Abstract
We previously identified a mutational hotspot upstream of the Ty1 U5-primer binding site (PBS) border and proposed a novel mechanism to account for this phenomenon during Ty1 replication. In this report, we verify key points of our model and show that in vivo RNase H cleavage of Ty1 RNA during minus-strand strong-stop synthesis creates heterogeneous 5′ RNA ends. The preferred cleavage sites closest to the PBS are 6 and 3 bases upstream of the U5-PBS border. Minus-strand cDNA synthesis terminates at multiple sites determined by RNase H cleavage, and DNA intermediates frequently contain 3′-terminal sequence changes at or near their template ends. These data indicate that nontemplated terminal base addition during reverse transcription is a real in vivo phenomenon and suggest that this mechanism is a major source of sequence variability among retrotransposed genetic elements.
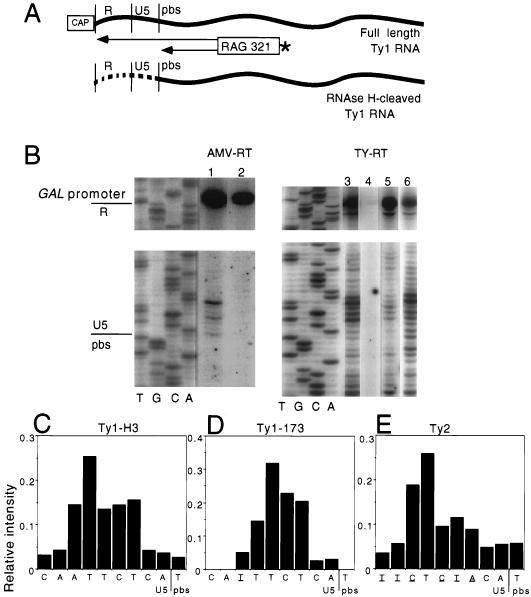
Ty1 is a well-characterized yeast retrotransposon which is structurally and mechanistically related to vertebrate retroviruses (16). Both entities contain long terminal repeats (LTRs) in their genomic double-stranded DNA form. Both replicate via reverse transcription of an RNA template, which requires independent plus- and minus-strand primers and multiple strand transfers. Our understanding of Ty1 replication is based on the paradigm of retrovirus replication (Fig. 1). According to this model (44), Ty1 reverse transcriptase (RT) initiates replication by copying the 5′ end of the RNA template, using an initiator methionine tRNA primer annealed at the primer binding site (PBS) just downstream of the U5 region. Reverse transcription proceeds to the 5′ terminus of the RNA, creating minus-strand strong-stop DNA (Fig. 1A). This intermediate has been observed as a product of endogenous RT reactions within Ty1 virus-like particles (VLPs) (9). Template RNA annealed to minus-strand cDNA is a substrate for RNase H (Fig. 1A). Although such an activity has never been directly demonstrated for Ty1, this enzyme is likely to be associated with Ty1 RT. The initial in vivo RNase H cleavage site on genomic RNA is typically assumed to be at the U5-PBS border, where there is a transition from a RNA-tRNA hybrid to a RNA-cDNA hybrid. However, this too is an untested presumption for both Ty1 and retroviruses. In fact, in vitro mapping of retroviral RNase H cleavage specificity, using model template-primer systems, has shown RNase H cleavage sites for both human immunodeficiency virus (HIV) and murine leukemia virus (MuLV) RT 1 to 5 bases upstream of the U5-PBS border, with heterogeneous minor sites up to 10 bases into the U5 region (2, 3, 21, 41).
FIG. 1.
Schematic diagram of the Ty1 replication cycle, based on the retrovirus model. Circles above Ty1 genomic DNA indicate the mutational hot spot detected in the fidelity study of 29 independent Ty1 transpositions (18). Positions of GAG and POL open reading frames are indicated under Ty1 full-length DNA (F). Boxes indicate intermediates addressed in this study. (A) Initial RNase H cleavage of the RNA template during minus-strand strong-stop synthesis; (B) minus-strand near-full-length DNA paused at the 5′ end of RNase H-cleaved RNA; (C) minus-strand near-full-length DNA paused at the 5′ end of the capped RNA transcript; (D) plus-strand strong-stop synthesis; (E) plus-strand strong-stop transfer to minus-strand DNA paused at the 5′ end of RNase H-cleaved RNA, mediated by complementarity between the PBS region and additional bases upstream of the U5-PBS border; (F) Ty1 full-length double-stranded DNA. rrr, ribonucleotides remaining upstream of the U5-PBS border after RNase H cleavage during minus-strand strong-stop synthesis; ddd, deoxyribonucleotides templated by RNA upstream of the U5-PBS border; (x), sequence changes at the 3′ end of near-full-length minus-strand DNA paused at the 5′ end of the RNase H-cleaved RNA.
RNase H cleavage of the 5′ end of the RNA exposes minus-strand cDNA sequences complementary to the repeat (R) region and plays a role in transfer of minus-strand strong-stop DNA to the R region found near the 3′ end of genomic RNA (4, 34, 43). Minus-strand strong-stop DNA may transfer from the 5′ to the 3′ end of the same RNA molecule (intramolecular transfer) or transfer to a different, full-length RNA template (intermolecular transfer). Continued minus-strand cDNA synthesis may also be accompanied by recombination between template RNAs (24, 49). Eventually RT reaches one of the two template termini; i.e., it returns either to the RNase H cleavage site generated during minus-strand strong-stop synthesis (Fig. 1B) or to the capped 5′ end of an uncleaved transcript (Fig. 1C). In either case, synthesis of the full-length minus-strand DNA, including U3, can continue only after plus-strand strong-stop DNA transfers to the 3′ end of the minus-strand DNA (Fig. 1D and E) and provides the template to complete minus-strand synthesis.
Retroviral RTs are known to have high error rates (38). We have recently determined that the process of Ty1 replication is highly error prone, with an in vivo mutation rate similar to that previously observed for retroviruses undergoing single rounds of replication (18, 37). Interestingly, the errors that we observed during Ty1 retrotransposition were not randomly distributed; 3 of 10 errors were just upstream of the U5-PBS border, while an additional mutation was at the 5′ U3-R border (Fig. 1). We postulated that the observed pattern of mutations might be due to their proximity to template ends, particularly since retroviral RTs have been shown capable, in vitro, of adding nontemplated bases at template ends (11, 31, 33) and of extending past terminal mismatches (35, 50). Such events have also been observed, in vivo, during MuLV minus-strand strong-stop synthesis and minus-strand transfer (25) and have been suggested by extra bases within circle junctions of HIV-1 and the retrotransposon Tnt1 (13a, 41a). To account for our observation that the hotspot comprised a region upstream of the U5-PBS border rather than a single base, we suggested that in vivo, Ty1 RNase H cuts heterogeneously upstream of the U5-PBS border.
To directly examine these hypotheses, we used primer extension analysis, ligation-mediated PCR (LM-PCR), and terminal deoxynucleotidyl transferase (TdT)-mediated PCR (TdT-PCR) to identify the in vivo generated ends of specific Ty1 replication intermediates. In this study, we demonstrate that the Ty1 RNase H cleavage sites closest to the PBS generated during minus-strand strong-stop synthesis are indeed heterogeneous, that minus-strand synthesis pauses at the RNase H-generated 5′ end of the RNA template, and that sequence errors are concentrated at or near these ends.
