Abstract
Retrotransposon Ty1 faces a formidable cell barrier during transposition—the yeast nuclear membrane which remains intact throughout the cell cycle. We investigated the mechanism by which transposition intermediates are transported from the cytoplasm (the presumed site of Ty1 DNA synthesis) to the nucleus, where they are integrated into the genome. Ty1 integrase has a nuclear localization signal (NLS) at its C terminus. Both full-length integrase and a C-terminal fragment localize to the nucleus. C-terminal deletion mutants in Ty1 integrase were used to map the putative NLS to the last 74 amino acid residues of integrase. Mutations in basic segments within this region decreased retrotransposition at least 50-fold in vivo. Furthermore, these mutant integrase proteins failed to localize to the nucleus. Production of virus-like particles, reverse transcriptase activity, and complete in vitro Ty1 integration resembled wild-type levels, consistent with failure of the mutant integrases to enter the nucleus.
Yeast retrotransposon Ty1 is similar in many aspects to retroviruses (7). Ty1 must generate more copies of itself for insertion into the genome of the host cell. Ty1 mRNA is transcribed and processed in the nucleus and then transported to the cytoplasm, where it is translated into Gag and Gag-Pol proteins. These are assembled into virus-like particles (VLPs) resembling retroviral core particles (34, 50). Reverse transcription of Ty1 RNA into Ty1 cDNA occurs in the VLPs, which also contain integrase (IN) (30). Unlike retroviruses, in which the viral core becomes enveloped and leaves the producer cell to infect a new cell and integrate into the target genome, Ty1 cDNA reenters the producer cell nucleus to integrate at a new target site. How extrachromosomal Ty1 cDNA enters the nucleus where it can access its target site DNA remains unresolved.
The question of how Ty1 enters the nucleus is especially important because in all fungi, including the Ty1 host Saccharomyces cerevisiae, the nuclear envelope (NE) does not break down during mitosis (43). This significant intracellular barrier prevents Ty1 DNA from accessing the genome via mitotic NE dissociation, a cellular process apparently exploited by many retroviruses to gain nuclear access (55). However, this opportunity exists only in cells that are undergoing growth and division. Lentiviruses like human immunodeficiency virus type 1 (HIV-1) are able to infect nondividing cells (15, 17), which, like growing yeast cells, do not offer a mitotic window of opportunity to the nucleus. Thus, an understanding of the mechanism used by Ty1 to enter the nucleus should shed light on the analogous process in lentiviruses.
Several possible mechanisms for nuclear entry can be envisioned. VLPs might enter the nucleus intact. However, VLPs are estimated to be 600 Å in diameter and thus seem too large to be transported through the nuclear pore complex (NPC) (13, 19, 34, 50). Maximal pore size estimates range from 90 Å (for proteins that enter the nucleus passively) to 260 Å (for NLS-bearing proteins of >∼40 kDa) (23, 29). Alternatively, VLPs might enter the nucleus by means of a conformational change allowing passage through pores. However, electron micrographs show that most if not all VLPs are cytoplasmic (34, 50). Another formal possibility is that VLPs dock near the pore and inject their contents into the nucleus (7) much like what has been proposed for adenovirus (21). A more likely possibility is that a subassembly of the particle (similar to retroviral preintegration complexes) is transported into the nucleus by host cell machinery.
IN is a Ty1 protein that must enter the nucleus since its intranuclear function of inserting a Ty1 cDNA copy into the chromosomal DNA is absolutely required for retrotransposition. In this study, we demonstrated that Ty1 IN indeed has a bipartite nuclear localization signal (NLS) that is essential for retrotransposition. This NLS differs from other bipartite signals in its long spacer region. An analysis of related protein sequences found in the complete set of yeast protein sequences suggests there may be other NLSs of this type.
MATERIALS AND METHODS
Strains and media.
The plasmids used are listed in Table 1. DNA cloning was performed by use of standard techniques described by Sambrook et al. (56). Selection against Ura+ strains was accomplished by culture on solid synthetic complete (SC) medium containing 1 mg of 5-fluoro-orotic acid (5-Foa) per ml (6).
TABLE 1.
Plasmids used in this study
| Plasmid | Markers | Comments | Source |
|---|---|---|---|
| pGEU414 | CEN TRP1 | GAL1 E1 | 28 |
| pGEU415 | CEN LEU2 | GAL1 E1 | 28 |
| pCAR224 | CEN LEU2 | C-terminal IN in p415GEU1 | This study |
| pCAR227 | CEN LEU2 | Full-length IN in p415GEU1 | This study |
| pCAR607 | CEN LEU2 | N-terminal IN in p415GEU1 | This study |
| pMAK60 | CEN TRP1 | Full-length IN in p414GEU1 | This study |
| pMAK61 | CEN TRP1 | INΔBglII-ClaI | This study |
| pMAK62 | CEN TRP1 | INΔEcoRI | This study |
| pMAK63 | CEN TRP1 | INΔClaI | This study |
| pMAK64 | CEN TRP1 | INΔClaI + EcoRI | This study |
| pMAK65 | CEN TRP1 | INΔBglII-ClaI/ΔEcoRI | This study |
| pMAK66 | CEN TRP1 | C-terminal IN in pGEU414 | This study |
| pMAK67 | CEN TRP1 | N-terminal IN in pGEU414 | This study |
| pX3 | CEN URA3 TRP1 | Ty1-TRP1, URA3 on plasmid | 58 |
| pMAK69 | CEN URA3 TRP1 | IN B1 in pX3 | This study |
| pMAK70 | CEN URA3 TRP1 | IN B2 in pX3 | This study |
| pGM315 | CEN URA3 TRP1 | Linker insertion mutation in IN | 51a |
| 90-1553 | CEN URA3 TRP1 | DD-YD change at YXDD domain in Ty1 RT | A. Gabriel (unpublished data) |
| pSD546 | 2 μm HIS3 bla | Target plasmid for in vitro IN assay | 26 |
The following yeast strains were used: W303a (MATa ade2-1 ura3-1 his3-11 trp1-1 leu2-3 can1-100), W303α (MATα ade2-1 ura3-1 his3-11 trp1-1 leu2-3 can1-100), YH50 (MATa his3Δ200 ura3-167 leu2Δ1 trp1Δ1 spt3-Δ202), and YM603 (MATa ura3-52 hisΔ200 ade2-101 lys2-801 met− reg1-501). W303a and -α were obtained from L. Davis. YM603 was obtained from M. Johnston. YH50 was constructed by H. Xu in this laboratory.
Indirect immunofluorescence.
The anti-IN monoclonal antibody (MAb) 8B11 (30) and polyclonal anti-E1 (raised against an avian coronavirus glycoprotein [53]) antibodies were used for immunofluorescence experiments essentially as described by Adams and Pringle (1). To visualize nuclear DNA, cells were stained with 0.5 μg of DAPI (4′,6-diamidino-2-phenylindole) per ml. Treated cells were mounted on glass slides in a 1-mg/ml concentration of p-phenylenediamine in 90% glycerol. The cells were examined by using fluorescence microscopy with a Zeiss Axioscope fitted with UV and fluorescein isothiocyanate (FITC) optics. Images were collected by a cooled charge-coupled device camera (Photometrix) by using IPLab Spectrum software from Signal Analytics (Vienna, Va.) and imported into Adobe Photoshop 3.0 for figure presentation. The following antibodies were used at the dilutions indicated in parentheses: 1 mg of protein G-Sepharose-purified MAb 8B11 (1:2,500 to 1:5,000) per ml, polyclonal R1-F serum (1:2,000), and polyclonal E1 serum (1:500). A diploid yeast strain made by mating W303a and W303α was used for all immunofluorescence experiments.
Construction of GAL-IN subclones: E1-tagged IN constructs.
