Abstract
Introduction
Biomarker testing is mandatory for the clinical management of patients with advanced non-small cell lung cancer (NSCLC). Myriads of technical platforms are now available for biomarker analysis with differences in terms of multiplexing capability, analytical sensitivity, and turnaround time (TAT). We evaluated the technical performance of the diagnostic workflows of 24 representative Italian institutions performing molecular tests on a series of artificial reference specimens built to mimic routine diagnostic samples.
Methods
Sample sets of eight slides from cell blocks of artificial reference specimens harboring exon 19 EGFR (epidermal growth factor receptor) p.E746_AT50del, exon 2 KRAS (Kirsten rat sarcoma viral oncogene homologue) p.G12C, ROS1 (c-ros oncogene 1)-unknown gene fusion, and MET (MET proto-oncogene, receptor tyrosine kinase) Δ exon 14 skipping were distributed to each participating institution. Two independent cell block specimens were validated by the University of Naples Federico II before shipment. Methodological and molecular data from reference specimens were annotated.
Results
Overall, a median DNA concentration of 3.3 ng/µL (range 0.1–10.0 ng/µL) and 13.4 ng/µL (range 2.0–45.8 ng/µL) were obtained with automated and manual technical procedures, respectively. RNA concentrations of 5.7 ng/µL (range 0.2–11.9 ng/µL) and 9.3 ng/µL (range 0.5–18.0 ng/µL) were also detected. KRAS exon 2 p.G12C, EGFR exon 19 p.E736_A750del hotspot mutations, and ROS1 aberrant transcripts were identified in all tested cases, whereas 15 out of 16 (93.7%) centers detected MET exon 14 skipping mutation.
Conclusions
Optimized technical workflows are crucial in the decision-making strategy of patients with NSCLC. Artificial reference specimens enable optimization of diagnostic workflows for predictive molecular analysis in routine clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40487-023-00252-5.
Keywords: Lung, Molecular pathology, Tumor biomarker
Key Summary Points
| Lung cancer still represents one of the most leading causes of death worldwide. In the last decades, target therapy has radically shifted clinical paradigm for Non-Small Cell Lung Cancer (NSCLC) implementing a clinically approved mandatory testing gene panel able to identify patients with NSCLC administrable with target drugs. |
| A wide series of diagnostic routine samples (small biopsy, surgical resection, direct smear, cell-block) are diagnostically available for molecular analysis. In this scenario, “scant” diagnostic specimens often represents the only source of nucleic acids for molecular testing. As a consequence, diagnostic testing strategy should be technically optimized in accordance with routinely available biological source. |
| In this heterogeneous scenario, harmonized procedures may support testing strategies of clinically relevant predictive biomarkers in clinical practice. As regards, in this multicentre trial an artificial reference slide set, mimicking diagnostic samples, was distributed to each participating institutions evaluating concordance rate and inter-laboratory reproducibility. |
| Among n=24 participating institutions, manual and fully automatized analytical procedures highlighted comparable results in terms of technical performance on nucleic acids extraction and molecular analysis. Particularly, DNA based hotspot mutations (KRAS (Kirsten Rat Sarcoma Viral Oncogene Homologue) exon 2 p.G12C, EGFR (Epidermal Growth Factor Receptor) exon 19 p.E746_A750del) and ROS1 (c-ros Oncogene 1) aberrant transcripts were successfully detected in all instances whereas MET (MET proto-oncogene, receptor tyrosine kinase) exon 14 skipping mutation was identified in 93.7% of institutions approaching this test. |
| RNA management still represents a hardly managing target in terms of quantity and quality. Optimized pre-analytical and analytical procedures are recommended to improve successful rate of molecular testing on this analyte. |
| Harmonized ring trials based on artificial samples mimicking diagnostic specimens may represent a key weapon to optimize diagnostic workflow in predictive molecular laboratories. |
Introduction
Lung cancer still remains one of the leading causes of death worldwide [1]. Over the past two decades, precision medicine has revolutionized the clinical management of patients with advanced lung cancer through the identification of clinically relevant predictive biomarkers [2–4]. In this scenario, an ever-increasing number of predictive biomarkers have emerged as novel targets for molecular analysis [3, 5]. Indeed, biomarkers testing is now highly recommended for the management of patients with non-small cell lung cancer (NSCLC). In this regard, the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASCL), and the Association for Molecular Pathology (AMP) have established a panel of “mandatory test genes” [6]. This panel covers DNA -based hotspot mutations including epidermal grow factor receptor (EGFR) and v-Raf murine sarcoma viral oncogene homolog B (BRAF), as well as RNA-based aberrant transcripts including anaplastic lymphoma kinase (ALK), proto-oncogene 1 (ROS1), proto-oncogene tyrosine-protein kinase receptor Ret (RET) and MET proto-oncogene, receptor tyrosine kinase (MET) Δ exon 14 skipping. In addition, KRAS (Kirsten rat sarcoma viral oncogene homologue) exon 2 p.G12C mutation has recently been approved as a novel molecular target for the selection of targeted treatments [7–9]. In view of this heterogeneous scenario, next-generation sequencing (NGS) platforms are increasingly being used and recommended in routine clinical practice to comprehensively analyze all clinically approved predictive biomarkers [10, 11].
Although singleplex technologies have a lower mutation detection coverage than multiplex technologies, most Italian molecular diagnostics laboratories still use real-time reverse transcriptase-polymerase chain reaction (RT-PCR)-based assays in their diagnostic workflows [12–14]. In the setting of NSCLC, a wide range of diagnostic specimens are routinely analyzed to detect hotspot mutations in predictive biomarkers. This procedure is highly complex because each sample type possesses distinct technical features requiring tailored approaches for accurate molecular analysis. Moreover, a significant proportion of cases rely on limited diagnostic material, an additional complexity that underscores the need for optimized protocols to maximize diagnostic yield [15–17]. Hence, many institutions conducting molecular tests should optimize their own diagnostic workflows for the analysis of clinically relevant predictive biomarkers on the basis of the available diagnostic specimen. Not surprisingly, different technical approaches have been observed across laboratories, giving rise to variations in success rates, turnaround time (TAT), and accuracy of molecular analysis [18]. Undeniably, developing harmonized procedures based on interlaboratory concordance across different institutions represents an open challenge that must be addressed to overcome the technical limitations in routine diagnostic practice. Accordingly, to evaluate the technical performance of these approaches we conducted a multicenter trial involving 24 representative Italian institutions for molecular testing. Each participating institution analyzed artificial reference slides built on engineered cell lines harboring representative clinically relevant mutations in approved biomarkers for patients with NSCLC. Then, concordance rates and interlaboratory reproducibility were determined by comparing the molecular data reported by each participating institution.
