Abstract
Interpersonal space (IPS) refers to the area surrounding the body in which we engage in social interactions while maintaining our comfort. Numerous previous studies have reported the psychological and physiological changes associated with the proximity of two people engaged in face-to-face interaction. Currently, there is limited knowledge about how the relative position between two socially intimate individuals affects their psychological and physiological states. This research measured the subjective discomfort and electrocardiographic responses of participants when standing static at various relative positions. The highest discomfort, lowest heart rate, and highest heart rate variability (HRV; parasympathetic activity index) were observed when the friend stood in the face-to-face position. Interestingly, heart rate also decreased when the friend stood on the right side, although HRV did not change. We interpreted the results as suggesting that the presence of a familiar person elicits the electrocardiographic responses associated with an increase in parasympathetic activity.
Subject terms: Psychology, Human behaviour
Introduction
Communication with others is achieved by maintaining different positions and interpersonal distances. The space surrounding the body when interacting with others is called interpersonal space (IPS)1–5. Having adequate IPS is important for better interactions with others6,7.
Studies have identified the shape of the IPS based on the feeling associated with avoidance behavior when approached by others8,9, such as fear or discomfort related to autonomic nervous system responses10–18. In terms of autonomic nervous system responses, Candini et al.19 showed that skin conductance responses were higher when others entered the IPS. Other research has reported increased heart rate and decreased HRV when others approach the IPS20. Because fear is often associated with increased heart rate, decreased heart rate variability (HRV), and increased skin conductance21,22, these physiological changes may be caused by sympathetic activity23,24 in response to potentially threatening situations.
In many previous studies, the IPS was examined in a stopping distance task in which a stranger approached or retreated19,20,25. However, the social relationship could influence psychological and physiological responses. For example, when considering a real-life situation in which acquaintances or friends are standing next to us, the threat level might be relatively low compared to when strangers are standing next to us. Given that there is evidence that smaller IPSs may be formed for more intimate others, such as family and friends26–28, psychological and physiological responses may differ.
Furthermore, due to task limitations, such as the visual information-based measurement of approaching and retreating others, previous studies have often only examined interpersonal distance in front of participants. There is little research on the shape of the IPS around the body, and the findings are inconclusive8,25,29. For instance, Hayduk8 found that the anterior IPS was larger than the posterior IPS, whereas Hecht et al.25 observed that the shape of the personal space was similar to a circular zone. Only a few studies have compared more than two directions.
In this study, we investigated changes in psychological and physiological responses to the presence of another person at various positions in the transverse plane. Instead of the stop distance task commonly used in previous studies on the IPS, we used a static standing task with a lower level of fear. The advantage of our task is that it allows the IPS to be examined in various directions. Participants maintained a static standing posture in various relative positions (8 position conditions) with another person. Heart rate and HRV were assessed by recording participants’ electrocardiogram (ECG) data. We also assessed the subjective report of discomfort for each trial.
Results
To test the psychological and physiological responses to the presence of another person at various positions, subjective discomfort using the visual analog scale (VAS) and R-R intervals (RRI) and HRV calculated from ECG time-series data were collected, see “Methods” section for details. The means without normalization for each condition for all variables (discomfort, RRI, and RMSSD) are shown in Table 1. The results of the two-way mixed ANOVAs for all variables are shown in Table 2. Statistical results of the original scores for all variables are presented in the Supplementary materials.
Table 1.
Mean (SD) values without normalization of discomfort, RRI, and RMSSD in each condition.
