Summary
Background
In the post-pandemic era, growing apprehension exists regarding the potential sequelae of COVID-19. However, the risks of respiratory diseases following SARS-CoV-2 infection have not been comprehensively understood. This study aimed to investigate whether COVID-19 increases the long-term risk of respiratory illness in patients with COVID-19.
Methods
In this longitudinal, population-based cohort study, we built three distinct cohorts age 37–73 years using the UK Biobank database; a COVID-19 group diagnosed in medical records between January 30th, 2020 and October 30th, 2022, and two control groups, a contemporary control group and a historical control group, with cutoff dates of October 30th, 2022 and October 30th, 2019, respectively. The follow-up period of all three groups was 2.7 years (the median (IQR) follow-up time was 0.8 years). Respiratory outcomes diagnosed in medical records included common chronic pulmonary diseases (asthma, bronchiectasis, chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), pulmonary vascular disease (PVD), and lung cancer. For the data analysis, we calculated hazard ratios (HRs) along with their 95% CIs using Cox regression models, following the application of inverse probability weights (IPTW).
Findings
A total of 3 cohorts were included in this study; 112,311 individuals in the COVID-19 group with a mean age (±SDs) of 56.2 (8.1) years, 359,671 in the contemporary control group, and 370,979 in the historical control group. Compared with the contemporary control group, those infected with SARS-CoV-2 exhibited elevated risks for developing respiratory diseases. This includes asthma, with a HR of 1.49 and a 95% CI 1.28–1.74; bronchiectasis (1.30; 1.06–1.61); COPD (1.59; 1.41–1.81); ILD (1.81; 1.38–2.21); PVD (1.59; 1.39–1.82); and lung cancer (1.39; 1.13–1.71). With the severity of the acute phase of COVID-19, the risk of pre-described respiratory outcomes increases progressively. Besides, during the 24-months follow-up, we observed an increasing trend in the risks of asthma and bronchiectasis over time. Additionally, the HR of lung cancer for 0–6 month follow-up was 3.07 (CI 1.73–5.44), and the association of lung cancer with COVID-19 disease disappeared at 6–12 month follow-up (1.06; 0.43–2.64) and at 12–24 months (1.02; 0.45–2.34). Compared to those with one SARS-CoV-2 infection, reinfected patients were at a higher risk of asthma (3.0; 1.32–6.84), COPD (3.07; 1.42–6.65), ILD (3.61; 1.11–11.8), and lung cancer (3.20; 1.59–6.45). Similar findings were noted when comparing with a historical cohort serving as a control group, including asthma (1.31; 1.13–1.52); bronchiectasis (1.53; 1.23–1.89); COPD (1.41; 1.24–1.59); ILD (2.53; 2.05–3.13); PVD (2.30; 1.98–2.66); and lung cancer (2.23; 1.78–2.79).
Interpretation
Our research suggests that patients with COVID-19 may have an increased risk of developing respiratory diseases, and the risk increases with the severity of infection and reinfection. Even during the 24-month follow-up, the risk of asthma and bronchiectasis continued to increase. Hence, implementing appropriate follow-up strategies for these individuals is crucial to monitor and manage potential long-term respiratory health issues. Additionally, the increased risk in lung cancer in the COVID-19 individuals was probably due to the diagnostic tests conducted and incidental diagnoses.
Funding
The National Natural Science Foundation of China of China Regional Innovation and Development Joint Foundation; National Natural Science Foundation of China; Program for High-level Foreign Expert Introduction of China; Natural Science Foundation for Distinguished Young Scholars of Guangdong Province; Guangdong Basic and Applied Basic Research Foundation; Climbing Program of Introduced Talents and High-level Hospital Construction Project of Guangdong Provincial People’s Hospital; VA Clinical Merit and ASGE clinical research funds.
Keywords: COVID-19 (coronavirus disease 2019), SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), Respiratory diseases, Epidemiological study
Research in context.
Evidence before this study
We searched PubMed for studies published until September 01th, 2023, using search terms “COVID-19” or “SARS-CoV-2” and “sequelae”, “complication”, “lung”, “respiratory” with search terms found in abstract, title or MESH headings, to identify studies evaluating the relationship between SARS-CoV-2 infection and respiratory diseases. We also conducted a search of the references listed in the identified papers. Previous studies have reported that the risks of several respiratory diseases (Asthma, ILD, and Chronic pulmonary disease) increased in COVID-19 cohorts, COVID-19 vaccine may affect the risk of complications. However, current research on the risk of respiratory diseases in patients infected with SARS-CoV-2 is limited to cohorts with relatively small sample sizes and short follow-up times, or has only analyzed a small subset of respiratory diseases, failing to effectively address the impact of vaccines on respiratory system. Besides, there is no study to determine whether reinfection with SARS-CoV-2 increases the risk of respiratory disease.
Added value of this study
Our analyses suggest that COVID-19 may be significantly associated with higher risk of developing respiratory diseases, such as asthma, bronchiectasis, COPD, ILD, PVD and lung cancer, with increased risk correlating to the severity of the infection, and the association with certain respiratory diseases remaining significant among non-hospitalized patients. During the 24-months follow-up, the risks of asthma and bronchiectasis increased, while other respiratory conditions like COPD and lung cancer showed decreasing trends. Reinfection with COVID-19 led to a greater risk of developing respiratory diseases such as asthma, COPD, lung diseases, and lung cancer compared to those with a single infection. Additionally, most risks of respiratory outcomes were increased before the availability of COVID-19 vaccination.