MATERIALS AND METHODS
Plasmids and yeast strains.
All Saccharomyces cerevisiae strains used contained plasmids carrying wild-type or mutant Ty elements downstream of the galactose-inducible GAL1 promoter (Table 1). Expression of endogenous genomic Ty1 elements was suppressed in YH51 strains due to a mutant SPT3 gene (6).
TABLE 1.
Yeast strains and plasmids used in this study
| Yeast straina | Plasmid(s)
|
Refer- ence | |
|---|---|---|---|
| Name | Description | ||
| AG51 | pJEF724 (pGTy1-H3) | 2μm URA3-marked plasmid containing wild-type Ty1-H3 | 17 |
| AG53 | pGTyΔSal (pGTy1-H3ΔRT) | Same as pJEF724 but deleted for most of POL open reading frame | 17 |
| AG1583 | pJEF1076 | Same as pJEF724 but containing Ty1-173 from XhoI to BstEII | 5 |
| AG1585 | pJEF1510 | Same as pJEF724 but Ty2-H556 | 4a |
The host strain was YH51 (MATa GAL+ ura3-52 spt3-202 his4-539 lys2-801).
VLP preparations.
Transposition was induced in yeast strains containing GAL1-Ty1 constructs by incubation in galactose-containing medium for 24 h at 22°C. Cells were disrupted, and VLPs were purified on linear sucrose gradients and then stored at −80°C, as previously described (7).
RNA preparations.
RNA for primer extension and LM-PCR was prepared from whole cells or VLPs by extraction with hot acidic phenol (1), followed by DNase treatment (100 mM sodium acetate [pH 5.0], 5 mM MgSO4, 0.1% 2-mercaptoethanol, RNase inhibitor [2 U/μl; Boehringer Mannheim], RNase-free DNase [0.5 U/μl; Boehringer Mannheim]) at 37°C for 1 h. All RNA preparations were denatured (100°C for 3 min) before reisolation on RNeasy columns (Qiagen).
Nucleic acids from VLPs.
Aliquots of VLP suspensions were incubated for 1 h in proteinase K (50 μg/ml)–25 mM EDTA–0.1% sodium dodecyl sulfate at 25°C, followed by two phenol-chloroform extractions (pH 7.9) and ethanol precipitation.
Primer extension.
Primer extensions were carried out in vitro by using avian myeloblastosis virus (AMV) RT or within VLPs (endogenously) by using Ty1 RT. A minus-strand primer (Table 2) was end labeled in a reaction containing 4.3 μM primer, 1× kinase buffer, 2.8 μM [γ-32P]ATP (NEG-035C; NEN) and 5 U of T4 polynucleotide kinase (New England Biolabs) for 45 min at 37°C. The reaction was stopped by incubation at 55°C for 5 min.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′) | Derivation | Strand | Function (restriction site on 5′ end) |
|---|---|---|---|---|
| RAG54 | CGTCTAGAAGCAGGTGTTGTTGTCTG | GAG, bp 453–472 | Minus | Primer for nested PCR (XbaI) |
| RAG198 | GGGGTACCGGAACGCTTCACGAA | Complementary to RNA linker cDNA and DNA linker | Linker PCR primer (KpnI) | |
| RAG199 | CATGGAGCTCTGAGGAGAGGCATGA | GAG, bp 497–511 | Minus | RT and PCR primer for RNase H-cleaved RNA, PCR primer for paused minus-strand DNA (SacI) |
| RAG208 | TTCGTGAAGCGTTCCGGTACCCCGC | DNA linker (5′ phosphorylated, 3′ blocked) | ||
| RAG321 | GCTGAAACGTCTAACGGATC | GAG, bp 384–403 (gel purified) | Minus | RT primer for primer extension of U5-PBS region |
| RAG340 | ACACGGTACCGGGGGGGGGG | Complementary to poly(C)-tailed templates | TdT-PCR poly(G) primer (KpnI) | |
| RAG343 | GACATACTCGAGACACGGTACCGG | Overlaps RAG340 | TdT-PCR primer used for nested PCR (KpnI) | |
| RAG347 | GGAACGCUUCACGAA | RNA linker (gel purified) | ||
| RAG400 | CATGGAGCTCAAGCAGGTTGAGGAG | GAG, bp 503–517 | Minus | PCR primer for full-length or paused minus-strand Ty2 DNA (SacI) |
| RAG478 | TACTCGAGACACGGTACCCCCCCCCC | Complementary to poly(G)-tailed templates | TdT-PCR poly(C) primer (KpnI) |
(i) In vitro.
Using prepared RNA from VLPs, primer extension was carried out as described by Whitcomb et al. (47). The RNA was denatured (100°C for 3 min) and then annealed to the α-32P-labeled primer in the reaction buffer at 42°C for 5 min, followed by addition of AMV RT (25 U; Promega), incubation at 42°C for 1 h, phenol-chloroform extraction, ethanol precipitation, and resuspension in 5 μl of Tris-EDTA and 2.5 μl of STOP mix (95% formamide, 10 mM EDTA, 0.1% each bromphenol blue and xylene cyanol).
(ii) Within VLPs.
Using VLP preparations directly, primer extension was performed as previously described (27), with the following changes: 12.5 μl of VLP suspension and labeled primer (final concentration, 0.3 μM) were preincubated at room temperature for 10 min, followed by addition of remaining reaction ingredients plus 50 μg of actinomycin D per ml and incubation at 22°C for 1 h. Primer-extended cDNA was extracted from VLPs as described above and reconstituted in 5 μl of Tris-EDTA and 2.5 μl of STOP mix. Reaction products were separated on 8% denaturing polyacrylamide gels.
LM-PCR. (i) RNA ligation.
5′-phosphorylated ends of cellular or VLP RNA were ligated to the RNA linker, RAG347, for 18 h at 14°C as previously described (14), then ethanol precipitated, and resuspended in 12 μl of ENT buffer (1 mM EDTA, 10 mM NaCl, 10 mM Tris-HCl [pH 8.0]). Three microliters of this resuspended ligation product was added to the reverse transcription reaction mixture, which included MuLV RT (10 U/μl; Gibco/BRL) and a Ty1 minus-strand primer. Conditions used were those recommended by Perkin-Elmer.
(ii) DNA ligation.
The DNA oligonucleotide, RAG208 (5′ phosphorylated and 3′ blocked by a C3 residue to eliminate self-ligation; Keystone Laboratories, Inc.), was ligated to the 3′ OH ends of denatured VLP-derived DNA for 18 h at 22°C (46).
(iii) PCR.
Either the cDNA (resuspended in ENT buffer) or the single-stranded DNA ligation reaction (resuspended in ligation termination buffer) were diluted 25-fold in a standard Taq polymerase PCR mixture, which included RAG198 (complementary to the RNA linker cDNA and the DNA linker) and the GAG-specific primer RAG199.
TdT-PCR.
VLP DNA was extracted as described above. 3′ tailing reactions using calf thymus TdT were performed as recommended by the manufacturer (Boehringer Mannheim). Subsequent PCR amplification conditions were as previously described (10, 23), using oligonucleotides listed in Table 2. Note that in some cases, the nested PCR strategy was not used.
