Summary
Background
Ultra-processed food (UPF) consumption continues to increase worldwide. However, evidences from meta-analyses are limited regarding the effects on cardiovascular events (CVEs).
Methods
A meta-analysis was performed to assess the dose–response relationship of UPF consumption and CVEs risk (including the morbidity and mortality of cardiovascular causes, and myocardial infarction, stroke, transient ischemic attack, coronary intervention). Databases (PubMed, EMBASE, Cochrane Library, and Web of Science) were searched for observational studies published in English language up to October 24, 2023. Generalized least squares regression and restricted cubic splines were used to estimate the linear/nonlinear relationship. PROSPERO CRD 42023391122.
Findings
Twenty studies with 1,101,073 participants and 58,201 CVEs cases with a median follow-up of 12.2 years were included. A positive linear relationship between UPF intake and CVEs risk was identified. In addition, positive correlation between coronary heart disease and UPF consumption in terms of daily serving and daily energy proportion. No significant association of UPF consumption with the risk of cerebrovascular disease was observed. Briefly, 10% increase of UPF by daily weight proportion was associated with a 1.9% increase of CVEs risk (RR = 1.019; 95% CI, 1.007–1.031; P = 0.002), an additional daily serving corresponding to 2.2% CVEs risk increase (RR = 1.022; 95% CI, 1.013–1.031; P < 0.001), and 10% increase by daily energy proportion corresponding to 1.6% CVEs risk increase (RR = 1.016; 95% CI, 1.002–1.030; P = 0.022).
Interpretation
UPF consumption were associated with a higher risk of CVEs in the positive linear relationship. Our findings highlight the importance of minimizing UPF consumption for cardiovascular health and might be help to pursue public health policies in control of UPF consumption.
Funding
This work was supported by the Key Research and Development Program of Shaanxi Province (2023-ZDLSF-22), the Innovative Talent Support Program of Shaanxi Province (2022KJXX-106), and the Key Research and Development Program of Shaanxi Province (2023-YBSF-424).
Keywords: Ultra-processed food, Cardiovascular events, Coronary heart disease, Dose–response, Meta-analysis, Risk
Research in context.
Evidence before this study
Diet has been recognized as a key modifiable risk factor for cardiovascular diseases at various prevention guidelines. The availability of food and diet is increasingly skewed towards ultra-processed food (UPF), which leads to an increased UPF intake. The extensive processing of UPF introduces new physical structures and chemical compositions, which may affect diet quality and lead to a cascade of healthy issues. In recent years, numerous high-quality studies on the association between UPF consumption and cardiovascular events (CVEs) risk have been published, but the conclusions are not consistent. Therefore, a comprehensive and up-to-date meta-analysis of the available evidence on the association between UPF consumption and CVEs is warranted.
Added value of this study
In present study, we conducted a systematic review and dose–response meta-analysis of 20 observational studies from nine countries, with approximately 1,101,073 participants and 58,201 cases of CVEs, including 24,086 cases of coronary heart disease and 7614 cases of cerebrovascular disease. To the best of our knowledge, our findings are the first to provide a dose–response meta-analysis of UPF consumption and CVEs risk based on the separate synthesis of effect sizes by different consumption units of UPF reported in corresponding studies, with further analyses about the coronary heart disease and cerebrovascular disease risk. We also estimated the relative risk of continuous exposure of UPF consumption, such as daily weight proportion, daily servings, and daily energy proportion from UPF consumption. Therefore, the best available evidence is provided, warning the need to effective control of UPF consumption to help reduce CVEs prevalence and adverse outcomes.
Implications of all the available evidence
Considering the increasing dominance of UPF in global food consumption over the past 20 years, limitations of UPF intake should be incorporated in dietary guidelines for the prevention of CVEs. Future research should explore the underlying mechanisms and deepen the understanding of racial or sex differences in observed associations. Food-marketing and front-of-package labeling legislation restricting UPF consumption should be developed and implemented in UPF high-consumption countries as soon as possible.
Introduction
Cardiovascular events (CVEs) remain the leading cause of mortality and an ongoing public health challenge on a global scale.1, 2, 3 Diet is highlighted as an important modifiable risk factor at all levels of guidelines for the prevention of cardiovascular disease (CVD).4,5 Due to significant transformations in contemporary fast-paced lifestyles and food processing techniques, a large number of ultra-processed food (UPF) agencies dominate the food environment.6, 7, 8 As the most processed category in the Nova food classification system, UPF primarily uses processed cooking ingredients and synthetic food compounds as sources of raw materials, and contains a large number of food additives (dyes, antioxidants and stabilizers). These make UPF consumable, appealing, and addictive.9 In different populations from multiple countries, individuals consume 16–67% of total energy per day with UPF, with the lowest intake among the Colombian population and the highest intake in among the younger population in the United States.10, 11, 12, 13, 14, 15 Additionally, excessive consumption of UPF (added sugars, processed meats, and refined grains) means decreased adherence to Mediterranean dietary patterns.16 Thus, investigating whether UPF intake is associated with cardiovascular disease risk has important public health implications.
Published meta-analyses have reported an association between UPF and cardiovascular events and cardio-cerebrovascular disease,17,18 though with some details not fully mined. For example, UPF dose–response on major types of CVDs and grouping by different consumption units of UPF were not fully analyzed. Moreover, a number of additional studies have been published after these meta-analyses.19,20 One recent prospective cohort study showed that total UPF consumption was not associated with the risk of CVD mortality.21 To further investigate the dose–response relationship between UPF consumption and the risk of CVEs, we conducted a systematic review and meta-analyses of articles that reported the CVEs in corresponding clinical studies.22
Methods
This systematic review and dose–response meta-analysis were carried out according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Supplementary 1). Our methods were described in the protocol established before the review and registered with PROSPERO (CRD42023391122).23
Search strategy and selection criteria
Two reviewers (YQ and WH) independently searched the PubMed, EMBASE, Cochrane Library, and Web of Science from inception to October 24, 2023 for observational studies that evaluated the association between UPF intake and risk of CVEs. The search strategy employed a combination of free text words and controlled vocabulary terms pertaining to the consumption of UPF and their association with CVEs. The search terms encompassed various concepts such as fast food, Nova food classification system, acute coronary syndrome, myocardial infarction, stable and unstable angina pectoris, revascularization, heart failure, and coronary stent thrombosis (detailed search strategies in Supplemental Table S1). The search was broadened to include reference lists of relevant review articles, contacts with study authors, manual review of primary studies and other grey articles. We restricted the search results to studies on humans and written in English. Any inconsistencies between the two reviewers were resolved through mutual agreement.
Study inclusion and exclusion
Studies were included in this meta-analysis if they met the following criteria: (1) observational studies including cohort, case–control, and cross-sectional studies; (2) general participants ≥18 years; (3) exposure is UPF consumption, and the original paper had classified food according to the Nova system; (4) the outcomes were CVEs (including the morbidity and mortality of cardiovascular causes, and myocardial infarction, stroke, transient ischemic attack, coronary intervention (including stent thrombosis), peripheral vascular intervention, hospitalization for unstable angina, and acute heart failure events)22; (5) effect estimates were in the form of odds ratio (OR), relative risk (RR) or hazard ratio (HR) with 95% confidence interval (95% CI). Studies were excluded if (1) study design or research subjects not related to present topic; (2) lack of effect sizes (HRs or ORs) in the relationship between UPF consumption and CVEs. Supplemental Table S2 provides Population, Intervention, Control, and Outcome (PICO) descriptions.