Methods
Study Design
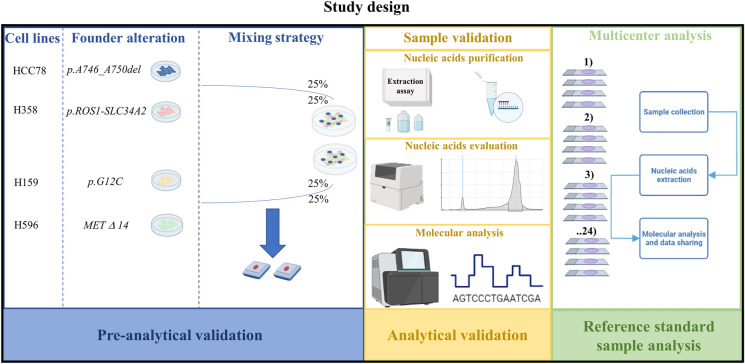
Each participating institution received a set of reference specimens from the coordinating center. The artificial control was built by mixing four engineered cell lines harboring clinically relevant mutations found in patients with NSCLC (Supplementary Table 1). Before shipment, each cell line was previously analyzed and validated by the laboratories of Federico II University. In brief, nucleic acids were first evaluated on the pellet samples from each cell line (Supplementary Fig. 1). Owing to the challenging management of “scant” RNA samples and heterogeneous technical approaches available for RNA testing, RNA-based biomarkers were additionally tested with an ultra-deep, long-read third-generation NGS platform (ONT-Nanopore, Oxford UK) (Supplementary Material). Then, cell block specimens were created from each cell line by generating formalin-fixed paraffin-embedded (FFPE) specimens [19]. This hybrid sampling approach, consisting of histological and cytological preparations, proved to be technically feasible and reproducible for this project. Nucleic acid evaluation was also assessed on cell blocks to verify the impact of sample preparation on the integrity of nucleic acids. In addition, molecular analysis was carried out with our own internal diagnostic workflow. Next, two independent cell block specimens were generated from mixed cell lines harboring all the referenced molecular alterations. A representative set of reference specimens from two independent batches were then internally validated before shipment to other institutions. Similarly, nucleic acid purification was carried out with our internal diagnostic workflow. NGS analysis was carried out with both semiautomated (Ion S5 system, Thermo Fisher Scientifics) and fully automated NGS platforms (Genexus, Thermo Fisher Scientifics). Overall, eight slides (four for DNA and RNA-based biomarkers, respectively) were distributed to each participating institution. After that, molecular analysis was carried out by each participating institution according to its internal diagnostic workflow. Finally, molecular alterations, DNA/RNA quantity (when technically available), and data reporting were shared with the coordinating center (Fig. 1).
Fig. 1.
Schematic representation of study design. Briefly, after internal validation of standard reference sample at University of Naples Federico II, a set of eight slides were distributed to each center. Here, own diagnostic workflow (from nucleic acid extraction to molecular data interpretation) was applied. Data were shared with the coordinating center. MET MET proto-oncogene, receptor tyrosine kinase, ROS1 c-ros oncogene 1, SLC34A2 solute carrier family 34 member 2
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Biological material was managed under the authorization of the Department of Public Health at the University of Naples Federico II, Naples. The cell lines used in this study were reference specimens and were not obtained from patients at the institutions. As such, patient informed consent was not applicable for this research. Institutional review board approval for this type of study on artificial samples is not required following the specification reported by The Italian Data Protection Authority and the Helsinki Declaration.
Standard Sample Generation and Validation
Cell Culture
Four cell lines harboring clinically relevant molecular alterations found in patients with NSCLC (Supplementary Table 1) were engineered to create artificial sample slides. In brief, HCC827 (Cat# CRL-2868) and H358 (Cat# CRL-5807) cell lines were purchased from the American Type Culture Collection (ATCC); HCC78 (Cat# ACC 563) was purchased from the Leibniz Institute DSMZ—German Collection of Microorganisms and Cell Cultures GmbH; H596 cell line was kindly provided by Dr. Miguel Angel Molina-Vila (Laboratory of Oncology, Pangaea Oncology, Spain). All cell lines were cultured in RPMI-1640 medium (Cat# 30-2001, ATCC) supplemented with 10% of inactivated FBS (fetal bovine serum) (Cat# 30-2020, ATCC), 2 mM glutamine (Cat# TCL012, HiMedia), 100 IU/mL of penicillin (100 IU/mL), and 100 μg/mL of streptomycin (Cat#A001, HiMedia) and maintained within a 5% CO2 incubator at 37 °C. All cell lines were validated morphologically and routinely tested for the presence of mycoplasma. Every 2–3 days, cells were split with trypsin–EDTA solution (Cat# TCL139, HiMedia) at a recommended splitting ratio. They were then washed twice in PBS (phosphate buffered saline), harvested via mechanical detachment, and counted in a cell counting chamber (Bürke). In particular, cells were gently detached with a rubber scraper. After harvest, all cell aliquots were washed in PBS once; then 10 μL of cell suspension was placed in a cell count chamber; afterwards, cells were counted in each of the three large squares. At the end of the procedure, the average number of viable cells and overall cell concentration were calculated according to the following standardized formula:
DNA and RNA Extraction
DNA was isolated and purified from HCC827 (exon 19 EGFR p.E746_AT50del), H358 (exon 2 KRAS p.G12C), and cell blocks of mixed cell lines with the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. In brief, after nucleic acid extraction and purification, DNA was resuspended at − 80 °C until molecular analysis.
RNA was instead extracted from HCC78 (ROS1-unknown gene fusion), H596 (MET Δ exon 14 skipping), and cell blocks of mixed cell lines by using the All-prep DNA/RNA mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions; then, RNA extracts were resuspended in 30 µL of nuclease-free water. The quantity and quality of nucleic acids were assessed with an automated electrophoresis system (TapeStation 4200, Agilent Technologies, Santa Clara, California, USA).
Molecular Analysis
After evaluation of nucleic acids, SiRe™ and SiRe fusion panels were applied to Ion S5™ plus (Thermo Fisher Scientifics) to assess the mutational status on the first cell block according to the manufacturer’s procedures [20–22].
An independent series of eight slides from a second cell block was analyzed on a fully automated NGS platform (Genexus, Thermo Fisher Scientifics) with the Oncomine Precision Assay (OPA) panel (Thermo Fisher Scientifics); this assay covers all the actionable genes in patients with NSCLC.
Results
Standard Sample Generation and Validation
Nucleic Acid Extraction
Overall, DNA quantification highlighted comparable levels between cell pellet (HCC827 8.9 ng/µL, H358 115.0 ng/µL) and cell block (HCC827 7.5 ng/µL, H358 145.0 ng/µL) preparations from corresponding cell lines. In addition, the DNA integrity number (DIN) of cell pellet preparations was similar to those of HCC827-derived and H358-derived cell block samples (6.9 and 7.9 vs 6.9 and 8.9, respectively). RNA evaluation from HCC78 and H596 samples on TapeStation 4200 (Agilent) revealed that cell block sampling, but not cell pellet preparations, affected RNA yield (HCC78, 12.5 ng/µL vs 15.3 ng/µL; H596, 6.3 ng/µL vs 9.5 ng/µL, respectively) and index fragmentation (2.6 vs 4.0 and 1.6 vs 3.9). Hence, nucleic acid extraction and qualification of cell block specimens from mixed engineered cell lines were conducted. As expected, DNA and RNA yields were consistent with previous data: [(DNA 8.2 ng/µL, DIN 2.0; RNA 37.2 ng/µL, RIN 1.0) block 1; (DNA 6.1 ng/µL, DIN 2.9; RNA 25.4 ng/µL, RIN 1.0) block 2] (Supplementary Fig. 2).
