Abstract
The objective of this study is to investigate the beneficial effects and underlying mechanism of dietary β-mannanase supplementation on the productive performance of laying hens fed with metabolic energy (ME)-reduced diets. A total of 448 Hy-Line gray laying hens were randomly assigned to seven groups. Each group had 8 replicates with 8 hens. The groups included a control diet (CON) with a ME of 2750 kcal/Kg, diets reduced by 100 kcal/Kg or 200 kcal/Kg ME (ME_100 or ME_200), and diets with 0.15 g/Kg or 0.2 g/Kg β-mannanase (ME_100+β-M_0.15, ME_100+β-M_0.2, ME_200+β-M_0.15, and ME_200+β-M_0.2). The productive performance, egg quality, intestinal morphology, inflammatory response, mRNA expression related to the Nuclear factor kappa B (NF-κB) and AMPK pathway, and cecum microbiome were evaluated in this study. ME-reduced diets negatively impacted the productive performance of laying hens. However, supplementation with β-mannanase improved FCR, decreased ADFI, and restored average egg weight to the level of the CON group. ME-reduced diets increased the levels of interleukin-1β (IL-1β) and IL-6 while decreasing the levels of IL-4 and IL-10 in the jejunum of laying hens. However, dietary β-mannanase supplementation improved jejunum morphology, reduced pro-inflammatory cytokine concentrations, and increased levels of anti-inflammatory factors in laying hens fed with ME-reduced diets. The mRNA levels of IL-6, IFN-γ, TLR4, MyD88, and NF-κB in the jejunum of ME-reduced diets were significantly higher than that in CON, dietary β-mannanase supplementation decreased these genes expression in laying hens fed with ME-reduced diets. Moreover, dietary β-mannanase supplementation also decreased the mRNA levels of AMPKα and AMPKγ, and increased the abundance of mTOR in the jejunum of laying hens fed with ME-reduced diets. Cecum microbiota analysis revealed that dietary β-mannanase increased the abundance of various beneficial bacteria (e.g., g_Pseudoflavonifractor, g_Butyricicoccus, and f_Lactobacillaceae) in laying hens fed with ME-reduced diets. In conclusion, dietary β-mannanase supplementation could improve the productive performance of laying hens fed with a ME-reduced diet by improving intestinal morphology, alleviating intestinal inflammation, changing energy metabolism-related signaling pathways, and increasing cecum-beneficial microbiota.
Key words: laying hens, β-Mannanase, low-energy diet, inflammatory response, cecum microbiota
INTRODUCTION
Non-starch polysaccharides (NSP) are recognized as the primary antinutritional factors in standard commercial poultry diets, reducing nutrient digestibility and growth performance (Choct, 2002; Choct et al., 2010). β-Mannan is one of a soluble NSP that is indigestible by poultry. It is commonly found in both dehulled and nondehulled soybean meals, with a content between 1.02%and 2.14% (Hsiao et al., 2006). Although the β-mannan content is relatively low in soybean meal, it receives more attention from nutritionists due to its highly anti-nutritive properties. β-Mannan can increase the viscosity of chyme, inhibit intestinal peristalsis, hinder digestion and absorption of nutrients, disrupt the microbial community in the digestive tract, and affect the feed conversion ratio (FCR) and body weight gain in broilers, as well as reduced the productive performance of laying hens (Patel and McGinnis, 1985; Daskiran et al., 2004; Ayoola et al., 2015; Shastak et al., 2015; Kim et al., 2017; Caldas et al., 2018).The addition of exogenous enzymes in poultry has been confirmed as an effective strategy to alleviate these anti-nutritive effects of NSP.
β-Mannanase is an exogenous enzyme that can cleave the internal glycosidic bonds of the mannan backbone, producing ß-1,4-manno-oligosaccharides, reducing its anti-nutritive properties (Chauhan et al., 2012). The positive effects of supplementing poultry diets with β-mannanase enzyme in the presence of β-mannan have been thoroughly reviewed. In broilers, studies have reported the beneficial effects of β-mannanase on improving body weight gain, FCR, nutrition digestibility, and digestible energy (Zou et al., 2006; Balasubramanian et al., 2018; Caldas et al., 2018; Latham et al., 2018; Mohammadigheisar et al., 2021). Data from studies on laying hens indicated that the addition of β-mannanase significantly improved FCR and egg production in older laying hens (Torki et al., 2016; Ryu et al., 2017; Hasani et al., 2019). Moreover, β-mannanase improved FCR and productive performance in laying hens fed with metabolic energy (ME)-reduced diets (-50∼-120 kcal/Kg ME) to the levels of hens fed with a high-energy diet without the enzyme (Jackson et al., 1999; Wu et al., 2005; Zheng et al., 2020; White et al., 2021). However, few studies have been conducted to investigate the effects of β-mannanase on the productive performance of laying hens fed a ME-reduced diet during a peak production period. Additionally, the detailed mechanism of dietary β-mannanase in improving the performance of laying hens fed with an ME-reduced diet is not completely understood.
Recently, evidence has shown that β-mannan can stimulate the innate immune system and lead to a nonproductive, energy-draining immune response (Zou et al., 2006; Latham et al., 2016; Lee et al., 2018). Nuclear factor kappa B (NF-κB) is a crucial transcription factor that controls the gene expression of numerous cytokines, innate immunity, and apoptosis. The change in the NF-κB signaling pathway in the intestinal may help us understand the beneficial effects of dietary β-mannanase supplementation on the productive performance of laying hens. Additionally, the adverse effects of β-mannan in poultry diets were associated with increased intestinal viscosity and altered gut microbiota composition (Shastak et al., 2015). The composition of gut microbiota is crucial for the intestinal health and growth performance of poultry. However, to our knowledge, few studies have been conducted to evaluate the effects of β-mannanase on cecum microbiota composition in laying hens. Therefore, the objectives of this study were: 1) to investigate the effects of β-mannanase on the productive performance and egg quality of laying hens fed with ME-reduced diets, and 2) to evaluate the effects of β-mannanase on the intestinal inflammatory response, morphology, NF-κB signaling pathway, and cecum microbiota composition of laying hens.
MATERIAL AND METHODS
Birds, Diets, Experiment Design, and Sample Collection
The research protocol and use of animals in this study were conducted in accordance with the guidelines for care and use of laboratory animals of Qingdao Agricultural University and approved by the animal care and use committee of Qingdao Agricultural University.
