Abstract
目的
探索携川芎嗪导电水凝胶(简称“TGTP”)修复脊髓损伤(spinal cord injury,SCI)的潜在机制。
方法
将72只雌性SD大鼠随机分为4组,假手术组(A组)、SCI组(B组)、SCI+导电水凝胶组(C组)和SCI+TGTP组(D组)。A组仅切除椎板,其余组构建切除2 mm长脊髓的全横断模型。通过BBB评分法和改良Rivlin-Tator斜板实验分别于术前及术后1、3、7、14、28 d评估大鼠后肢运动功能恢复情况。术后7、28 d动物取材,通过免疫荧光染色观察新生血管,采用Western blot评估血管生成素1(angiopoietin 1,Ang-1)、Tie-2蛋白表达;术后28 d采用Western blot进一步评估促血管新生相关蛋白PDGF-B、PDGF受体β(PDGF receptor β,PDGFR-β)、VEGF-A、VEGF受体2(VEGF receptor 2,VEGFR-2)的表达情况。术后28 d,采用Masson染色评估损伤区域瘢痕增生,Nissl染色观察神经元存活,LFB染色检测髓鞘分布和再生情况,免疫荧光染色和Western blot评估神经丝蛋白200(neurofilament 200,NF200)的表达。
结果
各组大鼠术后3 d起后肢运动功能逐渐恢复,D组术后3~28 d BBB评分和斜坡角度显著高于B组,术后14、28 d显著高于C组(P<0.05)。Masson染色示B~D组胶原容积分数显著高于A组,D组显著低于B、C组(P<0.05);D组断端可见少量散在黑色导电颗粒,周围胶原纤维成分少于C组。Nissl和LFB染色示D组损伤区域脊髓组织神经元及髓鞘结构相对完整、连续,尼氏体数量和髓鞘阳性表达面积明显优于B、C组(P<0.05)。NF200免疫荧光染色和Western blot检测示,D组损伤区域NF200蛋白相对表达量显著优于B、C组(P<0.05)。CD31免疫荧光染色示D组术后28 d荧光强度优于B、C组,可见管状或线性新生血管影。Western blot检测示术后7 d和28 d D组Ang-1、Tie-2蛋白相对表达量高于B、C组,差异均有统计学意义(P<0.05)。术后28 d D组PDGF-B、PDGFR-β蛋白相对表达量显著高于B、C组,B组显著高于C组,差异均有统计学意义(P<0.05)。术后28 d D组VEGF-A和VEGFR-2蛋白相对表达量优于B、C组,与B组比较差异有统计学意义(P<0.05),但与C组比较仅VEGF-A蛋白差异有统计学意义(P<0.05);B、C组间比较仅VEGFR-2蛋白差异有统计学意义(P<0.05)。
结论
TGTP可能增强损伤区域再血管化,同时发挥神经元保护作用,从而减轻脊髓组织结构损伤,促进大鼠SCI后神经功能恢复。
Keywords: 脊髓损伤, 川芎嗪, 水凝胶, 神经保护, 血管新生
Abstract
Objective
To explore the mechanisms for repairing spinal cord injury (SCI) with tetramethylpyrazine-loaded electroconductive hydrogel (hereinafter referred to as “TGTP”).
Mehtods
A total of 72 female Sprague-Dawley rats were randomly divided into 4 groups: sham operation group (group A), SCI group (group B), SCI+electroconductive hydrogel group (group C), and SCI+TGTP group (group D). Only the vertebral plate was removed in group A, while the remaining groups were subjected to a whole transection model of spinal cord with a 2 mm gap in the lesions. The recovery of hindlimb motor function was evaluated by Basso, Beattie, Bresnahan (BBB) score and modified Rivlin-Tator inclined plate test before operation and at 1, 3, 7, 14, and 28 days after operation, respectively. Animals were sacrificed at 7 days and 28 days after modeling. Neovascularisation was observed by immunofluorescence staining of CD31 and the expression levels of angiopoietin 1 (Ang-1) and Tie-2 were assessed by Western blot assay. At 28 days postoperatively, the expression levels of pro-angiogenic related proteins, including platelet-derived growth factor B (PDGF-B), PDGF receptor β (PDGFR-β), vascular endothelial growth factor A (VEGF-A), and VEGF receptor 2 (VEGFR-2), were also assessed by Western blot. The fibrous scar in the injured area was assessed using Masson staining, while neuronal survival was observed through Nissl staining. Furthermore, LFB staining was utilized to detect myelin distribution and regeneration. Immunofluorescence and Western blot assay were employed to evaluate the expression of neurofilament 200 (NF200).
Results
The hindlimb motor function of rats in each group gradually recovered from the 3rd day after operation. The BBB score and climbing angle in group D were significantly higher than those in group B from 3 to 28 days after operation, and significantly higher than those in group C at 14 days and 28 days after operation (P<0.05). Masson staining showed that the collagen volume fraction in groups B-D were significantly higher than that in group A, and that in group D was significantly lower than that in groups B and C (P<0.05); a small amount of black conductive particles were scattered at the broken end in group D, and the surrounding collagen fibers were less than those in group C. Nissl and LFB staining showed that the structure of neurons and myelin sheath in the injured area of spinal cord in group D was relatively complete and continuous, and the number of Nissl bodies and the positive area of myelin sheath in group D were significantly better than those in groups B and C (P<0.05). NF200 immunofluorescence staining and Western blot assay results showed that the relative expression of NF200 protein in group D was significantly higher than that in groups B and C (P<0.05). CD31 immunofluorescence staining showed that the fluorescence intensity of group D was better than that of groups B and C at 28 days after operation, and tubular or linear neovascularization could be seen. The relative expressions of Ang-1 and Tie-2 proteins in group D were significantly higher than those in groups B and C at 7 and 28 days after operation (P<0.05). The relative expressions of PDGF-B and PDGFR-β proteins in group D were significantly higher than those in groups B and C, and group B was significantly higher than group C at 28 days after operation (P<0.05). The relative expressions of VEGF-A and VEGFR2 proteins in group D were higher than those in groups B and C, showing significant difference when compared with group B (P<0.05), but only the expression of VEGF-A protein was significantly higher than that in group C (P<0.05). There was significant difference only in VEGFR-2 protein between groups B and C (P<0.05).
Conclusion
TGTP may enhance the revascularization of the injured area and protect the neurons, thus alleviating the injury of spinal cord tissue structure and promoting the recovery of neurological function after SCI in rats.