| Conditions | F-see | R-see | L-see | B-see | R-seen | L-seen | B-seen | Baseline |
|---|---|---|---|---|---|---|---|---|
| Discomfort | ||||||||
| Person | 4.35 (1.33) | 5.37 (0.98) | 5.52 (1.19) | 5.43 (1.02) | 5.51 (1.26) | 5.85 (1.13) | 5.93 (1.26) | 6.49 (1.30) |
| Object | 4.75 (1.36) | 5.39 (1.24) | 5.53 (1.37) | 5.48 (1.19) | 5.49 (1.16) | 5.41 (1.44) | 5.78 (1.45) | 6.01 (1.34) |
| RRI [ms] | ||||||||
| Person | 747.90 (92.87) | 742.89 (96.38) | 727.83 (94.22) | 723.91 (93.77) | 734.00 (100.07) | 725.01 (92.61) | 714.38 (93.55) | 710.75 (92.39) |
| Object | 732.94 (98.98) | 728.82 (102.16) | 734.70 (109.55) | 744.03 (123.80) | 722.39 (100.18) | 729.85 (108.19) | 739.15 (112.03) | 741.28 (119.95) |
| RMSSD [ms] | ||||||||
| Person | 27.66 (11.30) | 20.76 (9.61) | 20.74 (8.28) | 21.18 (10.07) | 22.15 (9.50) | 21.81 (8.63) | 21.57 (8.81) | 20.99 (8.89) |
| Object | 20.97 (12.79) | 19.75 (11.86) | 19.72 (11.02) | 22.41 (12.30) | 20.66 (12.06) | 20.98 (12.35) | 20.25 (12.63) | 20.48 (12.29) |
Table 2.
Statistical results of two-way mixed ANOVAs on discomfort, RRI, and RMSSD.
| Variables | Effect factor | F | df | p | ηp2 |
|---|---|---|---|---|---|
| Discomfort | Condition | 13.117 | 7, 308 | < 0.001 | 0.230 |
| Condition * Group | 1.074 | 7, 308 | 0.380 | 0.024 | |
| Group | 1.861 | 1, 44 | 0.179 | 0.041 | |
| RRI | Condition | 3.035 | 7, 294 | 0.004 | 0.067 |
| Condition * Group | 6.759 | 7, 294 | < 0.001 | 0.139 | |
| Group | 11.962 | 1, 42 | 0.001 | 0.222 | |
| RMSSD | Condition | 9.420 | 7, 294 | < 0.001 | 0.183 |
| Condition * Group | 7.739 | 7, 294 | < 0.001 | 0.156 | |
| Group | 1.763 | 1, 42 | 0.191 | 0.040 |
Subjective report of discomfort
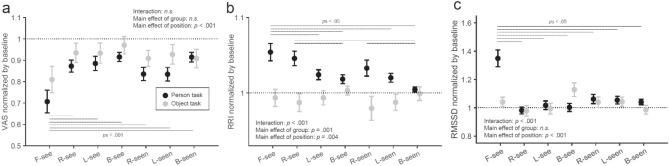
Figure 1a shows the ratio of the average discomfort scores for each condition to the baseline (back-to-back) condition. Lower scores indicate greater discomfort. A two-way mixed ANOVA revealed a significant main effect of the position condition (F[7, 308] = 13.117, p < 0.001, ηp2 = 0.230). A simple main effect test indicated that a significantly lower discomfort score (i.e., higher discomfort feeling) was observed in the F-see condition than those in all other conditions (pHolms < 0.001). The discomfort scores in the R-see, L-see, R-seen, L-seen, and B-seen conditions were significantly lower than that in the baseline condition (pHolms < 0.05). The main effect of the group (F[1, 44] = 1.861, p = 0.179, ηp2 = 0.041) and interaction (F[7, 308] = 1.074, p = 0.380, ηp2 = 0.024) were not significant.
Figure 1.
Plots of discomfort, RRI, and RMSSD ratio to the baseline condition. Error bars indicate the standard errors.
Heart rate
R-R intervals (RRI)
Figure 1b shows the average RRI scores for each condition to the baseline (back-to-back) condition. A two-way mixed ANOVA revealed a significant interaction (F[7, 294] = 6.759, p < 0.001, ηp2 = 0.139). Post-hoc analysis revealed that the RRI ratio of the F-see condition in the Person task was significantly higher than that of all conditions except those of the R-see and R-seen conditions in the Person task and those of all conditions in the Object task (pHolms < 0.05). The RRI ratio of the R-see condition in the Person task was significantly higher than those of the B-see, B-seen, and Baseline conditions in the Person task and those of all conditions in the Object task (pHolms < 0.05). The RRI ratio of the R-seen condition in the Person task was significantly higher than that of B-seen and Baseline conditions in the Person task and those of R-see, R-seen, and L-seen conditions in the Object task (pHolms < 0.05). The main effect of the position condition (F[7, 294] = 3.035, p = 0.004, ηp2 = 0.067) and that of the group (F[1, 42] = 11.962, p = 0.001, ηp2 = 0.222) were significant.
