Abstract
The investigation of peptide drugs has become essential in the development of innovative medications for hypertension. In this study, a sensitive high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) method was developed to determine the plasma concentration and stability of the antihypertensive peptide FR-6 in rats. An isotopically labeled peptide (with an unchanged sequence) was utilized as an internal standard (IS) for validation purposes. Subsequently, this assay was employed to examine the pharmacokinetics of different administration methods (tail vein and gavage) in Sprague Dawley (SD) rats. Extracted plasma samples underwent sample preparation through methanol protein precipitation, followed by elution of FR-6 on Wondasil C18 Superb column (4.6 × 150 mm, 5 μm), using a mobile phase consisting of formic acid (0.1%) in water (A) and formic acid (0.125%)-ammonium formate (2 mM) in methanol (B). Ion pairs corresponding to FR-6 and IS were monitored via multiple reaction monitoring (MRM) under positive ion mode: m/z 400.7 → 285.1 for FR-6 and m/z 406.1 → 295.1 for IS detection respectively. The method exhibited excellent linearity with respect to FR-6 concentrations. In addition, the inter-day and intra-day precision were 0.61–6.85% and 1.76–11.75%; the inter-day and intra-day accuracy were −7.28–0.13% and −7.20–2.28%, respectively. In conclusion, the matrix effect, extraction recovery, and stability data were validated according to FDA recommended acceptance criteria for bioanalytical methods. This validated method serves as a reliable tool for determining the concentration of antihypertensive peptide FR-6, and has been successfully applied in pharmacokinetic studies involving rats.
Keywords: Antihypertensive peptides, Biological sample preparation, LC-MS/MS method, Pharmacokinetic, Administration route
1. Introduction
Hypertension, the most prevalent cardiovascular disease worldwide is increasingly becoming a grave concern [1]. According to estimates from the International Society of Hypertension (ISH) and the World Health Organization (WHO), hypertension accounts for 4.5% of the global burden of illness. Approximately 972 million individuals globally, constituting 25%–35% of the population, suffer from hypertension. However, treatment and control rates remain suboptimal. The global medical community still faces an arduous task in effectively preventing and treating hypertension. Chemically synthesized medications, such as diuretics, beta-blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, and alpha-blockers, have gained widespread utilization in recent years for the prevention and treatment of hypertension. Nevertheless, these antihypertensive medications exhibit significant efficacy, they are also associated with several adverse effects including excessive blood pressure reduction, urological lesions, cutaneous eruptions, persistent coughing, andurotic edema. Recently, the discovery of novel and effective antihypertensive become imperative for hypertension and cardiovascular disorders [2]. Peptides offer advantages of high specificity, high biological activity, easy synthesis and side effects, especially the peptides derived from food exhibit lower risk profiles compared to chemically synthesized medications [3,4]. Numerous studies have successfully isolated, identified, and characterized several peptides possessing antihypertensive properties. The burgeoning field of peptide medicines in the international biotechnology arena holds great promise for medication research and development [[5], [6], [7], [8]]. Quinoa is internationally recognized by nutritionists as an ancient “nutritional gold”, a “super grain” and a “future food”. It is also hailed as the “King of Vegetarianism” among vegan enthusiasts, making it one of the most promising foods for the future [9,10]. In SHR rats, hydrolyzed quinoa protein extracts were found to effectively reduce blood pressure and exhibit significant inhibitory effects against ACE. Aluko and Monu [3] conducted in vitro evaluations on calcineurin-hydrolyzed quinoa proteins' ACE inhibition activity and demonstrated that low molecular weight peptides (less than 5 kDa), obtained through ultrafiltration, exhibited superior ACE inhibitory activity compared to high molecular weight peptides (greater than 5 kDa). In a study conducted by Zheng et al. [11], a novel ACE inhibitory peptide RGQVIYVL was identified from quinoa bran protein. The modified peptide exhibited potent ACE inhibitory activity (IC50 = 38.16 μM) and demonstrated significant antihypertensive effects when orally administered at a dose of 100–150 mg/kg. It has been shown that FR-6, a derived from quinoa extract also possess antihypertensive properties. Furthermore, further investigation is warranted to elucidate its pharmacokinetics and pharmacodynamics.
Due to their distinctive physicochemical characteristics, peptides pose greater challenges in vivo analysis compared to small molecule drugs. In addition, the limited dose and low potential interference of endogenous substances, the short half-life and the complex in vivo metabolic pathways further emphasize the need to select highly sensitive and specific analytical methods for accurate detection. Currently, commonly employed methods for peptide analysis include isotope labelling tracer, immunoassay, chromatography and in vivo imaging. Compared to electrophoresis and high performance liquid chromatography (HPLC), radioisotope tracing offers a direct, rapid and highly sensitive method with clear advantages in the study of tissue distribution and excretion of drugs [12]. Moreover, it enables successful differentiation between tracer pharmaceuticals and radioactive metabolic components. Appropriate specific activity is crucial for the detection of radioisotope tracer techniques. Insufficient specific activity can compromise sensitivity and accuracy of detection while excessive specific activity can impede the functionality of protein peptide drugs and lead to radioactive contamination. Enzyme-linked immunosorbent assay (ELISA) currently serves as the primary method for protein peptide pharmacokinetic studies [13]. However, its sensitivity and precision are compromised by endogenous substance interference, while the quantitative range is limited to 1–2 orders of magnitude. Additionally, it only allows measurement of one test substance at a time, precluding simultaneous assessment of multiple test substances. In vivo imaging is also a better method for in vivo analysis, which allows real-time dynamic monitoring of drug behaviors in organisms [14]. But the development is still in its nascent stage and faces challenges in meeting the demands for sensitivity, stability, and reproducibility required by pharmacokinetic assays. For the quantitative analysis of small compounds, LC-MS/MS has emerged as the “gold standard” [[15], [16], [17]]. It has also been employed for analyzing peptides with simple structures and high stability. Due to various interferences, challenging separation techniques, and enrichment difficulties, LC-MS/MS alone cannot fully meet the requirements of in vivo analysis. Peptides possess unique structural characteristics, low stability, and intricate internal metabolism processes. Currently, most antihypertensive peptides are in vitro or animal experimentation stages. Therefore, it is imperative to develop effective and sensitive analytical methods for conducting pharmacokinetic studies and safety assessments.
