Highlights
-
•
The bacterial diversity of inoculated sausages was decreased.
-
•
L. plantarum CQ01107 and S. simulans CD207 (CCA) improved sausages taste and aroma.
-
•
CCA culture had similar flavour improvement ability with commercial culture.
-
•
CCA was a desirable starter culture for flavour improvement of fermented sausages.
Keywords: Lactiplantibacillus plantarum, Staphylococcus simulans, Fermented sausage, Bacterial community, Taste, Aroma
Abstract
The aim of this study was to investigate and compare the effects of different mixed starter cultures (Lactiplantibacillus plantarum and Staphylococcus simulans) on the bacterial communities and flavor of fermented sausages. The results indicated that native starters grew well in fermented sausages and became dominant at the end of ripening. Among them, Lactobacillus spp. had the highest relative abundance, followed by Staphylococcus spp. In addition, the inoculation of the mixed starters promoted the formation of taste and aroma compounds that contribute to the overall flavor of the fermented sausages. Among them, the L. plantarum CQ01107 + S. simulans CD207 (CCA) treatment was found to have the highest umami amino acid, nucleotide, lactic acid, fatty acid and ketone contents (P < 0.05), as well as excellent sensory properties. In conclusion, the CCA starter may be a desirable starter culture to enhance the flavor of fermented sausages.
1. Introduction
Generally, fermented sausages are made by fermenting and ripening a mixture of pork, fat, sugar, nitrite, spices, and salt in a suitable environment (Chen, Kong, Han, Xia, & Xu, 2017). Fermented sausages have many desirable characteristics, including flavor, color and texture, that are favored by Chinese consumers. Flavor, including taste and aroma, is crucial to fermented sausages and determines consumer acceptability. Volatile flavor compounds, mainly aldehydes, acids and esters, are the source of aromas in fermented sausages (Gomez & Lorenzo, 2013). Additionally, the formation of taste in fermented sausages depends greatly on free amino acids (FAAs), nucleotides, and organic acids (Toldrá, 2006). The formation of these aroma and taste compounds involves complicated biochemical processes, mainly the degradation and oxidation of proteins, the hydrolysis and oxidation of lipids, and the hydrolysis of carbohydrates (Xiao, Liu, Chen, Xie, & Li, 2020).
Notably, apart from endogenous enzymes, the growth and metabolism of microbes also play a significant influence in the development of the flavor of fermented sausages (Ma et al., 2023). In fermented sausages from northeastern China, Hu et al. (2020) found Lactobacillus spp., Staphylococcus spp., Leuconostoc spp., Lactococcus spp., and Weissella spp. as dominant bacteria, where Lactobacillus spp. positively correlated the most with flavor production. However, the production of naturally fermented sausages depends on the original microorganisms in the raw material and the production environment, which is an uncontrolled process. Uncertain changes in microflora may occur in the same batch, resulting in inconsistencies in the flavor and safety of the product (Fadda, Lopez, & Vignolo, 2010). Selecting strains with desirable properties from fermented sausages and inoculating them into sausages is a feasible way to solve this problem.
Lactic acid bacteria (LAB) and coagulase-negative staphylococci (CNS) are the dominant bacteria in fermented meat products (Hu, Chen, Wen, Wang, Qin, & Kong, 2019). LAB can metabolize sugar into lactic acid, impede the proliferation of unwanted microorganisms, and improve the safety of fermented foods (Ge et al., 2019). Hu et al. (2022) found that LAB could also improve the flavor of fermented sausages. In addition, CNS can enhance the color of fermented sausages by promoting the formation of nitroso-myoglobin (Gotterup, Olsen, Knochel, Tjener, Stahnke, & Moller, 2008). Meanwhile, CNS also has a strong protein and lipid hydrolysis ability, which can form unique flavors (Wang et al., 2021). Notably, neither LAB nor CNS inoculation alone seems to improve the whole quality of fermented sausages, so it is necessary to prepare LAB and CNS as mixed starter cultures to access the desired properties of fermented sausages. In addition, the bacterial communities of fermented sausages have a significant effect on the aroma and taste characteristics (Xiao et al., 2020). Currently, many studies have focused on the relationship between the evolution of original microorganisms and flavor profiles (mainly volatile flavor compounds) in fermented foods ( Gao et al., 2023a, Hu et al., 2020, Li et al., 2023, Ma et al., 2023). However, the effect of artificial cultures on the microbial communities, aroma and taste compounds during sausage production is not clear, and it is necessary to comprehensively clarify the effect and mechanism.
The objective of this study was to elucidate the flavor characteristics of dry fermented sausages with various mixed starter cultures (Lactiplantibacillus plantarum and Staphylococcus simulans), which were isolated and selected from traditional Chinese fermented sausages. Meanwhile, alterations in bacterial communities at the end of ripening were also detected for the different treatments. The study’s findings enhance the comprehensive comprehension of mixed cultures’ impact on flavored fermented sausages and clear the path for picking and utilizing starter cultures that enhance flavors.
2. Materials and methods
2.1. Bacteria and fermented sausage preparation
Two strains of L. plantarum (L. plantarum KM119 and CQ01107) and two strains of S. simulans (S. simulans CQ02205 and CD207) were isolated from traditional Chinese fermented sausages and identified by 16S rDNA sequencing. They showed excellent flavor and quality formation potential in a previous study. L. plantarum and S. simulans strains were prepared and used for sausage production based on the procedure of Baka et al. (2011).
Fresh pork foreleg and back fat were procured from Suguo supermarket (Nanjing, China) following the 2010 Ethical Guidelines for Animal Care issued by the Ministry of Science and Technology of the People's Republic of China. The specimen was then transported on ice to the laboratory. The sausage formulation included lean (800 g), fat (200 g), sucrose (40 g), water (10 g), salt (30 g), liquor (20 mL), sodium erythorbate (0.5 g, food grade) and sodium nitrite (0.15 g, food grade). Mixtures were stuffed in natural porcine casings with a 3 cm diameter and 20 cm length. Three batches of meat batter were prepared, and from each of them, 6 different sausage formulations were manufactured. The sausage without inoculation was the control (CON) group, and inoculation with commercial starter THM-17 (including Pediococcus pentosaceus and S. xylosus; Clerici Sacco, Cadorago, Italy) was the positive control (PC) group. According to the descriptions of the THM-17, the starter was added at a level of 0.02 % of the meat. The remaining four formulations were prepared using different combinations of starters L. plantarum (KM119 and CQ01107) and S. simulans (CQ02205 and CD207). Formulation KCA included strains KM119 + CD207, formulation KCB included strains KM119 and CQ02205, formulation CCA included strains CQ01107 and CD207, and formulation CCB included strains CQ01107 and CQ02205. The initial inoculation numbers of the strains in the native mixed starters were all 107 CFU/g meat. All sausages underwent a 24-h fermentation process at 30 °C and 80 % relative humidity. The sausages were subsequently permitted to ripen for 9 days at 15 °C and 60 % relative humidity. The above process was conducted in a KBF 240 constant temperature and humidity chamber (Binder, Tuttlinggen, Germany). Two and five sausage samples (approximately 300 g) were taken at the initiation of fermentation (Day 0) and the end of ripening (Day 10), respectively, to measure microbial composition, flavor and aroma compounds.
2.2. Color determination
The sausage samples were sliced into 1 cm thick sections and their color was measured using a CR-400 colorimeter (Konia Minolta, Tokyo, Japan). L*, a* and b* were determined to indicate lightness, redness and yellowness, respectively.