Three plasmids encoding E1-IN (full length), E1–N-term IN, and E1–C-term IN (N-term and C-term IN are N- and C-terminal fragments of IN and are defined relative to the internal BglII site within the coding region of IN) were made. The E1 epitope tag is derived from the coronavirus glycoprotein (53). The IN regions were amplified by PCR and cloned into expression vector p415GEU1 (28). The fragment encoding full-length IN was amplified by using primer JB581 (nucleotides [nt] 2041 to 2063 with respect to the Ty1-H3 sequence [4]) incorporating a XhoI site 5′ to nt 2041 (see oligonucleotide list below) and primer JB584 (nt 4056 to 4077) incorporating a stop codon and a PstI site 3′ to nt 4077. The fragment encoding N-term IN was amplified by using primers JB581 and JB582 (nt 3286 to 3306) incorporating a stop codon and a PstI site 3′ to nt 3306. The fragment encoding C-term IN was amplified by using primer JB583 (nt 3301 to 3318) incorporating a XhoI site 5′ to nt 3301 and primer JB584. The PCR products were digested with XhoI and PstI, gel purified, and ligated to p415GEU1 digested with XhoI and PstI, creating pCAR153 (E1-IN), pCAR150 (E1–N-term IN), and pCAR151 (E1–C-term IN). Because these initial constructs did not faithfully reproduce the native C terminus of IN protein (amino acid sequence RIHLIA), we corrected the constructs encoding full-length and C-term IN as follows. The SalI-SmaI (SmaI is a polylinker site downstream of the PstI site used in cloning) fragment of IN in pCAR153 was replaced with the SalI-PvuII fragment of IN from pJEF724 (4, 5), creating pCAR226. pCAR224 was constructed in a similar fashion, with the BglII-SmaI fragment of pCAR151 being replaced with the BglII-PvuII fragment of IN from pJEF724. The BssHII fragments of pCAR224 and pCAR226 were then subcloned into pRS414 (CEN TRP), transferring the GAL-E1-IN cassettes and generating pMAK67 and pMAK60 (see Table 1). INΔBglII-ClaI was created by cutting pMAK60 with BglII and ClaI, filling in with Klenow fragment, and ligating the blunt ends, creating pMAK61. This created an in-frame fusion with the rest of the C terminus of IN. INΔEcoRI was created by digesting pMAK60 with EcoRI and religating, creating pMAK62. IN is truncated by a fortuitous stop codon within 3 amino acids of the EcoRI fusion point. INΔClaI was created by doubly digesting pMAK60 with ClaI and EcoRI. The ends were filled in, and the blunt ends were religated, generating pMAK63. This blunt ligation creates a new EcoRI site. In this construct, IN is truncated by a fortuitous stop codon 3 amino acids after the ClaI/EcoRI fusion point. INΔClaI+EcoRI was created by subcloning the 200-bp EcoRI fragment into the single EcoRI site that was generated by filling in and fusing ClaI to EcoRI, generating pMAK64. INΔBglII-ClaI/ΔEcoRI was created by digesting the INΔBglI-ClaI with EcoRI and religating, generating pMAK65.
Oligonucleotides.
The oligonucleotides used in the present study and their sequences were as follows: JB581, CGCGCTCGAGGAATGTCCATACAAGTGAAAGTAC; JB582, GCGCTGCAGTCAAGATCTCTTCTTTGTGGAAG; JB583, CGCGCTCGAGATCTAGCACCCCCCAA; JB584, GCGCTGCAGTCACAGTTGATTGACTTCTTTGTGG; JB1391, GCGGATCCAGCAGCGTTGATAGTAGTATAGGCATT; JB1392, GCGGATCCGCTGCTAATGAAACTGAAATTAAGGTA; JB1393, GCGGATCCAGCAGCTTCTAAACTACGCATATTCTT; JB1394, GCGGATCCGCTGCTCACCTGATTGCAGGGGGGATC; JB1395, GCGGATCCGCTGCTCACCTGATTGCAGCTGTAAA; JB1396, CAATATCCAGTACGGGTG; JB1397, CAGATGGTAAGCCCTCCC; JB1498, AGTTTGGGTGGTATTGGTGACTCTAATGCCTATACTACTATCTTAGA AGATAATGAAACTGAA; JB1499, AGTTTGGGTGGTATTGGTGACTCT AATGCCTATACTACTATCGCAGCAGGATCCGCAGCATTAGAAGATA ATGAAACTGAA; JB1500, AATTAACCCTCACTAAAA; JB1523, AATATG CGTAGTTTAGAACCTCCGGCAGCAGGATTCGCAGCACACCTGATTG CAGGGGGGATC; JB1524, GATCCCCCCTGCAATCAGGTGTGCTGCGGATCCTGCTGCCGGAGGTTCTAAACTACGCATATT; JB1618, AATGCCT ATACTACTATCAACGGTAAGAAGAGATCTAAGGCTGCTGGATCCGC TGCTAATG; JB1619, CATTAGCAGCGGATCCAGCAGCCTTAGATCTCTTCTTACCGTTGATAGTAGTATAGGCATT.
Site-directed mutagenesis.
Mutations (block substitutions or additions) in IN were made in one of two ways by utilizing fusion PCR strategies (38). (i) In the first strategy, the IN mutants in Ty1 were made in the parent vector pX3 (58) (see Fig. 4). Mutant DNA fragments of Ty1 IN were generated by PCR and directly ligated into pCRII (InVitrogen) and sequenced. Amino acid residues of interest (Fig. 1, underlined residues) were replaced by the sequence AAGSAA, which includes a diagnostic BamHI site (51). The first PCR fragment for the IN basic region 1 (B1) mutant was synthesized by using pX3 as the template and oligonucleotides JB1391 and JB1396 as primers. The second PCR fragment was synthesized by using pX3 as the template and oligonucleotides JB1392 and JB771 as primers. pCRII containing the 5′ segment was restricted with KpnI and BamHI. pCRII containing the 3′ segment was restricted with BamHI-AflII. Both insert fragments were ligated to pX3 previously digested with KpnI and AflII. The final construct was also completely sequenced in the regions derived from PCRs. The IN basic region 2 (B2) mutant was similarly assembled by using primer pairs JB1393-JB1396 and JB1395-JB771. (ii) To create block substitutions or insertion mutations, another PCR strategy was also employed (57). 3′ and 5′ primers homologous to the insertion site, each containing the desired insertion or substitution sequences, were designed. These were then used in separate amplification reactions, paired with the appropriate primer from either an upstream or downstream site. The two PCR products of IN thus generated overlap at the ends containing the mutation site; they were then used as the template to amplify the entire mutated construct with the two outside primers. The resulting fragment was cleaved with unique restriction sites and subcloned into the IN expression plasmid of choice.
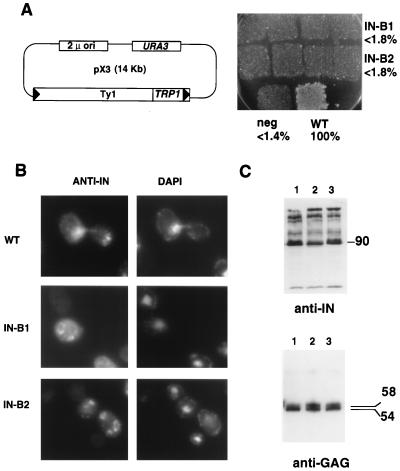
FIG. 4.
Two sites required for nuclear localization and transposition. (A) (Left) The pX3 GAL-Ty1-TRP1 plasmid (58) was used as the parent for making the IN B1 and B2 mutants (residues underlined in amino acid sequence of Fig. 1 were replaced with AAGSAA). Cells containing these pX3 derivatives were assayed for Ty1-TRP1 transposition. (Right) Transposition results for pX3 (wild-type IN [WT]) and mutants B1, B2, and RT (the negative control [neg]) are shown. Percentages of wild-type transposition frequency are indicated. (B) Indirect immunofluorescence. Anti-IN (MAb 8B11) panels are shown on the left (stained with FITC); DAPI staining is shown on the right. (C) (Top) Immunoblot with anti-IN (MAb 8B11) of whole-cell extracts prepared from wild-type (lane 1), IN B1 (lane 2), and IN B2 (lane 3) strains. The apparent molecular mass of fully processed IN is indicated on the right in kilodaltons. (Bottom) Immunoblot with R1-F (anti-Gag antibody). The apparent molecular masses of unprocessed and processed Gag proteins are indicated on the right in kilodaltons. Lanes are as described for the top panel.