Data extraction and synthesis
Two reviewers (YQ and WH) independently undertook data extraction and resolved any disagreements by discussion, or consulted a third reviewer (CX) to help resolve any differences. The following data were extracted from each study: the first author's name, year of publication, country, population, study type, follow-up years, dietary assessment tool, units of estimate, outcomes, definition of the outcomes, cases/participants, covariates used for adjustment in multivariate analysis, whether or not adjusted for diet quality, evaluation criteria and effect size for all categories of UPF consumption (including after adjusted for diet quality). For cohorts with published data on several CVEs outcomes, we chose morbidity and mortality of overall CVEs. Studies with only coronary heart disease (CHD) or cerebrovascular disease (CeVD) as the outcome were also included in the CVEs meta-analysis. After careful reviews of UPF intake for each category in each article, we re-categorized it according to a rigorous criterion. Specifically, only studies with three exposure categories and five exposure categories were included for re-categorization. For each of the included studies, the lowest and highest UPF intake categories corresponded to the lowest and highest groups, respectively. For studies with three exposure categories, the middle category was categorized to the group of the second or third highest in the meta-analysis, depending on its similarity to the second or third highest group in the meta-analysis. For studies with five exposure categories, besides the lowest and highest groups, the rest three groups were categorized either to the second or third highest groups in the meta-analysis based on their UPF consumption, which means two groups would be combined together according to the similarity.
We also extracted person-years of follow up, categories of UPF consumption, mean/median UPF consumption in each category to perform the dose–response meta-analysis. If the upper boundary for the category was not provided, it was assumed to be 1.5 times the lower boundary. If the lower boundary for the category was not provided, it was considered as zero. Studies lacking information on the number of person-years were estimated by multiplying the mean follow-up time by the total number of participants.
Quality assessment
The Newcastle–Ottawa Scale was used to assess the quality of the included studies. The cumulative score is obtained by adding the scores of each response. A study was deemed to be of high quality if the total score was no less than 7/9. The quality of the included studies was evaluated independently by two reviewers (YQ and WH), and any inconsistencies between the two reviewers were resolved by mutual agreement.
Statistical analysis
The outcome of this study were the associations between UPF intake and CVEs risk. We conducted meta-analysis and dose–response meta-analysis by different consumption units of UPF, including by daily weight proportion, daily servings, and daily energy proportion. We converted HR and OR to RR when pooling the estimates across the studies.24 Interstudy heterogeneity was assessed using Cochran Q test and I2 statistics. Given that we included diverse populations from multiple countries, pooled estimates of effect sizes and 95% CI were calculated using a random-effects model of the DerSimonian-Laird method.25 Forest plots were used to show results. Additionally, we performed the dose–response meta-analysis with STATA command GLST.26 Given the pre-estimated heterogeneity, we used a random-effects model for fitting. We firstly tested for potential non-linearity on CVEs in relation to UPF consumption using restricted cubic spline models with 4 knots (5%, 35%, 65% and 95%). Then, we used generalized least squares models to evaluate linear trends and calculate pooling dose–response RRs when the linear relation exists (P for non-linearity >0.050). In addition, we performed stratified analyses by evaluation of CHD and CeVD as the outcome, adjusted for dietary quality, publication year, country, dietary assessment method, sample size and follow-up years. We evaluated the robustness of pooled estimates via leave-one-out sensitivity analysis and trim and fill method. The pooled results were also validated by the fixed-effect model of the above analysis in the sensitivity analysis. Additionally, we used two-stage random-effects dose response models to combine studies that reported results for categorized UPF intake and studies with reported results for continuous UPF intake. Specifically, the RR of CVEs per unit increase of UPF consumption for each study was first estimated separately, and then the RRs from all of the studies were pooled together by a random-effects model. We assessed the potential for publication bias using the combination of visual inspection of funnel plots and Egger regression symmetry.27 Statistical significance was defined as P < 0.050. All analyses were conducted using STATA Version 14.0 (StataCorp, College Station, Lakeway, TX, USA) and SPSS v25.0 (IBM, Armonk, NY, USA).
Role of the funding source
The funder of the study had no role in accessing the raw data, study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
Characteristics of studies
Our initial search identified 43,502 potentially relevant citations and 19,659 were excluded for duplication. After screening titles and abstracts, we identified 42 studies for full paper assessment. Of them, we excluded two studies with the modeling study design or review, 11 studies due to unrelated outcomes, three studies with food classification standards not following the Nova food classification system; five studies due to unrelated exposures. We excluded one article reported UPF consumption based on the daily frequency, as this consumption unit of UPF was difficult to include in present meta-analysis with other studies.28 Finally, 20 studies (22 cohorts) were included in meta-analysis19, 20, 21,29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 (Fig. 1). Characteristics of these 20 studies are shown in Table 1. The excluded 22 studies are detailed in the Supplemental Table S3. The included studies comprised approximately 1,101,073 participants including 58,201 CVEs cases, 24,086 CHD cases and 7614 CeVD cases. Two studies had a cross-sectional study design,34,35 one had a case control study design,44 and the rest of studies were prospective cohort studies. Duration of follow-up for CVEs ranged from 5.2 to 27.0 years, with a median follow-up of 12.2 years. Eight cohorts were conducted in United States,32, 33, 34,36,39,40,45 four in Italy,19,31,37,42 and three in United Kingdom,38,41,45 one in France,29 one in Spain,30 one in Canada,35 one in Korea,21 one in Brazil,43 one in Iranian,44 and one contained participants from mixed countries (North and South America, Europe, Africa, Middle East, and Asia).20 Six cohorts assessed UPF consumption with the 24 h dietary recall29,34,35,38,41,45 and the rest with food frequency questionnaires. Two study modeled UPF consumption as a continuous variable,36,43 and the remaining studies modeled UPF consumption categorically. Ten studies estimating by daily weight proportion of UPF consumption,19,21,29,31,37,39,41, 42, 43,45 five by daily servings,20,30,32,36,40 and four by daily energy proportion.34,35,38,44 One study had data both by daily weight proportion and daily servings, and we use it separately in the analysis of different units.33 Two studies modeling UPF as a continuous exposure were only included in the sensitivity analysis and excluded in other analysis due to the difficulty of combining the risk estimate with those of other studies.36,43 Meta-analysis was performed with STATA command METAN, which used effect size, and lower-bound and upper-bound of 95% CI for each category in the process of pooling estimates without the requirement of participant numbers. Thus, 18 studies were included in the main analysis, while only 17 studies were included in the dose–response analysis between UPF intake and the CVEs risk, as one study was excluded due to omission the number of participants in each category.44 The scores of the NOS quality assessment ranged from 4 to 8, and 12 studies had scores of 7 or higher.
Fig. 1.
Study selection process of ultra-processed food consumption and risk of cardiovascular events.
Table 1.
Characteristics of included studies.