Subcloning of PCR products.
LM-PCR products were digested with KpnI and SacI and ligated to KpnI/SacI-digested pBluescript II. In some cases, PCR bands were gel purified before digestion to enrich for specific intermediates. TdT-PCR products were ligated directly into TA cloning vector pCRII (Invitrogen).
Sequencing.
Minipreps of pBluescript II and pCRII with PCR-generated inserts were sequenced directly by the double-stranded DNA cycle sequencing protocol recommended by Gibco BRL, using M13 −20 and reverse sequencing primers.
S. cerevisiae Ty sequences.
All full-length genomic Ty1 and Ty2 sequences were obtained through the Saccharomyces genome database (SGD) World Wide Web site (www.sgd.stanford.com). A listing of all full-length Ty elements, their locations, and their identifiers is available at www.public.iastate.edu/~voytas/ltrstuff/ltrtables/Bigtys.xls.
RESULTS
Primer extension analysis of Ty1 VLP RNA reveals heterogeneous stop sites near the U5-PBS border.
According to the retrovirus replication model (Fig. 1), RNase H cleavage of the RNA template initiates at the first base pair of the RNA-DNA hybrid, i.e., precisely at the U5-PBS border. Our previous analysis of in vivo Ty1 RT fidelity revealed mutations 2, 3, and 7 bases upstream of the U5-PBS border (18). We had postulated that this distribution of errors might be a consequence of imprecise Ty1 RNase H cleavage. To test this hypothesis, we performed primer extension analysis on Ty1 RNA extracted from VLPs derived from galactose-induced cells containing either wild-type RT (AG51) or ΔTy1 RT (AG53) plasmid-based GAL1-Ty elements. Since galactose-induced Ty1 retrotransposition takes place asynchronously within VLPs, intermediates from all stages of replication should be present in these particles (36). As shown in Fig. 2A, we annealed a gel-purified 5′-end-labeled Ty1 minus-strand primer to VLP-derived RNA downstream of the U5-PBS border and extended it with AMV RT. The major extension product from both sources was the size expected for the 5′ end of Ty1 RNA (see the band at the GAL1 promoter-R border in Fig. 2B, lanes 1 and 2). For the wild-type Ty1 element (Fig. 2B, lane 1), additional bands were clustered near the U5-PBS border. The most intense bands occurred 6 and 3 bases upstream of the PBS, with only a minor band at the actual U5-PBS border. These clustered bands were absent from the ΔTy1 RT sample (Fig. 2B, lane 2).
FIG. 2.
Primer extension analysis of the 5′ ends of RNAs derived from Ty VLPs. (A) Schematic diagram of the approach. An end-labeled minus-strand primer (RAG321), designed to maximize the band resolution in the U5-PBS region, was extended by using either AMV RT (in vitro) or Ty RT (endogenous reaction). The labeled primer extension products were separated on an 8% denaturing polyacrylamide gel. (B) Primer extension products map to both the U5-PBS border and the 5′ end of the RNA. Lanes represent in vitro primer extension along RNA extracted from wild-type Ty1-H3 (AG51) VLPs (lane 1) and from ΔTy1 RT (AG53) VLPs (lane 2) and endogenous primer extension using VLPs with their associated RNAs and Ty RTs extracted from yeast strains containing wild-type Ty1-H3 (lane 3), ΔTy1 RT (lane 4), Ty1-173–Ty1-H3 fusion (AG1583) (lane 5), and Ty2 (AG1585) (lane 6). Sequencing reactions using RAG321 were run as markers. The U5-PBS border is indicated to localize primer extension products representing the 5′ ends of RNase H-cleaved RNA. The plasmid-based GAL1 promoter-R border is indicated to localize the 5′ end of the RNA transcript. (C to E) Quantitative PhosphorImager analysis of band intensities based on endogenous primer extension reactions (lanes 3, 5, and 6). The U5-PBS sequence for each of the three elements is shown below the graphs, and bases of Ty1-173 and Ty2 that differ from those in Ty1-H3 are underlined.
The primer extension analysis suggested that a fraction of Ty1 RNA from wild-type Ty1 VLPs is variably cleaved just upstream of the PBS, consistent with heterogeneous RNase H cleavage of Ty1 RNA during minus-strand DNA synthesis. However, we were concerned that RNase H cleavage associated with AMV RT extension of preannealed endogenous tRNA primers (47) may have contributed to the cluster of bands upstream of the U5-PBS border. We reasoned that primer extensions using the Ty1 RT within VLPs would eliminate this potential artifact since the RNase H associated with Ty1 RT would be responsible for any observed cleavages. We therefore carried out endogenous primer extension reactions, by incubating the same end-labeled minus-strand primer with VLPs containing endogenous RT and RNA templates. We observed a similar heterogeneous pattern of bands in the U5-PBS region (Fig. 2B; compare lanes 1 and 3). PhosphorImager quantitation confirmed that the band present 6 bases upstream of the U5-PBS border was most intense, followed by the band 3 bases upstream (Fig. 2C). VLPs from the ΔTy1 RT construct showed no primer extension products, as would be expected in VLPs lacking an RT source (Fig. 2B, lane 4). These results were reproducible in assays using VLPs from several different preparations.
Endogenous primer extension analysis of other Ty elements reveals heterogeneous stop sites upstream of the U5-PBS border.
Since the sequences found upstream of the Ty1 U5-PBS border are variable among endogenous Ty1 and Ty2 elements (see below and reference 18), we next examined whether this heterogeneous pattern of primer extension products was specific to our particular cloned Ty1 element (Ty1-H3) or was generalizable to other Ty elements. We therefore performed endogenous primer extension analysis using VLPs derived from two different cloned Ty elements. Ty1-173 has a single substitution 7 bases upstream of the U5-PBS border relative to Ty1-H3 (compare the sequences in Fig. 2C and D). A cloned Ty2 element contains many sequence differences relative to Ty1-H3, including 7 of 12 bases at the 3′ end of U5. As with Ty1-H3, both of these Ty elements show a cluster of bands centered upstream of the PBS. The preferred cleavage sites were 5 bases (Ty1-173) and 6 bases (Ty2) upstream of the U5-PBS border (Fig. 2B, lanes 3, 5, and 6). Therefore, it appears that RNase H cleavage upstream of the U5-PBS is a general phenomenon for Ty1 and Ty2 elements. The frequency distribution of cleavage sites for the three Ty elements (compare histograms in Fig. 2C to E) suggests, however, that specific sequences upstream of the U5-PBS border can modify the cleavage site preference.
LM-PCR analysis confirms heterogeneous RNase H cleavage near the U5-PBS border.
While the primer extension analysis indicated stop sites upstream of the U5-PBS border, we wanted to determine if these represented real RNA termini rather than mere RT pause sites. Further, we wanted to precisely map the 5′ ends of the RNase H-cleaved RNA. We therefore made use of LM-PCR. As shown in Fig. 3A, an RNA linker was ligated to the 5′ end of phosphorylated RNA. Ty1 RNA ligation products were subsequently reverse transcribed, PCR amplified, cloned, and sequenced. The original RNA 5′ end could then be deduced by determining the base present next to the 3′ end of the linker.
FIG. 3.
