Introduction
Magnetic resonance imaging (MRI) of patients with an implantable device includes a potential safety risk, both for the patient and the implant. Cochlear implants use 2 magnets: 1 internal and 1 external.1 Although the external magnet located in the patient's headpiece is easily removable, the internal magnet implanted in the mastoid bone cannot be removed without surgical intervention and is particularly at risk during an MRI procedure. One approach for reducing this risk is to use a compression headwrap to fix the internal magnet. This technique has shown conditional compliance with 1.5 T and 3 T MRI but has not previously been tested at 0.35 T.1,2
Even with a compression headwrap on an MRI-conditional cochlear implant at 1.5 T, complications have been reported.2 These include patient discomfort because of physical magnetic pull, magnet dislocation, and occasionally complete dislodging of the internal cochlear implant component, requiring surgical intervention to reinstall the device.3 Many patients opt for surgical removal of the cochlear implant before undergoing an MRI procedure to prevent such complications.
One consideration when using the 0.35 T magnet is that even though the compression headwrap approach is approved for use at higher magnetic field strengths (1.5 and 3 T), data supporting safety at lower field strengths are not readily available. Gilk and Kanal have described the potential for untoward effects at lower magnetic field strengths for 2 reasons.4 First, although lower field strengths in general produce less torque on ferromagnetic implants, a difference in the orientation of the static field strength (ie, vertical versus parallel) could potentially induce a larger torque. Second, radiofrequency energy directionality in lower strength systems may also influence heating and current production, which may not necessarily be experienced to the same degree at higher field strengths.
In this report, we discuss our experience from the first reported use of MR-guided radiation therapy (MRgRT) to simulate and treat a patient with a cochlear implant. Additionally, we describe the workflow, clearance, and management of a patient treated with a cochlear device off-label on a low-field MR Linac system.
Methods and Materials
Case presentation
An 81-year-old woman with an unresectable intrahepatic cholangiocarcinoma (Bismuth IIIA) initially was treated with induction gemcitabine and cisplatin. Restaging positron emission tomography/computed tomography demonstrated a residual 13.0 cc tumor abutting the duodenum. After multidisciplinary discussion, the consensus recommendation was to treat with definitive stereotactic body radiation therapy. Stereotactic MR-guided adaptive radiation therapy (SMART) was identified as the preferred technological approach. To this end, the patient was triaged to receive 50 Gy in 5 fractions on the MRIdian system (ViewRay), which combines a low-field 0.35 T MR with a 6 MV Linac.
The patient had a cochlear implant as well as a pacemaker; both of which are Food and Drug Administration (FDA) cleared for use with 1.5 T and off-label use at 0.35 T has been performed. Given the limited data and the off-label use, we decided to craft a dedicated methodological approach performed on an institutional review board approved protocol.
Cochlear implant overview
The HiRes Ultra cochlear implant is an electronic device that enables hearing by delivering electrical stimuli to the auditory nerve. The implant is FDA approved for use with MRI, but validated only at a magnetic field strength of 1.5 T. No FDA-guidance exists for lower magnetic field strengths.
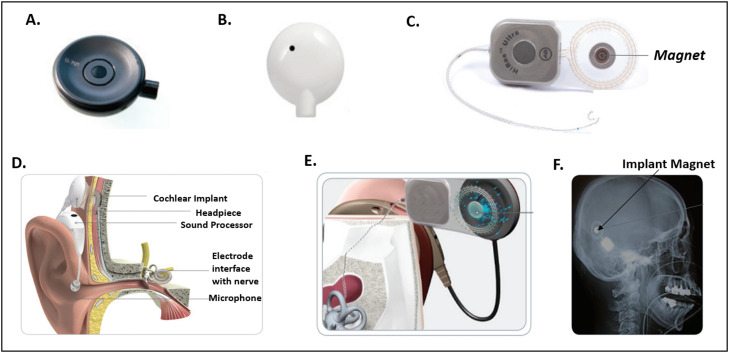
Figure 1 illustrates the components of the implant and their functionality. Specifically, Fig. 1D shows a cross sectional rendering of the cochlear implant installed in a patient. There are 2 components, an internal implant with an electrode array and a set of external devices, which function together to generate sound. The internal component, which consists of the implant and an electrode array is placed surgically. A combination of external devices, including the headpiece (Fig. 1B) and a sound processor, is worn on the patient's ear and attaches to the implant. The implant's magnet (Fig. 1C, F) holds the headpiece, which conveys sound to the internal device. The cochlear implant system (Fig. 1E), operates in the following manner: (1) sound is detected by the external microphone, (2) converted to digital signals by a sound processor, (3) signals sent to electrode interface of the inner ear of patient, and (4) electrode array stimulates the cochlear nerve, sending impulses to the brain. Before a patient enters the MRI room, the headpiece is removed and replaced with an MR safe coil cover (Fig. 1A).