Keywords: Spinal cord injury, tetramethylpyrazine, hydrogel, neuroprotection, angiogenesis
脊髓损伤(spinal cord injury,SCI)病理机制复杂,尤以继发性损伤为甚,如细胞程序性死亡、轴突脱髓鞘、炎症级联反应等。其中,微血管再生不足是限制神经组织再生和运动功能恢复不良的主要病理因素[1-2]。研究证实,SCI损伤后急性期,邻近区域灰质内内皮细胞特异性抗体标记的微血管密度持续性下降,损伤区域几乎无微血管存在,并伴有大量神经细胞死亡,其损伤程度与SCI严重程度密切相关[3-5]。损伤后慢性期,内源性微血管再生不足,形成无完整血管结构和功能的网络,具有高通透性及灌流不足等特征,加剧继发性损伤[5-6]。相关研究表明,损伤区域血管密度与神经功能的改善程度有密切关系,通过改善毛细血管血流量和促进血管新生可增强SCI后功能恢复[7-8]。同时,血管可能作为“支架”诱导受伤后轴突再生[9]。因此,增强微血管再生及重塑血管网络对于神经修复具有重要意义。
我们前期研究发现川芎嗪(tetramethylpyrazine,TMP)具有促血管新生和神经保护等多种药理作用[10-12],然而受限于给药途径和较低的生物利用率问题,导致其疗效不显著[12-13]。基于生物材料为载体的组合式手段可能是提高药物生物利用度,协同促进损伤区域再血管化,从而增强SCI修复效果的有效方式[14]。研究表明,以甲基丙烯酰化明胶(gelatin methacryloyl,GelMA)-聚吡咯(polypyrrole,Ppy)颗粒材料构建的导电水凝胶(简称“GTP”)通过仿细胞外基质结构,不仅具备与脊髓组织相匹配的力学特性,能较好地填充神经损伤后的空洞缺损,而且具有优良的导电性能,可诱导细胞黏附及迁移,为神经血管再生提供了良好微环境[15-16];还具有独特的三维网状结构可携载药物或种子细胞,局部缓释,调控继发性损伤。据此,我们将其作为载体,制备了携TMP的导电水凝胶(简称“TGTP”),初步研究证实其能实现药物局部富集与缓释,促进SCI大鼠运动功能恢复,但具体机制有待深入研究[17]。本研究拟以此为切入点,在前期研究基础上进一步深入探索TGTP修复SCI的作用机制。
1. 材料与方法
1.1. 实验动物及主要试剂、仪器
8周龄雌性SD大鼠72只,体质量220~240 g,由中国中医科学院骨伤研究所提供,造模前在标准屏障实验室(22℃,40%~50%湿度,12 h光/暗循环)适应性喂养1周,4只/笼,自由饮食,不限制活动。
吡咯单体、甲基丙烯酸酐、光引发剂Irgacure2959(Sigma公司,美国);明胶、六水合氯化铁(上海阿拉丁生化科技股份有限公司);盐酸TMP注射液(哈尔滨三联药业股份有限公司);PDGF-B抗体、CD31抗体、VEGF-A抗体(Abcam公司,美国);PDGF受体β(PDGF receptor β,PDGFR-β)抗体(Santa Cruz公司,美国);Tie-2(一种具有免疫球蛋白和EGF同源结构域的酪氨酸激酶受体)抗体、VEGF受体2(VEGF receptor 2,VEGFR-2)抗体、血管生成素1(angiopoietin 1,Ang-1)抗体(武汉三鹰生物技术有限公司);神经丝蛋白200(neurofilament 200,NF200;CST公司,美国)。激光共聚焦显微镜、倒置相差显微镜(Leica公司,德国)。
1.2. GTP和TGTP制备
TGTP构建方法与前期文献报道一致[17]。将适量光引发剂Irgacure2959和500 μg/mL盐酸TMP注射液在避光条件下共混,60℃水浴加热后,添加适量GelMA、Ppy等化合物;震荡混匀后再次水浴加热至完全溶解。避光条件下使用移液枪将混合液注入自制模具中,通过紫外线(6.9 mW/cm2,360~480 nm)光聚合制备TGTP;并将其浸泡于4℃ 75%乙醇溶液中消除凝胶内杂质,备用。GTP的制备除未添加盐酸TMP注射液外,其余步骤与TGTP一致。
1.3. 动物分组及造模方法
将72只SD大鼠通过随机数字表法分为假手术组(A组)、SCI模型组(B组)、SCI+GTP组(C组)和SCI+TGTP(D组),每组18只。术前所有大鼠均禁食、水8 h,造模方式与前期文献报道一致[17-18]。主要步骤:大鼠称重,以0.3%戊巴比妥(30 mg/kg)腹腔注射麻醉后,备皮消毒,俯卧位固定于手术台。以T9和T10的棘突为中心,依次分离皮肤、筋膜、肌肉层,暴露对应节段椎板和关节突关节,使用椎板钳沿上、下椎体间隙咬除T9、T10椎板,暴露脊髓。A组仅切除T9、T10椎板,其余3组使用自制显微双刃剪切除2 mm长脊髓,其中C、D组在损伤部位植入对应的水凝胶。分层缝合,闭合切口。术后每日3次进行膀胱按摩,帮助大鼠排尿、排便。
1.4. 观测指标
1.4.1. 行为学评分
采用BBB评分法和改良Rivlin-Tator斜板实验[19]评估大鼠术前及术后1、3、7、14、28 d后肢运动功能恢复情况。评估时间点固定于19∶00,大鼠自由活动;采用双人双盲法,观察大鼠5 min内的运动情况,评估3次,取均值。