LM-PCR analysis of 5′ ends of RNase H-cleaved RNA from Ty1 VLPs. (A) Scheme for ligation of an RNA linker to 5′-cleaved RNA ends. Arrows show relative positions of PCR primers. The capped RNA transcript is not a substrate for the T4 RNA ligase reaction. RAG347 was used as the RNA linker; RAG199 was used as the minus primer for in vitro reverse transcription; RAG198 and RAG199 were then used for PCR amplification. (B) Ethidium bromide-stained gel of PCR products obtained from LM-PCR of either wild-type (WT) Ty1-H3 or ΔTy1 RT VLP RNA. Sizes (in base pairs) of relevant marker fragments are indicated. (C) Sequence analysis of total PCR product observed in panel B, derived from LM-PCR of cellular RNA extracted after galactose induction of Ty1-H3. RAG199 was used as the sequencing primer. (D) Frequency distribution of the 5′ ends of Ty1-H3 VLP RNA ligated to the RNA linker, as derived from sequence analysis of cloned PCR products.
Initially we induced Ty1 expression in the wild-type Ty1 and control ΔTy1 RT yeast strains; we then extracted total cellular RNA and ligated the RNA linker to available 5′-phosphorylated RNA ends. After RT-PCR amplification of the ligation products using a Ty1-specific minus-strand primer and a linker-specific plus-strand primer, we observed an ∼210-bp PCR product for RNA from the wild-type Ty1-H3 strain (Fig. 3B). This is the size expected for ligation of the linker to Ty1 RNA cleaved near the U5-PBS border. Because capped RNA is not a substrate for T4 RNA ligase, a band representing full-length Ty1 RNA (304 bp) was neither expected nor observed. We sequenced the pool of PCR products by using a minus-strand primer in GAG. As shown in Fig. 3C, the sequence is unambiguously Ty1-H3 through the first base of the PBS. However, beyond this point, the sequence becomes unreadable because of multiple bands at each position. This is consistent with the RNA linker being ligated at multiple positions upstream of the U5-PBS border. As anticipated, no PCR products were obtained for the ΔTy1 RT strain.
We were concerned that total RNA might contain ends that had been degraded by cellular RNases other than RNase H. We therefore extracted RNA directly from wild-type Ty1 VLPs, performed LM-PCR, and then cloned and sequenced the PCR products. Analysis of the junctional sequences revealed that the distribution of RNA 5′ ends was similar to that obtained by the primer extension experiments described above (compare Fig. 3D and 2C). In the largest number of clones (16 of 33 [48%]), the linker was ligated 6 bases upstream of the U5-PBS border, while the next most common site, found in 7 (21%) of 33 clones, was 3 bases upstream of the U5-PBS border. In fact, the linker was ligated at the U5-PBS border in only 2 (6%) of 33 clones.
Minus-strand DNA synthesis pauses at multiple template ends.
We adapted the LM-PCR technique to examine the 3′ termini of minus-strand DNA (Fig. 4A). We expected the 3′ ends of minus-strand DNA paused near the U5-PBS region to reflect the location of RNase H cleavages closest to the PBS and to reveal any sequence changes that may have occurred during in vivo reverse transcription. As shown in Fig. 4A, we observed three strong PCR products after ligating a 5′-phosphorylated and 3′-blocked DNA linker to the 3′ ends of DNA extracted from VLPs and then amplifying the ligated products by using primers specific to the DNA linker and to a region of GAG downstream of the PBS. The sizes of the bands were consistent with (i) complete full-length minus-strand DNA, following plus-strand transfer (545 bp), (ii) a cDNA terminus at the 5′ end of the RNA transcript (304 bp), and (iii) a cDNA terminus near the U5-PBS border (∼210 bp). We observed no PCR products using nucleic acids extracted from ΔTy1 RT-derived VLPs.
FIG. 4.
LM-PCR and TdT-PCR of 3′ ends of minus-strand DNA from Ty VLPs. (A) Ethidium bromide-stained gel of PCR products derived from Ty1-H3 minus-strand DNA. The major PCR products, represented by A, B, and C, correspond with the diagram showing expected minus-strand DNA intermediates. RAG208 was used as a DNA linker oligonucleotide; RAG198 and RAG199 were used for PCR amplification. Sizes (in base pairs) of relevant marker fragments are indicated. (B) Frequency distribution of the DNA 3′ bases ligated to the DNA linker (black bars) or adjacent to TdT-mediated poly(C) additions (hatched bars), as derived from sequence analysis of cloned ∼210-bp PCR products from Ty1-H3 and Ty2 VLPs. Note that for PCR of Ty2 minus-strand DNA, RAG400, complementary to the Ty2 GAG sequence, was used. The minus-strand DNA sequence is indicated in uppercase letters, while the plus-strand RNA sequence is in lowercase letters. Bases in Ty2 that differ from those in Ty1 are underlined.
We sequenced a total of 39 cloned ∼210-bp fragments, derived from five different VLP preparations. Twenty-nine showed templated ends near the U5-PBS border; an additional 10 clones revealed anomalous sequences at their 3′ ends (see below). As expected, 62% (18 of 29) of the accurate minus-strands ended either 6 bases (15 of 29 [52%]) or 3 bases (3 of 29 [10%]) upstream of the U5-PBS border (Fig. 4B, Ty1-H3). Fourteen additional clones showed 3′ ends distributed randomly downstream of the U5-PBS border. None of these ends occurred near the PBS, which might have been expected if displacement of the tRNA primer were inefficient and blocked continued cDNA synthesis.
We wanted to determine if there was any systematic bias associated with the ligation step of the LM-PCR approach. Therefore we applied TdT-PCR as an alternative to examining these end sequences. Using TdT, we added either multiple dG or dC residues to the 3′ ends of purified VLP-derived DNA. This procedure marks the ends of Ty1 DNA replication intermediates by placing them subterminal to the resulting run of either G or C residues (10, 23). By using either a complementary poly(dC) or poly(dG) primer (Table 2) in conjunction with a downstream minus-strand primer, the minus-strand DNA ends were PCR amplified, cloned, and sequenced. Poly(dC) TdT-PCR clones allowed us to unambiguously identify the 3′ termini of minus-strand intermediates paused in the U5-PBS region, since no cytosines occur in this region of Ty1-H3 minus-strand sequence. From a total of 23 such TdT-PCR clones, 17 showed correctly templated ends in this region with a distribution entirely consistent with our LM-PCR-derived data (Fig. 4B). The remaining six clones showed 3′-terminal sequence changes (see below). Taken together with the primer extension and RNA LM-PCR data, these results are most consistent with heterogeneous RNase H cleavage during minus-strand strong-stop synthesis, followed by pausing of minus-strand DNA intermediates at these template ends.
Do the results obtained using LM-PCR of minus-strand DNA extend beyond Ty1-H3 to the cloned Ty2 element as well? After performing an equivalent ligation and PCR of Ty2-derived VLP nucleic acids, we observed PCR products of the same sizes as in Fig. 4A (data not shown). We sequenced 23 Ty2-derived PCR clones from minus-strand DNA paused in U5. As observed for Ty1, the major pause site (7 [35%] of 20 clones with no terminal errors) was 6 bases upstream of the U5-PBS border (Fig. 4B). These findings are consistent with the Ty2 RNA primer extension analysis (Fig. 2E) showing the predominant RNase H cleavage product at the same location.