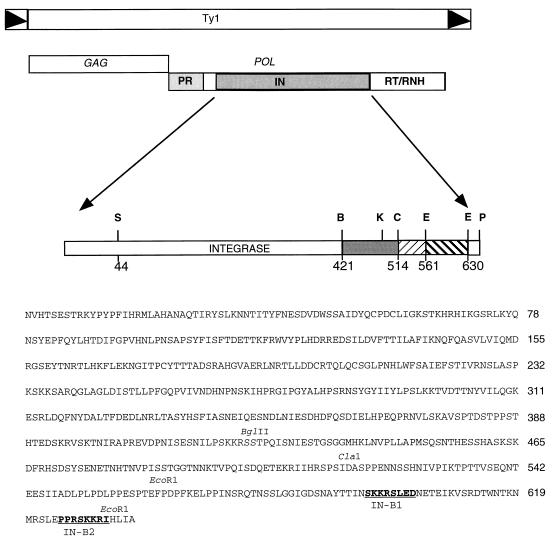
FIG. 1.
Ty1 and IN structure. The structure of the Ty1 retrotransposon is diagrammed. Black triangles indicate the long-terminal-repeat regions. The GAG and POL ORFs and the element-encoded proteins derived from POL are indicated. The complete sequence of Ty1 IN is shown. Restriction site abbreviations: S, SalI; B, BglII; C, ClaI; E, EcoRI; K, KpnI; P, PvuII. The amino acid number is indicated beneath each restriction site. Key restriction sites are indicated above the corresponding amino acid sequence. IN NLS mutations, B1 and B2, made in the GAL-Ty1 plasmid are in boldface type. The numbers to the right of the sequence represent the last amino acid residue on that line of sequence.
Protein analysis.
For fractionation studies, yeast strains were grown to mid-log phase and cells were turned into spheroplasts as described by Kalinich and Douglas (41). The spheroplasts were lysed by resuspension in 10 ml of ice-cold solution A (20 mM HEPES-HCl [pH 7.5], 5 mM MgCl2, protease inhibitors) per g of yeast cell pellet (41), followed by vortexing and incubation on ice for 5 min. Lysates were centrifuged at 9,000 rpm in an SA600 rotor for 45 min at 4°C. The supernatant (S1), representing soluble cellular components, was collected. The pellet was resuspended in 10 ml of ice-cold solution B (20 mM HEPES-HCl [pH 7.4], 5 mM MgCl2, 1 M NaCl, protease inhibitors) per g of yeast cell pellet, incubated on ice for 10 min, and centrifuged at 9,000 rpm for 45 min. This supernatant (S2) was collected. Eight hundred microliters of S1 fractions and 1,000 μl of S2 fractions were precipitated with trichloroacetic acid (TCA) for protein gel analysis and resuspended in Laemmli buffer (45). Proteins were separated electrophoretically on sodium dodecyl sulfate (SDS)–8% polyacrylamide gels as described below. Rabbit antihexokinase antibody was a gift from Rob Jensen’s lab. Mouse antinucleoporin (MAb 414) was a gift from Laura Davis.
Whole-cell yeast extracts were made by harvesting 5 ml of cells (A600, 0.8 to 1.0), washing the cells once with H2O, and resuspending them in 200 μl of H2O. This suspension was frozen in liquid N2 and stored at −80°C. Glass beads and 40 μl of 100% TCA were added, and the yeast cells were broken in a Mini Bead Beater 8 (Biospec Products) as directed by the manufacturer. One milliliter of 5% TCA was added, and samples were spun in a microcentrifuge for 20 min at 4°C. The supernatant was removed, and the pellet was resuspended in H2O and spun and washed as described for the previous step. Proteins were extracted by resuspending the pellet in sample buffer (6% SDS, 0.5 M Tris base) at 45 to 50°C for 10 min two times, pooling the supernatants. One-third volume of a solution of 0.25 M dithiothreitol, 50% glycerol, and 0.2% bromophenol blue was added prior to loading the gel.
Proteins were separated electrophoretically on SDS–8% or –10% polyacrylamide gels and transferred to nitrocellulose by use of a Bio-Rad semidry transfer apparatus. The following antibodies were used at the dilutions indicated in parentheses: 1 mg of protein G-Sepharose-purified MAb 8B11 (1:5,000) per ml, polyclonal R1-F serum (1:2,000), polyclonal E1 serum (1:500). Filters were incubated with the appropriate secondary antibodies and then incubated with an enhanced chemiluminescence reagent (Amersham) and exposed to film.
Transposition assays.
The transposition assay method is similar to that previously described (9). pX3 and its derivatives were transformed into YH50 (46). This strain is an spt3 mutant; hence, transposition events arising from endogenous Ty1 copies are eliminated (8). Colonies from each strain were patched onto SC−Ura−Trp (SC medium lacking uracil and tryptophan) and incubated at 30°C for 2 days. The cells from the plates were replica plated onto SC−Ura−Trp galactose and incubated for 3 days at 22°C for induction of transposition. The cells from the plates were replica plated onto yeast extract-peptone-dextrose (YPD) and incubated at 30°C for 24 h to allow for plasmid loss. After replica plating the cells onto YPD once more, the cells from the final YPD plates were replica plated onto SC+5-Foa−Trp and grown at 30°C. Cells that lost the URA3-based plasmid and contain transpositions grow on SC+5-Foa−Trp. To quantify results, patches from the second YPD replica were scraped into sterile H2O, and appropriate dilutions were plated for single colonies onto YPD plates and replica plated onto SC−Ura and SC−Trp plates. Transposition frequencies were determined by dividing the number of Trp+ Ura− derivatives by the total number of Ura− derivatives, and then converting the value to a percentage of wild-type frequency, normalized to 100%.
VLP preparations, RT assays, and in vitro IN assays.
VLPs were isolated from a reg1 mutant strain (YM603) which allows galactose induction in the presence of glucose (39). A single colony was grown in 10 ml of SC−Ura medium supplemented with 0.8 mM adenine and 2% glucose for 18 to 24 hours at 30°C. This culture was used to inoculate 200 ml of the same medium and was incubated at 30°C for another 24 h. For induction of VLPs, 500 ml of yeast nitrogen base medium without ammonium sulfate and amino acids (Difco) supplemented with 20 g of Casamino Acids per liter, 5 g of ammonium sulfate per liter, 0.8 mM adenine, 2% glucose, and 2% galactose in a 2-liter flask was inoculated with enough cells from the 200-ml culture to give a starting A600 of 0.25 to 0.5. This culture was incubated at 22°C in a floor shaker with vigorous shaking (350 rpm) for 36 h. The final A600 was between 4.0 and 6.0. The cells were harvested, and VLPs were prepared as described previously (10, 30). Reverse transcriptase (RT) activity was assayed as described previously (30, 34), and in vitro integration activity was performed as reported before (25, 26).
RESULTS
Ectopically expressed IN localizes to the nucleus.
The question of exactly how Ty1 cDNA crosses the yeast NE (which remains intact throughout the cell cycle) and then inserts itself at its target sites within the yeast genome is intriguing. Other than intact VLPs (30), preintegration complexes have not yet been defined for Ty1, but these should consist minimally of a cDNA-IN complex, since IN must cross the NE to direct integration of new Ty1 copies. This led us to examine whether Ty1 IN was nuclear localized.
Full-length Ty1 IN was fused at its N terminus to a GAL1-regulated E1 epitope (see Materials and Methods). Indirect immunofluorescence using an anti-IN MAb, 8B11 (30), shows that IN is nuclear localized (Fig. 2A, panels A and B). The IN-coding region was further subcloned to identify which region conferred nuclear localization. An N-terminal fusion consisting of residues 1 to 422 and a C-terminal fusion containing residues 423 to 636 were constructed and analyzed by immunofluorescence. The N-terminal fusion failed to localize to the nucleus, as assayed by MAb 8B11 staining (Fig. 2A, panels C and D). This finding suggests that an NLS resides at the C-terminal third of IN. To analyze the C-terminal construct, we used a polyclonal antibody against the E1 epitope (28, 53) because the MAb 8B11 epitope is not present on this fragment. The C-terminal IN fusion localized to the nucleus (Fig. 3A). Further mapping was carried out by using a series of deletion constructs. Figure 2 shows immunofluorescence of a key subset of these constructs. Since the deletion of C-terminal residues between BglII and ClaI (Fig. 1) had no effect on nuclear localization but the deletion of residues after the ClaI site did have an effect (Fig. 2A, panels G and H), we conclude that an NLS resides in the last 115 amino acids of IN.