1.4.2. 组织学染色观察
术后28 d每组取6只大鼠,同上法麻醉后,经4%多聚甲醛心脏灌注后,以损伤区域为中心取1.0 cm长脊髓组织,标注头、尾端。以4%多聚甲醛浸泡过夜,修块、脱水、石蜡包埋,制备5 μm厚切片,常规行组织学染色观察。① Masson染色观察损伤区域瘢痕增生情况,并行胶原容积分数半定量分析;② Nissl染色观察神经元存活情况,并行尼氏体数量半定量分析;③ LFB染色观察髓鞘分布和再生情况,并行髓鞘阳性表达面积半定量分析。
1.4.3. 免疫荧光染色观察
术后7 d同上法取材,制备脊髓切片,并取上述术后28 d部分切片脱蜡、乙醇梯度脱水后,EDTA修复液抗原修复。0.2%Triton X-100溶液避光条件下透膜10 min,10%FBS室温封闭1 h。去除封闭液后,滴加CD31(1∶2 000)、NF200(1∶200)一抗,4℃过夜。PBS漂洗3次,滴加对应二抗(Alexa Fluor® 594标记山羊抗兔IgG,Alexa Fluor® 488标记山羊抗小鼠IgG),4℃湿盒内孵育1 h。PBS漂洗后滴入含有DAPI的防淬灭封片剂,封片,激光共聚焦显微镜下观察内皮细胞标记分布,并通过Image J软件半定量分析CD31阳性表达荧光强度。
1.4.4. Western blot检测
术后7 d和28 d每组取3只大鼠同上法麻醉后,低温取材、组织研磨裂解,经BCA法评估浓度,并上样、转膜及封闭。分别在术后7 d切片滴加一抗[Ang-1(1∶2 000)、Tie-2(1∶2 000)];术后28 d切片滴加一抗 [Ang-1(1∶2 000)、PDGF-B (1∶1 000)、Tie-2(1∶2 000)、VEGF-A(1∶1 000)、VEGFR2(1∶500)、PDGFR-β(1∶500)、NF200 (1∶1 000)],所有切片低温过夜,二抗室温孵育。经ECL法曝光及定影。Image Pro Plus软件对扫描胶片图像进行处理,分析目的条带灰度,获得目的蛋白相对表达量(目的蛋白和GAPDH的比值)。
1.5. 统计学方法
采用SPSS25.0统计软件进行分析。计量资料经Shapiro-Wilk正态性检验,符合正态分布的数据以均数±标准差表示,组间比较采用单因素方差分析,两两比较采用LSD检验;不符合正态分布的数据以M(Q1,Q3)表示,组间比较采用Kruskal-Wallis H检验。检验水准α=0.05。
2. 结果
2.1. 行为学评分
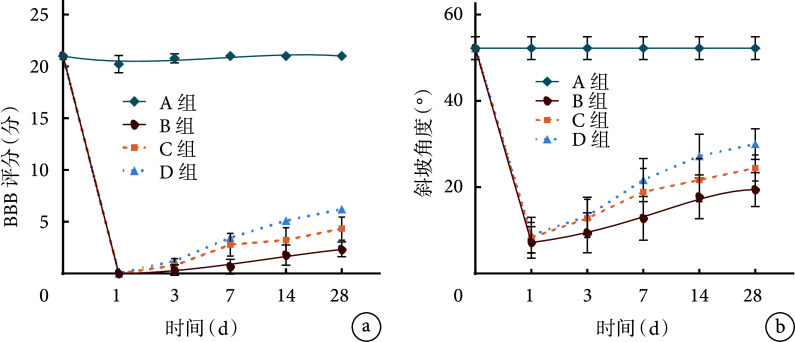
BBB评分和改良Rivlin-Tator斜板实验结果示,术前所有大鼠后肢功能均正常;术后1 d各组大鼠后肢完全瘫痪,并在3 d后逐渐恢复后肢运动功能。术后各时间点B~D组BBB评分和斜坡角度均显著低于A组,C组术后7~28 d及D组术后3~28 d各指标显著高于B组,D组术后14、28 d显著高于C组,差异均有统计学意义(P<0.05)。见图1。
图 1.
Variations of hind limb motor function at different time points in each group
各组各时间点后肢运动功能检测
a. BBB评分;b. 改良Rivlin-Tator斜板实验斜坡角度
a. BBB score; b. Climbing angle of modified Rivlin-Tator inclined plate test
2.2. 组织学染色观察
Masson染色示,A组灰质和白质组织结构排列紧密,未见蓝染胶原纤维;B组断端两侧组织结构与细胞排列杂乱,断端两侧组织溶解形成空洞囊腔,断端处严重纤维化,呈亮蓝色;C组断端处可见纤维瘢痕组织包裹的黑色导电颗粒,周围炎症细胞浸润,并存在少量空洞囊腔;D组断端可见少量散在黑色导电颗粒,周围胶原纤维成分相对较少。B~D组胶原容积分数显著高于A组,差异有统计学意义(P<0.05);D组低于B、C组,但仅与B组比较差异有统计学意义(P<0.05);B组高于C组,差异有统计学意义(P<0.05)。
Nissl染色示,A组灰、白质结构完整,灰质内神经元形态规整,尼氏体呈虎斑状;B组神经元变性严重,表现为尼氏体溶解、数量明显减少,形态异常;C组仅于损伤邻近部位观察到少数残存神经元,尼氏体形态欠规整;D组损伤邻近区域灰质内可见部分未凋亡的神经元,尼氏体固缩。B~D组损伤邻近区域尼氏体数量显著低于A组,差异有统计学意义(P<0.05);D组高于B、C组,但仅与B组比较差异有统计学意义(P<0.05);B、C组间差异无统计学意义(P>0.05)。
LFB染色示,A组脊髓组织髓鞘呈连续蓝色结构,排列致密有序,结构完整;B组损伤周边组织结构稀疏,白质出现溶解,神经纤维缺损;C、D组髓鞘结构损伤相对较轻,D组脊髓组织邻近区域可见相对完整、连续的蓝染髓鞘结构,髓鞘面积占比明显高于B、C组。同时,C、D组损伤中心可见散在分布的导电颗粒,其周围髓鞘密度高于B组。B~D组髓鞘阳性表达面积显著低于A组,差异有统计学意义(P<0.05);D组低于B、C组,但仅与B组比较差异有统计学意义(P<0.05);B、C组间差异无统计学意义(P>0.05)。见图2、表1。
图 2.