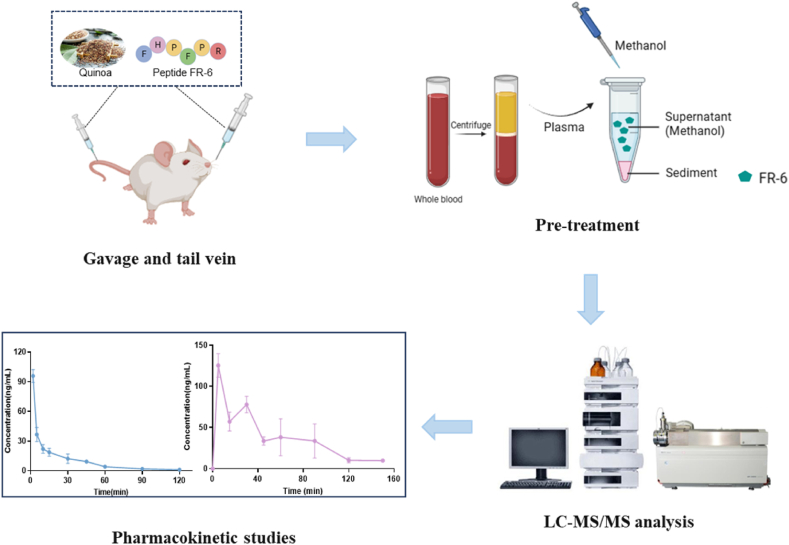
In summary, this study optimized the chromatographic and sample pretreatment conditions for the novel antihypertensive peptide FR-6 as the research target, leading to the development of an in vivo LC-MS/MS analytical technique with good sensitivity, high precision, and high specificity (Fig. 1). By assessing the stability of FR-6 in plasma, our method not only improved the pre-treatment of biological samples containing peptide drugs but also investigated the pharmacokinetic properties of FR-6 in SD rats through tail vein injection and oral administration. The development of this technique enhances both pharmacological and pharmacokinetic data on FR-6 while expediting its study, development, and non-clinical translation as a potential antihypertensive medication. These findings lay a solid foundation for future clinical introduction.
Fig. 1.
A sensitive and specific LC–MS/MS method for determination of a novel antihypertensive peptide FR-6 in rat plasma and pharmacokinetic study.
2. Materials and methods
2.1. Chemicals and reagents
FR-6 (purity >95%) and isotope-labeled internal standard (purity >95%, IS) were purchased from Shanghai Gill Biochemistry Co., LTD. (Shanghai, China). Ammonium formate (purity ≥98%, NH4FA) was purchased from Shanghai Lingfeng Chemical Reagent Co., LTD. (Shanghai, China). Heparin sodium and formic acid (purity ≥98%, FA) were purchased from Shanghai Aladdin Biochemical Technology Co., LTD. (Shanghai, China). The chromatographic grade methanol and acetonitrile were purchased from Merck KGaA (Darmstadt, Germany). Ultrapure water was obtained from a Milli-Q Reagent Water System (Millipore, MA, America). All other chemicals and solvents were of analytical grade and used without further purification.
2.2. Stock and working solution
Accurately weighed 10.0 mg of FR-6 into a 10 mL brown volumetric flask, added methanol to dissolve and mixed thoroughly to obtain the FR-6 stock solution (1 mg/mL). Precisely weighed 5.0 mg of internal standard into another 10 mL brown volumetric flask, added methanol to dissolve and mixed well to obtain the internal standard stock solution (500 μg/mL). After verifying the stock solution, diluted a certain amount of FR-6 stock solution with methanol to prepare a series of working solutions for standard curve samples (5, 20, 100, 500, 2000, 4000, 6000 ng/mL) and quality control samples (50, 1000, 5000 ng/mL). Stored these working solutions in transparent EP tubes and kept at −20 °C until use. Diluted a certain amount of the internal standard stock solution with methanol and configured the working solution (2000 ng/mL). Stored this working solution in transparent EP tubes at −20 °C until use.
2.3. Chromatographic and mass spectrometric conditions
The HPLC (Agilent1260, America) was equipped with high pressure binary pump (G1312B), vacuum degasser (G4225A), automatic sampler (G1367E) and column temperature control unit (G1330B). The chromatographic column was Wondasil C18 Superb (4.6 × 150 mm, 5 μm, Shimadzu, Japan) with the column temperature set at 35 °C and the injection volume was set at 5 μL. The mobile phase consisted of Water (0.1% formic acid, A) and MeOH (0.125% formic acid-2mM ammonium formate, B) at a flow rate of 0.5 mL/min. The 12 min gradient elution procedure included the following linear components: 0~1 min, 70~30% A; 1~9 min, 30% A; 9.1~12 min, 70% A.
API 4000 mass spectrometer (Applied Biosystems, Sciex, USA) was equipped with a triple quadrupole mass spectrometer, electrospray ion source in positive mode (ESI+) and Analyst 1.6.2 Data Acquisition System. Quantitative analysis was performed in multiple reaction monitoring (MRM) transitions. After optimizing the mass spectrometry parameters, the heating temperature (TEM) was 450 °C, the ion spray voltage (IS) was 5500v, the collision gas (CAD) was 6 psi, the curtain gas (CUR) was 10 psi, the ion source gas (GS1) was 17 psi, and the ion source gas 2 (GS2) was 20 psi. The dwell time was 300 ms. The declustering potential (DP) was 40 v, 32 v; entrance potential (EP) was 10 v, 9 v; collision energy (CE) was 21v, 20 v and collision cell exit potential (CXP) was 19 v, 7v for FR-6 and IS, respectively. The ion pairs chosen for the quantification of FR-6 and IS in multiple reactions monitoring (MRM) mode were m/z 400.7 → 285.1 for FR-6 and m/z 406.1 → 295.1 for IS.
