CONSPECTUS:
The cyclic GMP-AMP synthase-stimulator interferon gene (cGAS-STING) pathway is an emerging therapeutic target for the prophylaxis and therapy of a variety of diseases, ranging from cancer, infectious diseases, to autoimmune disorders. As a cytosolic double stranded DNA (dsDNA) sensor, cGAS can bind with relatively long dsDNA, resulting in conformational change and activation of cGAS. Activated cGAS catalyzes the conversion of adenosine triphosphate (ATP) and guanosine triphosphate (GTP) into cGAMP, a cyclic dinucleotide (CDN). CDNs, including 2′3′-cGAMP, stimulate adapter protein STING on the endoplasmic membrane, triggering interferon regulatory factor 3 (IRF3) phosphorylation and nuclear factor kappa B (NF-κB) activation. This results in antitumor and antiviral type I interferon (IFN-I) responses. Moreover, cGAS-STING overactivation and the resulting IFN-I responses have been associated with a number of inflammatory and autoimmune diseases. This makes cGAS-STING appealing immunomodulatory targets for the prophylaxis and therapy of various related diseases. However, drug development of CDNs and CDN derivatives is challenged by their limited biostability, difficult formulation, poor pharmacokinetics, and inefficient tissue accumulation and cytosolic delivery. Though recent synthetic small molecular CDN- or non-CDN-based STING agonists have been reported with promising preclinical therapeutic efficacy, their therapeutic efficacy and safety remain to be fully evaluated preclinically and clinically. Therefore, it is highly desirable and clinically significant to advance drug development for cGAS-STING activation by innovative approaches, such as drug delivery systems and drug development for pharmacological immunomodulation of cGAS. In this Account, we summarize our recent research in the engineering and delivery of immunostimulatory or immunoregulatory modulators for cGAS and STING for the immunotherapy of cancer and autoimmune diseases. To improve the delivery efficiency of CDNs, we developed ionizable and pH-responsive polymeric nanocarriers to load STING agonists, aiming to improve the cellular uptake and facilitate the endosomal escape to induce efficient STING activation. We also codelivered STING agonists with complementary immunostimulatants in nanoparticle-in-hydrogel composites to synergetically elicit potent innate and adaptive antitumor responses that eradicate local and distant large tumors. Further, taking advantage of the simplicity of manufacturing and the established nucleic acid delivery system, we developed oligonucleotide-based cGAS agonists as immunostimulant immunotherapeutics as well as adjuvants for peptide antigens for cancer immunotherapy. To suppress the overly strong proinflammatory responses associated with cGAS-STING overactivation in some of the autoimmune disorders, we devised nanomedicine-in-hydrogel (NiH) that codelivers a cGAS inhibitor and cell-free DNA (cfDNA)-scavenging cationic nanoparticles (cNPs) for systemic immunosuppression in rheumatoid arthritis (RA) therapy. Lastly, we discussed current drug development by targeting cGAS-STING for cancer, infectious diseases, and autoimmune diseases, as well as the potential opportunities for utilizing cGAS-STING pathway for versatile applications in disease treatment.
Graphical Abstract
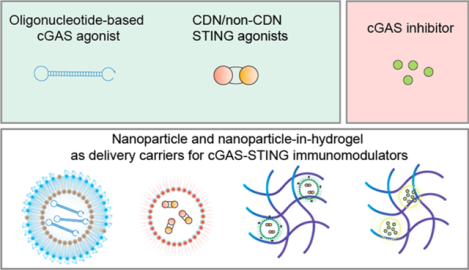
INTRODUCTION
cGAS is a cytosolic sensor and a pattern recognition receptor (PPR) for long dsDNA.5 cGAS is ubiquitously expressed in cytoplasm and nucleosomes in various prokaryotic and eukaryotic species, including bacteria,6 rodents,7 and humans.8 cGAS in the cell nucleus is restricted by nucleosomes and remains inactive.9 Cytosolic cGAS activation results in the synthesis of 2′3′-cGAMP from endogenous adenosine triphosphate (ATP) and guanosine triphosphate (GTP). 2′3′-cGAMP binds to and activates STING located on the endoplasmic reticulum. In the resting state, two STING molecules form a homodimer in a butterfly shape.10 Upon 2′3′-cGAMP binding, STING undergoes a series of structural rearrangements to form oligomerized STING, which is then ubiquitinated and translocated to the Golgi apparatus.11 Next, STING recruits TANK-binding kinase 1 (TBK1) to induce the transcription of interferon regulatory transcription factor 3 (IRF3) and nuclear factor kappa-B (NF-κB), resulting in the production of IFN-I and other proinflammatory cytokines. Moreover, the cytokines produced from cGAS-STING activation can promote antigen-specific adaptive immune responses.11 Particularly, by binding with IFN receptors alpha 1 and alpha 2,12,13 IFN-I promotes their antigen processing and presentation in antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages.14 As a result, IFN-I responses may potentiate the ability of antigens to elicit antigen-specific plasma and memory B cells,15 drive CD4+ T helper 1 (Th1) responses, and induce antigen-specific CD8+ T cell responses.16,17 Interestingly, there is crosstalk of cGAS-STING activation between cancer cells and tumor-associated immune cells via intercellular transporation of 2′3′-cGAMP through gap junctions between cells.18 cGAS-STING activation is also involved in various programmed cell death pathways.19,20 For example, STING activation triggered by endoplasmic reticulum stress causes cell apoptosis in a cell-specific manner, while apoptosis induced by caspase-3 activation can inhibit STING activation by cleavage of IRF3 and STING. IFN-I responses resulting from cGAS-STING activation further regulates cell death via such mechanisms as necroptosis and pyroptosis by interacting with its membrane receptors or triggering absence in melanoma 2 (AIM2) inflammasomes, respectively.21 Given the above immunomodulatory roles of cGAS-STING activation, on one hand, cGAS-STING activation and the resulting innate and adaptive immune responses hold the potential to benefit antiviral and anticancer prevention and therapy; on the other hand, cGAS-STING overactivation, by, for example, overly abundant cell-free self-dsDNA and dysfunctional cytosolic DNases,22 is involved in various autoimmune disorders, such as Aicardi–Goutières syndrome (AGS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE).23 Further, cGAS-STING activation and the resulting innate immunity have been recently shown to be involved in the pathogenesis of COVID-19.24 As such, the cGAS-STING pathway has become a promising immunomodulatory target for the prophylaxis and treatment of these diseases. To this end, various pharmacological agonists and antagonists for cGAS and STING have been tested. For instance, CDNs and derivatives have been extensively tested as immunotherapeutics and vaccine adjuvants for the prevention of various infectious diseases and for the immunotherapy of many types of cancers.25 Moreover, non-CDN-based agonists for STING have been developed as promising alternatives to CDNs. It has been reported that STING can be activated by multiple non-CDN molecular agonists, such as synthetic small molecules, lipids, and polymers. For example, benzothiazinone-like compounds, which are more hydrophobic than CDNs, function as selective and potent STING agonists.26 One of the small benzothiazinone-like molecules, C53, activates human STING by engaging at a cryptic pocket in its transmembrane domain, inducing efficient oligomerization of STING.27 Interestingly, STING activation was also found to be induced by ionizable polymers with cyclic or linear tertiary amines in the side chains.28–30 Specifically, a polymer named as PC7A with ring structures in the side chain efficiently activated STING by forming polyvalent protein condensation to facilitate STING oligomerization. Further, PC7A polymer nanoparticles loaded with antigens induce robust antigen-specific CD8+ T cell response and STING-dependent innate immune responses. Lastly, ionizable lipids with cyclic tertiary amine group also showed STING-activating ability.28
In addition, cGAS activation triggered by DNA release via radiotherapy or chemotherapy has been an appealing strategy to induce immune responses.31–33 Meanwhile, physical scavenging of the abundant cell-free dsDNA in autoimmune diseases holds the potential to alleviate the overly strong activation of cGAS-STING pathway for the prevention and therapy of these autoimmune diseases.34–36 To inhibit cGAS-STING, cGAS inhibitors have been reported and tested in murine and human systems.37–39
Like many other therapeutics, the formulation and delivery of cGAS-STING immunomodulators play a pivotal role in the prophylactic and therapeutic efficacies.40–42 This is especially critical for CDNs or oligonucleotides that often have poor biostability, suboptimal pharmacokinetics, and the resulting narrow therapeutic windows.43 Drug delivery systems have shown the abilities to protect pharmacological active ingredients from degradation, promote their tissue accumulation and cellular uptake, and reduce their unwanted dissemination and the associated adverse side effects.44–46 In this Account, we will review our recent research in the area of engineering and delivery of cGAS-STING immunomodulators for the immunotherapy of cancers as well as autoimmune diseases, such as RA.
STING Activation: CDN-Based Cancer Nanovaccines (NVs)
Natural STING agonists are CDNs such as c-di-GMP (CDG), c-di-AMP, and cGAMP, which have intrinsic small molecular weight, great hydrophilicity, and negative electrostatic charges.47,48 As a result, these CDNs are difficult to cross the cellular plasma membrane and endosomal membrane to access the endoplasmic reticulum in the cytosol where STING is located.49,50 In parallel to these cellular barriers, the susceptibility to enzymatic degradation also inhibits the delivery efficacy to target tissues, leading to poor therapeutic results.51,52 To address these challenges, drug delivery systems have been engineered for the optimal delivery, immunomodulatory, and therapeutic efficacies of CDNs.53,54 STING agonists have been extensively tested, most in preclinical studies, for the treatment of many types of cancers and infectious diseases.55–58 In this section, we will discuss our research on the applications of STING agonists for cancer combination immunotherapy.