Figure 1.
Overview of HiRes Ultra Cochlear Implant Device. (A) Coil cover for magnetic resonance imaging use, (B) patient headpiece, (C) cochlear implant with magnet, (D) anatomic implant view, (E) magnet device in the cochlea, and (F) example magnet implanted in patient. Used with permission from Advanced Bionics LLC.
Overview of MRgRT safety protocol for off-label device use
MRI screening workflow
We developed an institutional MR screening (Fig. 2) and MR safety triage workflow (Fig. 3) to manage and clear off-label implants for MRgRT. The general workflow is described below for all implants.
Figure 2.
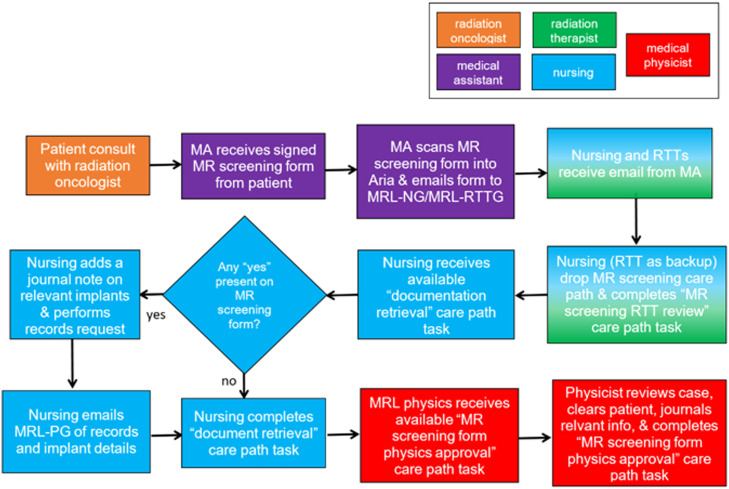
Overview of magnetic resonance (MR) screening workflow and documentation retrieval of implants for clearance for MR-guided radiation therapy. Hand-off in communication is noted in colored boxes for respective radiation therapist, nursing, and medical physicist. Abbreviations: MA = medical assistant; MRL-NG = MR Linac nursing; MRL-PG = MR Linac physics group; MRL-RTTG = MR Linac radiation therapist; RTT = radiation therapists.
Figure 3.
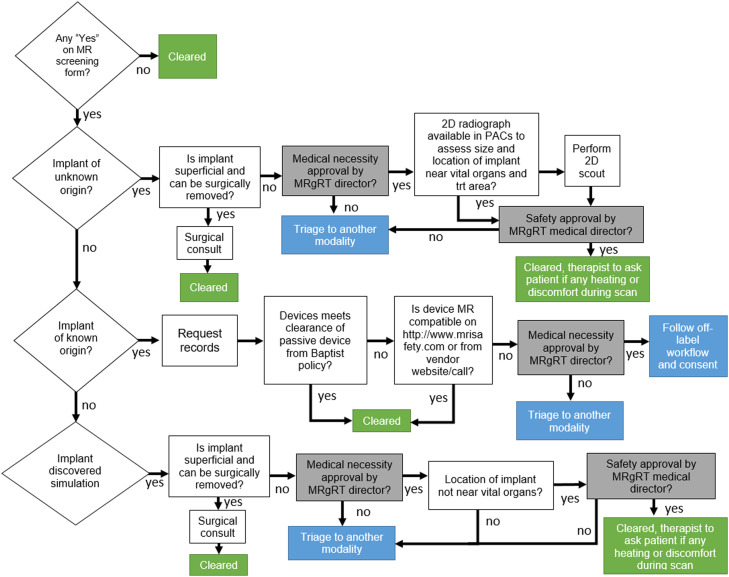
Overview of magnetic resonance clearance workflow demonstrating off-label usage and medical necessity by MRgRT director. Abbreviations: 2D = 2-dimensional; MRgRT = magnetic resonance-guided radiation therapy; PACS = picture archiving and communication system.