A total of 448 25-week-old Hy-Line Gray laying hens were divided into seven treatment groups, each consisting of 8 replicates, and placed into randomized parcels. Each replicate consisted of 8 cages, each containing 8 hens. The control (CON) group received a basal diet (Table 1) formulated to meet the nutrient requirements of Chinese feeding standard (NY/T33-2004). The ME-reduced diet groups received a diet with a ME reduction of 100 kcal/Kg (ME_100) and 200 kcal/Kg (ME_200), respectively (Table 1). The β-mannanase treatment groups were fed the ME_100 diet supplemented with 0.15 g/Kg (ME_100+β-M_0.15) or 0.2 g/Kg (ME_100+β-M_0.2) β-mannanase, and the ME_200 diet supplemented with 0.15 g/Kg (ME_200+β-M_0.15) or 0.2 g/Kg (ME_200+β-M_0.2) β-mannanase, respectively. The trial period was 8 wk. β-Mannanase were provided by Elanco (Shanghai) Animal Health Co., Ltd (Shanghai, China). The activity of the pure enzyme and the experimental diets were analyzed and verified by the manufacturer. The pure enzyme activity was not less than 3.6×108 U/Kg. The enzyme activity in the experimental diets was not less than 5.4×104 U/Kg for the 0.15 g/Kg β-mannanase supplement groups and 7.2×104 U/Kg for the 0.2 g/Kg β-mannanase supplement groups, respectively. One unit is defined as the amount of mannanase enzyme which generates 0.72 mg of reducing sugars per minute from a mannan-containing substrate at pH 7.0 and 40°C. All laying hens were provided with ad libitum access to food and water. Feed intake was recorded in the 4th and 8th weeks, respectively, and used to calculate the value of FCR. The FCR was calculated by dividing feed consumption by the produced egg. At the end of the experiment, egg quality was determined by using 16 egg (2 eggs per replicate) samples from each group.
Table 1.
Composition and nutrient levels of basal diets (%).
| Ingredients | CON diet | ME_100 | ME_200 |
|---|---|---|---|
| Corn | 61.5 | 61.5 | 60.3 |
| Soybean meal | 25.0 | 25.0 | 25.2 |
| Soybean oil | 2.0 | 0.8 | 0.0 |
| Limestone | 8.5 | 8.5 | 8.5 |
| Zeolite powder | 0 | 1.2 | 3.0 |
| Premix 1 | 3 | 3 | 3 |
| Total | 100 | 100 | 100 |
| Nutrient levels 2 | |||
| ME, kcal/Kg | 2750 | 2650 | 2550 |
| CP | 16.04 | 16.02 | 16.02 |
| Ca | 3.50 | 3.50 | 3.50 |
| Available P | 0.38 | 0.38 | 0.38 |
| Lys | 0.82 | 0.82 | 0.82 |
| Met | 0.38 | 0.38 | 0.38 |
| Thr | 0.61 | 0.61 | 0.61 |
| Try | 0.19 | 0.19 | 0.19 |
| β-mannan | 0.48 | 0.48 | 0.49 |
The premix provided the following per kg of diets:Vitamin A (trans-retinyl acetate) 10,000 IU; Vitamin D3 (cholecalciferol) 2,500 IU; Vitamin E (all-rac-a-tocopherol acetate) 30 IU; Vitamin K3 0.75 mg; Vitamin B1 (thiamin) 1.5 mg; Vitamin B2 (riboflavin) 3.8 mg; Vitamin B6 (pyridoxine HCl) 4.5 mg; Vitamin B12 (cobalamin) 0.01 mg; biotin 0.15 mg; folic acid 0.5 mg; D-pantothenic acid 10 mg; nicotinic acid 30 mg; choline (as choline chloride) 500 mg; Cu (as copper sulfate) 10 mg; Fe (as ferrous sulfate) 80 mg; Mn (as manganese sulfate) 80 mg; Zn (as zinc sulfate) 90 mg; I (as potassium iodide) 0.40 mg; Se (as sodium selenite) 0.30 mg; Met (as dl-Met) 0.12%; available P (as CaHPO4) 2.8%; NaCl 0.15%.
The nutrient levels were calculated values (except for CP) according to data obtained from China Feed Data (https://www.chinafeeddata.org.cn/admin/Login/index.html), for example, ME=corn ratio × ME of corn in chickens + soybean meal ratio of soybean meal in chickens+ Soybean oil ratio × ME of soybean in chickens. CP was determined by combustion (AOAC method 990.03). The β-mannan content was calculated based on previously published data. (Dierick, 1989; Upadhaya et al., 2016). The diets used in the research were isonitrogenic.
At the end of the experiment, after fasting for 12 h, one blood sample was collected from each replicate. The samples were taken from the wing vein and then centrifuged for 10 min (3,000 g, at 4°C) to separate the serum. Thereafter, the laying hens (1 bird per replicate) were humanely euthanized by cervical dislocation, and the jejunum samples were collected and mixed with 4% paraformaldehyde for analysis of intestinal histomorphology. Additionally, the mucosa of the jejunum was scraped away, frozen in liquid nitrogen, and stored at −80°C for mRNA extraction and cytokine determination. Cecum contents were collected into sterile cryotubes, immediately frozen in liquid nitrogen, and then stored at −80°C until analysis.
Measurement of Egg Quality
At the 8th week of the experiment, egg quality measurements include shell strength, shell thickness, yolk color score, albumen height, and Haugh unit. The strength of the eggshell was analyzed using an eggshell strength meter (ORKA, Ramat Hasharon, Israel). Shell thickness was measured with an electronic micrometer at 3 positions on the shell. Yolk color score, albumen height, and Haugh unit were determined using an egg Multi-Tester (ORKA, Ramat Hasharon, Israel).
Intestinal Histomorphology
Fixed jejunum tissue was embedded in paraffin and then sectioned into 5 μm sections for hematoxylin-eosin staining, following the protocol described in our previous report (Liu et al., 2022). The villus height and crypt depth were measured using optical microscopy (Olympus, Tokyo, Japan). Then, the villus height, crypt depth, and villus height to crypt depth ratio were calculated from these measurements.
Serum and Jejunum Cytokines Analysis
Jejunum mucosa (100 mg) was homogenized in 0.9 mL of saline, centrifuged at 1,000 g for 10 min at 4°C, and the supernatants were collected for subsequent analysis. Serum and jejunum mucosa concentrations of interleukin-1β (IL-1β), IL-4, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were determined using commercial assay kits (Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China), following the manufacturer's protocols. Additionally, the protein concentrations of the jejunum mucosa were analyzed using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the manufacturer's instructions.
Quantitative Real-Time PCR
Total RNA from jejunum mucosa was isolated using Trizol reagent (Tiangen Biotech Co., Ltd., Beijing, China). The purity and integrity of the RNA were evaluated using the OD 260/280 value and 1.2% gel electrophoresis. Reverse transcription and real-time quantitative PCR were performed using the CFX96 PCR System (Bio-Rad, Hercules, CA) with commercial kits (TaKaRa, Kusatsu, Japan), according to our previous report (Liu et al., 2022). Relative mRNA expression levels were calculated using the 2−ΔΔCt method. Primers are listed in Table S1.
Cecum Microbiome Analysis
Cecum microbiome analysis was performed as described in our previous report (Liu et al., 2023). Briefly, DNA samples were isolated from cecum contents using the TIANamp stool DNA kit (Tiangen Biotech Co., Ltd., Beijing, China). Subsequently, the DNA quality was assessed using 0.8% gel electrophoresis. The purified DNA was sequenced using a HiSeq 4,000 system (Illumina Inc., San Diego, CA) for 16S rRNA analysis. DNA samples were amplified by PCR using the primer pair 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′). High-quality clean tags were obtained by filtering of the raw tags using QIIME2. Operational taxonomic units (OTU) were clustered at a 97% sequence identity level using Uclust (https://arc.umich.edu/software-item/uclust). The SILVA database (https://www.arb-silva.de/documentation/release-132/) was used to assign taxonomy to the representative sequences of each cluster. Alpha diversity (including Chao1, Shannon, Simpson, and observed OUT) and beta diversity were calculated using QIIME2. Linear discriminant analysis effect size (LEfSe) analysis were performed using the OmicStudio tools (https://www.omicstudio.cn/tool).