2.4. Plasma stability
Male Sprague Dawley rats (license number SYXK (Su) 2020–0009), weighing 180g–220g, were subjected to a 24-h fasting period prior to the experiments. The experimental animals were sourced from the Animal Experimental Center of China Pharmaceutical University. They received adequate daily provision of food and water under controlled conditions of continuous temperature (24 ± 1 °C), relative humidity (50 ± 10%), and a 12-h light-dark cycle. All animal experiments adhered to the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised in 1978) and were approved by the Ethics Committee of China Pharmaceutical University (No. 2023-11-001).
Fresh blood was collected from the retro-orbital venous plexus of SD rats and immediately added to centrifuge containing 1.5% sodium heparin as an anticoagulant. The mixture was then centrifuged at 814g for 8 min, and the resulting plasma was collected for further analysis. LQC and HQC plasma samples with FR-6 concentrations set at 5 ng/mL and 500 ng/mL respectively were thoroughly mixed before use. Subsequently, the stability of FR-6 in rat plasma was assessed by incubating it at room temperature for various durations including 0, 10 min, 20 min, 40 min, 60 min, 120 min and 180 min. After each incubation period, plasma samples were promptly processed according to "2.5 Pretreatment of biological samples" and subjected to LC-MS/MS analysis. The ratio between the peak area of the incubated sample and that of the immediately processed sample served as an indicator for evaluating FR-6 stability in plasma.
2.5. Pretreatment of biological samples
We measured 100 μL of plasma containing the FR-6 into a 1.5 mL centrifuge tube, added 10 μL of internal standard working solution and 300 μL of methanol in turn. After vortexing for 5 min and centrifuging at 15300 g for 10 min, aspirated 300 μL supernatant into another centrifuge tube and blew dry it with nitrogen. And then added 100 μL mobile phase (0.125% formic acid-2mM ammonium formate - methanol: 0.1% formic acid-water = 7:3, V/V) into the centrifuge tube to re-dissolve. Finally, 80 μL of supernatant was taken for detection by LC-MS/MS after vortexing and centrifugation.
2.6. Method validation
We investigated the metabolism of the antihypertensive peptide FR6 in rats using an LC-MS/MS analytical method in this experiment. The method was performed in compliance with FDA guidelines for validation of industrial bioanalytical methods [[18], [19], [20], [21]].
2.6.1. Specificity
The blank plasma samples were collected and processed according to "2.5 Pretreatment of biological samples". Subsequently, LC-MS/MS analysis was performed on the prepared double-blank samples, blank plasma spiked with a specific concentration of FR-6 (600 ng/mL) after sample processing, blank plasma treated with an internal standard working solution after sample treatment. Finally, LC-MS/MS analysis was performed on plasma obtained 2 min post intravenous injection FR-6 at a dose of 10 mg/kg after sample processing. The chromatograms of the double blank plasma without FR-6 and internal standard were compared to determine if there were peaks corresponding to the analyte and internal standard at their respective retention times, as well as to assess for any potential interference.
2.6.2. Calibration curve
The FR-6 working solution (10 μL) and IS working solution (10 μL) were added to 90 μL of blank plasma, thoroughly mixed, and processed following the "2.5 Pretreatment of biological samples" protocol. Subsequently, LC-MS/MS analysis was performed on prepared samples (0.5, 2, 10, 50, 200, 400, and 600 ng/mL). The Analyst software was utilized to calculate the ratio of FR-6 to the peak area of the internal standard (As/Ai), enabling finalization of the linear regression equation.
2.6.3. Accuracy and precision
QC samples containing FR-6 at concentrations of 0.5, 100, and 500 ng/mL were prepared in three consecutive analytical batches to assess precision and accuracy. Each concentration was represented by five samples, while standard curve samples were also prepared. The prepared samples underwent processing as described in "2.5 Pretreatment of biological samples," followed by analysis using LC-MS/MS the concentration of the supernatant. Intra- and inter-batch precision and accuracy were calculated based on the measured concentration and accuracy, respectively.
2.6.4. Matrix effect
Six plasma samples were collected from different sources and treated by vortexing with 300 μL methanol for 5 min, followed by centrifugation at 15300g for 10 min. Subsequently, the upper organic phase (300 μL) was dried using nitrogen gas at room temperature. 100 μL solution containing a specific amount of FR-6 and the internal standard was added to the plasma and thoroughly mixed to ensure consistent concentrations with the LQC, MQC, and HQC samples. The peak area of FR(As) and internal standard (Ai) were recorded. Additionally, solutions with final concentrations matching those used in LQC, MQC, and HQC were prepared simultaneously to record respective FR-6 peak area (Asr) and internal standard peak area (Air). Finally, matrix factors for both FR-6 and the internal standard were calculated using sample matrix factor MF (%) = As/Asr × 100% and internal standard matrix factor MF (%) = Ai/Air × 100%, respectively. Furthermore, normalization of matrix factors was performed by dividing the matrix factor of FR-6 by that of the internal standard. Acceptable results within our assessment on matrix effects are indicated when normalized matrix factors have a maximum RSD value of 15%.
2.6.5. Recovery
Three concentration levels of quality control (QC) samples containing FR-6 at 5, 100, and 500 ng/mL were prepared. Five replicates of each concentration were analyzed using LC-MS/MS to determine the FR-6 peak area (As) and IS peak area (Ai). Fifteen blank plasma samples were processed by adding 300 μL of methanol, vortexing for 5 min, centrifuging at 15300 g for 10 min, collecting the upper organic phase (300 μL), and evaporating it with nitrogen blowing at room temperature. Subsequently, a solution containing a specific amount of FR-6 and the internal standard was added separately in volumes of 100 μL each and mixed thoroughly to achieve final concentrations equivalent to those in low (LQC), medium-quality control (MQC), and high-quality control (HQC). The peak areas for FR-6 (Asr) and isotopic internal standard (Air) were recorded. Finally, extraction recoveries for both FR-6 and the internal standard were calculated using sample extraction recovery = As/Asr × 100% and internal standard extraction recovery = Ai/Air × 100%, respectively.