During a patient's initial consult, the medical assistant has the patient complete an MR safety screening form. This is a standard questionnaire adopted from our radiology department for patients undergoing diagnostic MRI. The patient is asked to list any surgical implants or devices they may have, exposure to shrapnel, metal, tattoos, etc. An MR screening care path in our Aria Record and Verify system is initiated when the medical assistant uploads the completed MR screening form, which is then distributed to the care team, including the MR Linac nursing (MRL-NG) and MR Linac radiation therapist (MRL-RTTG). The MRL-NG then initiates the MR screening care path in Aria and retrieves documentation for all implanted devices indicated on the MR screening form from the patient's medical history and as necessary from the manufacturer. The MRL-RTTG verifies that the care path is launched to ensure promptness of screening workflow completion. When all appropriate records are acquired, MRL-NG uploads all documentation to Aria. In doing so, a “documentation retrieval” care path task is completed and the MRL-NG emails the implant-related MR compatibility data and documentation to the MR Linac physics group (MRL-PG). MRL-PG reviews the MR screening form and appropriate document records, denotes clearance status in a journal note in Aria and completes the MR screening review task in the care path. Before the day of simulation, the MRL-RTTG reviews all scheduled simulations to verify that upcoming patients have been cleared. This process ensures that at least 1 business day is available to address any screening concerns.
MR clearance of off-label device
The MR clearance workflow (Fig. 3) is designed to clear each implant and proceed with imaging/treatment based on known/unknown origin, passive/active nature of the implant,5 and vendor on/off-label usage instructions. We implemented this specific protocol as a foundational safety component of our MRgRT program. For off-label use on each patient, the clinical director is required to triage the patient based on the medical necessity for MRgRT.
For this case, the internal component of the cochlear implant was not surgically removed and therefore was triaged for MRgRT medical necessity approval. The HiRes Ultra cochlear implant is cleared for use at 1.5 T, and vendor recommendations for MR scanning were available. Knowing this, we were able to clear the device by institutional policy for off-label usage at 0.35 T. The off-label workflow and consent were initiated and performed. Details of the wrapping procedure regarding the preparation for MR scanning are noted below. The therapist also tracked and noted any level of patient discomfort or heating during the scan.
Cochlear implant: Imaging and radiation specifications
Table 1 lists the specifications for MR imaging and radiation conditions between the vendor approved protocol and MRIdian system. For intact magnets of HiRes Ultra implants, a 1.5 T MR system with the maximum spatial field gradient of 20 T/m and a whole-body maximum MR specific absorption rate average of ≤2.0 W/kg at 1.5 T is approved for use by Advanced Bionics. The MRIdian system met all imaging conditions except for static magnetic field strength. Therefore, the use of the device at 0.35 T is categorized off-label and requires patient consent. The planned radiation delivery used a lower energy and dose than the manufacturer's specifications for the implant, and therefore was in accordance with vendor usage.
Table 1.