| Study year/Country | Population | Study type/Follow-up years | Dietary assessment tool | Units of estimate | Outcome | Definition of the outcome | Associated with UPF consumption | Cases/Participants | Effect size (95% CI) (Highest vs. lowest consumption) | Confounders adjusted for | Adjusted for diet quality | Evaluation criteria | Effect size (95% CI) (Adjusted for diet quality) (Highest vs. lowest consumption) | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Srour et al., 2019/France16 | Healthy adults aged 18–73 years | Prospective cohort/5.2 | 24 h dietary recall | Daily weight proportion | CVD mortality | Death of overall CVD | Yes | 1409/105,159 | 1.23 (1.04, 1.45) | Age, sex, energy intake, number of 24 h dietary recall, smoking status, educational level, physical activity, BMI, alcohol intake, and family history of CVD | Yes | Healthy dietary pattern (derived by factor analysis) | 1.20 (1.01, 1.42) | +8/9 |
| CHD mortality | MI, acute coronary syndrome, and angioplasty. | Yes | 665/105,159 | 1.20 (0.93, 1.53) | 1.16 (0.90, 1.49) | |||||||||
| CeVD mortality | Stroke and TIA | Yes | 829/105,159 | 1.24 (1.00, 1.53) | 1.21 (0.98, 1.51) | |||||||||
| Rico-Campà et al., 2019/Spain17 | General adults aged 20–91 years | Prospective cohort/10.4 | FFQ | Daily servings | CVD mortality | Death of overall CVD | No | 71/19,899 | 2.16 (0.92, 5.06) | Age, sex, marital status, physical activity, smoking status, snacking, special diet at baseline, BMI, total energy intake, alcohol consumption, family history of CVD, diabetes, hypertension, self-reported hypercholesterolemia, CVD, cancer and depression at baseline, education level, lifelong smoking stratified by recruitment period, deciles of age, sedentary index, and television viewing | No | – | – | +8/9 |
| Bonaccio et al., 2020/Italy20 | General adults aged ≥35 years | Prospective cohort/8.2 | FFQ | Daily weight proportion | CVD mortality | The ICD-9 codes: 390–459 | Yes | 439/22,475 | 1.65 (1.29, 2.11) | Sex, age, energy intake, educational level, housing tenure, smoking, BMI, leisure-time physical activity, history of cancer, CVD, diabetes, hypertension, hyperlipidemia, and residence | Yes | Mediterranean Diet Score | 1.58 (1.23, 2.03) | +7/9 |
| Du et al., 2021/USA28 | Healthy adults aged 45–65 years | Prospective cohort/27.0 | FFQ | Daily servings | CHD mortality | The first occurrence of a definite or probable hospitalization due to myocardial infarction or definite CHD death | Yes | 2006/13,548 | 1.19 (1.05, 1.35) | Age, sex, race, total energy intake, education level, smoking status, drinking status, and physical activity score | No | – | – | +6/9 |
| Zhong et al., 2021/USA29 | Healthy adults aged 55–74 years | Prospective cohort/13.5 | FFQ | Daily servings | CVD mortality | The ICD-9 codes: 390–459 | Yes | 5490/91,891 | 1.50 (1.36, 1.64) | Age, sex, race, educational level, marital status, study center, aspirin use, history of hypertension, history of diabetes, smoking status, alcohol consumption, BMI, physical activity, and energy intake from diet | Yes | Healthy Eating Index (HEI-2005) | 1.48 (1.35, 1.63) | +7/9 |
| CHD mortality | The ICD-9 codes: 390–398, 402, 404, and 410–429 | Yes | 3985/91,891 | 1.68 (1.50, 1.87) | 1.67 (1.49, 1.86) | |||||||||
| CeVD mortality | The ICD-9 codes: 430–438 | No | 1126/91,891 | 0.94 (0.76, 1.17) | 0.94 (0.75, 1.16) | |||||||||
| FFQ | Daily energy proportion | CVD mortality | The ICD-9 codes: 390–459 | Yes | 5490/91,891 | 1.21 (1.07, 1.37) | Age, sex, race, educational level, marital status, study center, aspirin use, history of hypertension, history of diabetes, smoking status, alcohol consumption, BMI and physical activity | No | – | – | ||||
| CHD mortality | The ICD-9 codes: 390–398, 402, 404, and 410–429 | Yes | 3985/91,891 | 1.34 (1.16, 1.55) | ||||||||||
| CeVD mortality | The ICD-9 codes: 430–438 | No | 1126/91,891 | 0.87 (0.67, 1.13) | ||||||||||
| da Silva et al., 2021/USA30 | Adults with diagnosed at least one CVEs aged 45 years | Cross sectional/- | 24 h dietary recall | Daily energy proportion | PAD morbidity | Subjects with the presence of PAD only | Yes | 46/2274 | 2.02 (0.95, 4.31) | Sex, age, physical activity, smoking, medication use, overweight and high waist circumference | No | – | – | +5/9 |
| CHD + PAD + Strok morbidity | Subjects with the simultaneous presence of CHD, PAD, and stroke | No | 42/2274 | 0.25 (0.10, 0.61) | ||||||||||
| Nardocci et al., 2021/Canada31 | General adults aged ≥19 years | Cross-sectional/- | 24 h dietary recall | Daily energy proportion | CHD morbidity | Self-reported in the context of asking participants about long-term health conditions diagnosed by a health professional | No | 680/13,608 | 1.22 (0.87, 1.71) | Age, sex, smoking status, physical activity, alcohol consumption, education, income, residential area, immigrant status, and indigenous identity | No | – | – | +5/9 |
| Juul et al., 2021/USA32 | Adults free from CVD (mean age: 53.5 years) | Prospective cohort/26.0 | FFQ | Daily servings | Overall CVD morbidity | CHD, fatal, ischemic and hemorrhagic stroke, TIA, cerebral embolism, other CVD, PAD and congestive heart failure | Yes | 648/3003 | Each additional daily serving 1.05 (1.02, 1.08) | Age, sex, education, smoking status, alcohol intake, and physical activity | Yes | Dietary Guidelines for Americans Adherence Index 2010 | Each additional daily serving 1.04 (1.01–1.07) | +7/9 |
| CVD mortality | Yes | 108/3003 | Each additional daily serving 1.09 (1.02, 1.16) | Each additional daily serving 1.09 (1.02–1.16) | ||||||||||
| Bonaccio et al., 2022/Italy33 | General adults aged ≥35 years | Prospective cohort/12.2 | FFQ | Daily weight proportion | CVD mortality | The ICD-9 codes: 390–459 | Yes | 792/22,895 | 1.27 (1.02, 1.58) | Sex, age, energy intake, educational level, housing tenure, smoking, body mass index, leisure time physical activity, history of cancer, CVD, diabetes, hypertension and hyperlipidemia, and residence | No | – | – | +8/9 |
| Chen et al., 2022/United Kingdom34 | General adults aged ≥40 years | Prospective cohort/10.9 | 24 h dietary recall | Daily energy proportion | New-onset CVD morbidity | The ICD-10 codes: I20–I25, I48, I50, I60–I64 and I67–I69 | Yes | 6048/60,298 | 1.15 (1.07, 1.24) | Age, sex, ethnicity, education years, smoking status and Townsend deprivation index, obesity status, sleep duration, total energy intake, physical activity, protein, total fat, carbohydrates, alcohol, fiber, saturated fat, monounsaturated fat, polyunsaturated fat, trans-fat, hypertension, diabetes and dyslipidemia | No | – | – | +6/9 |
| New-onset CHD morbidity | The ICD-10 codes: I20–I25, I48 and I50 | Yes | 5327/60,298 | 1.18 (1.08, 1.28) | ||||||||||
| New-onset CeVD morbidity | The ICD-10 codes: I60–I64 and I67–I69 | Yes | 1519/60,298 | 1.29 (1.10, 1.51) | ||||||||||
