Abstract
Purpose
The purpose of this study is to investigate the efficacy and safety of immune checkpoint inhibitors (ICIs) or plus with chemotherapy in older patients.
Methods
We enrolled 110 older patients with non-small cell lung cancer (NSCLC ≥75 years) who received either chemotherapy alone (chemo), ICI plus chemotherapy (ICI + chemo), or ICI alone and ICI plus other therapies, which included anti-angiogenesis drugs or other novel ICI (ICIs). Patient characteristics, treatment response, survival, and toxicity were evaluated.
Results
In total population, the ICIs group has the highest disease control rate (DCR 75%). There were no significant differences in progression-free survival (PFS) and overall survival (OS) among older patients between ICI + chemo and ICIs groups (PFS: 5.3 months vs. 5.5 months, p = 0.70, OS: 10.7 months vs. 20.3 months, p = 0.995). Meanwhile, we observed ICIs had a longer PFS and OS than chemo group (PFS: 3.9 months vs. 5.5 months, p = 0.01, OS: 10.9 months vs. 20.3 months, p = 0.05). Subgroup analysis showed that patients with programmed death ligand-1 (PD-L1) ≥ 1% had a distinct longer trend toward OS in ICIs group compared to ICI + chemo group (22.4 months vs. 10.7 months, p = 0.605), even though there was no significant difference. In terms of safety, ICIs was more tolerable and had a lower discontinuation rate than ICI + chemo group.
Conclusion
In the real world, ICI + chemo is more likely to be discontinued due to adverse effects and does not significantly improve patient survival compared with ICIs treatment in total population and subgroup. Therefore, ICI alone or ICIs plus other therapies, such as anti-angiogenesis drugs or other novel ICI (ICIs) could be recommended for older cases with PD-L1 positive NSCLC.
Keywords: Non-small cell lung cancer, Older patients, Immunotherapy
1. Introduction
Lung cancer is the leading cause of cancer-related death worldwide [1]. With the unavoidable trend toward aging, the proportion of elderly patients those 75 years old and over with lung cancer is growing [2]. For senior patients’ poor performance status (PS), progressive organ functional reserve failure, the presence of multiple comorbidities, and a lack of sufficient social support, age creates a barrier to clinical trial enrollment. Additionally, there is no universally accepted standard of first-line treatment for older patients with advanced NSCLC [3,4].
Moreover, the development of immune checkpoint inhibitors has been rapid in recent years. Those inhibitors, represented by PD-L1/PD-1 inhibitors, have greatly improved the prognosis for NSCLC patients [[5], [6], [7]]. The combination of ICIs and chemotherapy has been recognized as a standard treatment option for advanced NSCLC with no target gene alterations, regardless of PD-L1 expression based on several phase III clinical trial results [[8], [9], [10]]. The treatment was beneficial even in NSCLC patients ≥75 years in the KEYNOTE-407 trial of pembrolizumab or placebo plus carboplatin and paclitaxel/nab-paclitaxel in previously untreated metastatic squamous lung cancar [8]. A separate meta-analysis also found that combinations of ICIs and chemotherapy in older patients (≥65 years) with NSCLC is as effective as it is in young patients [11]. However, older participants in clinical trials tend to have a good performance status, challengling the reflection of real-world data. According to a real-world study, older patients with NSCLC (≥75 years old) who received ICI plus chemotherapy had much lower PFS and OS rates than young patients [12].
With the need for large randomized trials designed specifically for older patients, retrospective cohort studies have tried to explore this issue with contradictory results [13,14]. Thus, in this retrospective study, we aim to investigate the efficacy and safety of immune checkpoint inhibitors (ICIs) or plus with chemotherapy in older NSCLC patients.
2. Materials and methods
2.1. Study patients
This study was a retrospective cohort study of 110 patients aged ≥75 years diagnosed with advanced NSCLC between March 8, 2013 and May 9, 2023 in TNM (Tumor Node Metastasis staging system) stage III or IV, with measurable disease (based on the Response Evaluation Criteria in Solid Tumors, version 1.1) at the Thoracic Oncology Department of Peking University Cancer Hospital. Other inclusion criteria were an Eastern Cooperative Oncology Group performance score (ECOG PS) ≤ 2, driver oncogenes, such as EGFR/ALK wild type status, and receiving at least one line of systemic anti-tumor therapy. The exclusion criteria were an ECOG PS > 3, a need for palliative treatment, refusal of anti-tumor therapy, lack of detailed clinical information, and receiving other first-line regimens (such as radiotherapy). We obtained the baseline characteristics of the enrolled patients through retrospective analysis of medical records, including age, gender, histological type, smoking status, comorbidity number, performance score (ECOG PS), tumor clinical stage, initial metastasis site and treatment. By using the PD-L1 IHC 22C3 pharmDx test to measure PD-L1 expression, the tumor proportion score (TPS), of live tumor cells showing partial or total membrane staining was calculated. TPS <1% was defined as negative and TPS ≥1% as positive.