Errors are observed at the 3′ ends of minus-strand DNA paused near the U5-PBS border.
In addition to the 29 LM-PCR-derived Ty1-H3 clones described above with templated ends near the U5-PBS border, we observed 10 additional clones (26%) with terminal or subterminal errors in this region (Fig. 5A). Five clones had a single terminal mismatch 10, 9, 7, 2, and 1 base, respectively, upstream of the U5-PBS border. Two additional clones exhibited multiple terminal mismatches. Two clones appeared to have subterminal base substitutions. Finally, a 10th aberrant clone showed a 6-base deletion just upstream of the U5-PBS border followed by 5 templated terminal bases. These terminal errors were not limited to Ty1-H3. Examination of the terminal sequences of the Ty2-derived clones revealed three with terminal base substitutions 7, 6, and 4 bases upstream of the U5-PBS border (Fig. 5B). Of 49 clones derived from the TdT-PCR approach, 10 (20%) exhibited errors at or near the 3′ minus-strand terminus (Fig. 5A). In addition to single terminal base substitutions 13, 12, and 7 (two independent clones) bases upstream of the U5-PBS border, we observed three clones with multiple terminal base substitutions, one clone with two subterminal substitutions, and two clones with apparent subterminal deletions. One of these deletions is identical to that observed among the LM-PCR-derived clones (compare OU 9 and EM 1922 in Fig. 5A).
FIG. 5.
Errors at the 3′ ends of minus-strand DNA paused near the U5-PBS border. Wild-type (WT) minus-strand sequences are listed above the mutant sequences in the 3′-to-5′ orientation. Identifying numbers are to the right of the sequences. Clones observed during LM-PCR analysis of the U5-PBS border are indicated by a boxed LINKER. Clones observed during TdT-PCR analysis are indicated by boxed mononucleotide runs. Mutations are in boldface. Deletions are represented by [DEL]. (A) Mutations observed during LM-PCR and TdT-PCR analysis of Ty1-H3 minus-strand DNA paused at the U5-PBS border. The same primers as used for Fig. 4 were used for LM-PCR and sequencing. For TdT-PCR after the poly(C) 3′ tailing reaction, RAG340 and RAG199 were used for the initial PCR amplification, followed by a nested amplification using RAG343 and RAG54. After the poly(G) 3′ tailing reaction, RAG478 and RAG199 were used for PCR amplification. (B) Mutations observed during LM-PCR analysis of Ty2 minus-strand DNA paused at the U5-PBS border. The same primers as used for Fig. 4 were used.
Thus, it appears that errors during Ty1 cDNA synthesis occur at or near template termini. Substitutions of adenine or thymine were much more frequent than either guanine or cytosine substitutions. A plausible interpretation of many of these results is that Ty1 RT can add one or more nontemplated bases after reaching the RNase H-determined template terminus upstream of the U5-PBS border. Since this cleavage site is variable, so are the positions of the nontemplated bases. As has been seen with retroviral RTs in vitro (11, 31, 33), there appears to be preferential addition of certain nucleotides over others. The propensity for terminal base addition may explain why the pattern of endogenous Ty1 RT-generated primer extension products just upstream of the U5-PBS border is less discrete than the distribution of RNA ends determined by LM-PCR (compare Fig. 2C and 3D). The primer extension pattern may reflect a combination of correct termination at multiple template ends, upon which is superimposed a population of aberrant products, extended by nontemplated bases at the template ends.
Genomic Ty1 and Ty2 elements show extensive sequence variation in the 3′ end of U5.
We next examined whether the sequences of LTRs in full-length Ty elements reflected the errors observed in minus-strand intermediates upstream of the U5-PBS border. We analyzed the U5 regions from both LTRs of all 42 full-length Ty1 and Ty2 elements identified in the SGD. By linking the 5′ and 3′ LTRs from each element, color coding the actual sequences, and grouping elements with similar patterns, it is clear that the 3′ half of U5 is highly polymorphic compared to the remainder of the R and U5 regions shown in Fig. 6. This result is consistent with the U5-PBS region being a mutational hotspot. To look more closely at the question of whether the specific intermediates that we have examined could serve as the source of this variability, we looked for asymmetry between the 5′ and 3′ U5 regions of each element. Of 29 full-length Ty1 elements, the 5′ and 3′ U5s were different from each other in 12 cases (41%). For 10 of these elements, the sequence variation between the two LTRs could be explained simply by a strand switch between U5s from two different elements, occurring from 5 to 15 bp upstream of the U5-PBS border. This result is compatible with transfer of minus-strand DNA intermediates paused at the 5′ end of varied RNase H-mediated template ends to heterologous templates, copackaged within the same VLP. In the case of Ty2 elements, 9 of 13 LTR pairs had differences in their U5 regions, and 3 of these could be explained by strand switching events. Interestingly we failed to detect interelement recombination between the U5s of any Ty1 and Ty2 elements.
FIG. 6.
Representation of R and U5 sequences of the 42 full-length Ty1 and Ty2 elements in the SGD, arranged so that the 5′ (left) LTR (indicated by 3L, etc.) is directly above the 3′ (right) LTR (indicated by 3R, etc.) of each element. 5′ LTRs are followed by PBS sequence; SGD identification numbers are to the right. Bases are color coded as follows: A, red; C, yellow; G, purple; T, green; gap, blue. Base substitutions unique to the 5′ LTR are circled. The single base substitution unique to the 3′ LTR is indicated by a diamond. The region of single base insertion in a 5′ LTR is boxed.
As highlighted in Fig. 6, seven elements (two Ty1s and five Ty2s) showed unique base substitutions; i.e., they had sequences not found in any of the other U5s within their family of elements at the equivalent position. In six of the seven cases, these unique polymorphisms were in the 5′ U5, occurring 11, 10, 7 (twice), and 6 bp upstream of the U5-PBS border. The sixth U5 exhibited two unique substitutions 4 and 9 bp upstream of the U5-PBS border. In addition, a different Ty2 5′ U5 contained a single T insertion 6 to 10 bp upstream of the U5-PBS border (Fig. 6, YNLCTy2-1L). This striking asymmetry in the location of sequence alterations strongly suggests that these changes occurred during Ty replication, most likely through extension of minus-strand near-full-length DNAs with terminal base additions that were paused at the variable RNase H-cleaved template ends. Finally, we noted two other unique changes in the highly conserved R region. While we do not have a cogent explanation for their origin, it is interesting that both appear in the 5′ LTR.
DISCUSSION
Our work demonstrates that nontemplated base addition at the 3′ end of Ty1 minus-strand intermediates is a real in vivo phenomenon. This study provides physical data that strongly support our model that the combination of heterogeneous RNase H-determined RNA ends and nontemplated base additions could be the source of the hotspot of errors upstream of the U5-PBS border in newly transposed Ty1 elements (18). Examination of the precise LTR sequences of 42 full-length Ty1 and Ty2 elements in the complete S. cerevisiae genome support our proposition that this mechanism of error generation is a source of Ty element variability. Whether this mechanism functions uniquely in yeast transposons or is generalizable to other LTR-containing elements which employ the same replication strategy is a question that we are now pursuing.
Specificity of RNase H cleavage at the U5-PBS border.