In one example, we developed a pH-responsive STING-NVs, in which CDG was loaded into poly(ethylene glycol)-block-poly(d,l-lactide) (PLA-b-PEG) micelles with cytosine (C)-rich i-motif DNA on the surface59 (Figure 1A). CDG was efficiently loaded via C:G base pairing at physiological pH, and the i-motifs with five consecutive Cs in the C domain showed a high CDG loading capacity (2.93% ± 0.17% w/w). In acidic environments such as endosomes, CDG was dissociated from i-motifs on STING-NVs by forming C-quadruplexes through protonated C:C+ base-pair formation. The formulation of STING-NVs protected CDG from enzymatic degradation at physiological conditions and enabled CDG release in the endosomes after endocytosis. In the murine B16F10 melanoma tumor model, STING-NVs modulated the tumor microenvironment by repolarizing immunosuppressive M2-like macrophages into antitumor M1-like macrophages. As a result, STING-NVs showed robust therapeutic efficacy of B16F10 melanoma, especially when combined with an immune checkpoint inhibitor against programmed death receptor 1 (PD-1).
Figure 1.
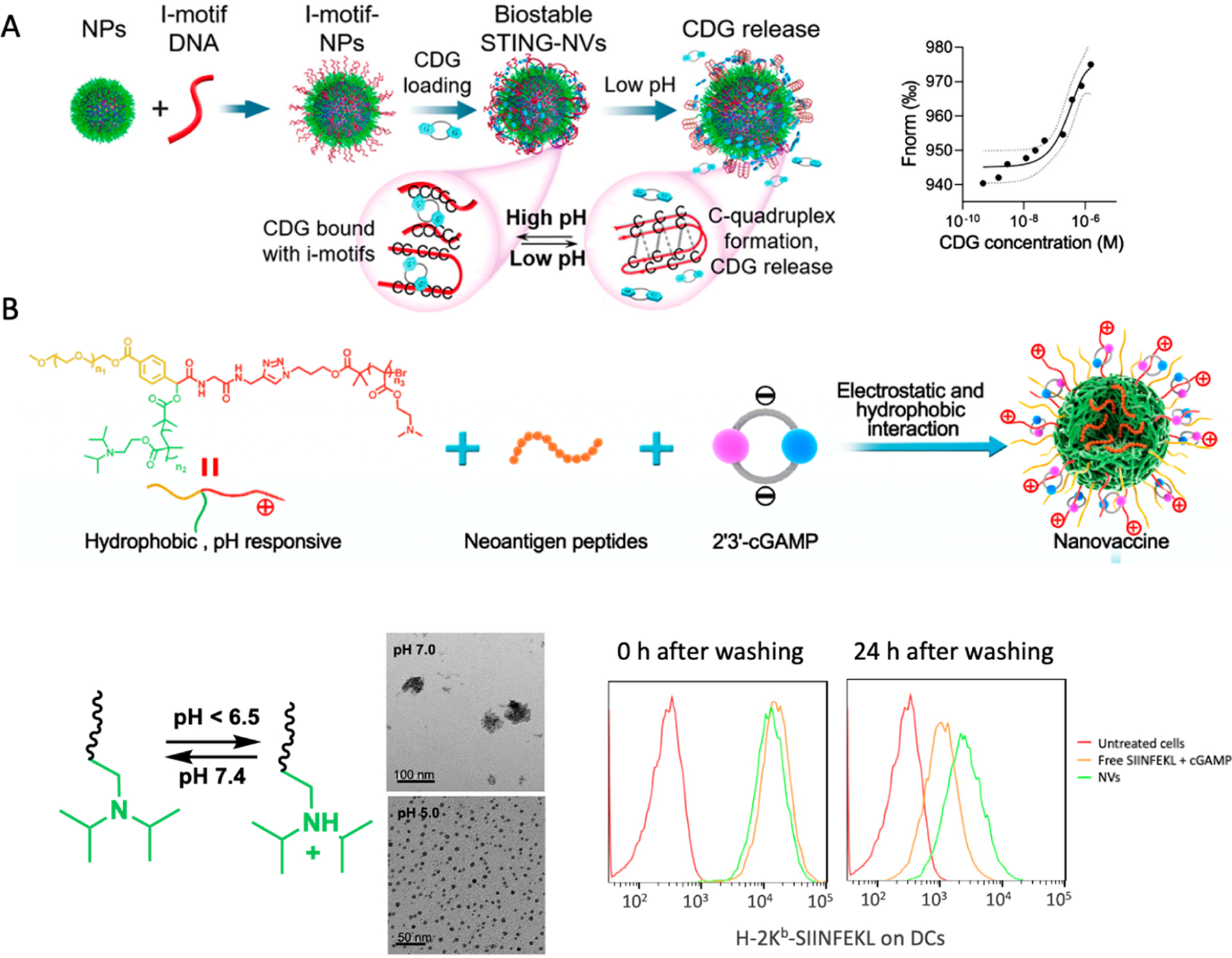
Development of STING agonist-based NVs for cancer immunotherapy. (A) Design of STING-NVs based on pH-responsive DNA i-motif-functionalized PEG-b-PLA NPs for CDG loading via hydrogen bonding. These STING-NVs efficiently loaded CDG by hydrogen bonding (i.e., G:C base pairing). Under acidic conditions, CDG was dissociated from i-motifs due to the formation of C-quadruplexes from i-motifs through protonated C:C+ base-pair formation. As the driving force of CDG loading in these NPs, the binding affinity (Kd) of CDG with NPs was determined to be 47 × 10−9 M, as measured by microscale thermophoresis (MST). (B) pH-responsive polymeric NVs for the codelivery of cGAMP and tumor neoantigen peptides through electrostatic and hydrophobic interactions, respectively. The pH-responsive PDPA moiety was protonated at acidic environment, such as endolysosome inside APCs, to induce NV dissociation and vaccine release from NPs. The codelivery of cGAMP and SIINFEKL peptide sustained the antigen presentation even after washing off extracellular vaccines as shown by flow cytometric analysis using a dye-labeled antibody for the SIINFEKL/H-2Kb complexes (H-2Kb is a subtype of murine MHC-I). The NVs potentiated the neoantigen-specific T cell responses, resulting in robust tumor immunotherapy. Adapted with permission from refs 1 and 59. Copyright 2022 for ref 1, 2020 for ref 59 by Wiley-VCH GmbH.
Nonviral nanocarriers have demonstrated instrumental for effective and safe gene and drug delivery.60 Polymeric nanocarriers are one of the most successful classes of nonviral nanocarriers thus far.61 Polymeric nanocarriers have shown great potential for STING agonists delivery in preclinical studies.41,47 For example, an in situ cross-linking endosomolytic polymersomes formed by a well-defined copolymer PEG-block-[(2-(diethylamino)ethyl methacrylate)-co-(butyl methacrylate)-co-(pyridyl disulfide ethyl methacrylate)] (DBP-b-PEG) showed great efficiency for cGAMP cytosolic delivery via endosomal escape.41 The polymer design highlights key distinctions between nucleic acid therapeutics and CDNs, which provided design insights for the delivery systems for STING agonists. In our recent study, we developed an ionizable pH-responsive polymer to codeliver STING agonists and peptide-based tumor neoantigens for tumor immunotherapy. We synthesized a series of star-shaped polymers with varying lengths of cationic poly((2-dimethylaminoethyl) methacrylate) (PDMA) in order to optimize the loading capacity of negatively charged cGAMP and biocompatibility (Figure 1B). Another arm in this polymer is the pH-responsive poly(2-(diisopropylamino)ethyl methacrylate) (PDPA) that works as hydrophobic core that drives the self-assembly of micellular nanoparticles and the loading of hydrophobic peptide neoantigens. The PDPA segment has shown ultra-pH-responsive in weak acidic condition (pH ≤ 6.3) upon protonation of the tertiary amines, which is ideal for the dissociation of nanoparticles and payload release in early endosomes (pH 5.9–6.2), as confirmed by dynamic light scattering (DLS) results. This pH-responsiveness in the acidic endolysosome facilitates endosomal escape of cGAMP and antigens, which enhanced STING activation by cGAMP in the cytosol and antigen–MHC complexation in the cytosol for presentation on APC surfaces. In syngeneic murine tumors, mice treated with these polymeric cGAMP/neoantigen-codelivering NVs showed potent neoantigen-specific CD8+ T-cell responses and antitumor therapeutic efficacy.