2.2. Treatment and response evaluation
According to the first-line treatment, the cohort of older patients was divided into three groups. Individuals that received a single agent treatment (n = 43), and those who received a platinum-doublet drug (n = 17) were both grouped into the chemotherapy alone group (chemo). Chemotherapy in this study includes pemetrexed, and gemcitabine with or without platinum. Patients who received ICI plus pemetrexed, gemcitabine, albumin-bound paclitaxel or platinum-doublet treatment were grouped as ICI + chemo. Patients who received either ICI alone or ICI in combination with other therapies, such as anti-angiogenesis drugs (bevacizumab, endostar) and other novel ICIs including Tigit antibody were grouped as ICIs. ICIs in this study included pembrolizumab, atezolizumab, camrelizumab, and infliximab.
The primary endpoint of the study was Progression-free survival (PFS), whereas the secondary endpoints were Overall survival (OS), objective response rate (ORR), disease control rate (DCR) and the incidence and severity of adverse events (AEs). PFS was calculated from the first day of treatment until progressive disease (PD) or death from tumor. OS was calculated from the initiation of treatment until death of tumor or was censored on the day of the last visit. Treatment responses were assessed according to Response Evaluation Criteria in Solid Tumors version 1.1. Tumor responses were classified as complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and not evaluated. ORR was defined as the rate of complete response plus partial response, and DCR was defined as the rate of CR, PR plus SD. The final follow-up time was on August 10, 2023. Adverse events were classified using National Cancer Institute Common Terminology Criteria for AEs (CTCAE), version 5.0.
2.3. Statistical analysis
All analyses were performed in SPSS 25.0 and Prism 9.0. Chi-square test and Fisher's exact test were used to compare categorical variables and continuous variables between groups. The Kaplan-Meier method and log-rank test were used for PFS and OS analysis to compare the prognosis of different groups. A p-value of less than 0.05 was considered statistically significant. The hazard ratios (HR) and their 95% confidence intervals (CI) were estimated using the COX proportional hazards model in univariate and multivariate analyses.
3. Results
3.1. Patient characteristics
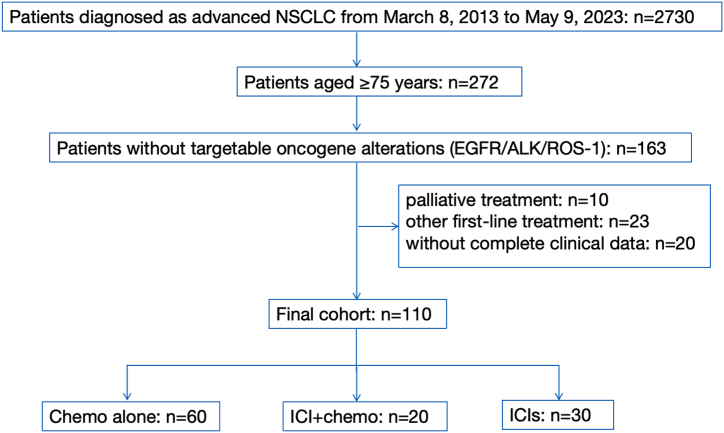
Among 272 older patients diagnosed with advanced NSCLC from March 8, 2013 to May 9, 2023, 163 did not have a targetable oncogene mutation. After excluding 10 patients receiving palliative treatment, 23 receiving other first-line regimens (such as radiotherapy), and 12 without complete clinical data, 110 were enrolled in the retrospective analysis finally (Fig. 1). Of the 110 older patients, 84 (76.4%) were aged 75–80 years, and 26 (23.6%) were aged 80 years or older. The median age of all patients was 77 years (ranging from 75 to 91 years). 42.7% of patients had a history of more than two combined chronic diseases, with the most common comorbidities being diabetes mellitus and hypertension, whereas 70% of the patients had stage IV disease. Baseline clinicopathologic characteristics were summarized in Table 1. Among them, 60 patients were treated with chemotherapy (chemo), 20 cases received immunotherapy plus chemotherapy (ICI + chemo), and 30 patients got ICI alone or ICI combined with other treatments (ICIs). There was no significant difference between the three groups in gender, histological type, smoking status, comorbidity number, clinical stage, and initial metastasis site. Age and ECOG differences exist between groups, because older and ECOG≥1 older patients tend to choose ICIs without chemotherapy in clinical practice.
Fig. 1.
Flow diagram of patient selection steps.
Table 1.