Molecular Analysis
NGS analysis was successfully carried out in all instances. Briefly, adequate technical parameters of DNA and RNA samples were obtained from semiautomated NGS systems [DNA sample: number of reads 566,693, mean read length 146, number of mapped reads 565,956, percent reads on target 99.6%, average reads per amplicon 13,415, uniformity of amplicon coverage 97.6%); RNA sample: number of reads 365,350, mean read length 117, mapped reads for SLC34A2(4)-OS1(32) 45,651, mapped reads for MET Δ exon 14 3066)]. Likewise, adequate parameters of DNA and RNA samples were obtained from fully automated NGS systems [DNA sample: number of reads 1,966,288, mean read length 97, number of mapped reads 1,951,334, percent reads on target 95.3%, average reads per amplicon 6828, uniformity of amplicon coverage 95.8%; RNA sample: number of reads 1,591,464, mean read length 101, mapped reads for SLC34A2(4)-OS1(32) 3327, mapped reads for MET Δ exon 14 349] (Table 1).
Table 1.
Validation step
| Sample | Platform | Reads | Mean read length | Number of mapped reads | Percent reads on target | Average reads per amplicon | Uniformity of amplicon coverage | Total mapped fusion panel reads |
|---|---|---|---|---|---|---|---|---|
| CB_1_DNA | S5plus | 566,693 | 146 | 565,956 | 99.6% | 13,415 | 97.6% | NA |
| CB_1_RNA | S5plus | 365,350 | 117 | NA | NA | NA | NA | 128,762 |
| CB_2_DNA | GENEXUS | 1,966,288 | 97 | 1,951,334 | 95.3% | 6828 | 95.8% | NA |
| CB_2_RNA | GENEXUS | 1,591,464 | 101 | NA | NA | NA | NA | NA |
Technical parameters from NGS analysis performed on two different platforms at University of Naples Federico II
CB cell block, NA not available
NGS analysis on two independent cell blocks from mixed engineered cell lines confirmed all the molecular alterations found in single-cell populations, showing no differences between semiautomated and fully automated NGS platforms (Supplementary Fig. 3A, 3B).
Standard Sample Analysis
Overall, series of eight reference 5-µm slide sets, generated from previously validated cell block samples, were successfully shared with all participating institutions. Molecular results were shared with the coordinating institution within 30 working days, as recommended by the study protocol. Regarding the geographical distribution of the centers, 14 out of 24 (58.4%) were located in Northern Italy, 5 out of 24 (20.8%) in Central Italy, and 5 out of 24 (20.8%) in Southern Italy. Only one participating institution (ID#22) (4.2%) was unable to perform the molecular analysis and to convey the results to the coordinating center.
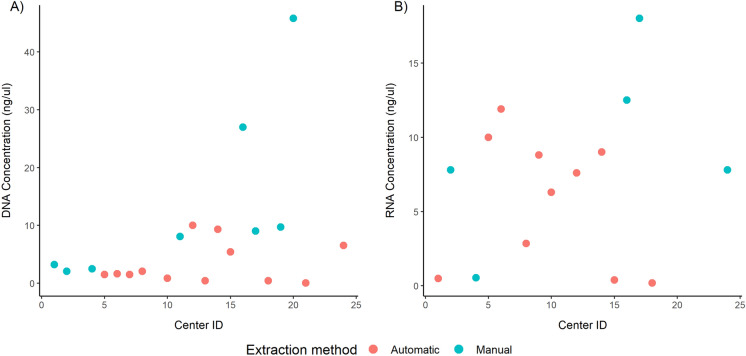
Regarding nucleic acids extraction, 21 out of 23 (91.3%) centers successfully carried out DNA purification, whereas 17 out of 23 (73.9%) participating centers successfully performed RNA extraction. Moreover, DNA and RNA purification was performed with manual strategies in 9 out of 21 (42.8%) and in 6 out of 17 (35.3%) institutions and with automated platforms in 12 out of 21 (57.2%) and in 11 out of 17 (64.7%) institutions. In a single case, neither DNA nor RNA purification was performed (ID#23). Owing to some technical issues, DNA purification failed in one automated test-based center. Furthermore, DNA and RNA quantification was performed by 20 out of 21 (95.2%) and 15 out of 17 (88.2%) participating institutions. In particular, a median DNA concentration of 3.3 ng/µL (range 0.1–10.0 ng/µL) was inspected with automated systems, whereas a median of 13.4 ng/µL (range 2.0–45.8 ng/µL) was inspected with manual procedures. Moreover, RNA concentrations of 5.7 ng/µL (range 0.2–11.9 ng/µL) and 9.3 ng/µL (range 0.5–18.0 ng/µL) were detected in the same settings (Table 2, Fig. 2).
Table 2.
List of DNA and RNA extraction kits used by participating institutions and matched nucleic acids quantification data
| Center ID | DNA extraction kit | DNA quantification Yes/No | DNA ng/μL | RNA extraction kit | RNA quantification Yes/No | RNA ng/μL |
|---|---|---|---|---|---|---|
| 1 | Qiaamp DNA Mini kit (Qiagen) | Yes | 3.2 | MagCore Total RNA FFPE One-Step Kit (Diatech Pharmacogenetics) | Yes | 0.5 |
| 2 | RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE. ThermoFisher Scientific | Yes | 2.1 | RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE. ThermoFisher Scientific | Yes | 7.8 |
| 3 | EasyPGX Extraction Kit | No | N/A | N/A | No RNA extracted | N/A |
| 4 | QIAamp DNA FFPE Tissue Kit | Yes | 2.5 | High Pure FFPET RNA Isolation Kit | Yes | 0.6 |
| 5 | Maxwell RSC DNA FFPE Kit (Promega) | Yes | 1.5 | Maxwell RSC RNA FFPE Kit (Promega) | Yes | 10.0 |
| 6 | Maxwell RSC DNA FFPE kit_Promega | Yes | 1.6 | Maxwell RSC RNA FFPE kit_Promega | Yes | 11.9 |
| 7 | MagCore Genomic DNA FFPE One-Step Kit | Yes | 1.5 | MagCore Total RNA FFPE One-Step Kit | No | N/A |
| 8 | Maxwell CSC DNA FFPE kit (estrazione di acidi nucleici totali) | Yes | 2.0 | Maxwell CSC DNA FFPE kit (estrazione di acidi nucleici totali) | Yes | 2.9 |
| 9 | QIACUBE | No DNA extracted | N/A | QIACUBE | Yes | 8.8 |
| 10 | Promega Maxwell RSC DNA FFPE kit | Yes | 0.9 | Promega Maxwell RSC RNA FFPE kit | Yes | 6.3 |
| 11 | QIAamp DNA FFPE Tissue Kit | Yes | 8.1 | N/A | No RNA extracted | N/A |
| 12 | Maxwell CSC System Promega. kit Maxwell CSC RNA FFPE | Yes | 10.0 | Maxwell CSC System Promega. kit Maxwell CSC RNA FFPE | Yes | 7.6 |
| 13 | MagCore Genomic DNA FFPE One-Step Kit | Yes | 0.4 | N/A | No RNA extracted | N/A |
| 14 | Maxwell RSC DNA FFPE Kit (Promega) | Yes | 9.3 | Maxwell RSC RNA FFPE Kit (Promega) | Yes | 9.0 |
| 15 | Maxwell RSC DNA FFPE Kit (Promega) | Yes | 5.4 | Maxwell RSC RNA FFPE Kit (Promega) | Yes | 0.4 |
| 16 | GeneReadDNA FFPE KIT QIAGEN | Yes | 27.0 | Roche FFPET RNA Isolation Kit | Yes | 12.5 |
| 17 | Qiagen QIAamp DNA FFPE Advanced UNG Kit | Yes | 9.0 | Qiagen RNeasy FFPE kit | Yes | 18.0 |
| 18 | MaGCore Nucleic Acid Extraction Kit | Yes | 0.4 | MaGCore Nucleic Acid Extraction Kit | Yes | 0.2 |
| 19 | QIAamp DNA FFPE Tissue Kit QIAGEN | Yes | 9.7 | RNA: Rneasy FFPE Kit QIAGEN | No | N/A |
| 20 | Mini amp kit FFPE qiagen | Yes | 45.8 | N/A | No RNA extracted | N/A |
| 21 | Maxwell RSC FFPE Plus DNA kit | Yes | 0.1 | N/A | No RNA extracted | N/A |
| 22 | – | – | – | – | – | – |
| 23 | N/D | No | N/A | N/D | No | N/A |
| 24 | MagCore® Genomic DNA FFPE One-Step Kit (Codice cartuccia 405) | Yes | 6.5 |
High Pure FFPET kit RNA Isolation Kit-Roche® |
Yes | 7.8 |
N/A not assessed, N/D not defined, FFPE formalin-fixed paraffin-embedded
Fig. 2.