| New-onset CVD mortality | The ICD-10 codes: I20–I25, I48, I50, I60–I64 and I67–I69 | No | 384/60,298 | 1.07 (0.77, 1.49) | ||||||||||
| Bonaccio et al., 2022/Italy35 | Adults with history of CVD aged ≥35 years | Prospective cohort/10.6 | FFQ | Daily weight proportion | CVD mortality | The ICD-9 codes: 390–459 | Yes | 178/1171 | 1.78 (1.16, 2.72) | Sex, age, energy intake, educational level, housing tenure, smoking, BMI, leisure-time physical activity, history of cancer, CVD, diabetes, hypertension and hyperlipidemia, and residence | Yes | Mediterranean Diet Score | 1.65 (1.07, 2.55) | +8/9 |
| Wang et al., 2023/USA36 | Adults free from CVD (mean age: 53.5 years) | Prospective cohort/12.2 | FFQ | Daily weight proportion | CHD mortality | The ICD-10 codes: 410–414 and I20–I25 | No | 2208/77,060 | 0.99 (0.87, 1.12) | Enrollment source, age, sex, race, education, income, deprivation index, marital status, smoking status, pack-years, alcohol drinking, physical activity, and sitting hours | Yes | Healthy Eating Index (HEI-2010) | 0.88 (0.76, 1.01) | +7/9 |
| Stroke mortality | The ICD-10 codes: 430–438 and I60–I69 | No | 867/77,060 | 1.26 (1.03, 1.54) | 1.15 (0.91, 1.44) | |||||||||
| Sullivan et al., 2023/USA37 | Adults free from CVD (mean age: 53.5 years) | Prospective cohort/7.0 | FFQ | Daily servings | CVD morbidity | A composite of MI, congestive heart failure, or stroke | No | 406/2616 | 1.09 (0.85, 1.40) | Age, sex, total energy intake, race/ethnicity, education, income, smoking status, physical activity, and study site | Yes | Healthy Eating Index (HEI-2015) | 1.01 (0.77, 1.32) | +7/9 |
| Dehghan et al., 2023/North and South America, Europe, Africa, the Middle East, and Asia38 | Adults without a history of CVD aged 35–70 years | Prospective cohort/10.2 | FFQ | Daily servings | CVD mortality | The ICD-10 codes: I00–I99 | Yes | 3073/138,076 | 1.17 (0.98, 1.41) | Age, sex, urban/rural location, education, wealth index, country income level, smoking, body mass index, physical activity, history of diabetes, cancer and hypertension, blood pressure medication, daily energy intake, and country | No | – | – | +7/9 |
| Major CVD morbidity | Composite of fatal CVD, and nonfatal MI, stroke, and heart failure | No | 7934/138,076 | 1.01 (0.90, 1.12) | ||||||||||
| Li et al., 2023/United Kingdom39 | General adults aged 40–69 years | Prospective cohort/10.0 | 24 h dietary recall | Daily weight proportion | CVD morbidity | The ICD-10 codes: I20–I25 and I60–I64, I69 | Yes | 7006/111,646 | 1.15 (1.07, 1.23) | Age, sex, BMI, ethnicity, education, Townsend Deprivation Index, physical activity, smoking status, drinking status, diabetes, and hypertension | Yes | Healthy diet score | 1.19 (1.11, 1.27) | +6/9 |
| CHD morbidity | The ICD-10 codes: I20–I25 | Yes | 5217/111,646 | 1.19 (1.09, 1.29) | 1.24 (1.14, 1.34) | |||||||||
| CeVD morbidity | The ICD-10 codes: I60–I64 and I60–I69 | Yes | 2147/111,646 | 1.09 (0.97, 1.23) | 1.10 (0.97, 1.24) | |||||||||
| CVD mortality | The ICD-10 codes: I00–I99 | Yes | 1124/111,646 | 1.39 (1.18, 1.64) | 1.46 (1.24, 1.73) | |||||||||
| Bonaccio et al., 2023/Italy40 | Adults with type 2 diabetes aged ≥35 years | Prospective cohort/11.6 | FFQ | Daily weight proportion | CVD mortality | Angina, MI, revascularization procedures, PAD, and CeVD | Yes | 129/1065 | 2.64 (1.59, 4.40) | Sex, age, residence, educational level, housing tenure, smoking, BMI, leisure-time physical activity, history of cancer, history of CVD, hypertension, hyperlipidemia, aspirin use, years since diagnosis of type 2 diabetes and special diet for blood glucose control | Yes | Mediterranean Diet Score | 2.55 (1.53, 4.24) | +7/9 |
| Kityo et al., 2023/Korea41 | General adults aged 40–69 years | Prospective cohort/10.6 | FFQ | Daily weight proportion | CVD mortality (men) | The ICD-10 codes: I00–I99 | No | 333/38,847 | 0.88 (0.64, 1.22) | Age, total energy intake, education level, monthly income, marital status, smoking, drinking, regular physical exercise, BMI, comorbidity score, menopausal status, use of oral contraceptives and hypercholesterolemia | No | – | – | +5/9 |
| CVD mortality (women) | The ICD-10 codes: I00–I99 | No | 206/74,729 | 0.80 (0.53, 1.19) | ||||||||||
| Silva et al., 2023/Brazil42 | General adults aged 35–74 years | Prospective cohort/8.0 | FFQ | Daily weight proportion | CVD mortality | The ICD-10 codes: I00–I99 | Yes | 143/14,459 | 10% increment of UPF in the diet 0.97 (0.82, 1.14) |
Sex, age, research center, educational level, physical activity, smoking, excessive alcohol use, diabetes, hypertension, hypercholesterolemia, CVD, and cancer | No | – | – | +6/9 |
| Ansari et al., 2023/Iranian43 | Adults with the stenosis of coronary artery in the cases. Adults with normal coronary artery in control group. | Case–control/- | FFQ | Daily energy proportion | CHD morbidity | Coronary a gigography and had a stenosis of at least single coronary artery equal and above 75% or left main coronary of equal or more than 50% | Yes | 2077/3248 | 2.84 (2.23, 3.62) | Age, sex, daily energy intake, smoking, level of education, different ethnicities and family history of CVD | Yes | Mediterranean Diet Score | 2.53 (1.98, 3.24) | +4/9 |
| Zhao et al., 2023/USA, United Kingdom44 | General adults aged 55–74 years. | Prospective cohort/16.9(PLCO)/12.4(UK Biobank)/9.3(NHANES) | FFQ (PLCO/UK Biobank)/24 h dietary recall (NHANES) | Daily weight proportion | CVD mortality (PLCO) | The ICD-10 codes: I00–I99 | Yes | 8099/108,714 | 1.12 (1.05, 1.19) | Age, sex, race, smoking status, alcohol drinking status, education, body mass index, total energy intake, history of hypertension, diabetes, cardiovascular disease and cancer, randomization arm (PLCO only), marital status (PLCO and NHANES), family income and physical activity (UK Biobank and NHANES), and sleep duration and Townsend deprivation index (UK Biobank only) | No | – | – | +7/9 |
| CVD mortality (UK Biobank) | The ICD-10 codes: I00–I99 | Yes | 1979/208,051 | 1.28 (1.13, 1.45) | ||||||||||
| CVD mortality (NHANES) | The ICD-10 codes: I00–I69 | Yes | 1749/41,070 | 1.11 (0.92, 1.34) |
Abbreviations: FFQ, food frequency questionnaires; UPF, ultra-processed food; CVD, cardiovascular disease; CI, confidence interval; BMI, body mass index; CHD, coronary heart disease; CeVD, cerebrovascular disease; PAD, peripheral artery disease; MI, myocardial infarction; TIA, transient ischemic attack.
UPF consumption and risk of CVEs
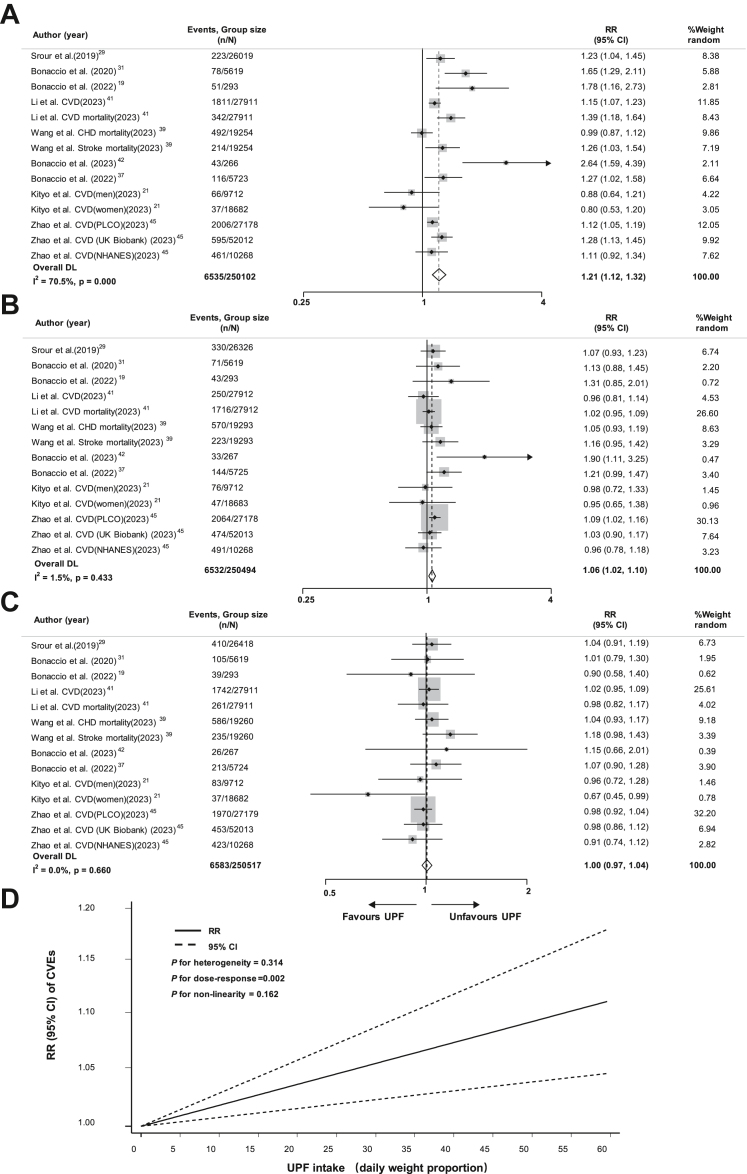
For the risk of CVEs with UPF consumption, compared with the lowest group in terms of daily weight proportion (mean: 7.5%/d; median: 6.2%/d), the pooled RR was 1.00 (95% CI, 0.97–1.04, I2 = 0.0%) for the third highest (mean: 14.9%/d; median: 11.3%/d), 1.06 (95% CI, 1.02–1.10, I2 = 1.5%) for the second highest (mean: 21.4%/d; median: 17.3%/d) and 1.21 (95% CI, 1.12–1.32, I2 = 70.5%) for the highest (mean: 35.4%/d; median: 31.2%/d) group (Fig. 2A–C).