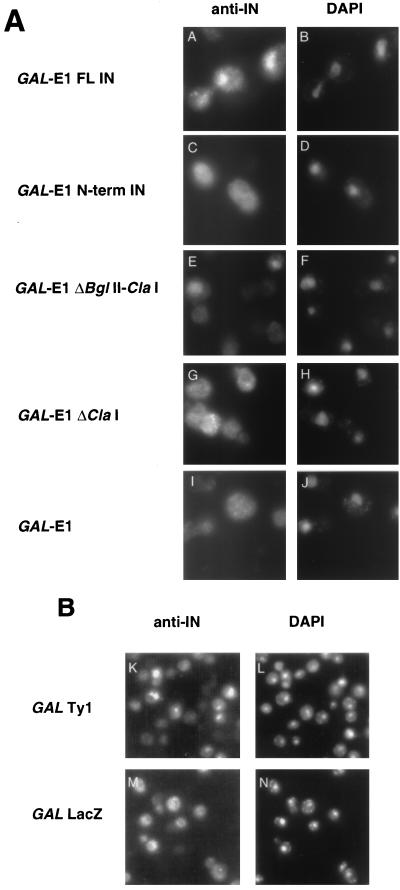
FIG. 2.
Ty1 IN localizes to the nucleus. Anti-IN antibody was used on cells induced for the production of E1-IN from the GAL1 promoter (A) or on cells induced for Ty1 transposition (B, panels K and L [panels M and N show GAL-LacZ negative controls]). FITC-stained panels are on the left; DAPI-stained panels are on the right. Constructs tested for IN localization are indicated to the left of the micrographs. FL, full length.
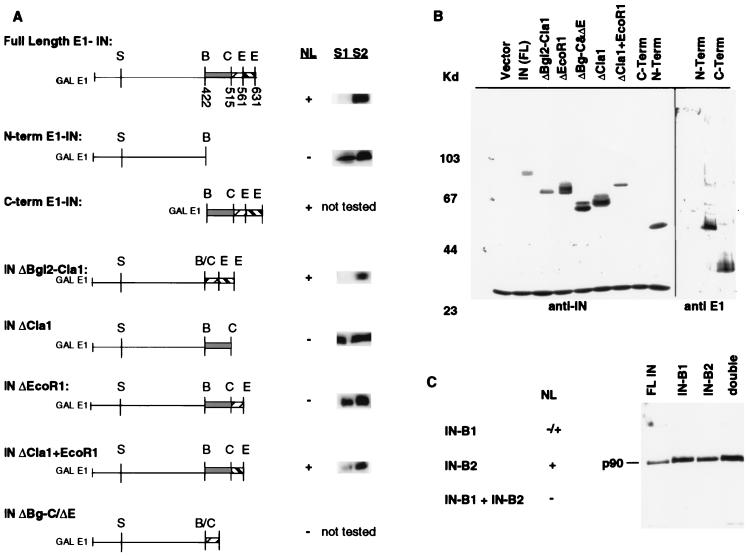
FIG. 3.
Analysis of E1-IN fusion proteins. (A) C-terminal deletion constructs map the NLS region within the last 74 amino acids of IN. Constructs tested are diagrammed, with restriction sites indicated; abbreviations for these are defined in the legend to Fig. 1. Numbers shown below restriction sites in full-length E1-IN represent amino acid residues. Nuclear localization (NL) is indicated as present (+) or absent (−); these results are from the indirect immunofluorescence experiments with anti-IN antibody described in the legend to Fig. 2, except for that for the C-terminal construct that necessitated the use of anti-E1 (see text). Cell fractionation experiments (described in Materials and Methods) were performed on strains bearing the E1-IN truncation constructs. Bands corresponding to the indicated E1-IN protein are shown to the right of the NL data. (B) Immunoblot analysis of the truncated IN proteins. Whole-cell extracts were electrophoresed on an SDS–8% polyacrylamide gel. Proteins were detected with either MAb 8B11 (1:5,000) or anti-E1 (1:500). Molecular mass markers are shown at the left in kilodaltons. MAb 8B11 also recognizes a non-Ty1 band of ∼30 kDa. Since the C-terminal truncation does not contain the epitope for MAb 8B11, this protein extract, along with the N-terminal one, was probed with anti-E1. (C) Similar immunoblot analysis of the substitution mutant GAL-E1-IN constructs B1 (NSKKRS→AAGSAA) and B2 (RSKKRI→AAGSAA). NL is indicated as present (+), partial (−/+), or absent (−).
Ty1 IN from cells undergoing transposition localizes to the nucleus.
Pol proteins are expressed as translational readthrough products from the GAG open reading frame (ORF) (3, 20, 49). The Ty1 protease (PR) processes the Gag/Pol polyprotein (190 to 200 kDa) to mature Ty1 proteins (p90 IN and p65 RT/RNase H [35]). We examined the localization of the IN expressed in this GAG-POL context. Cells containing a plasmid-borne Ty1 element under control of the GAL1 promoter were induced for transposition. IN localized to the nucleus of yeast cells, as demonstrated by MAb 8B11 immunofluorescence (Fig. 2B, panels K and L). We observed nuclear localization in about 40% of the cells and no signal in about 60% of the cells. The lack of signal in 60% of the cells is directly correlated with the high rate of plasmid loss of this GAL-Ty1 plasmid in galactose medium (even when cells are grown selectively; data not shown). Figure 2B, panels M and N, show a negative control of a GAL-lacZ plasmid. Several additional controls were used, including a comparison of uninduced and induced cells, an empty 2μm vector, or secondary antibody alone (data not shown). In each case, these negative controls failed to show nuclear staining, indicating that the observed nuclear staining was IN specific.
C-terminal deletions of IN identify a candidate NLS(s).
To more precisely map the regions of the C terminus representing the putative NLS, a series of C-terminal deletions were made in the GAL-E1-IN construct, and plasmids encoding these truncated proteins were transformed into yeast. The transformants were induced by growth on galactose and analyzed by immunoblotting and immunofluorescence. Figure 3B shows that all of the truncated forms of IN are expressed. Immunofluorescence with MAb 8B11 shows that IN is nuclear localized in all constructs that maintain the last 74 residues (encoded within the EcoRI-EcoRI fragment) (Fig. 3A). This fragment, when added back to the ΔClaI construct, restores nuclear localization (Fig. 3A; INΔClaI+EcoRI). Therefore, an NLS resides within the last 74 residues.
To biochemically confirm the immunofluorescence data, we fractionated protein extracts from several of the transformants into soluble and nuclear components. It has previously been shown that nuclear proteins such as nucleoporins fractionate with the nuclear pellet and can then be solubilized with 1 M NaCl (24). The subcellular localization of the IN fusions was crudely analyzed by separating soluble (S1) and particulate fractions by low-speed centrifugation of spheroplast lysates. The particulate fraction was then extracted with 1 M NaCl (S2) (see Materials and Methods). Immunoblot analysis of these two fractions showed that deletions removing the NLS region result in the release of a significant portion of the E1-IN construct into the S1 fraction (Fig. 3A, N-term E1-IN, INΔClaI, and INΔEcoRI). Constructs that retain this region (full-length IN, INΔBglII-ClaI, and INΔClaI+EcoRI) fractionate with the nuclear-enriched fraction and are released into the S2 supernatant only by treatment with 1 M NaCl. Although this crude fractionation is not sufficient to unambiguously determine subcellular localization, these data fully support the conclusions from the immunofluorescence experiments. The same immunoblot was reprobed with an antibody against a soluble protein, hexokinase, as a marker for the cytosolic fraction and with an antibody against a nucleoporin, MAb 414, as a marker for the nuclear fraction. These results (data not shown) indicate that the S1 and S2 fractions represent cytoplasmic and nuclear compartments, respectively.
Site-directed mutagenesis.
Canonical NLSs are most typically regions of consecutive basic amino acids. There are two basic segments in IN that somewhat resemble previously identified NLS motifs. We tested whether either basic region 1 (B1) or 2 (B2) (Fig. 1) acted as an NLS by replacing these basic regions with the neutral residues AAGSAA. Similar mutations were made in both the GAL-Ty1 and the GAL-E1-IN vectors.