cGAS-STING agonists have the potential to be combined with immunomodulators targeting alternative or synergistic PRRs, thereby eliciting potent and a broad spectrum of anticancer cytokines.62 This is clinically significant to overcome the interpatient heterogeneity of the expression level of a single PRR and the limited human cell subsets that express some particular PRRs such as TLR9.63 Moreover, while administration of STING agonists distant from tumors can result in lymph node draining and induce systemic antitumor immune response, intratumoral vaccination has been and will continue to represent a clinically appealing tumor therapeutic approach, especially for many types of surgically accessible tumors.64–66 Moreover, intratumoral drug injection may prolong the tumor retention of immunomodulators and reduce the systemic dissemination of immunomodulators, which would allow efficient antitumor modulation of the immunosuppressive TME while minimizing adverse side effects caused by overly strong systemic inflammatory responses.42 To achieve site-specific and sustained release of multiple combination immunostimulant adjuvants while reducing adverse side effects, we designed an injectable thermoresponsive nanovaccines-in-hydrogel (NvH) to codeliver STING agonist cGAMP with two Toll-like receptor (TLR) immunostimulants, TLR7/8 agonist R848 and TLR9 agonist CpG oligonucleotide (Figure 2A). Specifically, we loaded cGAMP, R848, and CpG into cationic groups modified poly(β-thioether esters) (PHD) through electrostatic and hydrophobic interactions, followed by encapsulating the resulting drug-loaded NVs, as well as immune checkpoint inhibitors (ICB) if applicable, into FDA-approved Pluronic F127 hydrogel to form NVs combined with ICBs in hydrogel (NvIH). We tuned the hydrogel concentration such that it was at salutation status on ice and rapidly transited to gel status at body temperature. Relative to free immunostimulants, this injectable NvH significantly prolonged their tumor retention for up to 11 days postadministration (Figure 2B). Interestingly, the in situ tumor vaccination of NvH captured endogenous tumor antigens and delivered these antigens together with immunostimulatory adjuvants to tumor draining lymph nodes and distal inguinal lymph nodes (Figure 2C), where the antigens could be taken up and presented by APCs to elicit potent and broad (against a broad spectrum of tumor antigens) antitumor adaptive immune responses. This was confirmed by the antigen-specific CD8+ T cell responses in the peripheral blood mononuclear cells (PBMCs) (Figure 2D), indicating the induction of systemic immune response after in situ tumor vaccination with NvH. While many preclinical immunotherapy approaches are effective to treat relatively small tumors, their therapeutic efficacies for large tumors, which resemble the tumor treatment situations in the clinic where tumors are often large when diagnosed, are suboptimal so far. To test the therapeutic efficacy of NvIH in large tumors, we initiated treatment in mice with established single or dual large (~200–300 mm3) subcutaneous syngeneic murine tumors, including B16F10 melanoma, 4T1 mammary carcinoma, and GL261 glioblastoma, all of which are poorly immunogenic and respond poorly to current immune checkpoint inhibitors. Single-dose intratumoral injection of the NvIH resulted in significant tumor inhibition, including complete regression of most of the tumors without recurrence during the course of the study. Moreover, in Balb/c mice with dual large syngeneic 4T1 mammary carcinoma tumors, NvIH-based tumor in situ vaccination of one tumor resulted in robust immunotherapy of not only the directly vaccinated tumors but also the unvaccinated distant tumors due to the abscopal efficacy mediated by the systemic antitumor innate and adaptive immunity. Interestingly, in another mouse model with dual syngeneic GL261 glioblastomas (one on the shoulder and another in the brain), in situ vaccination of the large tumor on the shoulder resulted in not only the complete regression of most of the vaccinated tumors but also the complete regression of most of the unvaccinated orthotopic GL261 tumors in the brain, again likely because in situ vaccination of peripheral tumors elicited robust and broad innate and adaptive antitumor immunity that penetrated blood–brain barriers (BBB) and blood–tumor barriers (BTB) for robust therapy of orthotopic glioblastoma. Worth noting, NvIH showed robust immunotherapeutic efficacy in multiple types of tumors of different tissue origins and tumor immune microenvironments, making this platform potentially widely applicable.
Figure 2.
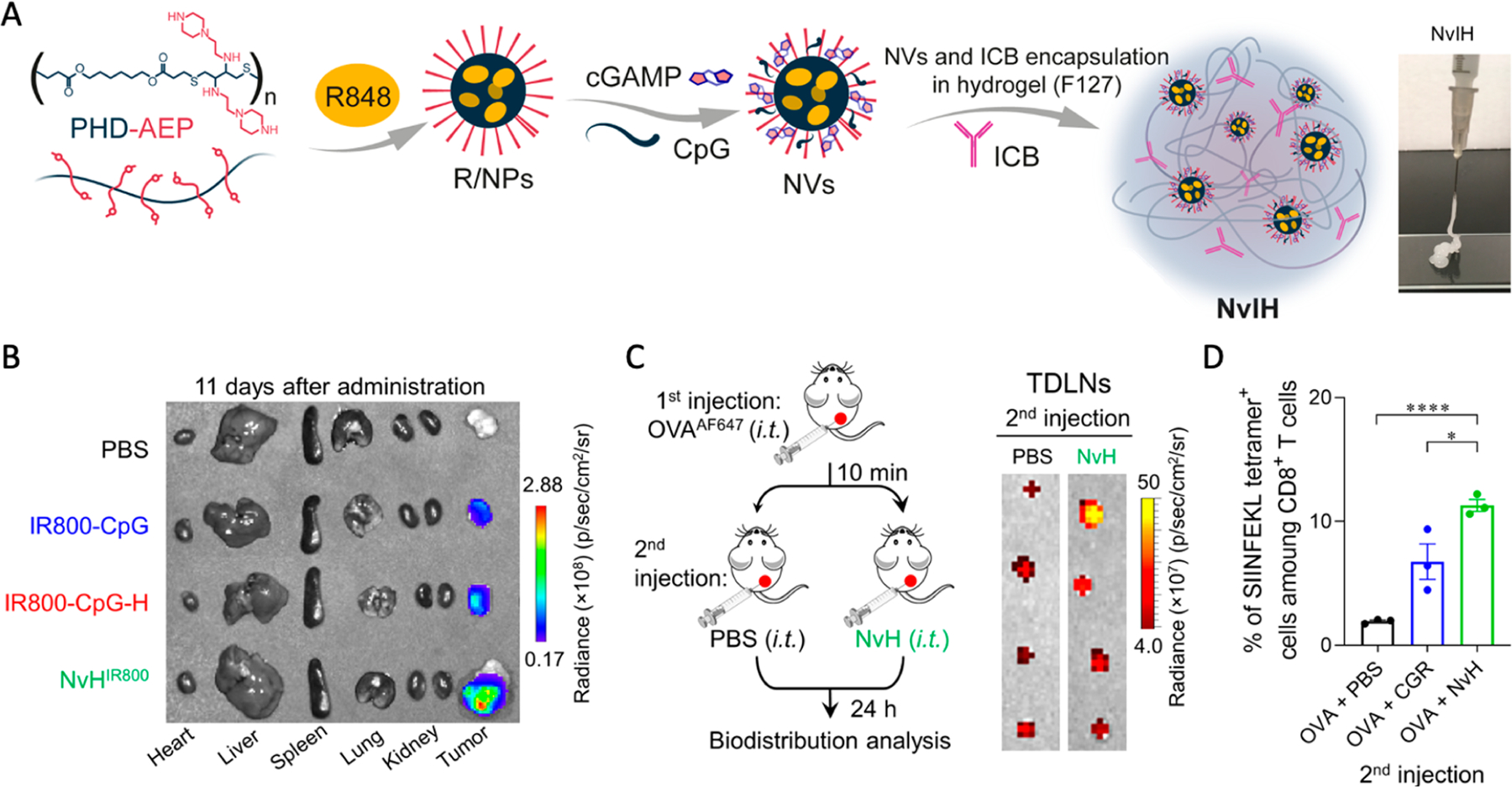
Design of injectable NVs combined immune checkpoint blockade (ICB) in hydrogel (NvIH) for single-dose intratumoral treatment of large tumors with abscopal effect. (A) Construction of NvIH:polymeric NVs was formed by poly(β-thioether esters) PHD modified with cationic groups (PHD–AEP) and loading triple immunostimulants and ICB. (B) NVs-in-hydrogel (NvH) prolonged tumor retention of immunostimulants at day 11 after single-dose intratumoral vaccination. (C) Antigen capturing design and delivery to the tumor-draining lymph nodes (TDLNs) by NvH. (D) Peripheral blood H-2Kb-SIINFEKL tetramer staining after in situ tumor vaccination with NvH of antigen capturing experiment at day 4. Adapted with permission from ref 2. Copyright 2023 by AAAS.
