Abstract
Background
Following decompression in anterior cervical discectomy and fusion (ACDF), reconstruction is typically done with structural allograft or a synthetic cage. Relative trends and factors associated with utilizing these implants have not been well characterized.
Methods
The PearlDiver 2011 to 2021 M157 database was used to identify adult patients undergoing 1- or 2-level ACDF. The incidence of structural allograft versus synthetic cage utilized was compared by year. Patient factors predictive of synthetic cage use as the structural interbody for ACDF were assessed with multivariable analysis. Further, the use of anterior plates was trended to provide a measure of usage of stand-alone devices (this comparison was made beginning with 2016 based on coding limitations).
Results
Of 173,833 isolated 1- or 2-level ACDF cases identified, structural allograft was used for 63,029 (36.3%) and synthetic cages were used for 110,804 (63.8%). The use of synthetic cages increased from 51.1% of cases in 2011 to 75.8% of cases in 2021 (p < 0.0001). Independent clinical predictors of synthetic cage use were: older age (odds ratio [OR] 1.02 per decade), female sex (OR 1.04), and greater ECI (OR 1.09 per 2-point increase).
Independent non-clinical predictors of synthetic cage use were: geographic region (Northeast OR 1.11, South OR 1.85, and West 2.08, each relative to Midwest), and provider specialty (orthopedic OR 1.06 relative to neurosurgeons). There was an increase in the percent of synthetic cases without separately coded plate (“stand-alone” interbody cages: 21.7% in 2016 to 35.3% in 2021, p < 0.001).
Conclusions
The usage of synthetic cages in 1- and 2- level ACDF has increased relative to structural allograft between 2011 and 2021 in the United States and more recently the use of “stand-alone” synthetic cages has been on the rise. Non-clinical as well as clinical factors were associated with implant choice, suggesting room for more consistent care algorithms.
Keywords: ACDF, Allograft, Anterior plate, Synthetic cage, Trends
Introduction
Anterior cervical discectomy and fusion (ACDF) is a common surgical procedure that is increasingly performed [1], [2], [3], [4]. Following decompression, the disc space is reconstructed with autologous iliac crest bone graft (ICBG, rarely used in recent years [5], [6], [7]), structural allograft, or synthetic cage, to maintain interbody height during fusion. The relative use of the latter 2 more commonly utilized structural interbody devices and factors associated with their use has not been well defined [8].
Structural allograft has long been utilized for ACDF. Compared to the historic gold stand of iliac crest autograft, similar fusion rates have been found [9]. Synthetic cages may also be considered for ACDF. These may be made of polyetheretherketone (PEEK), titanium, or other materials [10]. Comparisons of outcomes between synthetic cage and structural allografts in ACDF have shown mixed clinical results [6,11]. A systematic literature review comparing PEEK cages relative to structural allograft found fusion rates to be similar between the 2 interbody devices [12].
Based on prior mixed results in the literature, the relative utilization of different interbody spacer options was assessed with a 2017 survey sent to global members of AOSpine which found that synthetic cages were most commonly used worldwide, while allograft was used most commonly in North America [8]. In another study using private health insurance claims data from 2010 to 2017, one-level ACDF cases were found to utilize synthetic cages more commonly than structural allograft (57% relative to 43%) [13].
Following placement of an interbody device with ACDF, anterior cervical plates are routinely used to increase stability and decrease reliance on external orthoses [14]. To minimize prominence and potentially decrease dysphagia, synthetic cages with intercalary instrumentation have evolved [15]. A recent systematic review found such cages to be safe and effective [16]. The relative use of such “stand-alone” interbody devices relative to synthetic cages with plates has not been well established.
The present study was performed to characterize use of structural allograft relative to synthetic cages, related drivers, and trends of “stand-alone” interbody devices. A large, national, multi-insurance database was used for these characterizations.