For cells expressing the GAL-E1-IN construct, we found that the IN B1 mutation caused a clear but partial loss of nuclear signal, with some signal instead accumulating in the cytoplasm, as compared to cells expressing wild-type IN (Fig. 3C). The IN B2 mutation apparently had no detectable effect by itself on the nuclear localization of IN, since staining of the mutant with MAb 8B11 looked similar to staining of the wild type. However, when both mutations were introduced into the same full-length E1-IN construct, nuclear localization of IN was completely abolished. Thus, site-directed alterations to constructs expressing the E1-IN fusion protein reveal that these two regions are each important for nuclear localization, reminiscent of the bipartite nuclear targeting sequence of nucleoplasmin (27).
Nuclear localization and retrotransposition defects.
The advantage of the GAL-E1-IN system lies in the ease with which the immunofluorescence analysis can be performed, presumably because more IN protein is produced. However, the more relevant question is what happens to IN produced within the context of a native Ty1 element. In this natural context, in vivo function, transposition, and other aspects of the Ty1 life cycle can be assessed along with effects on nuclear localization.
When strains carrying IN B1 or B2 mutations in the GAL-Ty1-TRP1 plasmid were assayed by immunofluorescence, we detected no MAb 8B11 staining in the nucleus of cells of either mutant strain (Fig. 4B). In these mutants, we observed a punctate or patchy cytoplasmic signal. In some instances, we observed ring-like structures (also seen in cells with the wild-type plasmid but to a lesser extent); however, these ring structures are clearly not surrounding the nucleus, as judged by the location of the DAPI staining regions, and therefore do not represent a nuclear-rim staining pattern. Perhaps this staining reflects mutant IN trapped in the cytoplasm or in VLPs, unable to enter the nucleus. When strains with these plasmids were assayed for transposition, both the IN B1 and B2 mutants were severely defective in transposition, as compared to that of the wild type (Fig. 4A). Their transposition defect is as severe as that of a previously constructed RT mutant with a missense mutation in the active site region (FVDD→FVYD) (31a), and the level of transposition is at least 50-fold lower than wild-type Ty1-TRP1 transposition. Taken together, these data suggest that these two basic segments are key components of an unusually long bipartite NLS. The spacer region between the KKR motifs is 30 residues long. For example nucleoplasmin, the model for this class of NLSs, has a spacer of only 10 residues (54).
Mutant VLPs have normal PR, RT, and strand transfer activities.
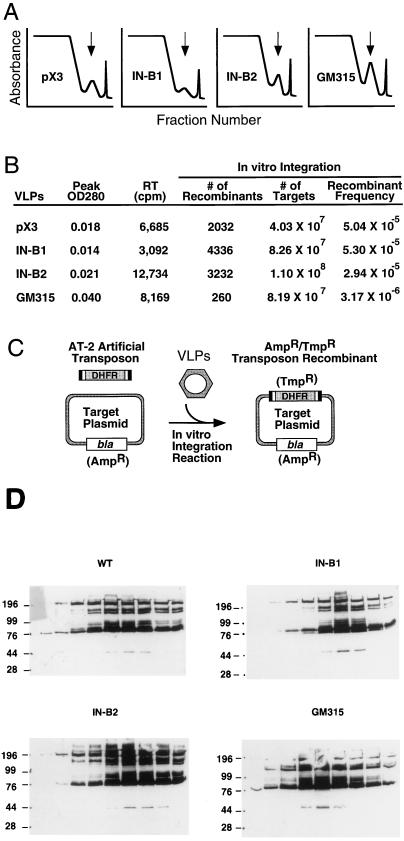
To ensure that these mutations were specific for a defect in nuclear transport of IN, we analyzed other retrotransposon functions. We first examined the IN protein by immunoblotting to evaluate expression level and whether it is properly released from the Gag-Pol precursor by the Ty1 PR. This is important because the IN B2 mutation lies very close to the IN/RT junction (Fig. 1). Figures 4C (anti-IN panel) and 5D show that IN is made at near wild-type levels and is processed properly. Additionally, similar levels of cDNA for the mutant and the wild-type INs were recovered (data not shown). We also probed with an anti-Gag antibody (R1-F [12]) and observed Gag proteins of the expected mobilities (p58 and p54), indicating that proper processing to mature Gag occurred in the mutant VLPs (Fig. 4C). VLPs were isolated by running 20 to 70% sucrose density gradients, and the VLP profiles were similar for the wild type and mutants (Fig. 5A). RT activity cofractionated with the VLP peak, and levels of encapsidated IN protein were similar in all of the samples (Fig. 5B). Most importantly, in vitro IN activity in the NLS mutants was nearly identical to that in the wild type (Fig. 5B). Previous experiments demonstrated that the C terminus is dispensable for the single-end joining function of IN (11). However, the assay used here closely mimics the in vivo integration reaction. The reaction consists of mixing an artificial transposon substrate with Ty1 termini, a target plasmid, and the VLPs to be tested (Fig. 5C). Formation of a recombinant target plasmid bearing the artificial transposon requires concerted joining of both transposition ends to target DNA, with appropriate formation of a 5-bp target site duplication (25, 26). The B1 and B2 mutant INs are catalytically indistinguishable from wild-type IN in this in vitro reaction (Fig. 5B). VLPs isolated from these mutants are fully functional transposition intermediates. Thus, an essential function of the basic C-terminal region of IN is to transport the IN protein and perhaps a larger preintegration complex into the nucleus.
FIG. 5.
Analysis of VLPs from wild-type and NLS mutant Ty1 strains. (A) Sucrose gradient fractionation profiles showing VLP peaks. The top of the gradient (20% sucrose) is presented at the left side of the x axis; the bottom of the gradient (70% sucrose) is at the right. The y axis represents absorbance at 254 nm. It is important to note that VLPs could be isolated from wild-type and NLS mutant Ty1 strains (small peaks under vertical arrows). The first peak at the top of the gradient is bulk protein, and the last peak at the right is the extreme bottom of the gradient. (B) Biochemical analysis of VLPs. The peak fraction from each VLP preparation was examined for RT and integration activities in vitro. All of the preparations showed normal RT activity. The activities of positive control avian myeloblastosis virus RT and the negative control, no enzyme (data not shown), were 93,500 and 465 cpm, respectively. VLPs isolated from the wild-type strain (pX3), or the NLS mutant strains (IN B1 and B2) supported normal levels of integration, whereas the negative control IN mutant strain (GM315) was 16-fold less active. OD280, optical density at 280 nm. (C) In vitro integration assay (25). The artificial transposon AT-2 serves as a substrate for Ty1 IN and is integrated into the target plasmid in vitro when VLPs are added. AT-2 carries the dhfr gene which confers trimethoprim resistance and thus serves as a selectable marker when the transposition reaction mixture is transformed into Escherichia coli. The target plasmid contains the bla gene conferring ampicillin resistance, while transposon recombinants carry both markers. The number of recombinants (Ampr Tmpr colonies) divided by the total number of targets recovered (Ampr colonies) provides the recombinant frequency. (D) Immunoblots of nine peak fractions from each gradient probed with MAb 8B11, showing similar yields of encapsidated IN protein in the four preparations. Molecular masses are indicated on the left in kilodaltons. WT, wild type; GM315, negative control IN mutant strain.
A new family of NLS sequences?
Since the Ty1 IN NLS appeared to consist of two repeats of the sequence SKKR separated by about 30 amino acid residues, we used this information to search the complete yeast genome sequence for possible additional members of this class of NLS. By using the search parameters (SKKR)N20–40(SKKR) with the program CoSMoS (prerelease version made available by D. Bassett and P. Hieter; the final version will be deposited on the XREF database, http://www.ncbi.nlm.nih.gov/XREFdb/) to search the complete set of yeast ORFs, we identified only IN sequences from Ty1 and Ty2 elements. By relaxing the stringency to (KKR)N20–40(KKR), we identified 16 additional single copy sequences (Table 2). Of these, 10 ORFs were sequences about which little is known, and six represented proteins of known or suspected function. Remarkably, five of the six known proteins are nuclear localized or very likely to be so. In several cases, the putative NLS is near the C terminus as in Ty1 IN.
TABLE 2.
