Abstract
Background
Adhesive capsulitis (also termed frozen shoulder) is commonly treated by manual therapy and exercise, usually delivered together as components of a physical therapy intervention. This review is one of a series of reviews that form an update of the Cochrane review, 'Physiotherapy interventions for shoulder pain.'
Objectives
To synthesise available evidence regarding the benefits and harms of manual therapy and exercise, alone or in combination, for the treatment of patients with adhesive capsulitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, CINAHL Plus, ClinicalTrials.gov and the WHO ICTRP clinical trials registries up to May 2013, unrestricted by language, and reviewed the reference lists of review articles and retrieved trials, to identify potentially relevant trials.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐randomised trials, including adults with adhesive capsulitis, and comparing any manual therapy or exercise intervention versus placebo, no intervention, a different type of manual therapy or exercise or any other intervention. Interventions included mobilisation, manipulation and supervised or home exercise, delivered alone or in combination. Trials investigating the primary or adjunct effect of a combination of manual therapy and exercise were the main comparisons of interest. Main outcomes of interest were participant‐reported pain relief of 30% or greater, overall pain (mean or mean change), function, global assessment of treatment success, active shoulder abduction, quality of life and the number of participants experiencing adverse events.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted the data, performed a risk of bias assessment and assessed the quality of the body of evidence for the main outcomes using the GRADE approach.
Main results
We included 32 trials (1836 participants). No trial compared a combination of manual therapy and exercise versus placebo or no intervention. Seven trials compared a combination of manual therapy and exercise versus other interventions but were clinically heterogeneous, so opportunities for meta‐analysis were limited. The overall impression gained from these trials is that the few outcome differences between interventions that were clinically important were detected only up to seven weeks. Evidence of moderate quality shows that a combination of manual therapy and exercise for six weeks probably results in less improvement at seven weeks but a similar number of adverse events compared with glucocorticoid injection. The mean change in pain with glucocorticoid injection was 58 points on a 100‐point scale, and 32 points with manual therapy and exercise (mean difference (MD) 26 points, 95% confidence interval (CI) 15 points to 37 points; one RCT, 107 participants), for an absolute difference of 26% (15% to 37%). Mean change in function with glucocorticoid injection was 39 points on a 100‐point scale, and 14 points with manual therapy and exercise (MD 25 points, 95% CI 35 points to 15 points; one RCT, 107 participants), for an absolute difference of 25% (15% to 35%). Forty‐six per cent (26/56) of participants reported treatment success with manual therapy and exercise compared with 77% (40/52) of participants receiving glucocorticoid injection (risk ratio (RR) 0.6, 95% CI 0.44 to 0.83; one RCT, 108 participants), with an absolute risk difference of 30% (13% to 48%). The number reporting adverse events did not differ between groups: 56% (32/57) reported events with manual therapy and exercise, and 53% (30/57) with glucocorticoid injection (RR 1.07, 95% CI 0.76 to 1.49; one RCT, 114 participants), with an absolute risk difference of 4% (‐15% to 22%). Group differences in improvement in overall pain and function at six months and 12 months were not clinically important.
We are uncertain of the effect of other combinations of manual therapy and exercise, as most evidence is of low quality. Meta‐analysis of two trials (86 participants) suggested no clinically important differences between a combination of manual therapy, exercise, and electrotherapy for four weeks and placebo injection compared with glucocorticoid injection alone or placebo injection alone in terms of overall pain, function, active range of motion and quality of life at six weeks, six months and 12 months (though the 95% CI suggested function may be better with glucocorticoid injection at six weeks). The same two trials found that adding a combination of manual therapy, exercise and electrotherapy for four weeks to glucocorticoid injection did not confer clinically important benefits over glucocorticoid injection alone at each time point. Based on one high quality trial (148 participants), following arthrographic joint distension with glucocorticoid and saline, a combination of manual therapy and supervised exercise for six weeks conferred similar effects to those of sham ultrasound in terms of overall pain, function and quality of life at six weeks and at six months, but provided greater patient‐reported treatment success and active shoulder abduction at six weeks. One trial (119 participants) found that a combination of manual therapy, exercise, electrotherapy and oral non‐steroidal anti‐inflammatory drug (NSAID) for three weeks did not confer clinically important benefits over oral NSAID alone in terms of function and patient‐reported treatment success at three weeks.