Material and methods
Database and cohorts
The current study utilized the 2010 through October 2021 PearlDiver M157Ortho administrative database (PearlDiver Technologies, Colorado Springs). This is a commercially used administrative US database containing 157 million patients [17], [18], [19], [20], [21]. As data from the database is output in deidentified, aggregated, and Health Insurance Portability and Accountability Act compliant fashion, our Institutional Review Board deemed studies using this database as exempt from review.
Adult patients undergoing ACDF were identified using the Current Procedural Terminology (CPT) code 22551. A single instance of the CPT code 22552 on the same day was used to query for 2-level ACDF cases (Table 1). Exclusion criteria included: age <18 years old, patients receiving ACDF for more than 2 levels, patients receiving other cervical spine surgeries on the same day as ACDF, and recent history of trauma/infection/neoplasm.
Table 1.
CPT codes queried.
| Description | CPT Codes |
|---|---|
| 1-level ACDF | CPT-22551, not CPT-22552 same date |
| 2-level ACDF | CPT-22551 and 1 instance of CPT-22552 on same date |
| Exclusions for ACDF | CPT-63081, CPT-63020, CPT-63001, CPT-63045, CPT-22840, CPT-22842, CPT-22943 Trauma, infections, neoplasms Age < 18 |
| Structural interbody (mutually exclusive) Structural allograft Synthetic cage |
CPT-20931 CPT-22851, CPT-22853 |
| Anterior plate | CPT-22845 |
Clinical factors abstracted from the dataset included: age, sex, and Elixhauser comorbidity index (ECI, a measure of comorbidity burden [22]). Non-clinical factors abstracted included: geographic region (Midwest, Northeast, South, West), insurance plan (Commercial, Medicare, Medicaid), and surgeon specialty (orthopedics and neurosurgery). Further, 1 versus 2 levels treated was tabulated.
Usage of different structural interbody spacers and anterior plate
The structural interbody device used with ACDF was determined based on CPT codes. Structural allograft was identified based on CPT 20931. Synthetic cage interbody device was identified based on usage of the CPT codes 22851 or 22853, the latter of which replaced the former in January of 2017 [23]. For the year 2021, since the database included patients up to October 31, 2021, the number of patients was extrapolated to obtain a number for the whole year, assuming that ACDFs were equally distributed throughout the year.
For cases with structural allograft, a distinct anterior plate was used based on CPT 22,845. In synthetic cage ACDFs, this separate anterior plate code was only utilized with CPT code 22,853, which was introduced in late 2015 [23]. Therefore, only data from 2016 onward was included for this particular analysis.
Statistical Analysis
The trends in use of structural allograft versus synthetic cages were assessed, and first to last year of analysis were compared with chi-squared tests. Similar comparison was done for cases of synthetic cages with or without distinct plate code.
Differences in patient characteristics among the structural allograft and synthetic cage cohort were assessed. Univariable analysis was done with Student's t tests and chi-square tests where appropriate. Multivariable logistic regression was then done to assess for odds ratios (OR) and 95% confidence intervals (95%CIs).
All statistical analysis was conducted within the PearlDiver Bellwether software. Statistical significance was achieved at a value of p<.05. Prism9 (GraphPad Software) and Microsoft PowerPoint (Microsoft Corporation, Redmond, WA) were used for figure and table development.
Results
Study cohort and trends in structural allografts versus synthetic cages
A total of 173,833 ACDF patients met the criteria for inclusion in the study. Of this cohort, 63,029 (36.3%) used structural allografts, while 110,804 (63.7%) used a synthetic cage (Table 2).
Table 2.
