Abstract
p202 is a primarily nuclear, interferon-inducible murine protein that is encoded by the Ifi 202 gene. Overexpression of p202 in transfected cells retards cell proliferation. p202 modulates the pattern of gene expression by inhibiting the activity of various transcription factors including NF-κB, c-Fos, c-Jun, E2F-1, and p53. Here we report that p202 was constitutively expressed in mouse skeletal muscle and that the levels of 202 RNA and p202 greatly increased during the differentiation of cultured C2C12 myoblasts to myotubes. When overexpressed in transfected myoblasts, p202 inhibited the expression of one muscle protein (MyoD) without affecting the expression of a second one (myogenin). Thus, the decrease in the level of MyoD (but not of myogenin) during muscle differentiation may be the consequence of the increase in p202 level. Overexpressed p202 also inhibited the transcriptional activity of both MyoD and myogenin. This inhibition was correlated with an interaction of p202 with both proteins, as well as the inhibition by p202 of the sequence-specific binding of both proteins to DNA. This inhibition of the expression of MyoD and of the transcriptional activity of MyoD and myogenin may account for the inhibition of the induction of myoblast differentiation by premature overexpression of p202.
p202 is a 52-kDa interferon-inducible, murine protein (19, 21). Upon induction by interferon, p202 accumulates in the cytoplasm, and after a delay the bulk of it appears in the nucleus. In metaphase cells, it is primarily chromatin associated. p202 is a member of the “200 family” of murine proteins (46). These share homologous 200-amino-acid segments which are adjacent to the C termini. The proteins are encoded by the six or more interferon-activatable genes of the “gene 200 cluster” that is located in the q21–q23 domain of murine chromosome 1 (55). Three human homologs of these murine proteins have also been described (11, 24, 25).
Overexpression of p202 in transfected cells retards cell proliferation (19, 20, 49). Studying the mechanism of this effect, we established that p202 modulates transcription (18, 49). It inhibits the activities of various transcription factors including NF-κB p50 and p65, AP-1 c-Fos and c-Jun, E2F-1, E2F-4, and p53 (16, 18, 22, 49). In c-Fos, c-Jun, E2F-1, and E2F-4, p202 impairs the sequence-specific DNA binding. This impairment is apparently a consequence, at least in part, of a direct interaction of p202 with the DNA binding segments of these transcription factors (49). p202 also binds the murine homolog of the human p53 binding protein 1 (p53PB1) (22). The binding to p202 of a segment from this murine homolog overcomes the p202-mediated inhibition of the transcriptional activities of p53 and of the AP-1 transcription factors (22). The pocket region of the hypophosphorylated form of the retinoblastoma growth suppressor protein is also bound by p202 (20). The functional consequences of this binding have not been explored. p202 can also increase the expression of particular genes. This was first noted in the case of a reporter gene driven by the 5′-flanking segment of the human metallothionein IIA gene (49).
The study reported here was started by examining the tissue distribution of 202 RNA in adult mice by Northern blotting. This revealed that skeletal muscle is among the tissues in which the constitutive level of 202 RNA is pronounced. Subsequent experiments indicated that the differentiation of the muscle precursor myoblasts from the murine C2C12 cultured thigh muscle cell line (73) to myotubes was accompanied by a delayed, severalfold increase in the level of 202 mRNA and protein.
The expression of p202 in skeletal muscle, together with the increase in its level during myotube formation, prompted us to examine whether p202 affects the expression of two muscle-specific mRNAs and proteins. The two proteins selected, MyoD (23) and myogenin (27, 71), are members of the myogenic basic helix-loop-helix (bHLH) protein family that also includes the Myf5 (10) and MRF4 (50, 60) proteins. Members of this family are transcription factors designated muscle regulatory factors (MRFs) (for recent reviews, see references 12, 44, 47, 51, 54, 58, 61, and 75). The MRFs can form heterodimers with various bHLH E proteins (43). The heterodimers serve as skeletal muscle-specific transcription factors that bind to DNA sites called E boxes (consensus sequence CANNTG), which are functionally important elements in transcriptional enhancers of muscle differentiation genes (e.g., the muscle creatine kinase gene) (13, 40, 43). When any member of the MRFs is ectopically expressed in some nonmyogenic cell types (e.g., 10T1/2 fibroblasts), expression of various muscle differentiation genes and in some instances fusion of the cells to myotubes result (2, 15, 23, 63). In general MyoD or Myf5 is expressed in proliferating myoblasts whereas myogenin and MRF4 are not expressed until the myoblasts exit the cell cycle (e.g., in response to mitogen depletion) (47, 54, 61). There are a large number of positive and negative regulators of skeletal muscle differentiation. Some of the negative regulators, e.g., Id proteins (1, 7, 39, 48) and I-mf protein (14), form heterodimers with MRF proteins; these do not bind DNA. Differentiation of skeletal muscle entails transcriptional activation of muscle-specific genes coupled with irreversible cell cycle withdrawal (44, 53, 61).
We established that overexpression of p202 in stably transfected C2C12 myoblasts inhibited the expression of MyoD (without affecting that of myogenin). The level of MyoD (but not of myogenin) was reported to decrease during the fusion of myoblasts to myotubes. This decrease in the MyoD level during muscle differentiation can thus be correlated with a pronounced increase in the p202 level. Overexpressed p202 also inhibited the transcriptional activity of both MyoD and myogenin. This inhibition was correlated with an interaction of p202 with both proteins, as well as with the inhibition by p202 of the sequence-specific binding of both proteins to DNA. Overexpression of p202 prior to the induction of muscle differentiation inhibited differentiation. This inhibition may be, at least in part, the consequence of the inhibition by p202 of the expression of MyoD and of the transcription factor activity of MyoD and myogenin.
MATERIALS AND METHODS
Antiserum, interferon, other reagents and cell lines.
The preparation of an antiserum to p202 was reported previously (17). The recombinant human alpha-2/alpha-1 interferon, 1-83, that is highly active in murine cells was a gift from H. Weber and C. Weissmann (68). Cell culture media, fetal bovine and horse sera, and insulin were from Gibco Life Science Technology. AKR-2B cloned murine embryo cells (33), C2C12 (ATCC-1172-CRL) murine thigh muscle myoblasts (73), and C3H 10T1/2 (ATCC-226-CCL) cloned murine embryo fibroblasts, designated 10T1/2 (59), were used.
Plasmids.
In the 4RCAT plasmid (70), chloramphenicol acetyltransferase (CAT) expression is driven by four MyoD binding (R) sites from the muscle-specific creatine kinase gene. In the MRF4(wt)CAT plasmid (9), CAT expression is driven by a 390-bp upstream segment from the mouse MRF4 gene. The MRF4(mut)CAT plasmid (9) is identical to the MRF4(wt)CAT plasmid, except that in the MRF4(mut)CAT plasmid one E box has been deleted and a second E box has been mutated (9). In the pSVβgal control plasmid (Promega), which served as the internal standard for transfection, the expression of the β-galactosidase gene is driven by the simian virus 40 enhancer and promoter. In the MyoD expression plasmid pCMV-MyoD (36), the expression of the murine MyoD gene is driven by a cytomegalovirus enhancer. In the mouse myogenin expression plasmid pEMSVscribe-myogenin (9), mouse myogenin cDNA was inserted into the pEMSVscribe expression vector.
Cell growth and differentiation, interferon treatment, labeling of cells, cell lysis, and fractionation of the lysate.