2.6.6. Residual effect
A double blank was injected after the upper limit of quantification sample, and the peak areas of FR-6 and IS in the double blank were recorded to evaluate system residue. The residual effect was determined using a specific formula. A FR-6 peak area response in a double blank that did not exceed 20% of the Lower Limit of Quantitation (LLOQ) peak area response for that analytical lot, along with an internal standard peak area in a double blank sample that did not surpass 5% of the LLOQ peak area response for that analytical lot, indicated absence of residue.
2.6.7. Stability
The QC plasma samples were prepared at two concentration levels, namely LQC and HQC, with FR-6 concentrations of 5 ng/mL and 500 ng/mL, respectively. The QC samples at each concentration level were thoroughly mixed. (i) Three plasma samples were immediately processed according to "2.5 Pretreatment of biological samples" after preparation and subsequently analyzed by LC-MS/MS. (ii) Three plasma samples underwent three freeze-thaw cycles in a −80 °C refrigerator, followed by processing according to "2.5 Pretreatment of biological samples", before being analyzed by LC-MS/MS. (iii) After the initial injection in the first analytical batch for precision and accuracy assessment, the remaining samples were stored in an autosampler (4 °C) for 12 h prior to a subsequent injection for LC-MS/MS analysis. (iv) Three plasma samples were prepared and stored in a −80 °C refrigerator for 21 days, then thawed following "2.5 Pretreatment of biological samples" being subjected to LC-MS/MS analysis.
2.7. Pharmacokinetic studies
Ten SD male rats were divided into tail vein group (iv) and gavage group (ig), five in each group. For iv group, blood was collected from the retro-orbital venous plexus at 0 min, 2 min, 5 min, 10 min, 15 min, 30 min, 45 min, 60 min, 90 min, and 120 min after the administration of tail vein injection (10 mg/kg). For ig group, blood was collected from the retro-orbital venous plexus at 0 min, 5 min, 15 min, 30 min, 45 min, 60 min, 90 min, 120 min, and 150 min after administration of the drug by gavage (50 mg/kg). 0.3 mL of blood was collected into EP tubes that had been infiltrated with 50 μL of 1.5% sodium heparin in advance. Shook up and down to mix them thoroughly, and then centrifuged at 814 g for 8 min to obtain plasma. Obtained plasma samples were processed according to "2.5 Pretreatment of biological samples".
2.8. Statistical methods
The data were expressed as Mean ± SD in this study, and the pharmacokinetic parameters of tail-vein and oral administration were analyzed and estimated using DAS software with a non-linear mathematical model. The key parameters included time to reach maximum (tmax), maximum peak concentration (Cmax), area under the plasma concentration-time curve (AUC0-t and AUC0-∞), and elimination half-life (t1/2). The absolute bioavailability (Fabs) of FR-6 was calculated based on the pharmacokinetic parameters obtained from FR-6 oral versus tail vein injection, using the following equation:
| Fabs(%)=(AUCio·Div)/(AUCiv·Dio) × 100% |
where AUC represents the area under the blood concentration-time curve, subscripts io and iv represent oral and tail vein injection, respectively, and D represents the administered dose.
3. Results and discussion
3.1. Condition optimization
3.1.1. Optimization of mass spectrometry conditions
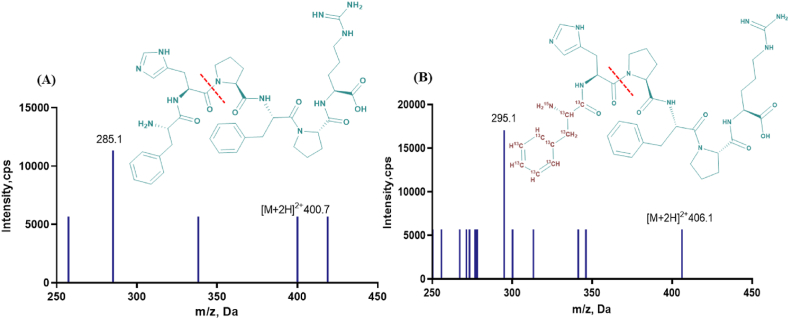
In the positive ion monitoring mode, solutions of FR-6 and IS were injected into the mass spectrometer via a flow injection pump for ion pair monitoring and optimization of mass spectrometry parameters, respectively. Initially, the Q1 mode was employed to scan the precursor ion of FR-6, revealing that the primary parent ion peak of FR-6 corresponded to [M+2H]2+ with m/z 400.7 (Fig. 2A). Subsequently, MS2 mode was selected to scan the daughter ions of FR-6, resulting in identification of a major fragmentation ion peak at m/z 285.1. The specific ion pair for FR-6 was determined as m/z 400.7 → 285.1 through careful analysis, followed by optimization of both ion source parameters and voltage parameters to enhance response efficiency towards this particular ion pair (m/z 400.7 → 285.1). Similarly, repeating these steps for IS revealed formation of [M+2H]2+ precursor ions at m/z 406.1 (Fig. 2B), with subsequent generation of product ions at m/z 295.1 upon scanning in MS2 mode. Consequently, the precise determination for IS's corresponding ion pair m/z 406.1 → 295.1, further optimization involving appropriate adjustment of both ion source parameter and voltage parameter facilitated enhanced response efficiency towards this specific ion pair (m/z 406.l →295.l).
Fig. 2.
Product ion scan and cleavage pathways of FR-6 (A) and IS (B).
