Abstract
Introduction
This retrospective study investigates the efficacy of cemiplimab, a monoclonal antibody targeting the PD-1 receptor, in treating squamous cell carcinoma (SCC) of the skin.
Methods
The study analyzes data from 50 patients with SCC, focusing on various clinical parameters, including patient demographics, tumor characteristics, treatment history, disease status at the beginning of therapy, and survival outcomes.
Results
Of the patients who received at least one cycle of cemiplimab, 42% showed a clinical response. Adverse reactions were generally low, with the safety profile deemed excellent. During a median follow-up of 9.6 months, 17 patients experienced progression or death. Among these, 15 patients had died at the time of the analysis. The median progression-free survival (PFS) for the entire cohort was approximately 20.8 months, while median overall survival (OS) was not reached. Univariate Cox regression analysis for PFS showed that tumors in the arms and legs were associated with higher progression risk, while age above 65 years was not statistically significant. Distant metastasis exhibited a trend towards improved PFS. In terms of OS, distant metastasis was a significant predictor of reduced survival, while age above 65 years was not statistically significant. In a multivariate model, only the absence of distant metastasis remained significant, with an adjusted odds ratio (OR) of 12.3 (95% confidence interval 1.3–112.1).
Conclusion
These findings provide valuable insights into the real-world effectiveness of cemiplimab in SCC management.
Keywords: Cemiplimab, Squamous cell carcinoma, Clinical response, Adverse reactions, Distant metastasis, Survival outcomes
Key Summary Points
| This retrospective study assessed the efficacy of cemiplimab, a PD-1 inhibitor, in treating squamous cell carcinoma (SCC) of the skin in a real-world setting. |
| Among 50 patients with SCC, 42% exhibited a clinical response to cemiplimab treatment, with a median progression-free survival (PFS) of approximately 20.8 months. |
| Multivariate analysis identified the absence of distant metastasis as a significant predictor of a clinical response to cemiplimab. |
| The study highlights the favorable safety profile of cemiplimab in this patient population and the relevance of cemiplimab in elderly patients often excluded from clinical trials. |
Introduction
Squamous cell carcinoma (SCC) of the skin poses a significant clinical challenge, impacting patients’ well-being and survival [1]. SCC is the second most common skin cancer, with risk factors including sun exposure, advanced age, and immunosuppression [2]. While most cases can be cured with surgery and/or radiotherapy, a small percentage becomes incurable as a result of metastasis or advanced progression [3]. These patients are considered for systemic therapy. In the quest to improve therapeutic outcomes while minimizing adverse effects, the landscape of SCC management has witnessed notable advancements over the years. Among these advancements, immune checkpoint inhibitors, particularly cemiplimab, have emerged as a promising avenue for the treatment of advanced SCC [4].
Cemiplimab, a monoclonal antibody targeting the programmed death 1 (PD-1) receptor, has exhibited substantial potential in the management of advanced SCC [5]. However, the real-world effectiveness of this therapeutic agent, especially within distinct patient populations and varied clinical scenarios, remains a subject of paramount interest [6].
This retrospective article embarks on an exploration of the efficacy of cemiplimab in SCC treatment. Drawing upon a dataset encompassing 50 patients diagnosed with SCC of the skin, we examine a comprehensive set of clinical variables. These variables encompass patient demographics, tumor characteristics, treatment history, disease status at the beginning of therapy, and survival outcomes. Our objective is to offer valuable insights into the practical application of cemiplimab in SCC management, shedding light on its efficacy profile within diverse clinical contexts.
Methods
Patient Population
This retrospective study was conducted at four participating medical institutions. A retrospective chart review retrieved data regarding eligible individuals aged 18 years or older with a confirmed diagnosis of SCC of the skin. Eligible patients must have undergone at least one cycle of cemiplimab treatment as part of their therapeutic regimen. Demographic information was gathered from each patient in this retrospective study of cemiplimab efficacy in SCC. Age, previous systemic therapy, previous radiotherapy, and previous surgery were assessed at baseline. Furthermore, this retrospective study also included information about conditions that may interact with cemiplimab effectiveness or toxicity, such as lymphoproliferative disorders and immunosuppressive or autoimmune conditions. Additionally, the binary variable “age ≥ 65” was used to distinguish patients aged 65 years or older at the initiation of cemiplimab therapy, allowing for the differentiation of older patient populations. Tumor location documented the anatomical regions affected, including the head and neck, arms and legs, trunk, and genitalia, providing insight into the primary site of SCC lesions. Disease status at the beginning of therapy at the time of cemiplimab initiation was stratified into “locally advanced progression only,” “regional metastasis only,” or “distant metastasis” at the initiation of cemiplimab therapy, reflecting the extent and nature of SCC progression.