Mouse C2C12 myoblasts and mouse 10T1/2 fibroblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum (growth medium), under 10% CO2–90% air at 37°C (36, 64). If so indicated, after reaching confluency, the cultures were shifted to DMEM supplemented with 2% horse serum and 12 μg of insulin per ml (differentiation medium). Mouse AKR-2B cells were grown in DMEM supplemented with 10% fetal bovine serum under 5% CO2–95% air at 37°C. All the media were changed every 24 h unless otherwise specified. If so indicated, AKR-2B and C2C12 cultures at 50 to 60% confluency were treated with 1,000 U of interferon per ml for the times indicated. The cells were labeled with [35S]methionine as described previously (19). Whole-cell lysates were prepared by lysing cells in RIPA buffer supplemented with a set of protease inhibitors: 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 1 mM sodium benzamidine, and 1 mM phenylmethylsulfonyl fluoride (3). To generate cell lysates for fractionation, the cells were lysed in buffer A (50 mM HEPES-NaOH [pH 7.6], 150 mM NaCl, 5 mM NaF, 1 mM Na3VO4, 0.5% Nonidet P-40) supplemented with the above set of protease inhibitors (3). The lysate was divided into nuclear and cytoplasmic fractions by centrifugation at 2,000 × g for 5 min (19).
Generation of stable cell lines.
Cells from the C2C12 and 10T1/2 lines were transfected with the type of plasmid indicated by using the calcium phosphate procedure followed by a glycerol shock (62). At 48 h after transfection, the cells were dissociated with trypsin and cultured in selective medium containing 1.2 mg of G418 per ml, the medium being changed every 5 days during the first 2 weeks and every 2 days thereafter, until individual colonies were picked or pools of at least 100 colonies were harvested. The cells were maintained in G418, and 3 days before the indicated experiments they were shifted to the appropriate medium without G418. The plasmids used encoded murine MyoD (plasmid pCMV-MyoD), murine myogenin (plasmid pEMSVscribe-myogenin), or p202 (plasmid pCMV-202).
Transient-transfection assays.
10T1/2 cells grown to confluency in growth medium in 100-mm dishes were transfected with the plasmids indicated by using the Lipofectamine procedure (Gibco/BRL). At 48 h after transfection, the cultures were confluent. At this time, the cultures were washed with phosphate-buffered saline, harvested, and lysed with 200 μl of 0.25 M Tris-Cl (pH 7.6) by four cycles of freezing in dry ice and thawing at 37°C followed by vortexing. After centrifugation in the microcentrifuge, the supernatant fractions of the lysates were stored at −70°C. β-Galactosidase and CAT activities were measured in 20-μl aliquots from the lysates, using the kits from Promega. Aliquots corresponding to equal amounts of internal standard reporter activity were assayed for test reporter activity.
Northern blotting, dot blotting, and Western blotting.
For Northern blotting and dot blotting, total cytoplasmic RNA was isolated by the guanidine HCl procedure (62). RNA aliquots (20 μg) were analyzed by electrophoresis on 1.5% agarose gels by the formaldehyde procedure (62), transferred to Hybond N membranes (Amersham), and hybridized with 32P-labeled probes (with the kit from Boehringer Mannheim). For dot blotting, 25-μg aliquots of total cytoplasmic RNA were spotted on Hybond N membranes and hybridized as described for Northern blotting. For Western blotting and immunoprecipitation, the cell cultures were lysed in buffer A supplemented, unless otherwise specified, with the indicated set of protease inhibitors (3). The protein concentration was determined by the Bio-Rad assay. Appropriate aliquots were used for Western blotting (i.e., sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting, with the indicated antisera and the enhanced chemiluminescence system [Amersham]).
GST-protein binding assay and coimmunoprecipitation.
The glutathione S-transferase (GST)–protein binding assays were performed as described by Datta et al. (22). GST-MyoD and GST-E47 were purchased from Santa Cruz Biotechnology. Coimmunoprecipitations from cell extracts were performed as described by Datta et al. (22), except that rabbit polyclonal p202 antiserum (49) was used for immunoprecipitation and immunoaffinity-purified rabbit polyclonal antibodies to MyoD (C20 from Santa Cruz Biotechnology) were used for immunoblotting.
Electrophoretic mobility shift assays with purified GST fusion proteins.
Electrophoretic mobility shift assays were performed with the MEF-1 consensus oligodeoxynucleotide and the mutated MEF-1 oligonucleotide (Santa Cruz Biotechnology) by the procedures of Lassar et al. (43), except that the identities and amounts of GST and GST fusion proteins were as indicated and the electrophoresis was performed at 140 V at room temperature for 3 to 4 h.
RESULTS
Tissue distribution of 202-specific RNA.
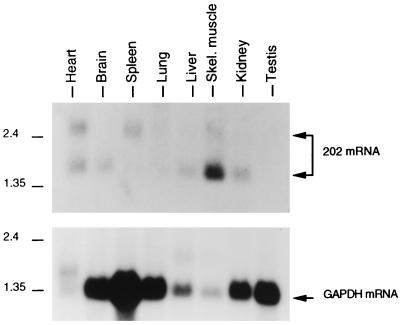
We examined the tissue distribution of 202 mRNA in adult BALB/c mice by hybridizing a blot of poly(A)+ RNA samples from various tissues with a labeled 202 cDNA probe (Fig. 1). 202 mRNA was constitutively expressed in at least six tissues: heart, brain, spleen, liver, skeletal muscle, and kidney. The detection of 202 RNA in the spleen is consistent with earlier findings (32).
FIG. 1.
Tissue distribution of 202 mRNA in adult mice. A multiple-tissue Northern (MTN) blot (from Clontech) with 2 μg of poly(A)+ RNA samples from various tissues of adult BALB/c mice was probed with labeled p202 cDNA. The probe from the blot was stripped off, and the blot was rehybridized with labeled glyceraldehyde phosphate dehydrogenase (GAPDH) cDNA. The positions of size markers (in kilobases) are shown. The positions of the 202 mRNA and GAPDH mRNA bands are indicated. It should be noted that the major 202 RNA band from cultured control or interferon-treated fibroblasts is 1.5 kb. It remains to be established whether the 2.5-kb 202 mRNA present in some tissues is due to readthrough of transcription, differential splicing, or other causes. The pronounced variation in GAPDH mRNA levels in the different lanes may be because the mRNA samples were from different tissues. For further details, see Materials and Methods.
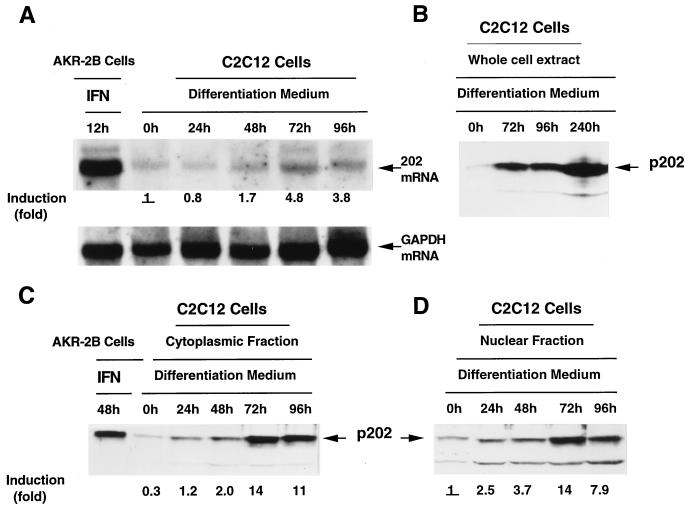
Increase in the 202 mRNA and p202 levels during the differentiation of C2C12 myoblasts to myotubes.