DNA and RNA concentration by participating center. Scatterplots showing a DNA and b RNA concentration obtained by each participating center, colored by the extraction method (red, automatic procedure; blue, manual procedure)
As shown in Fig. 2, manual procedures yielded higher concentrations than automated procedures. Center ID#20 yielded higher DNA concentrations probably because it used all the available input slides for DNA isolation. Moreover, a comparative analysis between the DNA and RNA yields obtained either by automated or manual procedures suggested once more that manual extraction yields higher DNA concentrations (p = 0.038) than automated systems (Table 3).
Table 3.
DNA concentration according to the extraction procedure (automatic or manual)
| DNA | Automatic (N = 12) | Manual (N = 7) | Total (N = 19) | p value |
|---|---|---|---|---|
| Median | 1.565 | 8.060 | 2.490 | 0.038 |
| Q1, Q3 | 0.739, 5.675 | 2.855, 9.355 | 1.490, 8.530 | |
| Range | 0.050–0.000 | 2.049–27.000 | 0.050–27.000 |
Table shows median, quartile 1, quartile 3, and range of each category. p value was obtained using Mann–Whitney U test
Q1 quartile 1, Q3 quartile 3, N number
However, no statistically significant difference was observed for RNA extraction (Table 4).
Table 4.
RNA concentration according to the extraction procedure (automatic or manual)
| RNA | Automatic (N = 10) | Manual (N = 5) | Total (N = 15) | p value |
|---|---|---|---|---|
| Median | 6.950 | 7.800 | 7.800 | 0.244 |
| Q1, Q3 | 1.080, 8.950 | 7.800, 12.500 | 1.700, 9.500 | |
| Range | 0.200–11.900 | 0.550–18.000 | 0.200–18.000 |
Table shows median, quartile 1, quartile 3, and range of each category. p value was obtained using Mann–Whitney U test
Q1 quartile 1, Q3 quartile 3, N number
Overall, DNA-based biomarker analysis was successfully carried out by 22 out of 23 (95.6%) participating institutions, whereas RNA-based molecular analysis was achieved in 18 out of 19 (94.7%) centers equipped with all the necessary technical support; of note, RNA molecular analysis was successfully carried by ID#13 by using a fully automated platform without previous RNA extraction.
Testing Strategies and Molecular Analysis
For the molecular analyses, participating centers adopted their own routine diagnostic method. For DNA-related biomarker testing, NGS, RT-PCR, and MassArray-based systems were employed in 14 out of 22 (63.6%), 5 out of 22 (22.8%), and 1 out of 22 (4.6%) institutions, respectively. Moreover, DNA-based biomarkers were tested with NGS plus RT-PCR platforms and pyrosequencing plus RT-PCR in two institutions (ID#8 and ID#20, respectively). Among the centers that adopted GS, one institution (ID#6) used two different NGS systems (hybridization and amplicon-based platforms) to validate the molecular results, whereas another institution (ID#5) used a commercially available NGS panel plus a customized NGS assay. Overall, hybridization-based and amplicon-based NGS systems were adopted in 12 out of 14 (85.7%) and 2 out of 14 (14.3%) participating centers, respectively. Among the institutions that implemented RT-PCR-based systems, 3 out of 5 (60.0%) adopted a semiautomated lyophilized system, whereas 2 out of 5 (40.0%) adopted a fully automated system. Concerning RNA-based biomarker testing, 7 out of 18 (38.9%) and 9 out of 18 (50.0%) participating institutions reported using NGS-based and RT-PCR-based solutions, respectively. Interestingly, two centers adopted a hybridization-based NGS system plus a semiautomated RT-PCR platform either to confirm their molecular results (ID#18) or to repeat their molecular analysis after failing with NGS (ID#17) (11.2%). As with DNA-based biomarkers, one participating laboratory employed two independent NGS systems (hybridization and amplicon-based platforms) to carry out RNA-based molecular analysis (ID#6). Overall, hybridization-based (5 out of 7, 71.4%) and amplicon-based strategies (2 out of 7, 28.6%) were assessed in the NGS group. Moreover, 2 out of 9 (22.2%) and 7 out of 9 (77.8%) fully automated and semiautomated automatized lyophilized RT-PCR systems were reported, respectively (Table 5).
Table 5.
