Abstract
Introduction
There has been a growing recognition on the importance of diversity in clinical trials. Existing research has highlighted a significant demographic imbalance. Amidst this renewed focus on diversity, it is crucial to acknowledge that Asia comprises over half of the world’s population. Given the region’s demographic significance, we sought to compare various characteristics and growth rates for trials with sites in Asia against those without any sites in Asia.
Methods
We performed comprehensive analyses of industry-sponsored phase 2 and 3 oncology trials registered at Clinicaltrials.gov, using drugs or biologics as investigational agents and executed between 1 January 2018 and 31 December 2022. We applied the compound annual growth rate (CAGR) as an analytical tool to track the trial growth rates over this 5-year period.
Results
We identified 894 industry-sponsored phase 2 and 3 cancer studies with available study location data. Out of these, 415 trials (46.42%) had study sites in Asia. Notably, these trials with sites in Asia were also more likely to be phase 3 trials (39.76% vs 6.47%, p < 0.001), include female and paediatric populations, and be randomised trials. Interestingly, lung and stomach cancers were more commonly studied in these trials, while myeloma was less commonly studied. The number of trial sites for liver cancer was not significantly higher for Asia, even though the incidence of the disease is much higher in this region. Despite an overall declining trend in the number of clinical trials in the last 5 years, we observed a transitional positive increase in the CAGR from 2020 to 2021 for trials with sites in Asia. However, East Asia, specifically China, exhibited a disproportionate overrepresentation in these trials.
Conclusions
There are notable characteristics of clinical trials with sites in Asia. Comprehending these disparities may aid in the strategic planning to enhance a balanced representation of ethnicities in trials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40487-023-00254-3.
Keywords: Asia, Clinical trials, Diversity, Industry-sponsored, Oncology
Key Summary Points
| There is increasing acknowledgement of the crucial role that diversity plays in clinical trials. |
| Despite accounting for more than half of the global population and experiencing an upsurge in cancer cases, it remains to be evaluated whether Asia is proportionately represented in recent oncology clinical trials. |
| An analysis of phase 2/phase 3 clinical trials over 5 years was conducted to understand the current landscape. |
| Approximately 46% of global trials have centres in Asia. While East Asia, specifically China, exhibited strong participation in these trials, other Asian countries were largely underrepresented. |
| Contrary to the higher prevalence of liver cancer in Asia, Asian populations are underrepresented in liver cancer trials. |
| Comprehending disparities in oncology clinical trials may aid in the strategic planning to promote a balanced representation of ethnicities in trials. |
Introduction
Clinical trials are necessary for the development of novel therapies and for establishing their safety and efficacy [1]. In the context of the evidence-based medicine framework, clinical trials hold a high level of preference due to the incorporation of randomisation of the intervention. This structure helps to diminish potential sources of bias, thereby maintaining a balance of potential confounders across study arms and preventing false conclusions [2]. A well-structured clinical trial defines the selected study population through explicit inclusion and exclusion criteria. Even though participants willingly give their informed consent to participate in a clinical trial, it is important to note that the demographic makeup of the enrolled study population might not accurately represent the wider population from which they were selected [3].
A report by the United States Food and Drug Administration (FDA) summarising and analysing clinical trial participation over a 5-year span from 2015 to 2019 emphasized the demographic disparity between the enrolled participants and the global population [4]. The report found that out of the 292,537 participants in global clinical trials, a considerable majority (76%) were white. In contrast, only 11% were Asian, 7% were Black/African American, those categorised as ‘other’ made up 4%, and American Indian or Alaska Native participants accounted for 1% [4]. Despite the fact that these demographics do not accurately mirror the global population, it has been widely acknowledged and recognised that the participants enrolled in clinical studies should closely reflect the real-world population(s) affected by the medical condition and require investigational treatment [5–7]. This ensures that the investigational product is being tested in a context that is as realistic as possible. The importance of this representation is amplified considering that over 60% of the world’s population currently resides in Asia, a region where population growth is projected to rise significantly in the forthcoming years [8]. This issue of the underrepresentation of Asians in clinical trials has recently been recognised. However, most of this recognition has been primarily within the context of research in the United States. It is noteworthy that nearly 60% of individuals of Asian descent residing in the United States were born overseas, which contributes to a highly diverse and heterogeneous population [9, 10].
Failure to achieve suitable and appropriate ethnic representation in clinical trials can undermine their universal applicability and the accuracy of information about drug responses in ethnic minority subgroups. Consequently, this may impact the measures of safety and efficacy. This lack of representation could lead to inaccurate estimations of response rates and flawed evaluations of treatment effectiveness or safety, potentially exacerbating health disparities [11]. Furthermore, the inadequate representation of specific ethnic groups in drug registration trials has led to diminished effectiveness and safety of the approved products. It has also triggered issues regarding trust with some medicines [12, 13]. The FDA has acknowledged the importance of addressing these issues and promoting diversity in clinical trials. The recent guidance highlights the significance of achieving greater diversity to mitigate disparities in cancer care [14].
Industry-sponsored trials often investigate novel drugs or treatments, providing insights into the latest advancements in oncology treatment strategies [15]. These trials are generally more international [16] and by examining these trials in recent years, we are also able to understand to what extent the research for the latest patented oncological therapies embraces diversity for Asian populations [17]. Prior research evaluating the proportion of industry-sponsored trials undertaken in Asia has been sparse, and the growth rate for cancer trials with sites in Asia has been limited to China, India, Japan and South Korea [18]. In order to determine the extent of industry-sponsored oncology clinical trials in Asia, understand their current status and examine recent trends, we reviewed clinical studies registered in ClinicalTrials.gov. This resource, provided by the US National Library of Medicine, houses a database of clinical studies conducted worldwide and funded both privately and publicly [19, 20]. Starting from 2005, all clinical trials must be registered on clinical trial databases before they can be considered for publication by peer-reviewed journals. This measure was implemented to control reporting bias from clinical trials [21].