On the basis of 25 clinically heterogeneous trials, we are uncertain of the effect of manual therapy or exercise when not delivered together, or one type of manual therapy or exercise versus another, as most reported differences between groups were not clinically or statistically significant, and the evidence is mostly of low quality.
Authors' conclusions
The best available data show that a combination of manual therapy and exercise may not be as effective as glucocorticoid injection in the short‐term. It is unclear whether a combination of manual therapy, exercise and electrotherapy is an effective adjunct to glucocorticoid injection or oral NSAID. Following arthrographic joint distension with glucocorticoid and saline, manual therapy and exercise may confer effects similar to those of sham ultrasound in terms of overall pain, function and quality of life, but may provide greater patient‐reported treatment success and active range of motion. High‐quality RCTs are needed to establish the benefits and harms of manual therapy and exercise interventions that reflect actual practice, compared with placebo, no intervention and active interventions with evidence of benefit (e.g. glucocorticoid injection).
Keywords: Adult; Female; Humans; Male; Middle Aged; Bursitis; Bursitis/therapy; Exercise Therapy; Exercise Therapy/methods; Glucocorticoids; Glucocorticoids/therapeutic use; Injections, Intra‐Articular; Musculoskeletal Manipulations; Musculoskeletal Manipulations/methods; Randomized Controlled Trials as Topic; Shoulder Pain; Shoulder Pain/therapy
Plain language summary
Manual therapy and exercise for frozen shoulder (adhesive capsulitis)
Background
Frozen shoulder is a common cause of shoulder pain and stiffness. The pain and stiffness can last up to two to three years before going away, and in the early stages it can be very painful.
Manual therapy comprises movement of the joints and other structures by a healthcare professional (e.g. physiotherapist). Exercise includes any purposeful movement of a joint, muscle contraction or prescribed activity. The aims of both treatments are to relieve pain, increase joint range and improve function.
Study characteristics
This summary of an updated Cochrane review presents what we know from research about the benefits and harms of manual therapy and exercise in people with frozen shoulder. After searching for all relevant studies published up to May 2013, we included 32 trials (1836 participants). Among the included participants, 54% were women, average age was 55 years and average duration of the condition was six months. The average duration of manual therapy and exercise interventions was four weeks.
Key results—manual therapy and exercise compared with glucocorticoid (a steroid that reduces inflammation) injection into the shoulder
Pain (higher scores mean worse pain)
People who had manual therapy and exercise for six weeks did not improve as much as people who had glucocorticoid injection—improvement in pain was 26 points less (ranging from 15 to 37 points less) at seven weeks (26% absolute less improvement).
• People who had manual therapy and exercise rated their change in pain score as 32 points on a scale of 0 to 100 points.
• People who had glucocorticoid injection rated their change in pain score as 58 points on a scale of 0 to 100 points.
Function (lower scores mean better function)
People who had manual therapy and exercise for six weeks did not improve as much as people who had glucocorticoid injection—improvement in function was 25 points less (ranging from 15 to 35 points less) at seven weeks (25% absolute less improvement).
• People who had manual therapy and exercise rated their change in function as 14 points on a scale of 0 to 100 points.
• People who had glucocorticoid injection rated their change in function as 39 points on a scale of 0 to 100 points.
Treatment success
31 fewer people out of 100 rated their treatment as successful with manual therapy and exercise for six weeks compared with glucocorticoid injection—31% absolute less improvement (ranging from 13% to 48% less improvement).
• 46 out of 100 people reported treatment success with manual therapy and exercise.
• 77 out of 100 people reported treatment success with glucocorticoid injection.
Side effects
Out of 100 people, three had minor side effects such as temporary pain after treatment with manual therapy and exercise for six weeks compared with glucocorticoid injection.
• 56 out of 100 people reported side effects with manual therapy and exercise.
• 53 out of 100 people reported side effects with glucocorticoid injection.
Quality of the evidence
Evidence of moderate quality shows that the combination of manual therapy and exercise probably improves pain and function less than glucocorticoid injection up to seven weeks, and probably does not result in more adverse events. Further research may change the estimate.