Statistical Analysis
Statistical analysis of data from this study was performed using ANOVA in the SPSS 26.0 software package (SPSS, Inc., Chicago, IL,). Duncan's multiple range test was used to compare differences among the groups. Significance was accepted at P < 0.05. Data were represented by the mean ± standard error of the mean, and graphs were prepared using GraphPad Prism 8.0 software (GraphPad Software Inc., La Jolla, CA). Alpha diversity and beta diversity of cecal microbiota were analyzed using the Kruskal-Wallis test. LEfSe analysis was filtered based on Kruskal-Wallis and Wilcoxon values < 0.05 and LDA score > 3.
RESULTS
Laying Performance and Egg Quality
The effects of β-mannanase supplementation on the productive performance of laying hens fed with ME-reduced diets are shown in Table 2. Compared to the CON group, the ADFI and FCR in ME_100 and ME_200 groups were 6% and 8% higher (P < 0.05) during the 1 to 4 wk period, 7% and 10% higher (P < 0.05) during the 5 to 8 wk period, and 6% and 9% higher (P < 0.05) during the 1 to 8 wk period, respectively. Moreover, when dietary ME was reduced by 100 kcal/Kg or 200 kcal/Kg, the average egg weight was 1 to 2% lower (P < 0.05) than that in the CON group. Compared with ME_100 group, supplementation of β-mannanase significantly decreased the ADFI and improved the FCR of laying hens fed a ME-reduced diet (P < 0.05). Furthermore, the average egg weight in the ME-reduced group was significantly lower (P < 0.05) than that in the CON group. However, β-mannanase supplementation significantly increased (P < 0.05) the average egg weight of laying hens fed with the ME-reduced diet, bring it to similar levels as the CON group. There was no significant difference (P > 0.05) in laying rate among the seven groups. The effects of β-mannanase supplementation on egg quality of laying hens fed a ME-reduced diet are shown in Table 3. There were no significant difference (P > 0.05) in shell strength, shell thickness, yolk color score, albumen height, and Haugh unit among the 7 groups.
Table 2.
Effects of β-mannanase supplementation on productive performance of laying hens fed with ME-reduced diets (n = 8).
| Group | ADFI (g) | Average laying rate (%) | Average egg mass (g) | Feed to egg ratio |
|---|---|---|---|---|
| 1 to 4 wk | ||||
| CON | 111.42±0.33e | 96.73±1.12 | 58.93±0.41a | 1.91±0.03e |
| ME_100 | 117.79±0.25c | 96.15±0.62 | 57.88±0.39bc | 2.07±0.01c |
| ME_200 | 121.05±0.29a | 95.71±0.98 | 57.54±0.70c | 2.15±0.03a |
| ME_100+β-M_150 | 116.66±0.27d | 95.78±0.52 | 58.22±0.69abc | 2.04±0.03cd |
| ME_100+β-M_200 | 116.25±0.37d | 95.93±0.69 | 58.34±0.59abc | 2.03±0.03d |
| ME_200+β-M_150 | 120.60±0.23a | 95.93±0.54 | 58.33±0.92abc | 2.11±0.03b |
| ME_200+β-M_200 | 119.86±0.31b | 96.37±0.68 | 58.70±0.57ab | 2.07±0.02c |
| P-value | 0.001 | 0.125 | 0.001 | 0.001 |
| 5 to 8 wk | ||||
| CON | 110.54±0.74f | 97.09±1.12 | 58.71±0.36ab | 1.98±0.04d |
| ME_100 | 118.13±0.19d | 96.08±0.60 | 57.92±0.43bc | 2.17±0.02b |
| ME_200 | 121.88±0.39a | 96.00±0.95 | 57.36±0.65c | 2.27±0.03a |
| ME_100+β-M_150 | 115.85±0.12e | 96.29±0.53 | 57.91±0.63bc | 2.13±0.03c |
| ME_100+β-M_200 | 115.89±0.21e | 96.08±0.60 | 58.25±0.69ab | 2.12±0.03c |
| ME_200+β-M_150 | 120.02±0.28b | 95.93±0.62 | 58.33±0.73ab | 2.20±0.03b |
| ME_200+β-M_200 | 119.12±0.19c | 96.15±0.76 | 58.84±0.60a | 2.15±0.03bc |
| P-value | 0.001 | 0.066 | 0.001 | 0.001 |
| 1–8 wk | ||||
| CON | 110.97±0.24f | 96.91±1.09 | 58.82±0.35a | 1.95±0.03e |
| ME_100 | 117.96±0.19d | 96.11±0.58 | 57.90±0.39bc | 2.12±0.02bc |
| ME_200 | 121.50±0.26a | 95.86±0.90 | 57.52±0.64c | 2.21±0.03a |
| ME_100+β-M_150 | 116.24±0.18e | 96.04±0.49 | 58.17±0.61abc | 2.09±0.03cd |
| ME_100+β-M_200 | 116.06±0.23e | 96.00±0.53 | 58.40±0.50ab | 2.07±0.03d |
| ME_200+β-M_150 | 120.30±0.24b | 95.93±0.54 | 58.42±0.67ab | 2.15±0.03b |
| ME_200+β-M_200 | 119.48±0.22c | 96.26±0.69 | 58.77±0.57a | 2.11±0.03bcd |
| P-value | 0.001 | 0.089 | 0.001 | 0.001 |
Different lowercase letters of peer shoulder notes indicate significant differences (P < 0.05).
Abbreviations: CON, control diet; ME_100 or ME_200, diets with ME reduced by 100 kcal/Kg or 200 kcal/Kg; ME_100+β-M_0.15, ME_100 diet + 0.15 g/Kg β-mannanase; ME_100+β-M_0.2, ME_100 diet + 0.2 g/Kg β-mannanase; ME_200+β-M_0.15, ME_200 diet + 0.15 g/Kg β-mannanase; ME_200+β-M_0.2, ME_200 diet + 0.2 g/Kg β-mannanase.
Table 3.
