Abstract
STUDY QUESTION
Does sperm cryopreservation serve as a feasible and effective method for preserving fertility in adult male patients with cancer?
SUMMARY ANSWER
Sperm cryopreservation is an effective fertility preservation method and may benefit patients with cancer.
WHAT IS KNOWN ALREADY
Sperm cryopreservation is the only way to efficiently preserve male fertility. It is an important procedure in ART. Recently, due to remarkable advances in cancer treatment, an increasing number of studies have reported the outcomes of sperm cryopreservation in patients with cancer.
STUDY DESIGN, SIZE, DURATION
We conducted an extensive literature search for relevant studies published through to 31 December 2021, in the following databases: CENTRAL, CNKI, Cochrane Systematic Reviews, EMBASE, MEDLINE, PUBMED, and Web of Science. The search terms used were ‘(cryopreservation OR freeze OR freezing OR banking OR cryostorage OR storage) AND (sperm OR semen OR spermatozoon) AND (cancer OR tumor OR malignancy OR neoplasm)’.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We included all studies that reported offering or attempting to cryopreserve sperm before or during cancer treatment in male patients considered at risk of treatment-related fertility impairment. We evaluated the eligibility of all data in each study. The major exclusion criteria were as follows: non-cancer patients; pediatric and adolescent cancer patients; not reporting the use of cryopreserved sperm; use of fresh semen for ART; not reporting the number of patients with cancer offered sperm cryopreservation or attempting to do so before or during treatment; using an experimental fertility preservation technique such as preservation of testicular tissue or spermatogonial stem cells; duplicate data; abstracts, case report, comments, reviews, or editorials; insufficient data reported. The quality of the included studies was assessed using the Newcastle–Ottawa scale and the Methodological Index for Non-Randomized Studies.
MAIN RESULTS AND THE ROLE OF CHANCE
This meta-analysis included 69 non-randomized studies, with 32 234 patients referred for sperm analysis and 23 178 patients cryopreserving at least one sperm sample. The pooled failed-to-cryopreserve rate was 10% (95% CI, 8–12%), and the sperm disposal and sperm use rates were 23% (95% CI, 16–30%) and 9% (95% CI, 8–10%), respectively. The pregnancy, miscarriage, and delivery rates were 28% (95% CI, 22–33%), 13% (95% CI, 10–17%), and 20% (95% CI, 15–25%), respectively. Subgroup analysis showed higher pregnancy and delivery rates, as well as a lower failed-to-cryopreserve rate, in recent studies compared to those released a decade ago. The studies from Asia reported higher sperm disposal and pregnancy rates than in other continents. Our analysis showed clinical pregnancy rates per cycle of 34% (27–41%), 24% (14–35%), and 9% (5–15%) and delivery rates per cycle of 23% (17–30%), 18% (11–26%), and 5% (1–9%) for ICSI, IVF, and IUI, respectively.
LIMITATIONS, REASONS FOR CAUTION
As with all meta-analyses, some limitations should be considered. The first limitation of our study is that the data span 36 years. During this time, the World Health Organization has revised its sperm analysis standards, and other important changes have been made. There is also a limitation in that the outcome does not analyze the correlation between the type of cancer and sperm quality. Many of the earlier studies were limited by small sample sizes and a lack of control groups. Furthermore, almost all studies did not consider the severity of the disease, which could potentially have a substantial impact on the results. Consequently, further research should evaluate the effect of the type of cancer and, in particular, the severity of the condition on sperm quality in order to draw more precise conclusions. Similarly, it is inappropriate that most studies failed to differentiate between patients with different types of tumors and instead drew generalized conclusions that are presumed to apply to all patients with cancer. In the present analysis, we did not have in-depth information on patients’ disease, and although extensive efforts were made to conduct a thorough systematic review and meta-analysis of the outcomes for patients with various types of tumors, the results must be acknowledged as being subject to bias. However, the use of average results obtained in each study, without the patient-level data, might also represent a source of bias.
WIDER IMPLICATIONS OF THE FINDINGS
Sperm cryopreservation is an effective fertility preservation method and may benefit patients with cancer. The observed utilization rate of frozen sperm at 9% may underestimate the actual usage, as the short follow-up period is inadequate for obtaining comprehensive data on the use of frozen sperm in young cancer survivors. ART plays an important role in fertility preservation and the achievement of pregnancy, with this meta-analysis showing that ICSI delivers better clinical outcomes than IVF or IUI in patients with cancer undergoing fertility preservation.
STUDY FUNDING/COMPETING INTERESTS
This work was supported by the National Natural Science Foundation of China (grant no. 82001634, 81960550), and the China Postdoctoral Science Foundation (2019M661521). There are no competing interests to declare.
REGISTRATION NUMBER
CRID 42022314460.
Keywords: male fertility preservation, cancer, sperm cryopreservation, reproductive outcome, meta-analysis, sperm use rate, pregnancy rate, miscarriage rate, delivery rate
WHAT DOES THIS MEAN FOR PATIENTS?
The increasing number of cancer survivors is attributed to advances in cancer treatment. However, these treatments can have adverse effects on male fertility, either temporarily or permanently. For adult males undergoing treatment for cancer who are concerned about their future fertility, sperm cryopreservation (storing sperm at a very low temperature) is the most effective method for preserving fertility. This procedure is integral to assisted reproductive technologies (e.g. IVF) and should be initiated before the onset of fertility-compromising oncological procedures. Therefore, our study aimed to evaluate the effectiveness of sperm cryopreservation in preserving fertility and reproductive outcomes in male cancer patients, with a particular emphasis on the effects of various assisted reproductive technology methods on reproductive outcomes when using cryopreserved sperm. Encompassing 69 studies and 32 234 patients referred for sperm analysis, as well as 23 178 patients cryopreserving at least one sperm sample, the results provide further support for the effectiveness of sperm cryopreservation as a method of fertility preservation in male cancer patients. Despite the fact that only 9% of frozen sperm is currently being utilized, the limited duration of follow-up does not provide enough data to draw conclusions about the use of frozen sperm in young cancer survivors. These preserved samples have been used successfully in various assisted reproductive technology procedures to achieve pregnancy, with intracytoplasmic sperm injection (ICSI) demonstrating better clinical outcomes compared to IVF and intrauterine insemination. Therefore, it is recommended that male cancer patients seeking to preserve their future fertility discuss sperm cryopreservation with their healthcare providers before commencing treatment, as this measure could be pivotal in preserving their ability to conceive after treatment.
Introduction
According to the World Health Organization (WHO), infertility has become the third most common disease affecting human life and health, after cardiovascular disease and cancer (Rhoton-Vlasak, 2000). Studies have reported that the distress related to infertility is more prevalent among male cancer survivors than unaffected males (Jacobs and Vaughn, 2012; Hibi et al., 2020). The number of cancer survivors has been increasing with recent improvements in cancer treatment modalities. However, cancer treatments, including surgery, radiotherapy, and chemotherapy, can have a transitory or permanent detrimental impact on male fertility. Their gonadotoxic side effects can severely impair fertility in an agent- and dose-dependent way (Del-Pozo-Lerida et al., 2019; Appiah, 2020), and combination treatments of radiotherapy and chemotherapy are more gonadotoxic than either modality alone (Vakalopoulos et al., 2015). Hence, a decline in fertility potential brought about by cancer diagnosis/treatment has one of the biggest impacts on the long-term quality of life of patients with cancer (Behboudi-Gandevani et al., 2021).
Sperm cryopreservation is the only way to efficiently preserve male fertility. It is an important procedure in ART. The importance of consistently addressing the issue of fertility preservation in the course of cancer diagnosis and treatment has been stressed by organizations such as the American Society for Reproductive Medicine (ASRM) (Practice Committee of the American Society for Reproductive Medicine, 2019) and the American Society of Clinical Oncology (ASCO) (Oktay et al., 2018) who have issued formal recommendations urging clinicians to inform their patients about the potential impact of cancer treatments on fertility and offer solutions for fertility preservation, including sperm cryopreservation when necessary. Nevertheless, it has been reported that the utilization rate of stored sperm remains low among male patients with cancer (van Casteren et al., 2008; Ferrari et al., 2016). The utilization rate of cryopreserved sperm is often under 10% and differs widely among studies, as was recently summarized (Ferrari et al., 2016). Recently, owing to remarkable advances in ART, an increasing number of studies have reported the outcomes of sperm cryopreservation in patients with cancer (Grin et al., 2021).
We performed this systematic review and meta-analysis to summarize the current evidence on sperm cryopreservation and reproductive outcomes in adult male patients with cancer, including the most recent contributions and particularly emphasizing the impact of different ART procedures on the reproductive outcomes when using sperm cryopreserved before starting cancer treatment. The main objective of the study was to assess whether sperm cryopreservation serves as a feasible and effective method for preserving fertility in adult male patients with cancer.
Methods
Literature search
This study was registered in the International Prospective Register of Systematic Reviews and Meta-analysis Protocols registry (PROSPERO; registration number: CRID 42022314460). We conducted this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) (Moher et al., 2015; Shamseer et al., 2015). Briefly, we searched the CENTRAL, Cochrane systematic reviews, EMBASE, MEDLINE, PUBMED, and Web of Science databases using the following terms: ‘(cryopreservation OR freeze OR freezing OR banking OR cryostorage OR storage) AND (sperm OR semen OR spermatozoon) AND (cancer OR tumor OR malignancy OR neoplasm)’. The last search update was on 31 December 2021. Filters were applied for English language, study type (to exclude reviews and case reports), studies conducted on humans, and male sex. The most recent or complete study was included in case of overlapping or duplicated data from the same researchers.
Selection criteria and outcomes of interest
All studies reporting on offering or attempting to cryopreserve sperm before or during cancer treatment in male patients considered at risk of treatment-related fertility impairment were included. We evaluated the eligibility of all data in each study. The major exclusion criteria were as follows: non-cancer patients; pediatric and adolescent cancer patients; not reporting the use of cryopreserved sperm; use of fresh semen for ART; not reporting the number of patients with cancer offered sperm cryopreservation or attempting to do so before or during treatment; using an experimental fertility preservation technique such as preservation of testicular tissue or spermatogonial stem cells; duplicating data; abstracts, case report, comments, reviews, or editorials; insufficient data reported (i.e. impossible to extract primary data).
Two investigators (QL and CH) established the inclusion and exclusion criteria. The retrieved studies were initially screened for potential eligibility based on the title, abstract, and content. Disagreements about study eligibility were resolved by discussion with a third author (Z.W.B.).
Data extraction
Two investigators (QL and CH) extracted the data from all included studies. Cases of disagreement were resolved by discussion to reach consensus. We developed a standardized data extraction sheet and extracted the baseline clinical and demographic characteristics from the studies. We recorded the following information from each study: name of the first author; publication year; country; years of follow-up; age at sperm storage; cancer type; failed-to-cryopreserve rate (number of failed-to-cryopreserve patients divided by the number of patients attempting cryopreservation); sperm disposal rate (number of patients who requested the disposal of their cryopreserved sperm for reasons other than unsuccessful cryopreservation divided by the number of patients with successfully cryopreserved samples), sperm use rate (number of patients whose cryopreserved sperm was used for ART divided by the number of patients with successfully cryopreserved samples), pregnancy rate (number of pregnancies divided by the number of ART cycles), miscarriage rate (number of miscarriages divided by the number of ART cycles); delivery rate (number of deliveries divided by the number of ART cycles).