Low‐quality evidence suggests that (1) the combination of manual therapy, exercise and electrotherapy (such as therapeutic ultrasound) may not improve pain or function more than glucocorticoid injection or placebo injection into the shoulder, (2) the combination of manual therapy, exercise, electrotherapy and glucocorticoid injection may not improve pain or function more than glucocorticoid injection alone and (3) the combination of manual therapy, exercise, electrotherapy and oral non‐steroidal anti‐inflammatory drug (NSAID) may not improve function more than oral NSAID alone. Further research is likely to change the estimate.
High‐quality evidence shows that following arthrographic joint distension, the combination of manual therapy and exercise does not improve pain or function more than sham ultrasound, but may provide greater patient‐reported treatment success and active range of motion. Further research is very unlikely to change our confidence in the estimate of effect.
No trial compared the combination of manual therapy and exercise versus placebo or no intervention.
Summary of findings
Summary of findings for the main comparison. Combination of manual therapy and exercise compared with glucocorticoid injection for adhesive capsulitis (frozen shoulder).
| Combination of manual therapy and exercise compared with glucocorticoid injection for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: general practices in high‐income countries Intervention: manual therapy plus exercise for six weeks Comparison: glucocorticoid injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Glucocorticoid injection | Manual therapy plus exercise | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Overall pain (change from baseline) 0‐100 visual analogue scale (lower score = less pain) Follow‐up: 7 weeks | Mean overall pain (change from baseline) in the control group was 58 points | Mean overall pain (change from baseline) in the intervention group was 26 points lower (36.8‐15.2 lower) | 107 (1 study) | ⊕⊕⊕⊝ moderatea | Absolute risk difference 26% (37% to 15% fewer); relative percentage change 30% (43% to 17% fewer) NNTB 3 (2 to 4) |
|
| Function (change from baseline) Shoulder Disabilty Questionnaire 0‐100 (lower scores = better function) Follow‐up: 7 weeks | Mean function (change from baseline) in the control group was 39 points | Mean function (change from baseline) in the intervention group was 25 points lower (35.24‐14.76 lower) | 107 (1 study) | ⊕⊕⊕⊝ moderatea | Absolute risk difference 25% (35% to 15% fewer); relative percentage change 38% (51% to 22% fewer) NNTB 3 (2 to 4) |
|
| Global assessment of treatment success Complete recovery or much improvement (self‐rated) Follow‐up: 7 weeks | Study populationb | RR 0.6 (0.44‐0.83) | 108 (1 study) | ⊕⊕⊕⊝ moderatea | Absolute risk difference 30% (48% to 13% fewer); relative percentage change 40% (56% to 17% fewer). NNTB 4 (3 to 8) |
|
| 769 per 1000 | 462 per 1000 (338‐638) | |||||
| Active shoulder abduction | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Adverse events | Study population | RR 1.07 (0.76‐1.49) | 114c (1 study) | ⊕⊕⊕⊝ moderatea | Absolute risk difference 4% (15% fewer to 22% more); relative percentage change 7% (24% fewer to 49% more) NNTH not applicable Adverse events recorded included pain after treatment < 1 day or > 2 days, facial flushing, irregular menstrual bleeding, fever and skin irritation |
|
| 526 per 1000 | 563 per 1000 (400‐784) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aParticipants were not blinded. bRisk of treatment success in the glucocorticoid injection group of van der Windt 1998 used as the assumed control group risk.
cIncludes 56 participants in the manual therapy and exercises group, who were treated according to protocol, 1 participant treated with both interventions, 52 participants in the glucocorticoid injection group who were treated according to protocol and 5 participants treated with both interventions.