Effects of β-mannanase supplementation on egg quality of laying hens fed with ME-reduced diets (n = 8).
| Group | Shell strength (kg/cm2) | Shell thickness (mm) | Yolk color score | Albumen height | Haugh unit |
|---|---|---|---|---|---|
| CON | 4.727±0.775 | 0.374±0.032 | 8.98±0.54 | 9.39±0.63 | 96.29±3.18 |
| ME_100 | 4.653±0.531 | 0.387±0.014 | 9.11±0.31 | 8.69±0.95 | 93.19±4.87 |
| ME_200 | 4.489±0.746 | 0.380±0.014 | 8.23±0.93 | 8.48±1.05 | 92.50±4.82 |
| ME_100+β-M_150 | 5.052±0.105 | 0.399±0.017 | 8.41±1.01 | 8.89±0.66 | 94.54±3.26 |
| ME_100+β-M_200 | 4.996±0.195 | 0.388±0.022 | 8.93±0.66 | 8.38±1.20 | 91.94±6.33 |
| ME_200+β-M_150 | 4.978±0.199 | 0.389±0.020 | 8.64±0.23 | 8.84±0.74 | 94.14±3.59 |
| ME_200+β-M_200 | 3.993±0.823 | 0.390±0.018 | 8.41±0.56 | 8.71±0.81 | 93.31±3.89 |
| P-value | 0.158 | 0.300 | 0.073 | 0.370 | 0.529 |
Abbreviations: CON, control diet; ME_100 or ME_200, diets with ME reduced by 100 kcal/Kg or 200 kcal/Kg; ME_100+β-M_0.15, ME_100 diet + 0.15 g/Kg β-mannanase; ME_100+β-M_0.2, ME_100 diet + 0.2 g/Kg β-mannanase; ME_200+β-M_0.15, ME_200 diet + 0.15 g/Kg β-mannanase; ME_200+β-M_0.2, ME_200 diet + 0.2 g/Kg β-mannanase.
Intestinal Histomorphology
The histological analysis of jejunum tissues (Figure 1A and 1B) showed that the intestinal villi of laying hens in the CON group were tightly packed and the epithelium was morphologically intact. In the ME_100 and ME_200 groups, the histological analysis of the jejunum in laying hens showed significant villus swelling and breakage. However, the addition of dietary β-mannanase improved the morphology of the villi in the ME-reduced groups. The villus height in the ME_100 group was 20% lower (P < 0.05) than that in the CON group. However, dietary supplementation with 0.2 g/Kg β-mannanase significantly increased (P < 0.05) the villus height of laying hens in the ME_100 group, restoring it to the CON levels. There was no significant difference in crypt depth among the CON, ME_100, and ME_200 groups (P > 0.05). However, dietary supplementation with 0.2 g/Kg β-mannanase significantly decreased (P < 0.05) the crypt depth of laying hens in the ME-reduced groups. Compared to the CON group, the ME_200 group had significantly lower (P < 0.05) values of villus height/crypt depth. This value significantly increased (P < 0.05) with the addition of 0.2 g/Kg β-mannanase to the diet. Compared to a 0.1 g/Kg β-mannanase supplement, a 0.2 g/Kg β-mannanase supplement has a greater (P < 0.05) impact on improving the jejunum morphology of laying hens that are fed with ME-reduced diets.
Figure 1.
Effects of β-mannanase on the jejunum morphology of laying hens fed with ME-reduced diets (n = 8). CON: control diet; ME_100 or ME_200: diets with ME reduced by 100 kcal/Kg or 200 kcal/Kg; ME_100+β-M_0.15: ME_100 diet + 0.15 g/Kg β-mannanase; ME_100+β-M_0.2: ME_100 diet + 0.2 g/Kg β-mannanase; ME_200+β-M_0.15: ME_200 diet + 0.15 g/Kg β-mannanase; ME_200+β-M_0.2: ME_200 diet + 0.2 g/Kg β-mannanase. Different lowercase letters of peer shoulder notes indicate significant differences (P < 0.05).
Serum and Jejunum Cytokines
As shown in Figure 2, the serum concentrations of IL-1β (ME_200 group) and IL-6 (ME_100 and ME_200 groups) were 22%∼48% higher (P < 0.05) than that in the CON group. While dietary supplementation with 0.15 g/Kg and 0.2 g/Kg β-mannanase significantly decreased (P < 0.05) the serum levels of IL-1β and IFN-γ in laying hens fed a ME-reduced diet. The serum levels of IL-4 and IL-10 in ME-reduced groups were 18%∼34% lower (P < 0.05) than that in the CON group, and β-mannanase supplementation significantly increased (P < 0.05) these levels in the serum of ME_100 or ME_200 groups. In the jejunum mucosa, compared with the CON group, the levels of proinflammatory cytokines (IL-1β and IL-6) in ME_100 and ME_200 groups were 18%∼52% higher (P < 0.05), and anti-inflammatory factors (IL-4 and IL-10) were 18%∼54% lower (P < 0.05). However, dietary supplementation with 0.15 g/Kg or 0.2 g/Kg of β-mannanase significantly decreased jejunum IL-1β and IL-6 levels (P < 0.05), and increased jejunum IL-4 and IL-10 concentrations of laying hens that were fed ME-reduced diets (P < 0.05). Serum and jejunum TNF-α concentrations showed no significant difference among the CON, ME_100, and ME_200 groups (P > 0.05). However, dietary supplementation with 0.15 g/Kg β-mannanase significantly decreased TNF-α levels in the serum and jejunum of laying hens that were fed a ME_100 diet (P < 0.05).
Figure 2.
Effects of β-mannanase on serum and jejunum cytokines levels in layihng hens fed with ME-reduced diets (n = 8). IL-1β: interleukin-1β; TNF-α: tumor necrosis factor-α; IFN-γ: interferon-γ; CON: control diet; ME_100 or ME_200: diet with ME reduced by 100 kcal/Kg or 200 kcal/Kg; ME_100+β-M_0.15: ME_100 diet + 0.15 g/Kg β-mannanase; ME_100+β-M_0.2: ME_100 diet + 0.2 g/Kg β-mannanase; ME_200+β-M_0.15: ME_200 diet + 0.15 g/Kg β-mannanase; ME_200+β-M_0.2: ME_200 diet + 0.2 g/Kg β-mannanase. Different lowercase letters of peer shoulder notes indicate significant differences (P < 0.05).
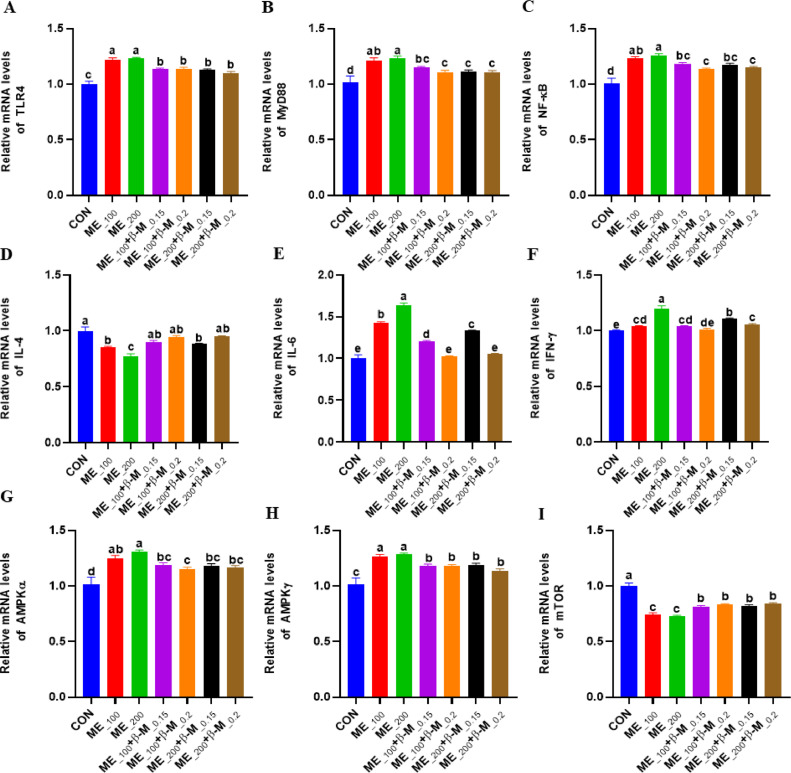
mRNA Expression Related to NF-κB and AMP-Activated Protein Kinase (AMPK)
As depicted in Figure 3, the levels of TLR4, MyD88, NF-κB, IL-6, and IFN-γ mRNA were significantly higher (P < 0.05) in the jejunum mucosa of laying hens in the ME_100 and ME_200 groups compared to the CON group. Conversely, the level of IL-4 mRNA was significantly lower (P < 0.05) in the ME_100 and ME_200 groups compared to the CON group. Dietary supplementation of β-mannanase significantly reduced (P < 0.05) the mRNA levels of TLR4, MyD88, NF-κB, IL-6, and IFN-γ, and increased (P < 0.05) the abundance of IL-4 mRNA in the jejunum mucosa of laying hens fed with ME-reduced diets. The dose of 0.2 g/Kg β-mannanase was more effective than 0.1 g/Kg in this regard. Moreover, laying hens fed with ME-reduced diets showed a significant increased in the mRNA expression of AMPKα and AMPKγ (P < 0.05), and a significant (P < 0.05) reduction in the mRNA expression of mTOR in the jejunum mucosa tissues. The groups supplemented with β-mannanase showed a significant decrease in the abundance of AMPKα and AMPKγ mRNA, while the mRNA expression level of mTOR was significantly increased (P < 0.05) compared to the CON group.