Assessment of study quality
The quality of the included studies was assessed using the Newcastle–Ottawa scale (Lo et al., 2014) and the Methodological Index for Non-Randomized Studies (Slim et al., 2003). The scale consists of 10 items: a clearly stated aim; a clearly defined study population; representativeness of the sample; report of excluded patients; ascertainment of exposure; prospective collection of data; presence of the outcome of interest; adequate assessment of the outcome of interest; loss to follow-up; and prospective calculation of the study size. The items are scored with one point if affirmative and zero points if negative. The maximum possible total in the scale is 10, the global ideal score being 10 points, representing studies of high quality (Supplementary Table S1). Two reviewers independently scored all studies. Disagreements were resolved by consensus.
Statistical analysis
The pooled data for cryopreservation of sperm and reproductive outcomes were used to calculate the failed-to-cryopreserve, sperm disposal, sperm use, pregnancy, miscarriage, and delivery rates. Pooling for meta-analysis was performed using the inverse variance method for calculating weights. Random-effects meta-analysis of single proportions was performed to obtain the overall proportions (Suh and Park, 2016). CIs were calculated using the Clopper–Pearson interval for individual studies. Double arcsine transform was performed in studies with data values of 0 or 100%, and the calculated effects were then back-transformed to proportions. Heterogeneity was assessed using Cochran’s Q test (P < 0.05 indicated significant heterogeneity) and the Higgins’ inconsistency index (I2; >50% indicating significant heterogeneity). Sensitivity analysis was conducted by removing one study at a time to evaluate the quality and consistency of the meta-analysis results. Subgroup analyses within the reproductive outcome cohorts (failed-to-cryopreserve, sperm disposal, sperm use, pregnancy, miscarriage, and delivery rates) included the following covariates: continent (Europe, North America, Oceania, and Asia); and publication year (1982–1991, 1992–2001, 2002–2011, 2012–2021). Furthermore, subgroup analyses were performed within the sperm use, pregnancy, miscarriage, and delivery cohorts for various ART approaches (IUI, IVF, and ICSI). All statistical analyses were conducted using Stata, Version 14.0 (Stata Corporation, College Station, TX, USA).
Results
Literature search and selection
We retrieved 1610 relevant articles. After removing duplicates and screening the titles, 564 articles remained. After reading the abstracts of these articles, we excluded 432 articles. We evaluated the remaining 132 articles based on the full text and selected 80 non-randomized studies for the systematic review. We excluded 11 of these 80 studies, seven because it was impossible to extract primary data, and four for using experimental fertility preservation techniques. Finally, 69 non-randomized studies were included in the meta-analysis (Fig. 1) (Rhodes et al., 1985; Scammell et al., 1985; Reed et al., 1986; Redman et al., 1987; Fossa et al., 1989; Milligan et al., 1989; Tournaye et al., 1991; Khalifa et al., 1992; Lass et al., 1998; Fitoussi et al., 2000; Keane et al., 2000; Ginsburg et al., 2001; Kelleher et al., 2001; Blackhall et al., 2002; Ragni et al., 2003; Spermon et al., 2003; Agarwal et al., 2004; Chung et al., 2004, 2013; Schmidt et al., 2004; Brydøy et al., 2005; Magelssen et al., 2005; Revel et al., 2005; Chang et al., 2006; Meseguer et al., 2006; Zorn et al., 2006; Girasole et al., 2007; Ishikawa et al., 2007; Knoester et al., 2007; Neal et al., 2007; Hourvitz et al., 2008; van Casteren et al., 2008; Crha et al., 2009; Selk et al., 2009; Ping et al., 2010, 2014; Babb et al., 2012; Bizet et al., 2012; Freour et al., 2012; Keene et al., 2012; Sheth et al., 2012; Botchan et al., 2013; Johnson et al., 2013; Dearing et al., 2014; Greaves et al., 2014; van der Kaaij et al., 2014; Záková et al., 2014; García et al., 2015; Sonnenburg et al., 2015; Tomlinson et al., 2015; Depalo et al., 2016; Muller et al., 2016; Kobayashi et al., 2017; Hamano et al., 2018; Levi-Setti et al., 2018; Machen et al., 2018; Negoro et al., 2018; Ukita et al., 2018; Fu et al., 2019; Noetzli et al., 2019; Song et al., 2019; Uçar et al., 2019; Ito et al., 2020; Ferrari et al., 2021; Lackamp et al., 2021; Liu et al., 2021; Papler et al., 2021; Stigliani et al., 2021; Yamashita et al., 2021). These studies included 32 234 patients referred for sperm analysis and 23 178 whose sperm was cryopreserved at least once. Patient age at storage was 16–51 years. Sixty-five studies reported on mixed tumor types. A total of 91.3% (63/69) studies focused on testicular cancer, leukemia, and lymphoma.
Figure 1.
Flowchart of the study screening and selection procedure for a systematic review and meta-analysis of outcomes following fertility preservation in adult male patients with cancer.
Characteristics of the included studies
The characteristics of the 69 included studies are presented in Table 1. All but nine studies have been published since 2000. Of these 60 studies, six were published in 2021. The studies were conducted in over 23 countries; 13 in the USA, eight in the UK, seven in Japan, six in China, six in Italy, four in France, and four in The Netherlands. Canada, Norway, and Israel recorded three articles each, while the remaining countries recorded 1 or 2 articles each. Among the studies, 62 reported sperm use, and 34 reported pregnancy outcomes. We evaluated the ART approaches in studies reporting pregnancy outcomes. As shown in Table 2, we conducted a systematic review and meta-analyses on 19 studies reporting IUI cycles, 16 reporting IVF cycles, and 22 reporting ICSI cycles. In most cases, more than one method was reported per study.
Table 1.
Characteristics of the studies included in a meta-analysis of ART outcomes following fertility preservation in adult male patients with cancer.
| Studies | Country | Number of patients | Date of study | Follow-up years | Age at storage | Cancer type | Failed-to-cryopreserve | Sperm discarded rate | Sperm used rate | Pregnancy rate | Miscarriage rate | Live births rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhodes et al. (1985) | USA | 24 | 1975–1984 | 9 | – | Hodgkin’s and non-Hodgkin’s lymphoma, testicular cancer, various hematologic or bone/connective tissue tumors | – | – | 16.7% (4/24) | – | – | – |
| Scammell et al. (1985) | UK | 22 | 1976–1984 | 8 | – | Hodgkin’s disease and testicular tumor | – | – | – | 7.0% (8/115) | 12.5% (1/8) | 6.1% (7/115) |
| Reed et al. (1986) | USA | 54 | – | – | – | Testicular carcinoma or lymphoma | – | 25.9% (14/54) | 13.0% (7/54) | – | – | – |
| Redman et al. (1987) | USA | 71 | 1975–1982 | 7 | – | Hodgkin’s disease | – | – | 15.5% (11/71) | 9.6% (7/73) | – | 4.1% (3/73) |
| Milligan et al. (1989) | UK | 2219 | 1977–1987 | 8 | – | – | – | – | 6.0% (133/2219) | – | – | – |
| Fossa et al. (1989) | Norway | 147 | 1979–1987 | 6 | – | Testicular cancer | 41.8% (38/91) | – | 7.5% (4/53) | – | – | – |
| Tournaye et al. (1991) | Belgium | 11 | 1985–1991 | 4 | 23 | Hodgkin’s disease | – | – | 45.5% (5/11) | 25.9% (7/27) | – | 22.2% (6/27) |
| Khalifa et al. (1992) | USA | 10 | 1986–1990 | 4 | 33.4 | Seminoma, testicular carcinoma, leiomyosarcoma of the prostate, Wegener’s granulomatosis, non-Hodgkin’s, and Hodgkin’s lymphoma. | – | – | – | 40% (4/10) | 25% (1/4) | 30% (3/10) |
| Lass et al. (1998) | UK | 231 | 1989–1997 | 8 | 28 | Testicular tumors, hematological malignancy, other cancer | 17.3% (40/231) | – | 3.1% (6/191) | 33.3% (5/15) | – | 20% (3/15)* |
| Keane et al. (2000) | Ireland | 58 | 1998 | – | 30 | Testicular carcinoma, lymphoma, leukemia, and other | 0% (0/58) | 1.7% (1/58) | 3.4% (2/58) | – | – | – |
| Fitoussi et al. (2000) | France | 316 | 1976–1996 | 20 | 27.5 | Hodgkin’s disease | – | – | 13.8% (13/94) | 10.2% (9/88) | 11.1% (1/9) | 2.3% (2/88) |
| Ginsburg et al. (2001) | USA | 19 | – | – | 31 | Hodgkin’s lymphoma, CML, brain tumor, testicular cancer, thyroid cancer, melamoma | – | – | – | – | 21.4% (3/14) | 27.5%(11/40) |
| Kelleher et al. (2001) | Australia | 930 | – | – | – | – | 10.4% (97/930) | 37.6% (313/833) | 8.2% (68/833) | 34.1% (29/85) | – | – |
| Blackhall et al. (2002) | UK | 122 | 1978–1990 | 12 | 24 | Hodgkin’s disease | 5.7% (7/122) | 25.2% (29/115) | 28.7% (33/115) | – | – | – |
| Ragni et al. (2003) | Italy | 776 | 1986–2001 | 15 | 28 | Testicular tumors, lymphoma, Leukemias, other | 11.6% (90/776) | 18.1% (124/686) | 5.2% (36/686) | 15.9% (14/88) | 14.3% (2/14) | 13.6% (12/88) |
| Spermon et al. (2003) | The Netherlands | 99 | 1982–1999 | 18 | – | Testicular tumors | 21.2% (21/99) | – | 16.7% (13/78) | – | – | – |
| Agarwal et al. (2004) | USA | 318 | 1982–2001 | 19 | 30 | Testicular cancer, Hodgkin’s disease, and other | – | – | 9.1% (29/318) | 18.4% (16/87) | – | 13.8% (12/87) |
| Chung et al. (2004) | USA | 164 | 1993–2003 | 10 | 29.5 | Testicular cancer, Hodgkin’s lymphoma, leukemia, and gastrointestinal cancers and other | – | 19.5% (32/164) | 4.7% (6/127) | 10% (2/20) | – | 10% (2/20) |
