Abstract
Purpose
The aim of this study was to evaluate the image quality and feasibility of a field map-based technique to correct for susceptibility-induced geometric distortions which are typical for diffusion EPI brain imaging.
Methods
We prospectively included 52 patients during clinical routine in this single-center study. All scans were performed on a 3T MRI. Patients’ indications for MRI mainly consisted of suspected stroke due to the clinical presentation. For the morphological comparison of the corrected and uncorrected EPI diffusion, three experienced radiologists assessed the image quality of the sequences in a blinded and randomized fashion using a Likert scale (1 being poor; 5 being excellent). To ensure comparability of the two methods, an additional quantitative analysis of the apparent diffusion coefficient (ADC) was performed.
Results
Corrected EPI diffusion was rated significantly superior in all the selected categories: overall level of artifacts (p < 0.001), degree of distortion at the frontal, temporal, occipital and brainstem levels (p < 0.001), conspicuousness of ischemic lesions (p < 0.001), image quality (p < 0.001), naturality (p < 0.001), contrast (p < 0.001), and diagnostic confidence (p < 0.001).
Conclusion
Corrected EPI diffusion offers a significant reduction of geometric distortion in all evaluated brain regions and an improved conspicuousness of ischemic lesions. Image quality, overall artifacts, naturality, contrast and diagnostic confidence were also rated superior in comparison to uncorrected EPI diffusion.
Keywords: Diffusion-weighted imaging, Field map correction, Geometric distortion, Stroke imaging, Image quality
Introduction
Diffusion-weighted imaging has long been an integral part of MRI examinations in clinical routine [1]. In neuroimaging, it plays a crucial role in the early detection of ischemic infarction within the window of opportunity by visualizing early signal changes in the affected brain tissue mainly due to intracellular shift of water [2, 3].
A commonly used sequence for the acquisition of diffusion-weighted images is echo planar imaging (EPI). It enables rapid image acquisition, reducing signal loss and artifacts due to motion, but it is coupled with a higher sensitivity to susceptibility-related inhomogeneities in the main magnetic field [4]. This frequently causes geometric distortion of anatomical structures in the reconstructed images and thus a lack of agreement with undistorted high-resolution MRI scans, ultimately impairing diagnostic accuracy [5, 6].
A variety of methods have already been developed to compensate for the resulting loss in image quality, such as acquisition of multiple EPI images with different phase encoding directions [7] or using a registration template from undistorted sequences to estimate the extent of distortion [8]. In this work, we adopted the suggestion to acquire a separate field map which provides spatially resolved information of the absolute resonance frequency variation [9], and to use this information in a subsequent image correction.
Therefore, the hypothesis of our prospective study is that the application of corrected EPI diffusion is feasible in a clinical setting and will demonstrate a significant improvement in distortion artifacts, conspicuousness of ischemic lesions, naturality and contrast, the general image quality as well as the diagnostic confidence.
Material and Methods
Study Design
This prospective single center study was approved by the local ethics committee and conformed to the principles of the Declaration of Helsinki. All patients gave written informed consent. The acquisition period was between February 2022 and August 2022.
The following inclusion and exclusion criteria were defined: patients were included if suspicion of a stroke was raised by a referring physician, either in the emergency room or the hospital. They should be between 18 and 85 years of age, they were not allowed to have MRI contraindications as well as existing pregnancy or lactation and should not cause excessive motion artifacts. Patients were not included if they were not able to give informed consent.
Image Acquisition
All measurements were performed on a 3T MAGNETOM Skyra (Siemens Healthcare, Erlangen, Germany) with a 64-channel head and neck coil. Corrected and uncorrected EPI diffusion data were obtained using research sequences acquired at the end of the patients’ clinically indicated examination. The relevant sequence parameters of the study sequences are listed in Table 1.
Table 1.