Baseline characteristics of the 110 patients.
| Characteristics | Total n% | chemo | ICI + chemo | ICIs | p value | |
|---|---|---|---|---|---|---|
| Gender | Male | 88(80.0) | 48(80) | 16(80) | 24(80) | 0.99 |
| Female | 22(20.0) | 12(20) | 4(20) | 6(20) | ||
| Age (year) | ≥75 <80 | 84(76.4) | 54(90) | 17(85) | 13(43.3) | <0.01 |
| >80 | 26(23.6) | 6(10) | 3(15) | 17(56.7) | ||
| Median(min,max) | 77(75,91) | 76(75,90) | 77(75,82) | 80(75,91) | – | |
| Histological type | Adenocarcinoma | 46(41.8) | 29(48.3) | 3(15) | 14(46.7) | 0.28 |
| Squamous carcinoma | 54(49.1) | 26(43.3) | 14(70) | 14(46.7) | ||
| Sarcomatoid carcinoma | 4 (3.7) | 2(3.3) | 2(10) | 0 | ||
| Adenosquamous carcinoma | 2 (1.8) | 1(1.7) | 0 | 1(3.3) | ||
| Large cell cancer | 1 (0.9) | 1(1.7) | 0 | 0 | ||
| Non-small cell lung cancer | 3 (2.7) | 1(1.7) | 1(5) | 1(3.3) | ||
| Smoking status | Yes | 88(80.0) | 47(78.3) | 18(90) | 23(76.7) | 0.46 |
| No | 22(20.0) | 13(21.7) | 2(10) | 7(23.3) | ||
| Comorbidity number | <2 | 63(57.3) | 37(61.7) | 10(50) | 16(53.3) | 0.58 |
| ≥2 | 47(42.7) | 23(38.3) | 10(50) | 14(46.7) | ||
| ECOG PS | 0 | 43(39.1) | 33(55) | 6(30) | 4(6.7) | <0.01 |
| 1 | 62(56.4) | 27(45) | 13(65) | 22(86.6) | ||
| 2 | 5(4.5) | 0 | 1(5) | 4(6.7) | ||
| Clinical stage | III | 33(30.0) | 17(28.3) | 8(40) | 8(26.7) | 0.55 |
| IV | 77(70.0) | 43(71.7) | 12(60) | 22(73.3) | ||
| Metastasis site | Lung | 21(19.1) | 12(10.2) | 5(25) | 4(13.3) | 0.95 |
| Pleura、pericardium | 31(28.2) | 14(23.3) | 7(35) | 10(33.3) | ||
| Liver | 6(5.5) | 4(6.6) | 1(5) | 1(3.3) | ||
| Bone | 30(27.3) | 17(28.3) | 5(25) | 8(26.7) | ||
| Brain | 10(9.1) | 6(10) | 2(10) | 2(6.7) | ||
| Soft tissue | 4(3.6) | 3(5) | 0 | 1(3.3) | ||
| Abdomen | 13(11.8) | 7(11.7) | 1(5) | 5(16.7) | ||
| Treatment | Chemo | 60(50.9) | – | – | – | |
| ICI + chemo | 20(16.9) | – | – | – | ||
| ICIs | 30(25.4) | – | – | – |
chemo:Chemotherapy ICI: Immune Checkpoint Inhibitor ECOG PS: Eastern Cooperative Oncology Group performance status.
3.2. Treatment response and survival analysis
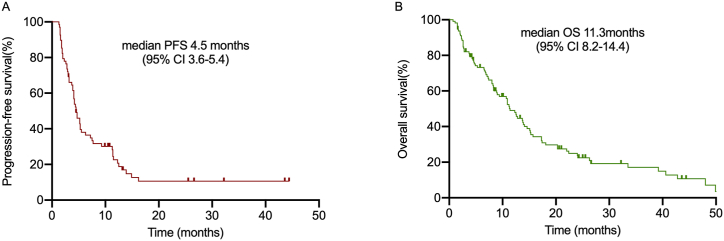
The median PFS of 68 patients and the median OS of 104 patients (4 people lost follow-up,2 drug-related deaths were as censored value) were 4.5 months (95% CI 3.6–5.4) and 11.3 months (95% CI 8.2–14.4), respectively (Fig. 2A–B). Treatment response was shown in Table 2. In total, 9 (25%), and 16 (44.4%) patients in the chemo group achieved PR and SD, respectively, whereas 11 (30.6%) patients developed PD. The ORR and DCR were 25% and 69.4%, respectively in total. In the ICI + chemo group, PR was observed in 6 (40%) patients, SD was noted in 5 (33.3%) cases, and PD was found in 4 (26.7%) patients, resulting in an ORR of 40% and DCR of 73.3%. Among the ICIs group, 4 (16.7%), 14 (58.3%), and 6 (25%) patients got PR, SD, and PD, respectively. Although the ORR (16.7%) was lower than the other groups, the DCR (75%) of ICIs group was the highest.
Fig. 2.
Kaplan–Meier analysis of progression-free survival and overall survival in total patients. (A) Kaplan–Meier analysis of PFS among 68 patients. (B) Kaplan–Meier analysis of OS among 104 patients.
Table 2.