List of technical platforms and molecular results for each participating institution
| Center ID | DNA analysis | DNA mutations | MAF% or Ct | RNA analysis | RNA mutations | Reads count or Ct |
|---|---|---|---|---|---|---|
| 1 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
36.3% 72.2% 22.8% |
NGS | ROS1 SLC34A2-ROS1 S4:R32 | 3277 reads |
| 2 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
18.0% 86.5% 5.6% |
RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
31.9 Ct 29.8 Ct |
| 3 | RealTime | EGFR Del Ex19 | 27.5 Ct | N/A | N/A | N/A |
| 4 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
33.3% 84.4% 28.1% |
RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
32.0 Ct 27.6 Ct |
| 5 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
17.8% 92.0% 6.4% |
NGS | ROS1 SLC34A2-ROS1 S4:R32 | 74,380 reads |
| 6 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
15.0% 88.0% 8.0% |
NGS |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
8340 reads 138,166 reads |
| 7 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
50.3% 85.8% 8.1% |
RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
32.5 Ct 27.9 Ct |
| 8 | NGS/RealTime |
KRAS p.G12C EGFR p.E746_A750del |
42.9%/35.2 Ct 72.4%/26.8 Ct |
NGS |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
468 reads 6101 reads |
| 9 | N/A | N/A | N/A | RealTime | Analysis failed | Analysis failed |
| 10 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
14.0% 85.0% 9.0% |
NGS |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
5966 reads 96,702 reads |
| 11 | NGS |
KRAS p.G12C EGFR p.E746_A750del ROS1 SLC34A2-ROS1 |
16.2% 78.5% 21.0% |
N/A | N/A | N/A |
| 12 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
37.0% 73.6% 24.2% |
NGS |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
37 reads 84 reads |
| 13 | RealTime |
KRAS p.G12C EGFR Del Ex19 |
29.1 Ct 25.5 Ct |
RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
30.5 Ct 31.1 Ct |
| 14 | RealTime |
KRAS p.G12C EGFR Del Ex19 |
27.4 Ct 24.9 Ct |
RealTime | ROS1 SLC34A2-ROS1 S4:R32 | 29.7 Ct |
| 15 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
15.7% 84.3% 6.4% |
NGS |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
95 reads 21,823 reads |
| 16 | Mass array |
KRAS p.G12C EGFR p.E746_A750del |
45.0% 61.0% |
RealTime | MET Δ ex14 | 35.0 Ct |
| 17 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
37.6% 73.6% 22.1% |
NGS/RealTime | MET Δ ex14 | 33.3 Ct |
| 18 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
52.0% 86.0% 7.0% |
NGS/RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
30.5 Ct 29.0 Ct |
| 19 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
35.4% 72.0% 24.3% |
RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
33.4 Ct 29.1 Ct |
| 20 | PyroSeq/RealTime |
KRAS p.G12C EGFR Del Ex19 |
20.0% N/D |
N/A | N/A | N/A |
| 21 | RealTime |
KRAS p.G12C EGFR Del Ex19 |
27.8 Ct 25.2 Ct |
N/A | N/A | N/A |
| 22 | – | – | – | – | – | – |
| 23 | RealTime |
KRAS p.G12C EGFR Del Ex19 |
N/D N/D |
RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
27.8 Ct 25.5 Ct |
| 24 | NGS |
KRAS p.G12C EGFR p.E746_A750del MET c.3082 + 1G > T |
14.7% 89.7% 10.3% |
RealTime |
MET Δ ex14 ROS1 SLC34A2-ROS1 S4:R32 |
30.8 Ct 27.7 Ct |
Ct cycle threshold, KRAS Kirsten rat sarcoma virus, MAF minor allele frequency, MET mesenchymal epithelial transition factor, N/A not assessed, N/D not defined, NGS next-generation sequencing, RET rearranged during transfection, ROS1 c-ros oncogene 1
Data Analysis
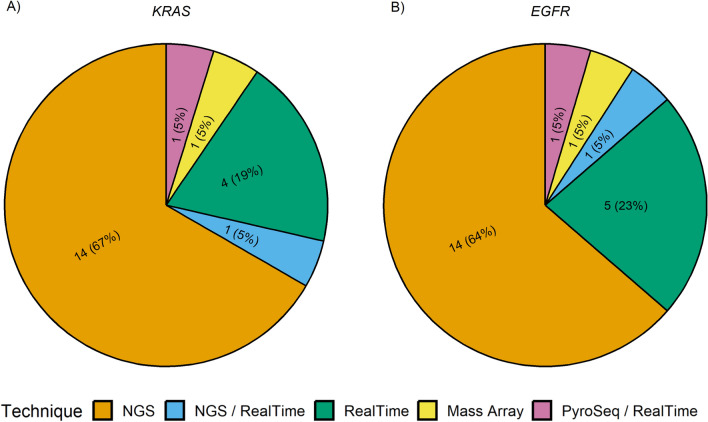
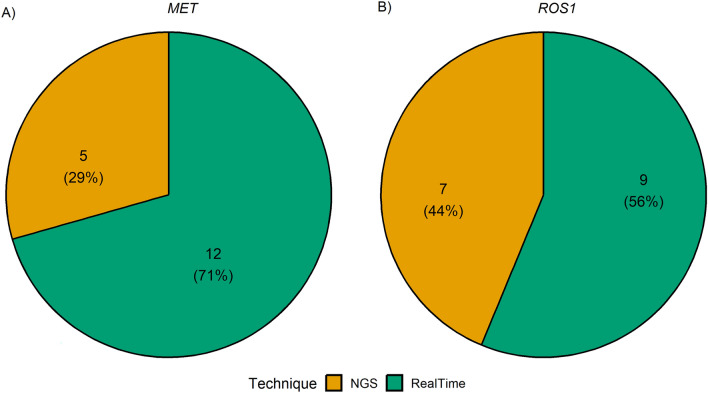
Overall, KRAS exon 2 p.G12C and EGFR exon 19 p.E746_A750del hotspot mutations were successfully detected by all participating institutions. Interestingly, for the detection of KRAS exon 2 p.G12C, 14 out of 21 (66.6%), 4 out of 21 (19.0%), 1 out of 21 (4.8%), 1 out of 21 (4.8%), and 1 out of 21 (4.8%) institutions adopted NGS, RT-PCR, NGS plus RT-PCR, MassArray, and pyrosequencing systems, respectively; instead, for the detection of EGFR exon 19 p.E746_A750del, 14 out of 22 (63.7%), 6 out of 22 (27.3%), 1 out of 22 (4.5%), and 1 out of 22 (4.5%) adopted NGS, RT-PCR, NGS plus RT-PCR, and MassArray systems, respectively (Fig. 3). In this regard, the sequencing platforms yielded median mutant allelic fraction (MAF) values of 29.5% (range 14.0–52.0%) and 80.3% (range 61.0–92.0%) for KRAS p.G12C and EGFR p.E746_A750del mutations, respectively. Similarly, RT-PCR-based technologies yielded a median Ct of 29.9 (range 27.4–35.1) and 26.0 (range 24.9–27.5) for the same genetic alterations. For RNA-related molecular results, 15 out of 16 (93.7%) participating institutions detected MET exon 14 skipping mutation, whereas all institutions detected ROS1 gene fusion using RT-PCR. In addition, 13 out of 18 (72.2%) centers successfully detected both ROS1 rearrangements and MET Δ exon 14 skipping alterations. Overall, 7 out of 16 (43.8%), 8 out of 16 (50.0%), 1 out of 16 (6.2%), 5 out of 15 (33.3%), 8 out of 15 (53.4%), and 2 out of 15 (13.3%) institutions adopted NGS, RT-PCR, and NGS plus RT-PCR systems for the detection of ROS1 aberrant transcript and MET Δ exon 14 skipping molecular alterations (Fig. 4). In addition, in ID#17, RT-PCR was used after NGS failed to detect MET Δ exon 14 skipping molecular alterations. In this regard, NGS platforms generated median readcounts of 48,647.6 (range 84.0–138,166.0) and 2981.2 (range 37.0–8340.0) for ROS-1 rearrangement and MET exon 14 skipping, respectively. Similarly, RT-PCR based systems generated a median Ct of 28.6 (range 25.5–31.1) and 31.8 (range 27.8–35.0) for ROS-1 rearrangement and MET Δ exon 14 skipping alterations, respectively. Moreover, 13 out of 18 (72.2%) institutions also evaluated MET Δ exon 14 skipping molecular alteration on DNA samples. Interestingly, 2 out 13 (15.4%) suggested DNA-based molecular analysis as an alternative to the RNA testing strategy (Fig. 5). Of note, 11 centers successfully detected MET Δ exon 14 skipping molecular alteration using both DNA- and RNA-based detection strategies. In a single case (ID#11), ROS1 rearrangement was successfully detected without previous RNA-based molecular analysis. A list of additional molecular alterations reported by the participating institutions is provided in Supplementary Table 2.
Fig. 3.
Pie charts showing the different techniques used for analysis of DNA a KRAS and b EGFR hotspot mutations. NGS next-generation sequencing, PyroSeq pyrosequencing, KRAS Kirsten rat sarcoma viral oncogene homologue, EGFR epidermal growth factor receptor
Fig. 4.