Putative bipartite NLSs in other yeast ORFs
| ORF name (gene name) | (Putative) NLS sequencea | Distance between KKR motifs (aa)b | Distance from C terminus (aa)c | Protein length (aa)d | Functione | Localization |
|---|---|---|---|---|---|---|
| Ty1 and Ty2 IN | KKRSLEDNETEIKVSRDTWNTK..........NMRSLEPPRSKKR | 29 | 5 | 635 | Ty1 integration | Nuclear |
| YIL126w (STH1) | KKRVYYDDGLTEEQFLEAVE............DDNMSLEDAIKKR | 27 | 274 | 1,359 | Helicase-related; SNF2 homolog | Nuclear |
| YBR142w (MAK5) | KKRGHNSIIGHEK...................TNALETLKKKKKR | 20 | 2 | 773 | DEAD box helicase; RNA splicing | Nuclearf |
| YOL109c (INO4) | KKRKRRSKKINKLTDG................QIRINHVSSEKKR | 23 | 94 | 151 | Transcription factor | Nuclearf |
| YKL114c (APN1) | KKRAGGTKRKKATAEP................SDNDILSQMTKKR | 23 | 5 | 368 | AP endonuclease; DNA repair | Nuclearf |
| YKL129c (MYO3) | KKRSAKIKKATFDANKKKEVGISDLTLLSK..ISDESINENLKKR | 37 | 1,212 | 1,272 | Myosin nonessential; synthetic lethal with myo5 | Cytoskeleton?f |
| YKL021c (MAK11) | KKRDAETADIGDQSEVESDTEELKKI......MFGEKKKLNKKKR | 33 | 13 | 468 | Essential; 60S ribosome subunit biogenesis | Nucleolar?f |
| YML034w | KKRKDPDSDDWSESNSKENKIDNKHLNLLSSDSEIEQDYQKAKKR | 39 | 438 | 657 | Unknown | Unknown |
| YML093w | KKRVIDDEDDKEVDTTLPGWGE..........WAGAGSKPKNKKR | 29 | 93 | 900 | Unknown | Unknown |
| YKL082c | KKRKRLESEQEQDQDEIASDSDME........DIDSDLENNSKKR | 31 | 186 | 435 | Unknown | Unknown |
| L8300.12 | KKRRRGNGKHLSR...................KEKRKMERADKKR | 20 | 840 | 899 | Unknown | Unknown |
| YOR004w | KKRKLGPKAPNPLSVKKKKKVNSPSDEV....KDKEDTSKEKKKR | 35 | 21 | 296 | Unknown | Unknown |
| YGL136c | KKRMQAVFTNVHKFKPDASRDE..........SKETYYIGLKKKR | 29 | 11 | 320 | Unknown | Unknown |
| YGL133w | KKRKPTEVNDSENNSSEEDKKK..........GQNVTSETHSKKR | 29 | 912 | 1,264 | Unknown | Unknown |
| YDL094c | KKRNGSMHNMYYASVPFLLFSNAYSID.....FSRHVNEFLEKKR | 34 | 49 | 169 | Unknown | Unknown |
| YGL141w | KKRLKSSLRFRKLEILLELPFFIPFEERV...DLFYMFIALDKKR | 36 | 380 | 910 | Unknown | Unknown |
The complete yeast genome sequence was probed by using the pattern matching program CoSMoS (2a) with the query (KKR)N20–40(KKR). The results are summarized in this table. KKR motifs are in boldface type, and acidic residues in the spacer region are underlined. The motifs are arbitrarily split 10 amino acid residues upstream of the downstream KKR motif. The proteins derived from known genes are listed first, with the anonymous ORFs listed below.
aa, amino acids.
Distance of putative NLS from predicted C terminus of protein. aa, amino acids.
Total length of protein (predicted). aa, amino acids.
Functional data obtained from Saccharomyces Genome Database (URL: http://genome-www.stanford.edu/Saccharomyces/).
Localization inferred from function.
DISCUSSION
Retrotransposition of Ty1 absolutely depends on IN. IN is responsible for the strand-joining reaction that unites Ty1 cDNA with host cell target DNA. The studies presented here indicate that IN must be transported to the nucleus for retrotransposition to occur. We have identified a bipartite NLS at the C terminus of IN which is required for both its nuclear localization and its in vivo retrotransposition function.
An essential NLS in Ty1 IN.
Several lines of evidence reveal the Ty1 IN NLS, including immunofluorescence studies on GAL-Ty1-produced IN and ectopically expressed tagged IN (GAL-E1-IN). Furthermore, independent studies at this laboratory (50a) and in the Garfinkel laboratory (51b) examined Ty1 IN fusions to green fluorescent protein (GFP). In all cases, IN was unambiguously nuclear localized.
Active transport of nuclear proteins depends on the presence of short stretches of basic amino acids that associate with the cytosolic receptor karyopherin (36). Although many proteins contain such sequences, no single consensus has emerged for NLSs. They have, however, been placed into the following two classes: (i) those with a single contiguous stretch of predominately arginine or lysine residues (e.g., simian virus 40 T antigen), and (ii) those having a bipartite structure (e.g., Xenopus nucleoplasmin) (54). Naturally occurring bipartite NLSs consist of two short stretches of basic residues separated by a spacer of about 10 nonconserved residues. Experiments with artificial bipartite NLSs suggest that they tolerate deletion, insertion, and amino acid changes (48, 54). Our results show that Ty1 IN represents a member of a new class of bipartite NLSs with a much longer spacer element; a similar element was recently reported for HIV-1 IN (32).
We have shown that site-specific block substitution mutations in basic regions of the C terminus of Ty1 IN identified functional NLS sequences. Two different systems were analyzed, GAL-E1-IN and GAL-Ty1, and two regions important for nuclear localization were revealed in both contexts. In the ectopically expressed IN (GAL-E1-IN), it is interesting to note that IN regions B1 and B2 must both be mutated to block nuclear import, suggesting that in this context each basic region is important but that neither is essential for simple nuclear import. However, defects in transposition and localization are evident with either B1 or B2 mutated in the context of the full transposon (GAL-Ty1), suggesting that the NLS functions as a single bipartite signal, relying on both basic regions. As shown in the accompanying paper (51b), Moore et al. performed mutagenesis studies similar to ours and identified the same two basic regions near the Ty1 IN C terminus as essential for NLS function.
The difference in behavior of the B1 and B2 mutations in the two different experimental systems used to analyze IN nuclear localization has several possible explanations. One possible explanation stems from the fact that subtly different mutations were made in our study in the GAL-E1 and GAL-Ty1 plasmids. However, this explanation seems unlikely given that Moore et al. (51b) have shown that a completely different set of specific NLS mutations in the same regions behave similarly; complete deletion of B2 has no deleterious effect in the GAL-GFP construct; a localization defect is observed only when this deletion is combined with a substitution mutation(s) in B1. More likely, the relatively subtle defects caused by mutating only B1 or B2 have relatively little effect when IN is expressed ectopically at high levels in the form of an E1-IN fusion protein (or IN-GFP) and much more of an effect in the context of the intact GAL-Ty1, from which less IN protein is synthesized. Moreover, in the latter case, the Ty1 IN may be burdened by association with Ty1 DNA (see below) and other preintegration complex components, whereas the E1-IN fusion protein is unlikely to be so encumbered. An interesting observation, noted by Moore et al., is that an acidic region (amino acids LED) immediately adjacent to the basic residue in IN B1 may contribute to this region’s ability to act as an NLS. We substituted this region in the IN B1 mutation in the GAL-Ty1 vector but not in the GAL-E1-IN vector. In fact, acidic amino acids, as well as other nonbasic components, have been shown to be important for NLS activity (22, 48). Thus, our understanding of the rules governing NLS behavior is incomplete. Mutations that inactivate a signal can sometimes be rescued by a secondary mutation at a remote site, and neutral and acidic residues can play critical roles when they flank a short cluster of basic residues (48).
The role of the Ty1 IN NLS may differ from that of conventional NLSs, which need only facilitate the import of a single protein. The IN NLS may facilitate the import of ∼6,000 bp of cDNA (and possibly additional preintegration complex proteins). This might explain why in the GAL-E1-IN constructs, mutations in just one of the basic clusters results in proper nuclear localization, but in the GAL-Ty1-TRP1 plasmid, a mutation in just one cluster is sufficient to prevent IN from localizing to the nucleus. Direct evidence for the ability of large nucleic acids to transit the NE comes from the influenza virus system. Influenza virus RNA is imported via an RNA-associated protein(s) containing an NLS and requires host cell transport factors (karyopherin, Ran, and p10) in an in vitro assay (52).