In addition, we explored the World Health Organization International Clinical Trials Registry Platform (WHO-ICTRP). It also provides access to a centralised database consisting of trial registration datasets from 18 different registries. Although the WHO-ICTRP includes some datasets from CT.gov, it does not include many industry-sponsored clinical trials [22]. To confirm our decision, we ran a pilot search in both databases and concluded that the CT.gov database most accurately captures industry-sponsored trials. This is likely because Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801) mandates registration in CT.gov for any investigational product requiring FDA approval [20]. Hence, it is noteworthy that 60.6% of trials listed on CT.gov are sponsored by industry, and also that 36.3% of trials include countries outside of the United States [23].
In this article, we showcase the pivotal results of our investigation, comparing trials performed in Asia against those from other regions. In our study, ‘diversity’ refers to the inclusion of a broad range of participants in clinical trials for various types of cancer, including different ethnicities, age groups and genders. This diverse representation ensures that the trial outcomes are applicable and beneficial to a broad section of the population, particularly within Asia, which has a complex array of genetic diversity and disease prevalence patterns [24].
Our focus was only on phase 2 and phase 3 studies as these clinical trials offer insights that are directly relevant to clinical practice. Specifically, these trials frequently analyse a new treatment against the existing standard of care, a comparison that offers regulatory and clinical implications [25, 26]. Additionally, they often include patient stratification based on ethnicities, a level of granularity typically absent in phase 1 trials [27–29]. For these reasons, we chose to omit phase 1 studies, as they did not align closely with the research questions we sought to answer.
As per our hypotheses, the characteristics of oncology trials conducted in Asia may differ significantly from those that do not include locations in Asia. The uniformity in coverage of oncology studies within different regions within Asia might also vary. Understanding these differences is crucial in generating valuable insights that could facilitate a fair demographic representation in clinical trials, and aid in developing cancer treatments that demonstrate efficacy on a global scale.
Methods
Trial Selection
Our study centred on interventional trials conducted globally and in Asian countries and fully or partially financially supported by the industry. The list of Asian countries was standardised, as defined by GLOBOCAN 2020 [30]. We performed a comprehensive search of Clinicaltrials.gov (CT.gov), focusing on phase 2/phase 3 clinical trials using drugs or biologics as investigational agents carried out over a 5-year span from 1 January 2018 to 31 December 2022. We subsequently refined the study pool by applying filters for cancer treatment and industry-sponsored studies. The search terms used to denote ‘cancer’ included cancer OR cancers OR neoplasm OR neoplasia OR malignancy OR tumour OR tumor, which were adapted from the synonymous entry terms listed under the Medical Subject Headings for neoplasms [31].
Inclusion and Exclusion Criteria
The scope of our study was narrowed down by inclusion and exclusion criteria. We included phase 2/phase 3 trials investigating drug or biological therapies for cancer. Clinical trials with the following status categories were included: ‘recruiting’, ‘not yet recruiting’, ‘active, not recruiting’, ‘completed’, ‘enrolling by invitation’, ‘suspended’, ‘terminated’ and ‘unknown status’. On the other hand, trials that were withdrawn or lacked information on the study location were excluded from our analysis.
We carried out an analysis of all trials held in Asia versus other cancer trials, focusing on several key aspects:
-
i.
Number and percentage of all trials with sites in Asia and the overall top 10 Asian countries with the most trial sites along with top five Asian countries contributing to most trials from each geographical region of Asia.
-
ii.
The median number of study sites, including the interquartile range (IQR).
-
iii.
The median number of subjects enrolled, along with the IQR.
-
iv.
Trials solely funded by the industry in Asia, including and top ten industry sponsors, categorised as global corporations and regional Asian biotechnology firms.
-
v.
The count and percentage of phase 2 or 3 trials.
-
vi.
The distribution of participants by gender.
-
vii.
The participant age distribution, categorised as paediatric (under 18 years), adult and older adult.
-
viii.
The design of interventional trials, categorised as randomised or non-randomised.
-
ix.
The types of cancers studied, (i) breast, (ii) lung, (iii) colorectal, (iv) stomach, (v) liver (top five most common solid tumours in Asia, as the incidence of stomach and liver cancers is higher in Asia than globally) and (iv) lymphoma, (v) leukaemia, (vi) myeloma (top three most common haematological malignancies in Asia) and (vii) ‘others’ to include all other cancers including multiple cancers’ studied [24]. Cancer with a location exclusively in the stomach also included the gastro-esophageal junction. Studies with cancer location exclusively in esophagus or gastrointestinal tract were classified under ‘others’.
We analysed the year-on-year rate of increase in the number of trials using the compound annual growth rate (CAGR). Ethical approval was not applicable since the research involved secondary use of data from ClinicalTrials.gov without any identifier(s).
Statistical Analysis
Categorical characteristics with expected frequency counts of greater than 5 in all possible cells of the cross-tabulation were compared between the trials with and without sites in Asia groups using the Chi-square test. Similarly, categorical characteristics with an expected frequency count less than or equal to 5 in any of the cells in cross-tabulation were compared using Fisher’s exact test. The Wilcoxon rank-sum test (Mann–Whitney U test) was used to compare the distribution of numerical characteristics between the two groups.
All statistical analyses were carried out using R version 4.2.2 for Windows.
Results
As per the search conducted on 27 March 2023, a total of 4071 phase 2 and 3 interventional studies with the status categories ‘recruiting’, ‘not yet recruiting’, ‘active, not recruiting’, ‘completed’, ‘enrolling by invitation’, ‘suspended’, ‘terminated’, or ‘unknown status’ were generated from CT.gov with a start date in the last 5 years. Of this number, around 40% (totalling 1623 studies) focused on cancer treatment. Among these cancer treatment studies, approximately 56% (constituting 912 studies) were sponsored by the industry.
Of these 912 industry-sponsored clinical trials for drugs or biologics in cancer treatment, 18 clinical trials did not have information on the study location. As a result, they were omitted from the final analysis, resulting in a final sample size of 894 trials for this study (Supplementary Fig. 1). These qualifying studies were divided into two categories: (i) trials without any locations in Asia, encompassing a total of 479 studies (53.58%), and (ii) trials with locations in Asia, consisting of 415 studies (46.42%). The second category consisted of clinical trials that had locations in Asia and in other countries outside Asia (211 trials, accounting for 23.60%), and trials that had locations exclusively in Asia (204 trials, making up 22.82% of the total).