| Schmidt et al. (2004) | Denmark | 67 | 1996–2003 | 7 | – | Testicular cancer, lymphomas | 17.9% (12/67) | – | – | 31.5% (23/73) | – | 24.7% (18/73) |
| Revel et al. (2005) | Israel | 21 | 1999–2002 | 3 | 33 | Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, sarcoma, seminoma, testicular teratoma, inguinal hystiocytoma, prostate carcinoma, and acute lymphocytic leukemia. | – | – | – | 41.9% (26/62) | 30.7% (8/26) | 29.0% (18/62) |
| Zorn et al. (2006) | Slovenia | 360 | 1996–2003 | 7 | 31.6 | Testicular cancer, Hodgkin’s disease, non-Hodgkin lymphoma, leukemia, and other | – | – | 5.6% (20/360) | 22.6% (7/31) | 28.6% (2/7) | 12.9% (4/31) |
| Magelssen et al. (2005) | Norway | 422 | 1983–2002 | 19 | – | Testicular cancer | – | – | 6.9% (29/422) | – | – | – |
| Brydøy et al. (2005) | Norway | 326 | 1998–2002 | 4 | – | Testicular cancer | – | – | 5.5% (18/326) | – | – | – |
| Girasole et al. (2007) | USA | 129 | 1994–2004 | 10 | 26.2 | Testicular carcinoma | – | – | 6.5% (2/31) | – | – | – |
| Chang et al. (2006) | China | 75 | 1995–2004 | 9 | 25.7 | Leukemia, lymphoma, testicular cancer, and other | – | 17.3% (13/75) | 4.0% (3/75) | 0% (0/5) | – | 0% (0/5) |
| Meseguer et al. (2006) | Spain | 186 | 1991–2004 | 13 | 27.1 | Testicular cancer, Hodgkin’s lymphoma, and other | – | 8.6% (16/186) | 16.1% (30/186) | 45.7% (16/35) | – | 34.3% (12/35) |
| Knoester et al. (2007) | USA | 8 | 2002–2005 | 3 | 50.1 | Prostate cancer | – | – | 12.5% (1/8) | – | – | – |
| Ishikawa et al. (2007) | Japan | 130 | 2002–2005 | 3 | 30.1 | Testicular cancer/lymphoma/leukemia/other | 9.2% (12/130) | – | 3.4% (4/118) | 42.9% (3/7) | – | 42.9% (3/7) |
| Neal et al. (2007) | Canada | 146 | 1995–2005 | 10 | – | Hodgkin lymphoma, testicular cancer | – | 15.1% (22/146) | 14.4% (21/146) | – | – | – |
| Hourvitz et al. (2008) | Israel | 118 | 1994–2005 | 11 | 38.5 | Testicular cancer, lymphomas, and prostate cancer and other | – | – | – | 56.8% (96/169) | 11.5% (11/96) | 50.3% (85/169) |
| van Casteren et al. (2008) | The Netherlands | 629 | 1983–2004 | 21 | 27 | Testicular germ cell tumors, hematological malignancies, extragonadal germ cell tumors, one melanoma, and two schwannomas | 11.4% (72/629) | 5.2% (29/557) | 7.5% (42/557) | 26.7% (27/101) | 7.4% (2/27) | 21.8% (22/101) |
| Selk et al. (2009) | Canada | 388 | 2002–2005 | 3 | – | Testicular cancer and Hodgkin lymphoma and other | 5.4% (21/388) | – | 8.4% (31/367) | 33.3% (16/48) | – | – |
| Crha et al. (2009) | Czech Republic | 619 | 1995–2006 | 11 | 26.2 | Malignant testicular cancer, malignant neoplasms of the lymphatic and hematopoietic tissues, and other | – | – | 16.6% (28/619) | – | – | – |
| Ping et al. (2010) | China | 30 | 2003–2008 | 5 | – | – | – | – | 6.7% (2/30) | 50% (1/2) | – | 50% (1/2) |
| Freour et al. (2012) | France | 1042 | 1997–2007 | 10 | 28.58 | Testicular cancer/lymphoma/leukemia/other | 5.3% (55/1042) | – | 8.3% (82/987) | 19.1% (34/178) | 14.6% (26/178) | – |
| Babb et al. (2012) | UK | 112 | 1979–2007 | 28 | 33 | Hematological malignancy | – | – | 59.5% (25/42) | – | – | – |
| Keene et al. (2012) | UK | 180 | 1995–2009 | 14 | 16.1 | Lymphoma, leukemia, bone tumors, testicular tumors, soft tissue sarcoma, brain tumor, germ cell tumors, and other cancers | 22.7% (35/154) | 16.8% (20/119) | 0.8% (1/119) | 50% (1/2) | – | – |
| Sheth et al. (2012) | USA | 4881 | 2002–2010 | 18 | – | – | 6.4% (17/266) | 14.9% (37/249) | 8.4% (21/249) | – | – | – |
| Bizet et al. (2012) | France | 1007 | 1995–2009 | 14 | 29.3 | Testicular cancer, lymphoma, other hematological cancers or other types of cancer | 9.5% (96/1007) | 18.7% (170/911) | 6.3% (57/911) | 25.6% (30/117) | 19.1% (22/115) | |
| Botchan et al. (2013) | Israel | 682 | – | 20 | 31 | Testicular cancer , lymphoma, and other types of cancer | – | – | 10.3% (170/682) | 21.7% (40/184) | 9.3% (4/43) | 16.3% (30/184) |
| Chung et al. (2013) | China | 130 | 1995–2012 | 17 | 27 | Testicular cancer, hematological malignancy, Gastro-intestinal malignancy, Musculoskeletal malignancy, Neurological malignancy, Nasopharyngeal malignancy, and other | 12.0% (15/125) | 37.3% (41/110) | 3.6% (4/110) | – | – | – |
| van der Kaaij et al. (2014) | France, Belgium, Netherlands, Italy, Switzerland | 902 | 1974–2004 | 30 | – | Hodgkin’s lymphoma | – | – | 21.5% (78/363) | – | – | – |
| Johnson et al. (2013) | USA | 423 | 1991–2010 | 19 | 29.8 | Testicular cancer, lymphoma, and leukemia and other | 10.6% (45/423) | 42.6% (161/378) | 9.5% (36/378) | – | – | – |
| Dearing et al. (2014) | UK | 4362 | 1976–2013 | 37 | 32 | Testicular cancer/lymphoma/leukemia/other | – | – | 6.0% (183/3062) | – | – | – |
| Greaves et al. (2014) | UK | 91 | 1957–2006 | 49 | – | Lymphoma/leukemia | – | – | 28.6% (26/91) | – | – | – |
| Záková et al. (2014) | Czech Republic | 523 | 1995–2012 | 17 | 28.5 | Testicular cancer | 6.1% (34/557) | – | 6.5% (34/523) | 34.8% (16/46) | – | 13.0% (6/46) |
| Ping et al. (2014) | China | 125 | 1996–2010 | 14 | 36.3 | Testicular cancer | – | – | 23.8% (5/21) | – | – | – |
| García et al. (2015) | Canada | 272 | 2008–2012 | 4 | 36.7 | Lymphoma, testicular cancer, leukemia, and other malignancies including sarcoma, gastrointestinal, and central nervous system malignancies | – | – | 10.7% (29/272) | – | – | – |
| Tomlinson et al. (2015) | Pakistan | 823 | – | – | – | Testicular cancer/lymphoma/leukemia/other | – | – | 5.5% (45/823) | – | – | – |
| Sonnenburg et al. (2015) | USA | 200 | – | 28.4 | Germ cell tumor | – | – | 18.0% (11/61) | – | – | – | |
| Muller et al. (2016) | The Netherlands | 942 | 1983–2013 | 20 | 29 | Testicular cancer, Hodgkin’s lymphoma, leukemia, non-Hodgkin’s lymphoma, and other | 4.7% (44/942) | 33.9% (304/898) | 10.7% (96/898) | 33.0% (95/288) | – | 28.0% (81/288) |
| Depalo et al. (2016) | Italy | 778 | 1999–2015 | 16 | 29.23 | Seminoma of the testis, Hodgkin’s lymphoma, mixed testicular tumors, germ cell tumors, other tumors, hematological tumors, and non-Hodgkin’s lymphoma | 7.3% (57/778) | 1.0% (7/721) | 2.6% (19/721) | 25% (5/20) | – | 25% (5/20) |
| Kobayashi et al. (2017) | Japan | 122 | 2006–2015 | 9 | 33.6 | Testicular, hematological, digestive, and other types | – | – | 9.8% (12/122) | – | – | – |
| Machen et al. (2018) | USA | 271 | 1988–2015 | 27 | – | – | – | 16.2% (44/271) | 1.5% (4/271) | 25.0% (1/4) | – | – |
| Negoro et al. (2018) | Japan | 257 | 1994–2013 | 19 | – | Germ cell tumor and hematological disorders | – | – | 9.7% (25/257) | – | – | – |
| Hamano et al. (2018) | Japan | 111 | 1999–2016 | 17 | 29 | Hematological malignancies, testicular cancer | – | – | 7.2% (8/111) | – | – | – |
| Noetzli et al. (2019) | Switzerland | 169 | 2002–2012 | 10 | 29.6 | Acute leukemia | 15.2% (5/33) | 64.3% (18/28) | 7.1% (2/28) | – | – | – |
| Levi-Setti et al. (2018) | Italy | 213 | 1998–2017 | 19 | 28.6 | Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, leukemia, and myelomas | 5.1% (8/156) | – | 17.9% (28/156) | 21.2% (11/52) | 9.1% (1/11) | 19.2% (10/52) |
| Ukita et al. (2018) | Japan | 31 | 2004–2017 | 13 | – | Testicular cancer, malignant lymphoma, leukemia, and other | – | – | 6.5% (2/31) | – | – | – |
| Fu et al. (2019) | China | 145 | 2006–2017 | 11 | 29.3 | Testicular cancer/lymphoma/leukemia/other | 19.4% (13/167) | 9.7% (14/145) | 51.5% (17/33) | 6.1% (2/33) | 30.3% (10/33) | |
| Song et al. (2019) | Korea | 721 | 1996–2016 | 20 | 27 | Leukemia, lymphoma, testis cancer, and other | – | 40.8% (294/721) | 6.1% (44/721) | – | – | – |
| Ito et al. (2020) | Japan | 91 | 1996–2016 | 20 | – | Testicular cancer /extragonadal germ cell tumors) | 17.3% (9/52) | 30.2% (13/43) | 23.3% (10/43) | 47.6% (10/21) | 9.5% (2/21) | 33.3% (7/21) |
| Uçar et al. (2019) | Turkey | 110 | 2000–2016 | 16 | 36 | Testicular cancer | – | – | 4.5% (5/110) | – | – | – |
| Ferrari et al. (2021) | Italy | 1524 | 1986–2009 | 23 | 29 | Testicular cancer, Hodgkin Lymphoma, non-Hodgkin lymphoma, leukemia, other solid tumors | 9.4% (158/1682) | 42.5% (648/1524) | 9.4% (144/1524) | – | – | – |
| Lackamp et al. (2021) | Germany | 91 | 1994–2017 | 23 | 38 | Testicular cancer and malignancies of the lymphatic and hematopoietic tissue and other | – | – | 17.6% (16/91) | – | – | – |
| Stigliani et al. (2021) | Italy | 682 | 2004–2019 | 15 | – | Leukemia and lymphoma, testicular cancer, and other | 7.3% (50/682) | – | 7.2% (26/632) | 33.3% (15/45) | 33.3% (5/15) | 28.9% (13/45) |
| Yamashita et al. (2021) | Japan | 567 | 2018–2019 | 1 | – | Testicular cancer | 13.1% (20/153) | – | 21.1% (28/133) | – | – | – |
| Papler et al. (2021) | Slovenia | 70 | 2004–2018 | 14 | – | Testicular cancer, Hodgkin lymphoma, Leukemia, and other | – | – | – | 35.8% (39/109) | 25.6% (10/39) | 26.6% (29/109) |
| Liu et al. (2021) | China | 339 | 2010–2019 | 9 | 26.7 | Germ cell tumors, hematological neoplasms, head and neck cancers, thoracic tumors, abdominal tumors, and others | 7.7% (26/339) | 34.5% (108/313) | 4.2% (13/313) | 66.7% (10/15) | 20.0% (2/10) | 33.4% (5/15) |
Not including two ongoing pregnancies.
Table 2.