Relevant MRI sequence parameters for corrected and uncorrected EPI diffusion
| Parameter | Corrected EPI diffusion | Uncorrected EPI diffusion |
|---|---|---|
| Diffusion mode | N‑scan trace | 3‑scan trace |
| b‑value 1 (averaging) | 0 s/mm2, (3) | 0 s/mm2, (3) |
| b‑value 2 (averaging) | 1000 s/mm2, (3) | 1000 s/mm2, (3) |
| Polarity | Bipolar | Bipolar |
| FOV (mm2) | 230 | 230 |
| TE | 94 ms | 94 ms |
| TR | 5900 ms | 5900 ms |
| Voxel size | 1.2 × 1.2 × 3 mm | |
| Partial Fourier | 6/8 | 6/8 |
| TA | 1.39 min | 1.39 min |
FOV field of view, TE echo time, TR repetition time, TA time of acquisition, EPI echo planar imaging
Field maps were acquired within 26 s using a triple-contrast gradient echo (GRE) sequence with the following parameters: field of view 320 × 320mm2; resolution 5.0 × 5.0 mm2; 58 slices (thickness 5 mm); echo times (TE) 2.4 ms, 4.6 ms, 7.1 ms; repetition time (TR) 10.9 ms. The orientation of the GRE scan was independent of the actual imaging scan, and position-matched, orientation-matched and resolution-matched field maps were interpolated directly on the scanner. Using an independent scan protocol for the field map enabled the same data to be reused for multiple subsequent imaging scans, without the need for repeated GRE acquisitions. Complex data from the three acquired contrasts were processed by the methods described in [10–12] to obtain absolute values of the local frequency offset. While the selected resolution of the field map limited the possibility to correct distortions on small spatial scales (e.g., in the temporal lobe), it allowed a robust and reliable estimation of actual absolute field variations.
Diffusion images were generated with a research application which combined a twice refocused spin-echo (TRSE) EPI acquisition with an integrated image correction algorithm. Using the spatially matched field maps, distortions of the diffusion-weighted magnitude images were corrected using interpolation and a Jacobian determinant-based density compensation as described in [13]. The apparent diffusion coefficient (ADC) maps were calculated based on diffusion-weighted images after distortion and density correction.
Image Analysis: Morphological Comparison
The extent of distortion with a focus on the frontal and occipital pole of the brain as well as the temporal lobe and brainstem was of particular interest for our study and was rated using a 5-point Likert scale (1 being non-diagnostic area due to distortions, 5 being no distortions). Image quality evaluation comprised the assessment of the overall level of artifacts, overall image quality, diagnostic confidence and naturality (1 being poor, 5 being excellent). If ischemic lesions were present, their conspicuousness was rated using the same 5‑point Likert scale. The comparison was performed in a randomized, blinded manner by three radiologists (2 radiologists with 4 years and 1 radiologist with 9 years professional experience). All reviewers were given an introduction using two examples not included in the analysis. Images were viewed in 2 sessions separated by at least 4 weeks.
Image Analysis: Comparison of ADC Values
Apparent diffusion coefficient (ADC) maps were automatically obtained from both corrected EPI diffusion and uncorrected EPI diffusion in all 52 patients. Measurements of ADC values were performed by placing a circular region of interest (ROI) of exactly 30 mm2 in 4 specific and corresponding regions of the provided ADC maps (left and right thalami as well as left and right precentral gyrus). Mean values in units of mm2/s were registered and saved to enable further statistical analysis.
Statistical Analysis
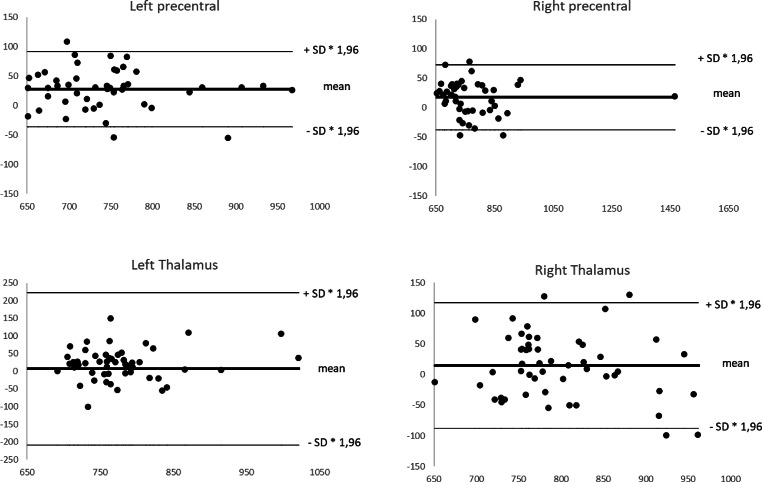
For the statistical analysis we used R 4.1 (A Language and Environment for Statistical Computing (2008), Vienna, Austria). The non-parametric values of Likert scales are characterized with median and interquartile range (IQR). To determine whether corrected EPI diffusion scored significantly higher than uncorrected EPI diffusion, Wilcoxon signed-rank test was performed for all image quality criteria. Inter-rater reliability was determined using interclass coefficient (ICC) in an absolute-agreement one-way model. It was interpreted as poor (< 0.5), moderate (0.5–0.75), good (0.76–0.9) or excellent (> 0.9). To demonstrate the distribution of ADC values between corrected and uncorrected EPI diffusion in all 52 cases mean values and standard deviations were calculated separately for all four regions of interest and plotted using the Bland-Altman method (Fig. 4).