Models of retrovirus replication typically show the initial RNase H cleavage of 5′ RNA during minus-strand strong-stop elongation to occur precisely at the U5-PBS border (12, 20, 28). Our findings provide the first direct in vivo data pertinent to this aspect of the retrovirus model. We used LM-PCR, TdT-PCR, and primer extension analysis to precisely define the ends of two intermediates in Ty1 replication. These intermediates were obtained directly from VLPs associated with in vivo transposition. Each experimental method was consistent in showing that the RNase H cleavage sites closest to the PBS are heterogeneous, with specific preferred cut sites. In vitro mapping results from HIV and MuLV RTs have shown that the major cleavage sites were 1 to 5 bases upstream of the U5-PBS border, with heterogeneous minor sites up to 10 bases into the U5 region (2, 3, 21, 41). In one case, the in vitro results were confirmed by performing endogenous reactions with activated MuLV virions (41). Our findings, taken together with these complementary in vitro studies, modify previous assumptions regarding this step in LTR-containing retroelement replication. More specifically, the results indicate that the 3′ minus-strand DNA end, which serves as the recipient of transferred plus-strand strong-stop DNA, is several bases longer than previously presumed.
What is the basis for specificity of RNase H cleavage site selection? Our results suggest that the interplay between potential structural determinants and specific sequences in the immediate vicinity of the cut affect cleavage site specificity. We observed the major cut site to be 5 to 6 bases upstream of the U5-PBS border, using three different U5 sequences and two different (though related) RT sources (Ty1 RT and Ty2 RT). These results imply that cleavage sites are selected more by an intrinsic structural determinant than by specific sequences. An analogous situation exists for HIV type 1 during plus-strand strong-stop synthesis, where RNase H cleavage of the tRNA primer occurs one base beyond the DNA-RNA junction rather than at the junction (15, 39, 42). Both AMV RNase H and MuLV RNase H use this same cleavage site on a model HIV primer-template (8). Since these heterologous RTs are unlikely to recognize specific sequence cues in HIV, it is more likely that particular secondary structures play a key role in defining the precise sites of cleavage. On the other hand, we found that a single base change 7 bases upstream of the U5-PBS border, which distinguished Ty1-H3 from Ty1-173, shifted the major cleavage site from 6 bases to 5 bases upstream of the PBS (compare Fig. 2C and D). Thus, while we do not yet understand the rules underlying cleavage site selection, it is apparent that specific sequence combinations do influence the process.
Errors in Ty1 minus-strand replication intermediates occur at 3′ termini.
By LM-PCR and TdT-PCR analysis of minus-strand DNA intermediates, we have detected pausing at the 5′ end of RNase H-generated RNA template ends, prior to plus-strand strong stop transfer. We found that base substitutions were relatively common at these 3′ DNA termini. The most likely basis for these errors is nontemplated nucleotide addition, a process recognized to occur in vitro for many RTs (11, 31), to be associated with sites of in vitro strand transfer (33, 34, 48), and to occasionally be present at sites of retrotransposon cDNA insertions (13, 19, 45). Our data suggest that such findings are not just biochemical oddities but also represent a normal consequence of retrotransposon replication in yeast. Furthermore, we found examples of subterminal base substitutions and small deletions which suggest that nontemplated base addition is not the sole mechanism by which errors are generated near template ends. It is of interest that two clones, from two independent experiments, showed the same 6-base deletion (Fig. 5). The borders of this deletion occur at the preferred RNase H cleavage site 6 bases upstream of the U5-PBS border and precisely at the U5-PBS border. Further, the 3′ end of the minus-strand intermediates occur 5 bases (Fig. 5, EM 1922) and 8 bases (Fig. 5, OU 9) beyond the deletion. Such features raise the possibility that these clones represent aberrant transfer to RNase H-generated RNA fragments after Ty1 RT comes to the 5′ end of the genomic RNA template, a mechanism similar to that proposed to explain certain in vivo errors identified in retroviral systems (30, 32).
The frequent errors that we identified at the 3′ ends of Ty1 DNA intermediates are not likely to be artifacts of our detection system. Because the potential exists for the introduction of spurious sequence during the single-strand DNA ligation step, we independently analyzed minus-strand replication intermediates by using TdT-PCR. The results from these data were very similar to those obtained with T4 RNA ligase (Fig. 5A). Furthermore, in the mononucleotide runs generated by TdT, incorrect bases were identified only two times in ∼1,100 added mononucleotides, indicating that spurious base addition at the 3′ terminus of a Ty1 intermediate by TdT is a rare event. Finally, in our sequence analysis of 137 Ty1 clones of 5′ RNA ends ligated to the RNA linker, where no RT-generated errors are expected, only two had unexpected sequences at the linker-ligation junction. Harada and Orgel (22) have reported that efficiency of substrate selection by T4 RNA ligase may be influenced by sequence. However, the similarity of T4 RNA ligase-generated data to data derived from TdT suggests that this bias probably plays little, if any, role in influencing our findings. Finally, the compatibility of our LM-PCR data with the independently derived primer extension data suggests that the ends ligated to the linker closely reflect the naturally occurring ends generated by the various steps of Ty1 replication. Therefore, we conclude that most, if not all, aberrations observed at the 3′ ends of Ty1 DNA intermediates were generated by Ty1 RT.
We are aware, however, that certain biases may be inherent to the system. In particular, in vivo Ty1 intermediates with mismatched 3′ ends are less likely, kinetically, to be extended than those with exact primer-template complementarity (29, 50). They may be temporarily stalled or may be replication dead ends. Ty1 VLPs may accumulate such aberrant replication products, and these will be more available than correctly synthesized intermediates as substrates for ligation to the linker. This is probably true not only at natural template ends but throughout the Ty1 element. For example, we occasionally observed apparently random 3′-terminal errors at regions in Ty1 not predicted to coincide with template ends (data not shown). These clones could represent base misincorporation by Ty1 RT followed by termination of synthesis along a continuous RNA template or termination of reverse transcription at a broken RNA end, followed by nontemplated base addition. Consequently, it is likely that the wealth of 3′ mismatched intermediates that we have observed comes from a naturally enriched pool of mutated cDNAs.
Heterogeneous sites of RNase H cleavage affect multiple steps in Ty1 replication.
What are the implications of heterogeneous RNase H cleavage upstream of the U5-PBS border? As Fig. 1 shows, the single step of RNase H cleavage during minus-strand strong-stop synthesis has implications for succeeding intermediates in replication; i.e., the 3′ end of paused minus-strand DNA and region of complementarity for plus-strand transfer. The major consequence is that minus-strand DNA synthesis pauses at variable sites upstream of the U5-PBS border rather than at a single site at the U5-PBS border. The retrovirus replication model predicts that plus-strand transfer to the 3′ end of near-full-length minus-strand DNA is mediated by complementarity between the tRNA-templated 3′ end of the plus strand and its complementary region at the 3′ end of the paused minus-strand DNA (Fig. 1E). In the case of retroviruses, this region of complementarity includes the 18 bases of the PBS. However, the PBS of Ty1 or Ty2 is only 10 bases, and that for Ty3 is only 8 bases. Lauermann et al. (26, 26a, 27) have demonstrated that plus-strand strong-stop transfer occurs prior to Ty1 RT reaching the seventh base of the tRNA template, further shortening the region of potential complementarity. For these reasons, pausing of minus-strand DNA intermediates at RNase H cleavage sites upstream of the PBS might be especially critical for Ty1 replication; it would result in an extended region of complementarity between the two 3′ ends. This could facilitate successful completion of Ty1 replication after plus-strand strong-stop transfer.