cGAS Activation: Development of Novel cGAS Agonists for Cancer Immunotherapy
While cGAS-STING pathway activation holds great potential for various prophylactic and therapeutic applications, current approaches of drug development for cGAS-STING activation, which are largely based on STING agonists such as CDNs, have been hampered by the intrinsic physicochemical properties as well as the restriction of human STING alleles. Specifically, CDNs are highly water-soluble, anionic, and difficult for site-specific modifications without affecting their pharmacological activities, which altogether lead to challenging large-scale formulation, poor pharmacokinetics, suboptimal safety, narrow therapeutic windows, and consequently limited therapeutic efficacy. Recently, a few non-CDN small molecular STING agonists have been tested in animal models that may address some of the challenges associated with CDNs. Aside from targeting STING, cGAS can be an appealing target for cGAS-STING activation. Inspired by the natural activation of cGAS by relatively long dsDNA (>45 bp) in a sequence-independent manner, we attempted to engineer oligonucleotide agonists for cGAS. We envision that oligonucleotide-based cGAS agonists can leverage the advancements of nucleic acid chemistry and delivery systems made in the past few decades that have contributed to the FDA approval of more than a dozen of nucleic acid therapeutics and vaccines thus far. Structural biology analysis reveals that cGAS monomer is able to bind dsDNA < 20 bp but is insufficient to induce conformational change for cGAS activity, which requires cGAS to form multiple dimers with a minimum unit of cGAS2–DNA2. dsDNA longer than 45 bp stabilizes cGAS dimer by forming a ladder-like cGAS–DNA complex, resulting in robust cGAS activation. On the other hand, cGAS can be activated by short dsDNA less than 20 bp if dsDNA contains flanking guanosine-rich overhang. Moreover, there are also other proteins regulating cGAS activation. For example, polylglutamine binding protein 1 (PQBP1) binds to the complementary DNA (cDNA) of human immunodeficiency virus (HIV) as well as the transmissible neurodegenerative disease protein tau, further facilitating the interaction of cGAS with the respective cDNA or protein. In addition, CCHC-type zinc-finger protein (ZCCHC3) improves cGAS activation by directly binding with DNA to enhance the binding of cGAS with DNA. Lastly, GTPase-activating protein SH3 domain-binding protein 1 (G3BP1) binds with cGAS to promote the formation of large cGAS complexes. Moreover, other cofactors, such as metal ions including Zn2+ and Mn2+, contribute to cGAS activation.67,68
We designed a series of short single-stranded DNAs (ssDNAs) with hairpin secondary structures, which have dsDNA stem lengths ranging from 10 bp to 24 bp and consecutive guanosines (G) flanking each of the 5′- and 3′- ends of the two DNA strands in dsDNA stems. These consecutive G(s) were verified to be critical for the ability of these DNA to activate cGAS. On the basis of their in vitro cGAS activation potency screening, we identified the most potent ssDNA, termed as Svg3, with a 21-bp dsDNA stem, a nine-nucleotide loop, and a GGG triplet adjacent to the stem in each of its four overhangs (Figure 3A).
Figure 3.
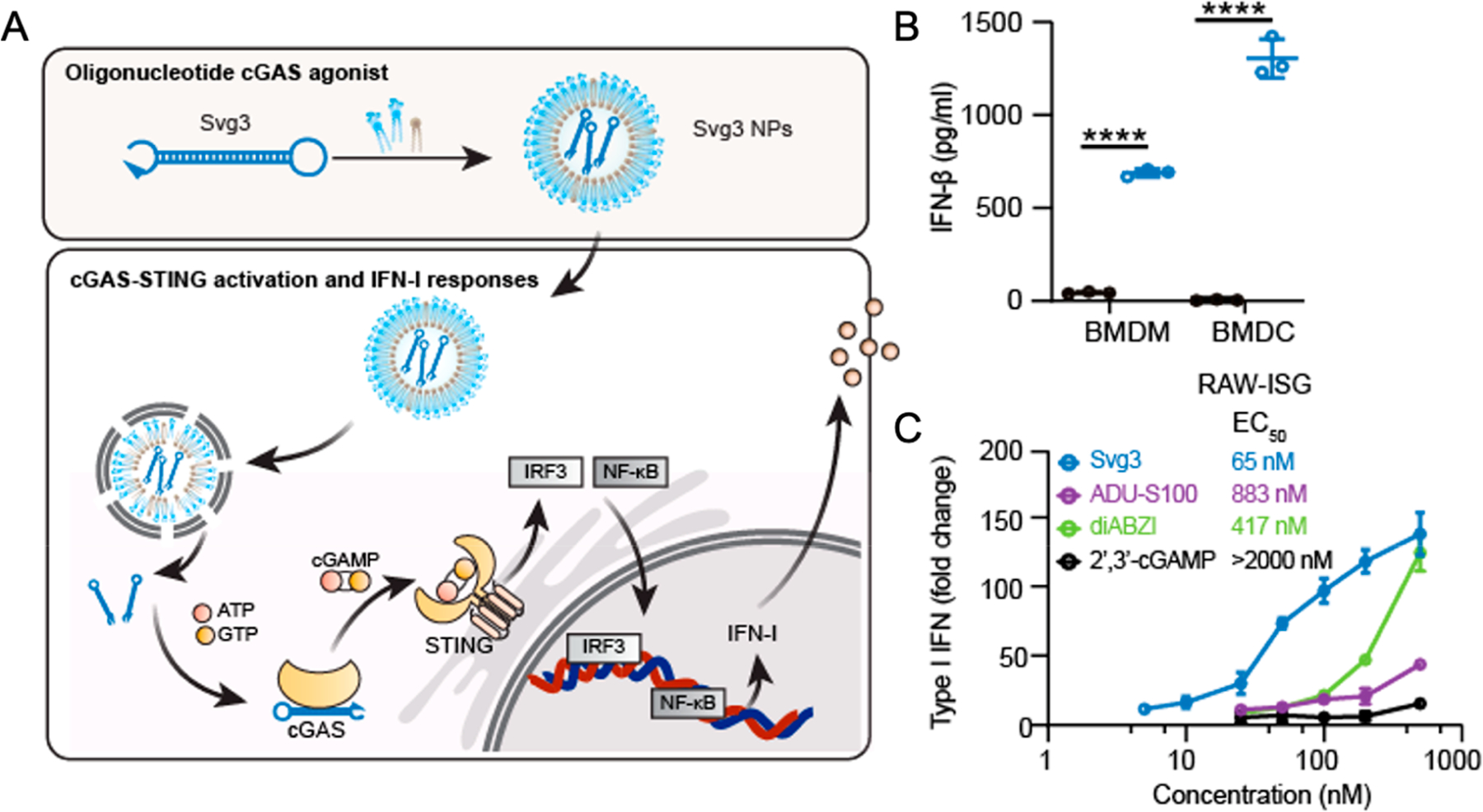
Developing novel oligonucleotide-based cGAS agonists for cancer immunotherapy. (A) Schematic illustration of Svg3 delivered via lipid-based nanoparticles as a cGAS agonist to activate the cGAS-STING signaling pathway. (B) IFN-I response induced by Svg3 stimulation in murine bone marrow-derived macrophages (BMDMs) and bone marrow-derived dendritic cells (BMDCs). (C) Comparison of IFN-I responses elicited by the cGAS agonist and benchmark STING agonists in murine macrophages. Adapted with permission from ref 3.
Svg3 had a high binding affinity with both mouse and human cGAS, which have structural differences. Previously, the complexes of cGAS with long dsDNA was shown to form cGAS–dsDNA condensates via a process of phase separation, which represents a significant biophysical feature of cGAS activation. We verified that Svg3 and cGAS binding formed cGAS–DNA protein condensates via phase separation. Svg3 induced strong IFN-I responses in murine (Figure 3B) and human immune cells, a variety of murine cancer cells, and surgically dissected human head and neck squamous cell carcinoma (HNSCC) tumor tissues. This is significant because it confirmed that Svg3 overcomes the interspecies heterogeneity of cGAS, providing the basis for the clinical translation of Svg3. Remarkably, Svg3 outperformed multiple current state-of-the-art STING agonists including 2′3′-cGAMP and CDN-derived ADU-S100 and diABZI (Figure 3C). The IFN-I responses elicited by Svg3 were cGAS-specific. While other DNA sensors such as AIM2 can also be activated by DNA, AIM2 activation requires dsDNA with a length above 80 bp. Indeed, we verified that Svg3 specifically activated cGAS, but not AIM2 inflammasome, which avoids potential confounding mechanisms. Lipid-based nanoparticles including liposomes and ionizable lipid nanoparticles efficiently delivered Svg3 at the cell and tissue levels and mediated efficient endosome escape for Svg3 delivery to the cytosol, where cGAS resides. Specifically, liposomal Svg3 prolonged the tumor retention of Svg3, relative to free Svg3, after intratumoral injection into 4T1 tumors in mice. Further, intratumoral administration of liposomal Svg3 synergized with immune checkpoint inhibitors to inhibit tumor growth, as shown in a 4T1 mammary carcinoma model and a B16F10 melanoma model in syngeneic mice. Moreover, Svg3 can also serve as a potent immunoadjuvant for peptide and protein antigens to induce antigen-specific immune responses, which were shown in mice using lipid nanoparticles that codelivered Svg3 with protein or peptide antigens. Compared to small molecule-based STING agonists, oligonucleotide-based cGAS agonists have the advantages of easy manufacturing, large-scale formulation by leveraging pre-existing well-established nucleic acid delivery systems, and the absence of human allele restriction for cGAS recognition.
cGAS-Inhibiting Drug Delivery Systems for RA Immunotherapy
While acute and localized activation of the cGAS-STING pathway often supports an adequate amount of immune activity for the elimination of disease, chronic cGAS activation by microbial DNA or self-DNA in the cytosol can cause the chronic and overly strong IFN-I, leading to a wide variety of disorders that are driven by inflammation.69 For example, overactivation of the cGAS-STING pathway in humans has been linked to autoimmune diseases including AGS, RA, and SLE.70 The emergence of small-molecule-based cGAS or STING inhibitors opened the door for the development of therapeutics targeting cGAS–STING pathway.71 For example, some cGAS inhibitors block cGAS in the active site for cGAMP production, thus competing with the ATP or GTP substrates; other cGAS inhibitors may compete with DNA for binding to cGAS, thus interfering with the initial activation of cGAS. STING inhibitors occupy the CDN binding site in STING, or bind to either the Cys88 or the Cys91 residues near the transmembrane domain of the STING that blocks the palmitoylation of STING and retains STING in a signaling incompetent state.72 However, these inhibitors are hydrophobic and have low bioavailability and limited intracellular delivery. In order to address these challenges, we loaded RU.521, a cGAS inhibitor, in cationic nanoparticles (cNPs) to fabricate nanomedicine cRNPs for RA treatment (Figure 4A). Next, nanomedicine cRNPs were loaded in hydrogel to again form nanoparticle-in-hydrogel (NiH) to prolong the release of cRNPs and RU.521. DIR was used as a model fluorescent probe to evaluate the ability of DIR-loaded cNPs-in-hydrogel (DIR/cNPs-H) for long LNs retention of drugs. DIR/cNPs-H and controls were s.c. injected at mouse tail base. Compared to free DIR, DIR/cNPs and DIR/cNPs-H significantly enhanced DIR accumulation in draining LNs 1–5 days after administration (Figure 4B,C). Specifically, relative to DIR/cNPs that showed rapid reduction of DIR fluorescence intensity after 2 days, DIR/cNPs-H prolonged DIR retention in draining LNs with 1.47- and 7.8-fold LN retention at day 5 relative to DIR/cNPs and free DIR, respectively. Consistently, on day 5, relative to DIR/cNPs, DIR/cNPs-H showed a 217% increase of DIR fluorescence intensity ratios in LNs over that in abdomen, indicating efficient drug delivery and retention in LNs as well as reduced systemic drug dissemination by cNPs-H (Figure 4D). On day 5, ex vivo imaging of resected LNs verified the efficient DIR retention in LNs by DIR/cNPs-H (Figure 4E).