Fig. 4.
Bland-Altman plot with the distribution of ADC values measured in both corrected and uncorrected EPI diffusion each in four different regions of the brain (left and right precentral gyrus and left and right thalamus). Measurements are plotted in units of mm2/s using the average (horizontal axis) and the difference between two corresponding measurements (vertical axis)
Results
Description of the Study Cohort
Corrected and uncorrected EPI diffusion images were successfully acquired in a total of 52 patients (28 male, 24 female) with a mean age of 59 years ranging between 21 and 85 years. In this cohort, a total of 18 patients revealed acute ischemic lesions, 7 patients with subacute ischemic lesions and a total of 4 patients with chronic infarction. Additional notable pathological findings included cerebral aneurysms, sinus thrombosis and cavernous hemangioma. A detailed description of the study cohort including indications for MR imaging is provided in Table 2.
Table 2.
Study population
| Patients, n (male/female) | 52 (28/24) |
| Patient age in years, mean ± SD (range) | 59 ± 17 (21–85) |
| Patient diagnosis, n |
Ischemic strokes in total, 28 multiple, acute, 11 singular, acute, 6 multiple, subacute, 4 singular, subacute, 3 multiple, old, 1 singular, old, 2 multiple, acute, and old, 1 No pathological findings, 13 Aneurysms (with coiling/with clip/without intervention), 3 (1/1/1) Sinus thrombosis, 2 Leukoencephalopathy, 2 Microangiopathy, 2 Subdural hygroma, 1 Cavernous hemangioma, 1 |
n number, SD standard deviation
Morphological Comparison
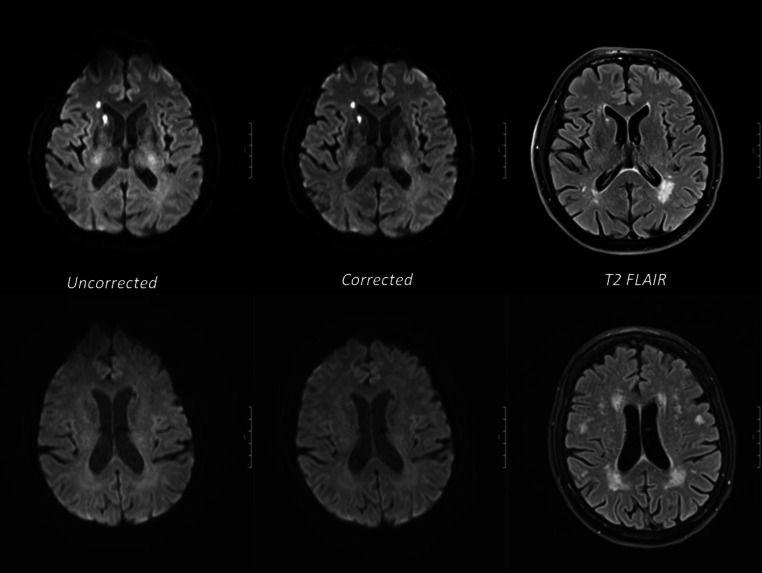
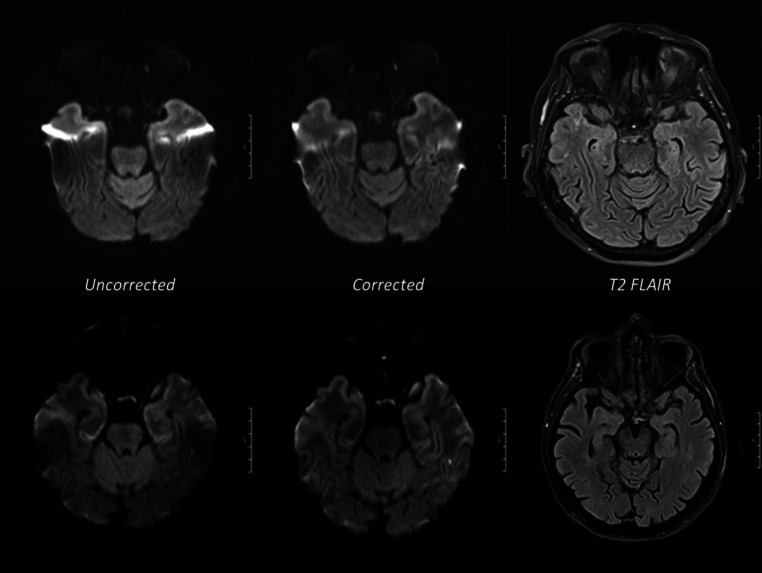