Implications of all the available evidence
Our study adds to the existing knowledge regarding the effects of COVID-19 on the respiratory system. Yet it's worth noting that the increased risk in lung cancer in the COVID-19 group was probably due to the diagnostic tests conducted and incidental diagnoses. Our findings emphasize the importance of providing extended care and attention to patients infected with SARS-CoV-2s, as well as implementing appropriate follow-up strategies to address potential post-acute complications in the respiratory system.
Introduction
Since 2019, the worldwide outbreak of coronavirus disease 2019 (COVID-19), initiated by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has escalated into a significant public health challenge.1,2 As we enter the post-pandemic era, the enduring consequences of COVID-19 are receiving increasing global attention.
Most patients recovered within the acute phase of COVID-19 (four weeks post-infection), while some cases developed long-term health complications associated with COVID-19.3 When symptoms persist or appear after 4 weeks of a verified SARS-CoV-2 diagnosis, it's termed as Post-Covid Syndrome or post-acute sequelae of SARS-CoV-2 (PASC).4 According to the US Centers for Disease Control and Prevention (CDC), Post-Covid Syndrome is a wide range of ongoing health problems5 that people who have been infected can display, spanning from asymptomatic or mild respiratory illness to severe and multiple organ impairments.3,6 Hence, it's necessary to focus more on the post-acute syndrome of COVID-19 and its related complications.
There is evidence that the risks of several respiratory diseases (Asthma,7 ILD,8 and Chronic pulmonary disease9) increased in COVID-19 cohorts. However, the risks of respiratory diseases in the post-acute phase of COVID-19 remain unclear. Current studies on the long-term risks of respiratory diseases in patients infected with SARS-CoV-2 have been limited to small sample sizes and comparatively brief follow-up time, and only a small fraction of respiratory diseases were analysed.7, 8, 9 Only two large studies have expanded the analysis to report long-term acute sequelae of patients with COVID-19, including multiple organ complications and mortality.10,11 However, only 2–3 of these respiratory complications were reported, and a comprehensive analysis of COVID-19's prolonged effects on the respiratory system is yet to be fully conducted. In addition, COVID-19 vaccination may affect the risk of complications,12, 13, 14 but its effect on respiratory diseases has not been adequately answered in earlier studies. Besides, there is no study to determine whether reinfection with SARS-CoV-2 increases the risk of respiratory disease. To overcome the previously mentioned shortcomings, we conducted a large-scale cohort study, drawing on continuous long-term follow-up data from the UK Biobank, to help us further comprehensively analyse the overall impact of COVID-19 on respiratory disease complications in this study.
This investigation aimed to assessed and characterized the long-term risk of respiratory system diseases among individuals who had survived the acute phase of COVID-19. Additionally, we assessed the risk based on the severity of the acute phase of COVID-19, different follow-up times and analysed the potential impact of COVID-19 vaccination on respiratory outcomes.
Methods
Data source
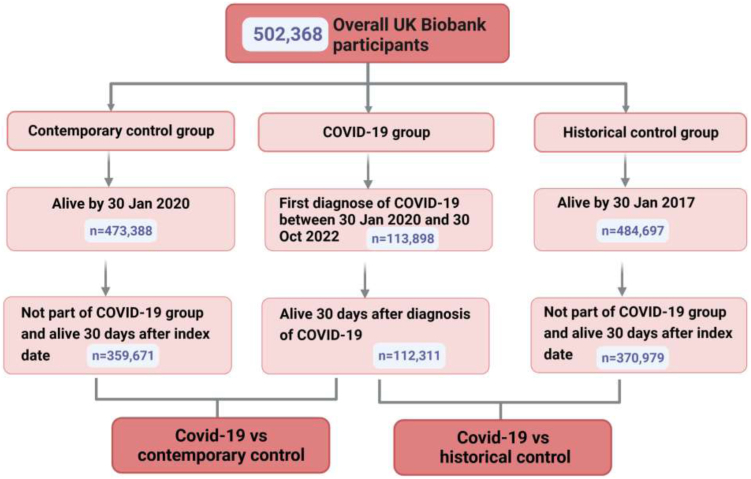
Our research drew from the UK Biobank database, a large-scale biomedical database contains medical records of 502,368 participants aged between 37 and 73 years, who were enrolled from 2006 to 2010. The database provided extensive health-related information through baseline or follow-up online questionnaires, verbal interviews, biological samples, and physical assessments. Approval for this study was granted by the North West Multi-Center Research Ethics Committee, with approval numbers: 11/NW/0382, 16/NW/0274, and 21/NW/0157, and written informed consent was obtained from each participant. The UK Biobank project is assigned the approval number 83339. Further details about the UK Biobank can be found in Supplementary Notes.