Fig. 2.
Forest plot of the association between the highest level of ultra-processed food consumption by daily weight proportion and risk of cardiovascular events compared to the lowest level (A); Forest plot of the association between the second highest level of ultra-processed food consumption by daily weight proportion and risk of cardiovascular events compared to the lowest level (B); Forest plot of the association between the third highest level of UPF consumption by daily weight proportion and risk of cardiovascular events compared to the lowest level (C); Dose–response association between ultra-processed food consumption by daily weight proportion and risk of cardiovascular events (D). Abbreviations: RR, relative risk; CI, confidence interval; CVD, cardiovascular disease; CHD, coronary heart disease; UPF, ultra-processed food; CVEs, cardiovascular events.
In terms of daily servings, compared with the lowest UPF intake group (mean: 1.7/d; median: 0.8/d), the pooled RR was 1.02 (95% CI, 0.98–1.07, I2 = 8.9%) for the third highest (mean: 2.1/d; median: 1.6/d), 1.11 (95% CI, 1.03–1.19, I2 = 25.3%) for the second highest (mean: 3.7/d; median: 3.3/d) and 1.21 (95% CI, 1.02–1.43, I2 = 84.8%) for the highest (mean: 5.9/d; median: 6.8/d) group (Supplemental Figure S1A–C).
Regarding daily energy proportion, compared with the lowest UPF consumption group (mean: 9.6%/d; median: 10.4%/d), the pooled RR was 1.04 (95% CI, 0.98–1.11, I2 = 0.0%) for the third highest (mean: 18.6%/d; median: 26.1%/d), 1.07 (95% CI, 0.98–1.17, I2 = 26.5%) for the second highest (mean: 29.3%/d; median: 37.2%/d) and 1.28 (95% CI, 0.98–1.69, I2 = 90.6%) for the highest (mean: 44.2%/d; median: 53.3%/d) group (Supplemental Figure S2A–C).
Dose–response analysis of UPF consumption with risk of CVEs
In the dose–response analyses, a positive linear relation was observed between UPF intake and the CVEs risk with no heterogeneity in study results. Specifically, 10% increase in UPF consumption daily weight proportion (P for dose–response = 0.002, P for non-linearity = 0.162, P for heterogeneity = 0.314) might result in a 1.9% higher risk of CVEs (RR = 1.019; 95% CI, 1.007–1.031) (Fig. 2D), each additional daily serving (P for dose–response <0.001, P for non-linearity = 0.836, P for heterogeneity = 0.893) corresponding to 2.2% CVEs risk increase (RR = 1.022; 95% CI, 1.013–1.031) (Supplemental Figure S1D), and 10% increase by daily energy proportion (P for dose–response = 0.022, P for non-linearity = 0.862, P for heterogeneity = 0.680) corresponding to 1.6% CVEs risk increase (RR = 1.016; 95% CI, 1.002–1.030) (Supplemental Figure S2D).
Stratified analyses
Stratified analyses were conducted with CHD and CeVD as the outcome, adjusted for dietary quality, publication year, region, dietary assessment tool, sample size and follow-up years.
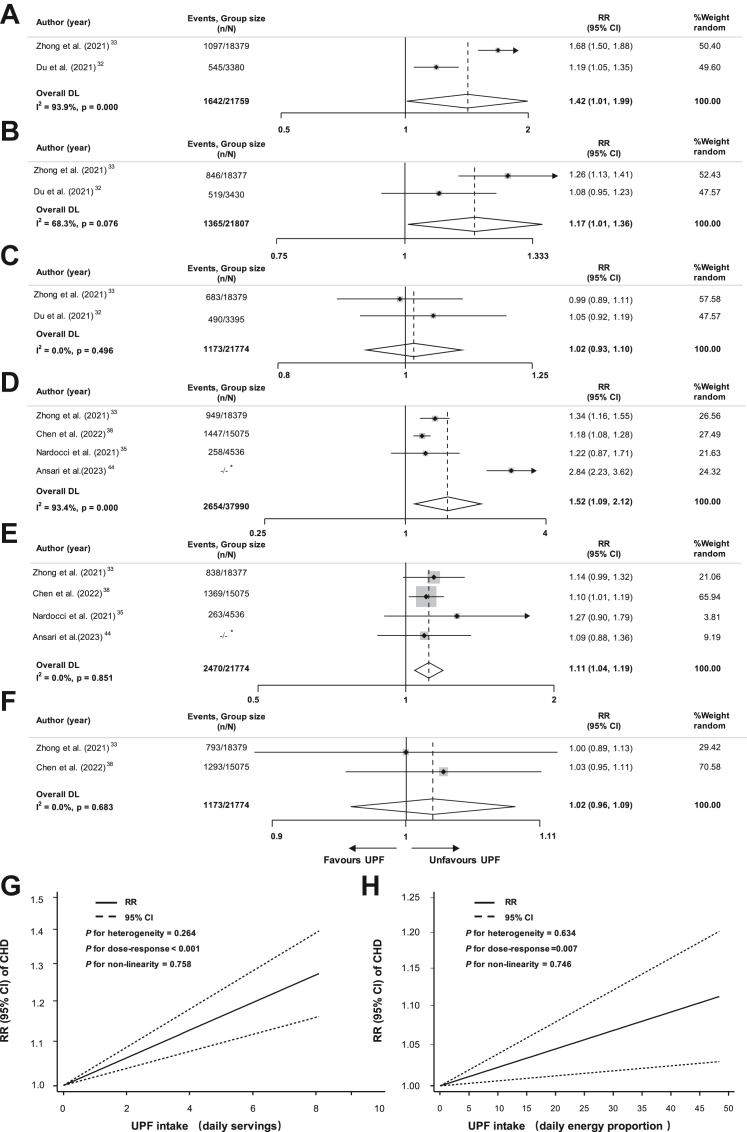
For the risk of CHD with UPF consumption in the scale of daily weight proportion, compared with the lowest group (mean: 10.2%/d; median: 8.3%/d), the pooled RR was 1.06 (95% CI, 1.00–1.13, I2 = 0.0%) for the third highest group (mean: 20.7%/d; median: 14.5%/d), 1.09 (95% CI, 1.02–1.16, I2 = 0.0%) for the second highest group (mean: 27.9%/d; median: 20.1%/d), and 1.11 (95% CI, 0.97–1.28, I2 = 66.0%) for the highest group (mean: 45.6%/d; median: 31.6%/d) (Supplemental Figure S3A–C). The corresponding RRs regarding daily servings were 1.02 (95% CI, 0.93–1.10, I2 = 0.0%) for the third group (mean/median: 3.5/d), 1.17 (95% CI, 1.01–1.36, I2 = 68.3%) for the second group (mean/median: 4.7/d), and 1.42 (95% CI, 1.01–1.99, I2 = 93.9%) for the highest group (mean/median: 8.3/d) (Fig. 3A–C). The corresponding RRs in daily energy proportion were 1.02 (95% CI, 0.96–1.09, I2 = 0.0%) for the third group (mean/median: 29.8%/d), 1.11 (95% CI, 1.04–1.19, I2 = 0.0%) for the second group (mean: 33.8%/d; median: 40.6%/d), and 1.52 (95% CI, 1.09–2.12, I2 = 93.4%) for the highest group (mean: 49.7%/d; median: 56.4%/d) (Fig. 3D–F).
Fig. 3.
