. Therefore, FKS2 induction in response to entry to stationary phase represents a fifth distinct regulatory input for the control of this gene. A summary of these inputs is presented in Fig. 9.
FIG. 9.
Known inputs for the regulation of FKS2 expression.
Expression of FKS1 and FKS2 are generally regulated in opposition to each other, with FKS1 predominating under optimal growth conditions. However, FKS2 is induced in response to calcium influx, mating pheromone, thermal stress, glucose starvation, entry to stationary phase, and mutation of FKS1. Induction of FKS2 is usually accompanied by a decrease in FKS1 expression so that the level of GS activity in cells is not appreciably altered in response to these conditions. Why then do cells shift from one form of GS to the other in response to stress? One possibility is that the two forms of GS may be functionally distinct in ways that have yet to be discovered. For example, GS derived from FKS2 may be more stable than that derived from FKS1, thereby enhancing survival in stasis and under conditions of stress.
ACKNOWLEDGMENTS
C.Z., U.S.J., and P.G.-E. contributed equally to this work.
We thank Marian Carlson and Mark Johnston for strains and for helpful discussions and Chris Python for GS assays.
This work was supported by grants from the NIH (GM48533 to D.E.L. and GM48729 to M.S.C.), the American Cancer Society (Faculty Research Award 446 to D.E.L.), the National Science Foundation (MCB-9357017 to M.S.C.), the Lucille P. Markey Charitable Trust (Biomedical Scholar Award 92-42 to M.S.C.), and funds from the Procter & Gamble Company (to M.S.C.).
REFERENCES
- 1.Boeke J D, LaCroute F, Fink G R. A positive selection for mutants lacking orotinine-5-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 2.Cairns B R, Ramer S W, Kornberg R D. Order of action of components in the yeast pheromone response pathway revealed with a dominant allele of the STE11 kinase and the multiple phosphorylation of the STE7 kinase. Genes Dev. 1992;6:1305–1318. doi: 10.1101/gad.6.7.1305. [DOI] [PubMed] [Google Scholar]
- 3.Celenza J L, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 4.Cid V J, Duran A, Rey F, Snyder M P, Nombela C, Sanchez M. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev. 1995;59:345–386. doi: 10.1128/mr.59.3.345-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costigan C, Gehrung S, Snyder M. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol Cell Biol. 1992;12:1162–1178. doi: 10.1128/mcb.12.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cyert M S, Thorner J. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol. 1992;12:3460–3469. doi: 10.1128/mcb.12.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodou E, Treisman R. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1848–1859. doi: 10.1128/mcb.17.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas C M, Foor F, Marrinan J A, Morin N, Nielsen J B, Dahl A M, Mazur P, Baginsky W, Li W, El-Sherbeini M, Clemas J A, Mandala S M, Frommer B R, Kurtz M B. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc Natl Acad Sci USA. 1994;91:12907–12911. doi: 10.1073/pnas.91.26.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drgonova J, Drgon T, Tanaka K, Kollar R, Chen G-C, Ford R A, Chan C S M, Takai Y, Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- 10.Franz B, Norby E C, Bren G, Steffen N, Paya C V, Kincaid R L, Tocci M J, O’Keefe S J, O’Neill E A. Calcineurin acts in synergy with PMA to inactivate IκB/MAD3, an inhibitor of NF-κB. EMBO J. 1994;13:861–870. doi: 10.1002/j.1460-2075.1994.tb06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett-Engele P, Moilanen B, Cyert M S. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol Cell Biol. 1995;15:4103–4114. doi: 10.1128/mcb.15.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarente L, Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983;32:1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- 13.Hill J E, Muers A M, Koerner T J, Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- 14.Igual J C, Johnson A L, Johnston L H. Coordinated regulation of gene expression by the cell cycle transcription factor SWI4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 1996;15:5001–5013. [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue S B, Takewaki N, Takasuka T, Mio T, Adachi M, Fujii Y, Miyamoto C, Arisawa M, Furuichi Y, Watanabe T. Characterization and gene cloning of 1,3-β-d-glucan synthase from Saccharomyces cerevisiae. Eur J Biochem. 1995;231:845–854. doi: 10.1111/j.1432-1033.1995.tb20770.x. [DOI] [PubMed] [Google Scholar]