To demonstrate relevant differences between corrected and uncorrected EPI diffusion examples are provided in Figs. 1, 2 and 3. Among these images Fig. 2 clearly illustrates a reduction of signal pile up when using distortion correction. Wilcoxon-signed rank test demonstrated that corrected EPI diffusion was rated significantly better (p < 0.001) than uncorrected EPI diffusion in all aspects except for contrast in reader 1 and 3 (p = 0.014; p = 0.0017) and occipital distortion in reader 2 (p = 0.001). The overall median and interquartile range for corrected EPI diffusion were as follows: Image contrast 4 (3–4), naturality 3 (3–4), overall artifacts 3 (3–4), image quality 3 (3–4), ischemia conspicuity 4 (3–4), diagnostic confidence 4 (3–4), frontal distortions 4 (3–4), temporal distortions 4 (4–4), occipital distortions 4 (4–4) and brainstem distortions 4 (4–4). In contrast, median and interquartile range for uncorrected EPI distortions listed in the same order were as follows: image contrast 3 (3–3), naturality 3 (3–3), overall artifacts 3 (2–3) image quality 3 (3–3), ischemia conspicuity 3 (3–4) diagnostic confidence 3 (3–3), frontal distortions 2 (2–3), temporal distortions 3 (2–3), occipital distortions 3 (3–4) and brainstem distortions 3 (3–4). The majority of results demonstrated moderate interrater agreement (0.51–0.75). Agreement below 0.5 was present in image contrast (0.43). The medians with their interquartile ranges and interrater agreement are summarized in Table 3 for each image quality criterion.
Fig. 1.
Examples axial MRI scans of two different patients (upper and lower rows): corrected EPI diffusion demonstrates a clear reduction of geometric distortion of the frontal lobe in both cases. Matched T2 FLAIR images are provided for comparison
Fig. 2.
Examples of axial MRI scans of two different patients (upper row). Corrected EPI diffusion with a clear reduction in distortion artifacts affecting the temporal lobes. (lower row): corrected EPI diffusion showing improved conspicuousness of an ischemic lesion in the left temporal lobe. Matched T2 FLAIR images are provided for comparison
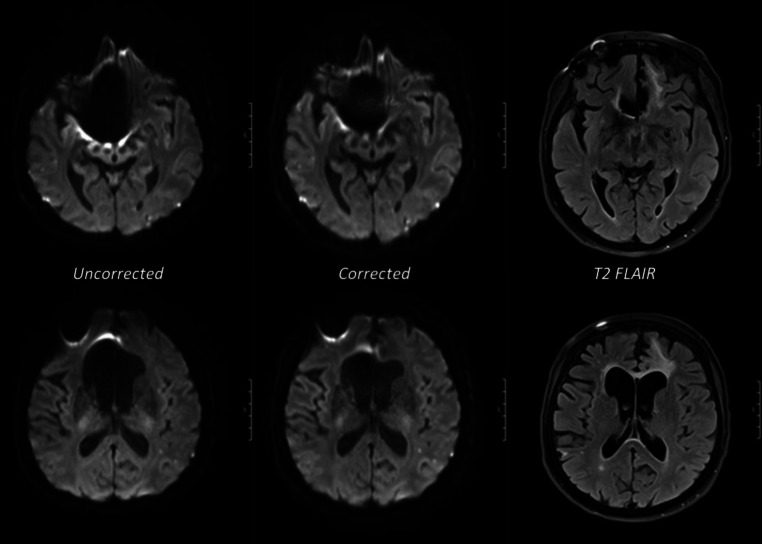
Fig. 3.