MR imaging and radiation specifications between HiRes Ultra cochlear implant and off-label usage on MRIdian
| Condition | Advanced Bionic HiRes Ultra MR specification | ViewRay MRIdian Linac specification |
|---|---|---|
| Static B0 field | 1.5 T | 0.35 T |
| Maximum spatial gradient | 20 T/m | 7 T/m (static gradient outside covers) 18 mT/m (dynamic gradient field strength) |
| Max SAR | 2.0 W/kg | 1.2 W/kg |
| Max slew rate | 200 T/m/sec per axis | 160 T/m/sec per axis |
| Duration after implantation | 4 weeks after implant | N/A |
| Radiation maximum energy | 15 MV | 6 MV |
| Radiation maximum dose | 250 Gy | N/A |
Abbreviations: MR = magnetic resonance; SAR = specific absorption rate.
Consent for off-label usage
The patient was consented regarding the safety and risks associated with undergoing off-label MR scanning with cochlear implant. Per the vendor, verbiage was provided regarding the risks of off-label device scanning of the implant for patients entering a 0.35 T field. The radiation oncologist presented the off-label risks to the patient before the MRgRT simulation, and the patient understood the risks, accepted them, and consented.
Additionally, the patient had an MR-compatible pacemaker (Abbott PM2272 model, Assurity MRI dual-chamber pacemaker), which has been designated as safe for use with 1.5 T and 3 T fields; this device was also cleared by our team for off-label pacemaker workflow (0.35 T).
Management of cochlear implant for MRgRT
Wrapping procedure
Per the vendor protocol (Advanced Bionics), a bandaging technique6 should be performed when a patient undergoes MRI with an intact magnetic implant. The wrapping procedure prevents displacement of the magnet and impedes magnet torque during MRI.1,2,3,6, 7, 8 The technique is performed with the patient in a sitting position to best access the implant. Figure 4 displays the required materials, which includes a self-adhesive 3-inch width elastic bandage, plastic MR antenna coil cover, patient's headpiece (external headpiece removed after marking respective location), and a marking pen. The patient brought 6 single-use MR antenna coil covers for each MR-guided session (one simulation and 5 radiation therapy procedures).
Figure 4.
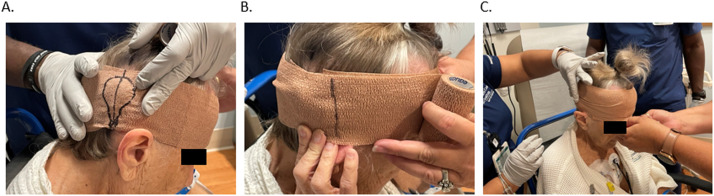
Example of wrapping technique on patient case showing (A) marking of headpiece before removal, (B) circumference measurement with line indicating 1 full rotation, and (C) compression of wrapping with 150% tension.
For the wrapping technique, the patient is first instructed to remove the external sound processor. We found it best to have the patient's hair in a ponytail on top of head to ease accessibility (Fig. 4). The circumference of the head is measured for the first bandage, which secures the coil cover. The bandage is then wrapped around the head, covering the patient's headpiece at nominal tension. The headpiece is marked, indicating its position (Fig. 4A). A mark on the scalp at the midline of the headpiece (Fig. 4A) enables a visual check verifying that the wrap and coil cover did not twist with respect to patient's head/internal magnet. The headpiece is then carefully removed. With the bandage still intact, the coil cover is slid under the first bandage and aligned with the marked outline of the headpiece. Any remnant of the bandage is wrapped around the head while maintaining nominal compression tension.
Once the first bandage is secure, a second bandage is then used as a compression wrap. The circumference of the head starting behind the ear is measured and marked with a pen (Fig. 4B). The bandage is removed and folded over itself to double the length (ie, resulting bandage length is twice head circumference). This bandage is then tightly wrapped with the marked line positioned at the half turn around the head. After 1.5 turns, the marked line should be on the opposite side of the head from the start of the wrap. The rotations of the bandage should continue for an additional 1.5 turns resulting in 3 full turns so that the bandage ends where the wrap started (Fig. 4C). Once the MRI procedure is completed, the wrap and antenna coil cover are removed, and all components are discarded.