Total number of 1 and 2-Level ACDF using structural allograft and synthetic cage by year.
| Year | Structural allograft | Synthetic cage |
|---|---|---|
| 2011 | 6,449 (48.9%) | 6,733 (51.1%) |
| 2012 | 6,695 (45.2%) | 8,126 (54.8%) |
| 2013 | 6,720 (42.2%) | 9,191 (47.8%) |
| 2014 | 6,638 (42.4%) | 9,035 (57.6%) |
| 2015 | 6,027 (39.3%) | 9,300 (60.7%) |
| 2016 | 6,422 (36.2%) | 11,307 (63.8%) |
| 2017 | 5,831 (34.9%) | 10,877 (65.1%) |
| 2018 | 5,141 (31.8%) | 11,019 (68.2%) |
| 2019 | 5,366 (29.4%) | 12,878 (70.6%) |
| 2020 | 4,359 (27.0%) | 11,760 (73.0%) |
| 2021 | 4,057 (24.2%) | 12,694 (75.8%) |
| Total | 63,029 (36.3%) | 110,804 (63.7%) |
Overall, the number of ACDFs with structural allografts decreased over the years included in the study, with the peak number performed in 2013. The number of ACDFs with synthetic cages as the interbody spacer steadily increased over the years studied (Figure 1). In 2011, the first year investigated in the present study, synthetic cages comprised 51.1% of ACDFs with a structural interbody. By 2021, the last year included in the present study, synthetic cages represented 75.8%, a significant increase (p < 0.001, Figure 2).
Fig. 1.
Trends in the total number of 1 and 2-level ACDF using synthetic cage compared to structural allograft. Numbers for 2021 were extrapolated to include the full year.
Fig. 2.
Trends in the relative utilization of synthetic cages compared to allografts in single 1 and 2-level ACDF procedures.
Factors associated with synthetic cage use compared to structural allograft
According to univariable analysis, all factors analyzed – age, sex, ECI, geographic region, insurance plan, physician specialty, and number of levels treated—were significantly associated with utilization of synthetic cage (p<.001 for each, Table 3).
Table 3.
Univariable analysis of demographics for structural allograft and synthetic cage use and multivariable analysis of patient characteristics predicting synthetic cage use in 1- and 2-level ACDF.
| Univariable analysis |
Multivariable factors predictive of cage |
||||
|---|---|---|---|---|---|
| Structural allograft | Synthetic cage | p-value | OR (95% CI) | p-value | |
| N | 63,029 | 110,804 | 173,833 | ||
| Age (mean ± SD) (per decade increase) |
54.20 ± 11.47 | 55.11 ± 11.54 | p<.001 | 1.02 (1.01, 1.03) | p<.001 |
| Sex Male (%) (referent) Female (%) |
28,930 (45.9%) 34,040 (54.1%) |
49,154 (44.4%) 61,507 (55.6%) |
p<.001 | 1.04 (1.02, 1.06) | p<.001 |
| ECI (Mean ± SD) (per 2 point increase) |
3.50 ± 3.13 | 3.95 ± 3.33 | p<.001 | 1.09 (1.09, 1.10) | p<.001 |
| Region Midwest (referent) Northeast South West |
20,548 (32.6%) 11,136 (17.7%) 25,375 (40.3%) 5,723 (9.1%) |
24,582 (22.2%) 15,082 (13.6%) 55,812 (50.4%) 14,520 (13.1%) |
p<.001 | 1.11 (1.07, 1.14) 1.85 (1.81, 1.89) 2.08 (2.01, 2.16) |
p<.001 p<.001 p<.001 |
| Insurance plan Commercial (referent) Medicare Medicaid |
47,598 (75.5%) 9,361 (14.9%) 3,684 (5.8%) |
82,144 (74.1%) 18,104 (16.3%) 6,398 (5.8%) |
p<.001 | 1.00 (0.97, 1.03) 1.00 (0.96, 1.05) |
p=.944 p=.924 |
| Physician specialty Neurosurgery (referent) Orthopedics |
36,234 (57.5%) 16,433 (26.1%) |
58,713 (53.0%) 27,940 (25.2%) |
p<.001 | 1.06 (1.03, 1.08) | p<.001 |
| Number of levels treated Single-level (referent) 2-level |
44,081 (69.9%) 18,958 (30.1%) |
76,558 (69.1%) 34,268 (30.9%) |
p<.001 | 0.99 (0.97, 1.01) | p=.338 |
To control for potential confounding, multivariable analysis was then performed to determine independent factors predictive of synthetic cage relative to allograft use (Table 3). Independent clinical predictors of synthetic cage use were: older age (odds ratio [OR] 1.02 per decade), female sex (OR 1.04), and greater ECI (OR 1.09 per 2-point increase). Independent nonclinical predictors of synthetic cage use were: geographic region (Northeast OR 1.1, South OR 1.85, and West 2.08, each relative to Midwest), and provider specialty (orthopedic OR 1.06 relative to neurosurgeons). Insurance plan and number of levels treated were no longer found as statistically significant predictors of synthetic cage use.