Examples of axial MRI scans of two different slice positions in the same patient with a surgically clipped aneurysm of the right anterior cerebral artery (upper row): substantially reduced artifact around the metal clip in corrected EPI diffusion. (lower row): consequentially improved geometry of the right anterior horn of the lateral ventricle due to the reduced metal-induced artefact. Matched T2 FLAIR images are provided for comparison
Table 3.
Results of image quality evaluation
| Reader 1 | Reader 2 | Reader 3 | Overall | Agreement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cor. Diff. Median (IQR) |
Uncor. Diff. Median (IQR) |
Wilcoxon-rank-sum test |
Cor. Diff. Median (IQR) |
Uncor. Diff. Median (IQR) |
Wilcoxon-rank-sum test |
Cor. Diff. Median (IQR) |
Uncor. Diff. Median (IQR) |
Wilcoxon-rank-sum test |
Cor. Diff. Median (IQR) |
Uncor. Diff. Median (IQR) |
Wilcoxon-rank-sum test | ICC | |
| Naturality | 3 (3–4) | 3 (2–3) | p < 0.001 | 4 (3–4) | 3 (3–3) | p < 0.001 | 4 (3–4) | 3 (3–3) | p < 0.001 | 3 (3–4) | 3 (3–3) | p < 0.001 | 0.53 |
| Contrast | 3 (3–4) | 3 (2–4) | p = 0.014 | 4 (4–4) | 3 (3–3) | p < 0.001 | 3 (3–4) | 3 (3–3) | p = 0.017 | 4 (3–4) | 3 (3–3) | p < 0.001 | 0.43 |
| Artifacts | 3 (3–4) | 3 (2–3) | p < 0.001 | 3 (3–4) | 3 (2–3) | p < 0.001 | 4 (3–4) | 3 (3–3) | p < 0.001 | 3 (3–4) | 3 (2–3) | p < 0.001 | 0.60 |
| IQ | 3 (3–4) | 3 (2–3) | p < 0.001 | 4 (3–4) | 3 (3–3) | p < 0.001 | 3 (3–4) | 3 (3–3) | p < 0.001 | 3 (3–4) | 3 (3–3) | p < 0.001 | 0.51 |
| IC | 4 (4–4) | 3 (3–4) | p < 0.001 | 4 (4–4) | 3 (3–4) | p < 0.001 | 4 (3–4) | 3 (3–4) | p < 0.001 | 4 (3–4) | 3 (3–4) | p < 0.001 | 0.57 |
| DC | 4 (3–4) | 3 (3–3) | p < 0.001 | 4 (4–4) | 3 (3–3) | p < 0.001 | 4 (3–4) | 3 (3-3) | p < 0.001 | 4 (3–4) | 3 (3–3) | p < 0.001 | 0.63 |
| Frontal D | 4 (3–4) | 2 (2–3) | p < 0.001 | 3 (3–4) | 2 (2–3) | p < 0.001 | 4 (3–4) | 2 (2–3) | p < 0.001 | 4 (3–4) | 2 (2–3) | p < 0.001 | 0.75 |
| Temporal D | 4 (3–4) | 2 (2–3) | p < 0.001 | 4 (3–4) | 2 (2–3) | p < 0.001 | 4 (4–4) | 3 (3–3) | p < 0.001 | 4 (4–4) | 3 (2–3) | p < 0.001 | 0.61 |
| Occipital D | 4 (4–4) | 3 (3–4) | p < 0.001 | 4 (4–4) | 3 (3–4) | p = 0.001 | 4 (4–4) | 3 (3–4) | p < 0.001 | 4 (4–4) | 3 (3–4) | p < 0.001 | 0.61 |
| Brainstem D | 4 (4–4) | 3 (3–4) | p < 0.001 | 4 (4–4) | 3 (3–4) | p < 0.001 | 4 (3–4) | 3 (3–3) | p < 0.001 | 4 (4–4) | 3 (3–4) | p < 0.001 | 0.69 |
Cor. Diff. corrected diffusion, Uncor. Diff. uncorrected diffusion, IQ image quality, DC diagnostic confidence, IC ischemia conspicuousness, D distortion, IQR interquartile range, ICC interclass coefficient
Quantitative Comparison of ADC Values
The ADC values plotted separately for the four selected brain regions showed a distribution generally within the limits of agreement using a Bland-Altman plot as shown in Fig. 4. Three of the four analyzed regions (left and right precentral gyrus, right thalamus) included a maximum of four outliers.