To examine whether the expression of the 202 gene changes in the course of skeletal muscle differentiation, we prepared RNA samples from cells of the murine thigh muscle line, C2C12 (73), proliferating in growth medium in the form of myoblasts, and also from cells of the same line that were shifted to differentiation medium, stopped proliferating, and were undergoing fusion to multinucleated myotubes. The Northern blot in Fig. 2A reveals that the density of the 202 RNA-specific band (of the same mobility as that of 202 RNA induced by interferon in AKR-2B cells) had increased by 48 h after being shifted to differentiation medium, peaked at about 72 h (the time by which the conversion to myotubes was complete) at a 4.8-fold-higher level than that in myoblasts, and slightly decreased by 96 h. An examination of p202 levels during myotube formation by immunoblotting revealed corresponding changes: the density of the p202 specific band (of the same mobility as that induced by interferon in AKR-2B cells) started to increase after the C2C12 cultures were shifted to differentiation medium, peaked at 72 h, and decreased slightly by 96 h. This was the case for p202 in the whole-cell extract (Fig. 2B), the cytoplasmic fraction (Fig. 2C), and the nuclear fraction (Fig. 2D), in which the peak level of p202 was 14-fold higher than that in myoblasts. The level of p202 in the whole-cell extract remained high, even increased, in myotubes that were kept in culture for 10 days (Fig. 2B). In C2C12 myoblasts in growth medium (Fig. 2C and D, indicated as 0 h), the level of p202 was approximately 3 times higher in the nuclear fraction than in the cytoplasmic fraction, whereas during differentiation to myotubes, the level of p202 rose to similar, high levels in the two fractions. Immunocytochemical localization in C2C12 cells revealed that the concentration of p202 was higher in the nuclei than in the cytoplasm (data not shown), suggesting some leakage of p202 from the nuclei during the preparation and fractionation of the cell extract.
FIG. 2.
Increase in the 202 mRNA and protein levels during the differentiation of C2C12 myoblasts into myotubes. (A) Increase in the 202 mRNA level. C2C12 cells were grown in growth medium and, upon reaching confluency, were shifted to differentiation medium (0 h). After incubation for the times indicated, cytoplasmic RNA was isolated and 20-μg samples were assayed for 202 mRNA by Northern blotting with a labeled p202 cDNA probe. The blot was then stripped and reassayed with a labeled GAPDH cDNA probe. 202 mRNA (serving as a size marker) was induced in AKR-2B cells by treatment with interferon. The 202 mRNA and GAPDH mRNA bands are indicated. The bands were scanned, the 202 mRNA levels were standardized in terms of GAPDH mRNA, and the extent of 202 mRNA induction is indicated. (The level of 202 mRNA at 0 h, indicated as 1, was taken as the basis of the comparison.) (B) Increase in the p202 level. C2C12 cells were grown and shifted to differentiation medium as in panel A. After incubation for the times indicated, the cells were lysed, and 40-μg protein samples were assayed for p202 by Western blotting with p202 antiserum. The p202 band is indicated. (C and D) Increase in the p202 level in the cytoplasmic and nuclear fractions. C2C12 cells were grown and shifted to differentiation medium as in panel A. At the times indicated, the cells were lysed, and the lysate was divided into cytoplasmic and nuclear fractions. Protein samples (40 μg) from the cytoplasmic fraction (C) and the nuclear fraction (D) were assayed for p202 by Western blotting with p202 antiserum. p202 (serving as a size marker) was induced in AKR-2B cells by treatment with interferon. The bands were scanned, and the extent of p202 induction is indicated. (The level of p202 in the nuclear fraction at 0 h, indicated as 1, was taken as the basis for comparison for both the nuclear and the cytoplasmic fractions.) The p202 bands are indicated. For further details, see Materials and Methods.
It is noteworthy that during differentiation the level of p202 increased much more (14-fold in the nuclear fraction [Fig. 2D] and even more in the cytoplasmic fraction) than the level of 202 mRNA (4.8-fold) (Fig. 2A). This indicates that the increase in the level of p202 during differentiation may be a consequence, in part, of an enhancement in the yield of posttranscriptional processes (e.g., increased efficiency of translation and/or diminished protein turnover).
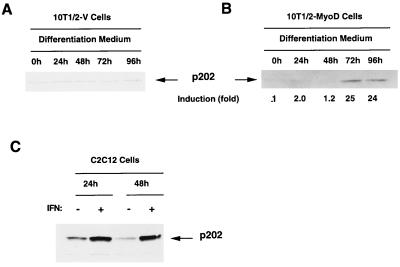
Only after transfection of a MyoD expression plasmid does incubation of 10T1/2 fibroblasts in differentiation medium induce myotube formation and p202 accumulation; p202 also accumulates in C2C12 myoblasts treated with alpha/beta interferon.
Myotube formation in C2C12 cultures, which was accompanied by an increase in 202 RNA and p202 levels, was elicited by shifting the cultures from growth medium to differentiation medium. We wanted to establish whether such a shift in medium suffices to induce the enhanced expression of the 202 gene or if there is a need for differentiation. For this purpose, we compared the effect on the p202 level of shifting from growth medium to differentiation medium for 10T1/2 fibroblasts, which do not form myotubes under such conditions (10T1/2-V), and 10T1/2 fibroblasts, which carry a transfected MyoD expression plasmid (10T1/2-MyoD) and do form myotubes (23, 69). The experiments in Fig. 3A and B reveal that only for the 10T1/2-MyoD culture did the shift to differentiation medium result in an increase in the p202 level (25-fold at the peak) as well as myotube formation (data not shown). Under the same conditions, there was no change in the p202 level (or the morphology) in the 10T1/2-V culture. We also established that interferon, which is known to induce p202 in various murine cell lines, including AKR-2B fibroblasts (19), also induced p202 in C2C12 cells (Fig. 3C).
FIG. 3.
Only after transfection with a MyoD expression plasmid does incubation of 10T1/2 fibroblasts in differentiation medium induce myotube formation and p202 accumulation. p202 also accumulates in C2C12 myoblasts in response to interferon. (A and B) 10T1/2 cells which had been stably transfected with the pCMV vector (10T1/2-V cells) (A) or the pCMV-MyoD expression plasmid (10T1/2-MyoD cells) (B) were grown in growth medium to confluency and shifted to differentiation medium (0 h). After incubation for the times indicated, the cells were lysed, and 40-μg protein samples were assayed for p202 by Western blotting with p202 antiserum. The extent of p202 induction (B) is indicated. (The level of p202 at 0 h indicated as 1 was taken as the basis for comparison.) (C) Induction of p202 by interferon. Cultures of C2C12 cells in growth medium at 50 to 60% confluency were treated with 1,000 U of interferon (IFN) per ml for the times indicated. p202 was assayed in 40-μg protein samples by Western blotting with p202 antiserum. The p202 band is indicated. For further details, see Materials and Methods.
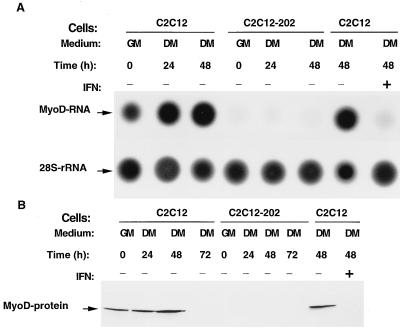
p202 inhibits the expression of endogenous MyoD RNA and protein without affecting the expression of endogenous myogenin RNA.
In accord with earlier results (26, 31, 34, 42, 66, 72), we observed that (after a transient increase) the endogenous MyoD RNA and protein levels diminished below detectability by 72 h after the C2C12 culture was shifted from growth medium to differentiation medium (Fig. 4B and data not shown). The finding that the level of p202 greatly increased during muscle differentiation (Fig. 2) and our previous observation that an increase in the level of p202 strongly impaired the activities of various enhancers and transcription factors (18, 22, 49) prompted us to examine whether this decrease in the endogenous MyoD RNA and protein levels during muscle differentiation might be a consequence of the increase in the p202 level. For this study, we generated stable cell lines constitutively overexpressing 202 RNA. In cells from the stable C2C12 myoblast line carrying a 202 RNA expression plasmid (cell line C2C12-202), the level of p202 was approximately 3.5-fold higher than in the parental cell line (data not shown). In this C2C12-202 line MyoD RNA (Fig. 4A) and MyoD protein (Fig. 4B) were below the detectable level in growth medium and remained below this level after the shift to differentiation medium for at least 48 h for MyoD RNA and for at least 72 h for MyoD protein (Fig. 4). Moreover, the treatment of C2C12 cultures with interferon (1,000 U per ml for 48 h), which is known to increase the level of p202 10-fold (Fig. 3C), also resulted in the disappearance of detectable MyoD RNA and protein (Fig. 4, lanes IFN+).