2.3. Bacterial communities
2.3.1. Bacterial counts
Bacterial counts of fermented sausages were performed in accordance with the procedure of Shao et al. (2021) with minor adjustments. Total bacterial counts, LAB counts and Staphylococcus counts were incubated on plate count agar, MRS agar and mannitol salt agar (Haibo Biological Sci. & Tech. Co., Ltd., Qingdao, China) at 37 °C for 48 h each and subsequently counted using a Scan500 automatic colony counter (Interscience, Mourjou, France).
2.3.2. High-throughput sequencing
Following the manufacturer’s instructions, genomic DNA was extracted from fermented sausages using the DNeasy® PowerSoil® Pro kit (MP Biomedicals, Santa Ana, GA, USA). The V3-V4 hypervariable region of the bacterial 16S rRNA gene was amplified by an ABI GeneAmp® 9700 PCR Thermocycler (ABI, CA, USA) using primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Illumina sequencing and bioinformatics processing were based on the method of Ma et al. (2023).
2.4. Taste compounds
The analysis of FAAs in fermented sausages used the technique developed by Xu et al. (2023) with slight modifications. Briefly, minced sausage (4 g) was mixed with 20 mL of sulfosalicylic acid (3 %, w/v). After homogenization (10,000 rpm, 1 min) and centrifugation (4 °C, 12,000 × g, 15 min), 4 mL of the supernatant was taken and mixed with 2 mL of hexane. The water phase was then filtered using a 0.22 μm filter membrane after allowing it to rest and stratify. The determination of FAAs was conducted with an automated amino acid analyzer from Hitachi (Tokyo, Japan).
The determination of nucleotides and organic acids was performed in congruence with the procedure of Jin et al. (2023) with some modifications. Five grams of minced sausage was mixed with 20 mL of 5 % cold perchloric acid and homogenized (5000 rpm) for 1 min. The supernatant was collected by centrifugation (4 °C, 10,000 g, 10 min) and the pH was adjusted to 5.7 with 1 M potassium hydroxide. The sample was made up to 50 mL with ultrapure water, then filtered through a 0.22 μm filter membrane and analyzed by a LC-20 A high-performance liquid chromatography (Shimadzu Corporation, Kyoto, Japan) equipped with a reversed-phase C18 column (250 mm × 4.6 mm, 5.0 μm, Agilent Technologies Co. Ltd., California, US) and an UV detector. The detection wavelength was 254 nm, and the column temperature was 30 °C. The flow rate and injection volume were 1.0 mL/min and 10.0 µL, respectively. Mobile phase A was a mixture of 0.015 M KH2PO4 and K2HPO4 (V:V = 1:1, pH 5.7) and mobile phase B was methanol. Elution was performed using the following gradient: 0 min, 100 % A; 15 min, 85 % A; 23 min, 70 % A; and 30 min, 100 % A. Standard curves of the nucleotides were performed with standard solutions of 0, 10, 50, 100, 200, and 500 μg/mL under the above conditions. For organic acids, minced sausage (1 g) was homogenized (5000 rpm) in 4 mL of purified water for 5 min. Mixed samples were centrifuged (4 °C, 10,000 g, 20 min) and filtered through 0.22 μm filter membrane for HPLC analysis. The HPLC conditions were similar to those employed for nucleotides, except the detector wavelength of 215 nm.
2.5. Electronic tongue
The taste of fermented sausages was measured using an SA402B electronic tongue meter (Insent, Atsugi City, Japan) according to the method of previous literature (Chen, Hu, Wen, Wang, Qin, & Kong, 2021).
2.6. Determination of fatty acids
Fatty acids (FAs) were determined according to the method of Feng, Tjia, Zhou, Liu, Fu, and Yang. (2020). A mixture of 37 fatty acid methyl ester standards (Sigma Aldrich Chemical Co., St. Louis, MO, USA) was used as the external standard for characterization, and heptadecanoic acid methyl ester was used as the internal standard for quantification.
2.7. Electronic nose
A PEN3 electronic nose (Airsense Analytics GmbH, Schwerin, Germany) was employed to determine the flavor profiles of fermented sausages following the method of Barbosa-Pereira, Rojo-Poveda, Ferrocino, Giordano, and Zeppa. (2019).
2.8. Determination of volatile flavor compounds
Volatile flavor compounds of fermented sausages were extracted using headspace-solid phase microextraction (HS-SPME) and analyzed using a Thermo gas chromatography and mass spectrometry (GC/MS) system (Thermo Scientific, Waltham, MA, USA) equipped with a DB-WAX column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies, USA) according to the method of Qi et al. (2018). Minced sausage (4 g) was taken and placed in a 20 mL headspace vial. As an internal standard, 20 μL of a 1,2-dichlorobenzene solution (100 mg/L) was then added to the sample. Volatile substances were collected at 50 °C for 30 min and then desorbed at the GC inlet (250 °C) for 3 min. The oven temperature was maintained at 40 °C for 3 min, ramped up to 200 °C at 5 °C/min, then to 230 °C at 10 °C/min and held for 2 min. The carrier gas (helium, 99.999 %) flow rate was 1 mL/min. The mass selective detector was operating in full scan mode (45–450 m/z) and electron collision mode (70 eV). Volatile chemicals were identified via comparison to the NIST 14 mass spectrometry database and confirmed by linear retention indices (LRI) using standard alkanes (C7-C40) (Sigma, Shanghai, China). The content of each compound was calculated by comparing its area with the internal.
2.9. Sensory evaluation
Sensory evaluation of fermented sausages was performed at the end of ripening. The sensory evaluation team consisted of 10 food professionals (50 % female and 50 % male, aged between 20 and 30 years, from the College of Food Science and Technology, Nanjing Agricultural University). They were chosen based on their completion of a sensory analysis course and adherence to ISO standard 8586:2012 for trained and selected. Moreover, they underwent three basic training sessions to develop discriminatory skills prior to sensory evaluation (Deng, Liu, Li, Xu, & Zhou, 2022). Slices (approximately 3 mm thick) were marked with three numerals and randomly delivered to the sensory evaluators for assessment. Five properties of fermented sausages were assessed: color, aroma, chewiness, taste, and overall acceptability. Sensory evaluation was conducted via a 9-point intensity scale: color (1 = dark and dull; 9 = red and shiny), aroma (1 = no meaty and fermented aroma; 9 = strong meaty and fermented aroma), chewiness (1 = strong grainy and astringent; 9 = no grainy and astringent, chewy), flavor (1 = very bitter, sour and salty; 9 = appropriately bitter, acidic and salty, desired umami) and overall acceptability (1 = low; 9 = high) (Chen et al., 2021). The evaluators rinsed their mouths with water in between samples. The assessments were carried out under standard lighting and room temperature.
2.10. Statistical analysis
One-way analysis of variance was performed by Duncan’s multiple comparisons test using SPSS 16.0 (International Business Machines Corp., USA) to analyze significant differences. Each replicate was included as a random effect, and the sampling time was included as a fixed effect. Three independent batches of fermented sausages were prepared, and the indicator was measured in triplicate for each batch (triplicate observations). The results are expressed as the mean ± standard error (SE), with SE representing the error around the mean for all figures. Principal component analysis (PCA) of volatile compounds were performed using the Metaboanalyst tool (https://www.metaboanalyst.ca/). The heatmaps were performed via the online platform provided by Shanghai Majorbio Bio-pharm Technology Co., ltd. (https://cloud.majorbio.com/page/tools/) after the data had been Z-score standardized.