Table 1 provides the comparative analysis of key trial characteristics for oncology clinical trials conducted with sites in Asia (415 trials) and without sites in Asia (479 trials). The median number of sites was significantly higher for trials with sites in Asia, at 17.0, versus trials without sites in Asia at 4.0. This difference was statistically significant (p < 0.001). Target enrolment was also significantly larger for trials with sites in Asia, with a median of 144 participants, versus a median of 51 participants for trials without sites in Asia (p < 0.001). For the funding type, the industry was the sole funder for a significantly higher proportion of trials with sites in Asia (80.48%) than for those without sites in Asia (53.03%, p < 0.001). The distribution of trial phases was also significantly different between the two groups. Phase 2 trials were more common in trials without sites in Asia (94.15%) than in trials with sites in Asia (62.41%, p < 0.001). Conversely, phase 3 trials were more prevalent in trials with sites in Asia (39.76%) than in trials without sites in Asia (6.47%, p < 0.001).
Table 1.
Industry-sponsored cancer trials by geographic location: trials with sites in Asia versus trials without sites in Asia (N = 894)a
| Trial characteristics | Trials with sites in Asia (n = 415) | Trials without sites in Asia (n = 479) | p value (trials with sites in Asia vs trials without sites in Asia) |
|---|---|---|---|
| Number of sites, median (Q1, Q3) | 17.0 (1.0, 74.5) | 4.0 (1.0, 13.0) | < 0.001b |
| Target enrolment, median (Q1, Q3) | 144.0 (54.0, 480.0) | 51.0 (25.0, 114.5) | < 0.001b |
| Funding type, n (%) | |||
| Industry as the sole funder | 334 (80.48) | 254 (53.03) | < 0.001c |
| Industry with other funders | 81 (19.52) | 225 (46.97) | < 0.001c |
| Trial phase, n (%) | |||
| Phase 2 | 259 (62.41) | 451 (94.15) | < 0.001c |
| Phase 3 | 165 (39.76) | 31 (6.47) | < 0.001c |
| Gender, n (%) | |||
| All | 347 (83.61) | 424 (88.52) | 0.0428c |
| Female | 56 (13.49) | 29 (6.05) | < 0.001c |
| Male | 12 (2.89) | 26 (5.43) | 0.0875c |
| Study population, n (%) | |||
| Adults (18–64 years) | 413 (99.52) | 476 (99.37) | > 0.999d |
| Older adults (≥ 65 years) | 379 (91.33) | 461 (96.24) | 0.0033c |
| Paediatric (birth–17 years) | 50 (12.05) | 37 (7.72) | 0.0392c |
| Trial design, n (%) | |||
| Non-randomised | 186 (44.82) | 348 (72.65) | < 0.001c |
| Randomised | 229 (55.18) | 131 (27.35) | < 0.001c |
aPhase 2 and 3 interventional trials (drugs or biological use in cancer) with a start date between 1 January 2018 and 31 December 2022 in ClinicalTrials.gov. The percentage values in the table represent column percentages based on the number of observations (n) in each column
bp-value based on Wilcoxon rank-sum test; cp-value based on Chi-square test; dp-value based on Fisher’s exact test
In terms of gender distribution, a larger proportion of trials without sites in Asia included both genders (88.52%) than trials with sites in Asia (83.61%, p = 0.0428). Furthermore, the representation of females was significantly higher in trials with sites in Asia (13.49%) than in those without (6.05%, p < 0.001). In addition, the study population was predominantly adults (18–64 years) and older adults (≥ 65 years) in both groups. However, older adults were more highly represented in trials without sites in Asia (96.24%) than in trials with sites in Asia (91.33%, p = 0.0033). An opposite trend was observed for the paediatric population where trials with sites in Asia included more younger populations from birth to 17 years of age (12.05% vs 7.72%, p = 0.0392). Lastly, in terms of trial design, non-randomised trials were more common in trials without sites in Asia (72.65%), while trials with sites in Asia had a higher proportion of randomised trials (55.18%). This difference was also statistically significant (p < 0.001). Supplementary Table 1 provides a breakdown of these characteristics for trials with sites in Asia only and trials with sites in Asia and other countries outside Asia. Among the top 10 industry sponsors for trials with a location in Asia (n = 415), global corporations funded a significant proportion of these trials, sponsoring 177/415 trials or 42.65% of the total. In contrast, regional Asian biotechnology firms were involved to a lesser extent, sponsoring 54/415 trials, which accounted for 13.01% of the trials in Asia.
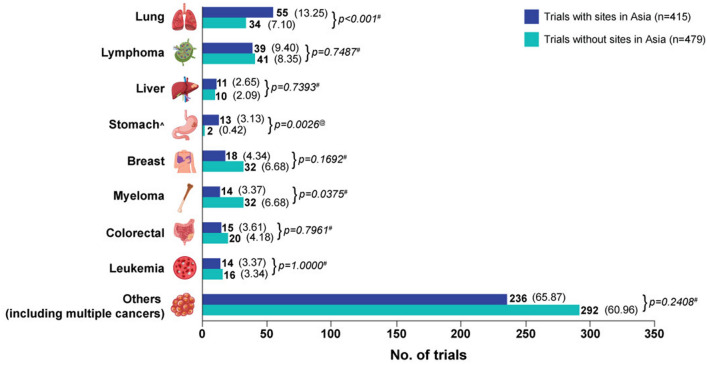
Figure 1 illustrates the comparison of cancer types under investigation in the two study groups. For trials with sites in Asia, 13.25% investigated lung cancer, 9.40% focused on lymphoma, 4.34% were for breast cancer, 3.37% for myeloma, and another 3.37% for leukaemia, 3.61% investigated colorectal cancer, 3.13% studied stomach cancer and 2.65% investigated liver cancer. A significant majority (65.87%) investigated other types of cancer or multiple cancers. For trials without sites in Asia, 7.10% investigated lung cancer, 8.35% focused on lymphoma, 6.68% on breast cancer, 6.68% on myeloma, 4.18% on colorectal cancer, 3.34% on leukaemia, 2.09% investigated liver cancer and 0.42% studied stomach cancer. Most trials (60.96%) however investigated other types of cancer or multiple cancers, similar to those with sites in Asia. However, statistically significant differences between the two groups were observed for lung and stomach cancers and myeloma clinical trials. Lung cancer was more frequently studied in trials with sites in Asia (13.25%) than in those without sites in Asia (7.10%), with a p-value of < 0.001, indicating a significant difference. Similarly, stomach cancer was more frequently studied in trials with sites in Asia (3.13%) than in those without sites in Asia (0.42%), with a p-value of 0.0026, indicating a significant difference. Myeloma was less frequently studied in trials with sites in Asia (3.37%) than in trials without sites in Asia (6.68%), with a p-value of 0.0375, indicating a significant difference here as well. There were no significant differences in other types of cancers between the two groups.