Root mean square of successive RRI differences (RMSSD)
Figure 1c shows the average RMSSD scores for each condition to the baseline (back-to-back) condition. A two-way mixed ANOVA revealed significant interactions (F[7, 294] = 7.739, p < 0.001, ηp2 = 0.156). Post-hoc analyses indicated that RMSSD of the F-see condition in the Person task were significantly greater than those of all other conditions (pHolms < 0.001). The main effect of the position condition was significant (F[7, 294] = 9.420, p < 0.001, ηp2 = 0.183). The main effect of the group was not significant (F[1, 42] = 1.763, p = 0.191, ηp2 = 0.040).
Discussion
The results showed that the greatest discomfort, the greatest decrease in heart rate, and the greatest increase in HRV occurred when others were standing in front. In addition, the decrease in heart rate was observed when a person was on the right side. The changes in electrocardiographic responses occurred when a person, not an object, was present.
We found that heart rate decreased when another person was present in specific relative positions (front and right). In addition, HRV tended to increase, i.e., parasympathetic activity increased when standing facing another person. This result could be raised by the social relationship and social situation in the current experimental setting. Given the evidence that the presence of a romantic partner or spouse in a good relationship activates parasympathetic activity, such as low resting blood pressure and higher HF-HRV30,31, the observed decrease in heart rate may have been due to increased parasympathetic activity during interactions with others who were not as close as romantic partners and spouse but still had relatively close relationship with them. It has been suggested that invasion of IPS induces negative emotions associated with avoidance behaviors such as fear and discomfort8,9 and an increased skin conductance response and decreased heart rate variability was observed19,20. In the social situation with an approaching stranger used in many previous studies, people may experience discomfort associated with increased sympathetic responses. Candini et al.19 reported that an increase in skin conductance response was not observed in social situations with a relatively low degree of threat, such as a stranger moving away (withdrawal condition). In the current study, two people were standing stationary, which may have been a low threat level situation compared to approaching situation, as in the withdrawal condition. It is possible that activities of automatic nervous system are sensitive to the social context of social intimacy or the type of situation in which they are interacting.
Interestingly, a decrease in heart rate was also observed when another person was on the right side. One possible explanation for this asymmetry could be the influence of handedness. Gérin-Lajoie et al.32 reported that the physical distance between a person and an object was shorter on the dominant side of the hand than on the non-dominant side when bypassing an object. Since all but one of the participants in this study was also right-handed, the IPS on the right side might be smaller than on the left side. This asymmetry in IPS might have produced differences in the electrocardiographic responses received when standing with others. However, since the increased parasympathetic activity was not observed when others were on the right side, there might be a different mechanism of the autonomic activity underlying the right-side specific electrocardiographic response than when others were in the front. To explore this possibility, autonomic activity would need to be examined in terms of multiple physiological indices. Our study could not quantify sympathetic activity because the data length of this study was not sufficient to estimate sympathetic activity. Future studies should investigate this possibility by simultaneously measuring sympathetic activity, which can be examined from electrodermal response and long-term ECG data, indices to follow the relationship between the IPS and the autonomic nervous system. Furthermore, considering that the activity of the autonomic nervous system is influenced by body movements33,34, the possibility of body movements depending on the relative position conditions could not be ruled out. Simultaneous measurement of body movements would help to clarify whether the increase in parasympathetic activity in a particular relative position is due to body movement or not. In addition, the back-to-back condition was set as the baseline condition in both the person and object tasks to normalize the data in the current study. Given that potential differences between the baseline conditions for both tasks could not be eliminated due to the differences in what was behind the two tasks, it is necessary to measure the resting autonomic nervous system and set it as the baseline condition in future studies.