3. Results and discussion
3.1. Color
Color is a critical quality attribute of sausages due to its impact on consumer acceptance. As presented in Table 1, the L* value of the sausages exhibited a significant decrease following the fermentation and ripening stages (P < 0.05), which may be ascribed to water loss during production (Huang et al., 2023). Importantly, the starter had no discernible impact on the L* and b* values of the sausages at the end of ripening process, aligning with prior findings by Essid and Hassouna. (2013). Notably, the highest a* value was observed in the PC and CCB groups, suggesting that commercial and CCB starters contribute to the desirable coloration of the sausages.
Table 1.
Effects of different mixed starter cultures on color of fermented sausages.
| CON-0 | CON-10 | PC-10 | KCA-10 | KCB-10 | CCA-10 | CCB-10 | |
|---|---|---|---|---|---|---|---|
| L* | 54.70 ± 1.69a | 51.06 ± 1.09b | 51.85 ± 1.19b | 50.26 ± 0.87b | 50.96 ± 0.45b | 51.76 ± 1.15b | 51.16 ± 0.59b |
| a* | 6.58 ± 0.29e | 8.96 ± 0.36 cd | 11.47 ± 0.47a | 9.18 ± 0.27 cd | 8.46 ± 0.38d | 9.71 ± 0.31bc | 10.29 ± 0.44b |
| b* | 7.76 ± 0.34b | 7.89 ± 0.41ab | 8.77 ± 0.48a | 8.42 ±.38ab | 8.31 ±.26ab | 8.56 ± 0.25ab | 8.65 ± 0.46a |
The means with different lowercase letters (a-e) indicate significant differences between different treatments. CON: uninoculated, PC: commercial starter, KCA: L. plantarum KM119 and S. simulans CD207, KCB: and L. plantarum KM119 and S. simulans CQ02205, CCA: L. plantarum CQ01107 and S. simulans CD207, CCB: L. plantarum CQ01107 and S. simulans CQ02205.
3.2. Bacterial communities
3.2.1. Bacterial counts
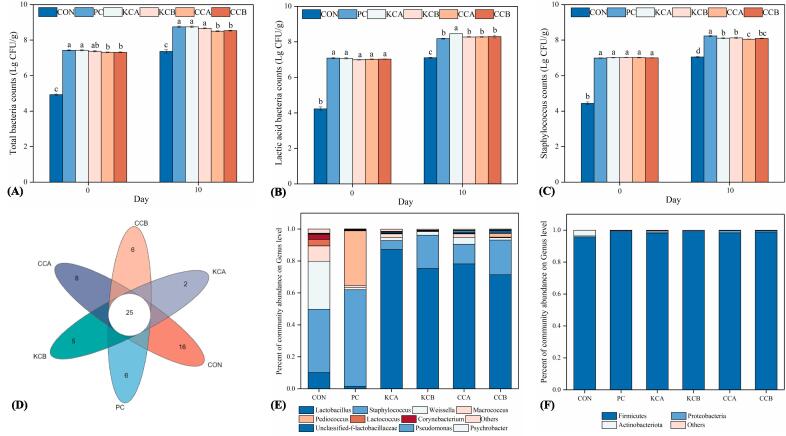
As shown in Fig. 1A, the total bacterial counts in the CON, PC, KCA, KCB, CCA, and CCB sausages rapidly increased from 4.93, 7.43, 7.43, 7.37, 7.32, and 7.31 Lg CFU/g on Day 0 to 7.36, 8.75, 8.75, 8.66, 8.51, and 8.54 Lg CFU/g on Day 10, respectively. The total bacterial counts in the inoculated group were markedly higher than those in the CON group (P < 0.05), indicating that the mixed cultures were able to adapt to the fermented sausage production situation and grow well.
Fig. 1.
Total bacterial counts (A), lactic acid bacteria counts (B) and Staphylococcus counts (C) of fermented sausages on day 0 and day 10, and OUT Venn diagram (D), relative abundance at the phylum (E) and genus (F) level of fermented sausages on day 10. The means with different lowercase letters (a-d) indicate significant differences between different treatments at the same time points (P < 0.05). CON: uninoculated, PC: commercial starter, KCA: L. plantarum KM119 and S. simulans CD207, KCB: and L. plantarum KM119 and S. simulans CQ02205, CCA: L. plantarum CQ01107 and S. simulans CD207, CCB: L. plantarum CQ01107 and S. simulans CQ02205.
LAB grew rapidly in all sausages and became the dominant microflora at the end of ripening (Fig. 1B), probably due to their good adaptation to the meat matrices (Xiao et al., 2020). LAB, which can suppress the development of undesirable microbes by lowering pH or producing antibacterial compounds, are desirable microorganisms in fermented sausages (Lorenzo, Gómez, & Fonseca, 2014). Interestingly, the LAB counts were remarkably greater in the KCA group than in the KCB group (P < 0.05), indicating that S. simulans CQ02205 and S. simulans CD207 have different interactions on L. plantarum KM119, resulting in different growth states.
Staphylococcus is also a group of favorable bacteria in fermented sausages, contributing to color and flavor improvement (Wang, et al., 2021). Staphylococcus had a similar growth trend as LAB during the production of fermented sausages (Fig. 1C). Higher levels of Staphylococcus were observed in the PC group, reaching 8.23 Lg CFU/g at the end of ripening. Notably, Staphylococcus counts were lower than LAB counts in the same sausages, which may be due to the acidification caused by LAB inhibiting the growth of Staphylococcus (Xiao et al., 2020).
3.2.2. Bacterial diversity
Bacterial diversity at the end of sausage ripening was determined by high-throughput sequencing. The six samples yielded a total of 846,445 high-quality sequences, with an average length of 427 bp. As shown in Fig. S1, the coverage of all groups was > 0.992, which showed that almost all bacteria were detected in the samples. The Shannon, Chao and Ace indices of all inoculated sausages were significantly lower than those of the control sausage (P < 0.05), while the Simpson index was significantly higher than that of the control sausage (P < 0.05). Moreover, there were 25 common operational taxonomic units (OTUs) in the six groups of fermented sausages, the control group had the highest number of unique OTUs (16), and the KCA group had only 2 unique OTUs (Fig. 1D). These results indicated that starter cultures significantly decreased the diversity of bacterial communities in the fermented sausages, in line with the findings of Xiao et al (2020).
As shown in Fig. 1E, Firmicutes was the most abundant phylum among all treatments at the end of sausage ripening, which may be because bacteria such as Weisseria spp., Lactobacillus spp., Staphylococcus spp. and Lactococcus spp. commonly present in fermented sausages belong to Firmicutes. Additionally, the abundance of Firmicutes was significantly higher in the inoculated sausages than that in the uninoculated sausages, which may indicate that the starter cultures grew well in the fermented sausages. The bacterial community of the CON sausages was more diversified (Fig. 1F). Remarkably, starter inoculation led to remarkable modifications in the bacterial community. Pediococcus spp. (34.15 %) and Staphylococcus spp. (60.50 %) were the dominant bacteria in the commercial culture treatment, and Lactobacillus spp. (71.40 %-81.20 %) and Staphylococcus spp. (5.60–21.70 %) were the dominant bacteria in the four native culture groups, which was consistent with the results of bacterial counts. Notably, Staphylococcus spp. is biogenic amines-producing bacterial, improper control of the bacterial in sausage may lead to food safety issue (Gao et al., 2023b). Meanwhile, Lactobacillus spp. and Staphylococcus spp. are also desirable bacteria in food, as they are the key bacteria involved in lipid and protein hydrolysis (Xiao et al., 2020). Moreover, Lactobacillus spp. can produce lactic acid to limit the growth of some undesirable bacteria (Ge et al., 2019). Staphylococcus spp. can promote the formation of nitroso-myoglobin and improve the color of fermented sausages (Gotterup et al., 2008). Therefore, improving the abundance of prevalent bacteria (Lactobacillus spp. and Staphylococcus spp.) properly is essential to improve the flavor and safety of sausages.