Pie charts showing the different techniques used for analysis of RNA a ROS1 gene fusions and b MET exon 14 skipping. NGS next-generation sequencing, MET MET proto-oncogene, receptor tyrosine kinase, ROS1 c-ros oncogene 1
Fig. 5.
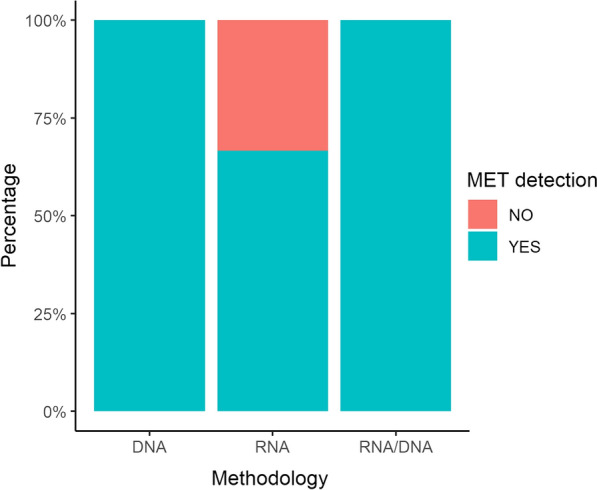
Bar chart showing the percentage of cases in which MET exon 14 skipping was detected using DNA-based technologies, RNA-based technologies, or both. MET MET proto-oncogene, receptor tyrosine kinase
Discussion
Today, biomarker testing plays a pivotal role in guiding therapy choices for patients with solid tumors, including NSCLC [23]. However, two main critical issues hinder the widespread adoption of biomarker testing in routine clinical practice. A primary limitation is that in a substantial number of advanced cases of NSCLC, the diagnostic material is oftentimes insufficient for molecular testing. A secondary but equally limiting issue is the considerable heterogeneity among laboratories in managing the pre-analytical steps. This factor contributes to reducing even further the already scarce availability of diagnostic samples for the identification of DNA and RNA-based biomarkers [25, 26]. In this scenario, harmonization of the analytical procedures is key to achieving timely and accurate analysis of clinically relevant biomarkers starting from very challenging diagnostic specimens [27]. In the present study, we strove to evaluate interlaboratory performance and reproducibility between 24 Italian laboratories by using a standard reference specimen built ad hoc for this study. Overall, we observed no sharp distinction between the molecular testing approaches employed by the various participating institutions. In fact, RNA analysis was feasible in 19 out of 23 institutions, albeit technically equipped for DNA-based molecular testing. However, RNA-based molecular analyses appeared to be quite complex owing to RNA pre-analytical and analytical procedures, suggesting that well-trained personnel and technically equipped laboratories are indispensable to optimize the application of molecular testing in routine clinical practice.
As regards, purification of nucleic acids is another crucial factor to ensure accurate identification of predictive biomarkers [26]. Of note, we observed that a comparable number of institutions adopted manual and automated technical procedures for DNA and RNA purification. Regarding extraction efficiency, our analysis revealed a statistically significant difference between manual and automated extraction procedures for DNA-based biomarkers (p = 0.038) but not for RNA-based biomarkers. These findings highlight that scant and not easily manageable samples from diagnostic routine practice could benefit from manual procedures built on specific technical parameters. Moreover, we observed no statistically significant results for RNA-derived biomarkers owing to the low and non-comparable number of institutions adopting manual and automated procedures in the RNA analysis scenario. Interestingly, DNA purification failed in one institution adopting an automated purification platform. Accordingly, some studies suggest that establishing tailored pre-analytical diagnostic procedures for challenging samples can improve sample adequacy for molecular testing [28].
Given the critical limitations of NSCLC diagnostic specimens, we advance the hypothesis that automated extraction platforms seem less adaptable to scant diagnostic sample. Indeed, our findings evidenced that manual extraction procedures improved DNA/RNA yields from scant diagnostic specimens (13.4 vs 3.3 ng/µL DNA, 9.3 vs 5.7 ng/µL RNA).
Regarding the types of technical platforms employed, NGS and RT-PCR platforms were most commonly used (63.6% vs 22.8% and 56.2–60.0%) for DNA and RNA-derived biomarkers, respectively. In terms of reference range, all RT-PCR based institutions (6 out of 22) named EGFR deletion “delex19” in accordance with RT-PCR proprietary software assignments. To date, identifying and evaluating the different types of EGFR clinically relevant mutations is crucial as clinical responses to EGFR tyrosine kinase inhibitors may vary depending on the type of activating mutation [29].
Another case in point is the recent approval of KRAS exon 2 p.G12C hotspot mutation. Indeed, patients harboring this mutation can now benefit from p.G12 C-targeted therapies [30]. However, because of the ever-increasing number of predictive biomarkers, some technologies may present some limitations owing to the small-sized input material available in most NSCLC cases. For instance, the RT-PCR testing strategy may fail because of the sequential analytical procedures required to cover all the clinically approved biomarkers. For this reason, in some cases, a “selection” of biomarker tests is necessary to circumvent the issue of inadequate amounts of nucleic acids, oftentimes too scarce to cover all the diagnostically relevant genes [24]. In this context, the implementation of NGS platforms has proven useful to overcome these limitations as they can analyze all druggable tumor-specific mutations simultaneously. Remarkably, another major advantage of this high-throughput sequencing technology is that it can successfully analyze molecular biomarkers starting from very low concentrations of nucleic acids, as demonstrated in this study (ID#9). Interestingly, 14 out of 23 participating institutions adopting NGS platforms identified molecular alterations that were not included in this project. Despite such outstanding analytical performance, some cases were not adequately profiled. Specifically, these cases were characterized by limited yields of tumor-derived nucleic acids (ID#11). Thus, an integrated diagnostic approach combining RT-PCR and NGS platforms may represent a robust technical strategy to identify molecular targets in scant diagnostic specimens [10]. Accordingly, orthogonal technologies are required as confirmatory testing approaches for samples characterized by a low percentage of tumor cells [31]. Similarly, integrating biological matrices in diagnostic algorithms may help to overcome clinical limitations (insufficient diagnostic material) and analytical issues (low sensitive molecular testing platforms and critical pre-analytical managing steps) [32]. Regarding MET Δ exon 14 skipping mutations, studies have shown that both DNA- and RNA-based approaches can be used to detect these variants in patients who could benefit from selective MET inhibitors [33, 34]. Of note, in ID#1 and in five other institutions, MET Δ exon 14 skipping was successfully detected in DNA input material. On the other hand, RNA was not sequenced because of the lack of sufficient material; interestingly, the institutions that used both DNA- and RNA-based approaches reported no statistically significant difference in MET detection rates (Fig. 5 and Table 5). Of note, in line with Davies et al.’s results, our findings suggest implementing DNA as input starting material when RNA procedures fail owing to poor-quality RNA. In addition, we found no statistically significant differences in MET detection rates between NGS and RTqPCR. This finding should, however, be further confirmed in larger studies, as it could have derived from the limited sample size.