- 16.Irie K, Takase M, Lee K S, Levin D E, Araki H, Matsumoto K, Oshima Y. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol Cell Biol. 1993;13:3076–3083. doi: 10.1128/mcb.13.5.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada Y, Jung U S, Piotrowski J, Levin D E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- 19.Kamada Y, Qadota H, Python C P, Anraku Y, Ohya Y, Levin D E. Activation of yeast protein kinase C by Rho1 GTPase. J Biol Chem. 1996;271:9193–9195. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- 20.Klis F M. Review: cell wall assembly in yeast. Yeast. 1994;10:851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- 21.Lee K S, Irie K, Gotoh Y, Watanabe Y, Araki H, Nishida E, Matsumoto K, Levin D E. A yeast mitogen-activated protein kinase homolog (Mpk1) mediates signaling by protein kinase C. Mol Cell Biol. 1993;13:3067–3075. doi: 10.1128/mcb.13.5.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K S, Levin D E. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol Cell Biol. 1992;12:172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin D E, Bowers B, Chen C, Kamada Y, Watanabe M. Dissecting the protein kinase C/MAP kinase signalling pathway of Saccharomyces cerevisiae. Cell Mol Biol Res. 1994;40:229–239. [PubMed] [Google Scholar]
- 24.Levin D E, Bartlett-Heubusch E. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol. 1992;116:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin D E, Fields F O, Kunisawa R, Bishop J M, Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990;62:213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- 26.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 27.Marchler G, Schuler C, Adams G, Ruis H. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 1993;12:1997–2003. doi: 10.1002/j.1460-2075.1993.tb05849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazur P, Morin N, Baginsky W, El-Sherbeini M, Clemas J A, Nielsen J B, Foor F. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol Cell Biol. 1995;15:5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mol P C, Park H M, Mullins J T, Cabib E. A GTP-binding protein regulates the activity of (1→3)-β-d-glucan synthase, an enzyme directly involved in yeast cell wall morphogenesis. J Biol Chem. 1994;269:31267–31274. [PubMed] [Google Scholar]
- 30.Munro S, Pelham H. What turns on heat shock genes? Nature. 1985;317:476–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- 31.Nehlin O N, Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms’ tumor finger proteins. EMBO J. 1990;9:2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nehlin O N, Carlberg M, Ronne H. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 1991;10:3373–3377. doi: 10.1002/j.1460-2075.1991.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 1995;14:5931–5938. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paravicini G, Cooper M, Friedli L, Smith D J, Carpentier J-L, Klig L S, Payton M A. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol Cell Biol. 1992;12:4896–4905. doi: 10.1128/mcb.12.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qadota H, Python C P, Inoue S B, Arisawa M, Anraku Y, Zheng Y, Watanabe T, Levin D E, Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- 36.Ram A F J, Brekelmans S S C, Oehlen L J W M, Klis F M. Identification of two cell cycle regulated genes affecting the β-1,3-glucan content of cell wall in Saccharomyces cerevisiae. FEBS Lett. 1995;358:165–170. doi: 10.1016/0014-5793(94)01418-z. [DOI] [PubMed] [Google Scholar]
- 37.Rose M D, Winston F, Hieter P. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 38.Shore P, Sharrocks A D. The MADS-box family of transcription factors. Eur J Biochem. 1995;229:1–13. doi: 10.1111/j.1432-1033.1995.tb20430.x. [DOI] [PubMed] [Google Scholar]
- 39.Stewart A A, Ingebritsen T S, Manalan A, Klee C B, Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase. FEBS Lett. 1982;137:80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- 40.Torres L, Martin H, Garcia-Saez M I, Arroyo J, Molina M, Sanchez M, Nombela C. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol Microbiol. 1991;5:2845–2854. doi: 10.1111/j.1365-2958.1991.tb01993.x. [DOI] [PubMed] [Google Scholar]
- 41.Trumbly R J. Glucose repression in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1992;6:15–21. doi: 10.1111/j.1365-2958.1992.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe Y, Irie K, Matsumoto K. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol Cell Biol. 1995;15:5740–5749. doi: 10.1128/mcb.15.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe Y, Takaesu G, Hagiwara M, Irie K, Matsumoto K. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:2615–2623. doi: 10.1128/mcb.17.5.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]