Cohorts
COVID-19 infection was defined as the first positive result on COVID-19 polymerase chain reaction (PCR) testing or patients being initially diagnosed with COVID-19 (U07.1 and U07.2) in medical records between January 30th, 2020 and October 30th, 2022. Then, we focused on individuals who survived for over 30 days after the COVID-19 diagnosis (n = 112,311) to investigate outcomes during the post-acute phase, which constituted the COVID-19 group. Further details about the cohorts can be found in Supplementary Notes.
To explore the impact of COVID-19 on respiratory disease, two control groups were constructed to examine the associations—a contemporary control group and a historical control group. The contemporary control group was constituted by individuals who were alive by January 30th, 2020 and but not included in the COVID-19 group. To further compare the impact of COVID-19 with a control group unaffected by the pandemic, the historical control group was comprising by individuals who were started from January 31st, 2017 (3 years prior to the pandemic) after being excluded in the COVID-19 group. Participants who died before January 30th, 2020, the COVID-19 outbreak (n = 28,980) and those who were lost to follow-up (n = 1298) were omitted.
To ensure comparable follow-up time distributions, the start time of follow-up for the contemporary control group was randomly assigned based on the start time of follow-up for the COVID-19 group. Simultaneously, for the historical control group, the start time of follow-up was assigned randomly, using a similar distribution as the start of follow-up minus three years in the COVID-19 group.
For each patient, the follow-up deadline was until the first date of outcome, mortality, or October 30th, 2022 for contemporary cohort and October 30th, 2019 for historical cohort, whichever occurred first.
Outcomes
The selection of respiratory outcomes was primarily based on prior literature evidence12,13 and data obtained from the UK Biobank's records on respiratory system diseases. Elaborate explanations of the definitions for all outcomes, aligned with the 10th revision of the International Classification of Diseases (ICD-10), can be found in Supplementary Table S1. The specific outcomes encompassed in the analysis are as follows: (1) asthma, (2) bronchiectasis, (3) chronic obstructive pulmonary disease (COPD), (4) interstitial lung disease (ILD), which includes pulmonary eosinophilia, sarcoidosis of the lung, and other ILD, and (5) pulmonary vascular disease (PVD), which includes pulmonary embolism, pulmonary heart disease, pulmonary edema, and other diseases of pulmonary vessels. Other respiratory disorders with known causes or that are known complications of the COVID-19, such as infectious respiratory diseases, lung diseases due to external agents, acute respiratory distress syndrome, respiratory failure etc., were not included in this study.
Each respiratory outcome was defined as the diagnosis 30 days or beyond post the onset of SARS-CoV-2 infection or the index date.
Covariates (see Supplementary Notes)
Statistical analyses
The baseline characteristics of the COVID-19 group, along with the contemporary and historical control groups, were illustrated by mean, standard deviation (SD) (continuous variables) and percentage (categorical variables) as suitable.
To mitigate the influence of confounding factors, inverse probability weights (IPTW) were computed for each participant. The effectiveness of the weighting was assessed by evaluating the SMD of covariates between the weighted populations, with SMD< 0.1 indicating sufficient balance of covariates between groups. For evaluating the long-term impact of COVID-19 infection on the respiratory outcomes, we constructed cox regression models using inverse probability weights and made additional adjustments for the unbalanced variables.
Due to the unavailability of complete data of UK Biobank on vaccination status or vaccine doses for the participants in our study period, we approached the sensitive analysis by restricting the inclusion period for the COVID-19 cohort to dates before December 2020. Additional analyses were further conducted (See Supplementary Notes).
We carried out all statistical evaluations utilizing RStudio and R 4.2.1 software. To address multiplicity, a P value below 0.05/6 was deemed statistically significant. This study is reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MJM, YJW and RJZ had access to and verify the underlying study data. HC is responsible for the decision to submit the manuscript.
Results
Baseline characteristics
Fig. 1 illustrates the selection process for the three cohorts. The groups were comprised of 112,311 participants for COVID-19, 359,671 for the contemporary control, and 370,979 for the historical control. The median follow-up time for all three groups was 300 days (interquartile range [IQR] 184–364). Supplementary Table S2 displays the number and percentage of missing covariates. Table 1 and Supplementary Table S3 detail the baseline characteristics of the study population before and after weighting. After applying weighting, all characteristics were approximately balanced between the groups.
Fig. 1.
Flow chart showing selection of cohort.
Table 1.
Baseline characteristics of COVID-19 group and contemporary controls after weighting.