Fig. 1.
Comparison of cancer types under investigation: trials with sites in Asia versus trials without sites in Asia, n (%). ^Cancer with location exclusively in the stomach also includes the gastro-esophageal junction. Studies with cancer location exclusively in the esophagus or gastrointestinal tract were classified under ‘others’. #p-value based on Chi-square test. @p-value based on Fisher’s exact test
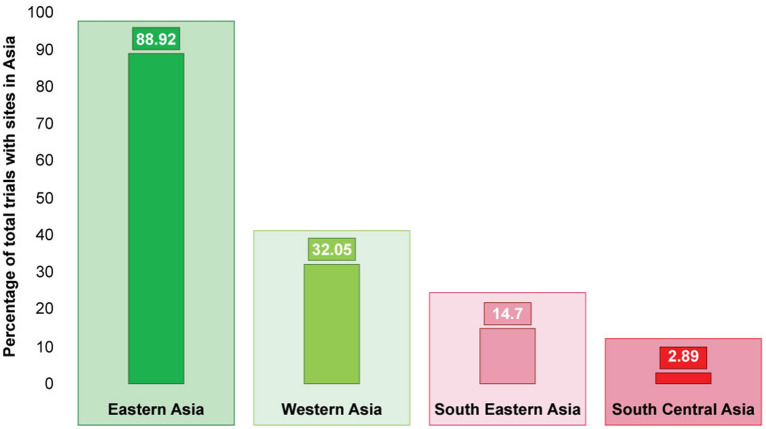
The geographic variations in the distribution of oncology clinical trial sites across different regions in Asia are illustrated in Fig. 2. The overwhelming majority, 88.92%, of the trials included in our study had sites located in Eastern Asia while a significant portion, 32.05%, also had sites in Western Asia. South East Asia contributed to far fewer trials, at 14.7%, while South Central Asia was involved in a marginal proportion of only 2.89%, of trial sites in Asia. Supplementary Table 2 provides a snapshot, of the top 10 Asian countries’ trials with at least one site in Asia. China emerged as the most prominent country, hosting 234 trials, which constitutes 56.39% of the trials with sites in Asia. It was followed by the Republic of Korea with 131 trials (31.57%) and Japan with 105 trials (25.30%).
Fig. 2.
Percentage participation of various Asian geographical regions in cancer clinical trials. Top countries according to number of trials having at least one site in Eastern Asia (n = 369, 88.92%): China (n = 234, 56.39%), Republic of Korea (n = 131, 31.57%), Japan (n = 105, 25.30%), Taiwan (n = 93, 22.41%) and Hong Kong (n = 33, 7.95%). Top countries according to number of trials having at least one site in Western Asia (n = 133, 32.05%: Israel (n = 97, 23.37%), Turkey (n = 67, 16.14%) and Georgia (n = 13, 3.13%). Top countries according to number of trials having at least one site in South East Asia (n = 61, 14.70%): Singapore (n = 31, 7.47%), Thailand (n = 18; 4.34%), Malaysia (n = 16, 3.86%), Philippines (n = 7, 1.69%), Indonesia (n = 2, 0.48%) and Vietnam (n = 1, 0.24%). Top countries according to number of trials having at least one site in South Central Asia (n = 12, 2.89%): India (n = 10, 2.41%), Bangladesh and the Islamic Republic of Iran (each, n = 1, 0.24%)
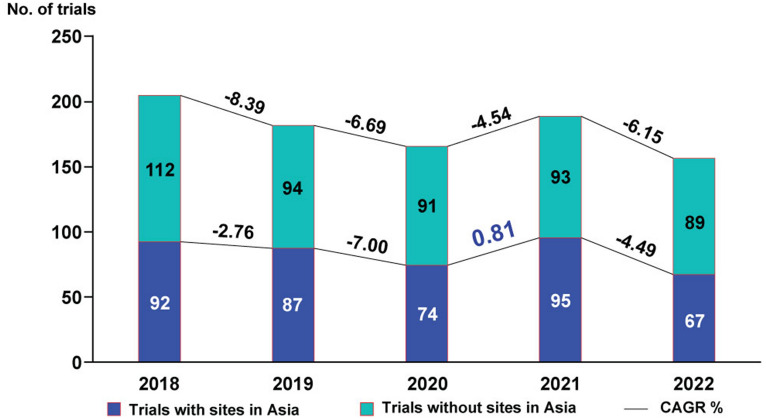
Figure 3 illustrates the year-on-year growth (CAGR) for industry-sponsored clinical trials from 2018 to 2022, comparing trials with sites in Asia and trials without sites in Asia. In 2018, there were 92 trials with sites in Asia, and this number fluctuated over the following years, reaching 67 in 2022, indicating a CAGR of −6.15%. On the other hand, the number of trials without sites in Asia started at 112 in 2018 and dropped to 89 in 2022, with a slightly less decline in CAGR at −4.49%. The downward trend in the number of clinical trials was also observed in clinical trials conducted both exclusively in Asia and in Asia in conjunction with other countries (Supplementary Table 3). However, from 2020 to 2021, there was a noticeable upward growth in the number of trials with sites in Asia. As a result, the CAGR showed a positive increase of 0.81% for trials conducted in Asia during this period. This is a prominent shift from the previous years, which displayed a declining trend (Fig. 3).
Fig. 3.
Annual trend since last 5 years in industry-sponsored cancer clinical trials categorized by the geographical locations of recruitment sites. CAGR compound annual growth rate
Discussion
Clinical research directed towards understanding variations in cancer outcomes across different ethnicities is essential for understanding risk factors and enabling accurate interpretation of treatment efficacy, safety and tailored management with novel therapies [32]. In the evolving era of precision medicine within oncology, variables such as ethnic pharmacogenomic differences, individual patient characteristics and lifestyle factors should be considered in clinical trials to optimise the accuracy of outcomes [33].