Forest plot of the association between the highest level of ultra-processed food consumption by daily servings and risk of coronary heart disease compared to the lowest level (A); Forest plot of the association between the second highest level of ultra-processed food consumption by daily servings and risk of coronary heart disease compared to the lowest level (B); Forest plot of the association between the third highest level of ultra-processed food consumption by daily servings and risk of coronary heart disease compared to the lowest level (C); Forest plot of the association between the highest level of ultra-processed food consumption by daily energy proportion and risk of coronary heart disease compared to the lowest level (D); Forest plot of the association between the second highest level of ultra-processed food consumption by daily energy proportion and risk of coronary heart disease compared to the lowest level (E); Forest plot of the association between the third highest level of ultra-processed food consumption by daily energy proportion and risk of coronary heart disease compared to the lowest level (F); Dose–response association between ultra-processed food consumption by daily servings and risk of coronary heart disease (G); Dose–response association between ultra-processed food consumption by daily energy proportion and risk of coronary heart disease (H). −/−∗: not reported in the original article. Abbreviations: CHD, coronary heart disease; RR, relative risk; CI, confidence interval; UPF, ultra-processed food.
We observed a potential linear dose–response relationship between UPF consumption and CHD by daily serving and daily energy proportion. One additional daily serving of UPF consumption was associated with a 3.0% increase of CHD risk (RR = 1.030, 95% CI, 1.019–1.042; P for dose–response <0.001, P for non-linearity = 0.264, P for heterogeneity = 0.758). And 10% increase of UPF consumption by daily energy proportion was related with a 2.2% increase of CHD risk (RR = 1.022; 95% CI, 1.006–1.039; P for dose–response = 0.007, P for non-linearity = 0.746, P for heterogeneity = 0.634) (Fig. 3G and H).
For the risk of CeVD with UPF consumption, compared with the lowest group in terms of daily weight proportion (mean: 10.2%/d; median: 8.3%/d), the pooled RR was 1.00 (95% CI, 0.85–1.19, I2 = 71.0%) for the third highest group (mean: 20.7%/d; median: 14.5%/d), 0.99 (95% CI, 0.84–1.16, I2 = 62.9%) for the second highest group (mean: 27.9%/d; median: 20.1%/d), and 1.15 (95% CI, 1.05–1.27, I2 = 2.8%) for the highest group of UPF consumption (mean: 45.6%/d; median: 31.6%/d) (Supplemental Figure S4A–C). The corresponding RRs in daily energy proportion were 0.90 (95% CI, 0.64–1.26, I2 = 81.0%) for the third group (mean/median: 26.4%/d), 0.92 (95% CI, 0.72–1.17, I2 = 64.6%) for the second group (mean/median: 40.6%/d), and 1.07 (95% CI, 0.73–1.58, I2 = 84.3%) for the highest group (mean/median: 56.4%/d) (Supplemental Figure S4D–F). No dose–response associations of the UPF consumption with the CeVD risk were observed. The effects for the UPF consumption in terms of daily servings was not analyzed because the number of corresponding studies was insufficient (only one study).
We found that region and sample size may be sources of heterogeneity in the association between UPF intake in daily weight proportion and CVEs risk (all P for interactions <0.050). When stratified by region, the highest group increased the risk of CVEs among non- United States (RR: 1.28; 95% CI, 1.14–1.44) and is higher than in the United States (RR: 1.10; 95% CI, 1.02–1.19). RR of CVEs was much smaller for studies with sample size ≥30,000 than <30,000 (1.15, 95% CI, 1.07–1.22, vs. 1.66, 95% CI, 1.28–2.16). In the UPF consumption estimating by daily servings, publication year may be sources of heterogeneity in relation to CVEs risk (P for interactions <0.050). Comparing the highest with the lowest intakes, the RR of CVEs was 1.38 (95% CI, 1.11–1.72) for publication year before 2022, but 1.06 (95% CI, 0.97–1.15) for publication year after 2022. No interactions between UPF intake and the remaining stratification variables in relation to CVEs risk were observed (all P for interactions >0.050) (Table 2).
Table 2.
Stratified analyses for the association between ultra-processed food consumption and risk of cardiovascular events.
| Groups | Estimating by daily weight proportion |
Estimating by daily servings |
Estimating by daily energy proportion |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N |
RR (95%CI) |
I2 (%) |
Pa |
Pb |
N |
RR (95%CI) |
I2 (%) |
Pa |
Pb |
N |
RR (95%CI) |
I2 (%) |
Pa |
Pb |
|
| Highest vs. lowest category | Highest vs. lowest category | Highest vs. lowest category | |||||||||||||
| Overall | 9 | 1.21 (1.12, 1.31) | 69.9 | <0.001 | 5 | 1.21 (1.02, 1.43) | 84.8 | <0.001 | 5 | 1.28 (0.98, 1.69) | 90.6 | <0.001 | |||
| Publication year | |||||||||||||||
| <2022 | 3 | 1.40 (1.05, 1.87) | 73.4 | 0.052 | 0.255 | 3 | 1.38 (1.11, 1.72) | 78.8 | 0.009 | 0.026 | 3 | 1.05 (0.68, 1.64) | 78.0 | 0.003 | 0.334 |
| ≥2022 | 6 | 1.18 (1.09, 1.28) | 68.2 | <0.001 | 2 | 1.06 (0.97, 1.15) | 0.0 | 0.383 | 2 | 1.52 (0.84, 2.75) | 96.0 | <0.001 | |||
| Region | |||||||||||||||
| The United States | 2 | 1.10 (1.02, 1.19) | 36.5 | 0.193 | 0.036 | 3 | 1.27 (1.05, 1.55) | 82.7 | 0.003 | 0.332 | 3 | 1.44 (0.92, 2.27) | 94.0 | <0.001 | 0.362 |
| Non- the United States | 7 | 1.28 (1.14, 1.44) | 71.2 | <0.001 | 2 | 1.11 (0.92, 1.34) | 56.2 | 0.102 | 2 | 0.90 (0.37, 2.22) | 85.3 | 0.001 | |||
| Dietary assessment method | |||||||||||||||
| 24 h dietary recall | 3 | 1.20 (1.10, 1.31) | 39.7 | 0.174 | 0.823 | 0 | – | – | – | – | 3 | 1.07 (0.80, 1.44) | 70.5 | 0.009 | 0.233 |
| FFQ | 6 | 1.22 (1.08, 1.38) | 76.1 | <0.001 | 5 | 1.21 (1.02, 1.43) | 84.8 | <0.001 | – | 2 | 1.84 (0.80, 4.25) | 97.4 | <0.001 | ||
| Sample size | |||||||||||||||
| <30,000 | 4 | 1.66 (1.28, 2.16) | 62.7 | 0.045 | 0.007 | 3 | 1.18 (1.03, 1.36) | 14.5 | 0.311 | 0.866 | 3 | 1.24 (0.57, 2.74) | 91.9 | <0.001 | 0.865 |
| ≥30,000 | 5 | 1.15 (1.07, 1.22) | 55.2 | 0.017 | 2 | 1.21 (0.92, 1.59) | 93.3 | <0.001 | 2 | 1.16 (1.09, 1.24) | 0.0 | 0.695 | |||
| Follow-up years | |||||||||||||||
| <11 | 5 | 1.22 (1.07, 1.38) | 68.6 | 0.002 | 0.910 | 3 | 1.09 (0.96, 1.24) | 34.9 | 0.203 | 0.120 | 3 | 1.24 (0.57,2.74) | 91.9 | <0.001 | 0.865 |
| ≥11 | 4 | 1.21 (1.07, 1.36) | 74.4 | 0.002 | 2 | 1.34 (1.07, 1.68) | 88.1 | 0.004 | 2 | 1.16 (1.09, 1.24) | 0.0 | 0.695 | |||
| Adjusted for dietary quality | |||||||||||||||
| Yes | 6 | 1.29 (1.11, 1.51) | 82.0 | <0.001 | 0.844 | 2 | 1.25 (0.86, 1.81) | 85.5 | 0.009 | 0.856 | – | – | – | – | – |
| No | 3 | 1.32 (1.15, 1.51) | 78.5 | <0.001 | 3 | 1.31 (0.96, 1.78) | 81.9 | 0.019 | – | – | – | – | – | ||
Abbreviations: N, number; RR, relative risk; CI, confidence interval; FFQ, food frequency questionnaire; Pa: P value for heterogeneity within each subgroup; Pb: P value for heterogeneity between subgroups.