| Baseline characteristics | COVID-19 group (n = 112,311) | Contemporary control (n = 359,671) | SMDa |
|---|---|---|---|
| Sex, female, n (%) | 61,659 (54.9) | 198,538 (55.2) | 0.007 |
| Age, mean (SD), years | 56.2 (8.1) | 56.2 (8.1) | 0.001 |
| BMI, mean (SD), kg/m2 | 27.4 (4.7) | 27.4 (4.8) | 0.001 |
| Ethnicity | 0.001 | ||
| White, n (%) | 106,134 (94.5) | 339,889 (94.5) | |
| Other ethnicities, n (%) | 6177 (5.5) | 19,782 (5.5) | |
| Townsend deprivation index, mean (SD) | −1.3 (3.0) | −1.3 (3.1) | 0.002 |
| Physical activity, mean (SD), MET | 2643.2 (2701.1) | 2649.9 (2708.0) | 0.002 |
| Household income | 0.004 | ||
| <18,000, n (%) | 24,259 (21.6) | 78,049 (21.7) | |
| 18,000–30,999, n (%) | 28,527 (25.4) | 91,716 (25.5) | |
| 31,000–51,999, n (%) | 29,650 (26.4) | 94,953 (26.4) | |
| 52,000–100,000, n (%) | 23,473 (20.9) | 74,452 (20.7) | |
| >100,000, n (%) | 6514 (5.8) | 20,501 (5.7) | |
| Alcohol consumption | 0.005 | ||
| Daily or almost daily, n (%) | 22,799 (20.3) | 72,654 (20.2) | |
| Three or four times a week, n (%) | 26,281 (23.4) | 83,803 (23.3) | |
| Once or twice a week, n (%) | 29,089 (25.9) | 93,514 (26) | |
| One to three times a month, n (%) | 12,579 (11.2) | 40,283 (11.2) | |
| Special occasions only or never, n (%) | 12,691 (11.3) | 41,002 (11.4) | |
| Never, n (%) | 8760 (7.8) | 28,414 (7.9) | |
| Smoking status | 0.002 | ||
| Never smoker, n (%) | 62,557 (55.7) | 200,696 (55.8) | |
| Previous smoker, n (%) | 38,523 (34.3) | 123,007 (34.2) | |
| Current smoker, n (%) | 11,231 (10) | 35,967 (10) | |
| Hypertension, n (%) | 40,544 (36.1) | 129,841 (36.1) | 0.001 |
| Heart failure, n (%) | 112 (0.1) | 360 (0.1) | 0.003 |
| Renal failure, n (%) | 4717 (4.2) | 15,106 (4.2) | 0.001 |
| Dementia, n (%) | 786 (0.7) | 2518 (0.7) | 0.002 |
| Myocardial infarction, n (%) | 4829 (4.3) | 15,466 (4.3) | 0.002 |
| Stroke, n (%) | 2920 (2.6) | 9351 (2.6) | 0.002 |
| Diabetes, n (%) | 8536 (7.6) | 27,335 (7.6) | 0.001 |
| Hypercholesterolemia, n (%) | 16,510 (14.7) | 52,872 (14.7) | 0.002 |
| Chronic Liver Disease, n (%) | 1348 (1.2) | 4316 (1.2) | 0.004 |
| Hospital admission frequency (past three years), mean (SD), times | 0.9 (1.7) | 0.9 (1.8) | 0.009 |
| History of previous respiratory diseases, n (%) | 22,350 (19.9) | 71,215 (19.8) | 0.003 |
SD, standard deviation; BMI, body mass index; MET, metabolic equivalent task; SMD, standardized mean difference.
SMD ≤0.10 is considered good balance.
Risks of respiratory diseases (COVID-19 group versus contemporary control group)
Long-term risks of respiratory diseases in patients infected with SARS-CoV-2
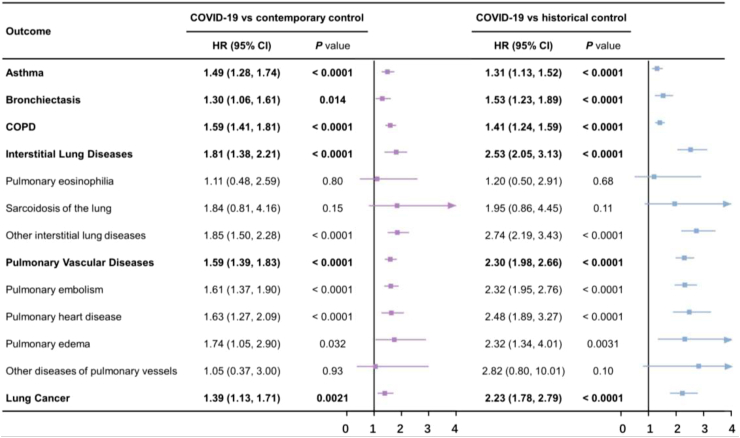
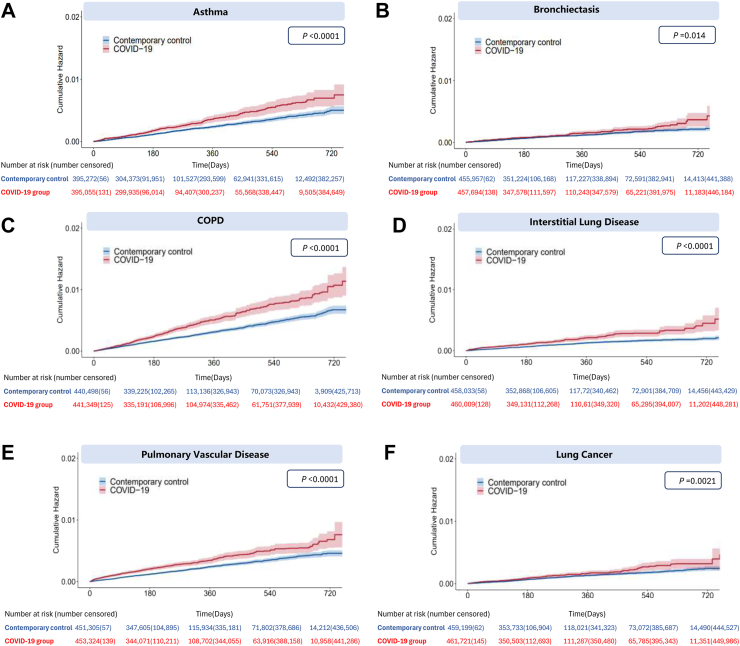
Fig. 2 illustrates the comparison of the risks of respiratory outcomes in the COVID-19 group with those in the contemporary control group. In comparison to the contemporary control group, individuals who survived the first 30 days of COVID-19 exhibited an increased risk of asthma (hazard ratio (HR) 1.49 [95% confidence interval (CI) [1.28–1.74], P < 0.0001); bronchiectasis (HR 1.30 [1.06–1.61], P = 0.014); COPD (HR 1.59 [1.41–1.81], P < 0.0001); ILD (HR 1.81 [1.38–2.21], P < 0.0001); PVD (HR 1.59 [1.39–1.83], P < 0.0001); and lung cancer (HR 1.39 [1.13–1.71], P = 0.0021). Fig. 3 presents the plots for weighted cumulative hazards.