Figure 4.
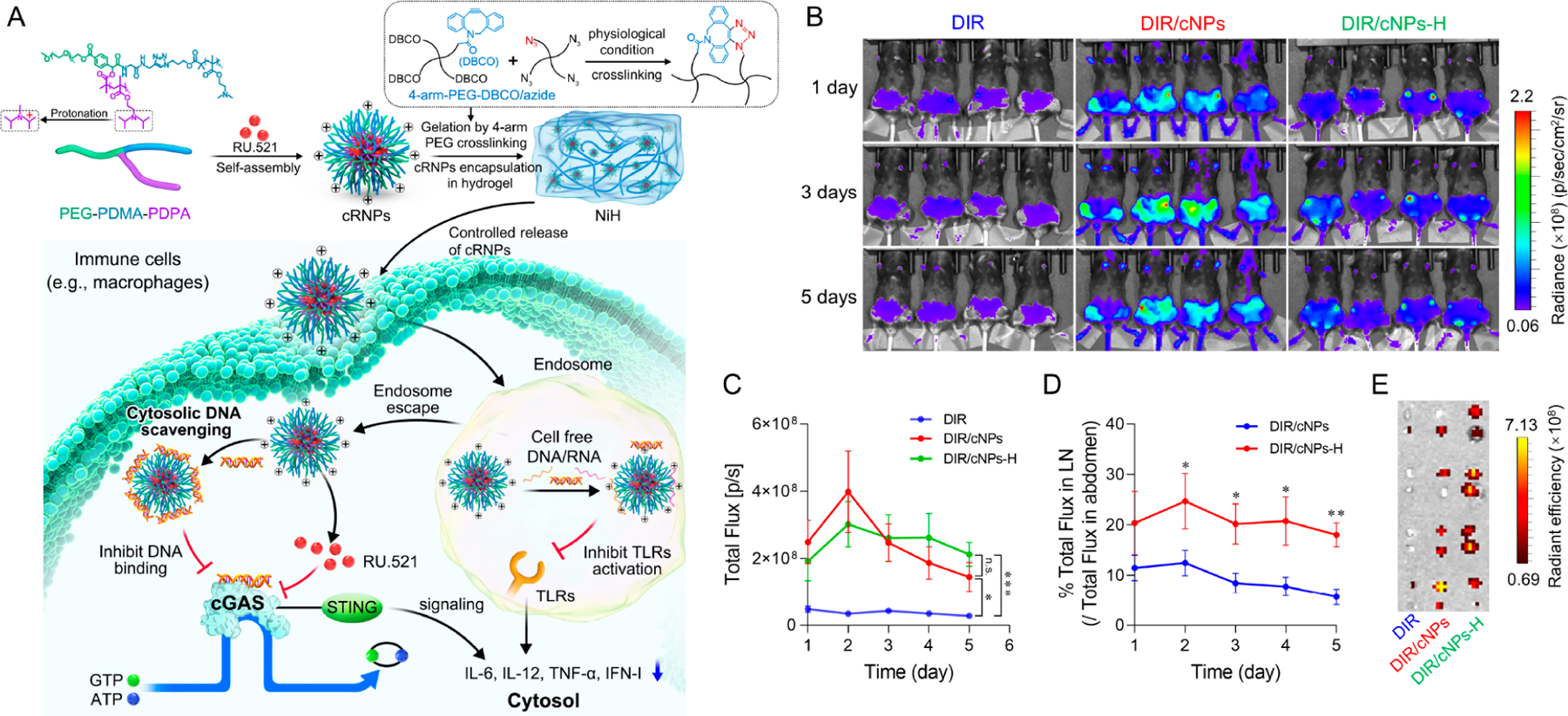
Injectable NiH codelivered cfDNA-scavenging cNPs and cGAS inhibitors to draining LNs for systemic immunosuppression in RA immunotherapy. (A) NiH is composed of injectable hydrogel encapsulated with RU-loaded cfDNA-scavenging cNPs (i.e., cRNPs). Upon sc injection, NiH prolonged the retention of cRNPs in draining LNs, where they concurrently scavenged cfDNA and pharmacologically inhibited cGAS, which inhibited cGAS activation and IFN-I responses. This results in systemic immunosuppression at the molecular and cellular levels, thereby inhibiting RA progression as shown in a mouse model. (B) IVIS images of C57BL/6 mice over 1–5 days after sc injection of DIR/cNPs-H and controls (n = 4). (C) Quantification of DIR fluorescence intensities in the draining inguinal LNs of the above mice. (D) The percentage of total fluorescence in LNs relative to that in abdomen from the above mice. (E) IVIS images of ex vivo LNs from the above mice 5 days after administration. Adapted with permission from ref 4. Copyright 2023 by Wiley-VCH GmbH.
cRNPs accumulated in draining lymph nodes and captured extracellular/internalized immunostimulatory DNA by cNPs while pharmacologically inhibiting cGAS by RU.521, resulting in decreased production of pro-inflammatory cytokines and reduced expression of costimulatory factors, both of which are essential to present antigens from APCs to naïve T or B cells to elicit antigen-specific effector T or B cells. As a result, we expect that antigen presentation without costimulatory signals would induce the apoptosis and deletion of T or B cells, which would inhibit the induction and autoantigen-specific adaptive immunity and hence promote immune tolerance. In a collagen-induced arthritis (CIA) mouse model, subcutaneous administration of NiH retarded RA progression and reduced arthritis severity due to the controlled drug release and prolonged drug retention in immunomodulatory tissues and cells. Flow cytometry analysis revealed that NiH repolarized systemic macrophages toward immunosuppression, as accompanied by reduced production of proinflammatory cytokines. Meanwhile, NiH decreased the percentage of CD4+ T cells and Th17 cells, while increasing systemic immunosuppressive Tregs, MDSCs, and M2/M1-like macrophage ratios. Interestingly, subcutaneous administration of the above NiH formulation reduced the proinflammatory responses and enhanced the immunoregulatory cell abundances, not only in draining lymph nodes but also systemically in peripheral blood. This can be particularly significant for the systemic immunotherapy of RA, which has been often treated by highly invasive and local intraarticular administration into the joints in current clinical practice.
Recently, Wang et al. developed polyethyleimine-polydopamine (PEI–PDA)@C-176 NPs for DNA adsorption and STING in RA therapy.73 In our study, we showed that, by local administration of our NiH, the combinatorial cGAS inhibition and cfDNA scavenging (and its resulting immunosuppression of the corresponding cfDNA-associated PRRs) efficiently suppressed proinflammation not only locally but also systemically, which is critical for the treatment of autoimmune diseases such as RA that are associated with systemic overly active proinflammatory responses.
DISCUSSION AND CONCLUSION
cGAS and STING are cytosolic PRRs that play important roles in various physiological and pathological conditions. The activation of the cGAS-STING signaling pathway results in IFN-I responses, which can be a double-edge sword. On one hand, enhancing IFN-I responses in some cell subsets (e.g., DCs, macrophages, and many types of tumor cells) may promote innate and adaptive antitumor immune responses. On the other hand, cGAS-STING overactivation has been associated with a number of autoimmune diseases, making cGAS-STING an appealing therapeutic target for the immunosuppressive treatment of these autoimmune diseases. Cytosolic delivery of cGAS or STING agonists is critical for the activation of intracellular cGAS and STING. Current approaches of cGAS-STING modulators rely on small molecules,74 primarily STING agonistic CDNs. Though more recent STING agonists such as ADU-S100 and diABZI exhibit great pharmacokinetic profiles and biostability with promising preclinical tumor therapeutic efficacy, their therapeutic efficacy and safety remain to be fully examined preclinically and clinically. Here, we summarize our recent attempt to advance the drug development for cGAS-STING immunomodulation by approaches such as novel drug delivery systems and targeting cGAS, instead of directly targeting STING, for the immunotherapy of cancer and autoimmune disorders.75 In some examples, we developed physiologically responsive drug delivery systems for efficient delivery and conditional release of STING agonists in robust cancer immunotherapy. Moreover, the drug development of CDNs, such as 2′3′-cGAMP, is largely limited by its limited biostability and poor cytosolic delivery. To address this issue, we developed oligonucleotide-based cGAS agonists that activate cGAS, resulting in the in situ production of 2′3′c-GAMP in the cytosol and thus bypassing the cellular uptake barrier for CDN delivery. Worth noting, we envision that oligonucleotide therapeutics, such as the above cGAS agonists, could leverage the current industry advancement of oligonucleotide therapeutics development through well-established large-scale cGMP manufacturing capacity, pre-existing oligonucleotide delivery systems, and versatile nucleotide modifications that could further improve the therapeutic efficacy and safety of these cGAS agonistic oligonucletoides.