A different consequence of preferred RNase H cleavage upstream of the U5-PBS border is that the two most 3′ bases of U5 are relatively protected from nontemplated base errors. The terminal U5 bases CA at the 3′ end of the downstream LTR are canonical signals for LTR-containing retroelement integration. Because the 5′ U5 provides the template for both U5s in the next generation, a mutation affecting either one of these two required bases would adversely influence future integrations. By virtue of the CA placement proximal to the preferred RNase H cleavage sites, template end-induced errors would be less likely to interfere with the integration process.
Finally, since much of U5 encodes the amino terminus of the first Ty1 open reading frame, a consequence of base substitutions upstream of the U5-PBS border is that the amino acid composition of this segment of Gag is not fixed. While nothing is known about this region of Gag, it is plausible that the variability could provide a selective advantage or disadvantage to different individual Ty elements as they replicate within the yeast genome.
Polymorphisms within genomic Ty elements correlate with sites of RNase H-generated template ends.
Are minus-strand intermediates with terminal errors viable? Previous work with retrovirus RTs has demonstrated that these enzymes are capable of extending mismatched 3′ DNA termini both in vitro and in vivo (25, 35, 40, 50). Our earlier analysis of mutations generated during single cycles of Ty1 transposition revealed 3 (of 29) new events with base changes near the U5-PBS border (18). In fact, in the present study, we found one clone (Fig. 5A, OU 1185) with the identical error (TC to CA, bp 332 to 333) observed in the previous study. Further, in our global analysis of 42 full-length Ty1 and Ty2 elements in the SGD, both a high degree of variability near the U5-PBS border and a strong bias for unique changes in the 5′ LTR were noted. It is tempting to speculate that these changes originated as minus-strand near-full-length DNAs paused at RNase H-cleaved template ends. To extend this hypothesis to its fullest implication, recombination and errors generated in the 5′ U5 by this mechanism would, over time, be copied to both LTRs and might very likely account for the high degree of polymorphism in this region.
ACKNOWLEDGMENTS
We thank J. Boeke, T. Heyman, D. Voytas, F. X. Wilhelm, and H. Xu for helpful discussions. We are grateful to J. Boeke for providing plasmids and to J. Dougherty and M. Roth for critical reading of the manuscript. We thank S. Vanguri and M. Kim for technical assistance.
This work was supported in part by the Lucille P. Markey Charitable Trust and the Charles and Johanna Busch Endowment.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R L, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Ben-Artzi H, Shemesh J, Zeelon E, Amit B, Kleiman L, Gorecki M, Panet A. Molecular analysis of the second template switch during reverse transcription of the HIV RNA template. Biochemistry. 1996;35:10549–10557. doi: 10.1021/bi960439x. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Artzi H, Shemesh J, Zeelon E, Amit B, Kleiman L, Gorecki M, Panet A. Ribonuclease H activity during initiation of reverse transcription using tRNA(lys)/RNA primer/template of human immunodeficiency virus. Arch Biochem Biophys. 1996;325:209–216. doi: 10.1006/abbi.1996.0026. [DOI] [PubMed] [Google Scholar]
- 4.Blain S W, Goff S P. Effects on DNA synthesis and translocation caused by mutations in the RNase H domain of Moloney murine leukemia virus reverse transcriptase. J Virol. 1995;69:4440–4452. doi: 10.1128/jvi.69.7.4440-4452.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Boeke, J. Unpublished data.
- 5.Boeke J D, Eichinger D, Castrillon D, Fink G R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988;8:1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeke J D, Styles C A, Fink G R. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986;6:3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braiterman L T, Manokian G M, Eichinger D J, Merbs S L, Gabriel A, Boeke J D. In-frame linker insertion mutagenesis of yeast transposon Ty1: phenotypic analysis. Gene. 1994;139:19–26. doi: 10.1016/0378-1119(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 8.Champoux J J. Roles of ribonuclease H in reverse transcription. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 103–117. [Google Scholar]
- 9.Chapman K B, Byström A S, Boeke J D. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc Natl Acad Sci USA. 1992;89:3236–3240. doi: 10.1073/pnas.89.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 11.Clark J M. Novel non-templated nucleotide addition reactions catalyzed by procaryotic and eucaryotic DNA polymerases. Nucleic Acids Res. 1988;16:9677–9686. doi: 10.1093/nar/16.20.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffin J M. Retroviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1767–1847. [Google Scholar]
- 13.Dombroski B A, Feng Q, Mathias S L, Sassaman D M, Scott A F, Kazazian H H, Boeke J D. An in vivo assay for the reverse transcriptase of human retrotransposon L1 in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:4485–4492. doi: 10.1128/mcb.14.7.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Feuerbach F, Drouaud J, Lucas H. Retrovirus-like end processing of the tobacco Tnt1 retrotransposon linear intermediates of replication. J Virol. 1997;71:4005–4015. doi: 10.1128/jvi.71.5.4005-4015.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fromont-Racine M, Bertrand E, Picket R, Grange T. A highly sensitive method for mapping the 5′ termini of mRNAs. Nucleic Acids Res. 1993;21:1683–1684. doi: 10.1093/nar/21.7.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furfine E S, Reardon J E. Reverse transcriptase-RNase H from the human immunodeficiency virus. Relationship of the DNA polymerase and RNA hydrolysis activities. J Biol Chem. 1991;266:406–412. [PubMed] [Google Scholar]
- 16.Gabriel A, Boeke J D. Retrotransposon reverse transcription. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 275–328. [Google Scholar]
- 17.Gabriel A, Boeke J D. Reverse transcriptase encoded by a retrotransposon from the trypanosomatid Crithidia fasciculata. Proc Natl Acad Sci USA. 1991;88:9794–9798. doi: 10.1073/pnas.88.21.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriel A, Willems M, Mules E H, Boeke J D. Replication infidelity during a single cycle of Ty1 retrotransposition. Proc Natl Acad Sci USA. 1996;93:7767–7771. doi: 10.1073/pnas.93.15.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George J A, Burke W D, Eickbush T H. Analysis of the 5′ junctions of R2 insertions with the 28S gene: implications for non-LTR retrotransposition. Genetics. 1996;142:853–863. doi: 10.1093/genetics/142.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilboa E, Mitra S W, Goff S, Baltimore D. A detailed model of reverse transcription and a test of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- 21.Gotte M, Fackler S, Hermann T, Perola E, Cellai L, Gross H J, Le Grice S F J, Heumann H. HIV-1 reverse transcriptase-associated RNase H cleaves RNA/RNA in arrested complexes: implications for the mechanism by which RNase H discriminates between RNA/RNA and RNA/DNA. EMBO J. 1995;14:833–841. doi: 10.1002/j.1460-2075.1995.tb07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada K, Orgel L E. In vitro selection of optimal DNA substrates for T4 RNA ligase. Proc Natl Acad Sci USA. 1993;90:1576–1579. doi: 10.1073/pnas.90.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyman T, Agoutin B, Friant S, Wilhelm F X, Wilhelm M L. Plus-strand DNA synthesis of the yeast retrotransposon Ty1 is initiated at two sites, PPT1 next to the 3′ LTR and PPT2 within the pol gene. PPT1 is sufficient for Ty1 transposition. J Mol Biol. 1995;253:291–303. doi: 10.1006/jmbi.1995.0553. [DOI] [PubMed] [Google Scholar]