3.3. Taste characteristics
3.3.1. FAAs
Fermented sausages are a class of high protein foods, and these proteins are converted to FAAs by the combination of microbial and endogenous enzymes (Xiao et al., 2020). Some FAAs have unique flavors, and others are precursors of taste and aroma compounds (Toldrá, 2006). As shown in Fig. 2A and Table S1, a total of 17 FAAs were found in all sausages. Apart from the CCB group, the total FAA concentration was markedly higher in the inoculation treatment than in the control (P < 0.05), which could be attributed to the high proteolytic activity of the starters (Xiao et al., 2020).
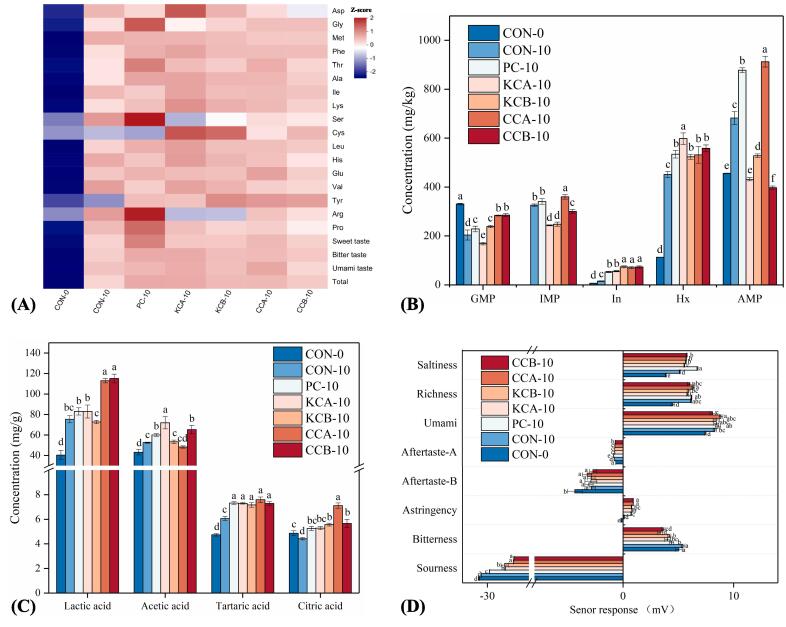
Fig. 2.
Effects of different mixed starter cultures on free amino acids (A), nucleotides (B), organic acids (C) and electron tongue response values (D) of fermented sausages. The means with different lowercase letters (a-f) indicate significant differences between different treatments. CON: uninoculated, PC: commercial starter, KCA: L. plantarum KM119 and S. simulans CD207, KCB: and L. plantarum KM119 and S. simulans CQ02205, CCA: L. plantarum CQ01107 and S. simulans CD207, CCB: L. plantarum CQ01107 and S. simulans CQ02205.
Glutamic acid (Glu), leucine (Leu), lysine (Lys), alanine (Ala), and threonine (Thr) were the predominant FAAs in both inoculated and uninoculated sausages. Remarkably, the Glu level was higher in the PC and CCA treatment group than in the other groups (P < 0.05). Glu is an umami-tasting FAA, that not only enhances the taste of meat products but also promotes the formation of some flavor compounds associated with the Maillard reaction (Zhang, Chen, Liu, Xia, Wang, & Kong, 2022). The maximum total FAA and sweet FAA (serine, proline, glycine, Thr, and Ala) levels were detected in the PC group. Nevertheless, it also had the highest content of bitter FAA (valine, methionine, isoleucine, phenylalanine (Phe), Lys, tyrosine, arginine, histidine, and Leu), which may have an undesirable effect on the sausage taste (Huang et al., 2023). The CCA treatment was followed only by the PC group in terms of sweet FAA and had the highest content of umami FAA (aspartic acid and Glu). Therefore, CCA culture may be more favorable for the formation of desirable tastes in fermented sausages.
3.3.2. Nucleotides
Nucleotides are mainly derived from nucleotide metabolism, are essential taste components in fermented sausages (Feng, Jo, Nam, & Ahn, 2019). As shown in Fig. 3B, the hypoxanthine nucleotide (IMP), inosine and hypoxanthine (Hx) at the end of ripening were considerably greater than those at the initial stage of fermentation (P < 0.05), indicating that adenosine triphosphate was continuously degraded during fermentation and ripening, promoting the formation and accumulation of related nucleotides. Additionally, the levels of adenosine monophosphate (AMP, 912.67 mg/kg) and IMP (359.78 mg/kg) were considerably greater in the CCA group compared to the other groups (P < 0.05). Moreover, no significant difference in the guanosine monophosphate (GMP) levels was observed between the CCA and CCB groups (P > 0.05). GMP and IMP are umami enhancers in food, AMP is associated with sweetness improvement, all playing a crucial role in forming the overall flavor of food (Jin, et al., 2023). The maximum level of Hx was detected in the KCA group, but Hx is a tasteless nucleotide that rarely contributes to the formation of sausage flavor (Xu et al., 2021).
Fig. 3.
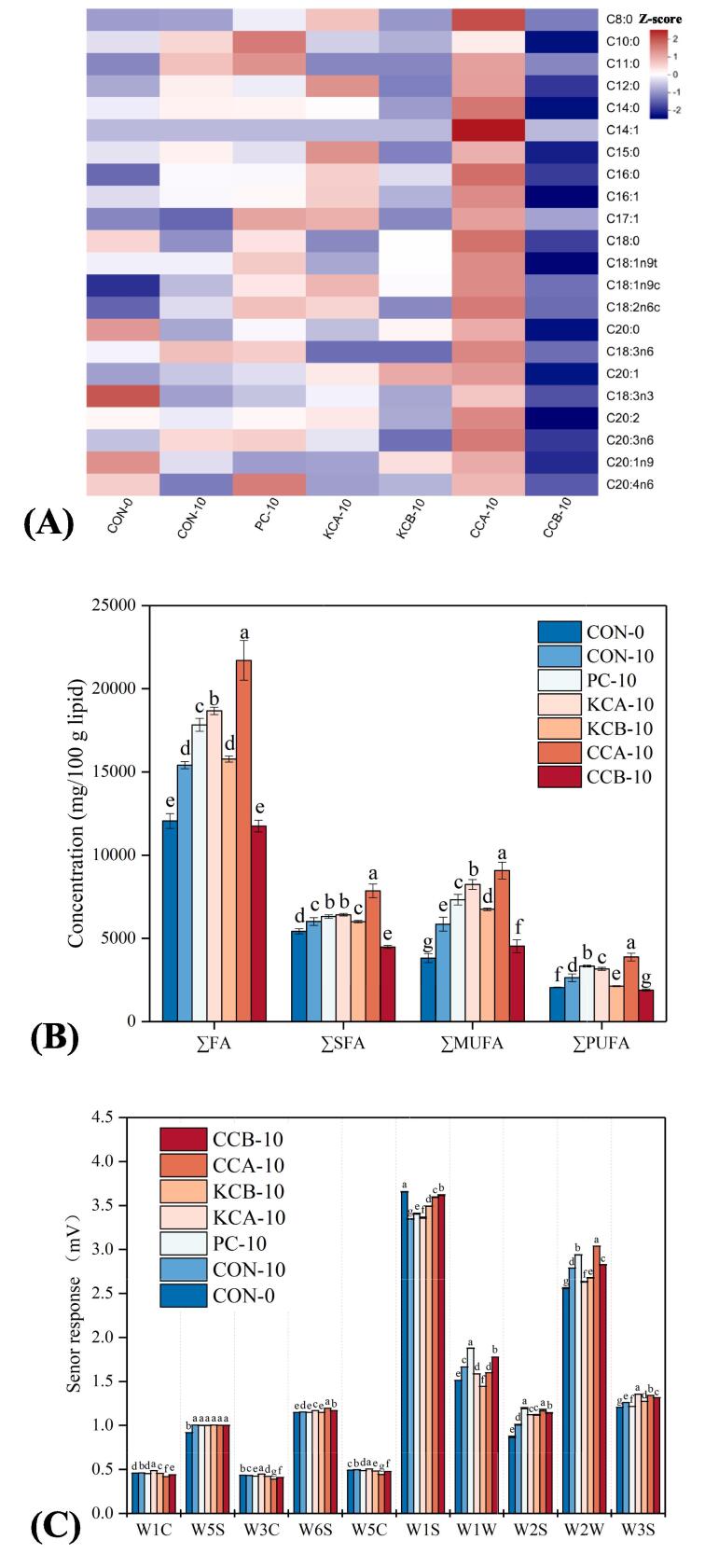
Effects of different mixed starter cultures on fatty acids (A, B) and electronic nose response values (C) of fermented sausages. The means with different lowercase letters (a-g) indicate significant differences between different treatments. CON: uninoculated, PC: commercial starter, KCA: L. plantarum KM119 and S. simulans CD207, KCB: and L. plantarum KM119 and S. simulans CQ02205, CCA: L. plantarum CQ01107 and S. simulans CD207, CCB: L. plantarum CQ01107 and S. simulans CQ02205.