FIG. 4.
Overexpression of p202 in stable C2C12 lines, or treatment of C2C12 cells with interferon, decreases the level of endogenous MyoD RNA and MyoD protein. Dot blot and Western blot analyses are shown. Pairs of dishes of cells from pools of at least 100 clones of stable C2C12 lines carrying an expression vector (C2C12) and cells from pools of at least 100 clones of stable C2C12 lines overexpressing p202 (C2C12-202), as indicated, were cultured in growth medium (GM). When reaching confluency (GM, 0) some of the dishes were further incubated in differentiation medium (DM) for 24, 48, or 72 h as indicated. If so specified (IFN+), when being shifted to differentiation medium the cultures were also supplemented with 1,000 U of interferon per ml. (A) At the times indicated, total cytoplasmic RNA was extracted from one set of culture dishes, and 25-μg RNA samples from each dish were analyzed by dot blotting and probing with a labeled MyoD cDNA probe. The filters were stripped and rehybridized with labeled 28S rDNA. The MyoD RNA and 28S rDNA dots are indicated. (B) At the times indicated, total protein was extracted from a second set of culture dishes and the levels of MyoD protein were assayed in 40-μg protein samples by Western blotting with anti-MyoD antibodies. The MyoD protein band is indicated. For further details, see Materials and Methods.
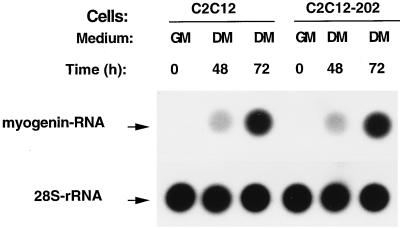
We also examined the effect of the overexpression of p202 on the endogenous myogenin RNA level. Cultures of the control C2C12 line and the C2C12-202 line (stably overexpressing p202) grown to confluency in growth medium did not contain detectable amounts of myogenin RNA (Fig. 5). Shifting the two cultures to differentiation medium resulted, however, in a similar moderate increase in the myogenin RNA level by 48 h and a similar strong increase by 72 h. These findings indicate that an increase in the p202 level in C2C12 myoblasts results in the inhibition of the expression of MyoD RNA and protein without affecting the expression of myogenin RNA.
FIG. 5.
Overexpression of p202 in stable C2C12 lines does not affect the increase in the level of endogenous myogenin RNA during incubation in differentiation medium. A dot blot analysis is shown. Pairs of dishes of cells from pools of at least 100 clones of stable C2C12 lines carrying an expression vector (C2C12) and cells from pools of at least 100 clones of stable C2C12 lines overexpressing p202 (C2C12-202), as indicated, were cultured in growth medium (GM). When reaching confluency (GM, 0) some of the dishes were further incubated in differentiation medium (DM) for 48 or 72 h as indicated. At the time specified, total cytoplasmic RNA was extracted and 25-μg RNA samples from each dish were analyzed by dot blotting and probing with the labeled segment of myogenin DNA. The filters were stripped and rehybridized with 28S rDNA. The myogenin and 28S rRNA dots are indicated. For further details, see Materials and Methods.
p202 inhibits the transcriptional activity of both MyoD and myogenin.
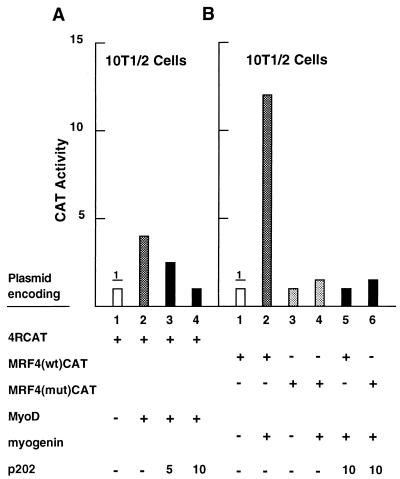
To establish whether p202 affects not only the expression but also the transcriptional activity of the MyoD transcription factor, we measured the effect of transfected p202 on the activity of the 4RCAT reporter plasmid, in which CAT expression is driven by four MyoD binding (R) sites (70). The activity of this reporter in 10T1/2 murine fibroblasts was low; it was increased three- to fourfold upon transfection with a MyoD expression plasmid (Fig. 6A; compare lanes 1 and 2). Cotransfection of a p202 expression plasmid with the MyoD expression plasmid inhibited the MyoD activity in a concentration-dependent manner (compare lanes 3 and 4 with lane 2). Exposure to interferon for 24 or 48 h, which had been shown to result in an increase in the p202 level, also strongly inhibited the MyoD transcription factor activity in 10T1/2 cells stably transfected with the MyoD expression plasmid (data not shown).
FIG. 6.
Overexpression of p202 inhibits the transcriptional activities of MyoD and of myogenin in transient-transfection assays. (A) Cultures of 10T1/2 cells were transfected with the 4RCAT reporter plasmid (2.5 μg) in which CAT expression is driven by four MyoD binding (R) sites from the muscle-specific creatine kinase gene (lanes 1 to 4), together with 10 μg of the MyoD expression plasmid (pCMV-MyoD) (lanes 2 to 4) and the p202 expression plasmid (pCMV-202) in the amounts indicated in micrograms (lanes 3 and 4). In each case, a pSVgal internal control plasmid (3.6 μg) was cotransfected and the expression vector (pCMV) was used to bring the total amount of DNA transfected to 30 μg. At 48 h after starting the transfection, the reporter activities were assayed in the cell lysates after normalization with the internal standard. Normalized CAT activity in the experiment in lane 1 was defined as 1. (B) Cultures of 10T1/2 cells were transfected with the MRF4(wt)CAT reporter plasmid (5 μg) (lanes 1, 2, and 5), in which CAT expression is driven by a 390-bp segment from the MRF4 gene, or the MRF4(mut)CAT reporter plasmid (5 μg) (lanes 3, 4, and 6), which differs from MRF4(wt)CAT by deletion of one E box and mutation of a second one. If so indicated, the myogenin expression plasmid (pEMSVscribe-myogenin) (10 μg) (lanes 2, 4, 5, and 6) and the p202 expression plasmid (pCMV-202) (10 μg) (lanes 5 and 6) were also transfected. In each case, pSVgal (internal control plasmid) (3.6 μg) was cotransfected. Salmon sperm DNA was used to bring the total amount of DNA transfected to 30 μg. At 48 h after starting the transfection, cell lysates were prepared and assayed for CAT activity as described for panel A. For further details, see Materials and Methods.
To test whether p202 affects the transcriptional activity of the myogenin transcription factor, we examined the effect of transfected p202 on the activity of the MRF(wt)CAT reporter plasmid. CAT expression is driven in this plasmid by a 390-bp upstream segment from the mouse MRF4 gene that was shown to be transactivated by myogenin (9). The activity of this reporter plasmid in 10T1/2 murine fibroblasts was very low; it was increased approximately 12-fold upon transfection with a myogenin expression plasmid (Fig. 6B, compare lanes 1 and 2). Cotransfection of a p202 expression plasmid with the myogenin expression plasmid inhibited the myogenin transcription factor activity approximately 12-fold (compare lanes 2 and 5). As a control plasmid, we used MRF4(mut)CAT (9), in which one E box has been deleted and a second E box has been mutated. The low activity of this reporter in 10T1/2 cells was only slightly (twofold) enhanced by a transfected myogenin expression plasmid (compare lanes 3 and 4), and the enhanced activity was not affected by p202 (compare lanes 4 and 6). These results indicate that p202 can inhibit the transcriptional activities of both MyoD and myogenin.