The Ty1 IN has a long spacer region and is flanked by identical SKKR motifs. Does this represent a special class of NLS or will a generic NLS function equally well? Placing the NLS from the yeast histone H2B gene directly upstream of the inactivated IN B1 mutant in Ty1 failed to restore its transposition (data not shown), suggesting that the specific sequence of the Ty1 IN NLS (or its immediate context) may be important. While it is tempting to speculate about the exact nature of these signals, the main purpose for using the GAL-E1-IN system was to facilitate indirect immunofluorescence assays. The more relevant information comes from the situation in which IN is produced from an intact Ty1 element, and we reached similar conclusions by analyzing both systems; both basic clusters are important for the correct nuclear localization of IN.
Relationship to retroviral mechanisms.
Ty1 and retroviruses are often compared because of the similarity between their life cycles and their mechanism of integrating cDNA into the respective host genomes. One major difference is that Ty1 is not infectious and does not exit the host cell. But the mechanism by which Ty1 enters the nucleus of yeast in which the NE does not break down may represent an important functional similarity to lentiviruses such as HIV-1.
HIV-1 can infect both dividing and nondividing cells (47). During the course of infecting nondividing cells, for example, terminally differentiated macrophages, the HIV-1 preintegration complex is actively transported across the intact interphase NE (15). The matrix (MA) protein (17 kDa) contains an NLS capable of targeting reporter proteins to the nuclei. Viruses with mutations in the MA NLS and the Vpr gene can be propagated only in dividing cells, not in cell cycle-arrested cells (14). Vpr was subsequently shown to contain an NLS that is sufficient for HIV-1 import in a strain containing a defective MA NLS (37). MA associates with karyopherin α (Rch1) (33). Thus, it appears that MA and Vpr proteins contain redundant NLSs that are sufficient but not individually necessary for HIV-1 nuclear transport. There are conflicting reports regarding the possible presence of an NLS in HIV-1 IN (33, 40, 44). Gallay et al. (33) have suggested that at least one other NLS-bearing protein plays a role in import of HIV-1 and that HIV-1 IN has sequences that resemble karyophilic motifs. Recent evidence suggests that HIV-1 IN does have a C-terminal, bipartite NLS, mutations in which block nuclear import and association with karyopherin. However, viruses mutated in this region appear to have a generalized replication defect, even in dividing cells (32). Thus, the relevance of the HIV-1 IN NLS to HIV-1 replication is not clear at the present time.
The avian sarcoma virus (ASV) retrovirus IN contains a potent NLS which also somewhat resembles the Ty1 NLS in that it has lysine residues at the ends of a ∼20-amino-acid-residue signal (44). However, because ASV replicates only in dividing cells, it is not clear whether the NLS is actually required for viral replication because ASV preintegration complexes can gain nuclear access during mitosis. Perhaps the NLS in ASV IN allows it to transit the NE during nonmitotic parts of the cell cycle or allows replication in some select subset of cell types. In contrast to the retroviral cases, the importance of the IN NLS is very clear in the Ty1 system; the yeast NE never breaks down and IN is the only element-encoded protein whose function is required in the nucleus. Furthermore, IN is the only Ty1 protein known to contain an NLS. The reports of NLSs in retroviral INs hint at the possibility of a conserved import mechanism.
The finding that IN has an NLS that specifies its nuclear localization predicts that it interacts with the host cell protein import machinery and the NPC. In an initial survey to identify genes encoding host factors important for IN uptake, we measured transposition rates in several mutants affected in NPC components and cytoplasmic nuclear transport factors (data not shown). As expected, several mutants did indeed show reductions in transposition rates, but since these mutants were also defective in nuclear import or RNA export, it is possible that the effect on transposition is indirect. At least one mutant, nup170Δ, showed a significant reduction in transposition. Other studies have shown that nup170Δ has no effect on gross import or RNA export (2, 42), suggesting that this effect on transposition may be rather specific to IN uptake. Nevertheless, these data support the idea that the IN B1 and B2 sequences serve as NLSs that interact with import machinery rather than simple nuclear retention signals.
Cell biology of the Ty1 integration machinery.
Ty1 VLPs apparently serve as the center for organizing the complex steps of reverse transcription, a process assumed to occur in the cytoplasm, where most if not all VLPs are found (34, 50). Supporting this, a Gag-LacZ fusion protein was shown to localize to ∼50 cytoplasmic dots, consistent with the number of VLPs expected per cell (18). Furthermore, VLPs contain the components necessary for an in vitro integration reaction (30). Thus far, it has not been possible to isolate a complex capable of efficiently mediating Ty1 integration other than the VLP itself. However, since IN is expected to be inside the VLP, together with the Ty1 cDNA, and IN contains an NLS, some specific event must expose the IN NLS to the nuclear import machinery. The actual identity and composition of the species of preintegration complex that transits the NE are not clear; at a minimum, the complex must include cDNA and IN, and at a maximum, it could include the entire VLP. The latter possibility seems unlikely for several reasons: (i) the IN NLS is probably inaccessible in intact VLPs, (ii) VLPs are very large, and (iii) in HIV-1, there is good evidence for a preintegration complex intermediate containing a subset of the core virion proteins and viral cDNA (16, 31). Two possible processes that seem likely are the following: (i) docking of Ty1 VLPs at a nuclear pore, coupled with transfer of the preintegration complex into the nucleus, requiring the NLS, and (ii) a specific VLP disassembly process that releases a preintegration complex competent for subsequent nuclear uptake. A third possibility is that IN and the DNA are transported to the nucleus independently, but this seems unlikely based on retroviral precedents. The discovery and characterization of the IN NLS provide a useful new tool to further explore mechanistic aspects of preintegration complex uptake into yeast nuclei as well as the further steps involved in defining and acquiring targets in chromosomal DNA.
ACKNOWLEDGMENTS
We thank Y. Eby for assistance with DNA sequencing, L. Davis for generously supplying strains and antibodies, and S. Moore and D. Garfinkel for communicating data prior to publication. We thank A. Davis and R. Jensen for supplying the antihexokinase antibody. We are especially grateful to D. Bassett and P. Hieter for assistance with the prerelease CoSMoS program and members of the Boeke lab for their helpful comments, especially J. Smith and E. Hoff. M.A.K. thanks R. Skibbens for suggestions and support.
This work was supported in part by NIH grants 5-T-32-CA09139-22 to M.A.K. and PO1-CA16519 to J.D.B.
REFERENCES
- 1.Adams A E, Pringle J R. Staining of actin with fluorochrome-conjugated phalloidin. Methods Enzymol. 1991;194:729–731. doi: 10.1016/0076-6879(91)94054-g. [DOI] [PubMed] [Google Scholar]
- 2.Aitchison J D, Rout M P, Marelli M, Blobel G, Wozniak R W. Two novel related yeast nucleoporins, Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J Cell Biol. 1995;131:1133–1148. doi: 10.1083/jcb.131.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Bassett, D. Personal communication.