3.1.2. Optimization of chromatographic conditions
In the process of establishing chromatographic conditions for peptide FR-6, we systematically investigated various options for the mobile phase, including methanol-water, acetonitrile-water, methanol-water (both with formic acid added), and methanol (formic acid-ammonium formate)-water (formic acid). Due to the relatively high solubility of FR-6 in methanol compared to acetonitrile, we observed peak fronting when using acetonitrile-water separation (Fig. 3A). Consequently, we selected methanol as the organic phase. During, it was discovered that incorporating formic acid into the mobile phase under positive ionization conditions enhanced ionization efficiency and resulted in sharper peak shapes and significantly higher response compared to a pure methanol-pure water system. However, severe peak fronts were observed (Fig. 3B). By of ammonium formate-formic acid into the organic phase at equivalent concentrations, both peak shape and ionic response improved significantly (Fig. 3C). Therefore, the mobile phases were determined as 0.125% formic acid- 2 mM ammonium formate-methanol (B) and 0.1% formic acid-water (A).
Fig. 3.
Liquid chromatography was performed using different mobile phase for FR-6 separations: (A) the mobile phase consisted of acetonitrile-water; (B) the mobile phase comprised methanol (0.1% formic acid)-Water (0.1% formic acid); (C) the mobile phase consisted of methanol (0.125% formic acid- 2 mM ammonium formate)-Water (0.1% formic acid); (D) Investigation on FR-6 stability in blank plasma of SD rats placed at room temperature for 180 min (n = 3, Mean ± SD).
3.1.3. Optimization of pre-treatment conditions for biological samples
The present study investigated various pretreatment methods for biological samples, including ethyl acetate extraction, methanol protein precipitation, and acetonitrile protein precipitation. Given the relatively high polarity of FR-6, which was soluble in methanol but insoluble in acetonitrile and ethyl acetate, the recovery of FR-6 was examined. It was found that the recovery using methanol protein precipitation (83.33 ± 2.63%) was significantly higher than that using acetonitrile (29.60 ± 3.66%) or ethyl acetate (22.34 ± 2.76%). The protein precipitation method offers a simplified procedure with ease of operation and improved pre-processing efficiency. Considering all factors comprehensively, the methanol ultimately selected for biological sample pretreatment in this study.
3.1.4. Plasma stability and pretreatment of plasma sample
The results of the investigation on FR-6 stability in blank plasma of SD rats placed at room temperature for 180 min are presented in Fig. 3D. After being incubated in blank plasma at room temperature for 10 min, the stability of FR-6 decreased to 52.68 ± 12.93% (5 ng/mL) and 35.01 ± 9.18% (500 ng/mL), respectively. These findings indicate that FR-6 exhibits poor stability in plasma and is susceptible to enzymatic metabolism and degradation by various enzymes present [[22], [23], [24]]. To enhance its stability in plasma, we attempted to pre-add 10% H2SO4 into whole blood during the experiment followed by thorough mixing [5], after which was obtained through centrifugation for subsequent. However, it was observed that while the addition of an appropriate amount of acid improved stability, it also had a detrimental effect on red blood cells, compromising their anticoagulant function and leading hemolysis. Therefore, to minimize enzymatic degradation rate of FR-6, immediate centrifugation was performed after obtaining blood samples during rat pharmacokinetic assays, with immediate addition of internal standard (IS) for sample processing purposes as well adjustments were made to the order of sample addition during preparation: descending peptide and IS were added first followed by protein precipitant (methanol), with plasma being added last.
3.2. Method validation
3.2.1. Specificity
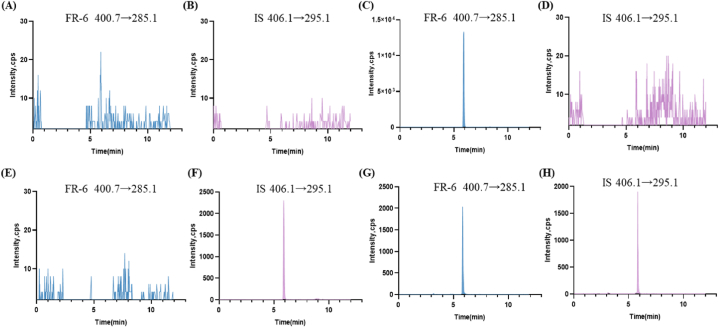
The specificity results were depicted in Fig. 4. Notably, no discernible interference from blank plasma was observed at the positions corresponding to FR-6 (Fig. 4A) and IS (Fig. 4B). where the retention time of FR-6 was approximately 5.84 min (Fig. 4C and D), similar to that of the isotope internal standard (Fig. 4E and F). Furthermore, both FR-6 and IS exhibited well-defined peak shapes without any interference at their respective positions, thereby confirming the excellent specificity of the method (Fig. 4G and H).
Fig. 4.
Representative MRM chromatograms were obtained for the following conditions: (A) FR-6 and (B) IS in blank plasma; (C) FR-6 and (D) IS in plasma with only FR-6 at a concentration of 600 ng/mL; (E) FR-6 and (F) IS in plasma with only IS added; (G) FR-6 and (H) IS in plasma samples determined 2 min after intravenous injection of FR-6.
3.2.2. Linearity and lower quantitation limit
The standard curve samples were freshly prepared on a daily basis and transformed into standard curve samples containing FR-6 at concentrations of 0.5, 2, 10, 50, 200, 400, and 600 ng/mL. The internal standard method was employed for quantifying the concentration of analyte under investigation (x) and determining the ratio between the peak area of x and that of the internal standard (y). Weighted least squares regression analysis (1/x2) was utilized to derive the regression equation: y = 0.0105 x + 0.0631 (R2 = 0.9961), with a lower limit of quantification set at 0.5 ng/mL.
3.2.3. Accuracy and precision
The precision (RSD) and accuracy (RE) in Table 1. For FR-6, both intra- and inter-batch QC samples exhibited RSD values below 15%, while the RE remained within ±15%. Additionally, the RSD for LLOQ samples was less than 20%, with a RE no more than ±20%.
Table 1.