Summary of IUI, IVF, and ICSI results in cycles using cryopreserved semen in the 69 studies included in the meta-analysis.
| Studies | IUI |
IVF |
ICSI |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy rate | Miscarriage rate | Live births rate | Pregnancy rate | Miscarriage rate | Live births rate | Pregnancy rate | Miscarriage rate | Live births rate | |
| Redman et al. (1987) | 7.0% (8/115) | 12.5% (1/8) | 6.1% (7/115) | – | – | – | – | – | – |
| Tournaye et al. (1991) | – | – | – | 15.8% (3/19) | – | 10.5% (2/19) | – | – | – |
| Khalifa et al. (1992) | – | – | – | 50.0% (4/8) | 25% (1/4) | 37.5% (3/8) | 0% (0/2) | – | 0% (0/2) |
| Lass et al. (1998) | 100% (2/2) | – | 100% (2/2) | 11.1% (1/9) | – | 11.1% (1/9) | 50.0% (2/4) | – | – |
| Fitoussi et al. (2000) | – | – | 2.5% (2/80) | – | – | – | 0% (0/8) | – | 0% (0/8) |
| Kelleher et al. (2001) | 31.4% (11/35) | – | – | 21.4% (6/28) | – | – | 54.5% (12/22) | – | – |
| Ragni et al. (2003) | 7.5% (3/40) | – | – | 0% (0/6) | – | 0% (0/6) | 26.2% (11/42) | – | – |
| Agarwal et al. (2004) | 7.1% (3/42) | – | 7.1% (3/42) | 23.1% (6/26) | – | 19.2% (5/26) | 36.8% (7/19) | – | 21.1% (4/19) |
| Chung et al. (2004) | 0% (0/12) | – | 20.0% (1/5) | – | 20.0% (1/5) | 33.3% (1/3) | – | 33.3% (1/3) | |
| Schmidt et al. (2004) | 16.7% (4/24) | – | 16.7% (4/24) | – | – | – | 38.8% (19/49) | – | 30.6% (15/49) |
| Zorn et al. (2006) | – | – | – | – | – | – | 22.6% (7/31) | 28.6% (2/7) | 12.9% (4/31) |
| Chang et al. (2006) | 0% (0/2) | – | 0% (0/2) | – | – | – | 0% (0/3) | – | 0% (0/3) |
| Meseguer et al. (2006) | 20.0% (1/5) | – | 0% (0/5) | – | – | – | 50.0% (15/30) | – | 40.0% (12/30) |
| Ishikawa et al. (2007) | 0% (0/1) | – | 0% (0/1) | – | – | – | 50.0% (3/6) | – | 50.0% (3/6) |
| van Casteren et al. (2008) | 14.3% (1/7) | 0% (0/1) | 14.3% (1/7) | 25.0% (8/32) | 0% (0/8) | 25.0% (8/32) | 30.2% (16/53) | 6.3% (1/16) | 28.3% (15/53) |
| Crha et al. (2009) | 22.2% (2/9) | – | 22.2% (2/9) | – | – | – | – | – | – |
| Ping et al. (2010) | – | – | – | 0% (0/1) | – | 0% (0/1) | 100% (1/1) | – | 100% (1/1) |
| Freour et al. (2012) | 12.1% (8/66) | – | 12.1% (8/66) | – | – | – | – | – | – |
| Keene et al. (2012) | – | – | – | – | – | – | 50.0% (1/2) | – | – |
| Bizet et al. (2012) | 12.8% (5/39) | – | 10.3% (4/39) | 28.6% (2/7) | – | 28.6% (2/7) | 32.4% (23/71) | – | 23.2% (16/69) |
| Botchan et al. (2013) | 10.0% (8/81) | 0% (0/8) | 8.6% (7/81) | 0% (0/12) | – | 0% (0/12) | 35.2% (32/91) | 12.5% (4/32) | 25.3% (23/91) |
| Záková et al. (2014) | 50.0% (3/6) | – | 16.7% (1/6) | 0% (0/2) | – | 0% (0/2) | 34.2% (13/38) | – | 13.2% (5/38) |
| Muller et al. (2016) | 13.9% (15/108) | – | 13.0% (14/108) | 41.8% (33/79) | – | 34.2% (27/79) | 46.5% (47/101) | – | 39/.6% (40/101) |
| Levi-Setti et al. (2018) | – | – | – | – | – | – | 21.2% (11/52) | 9.1% (1/11) | 19.2% (10/52) |
| Fu et al. (2019) | 42.9% (6/14) | – | 28.6% (4/14) | 57.1% (8/14) | – | 28.6% (4/14) | 60.0% (3/5) | – | 40.0% (2/5) |
| Ito et al. (2020) | – | – | – | 47.6% (10/21) | 20.0% (2/10) | 33.3% (7/21) | – | – | – |
| Liu et al. (2021) | 0% (0/1) | – | 0% (0/1) | 60.0% (3/5) | 66.7% (2/3) | 20.0% (1/5) | 77.8% (7/9) | 0% (0/7) | 44.4% (4/9) |
Overall semen cryopreservation and reproductive outcomes
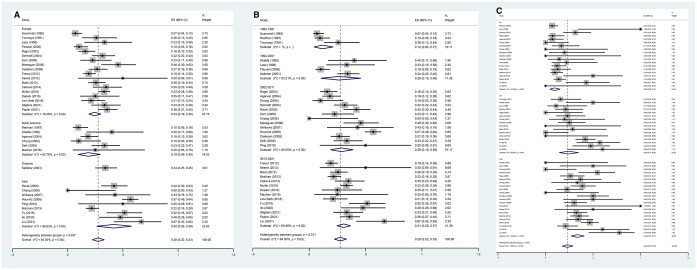
Among the studies that investigated fertility preservation in male patients with cancer, the pooled failed-to-cryopreserve rate was 10% (95% CI, 8–12%). We used a random-effects model as high heterogeneity was found (P-value <0.001, I2=88.1%; Fig. 2A). The pooled sperm disposal rate was 23% (95% CI, 16–30%). We used a random-effects model as high heterogeneity was found (P < 0.001, I2 = 98.4%; Fig. 2B). The pooled sperm use rate was 9% (95% CI, 8–10%). We used a random-effects model as high heterogeneity was found (P < 0.001, I2=88.1%; Fig. 2C). The pooled pregnancy rate was 28% (95% CI, 22–33%). We used a random-effects model as high heterogeneity was found (P < 0.001, I2=84.0%; Fig. 2D). The pooled miscarriage rate was 13% (95% CI, 10–17%), and a random-effects model was used (P = 0.30, I2=12.9%; Fig. 2E). The pooled delivery rate was 20% (95% CI, 15–25%), and a random-effects model was used (P < 0.001, I2=84.2%; Fig. 2F).
Figure 2.
Forest plot of overall semen cryopreservation in adult male patients with cancer and reproductive outcomes. Forest plots of: (A) the failed-to-cryopreserve rate; (B) the disposed sperm rate; (C) the sperm use rate; (D) the pregnancy rate when using cryopreserved sperm; (E) the miscarriage rate after using cryopreserved sperm; and (F) the delivery rate after using cryopreserved sperm.
Sperm cryopreservation in male patients with cancer
Subgroup analysis of the failed-to-cryopreserve rate by continent found no differences across continents (P = 0.475). However, as shown in Supplementary Fig. S1b, significant differences were noted across publication years (8% (95% CI, 2–17%) vs 11% (95% CI, 8–14%) vs 9% (95% CI, 7–10%), P < 0.001).
The numerically highest sperm disposal rate among continents was in Asia (33% (26–40%)), while the lowest was in Europe (18% (9–30%); Supplementary Fig. S2a). Statistically significant differences across publication years were noted in the sperm disposal rates (34% (95% CI, 31–37%) vs 15% (95% CI, 9–21%) vs 28% (95% CI, 18–39%), P < 0.001).
Similar sperm use rates were noted across continents (P = 0.556) and publication years (P = 0.347; Supplementary Fig. S3).
Reproductive outcomes in male patients with cancer
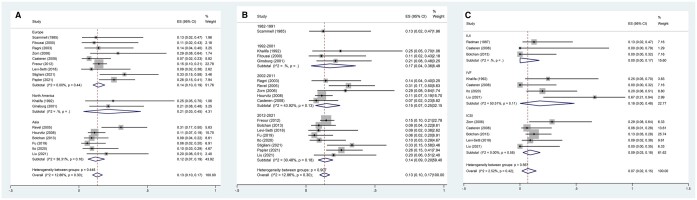
The numerically highest pregnancy rate among the ART approaches with frozen sperm from patients with cancer was obtained with ICSI (34% (CI, 27–41%)), followed by IVF (24% (CI, 14–35%)) and IUI (9% (CI, 5–15%), Fig. 3C). Subgroup analysis of the pregnancy rate by continent found significant differences among continents (Europe vs North America vs Asia: 24% (CI, 19–30%) vs 19% (CI, 9–30%) vs 42% (CI, 26–58%), P < 0.001) (Fig. 3A) and publication years (12% (CI, 4–21%) vs 26% (CI, 10–46%) vs 29% (CI, 19–39%) vs 31% (CI, 25–37%), P = 0.011) (Fig. 3B). Importantly, the more recent the publication year, the higher the reported pregnancy rate.
Figure 3.
The subgroup analysis of pregnancy rate in partners of adult male patients with cancer. Forest plots of: (A) the pregnancy rate by continent after using cryopreserved sperm, (B) the pregnancy rate by publication year after using cryopreserved sperm, and (C) the pregnancy rate by ART method after using cryopreserved sperm.
Similar miscarriage rates were noted among continents (P = 0.448), publication years (P = 0.907), and ART approach (P = 0.567; Fig. 4).
Figure 4.
The subgroup analysis of miscarriage rate in partners of adult male patients with cancer. Forest plots of: (A) the miscarriage rate by continent after using cryopreserved sperm, (B) the miscarriage rate by publication year after using cryopreserved sperm, and (C) the miscarriage rate by ART method after using cryopreserved sperm.
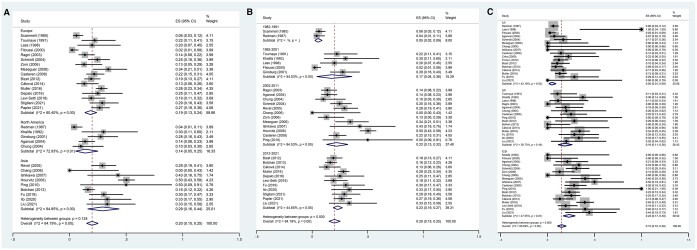
The numerically highest delivery rate among the different ART approaches using frozen sperm from patients with cancer was obtained with ICSI (23% (CI, 17–30%)), followed by IVF (18% (CI, 11–26%)) and IUI (5% (CI, 1–9%), Fig. 5C). More recent studies reported higher delivery rates (5% (CI, 2–9%) vs 17% (CI, 4–36%) vs 22% (CI, 13–32%) vs 23% (CI, 19–27%), P < 0.001, Fig. 5B). However, as shown in Fig. 5A, similar delivery rates were noted across continents (P = 0.124).
Figure 5.
The subgroup analysis of delivery rate in partners of adult male patients with cancer. Forest plots of: (A) the delivery rate by continent after using cryopreserved sperm, (B) the delivery rate by publication year after using cryopreserved sperm, and (C) the delivery rate by ART method after using cryopreserved sperm.
Meanwhile, as shown in Table 3, the included studies reveal that lymphoma patients experience lower pregnancy (lymphoma vs leukemia vs testicular tumors vs other solid tumors: 17.1% vs 32.6% vs 28.3% vs 33.3%, respectively) and delivery rates (lymphoma vs leukemia vs testicular tumors vs other solid tumors: 10.6% vs 58.8% vs 29.6% vs 35.5%, respectively).
Table 3.