- 3.Belcourt M F, Farabaugh P J. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell. 1990;62:339–352. doi: 10.1016/0092-8674(90)90371-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke J D, Eichinger D J, Castrillon D, Fink G R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988;8:1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeke J D, Garfinkel D J, Styles C A, Fink G R. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- 6.Boeke J D, Lacroute F, Fink G R. A positive selection for mutants lacking orotidine 5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 7.Boeke J D, Sandmeyer S B. Yeast transposable elements. In: Broach J R, Jones E, Pringle J, editors. The molecular and cellular biology of the yeast Saccharomyces: genome dynamics, protein synthesis, and energetics. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 193–261. [Google Scholar]
- 8.Boeke J D, Styles C A, Fink G R. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986;6:3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeke J D, Xu H, Fink G R. A general method for the chromosomal amplification of genes in yeast. Science. 1988;239:280–282. doi: 10.1126/science.2827308. [DOI] [PubMed] [Google Scholar]
- 10.Braiterman L T, Boeke J D. In vitro integration of retrotransposon Ty1: a direct physical assay. Mol Cell Biol. 1994;14:5719–5730. doi: 10.1128/mcb.14.9.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braiterman L T, Boeke J D. Ty1 in vitro integration: effects of mutations in cis and in trans. Mol Cell Biol. 1994;14:5731–5740. doi: 10.1128/mcb.14.9.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braiterman L T, Monokian G M, Eichinger D J, Merbs S L, Gabriel A, Boeke J D. In-frame linker insertion mutagenesis of yeast transposon Ty1: phenotypic analysis. Gene. 1994;139:19–26. doi: 10.1016/0378-1119(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 13.Brookman J L, Stott A J, Cheeseman P J, Burns N R, Adams S E, Kingsman A J, Gull K. An immunological analysis of Ty1 virus-like particle structure. Virology. 1995;207:59–67. doi: 10.1006/viro.1995.1051. [DOI] [PubMed] [Google Scholar]
- 14.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukrinsky M I, Stanwick T L, Dempsey M P, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns N, Grimwade B, Ross-Macdonald P B, Choi E Y, Finberg K, Roeder G S, Snyder M. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 19.Burns N R, Saibil H R, White N S, Pardon J F, Timmins P A, Richardson S M H, Richards B M, Adams S E, Kingsman S M, Kingsman A J. Symmetry, flexibility and permeability in the structure of yeast retrotransposon virus-like particles. EMBO J. 1992;11:1155–1164. doi: 10.1002/j.1460-2075.1992.tb05156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clare J J, Belcourt M, Farabaugh P J. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty1 transposon. Proc Natl Acad Sci USA. 1988;85:6816–6820. doi: 10.1073/pnas.85.18.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dales S, Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973;56:465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- 22.Dang C V, Lee W M F. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988;8:4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis L I. The nuclear pore complex. Annu Rev Biochem. 1995;64:865–896. doi: 10.1146/annurev.bi.64.070195.004245. . (Review.) [DOI] [PubMed] [Google Scholar]
- 24.Davis L I, Fink G R. The NUP1 gene encodes an essential component of the yeast nuclear pore complex. Cell. 1990;61:965–978. doi: 10.1016/0092-8674(90)90062-j. [DOI] [PubMed] [Google Scholar]
- 25.Devine S E, Boeke J D. Efficient integration of artificial transposons into plasmid targets in vitro: a useful tool for DNA mapping, sequencing and genetic analysis. Nucleic Acids Res. 1994;18:3765–3772. doi: 10.1093/nar/22.18.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devine S E, Boeke J D. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes Dev. 1996;10:620–633. doi: 10.1101/gad.10.5.620. [DOI] [PubMed] [Google Scholar]
- 27.Dingwall C, Robbins J, Dilworth S M, Roberts B, Richardson W D. The nucleoplasmin nuclear location sequence is larger and more complex than that of SV-40 large T antigen. J Cell Biol. 1988;107:841–849. doi: 10.1083/jcb.107.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doheny K F, Sorger P K, Hyman A A, Tugendreich S, Spencer F, Hieter P. CTF13 is an essential component of the Saccharomyces cerevisiae kinetochore. Cell. 1993;73:761–774. doi: 10.1016/0092-8674(93)90255-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dworetzky S I, Feldherr C M. Translocation of RNA-coated gold particles through the nuclear pores of oocytes. J Cell Biol. 1988;106:575–584. doi: 10.1083/jcb.106.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichinger D J, Boeke J D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988;54:955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- 31.Farnet C M, Haseltine W A. Determination of viral proteins present in the human immunodeficiency virus type 1 preintegration complex. J Virol. 1991;65:1910–1915. doi: 10.1128/jvi.65.4.1910-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Gabriel, A., and J. D. Boeke. Unpublished data.
- 32.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garfinkel D J, Boeke J D, Fink G R. Ty element transposition: reverse transcriptase and virus-like particles. Cell. 1985;42:507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- 35.Garfinkel D J, Hedge A-M, Youngren S D, Copeland T D. Proteolytic processing of pol-TYB proteins from the yeast retrotransposon Ty1. J Virol. 1991;65:4573–4581. doi: 10.1128/jvi.65.9.4573-4581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 37.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higuchi R, Krummel B, Saiki R. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hovland P, Flick J, Johnston M, Sclafani R A. Galactose as a gratuitous inducer of GAL gene expression in yeasts growing on glucose. Gene. 1989;83:57–64. doi: 10.1016/0378-1119(89)90403-4. [DOI] [PubMed] [Google Scholar]
- 40.Jones K S, Kulkosky J, Skalka A M. Analyses of HIV integration components. In: Kumar A, editor. Advances in molecular biology and targeted treatment for AIDS. New York, N.Y: Plenum Press; 1991. pp. 21–26. [Google Scholar]
- 41.Kalinich J F, Douglas M G. In vitro translocation through the yeast nuclear envelope. Signal-dependent transport requires ATP and calcium. J Biol Chem. 1989;264:17979–17989. [PubMed] [Google Scholar]
- 42.Kenna M A, Petranka J G, Reilly J L, Davis L I. Yeast N1e3p/Nup170p is required for normal stoichiometry of FG nucleoporins within the nuclear pore complex. Mol Cell Biol. 1996;16:2025–2036. doi: 10.1128/mcb.16.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubai D F. The evolution of the mitotic spindle. Int Rev Cytol. 1975;43:167–227. doi: 10.1016/s0074-7696(08)60069-8. [DOI] [PubMed] [Google Scholar]
- 44.Kukolj G, Jones K S, Skalka A M. Subcellular localization of avian sarcoma virus and human immunodeficiency virus type 1 integrases. J Virol. 1997;71:843–847. doi: 10.1128/jvi.71.1.843-847.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 46.Lauermann V, Nam K, Trambley J, Boeke J D. Plus-strand strong-stop DNA in retrotransposon Ty1. J Virol. 1995;69:7845–7850. doi: 10.1128/jvi.69.12.7845-7850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makkerh J P S, Dingwall C, Laskey R A. Comparative mutagenesis of nuclear localization signals reveals the importance of neutral and acidic amino acids. Curr Biol. 1996;6:1025–1027. doi: 10.1016/s0960-9822(02)00648-6. [DOI] [PubMed] [Google Scholar]
- 49.Mellor J, Fulton A M, Dobson M J, Wilson W, Kingsman S M, Kingsman A J. A retrovirus-like strategy for the expression of a fusion protein encoded by yeast transposon Ty1. Nature. 1985;313:243–246. doi: 10.1038/313243a0. [DOI] [PubMed] [Google Scholar]
- 50.Mellor J, Malim M H, Gull K, Tuite M F, McCready S, Dibbayawan T, Kingsman S M, Kingsman A J. Reverse transcriptase activity and Ty RNA are associated with virus-like particles in yeast. Nature. 1985;318:583–586. doi: 10.1038/318583a0. [DOI] [PubMed] [Google Scholar]
- 50a.Merkulov, G., and J. D. Boeke. Submitted for publication.
- 51.Merkulov G V, Swiderek K M, Brachmann C B, Boeke J D. A critical proteolytic cleavage site near the C terminus of the yeast retrotransposon Ty1 Gag protein. J Virol. 1996;70:5548–5556. doi: 10.1128/jvi.70.8.5548-5556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.Monokian G M, Braiterman L T, Boeke J D. In-frame linker insertion mutagenesis of yeast transposon Ty1: mutations, transposition, and dominance. Gene. 1994;139:9–18. doi: 10.1016/0378-1119(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 51b.Moore S P, Rinckel L A, Garfinkel D J. A Ty1 integrase nuclear localization signal required for retrotransposition. Mol Cell Biol. 1998;18:1105–1114. doi: 10.1128/mcb.18.2.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Neill R E, Jaskunas R, Blobel G, Palese P, Moroianu J. Nuclear import of influenza virus RNA can be mediated by viral nucleoprotein and transport factors required for protein import. J Biol Chem. 1995;270:22701–22704. doi: 10.1074/jbc.270.39.22701. [DOI] [PubMed] [Google Scholar]
- 53.Pluta A F, Saitoh N, Goldberg I, Earnshaw W C. Identification of a subdomain of CENP-B that is necessary and sufficient for localization to the human centromere. J Cell Biol. 1992;116:1081–1093. doi: 10.1083/jcb.116.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robbins J, Dilworth S M, Laskey R A, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- 55.Roe T, Reynolds T C, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Vallette F, Merge E, Reiss A, Adesnik M. Construction of mutant and chimeric genes using the polymerase chain reaction. Nucleic Acids Res. 1989;17:723–733. doi: 10.1093/nar/17.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H, Boeke J D. High frequency deletion between homologous sequences during retrotransposition of Ty elements in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1987;84:8553–8557. doi: 10.1073/pnas.84.23.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]