Staff training: Cochlear implant and general MR safety education
Our institution-specific MR safety training requires all MRgRT staff, responsible for safety in zones III or IV, collectively called MR Personnel by American College of Radiology (ACR) guidelines9 to complete an annual MR safety and operations online training program. The annual education frequency is in accordance with the ACR and the Joint Commission standards, and we have specifically implemented level 2 training in accordance with ACR for MR Personnel. Level 2 MR training includes the broader aspects of MR safety, including principles related to the potential for RF-related thermal heating or burns and neuromuscular excitation from changing gradients.9 The definition of MR Personnel at our institution includes all physicians, medical physicists, radiation therapists (RTT), and nursing participating in the MRgRT program. Completion of annual required MR safety education is internally documented. Further medical credentials such as active ABR certification or specialty specific licensing (ie, ARRT for RTT, etc) are required with active status of maintenance of certification.
For cochlear implant safety education, a specialized audiologist from Advanced Bionics presented simulation training for the MRL-RTTG and MRL-PG, demonstrating the wrapping technique for immobilizing the internal magnet. The team practiced the wrapping protocol on a staff member with the audiologist present. A stepwise written overview of the procedure with pictures was reviewed with the team and adopted for internal procedure documentation. Vendor training was internally documented for commissioning and future cases.
For each head wrapping procedure, 2 RTTs and a medical physicist were required to be present. The 2 RTTs performed the wrapping, and the medical physicist assisted with verifying the coil cover positioning, sequence of steps, and wrap tension. All 3 members were required to have completed the previously mentioned in-service vendor training from Advanced Bionics and be up to date with level 2 MR training.
MR-guided radiation therapy
Simulation and initial plan creation
Patient simulation was performed under breath hold in the supine position with both arms at the sides. Simulation included a planning MR scan acquired on MRIdian at 45 × 45 × 24 cm3 field of view with resolution 1.6 × 1.6 × 3.0 mm3. The primary scan used for both simulation and each fractional MR scan for adaptive replanning was a balanced steady-state free precession sequence (TrueFISP). The TrueFISP spatial fidelity has previously been characterized, and the maximum distortion is <2.0 mm within 17.5 cm of isocenter.10 Minimal immobilization (foam pad and wing board) was used for simulation and treatment because MRgRT delivery was performed under continuous MR imaging11 for motion management.
Segmentation and treatment planning was performed on the MR simulation scan per institutional technique.12 The gross target volume was defined as the tumor visualized on diagnostic imaging and the TrueFISP MR, with a 3 mm margin to delineate the clinical target volume (CTV). The CTV was uniformly expanded by a 3 mm setup margin (SM) to create the planning target volume (PTV). Relevant organs at risk (OAR) segmented were stomach, duodenum, small bowel, large bowel, kidneys, liver, and spinal cord.
A 15-field beam step and shoot IMRT arrangement with 40 segments was performed with a Monte Carlo dose calculation algorithm using a 2 mm isotropic dose grid size. A bulk density approach was used for electron density based on an override to the vertebral bodies as bone, lung as lung, and gastrointestinal luminal air as air with the remaining body being defined as water.
On-table adaptation
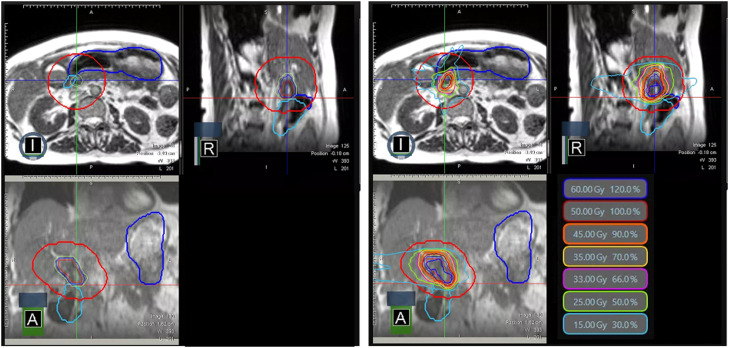
Our on-table adaptive MRgRT workflow has been previously reported.13 Target volumes were rigidly registered, and OARs were deformably registered from the simulation MR to the daily volumetric MR scan. The radiation oncologist reviewed and manually edited all OARs within 2 cm axially and 3 cm craniocaudally of the PTV surface (Fig. 5, left). The partial OAR segmentation technique, in line with the community practice,14 is used during online adaptive radiation therapy to accelerate segmentation editing/review to only OARs in the intermediate and high dose regions. Because the dose distribution falls off faster in the superior-inferior region with coplanar beam geometry, a smaller region of 2 cm is used for this direction.