Histological staining of spinal cord in each group at 28 days postoperatively (×20)
术后28 d各组组织学染色观察(×20)
箭头示导电颗粒,从左至右依次为A、B、C、D组 a. Masson染色;b. Nissl染色;c. LFB染色(黑色虚线示损伤区域和邻近正常脊髓组织界线)
Arrow showed conductive particles; from left to right for groups A, B, C, and D, respectively a. Masson staining; b. Nissl staining; c. LFB staining (Black dotted line showed the boundary between the area of injury and the adjacent normal spinal cord tissue)
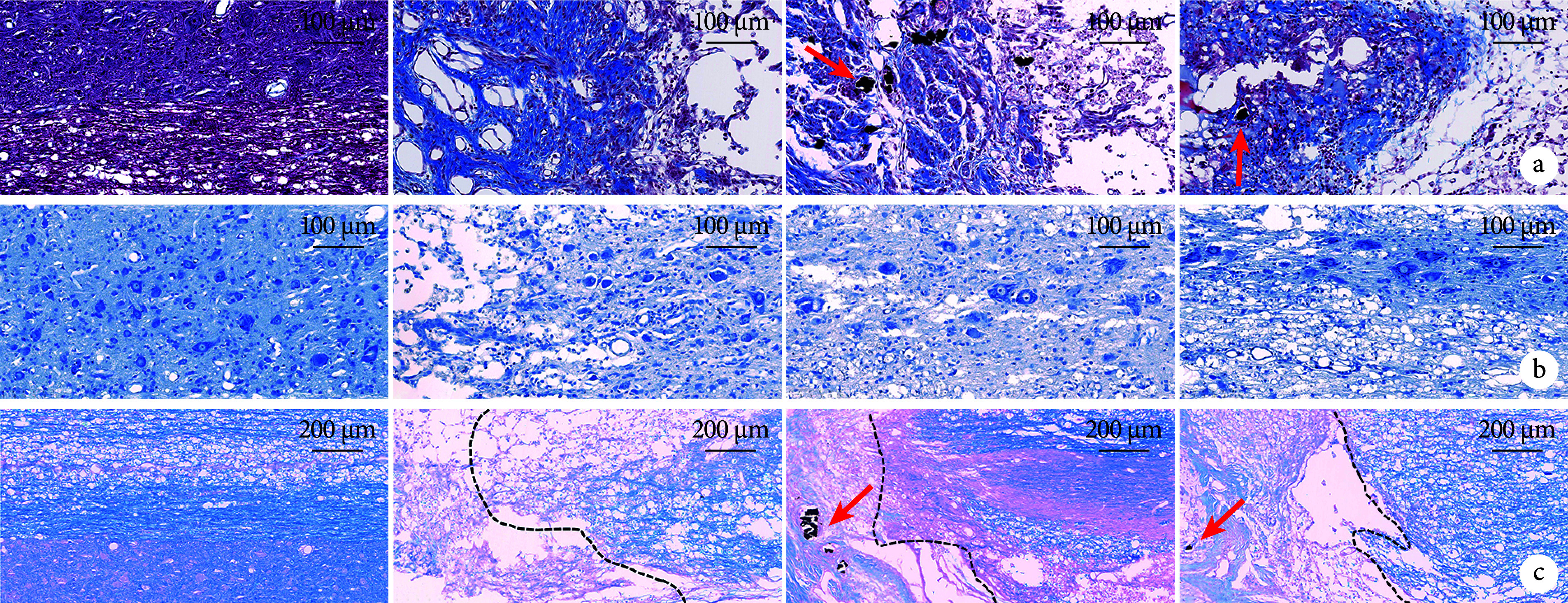
表 1.
Comparison of each indicator between groups after operation (n=6, 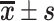 )
)
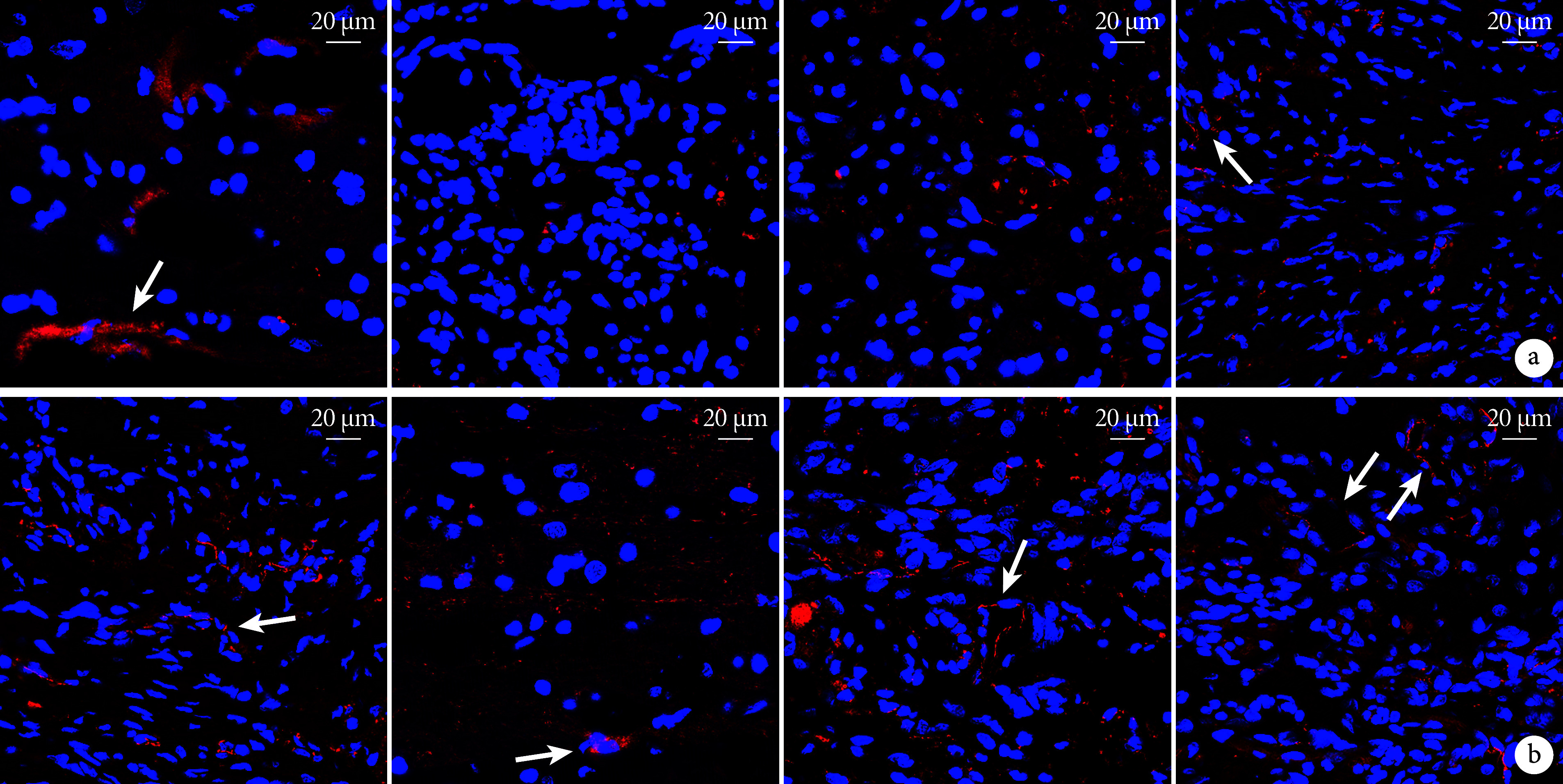
术后各组各检测指标比较(n=6,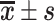 )
)
| 组别 Group |
胶原容积分 数(%) Collagen volume fraction (%) |
尼氏体数量 (个/视野) Number of Nissl- bodies (/field) |
髓鞘阳性表达 面积(%) Positive area of myelin (%) |
NF200蛋白 相对表达量 Relative expre- ssion levels of NF200 |
CD31阳性表达荧光强度 Fluorescence intensity of CD31 |
VEGFR-2蛋白相 对表达量 Relative expre- ssion levels of VEGFR-2 |
|
| 7 d | 28 d | ||||||
| A | 3.8±0.9#△ | 51.3±10.3#△ | 69.5±8.5#△ | 1.5±0.2#△ | 1.00(1.00,1.00) | 1.00(1.00,1.00)#△ | 0.3±0.0#△ |
| B | 45.6±3.8*△ | 9.7±5.0* | 20.6±5.0* | 0.6±0.1* | 0.30(0.29,0.46) | 0.62(0.56,0.68)* | 0.7±0.0* |
| C | 40.8±2.9*# | 11.7±5.8* | 28.6±2.8* | 0.8±0.1* | 0.40(0.38,0.45) | 0.64(0.58,0.65)* | 0.9±0.1*# |
| D | 37.7±2.8*# | 15.8±5.7*# | 35.5±4.5*# | 1.1±0.1*#△ | 0.52(0.48,0.57) | 0.76(0.76,0.81)*△ | 1.0±0.1*# |
| 统计量 | F=274.064 | F=47.101 | F=88.986 | F=21.792 | H=7.775 | H=9.492 | F=48.013 |
| P值 | <0.001 | <0.001 | <0.001 | <0.001 | 0.051 | 0.023 | <0.001 |
表 1-1.
| 组别 Group |