Premature overexpression of p202 in C2C12 myoblasts inhibits their differentiation to myotubes.
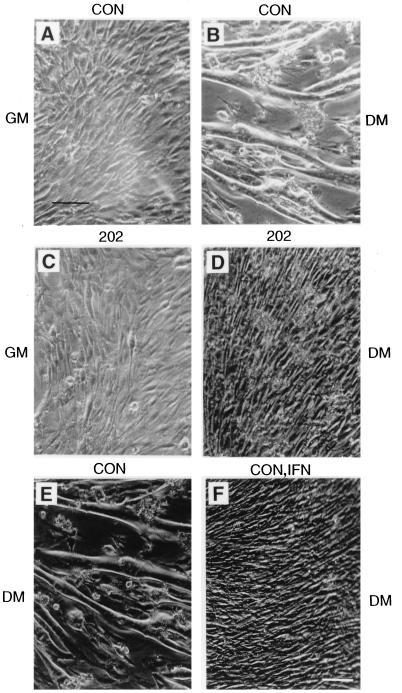
MyoD and myogenin are among the proteins involved in the differentiation of myoblasts to myotubes (12, 44, 47, 51, 54, 61, 75). The inhibition of MyoD expression and transcriptional activity, as well as myogenin transcriptional activity, by p202 prompted us to test whether C2C12 myoblasts constitutively overexpressing p202 can be induced to differentiate to myotubes. The photographs (Fig. 7) illustrate that, as expected, shifting of a confluent culture of C2C12 (control) myoblasts from growth medium (Fig. 7A) to differentiation medium (Fig. 7B) resulted in the fusion of the myoblasts to myotubes. However, a confluent culture of C2C12 myoblasts stably overexpressing p202 in growth medium (Fig. 7C) did not form myotubes after being shifted to differentiation medium (Fig. 7D). These results indicate that overexpression of p202 in myoblasts prior to differentiation inhibits differentiation. It is in accord with this finding that shifting to differentiation medium failed to induce myotube formation in C2C12 cultures which had been treated with interferon (1,000 U per ml for 48 h in growth medium), a treatment known to increase the p202 level at least 10-fold (compare Fig. 7E and F).
FIG. 7.
Constitutive overexpression of p202, or treatment with interferon, inhibits the differentiation of C2C12 myoblasts into myotubes. Pooled stable C2C12 cell lines carrying a pCMV expression vector (serving as control [CON]) (A and B) or pCMV-202, a 202 RNA expression plasmid (202) (C and D), were cultured in growth medium (GM) to confluency (A and C) and were shifted to differentiation medium (DM) for 72 h (B and D). Two more dishes of C2C12 cells were cultured in growth medium to 30 to 40% confluency. Thereafter, one of the dishes was supplemented with 1,000 U of interferon per ml (CON, IFN) (F), and the other dish served as control (CON) (E); both dishes were incubated for 48 h in growth medium and then shifted to differentiation medium for 72 h. The cultures were examined under the microscope at a 400-fold magnification (372-fold magnificaion shown in figure). Bar, 200 μm. For further details, see Materials and Methods.
Interferon treatment of chicken myoblast cultures was reported to inhibit cell fusion and myoblast formation (65). However, interferon treatment of myoblast cultures from human mature skeletal muscle in enriched medium was found to accelerate myotube formation (29). The basis of this apparent discrepancy in the effect of interferon on muscle differentiation is not clear.
p202 binds MyoD in vitro and in vivo.
As mentioned above, inhibition of the activity of particular transcription factors by p202 was correlated in some cases with a direct interaction between the transcription factors and p202 (18, 49). This consideration, together with the inhibition of the transcription factor activity of MyoD and myogenin by p202, prompted us to test for an interaction between these muscle proteins and p202.
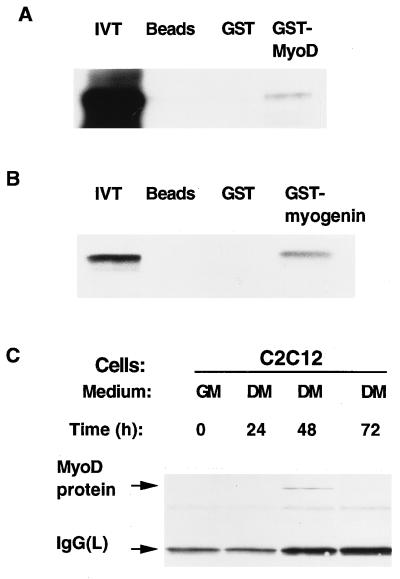
To test whether p202 can bind MyoD in vitro, we used a GST-MyoD fusion protein. When immobilized on glutathione beads, GST-MyoD retained labeled p202, whereas neither unloaded beads nor the beads loaded with GST retained it (Fig. 8A). This indicated an interaction between MyoD and p202 in vitro. To test for an interaction between p202 and MyoD in vivo we lysed C2C12 myoblast cultures, immunoprecipitated the lysates with immunopurified anti-p202 antiserum, and examined the washed immunoprecipitate for the presence of MyoD by Western blotting with anti-MyoD antibodies. We detected coimmunoprecipitation of MyoD protein with p202 in a lysate from a C2C12 myoblast culture which had been incubated in differentiation medium for 48 h (Fig. 8C). This indicated an interaction between MyoD and p202 in vivo. The lysates from C2C12 cells in growth medium or differentiation medium for 24 or 72 h revealed no coprecipitation. The lack of coprecipitation under these conditions is likely to be because in growth medium and also in differentiation medium for 24 h the level of p202 was too low (Fig. 2C and D) and by 72 h in differentiation medium the level of MyoD protein diminished to a level that was too low (Fig. 4B). An interaction between p202 and MyoD in vivo was also revealed by the results of a yeast two-hybrid assay. For this assay (28, 38, 67), we introduced constructs encoding (i) p202 linked to a DNA binding segment and (ii) MyoD linked to a transactivator segment into a yeast culture carrying a β-galactosidase reporter construct (38). An interaction between p202 and MyoD in the yeast cells was revealed by a strong β-galactosidase activity (data not shown). A control experiment in which a negative control protein moiety (i.e., proton pump subunit A1 [37]) was substituted for the MyoD moiety gave rise to only barely detectable activity.
FIG. 8.
Interaction of p202 with MyoD and with myogenin in in vivo and in vitro assays. (A and B) GST-protein binding assay for testing the binding in vitro of [35S]methionine-labeled p202 translated in a rabbit reticulocyte lysate (10 μl) to glutathione beads (Beads), to GST (0.5 μg) bound to glutathione beads (GST), or to GST-MyoD (0.5 μg) bound to glutathione beads (GST-MyoD) (A) or GST-myogenin (0.5 μg) bound to glutathione beads (GST-myogenin) (B). After the beads were washed, the bound protein was eluted and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography. (C) Binding of p202 to MyoD protein as assayed by coimmunoprecipitation. C2C12 cultures were grown to confluency in growth medium (GM), shifted to differentiation medium (DM), and further incubated. At the times indicated (in hours), the cells were lysed in the presence of protease inhibitors, and 100-μg aliquots were used for immunoprecipitation assays with the anti-p202 antiserum. The immunoprecipitates were analyzed by Western blotting with an immunoaffinity-purified anti-MyoD antiserum. The MyoD and immunoglobulin G light-chain [IgG(L)] protein bands are indicated. For further details, see Materials and Methods.
The positive results of the yeast two-hybrid assay, GST pulldown assay, and immunoprecipitation assay indicate that p202 can bind MyoD protein both in vivo and in vitro. The results of a GST pulldown assay suggest that myogenin may also bind p202, at least in vitro (Fig. 8B).
p202 inhibits the sequence-specific binding of MyoD and myogenin to DNA.