Pregnancy rate for ART cycles according to type of cancer in the included studies.
| Studies | Pregnancy rate |
Miscarriage rate |
Live births rate |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoma |
Leukemia | Testicular tumors | Other solid tumors | Lymphoma |
Leukemia | Testicular tumors | Other solid tumors | Lymphoma |
Leukemia | Testicular tumors | Other solid tumors | ||||
| Hodgkin’s | Non-Hodgkin’s | Hodgkin’s | Non-Hodgkin’s | Hodgkin’s | Non-Hodgkin’s | ||||||||||
| Redman et al. (1987) | 9.6% (7/73) | _ | _ | _ | _ | _ | _ | _ | _ | _ | 4.1% (3/73) | _ | _ | _ | _ |
| Tournaye et al. (1991) | 25.9% (7/27) | _ | _ | _ | _ | _ | _ | _ | _ | _ | 22.2% (6/27) | _ | _ | _ | _ |
| Lass et al. (1998) | 18.2% (2/11) | 100% (1/1) | 100% (1/1) | 50% (1/2) | _ | _ | _ | _ | _ | _ | 9.1% (1/11)* | 100% (1/1) | 100% (1/1) | 0% (0/2)* | _ |
| Fitoussi et al. (2000) | 10.2% (9/88) | _ | _ | _ | _ | 11.1% (1/9) | _ | _ | _ | _ | 2.3% (2/88) | _ | _ | _ | _ |
| Agarwal et al. (2004) | 13.5% (5/37) | _ | _ | 18.8% (6/32) | _ | _ | _ | _ | _ | _ | 8.1% (3/37) | _ | _ | 12.5% (4/32) | _ |
| Chung et al. (2004) | 9.1% (1/11) | _ | _ | 12.5% (1/8) | 0% (0/1) | _ | _ | _ | _ | _ | 9.1% (1/11) | _ | _ | 12.5% (1/8) | _ |
| Revel et al. (2005) | 27.8% (5/18) | 45.5% (5/11) | 100% (1/1) | 38.9% (7/18) | 57.1% (8/14) | 20% (1/5) | 40% (2/5) | 0% (0/1) | 57.1% (4/7) | 12.5% (1/8) | 22.2% (4/18) | 27.3% (3/11) | 100% (1/1) | 16.7% (3/18) | 50% (7/14) |
| Ishikawa et al. (2007) | 0% (0/4) | 100% (2/2) | 100% (1/1) | _ | _ | _ | _ | _ | 0% (0/4) | 100% (2/2) | 100% (1/1) | _ | |||
| Hourvitz et al. (2008) | _ | _ | _ | 57.9% (40/69) | _ | _ | _ | _ | _ | _ | _ | _ | _ | 52.2%(36/69) | _ |
| Ping et al. (2010) | _ | _ | _ | 50% (1/2) | _ | _ | _ | _ | _ | _ | _ | _ | _ | 50% (1/2) | _ |
| Freour et al. (2012) | 18.8% (6/32) | 25% (18/72) | 11.8% (8/68) | 7.1% (2/28) | _ | _ | _ | _ | _ | _ | _ | _ | _ | ||
| Botchan et al. (2013) | 22.2% (16/72) | _ | 29.6% (16/54) | _ | _ | _ | _ | _ | 18.1% (13/72) | _ | 29.6% (16/54) | _ | |||
| Záková et al. (2014) | _ | _ | _ | 34.8% (16/46) | _ | _ | _ | _ | _ | _ | _ | _ | _ | 13.0% (6/46) | _ |
| Fu et al. (2019) | 66.7% (2/3) | 45.5% (5/11) | 55.6% (5/9) | 50% (5/10) | _ | _ | _ | _ | 33.3% (1/3) | 36.4% (4/11) | 22.2% (2/9) | 30% (3/10) | |||
| Liu et al. (2021) | 25% (1/4) | 100% (2/2) | 100% (2/2) | 71.4% (5/7) | _ | _ | _ | 40.0% (2/5) | 0% (0/4) | 100% (2/2) | 100% (2/2) | 14.3% (1/7) | |||
| Total | 17.1% (67/392) | 32.6% (29/89) | 28.3% (88/311) | 33.3% (20/60) | 21.1% (4/19) | 0% (0/1) | 57.1% (4/7) | 23.1% (3/13) | 10.6% (38/359) | 58.8% (10/17) | 29.6% (72/243) | 35.5% (11/31) | |||
Not including one ongoing pregnancy.
Sensitivity analysis
Sensitivity analysis assessed the failed-to-cryopreserve, sperm disposal, sperm use, pregnancy, miscarriage, and delivery rates (Supplementary Fig. S4). The sensitivity assessment found the results to be stable.
Discussion
Fertility preservation is the only option for patients with cancer who are hoping to maintain their fertility despite cancer treatments. Advancements in early diagnosis and new treatments have greatly lowered the mortality rate of young (aged 20–39 years) patients with cancer, and the overall all-cancer incidence and mortality rates in the USA showed a clear downward trend, declining more rapidly in males (16% and 21%, respectively) than females (2% and 17%) (Feng et al., 2019). Despite a slight increase in the incidence rate in the UK from 2000 to 2012, the overall all-cancer mortality rate has declined by 11% in females and 15% in males (Feng et al., 2019). Evidence suggests that most cancer survivors younger than 40 years expect their fertility to be maintained or their endocrine function to be restored (Donnez et al., 2013). Sperm cryopreservation remains the only method to effectively preserve the reproductive potential of adult or adolescent male patients with cancer (Barak, 2019). The study of Huyghe showed that the fertility in patients with testicular cancer decreased by 30% after treatments: only 67.1% of patients get their partners pregnant successfully (Huyghe et al., 2004). Data for cancer survivors failing to father children was limited; however, men with a history of cancer had two to three times higher odds of using IVF/ICSI treatments to father a child compared to those not being treated for cancer (Kitlinski et al., 2023). One study reported that the chance of obtaining fatherhood was statistically significantly decreased after chemotherapy for testicular germ cell cancer, and the risk of needing ART to achieve fatherhood was increased after all treatment modalities (Bandak et al., 2022). Moreover, it should be noted that clinical practice for fertility preservation recommendations and oncological treatment strategies has changed over the years. Unfortunately, we saw that, despite these changes, the numbers of cryopreservation procedures in cancer patients remained low. Meanwhile, surveys indicate a limited knowledge of guidelines and poor compliance with recommendations by medical professionals (Tholeti et al., 2021; Huang et al., 2022). Many patients also express dissatisfaction with the information provided by healthcare professionals regarding fertility risks and available options. Nevertheless, a significant obstacle that hampers the implementation of ideal fertility preservation practices is the absence of a structured and co-ordinated program (Brannigan et al., 2021; Chen et al., 2023). Some institutions have successfully implemented formal fertility preservation programs, which has led to a rise in the number of patients receiving fertility preservation consultations and using sperm cryopreservation services (Behl et al., 2021).
The cancer types among male patients requiring fertility preservation include, for example, testicular germ cell tumors, lymphoma, leukemia, hematological neoplasms, and head and neck cancers. Cancer of the prostate (29%), lung and bronchus (12%), and colorectal (8%) were the most common forms of cancer diagnosed in men in 2023, while the greatest number of deaths were caused by lung (21%), prostate (11%), and colorectal (9%) cancer in male patients. The risk of developing invasive cancer varies significantly among different age groups, with the highest incidence in individuals aged 70 years or older. Furthermore, the rate of cancer among individuals from birth to 49 years is about 3.5%, including 0.4% for colorectal cancer, 0.3% for kidney cancer, 0.3% for lymphoma, 1% for lung cancer, 0.4% for skin melanoma, 0.3% for non-Hodgkin lymphoma, 0.2% for prostate cancer, and 0.2% for thyroid cancer (Siegel et al., 2023). The highest survival rate is seen for cancers of the thyroid (98%), prostate (97%), testis (95%), and melanoma (94%), while the lowest survival rate is seen for cancers of the pancreas (12%), liver, and esophagus (21%) (Siegel et al., 2023). Almost all studies in this meta-analysis included testicular germ cell tumors, Hodgkin’s or non-Hodgkin’s lymphoma, and leukemia because early-onset age and a good prognosis are the most common feature of these tumors (Pulte et al., 2009; Ghazarian and McGlynn, 2020). Consequently, patients with these tumors will be concerned about their future fertility capacity. Negative effects on sperm quality, leading to defective spermatogenesis, are common in testicular malignancies (van Casteren et al., 2010) and Hodgkin’s lymphoma (Paoli et al., 2016). There is evidence that gonadotoxic treatment results in a significant reduction in sperm quality. The type of cancer and the pre-treatment sperm concentrations were found to be the most significant factors governing post-treatment semen quality and recovery of spermatogenesis (Bahadur et al., 2005). Sperm quality varies with the type of underlying disease, with patients with testicular malignancy and hematological malignancies showing the lowest sperm counts (Vomstein et al., 2021). However, some studies have not observed differences in sperm parameters among cancer patients (Auger et al., 2016; Song et al., 2019; Liu et al., 2021). In the present study, failed-to-cryopreserve occurred in 10% (95% CI, 8–12%) of the patients. One of the most important causes was azoospermia, and it is important to consider this factor in future cost–benefit analysis. While we are still uncertain about the specific type of cancer affecting sperm quality, it is important to consider the potential impact of certain oncological treatments on the rate of azoospermia. The impact of cancer treatment on fertility is contingent upon the age of the patient at the time of diagnosis and treatment, as well as the type, duration, and dose intensity of treatment. Chemotherapy based on alkylating agents is linked to a higher risk of infertility in patients. Azoospermia is associated with chemotherapy and radiation, and whether it is temporary or permanent depends on the type of treatment, with radiation and alkylating agents posing the greatest risk for long-term damage (Meistrich, 2013). It is critical for clinicians to communicate effectively with their patients to guarantee that they freeze sperm before cancer treatment. This could potentially result in an increased use of ART.
This was the first meta-analysis to evaluate an association between male fertility preservation by way of sperm cryopreservation and reproductive outcomes. In this systematic review and meta-analysis, the inclusion of a significantly larger number of papers (69 compared with 30) and patients (32 234 compared with 11 798) did not contribute to outcomes that were significantly different from previous reviews (Ferrari et al., 2016). The rate of use of frozen sperm remained low (9% compared with the previously reported 7.5% (van Casteren et al., 2008) and 8% (Ferrari et al., 2016)). We also noted that previously, fertility preservation was mostly performed in North America and Europe but is now performed globally, except in Africa, suggesting that economic development level might be related to the use of fertility preservation. Consequently, an in-depth analysis is needed to investigate the economic cost of sperm cryopreservation in male patients with cancer. From an economic point of view, the costs of the freezing process, long-term maintenance in a biobank, and the subsequent (when needed) use in ART are not trivial. Furthermore, only one-quarter of patients in the present study discarded their banked sperm (23%, 95% CI, 16–30%), indicating that most patients do not rule out the possibility of using their frozen semen in the future. However, spermatogenesis may have resumed in some of the patients who discard their banked spermatozoa. Radiotherapy and chemotherapy affect sperm concentration, and irradiation also increases sperm DNA fragmentation, which might be sustained for up to 2 years after treatment, affecting fertilization rates even after spermatogenesis recovery (Stahl et al., 2006). In fact, more patients seem to dispose of sperm for no specific reason. The elapsed time between sperm freezing and the follow-up assessment is a fundamental factor that determines the utilization rate. A higher frozen sperm use rate might be related to a longer follow-up. Indeed, short follow-up is insufficient to draw information on frozen sperm use in young cancer survivors. Hence, the low rate for use of frozen sperm might be misleading. The observed 9% frozen sperm use might underestimate the real use rate.