All above the analyses have also been performed using the fix-effect model, the results didn't change corresponding relationships (Supplemental Figure S5–S7 and Supplemental Table S4).
Sensitivity analysis
With one study excluded each time, as well as trim and fill method, the results did not change substantially. Notably, there were two studies with UPF consumption modeled as a continuous variable excluded from the main analysis. Addition of the RR from these study to the meta-analysis did not substantially change the results (Supplemental Figure S8).
Publication bias
The Egger regression symmetry and funnel plots didn't find any publication bias on CVEs in relation to UPF consumption (Supplemental Figure S9).
Discussion
The findings from this meta-analysis based on 1,101,073 participants, including 58,201 CVEs cases, 24,086 CHD cases and 7614 CeVD cases, demonstrate a positive linear relationship between UPF intake and risk of CVEs. A 10% increase of UPF by daily weight proportion was associated with a 1.9% increase of CVEs risk, each additional daily serving corresponding to 2.2% CVEs risk increase, and per 10% increase by daily energy proportion corresponding to 1.6% CVEs risk increase. Our meta-analysis has several strengths. First, our meta-analysis included 22 cohorts and 1,101,073 participants, which provided sufficient power to detect modest associations. Second, the inclusion of considerable references (9/20) of no association or opposite association between CVEs and UPF, which is of the largest negative sample sizes in similar analysis, largely reducing the bias. Third, the detailed and stratified analysis for the major type of CVDs, i.e., CHD and CeVD, which providing the detailed corresponding estimated risks for the first time.
A previous meta-analysis summarizing eight prospective cohort studies found a linear upward trend between UPF intake and the risk of CVEs,19 demonstrated that each additional daily serving of UPF was associated with a 4% higher risk of CVEs, which is much higher than our results. This may be due to the fact that some newly published articles revealed no association or opposite association between CVEs and UPF consumption. In addition, we did not include articles that estimated UPF consumption by daily frequency to ensure the accuracy of our pooled results.27 From this perspective, future researches to set standard units/methods and to provide quantitative estimates of absolute UPF intake are recommended to increase inter-study comparability and confirm our findings.
In our study, we found that there was a positive linear relationship between UPF consumption and the CHD risk in daily servings and energy proportion classification, while no significant association between UPF intake and CeVD risk. Guo et al. reported a non-linear dose–response association between UPF consumption and the risk of cardio-cerebrovascular diseases.20 The inconsistent results may be due to the differences of included studies and the absolute small number of participants for CeVD in the original studies. Moreover, CeVD have different mechanisms from CHD, and the effects of UPF on CeVD could be different from that on CHD.
Differences among studies in the characteristics of the study populations, study design, sample size, and statistical adjustments may have contributed to heterogeneity. When we stratified daily weight proportion studies by region, we found that the RR of CVEs in United States was lower than in non-United States regions. However, in terms of daily serving and energy proportion studies, the RR of United States is higher than that of non-United States. This may be due to the large difference in the number of daily weight proportion studies grouped by region, which does not accurately reflect the regional differences in UPF intake. In stratified analysis, we also found that daily weight proportion studies with larger sample sizes had lower RR, and daily servings studies published before 2022 had higher RR. Additionally, the dose–response RRs in daily servings and weight proportion studies were higher than that in daily energy proportion, which can be attributed to the non-nutritional factors pertaining to food. The most prominent features of UPF are poor dietary quality, as well as lower food nutrient density and higher food energy density.46,47 However, our stratified analysis with diet quality adjusted concerned revealed that UPF intake was still significantly associated with CVEs risk. Therefore, it was suggested that beyond dietary quality, food processing may be another factor that cannot be ignored. Large amounts of dietary lipid oxidation products are formed during high-temperature cooking, a class of new carcinogens and contaminants highly associated with CVD risk.48 To extend shelf life, most UPF have excessive food packaging that releases trace chemicals. UPF also add high concentrations of cosmetic additives and no/low-calorie sweeteners, which are also associated with an increased risk of CVD.49
It is important to note that our study also had several limitations. First, the use of observational data is always subject to bias. In particular, the use of different covariate adjustment across studies is a particular limitation of this meta-analyses. Second, the CVEs endpoint contains different events which have differing levels of importance, and the effect of UPF consumption on each of these individual events may differ. Third, due to the type of data reported in original articles, the dose of reference groups was not zero (no consumption of UPF). Notably, our procedure for re-categorising the UPF intake might introduce some misclassification of the exposure as different studies categorized UPF intake differently, which should be another important limitation of the study.
Our findings highlight that UPF consumption significantly increases the burden of cardiovascular diseases. These specific data need to be fully translated into public health policies to mitigate the adverse impacts of UPF in a sustainable way. National public health authorities should incorporate specific recommendations to limit UPF into their national dietary guidelines, especially in densely populated middle-income countries where sales of UPF are increasing rapidly.50 In addition, cardiologists and dietitians need to work together to give adequate attention and timely advice on the patients' UPF intake.
Contributors
YQ and WH developed the search strategy and wrote the manuscript. JH, BT, and FM checked the search and reviewed the manuscript. YQ and WH performed articles screening and data extraction and conducted the quality assessment of the included studies. HW and BT carried out the data analysis. CX and LY designed the project, reviewed the manuscript, and finally approved the version to be published. All authors contributed to the article and approved the submitted version.
Data sharing statement
The study protocol and statistical analysis plan are available upon request to the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgements
Thanks to Prof. Ling Wang from the Air Force Medical University for the great help regarding the statistics. Thanks to Prof. Li Liu and Yingying Zhao from Tongji Medical College for providing unpublished data in the article.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102484.
Contributor Information
Changyang Xing, Email: xingcy712@163.com.
Lijun Yuan, Email: yuanlj@fmmu.edu.cn.