Figure 5.
Targets with abutting gastrointestinal organs at risk for fraction 5 (left) and adaptive dose distribution (right).
After segmentation, a predicted plan was calculated using the original plan, generated using the simulated anatomy, superimposed on the current anatomy and contours of the day to evaluate whether there was any indication for adaptation. The predicted plan was considered sufficient if all organs at risk and target coverage metrics were achieved. If the predicted plan failed for any metric, an adaptive plan was generated (Fig. 5, right). The priority was to ensure that OAR constraints were met, and target coverage was a secondary goal.
For treatment delivery, real-time tracking was performed based on a 2-dimensional sagittal plane tracking at 4 frames per second with a tracking region of interest approximating the gross tumor volume. Automatic beam hold was implemented when >5% of the tracking region of interest area was outside the 3 mm static boundary.
Nonverbal communication
Because the patient was legally deaf, audio communication during the treatment workflow was not possible and therefore nonverbal communication techniques were developed. A light switch instructed the patient to initiate her breath hold. When the light was switched off, the patient was instructed to hold her breath. To communicate the need for a deeper breath, lights were flashed twice. In addition, staff would enter the vault during pauses throughout the treatment process to communicate further instructions, if necessary, with their lips visible to the patient through a mirror mounted to display behind the gantry.
Results
The patient did not report any discomfort beyond mild soreness from the pressure of wrapping. No adverse reactions or safety concerns were identified during MRI or radiation delivery. Specifically, there were no instances of device stimulation, device malfunction, or excessive heating. No magnet tilt or displacement occurred.
The patient had no acute or late severe treatment-related toxicity. The patient continued care through her audiologist and had no long-term device complications with HiRes Ultra post-MRgRT. The patient achieved a favorable radiographic response in the treated area but expired 10 months after completing radiation therapy due to multifocal out-of-field intrahepatic progression.
Discussion
Our first-in-human case demonstrates the feasibility of using SMART for a patient with a cochlear implant on a 0.35 T MR Linac. MRgRT offers a multitude of benefits for radiation therapy of cholangiocarcinoma with abutting gastrointestinal organs at risk. Although the implanted magnet can be surgically removed before radiation therapy treatment, removal and reimplantation is cumbersome and a nonsurgical option would be preferred by patients.
To our knowledge, this is the first report demonstrating viability of repeated daily MR imaging sessions at 0.35 T with off-label scanning of a cochlear implant. Compared with prior studies2,5 that demonstrated feasibility on diagnostic MR scanners with cochlear implants, patients treated with MRgRT are potentially at higher risk because of the requirement to undergo multiple MRI scans at the time of simulation as well as before each treatment day. As such, minimizing the number of fractions is particularly advantageous. Additionally, there was an extended time for each treatment session of the patient being present in the magnetic field, due to the adaptive workflow and gated delivery. Such additional risk could be mitigated by treating the patient in a single fraction course, as has been previously shown feasible with MRIdian.15,16
Because the MRIdian system is a 0.35 T onboard MR scanner, we had to clear and consent our patient to off-label scanning at 0.35 T. Scanning implanted devices at low-field versus high-field strength is expedient because of the reduced specific absorption rate,17 reduced null band artifacts,18 reduced magnetic susceptibility,19 and overall reduced ferromagnetic pull on the magnetic implant. Additionally, there are fewer spatial distortion effects due to less perturbation of magnetic susceptibility from the introduced implant.19 Our report demonstrates both an MR-Linac triage and clearance workflow based on off-label usage and MRgRT medical necessity. Note that for our case, there was a significant distance from the treatment area (ie, abdomen) to the implant location. If the disease site was more proximal to the cochlear implant (ie, brain or head and neck cancers), further complications would be induced that may limit the feasibility of providing MRgRT.