Our analysis revealed significant differences in the characteristics of clinical trials conducted with sites in Asia versus those without. Trials with sites in Asia tend to have more locations and larger target enrolments, and are more often funded solely by the industry. They also exhibit a higher proportion of phase 3 trials and greater female representation. These findings are comparable to a previous study analysing industry-sponsored clinical research registered on CT.gov conducted between 2006 and 2014, showing that countries in Asia were the most likely to recruit exclusively outside high-income countries, and studies tended to be larger, of longer duration, and later phase interventions than studies performed solely in high-income countries [34].
We also found that trials without sites in Asia show a higher prevalence of phase 2 trials, a non-randomized design and a greater representation of older adults. These differences could be influenced by various factors, such as regional health policies, demographic characteristics, disease prevalence and industry strategies. Our data also indicate that global corporations play a dominant role in the sponsorship of oncology clinical research in Asia. Regional Asian biotechnology firms, although active, have a considerably smaller footprint in this regard. Understanding these discrepancies can provide valuable insights for future research planning and policy-making to ensure more equitable and representative clinical trials across different regions [35, 36].
Our analysis of the types of cancers studied in clinical trials also revealed a varied landscape depending on whether the trials include sites in Asia. Notably, lung and stomach cancer studies were significantly more common in trials with Asian sites than in those without (p < 0.001 and p = 0.0026, respectively). Myeloma also showed a significant difference, with less representation in trials with sites in Asia (p = 0.0375). These findings highlight the potential influence of regional disease prevalence and research focus on selecting cancer types studied in clinical trials which however does not match with the demographic trend in cancer incidence [37–39]. The higher prevalence of stomach cancer in Asia is mirrored in the types of trials conducted; the same was not found for liver cancer. This clearly suggests a research gap and possibly competing priorities in cancer research funding and focus in Asia.
The CAGR data suggest a general downward trend in the number of clinical trials conducted both in and outside of Asia over the 5-year period analysed. The overall downward trend in the number of clinical trials conducted in Asia was uniform and did not vary irrespective of whether the trials were carried out exclusively in Asia or in conjunction with other countries. However, this trend is contrary to previous analyses of registered clinical trial information investigating the participation of Asian countries. A study of phase 2 and phase 3 global clinical trials registered on CT.gov between 2008 and 2015 showed larger increases in East Asia than in the United States and Europe [40]. Similarly, clinical trial information from the WHO’s ICTRP between 2008 and 2017 showed a sevenfold increase in the number of registered clinical trials in Asia over the 10-year period and the average annual increase in the number of registered trials was generally higher in Asia than in the USA, Canada, EU countries, or Australia [41]. This has also been demonstrated specifically for oncology clinical trials in China, India, Japan and South Korea, where the annual number of registered cancer trials from 2005 to 2018 in the ICTRP increased substantially, with an almost two-fold increase of registered interventional trials between 2005 and 2018 [18]. Only the study analysing industry-sponsored clinical research with countries categorised by income as used in the Global Burden of Disease project showed a stable rate of industry-funded clinical studies outside high-income countries over the 7-year investigation period (2006–2014) [34]. The declining trend identified in our analysis could reflect the impact of the global COVID-19 pandemic, which has disrupted research activities worldwide, particularly during 2020 and 2021 [42, 43].
Our study further demonstrated a less pronounced decline in the number of trials conducted at Asian sites. Despite a general downtrend in the overall trial count, the proportion of trials taking place in Asia witnessed a relative upward swing, as revealed by a 0.81% spike in the CAGR from 2020 to 2021. This observation is in harmony with earlier research that also signalled a more pronounced upward movement for clinical trial sites in Asia [18, 34, 41]. This increase could potentially signify a revitalisation of clinical research activities in the region after the disruptions caused by the COVID-19 pandemic. It might also reflect the increasing recognition of the importance of including diverse populations in clinical trials and the substantial patient pools and improved clinical trial infrastructure in many Asian countries. This positive trend underscores the growing role of Asia in the global clinical trials landscape.
Although the median for the number of sites was higher for trials with sites in Asia, we found a concentration of clinical trials primarily in East Asia, with China leading among trials with sites in Asia. Conversely, the United States dominates the trials without sites in Asia. This uneven distribution indicates a significant geographical disparity in the global conduct of clinical trials, with East Asia, particularly China, being highly represented, and without a significant contribution from other regions in Asia. This highlights an opportunity to expand the scope of clinical trials to encompass more diverse regions in Asia, fostering a more representative and inclusive research landscape. Such an expansion could provide richer and more generalised data, potentially enhancing the efficacy and safety of oncology treatment strategies across diverse populations.
Market considerations and regulatory obligations might account for the higher prevalence of clinical trial sites in East Asian countries, especially China. The National Medical Product Administration (NMPA) in China encourages early clinical trial participation in China or including China in multi-regional clinical trials and implementing an ethnic sensitivity analysis in the drug development plan [44, 45]. The preference and requirement for local patient data in new oncology drug applications is also a norm in Japan, South Korea and Taiwan, albeit with varying regulations based on the trial phase and scale and existing FDA/European medicines Agency (EMA) approval [46]. Simultaneously, participation in global phase 3 studies and demonstrating consistency with the overall study population can facilitate smooth approval timelines, enabling swifter access to innovative oncology medicines in Asia [47]. This aligns with the recent FDA guidance that provides recommendations and techniques to clinical trial sponsors for enhancing the diversity of clinical trial populations [48, 49].
Several barriers, however, often limit the execution of clinical trials in Asia. These include the potentially lengthy duration associated with obtaining regulatory approval for trials [50], along with regulatory differences in review processes, and inconsistencies in dossiers and other document requirements and management procedures [46]. In the field of oncology, various personal and geographical hurdles may interfere with the successful execution of clinical trials. Personal barriers include a need for more experienced healthcare personnel capable of conducting trials. Geographical challenges may arise due to the concentration of specialised centres in cities, making them inaccessible to individuals residing in rural areas [51]. Additionally, variations in health literacy across Asia can also pose challenges. This may manifest as a cautious approach towards enrolling in a trial due to unfamiliarity or misconceptions and a cultural tendency to lean on family support when making healthcare decisions [51].