Figure 3.
Effects of β-mannanase on NF-κB and AMPK pathway mRNA expression in the jejunum mucosa of laying hens fed with ME-reduced diets (n = 8). TLR4: Toll-like receptor 4; MyD88: Myeloid differentiation factor 88; NF-κB: Nuclear Factor Kappa B; IL4: interleukin-4; IFN-γ: interferon-γ; AMPK: AMP-activated protein kinase; mTOR: Mechanistic target of rapamycin complex 1; CON: control diet; ME_100 or ME_200: diets with ME reduced by 100 kcal/Kg or 200 kcal/Kg; ME_100+β-M_0.15: ME_100 diet + 0.15 g/Kg β-mannanase; ME_100+β-M_0.2: ME_100 diet + 0.2 g/Kg β-mannanase; ME_200+β-M_0.15: ME_200 diet + 0.15 g/Kg β-mannanase; ME_200+β-M_0.2: ME_200 diet + 0.2 g/Kg β-mannanase. Different lowercase letters of peer shoulder notes indicate significant differences (P < 0.05).
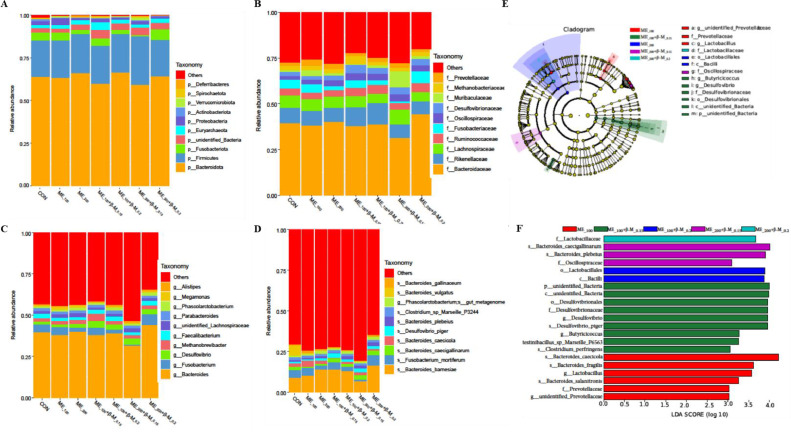
Cecum Microbiome Composition
Alpha diversity of the cecum microbiota was determined using Chao1, Shannon, Simpson, and OUT indices (Figures 4A–4D). There were no significant differences in the α-diversity among the groups (P > 0.05). PCoA analysis (Figure 4E) showed that β-diversity analysis of the cecum microbiota composition was not distinct among the groups (P > 0.05). Figures 5A–5D and Table S2 (Supplementary file) indicate the specific differences in cecum microbiota at phylum, family, genus, and species levels. When compared to the ME-reduced group, the phylum-level abundance of Synergistota was higher (P = 0.011) and the abundance of Actinobacteria and Spirochaetota was lower (P < 0.05) with β-mannanase supplementation. Within the family, the laying hens fed diets supplemented with β-mannanase had lower (P < 0.05) and greater (P < 0.05) abundances of Marinifilaceae and Synergistaceae, respectively, than the ME-reduced group. At the genus level, the ME_100 group had a higher abundance of unidentified Prevotellaceae (P = 0.006) than the CON group. On the other hand, the abundance of unidentified Prevotellaceae in laying hens treated with the ME_100 diet was significantly reduced (P < 0.05) by dietary supplementation with 0.15 g/Kg and 0.2 g/Kg β-mannanase. LEfSe analysis was used to detect taxonomic indicators in the cecum microbiota, as shown in Figures 5E and 5F. In the ME_100 group, there was an increase in the relative abundance of f_Prevotellaceae, g_unidentified_Prevotellaceae, and g_Lactobacillus, as well as s_caecicola, s_fragilts, and s_salanitronts. In laying hens fed a ME_100 diet, dietary supplementation with β-mannanase increased (P < 0.05) the relative abundance of f_desulfovibrionaceae, g_desulfovibrio, s_desulfovibrio_piger, g_Butyricicoccus, and s_perfringens. Furthermore, the abundance of f_Oscillospiraceae, f_Lactobacillaceae, s_Plebeius, and s_Caecigallinarum in β-mannanase supplemented diets was higher (P < 0.05) compared to laying hens fed a ME_200 diet.
Figure 4.
Effects of β-mannanase on α-diversity and β-diversity of cecum microbiota in laying hens fed with ME-reduced diets (n = 8). CON: control diet; ME_100 or ME_200: diets with ME reduced by 100 kcal/Kg or 200 kcal/Kg; ME_100+β-M_0.15: ME_100 diet + 0.15 g/Kg β-mannanase; ME_100+β-M_0.2: ME_100 diet + 0.2 g/Kg β-mannanase; ME_200+β-M_0.15: ME_200 diet + 0.15 g/Kg β-mannanase; ME_200+β-M_0.2: ME_200 diet + 0.2 g/Kg β-mannanase.
Figure 5.
Effects of β-mannanase on the abundance of cecum microbiota in laying hens fed with ME-reduced diets (n = 8). CON: control diet; ME_100 or ME_200: diets with ME reduced by 100 kcal/Kg or 200 kcal/Kg; ME_100+β-M_0.15: ME_100 diet + 0.15 g/Kg β-mannanase; ME_100+β-M_0.2: ME_100 diet + 0.2 g/Kg β-mannanase; ME_200+β-M_0.15: ME_200 diet + 0.15 g/Kg β-mannanase; ME_200+β-M_0.2: ME_200 diet + 0.2 g/Kg β-mannanase.