To this end, first, we developed polymer-based drug delivery systems to improve the delivery of CDN-based STING agonists. We showed that these STING agonist delivery carriers improved antitumor responses and enhanced antitumor immunity in the tumor microenvironment. Second, to the end of cGAS-STING activation, cGAS remains an attractive alternative therapeutic target for the development of cGAS agonists that activate the cGAS-STING signaling pathway and elicit IFN-I responses. Pharmacological cGAS activation expands the repertoire of potential therapeutics, offering great opportunities to develop oligonucleotide-based cGAS agonists as immunotherapeutics or immunostimulatory vaccine adjuvants. While cGAS-STING activation in APCs, myeloid cells, and tumor cells often promote antitumor responses, cGAS-STING overactivation is associated with chronic inflammation and a number of autoimmune diseases. Thus, cGAS-STING emerges as a promising therapeutic target to suppress the associated pathogenic IFN-I responses for the immunotherapy of related autoimmune diseases. To this end, we attempted to develop multifunctional injectable nanoparticle-in-hydrogel (NiH) composites that codelivered dsDNA-scavenging cationic polymeric NPs and a cGAS pharmacological inhibitor for RA treatment. Upon subcutaneous injection at the tail base which is distant from RA disease sites in an RA mouse model, NiH prolonged the retention of the NPs in draining LNs. Interestingly, this system inhibited cGAS overreaction, reduced systemic proinflammatory responses, and induced systemic immunosuppression, not only locally in adjacent RA joints but also in distant RA joints. This essentially provides an approach to systemic RA treatment without invasive intraarticular injection as often used in current clinic practice. This study highlights the potential of cGAS-STING inhibition for the immunotherapy of related autoimmune diseases. Note that, even though the implications of cGAS-STING activation in various nonimmune cells and organs are beyond the scope of discussion in this Account, cGAS-STING may provide unique opportunities to uncover the fundamental biological mechanisms and develop prophylactic and therapeutic interventions for many other types of diseases. Overall, our studies so far have demonstrated the potential to develop drugs and drug delivery systems for the immunostimulatory therapy of many types of cancer as well as the immunosuppressive therapy of related autoimmune diseases.
ACKNOWLEDGMENTS
G.Z. acknowledges funding support for the related research from US National Institutes of Health (R01CA266981), American Cancer Society Research Scholar Grant (RSG-22-055-01-IBCD), and startup fund from University of Michigan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies
Shurong Zhou obtained her B.S. (2017) and M.S. degrees (2019) in Pharmaceutical Sciences from Peking University in China and is currently a Ph.D. student in Dr. Guizhi Zhu’s lab at University of Michigan. Her research interests focus on engineering and delivery of oligonucleotide and circular RNA therapeutics for cancer immunotherapy.
Furong Cheng received his B.S. (2013) in Biomedical Materials from Beijing University of Chemical Technology, China, and M.S. (2016) and Ph.D. (2019) degrees in Biomedical Engineering from Sichuan University, China. He conducted postdoctoral training with Dr. Guizhi Zhu. His current research interest is the development of nanocarriers for cancer immunotherapy. Dr. Cheng is now an Assistant Researcher at Shanghai Cancer Institute.
Yu Zhang received his B.S. (2011) in Chemistry from Hunan University, China, and Ph.D. degree (2016) in Inorganic Chemistry at the Lanzhou University, China. Dr. Zhang conducted postdoctoral training with Dr. Guizhi Zhu from 2019 to 2023. His research interests involve novel circular mRNA for cancer immunotherapy and infectious disease prophylaxis. He is currently Assistant Researcher at Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, China.
Ting Su received her B.S. degree (2012) in Materials Chemistry from Tianjin University and Ph.D. degree (2018) in Biomedical Engineering at Sichuan University, China. Then she conducted her postdoctoral training with Dr. Guizhi Zhu. Her research interests include the development of biomaterials and immunotherapeutics for drug delivery in cancer therapy.
Guizhi Zhu received his B.S. degrees (2008) in Biotechnology from Nankai University in China, and Ph.D. degree (2013) in Medical Sciences from the University of Florida, USA. Dr. Zhu conducted postdoctoral training at National Institutes of Health (NIH), USA. His research interests are engineering and delivery of nucleic acid therapeutics and vaccines for the immunotherapy and prophylaxis of cancer, infectious diseases, and autoimmune diseases. Dr. Zhu is currently Ara G. Paul Associate Professor of Pharmaceutical Sciences at University of Michigan.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.accounts.3c00394
The authors declare the following competing financial interest(s): All the authors were listed as inventors in related patent applications.
Contributor Information
Shurong Zhou, Department of Pharmaceutical Sciences, College of Pharmacy; Biointerfaces Institute, University of Michigan, Ann Arbor, Michigan 48109, United States.
Furong Cheng, State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200032, China.
Yu Zhang, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, Zhejiang 31002, China;.
Ting Su, Department of Pharmaceutics, School of Pharmacy, Virginia Commonwealth University, Richmond, Virginia 23298, United States;.
Guizhi Zhu, Department of Pharmaceutical Sciences, College of Pharmacy; Biointerfaces Institute, University of Michigan, Ann Arbor, Michigan 48109, United States;.
REFERENCES
- (1).Su T; Cheng F; Qi J; Zhang Y; Zhou S; Mei L; Fu S; Zhang F; Lin S; Zhu G Responsive Multivesicular Polymeric Nanovaccines That Codeliver STING Agonists and Neoantigens for Combination Tumor Immunotherapy. Adv. Sci 2022, 9 (23), 2201895. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, we reported a pH-responsive ionizable polymeric nanovaccines to codeliver STING agonists and neoantigen peptides for combination tumor immunotheraopy.
- (2).Cheng F; Su T; Zhou S; Liu X; Yang S; Lin S; Guo W; Zhu G Single-Dose Injectable Nanovaccine-in-Hydrogel for Robust Immunotherapy of Large Tumors with Abscopal Effect. Sci. Adv 2023, 9, No. eade6257. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper presented a single-dose injectable nanovaccine-in-hydrogel that codelivers triple immunostimulants, including STING agonists, and immune checkpoint inhibitors, to eradicate large tumors with abscopal effect.
- (3).Zhou S; Su T; Cheng F; Cole J; Liu X; Zhang B; Alam S; Liu J; Zhu G Engineering cGAS-agonistic oligonucleotides as therapeutics and vaccine adjuvants for cancer immunotherapy. bioRxiv 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reported the engineering of an oligonucleotide-based cGAS agonist as immunostimulant immunotherapeutics as well as adjuvants for peptide neoantigens for cancer combination immunotherapy.
- (4).Cheng F; Su T; Liu Y; Zhou S; Qi J; Guo W; Zhu G Targeting Lymph Nodes for Systemic Immunosuppression Using Cell-Free-DNA-Scavenging And CGAS-Inhibiting Nanomedicine-In-Hydrogel for Rheumatoid Arthritis Immunotherapy. Adv. Sci 2023, 10, 2302575. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper described NiH to codeliver a cGAS inhibitor RU.521 and cfDNA-scavenging cNPs for systemic immunosuppression in RA therapy.