3.3.3. Organic acids
Organic acids, derived from carbohydrate metabolism, have a unique sour taste, and their amount and type can significantly affect the flavor of fermented sausages (Huang et al., 2023). Four kinds of organic acids (lactic acid, acetic acid, citric acid and tartaric acid) were detected in all sausages (Fig. 2C). The highest organic acid was lactic acid (40.37–115.13 mg/g), followed by acetic acid (43.16–71.96 mg/g). Lactic acid is the main source of sourness in fermented sausages, and it can also enhance the formation of umami (Dashdorj, Amna, & Hwang, 2015). The highest levels of lactic acid were detected in the CCA and CCB treatments, indicating a strong lactic acid-forming capability of L. plantarum CQ 01107. Additionally, the contents of acetic acid and tartaric acid were highest in KCA and CCA sausages, respectively. For citric acid, there was no significant difference between sausages inoculated with starter cultures (P > 0.05).
3.3.4. Electronic tongue analysis
The electronic tongue, a tool used to mimic the human tongue to obtain taste information, has been widely used for the identification of taste characteristics of food (Ismail, Hwang, & Joo, 2020). As shown in Fig. 2D, the response values of the sourness, saltiness, umami, astringency, aftertaste-bitterness (aftertaste-B) and richness increased (P < 0.05), and the response value of aftertaste-astringency (aftertaste-A) and bitterness decreased (P < 0.05) in the sausage from 0 d to 10 d. The reasons for the increased sourness may be attributed to the metabolism of carbohydrates during the production of fermented sausages. Notably, CCA and CCB sausages had the highest acidity response values, which was consistent with the results for organic acids (Fig. 2C). Additionally, inoculation of starter cultures significantly reduced the bitterness of the fermented sausages, especially CCA treatment, which contributed to the desired flavor of the sausages. Furthermore, the highest umami and richness response values were also found in CCA sausages, which may be due to the higher Glu and flavor nucleotide content of CCA sausages (Fig. 2A and B).
3.4. Aroma characteristics
3.4.1. FAs
FAs are products of lipolysis and substrates of lipid oxidation and are critical to the flavor of fermented sausages (Xiao et al., 2020). The contents and composition of FAs in the inoculated and uninoculated groups are shown in Table S2 and Fig. 3A. A total of 22 FAs were identified in all sausages, including 9 saturated FAs (SFA), 7 monounsaturated FAs (MUFA) and 6 polyunsaturated FAs (PUFA). Among these FAs, the major FAs were C16:0 (2654.17–5007.35 mg/100 g lipid), C18:0 (1687.40–2422.45 mg/100 g lipid), C18:1n9c (3344.88–8392.85 mg/100 g lipid) and C18:2n6c (1613.04–3426.20 mg/100 g lipid). Notably, C16:0, C18:0, C18:1n9c and C18:2n6c were significantly higher in the CCA group than in the other groups (P < 0.05), among them, C16:0 and C18:2n6c are commonly regarded as important precursors for the flavor compounds of fermented sausages, mainly decomposing to produce aldehydes and ketones, respectively (Al-Dalali, Li, & Xu, 2022). The ∑FA content of all sausages (excluding the CCB group) on Day 10 was significantly higher than that on Day 0 (P < 0.05, Fig. 3B), indicating that remarkable lipid hydrolysis occurred during sausage fermentation and ripening. Moreover, the highest levels of ∑FA, ∑SFA, ∑MUFA and ∑PUFA were detected in the CCA treatment at the end of ripening (P < 0.05), followed by the KCA and PC treatments. Interestingly, CCA and KCA cultures contained S. simulans CD207, and both had high FA contents. In contrast, CCA and CCB cultures had L. plantarum CQ01107, but the CCB group had the lowest FA content. These results suggested that S. simulans was the main contributor to lipolysis, promoting the formation of FAs in fermented sausages. This was consistent with the results of Cruxen et al. (2017) and Xiao et al. (2020).
3.4.2. Electronic nose analysis
The electronic nose is a rapid detection tool for characterizing the overall volatile aroma of foods by simulating the human olfactory system. On Day 0, the W1S (sensitive to methyl) response value in fermented sausages was stronger and significantly higher than that on Day 10 (P < 0.05, Fig. 3C), indicating that the sausages may initially contain more volatile compounds with methyl groups. Inoculation with various starter cultures showed significant differences in response values for W1S, W1W (sensitive to sulfides), W2S (sensitive to alcohols, aldehydes, and ketones), W2W (sensitive to organic sulfides and W3S (sensitive to long-chain alkanes). The PC and CCA groups had similar aromas with higher W1W, W2S and W2W values, suggesting that PC and CCA cultures promote the formation of sulfur-containing compounds, alcohols, aldehydes and ketones in fermented sausages. In addition, KCA sausages were the most sensitive to the W3S sensor (P < 0.05). However, it is difficult to know the differences in the specific volatile components of these samples by the electronic nose.
3.4.3. Volatile flavor compounds
To better elucidate the flavor characteristics of the sausages, the volatile flavor compounds were identified using GC–MS. As shown in Fig. 4A and Table S3, a total of 43 volatile flavor compounds were found in all groups, including 6 alcohols, 5 aldehydes, 7 acids, 4 ketones, 15 esters, and 6 other classes of compounds. These compounds are mainly produced by lipid oxidation, amino acid metabolism, esterification reactions and carbohydrate metabolism (Hu et al., 2022). During sausage fermentation and ripening, alcohols declined and aldehydes, acids, esters and ketones increased significantly (P < 0.05, Fig. 4B). At the end of ripening, all inoculation treatments significantly increased aldehydes, esters and acids (P < 0.05), indicating that starters were helpful to improve the flavor of fermented sausages. Among them, the PC treatment was found to have the highest levels of aldehydes, and the KCA group had the highest amounts of acids (P < 0.05). Notably, the esters content was significantly higher in the CCA and CCB treatments (P < 0.05), and no significant differences were observed between the two groups (P > 0.05).