The inhibition of the transcriptional activity of the c-Fos, c-Jun, and E2F-1 transcription factors by p202 was correlated with an inhibition of their sequence-specific binding to DNA (18, 49). This prompted us to establish whether the inhibition of the transcriptional activity of MyoD and of myogenin by p202 can also be correlated with an inhibition of the sequence-specific binding of MyoD and myogenin to DNA by p202. The electrophoretic mobility shift assays with the MyoD- and myogenin-specific MEF-1 oligodeoxynucleotide probe (Fig. 9) indicate that it can.
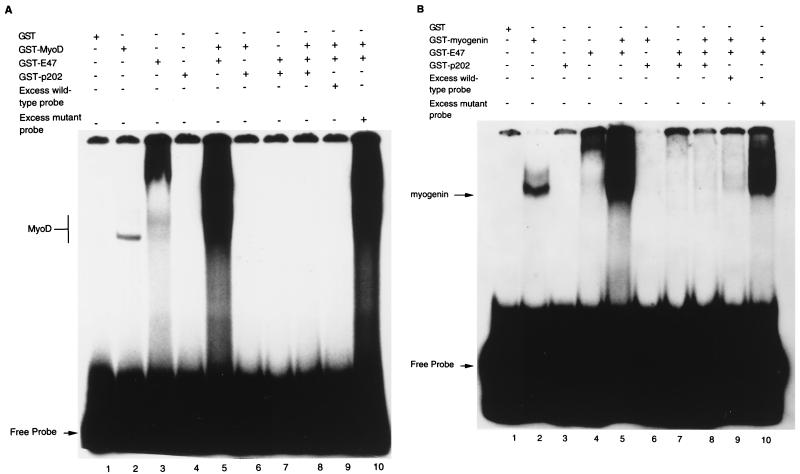
FIG. 9.
p202 inhibits the sequence-specific DNA binding of MyoD and of myogenin in electrophoretic mobility shift assays. (A) Assays involving the binding of GST-MyoD fusion protein to the MEF-1 oligodeoxynucleotide. The proteins indicated, i.e., GST (0.5 μg), GST-MyoD (0.5 μg), GST-E47 (1.0 μg), and GST-p202 (0.5 μg), were added, and the reaction mixtures were incubated for 20 min before the addition of the labeled MEF-1 probe (1.5 ng). After a further 10-min incubation, the reaction mixtures were analyzed by gel electrophoresis and autoradiography. For competition experiments, a 20-fold excess of either unlabeled wild-type MEF-1 probe or unlabeled mutant MEF-1 probe, as indicated, was added to the reaction mixtures. The MyoD band and the labeled free probe are indicated. (B) Assays involving the binding of GST-myogenin fusion protein to the MEF-1 oligodeoxynucleotide. The assays were performed as described for panel A, except that GST-myogenin (0.5 μg) was used instead of GST-MyoD. The labeled myogenin band and the free probe are indicated. For further details, see Materials and Methods.
GST or GST-p202 did not retain the MyoD-specific MEF-1 probe (Fig. 9A, lanes 1 and 4), whereas GST-MyoD and GST-E47 (an E protein capable of forming a homodimer and also a heterodimer with MyoD) (43) did (lanes 2 and 3). GST-p202 inhibited the retention of the MEF-1 probe by both GST-MyoD (lane 6) and GST-E47 (lane 7). A GST-MyoD-GST-E47 heterodimer retained much more MEF-1 probe than did either GST-MyoD or GST-E47 (lane 5). This retention by the heterodimer was also inhibited by GST-p202 (lane 8). As controls, an excess (20-fold) of unlabeled wild-type probe greatly diminished the retention of the labeled MEF-1 probe by the heterodimer (compare lanes 5 and 9) whereas the same excess of unlabeled mutant probe did not (compare lanes 5 and 10).
The data in Fig. 9 reveal that in the electrophoretic mobility shift assays GST-myogenin (Fig. 9B) behaved similarly to GST-MyoD (Fig. 9A). Thus, GST-myogenin retained the labeled MEF-1 probe (Fig. 9B, lane 2). This retention was overcome by GST-202 (lane 6) and was greatly increased by GST-E47 (lane 5). This increased retention by the GST-myogenin–GST-E47 heterodimer was also inhibited by GST-202 (lane 8) and was greatly diminished by excess unlabeled wild-type probe (lane 9) but not by excess mutant probe (lane 10). The data in Fig. 9 indicate that purified GST-p202 can inhibit the sequence-specific binding of purified GST-MyoD and GST-myogenin to DNA.
DISCUSSION
The data presented above indicate a strong (over 14-fold) increase in the level of p202 during the differentiation of cultured C2C12 myoblasts to myotubes. Part of this increase depended on induction by endogenous interferon: incubation in differentiation medium of secondary thigh muscle cultures from mice lacking functional alpha/beta interferon receptors (IFN-α/β R0/0 mice from M. Aguet [52]) resulted in only one-third as much increase in the p202 level as did incubation of such cultures from control mice (not shown). This involvement of endogenous interferon in increasing the level of p202 during skeletal muscle differentiation is in line with earlier findings; i.e., different hematopoietic cells were found to produce minute amounts of beta (related) interferon following induction of differentiation by various natural inducers (30, 74).
p202 is not the only interferon-inducible protein whose level increases during muscle differentiation. The activities of some other interferon-inducible proteins, e.g., 2′,5′-oligoadenylate synthetases and double-stranded RNA-activatable protein kinase, were reported to increase transiently during the fusion of rat thigh muscle cells in vitro (8).
The ability of p202 to inhibit the activity of various transcription factors (18, 22, 49) prompted us to test whether p202 affects the expression of muscle-specific genes. For this purpose, we generated stable C2C12 lines in which the level of p202 was overexpressed in consequence of the transfection of a p202 expression plasmid. In cultures from these lines (proliferating in growth medium or incubated in differentiation medium), the levels of MyoD RNA and protein were diminished below detectability. Such a strong decrease in the MyoD RNA and protein levels was reported previously to occur during skeletal muscle differentiation (26, 31, 34, 42). Our data suggest that this decrease may be the consequence of the pronounced increase in the level of p202 during differentiation together with the inhibitory effect of p202 on MyoD expression. The enhancers in the MyoD gene whose activity is susceptible to inhibition by p202 and which account for the inhibition of MyoD expression by the increased level of p202 remain to be identified. The identification will be facilitated by the fact that overexpression of p202 also inhibited the expression of reporter constructs in which CAT expression was driven by the 5′-flanking region of the mouse MyoD gene (data not shown). The identification of the MyoD enhancer(s) in question will make it possible to establish whether p202 inhibits the binding of transcription factors to the enhancer(s) (as it does, e.g., for c-Fos, c-Jun, and E2F-1) or whether it acts in a different manner (e.g., as for NF-κB [18, 49]). p202 does not uniformly inhibit the expression of muscle-specific genes, since the expression of myogenin (whose level strongly increases during differentiation) was unaffected by the overexpression of p202.
Whereas p202 inhibited the expression of MyoD but not of myogenin, it inhibited the transcriptional activity of both MyoD and myogenin. This inhibition of transcriptional activity was correlated with a protein-protein interaction, both in vitro and in vivo for p202 and MyoD and at least in vitro for p202 and myogenin. Moreover, p202 inhibited the sequence-specific binding to DNA of both MyoD (also in complex with E47) and myogenin (also in complex with E47). It is likely that the inhibition of the transcriptional activity of both MyoD and myogenin by p202 is the consequence of this inhibition of their sequence-specific binding to DNA.
MyoD and myogenin can thus be added to the set of proteins found to bind p202. This set also includes pRb, c-Fos, c-Jun, NF-κB p50 and p65, E2F-1, E2F-4, and p53 binding protein 1 (16, 18, 20, 22, 49). With the exception of pRb and p53 binding protein 1, all of the above are transcription factors, and p202 was shown to inhibit their transcriptional activity. Furthermore, all these transcription factors including MyoD and myogenin are bHLH proteins.