Clinical outcomes were evaluated through progression-free survival (PFS), defined as the time in months from cemiplimab treatment initiation until disease progression or death, and overall survival (OS), measuring the period in months from initial SCC diagnosis to death or last follow-up. Lastly, response rate was defined as the number of patients showing either a partial or complete response based on clinical criteria divided by the total number of evaluable patients. These variables collectively provide a comprehensive understanding of the patient population and the study’s evaluation of cemiplimab’s effectiveness in SCC treatment.
Statistical Analysis
The primary end point of the study was clinical response rate. Secondary end points included the number of delivered cycles of cemiplimab, PFS, and OS. Descriptive statistics was used to provide a comprehensive overview of the patient cohort and treatment outcomes. Measures such as median and interquartile range were calculated for continuous variables like age and the number of treatment cycles. Categorical variables such as gender, tumor location, prior systemic therapy, radiotherapy, surgery, and disease status at the beginning of therapy were summarized using percentages. To assess survival outcomes, the Kaplan–Meier method was employed. PFS and OS were analyzed using Kaplan–Meier survival curves. As a result of the retrospective nature of the study and the utilization of anonymized data, formal permission from an ethical committee was not required for this research according to the Italian Medicines Agency (AIFA) guidelines. However, it adhered to the Helsinki Declaration.
Results
Study Cohort Characteristics
The patient cohort consisted of 50 patients, with a median age of 83 years. Notably, nearly all patients (96%) were aged 65 years or older at the initiation of cemiplimab therapy. In terms of gender, the majority of patients were male (70%). In the studied population, comorbidities were observed as follows: 20% of individuals had diabetes, 6% had severe renal insufficiency, 2% had alcoholic cirrhosis, while autoimmune diseases, lymphoproliferative disorders, and immunosuppressive medications were not reported in any cases, indicating a low prevalence of these conditions within the cohort. Tumor locations varied, with some patients having tumors in the head and neck (70%) and others in the trunk (12%). A smaller proportion had tumors in the arms or legs (16%) or the genitalia (2%). Twenty patients had received previous systemic therapy (40%) for SCC before starting cemiplimab treatment. Radiotherapy and surgery were also part of the treatment landscape, with 58% of patients receiving radiotherapy and 54% undergoing surgical intervention during their therapeutic journey. Among the observed disease status at the beginning of therapy, 16% of patients presented with locally advanced progression only. The majority (64%) had regional metastasis only, while 20% of patients exhibited distant metastasis (Table 1).
Table 1.
Characteristics of the study population
| Variable | Value |
|---|---|
| Age, years, median [IQR] | 83 [80–89] |
| Age ≥ 65 | 96% |
| Gender (male) | 70% |
| Relevant comorbidities | |
| Diabetes | 20% |
| Severe renal insufficiency | 6% |
| Autoimmune diseases | 0% |
| Lymphoproliferative disorders | 0% |
| Alcoholic cirrhosis | 2% |
| Immunosuppressive medications | 0% |
| Tumor location | |
| Head and neck | 70% |
| Arm/leg | 16% |
| Trunk | 12% |
| Genitalia | 2% |
| Previous systemic therapy | 40% |
| Radiotherapy | 58% |
| Surgery | 54% |
| Disease status at the beginning of therapy | |
| Locally advanced only | 16% |
| Regional metastasis only | 64% |
| Distant metastasis | 20% |
Data are presented as a percentage unless otherwise specified#
IQR interquartile range
Treatment Safety and Effectiveness and Exploratory Analysis
The median number of treatment cycles was 10 (interquartile range 4–21). Of the 50 patients who received at least one cycle of cemiplimab, 42% demonstrated a clinical partial or complete response to cemiplimab.
Several adverse reactions were reported. Nausea was observed in 1 out of 50 participants (2%), while asthenia was reported in 9 out of 50 individuals (18%). Xerostomia was reported in 1 out of 50 participants (2%), as was dizziness (2%), rectorrhagia/urothelial carcinoma (UC) (2%), itch (2%), pneumonia (2%), and neutropenia (2%). Additionally, biliary lithiasis (gallstones) was observed in 1 out of 50 individuals (2%) (Table 2). The overall safety profile of the study appeared to be excellent (Table 2).