- 24.Hu W-S, Temin H M. Retroviral recombination and reverse transcription. Science. 1990;250:1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- 25.Kulpa D, Topping R, Telesnitsky A. Determination of the site of first strand transfer during Moloney murine leukemia virus reverse transcription and identification of strand transfer-associated reverse transcriptase errors. EMBO J. 1997;16:856–865. doi: 10.1093/emboj/16.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauermann V, Boeke J D. The primer tRNA sequence is not inherited during Ty1 retrotransposition. Proc Natl Acad Sci USA. 1994;91:9847–9851. doi: 10.1073/pnas.91.21.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Lauermann V, Boeke J D. Plus strand strong-stop DNA transfer in yeast Ty retrotransposons. EMBO J. 1997;16:6603–6612. doi: 10.1093/emboj/16.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauermann V, Nam K, Trambley J, Boeke J D. Plus-strand strong-stop DNA synthesis in retrotransposon Ty1. J Virol. 1995;69:7845–7850. doi: 10.1128/jvi.69.12.7845-7850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodish H, Baltimore D, Berk A, Zipursky S L, Matsudaira P, Darnell J. Molecular cell biology. 3rd ed. New York, N.Y: W. H. Freeman and Company; 1995. [Google Scholar]
- 29.Mendelman L V, Petruska J, Goodman M F. Base mispair extension kinetics. J Biol Chem. 1990;265:2338–2346. [PubMed] [Google Scholar]
- 30.Parthasarathi S, Varela-Echavarria A, Ron Y, Preston B D, Dougherty J P. Genetic rearrangements occurring during a single cycle of murine leukemia virus vector replication: characterization and implications. J Virol. 1995;69:7991–8000. doi: 10.1128/jvi.69.12.7991-8000.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P H, Preston B D. Marked infidelity of human immunodeficiency virus type 1 reverse transcriptase at RNA and DNA template ends. Proc Natl Acad Sci USA. 1994;91:549–553. doi: 10.1073/pnas.91.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathak V K, Temin H M. Broad spectrum of in vivo forward mutations, hypermutations, and mutational hotspots in a retroviral shuttle vector after a single replication cycle: deletions and deletions with insertions. Proc Natl Acad Sci USA. 1990;87:6024–6028. doi: 10.1073/pnas.87.16.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peliska J A, Benkovic S J. Fidelity of in vitro DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Biochemistry. 1994;33:3890–3895. doi: 10.1021/bi00179a014. [DOI] [PubMed] [Google Scholar]
- 34.Peliska J A, Benkovic S J. Mechanism of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Science. 1992;258:1112–1118. doi: 10.1126/science.1279806. [DOI] [PubMed] [Google Scholar]
- 35.Perrino F W, Preston B D, Sandell L L, Loeb L A. Extension of mismatched 3′ termini of DNA is a major determinant of infidelity of human immunodeficiency virus type 1 reverse transcriptase. Proc Natl Acad Sci USA. 1989;86:8343–8347. doi: 10.1073/pnas.86.21.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pochart P, Agoutin B, Rousset S, Chanet R, Doroszkiewicz V, Heyman T. Biochemical and electron microscope analyses of the DNA reverse transcripts present in the virus-like particles of the yeast transposon Ty1. Identification of a second origin of Ty1 DNA plus strand synthesis. Nucleic Acids Res. 1993;21:3513–3520. doi: 10.1093/nar/21.15.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston B D. Error-prone retrotransposition: rime of the ancient mutators. Proc Natl Acad Sci USA. 1996;93:7427–7431. doi: 10.1073/pnas.93.15.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston B D, Dougherty J P. Mechanisms of retroviral mutation. Trends Microbiol. 1996;4:16–21. doi: 10.1016/0966-842x(96)81500-9. [DOI] [PubMed] [Google Scholar]
- 39.Pullen K A, Ishimoto L K, Champoux J J. Incomplete removal of RNA primer for minus-strand DNA synthesis by human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1992;66:367–373. doi: 10.1128/jvi.66.1.367-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulsinelli G A, Temin H M. High rate of mismatch extension during reverse transcription in a single round of retrovirus replication. Proc Natl Acad Sci USA. 1994;91:9490–9494. doi: 10.1073/pnas.91.20.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz S J, Whiting S H, Champoux J J. Cleavage specificities of Moloney murine leukemia virus RNase H implicated in the second strand transfer during reverse transcription. J Biol Chem. 1995;270:24135–24145. doi: 10.1074/jbc.270.41.24135. [DOI] [PubMed] [Google Scholar]
- 41a.Smith J S, Kim S, Roth M J. Analysis of long terminal repeat circle junctions of human immunodeficiency virus type 1. J Virol. 1990;64:6286–6290. doi: 10.1128/jvi.64.12.6286-6290.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith J S, Roth M J. Specificity of human immunodeficiency virus-1 reverse transcriptase-associated ribonuclease H in removal of the minus-strand primer, tRNALys3. J Biol Chem. 1992;267:15071–15079. [PubMed] [Google Scholar]
- 43.Tanese N, Telesnitsky A, Goff S P. Abortive reverse transcription by mutants of Moloney murine leukemia virus deficient in the reverse transcriptase-associated RNase H function. J Virol. 1991;65:4387–4397. doi: 10.1128/jvi.65.8.4387-4397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Telesnitsky A, Goff S P. Strong-stop strand transfer during reverse transcription. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 49–83. [Google Scholar]
- 45.Teng S-C, Kim B, Gabriel A. Retrotransposon reverse transcriptase-mediated repair of chromosomal breaks. Nature. 1996;383:641–644. doi: 10.1038/383641a0. [DOI] [PubMed] [Google Scholar]
- 46.Tessier D C, Brousseau R, Vernet T. Ligation of single-stranded oligodeoxyribonucleotides by T4 RNA ligase. Anal Biochem. 1986;158:171–178. doi: 10.1016/0003-2697(86)90606-8. [DOI] [PubMed] [Google Scholar]
- 47.Whitcomb J M, Ortiz-Conde B A, Hughes S H. Replication of avian leukosis viruses with mutations at the primer binding site: use of alternative tRNAs as primers. J Virol. 1995;69:6228–6238. doi: 10.1128/jvi.69.10.6228-6238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu W, Blumberg B M, Fay P J, Bambara R A. Strand transfer mediated by human immunodeficiency virus reverse transcriptase in vitro is promoted by pausing and results in misincorporation. J Biol Chem. 1995;270:325–332. doi: 10.1074/jbc.270.1.325. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Boeke J D. High-frequency deletion between homologous sequences during retrotransposition of Ty elements in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1987;84:8553–8557. doi: 10.1073/pnas.84.23.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zinnen S, Hsieh J-C, Modrich P. Misincorporation and mispaired primer extension by human immunodeficiency virus reverse transcriptase. J Biol Chem. 1994;269:24195–24202. [PubMed] [Google Scholar]