Tumor control and 104 patient survival.
| Efficacy | chemo | ICI + chemo | ICIs |
|---|---|---|---|
| CR, n (%) | 0 | 0 | 0 |
| PR, n (%) | 9(25) | 6(40) | 4(16.7) |
| ORR, n (%) | 9(25) | 6(40) | 4(16.7) |
| SD, n (%) | 16(44.4) | 5(33.3) | 14(58.3) |
| DCR, n (%) | 25(69.4) | 11(73.3) | 11(75) |
| PD, n (%) | 11(30.6) | 4(26.7) | 6(25) |
| Could be evaluated,n (%) | 36(64.3) | 15(75) | 24(80) |
| Could not be evaluated,n (%) | 20(35.7) | 5(25) | 6(20) |
| PFS | |||
| Events, n | 32 | 13 | 23 |
| Median, months (95% CI) | 3.9(2.7–5.1) | 5.3(0–10.7) | 5.5(1.4–9.6) |
| OS | |||
| Events, n | 55 | 19 | 30 |
| Median, months (95% CI) | 10.9 (6.0–15.8) | 10.7 (7.3–14.1) | 20.3 (7.1–33.5) |
CR: Complete Response PR: Partial Response, SD: Stable Disease, PD: Progressive Disease.
ORR: Objective Response Rate DCR: Disease Control Rate PFS: Progression Free Survival.
OS: Overall Survival.
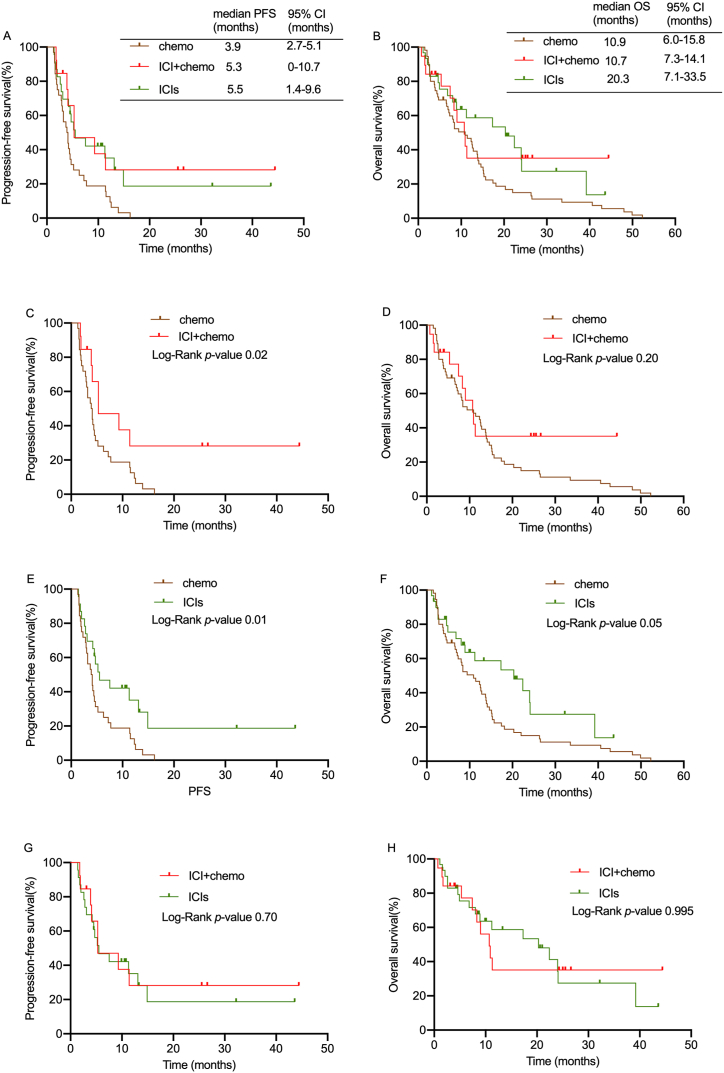
The median PFS was similar among the ICI + chemo and ICIs groups (5.3 months vs. 5.5 months, p = 0.70) (Fig. 3A,G, Table 2). However, the chemo alone group had shorter a median PFS (3.9 months) than the other groups (Fig. 3A,C,3E, p <0.05). Next, we investigated the prognostic factors of PFS in elderly individuals. Univariate Cox proportional hazard regression analysis revealed that histological type, clinical stage, and treatment affect the prognosis of patients. A multivariate Cox proportional hazards regression analysis with five variables (age, histological type, ECOG, clinical stage, and treatment). Early clinical stage and adenocarcinoma and squamous cancer were significantly associated with a better PFS (Table 3).
Fig. 3.
The PFS and OS curve of 3 groups. (A–B) Kaplan–Meier analysis of PFS and OS among 3 groups. (C–D) Kaplan–Meier analysis of PFS and OS among chemo and ICI + chemo group. (E–F) Kaplan–Meier analysis of PFS and OS among chemo and ICIs group. (G–H) Kaplan–Meier analysis of PFS and OS among ICI + chemo and ICIs group.
Table 3.