Ang-1蛋白相对表达量 Relative expression level of Ang-1 |
Tie-2蛋白相对表达量 Relative expression level of Tie-2 |
PDGF-B蛋白 相对表达量 Relative expre- ssion level of PDGF-B |
PDGFR-β蛋白 相对表达量 Relative expre- ssion level of PDGFR-β |
VEGF-A蛋白 相对表达量 Relative expre- ssion level of VEGF-A |
|||
| 7 d | 28 d | 7 d | 28 d | |||||
|
*与A组比较P<0.05,#与B组比较P<0.05,△与C组比较P<0.05 *Compared with group A, P<0.05; #compared with group B, P<0.05; △compared with group C, P<0.05 | ||||||||
| A | 0.5±0.1#△ | 0.2±0.0#△ | 0.4±0.1#△ | 0.3±0.0#△ | 0.4±0.0 | 0.4±0.0 | 0.2±0.0#△ | |
| B | 0.7±0.2* | 0.6±0.1* | 0.7±0.1* | 0.7±0.1* | 0.7±0.1△ | 0.8±0.1△ | 0.5±0.0* | |
| C | 0.6±0.0* | 0.5±0.2* | 0.7±0.0* | 0.5±0.1* | 0.4±0.0# | 0.4±0.1# | 0.6±0.1* | |
| D | 1.1±0.3*#△ | 0.9±0.1*#△ | 1.0±0.2*#△ | 1.3±0.4*#△ | 1.0±0.1#△ | 1.2±0.1#△ | 2.0±0.2*#△ | |
| 统计量 | F=5.528 | F=18.192 | F=11.546 | F=13.360 | F=82.388 | F=82.763 | F=183.798 | |
| P值 | 0.024 | <0.001 | 0.003 | 0.002 | <0.001 | <0.001 | <0.001 | |
2.3. 免疫荧光染色观察
NF200免疫荧光染色示,A组脊髓组织结构完整,神经元形态规整,排列致密;B组NF200阳性神经丝呈点状分布,邻近区域几乎未见完整神经元;C组损伤部位可见少量神经纤维生长;D组损伤部位NF200阳性纤维密度优于B、C组。见图3。
图 3.
Immunofluorescence staining for NF200 in each group at 28 days postoperatively (×40)
术后28 d各组NF200免疫荧光染色观察(×40)
从左至右依次为A、B、C、D组
From left to right for groups A, B, C, and D, respectively
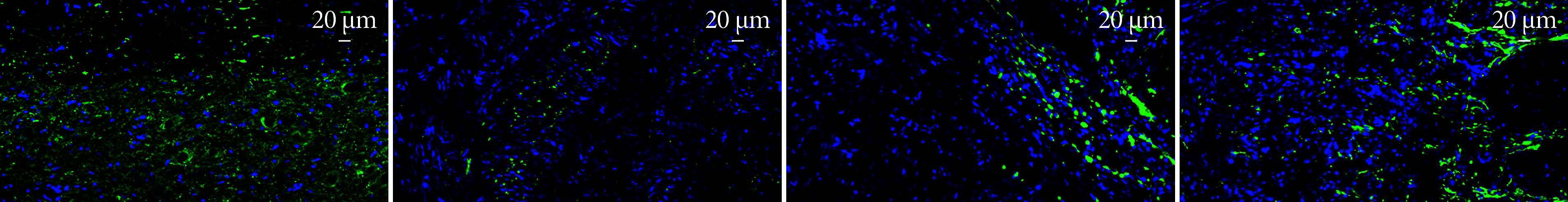
CD31免疫荧光染色示,A组术后7、28 d均可见红色荧光标记的内皮细胞,呈连续“线性”或类圆形形态;B、C组术后7 d损伤区域可见零星点状分布的内皮细胞,未见明显新生血管,术后28 d可见少量新生血管;D组术后7 d损伤区域周围可见少量新生血管影,术后28 d可见管状新生血管影。术后7 d各组间损伤区域CD31阳性表达荧光强度差异均无统计学意义(P>0.05);28 d,B~D组损伤区域CD31阳性表达荧光强度均显著低于A组,差异有统计学意义(P<0.05);D组显著高于B、C组,但仅与28 d的C组差异有统计学意义(P<0.05);B、C组间差异均无统计学意义(P>0.05)。见图4、表1。
图 4.