Appendix A. Supplementary data
References
- 1.Townsend N., Kazakiewicz D., Lucy Wright F., et al. Epidemiology of cardiovascular disease in Europe. Nat Rev Cardiol. 2022;19:133–143. doi: 10.1038/s41569-021-00607-3. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai H., Much A.A., Maor E., et al. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990-2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2022;8:50–60. doi: 10.1093/ehjqcco/qcaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgoine T., Forouhi N.G., Griffin S.J., Wareham N.J., Monsivais P. Associations between exposure to takeaway food outlets, takeaway food consumption, and body weight in Cambridgeshire, UK: population based, cross sectional study. BMJ. 2014;348:g1464. doi: 10.1136/bmj.g1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuevas García-Dorado S., Cornselsen L., Smith R., Walls H. Economic globalization, nutrition and health: a review of quantitative evidence. Glob Health. 2019;15:15. doi: 10.1186/s12992-019-0456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung M.M.S., Bao Y., Zhang B.Y., Le T.M., Huang J.Y. Life cycle assessment on environmental sustainability of food processing. Annu Rev Food Sci Technol. 2022;13:217–237. doi: 10.1146/annurev-food-062420-014630. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro C.A., Cannon G., Moubarac J.C., Levy R.B., Louzada M.L.C., Jaime P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21:5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srour B., Kordahi M.C., Bonazzi E., Deschasaux-Tanguy M., Touvier M., Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. 2022;7:1128–1140. doi: 10.1016/S2468-1253(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 11.Juul F., Parekh N., Martinez-Steele E., Monteiro C.A., Chang V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am J Clin Nutr. 2022;115:211–221. doi: 10.1093/ajcn/nqab305. [DOI] [PubMed] [Google Scholar]
- 12.Wang L., Martínez Steele E., Du M., et al. Trends in consumption of ultraprocessed foods among US youths aged 2-19 years, 1999-2018. JAMA. 2021;326:519–530. doi: 10.1001/jama.2021.10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whatnall M., Clarke E., Collins C.E., Pursey K., Burrows T. Ultra-processed food intakes associated with 'food addiction' in young adults. Appetite. 2022;178 doi: 10.1016/j.appet.2022.106260. [DOI] [PubMed] [Google Scholar]
- 14.Bertoni Maluf V.A., Bucher Della Torre S., Jotterand Chaparro C., et al. Description of ultra-processed food intake in a Swiss population-based sample of adults aged 18 to 75 years. Nutrients. 2022;14:4486. doi: 10.3390/nu14214486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero Ferreiro C., Cancelas Navia P., Lora Pablos D., Gómez de la Cámara A. Geographical and temporal variability of ultra-processed food consumption in the Spanish population: findings from the DRECE study. Nutrients. 2022;14:3223. doi: 10.3390/nu14153223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinu M., Tristan Asensi M., Pagliai G., et al. Consumption of ultra-processed foods is inversely associated with adherence to the mediterranean diet: a cross-sectional study. Nutrients. 2022;14:2073. doi: 10.3390/nu14102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan L., Hu H., Li T., et al. Dose-response meta-analysis of ultra-processed food with the risk of cardiovascular events and all-cause mortality: evidence from prospective cohort studies. Food Funct. 2023;14:2586–2596. doi: 10.1039/d2fo02628g. [DOI] [PubMed] [Google Scholar]
- 18.Guo L., Li F., Tang G., et al. Association of ultra-processed foods consumption with risk of cardio-cerebrovascular disease: a systematic review and meta-analysis of cohort studies. Nutr Metabol Cardiovasc Dis. 2023;33:2076–2088. doi: 10.1016/j.numecd.2023.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Bonaccio M., Costanzo S., Di Castelnuovo A., et al. Ultra-processed food intake and all-cause and cause-specific mortality in individuals with cardiovascular disease: the Moli-sani Study. Eur Heart J. 2022;43:213–224. doi: 10.1093/eurheartj/ehab783. [DOI] [PubMed] [Google Scholar]
- 20.Dehghan M., Mente A., Rangarajan S., et al. Ultra-processed foods and mortality: analysis from the prospective urban and rural epidemiology study. Am J Clin Nutr. 2023;117:55–63. doi: 10.1016/j.ajcnut.2022.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Kityo A., Lee S.A. The intake of ultra-processed foods, all-cause, cancer and cardiovascular mortality in the Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) cohort. PLoS One. 2023;18 doi: 10.1371/journal.pone.0285314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks K.A., Tcheng J.E., Bozkurt B., et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of cardiology/American heart association task Force on clinical data standards (writing committee to develop cardiovascular endpoints data standards) J Am Coll Cardiol. 2015;66:403–469. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symons M.J., Moore D.T. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55:893–899. doi: 10.1016/s0895-4356(02)00443-2. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S., Longnecker M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 27.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H., Hu E.A., Rebholz C.M. Ultra-processed food intake and mortality in the USA: results from the third national health and nutrition examination survey (NHANES III, 1988-1994) Public Health Nutr. 2019;22:1777–1785. doi: 10.1017/S1368980018003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srour B., Fezeu L.K., Kesse-Guyot E., et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé) BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rico-Campà A., Martínez-González M.A., Alvarez-Alvarez I., et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. doi: 10.1136/bmj.l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonaccio M., Di Castelnuovo A., Costanzo S., et al. Ultra-processed food consumption is associated with increased risk of all-cause and cardiovascular mortality in the Moli-sani Study. Am J Clin Nutr. 2021;113:446–455. doi: 10.1093/ajcn/nqaa299. [DOI] [PubMed] [Google Scholar]
- 32.Du S., Kim H., Rebholz C.M. Higher ultra-processed food consumption is associated with increased risk of incident coronary artery disease in the atherosclerosis risk in communities study. J Nutr. 2021;151:3746–3754. doi: 10.1093/jn/nxab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong G.C., Gu H.T., Peng Y., et al. Association of ultra-processed food consumption with cardiovascular mortality in the US population: long-term results from a large prospective multicenter study. Int J Behav Nutr Phys Activ. 2021;18:21. doi: 10.1186/s12966-021-01081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Silva A., Brum Felício M., Caldas A.P.S., et al. Ultra-processed foods consumption is associated with cardiovascular disease and cardiometabolic risk factors in Brazilians with established cardiovascular events. Int J Food Sci Nutr. 2021;72:1128–1137. doi: 10.1080/09637486.2021.1908963. [DOI] [PubMed] [Google Scholar]
- 35.Nardocci M., Polsky J.Y., Moubarac J.C. Consumption of ultra-processed foods is associated with obesity, diabetes and hypertension in Canadian adults. Can J Public Health. 2021;112:421–429. doi: 10.17269/s41997-020-00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juul F., Vaidean G., Lin Y., Deierlein A.L., Parekh N. Ultra-processed foods and incident cardiovascular disease in the framingham offspring study. J Am Coll Cardiol. 2021;77:1520–1531. doi: 10.1016/j.jacc.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 37.Bonaccio M., Di Castelnuovo A., Ruggiero E., et al. Joint association of food nutritional profile by Nutri-Score front-of-pack label and ultra-processed food intake with mortality: moli-sani prospective cohort study. BMJ. 2022;378 doi: 10.1136/bmj-2022-070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Chu J., Hu W., et al. Associations of ultra-processed food consumption with cardiovascular disease and all-cause mortality: UK Biobank. Eur J Public Health. 2022;32:779–785. doi: 10.1093/eurpub/ckac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Pan X.F., Munro H.M., Shrubsole M.J., Yu D. Consumption of ultra-processed foods and all-cause and cause-specific mortality in the Southern Community Cohort Study. Clin Nutr. 2023;42:1866–1874. doi: 10.1016/j.clnu.2023.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan V.K., Appel L.J., Anderson C.A.M., et al. Ultraprocessed foods and kidney disease progression, mortality, and cardiovascular disease risk in the CRIC study. Am J Kidney Dis. 2023;82:202–212. doi: 10.1053/j.ajkd.2023.01.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Li S., Yang H., et al. Association of ultra-processed food intake with cardiovascular and respiratory disease multimorbidity: a prospective cohort study. Mol Nutr Food Res. 2023;67 doi: 10.1002/mnfr.202200628. [DOI] [PubMed] [Google Scholar]
- 42.Bonaccio M., Di Castelnuovo A., Costanzo S., et al. Ultraprocessed food consumption is associated with all-cause and cardiovascular mortality in participants with type 2 diabetes independent of diet quality: a prospective observational cohort study. Am J Clin Nutr. 2023;118:627–636. doi: 10.1016/j.ajcnut.2023.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Silva F.M., Giatti L., Fonseca M., et al. Consumption of ultra-processed foods and eight-year risk of death from all causes and noncommunicable diseases in the ELSA-Brasil cohort. Int J Food Sci Nutr. 2023;74:845–854. doi: 10.1080/09637486.2023.2267797. [DOI] [PubMed] [Google Scholar]
- 44.Ansari S., Mohammadifard N., Haghighatdoost F., et al. The relationship between ultra processed food consumption and premature coronary artery disease: Iran premature coronary artery disease study (IPAD) Front Nutr. 2023;10 doi: 10.3389/fnut.2023.1145762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y., Chen W., Li J., et al. Ultra-processed food consumption and mortality: three cohort studies in the United States and United Kingdom. Am J Prev Med. 2023;66(2):315–323. doi: 10.1016/j.amepre.2023.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Shim J.S., Shim S.Y., Cha H.J., Kim J., Kim H.C. Association between ultra-processed food consumption and dietary intake and diet quality in Korean adults. J Acad Nutr Diet. 2022;122:583–594. doi: 10.1016/j.jand.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Juul F., Deierlein A.L., Vaidean G., Quatromoni P.A., Parekh N. Ultra-processed foods and cardiometabolic health outcomes: from evidence to practice. Curr Atherosclerosis Rep. 2022;24:849–860. doi: 10.1007/s11883-022-01061-3. [DOI] [PubMed] [Google Scholar]
- 48.Peng C.Y., Lan C.H., Lin P.C., Kuo Y.C. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. J Hazard Mater. 2017;324:160–167. doi: 10.1016/j.jhazmat.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 49.Srour B., Touvier M. Ultra-processed foods and human health: what do we already know and what will further research tell us? eClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker P., Machado P., Santos T., et al. Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. 2020;21 doi: 10.1111/obr.13126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.