Fig. 2.
Risks of incident respiratory outcomes in COVID-19 group during the post-acute phase compared with contemporary and historical controls. HR: hazard ratios; CI: confidence interval. Outcomes were ascertained 30 days after the COVID-19-positive test until the end of follow-up. Weighted HRs after IPTW and 95% CIs are presented.
Fig. 3.
Cumulative hazards of incident respiratory outcomes in COVID-19 group compared with contemporary control group. (A) Asthma outcome; (B) Bronchiectasis outcome; (C) Chronic obstructive pulmonary disease outcome; (D) Interstitial lung disease outcome; (E) Pulmonary vascular disease outcome; (F) Lung cancer outcome. Outcomes were ascertained 30 days after the initial SARS -CoV-2 positive test result until end of follow-up. Shaded areas are 95% confidence intervals. P values were calculated with the use of the stratified log-rank test. Numbers of participants at risk across groups are also presented.
Risks of respiratory outcomes by different care settings and follow-up periods
We explored the association between varying severity levels of COVID-19 and the risks of incident respiratory diseases based on the care setting during the acute phase (first 30 days) of the infection. Among the COVID-19 group (n = 112,311), a total of 8111 individuals were hospitalized for COVID-19, while 104,200 people were not admitted to hospital. Before applying weighting, most demographic and health characteristics were not balanced (SMD >0.1), suggesting there were differences between the groups (Supplementary Table S4). The baseline characteristics after weighting for these groups can be found in Supplementary Table S5, which shows that hospital admission frequency and hypercholesterolemia exhibit SMD values greater than 0.10 even after weighting. SMD suggested that hospital admission frequency and hypercholesterolemia were adjusted while other covariates were well balanced. In comparison to the contemporary control group, the increased risks of asthma and COPD were evident in those who were not admitted to hospital, while the increased risks of all major respiratory outcomes–asthma, bronchiectasis, COPD, ILD, PVD, and lung cancer—were evident in hospitalized patients with COVID-19 (Supplementary Figure S2). Overall, the risks of the predetermined respiratory outcomes in the COVID-19 group were found to be generally higher among individuals who hospitalized during the acute phase of the disease.
In order to examine the risks over different follow-up periods, a sensitivity analysis was conducted, comparing the HRs of the COVID-19 group with the contemporary control group at various follow-up times (Supplementary Table S6). Risk of asthma and bronchiectasis became evident at 12–24-months follow-up period, whereas the risks of developing COPD, ILD, PVD, and lung cancer were evident at an early follow-up period (within 6 months follow-up). Generally, we observed an increasing trend in the risks of asthma and bronchiectasis over time, and a decreasing trend in the risks of COPD, ILD, PVD, and lung cancer.
Risk of respiratory outcomes in SARS-CoV-2 reinfection and pre-vaccination
In the subgroup analysis of respiratory outcome risk associated with SARS-CoV-2 reinfection, the cohort included non-infected group (n = 359,671), single SARS-CoV-2 infection group (n = 107,950) and reinfected group (two or more infections) (n = 4361). Supplementary Tables S7 and S8 shows the baseline characteristics of these groups before and after weighting. Compared with the non-infected controls, the risks of asthma (HR 3.0 [1.32–6.84], P = 0.0083), COPD (HR 3.07 [1.42–6.65], P = 0.0042), ILD (HR 3.61 [1.11–11.80], P = 0.033), and lung cancer (HR 3.2 [1.59–6.45], P = 0.0012) were significantly higher among reinfected patients than that among patients with a single infection (Supplementary Table S9). For head-to-head comparison, reinfected patients were at an even higher risk for lung cancer (HR 2.49 [1.38–4.51], P = 0.0023) compared with those only experienced a single infection (Supplementary Table S10).