Trends in “stand-alone” versus separately coded anterior cervical plates with synthetic cages
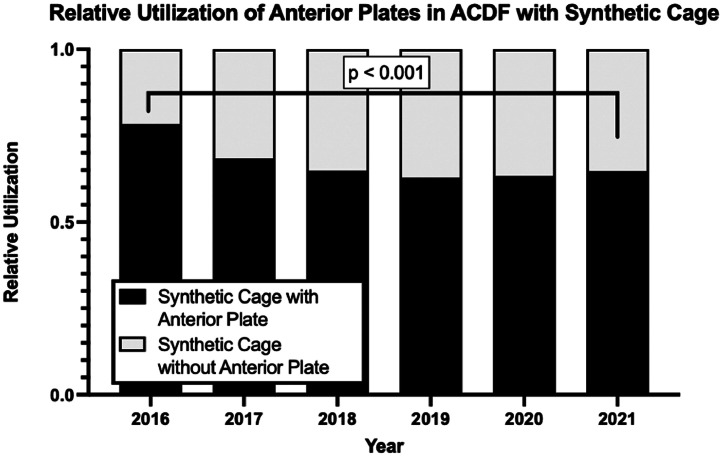
ACDFs with synthetic cages, anterior plates were used for 45,970 out of 68,419 (67.2%) procedures (Table 4). Over the years of the study, there was an increase in the percent of synthetic cases without separately coded plate (“stand-alone” interbody cages: 21.7% in 2016 to 35.3% in 2021, p<.001) (Fig. 3).
Table 4.
Trends in total number of 1 and 2-level ACDF grafts using separate anterior plate over time.
| Year | Synthetic Cage | Synthetic Cage with Anterior Plate | “Stand-alone” Synthetic Cage |
|---|---|---|---|
| 2016 | 11,307 | 8,858 (78.3%) | 2,449 (21.7%) |
| 2017 | 10,877 | 7,426 (68.3%) | 3,451 (31.7%) |
| 2018 | 11,019 | 7,140 (64.8%) | 3,879 (35.2%) |
| 2019 | 12,878 | 8,090 (62.8%) | 4,788 (37.2%) |
| 2020 | 11,760 | 7,427 (63.2%) | 4,333 (36.8%) |
| 2021 | 12,694 | 8,216 (64.7%) | 3,731 (35.3%) |
| Total | 70,535 | 47,157 (67.2%) | 23,378 (32.8%) |
Fig. 3.
Trends in the relative utilization of synthetic cages using anterior plate in single and 2-level ACDF procedures from 2016 onward.
Discussion
ACDF is a common surgical procedure with which reconstruction is typically done with structural allograft or a synthetic cage. Relative trends and factors associated with utilizing these implants have not been well characterized. The present study assessed trends in synthetic cage relative to structural allografts for ACDF from 2011 to 2021, predictive factors for receiving 1 interbody spacer relative to the other, as well as trends in “stand-alone” anterior synthetic cages from 2016 to 2021.
A large cohort of 173,833 1- and 2- level ACDF patients were identified for the study. When compared with structural allograft utilization, synthetic cage use increased from 51.1% of procedures performed in 2010 to 75.8% in 2021. This is interesting in light of the mixed literature regarding usage of one versus the other [8,13,24,25]. Hypothesized reasons for these changes could range from surgeon preference to evolution in technology.