Conclusion
Although a limitation of this study is the partial representation of clinically approved biomarkers for the management of patients with NSCLC, it does provide a brief but informative overview of the molecular testing strategies implemented in Italy. Notably, our present findings suggest that optimizing diagnostic algorithms by integrating technical platforms for molecular testing may improve the success rates of molecular analysis. Moreover, given that molecular testing procedures may vary across different laboratories, this study also suggests that the implementation of artificial controls in harmonized ring trials could be a valid tool for evaluating the technical efficiency of diagnostic workflows across molecular diagnostic laboratories. Indeed, our engineered cell lines harboring known genomic alterations could be instrumental in validating an ever-growing number of available molecular platforms both on DNA-based and RNA-based biomarkers. Finally, further investigation is warranted to evaluate how pre-analytical and analytical factors, as well as data analysis, can influence the molecular analysis of diagnostic routine samples.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Conceptualization: Francesco Pepe, Gianluca Russo, Giancarlo Troncone and Umberto Malapelle. Methodology: Francesco Pepe, Gianluca Russo, Alessandro Venuta, Claudia Scimone, Mariantonia Nacchio, Pasquale Pisapia, Gaia Goteri, Francesca Barbisan, Caterina Chiappetta, Angelina Pernazza, Domenico Campagna, Marco Giordano, Giuseppe Perrone, Giovanna Sabarese, Annalisa Altimari, Dario de Biase, Giovanni Tallini, Daniele Calistri, Elisa Chiadini, Laura Capelli, Alfredo Santinelli, Anna Elisa Gulini, Elisa Pierpaoli, Manuela Badiali, Stefania Murru, Riccardo Murgia, Elena Guerini Rocco, Konstantinos Venetis, Nicola Fusco, Denise Morotti, Andrea Gianatti, Daniela Furlan, Giulio Rossi, Laura Melocchi, Maria Russo, Caterina De Luca, Lucia Palumbo, Saverio Simonelli, Antonella Maffè, Paola Francia di Celle, Tiziana Venesio, Maria Scatolini, Enrico Grosso, Sara Orecchia, Matteo Fassan, Mariangela Balistreri, Elisabetta Zulato, Daniela Reghellin, Elena Lazzari, Maria Santacatterina, Maria Liliana Piredda, Manuela Riccardi, Licia Laurino, Elena Roz, Domenico Longo, Daniela Petronilla Romeo, Carmine Fazzari, Andrea Moreno-Manuel, Giuseppe Diego Puglia, Andrey D. Prjibelski, Daria Shafranskaya, Luisella Righi, Angela Listì, Domenico Vitale, Antonino Iaccarino, Umberto Malapelle, Giancarlo Troncone. Software: Francesco Pepe, Gianluca Russo and Andrea Moreno Manuel. Validation: All the authors. Formal analysis: All the authors. Data Curation: Francesco Pepe, Gianluca Russo and Umberto Malapelle. Writing—original draft preparation: Francesco Pepe, Gianluca Russo. Writing—review and editing: Giancarlo Troncone and Umberto Malapelle. Visualization: All the authors. Supervision: Giancarlo Troncone and Umberto Malapelle. Project administration: Giancarlo Troncone and Umberto Malapelle. All authors have read and agreed to the published version of the manuscript.
Funding
This study has partly been supported by the following grants: 1. Monitoraggio ambientale, studio ed approfondimento della salute della popolazione residente in aree a rischio—In attuazione della D.G.R. Campanian.180/2019. 2. POR Campania FESR 2014–2020 Progetto “Sviluppo di Approcci Terapeutici Innovativi per patologie Neoplastiche resistenti ai trattamenti—SATIN” 3. The Italian Health Ministry’s research program (ID: NET-2016-02363853). National Center for Gene Therapy and Drugs based on RNA Technology MUR-CN3 CUP E63C22000940007 to DS. No funding or sponsorship was received for the publication of this article.
Data Availability
Data are available on request from the corresponding author. All data relevant to the study are included in the article or uploaded as supplementary information. All data that are publicly available are used in the writing of this article in the text and the reference list.
Declarations
Conflict of Interest
Pasquale Pisapia has received personal fees as speaker bureau from Novartis for work performed outside of the current study. Giuseppe Perrone has received personal fees (as consultant and/or speaker bureau) from Boehringer Ingelheim, Roche, MSD, Amgen, Diaceutics, Merck, AstraZeneca, Novartis, Daiichi Sankyo, Exact Sciences, Diatech Pharmacogenetics unrelated to the current work. Elena Guerini-Rocco has relevant relationship (advisory fees, honoraria, travel accommodation and expenses, grants and non-financial support) with AstraZeneca, Exact Sciences, GlaxoSmithKline (GSK), Novartis, Roche, Thermo Fisher Scientific unrelated to the current work. Nicola Fusco reports honoraria from Merck Sharp and Dohme (MSD), Boehringer Ingelheim, Novartis, AstraZeneca and Daiichi Sankyo unrelated to the current work. Matteo Fassan reports research funding (to the institution) from QED, Macrophage pharma, Astellas, Diaceutics; personal honoraria as invited speaker from Roche, Astellas, AstraZeneca, Incyte, Bristol Myers Squibb (BMS), Merck Serono, Pierre Fabre, GSK, Novartis, Amgen; participation in advisory board for Amgen, Astellas, Roche, Merck Serono, GSK, Novartis, Janssen unrelated to the current work. Umberto Malapelle has received personal fees (as consultant and/or speaker bureau) from Boehringer Ingelheim, Roche, MSD, Amgen, Thermo Fisher Scientific, Eli Lilly, Diaceutics, GSK, Merck and AstraZeneca, Janssen, Diatech, Novartis and Hedera unrelated to the current work. Giancarlo Troncone reports personal fees (as speaker bureau or advisor) from Roche, MSD, Pfizer, Boehringer Ingelheim, Eli Lilly, BMS, GSK, Menarini, AstraZeneca, Amgen and Bayer, unrelated to the current work. Francesco Pepe, Gianluca Russo, Alessandro Venuta, Claudia Scimone, Mariantonia Nacchio, Gaia Goteri, Francesca Barbisan, Caterina Chiappetta, Angelina Pernazza, Domenico Campagna, Marco Giordano, Giovanna Sabarese, Annalisa Altimari, Dario de Biase, Giovanni Tallini, Daniele Calistri, Elisa Chiadini, Laura Capelli, Alfredo Santinelli, Anna Elisa Gulini, Elisa Pierpaoli, Manuela Badiali, Stefania Murru, Riccardo Murgia, Konstantinos Venetis, Denise Morotti, Andrea Gianatti, Daniela Furlan, Giulio Rossi, Laura Melocchi, Maria Russo, Caterina De Luca, Lucia Palumbo, Saverio Simonelli, Antonella Maffè, Paola Francia di Celle, Tiziana Venesio, Maria Scatolini, Enrico Grosso, Sara Orecchia, Mariangela Balistreri, Elisabetta Zulato, Daniela Reghellin, Elena Lazzari, Maria Santacatterina, Maria Liliana Piredda, Manuela Riccardi, Licia Laurino, Elena Roz, Domenico Longo, Daniela Petronilla Romeo, Carmine Fazzari, Andrea Moreno-Manuel, Giuseppe Diego Puglia, Andrey D. Prjibelski, Daria Shafranskaya, Luisella Righi, Angela Listì, Domenico Vitale, Antonino Iaccarino have nothing to disclose.
Ethical Approval
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Biological material was managed under the authorization of the Department of Public Health at the University of Naples Federico II, Naples. The cell lines used in this study were reference specimens and were not obtained from patients at the institutions. As such, patient informed consent was not applicable for this research. Institutional review board approval for this type of study on artificial samples is not required following the specification reported by The Italian Data Protection Authority.