Although we measured the changes in electrocardiographic responses at only one interpersonal distance in the current study, it has been suggested that individual differences exist in comfortable spaces with others, and that psychological and physiological responses may differ even when another person is physically present at the same distance19. One possible individual characteristic behind these individual differences in IPS is interoceptive accuracy35,36. Ferri et al.35 reported that individuals with high and low interoceptive accuracy differ in their autonomic responses to the presence of others around their peripersonal space. Although the relationship between peripersonal space and IPS is still on the table37, if both have the meaning of boundaries that include the self and others, it is possible that there is an association between personal characteristics such as interoceptive accuracy and the IPS.
Our findings suggest that electrocardiographic responses elicited by the invasion of the IPS by others have the potential to define the boundaries between self and others. We also believe that our reports that the physical reactions can vary in social contexts provide a new perspective on a number of studies of IPS that have primarily examined explicitly psychological variables.
Conclusion
This study showed that increased discomfort, decreased heart rate, and increased HRV occurred when others with high social closeness were present in front. These results may have been caused by the presence of a familiar person increasing the parasympathetic activity. Although a previous study reported that the approach of a stranger elicits sympathetic activity, the present study suggests that the physiological responses elicited differ depending on the social context.
Methods
Before conduction the experiment, the study was approved by the Ethics Committee of Nagoya University (No. NUPSY-210901-L-01) and all study methods were performed in accordance with the relevant guidelines and regulations approved by the Research Ethics Committee. Informed consent was obtained from all participants prior to their participation in this study.
Experimental task
Two tasks were used to investigate the effect of the presence of another person on one’s psychological and physiological state. One was a Person task in which participants were paired with a human partner. The other was an Object task in which an object was present in the surroundings instead of a human partner.
Person task
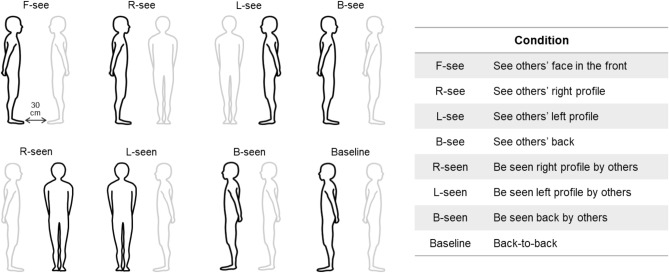
Two paired participants (friend pair) maintained a static standing posture for 60 s. They crossed their arms behind their backs to reduce fatigue from prolonged standing posture. Eight conditions were set regarding the positional relationship between the two participants (Fig. 2), as follows. In the F-see condition, participants were required to maintain a static standing posture face-to-face with friends, with their eyes fixed on the center of the partner’s eyes. In the L-see, R-see, and B-see conditions, they stood while looking at the friends' right profiles, left profiles, and backs with their eyes fixed on a point on the friends' heads, respectively. In the F-seen, L-seen, and B-seen conditions, they stood while friends looked at their right profiles, left profiles, and backs, respectively. In the baseline condition, they stood back-to-back with their friends. In the F-seen, L-seen, B-seen, and baseline conditions, they kept their eyes on the fixation point 30 cm in front of them. In all conditions, the distance between paired participants was 30 cm.
Figure 2.
Experimental conditions for the Person task. Paired participants maintained a static standing posture with their hands crossed behind their backs for 60 s. Eight conditions were set regarding the positional relationship between the two paired participants.
Object task
Participants maintained a static standing posture under the same conditions as in the Person task with an object placed in the same location. The object was a cylinder of the same height as each participant. The width of the object was 20 cm (circumference: 63 cm). In all conditions, participants were required to fixate a fixation point 30 cm in front of them while performing the Object task.
For both tasks, participants provided subjective ratings of comfort/discomfort using a visual analog scale (VAS) (0: lowest comfort—10: greatest comfort) after performing each trial. Participants' ECGs during the task were recorded with MP160 and Acknowledge (BIOPAC System Inc., US) at a sampling rate of 2000 Hz.