Cox proportional hazard models for progression-free survival in 68 patients. Univariable and multivariable analysis.
| Parameter | Category | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
| Gender | Male | 0.638 (0.358–1.138) | 0.128 | ||
| Female | Ref | ||||
| Age (year) | ≥75 <80 | 1.153 (0.606–2.193) | 0.664 | 0.616 (0.289–1.309) | 0.208 |
| >80 | Ref | ||||
| Histological type | Adenocarcinoma | 0.091 (0.019–0.426) | 0.002 | 0.135 (0.027–0.660) | 0.013 |
| Squamous carcinoma | 0.094 (0.020–0.445) | 0.003 | 0.197 (0.041–0.959) | 0.044 | |
| others | Ref | ||||
| Smoking status | Yes | 1.135 (0.595–2.164) | 0.700 | ||
| No | Ref | ||||
| Comorbidity number | <2 | 0.879 (0.569–1.358) | 0.561 | ||
| ≥2 | Ref | ||||
| ECOG PS | 0 | 0.837 (0.249–2.821) | 0.775 | 0.607 (0.163–2.265) | 0.457 |
| \ | 1 | 0.589 (0.177–1.963) | 0.389 | 0.386 (0.108–1.377) | 0.142 |
| 2 | Ref | ||||
| Clinical stage | III | 0.333 (0.164–0.675) | 0.002 | 0.293 (0.134–0.642) | 0.002 |
| IV | Ref | ||||
| Treatment | Chemo | 2.024 (1.105–3.708) | 0.022 | 1.704 (0.840–3.456) | 0.140 |
| ICI + chemo | 0.824 (0.351–1.932) | 0.656 | 0.795 (0.306–2.065) | 0.638 | |
| ICIs | Ref | ||||
The median OS for the chemo, ICI + chemo and ICIs groups were 10.9 months, 10.7 months, and 20.3 months, respectively (Fig. 3B, Table 2). Notably, patients who got chemotherapy had a shorter median OS than patients in ICIs groups (Fig. 3F, p = 0.05), while there was no significant difference between the chemo and ICI + chemo groups (Fig. 3D, p = 0.2). ICIs had a longer median OS than ICI + chemo, even though this difference was not statistically significant (Fig. 3H, p = 0.995). Univariate and Multivariate Cox proportional hazard regression analysis revealed that the treatment is an independent risk factor for the prognosis of elderly lung cancer patients (Table 4). Compared with chemotherapy, patients received ICIs had a low risk of death (HR = 2.300, 95% CI 1.148–4.605 p = 0.019). The results above showed that ICI + chemo group had no more significant benefit in improving survival of elderly patients over 75 years than ICIs group.
Table 4.
Cox proportional hazard models for overall survival in 104 patients. Univariable and multivariable analysis.
| Parameter | Category | Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
| Gender | Male | 0.834 (0.496–1.401) | 0.492 | ||
| Female | Ref | ||||
| Age (year) | ≥75 <80 | 0.938 (0.563–1.563) | 0.807 | 0.749 (0.395–1.421) | 0.376 |
| >80 | Ref | ||||
| Histological type | Adenocarcinoma | 0.509 (0.239–1.083) | 0.079 | ||
| Squamous carcinoma | 0.620 (0.294–1.311) | 0.211 | |||
| others | Ref | ||||
| Smoking status | Yes | 1.164 (0.690–1.964) | 0.569 | ||
| No | Ref | ||||
| Comorbidity number | <2 | 0.838 (0.539–1.302) | 0.431 | ||
| ≥2 | Ref | ||||
| ECOG PS | 0 | 0.684 (0.242–1.937) | 0.474 | 0.441 (0.133–1.467) | 0.182 |
| 1 | 0.661 (0.236–1.855) | 0.432 | 0.496 (0.165–1.489) | 0.211 | |
| 2 | Ref | ||||
| Clinical stage | III | 0.680 (0.408–1.134) | 0.140 | 0.750 (0.432–1.303) | 0.307 |
| IV | Ref | ||||
| Treatment | Chemo | 1.695 (0.977–2.941) | 0.061 | 2.278 (1.128–4.603) | 0.022 |
| ICI + chemo | 1.053 (0.482–2.302) | 0.897 | 1.452 (0.615–3.427) | 0.395 | |
| ICIs | Ref | ||||
3.3. Subgroup survival analysis
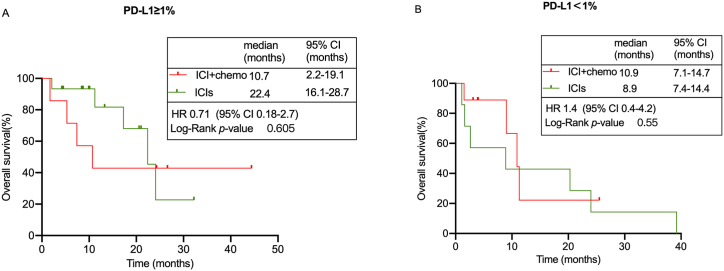
As shown in Table 5, there were much more PD-L1 positive (TPS ≥1%) (50% vs. 35%) patients in the ICIs group, whereas the ICI + chemo contained notably more PD-L1 negative (TPS < 1%) (45% vs. 23.3%) patients. Therefore, we investigated the ICI + chemo and ICIs subgroup survival differences according to the PD-L1 expression. Considering the limited number of subgroups, we only evaluate the median OS. In PD-L1 positive patients, we observed a distinct trend toward a longer OS in the ICIs group than those in the ICI + chemo group (22.4 months vs. 10.7 months, p = 0.605, Fig. 4A), but this difference was not statistically significant probably due to the small number of subgroup (p > 0.05). Meanwhile, there was no significant difference among PD-L1 negative patients between ICIs and ICI + chemo group (8.9 months vs. 10.9 months, p = 0.55, Fig. 4B). The above findings indicated that ICI + chemo did not significantly improve patient survival among PD-L1 positive subgroup compared to ICIs treatment alone.