Table 2.
Adverse events associated with cemiplimab
| Adverse events N = 50 | G1 | G2 | G3 | G4 |
|---|---|---|---|---|
| Nausea | 1/50 (2%) | |||
| Asthenia | 9/50 (18%) | 1/50 (2%) | ||
| Xerostomia | 1/50 (2%) | |||
| Dizziness | 1/50 (2%) | |||
| Rectorrhagia/UC | 1/50 (2%) | |||
| Dermatitis | 1/50 (2%) | |||
| Itch | 1/50 (2%) | |||
| Pneumonia | 1/50 (2%) | 2/50 (4%) | ||
| Neutropenia | 1/50 (2%) | |||
| Biliary lithiasis | 1/50 (2%) |
UC urothelial carcinoma
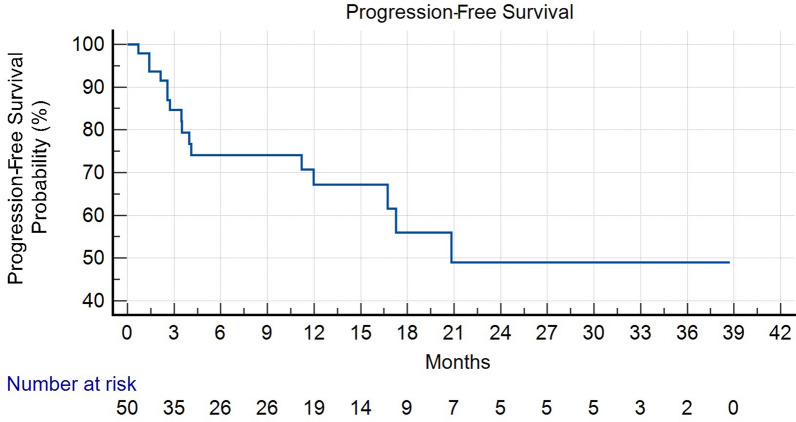
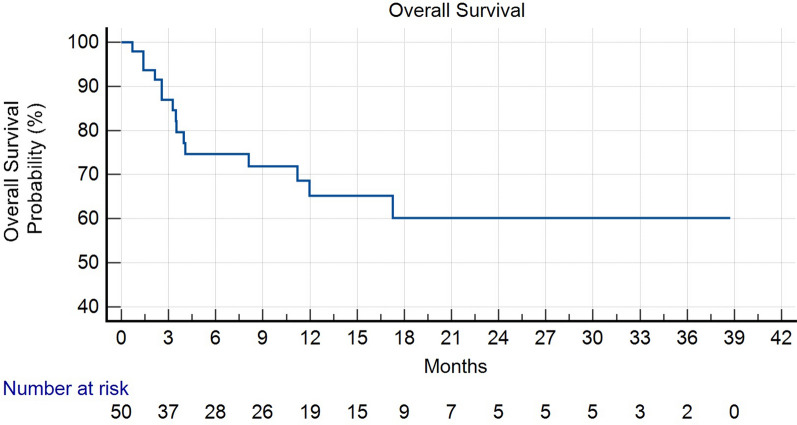
During a median follow-up time of 9.6 months, 17 patients experienced events of progression and/or death. Among these, 15 patients had died at the time of the analysis. The mean OS was 25.8 months (95% confidence interval (CI) for the mean OS 20.6–31.1 months), while the median OS was not reached. The mean PFS was approximately 23.24 months (95% CI for the mean PFS 18.2–29.4 months), while the median PFS for the entire study cohort was approximately 20.8 months) (Figs. 1 and 2). Table 3 presents the results of univariate logistic regression analysis examining the odds of achieving a clinical response. Notably, patients with tumors located in the arms and legs had significantly lower odds of a clinical response (odds ratio (OR) 0.21, 95% CI 0.04–0.98, p = 0.042). Similarly, age above 65 was associated with reduced odds of a clinical response (OR 0.24, 95% CI 0.09–0.61, p = 0.004), while the absence of distant metastasis significantly increased the odds of a clinical response (OR 5.31, 95% CI 1.77–15.94, p = 0.003). Importantly, when these three factors were entered into a multivariate model, only the absence of distant metastasis remained statistically significant, with an adjusted OR of 12.38 (95% CI 1.36–112.10). Notably, our analysis revealed that patients with tumors located in the arms and legs exhibited a significantly higher risk of progression (hazard ratio (HR) 8.09, 95% CI 1.66–39.33, p = 0.0095). Conversely, age above 65 (arbitrary cutoff) or above the median value of 83 years did not exhibit a statistically significant association with PFS (HR 0.50, 95% CI 0.06–3.79, p = 0.50 and HR 0.47, 95% CI 0.16–1.37, p = 0.16, respectively).