Participants
Fifteen Japanese friend pairs (30 participants, 16 females, mean age = 20.1 ± 1.4 years, mean height = 163.5 ± 7.3 cm) participated in the Person task. Sixteen Japanese adults (15 females, mean age = 20.4 ± 1.8 years, mean height = 161.6 ± 6.5 cm) participated in the Object task. There was no overlap of participants between the two experiments. The average height difference between the two paired participants in the Person task was 4.5 cm. The dominant hand of all participants was right-handed, except for one participant in the Person task. All had normal or corrected-to-normal vision.
Procedure
Participants were instructed to maintain a stationary standing posture for 60 s, facing the direction specified for each condition. Participants completed a total of 32 trials, including 4 trials in eight conditions. The order of trials was pseudo-randomized with a constraint that the same condition could not appear two or more times in a row. The duration of the trials was approximately one and a half hours (Person task) or one hour (Object task), including breaks.
Data analysis
The participants’ ECG time series data were high-pass filtered at 1 Hz and low-pass filtered at 35 Hz. For analysis, we used ECG data for a length of 60 s. Using the participant's ECG data, we detected the R wave's peak values and calculated the mean R–R interval (RRI) for each condition. Errors and noise were visually inspected during the RRI detection process. The subsequent analysis included ECG data of 14 pairs (28 participants) in the Person task group and 16 participants in the Object task group, except for one pair whose ECG data was not recorded successfully.
Moreover, HRV was evaluated employing root mean square of successive RRI differences (RMSSD)38–42. This methods assess parasympathetic activity of the autonomic nervous system using only a small amount of data40–42. The RMSSD was calculated by the following formula (1):
| 1 |
RMSSD reflects rapid beat-to-beat variability and is primarily associated with parasympathetic activity40. RMSSD were calculated from data points for 60 s on each trial, as was RRI.
Statistical analysis
Since there was no significant difference in baseline condition between the person and object conditions (see Supplementary materials), the ratio to the baseline condition was calculated by dividing the values of all variables (discomfort, RRI, and RMSSD) for each condition by the value of each participant’s baseline condition in order to control individual differences in psychological and electrocardiographic responses. Two-way mixed ANOVAs (group [2: Person vs. Object] × position condition [8]) were performed. All statistical analyses were performed with JASP 0.16.4.043. The level of statistical significance was set at p < 0.05.
Supplementary Information
Acknowledgements
This study was supported by Moonshot Research and Development (JPMJMS2012) from JST and KAKENHI (19K20651, 22H00090, 22H03494, 20K22293, and 21J01257) from JSPS. We also thank Sakina Ubukata for helping of data collection.
Author contributions
K.M., T.I., R.O., and K.W. designed the experiments. K.M. prepared the experimental task and performed the experiment. K.M. programmed and analyzed the results. All authors interpreted the results. K.M. wrote the first paper. T.I., R.O., and K.W. revised the paper. All authors confirmed and approved the final version of the paper.
Data availability
Analyzed data and commented analysis script are available on the Open Science Framework (OSF): https://osf.io/txyfc/?view_only=41f08b5f6aa144a9a0e1b13f8af3200c.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-54439-5.