Despite the barriers faced in conducting clinical trials in Asia, there are noteworthy opportunities in conducting oncology clinical trials in Asia that warrant exploration. These include potentially lower trial costs [52], higher chances for recruiting subjects for cancers with a higher prevalence in Asia (like gastric cancer) [50], higher representativeness for characteristics of cancers in Asia that differ from non-Asian populations [53, 54] and an availability of a larger pool of treatment-naïve patients for recruitment [55]. These concerted factors may have also contributed to the higher proportion of randomised trials in Asia, as observed in our study. Notably, conducting oncology clinical trials, including industry-sponsored studies, leads to the strengthening of local research capability [50, 51], enhancing regional research network capabilities [56] and establishing local clinical trial databases [57, 58]. These advancements ultimately support local oncologists in making referrals for patient enrolment, which not only advances the field of clinical research in the region but also facilitates early access to innovative therapies for life-threatening diseases.
The approval of cancer treatments marks a significant milestone; however, the lack of racial and ethnic representation in clinical trials poses barriers to developing evidence-based medicines. As shown in our study, studies with sites in Asia have a significantly higher rate of industry involvement as sole funder. This also puts the onus on the pharmaceutical industry to collaborate with the broader oncology community to implement effective changes [32, 59]. Several best practices for biopharmaceutical companies for enhancing ethnic diversity in clinical trials have also been discussed [7]. These include forming partnerships with diversity-focused professional bodies, selecting investigators and sites where diverse populations exist, creating referral maps that incorporate census data on diversity, consulting specialised advisory boards for diversity improvement, and implementing telemedicine approaches for broader participation. By working together in implementing these solutions, we can make significant strides in addressing systemic race-related barriers and promoting more equitable healthcare outcomes for all individuals, irrespective of their racial or ethnic background [60–62].
Our study has limitations, including reliance solely on entries from CT.gov, potentially leading to a sample bias that could affect the accuracy and generalizability of our results. Despite these limitations, we believe that they have not significantly impacted our overall findings regarding industry-sponsored trials. While the WHO-ICTRP includes some datasets from CT.gov, it often excludes many industry-sponsored clinical trials. Thus, our reliance on CT.gov primarily ensures the inclusion of these industry-sponsored studies in our analysis.
Conclusion
Our study uncovered notable disparities in the features of clinical trials that included Asian locations versus those that did not. The trials conducted with sites in Asia typically involved more locations, had larger recruitment targets, were predominantly funded solely by the industry, and displayed a greater frequency of phase 3 trials and higher inclusion of female and paediatric participants.
Although there was an overall relatively less steep decline and focal upward movement for clinical trial sites in Asia, there was also a geographical imbalance in global clinical trials, with an overrepresentation of East Asia, particularly China, in trials with Asian sites, and a dominant presence of the United States in trials without Asian sites. This suggests an opportunity for broader inclusivity by expanding trials to diverse Asian regions, thereby improving confidence in data for these populations and improving outcomes for novel oncology treatments across varied populations. Targeted recruitment strategies in future trials for liver cancer should also be encouraged to ensure that trial outcomes are relevant to those most affected by the disease. Awareness and understanding of current disparities are essential for future research strategies and robust policymaking aimed at achieving more balanced and representative conduct of clinical trials across diverse regions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing/Editorial Assistance
The authors sincerely appreciate the scientific rationale and medical writing provided by Dr Sajita Setia and editorial support by Dr Daniel Furtner from Transform Medical Communications, New Zealand and data analytics support provided by the Pharma-Stats team on behalf of Transform Medical Communications.
Author Contributions
Dany Habr, Manmohan Singh, and Roberto Uehara made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study and the rapid service fee were funded by Pfizer.
Data Availability
All supplementary tables and figures generated during this study are included in this published article as supplementary information files. Other datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Dany Habr, Manmohan Singh and Roberto Uehara are employees of Pfizer. They report no other conflicts of interest in this work. The results and discussions in this article do not represent or reflect in any way the official policy or position of the current or previous employers of the authors.
Ethical Approval
Not applicable; research involved secondary use of data from ClinicalTrials.gov without any identifier(s) which could allow access to private information of any individual. This article does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Spreafico A, Hansen AR, Abdul Razak AR, Bedard PL, Siu LL. The future of clinical trial design in oncology. Cancer Discov. 2021;11(4):822–837. doi: 10.1158/2159-8290.Cd-20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja AS. Should RCT’s be used as the gold standard for evidence based medicine? Integr Med Res. 2019;8(1):31–32. doi: 10.1016/j.imr.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umscheid CA, Margolis DJ, Grossman CE. Key concepts of clinical trials: a narrative review. Postgrad Med. 2011;123(5):194–204. doi: 10.3810/pgm.2011.09.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. 2015–2019 Drug trials snapshots summary report. Five-Year summary and analysis of clinical trial participation and demographics. [updated November 2020; cited 2023 May 14]. Available from https://www.fda.gov/media/143592/download.
- 5.Schwartz AL, Alsan M, Morris AA, Halpern SD. Why diverse clinical trial participation matters. N Engl J Med. 2023;388(14):1252–1254. doi: 10.1056/NEJMp2215609. [DOI] [PubMed] [Google Scholar]
- 6.Habr D, McRoy L, Papadimitrakopoulou VA. Age is just a number: considerations for older adults in cancer clinical trials. J Natl Cancer Inst. 2021;113(11):1460–1464. doi: 10.1093/jnci/djab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habr D, Corsaro M. Reimagining diversity in multiple myeloma clinical trials. Hematol Oncol. 2022;40(4):689–694. doi: 10.1002/hon.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadigov R. Rapid growth of the world population and its socioeconomic results. Sci World J. 2022;2022:8110229. doi: 10.1155/2022/8110229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niles PM, Jun J, Lor M, Ma C, Sadarangani T, Thompson R, et al. Honoring Asian diversity by collecting Asian subpopulation data in health research. Res Nurs Health. 2022;45(3):265–269. doi: 10.1002/nur.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen HT, Zheng A, Gugel A, Kistin CJ. Asians and Asian subgroups are underrepresented in medical research studies published in high-impact generalist journals. J Immigr Minor Health. 2021;23(3):646–649. doi: 10.1007/s10903-021-01142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn JM, Gray DM, 2nd, Oliveri JM, Washington CM, DeGraffinreid CR, Paskett ED. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer. 2022;128(2):216–221. doi: 10.1002/cncr.33905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. Meta-analysis group in cancer. J Clin Oncol. 1998;16(11):3537–41. 10.1200/JCO.1998.16.11.3537. [DOI] [PubMed]
- 13.Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107(3):904–906. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. FDA takes important steps to increase racial and ethnic diversity in clinical trials [cited 2023 May 27]. Available from https://www.fda.gov/news-events/press-announcements/fda-takes-important-steps-increase-racial-and-ethnic-diversity-clinical-trials.