Immunofluorescence staining for CD31 in each group at 7 and 28 days postoperatively (Laser confocal microscope×60)
术后7、28 d各组CD31免疫荧光染色观察(激光共聚焦显微镜×60)
箭头示新生血管,从左至右依次为A、B、C、D组 a. 术后7 d;b. 术后28 d
Arrow showed angiogenesis; from left to right for groups A, B, C, and D, respectively a. At 7 days postoperatively; b. At 28 days postoperatively
2.4. Western blot检测
Western blot检测示术后28 d B~D组NF200蛋白相对表达量显著低于A组,D组显著高于B、C组,差异均有统计学意义(P<0.05);B、C组间差异无统计学意义(P>0.05)。
术后7、28 d B~D组Ang-1、Tie-2蛋白相对表达量高于A组,D组高于B、C组,差异有统计学意义(P<0.05);B、C组间差异无统计学意义(P>0.05)。术后28 d D组PDGF-B、PDGFR-β蛋白相对表达量显著高于B、C组,B组显著高于C组,差异有统计学意义(P<0.05)。术后28 d B~D组VEGF-A、VEGFR-2蛋白相对表达量高于A组,差异均有统计学意义(P<0.05);D组高于B、C组,D组与B组比较差异有统计学意义(P<0.05),与C组比较仅VEGF-A蛋白差异有统计学意义(P<0.05);B、C组间比较仅VEGFR-2蛋白差异有统计学意义(P<0.05)。见图5、表1。
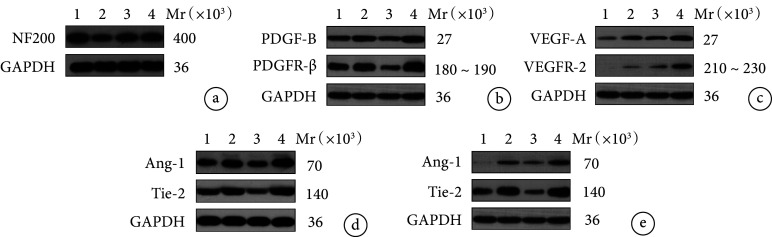
图 5.
The gel electrophoresis of different protein expressions in each group detected by Western blot assay at different postoperative time
Western blot检测各组术后各时间点各蛋白表达凝胶电泳图
Mr:相对分子质量 1:A组 2:B组 3:C组 4:D组 a. NF200;b. PDGF-B和PDGFR-β;c. VEGF-A和VEGFR-2;d. 术后7 d Ang-1和Tie-2;e. 术后28 d Ang-1和Tie-2
Mr: Relative molecular mass 1: Group A 2: Group B 3: Group C 4: Group D a. NF200; b. PDGF-B and PDGFR-β; c. VEGF-A and VEGFR-2; d. Ang-1 and Tie-2 at 7 days postoperatively; e. Ang-1 and Tie-2 at 28 days postoperatively
3. 讨论
近年来,通过多学科交叉协作,构建以水凝胶为基础的组合式神经修复策略,在促进SCI后再神经化和血管化方面取得重要进展[20]。GelMA水凝胶是一种典型的光固化水凝胶,在光引发剂作用下迅速成胶,并具备细胞生长与分化的三维空间[21]。GelMA水凝胶中甲基丙烯酸酯基团与lysine基团的结合,使其具有相对良好的机械强度与溶胀率,同时能为细胞黏附提供合适位点[21]。通过化学交联高导电性Ppy有机聚合物制备的新型高电活性水凝胶,不仅能有效加强细胞间生物电信号传递,诱导内源性神经元向水凝胶中迁移、存活,而且能够较好地填充神经损伤后的空洞缺损,抑制瘢痕增生[15-16]。据此,我们以GelMA/Ppy为“骨架”,通过TMP中的共轭双键与Ppy中的共轭双键产生π-π堆积效应,携载TMP,制备了携载TMP药物的导电水凝胶,初步表明其能有效缓释TMP,调节炎症反应,从而促进SCI后运动功能恢复[17],但其具体调控机制尚不明确。
本研究在此基础上,进一步探索了TGTP修复SCI的有效性及作用机制。行为学评估显示,D组相较于B、C组在改善SCI大鼠运动方面疗效显著。但D组尚未观察到大鼠后足掌面着地的现象(BBB评分>8分),这可能与脊髓全横断模型创伤过大及观察时间较短有关。我们进一步对损伤区域行组织学染色分析,以揭示D组运动功能恢复最佳的机制。纤维瘢痕大量增生是神经功能恢复受限的重要因素,不同于星形胶质细胞所产生的胶质瘢痕,局部上调的炎症反应会诱导并加速纤维瘢痕的发生[22-23]。前期研究中,我们证实了TGTP植入或TMP腹腔注射均能有效抑制TNF-α等炎症因子表达[11,17]。本研究中,我们通过Masson染色发现TGTP植入能够有效降低损伤区域胶原容积分数,抑制纤维结缔组织增生,从而减少纤维瘢痕形成,这可能与局部缓释的TMP抑制炎症反应密切相关。此外,尼氏体作为中枢神经元的特征性结构,其形态结构及数量的改变可评估神经元的功能状态[24]。本研究中D组脊髓断端周围尼氏体数量明显优于B、C组,尼氏体结构趋于正常,表明TGTP具有一定神经细胞保护作用。LFB染色结果也发现D组神经纤维排列相对有序,髓鞘密集紧致,髓鞘阳性表达面积优于B、C组。这与我们前期研究结果一致[15-17],即导电聚合物可能恢复局部电信号传导,诱导轴突再生。同时,NF200阳性纤维相对表达量与分布情况进一步提示了TGTP促进神经修复的有效性。NF200作为神经丝的成分参与了轴突构成,能有效反映神经元结构完整性和再生情况。本研究结果也发现D组损伤区域NF200表达量优于B、C组。上述结果提示TGTP不仅能发挥邻近组织的神经保护作用,在促进神经再生方面也有一定疗效。
早期重建损伤区域血运与加速血管化进程,对于神经元存活至关重要。损伤区域微血管密度与损伤后运动功能恢复程度密切相关[7-8]。本研究发现,D组在损伤后7 d和28 d,血管内皮细胞特异性标记物CD31表达强度呈上升趋势,并优于B、C组。同时,我们发现局部微血管密度动态变化过程和运动功能改善时间窗密切重合,即D组大鼠在损伤后3 d开始出现部分运动功能恢复,7 d时出现显著运动功能改善,28 d时达到相对稳定状态,与CD31阳性表达变化过程相似,与既往研究结果一致[6,25-26]。这进一步提示TGTP可能通过促进血管新生和重塑,改善局部血流量,从而促进神经功能的恢复。损伤部位再血管化受局部微环境影响,邻近细胞或组织通过直接作用或旁分泌多种相关信号因子调控微血管新生进程[27]。其中,促血管生成因子、血管生成素及PDGF等分子与SCI后血管新生密切相关。VEGF-A作为神经血管构建的主要初始驱动因子,在脉管系统形成和功能维持中起着关键作用[28]。VEGF-A可诱导尖端细胞迁移方向与茎细胞增殖,促进血管新生;与下游VEGFR-2结合,介导血管内皮细胞增殖与肌动蛋白重组,维持微管稳定及加速血管管腔形成等多种生物学进程[29-31]。本研究结果显示D组VEGF-A和VEGFR-2表达同步上调,并优于B、C组,提示TGTP可能通过调控此信号轴促进血管新生。VEGF-A/VEGFR-2轴可串扰内皮细胞中αvβ3整合素表达,调控血管新生,并受PDGFR-β蛋白负向调控[32]。本研究也发现C、D组PDGFR-β蛋白表达趋势与VEGFR-2相反,与既往研究结果一致。此外,PDGF-B为PDGF家族成员,是一种多功能的调节性多肽,主要来源于血小板、损伤内皮细胞及巨噬细胞;其与周细胞表面受体PDGFR-β相结合二聚化,募集下游信号,促进周细胞迁移、纤维转化和内皮小管成熟,在中枢系统微血管成熟和稳定中起重要作用[33]。Duan等[30]发现通过上调PDGF-B/PDFGR-β轴表达,能有效提升损伤区域新生血管周细胞覆盖率,促进功能化血管形成,从而增强SCI后修复疗效。Menezes等[34]发现上调PDGFR-β蛋白表达,可募集损伤邻近区域周细胞向新生血管迁移,促进血管成熟。本研究中,C组PDGF-B、PDFGR-β蛋白表达明显低于B组,而D组相关蛋白表达水平明显上调,显著优于B、C组。提示TMP药物和导电水凝胶协同应用优于单一手段的治疗模式,能有效正向调控PDGF-B/PDFGR-β信号通路,从而促进血管化。血管成熟和重塑过程中,血管生成素也起着关键作用。Ang-1是血管生成素家族中典型代表,主要由血管壁细胞表达,正常组织内表达含量较低,与酪氨酸受体Tie-2结合,通过调控内皮细胞间、内皮细胞与基质之间的紧密连接蛋白表达,减弱VEGF-A诱导的血管通透性改变以及募集内皮细胞迁移和管状结构形成,促进血管成熟[35-36]。Herrera等[37]发现在损伤急性期Ang-1蛋白靶向递送能增强内源性VEGF-A蛋白表达,促进损伤区域血管新生。疾病慢性期,注射Ang-1因子可有效促进内源性Ang-1/Tie-2蛋白过表达,增强血管重塑,同时抑制炎症因子表达,减轻其对血管通透性的影响。因此,增强Ang-1的内源性表达,可能是加速血管重塑的关键蛋白。本研究中,损伤后7 d和28 d C组Ang-1和Tie-2蛋白相对表达量均低于B组,与荧光染色结果较一致,提示GTP可能抑制血管新生进程。既往研究发现水凝胶中导电颗粒成分,即Ppy可能诱导炎症反应[16,38],本研究也发现导电颗粒周围存在炎症细胞浸润。研究证实炎症反应程度与血管网络重塑过程密切相关[39-40],我们前期研究发现与GTP相比,TGTP能有效抑制炎症反应,促进神经功能恢复[17]。本研究中D组血管新生相关蛋白表达显著上调,且CD31荧光强度也明显优于C组,提示TGTP可能抑制了Ppy诱导的炎症反应,增强了水凝胶对血管新生相关蛋白的调控作用,促进血管新生和重塑过程,从而加速SCI修复。