References
- 1.Becker FD, Mayo C. Delineating personal distance and territoriality. Environ. Behav. 1971;3:375. [Google Scholar]
- 2.Knowles ES. Boundaries around social space: "Dyadic responses to an invader". Environ. Behav. 1972;4:437. [Google Scholar]
- 3.Lloyd DM. The space between us: A neurophilosophical framework for the investigation of human interpersonal space. Neurosci. Biobehav. Rev. 2009;33:297–304. doi: 10.1016/j.neubiorev.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Hall ET. Distances in Man: The Hidden Dimension. Double Day; 1966. [Google Scholar]
- 5.Sommer R. Studies in personal space. Sociometry. 1959;22:247–260. doi: 10.2307/2785668. [DOI] [Google Scholar]
- 6.Costa M. Interpersonal distances in group walking. J. Nonverbal Behav. 2010;34:15–26. doi: 10.1007/s10919-009-0077-y. [DOI] [Google Scholar]
- 7.Thompson DE, Aiello JR, Epstein YM. Interpersonal distance preferences. J. Nonverbal Behav. 1979;4:113–118. doi: 10.1007/BF01006355. [DOI] [Google Scholar]
- 8.Hayduk LA. The shape of personal space: An experimental investigation. Can. J. Behav. Sci./Revue. 1981;13:87–93. doi: 10.1037/h0081114. [DOI] [Google Scholar]
- 9.Hayduk LA. Personal space: Where we now stand. Psychol. Bull. 1983;94:293–335. doi: 10.1037/0033-2909.94.2.293. [DOI] [Google Scholar]
- 10.Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006;10:229–240. doi: 10.1037/1089-2680.10.3.229. [DOI] [Google Scholar]
- 11.Collet C, Vernet-Maury E, Delhomme G, Dittmar A. Autonomic nervous system response patterns specificity to basic emotions. J. Auton. Nerv. Syst. 1997;62:45–57. doi: 10.1016/S0165-1838(96)00108-7. [DOI] [PubMed] [Google Scholar]
- 12.Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- 13.Hagemann D, Waldstein SR, Thayer JF. Central and autonomic nervous system integration in emotion. Brain Cogn. 2003;52:79–87. doi: 10.1016/S0278-2626(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 14.Khalfa S, Isabelle P, Jean-Pierre B, Manon R. Event-related skin conductance responses to musical emotions in humans. Neurosci. Lett. 2002;328:145–149. doi: 10.1016/S0304-3940(02)00462-7. [DOI] [PubMed] [Google Scholar]
- 15.Kreibig SD. Autonomic nervous system activity in emotion: A review. Biol. Psychol. 2010;84:394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Lang PJ, Bradley MM, Cuthbert BN. Emotion and motivation: Measuring affective perception. J. Clin. Neurophysiol. 1998;15:397–408. doi: 10.1097/00004691-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Levenson RW. The autonomic nervous system and emotion. Emot. Rev. 2014;6:100–112. doi: 10.1177/1754073913512003. [DOI] [Google Scholar]
- 18.Lynar E, Cvejic E, Schubert E, Vollmer-Conna U. The joy of heartfelt music: An examination of emotional and physiological responses. Int. J. Psychophysiol. 2017;120:118–125. doi: 10.1016/j.ijpsycho.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Candini M, Battaglia S, Benassi M, Pellegrino G, Frassinetti F. The physiological correlates of interpersonal space. Sci. Rep. 2021;11:2611. doi: 10.1038/s41598-021-82223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farkas K, et al. Altered interpersonal distance regulation in autism spectrum disorder. PLoS One. 2023;18:e0283761. doi: 10.1371/journal.pone.0283761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aue T, Flykt A, Scherer KR. First evidence for differential and sequential efferent effects of stimulus relevance and goal conduciveness appraisal. Biol. Psychol. 2007;74:347–357. doi: 10.1016/j.biopsycho.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Damasio AR, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 23.Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur. J. Appl. Physiol. 2003;90:317–325. doi: 10.1007/s00421-003-0953-9. [DOI] [PubMed] [Google Scholar]
- 24.van Dooren M, de Vries JJGG-J, Janssen JH. Emotional sweating across the body: comparing 16 different skin conductance measurement locations. Physiol. Behav. 2012;106:298–304. doi: 10.1016/j.physbeh.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Hecht H, Welsch R, Viehoff J, Longo MR. The shape of personal space. Acta Psychol. 2019;193:113–122. doi: 10.1016/j.actpsy.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Duke MP, Nowicki S. A new measure and social-learning model for interpersonal distance. J. Exp. Res. Personality. 1972;6:119–132. [Google Scholar]