DISCUSSION
It is widely acknowledged that laying hens can adjust their feed intake based on energy levels in their diet. A decrease in dietary energy level results in an increase in feed intake and decrease in FCR (Harms et al., 2000; Zhang and Kim, 2013; Mikulski et al., 2020). Similarly, the results of the current study showed that ME-reduced diets (-100 or 200 kcal/Kg ME) significantly increased the ADFI and FCR, while significantly decreasing the average egg weight. β-Mannan is a primary anti-nutritive factor in soybean meal that reduces energy utilization (Shastak et al., 2015). Inclusion of exogenous enzymes in laying hen diets to enhance egg production performance and decrease feed costs is a commonly practice within the industry. β-Mannanase supplementation has been shown to improve egg production performance and nutrient availability in hens that were fed ME-reduced diets (Zhang and Kim, 2013; Shim et al., 2018; White et al., 2021). In line with these studies, the addition of β-mannanase improved egg weight, reduced ADFI, and improved FCR in laying hens fed with ME-reduced diets.
The beneficial effects of β-mannanase on the productive performance of laying hens fed with ME-reduced diets cannot be simply explained by making β-mannan as a potential energy source. The study found that laying hens fed with ME-reduced diets exhibited impaired intestinal morphology, higher levels of proinflammatory cytokines (IL-1β and IL-6), and lower levels of anti-inflammatory factors (IL-4 and IL-10). Consistent with our present results, Zhang et al. (2024) found that broilers fed with ME-reduced diets had higher amounts of proinflammatory cytokines (IL-1β, IL-8, IFN-γ, and TNF-α) and lower amounts of anti-inflammatory factors (IL-4, IL-10, and TGF-β). These findings support the research by Yang et al.(2015), who found that a high dietary energy intake helps to alleviate the immunosuppressive effects of corticosterone in broiler chickens. The addition of β-mannanase can reduce the ability of β-mannan to stimulate the immune response of intestinal mucosa, thereby promoting intestinal health. A meta-analysis conducted on broilers showed that the use of β-mannanase improved intestinal integrity and reduced various conditions related to intestinal health (Poulsen et al., 2023). Scapini et al. (2019) demonstrated that adding β-mannanase reduced crypt depth and increased the villus/crypt ratio of Eimeria sp. challenged broilers. Our findings consistently indicate that laying hens fed with β-mannanase (ME_100+β-M_0.2) have a lower crypt depth and a higher villus/crypt proportion compared to those on CON and ME-reduced diets. Additionally, our findings showed that adding β-mannanase to ME-reduced diets significantly reduced the levels of pro-inflammatory factors (IL-1β and IL-6) levels and increased the concentration of IL-10 in the jejunum mucosa. According to Zou et al. (2006), similar to the findings of this study, β-mannanase has been shown to significantly increase the serum IgM concentration and T lymphocytes proliferation in broilers. Poulsen et al. (2023) suggest that β-mannanase can reduce intestinal inflammation in chickens by decreasing levels of IL-1β and IL-6. The positive effects of β-mannanase on laying hens fed with ME-reduced diets may be attributed to its ability to reduce intestinal inflammation. NF-κB is a transcription factor that regulates several cytokines, e.g., IL-1β, IL-6, and TNF-α. Cheng et al. (2021) reported that β-manana induced immune response may be attributed to its binding activity with TLR4/MD-2 complex. According to our current findings and previous research, we believe that β-mannanase reduces the release of proinflammatory cytokines by inhibiting the overactivation of the TLR4-MyD88-NF-κB signaling pathway in the jejunum.
To better understand how β-mannanase improves the performance of laying hens fed with ME-reduced diets, we analyzed the expression of crucial genes involved in energy metabolism. Our findings showed that laying hens fed with ME-reduced diets had significantly higher mRNA levels of AMPKα and AMPKγ in the jejunum mucosa, and lower levels of mTOR compared to the CON group. These findings are consistent with those of Hu et al. (2019) who reported that ME-reduced diets significantly increased the mRNA levels of AMPKα in broilers. AMPK is a vital cellular nutrient and energy sensor that regulates energy balance homeostasis (Hardie et al., 2012). AMPK can be activated by cellular energy depletion, leading to increased energy production pathways and inhibition of biosynthetic pathways, including the mTOR pathway. Interestingly, dietary supplementation of β-mannanase significantly reduced the expression of AMPKα and AMPKγ, while upregulating mTOR mRNA levels in the jejunum mucosa of laying hens that were fed ME-reduced diets. These results showed that β-mannanase decreased the mRNA levels of the AMPK signaling pathway and increased the mRNA expression of mTOR in the jejunum mucosa. This led to improved energy utilization in laying hens fed with ME-reduced diets.
The gut microbiota plays a crucial role in the performance and health of poultry (Brisbin et al., 2008). A previous report has shown that supplementing the diet with β-mannanase increases the abundance of lactobacillus in the feces, which contributes to improved growth performance in broilers (Mohammadigheisar et al., 2021). A recent study found that adding dietary β-mannanase improved the gut microbiota composition and performance of broilers that were fed a high galactomannans diet (de Souza et al., 2023). Our findings indicate that dietary supplementation of β-mannanase significantly increased the abundance of f_Barnesiellaceae, g_Barnesiella, s_caecigallinarum, g_Pseudoflavonifractor, g_Butyricicoccus, and f_Lactobacillaceae, while decreasing the abundance of p_Spirochaetota, f_Prevotellaceae, and f_Marinifilaceae in laying hens fed with ME-reduced diets. Previous studies have shown that g_Barnesiella can enhance the anti-inflammatory capacity of the intestinal mucosa in mice and is positively associated with the growth performance of broilers (Weiss et al., 2014; Zhu et al., 2020). Studies conducted on laying hens have shown that a higher abundance of Bacteroides_caecigallinarum is positively correlated with laying rate, while p_Spirochaetota is negatively correlated with the productive performance of hens (Xiao et al., 2022; Zhang et al., 2023). g_Pseudoflavonifractor and g_Butyricicoccus are 2 genera known for their positive impact on performance, intestinal health, and production of butyrate (Eeckhaut et al., 2016; Alam et al., 2021). The cecum of broilers challenged with oxidative stress or coccidia vaccine showed higher levels of f_Prevotellaceae and f_Marinifilaceae (Emami et al., 2020; Wang et al., 2021). The results suggest that the positive effects of β-mannanase on productivity and intestinal health may be partially explain by its ability to alter the composition of cecum microbiota. Specifically, it increases the abundance of beneficial bacteria, such as g_Pseudoflavonifractor, g_Butyricicoccus, and f_Lactobacillaceae. β-Mannan can be broken down by β-Mannanase into mannan-oligosaccharides, which function as a prebiotic and indirectly regulate metabolism, immunity, and intestinal microecology by promoting the growth of Bifidobacterium and Lactobacillus and inhibiting the attachment of pathogenic bacteria such as Escherichia coli to the animal intestine. (Gutierrez et al., 2008; Lai et al., 2015).