Table 5.
PD-L1 expression of chemo + ICI and ICIs group.
| PD-L1 expression,n (%) | ICI + chemo | ICIs |
|---|---|---|
| <1%, | 9(45) | 7(23.3) |
| ≥1% | 7(35) | 15(50) |
| Unmeasured | 4(20) | 8(26.7) |
PD-L1: Programmed Death Ligand-1.
Fig. 4.
Subgroup survival analysis of chemo + ICIs and ICIs group. (A) OS curve of patients with TPS≥1% among ICI + chemo and ICIs group. (B) OS curve of patients with TPS<1% among ICI + chemo and ICIs group.
3.4. Safety and toxicity profile
The presence of treatment-related AEs was assessed in all 110 patients. The safety and toxicity profile is demonstrated in Table 6. In the chemo group, the common treatment-related AEs were digestive tract reaction, myelosuppression, and infection. A grade 3–4 decrease of platelets and white blood cells were in 3.3% and 21.7% of patients respectively. However, except for the same adverse reactions that occurring in the chemo group, patients in the ICI + chemo developed immune-related AEs such as skin rash (5%), hypothyroidism (5%), hepatitis (5%), pneumonitis (20%), and increased cTnI (10%). Compared with ICI + chemo group, patients in the ICIs mainly experienced immune-related effects, and there was no significant difference in AEs incidence rate. The discontinuation rates of chemo (46.7%) and ICI + chemo (40%) were much higher than ICIs (20%). Two patients, one patient in the chemo group and the other in the ICI + chemo group had treatment-related deaths due to fever and infection. Overall, the safety and toxicity profile was tolerable and manageable in the patients who received ICIs alone or ICIs combined with other treatments.
Table 6.
Treatment-related adverse events of 110 patients.
| chemo n (%) |
ICI + chemo n (%) |
ICIs n (%) |
||||
|---|---|---|---|---|---|---|
| Adverse events | Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 |
| Fatigue | 23(38.3) | 10(16.7) | 10(50) | 1(5) | 0 | 0 |
| Nausea/vomiting | 20(33.3) | 8(13.3) | 9(45) | 0 | 0 | 0 |
| Decreased appetite | 17(28.3) | 4(16) | 10(50) | 0 | 1(3.3) | 0 |
| Leukopenia | 24(40) | 13(21.7) | 10(50) | 6(30) | 0 | 0 |
| Thrombocytopenia | 2(3.3) | 2(3.3) | 2(10) | 2(10) | 0 | 0 |
| Anemia | 1(1.7) | 0 | 2(10) | 0 | 0 | 0 |
| Diarrhea | 1(1.7) | 0 | 0 | 0 | 1(3.3) | 0 |
| fever | 9(15) | 0 | 1(5) | 0 | 2(6.7) | 0 |
| Pneumonitis | 4(16) | 3(5) | 4(20) | 0 | 5(16.7) | 1(3.3) |
| Rash | 1(1.7) | 0 | 1(5) | 1(5) | 2(6.7) | 0 |
| Hypothyroidism | 0 | 0 | 1(5) | 0 | 1(3.3) | 0 |
| Hepatitis | 1(1.7) | 0 | 1(5) | 0 | 0 | 0 |
| abnormal renal function | 0 | 0 | 0 | 0 | 0 | |
| increased cTnI | 0 | 0 | 2(10) | 0 | 0 | 0 |
| Led to discontinuation | 28(46.7) | 8(40) | 6(20) | |||
| Led to death | 1(1.7) | 0 | 1(5) | 0 | ||
4. Discussion
In this study, we investigated the efficacy and safety of ICIs alone or with chemotherapy in older individuals with NSCLC. We observed that patients who received ICI alone or ICI with other treatment were much older. Because older individuals, especially those over the age of 80 and ECOG≥1, are reluctant to accept chemotherapy in a clinical setting. Though there had a difference in age and ECOG between ICIs and ICI + chemo, the univariate and Multivariate Cox proportional hazard regression analysis revealed that age and ECOG were not independent risk factors for the prognosis of elderly lung cancer patients. Immunotherapy targeting PD-1 or PD-L1 have recently been introduced and being integrated into standard treatment [[15], [16], [17]]. Older patients have more therapeutic options to prolong the survival. Therefore, as long as their physical condition permits, elderly patients should not give up receiving anticancer treatment.