Accuracy, intra-batch and inter-batch precisions for FR-6 (n = 5).
| Sample | Spiked conc. (ng/mL) | Measured conc. (ng/mL) | Precision (RSD, %) |
Accuracy (RE, %) |
||
|---|---|---|---|---|---|---|
| Intra-batch | Inter-batch | Intra-batch | Inter-batch | |||
| FR-6 | 0.5 | 0.51 ± 0.06 | 11.75 | 2.74 | 2.28 | 0.13 |
| 5 | 4.76 ± 0.32 | 6.76 | 6.85 | −4.72 | −1.81 | |
| 100 | 92.80 ± 1.64 | 1.76 | 0.61 | −7.20 | −7.28 | |
| 500 | 477.40 ± 27.84 | 5.83 | 2.72 | −4.52 | −6.61 | |
Note: conc., concentration; SD, standard deviation; RSD, relative standard deviation; RE, relative error; n, number of replicates.
3.2.4. Matrix effect
The results of the matrix effects are presented in Table 2, and the relative standard deviation (RSD) of the matrix effect at low quality control (LQC), medium quality control (MQC), and high-quality control (HQC) concentrations after normalization with an internal standard did not exceed 15%. This compliance with regulatory guidelines for assessing matrix effects indicates that the method employed maintains acceptable levels of matrix interference.
Table 2.
FR-6 matrix effects in plasma (Internal standard normalized matrix factor).
| Spiked conc.(ng/mL) | FR-6 |
||
|---|---|---|---|
| 5 | 100 | 500 | |
| Internal standard normalized matrix factor (%) | 105.91 | 113.78 | 97.21 |
| 110.91 | 106.63 | 104.56 | |
| 101.74 | 110.53 | 106.62 | |
| 100.90 | 99.48 | 109.95 | |
| 95.90 | 94.27 | 103.98 | |
| 103.40 | 111.83 | 99.51 | |
| Mean (%) | 103.13 | 106.09 | 103.64 |
| RSD (%) | 4.90 | 7.25 | 4.49 |
Note: conc., concentration; RSD, relative standard deviation.
3.2.5. Recovery
The recovery results are presented in Table 3, indicating good method performance with recovery of 89.37 ± 6.38%, 83.33 ± 2.63%, and 81.16 ± 8.98% for FR-6 at LQC, MQC, and HQC levels, respectively. The internal standard (IS) showed a recovery of 85.59 ± 4.90%. The recoveries of both FR-6 and internal standard were stable and reproducible.
Table 3.
Extraction recovery rate of FR-6 and IS in plasma (n = 5).
| Spiked conc. (ng/mL) | FR-6 |
IS |
||
|---|---|---|---|---|
| 5 | 100 | 500 | 2000 | |
| Recovery rate (%) | 88.19 | 79.31 | 80.53 | 83.82 |
| 96.06 | 80.55 | 79.21 | 94.12 | |
| 81.10 | 83.02 | 71.63 | 77.94 | |
| 80.31 | 83.02 | 93.28 | 87.50 | |
| 92.13 | 86.74 | 84.60 | 84.56 | |
| Mean (%) | 89.37 | 83.33 | 81.16 | 85.59 |
| RSD (%) | 6.38 | 2.63 | 8.98 | 4.90 |
Note: conc., concentration; RSD, relative standard deviation.
3.2.6. Residual effect and stability
Following the injection of quantitative upper limit samples, the peak areas of FR-6 and IS in the double blank samples were determined to be 0.00e+000, indicating no detectable residual effects for both FR-6 and IS. The stability results are presented in Table 4, where the relative error (RE) for FR-6 stability remained consistently below 15%, irrespective of whether the samples underwent three cycles of freeze-thaw at −80 °C, were stored at −80 °C for 21 days, or were kept in the injector (4 °C) for 12 h post-treatment.
Table 4.
The stability study of FR-6 (n = 3).
| Conditions | Spied conc. (ng/mL) | Measured conc. (mean ± SD, ng/mL) | RE (%) |
|---|---|---|---|
| Immediately determine | 5 | 4.98 ± 0.62 | – |
| 500 | 489.67 ± 30.07 | – | |
| Three freeze/thaw cycles (−80 °C) | 5 | 5.73 ± 0.76 | 7.65 |
| 500 | 452.80 ± 13.16 | −8.44 | |
| Autosampler for12h (4 °C) | 5 | 5.29 ± 0.40 | 4.71 |
| 500 | 506.37 ± 30.75 | 0.46 | |
| Freezing for 21 days (−80 °C) | 5 | 5.69 ± 0.56 | 6.93 |
| 500 | 477.40 ± 20.84 | −6.94 |
Note: conc., concentration; RE, relative error.
3.3. Pharmacokinetic study
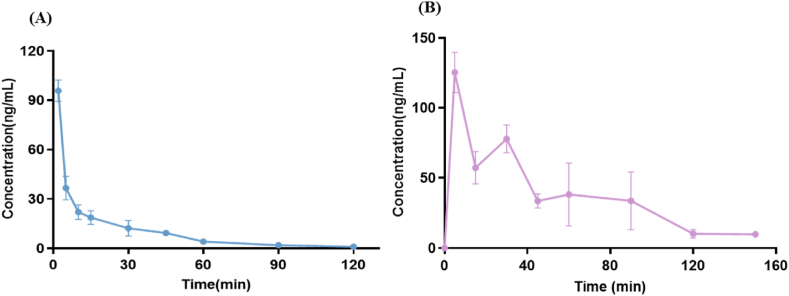
The mean blood concentration-time profiles of FR-6 following tail vein injection (Fig. 5A) and gavage (Fig. 5B) in SD rats. As depicted in Table 5, the plasma concentration of FR-6 reached its maximum (95.82 ± 14.49 ng/mL) after a 2 min (Tmax) administration via the tail vein, with a half-life (t1/2) of 6.77 ± 2.65 min. The AUC0-120 was calculated as 1351.94 ± 384.14 ng/mL·min, and AUC0-∞ was determined as 1352.36 ± 384.93 ng/mL·min, respectively. Following gavage for a duration of 5 min (Tmax), the plasma concentration of FR-6 peaked at Cmax (125.36 ± 32.19 ng/mL), exhibiting a t1/2 value of 33.66 ± 18.68 min. The AUC0-150 was calculated as 5646.22 ± 1852.98 ng/mL·min, and the AUC0-∞was 5963.46 ± 1731.73 ng/mL·min. The absolute bioavailability of FR-6 was determined as 88.19%。Peptide drugs possess distinctive advantages, characterized primarily by their elevated biological activity, cost-effectiveness, and enhanced stability in comparison to macromolecular protein drugs. Owing to the rapid metabolism of peptides into amino acids within the body [25,26], they exhibit swift clearance kinetics and minimal potential for accumulation. Consequently, peptide drugs have emerged as a prominent area of research interest. Although most commercially available peptide drugs are administered via injection (e.g., insulin) due to susceptibility to enzymatic degradation upon gastrointestinal entry, oral drug delivery has gained preference over injection-based delivery owing to its convenience and safety profile as the primary mode for protein peptide drug administration [27]. According to the pharmacokinetic parameters of FR-6, the t1/2 was 6.77 ± 2.65 min for tail vein administration and 33.66 ± 18.68 min for gavage. The t1/2 of oral administration was longer, and the AUC0-∞ was significantly higher than that of tail vein administration, indicating that FR-6 may be more suitable for oral administration with an absolute bioavailability of 88.19%.