The finding that an alteration in the p202 level changed the expression as well as the transcriptional activity of muscle-specific proteins prompted us to examine the effect of an increase in the p202 level on the induction of differentiation of cultured C2C12 myoblasts. Overexpression of p202 in stably transfected myoblasts inhibited myotube formation as triggered by shifting the culture from growth medium to differentiation medium. This inhibition was in line with the finding that treatment with interferon—which induces p202—also inhibited myotube formation. The inhibition of MyoD expression as well as of MyoD and myogenin transcription factor activity by overexpressed p202 and conceivably also the binding of p202 to the retinoblastoma protein (pRB) (20), reported to be involved in muscle differentiation, might account for this inhibition of the induction of muscle differentiation by p202 (35, 36, 64). It seems paradoxical that an increase in the p202 level blocks the induction of muscle differentiation, since the p202 level is greatly increased during the differentiation. However, it starts to increase only about 24 h after the induction of differentiation (Fig. 2A), whereas the high p202 level inhibiting differentiation was established prior to the induction of differentiation.
More remains to be learned about the biological significance of the increase in the p202 level during muscle differentiation. The available data suggest that one of the prerequisites for skeletal muscle differentiation is that the myoblasts terminally exit from the cell cycle. p202, whose overexpression was found to inhibit the proliferation of various cell lines, is likely to contribute to this exit. Moreover, the increase in the p202 level can account for the decrease in the MyoD protein level and activity occurring in the course of differentiation. It is probable, however, that p202 will also turn out to affect muscle differentiation in further, as yet unexpected, ways.
The data presented make it conceivable that the interferon system may have coopted for its own purposes p202, a protein involved in processes (e.g., muscle differentiation) apparently distinct from interferon action. The situation might resemble that of the double-stranded RNA unwindase-deaminase (4, 5). This enzyme, which was shown to function in RNA editing (41), is also strongly induced by interferon (56, 57), and when overexpressed in consequence of the induction, it is thought to play a role in blocking RNA virus multiplication (6, 57).
However, several interferon-inducible proteins (other than p202), e.g., 2′-5′ oligoadenylate synthetase and RNA-activatable protein kinase, are induced during differentiation (e.g., of muscle), presumably by endogenous interferon (8; see also references 11 and 24). Furthermore, similarly to p202, 2′-5′ oligoadenylate synthetase and RNA-activatable protein kinase may contribute to the inhibition of cell proliferation (45), which may be a prerequisite for differentiation. These facts make it more likely that the interferon system is a component of the machinery of differentiation.
ACKNOWLEDGMENTS
We are grateful to M. Aguet and Genentech Inc. for alpha/beta interferon R0/0 mice lacking active alpha/beta interferon receptors; to C. Weissmann and H. Weber for human alpha-2/alpha-1 interferon 1-83; to M. Horwitz for the 4RCAT plasmid; to A. Lassar for the pCMV-MyoD plasmid; to E. N. Olson and B. L. Black for the MRF(wt)CAT, MRF4(mut)CAT, and pEMSVscribe-myogenin plasmids; and to N. Stewart for preparing the manuscript for publication. We also like to thank the reviewers of the original version of the manuscript for their valuable suggestions and G. Chatterjee, H. Wang, and T. Williams for reading the manuscript.
This work was supported by the NIH NIAID research grant R37-AI12320.
Footnotes
B.D. and P.L. dedicate this study to the memory of Naba K. Gupta.
REFERENCES
- 1.Atherton G T, Travers H, Deed R, Norton J D. Regulation of cell differentiation in C2C12 myoblasts by the Id3 helix-loop-helix protein. Cell Growth Differ. 1996;7:1059–1066. [PubMed] [Google Scholar]
- 2.Aurade F, Pinset C, Chafey P, Gros F, Montarras D. Myf5, MyoD, myogenin, and MRF4 myogenic derivatives of the embryonic mesenchymal cell line C3H10T1/2 exhibit the same adult muscle phenotype. Differentiation. 1994;55:185–192. doi: 10.1046/j.1432-0436.1994.5530185.x. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 2. New York, N.Y: Greene Publishing Co.; 1992. [Google Scholar]
- 4.Bass B L, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- 5.Bass B L, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988;58:1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- 6.Bass B L, Weintraub H, Cattaneo R, Billeter M A. Biased hypermutation of viral RNA genomes could be due to unwinding/modification of double-stranded RNA. Cell. 1989;56:331. doi: 10.1016/0092-8674(89)90234-1. [DOI] [PubMed] [Google Scholar]
- 7.Benezra R, Davis R, Lockson D, Turner D, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum M, Trink B, Shainberg A, Salzberg S. Activation of the interferon system during myogenesis in vitro. Differentiation. 1990;45:138–145. doi: 10.1111/j.1432-0436.1990.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 9.Black B L, Martin J F, Olson E N. The mouse MRF4 promoter is transactivated directly and indirectly by muscle specific transcription factors. J Biol Chem. 1995;270:2889–2892. doi: 10.1074/jbc.270.7.2889. [DOI] [PubMed] [Google Scholar]
- 10.Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold H H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989;8:701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs J A, Burrus G R, Stickney B D, Briggs R C. Cloning and expression of the human myeloid cell nuclear differentiation antigen: regulation by interferon alpha. J Cell Biochem. 1992;49:82–92. doi: 10.1002/jcb.240490114. [DOI] [PubMed] [Google Scholar]
- 12.Buckingham M. Muscle: the regulation of myogenesis. Curr Opin Genet Dev. 1994;4:745–751. doi: 10.1016/0959-437x(94)90142-p. [DOI] [PubMed] [Google Scholar]
- 13.Buskin J N, Hauschka S D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989;9:2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C-M A, Kraut N, Groudine M, Weintraub H. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell. 1996;86:731–741. doi: 10.1016/s0092-8674(00)80148-8. [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Costa M L, Mermelstein C S, Chagas C, Holtzer S, Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci USA. 1990;87:7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choubey D, Gutterman J. Inhibition of E2F-4/DP-1 stimulated transcription by p202. Oncogene. 1997;15:291–301. doi: 10.1038/sj.onc.1201184. [DOI] [PubMed] [Google Scholar]
- 17.Choubey D, Lengyel P. Interferon action: nucleolar and nucleoplasmic localization of the interferon-inducible 70-kilodalton protein that is encoded by the Ifi204 gene from the gene 200 cluster. J Cell Biol. 1992;116:1333–1341. doi: 10.1083/jcb.116.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choubey D, Li S-J, Datta B, Gutterman J U, Lengyel P. Inhibition of E2F-mediated transcription by p202. EMBO J. 1996;15:5668–5678. [PMC free article] [PubMed] [Google Scholar]
- 19.Choubey D, Lengyel P. Interferon action: cytoplasmic and nuclear localization of the interferon-inducible 52-kilodalton protein that is encoded by the Ifi202 gene from the gene 200 cluster. J Interferon Res. 1993;13:43–52. doi: 10.1089/jir.1993.13.43. [DOI] [PubMed] [Google Scholar]
- 20.Choubey D, Lengyel P. Binding of an interferon-inducible protein (p202) to the retinoblastoma protein. J Biol Chem. 1995;270:6134–6140. doi: 10.1074/jbc.270.11.6134. [DOI] [PubMed] [Google Scholar]
- 21.Choubey D, Snoddy J, Chaturvedi V, Toniato E, Opdenakker G, Thakur A, Samanta H, Engel D, Lengyel P. Interferons as gene activators: indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989;264:17182–17189. [PubMed] [Google Scholar]
- 22.Datta B, Li B, Choubey D, Nallur G, Lengyel P. p202, an interferon-inducible modulator of transcription, inhibits transcription activation by the p53 tumor suppressor protein, and a segment from the p53-binding protein 1 that binds to p202 overcomes this inhibition. J Biol Chem. 1996;271:27544–27555. doi: 10.1074/jbc.271.44.27544. [DOI] [PubMed] [Google Scholar]