The success rate and safety of ART using semen frozen before and during cancer treatments are issues of the greatest concern for reproductive medicine clinicians and oncologists. The documented pregnancy and delivery rates using thawed sperm collected before cancer therapy ranges from 7.0% to 66.7%, and 2.3% to 50.0%, respectively. The pregnancy and delivery rates after ICSI were higher than for IVF and IUI in the present study. According to an early study, it took a median of three cycles to achieve pregnancy in ICSI, whereas eight cycles were required in IVF (Kelleher et al., 2001). Similar pregnancy rates have been observed compared with non-cancer controls or with fresh sperm (Schmidt et al., 2004; García et al., 2015). Indeed, it was disappointing to find that the clinical pregnancy and delivery rates were lower than theoretically expected (Boulet et al., 2015; Hu et al., 2020). This discrepancy could be because the included studies cover a wide timespan, with publication dates ranging from 1985 to 2021 (36 years). After all, the first successful treatment with conventional IVF was in 1978 (Steptoe and Edwards, 1978). In many respects, ART is still an immature field in its early stages of development. Our analysis also showed that more recent publications reported higher pregnancy and delivery rates, which may not be unexpected owing to improvements in ART over the years.
Our study has yielded some interesting insights into the impact of different tumor types on pregnancy and delivery rates. Notably, the pregnancy and delivery rates of lymphoma patients were lower than those of other cancer patients in this study. Indeed, there are many different subtypes of lymphatic tumors, each subtype having its own behavior, rate of progression, and response to treatment. The diffuse large B-cell lymphoma (DLBCL) is the most prevalent subtype of lymphoma among male patients (Ekberg et al., 2020). A study from Sweden revealed that the cumulative rate of childbirth showed a decrease in the 10-year follow-up period among patients diagnosed with DLBCL, whereas no difference in fertility was observed among those with other types of lymphoma (Entrop et al., 2023). In fact, different tumor types have distinct subtypes, and it may be biased to generalize the findings by pooling data from patients with heterogeneous tumor types, as these generalizations may not hold true for all tumor populations. Furthermore, this difference also may result from various ART approaches. Some studies have also reported decreased sperm counts and anti-Müllerian hormone concentration among lymphoma patients prior to treatment, suggesting that spermatogenesis was also affected by the disease (Pallotti et al., 2021; Drechsel et al., 2023).
The results of our meta-analysis also revealed that the pooled miscarriage rate after using frozen sperm was 13% (95%, CI, 10–17%) and, as ∼15–20% of all clinically confirmed pregnancies end in a miscarriage (Hooker et al., 2014), our data align with data from the general population. A critical indicator of miscarriage is the absence of fetal heart activity. However, most included studies did not provide data on fetal heart activity. It is important to note that a single ultrasound indicating the absence of a fetal heartbeat is not immediately conclusive. Depending on the circumstances and the specific timing during the pregnancy, waiting and performing another ultrasound a week later to verify the absence of fetal heart activity before definitively diagnosing a miscarriage is recommended. Therefore, the absence of data on fetal heart activity may introduce potential bias in the miscarriage data.
In fact, ART plays a key role in future fertility planning of patients with cancer aiming for fertility preservation. However, it should be noted that there is heterogeneity among human sperm banking services based on geographical location or setting. The decision of patients to bank or use sperm, as well as the follow-up rate, may be influenced by national legislation or requirements. For example, the availability and coverage of costs for banking and IVF procedures could be influenced by the national health system or insurance companies. Additionally, local guidelines play a role, such as whether fertility centers regularly follow-up with patients. These variations can further impact the interpretation of data regarding the success rate and rate of use of frozen sperm.
As with all meta-analyses, some limitations should be considered. The first limitation of our study is that the included data span 36 years. During this time, the WHO has revised its sperm analysis standards, and other important changes have been seen, including improved cancer survival rates, modifications of cancer treatment regimens, increased incidence of malignancies during reproductive age, and increased awareness among oncologists of the availability of sperm cryopreservation for male fertility preservation. Another limitation is that our aim was limited to the failed-to-cryopreserve rate, sperm disposal rate, sperm use rate, pregnancy rate, miscarriage rate, and delivery rate as the outcome of fertility preservation. However, it is important to note though, that these rates represent only some of the potential factors analyzed for the beneficial effects in male cancer patients and that these findings do not extend to an analysis of the impact of different types of cancer on sperm quality. Many of the earlier studies were limited by small sample sizes and a lack of control groups. Furthermore, almost all of studies did not consider the severity of the disease, which could potentially have a substantial impact on the results. Consequently, further research should evaluate the effect of the type of cancer and, in particular, the severity of the condition on sperm quality in order to draw more precise conclusions. Similarly, it is inappropriate that most studies failed to differentiate between types of tumors and, instead, drew generalized conclusions that are presumed to apply to all cancer patients. We did not have in-depth information on the patients’ disease and, although extensive efforts were made to conduct a thorough systematic review and meta-analysis of the outcomes in terms of the various tumor types, the results must be acknowledged as being subject to bias. On the other hand, the use of average results obtained in each included study, without the individual patient-level data, might also represent a source of bias. Finally, almost no studies provided in-depth information regarding the semen and its usage from the same patient after multiple ejaculations. Such information is valuable, as it can be used to customize the care model to the individual.
In conclusion, our study supported previous reports that sperm cryopreservation is an effective method of fertility preservation in male patients with cancer. The rate of use of frozen sperm in our review underestimated the actual rate, making it meaningful to actively recommend fertility preservation to patients with cancer. ART plays an important role in fertility preservation and pregnancy achievement, with ICSI resulting in better clinical outcomes than IVF and IUI in patients with cancer. Hence, fertility preservation still requires the involvement of oncologists, reproductive medicine clinicians, andrologists, and embryologists.
Supplementary Material
Contributor Information
Qing Li, Department of Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China.
Qiong-Yu Lan, Department of Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China.
Wen-Bing Zhu, Human Sperm Bank, Reproductive & Genetic Hospital of CITIC-Xiangya, Changsha, Hunan, People’s Republic of China; The Institute of Reproductive and Stem Cell Engineering, Basic Medicine College, Central South University, Changsha, Hunan, People’s Republic of China.
Li-Qing Fan, Human Sperm Bank, Reproductive & Genetic Hospital of CITIC-Xiangya, Changsha, Hunan, People’s Republic of China; The Institute of Reproductive and Stem Cell Engineering, Basic Medicine College, Central South University, Changsha, Hunan, People’s Republic of China.
Chuan Huang, Human Sperm Bank, Reproductive & Genetic Hospital of CITIC-Xiangya, Changsha, Hunan, People’s Republic of China; The Institute of Reproductive and Stem Cell Engineering, Basic Medicine College, Central South University, Changsha, Hunan, People’s Republic of China.
Data availability
Literature research results are available from the authors upon reasonable request.
Authors’ roles
Q.L. contributed to study conception and design, data analysis, and manuscript writing. C.H. contributed to study conception and design, execution, data analysis, and manuscript writing. W.-B.Z. contributed to data analysis. Q.-Y.L. contributed analysis tools and assessed the quality of included studies. L.-Q.F. contributed analysis tools and assessed the quality of included studies. All authors approved the final version of the article.
Funding
National Natural Science Foundation of China (82001634, 81960550); China Postdoctoral Science Foundation (2019M661521).
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Agarwal A, Ranganathan P, Kattal N, Pasqualotto F, Hallak J, Khayal S, Mascha E.. Fertility after cancer: a prospective review of assisted reproductive outcome with banked semen specimens. Fertil Steril 2004;81:342–348. [DOI] [PubMed] [Google Scholar]
- Appiah LC. Fertility preservation for adolescents receiving cancer therapies. Clin Obstet Gynecol 2020;63:574–587. [DOI] [PubMed] [Google Scholar]
- Auger J, Sermondade N, Eustache F.. Semen quality of 4480 young cancer and systemic disease patients: baseline data and clinical considerations. Basic Clin Androl 2016;26:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb A, Farah N, Lyons C, Lindsay K, Reddy N, Goldman J, Apperley JF, Salooja N.. Uptake and outcome of assisted reproductive techniques in long-term survivors of SCT. Bone Marrow Transplant 2012;47:568–573. [DOI] [PubMed] [Google Scholar]
- Bahadur G, Ozturk O, Muneer A, Wafa R, Ashraf A, Jaman N, Patel S, Oyede AW, Ralph DJ.. Semen quality before and after gonadotoxic treatment. Hum Reprod 2005;20:774–781. [DOI] [PubMed] [Google Scholar]
- Bandak M, Jensen A, Dehlendorff C, Lauritsen J, Kreiberg M, Wagner T, Rosenvilde J, Daugaard G.. Paternity after treatment for testicular germ cell cancer: a Danish nationwide population-based cohort study. J Natl Cancer Inst 2022;114:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S. Fertility preservation in male patients with cancer. Best Pract Res Clin Obstet Gynaecol 2019;55:59–66. [DOI] [PubMed] [Google Scholar]
- Behboudi-Gandevani S, Bidhendi-Yarandi R, Panahi MH, Vaismoradi M.. A systematic review and meta-analysis of male infertility and the subsequent risk of cancer. Front Oncol 2021;11:696702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl S, Joshi VB, Hussein RS, Walker DL, Lampat KL, Krenik AG, Barud KM, Fredrickson JR, Galanits TM, Rian KJ. et al. Consult and procedure incidence outcomes following establishment of a fertility preservation program for children with cancer. J Assist Reprod Genet 2021;38:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizet P, Saias-Magnan J, Jouve E, Grillo JM, Karsenty G, Metzler-Guillemain C, Perrin J.. Sperm cryopreservation before cancer treatment: a 15-year monocentric experience. Reprod Biomed Online 2012;24:321–330. [DOI] [PubMed] [Google Scholar]