While univariable analysis found all analyzed factors to be significantly associated with synthetic cage use, multivariable analysis revealed a smaller list of factors predictive of receiving ACDF with synthetic cage as opposed to ACDF with structural allograft. Independent clinical predictors of synthetic cage use were: older age (odds ratio [OR] 1.02 per decade), female sex (OR 1.04), and greater ECI (OR 1.09 per 2-point increase). Given the small magnitude of the OR and the absolute values for clinical predictors such as sex and age, it is possible that this significance is clinically irrelevant and the effect of random variation from the large sample size. However, as the ACDF surgical population in the United States is expected to increase in age and comorbidity in the future, these results suggest that it is possible that relative synthetic cage use may only continue to increase if these are considered important factors [26].
Independent nonclinical predictors of synthetic cage use were: geographic region (Northeast OR 1.1, South OR 1.85, and West 2.08, each relative to Midwest), and provider specialty (orthopedic OR 1.06 relative to neurosurgeons). Interestingly, the odds ratios for these nonclinical factors were generally greater than for the clinical factors. These provide further support that surgical specialty and geographic region may be more predictive of implant choice than perceived patient-specific factors. These results mirror similar findings in spine surgery of regional variation and training differences resulting in differential usage different techniques [17,27].
The increase in relative usage of synthetic cages in ACDF over structural allografts may be influenced by how the different implants are billed. According to the most recent US Department of Labor fee schedule, CPT-22853 for synthetic cage is worth 4.25 work relative value units (wRVUs), compared to 1.81 wRVUs for CPT-20931 for a structural allograft [28]. Given that some studies have found higher rates of complications such as pseudoarthrosis in ACDF done with synthetic cages compared to structural allografts, this disparity in wRVUs for 2 techniques of similar technical complexity is concerning, especially if such differences are the baseline for surgical decision making [29].
With synthetic cages, the use of “stand-alone” cages without separately coded anterior plates also increased in usage from 2016 to 2021 (21.7% to 35.3%). Recent meta-analyses have suggested “stand-alone” cages to have reduced operative time, intraoperative bleeding, rates of dysphagia, and rates of adjacent segment degeneration than cage-plate constructs, while maintaining similar rates of fusion and cage subsidence [16,30]. On the contrary, some studies have found that in long-term follow up, “stand-alone” cages had greater loss of cervical lordosis [30,31].
The current study does have limitations. As with any study using administrative data, the accuracy of findings is limited by the data coded. As there is no specific CPT code for stand-alone cages, the present study assumes that all synthetic cages without an anterior plate coding were stand-alone. However, this may not be the case for all stand-alone cages counted, as some may involve the placement of a regular cage without plating but with a hard collar [32]. The specific implants and bone graft materials utilized could not be determined from the administrative data. Even more importantly, the drivers behind these decisions could not be directly assessed. Further, clinical outcomes could not be determined.
Conclusion
Overall, the usage of synthetic cages in 1- and 2- level ACDF has increased relative to structural allograft between 2011 and 2021 in the United States and more recently the use of “stand-alone” synthetic cages has been on the rise. Non-clinical as well as clinical factors were associated with implant choice, suggesting room for more consistent care algorithms.
Declaration of competing interest
Lucas Y Kim received $5,203.00 USD from the Richard K. Gershon Yale School of Medicine Medical Student Research Fellowship as a grant directly to the author in support of medical student research unrelated to the current study. Jonathan N Grauer is Editor-in-Chief of North American Spine Society Journal and a member of the North American Spine Society Board of Directors.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: LK: Fellowship Support: Richard K. Gershon and Yale School of Medicine Fellowship for Medical Student Research (B). JNG: Other: NASSJ (D).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2024.100310.