Fig. 5.
Mean plasma concentration-time curves after tail vein injection (10 mg/kg, A) and gavage (50 mg/kg, B), Mean ± SD, n = 5.
Table 5.
The pharmacokinetic parameter of FR-6 in plasma after tail vein injection (iv) and gavage (ig) (n = 5).
| Pharmacokinetic parameters | iv | ig |
|---|---|---|
| Cmax (ng/mL) | 95.82 ± 14.49 | 125.36 ± 32.19 |
| Tmax (min) | 2 | 5 |
| K (1/min) | 0.11 ± 0.03 | 0.03 ± 0.01 |
| t1/2 (min) | 6.77 ± 2.65 | 33.66 ± 18.68 |
| MRT0-t (min) | 20.63 ± 1.91 | 47.88 ± 10.27 |
| MRT0-∞ (min) | 20.65 ± 1.89 | 58.13 ± 17.68 |
| CL (L/min/kg) | 7.83 ± 1.95 | 9.01 ± 2.74 |
| Vd (L/kg) | 72.01 ± 13.53 | 461.92 ± 318.68 |
| AUC0-t (ng/mL·min) | 1351.94 ± 384.14 | 5646.22 ± 1852.98 |
| AUC0-∞ (ng/mL·min) | 1352.36 ± 384.93 | 5963.46 ± 1731.73 |
| Absolute bioavailability (%) | 88.19 | |
4. Conclusion
In this study, we developed an LC-MS/MS method for the analysis of novel antihypertensive peptides in plasma, characterized by exceptional selectivity, high sensitivity, rapidity, and simplicity. The recovery rate was excellent and the linearity demonstrated outstanding performance within a concentration range of 0.5–600 ng/mL, meeting the stringent criteria for analyzing biological samples. By investigating peptide stability in plasma samples and exploring pre-treatment techniques for biological samples, we established a robust analytical approach that complies with FDA regulations. Subsequently we successfully applied this method to conduct pharmacokinetic experiments in rats. This technological breakthrough lays a solid foundation for future investigations into the pharmacokinetics of new antihypertensive peptides and related medications as well development of innovative therapeutic pharmaceuticals.
Data availability
Data will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Yu Yang: Writing – original draft, Software, Methodology, Investigation, Formal analysis, Conceptualization. Xingyan Bao: Writing – original draft, Data curation. Jiangyue Ning: Visualization, Investigation. Ruiyan Huang: Supervision, Resources. Yuan Liang: Validation, Software. Zelong Yan: Writing – review & editing. Haotian Chen: Investigation. Li Ding: Supervision. Chang Shu: Writing – review & editing, Supervision, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by the China Pharmaceutical University's “Double First Class” initiative (No. CPU2022QZ16).
References
- 1.Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The global burden of cardiovascular diseases and risk: a compass for future Health. J. Am. Coll. Cardiol. 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Piepho R.W. Overview of the angiotensin-converting-enzyme inhibitors. Am. J. Health Syst. Pharm. 2000;57(Suppl 1) doi: 10.1093/ajhp/57.suppl_1.S3. S3-7. [DOI] [PubMed] [Google Scholar]
- 3.Aluko R.E., Monu E. Functional and bioactive properties of quinoa seed protein hydrolysates. J. Food Sci. 2003;68:1254–1258. doi: 10.1111/j.1365-2621.2003.tb09635.x. [DOI] [Google Scholar]
- 4.Lan X., Liao D., Wu S., Wang F., Sun J., Tong Z. Rapid purification and characterization of angiotensin converting enzyme inhibitory peptides from lizard fish protein hydrolysates with magnetic affinity separation. Food Chem. 2015;182:136–142. doi: 10.1016/j.foodchem.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Pei J., Hua Y., Zhou T., Gao X., Dang Y., Wang Y. Transport, in vivo antihypertensive effect, and pharmacokinetics of an angiotensin-converting enzyme (ACE) inhibitory peptide LVLPGE. J. Agric. Food Chem. 2021;69:2149–2156. doi: 10.1021/acs.jafc.0c07048. [DOI] [PubMed] [Google Scholar]
- 6.Dang Y., Zhou T., Hao L., Cao J., Sun Y., Pan D. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. J. Agric. Food Chem. 2019;67:6757–6764. doi: 10.1021/acs.jafc.9b01137. [DOI] [PubMed] [Google Scholar]
- 7.Feng X., Liao D., Sun L., Feng S., Wu S., Lan P., Wang Z., Lan X. Exploration of interaction between angiotensin I-converting enzyme (ACE) and the inhibitory peptide from Wakame (Undaria pinnatifida) Int. J. Biol. Macromol. 2022;204:193–203. doi: 10.1016/j.ijbiomac.2022.01.114. [DOI] [PubMed] [Google Scholar]
- 8.Shobako N., Ogawa Y., Ishikado A., Harada K., Kobayashi E., Suido H., Kusakari T., Maeda M., Suwa M., Matsumoto M., Kanamoto R., Ohinata K. A novel antihypertensive peptide identified in thermolysin-digested rice bran. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700732. [DOI] [PubMed] [Google Scholar]
- 9.Dakhili S., Abdolalizadeh L., Hosseini S.M., Shojaee-Aliabadi S., Mirmoghtadaie L. Quinoa protein: composition, structure and functional properties. Food Chem. 2019;299 doi: 10.1016/j.foodchem.2019.125161. [DOI] [PubMed] [Google Scholar]