CONCLUSION
The study concluded that ME-reduced diets (ME: 2650 and 2550 kcal/Kg) led to decreased productive performance (average egg weight and FCR), increased intestinal inflammation, and disrupted energy metabolism by activating AMPK expression and downregulating mTOR expression in laying hens. Dietary supplementation of β-mannanase may aid in the recovery of the layer hens from ME-reduced diet. This supplementation can improve productive performance and intestinal morphology, reduce intestinal inflammation, inhibit the AMPK pathway in the jejunum, and increase the abundance of beneficial microbiota in the cecum.
ACKNOWLEDGMENTS
This study was supported by the Shandong Provincial Natural Science Foundation (ZR2021MC118 and ZR2021QC036); the Qingdao Science and Technology Program (22-3-7-xdny-11-nsh; 23-2-8-xdny-8-nsh); the Talents of High-Level Scientific Research Foundation of Qingdao Agricultural University (Grant No. 665/1120026); the Postgraduate Innovation Program of Qingdao Agricultural University (grant no. QNYCX22007).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103521.
Appendix. Supplementary materials
REFERENCES
- Alam M.S., Gangiredla J., Hasan N.A., Barnaba T., Tartera C. Aging-induced dysbiosis of gut microbiota as a risk factor for increased listeria monocytogenes infection. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.672353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola A.A., Malheiros R.D., Grimes J.L., Ferket P.R. Effect of dietary exogenous enzyme supplementation on enteric mucosal morphological development and adherent mucin thickness in turkeys. Front. Vet. Sci. 2015;2:45. doi: 10.3389/fvets.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian B., Ingale S., Park J.H., Rathi P., Shanmugam S., Kim I. Inclusion of dietary β-mannanase improves performance and ileal digestibility and reduces ileal digesta viscosity of broilers fed corn-soybean meal based diet. Poult. Sci. 2018;97:3097–3101. doi: 10.3382/ps/pey157. [DOI] [PubMed] [Google Scholar]
- Brisbin J.T., Gong J., Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008;9:101–110. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- Caldas J.V., Vignale K., Boonsinchai N., Wang J., Putsakum M., England J.A., Coon C.N. The effect of β-mannanase on nutrient utilization and blood parameters in chicks fed diets containing soybean meal and guar gum. Poult. Sci. 2018;97:2807–2817. doi: 10.3382/ps/pey099. [DOI] [PubMed] [Google Scholar]
- Chauhan P.S., Puri N., Sharma P., Gupta N. Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012;93:1817–1830. doi: 10.1007/s00253-012-3887-5. [DOI] [PubMed] [Google Scholar]
- Cheng T.Y., Lin Y.J., Saburi W., Vieths S., Scheurer S., Schülke S., Toda M. β-(1→ 4)-Mannobiose acts as an immunostimulatory molecule in murine dendritic cells by binding the TLR4/MD-2 complex. Cells. 2021;10:1774. doi: 10.3390/cells10071774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choct M., Non-starch polysaccharides: effect on nutritive value, In: McNab J.M. and Boorman K.N., Poultry Feedstuffs: Supply, Composition and Nutritive Value, 26, 2002, Poultry Science Symposium, CABI Publishing; Wallingford, Oxon OX10 8D, UK, 221–235.
- Choct M., Dersjant-Li Y., McLeish J., Peisker M. Soy oligosaccharides and soluble non-starch polysaccharides: a review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas. J. Anim. Sci. 2010;23:1386–1398. [Google Scholar]
- Daskiran M., Teeter R., Fodge D., Hsiao H. An evaluation of endo-β-D-mannanase (Hemicell) effects on broiler performance and energy use in diets varying in β-mannan content. Poult. Sci. 2004;83:662–668. doi: 10.1093/ps/83.4.662. [DOI] [PubMed] [Google Scholar]
- de Souza M., Eeckhaut V., Goossens E., Ducatelle R., Van Nieuwerburgh F., Poulsen K., Baptista A.A.S., Bracarense A.P.F.R.L., Van Immerseel F. Guar gum as galactomannan source induces dysbiosis and reduces performance in broiler chickens and dietary β-mannanase restores the gut homeostasis. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick N.A. Biotechnology aids to improve feed and feed digestion: enzymes and fermentation. Arch. Anim. Nutr. 1989;39:241–261. doi: 10.1080/17450398909429530. [DOI] [PubMed] [Google Scholar]
- Eeckhaut V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Van Immerseel F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Calik A., White M.B., Kimminau E.A., Dalloul R.A. Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.572142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez O., Zhang C., Caldwell D.J., Carey J.B., Cartwright A.L., Bailey C.A. Guar meal diets as an alternative approach to inducing molt and improving Salmonella enteritidis resistance in late-phase laying hens. Poult. Sci. 2008;87:536–540. doi: 10.3382/ps.2007-00337. [DOI] [PubMed] [Google Scholar]
- Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mole. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms R., Russell G., Sloan D. Performance of four strains of commercial layers with major changes in dietary energy. J. Appl. Poult. Res. 2000;9:535–541. [Google Scholar]
- Hasani M., Rezaei M., Pirsaraei Z.A., Kelarikolaei K.Y. Effects of different levels of guar meal and β-mannanase on performance, yolk cholesterol concentration and blood lipid parameters of laying hens in second-cycle of production. Iran. J. Appl. Anim. Sci. 2019;9:309–313. [Google Scholar]
- Hsiao H.-Y., Anderson D., Dale N. Levels of β-mannan in soybean meal. Poult. Sci. 2006;85:1430–1432. doi: 10.1093/ps/85.8.1430. [DOI] [PubMed] [Google Scholar]
- Hu X., Wang Y., Sheikhahmadi A., Li X., Buyse J., Lin H., Song Z. Effects of dietary energy level on appetite and central adenosine monophosphate-activated protein kinase (AMPK) in broilers. J. Anim. Sci. 2019;97:4488–4495. doi: 10.1093/jas/skz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M., Fodge D., Hsiao H. Effects of beta-mannanase in corn-soybean meal diets on laying hen performance. Poult. Sci. 1999;78:1737–1741. doi: 10.1093/ps/78.12.1737. [DOI] [PubMed] [Google Scholar]
- Kim M.C., Kim J.H., Pitargue F.M., Choi H.S., Kil D.Y. Effect of dietary β-mannanase on productive performance, egg quality, and utilization of dietary energy and nutrients in aged laying hens raised under hot climatic conditions. Asian-Australas. J. Anim. Sci. 2017;30:1450–1455. doi: 10.5713/ajas.17.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L.P., Lee M.T., Chen C.S., Yu B., Lee T.T. Effects of co-fermented Pleurotus eryngii stalk residues and soybean hulls by Aureobasidium pullulans on performance and intestinal morphology in broiler chickens. Poult. Sci. 2015;94:2959–2969. doi: 10.3382/ps/pev302. [DOI] [PubMed] [Google Scholar]
- Latham R., Williams M., Smith K., Stringfellow K., Clemente S., Brister R., Lee J. Effect of β-mannanase inclusion on growth performance, ileal digestible energy, and intestinal viscosity of male broilers fed a reduced-energy diet. J. Appl. Poult. Res. 2016;25:40–47. [Google Scholar]