Fig. 1.
Kaplan–Meier plot illustrating progression-free survival (PFS) among patients with squamous cell carcinoma (SCC) treated with cemiplimab
Fig. 2.
Kaplan–Meier plot depicting overall survival (OS) in patients with squamous cell carcinoma (SCC) who received cemiplimab therapy
Table 3.
Univariate logistic regression analysis for clinical response
| Variable | OR for clinical response | 95% CI | p value |
|---|---|---|---|
| Tumor located in the arms and legs | 0.211 | 0.045–0.982 | 0.042 |
| Age ≥ 65 | 0.244 | 0.097–0.614 | 0.004 |
| No distant metastasis | 5.318 | 1.776–15.943 | 0.003 |
| Tumor located in head and neck | 3.042 | 0.921–10.039 | 0.067 |
| Locally advanced progression only | 3.042 | 0.921–10.039 | 0.067 |
| Female | 0.577 | 0.208–1.595 | 0.292 |
| No previous systemic therapy | 1.419 | 0.422–4.773 | 0.578 |
| Received radiotherapy | 1.105 | 0.397–3.076 | 0.862 |
| Regional metastasis only | 2.265 | 0.855–6.002 | 0.100 |
| No surgery performed | 0.624 | 0.197–1.980 | 0.413 |
| Tumor located in the trunk | 0.285 | 0.047–1.736 | 0.167 |
OR odds ratio, CI confidence interval
Interestingly, the absence of distant metastasis showed a trend towards improved PFS (HR 0.17, 95% CI 0.02–1.29, p = 0.0870). Notably, the presence of distant metastasis emerged as a significant predictor of reduced OS (HR 4.7, 95% CI 1.6–13.7, p = 0.003). Conversely, age above 65 (arbitrary cutoff) and above the median value of 83 years did not exhibit a statistically significant association with OS (HR 0.95, 95% CI 0.36–2.48, p = 0.925 and HR 0.38, 95% CI 0.12–1.22, p = 0.105, respectively).
Discussion
Squamous cell carcinoma of the skin is a clinical challenge, affecting both the well-being and survival of patients. Our study explores the real-world effectiveness of cemiplimab, a monoclonal antibody targeting the PD-1 receptor, in the treatment of advanced SCC. Through a retrospective analysis of a dataset encompassing 50 patients with SCC, we aimed to shed light on the practical application and effectiveness of cemiplimab within diverse clinical contexts. Our patient cohort consisted of 50 individuals, primarily elderly with a median age of around 83 years, and predominantly male (66%). Importantly, almost all patients (96%) were aged 65 years or older at the initiation of cemiplimab therapy. This demographic distribution underscores the relevance of our study in the context of a patient population often underrepresented in clinical trials. Encouragingly, our findings demonstrated that 42% of the patients who received at least one cycle of cemiplimab exhibited a clinical partial or complete response, with a median PFS of approximately 20 months and a median OS that was not reached. A central aspect of our analysis involved conducting univariate analyses to better understand the interplay of multiple factors in predicting treatment outcomes. When considering the influence of tumor location, age, and distant metastasis on clinical response, we found that the absence of distant metastasis was a significant predictor of a clinical response even after adjusting for other significant variables. Patients without distant metastasis had significantly higher odds of responding to cemiplimab treatment. This multivariate analysis reaffirms the importance of distant metastasis as a critical predictor of cemiplimab response in advanced SCC. Importantly, our analysis revealed that age, whether analyzed using arbitrary or median cutoffs, was not associated with either PFS or OS. This observation suggests that cemiplimab demonstrates effectiveness and tolerability across a wide age range, including the elderly. The lack of an association with age implies that the primary determinant of progression and mortality is not age itself but rather the underlying disease.