We also compared responses to the multiple treatment options of this study. We found that the ICIs group had the highest DCR (75%) and lowest ORR (16.7%) among 68 patients. Recent studies have shown that chemotherapy shrinks tumor rapidly, whereas immunotherapy has a long-term effect that prolong survival. KEYNOTE 189 and KEYNOTE 407 further demonstrated that compared with chemotherapy, ICI + chemotherapy significantly improved PFS and OS in patients with metastatic non-squamous and squamous NSCLC, respectively (non-squamous: PFS: 9.0 months vs. 4.9 months; OS: 22.0 months vs. 10.7 months; squamous: PFS: 8.0 months vs. 5.1 months; OS: 17.1 months vs. 11.6 months) [8,10]. The Impower150 [18] and Impower131 [19] studies compared ICIs plus chemotherapy with chemotherapy in four age groups (65, ≥65 to <75, ≥75 to <85, and ≥85 years). The HR of patients in this trials, who were 75–84 years old, was 0.78 (0.50–1.76) in the Impower 150 study and 0.74 (0.45–1.23) in the Impower 131 study, not indicating a significant OS benefit in patients ≥75 years old [20]. In our study, the median PFS and OS for the ICI + chemotherapy group were 5.3 and 10.7 months, respectively, which had no obvious advantage in improving survival in older patients compared with chemotherapy. This could be due to several reasons: 1) given the fact that elderly patients are frequently excluded from clinical trials, individuals enrolled in clinical trials have had a better ECOG scores with fewer comorbidities than those in the real world; 2) a considerable percentage of older patients in the ICI + chemotherapy group found it hard to tolerate the side effects, leading to high a discontinuation rate in our study; 3) numerous studies suggested that ICI plus chemotherapy has no significant advantages in PFS. These considerations may imply that immunotherapy has long-term benefits and could influence subsequent treatments [21,22]. In addition, this may be due to the small sample size of the group.
KEYNOTE 042 indicated that ICIs alone significantly improved PFS and OS compared to chemotherapy in NSCLC patients with PD-L1 ≥1% (PFS: 5.6 months vs. 6.8 months; OS: 16.4 months vs. 12.1 months) [23]. All the patients included in the KEYNOTE 042 trial had ECOG-PSs of 0–1 score, whereas 93.3% of patients in our study had ECOG-PSs of 1–2. Nonetheless, patients who received ICI alone or ICI in combination with other therapies, such as anti-angiogenesis drugs (bevacizumab, endostar) and other novel ICIs such as Tigit antibody had a median OS of 20.3 months. Of those patients, half are still receiving follow-up appointments.
The expression of PD-L1 has a significant impact on the effectiveness of immunotherapy [24,25]. Fig. 3's OS curve for the total population shows that older patients are more likely to benefit from immunotherapy alone or immunotherapy combined with other treatments. In clinical practice, older patients with a high PD-L1 expression tend to receive the ICIs. Table 5 demonstrats that the PD-L1 expression was not similar between ICI + chemo and ICIs group. Therefore, a subgroup analysis using PD-L1 expression was necessary. Interestingly, in PD-L1 positive (TPS ≥1%) patients, ICIs had a longer OS than those in ICI + chemo, even though this difference was not statistically significant. The subgroup analysis showed no significant difference in the OS benefit between ICI + chemo and ICIs among PD-L1 negative patients (TPS <1%). A multicenter retrospective study reported in 2023 ASCO showed that in PD-L1 TPS ≥1%, PD-L1 1–49%and PD-L1 ≥ 50% subgroup,there was no difference in median OS and PFS between ICI alone and ICI plus chemotherapy among elderly patients (NEJ057, Abstract 9012). Our result also indicated that ICI + chemotherapy did not significantly improve the prognosis of older patients in total population and subgroup, therefore first-line ICI alone or ICI plus other treatments could be considered in older patients with PD-L1≥ 1%. However, these findings require further validation in larger, multi-institutional cohorts.
Our research has several limitations. First, as a retrospective single-institution study, a potential selection bias was inevitable. However, our sample size was relatively small, particularly in the older subgroup, therefore our findings may need further validation in larger cohorts. Second, most cases in our cohort did not have a TMB analysis and a proportion of patients did not undergo PD-L1 testing. In the future, enough biomarker data and a better selection of patients can help to determine beneficial therapy options for older patients, thereby avoiding exposure to potentially toxic and effective drugs.
In today's aging society, with an increasing number of older patients aged ≥75 years, it is imperative to understand the risks and benefits of various anticancer treatments among older adults and select optimal clinical approaches [26]. In conclusion, our study evaluated first-line therapies for older patients with advanced NSCLC without targetable oncogene alterations. Our results show that older patients using ICI plus chemotherapy are more likely to discontinue treatment due to adverse effects and does not improve older patients' survival compared with ICIs treatment in total population and subgroup. Therefore, ICI alone or ICI plus other therapies could be recommended for elderly cases, especially in PD-L1 (TPS ≥1%) patients. In the future, it is necessary to conduct larger, prospective studies to verify and validate our findings.
Funding
The work was supported by National Natural Science Foundation of China (Project No. 82103497 and 82002900).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Ethics approval
This study was conducted with approval from the Ethics Committee of Peking University Cancer Hospital (No.2022KT01). Consent was obtained from all participants.