综上述,TGTP可能通过促进SCI后血管新生,同时保护神经元,从而改善损伤后神经功能的恢复。本研究也存在一定局限性,VEGF-A/VEGFR-2等相关蛋白可能与多条生物学过程相互串扰,其与血管生成的因果关系尚不明确。而本研究未进行细胞层面的探索,仅从动物及组织层面初步分析了水凝胶修复SCI的潜在机制,后续将进一步深入研究,明确其确切调控作用。
利益冲突 在课题研究和文章撰写过程中不存在利益冲突
伦理声明 研究方案经北京中医药大学实验动物伦理委员会批准(BUCM-4-2021040802-2021);实验动物生产许可证号:SCXK(京)2017-0005,实验动物使用许可证号:SYXK(京)2020-0013
作者贡献声明 邓博文、徐林、穆晓红:研究设计及协助研究开展;蒋昇源、刘港、霍路遥、白惠中:研究实施及文章撰写;李筱叶、徐杰:数据收集整理及统计分析
References
- 1.Li L, Mu J, Zhang Y, et al Stimulation by exosomes from hypoxia preconditioned human umbilical vein endothelial cells facilitates mesenchymal stem cells angiogenic function for spinal cord repair. ACS Nano. 2022;16(7):10811–10823. doi: 10.1021/acsnano.2c02898. [DOI] [PubMed] [Google Scholar]
- 2.You Z, Gao X, Kang X, et al Microvascular endothelial cells derived from spinal cord promote spinal cord injury repair. Bioact Mater. 2023;29:36–49. doi: 10.1016/j.bioactmat.2023.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dray C, Rougon G, Debarbieux F Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci U S A. 2009;106(23):9459–9464. doi: 10.1073/pnas.0900222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedorova J, Kellerova E, Bimbova K, et al The histopathology of severe graded compression in lower thoracic spinal cord segment of rat, evaluated at late post-injury phase. Cell Mol Neurobiol. 2022;42(1):173–193. doi: 10.1007/s10571-021-01139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figley SA, Khosravi R, Legasto JM, et al Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J Neurotrauma. 2014;31(6):541–552. doi: 10.1089/neu.2013.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Wu T, Yuan Z, et al Three-dimensional imaging of microvasculature in the rat spinal cord following injury. Sci Rep. 2015;5:12643. doi: 10.1038/srep12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Lucas-Osma AM, Black S, et al Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med. 2017;23(6):733–741. doi: 10.1038/nm.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu SM, Xiao ZF, Li X, et al Vascular endothelial growth factor activates neural stem cells through epidermal growth factor receptor signal after spinal cord injury. CNS Neurosci Ther. 2019;25(3):375–385. doi: 10.1111/cns.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai HH, Niu J, Munji R, et al Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351(6271):379–384. doi: 10.1126/science.aad3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.陶经纬, 周婧雅, 赵毅, 等 川芎嗪对脊髓损伤大鼠铁死亡的调控作用及机制. 中国组织工程研究. 2024;28(26):4158–4163. [Google Scholar]
- 11.张厚君, 蒋昇源, 邓博文, 等 川芎嗪改善脊髓损伤模型大鼠炎性微环境的机制. 中国组织工程研究. 2023;27(11):1701–1707. [Google Scholar]
- 12.蒋昇源, 邓博文, 徐林, 等 川芎嗪修复脊髓损伤的作用及机制. 中国组织工程研究. 2022;26(11):1799–1804. doi: 10.12307/2022.369. [DOI] [Google Scholar]
- 13.Li G, Sng KS, Shu B, et al Effects of tetramethylpyrazine treatment in a rat model of spinal cord injury: A systematic review and meta-analysis. Eur J Pharmacol. 2023;945:175524. doi: 10.1016/j.ejphar.2023.175524. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Wei J, Wan Y, et al TAT-modified tetramethylpyrazine-loaded nanoparticles for targeted treatment of spinal cord injury. J Control Release. 2021;335:103–116. doi: 10.1016/j.jconrel.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Fan L, Yi X, et al Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano. 2018;12(11):10957–10967. doi: 10.1021/acsnano.8b04609. [DOI] [PubMed] [Google Scholar]
- 16.Fan L, Liu C, Chen X, et al Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv Sci (Weinh) 2022;9(13):e2105586. doi: 10.1002/advs.202105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.蒋昇源, 邓博文, 刘港, 等 携载川芎嗪缓释微粒导电水凝胶修复脊髓损伤实验研究. 中国修复重建外科杂志. 2023;37(1):65–73. [Google Scholar]
- 18.贺丰, 俞兴, 穆晓红, 等 新型脊髓完全横断缺损模型大鼠的建立. 中国组织工程研究. 2016;20(5):635–639. [Google Scholar]
- 19.Rivlin AS, Tator CH Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47(4):577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Lv HQ, Chao X, et al Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil Med Res. 2022;9(1):16. doi: 10.1186/s40779-022-00376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan L, Liu C, Chen X, et al Directing induced pluripotent stem cell derived neural stem cell fate with a three-dimensional biomimetic hydrogel for spinal cord injury repair. ACS Appl Mater Interfaces. 2018;10(21):17742–17755. doi: 10.1021/acsami.8b05293. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Liu H, Tan Z, et al Remodeling microenvironment for endogenous repair through precise modulation of chondroitin sulfate proteoglycans following spinal cord injury. Small. 2023;19(6):e2205012. doi: 10.1002/smll.202205012. [DOI] [PubMed] [Google Scholar]