- Latham R., Williams M., Walters H., Carter B., Lee J. Efficacy of β-mannanase on broiler growth performance and energy utilization in the presence of increasing dietary galactomannan. Poult. Sci. 2018;97:549–556. doi: 10.3382/ps/pex309. [DOI] [PubMed] [Google Scholar]
- Lee B.B., Yang T.S., Goo D., Choi H.S., Pitargue F.M., Jung H., Kil D.Y. Effects of dietary β-mannanase supplementation on the additivity of true metabolizable energy values for broiler diets. Asian-Australas. J. Anim. Sci. 2018;31:564. doi: 10.5713/ajas.17.0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Li X., Shi S., Zhou Y., Zhang K., Wang Y., Zhao J. Chlorogenic acid improves growth performance and intestinal health through autophagy-mediated nuclear factor erythroid 2-related factor 2 pathway in oxidatively stressed broilers induced by dexamethasone. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Li X., Zhang K., Lv X., Zhang Q., Chen P., Wang Y., Zhao J. Integrated multi-omics reveals the beneficial role of chlorogenic acid in improving the growth performance and immune function of immunologically-stressed broilers. Anim. Nutr. 2023;14:383–402. doi: 10.1016/j.aninu.2023.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulski D., Jankowski J., Mikulska M., Demey V. Effects of dietary probiotic (Pediococcus acidilactici) supplementation on productive performance, egg quality, and body composition in laying hens fed diets varying in energy density. Poult. Sci. 2020;99:2275–2285. doi: 10.1016/j.psj.2019.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadigheisar M., Shouldice V.L., Balasubramanian B., Kim I.H. Effect of dietary supplementation of β-mannanase on growth performance, carcass characteristics, excreta microflora, blood constituents, and nutrient ileal digestibility in broiler chickens. Anim. Biosci. 2021;34:1342. doi: 10.5713/ab.20.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., McGinnis J. The effect of autoclaving and enzyme supplementation of guar meal on the performance of chicks and laying hens. Poult. Sci. 1985;64:1148–1156. doi: 10.3382/ps.0641148. [DOI] [PubMed] [Google Scholar]
- Poulsen K., Mathlouthi N., Bargen J. Meta-analysis on the effect of dietary β-mannanase on intestinal integrity in broiler chickens. J. Appl. Poult. Res. 2023;32 [Google Scholar]
- Ryu M.H., Hosseindoust A., Kim J.S., Choi Y.H., Lee S.H., Kim M.J., Lee J.H., Chae B.J. β-Mannanase derived from Bacillus subtilis WL-7 improves the performance of commercial laying hens fed low or high mannan-based diets. J. Poult. Sci. 2017;54:212–217. doi: 10.2141/jpsa.0160021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapini L., de Cristo A., Schmidt J., Buzim R., Nogueira L., Palma S., Fernandes J. Effect of β-mannanase supplementation in conventional diets on the performance, immune competence and intestinal quality of broilers challenged with Eimeria sp. J. Appl. Poult. Res. 2019;28:1048–1057. [Google Scholar]
- Shastak Y., Ader P., Feuerstein D., Ruehle R., Matuschek M. ß-Mannan and mannanase in poultry nutrition. Worlds. Poult. Sci. J. 2015;71:161–174. [Google Scholar]
- Shim Y., Ryu M., Hosseindoust A., Kim J., Choi Y., Kim M., Lee J., Chae B. Evaluation of β-mannanase and different dietary energy levels on egg production, apparent digestibility and blood metabolites of laying hen. Anim. Nutr. Feed Technol. 2018;18:131–140. [Google Scholar]
- Torki M., Mirzaee M., Habibian M. Effects of barley cultivar and dietary supplemental enzyme on performance, egg quality traits, and selected blood parameters of laying hens. Poult. Sci. J. 2016;4:1–12. [Google Scholar]
- Upadhaya S.D., Park J.W., Lee J.H., Kim I.H. Ileal digestibility of nutrients and amino acids in low quality soybean meal sources treated with β-mannanase for growing pigs. Animal. 2016;10:1148–1154. doi: 10.1017/S1751731116000082. [DOI] [PubMed] [Google Scholar]
- Wang J., Jia R., Gong H., Celi P., Zhuo Y., Ding X., Bai S., Zeng Q., Yin H., Xu S. The effect of oxidative stress on the chicken ovary: Involvement of microbiota and melatonin interventions. Antioxidants. 2021;10:1422. doi: 10.3390/antiox10091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G.A., Chassard C., Hennet T. Selective proliferation of intestinal Barnesiella under fucosyllactose supplementation in mice. Br J. Nutr. 2014;111:1602–1610. doi: 10.1017/S0007114513004200. [DOI] [PubMed] [Google Scholar]
- White D., Adhikari R., Wang J., Chen C., Lee J.H., Kim W.K. Effects of dietary protein, energy and β-mannanase on laying performance, egg quality, and ileal amino acid digestibility in laying hens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bryant M., Voitle R., Sr D.R. Effects of β-mannanase in corn-soy diets on commercial leghorns in second-cycle hens. Poult. Sci. 2005;84:894–897. doi: 10.1093/ps/84.6.894. [DOI] [PubMed] [Google Scholar]
- Xiao G., Zheng L., Yan X., Gong L., Yang Y., Qi Q., Zhang X., Zhang H. Effects of dietary essential oils supplementation on egg quality, biochemical parameters, and gut microbiota of late-laying hens. Animals. 2022;12:2561. doi: 10.3390/ani12192561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu L., Sheikhahmadi A., Wang Y., Li C., Jiao H., Lin H., Song Z. Effects of corticosterone and dietary energy on immune function of broiler chickens. PLoS. One. 2015;10 doi: 10.1371/journal.pone.0119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Kim I. Effects of probiotic supplementation in different energy and nutrient density diets on performance, egg quality, excreta microflora, excreta noxious gas emission, and serum cholesterol concentrations in laying hens. J. Anim. Sci. 2013;91:4781–4787. doi: 10.2527/jas.2013-6484. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li M., Zhang K., Ding X., Bai S., Zeng Q., Chu L., Hou D., Xuan Y., Yin H. Effect of benzoic acid, Enterococcus faecium, and essential oil complex on intestinal microbiota of laying hens under coccidia and clostridium perfringens challenge. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu H., Gong L., Wang J., Fu J., Lv Z., Zhou L., Li X., Liu Q., Xia P., Guo Y. Mannanase improves the growth performance of broilers by alleviating inflammation of the intestinal epithelium and improving intestinal microbiota. Anim. Nutr. 2024;16:376–394. doi: 10.1016/j.aninu.2023.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Cho S., Kang C., Lee K., Kim K., An B. Effects of β-mannanase on egg production performance, egg quality, intestinal microbiota, viscosity, and ammonia concentration in laying hens. Braz. J. Poult. Sci. 2020;22:1–8. [Google Scholar]
- Zhu C., Gong L., Huang K., Li F., Tong D., Zhang H. Effect of heat-inactivated compound probiotics on growth performance, plasma biochemical indices, and cecal microbiome in yellow-feathered broilers. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.585623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Qiao X., Xu Z. Effect of β-mannanase (Hemicell) on growth performance and immunity of broilers. Poult. Sci. 2006;85:2176–2179. doi: 10.1093/ps/85.12.2176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.