Overall, our results align with the growing body of evidence supporting the effectiveness of cemiplimab in the treatment of advanced SCC [7, 8]. Furthermore, the toxicity profile of cemiplimab in our study was notably favorable, with a low incidence of adverse events, suggesting its tolerability and safety in this patient population. In a pivotal phase 2, single-arm trial [9] conducted across 25 outpatient clinics in Australia, Germany, and the USA, cemiplimab demonstrated significant antitumor activity and an acceptable safety profile in patients diagnosed with locally advanced SCC of the skin. A total of 78 eligible patients aged 18 years or older, with histologically confirmed locally advanced cutaneous squamous cell carcinoma (cSCC) and an Eastern Cooperative Oncology Group performance status of 0–1, received cemiplimab intravenously at a dose of 3 mg/kg every 2 weeks for a maximum of 96 weeks. Findings from this study revealed a notable objective response in 44% (34 of 78) of the enrolled patients. Within this responsive group, 13% achieved a complete response, while 31% exhibited a partial response. Notably, grade 3–4 treatment-emergent adverse events were documented in 44% of patients, with hypertension affecting 8% and pneumonia occurring in 5% of cases. Furthermore, serious treatment-emergent adverse events were noted in 29% of patients. Importantly, one treatment-related death was reported, which was attributed to aspiration pneumonia. Importantly, the final analysis of the study [7] confirmed consistent and durable responses. The results displayed a remarkable OS rate at 48 months, with 61.8% (95% CI 54.0–68.7), underscoring the long-term effectiveness of cemiplimab in treating advanced cSCC. The median duration of response (DOR) was also substantial, with an overall median of 41.3 months.
Conversely, in a retrospective observational study [10], Baggi and colleagues examined the real-world data of cemiplimab in the treatment of locally advanced and metastatic SCC of the skin. The study encompassed patient records from 17 referral Italian centers, covering the period between May 2019 and February 2020. A total of 131 patients, with a median age of 79 years, were included in the analysis. Among them, 9.2% had concurrent chronic lymphoproliferative diseases, and 8.5% had concomitant autoimmune diseases. The safety profile was assessed on the basis of Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE v 5.0), with 42.7% of patients experiencing at least one treatment-related adverse event (AE). Notably, 9.2% of patients encountered grade 3–4 adverse events, and two fatal adverse events were reported. The study revealed an overall response rate (ORR) of 58% and a disease control rate (DCR) of 71.7% among the patients. In another recent study, Denaro et al. [8] investigated the use of cemiplimab in ultra-octogenarian patients with cSCC at a tertiary referral center. The study included 20 patients, with a median age of 86.9 years, and assessed the efficacy and safety of cemiplimab treatment. Despite advanced age and comorbidities, the results indicated that cemiplimab was effective in this patient population, with a 65% response rate, including partial responses and long-lasting stable disease. The median duration of response was 14 months, and no treatment-related adverse events of grade 3 or higher were reported [8].
Importantly, in a phase 2 study exploring the neoadjuvant use of cemiplimab in resectable stage II–IV SCC of the skin [11], Gross and colleagues observed a substantial pathological complete response rate of 51%, indicating the potential effectiveness of cemiplimab in this context. In another study, multivariate analysis revealed a notable correlation between elevated serum interleukin (IL)-6 levels and shorter PFS, establishing IL-6 as an independent predictive factor for cemiplimab response [12]. Another clinical trial examining first-line pembrolizumab monotherapy for unresectable SCC of the skin suggested that PD-L1 positivity might serve as a predictive factor for a positive response to anti-PD-1 agents, such as cemiplimab. Specifically, the trial demonstrated that patients who were PD-L1-positive exhibited a significantly higher response rate (55%) compared to their PD-L1-negative counterparts (17%) [13].
It is crucial to recognize and address the limitations of our study. First, the retrospective nature of our research introduces inherent biases and prevents the establishment of causal relationships. Notably, the retrospective design may have led to an underestimation of toxicity. Furthermore, it is important to emphasize that this retrospective study lacked the availability of biomarkers for investigation. However, despite these limitations, the study’s strengths, including the sample size for a rare condition and the reflection of a real-world scenario, provide valuable insights into the practical application of cemiplimab.
Conclusion
Our study adds to the growing body of evidence supporting the potential benefit of cemiplimab in the treatment of advanced SCC. Multivariate analysis has shed light on the importance of factors such as distant metastasis, tumor location, and patient age in predicting treatment outcomes. While limitations exist, our findings underscore the relevance of cemiplimab, especially within the context of elderly and frail patients who are often excluded from clinical trials. Further research, including larger prospective trials with toxicity data and a broader patient population, is warranted to validate our observations and provide comprehensive guidance on treatment decisions for patients with advanced SCC in real-world settings.