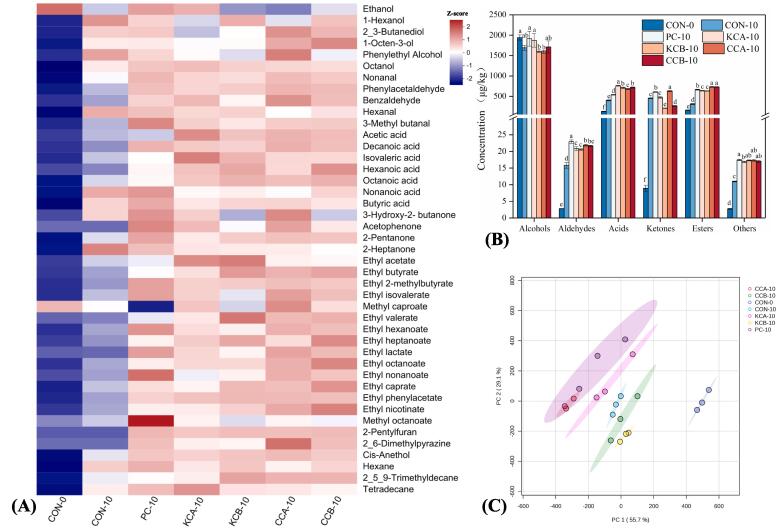
Fig. 4.
Effect of different mixed starter cultures on volatile flavor compounds of fermented sausages (A) and (B). Principal component analysis score plot of volatile flavor compounds in fermented sausages inoculated with different mixed starter cultures (C). The means with different lowercase letters (a-f) indicate significant differences between different treatments. CON: uninoculated, PC: commercial starter, KCA: L. plantarum KM119 and S. simulans CD207, KCB: and L. plantarum KM119 and S. simulans CQ02205, CCA: L. plantarum CQ01107 and S. simulans CD207, CCB: L. plantarum CQ01107 and S. simulans CQ02205.
Alcohols, which are primarily obtained from the reduction of methyl ketones, metabolism of carbohydrates and amino acids, as well as oxidation of lipids, are crucial for determining the flavor of fermented sausages (Sidira, Kandylis, Kanellaki, & Kourkoutas, 2015). Ethanol was the most abundant flavor compound, primarily from carbohydrate metabolism and liquor added during sausage preparation. The ethanol content of fermented sausages was the highest on Day 0, probably attributed to the evaporation of liquor during sausage production. 2,3-Butanediol is a byproduct of carbohydrate metabolism (Hu et al., 2022), and its content was significantly higher in the CCA and CCB treatments than in other treatments (P < 0.05). In addition, 1-octen-3-ol, derived from fat oxidation, has a distinctive mushroom-like aroma and a low odor threshold and is a characteristic flavor compound in fermented sausages (Ansorena, Gimeno, Astiasarán, & Bello, 2001). It was also found to be highest in CCA and CCB sausages. It is noteworthy that the content of phenethyl alcohol (violet-like, rose, floral, honey, and spicy aromas) from amino acid metabolism in CCA sausage is significantly higher than that in other sausages (P < 0.05).
Aldehydes have a low odor threshold and are considered critical flavor compounds in foods. Hexanal and nonanal are produced by the oxidation of linoleic acid and PUFA, respectively (Xiao et al., 2020). Among them, hexanal was chosen as an indicator of lipid oxidation since it is a characteristic product of lipid oxidation (Sidira et al., 2015). The content of hexanal in the inoculation group (except the PC group) was significantly lower than that in the control group, especially in the CCA group, suggesting that the inoculation of native cultures was able to inhibit lipid oxidation. The contents of benzaldehyde and phenylacetaldehyde in the inoculated group were remarkably greater than those in the uninoculated group (P < 0.05). These aldehydes, which may originate from the oxidative degradation of Phe, have a strong fruit aroma and an important role in the flavor of fermented sausages. Additionally, 3-methyl-1-butanal, mainly from branched-chain amino acid metabolism, was found to be the most abundant in PC sausages, followed by CCB and CCA sausages.
Short-chain FAs (<C6), such as acetic acid, butyric acid and isovaleric acid, are associated with sour and cheesy flavors in fermented sausages (Hu et al., 2022). The acetic acid and isovaleric acid contents of the inoculated groups were significantly higher than those of the uninoculated group (P < 0.05). Acetic acid, which is mainly metabolized by LAB and contributes to the aroma of fermented sausages, was the highest in KCA sausages, followed by CCA sausages. Medium-chain (C6-C12) and long-chain (C14-C18) FAs, such as octanoic acid and nonanoic acid, are mainly produced from the degradation of lipids. Although these acids have no immediate influence on the flavor of fermented sausages owing to their high odor threshold, they have an essential role in the formation of esters (Hu et al., 2019).
Four ketones were detected in all fermented sausages. Among them, 3-hydroxy-2-butanone was the predominant ketone and was markedly higher in CCA sausages than in other sausages (P < 0.05). 3-Hydroxy-2-butanone is mainly formed by carbohydrate metabolism and is also related to the chemical oxidation of 2,3-butanediol, contributing to dairy and fruity aromas (Marco, Navarro, & Flores, 2007). In addition, 2-pentanone, derived from β-oxidation of FAs, was significantly higher in the inoculated group than in the uninoculated group (P < 0.05), possibly due to the presence of Staphylococcus (Leroy, Verluyten, & De Vuyst, 2006). Furthermore, acetophenone with floral and almond aromas was found to be highest in the PC treatment, followed by the CCA treatment.
Esters are highly aromatic chemicals with a low odor threshold that impart a fruity flavor to fermented sausages (Marco et al., 2007). A total of 15 esters were detected in all treatments, the highest abundance being ethyl lactate, ethyl caprylate, ethyl caproate, ethyl decanoate and ethyl acetate. Ethyl esters, the main esters found, are considered necessary to obtain the desired aroma by adding fruitiness and masking sour rot aromas (Sidira et al., 2015). Except for methyl caproate, the concentrations of all esters were significantly higher in the inoculated group than in the uninoculated group (P < 0.05), especially in the CCA and CCB groups.
Notably, 2-pentylfuran and 2,6-dimethylpyrazine were also detected in the inoculated group of fermented sausages, which are derived from the Maillard reaction and are favored for the flavor improvement of fermented sausages. Hydrocarbons, such as tetradecane and hexane, are mainly from lipid autoxidation (Xiao et al., 2020). They are considered to play a minor role in the overall flavor of meat due to their high threshold.
According to the results of volatile compounds, the inoculation of native cultures improved the flavor of fermented sausages. Interestingly, the KCA and KCB cultures contained the same LAB, while the CNS cultures were from different strains of the same species, but there were significant differences in flavor compound content. Similar results were found in the KCA and CCA cultures, this indicated that the ability of starter cultures to form flavors is strain specific, which may be related to their growth characteristics and metabolic capacity (Gong et al., 2023). Among the four native cultures, CCA and CCB were able to promote the formation of flavor compounds that contribute to the flavor development of fermented sausages.
To explore the differences between sausages, PCA of volatile flavor compounds was performed (Fig. 4C). The first two components accounted for 55.7 and 29.1 of the total variances, respectively. A clear distinction was found between Day 0 and Day 10 fermented sausages, which was attributed to the amino acid metabolism, carbohydrate metabolism and fat oxidation that occurred during the production of the sausages. Interestingly, PC and CCA sausages were identified at the closest distances, indicating that both have similar volatile flavor profiles, which were mainly reflected in compounds such as nonanal, 3-hydroxy-2-butanone, acetophenone, ethyl lactate and ethyl isovalerate.