Summary of findings 2. Combination of manual therapy, exercise, electrotherapy and placebo injection compared with glucocorticoid injection for adhesive capsulitis (frozen shoulder).
| Combination of manual therapy, exercise, electrotherapy and placebo injection compared with glucocorticoid injection for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: outpatient rheumatology clinic and general practices in high‐income countries Intervention: manual therapy plus exercise plus electrotherapy for four weeks plus placebo injection Comparison: glucocorticoid injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Glucocorticoid injection | Manual therapy plus exercise plus electrotherapy plus placebo injection | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Overall pain (change from baseline) Shoulder Pain and Disability Questionnaire (SPADI, 0‐100) (lower scores = less pain)a Follow‐up: mean 6 weeks | Mean overall pain (change from baseline) in the control groups was 39.1b | Mean overall pain (change from baseline) in the intervention groups was 3.78 lower (19.26 lower‐11.7 higher)c | 86 (2 studies) | ⊕⊕⊝⊝ lowd,e,f | Absolute risk difference 4% (19% fewer to 12% more);
relative percentage change 5% (27% fewer to 17% more). NNTB not applicable SMD 0.21 (‐0.65 to 1.07) |
|
| Function (change from baseline) Shoulder Pain and Disability Questionnaire (SPADI, 0‐100) (lower scores = better function)g Follow‐up: mean 6 weeks | Mean function (change from baseline) in the control groups was 34.2h | Mean function (change from baseline) in the intervention groups was 8.56 lower (0.56‐16.56 lower)i | 86 (2 studies) | ⊕⊕⊝⊝ lowd,j | Absolute risk difference 9% (1% to 16% fewer);
relative percentage change 14% (1% to 26% fewer) NNTB 5 (3 to 68) SMD 0.46 (0.03 to 0.89) |
|
| Global assessment of treatment success | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Active shoulder abduction | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
|
Quality of life (change from baseline) SF‐36 Physical Component Score (0‐100) (lower scores = worse quality of life) Follow‐up: 6 weeks |
Mean quality of life (change from baseline) in the control group was 4.4 |
Mean quality of life (change from baseline) in the intervention group was 3.3 lower (8.57 lower‐1.97 higher) |
49 (1 study) |
⊕⊕⊝⊝ lowk,l | Absolute risk difference 3% (9% fewer to 2% more);
relative percentage change 9% (23% fewer to 5% more) NNTB not applicable |
|
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; SD: standard deviation; SF: Short Form; SMD: standardised mean difference; SPADI: Shoulder Pain and Disability Index; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aPain measured using the SPADI in Carette 2003 and daytime rest pain (VAS 0‐100) in Ryans 2005. Results analysed using SMDs and back‐transformed to the 0‐100 SPADI scale units. bGlucocorticoid injection group mean reported in Carette 2003 used as the assumed control group risk. cTo convert SMDs in the Carette 2003 and Ryans 2005 meta‐analyses of overall pain scores, the pooled baseline SD in Carette 2003 (SD = 18) was multiplied by the SMDs and the 95% CIs to convert values to a 100‐point SPADI pain score. dParticipants were not blinded in either trial, and risk of attrition bias was high in 1 trial (Ryans 2005). eStatistical heterogeneity was high (I2 = 75%, with the direction of effect differing between trials).
f95% CIs relatively wide, incorporating (1) a clinically insignificant difference favouring combined intervention, (2) no difference between groups and (3) a minimal clinically important difference favouring glucocorticoid injection as possible estimates of effect. gFunction measured using the SPADI in Carette 2003 and using the Croft Shoulder Disability Questionnaire in Ryans 2005. Results analysed using SMDs and back‐transformed to the 0‐100 SPADI scale units.
hGlucocorticoid injection group mean reported in Carette 2003 used as the assumed control group risk.
iTo convert SMDs in the Carette 2003 and Ryans 2005 meta‐analyses of function scores, the pooled baseline SD in Carette 2003 (SD = 18.6) was multiplied by the SMDs and the 95% CIs to convert values to a 100‐point SPADI disability score.
j95% CIs relatively wide, incorporating both a clinically insignificant difference and a minimal clinically important difference favouring glucocorticoid injection as possible estimates of effect.
kParticipants not blinded.
l95% CIs relatively wide, incorporating (1) a clinically insignificant difference favouring combined intervention, (2) no difference between groups and (3) a clinically insignificant difference favouring glucocorticoid injection as possible estimates of effect.