Footnotes
Francesco Pepe and Gianluca Russo contributed equally.
References
- 1.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 2.Mok TS. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol. 2011;8:661–668. doi: 10.1038/nrclinonc.2011.126. [DOI] [PubMed] [Google Scholar]
- 3.Rebuzzi SE, Zullo L, Rossi G, et al. Novel emerging molecular targets in non-small cell lung cancer. Int J Mol Sci. 2021;22:2625. doi: 10.3390/ijms22052625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadik H, Pritchard D, Keeling DM, et al. Impact of clinical practice gaps on the implementation of personalized medicine in advanced non-small-cell lung cancer. JCO Precis Oncol. 2022 doi: 10.1200/PO.22.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Šutić M, Vukić A, Baranašić J, et al. Diagnostic, predictive, and prognostic biomarkers in non-small cell lung cancer (NSCLC) management. J Pers Med. 2021;11:1102. doi: 10.3390/jpm11111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the college of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142:321–346. doi: 10.5858/arpa.2017-0388-CP. [DOI] [PubMed] [Google Scholar]
- 7.Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2021;157:103194. doi: 10.1016/j.critrevonc.2020.103194. [DOI] [PubMed] [Google Scholar]
- 8.Conde E, Rojo F, Gómez J, et al. Molecular diagnosis in non-small-cell lung cancer: expert opinion on ALK and ROS1 testing. J Clin Pathol. 2022;75:145–153. doi: 10.1136/jclinpath-2021-207490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 10.De Maglio G, Pasello G, Dono M, et al. The storm of NGS in NSCLC diagnostic-therapeutic pathway: how to sun the real clinical practice. Crit Rev Oncol Hematol. 2022;169:103561. doi: 10.1016/j.critrevonc.2021.103561. [DOI] [PubMed] [Google Scholar]
- 11.Kerr KM, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161–175. doi: 10.1016/j.lungcan.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Shen CI, Chiang CL, Shiao TH, et al. Real-world evidence of the intrinsic limitations of PCR-based EGFR mutation assay in non-small cell lung cancer. Sci Rep. 2022;12:13566. doi: 10.1038/s41598-022-17394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behnke A, Cayre A, De Maglio G, et al. FACILITATE: a real-world, multicenter, prospective study investigating the utility of a rapid, fully automated real-time PCR assay versus local reference methods for detecting epidermal growth factor receptor variants in NSCLC. Pathol Oncol Res. 2023;29:1610707. doi: 10.3389/pore.2023.1610707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the international association for the study of lung cancer. J Thorac Oncol. 2021;16:1647–1662. doi: 10.1016/j.jtho.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Vigliar E, Malapelle U, Bellevicine C, de Luca C, Troncone G. Outsourcing cytological samples to a referral laboratory for EGFR testing in non–small cell lung cancer: does theory meet practice? Cytopathology. 2015;26:312–317. doi: 10.1111/cyt.12221. [DOI] [PubMed] [Google Scholar]
- 16.Roy-Chowdhuri S, Stewart J. Preanalytic variables in cytology: lessons learned from next-generation sequencing—the MD Anderson experience. Arch Pathol Lab Med. 2016;140:1191–1199. doi: 10.5858/arpa.2016-0117-RA. [DOI] [PubMed] [Google Scholar]
- 17.Lozano MD, Zulueta JJ, Echeveste JI, et al. Assessment of epidermal growth factor receptor and K-ras mutation status in cytological stained smears of non-small cell lung cancer patients: correlation with clinical outcomes. Oncologist. 2011;16:877–885. doi: 10.1634/theoncologist.2010-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisapia P, Pepe F, Baggi A. Next generation diagnostic algorithm in non-small cell lung cancer predictive molecular pathology: the KWAY Italian multicenter cost evaluation study. Crit Rev Oncol Hematol. 2022;169:103525. doi: 10.1016/j.critrevonc.2021.103525. [DOI] [PubMed] [Google Scholar]
- 19.Vigliar E, Iaccarino A, Campione S, et al. PD-L1 expression in cell-blocks of non-small cell lung cancer: the impact of prolonged fixation. Diagn Cytopathol. 2020;48:595–603. doi: 10.1002/dc.24439. [DOI] [PubMed] [Google Scholar]
- 20.Malapelle U, Mayo de-Las-Casas C, Rocco D. Development of a gene panel for next-generation sequencing of clinically relevant mutations in cell-free DNA from cancer patients. Br J Cancer. 2017;116:802–810. doi: 10.1038/bjc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Luca C, Pepe F, Iaccarino A, et al. RNA-based assay for next-generation sequencing of clinically relevant gene fusions in non-small cell lung cancer. Cancers (Basel) 2021;13:139. doi: 10.3390/cancers13010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pepe F, De Luca C, Smeraglio R, et al. Performance analysis of SiRe next-generation sequencing panel in diagnostic setting: focus on NSCLC routine samples. J Clin Pathol. 2019;72:38–45. doi: 10.1136/jclinpath-2018-205386. [DOI] [PubMed] [Google Scholar]
- 23.Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:8. 10.1186/s13073-019-0703-1. [DOI] [PMC free article] [PubMed]
- 24.Van de Ven M, Koffijberg H, Retèl V, et al. Real-world utilization of biomarker testing for patients with advanced non-small cell lung cancer in a tertiary referral center and referring hospitals. J Mol Diagn. 2021;23:484–494. doi: 10.1016/j.jmoldx.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:863–870. doi: 10.1093/annonc/mdy474. [DOI] [PubMed] [Google Scholar]
- 26.Dolinar A, Grubelnik G, Srebotnik-Kirbiš I, Strojan Fležar M, Žlajpah M. Optimization of pre-analytical and analytical steps for DNA and RNA analysis of fresh cytology samples. Cancer Med. 2022;11:4021–4032. doi: 10.1002/cam4.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ascierto PA, Bifulco C, Palmieri G, Peters S, Sidiropoulos N. Preanalytic variables and tissue stewardship for reliable next-generation sequencing (NGS) clinical analysis. J Mol Diagn. 2019;21:756–767. doi: 10.1016/j.jmoldx.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Dagher G, Becker KF, Bonin S, et al. Pre-analytical processes in medical diagnostics: new regulatory requirements and standards. N Biotechnol. 2019;52:121–125. doi: 10.1016/j.nbt.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Gristina V, Malapelle U, Galvano A, et al. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: a systematic review and critical appraisal. Cancer Treat Rev. 2020;85:101994. doi: 10.1016/j.ctrv.2020.101994. [DOI] [PubMed] [Google Scholar]
- 30.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennings LJ, Arcila ME, Corless C, et al. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017;19:341–365. doi: 10.1016/j.jmoldx.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson J, Young S, Leng B, Zreik R, Rao A. Molecular diagnostic testing of cytology specimens: current applications and future considerations. J Am Soc Cytopathol. 2014;3:280–294. doi: 10.1016/j.jasc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Davies KD, Lomboy A, Lawrence CA, et al. DNA-based versus RNA-based detection of MET exon 14 skipping events in lung cancer. J Thorac Oncol. 2019;14:737–741. doi: 10.1016/j.jtho.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the corresponding author. All data relevant to the study are included in the article or uploaded as supplementary information. All data that are publicly available are used in the writing of this article in the text and the reference list.