Appendix. Supplementary materials
References
- 1.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. JBJS. 1958;40(3):607. [PubMed] [Google Scholar]
- 2.Cowan JA, Dimick JB, Wainess R, Upchurch GR, Chandler WF, La Marca F. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59(1):15–20. doi: 10.1227/01.NEU.0000219836.54861.CD. discussion 15-20. [DOI] [PubMed] [Google Scholar]
- 3.Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 4.Salzmann SN, Derman PB, Lampe LP, et al. Cervical Spinal Fusion: 16-Year Trends in Epidemiology, Indications, and In-Hospital Outcomes by Surgical Approach. World Neurosurg. 2018;113:e280–e295. doi: 10.1016/j.wneu.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs W, Willems PC, Kruyt M, et al. Systematic review of anterior interbody fusion techniques for single- and double-level cervical degenerative disc disease. Spine. 2011;36(14):E950–E960. doi: 10.1097/BRS.0b013e31821cbba5. [DOI] [PubMed] [Google Scholar]
- 6.Goz V, Buser Z, D'Oro A, et al. Complications and risk factors using structural allograft versus synthetic cage: analysis 17 783 anterior cervical discectomy and fusions using a National Registry. Glob Spine J. 2019;9(4):388–392. doi: 10.1177/2192568218797096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudisill KE, Ratnasamy PP, Maloy GC, Grauer JN. Decline in separate incision autograft for spine surgery over the past decade: a fading “gold standard. JAAOS - J Am Acad Orthop Surg. 2023;31(17):938. doi: 10.5435/JAAOS-D-22-01029. [DOI] [PubMed] [Google Scholar]
- 8.Yoon ST, Konopka JA, Wang JC, et al. ACDF graft selection by surgeons: survey of AOSpine members. Glob Spine J. 2017;7(5):410–416. doi: 10.1177/2192568217699200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg JL, Meaden RM, Hussain I, et al. Titanium versus polyetheretherketone versus structural allograft in anterior cervical discectomy and fusion: a systematic review. Brain Spine. 2022;2 doi: 10.1016/j.bas.2022.100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon N, Turcotte J, Patton C. Structural allograft versus synthetic interbody cage for anterior cervical discectomy and fusion: a comparison of 1-year outcomes from a National Database. Glob Spine J. 2021;11(8):1215–1222. doi: 10.1177/2192568220942217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirkle S, Kaskovich S, Cook DJ, Ho A, Shi LL, Lee MJ. Cages in ACDF are associated with a higher nonunion rate than allograft: a stratified comparative analysis of 6130 patients. Spine. 2019;44(6):384–388. doi: 10.1097/BRS.0000000000002854. [DOI] [PubMed] [Google Scholar]
- 12.Jain A, Marrache M, Harris A, et al. Structural allograft versus PEEK implants in anterior cervical discectomy and fusion: a systematic review. Glob Spine J. 2020;10(6):775–783. doi: 10.1177/2192568219883256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrache M, Bronheim R, Harris AB, et al. Synthetic cages associated with increased rates of revision surgery and higher costs compared to allograft in ACDF in the nonelderly patient. Neurospine. 2020;17(4):896–901. doi: 10.14245/ns.2040216.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser MG, Haid RWJ, Subach BR, Barnes B, Rodts GEJ. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50(2):229. doi: 10.1097/00006123-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine. 2007;32(21):2310. doi: 10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Yang S, Huo Y, Li Z, Yang D, Ding W. Locking stand-alone cage versus anterior plate construct in anterior cervical discectomy and fusion: a systematic review and meta-analysis based on randomized controlled trials. Eur Spine J. 2020;29(11):2734–2744. doi: 10.1007/s00586-020-06561-x. [DOI] [PubMed] [Google Scholar]