- Blackhall FH, Atkinson AD, Maaya MB, Ryder WD, Horne G, Brison DR, Lieberman BA, Radford JA.. Semen cryopreservation, utilisation and reproductive outcome in men treated for Hodgkin’s disease. Br J Cancer 2002;87:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan A, Karpol S, Lehavi O, Paz G, Kleiman SE, Yogev L, Yavetz H, Hauser R.. Preservation of sperm of cancer patients: extent of use and pregnancy outcome in a tertiary infertility center. Asian J Androl 2013;15:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ.. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. Jama 2015;313:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannigan RE, Fantus RJ, Halpern JA.. Fertility preservation in men: a contemporary overview and a look toward emerging technologies. Fertil Steril 2021;115:1126–1139. [DOI] [PubMed] [Google Scholar]
- Brydøy M, Fosså SD, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, Dahl O.. Paternity following treatment for testicular cancer. J Natl Cancer Inst 2005;97:1580–1588. [DOI] [PubMed] [Google Scholar]
- Chang HC, Chen SC, Chen J, Hsieh JT.. Initial 10-year experience of sperm cryopreservation services for cancer patients. J Formos Med Assoc 2006;105:1022–1026. [DOI] [PubMed] [Google Scholar]
- Chen L, Dong Z, Chen X.. Fertility preservation in pediatric healthcare: a review. Front Endocrinol (Lausanne) 2023;14:1147898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JP, Haines CJ, Kong GW.. Sperm cryopreservation for Chinese male cancer patients: a 17-year retrospective analysis in an assisted reproductive unit in Hong Kong. Hong Kong Med J 2013;19:525–530. [DOI] [PubMed] [Google Scholar]
- Chung K, Irani J, Knee G, Efymow B, Blasco L, Patrizio P.. Sperm cryopreservation for male patients with cancer: an epidemiological analysis at the University of Pennsylvania. Eur J Obstet Gynecol Reprod Biol 2004;113(Suppl 1):S7–S11. [DOI] [PubMed] [Google Scholar]
- Crha I, Ventruba P, Záková J, Huser M, Kubesova B, Hudecek R, Jarkovsky J.. Survival and infertility treatment in male cancer patients after sperm banking. Fertil Steril 2009;91:2344–2348. [DOI] [PubMed] [Google Scholar]
- Dearing C, Breen D, Bradshaw A, Ramsay J, Lindsay K.. Trends and usage in a London National Health Service Sperm Bank for cancer patients. Hum Fertil (Camb) 2014;17:289–296. [DOI] [PubMed] [Google Scholar]
- Del-Pozo-Lerida S, Salvador C, Martinez-Soler F, Tortosa A, Perucho M, Gimenez-Bonafe P.. Preservation of fertility in patients with cancer (Review). Oncol Rep 2019;41:2607–2614. [DOI] [PubMed] [Google Scholar]
- Depalo R, Falagario D, Masciandaro P, Nardelli C, Vacca MP, Capuano P, Specchia G, Battaglia M.. Fertility preservation in males with cancer: 16-year monocentric experience of sperm banking and post-thaw reproductive outcomes. Ther Adv Med Oncol 2016;8:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY.. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril 2013;99:1503–1513. [DOI] [PubMed] [Google Scholar]
- Drechsel KCE, Pilon MCF, Stoutjesdijk F, Meivis S, Schoonmade LJ, Wallace WHB, van Dulmen-den Broeder E, Beishuizen A, Kaspers GJL, Broer SL. et al. Reproductive ability in survivors of childhood, adolescent, and young adult Hodgkin lymphoma: a review. Hum Reprod Update 2023;29:486–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekberg S, K ES, Glimelius I, Nilsson-Ehle H, Goldkuhl C, Lewerin C, Jerkeman M, Eloranta S.. Trends in the prevalence, incidence and survival of non-Hodgkin lymphoma subtypes during the 21st century—a Swedish lymphoma register study. Br J Haematol 2020;189:1083–1092. [DOI] [PubMed] [Google Scholar]
- Entrop JP, Weibull CE, Smedby KE, Jakobsen LH, Ovlisen AK, Glimelius I, Marklund A, Larsen TS, Holte H, Fossa A. et al. Reproduction patterns among non-Hodgkin lymphoma survivors by subtype in Sweden, Denmark and Norway: a population-based matched cohort study. Br J Haematol 2023;202:785–795. [DOI] [PubMed] [Google Scholar]
- Feng RM, Zong YN, Cao SM, Xu RH.. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Paffoni A, Filippi F, Busnelli A, Vegetti W, Somigliana E.. Sperm cryopreservation and reproductive outcome in male cancer patients: a systematic review. Reprod Biomed Online 2016;33:29–38. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Paffoni A, Reschini M, Noli S, Dallagiovanna C, Guarneri C, Filippi F, Somigliana E.. Variables affecting long-term usage rate of sperm samples cryopreserved for fertility preservation in cancer patients. Andrology 2021;9:204–211. [DOI] [PubMed] [Google Scholar]
- Fitoussi O, Eghbali H, Tchen N, Berjon JP, Soubeyran P, Hoerni B.. Semen analysis and cryoconservation before treatment in Hodgkin's disease. Ann Oncol 2000;11:679–684. [DOI] [PubMed] [Google Scholar]
- Fossa SD, Aass N, Molne K.. Is routine pre-treatment cryopreservation of semen worthwhile in the management of patients with testicular cancer? Br J Urol 1989;64:524–529. [DOI] [PubMed] [Google Scholar]
- Freour T, Mirallie S, Jean M, Barriere P.. Sperm banking and assisted reproductive outcome in men with cancer: a 10 years’ experience. Int J Clin Oncol 2012;17:598–603. [DOI] [PubMed] [Google Scholar]
- Fu L, Zhou F, An Q, Zhang K, Wang X, Xu J, Guo Y, Lu W, Liang X, Gu Y.. Sperm cryopreservation for male cancer patients: more than 10 years of experience, in Beijing China. Med Sci Monit 2019;25:3256–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A, Herrero MB, Holzer H, Tulandi T, Chan P.. Assisted reproductive outcomes of male cancer survivors. J Cancer Surviv 2015;9:208–214. [DOI] [PubMed] [Google Scholar]
- Ghazarian AA, McGlynn KA.. Increasing incidence of testicular germ cell tumors among racial/ethnic minorities in the United States. Cancer Epidemiol Biomarkers Prev 2020;29:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg ES, Yanushpolsky EH, Jackson KV.. In vitro fertilization for cancer patients and survivors. Fertil Steril 2001;75:705–710. [DOI] [PubMed] [Google Scholar]
- Girasole CR, Cookson MS, Smith JA Jr, Ivey BS, Roth BJ, Chang SS.. Sperm banking: use and outcomes in patients treated for testicular cancer. BJU Int 2007;99:33–36. [DOI] [PubMed] [Google Scholar]
- Greaves P, Sarker SJ, Chowdhury K, Johnson R, Matthews J, Matthews R, Smith M, Korszun A, Gribben JG, Lister TA.. Fertility and sexual function in long-term survivors of haematological malignancy: using patient-reported outcome measures to assess a neglected area of need in the late effects clinic. Br J Haematol 2014;164:526–535. [DOI] [PubMed] [Google Scholar]
- Grin L, Girsh E, Harlev A.. Male fertility preservation – methods, indications and challenges. Andrologia 2021;53:e13635. [DOI] [PubMed] [Google Scholar]
- Hamano I, Hatakeyama S, Nakamura R, Fukuhara R, Noro D, Tanaka T, Yoneyama T, Yamamoto H, Yoneyama T, Hashimoto Y. et al. Differences in semen characteristics between patients with testicular cancer and other malignancies using various cut-off values. Int J Urol 2018;25:817–824. [DOI] [PubMed] [Google Scholar]
- Hibi H, Sugie M, Ohori T, Fukunaga N, Sonohara M, Asada Y.. Male infertility treatment for cancer survivors: does anticancer treatment affect infertility treatment? Nagoya J Med Sci 2020;82:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer BC, Brolmann HA, Mol BW, Huirne JA.. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update 2014;20:262–278. [DOI] [PubMed] [Google Scholar]
- Hourvitz A, Goldschlag DE, Davis OK, Gosden LV, Palermo GD, Rosenwaks Z.. Intracytoplasmic sperm injection (ICSI) using cryopreserved sperm from men with malignant neoplasm yields high pregnancy rates. Fertil Steril 2008;90:557–563. [DOI] [PubMed] [Google Scholar]
- Hu L, Bu Z, Huang G, Sun H, Deng C, Sun Y.. Assisted reproductive technology in China: results generated from data reporting system by CSRM from 2013 to 2016. Front Endocrinol (Lausanne) 2020;11:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Xu YC, Kuang LH, Lan QY, Hu J, Zhu W, Fan L, Li Q.. Practices, attitudes, and knowledge among healthcare providers and oncologists in china regarding male fertility preservation. Front Reprod Health 2022;4:801378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe E, Matsuda T, Daudin M, Chevreau C, Bachaud JM, Plante P, Bujan L, Thonneau P.. Fertility after testicular cancer treatments: results of a large multicenter study. Cancer 2004;100:732–737. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Kaneko S, Miyaji K, Takamatsu K.. Cryopreservation of human sperm in patients with malignancy: first 2 years’ experience. Reprod Med Biol 2007;6:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ichioka K, Dahal S, Matsui Y, Nakayama T, Hatayama H, Ogawa O, Negoro H.. Barriers for sperm cryopreservation in advanced germ cell tumor patients: a 20-year experience. Int J Clin Oncol 2020;25:906–911. [DOI] [PubMed] [Google Scholar]
- Jacobs LA, Vaughn DJ.. Hypogonadism and infertility in testicular cancer survivors. J Natl Compr Canc Netw 2012;10:558–563. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Cooper AR, Jungheim ES, Lanzendorf SE, Odem RR, Ratts VS.. Sperm banking for fertility preservation: a 20-year experience. Eur J Obstet Gynecol Reprod Biol 2013;170:177–182. [DOI] [PubMed] [Google Scholar]
- Keane D, Naughton S, Waite K, Harrison RF.. The provision of a semen cryopreservation service for male cancer sufferers in the Republic of Ireland. Ir J Med Sci 2000;169:22–25. [DOI] [PubMed] [Google Scholar]
- Keene DJ, Sajjad Y, Makin G, Cervellione RM.. Sperm banking in the United Kingdom is feasible in patients 13 years old or older with cancer. J Urol 2012;188:594–597. [DOI] [PubMed] [Google Scholar]
- Kelleher S, Wishart SM, Liu PY, Turner L, Di Pierro I, Conway AJ, Handelsman DJ.. Long-term outcomes of elective human sperm cryostorage. Hum Reprod 2001;16:2632–2639. [DOI] [PubMed] [Google Scholar]
- Khalifa E, Oehninger S, Acosta AA, Morshedi M, Veeck L, Bryzyski RG, Muasher SJ.. Successful fertilization and pregnancy outcome in in-vitro fertilization using cryopreserved/thawed spermatozoa from patients with malignant diseases. Hum Reprod 1992;7:105–108. [DOI] [PubMed] [Google Scholar]
- Kitlinski M, Giwercman A, Elenkov A.. Paternity through use of assisted reproduction technology in male adult and childhood cancer survivors: a nationwide register study. Hum Reprod 2023;38:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester PA, Leonard M, Wood DP, Schuster TG.. Fertility issues for men with newly diagnosed prostate cancer. Urology 2007;69:123–125. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Tamura K, Tai T, Nagao K, Nakajima K.. Semen cryopreservation as an oncofertility treatment in 122 Japanese men with cancer: a decade-long study. Reprod Med Biol 2017;16:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackamp N, Wilkemeyer I, Jelas I, Keller U, Bullinger L, Stintzing S, Le Coutre P.. Survey of long-term experiences of sperm cryopreservation in oncological and non-oncological patients: usage and reproductive outcomes of a large monocentric cohort. Front Oncol 2021;11:772809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Akagbosu F, Abusheikha N, Hassouneh M, Blayney M, Avery S, Brinsden P.. A programme of semen cryopreservation for patients with malignant disease in a tertiary infertility centre: lessons from 8 years’ experience. Hum Reprod 1998;13:3256–3261. [DOI] [PubMed] [Google Scholar]