In pre-vaccination analysis, there were 8431 participants in the COVID-19 group and 368, 102 participants in the contemporary control group. Supplementary Tables S11 and S12 display the baseline characteristics of the COVID-19 group and contemporary control group from the period prior to the availability of vaccination. The standardized mean differences indicated that post-weighting, all covariates achieved a balanced distribution. Interestingly, asthma was not evident in patients without vaccination, whereas bronchiectasis, COPD, ILD, PVD and lung cancer were all evident, and displaying much higher hazard ratios compared to the main analysis (Supplementary Table S13).
Subgroup analyses
Subgroup analyses including individuals with and without a history of respiratory illness and other prespecified subgroups, can be found in Supplementary Notes.
Risks of respiratory diseases (COVID-19 group versus historical control group)
We evaluated the robustness of our study design by utilizing a historical control group, derived from a time period preceding the COVID-19 pandemic, as a reference category. Baseline characteristics before and after weighting in Supplementary Tables S14–S19 suggested that most covariates were balanced after weighting. Unbalanced covariates were further adjusted. Similar trend of risks were noted when comparing with a historical cohort serving as a control group, including asthma (HR 1.31 [1.13–1.52], P < 0.0001); bronchiectasis (HR 1.53 [1.23–1.89], P < 0.0001); COPD (HR 1.41 [1.24–1.59], P < 0.0001); ILD (HR 2.53 [2.05–3.13], P < 0.0001); PVD (HR 2.30 [1.98–2.66], P < 0.0001); and lung cancer (HR 2.23 [1.78–2.79], P < 0.0001) (Fig. 2). The risk trends in other analyses were also similar to those observed in contemporary control group analyses (Supplementary Figure S3 and Supplementary Table S13).
Discussion
This research found that the occurrences of respiratory disorders among patients who survived for 30 days after the COVID-19 diagnosis continued to rise consistently, including asthma, bronchiectasis, COPD, ILD, PVD, and lung cancer. With the severity of the acute phase of COVID-19, the risk of all respiratory diseases increases progressively. Besides, during the 24-months follow-up, we observed an increasing trend in the risks of asthma and bronchiectasis over time, which indicates that long-term monitoring and meticulous follow-up of these patients is essential. These findings contribute to a more complete understanding of the impact of COVID-19 on the respiratory system and highlight the importance of prevention and early intervention of these respiratory sequelae of COVID-19.
In this study, several key findings have been further identified. Firstly, our research demonstrates a significant association between COVID-19 and an increased long-term risk of developing various respiratory diseases. In previous studies, Lam et al.11 used data from the Hong Kong Hospital Authority (HKHA) and UK biobank databases to investigate various diseases risk of post-acute sequelae of SARS-CoV-2. The study found increased risk for several respiratory outcomes in the COVID-19 cohort, which is consistent with our results. While it's important to note that only a subset of respiratory diseases (chronic pulmonary diseases, acute respiratory distress syndrome, and ILD) were analysed, and the follow-up time was relatively short (138–150 days follow-up in HKHA cohorts, 212–294 days follow-up in UK Biobank cohorts). Similarly, Wan et al.10 found that patients with COVID-19 over 65 years old had an increased risk of ILD and chronic lung disease, using UK Biobank and HKHA databases, but their maximum follow-up period was only 18 months. Compared with the previous two studies, this study has more types of respiratory diseases, longer follow-up time, and more comprehensive and multi-angle assessment of the impact of COVID-19 on respiratory diseases.
Secondly, we found that the risk of respiratory disease increases with severity in patients with COVID-19, indicating that it is necessary to pay attention to respiratory COVID-19 sequelae in patients, especially those hospitalized during the acute stage of infection. This is consistent with the findings of Lam et al.,11 who found that the risk of some respiratory diseases (including chronic pulmonary disease, acute respiratory distress syndrome and ILD) increased with the severity of COVID-19. Notably, however, our study found that asthma and COPD remained evident even in the non-hospitalized population. This emphasizes that even in cases of mild COVID-19, the healthcare system should remain vigilant.
Thirdly, we investigated differences in risk across time periods, as well as the long-term effects of COVID-19 on respiratory disease. During the 2-years follow-up period, the risks of COPD, ILD, PVD and lung cancer decreased, while risks of asthma and bronchiectasis increased. Moreover, due to the long follow-up time of our study, it is a meaningful extension of the results of previous studies with short follow-up time.7, 8, 9 Overall, improving our understanding of the risk of respiratory outcomes in patients with COVID-19 at different times is critical for developing appropriate care strategies after the acute phase and long-term clinical follow-up after recovery.
Fourthly, our study showed a significant increase of the long-term risk of developing asthma, COPD, ILD, and lung cancer diseases among individuals who suffered SARS-CoV-2 reinfection. This finding emphasizes the importance of preventing reinfection of COVID-19 in order to protect public health and reduce the potential burden of SARS-CoV-2 reinfection. By identifying specific diseases with higher long-term risks in reinfected individuals, heightened awareness and tailored prevention and treatment strategies can be implemented in a targeted manner for those at risk.