Summary of findings 3. Combination of manual therapy, exercise, electrotherapy and placebo injection compared with placebo injection for adhesive capsulitis (frozen shoulder).
| Combination of manual therapy, exercise, electrotherapy and placebo injection compared with placebo injection for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: outpatient rheumatology clinic and general practices in high‐income countries Intervention: manual therapy plus exercise plus electrotherapy for four weeks plus placebo injection Comparison: placebo injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo injection | Manual therapy plus exercise plus electrotherapy plus placebo injection | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Overall pain (change from baseline) Shoulder Pain and Disability Questionnaire (SPADI, 0‐100) (lower scores = less pain)a Follow‐up: mean 6 weeks | Mean overall pain (change from baseline) in the control groups was 17.3b | Mean overall pain (change from baseline) in the intervention groups was 4.32 higher (3.24 lower‐12.06 higher)c | 86 (2 studies) | ⊕⊕⊝⊝ lowd,e | Absolute risk difference 4% (3% fewer to 12% more);
relative percentage change 6% (5% fewer to 17% more) NNTB not applicable SMD ‐0.24 (‐0.67 to 0.18) |
|
| Function (change from baseline) Shoulder Pain and Disability Questionnaire (SPADI, 0‐100) (lower scores = better function)f Follow‐up: mean 6 weeks | Mean function (change from baseline) in the control groups was 20.4g | Mean function (change from baseline) in the intervention groups was 1.67 higher (6.14 lower‐9.67 higher)h | 86 (2 studies) | ⊕⊕⊝⊝ lowd,e | Absolute risk difference 2% (6% fewer to 10% more);
relative percentage change 3% (9% fewer to 15% more) NNTB not applicable SMD ‐0.09 (‐0.52 to 0.33) |
|
| Global assessment of treatment success | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Active shoulder abduction | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
|
Quality of life (change from baseline) SF‐36 Physical Component Score (0‐100) (lower scores = worse quality of life) Follow‐up: 6 weeks |
Mean quality of life (change from baseline) in the control group was 2.5 |
Mean quality of life (change from baseline) in the intervention group was 1.4 lower (6.67 lower‐3.87 higher) |
49 (1 study) |
⊕⊕⊝⊝ lowi,j | Absolute risk difference 1% (7% fewer to 4% more);
relative percentage change 4% (18% fewer to 11% more) NNTB not applicable |
|
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; SMD: standardised mean difference; SPADI: Shoulder Pain an Disability Index. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aPain measured using the SPADI in Carette 2003 and daytime rest pain (VAS 0‐100) in Ryans 2005. Results analysed using SMDs and back‐transformed to 0‐100 SPADI scale units. bPlacebo injection group mean reported in Carette 2003 used as the assumed control group risk. cTo convert SMDs in the Carette 2003 and Ryans 2005 meta‐analyses of overall pain scores, the pooled baseline SD in Carette 2003 (SD = 18) was multiplied by the SMDs and the 95% CIs to convert values to a 100‐point SPADI pain score. dParticipants were not blinded in either trial, and risk of attrition bias was high in 1 trial (Ryans 2005). e95% CIs relatively wide, incorporating (1) a clinically insignificant difference favouring combined intervention, (2) no difference between groups and (3) a clinically insignificant difference favouring placebo injection as possible estimates of effect. fFunction measured using the SPADI in Carette 2003 and using the Croft Shoulder Disability Questionnaire in Ryans 2005. Results analysed using SMDs and back‐transformed to 0‐100 SPADI scale units.
gPlacebo injection group mean reported in Carette 2003 used as the assumed control group risk.
hTo convert SMDs in the Carette 2003 and Ryans 2005 meta‐analyses of function scores, the pooled baseline SD in Carette 2003 (SD = 18.6) was multiplied by the SMDs and the 95% CIs to convert values to a 100‐point SPADI disability score.
iParticipants not blinded.
j95% CIs relatively wide, incorporating (1) a clinically insignificant difference favouring combined intervention, (2) no difference between groups and (3) a clinically insignificant difference favouring placebo injection as possible estimates of effect.