- 10.Guo H., Hao Y., Yang X., Ren G., Richel A. Exploration on bioactive properties of quinoa protein hydrolysate and peptides: a review. Crit. Rev. Food Sci. Nutr. 2021;63:2896–2909. doi: 10.1080/10408398.2021.1982860. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Y., Wang X., Zhuang Y., Li Y., Tian H., Shi P., Li G. In Vivo Antihypertension, and Molecular Docking. Molecules; 2019. Isolation of novel ACE-inhibitory and antioxidant peptides from quinoa bran albumin assisted with an in silico approach: characterization; p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cossum P.A., Dwyer K.A., Roth M., Chen S.A., Moffat B., Vandlen R., Ferraiolo B.L. The disposition of a human relaxin (hRlx-2) in pregnant and nonpregnant rats. Pharm. Res. (N. Y.) 1992;9:419–424. doi: 10.1023/a:1015863507496. [DOI] [PubMed] [Google Scholar]
- 13.Idowu O.S., Craigen J.L., Veal G.J., Jamieson D. Development and validation of an ELISA method for quantification of the anti-HER3 antibody HMBD-001 in human serum. Bioanalysis. 2022;14:1241–1249. doi: 10.4155/bio-2022-0141. [DOI] [PubMed] [Google Scholar]
- 14.Brutkiewicz S., Mendonca M., Stantz K., Comerford K., Bigsby R., Hutchins G., Goebl M., Harrington M. The expression level of luciferase within tumour cells can alter tumour growth upon in vivo bioluminescence imaging. Luminescence. 2007;22:221–228. doi: 10.1002/bio.953. [DOI] [PubMed] [Google Scholar]
- 15.Lin J.H. Pharmacokinetics of biotech drugs: peptides, proteins and monoclonal antibodies. Curr. Drug Metabol. 2009;10:661–691. doi: 10.2174/138920009789895499. [DOI] [PubMed] [Google Scholar]
- 16.Yang J.Z., Bastian K.C., Moore R.D., Stobaugh J.F., Borchardt R.T. Quantitative analysis of a model opioid peptide and its cyclic prodrugs in rat plasma using high-performance liquid chromatography with fluorescence and tandem mass spectrometric detection. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2002;780:269–281. doi: 10.1016/s1570-0232(02)00536-6. [DOI] [PubMed] [Google Scholar]
- 17.Chang D., Kolis S.J., Linderholm K.H., Julian T.F., Nachi R., Dzerk A.M., Lin P.P., Lee J.W., Bansal S.K. Bioanalytical method development and validation for a large peptide HIV fusion inhibitor (Enfuvirtide, T-20) and its metabolite in human plasma using LC-MS/MS. J. Pharm. Biomed. Anal. 2005;38:487–496. doi: 10.1016/j.jpba.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Xu R.-a., Lin Q., Qiu X., Chen J., Shao Y., Hu G., Lin G. UPLC-MS/MS method for the simultaneous determination of imatinib, voriconazole and their metabolites concentrations in rat plasma. J. Pharmaceut. Biomed. Anal. 2019;166:6–12. doi: 10.1016/j.jpba.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Qiu X., Zhao J.-l., Hao C., Yuan C., Tian N., Xu Z.-s., Zou R.-m. Simultaneous determination of mangiferin and neomangiferin in rat plasma by UPLC–MS/MS and its application for pharmacokinetic study. J. Pharmaceut. Biomed. Anal. 2016;124:138–142. doi: 10.1016/j.jpba.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Tang C., Niu X., Shi L., Zhu H., Lin G., Xu R.A. In vivo pharmacokinetic drug-drug interaction studies between fedratinib and antifungal agents based on a newly developed and validated UPLC/MS-MS method. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.626897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Liu Y.N., Xie S., Xu X., Xu R.A. Evaluation of the inhibitory effect of quercetin on the pharmacokinetics of tucatinib in rats by a novel UPLC-MS/MS assay. Pharm. Biol. 2022;60:621–626. doi: 10.1080/13880209.2022.2048862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allderdice P.W., Gardner H.A., Galutira D., Lockridge O., LaDu B.N., McAlpine P.J. The cloned butyrylcholinesterase (BCHE) gene maps to a single chromosome site, 3q26. Genomics. 1991;11:452–454. doi: 10.1016/0888-7543(91)90154-7. [DOI] [PubMed] [Google Scholar]
- 23.Perham R.N. The fructose-1,6-bisphosphate aldolases: same reaction, different enzymes. Biochem. Soc. Trans. 1990;18:185–187. doi: 10.1042/bst0180185. [DOI] [PubMed] [Google Scholar]
- 24.Jeon Y.H., Heo Y.S., Kim C.M., Hyun Y.L., Lee T.G., Ro S., Cho J.M. Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell. Mol. Life Sci. 2005;62:1198–1220. doi: 10.1007/s00018-005-4533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shobako N. Hypotensive peptides derived from plant proteins. Peptides. 2021;142 doi: 10.1016/j.peptides.2021.170573. [DOI] [PubMed] [Google Scholar]
- 26.Okagu I.U., Ezeorba T.P.C., Aham E.C., Aguchem R.N., Nechi R.N. Recent findings on the cellular and molecular mechanisms of action of novel food-derived antihypertensive peptides. Food Chem. 2022;4 doi: 10.1016/j.fochms.2022.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman M.S., Hossain K.S., Das S., Kundu S., Adegoke E.O., Rahman M.A., Hannan M.A., Uddin M.J., Pang M.G. Role of insulin in Health and disease: an update. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.