Discussion
EPI diffusion with field map-based correction was developed with the aim to decrease susceptibility-induced distortion and inhomogeneities typical for EPI-based diffusion imaging. The results demonstrate a clear improvement of image distortion, particularly at locations that tend to cause such severe artifacts regularly. These mainly include areas where bone, soft tissues or air interface and thereby can cause large differences in magnetic susceptibility [14, 15]. As a notable consequence, this can lead to a more difficult delineation or underestimation of ischemic changes in brain MRI [16–18]. Crucially, results of this study show that the conspicuousness of ischemic lesions improved significantly in the corrected EPI diffusion. Importantly, an additional review of our results revealed that no ischemic lesions were missed when viewing the corrected images. Otherwise, this could have indicated errors occurring in the field map used for image correction, which so far have not been reported in previous work using this method [13].
In addition to its influence on lesion detectability, geometric distortion in diffusion-weighted imaging can be problematic when pathological findings in distorted areas cannot be adequately associated with signal abnormalities in other sequences of a clinical MRI protocol [19, 20]. Specifically, matching diffusion-weighted images correctly with contrast enhanced sequences plays an important role in neuro-oncology, where correlation of sometimes very subtle changes can help to differentiate between malignancy or possible tissue necrosis after local radiation [21, 22]. Therefore, in a next step, the inclusion of additional MRI scans with a wider range of neurologic pathologies for which diffusion imaging is needed might be helpful to show whether the proposed correction method indeed offers diagnostic advantages in these cases as well.
Finally, overall artifacts, image quality, naturality and diagnostic confidence were rated superior in corrected EPI diffusion imaging. These results are in accordance with previous applications of the correction technique to improve the quality of prostate diffusion imaging [13]. We are therefore confident that the field map-corrected EPI diffusion is feasible in a clinical setting and can improve diagnosis in stroke imaging.
Limitations
The study has some limitations. Small sample size limits the generalizability of the results. In addition, not all patients revealed ischemic lesions. Hence, inclusion of more cases with ischemic lesions in areas affected by distortion may further highlight the role of corrected EPI diffusion. Furthermore, consensus reading prior to subjective image evaluation consisted of only two examples, which may have impacted the inter-rater agreement with the majority of ICC values ranging between 0.51 and 0.75 and with 1 value below 0.5.
Conclusion
Corrected EPI diffusion proved to be feasible in clinical routine MRI. Distortion artifacts were significantly reduced. Image quality, naturality, contrast, conspicuousness of ischemic lesions and diagnostic confidence were rated superior compared to uncorrected EPI diffusion.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
T. Feiweier is an employee of Siemens Healthcare GmbH, owns stocks in Siemens (Healthineers) AG and holds patents filed by Siemens. N.F. Grauhan, N. Grünebach, L. Brockstedt, A. Sanner, V. Schöffling, M.A. Brockmann and A.E. Othman declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45(3):169–184. doi: 10.1016/S0720-048X(02)00303-0. [DOI] [PubMed] [Google Scholar]
- 2.Roberts TP, Rowley HA. Diffusion weighted magnetic resonance imaging in stroke. Eur J Radiol. 2003;45(3):185–194. doi: 10.1016/S0720-048X(02)00305-4. [DOI] [PubMed] [Google Scholar]
- 3.DiBella E, Sharma A, Richards L, Prabhakaran V, Majersik J, HashemizadehKolowri S. Beyond diffusion tensor MRI methods for improved characterization of the brain after ischemic stroke: a review. Am J Neuroradiol. 2022;43(5):661–669. doi: 10.3174/ajnr.A7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhushan C, Haldar JP, Joshi AA, Leahy RM. Correcting susceptibility-induced distortion in diffusion-weighted MRI using constrained nonrigid registration. Proceedings of the 2012 Asia Pacific Signal and Information Processing Association Annual Summit and Conference. IEEE. 2012; 1–9. PMID: 26767197; PMCID: PMC4708288. [PMC free article] [PubMed]