Acknowledgements
The lead author conceived the initial idea for this study during ORA’s regular scientific meetings. ORA ETS provided financial support for obtaining statistical software licenses and made significant contributions to disseminating the research results.
Author Contributions
Aieta Michele, Leo Silvana, Domenico Bilancia, Rossella Di Trolio, Michela Rosaria, Iuliucci, Concetta Ingenito, Roberta Rubino, Arianna Piscosquito, Michele Caraglia, Marianna Donnarumma, Ferdinando Costabile, Raffaele Conca, Marco Pisino, Angelo Vaia, Luca Scafuri and Antonio Verde contributed to data collection and revision. Carlo Buonerba and Giuseppe Di Lorenzo were involved in the concept and design. Carlo Buonerba and Michela Rosaria Iuliucci were responsible for statistical analysis and drafting the paper.
Funding
No funding was received for this study or its publication.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Giuseppe Di Lorenzo, Aieta Michele, Leo Silvana, Domenico Bilancia, Rossella Di Trolio, Michela Rosaria Iuliucci, Concetta Ingenito, Roberta Rubino, Arianna Piscosquito, Michele Caraglia, Marianna Donnarumma, Ferdinando Costabile, Raffaele Conca, Marco Pisino, Angelo Vaia, Luca Scafuri, Antonio Verde and Carlo Buonerba have nothing to disclose.
Ethical Approval
As a result of the retrospective nature of the study and the utilization of anonymized data, formal permission from an ethical committee was not required for this research according to the Italian Medicines Agency (AIFA) guidelines. However, it adhered to the Helsinki Declaration.
References
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Wysong A. Squamous-cell carcinoma of the skin. N Engl J Med. 2023;388:2262–2273. doi: 10.1056/NEJMra2206348. [DOI] [PubMed] [Google Scholar]
- 3.Hillen U, Leiter U, Haase S, et al. Advanced cutaneous squamous cell carcinoma: a retrospective analysis of patient profiles and treatment patterns—results of a non-interventional study of the DeCOG. Eur J Cancer. 2018;96:34–43. doi: 10.1016/j.ejca.2018.01.075. [DOI] [PubMed] [Google Scholar]
- 4.Goodman DT. Cemiplimab and cutaneous squamous cell carcinoma: from bench to bedside. JPRAS Open. 2022;33:155–160. doi: 10.1016/j.jpra.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 6.Bailly-Caillé B, Kottler D, Morello R, et al. real-life study of the benefit of concomitant radiotherapy with cemiplimab in advanced cutaneous squamous cell carcinoma (cSCC): a retrospective cohort study. Cancers (Basel) 2023;15:495. doi: 10.3390/cancers15020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migden MR, Schmults C, Khushanlani N, et al. 814P Phase II study of cemiplimab in patients with advanced cutaneous squamous cell carcinoma (CSCC): final analysis from EMPOWER-CSCC-1 groups 1, 2 and 3. Ann Oncol. 2022;33:S918–S919. doi: 10.1016/j.annonc.2022.07.940. [DOI] [Google Scholar]
- 8.Denaro N, Passoni E, Indini A, et al. Cemiplimab in ultra-octogenarian patients with cutaneous squamous cell carcinoma: the real-life experience of a tertiary referral center. Vaccines (Basel) 2023;11:1500. doi: 10.3390/vaccines11091500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21:294–305. doi: 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggi A, Quaglino P, Rubatto M, et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur J Cancer. 2021;157:250–258. doi: 10.1016/j.ejca.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Gross ND, Miller DM, Khushalani NI, et al. Neoadjuvant cemiplimab for stage II to IV cutaneous squamous-cell carcinoma. N Engl J Med. 2022;387:1557–1568. doi: 10.1056/NEJMoa2209813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallardo D, Simeone E, Festino L, et al. IL-6 as new prognostic factor in patients with advanced cutaneous squamous cell carcinoma treated with cemiplimab. J Transl Med. 2023;21:140. doi: 10.1186/s12967-023-03971-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maubec E, Boubaya M, Petrow P, et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol. 2020;38:3051–3061. doi: 10.1200/JCO.19.03357298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.