- 23.Ruschel J, Hellal F, Flynn KC, et al Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348(6232):347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, Xu W, Ren Y, et al Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245. doi: 10.1038/s41392-023-01477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheaton BJ, Sena J, Sundararajan A, et al Identification of regenerative processes in neonatal spinal cord injury in the opossum (Monodelphis domestica): A transcriptomic study. J Comp Neurol. 2021;529(5):969–986. doi: 10.1002/cne.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D, Huang Y, Jia C, et al Administration of antagomir-223 inhibits apoptosis, promotes angiogenesis and functional recovery in rats with spinal cord injury. Cell Mol Neurobiol. 2015;35(4):483–491. doi: 10.1007/s10571-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouellette J, Lacoste B From neurodevelopmental to neurodegenerative disorders: The vascular continuum. Front Aging Neurosci. 2021;13:749026. doi: 10.3389/fnagi.2021.749026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons M, Gordon E, Claesson-Welsh L Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17(10):611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 29.Ruhrberg C, Gerhardt H, Golding M, et al Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16(20):2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan YY, Chai Y, Zhang NL, et al Microtubule stabilization promotes microcirculation reconstruction after spinal cord injury. J Mol Neurosci. 2021;71(3):583–595. doi: 10.1007/s12031-020-01679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duran CL, Howell DW, Dave JM, et al Molecular regulation of sprouting angiogenesis. Compr Physiol. 2017;8(1):153–235. doi: 10.1002/cphy.c160048. [DOI] [PubMed] [Google Scholar]
- 32.Borges E, Jan Y, Ruoslahti E Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J Biol Chem. 2000;275(51):39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- 33.Dias DO, Kalkitsas J, Kelahmetoglu Y, et al Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun. 2021;12(1):5501. doi: 10.1038/s41467-021-25585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menezes K, Rosa BG, Freitas C, et al Human mesenchymal stromal/stem cells recruit resident pericytes and induce blood vessels maturation to repair experimental spinal cord injury in rats. Sci Rep. 2020;10(1):19604. doi: 10.1038/s41598-020-76290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace RG, Rochfort KD, Barabas P, et al COMP-Ang1: Therapeutic potential of an engineered Angiopoietin-1 variant. Vascul Pharmacol. 2021;141:106919. doi: 10.1016/j.vph.2021.106919. [DOI] [PubMed] [Google Scholar]
- 36.Lee SW, Kim WJ, Jun HO, et al Angiopoietin-1 reduces vascular endothelial growth factor-induced brain endothelial permeability via upregulation of ZO-2. Int J Mol Med. 2009;23(2):279–284. [PubMed] [Google Scholar]
- 37.Herrera JJ, Sundberg LM, Zentilin L, et al Sustained expression of vascular endothelial growth factor and angiopoietin-1 improves blood-spinal cord barrier integrity and functional recovery after spinal cord injury. J Neurotrauma. 2010;27(11):2067–2076. doi: 10.1089/neu.2010.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Zhou X, Sarkar B, et al Recent progress on self-healable conducting polymers. Adv Mater. 2022;34(24):e2108932. doi: 10.1002/adma.202108932. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Joshi HP, Sheen SH, et al Resolvin D3 promotes inflammatory resolution, neuroprotection, and functional recovery after spinal cord injury. Mol Neurobiol. 2021;58(1):424–438. doi: 10.1007/s12035-020-02118-7. [DOI] [PubMed] [Google Scholar]
- 40.许子星, 许卫红, 陈薛敏, 等 急性脊髓损伤大鼠血管重构与炎症反应的相关性研究. 中国修复重建外科杂志. 2020;34(11):1429–1437. [Google Scholar]