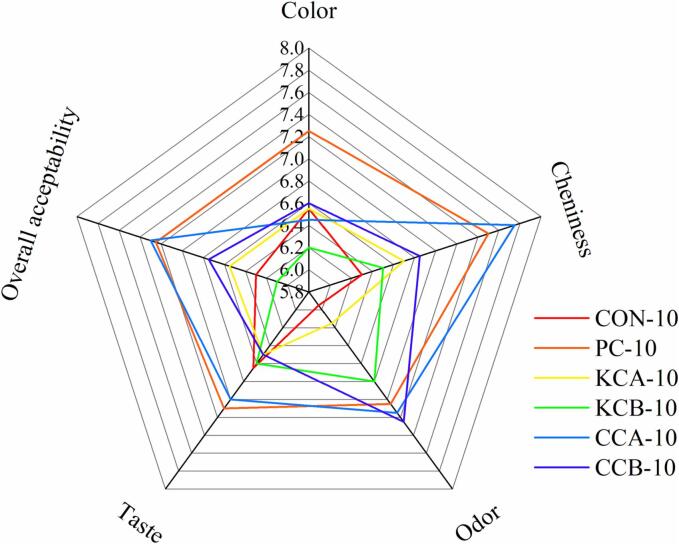
3.5. Sensory evaluation
Sensory evaluation is essential to estimate the acceptability of a product. Starters had a significant effect on the sensory characteristics of the fermented sausages (Fig. 5). The aroma score was highest in the CCB group, probably because CCB culture promoted the formation of desirable volatile flavor compounds such as esters. In addition, color score was highest in the PC treatment, which was consistent with the instrumental color results. Remarkably, CCA sausages had the highest overall acceptability and chewiness scores, with taste and aroma second only to the PC and CCB groups, which supports the e-tongue and GC–MS results. Overall, the CCA culture contributed more to the development of favorable sensory properties of the fermented sausages and was more suitable as a meat starter culture.
Fig. 5.
Effects of different mixed starter cultures on sensory characteristics of fermented sausages. CON: uninoculated, PC: commercial starter, KCA: L. plantarum KM119 and S. simulans CD207, KCB: and L. plantarum KM119 and S. simulans CQ02205, CCA: L. plantarum CQ01107 and S. simulans CD207, CCB: L. plantarum CQ01107 and S. simulans CQ02205.
4. Conclusions
In this study, the effect of mixed starter cultures on the bacterial communities and flavor of fermented sausages was investigated. During sausage production, LAB and CNS showed good growth and became the dominant bacteria at the end of ripening. Sausage inoculation with a mixed starter facilitated the development of the intended color. In addition, starter cultures can stimulate the formation of taste compounds, including FAAs, organic acids and nucleotides. Among them, CCA culture promoted the formation of umami amino acids, which was corroborated by e-tongue results. Furthermore, inoculation with starters, especially KCA and CCA, promoted the formation of FAs such as C16:0, C18:1n9c and C18:2n6c. Higher levels of volatile flavor compounds (such as ethyl 2-methylbutyrate, ethyl isovalerate, phenylacetaldehyde, and 3-hydroxy-2-butanone) were found in CCA and CCB sausages. In conclusion, the addition of mixed starter cultures had a significant effect on the flavor improvement of fermented sausages. Among them, CCA culture (L. plantarum CQ01107 + S. simulans CD207) was the most effective and may be the desirable starter culture to enhance the flavor of fermented sausages.
CRediT authorship contribution statement
Xuefei Shao: Writing – original draft, Methodology, Formal analysis, Data curation. Huhu Wang: Resources, Funding acquisition. Xiangyu Song: Writing – review & editing, Methodology. Na Xu: Writing – review & editing, Resources. Jian Sun: Resources. Xinglian Xu: Writing – review & editing, Supervision, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101225.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Al-Dalali S., Li C., Xu B. Insight into the effect of frozen storage on the changes in volatile aldehydes and alcohols of marinated roasted beef meat: Potential mechanisms of their formation. Food Chemistry. 2022;385 doi: 10.1016/j.foodchem.2022.132629. [DOI] [PubMed] [Google Scholar]
- Ansorena D., Gimeno O., Astiasarán I., Bello J. Analysis of volatile compounds by GC–MS of a dry fermented sausage: Chorizo de Pamplona. Food Research International. 2001;34(1):67–75. doi: 10.1016/S0963-9969(00)00133-2. [DOI] [Google Scholar]
- Baka A.M., Papavergou E.J., Pragalaki T., Bloukas J.G., Kotzekidou P. Effect of selected autochthonous starter cultures on processing and quality characteristics of Greek fermented sausages. LWT-Food Science and Technology. 2011;44(1):54–61. doi: 10.1016/j.lwt.2010.05.019. [DOI] [Google Scholar]
- Barbosa-Pereira L., Rojo-Poveda O., Ferrocino I., Giordano M., Zeppa G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Research International. 2019;123:684–696. doi: 10.1016/j.foodres.2019.05.041. [DOI] [PubMed] [Google Scholar]
- Chen Q., Hu Y., Wen R., Wang Y., Qin L., Kong B. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Science. 2021;172 doi: 10.1016/j.meatsci.2020.108338. [DOI] [PubMed] [Google Scholar]
- Chen Q., Kong B., Han Q., Xia X., Xu L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT-Food Science and Technology. 2017;77:389–396. doi: 10.1016/j.lwt.2016.11.075. [DOI] [Google Scholar]
- Cruxen C.E.d.S., Funck G.D., Dannenberg G.d.S., Haubert L., Marques J.d.L., Kroning I.S., et al. Characterization of Staphylococcus xylosus LQ3 and its application in dried cured sausage. LWT-Food Science and Technology. 2017;86:538–543. doi: 10.1016/j.lwt.2017.08.045. [DOI] [Google Scholar]
- Dashdorj D., Amna T., Hwang I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. European Food Research and Technology. 2015;241(2):157–171. doi: 10.1007/s00217-015-2449-3. [DOI] [Google Scholar]
- Deng S., Liu R., Li C., Xu X., Zhou G. Meat quality and flavor compounds of soft-boiled chickens: Effect of Chinese yellow-feathered chicken breed and slaughter age. Poultry Science. 2022;101(12) doi: 10.1016/j.psj.2022.102168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essid I., Hassouna M. Effect of inoculation of selected Staphylococcus xylosus and Lactobacillus plantarum strains on biochemical, microbiological and textural characteristics of a Tunisian dry fermented sausage. Food Control. 2013;32(2):707–714. doi: 10.1016/j.foodcont.2013.02.003. [DOI] [Google Scholar]
- Fadda S., Lopez C., Vignolo G. Role of lactic acid bacteria during meat conditioning and fermentation: Peptides generated as sensorial and hygienic biomarkers. Meat Science. 2010;86(1):66–79. doi: 10.1016/j.meatsci.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Feng X., Jo C., Nam K.C., Ahn D.U. Impact of electron-beam irradiation on the quality characteristics of raw ground beef. Innovative Food Science & Emerging Technologies. 2019;54:87–92. doi: 10.1016/j.ifset.2019.03.010. [DOI] [Google Scholar]
- Feng X., Tjia J.Y.Y., Zhou Y., Liu Q., Fu C., Yang H. Effects of tocopherol nanoemulsion addition on fish sausage properties and fatty acid oxidation. LWT-Food Science and Technology. 2020;118 doi: 10.1016/j.lwt.2019.108737. [DOI] [Google Scholar]
- Gao X., Li C., He R., Zhang Y., Wang B., Zhang. Z., et al. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chemistry. 2023;405(Part:A), 134911. doi: 10.1016/j.foodchem.2022.134911. [DOI] [Google Scholar]