- 27.Morgado N, Muller D, Gentaz E, Palluel-Germain R. Close to me? The influence of affective closeness on space perception. Perception. 2011;40:877–879. doi: 10.1068/p6830. [DOI] [PubMed] [Google Scholar]
- 28.Sundstrom E, Altman I. Interpersonal relationships and personal space: Research review and theoretical model. Hum. Ecol. Interdiscip. J. 1976;4:47–67. doi: 10.1007/BF01531456. [DOI] [Google Scholar]
- 29.Newman RC, Pollack D. Proxemics in deviant adolescents. J. Consult. Clin. Psychol. 1973;40:6–8. doi: 10.1037/h0033961. [DOI] [PubMed] [Google Scholar]
- 30.Smith TW, et al. Matters of the variable heart: Respiratory sinus arrhythmia response to marital interaction and associations with marital quality. J. Pers. Soc. Psychol. 2011;100:103–119. doi: 10.1037/a0021136. [DOI] [PubMed] [Google Scholar]
- 31.Uchino BN, Sanbonmatsu DM, Birmingham W. Knowing your partner is not enough: Spousal importance moderates the link between attitude familiarity and ambulatory blood pressure. J. Behav. Med. 2013;36:549–555. doi: 10.1007/s10865-012-9437-x. [DOI] [PubMed] [Google Scholar]
- 32.Gérin-Lajoie M, Richards CL, Fung J, McFadyen BJ. Characteristics of personal space during obstacle circumvention in physical and virtual environments. Gait Posture. 2008;27:239–247. doi: 10.1016/j.gaitpost.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Kowalewski MA, Urban M. Short- and long-term reproducibility of autonomic measures in supine and standing positions. Clin. Sci. 2004;106:61–66. doi: 10.1042/CS20030119. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe N, Reece J, Polus BI. Effects of body position on autonomic regulation of cardiovascular function in young, healthy adults. Chiropr. Osteopat. 2007;15:19. doi: 10.1186/1746-1340-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferri F, Ardizzi M, Ambrosecchia M, Gallese V. Closing the gap between the inside and the outside: Interoceptive sensitivity and social distances. PLoS One. 2013;8:e75758. doi: 10.1371/journal.pone.0075758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isomura T, Watanabe K. Direct gaze enhances interoceptive accuracy. Cognition. 2020;195:104113. doi: 10.1016/j.cognition.2019.104113. [DOI] [PubMed] [Google Scholar]
- 37.Bogdanova OV, Bogdanov VB, Dureux A, Farnè A, Hadj-Bouziane F. The Peripersonal Space in a social world. Cortex. 2021;142:28–46. doi: 10.1016/j.cortex.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 38.DeGiorgio CM, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: The SUDEP-7 Inventory. Epilepsy Behav. 2010;19:78–81. doi: 10.1016/j.yebeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeppesen J, Beniczky S, Johansen P, Sidenius P, Fuglsang-Frederiksen A. Detection of epileptic seizures with a modified heart rate variability algorithm based on Lorenz plot. Seizure. 2015;24:1–7. doi: 10.1016/j.seizure.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Munoz ML, et al. Validity of (ultra-)short recordings for heart rate variability measurements. PLoS One. 2015;10:e0138921. doi: 10.1371/journal.pone.0138921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salahuddin L, Cho J, Jeong MG, Kim D. Ultra short term analysis of heart rate variability for monitoring mental stress in mobile settings. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007;2007:4656–4659. doi: 10.1109/IEMBS.2007.4353378. [DOI] [PubMed] [Google Scholar]
- 42.Toichi M, Sugiura T, Murai T, Sengoku A. A new method of assessing cardiac autonomic function and its comparison with spectral analysis and coefficient of variation of R-R interval. J. Auton. Nerv. Syst. 1997;62:79–84. doi: 10.1016/S0165-1838(96)00112-9. [DOI] [PubMed] [Google Scholar]
- 43.Love J, et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019;88:1–17. doi: 10.18637/jss.v088.i02. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Analyzed data and commented analysis script are available on the Open Science Framework (OSF): https://osf.io/txyfc/?view_only=41f08b5f6aa144a9a0e1b13f8af3200c.