Consent for publication
Informed consent was obtained from the authors.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Panpan Zhang: Writing – original draft, Writing – review & editing, Project administration, Methodology, Data curation. Minting Ma: Writing – original draft, Writing – review & editing, Data curation. Jun Nie: Validation, Supervision, Data curation. Ling Dai: Visualization, Data curation. Weiheng Hu: Visualization, Validation, Data curation. Jie Zhang: Methodology, Investigation. Di Wu: Resources, Methodology. Xiaoling Chen: Validation, Supervision. Xiangjuan Ma: Supervision, Methodology. Guangming Tian: Methodology, Investigation. Sen Han: Formal analysis. Jieran Long: Methodology. Yang Wang: Methodology. Ziran Zhang: Investigation. Qianyun Hao: Investigation. Jian Fang: Writing – review & editing, Visualization, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Dr. Daxiang Na for English language editing. Thanks to all patients, their families, and all investigators for their contributions to this research.
References
- 1.Siegel R.L., et al. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., et al. Cancer treatment and survivorship statistics. CA A Cancer J. Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. 2016. [DOI] [PubMed] [Google Scholar]
- 3.Presley C., Lilenbaum R. The treatment of advanced lung cancer in the elderly: the role of a comprehensive geriatric assessment and doublet chemotherapy. Cancer J. 2015;21(5):392–397. doi: 10.1097/PPO.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 4.Gridelli C., Sgambato A. Elderly patients and PD-L1-positive advanced non-small cell lung cancer: is pembrolizumab monotherapy effective and safe? Ann. Transl. Med. 2019;7(Suppl 8):S282. doi: 10.21037/atm.2019.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 6.Bordoni R., et al. Patient-reported outcomes in OAK: a phase III study of atezolizumab versus docetaxel in advanced non-small-cell lung cancer. Clin. Lung Cancer. 2018;19(5):441–449 e4. doi: 10.1016/j.cllc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Uprety D. Chemo-immunotherapy: the beginning of a new era in lung cancer. Clin. Lung Cancer. 2019;20(2):63–65. doi: 10.1016/j.cllc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Novello S., et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J. Clin. Oncol. 2023;41(11):1999–2006. doi: 10.1200/JCO.22.01990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paz-Ares L., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 10.Gadgeel S., et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2020;38(14):1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 11.Yan X., et al. Impact of age on the efficacy of immune checkpoint inhibitor-based combination therapy for non-small-cell lung cancer: a systematic review and meta-analysis. Front. Oncol. 2020;10:1671. doi: 10.3389/fonc.2020.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wozniak A.J., et al. Clinical outcomes in elderly patients with advanced non-small cell lung cancer: results from ARIES, a bevacizumab observational cohort study. Clin. Oncol. 2015;27(4):187–196. doi: 10.1016/j.clon.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Gridelli C., et al. Immunotherapy in the first-line treatment of elderly patients with advanced non-small-cell lung cancer: results of an International Experts Panel Meeting by the Italian Association of Thoracic Oncology (AIOT) ESMO Open. 2023;8(2) doi: 10.1016/j.esmoop.2023.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naltet C., Besse B. Immune checkpoint inhibitors in elderly patients treated for a lung cancer: a narrative review. Transl. Lung Cancer Res. 2021;10(6):3014–3028. doi: 10.21037/tlcr-20-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmer J., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garon E.B., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 17.Spagnuolo A., Gridelli C. The role of immunotherapy in the first-line treatment of elderly advanced non-small cell lung cancer. Cancers. 2023;15(8) doi: 10.3390/cancers15082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Socinski M.A., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 19.Jotte R., et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J. Thorac. Oncol. 2020;15(8):1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Takigawa N., et al. Do elderly lung cancer patients aged >/=75 Years benefit from immune checkpoint inhibitors? Cancers. 2020;12(7) doi: 10.3390/cancers12071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macedo-Perez E.O., et al. Long progression-free survival with first-line paclitaxel plus platinum is associated with improved response and progression-free survival with second-line docetaxel in advanced non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2014;74(4):681–690. doi: 10.1007/s00280-014-2522-9. [DOI] [PubMed] [Google Scholar]
- 22.Martinez P., et al. Immunotherapy for the first-line treatment of patients with metastatic non-small cell lung cancer. Clin. Cancer Res. 2019;25(9):2691–2698. doi: 10.1158/1078-0432.CCR-18-3904. [DOI] [PubMed] [Google Scholar]
- 23.de Castro G., Jr., et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score >/= 1% in the KEYNOTE-042 study. J. Clin. Oncol. 2023;41(11):1986–1991. doi: 10.1200/JCO.21.02885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis A.A., Patel V.G. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z., et al. Effect of PD-L1 expression for the PD-1/L1 inhibitors on non-small cell lung cancer: a meta-analysis based on randomised controlled trials. Clin. Oncol. 2023;35(10):640–651. doi: 10.1016/j.clon.2023.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Sacco P.C., et al. Treatment of advanced non-small cell lung cancer in the elderly. Expet Rev. Respir. Med. 2018;12(9):783–792. doi: 10.1080/17476348.2018.1510322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.