- Gao X., Zhao X., Hu F., Fu J., Zhang Z., Liu Z., et al. The latest advances on soy sauce research in the past decade: Emphasis on the advances in China. Food Research Internation. 2023;173(part 2) doi: 10.1016/j.foodres.2023.113407. [DOI] [PubMed] [Google Scholar]
- Ge Q., Pei H., Liu R., Chen L., Gao X., Gu Y., et al. Effects of Lactobacillus plantarum NJAU-01 from Jinhua ham on the quality of dry-cured fermented sausage. LWT-Food Science and Technology. 2019;101:513–518. doi: 10.1016/j.lwt.2018.11.081. [DOI] [Google Scholar]
- Gomez M., Lorenzo J.M. Effect of fat level on physicochemical, volatile compounds and sensory characteristics of dry-ripened “chorizo” from Celta pig breed. Meat Science. 2013;95(3):658–666. doi: 10.1016/j.meatsci.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Gong X., Mi R., Chen X., Zhu Q., Xiong S., Qi B., et al. Evaluation and selection of yeasts as potential aroma enhancers for the production of dry-cured ham. Food Science and Human Wellness. 2023;12(1):324–335. doi: 10.1016/j.fshw.2022.07.022. [DOI] [Google Scholar]
- Gotterup J., Olsen K., Knochel S., Tjener K., Stahnke L.H., Moller J.K. Colour formation in fermented sausages by meat-associated staphylococci with different nitrite- and nitrate-reductase activities. Meat Science. 2008;78(4):492–501. doi: 10.1016/j.meatsci.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Hu Y., Chen Q., Wen R., Wang Y., Qin L., Kong B. Quality characteristics and flavor profile of Harbin dry sausages inoculated with lactic acid bacteria and Staphylococcus xylosus. LWT-Food Science and Technology. 2019;114 doi: 10.1016/j.lwt.2019.108392. [DOI] [Google Scholar]
- Hu Y., Wang J., Liu Q., Wang Y., Ren J., et al. Unraveling the difference in flavor characteristics of dry sausages inoculated with different autochthonous lactic acid bacteria. Food Bioscience. 2022;47 doi: 10.1016/j.fbio.2022.101778. [DOI] [Google Scholar]
- Hu Y., Zhang L., Liu Q., Wang Y., Chen Q., Kong B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiology. 2020;91 doi: 10.1016/j.fm.2020.103505. [DOI] [PubMed] [Google Scholar]
- Huang X., You Y., Liu Q., Dong H., Bai W., Lan B., et al. Effect of gamma irradiation treatment on microstructure, water mobility, flavor, sensory and quality properties of smoked chicken breast. Food Chemistry. 2023;421 doi: 10.1016/j.foodchem.2023.136174. [DOI] [PubMed] [Google Scholar]
- Ismail I., Hwang Y.H., Joo S.T. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chemistry. 2020;320 doi: 10.1016/j.foodchem.2020.126656. [DOI] [PubMed] [Google Scholar]
- Jin W., Fan X., Jiang C., Liu Y., Zhu K., Miao X., et al. Characterization of non-volatile and volatile flavor profiles of Coregonus peled meat cooked by different methods. Food Chemistry X. 2023;17 doi: 10.1016/j.fochx.2023.100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F., Verluyten J., De Vuyst L. Functional meat starter cultures for improved sausage fermentation. International Journal of Food Microbiology. 2006;106(3):270–285. doi: 10.1016/j.ijfoodmicro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Li Y., Li W., Li C., Li L., Yang D., Wang Y., et al. Novel insight into flavor and quality formation in naturally fermented low-salt fish sauce based on microbial metabolism. Food Research International. 2023;166 doi: 10.1016/j.foodres.2023.112586. [DOI] [PubMed] [Google Scholar]
- Lorenzo J.M., Gómez M., Fonseca S. Effect of commercial starter cultures on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control. 2014;46:382–389. doi: 10.1016/j.foodcont.2014.05.025. [DOI] [Google Scholar]
- Ma Y., Gao Y., Xu Y., Zhou H., Zhou K., Li C., et al. Microbiota dynamics and volatile metabolite generation during sausage fermentation. Food Chemistry. 2023;423 doi: 10.1016/j.foodchem.2023.136297. [DOI] [PubMed] [Google Scholar]
- Marco A., Navarro J.L., Flores M. Quantitation of Selected Odor-Active Constituents in Dry Fermented Sausages Prepared with Different Curing Salts. Journal of Agricultural and Food Chemistry. 2007;55(8):3058–3065. doi: 10.1021/jf0631880. [DOI] [PubMed] [Google Scholar]
- Qi J., Wang H., Zhou G., Xu X., Li X., Bai Y., et al. Evaluation of the taste-active and volatile compounds in stewed meat from the Chinese yellow-feather chicken breed. International Journal of Food Properties. 2018;20(sup3):S2579–S2595. doi: 10.1080/10942912.2017.1375514. [DOI] [Google Scholar]
- Shao X., Zhu M., Zhang Z., Huang P., Xu B., Chen C., Li P. N-nitrosodimethylamine reduction by Lactobacillus pentosus R3 in fermented cooked sausages. Food Control. 2021;124 doi: 10.1016/j.foodcont.2021.107869. [DOI] [Google Scholar]
- Sidira M., Kandylis P., Kanellaki M., Kourkoutas Y. Effect of immobilized Lactobacillus casei on the evolution of flavor compounds in probiotic dry-fermented sausages during ripening. Meat Science. 2015;100:41–51. doi: 10.1016/j.meatsci.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Toldrá F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends in Food Science & Technology. 2006;17(4):164–168. doi: 10.1016/j.tifs.2005.08.007. [DOI] [Google Scholar]
- Wang M., Wang C., Yang C., Peng L., Xie Q., Zheng R., et al. Effects of Lactobacillus plantarum C7 and Staphylococcus warneri S6 on flavor quality and bacterial diversity of fermented meat rice, a traditional Chinese food. Food Research Internation. 2021;150(Pt A) doi: 10.1016/j.foodres.2021.110745. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Liu Y., Chen C., Xie T., Li P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Research Internation. 2020;135 doi: 10.1016/j.foodres.2020.109247. [DOI] [PubMed] [Google Scholar]
- Xu N., Ye J., Li L., Wang X., Wang P., Han M., et al. Exploration of flavor and taste of soft-boiled chicken at different post-mortem aging time: Based on GC-IMS and multivariate statistical analysis. Food Bioscience. 2021;43 doi: 10.1016/j.fbio.2021.101326. [DOI] [Google Scholar]
- Xu N., Zeng X., Li L., Zhang X., Wang P., Han M., et al. Effects of post-mortem aging process on characteristic water-soluble taste-active precursors in yellow-feathered broilers. Food Science and Human Wellness. 2023;12(1):242–253. doi: 10.1016/j.fshw.2022.07.004. [DOI] [Google Scholar]
- Zhang L., Chen Q., Liu Q., Xia X., Wang Y., Kong B. Effect of different types of smoking materials on the flavor, heterocyclic aromatic amines, and sensory property of smoked chicken drumsticks. Food Chemistry. 2022;367 doi: 10.1016/j.foodchem.2021.130680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.