- 23.Davis R L, Weintraub H, Lassar A B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 24.Dawson M J, Trapani J A. IFI16 gene encodes a nuclear protein whose expression is induced by interferons in human myeloid leukemia cell lines. J Cell Biochem. 1995;57:39–51. doi: 10.1002/jcb.240570106. [DOI] [PubMed] [Google Scholar]
- 25.De Young K L, Ray M E, Su Y A, Anzick S L, Johnstone R W, Trapani J A, Meltzer P S, Trent J M. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity. Oncogene. 1997;15:453–457. doi: 10.1038/sj.onc.1201206. [DOI] [PubMed] [Google Scholar]
- 26.Dias P, Dilling M, Houghton P. The molecular basis of skeletal muscle differentiation. Semin Diagn Pathol. 1994;11:3–14. [PubMed] [Google Scholar]
- 27.Edmondson D G, Olson E N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 28.Fields S, Song O K. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:243–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 29.Fisher P B, Miranda A F, Babis L E, Pestka S, Weinstein I B. Opposing effects of interferon produced in bacteria and of tumor promoters on myogenesis in human myoblast cultures. Proc Natl Acad Sci USA. 1983;80:2961–2965. doi: 10.1073/pnas.80.10.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman-Einat M, Revel M, Kimchi A. Initial characterization of spontaneous interferon secreted during growth and differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1982;2:1472–1480. doi: 10.1128/mcb.2.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchtbauer E-M, Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn. 1992;193:34–39. doi: 10.1002/aja.1001930106. [DOI] [PubMed] [Google Scholar]
- 32.Gariglio M, Panico S, Cavallo G, Choubey D, Lengyel P, Landolfo S. Impaired transcription of the poly rI:rC and interferon-activatable 202 gene in mice and cell lines from the C57BL/6 strain. Virology. 1992;187:115–123. doi: 10.1016/0042-6822(92)90300-e. [DOI] [PubMed] [Google Scholar]
- 33.Getz M J, Elder P K, Benz E W, Jr, Stephens R E, Moses H L. Effect of cell proliferation on levels and diversity of poly(A)-containing mRNA. Cell. 1976;7:255–265. doi: 10.1016/0092-8674(76)90025-8. [DOI] [PubMed] [Google Scholar]
- 34.Grounds M D, Garrett K L, Lai M C, Wright W E, Beilharz M W. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- 35.Gu W, Schneider J W, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 36.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 37.Hernando N, Bartkiewicz M, Collin-Osdoby P, Osdoby P, Baron R. Alternative splicing generates a second isoform of the catalytic A subunit of the vacuolar H(+)-ATPase. Proc Natl Acad Sci USA. 1995;92:6087–6091. doi: 10.1073/pnas.92.13.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollenberg S M, Sternglanz R, Cheng P F, Weintraub H. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15:3813–3822. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992;6:1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- 40.Kadesch T. Helix-loop-helix proteins in the regulation of immunoglobulin gene transcription. Immunol Today. 1992;13:31–36. doi: 10.1016/0167-5699(92)90201-h. [DOI] [PubMed] [Google Scholar]
- 41.Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci USA. 1994;91:11457–11461. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koishi K, Zhang M, McLennan I S, Harris A J. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn. 1995;202:244–254. doi: 10.1002/aja.1002020304. [DOI] [PubMed] [Google Scholar]
- 43.Lassar A B, Davi R L, Wright W E, Kadesch T, Murre C, Voronova A, Baltimore D, Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991;66:305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- 44.Lassar A B, Skapek S X, Bennett N. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr Opin Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 45.Lengyel P. Tumor suppressor genes: news about the interferon connection. Proc Natl Acad Sci USA. 1993;90:5893–5895. doi: 10.1073/pnas.90.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lengyel P, Choubey D, Li S-J, Datta B. The interferon-activatable gene 200 cluster: from structure toward function. Semin Virol. 1995;6:203–213. [Google Scholar]
- 47.Megeney L A, Rudnicki M A. Determination versus differentiation and the MyoD family of transcription factors. Biochem Cell Biol. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- 48.Melnikova I N, Christy B A. Muscle cell differentiation is inhibited by the helix-loop-helix protein Id3. Cell Growth Differ. 1996;7:1067–1079. [PubMed] [Google Scholar]
- 49.Min W, Ghosh S, Lengyel P. The interferon-inducible p202 protein as a modulator of transcription: inhibition of NF-κB, c-Fos, and c-Jun activities. Mol Cell Biol. 1996;16:359–368. doi: 10.1128/mcb.16.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miner J H, Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci USA. 1990;87:1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molkentin J D, Olson E N. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- 52.Muller U, Steinhoff U, Luiz F L, Reis S H, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 53.Olson E N. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154:261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- 54.Olson E N, Klein W H. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;8:1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- 55.Opdenakker G, Snoddy J, Choubey D, Toniato E, Pravtcheva D D, Seldin M F, Ruddle F H, Lengyel P. Interferons as gene activators: a cluster of six interferon-activatable genes is linked to the erythroid alpha spectrin locus on murine chromosome 1. Virology. 1989;171:568–578. doi: 10.1016/0042-6822(89)90626-0. [DOI] [PubMed] [Google Scholar]
- 56.Patterson J B, Samuel C E. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson J B, Thomis D C, Hans S L, Samuel C E. Mechanism of interferon action: double-stranded RNA-specific adenosine deaminase from human cells is inducible by alpha and gamma interferons. Virology. 1995;210:508–511. doi: 10.1006/viro.1995.1370. [DOI] [PubMed] [Google Scholar]
- 58.Rawls A, Olson E N. MyoD meets its maker. Cell. 1997;89:5–8. doi: 10.1016/s0092-8674(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 59.Reznikoff C A, Brankow D W, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- 60.Rhodes S J, Konieczny S F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- 61.Rudnicki M A, Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- 62.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 63.Schafer B W, Blakely B T, Darlington G J, Blau H M. Effect of cell history on response to helix-loop-helix family of myogenic regulators. Nature. 1990;344:454–458. doi: 10.1038/344454a0. [DOI] [PubMed] [Google Scholar]
- 64.Skapek S X, Rhee J, Spicer D B, Lassar A B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 65.Tomita Y, Hasegawa S. Multiple effect of interferon on myogenesis in chicken myoblast cultures. Biochim Biophys Acta. 1984;804:370–376. doi: 10.1016/0167-4889(84)90141-1. [DOI] [PubMed] [Google Scholar]
- 66.Trouche D, Grigoriev M, Lenormand J L, Robin P, Leibovitch S A, Sassone-Corsi P, Harel-Bellan A. Repression of c-fos promoter by MyoD on muscle cell differentiation. Nature. 1993;363:79–82. doi: 10.1038/363079a0. [DOI] [PubMed] [Google Scholar]
- 67.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 68.Weber H, Valenzuela D, Lujber G, Gubler M, Weissmann C. Single amino acid changes that render human IFN-alpha 2 biologically active on mouse cells. EMBO J. 1987;6:591–598. doi: 10.1002/j.1460-2075.1987.tb04795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell T K, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 70.Weintraub H, Hauschka S D, Tapscott S J. The MCK enhancer contains a p53 responsive element. Proc Natl Acad Sci USA. 1991;88:4570–4571. doi: 10.1073/pnas.88.11.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright W E, Sassoon D A, Lin V K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 72.Yablonka-Reuveni Z, Rivera A J. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yaffe D, Saxe O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 74.Yarden A, Shure-Gottlieb H, Chebath J, Revel M, Kimchi A. Autogenous production of interferon-beta switches on HLA genes during differentiation of histiocytic lymphoma U937 cells. EMBO J. 1984;3:969–973. doi: 10.1002/j.1460-2075.1984.tb01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yun K, Wold B. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr Opin Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]