- 17.Ratnasamy PP, Rudisill KE, Maloy GC, Grauer JN. Cervical disc arthroplasty usage has leveled out from 2010 to 2021. Spine. 2022;28:E342–E348. doi: 10.1097/BRS.0000000000004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day W, Ch’en PY, Ratnasamy PP, Jeong S, Varthi AG, Grauer JN. The correlation of psoriasis and its treatment medications with lumbar discectomy postoperative infections. Spine J Off J North Am Spine Soc. 2023;23(11):1623–1629. doi: 10.1016/j.spinee.2023.06.392. [DOI] [PubMed] [Google Scholar]
- 19.Dhodapkar MM, Oghenesume OP, Halperin SJ, Modrak M, Yoo BJ, Grauer JN. Adverse events after ankle fracture open reduction internal fixation among patients with and without documented cannabis and tobacco use. Foot Ankle Int. 2023;44(10):941–948. doi: 10.1177/10711007231189698. 10711007231189698. [DOI] [PubMed] [Google Scholar]
- 20.Jayaram RH, Joo PY, Gouzoulis MJ, Ratnasamy PP, Caruana DL, Grauer JN. Single-level anterior cervical discectomy and fusion results in lower five-year revisions than posterior cervical foraminotomy in a large national cohort. Spine. 2023;48(18):1266–1271. doi: 10.1097/BRS.0000000000004754. [DOI] [PubMed] [Google Scholar]
- 21.Halperin SJ, Dhodapkar MM, Radford ZJ, Li M, Rubin LE, Grauer JN. Total knee arthroplasty: variables affecting 90-day overall reimbursement. J Arthroplasty. 2023;38(11):2259–2263. doi: 10.1016/j.arth.2023.05.072. [DOI] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.ISASS – The International Society for the Advancement of Spine Surgery. A closer look at biomechanical cage & device coding. Accessed October 5, 2023. https://isass.org/a-closer-look-at-biomechanical-cage-device-coding/.
- 24.Spanos SL, Siasios ID, Dimopoulos VG, Fountas KN. Anterior cervical discectomy and fusion: practice patterns among greek spinal surgeons. J Clin Med Res. 2016;8(7):506–512. doi: 10.14740/jocmr2572w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baweja RK, Bennardo M, Farrokhyar F, Martyniuk A, Reddy K. A Canadian perspective on anterior cervical discectomies: practice patterns and preferences. J Spine Surg Hong Kong. 2018;4(1):72–78. doi: 10.21037/jss.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neifert SN, Martini ML, Yuk F, et al. Predicting trends in cervical spinal surgery in the United States from 2020 to 2040. World Neurosurg. 2020;141:e175–e181. doi: 10.1016/j.wneu.2020.05.055. [DOI] [PubMed] [Google Scholar]
- 27.Nesterenko SO, Riley LH, Skolasky RL. Anterior cervical discectomy and fusion versus cervical disc arthroplasty: current state and trends in treatment for cervical disc pathology. Spine. 2012;37(17):1470–1474. doi: 10.1097/BRS.0b013e31824ee623. [DOI] [PubMed] [Google Scholar]
- 28.Download OWCP Fee schedule Effective 2023. DOL. Accessed January 2, 2024. http://www.dol.gov/agencies/owcp/regs/feeschedule/fee/feeJuly092023/download.
- 29.D'Antonio N, Lambrechts MJ, Heard J, et al. Effect of interbody composition on the development of pseudarthrosis following anterior cervical discectomy and fusion. Asian Spine J. 2023;17(3):518–528. doi: 10.31616/asj.2022.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Ju J, Wu J. Self-locking stand-alone cage versus cage-plate fixation in monosegmental anterior cervical discectomy and fusion with a minimum 2-year follow-up: a systematic review and meta-analysis. J Orthop Surg. 2023;18:403. doi: 10.1186/s13018-023-03885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Yang Y, Lan J, Xu H, Zhang Z, Miao J. Changes in cervical alignment of Zero-profile device versus conventional cage-plate construct after anterior cervical discectomy and fusion: a meta-analysis. J Orthop Surg. 2022;17:510. doi: 10.1186/s13018-022-03400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bębenek A, Godlewski B. Anterior cervical discectomy and fusion (ACDF) with and without plating: a comparison of radiological and clinical outcomes. Adv Clin Exp Med. 2023 doi: 10.17219/acem/172062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.