Finally, we found that the risk of most respiratory diseases was higher before COVID-19 vaccination was available. This finding suggests that nationwide COVID-19 vaccination has a protective effect against long-term respiratory outcomes. Interestingly, vaccination appears to have a potentially worsening effect on asthma morbidity compared with other outcomes. This observation aligns with some previous studies that have suggested a possible induction of asthma onset or exacerbation by COVID-19 vaccination.15,16 It suggests that more care may be necessary for patients with asthma on taking the COVID vaccines.
The underlying mechanisms associated with COVID and respiratory outcomes are not fully understood, but several hypotheses have been proposed. First, SARS-CoV-2 can persist in tissues (including the respiratory tract),17,18 as well as the circulating system19 for an extended period of time after the initial infection. This prolonged presence of the virus could directly contribute to long-term damage of the respiratory tissues, consequently leading to the development of various respiratory diseases. Second, it has been observed that SARS-CoV-2 infection can lead to prolonged immunological dysfunctions, including highly activated innate immune cells, a deficiency in naive T and B cells, and increased expression of interferons and other pro-inflammatory cytochines.20,21 These immune system abnormalities are closely associated with common chronic respiratory diseases–asthma,22 bronchiectasis,23 COPD,24 as well as the development of lung cancer.25 Next, SARS-CoV-2 itself has been shown to drive cross-reactive antibody responses, and a range of autoantibodies were found in patients with COVID-19.26,27 The molecular mimicry between the virus and host proteins is considered a significant initiator of many ILD, especially ILD in connective tissue disease (CTD-ILD).28 Finally, SARS-CoV-2 infection causes endothelial dysfunction,27,29,30 which may contribute to the increased risk of PVD.
Our research has multiple strengths, bolstering both the reliability and validity of our conclusions. Firstly, our study spanned a lengthy follow-up period of up to 2.5 years (January 30th, 2020–October 30th, 2022), allowing us to investigate the sustained impacts of COVID-19 on respiratory outcomes. Secondly, to specifically examine the long-term sequelae of COVID-19 on respiratory disorders, we intentionally excluded infection-related diseases that might overlap with COVID-19. Instead, our study focused on chronic respiratory conditions such as asthma, bronchiectasis, COPD, ILD, PVD, and lung cancer. This targeted approach allows for a more comprehensive investigation into the enduring impact of COVID-19 on respiratory health. Thirdly, to ensure comprehensive analysis, we incorporated two control cohorts: contemporary and historical groups. This approach enhances the robustness of our results. Additionally, we performed subgroup analyses (especially the subgroup of SARS-CoV-2 reinfection and the subgroup with or without a family history of respiratory disease), which are aspects that have not been fully studied in previous studies.7, 8, 9 This may facilitate future implementation of targeted prevention and treatment strategies for those at risk. Moreover, to further eliminate the impact of the vaccine, we set up pre-vaccination groupings based on the time when the COVID-19 vaccine was rolled out in the UK, allowing us to obtain large sample sizes and increase the reliability of the analysis results. Lastly, we investigated the dose–response relationship between the severity of COVID-19 and the occurrence of respiratory diseases, further enriching our understanding of this association.
Although our research offers critical perspectives on the association between COVID-19 and respiratory system diseases, it is important to acknowledge its limitations. Firstly, a significant proportion of our research participants were of European ethnicity and are aged between 37 and 73 years, potentially restricting the generalizability of our results to other ethnic backgrounds and other age groups. Secondly, due to the observational nature of our study, we are unable to definitively establish a cause-and-effect link between COVID-19 and the susceptibility to respiratory diseases. However, the noticeable dose–response correlation between COVID-19 severity and respiratory outcome risks suggested a potential causal link. Thirdly, although we employed multiple imputation to address missing covariate data, there is still a possibility of bias if confounding covariates were omitted from the model. However, the percentage of missing data was generally low, mitigating the potential impact of this limitation. Fourthly, “severe COVID-19” cases were identified as patients who required critical care admission within 7 days of a COVID-19 diagnosis and/or received invasive or non-invasive mechanical ventilation or other respiratory support treatments, which includes continuous positive airway pressure (CPAP) and oxygen therapy in previous studies.10 Due to the limited data in UK Biobank, we could not further analyse this part of the population. Future studies with larger and more comprehensive populations are needed to confirm and expand upon our results. Fifthly, our study found a significant association between COVID-19 and lung cancer, but we fully recognize that this may be due to large-scale chest CT scans performed on a large proportion of suspected or confirmed patients with COVID-19, leading to more early tumor cases being detected. Therefore, clinically, more caution is required when interpreting the association between them.
In conclusion, our research adds to the existing knowledge regarding the effects of COVID-19 on the respiratory system. Specifically, it shows that the risk of respiratory illness increases with the severity of infection and reinfection. Our findings emphasize the importance of providing extended care and attention to patients previously infected with SARS-CoV-2. At the same time, a comprehensive understanding of the risk of respiratory outcomes in patients with COVID-19 changing over time is significant for developing appropriate follow-up strategies.
Contributors
MJM and YJW contributed to data extraction, data analyses, and manuscript drafting. MJM, RW and HC contributed to study design, data visualization and manuscript drafting. RJZ, DLL, YYM, LJZ, WTH, and HSZ contributed to manuscript drafting. HC, WHS, XQQ, and FWL contributed to data interpretation, and final approval of the manuscript. HC, WHS, XQQ, and FWL are senior and corresponding authors who contributed equally to this study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. MJM, YJW and RJZ had access to and verify the underlying study data. HC is responsible for the decision to submit the manuscript.