- Levi-Setti PE, Negri L, Baggiani A, Morenghi E, Albani E, Parini V, Cafaro L, Dioguardi CMC, Cesana A, Smeraldi A. et al. Delayed childbearing and female ageing impair assisted reproductive technology outcome in survivors of male haematological cancers. J Assist Reprod Genet 2018;35:2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu B, Liu S, Xian Y, Zhao W, Zhou B, Xiao X, Wang L, Zhu X, Shu B. et al. Male cancer patient sperm cryopreservation for fertility preservation: 10-year monocentric experience. Basic Clin Androl 2021;31:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CK, Mertz D, Loeb M.. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machen GL, Harris SE, Bird ET, Brown ML, Ingalsbe DA, East MM, Reyes M, Kuehl TJ.. Utilization of cryopreserved sperm cells based on the indication for storage. Investig Clin Urol 2018;59:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magelssen H, Haugen TB, von During V, Melve KK, Sandstad B, Fossa SD.. Twenty years experience with semen cryopreservation in testicular cancer patients: who needs it? Eur Urol 2005;48:779–785. [DOI] [PubMed] [Google Scholar]
- Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril 2013;100:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer M, Molina N, Garcia-Velasco JA, Remohi J, Pellicer A, Garrido N.. Sperm cryopreservation in oncological patients: a 14-year follow-up study. Fertil Steril 2006;85:640–645. [DOI] [PubMed] [Google Scholar]
- Milligan DW, Hughes R, Lindsay KS.. Semen cryopreservation in men undergoing cancer chemotherapy—a UK survey. Br J Cancer 1989;60:966–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I, Oude Ophuis RJ, Broekmans FJ, Lock TM.. Semen cryopreservation and usage rate for assisted reproductive technology in 898 men with cancer. Reprod Biomed Online 2016;32:147–153. [DOI] [PubMed] [Google Scholar]
- Neal MS, Nagel K, Duckworth J, Bissessar H, Fischer MA, Portwine C, Tozer R, Barr RD.. Effectiveness of sperm banking in adolescents and young adults with cancer: a regional experience. Cancer 2007;110:1125–1129. [DOI] [PubMed] [Google Scholar]
- Negoro H, Matsui Y, Nakayama T, Hatayama H, Ogawa O, Ichioka K.. Sperm cryopreservation: clinical and fertility outcomes in male oncological patients with germ cell tumors or hematological disorders. Reprod Med Biol 2018;17:500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzli J, Voruz S, Wunder D, Primi MP, Spertini O, Blum S.. Ten-year single-centre experience in fertility preservation of 459 patients suffering from acute leukaemia. Br J Haematol 2019;184:969–973. [DOI] [PubMed] [Google Scholar]
- Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW.. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2018;36:1994–2001. [DOI] [PubMed] [Google Scholar]
- Pallotti F, Pelloni M, Faja F, Di Chiano S, Di Rocco A, Lenzi A, Lombardo F, Paoli D.. Semen quality in non-Hodgkin lymphoma survivors: a monocentric retrospective study. Hum Reprod 2021;36:16–25. [DOI] [PubMed] [Google Scholar]
- Paoli D, Rizzo F, Fiore G, Pallotti F, Pulsoni A, Annechini G, Lombardo F, Lenzi A, Gandini L.. Spermatogenesis in Hodgkin’s lymphoma patients: a retrospective study of semen quality before and after different chemotherapy regimens. Hum Reprod 2016;31:263–272. [DOI] [PubMed] [Google Scholar]
- Papler TB, Vrtacnik-Bokal E, Drobnic S, Stimpfel M.. The outcome of IVF/ICSI cycles in male cancer patients: retrospective analysis of procedures from 2004 to 2018. Radiol Oncol 2021;55:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P, Gu BH, Li P, Huang YR, Li Z.. Fertility outcome of patients with testicular tumor: before and after treatment. Asian J Androl 2014;16:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping P, Zhu WB, Zhang XZ, Yao KS, Xu P, Huang YR, Li Z.. Sperm banking for male reproductive preservation: a 6-year retrospective multi-centre study in China. Asian J Androl 2010;12:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 2019;112:1022–1033. [DOI] [PubMed] [Google Scholar]
- Pulte D, Gondos A, Brenner H.. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood 2009;113:1408–1411. [DOI] [PubMed] [Google Scholar]
- Ragni G, Somigliana E, Restelli L, Salvi R, Arnoldi M, Paffoni A.. Sperm banking and rate of assisted reproduction treatment: insights from a 15-year cryopreservation program for male cancer patients. Cancer 2003;97:1624–1629. [DOI] [PubMed] [Google Scholar]
- Redman JR, Bajorunas DR, Goldstein MC, Evenson DP, Gralla RJ, Lacher MJ, Koziner B, Lee BJ, Straus DJ, Clarkson BD.. Semen cryopreservation and artificial insemination for Hodgkin’s disease. J Clin Oncol 1987;5:233–238. [DOI] [PubMed] [Google Scholar]
- Reed E, Sanger WG, Armitage JO.. Results of semen cryopreservation in young men with testicular carcinoma and lymphoma. J Clin Oncol 1986;4:537–539. [DOI] [PubMed] [Google Scholar]
- Revel A, Haimov-Kochman R, Porat A, Lewin A, Simon A, Laufer N, Gino H, Meirow D.. In vitro fertilization-intracytoplasmic sperm injection success rates with cryopreserved sperm from patients with malignant disease. Fertil Steril 2005;84:118–122. [DOI] [PubMed] [Google Scholar]
- Rhodes EA, Hoffman DJ, Kaempfer SH.. Ten years of experience with semen cryopreservation by cancer patients: follow-up and clinical considerations. Fertil Steril 1985;44:512–516. [DOI] [PubMed] [Google Scholar]
- Rhoton-Vlasak A. Infections and infertility. Prim Care Update Ob Gyns 2000;7:200–206. [DOI] [PubMed] [Google Scholar]
- Scammell GE, White N, Stedronska J, Hendry WF, Edmonds DK, Jeffcoate SL.. Cryopreservation of semen in men with testicular tumour or Hodgkin’s disease: results of artificial insemination of their partners. Lancet 1985;2:31–32. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Larsen E, Bangsboll S, Meinertz H, Carlsen E, Andersen AN.. Assisted reproduction in male cancer survivors: fertility treatment and outcome in 67 couples. Hum Reprod 2004;19:2806–2810. [DOI] [PubMed] [Google Scholar]
- Selk A, Belej-Rak T, Shapiro H, Greenblatt E.. Use of an oncology sperm bank: a Canadian experience. Can Urol Assoc J 2009;3:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P, PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- Sheth KR, Sharma V, Helfand BT, Cashy J, Smith K, Hedges JC, Kohler TS, Woodruff TK, Brannigan RE.. Improved fertility preservation care for male patients with cancer after establishment of formalized oncofertility program. J Urol 2012;187:979–986. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Wagle NS, Jemal A.. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. [DOI] [PubMed] [Google Scholar]
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J.. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712–716. [DOI] [PubMed] [Google Scholar]
- Song SH, Kim DK, Sung SY, Her YS, Lee OH, Choi MH, Kim HK, Lyu SW, Kim DS.. Long-term experience of sperm cryopreservation in cancer patients in a single fertility center. World J Mens Health 2019;37:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg DW, Brames MJ, Case-Eads S, Einhorn LH.. Utilization of sperm banking and barriers to its use in testicular cancer patients. Support Care Cancer 2015;23:2763–2768. [DOI] [PubMed] [Google Scholar]
- Spermon JR, Kiemeney LA, Meuleman EJ, Ramos L, Wetzels AM, Witjes JA.. Fertility in men with testicular germ cell tumors. Fertil Steril 2003;79(Suppl 3):1543–1549. [DOI] [PubMed] [Google Scholar]
- Stahl O, Eberhard J, Jepson K, Spano M, Cwikiel M, Cavallin-Stahl E, Giwercman A.. Sperm DNA integrity in testicular cancer patients. Hum Reprod 2006;21:3199–3205. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG.. Birth after the reimplantation of a human embryo. Lancet 1978;2:366. [DOI] [PubMed] [Google Scholar]
- Stigliani S, Massarotti C, De Leo C, Maccarini E, Sozzi F, Cagnacci A, Anserini P, Scaruffi P.. Fifteen year regional center experience in sperm banking for cancer patients: use and reproductive outcomes in survivors. Cancers (Basel) 2021;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh CH, Park SH.. Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol 2016;17:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholeti P, Uppangala S, Bhat V, Udupa KS, Kumar V, Patted S, Natarajan P, Spears N, Kalthur G, Woodruff TK. et al. Oncofertility: knowledge, attitudes, and barriers among indian oncologists and gynecologists. J Adolesc Young Adult Oncol 2021;10:71–77. [DOI] [PubMed] [Google Scholar]
- Tomlinson M, Meadows J, Kohut T, Haoula Z, Naeem A, Pooley K, Deb S.. Review and follow-up of patients using a regional sperm cryopreservation service: ensuring that resources are targeted to those patients most in need. Andrology 2015;3:709–716. [DOI] [PubMed] [Google Scholar]
- Tournaye H, Camus M, Bollen N, Wisanto A, Van Steirteghem AC, Devroey P.. In vitro fertilization techniques with frozen-thawed sperm: a method for preserving the progenitive potential of Hodgkin patients. Fertil Steril 1991;55:443–445. [DOI] [PubMed] [Google Scholar]
- Uçar MA, Arikan F, Coşkun HŞ, Kondak Y, Tatlı AM, Göksu SS.. Fertility in testicular cancer patients: a single-centre study in Turkey. Int J Clin Oncol 2019;25:495–500. [DOI] [PubMed] [Google Scholar]
- Ukita Y, Wakimoto Y, Sugiyama Y, Fujii Y, Fukui A, Hasegawa A, Kondoh N, Yamamoto S, Shibahara H.. Fertility preservation and pregnancy outcomes in adolescent and young adult male patients with cancer. Reprod Med Biol 2018;17:449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakalopoulos I, Dimou P, Anagnostou I, Zeginiadou T.. Impact of cancer and cancer treatment on male fertility. Hormones (Athens) 2015;14:579–589. [DOI] [PubMed] [Google Scholar]
- van Casteren NJ, Boellaard WP, Romijn JC, Dohle GR.. Gonadal dysfunction in male cancer patients before cytotoxic treatment. Int J Androl 2010;33:73–79. [DOI] [PubMed] [Google Scholar]
- van Casteren NJ, van Santbrink EJ, van Inzen W, Romijn JC, Dohle GR.. Use rate and assisted reproduction technologies outcome of cryopreserved semen from 629 cancer patients. Fertil Steril 2008;90:2245–2250. [DOI] [PubMed] [Google Scholar]
- van der Kaaij MAE, van Echten-Arends J, Heutte N, Meijnders P, Abeilard-Lemoisson E, Spina M, Moser EC, Allgeier A, Meulemans B, Lugtenburg PJ, European Organisation for Research and Treatment of Cancer Lymphoma Group and the Groupe d’Étude des Lymphomes de l’Adulte et al. Cryopreservation, semen use and the likelihood of fatherhood in male Hodgkin lymphoma survivors: an EORTC-GELA Lymphoma Group cohort study. Hum Reprod 2014;29:525–533. [DOI] [PubMed] [Google Scholar]
- Vomstein K, Reiser E, Pinggera GM, Toerzsoek P, Deininger S, Kriesche T, Biasio W, Lusuardi L, Toth B.. Sperm banking before gonadotoxic treatment: is it worth the effort? Asian J Androl 2021;23:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Kakimoto K, Uemura M, Kishida T, Kawai K, Nakamura T, Goto T, Osawa T, Yamada S, Nishimura K. et al. Fertility and reproductive technology use in testicular cancer survivors in Japan: a multi-institutional, cross-sectional study. Int J Urol 2021;28:1047–1052. [DOI] [PubMed] [Google Scholar]
- Záková J, Lousová E, Ventruba P, Crha I, Pochopová H, Vinklárková J, Tesařová E, Nussir M.. Sperm cryopreservation before testicular cancer treatment and its subsequent utilization for the treatment of infertility. ScientificWorldJournal 2014;2014:575978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn B, Virant-Klun I, Stanovnik M, Drobnic S, Meden-Vrtovec H.. Intracytoplasmic sperm injection by testicular sperm in patients with aspermia or azoospermia after cancer treatment. Int J Androl 2006;29:521–527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Literature research results are available from the authors upon reasonable request.