The workflow presented here can be adopted for the triage and clearance of any patient for MR Linac. Particularly, the patient triage for off-label scanning has previously been limited to CIED in the literature.18 We also present a framework for discussion of medical necessity and off-label consent which can be applied broadly to any implantable device for clearance on MR Linac.
This case demonstrates several challenges with MRgRT for patients with cochlear implants. These include mastering the wrapping technique procedure for the specific nonsurgical stabilization of the implant. Additional challenges included adjusting imaging protocols for nonverbal communication with the patient who was deaf without the external component of the implant. Alternative methods including light switch actions and mirror motions were used. ViewRay has recently released MRIdian A3i, which includes full capabilities of nonverbal communication. Specially, the patient is informed of duration of breath hold scan time and can visually observe their tumor real-time position and ideal static position during treatment delivery. The console on A3i also enables the ability to have the user write out sentences and abbreviated phrases to further communicate with the patient at any given time during the workflow.
Conclusion
The first-in-human treatment of MRgRT with on-table adaptive workflow, real-time tracking and beam gating was demonstrated to be safe and feasible in a patient with unresectable intrahepatic cholangiocarcinoma and a magnetic cochlear implant. Special provisions were taken to ensure the patient's safety and minimize damage to the cochlear device resulting from the use of low-field 0.35 T MR Linac. We describe workflow features deployed to ensure MR safety including off-label clearance, off-label consent, and implementation of daily wrapping technique for immobilization of cochlear implant.
Disclosures
Nema Bassiri reports grants from ViewRay Inc; Alonso N. Gutierrez reports honoraria from ViewRay, Inc, Elekta AB, IBA AB. Ownership interest of Atlantic Health Solutions (uPlanÔ); Minesh P. Mehta reports consulting fees from Karyopharm, KAzia Therapeutics, Sapience, Zap, Mevion, Xoft; BOD Oncoceutics; Stock in Chimerix; Michael D. Chuong reports honoraria from ViewRay, Sirtex, IBA and institutional research funding from ViewRay, Novocure, StratPharma; Kathryn E. Mittauer reports ownership interest in a company that provides consulting services on image guided radiation therapy technology (MR Guidance, LLC.). She received travel reimbursement/consulting fees/speaking fees/grants from ViewRay Inc; Kevin J. Abrams is a consultant for Keystone Heart, Viz.Ai Stock: Keystone Heart, Viz.Ai, Cleerly. All remaining authors declare no conflicts of interest.
Acknowledgment
Deborah Mittauer of AdventHealth and Lindsey Martin of Advanced Bionics for their assistance with the case and head wrapping training.
Footnotes
Sources of support: This work had no specific funding.
Research data are not available at this time.