- 5.Polimeni JR, Bhat H, Witzel T, Benner T, Feiweier T, Inati SJ, et al. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn Reson Med. 2016;75(2):665–679. doi: 10.1002/mrm.25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donato F, Jr, Costa DN, Yuan Q, Rofsky NM, Lenkinski RE, Pedrosa I. Geometric distortion in diffusion-weighted MR imaging of the prostate—contributing factors and strategies for improvement. Acad Radiol. 2014;21(6):817–823. doi: 10.1016/j.acra.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 8.Studholme C, Constable RT, Duncan JS. Accurate alignment of functional EPI data to anatomical MRI using a physics-based distortion model. IEEE Trans Med Imaging. 2000;19(11):1115–1127. doi: 10.1109/42.896788. [DOI] [PubMed] [Google Scholar]
- 9.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 10.Fautz H‑P, Gross P, Gumbrecht R. Method and computer to determine a b0 field map with a magnetic resonance apparatus. Google Patents. U.S. Patent Application Nr. 14/487,541. 2015.
- 11.Gumbrecht R, Koehler M, Schneider R. Method and magnetic resonance apparatus for correction of a B0 map for chemical shifts. Google Patents. US patent Nr. 9.830.711.B2. 2015.
- 12.Bredfeldt JS, Miao X, Kaza E, Schneider M, Requardt M, Feiweier T, et al. Patient specific distortion detection and mitigation in MR images used for stereotactic radiosurgery. Phys Med Biol. 2022;67(6):065009. doi: 10.1088/1361-6560/ac508e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong A, Lemberskiy G, Huang C, Shanbhogue K, Feiweier T, Rosenkrantz AB. Exploratory study of geometric distortion correction of prostate diffusion-weighted imaging using B0 map acquisition. J Magn Reson Imaging. 2019;50(5):1614–1619. doi: 10.1002/jmri.26751. [DOI] [PubMed] [Google Scholar]
- 14.Okuchi S, Fushimi Y, Yoshida K, Nakajima S, Sakata A, Hinoda T, et al. Comparison of TGSE-BLADE DWI, RESOLVE DWI, and SS-EPI DWI in healthy volunteers and patients after cerebral aneurysm clipping. Sci Rep. 2022;12(1):1–9. doi: 10.1038/s41598-022-22760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duong ST, Phung SL, Bouzerdoum A, Schira MM. An unsupervised deep learning technique for susceptibility artifact correction in reversed phase-encoding EPI images. Magn Reson Imaging. 2020;71:1–10. doi: 10.1016/j.mri.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Choi NY, Park S, Lee CM, Ryu C-W, Jahng G-H. The role of double inversion recovery imaging in acute ischemic stroke. Investig Magn Reson Imaging. 2019;23(3):210–219. doi: 10.13104/imri.2019.23.3.210. [DOI] [Google Scholar]
- 17.Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil AA, Hohenhaus M, Kunze C, Schmidt W, Brunecker P, Villringer K, et al. Sensitivity of diffusion-weighted STEAM MRI and EPI-DWI to infratentorial ischemic stroke. PLoS ONE. 2016;11(8):e0161416. doi: 10.1371/journal.pone.0161416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irfanoglu MO, Walker L, Sarlls J, Marenco S, Pierpaoli C. Effects of image distortions originating from susceptibility variations and concomitant fields on diffusion MRI tractography results. Neuroimage. 2012;61(1):275–288. doi: 10.1016/j.neuroimage.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Embleton KV, Haroon HA, Morris DM, Ralph MAL, Parker GJ. Distortion correction for diffusion-weighted MRI tractography and fMRI in the temporal lobes. Hum Brain Mapp. 2010;31(10):1570–1587. doi: 10.1002/hbm.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134(3):495–504. doi: 10.1007/s11060-017-2375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park YW, Choi D, Park JE, Ahn SS, Kim H, Chang JH, et al. Differentiation of recurrent glioblastoma from radiation necrosis using diffusion radiomics with machine learning model development and external validation. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-82467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]