- (5).Cai X; Chiu Y-H; Chen ZJ The CGAS-CGAMP-STING Pathway of Cytosolic DNA Sensing and Signaling. Mol. Cell 2014, 54 (2), 289–296. [DOI] [PubMed] [Google Scholar]
- (6).Liu N; Pang X; Zhang H; Ji P The CGAS-STING Pathway in Bacterial Infection and Bacterial Immunity. Front. Immunol 2022, 12, 814709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wang X; Zhang H; Li W DNA-Binding Mechanisms of Human and Mouse CGAS: A Comparative MD and MM/GBSA Study. Phys. Chem. Chem. Phys 2020, 22 (45), 26390–26401. [DOI] [PubMed] [Google Scholar]
- (8).Motwani M; Pesiridis S; Fitzgerald KA DNA Sensing by the CGAS-STING Pathway in Health and Disease. Nat. Rev. Genet 2019, 20 (11), 657–674. [DOI] [PubMed] [Google Scholar]
- (9).Glück S; Guey B; Gulen MF; Wolter K; Kang T-W; Schmacke NA; Bridgeman A; Rehwinkel J; Zender L; Ablasser A Innate Immune Sensing of Cytosolic Chromatin Fragments through CGAS Promotes Senescence. Nat. Cell Biol 2017, 19 (9), 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Huang Y-H; Liu X-Y; Du X-X; Jiang Z-F; Su X-D The Structural Basis for the Sensing and Binding of Cyclic Di-GMP by STING. Nat. Struct. Mol. Biol 2012, 19 (7), 728–730. [DOI] [PubMed] [Google Scholar]
- (11).Zhang X; Bai X; Chen ZJ Structures and Mechanisms in the CGAS-STING Innate Immunity Pathway. Immunity 2020, 53 (1), 43–53. [DOI] [PubMed] [Google Scholar]
- (12).Gray PM; Forrest G; Wisniewski T; Porter G; Freed DC; DeMartino JA; Zaller DM; Guo Z; Leone J; Fu T-M; Vora KA Evidence for Cyclic Diguanylate as a Vaccine Adjuvant with Novel Immunostimulatory Activities. Cell. Immunol 2012, 278 (1–2), 113–119. [DOI] [PubMed] [Google Scholar]
- (13).Diamond MS; Kinder M; Matsushita H; Mashayekhi M; Dunn GP; Archambault JM; Lee H; Arthur CD; White JM; Kalinke U; Murphy KM; Schreiber RD Type I Interferon Is Selectively Required by Dendritic Cells for Immune Rejection of Tumors. J. Exp. Med 2011, 208 (10), 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zhou Y; Fei M; Zhang G; Liang W-C; Lin W; Wu Y; Piskol R; Ridgway J; McNamara E; Huang H; Zhang J; Oh J; Patel JM; Jakubiak D; Lau J; Blackwood B; Bravo DD; Shi Y; Wang J; Hu H-M; Lee WP; Jesudason R; Sangaraju D; Modrusan Z; Anderson KR; Warming S; Roose-Girma M; Yan M Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2 × 7R-Dependent STING Activation by Tumor-Derived CGAMP. Immunity 2020, S107476132030042X. [DOI] [PubMed] [Google Scholar]
- (15).Vinuesa CG; Linterman MA; Yu D; MacLennan ICM Follicular Helper T Cells. Annu. Rev. Immunol 2016, 34, 335–368. [DOI] [PubMed] [Google Scholar]
- (16).An J; Woodward JJ; Sasaki T; Minie M; Elkon KB Cutting Edge: Antimalarial Drugs Inhibit IFN-β Production through Blockade of Cyclic GMP-AMP Synthase-DNA Interaction. J. Immunol 2015, 194 (9), 4089–4093. [DOI] [PubMed] [Google Scholar]
- (17).Fuertes MB; Kacha AK; Kline J; Woo S-R; Kranz DM; Murphy KM; Gajewski TF Host Type I IFN Signals Are Required for Antitumor CD8+ T Cell Responses through CD8α+ Dendritic Cells. J. Exp. Med 2011, 208 (10), 2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chen Q; Boire A; Jin X; Valiente M; Er EE; Lopez-Soto A; Jacob LS; Patwa R; Shah H; Xu K; Cross JR; Massagué J Carcinoma-Astrocyte Gap Junctions Promote Brain Metastasis by CGAMP Transfer. Nature 2016, 533 (7604), 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chen D; Tong J; Yang L; Wei L; Stolz DB; Yu J; Zhang J; Zhang L PUMA Amplifies Necroptosis Signaling by Activating Cytosolic DNA Sensors. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (15), 3930–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang-Bishop L; Wehbe M; Shae D; James J; Hacker BC; Garland K; Chistov PP; Rafat M; Balko JM; Wilson JT Potent STING Activation Stimulates Immunogenic Cell Death to Enhance Antitumor Immunity in Neuroblastoma. J. Immunother. Cancer 2020, 8 (1), No. e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Murthy AMV; Robinson N; Kumar S Crosstalk between CGAS-STING Signaling and Cell Death. Cell Death Differ. 2020, 27 (11), 2989–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hashimoto T; Yoshida K; Hashiramoto A; Matsui K Cell-Free DNA in Rheumatoid Arthritis. Int. J. Mol. Sci 2021, 22 (16), 8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gao D; Li T; Li X-D; Chen X; Li Q-Z; Wight-Carter M; Chen ZJ Activation of Cyclic GMP-AMP Synthase by Self-DNA Causes Autoimmune Diseases. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Domizio JD; Gulen MF; Saidoune F; Thacker VV; Yatim A; Sharma K; Nass T; Guenova E; Schaller M; Conrad C; Goepfert C; de Leval L; von Garnier C; Berezowska S; Dubois A; Gilliet M; Ablasser A The CGAS-STING Pathway Drives Type I IFN Immunopathology in COVID-19. Nature 2022, 603 (7899), 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Le Naour J; Zitvogel L; Galluzzi L; Vacchelli E; Kroemer G Trial Watch: STING Agonists in Cancer Therapy. OncoImmunology 2020, 9 (1), 1777624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Pryde DC; Middya S; Banerjee M; Shrivastava R; Basu S; Ghosh R; Yadav DB; Surya A The Discovery of Potent Small Molecule Activators of Human STING. Eur. J. Med. Chem 2021, 209, 112869. [DOI] [PubMed] [Google Scholar]
- (27).Lu D; Shang G; Li J; Lu Y; Bai X; Zhang X Activation of STING by Targeting a Pocket in the Transmembrane Domain. Nature 2022, 604 (7906), 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Miao L; Li L; Huang Y; Delcassian D; Chahal J; Han J; Shi Y; Sadtler K; Gao W; Lin J; Doloff JC; Langer R; Anderson DG Delivery of MRNA Vaccines with Heterocyclic Lipids Increases Anti-Tumor Efficacy by STING-Mediated Immune Cell Activation. Nat. Biotechnol 2019, 37, 1174. [DOI] [PubMed] [Google Scholar]
- (29).Li S; Luo M; Wang Z; Feng Q; Wilhelm J; Wang X; Li W; Wang J; Cholka A; Fu Y; Sumer BD; Yu H; Gao J Prolonged Activation of Innate Immune Pathways by a Polyvalent STING Agonist. Nat. Biomed. Eng 2021, 5 (5), 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Luo M; Wang H; Wang Z; Cai H; Lu Z; Li Y; Du M; Huang G; Wang C; Chen X; Porembka MR; Lea J; Frankel AE; Fu Y-X; Chen ZJ; Gao J A STING-Activating Nanovaccine for Cancer Immunotherapy. Nat. Nanotechnol 2017, 12 (7), 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Marill J; Mohamed Anesary N; Paris S DNA Damage Enhancement by Radiotherapy-Activated Hafnium Oxide Nanoparticles Improves CGAS-STING Pathway Activation in Human Colorectal Cancer Cells. Radiother. Oncol 2019, 141, 262–266. [DOI] [PubMed] [Google Scholar]
- (32).McLaughlin M; Patin EC; Pedersen M; Wilkins A; Dillon MT; Melcher AA; Harrington KJ Inflammatory Microenvironment Remodelling by Tumour Cells after Radiotherapy. Nat. Rev. Cancer 2020, 20 (4), 203–217. [DOI] [PubMed] [Google Scholar]
- (33).Parkes EE; Savage KI; Lioe T; Boyd C; Halliday S; Walker SM; Lowry K; Knight L; Buckley NE; Grogan A; Logan GE; Clayton A; Hurwitz J; Kirk SJ; Xu J; Sidi FA; Humphries MP; Bingham V; Ang M; Askin C; Bamford L; Boyd R; Buckley M; Clarke J; Darragh L; Davis E; Foreman J; Gallagher R; Gill J; Hanna M; Hill N; Irwin G; Mallon P; McAleer S; McAllister J; Morris M; Pierce N; Refsum S; Sloan S; Treanor S; James JA; James CR; Paul Harkin D; Kennedy RD; McIntosh SA Neo-DDIR Investigators. Activation of a CGAS-STING-Mediated Immune Response Predicts Response to Neoadjuvant Chemotherapy in Early Breast Cancer. Br. J. Cancer 2022, 126 (2), 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Liang H; Peng B; Dong C; Liu L; Mao J; Wei S; Wang X; Xu H; Shen J; Mao H-Q; Gao X; Leong KW; Chen Y Cationic Nanoparticle as an Inhibitor of Cell-Free DNA-Induced Inflammation. Nat. Commun 2018, 9 (1), 4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lee J; Sohn JW; Zhang Y; Leong KW; Pisetsky D; Sullenger BA Nucleic Acid-Binding Polymers as Anti-Inflammatory Agents. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (34), 14055–14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Xie B; Du K; Huang F; Lin Z; Wu L Cationic Nanomaterials for Autoimmune Diseases Therapy. Front. Pharmacol 2022, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wiser C; Kim B; Vincent J; Ascano M Small Molecule Inhibition of Human CGAS Reduces Total CGAMP Output and Cytokine Expression in Cells. Sci. Rep 2020, 10 (1), 7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Vincent J; Adura C; Gao P; Luz A; Lama L; Asano Y; Okamoto R; Imaeda T; Aida J; Rothamel K; Gogakos T; Steinberg J; Reasoner S; Aso K; Tuschl T; Patel DJ; Glickman JF; Ascano M Small Molecule Inhibition of CGAS Reduces Interferon Expression in Primary Macrophages from Autoimmune Mice. Nat. Commun 2017, 8 (1), 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lama L; Adura C; Xie W; Tomita D; Kamei T; Kuryavyi V; Gogakos T; Steinberg JI; Miller M; Ramos-Espiritu L; Asano Y; Hashizume S; Aida J; Imaeda T; Okamoto R; Jennings AJ; Michino M; Kuroita T; Stamford A; Gao P; Meinke P; Glickman JF; Patel DJ; Tuschl T Development of Human CGAS-Specific Small-Molecule Inhibitors for Repression of DsDNAT-riggered Interferon Expression. Nat. Commun 2019, 10 (1), 2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Skopelja-Gardner S; An J; Elkon KB Role of the CGAS-STING Pathway in Systemic and Organ-Specific Diseases. Nat. Rev. Nephrol 2022, 18 (9), 558–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Shae D; Becker KW; Christov P; Yun DS; Lytton-Jean AKR; Sevimli S; Ascano M; Kelley M; Johnson DB; Balko JM; Wilson JT Endosomolytic Polymersomes Increase the Activity of Cyclic Dinucleotide STING Agonists to Enhance Cancer Immunotherapy. Nat. Nanotechnol 2019, 14 (3), 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lu X; Miao L; Gao W; Chen Z; McHugh KJ; Sun Y; Tochka Z; Tomasic S; Sadtler K; Hyacinthe A; Huang Y; Graf T; Hu Q; Sarmadi M; Langer R; Anderson DG; Jaklenec A Engineered PLGA Microparticles for Long-Term, Pulsatile Release of STING Agonist for Cancer Immunotherapy. Sci. Transl. Med 2020, 12 (556), No. eaaz6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Draghici B; Ilies MA Synthetic Nucleic Acid Delivery Systems: Present and Perspectives. J. Med. Chem 2015, 58 (10), 4091–4130. [DOI] [PubMed] [Google Scholar]
- (44).Paunovska K; Loughrey D; Dahlman JE Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet 2022, 23 (5), 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Dong Y; Siegwart DJ; Anderson DG Strategies, Design, and Chemistry in SiRNA Delivery Systems. NanoDDS 2018 Eng. Wave Nanomedicine Drug Delivery Syst 2019, 144, 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Li Y; Maciel D; Rodrigues J; Shi X; Tomás H Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev 2015, 115 (16), 8564–8608. [DOI] [PubMed] [Google Scholar]
- (47).Su T; Zhang Y; Valerie K; Wang X-Y; Lin S; Zhu G STING Activation in Cancer Immunotherapy. Theranostics 2019, 9 (25), 7759–7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Van Herck S; Feng B; Tang L Delivery of STING Agonists for Adjuvanting Subunit Vaccines. Adv. Drug Delivery Rev 2021, 179, 114020. [DOI] [PubMed] [Google Scholar]
- (49).Koshy ST; Cheung AS; Gu L; Graveline AR; Mooney DJ Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Adv. Biosyst 2017, 1 (1–2), 1600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zheng Y-F; Wu J-J Overcoming STING Agonists Barriers: Peptide, Protein, and Biomembrane-Based Biocompatible Delivery Strategies. Chem. - Asian J 2022, 17 (6), No. e202101400. [DOI] [PubMed] [Google Scholar]
- (51).Chen S; Peng A; Chen M; Zhan M Nanomedicines Targeting Activation of STING to Reshape Tumor Immune Microenvironment and Enhance Immunotherapeutic Efficacy. Front. Oncol 2023, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Su M; Zheng J; Gan L; Zhao Y; Fu Y; Chen Q Second Messenger 2′3′-Cyclic GMP-AMP (2′3′-CGAMP): Synthesis, Transmission, and Degradation. Biochem. Pharmacol 2022, 198, 114934. [DOI] [PubMed] [Google Scholar]
- (53).Zhou Q; Zhou Y; Li T; Ge Z Nanoparticle-Mediated STING Agonist Delivery for Enhanced Cancer Immunotherapy. Macromol. Biosci 2021, 21 (8), 2100133. [DOI] [PubMed] [Google Scholar]
- (54).Doshi AS; Cantin S; Prickett LB; Mele DA; Amiji M Systemic Nano-Delivery of Low-Dose STING Agonist Targeted to CD103+ Dendritic Cells for Cancer Immunotherapy. J. Controlled Release 2022, 345, 721–733. [DOI] [PubMed] [Google Scholar]
- (55).Gadkaree SK; Fu J; Sen R; Korrer MJ; Allen C; Kim YJ Induction of Tumor Regression by Intratumoral STING Agonists Combined with Anti-Programmed Death-L1 Blocking Antibody in a Preclinical Squamous Cell Carcinoma Model. Head Neck 2017, 39 (6), 1086–1094. [DOI] [PubMed] [Google Scholar]
- (56).Corrales L; Glickman LH; McWhirter SM; Kanne DB; Sivick KE; Katibah GE; Woo S-R; Lemmens E; Banda T; Leong JJ; Metchette K; Dubensky TW; Gajewski TF Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11 (7), 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Meric-Bernstam F; Sweis RF; Hodi FS; Messersmith WA; Andtbacka RHI; Ingham M; Lewis N; Chen X; Pelletier M; Chen X; Wu J; Dubensky TW; McWhirter SM; Müller T; Nair N; Luke JJ Phase I Dose-Escalation Trial of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients with Advanced/Metastatic Solid Tumors or Lymphomas. Clin. Cancer Res 2022, 28 (4), 677–688. [DOI] [PubMed] [Google Scholar]
- (58).Meric-Bernstam F; Sweis RF; Kasper S; Hamid O; Bhatia S; Dummer R; Stradella A; Long GV; Spreafico A; Shimizu T; Steeghs N; Luke JJ; McWhirter SM; Müller T; Nair N; Lewis N; Chen X; Bean A; Kattenhorn L; Pelletier M; Sandhu S Combination of the STING Agonist MIW815 (ADU-S100) and PD-1 Inhibitor Spartalizumab in Advanced/Metastatic Solid Tumors or Lymphomas: An Open-Label, Multicenter, Phase Ib Study. Clin. Cancer Res 2023, 29 (1), 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Zhang Y; Shen T; Zhou S; Wang W; Lin S; Zhu G PH-Responsive STING-Activating DNA Nanovaccines for Cancer Immunotherapy. Adv. Ther 2020, 3 (9), 2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Jafari M; Soltani M; Naahidi S; Karunaratne DN; Chen P Nonviral Approach for Targeted Nucleic Acid Delivery. Curr. Med. Chem 2012, 19 (2), 197–208. [DOI] [PubMed] [Google Scholar]
- (61).Sung YK; Kim SW Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res 2020, 24 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Zhu S; Zhang T; Zheng L; Liu H; Song W; Liu D; Li Z; Pan C Combination Strategies to Maximize the Benefits of Cancer Immunotherapy. J. Hematol. Oncol 2021, 14 (1), 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Re F; Strominger JL Heterogeneity of TLR-Induced Responses in Dendritic Cells: From Innate to Adaptive Immunity. Immunobiology 2004, 209 (1), 191–198. [DOI] [PubMed] [Google Scholar]
- (64).Marabelle A; Tselikas L; de Baere T; Houot R Intratumoral Immunotherapy: Using the Tumor as the Remedy. New Front. Immunother 2017, 28, xii33–xii43. [DOI] [PubMed] [Google Scholar]
- (65).Chen J; Qiu M; Ye Z; Nyalile T; Li Y; Glass Z; Zhao X; Yang L; Chen J; Xu Q In Situ Cancer Vaccination Using Lipidoid Nanoparticles. Sci. Adv 2021, 7 (19), No. eabf1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Lizotte PH; Wen AM; Sheen MR; Fields J; Rojanasopondist P; Steinmetz NF; Fiering S In Situ Vaccination with Cowpea Mosaic Virus Nanoparticles Suppresses Metastatic Cancer. Nat. Nanotechnol 2016, 11 (3), 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Sun X; Zhang Y; Li J; Park KS; Han K; Zhou X; Xu Y; Nam J; Xu J; Shi X; Wei L; Lei YL; Moon JJ Amplifying STING Activation by Cyclic Dinucleotide-Manganese Particles for Local and Systemic Cancer Metalloimmunotherapy. Nat. Nanotechnol 2021, 16 (11), 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Du M; Chen ZJ DNA-Induced Liquid Phase Condensation of CGAS Activates Innate Immune Signaling. Science 2018, 361 (6403), 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Decout A; Katz JD; Venkatraman S; Ablasser A The CGAS-STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol 2021, 21 (9), 548–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Duvvuri B; Lood C Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front. Immunol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Ding C; Song Z; Shen A; Chen T; Zhang A Small Molecules Targeting the Innate Immune CGAS-STING-TBK1 Signaling Pathway. Acta Pharm. Sin. B 2020, 10 (12), 2272–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Hall J; Brault A; Vincent F; Weng S; Wang H; Dumlao D; Aulabaugh A; Aivazian D; Castro D; Chen M; et al. Discovery of PF-06928215 as a High Affinity Inhibitor of CGAS Enabled by a Novel Fluorescence Polarization Assay. PloS One 2017, 12 (9), No. e0184843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Padilla-Salinas R; Sun L; Anderson R; Yang X; Zhang S; Chen ZJ; Yin H Discovery of Small-Molecule Cyclic GMP-AMP Synthase Inhibitors. J. Org. Chem 2020, 85 (3), 1579–1600. [DOI] [PubMed] [Google Scholar]
- (74).Haag SM; Gulen MF; Reymond L; Gibelin A; Abrami L; Decout A; Heymann M; van der Goot FG; Turcatti G; Behrendt R; Ablasser A Targeting STING with Covalent Small-Molecule Inhibitors. Nature 2018, 559 (7713), 269–273. [DOI] [PubMed] [Google Scholar]
- (75).Shen H; Jin L; Zheng Q; Ye Z; Cheng L; Wu Y; Wu H; Jon TG; Liu W; Pan Z; Mao Z; Wang Y Synergistically Targeting Synovium STING Pathway for Rheumatoid Arthritis Treatment. Bioact. Mater 2023, 24, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