References
- 1.Azadarmaki R, Tubbs R, Chen DA, Shellock FG. MRI information for commonly used otologic implants: Review and update. Otolaryngol Head Neck Surg. 2014;150:512–519. doi: 10.1177/0194599813518306. [DOI] [PubMed] [Google Scholar]
- 2.Jaimes C, Biaggotti D, Sreedher G, Chaturvedi A, Moore MM, Danehy AR. Magnetic resonance imaging in children with implants. Pediatr Radiol. 2021;51:748–759. doi: 10.1007/s00247-021-04965-5. [DOI] [PubMed] [Google Scholar]
- 3.Mayinger M, Kovacs B, Tanadini-Lang S, et al. First magnetic resonance imaging-guided cardiac radioablation of sustained ventricular tachycardia. Radiother Oncol. 2020;152:203–207. doi: 10.1016/j.radonc.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Gilk T, Kanal E. MRI safety considerations associated with low-field MRI: Mostly good news. MAGMA. 2023;36:427–428. doi: 10.1007/s10334-023-01079-x. [DOI] [PubMed] [Google Scholar]
- 5.Aissani S, Laistler E, Felblinger J. MR safety assessment of active implantable medical devices. Radiologe. 2019;59(Suppl 1):40–45. doi: 10.1007/s00117-019-0541-6. [DOI] [PubMed] [Google Scholar]
- 6.Leinung M, Loth AG, Kroth M, Burck I, Stöver T, Helbig S. Comparison of bandaging techniques to prevent cochlear implant magnet displacement following MRI. Eur Arch Otorhinolaryngol. 2021;278:4209–4216. doi: 10.1007/s00405-020-06504-8. [DOI] [PubMed] [Google Scholar]
- 7.Stecco A, Saponaro A, Carriero A. Patient safety issues in magnetic resonance imaging: State of the art. Radiol Med. 2007;112:491–508. doi: 10.1007/s11547-007-0154-4. [DOI] [PubMed] [Google Scholar]
- 8.Jansson KJ, Håkansson B, Reinfeldt S, Taghavi H. Eeg-Olofsson M. MRI induced torque and demagnetization in retention magnets for a bone conduction implant. IEEE Trans Biomed Eng. 2014;61:1887–1893. doi: 10.1109/TBME.2014.2309978. [DOI] [PubMed] [Google Scholar]
- 9.ACR Committee on MR Safety. ACR Manual on MR Safety, version 1.0, 2020
- 10.Ginn JS, Agazaryan N, Cao M, et al. Characterization of spatial distortion in a 0.35 T MRI-guided radiotherapy system. Phys Med Biol. 2017;62:4525–4540. doi: 10.1088/1361-6560/aa6e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuong MD, Bryant JM, Herrera R, et al. Dose-Escalated Magnetic Resonance Image-Guided Abdominopelvic Reirradiation With Continuous Intrafraction Visualization, Soft Tissue Tracking, and Automatic Beam Gating. Adv Radiat Oncol. 2021;7(2) doi: 10.1016/j.adro.2021.100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittauer KE, Yarlagadda S, Bryant JM, et al. Online adaptive radiotherapy: Assessment of planning technique and its impact on longitudinal plan quality robustness in pancreatic cancer. Radiother Oncol. 2023;188 doi: 10.1016/j.radonc.2023.109869. [DOI] [PubMed] [Google Scholar]
- 13.Chuong MD, Bryant J, Mittauer KE, et al. Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract Radiat Oncol. 2021;11(2):134–147. doi: 10.1016/j.prro.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Price AT, Zachary CJ, Laugeman E, et al. Patient specific contouring region of interest for abdominal stereotactic adaptive radiotherapy. Phys Imaging Radiat Oncol. 2023;25 doi: 10.1016/j.phro.2023.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finazzi T, van Sörnsen de Koste JR, Palacios MA, et al. Delivery of magnetic resonance-guided single-fraction stereotactic lung radiotherapy. Phys Imaging Radiat Oncol. 2020;14:17–23. doi: 10.1016/j.phro.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuong MD, Kotecha R, Mehta MP, et al. Case report of visual biofeedback-driven, magnetic resonance-guided single-fraction SABR in breath hold for early stage non-small-cell lung cancer. Med Dosim. 2021;46(3):247–252. doi: 10.1016/j.meddos.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Bottomley PA. Turning up the heat on MRI. J Am Coll Radiol. 2008;5(7):853–855. doi: 10.1016/j.jacr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gach HM, Green OL, Cuculich PS, et al. Lessons learned from the first human low-field MRI guided radiation therapy of the heart in the presence of an implantable cardiac defibrillator. Pract Radiat Oncol. 2019;9:274–279. doi: 10.1016/j.prro.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Stanescu T, Wachowicz K, Jaffray DA. Characterization of tissue magnetic susceptibility-induced distortions for MRIgRT. Med Phys. 2012;39:7185–7193. doi: 10.1118/1.4764481. [DOI] [PubMed] [Google Scholar]