- 15.Verma R, Khanna P, Chawla S. Vaccines for the elderly need to be introduced into the immunization program in India. Hum Vaccin Immunother. 2014;10(8):2468–2470. doi: 10.4161/hv.29254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atal I, Trinquart L, Porcher R, Ravaud P. Differential globalization of industry- and non-industry-sponsored clinical trials. PLoS One. 2015;10(12):e0145122. doi: 10.1371/journal.pone.0145122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosi G, Palagi E, Roventini A, Russo E. Do patents really foster innovation in the pharmaceutical sector? Results from an evolutionary, agent-based model. J Econ Behav Organ. 2023;212:564–589. doi: 10.1016/j.jebo.2023.05.039. [DOI] [Google Scholar]
- 18.Doi M, Yukawa K, Sato H. Characteristics of Asian 4 countries on cancer clinical trials registered in the International Clinical Trials Registry Platform between 2005 and 2018. Chin Clin Oncol. 2021;10(3):28. doi: 10.21037/cco-21-17. [DOI] [PubMed] [Google Scholar]
- 19.U.S. National Library of Medicine. ClinicalTrials.gov. Available from https://clinicaltrials.gov/.
- 20.U.S. National Library of Medicine. FDAAA 801 and the final rule [cited 2023 May 11]. Available from https://clinicaltrials.gov/ct2/manage-recs/fdaaa#WhichTrialsMustBeRegistered.
- 21.De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Lancet. 2004;364(9438):911–912. doi: 10.1016/s0140-6736(04)17034-7. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. International clinical trials registry platform—search portal [cited 2023 May 11]. Available from https://trialsearch.who.int/.
- 23.Zarin DA, Fain KM, Dobbins HD, Tse T, Williams RJ. 10-Year update on study results submitted to ClinicalTrials.gov. N Engl J Med. 2019;381(20):1966–1974. doi: 10.1056/NEJMsr1907644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajappa S, Singh M, Uehara R, Schachterle SE, Setia S. Cancer incidence and mortality trends in Asia based on regions and human development index levels: an analyses from GLOBOCAN 2020. Curr Med Res Opin. 2023;39(8):1127–1137. doi: 10.1080/03007995.2023.2231761. [DOI] [PubMed] [Google Scholar]
- 25.Jemielita T, Tse A, Chen C. Oncology phase II proof-of-concept studies with multiple targets: randomized controlled trial or single arm? Pharm Stat. 2020;19(2):117–125. doi: 10.1002/pst.1972. [DOI] [PubMed] [Google Scholar]
- 26.Buyse M. Phase III design: principles. Chin Clin Oncol. 2016;5(1):10. doi: 10.3978/j.issn.2304-3865.2014.08.05. [DOI] [PubMed] [Google Scholar]
- 27.Dillman RO, Koziol JA. Phase I cancer trials: limitations and implications. Mol Biother. 1992;4(3):117–121. [PubMed] [Google Scholar]
- 28.Jung SH. Phase II cancer clinical trials for biomarker-guided treatments. J Biopharm Stat. 2018;28(2):256–263. doi: 10.1080/10543406.2017.1372777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung SH. Design and analysis of cancer clinical trials for personalized medicine. J Pers Med. 2021;11(5):376. doi: 10.3390/jpm11050376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization—International Agency for Research on Cancer. Data & methods [cited 2023 May 14]. Available from https://gco.iarc.fr/today/data-sources-methods.
- 31.National Library of Medicine. MeSH. Neoplasms [cited 2023 March 27]. Available from https://www.ncbi.nlm.nih.gov/mesh/68009369.