Data sharing statement
The UK Biobank resource is open to all researchers (https://www.ukbiobank.ac.uk). The authors thank the UK Biobank for the access of data, and this research has been conducted under Application Number 83339.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This work was funded by the National Natural Science Foundation of China Regional Innovation and Development Joint Foundation (U23A20408), the National Natural Science Foundation of China (82171698, 81300279, 81741067, 82170561, 82202058), the Natural Science Foundation for Distinguished Young Scholars of Guangdong Province (2021B1515020003), the Program for High-level Foreign Expert Introduction of China (G2022030047L), the Guangdong Basic and Applied Basic Research Foundation (2022A1515012081), the Climbing Program of Introduced Talents and High-level Hospital Construction Project of Guangdong Provincial People's Hospital (DFJH201803, KJ012019099, KJ012021143, KY012021183), and in part by VA Clinical Merit and ASGE clinical research funds (FWL).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102500.
Contributor Information
Felix W. Leung, Email: Felix.Leung@va.gov.
Xinqi Qiu, Email: qiuxq1@sysucc.org.cn.
Weihong Sha, Email: shaweihong@gdph.org.cn.
Hao Chen, Email: chenhao@gdph.org.cn.
Appendix A. Supplementary data
References
- 1.Whitehead M., Taylor-Robinson D., Barr B. Poverty, health, and covid-19. BMJ. 2021;372:n376. doi: 10.1136/bmj.n376. [DOI] [PubMed] [Google Scholar]
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizrahi B., Sudry T., Flaks-Manov N., et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023;380 doi: 10.1136/bmj-2022-072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE); 2020. http://www.ncbi.nlm.nih.gov/books/NBK567261/ [PubMed] [Google Scholar]
- 5.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC . 2023. Post-COVID conditions. Centers for disease control and prevention.https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html [Google Scholar]
- 7.Thompson E.J., Williams D.M., Walker A.J., et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13:3528. doi: 10.1038/s41467-022-30836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wild J.M., Porter J.C., Molyneaux P.L., et al. Understanding the burden of interstitial lung disease post-COVID-19: the UK Interstitial Lung Disease-Long COVID Study (UKILD-Long COVID) BMJ Open Respir Res. 2021;8(1) doi: 10.1136/bmjresp-2021-001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fumagalli C., Zocchi C., Tassetti L., et al. Factors associated with persistence of symptoms 1 year after COVID-19: a longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med. 2022;97:36–41. doi: 10.1016/j.ejim.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan E.Y.F., Zhang R., Mathur S., et al. Post-acute sequelae of COVID-19 in older persons: multi-organ complications and mortality. J Travel Med. 2023;30(5) doi: 10.1093/jtm/taad082. [DOI] [PubMed] [Google Scholar]
- 11.Lam I.C.H., Wong C.K.H., Zhang R., et al. Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK. eClinicalMedicine. 2023;60 doi: 10.1016/j.eclinm.2023.102000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuodi P., Gorelik Y., Zayyad H., et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020-21, Israel. NPJ Vaccines. 2022;7(1):101. doi: 10.1038/s41541-022-00526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28(7):1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notarte K.I., Catahay J.A., Velasco J.V., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. eClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando M., Satonaga Y., Takaki R., et al. Acute asthma exacerbation due to the SARS-CoV-2 vaccine (Pfizer-BioNTech BNT162b2 messenger RNA COVID-19 vaccine [ComirnatyⓇ]) Int J Infect Dis. 2022;124:187–189. doi: 10.1016/j.ijid.2022.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colaneri M., De Filippo M., Licari A., et al. COVID vaccination and asthma exacerbation: might there be a link? Int J Infect Dis. 2021;112:243–246. doi: 10.1016/j.ijid.2021.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein S.R., Ramelli S.C., Grazioli A., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612(7941):758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swank Z., Senussi Y., Manickas-Hill Z., et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis. 2023;76(3):e487–e490. doi: 10.1093/cid/ciac722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phetsouphanh C., Darley D.R., Wilson D.B., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 21.Peluso M.J., Lu S., Tang A.F., et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;224(11):1839–1848. doi: 10.1093/infdis/jiab490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holgate S.T. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers J.D., Chang A.B., Chotirmall S.H., Dhar R., McShane P.J. Bronchiectasis. Nat Rev Dis Primer. 2018;4(1):45. doi: 10.1038/s41572-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 24.Barnes P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 26.Wang E.Y., Mao T., Klein J., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 27.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagiannis D., Steinestel J., Hackenbroch C., et al. Clinical, serological, and histopathological similarities between severe COVID-19 and acute exacerbation of connective tissue disease-associated interstitial lung disease (CTD-ILD) Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.587517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haffke M., Freitag H., Rudolf G., et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) J Transl Med. 2022;20(1):138. doi: 10.1186/s12967-022-03346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pretorius E., Venter C., Laubscher G.J., et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 2022;21(1):148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.