- 32.Habr D, Ferdinand R. Addressing racial/ethnic disparities in cancer clinical trials: everyone has a role to play. Cancer. 2021;127(18):3282–3289. doi: 10.1002/cncr.33600. [DOI] [PubMed] [Google Scholar]
- 33.Fountzilas E, Tsimberidou AM, Vo HH, Kurzrock R. Clinical trial design in the era of precision medicine. Genome Med. 2022;14(1):101. doi: 10.1186/s13073-022-01102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy S, Mandl KD, Bourgeois FT. Industry-sponsored clinical research outside high-income countries: an empirical analysis of registered clinical trials from 2006 to 2013. Health Res Policy Syst. 2015;13:28. doi: 10.1186/s12961-015-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Why diverse representation in clinical research matters and the current state of representation within the clinical research ecosystem. In: Bibbins-Domingo K, Helman A, editors. Improving representation in clinical trials and research: building research equity for women and underrepresented groups. Washington DC: National Academies Press (US); 2022. [PubMed]
- 36.Washington V, Franklin JB, Huang ES, Mega JL, Abernethy AP. Diversity, equity, and inclusion in clinical research: a path toward precision health for everyone. Clin Pharmacol Ther. 2023;113(3):575–584. doi: 10.1002/cpt.2804. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W, Christiani DC. East meets west: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30(5):287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanapuru B, Fernandes LL, Fashoyin-Aje LA, Baines AC, Bhatnagar V, Ershler R, et al. Analysis of racial and ethnic disparities in multiple myeloma US FDA drug approval trials. Blood Adv. 2022;6(6):1684–1691. doi: 10.1182/bloodadvances.2021005482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Liu L. Changes in the epidemiology of hepatocellular carcinoma in Asia. Cancers (Basel) 2022;14(18):4473. doi: 10.3390/cancers14184473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazaki K, Sato Y, Hanaoka H, Uyama Y. Current status and open issues concerning global clinical trials (GCTs) in Japan and East Asia. Clin Transl Sci. 2017;10(6):503–508. doi: 10.1111/cts.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali S, Egunsola O, Babar ZUD, Hasan SS. Clinical trials in Asia: a World Health Organization database study. Perspect Clin Res. 2019;10(3):121–124. doi: 10.4103/picr.PICR_109_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard-Lucey VM. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov. 2020;19(6):376–377. doi: 10.1038/d41573-020-00093-1. [DOI] [PubMed] [Google Scholar]
- 43.Sathian B, Asim M, Banerjee I, Pizarro AB, Roy B, van Teijlingen ER, et al. Impact of COVID-19 on clinical trials and clinical research: a systematic review. Nepal J Epidemiol. 2020;10(3):878–887. doi: 10.3126/nje.v10i3.31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang W, Huang Y, Zhou D, Huang Y, Chen Y, Ren S, et al. Evolving drug regulatory landscape in China: a clinical pharmacology perspective. Clin Transl Sci. 2021;14(4):1222–1230. doi: 10.1111/cts.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ge Q, Xu L, DiMasi JA, Kaitin KI, Shao L. Impact of regulatory system changes on the availability of innovative drugs in China. Nat Rev Drug Discov. 2023;22(5):344–345. doi: 10.1038/d41573-023-00058-0. [DOI] [PubMed] [Google Scholar]
- 46.Hata T, Nakamura K, Yonemori K, Noguchi E, Watanabe M, Sohn J, et al. Regulatory and operational challenges in conducting Asian international academic trial for expanding the indications of cancer drugs. Clin Transl Sci. 2021;14(3):1015–1025. doi: 10.1111/cts.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajman I, Hirano M, Honma W, Zhao S. New paradigm for expediting drug development in Asia. Drug Discov Today. 2020;25(3):491–496. doi: 10.1016/j.drudis.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Nephew LD. Accountability in clinical trial diversity: The buck stops where? EClin Med. 2021;36:100906. doi: 10.1016/j.eclinm.2021.100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Food and Drug Administration. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry (draft guidance). Apr 2022 [cited 2023 May 10]. Available from https://www.fda.gov/media/157635/download
- 50.Voon P-J, Lai W-H, Bustaman RS, Siu LL, Razak ARA, Yusof A, et al. Early phase oncology clinical trials in Malaysia: current status and future perspectives. Asia Pac J Clin Oncol. 2023;19(3):296–304. doi: 10.1111/ajco.13886. [DOI] [PubMed] [Google Scholar]
- 51.Eala MAB, Basilio PAS, Dee EC, Cereno REP, Ang CD, Magsanoc NJ, et al. Cancer clinical trials in the Philippines. Cancer Causes Control. 2022;33(10):1273–1275. doi: 10.1007/s10552-022-01611-9. [DOI] [PubMed] [Google Scholar]
- 52.Ooi A, Khalid K. Establishing early phase capabilities in Malaysia. Applied Clinical Trials. 2019;28(11):10–16. [Google Scholar]
- 53.Lam WK, White NW, Chan-Yeung MM. Lung cancer epidemiology and risk factors in Asia and Africa. Int J Tuberc Lung Dis. 2004;8(9):1045–1057. [PubMed] [Google Scholar]
- 54.Wong N, Chan KY, Macgregor PF, Lai PB, Squire JA, Beheshti B, et al. Transcriptional profiling identifies gene expression changes associated with IFN-alpha tolerance in hepatitis C-related hepatocellular carcinoma cells. Clin Cancer Res. 2005;11(3):1319–1326. doi: 10.1158/1078-0432.1319.11.3. [DOI] [PubMed] [Google Scholar]
- 55.Yathindranath S, Kureishi A, Singh S, Yeow S, Geng G, Wai K, et al. Evolution of the clinical trial landscape in Asia Pacific. Open Access J Clin Trials. 2014;6:75–84. [Google Scholar]
- 56.Sonoda M, Urbiztondo MRU, Siburian MD, Kerdsakundee N, Muchanga SMJ, Iiyama T. Boosting multiregional clinical trials (MRCT) in Asia through the establishment of the Japan-led network for clinical research, the ARO alliance for ASEAN & East Asia (ARISE) Glob Health Med. 2022;4(4):247–249. doi: 10.35772/ghm.2022.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esteban D, Laudico A, Uy N, Benabay L. Cancer registration in the Philippines. Asian Pac J Cancer Prev. 2001;2:55–60. [Google Scholar]
- 58.Ghersi D, Pang T. En route to international clinical trial transparency. Lancet. 2008;372(9649):1531–1532. doi: 10.1016/s0140-6736(08)61635-9. [DOI] [PubMed] [Google Scholar]
- 59.Habr D, Wolf Gianares B, Schuler KW, Chari D. Patients at the heart of the scientific dialogue: an industry perspective. Oncol Ther. 2023;11(1):15–24. doi: 10.1007/s40487-023-00220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The Pfizer corporate affairs multicultural center of excellence a catalyst for change [cited 2023 May 27]. Available from https://pfe-pfizercom-prod.s3.amazonaws.com/health/Pfizer_MCoE_One_Pager.pdf.
- 61.American Cancer Society. The American Cancer Society and Pfizer launch community grants focused on addressing systemic race-related barriers that contribute to disparities in care among black men and women with cancer. Nov 17, 2020 [cited 2023 May 27]. Available from http://pressroom.cancer.org/2020-11-17-The-American-Cancer-Society-and-Pfizer-Launch-Community-Grants-Focused-on-Addressing-Systemic-Race-Related-Barriers-that-Contribute-to-Disparities-in-care-Among-Black-Men-and-Women-with-Cancer.
- 62.Columbia University. Columbia University and Pfizer to establish clinical trials diversity initiative. Sep 09, 2021 [cited 2023 May 27]. Available from https://www.cuimc.columbia.edu/news/columbia-university-and-pfizer-establish-clinical-trials-diversity-initiative.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supplementary tables and figures generated during this study are included in this published article as supplementary information files. Other datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.