Summary of findings 4. Combination of manual therapy, exercise, electrotherapy and glucocorticoid injection compared with glucocorticoid injection for adhesive capsulitis (frozen shoulder).
| Combination of manual therapy, exercise, electrotherapy and glucocorticoid injection compared with glucocorticoid injection for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: outpatient rheumatology clinic and general practices in high‐income countries Intervention: manual therapy plus exercise plus electrotherapy for four weeks plus glucocorticoid injection Comparison: glucocorticoid injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Glucocorticoid injection | Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Overall pain (change from baseline) Shoulder Pain and Disability Questionnaire (SPADI, 0‐100) (lower scores = less pain)a Follow‐up: mean 6 weeks | Mean overall pain (change from baseline) in the control groups was 39.1b | Mean overall pain (change from baseline) in the intervention groups was 5.76 lower (13.86 lower‐2.34 higher)c | 86 (2 studies) | ⊕⊕⊝⊝ lowd,e | Absolute risk difference 6% (14% fewer to 2% more);
relative percentage change 8% (20% fewer to 3% more) NNTB not applicable SMD ‐0.32 (‐0.77 to 0.13) |
|
| Function (change from baseline) Shoulder Pain and Disability Questionnaire (SPADI, 0‐100) (lower scores = better function)f Follow‐up: mean 6 weeks | Mean function (change from baseline) in the control groups was 34.2g | Mean function (change from baseline) in the intervention groups was 6.51 lower (14.88 lower‐1.86 higher)h | 86 (2 studies) | ⊕⊕⊝⊝ lowd,e | Absolute risk difference 7% (15% fewer to 2% more);
relative percentage change 10% (24% fewer to 3% more) NNTB not applicable SMD ‐0.35 (‐0.80 to 0.10) |
|
| Global assessment of treatment success | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Active shoulder abduction | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
|
Quality of life (change from baseline) SF‐36 Physical Component Score (0‐100) (lower scores = worse quality of life) Follow‐up: 6 weeks |
Mean quality of life (change from baseline) in the control group was 4.4 |
Mean quality of life (change from baseline) in the intervention group was 2 higher (3.27 lower‐7.27 higher) |
44 (1 study) |
⊕⊕⊝⊝ lowi,j | Absolute risk difference 2% (3% fewer to 7% more);
relative percentage change 5% (9% fewer to 19% more) NNTB not applicable |
|
| Adverse events | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; SF: Short Form; SMD: standardised mean difference; SPADI: Shoulder Pain and Disability Index. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aPain measured using the SPADI in Carette 2003 and daytime rest pain (VAS 0‐100) in Ryans 2005. Results analysed using SMDs and back‐transformed to 0‐100 SPADI scale units. bGlucocorticoid injection group mean reported in Carette 2003 used as the assumed control group risk. cTo convert SMDs in the Carette 2003 and Ryans 2005 meta‐analyses of overall pain scores, the pooled baseline SD in Carette 2003 (SD = 18) was multiplied by the SMDs and the 95% CIs to convert values to a 100‐point SPADI pain score. dParticipants were not blinded in either trial, and risk of attrition bias was high in 1 trial (Ryans 2005). e95% CIs relatively wide, incorporating (1) a minimal clinically important difference favouring combined intervention, (2) no difference between groups and (3) a clinically insignificant difference favouring glucocorticoid injection alone as possible estimates of effect. fFunction measured using the SPADI in Carette 2003 and using the Croft Shoulder Disability Questionnaire in Ryans 2005. Results analysed using SMDs and back‐transformed to 0‐100 SPADI scale units.
gGlucocorticoid injection group mean reported in Carette 2003 used as the assumed control group risk.
hTo convert SMDs in the Carette 2003 and Ryans 2005 meta‐analyses of function scores, the pooled baseline SD in Carette 2003 (SD = 18.6) was multiplied by the SMDs and the 95% CIs to convert values to a 100‐point SPADI disability score.
iParticipants not blinded.
j95% CIs relatively wide, incorporating (1) a clinically insignificant difference favouring combined intervention, (2) no difference between groups and (3) a clinically insignificant difference favouring glucocorticoid injection as possible estimates of effect.
Summary of findings 5. Combination of manual therapy and exercise following joint distension compared with sham ultrasound following joint distension for adhesive capsulitis (frozen shoulder).
| Combination of manual therapy and exercise following joint distension compared with sham ultrasound following joint distension for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: primary care and specialist practice in high‐income country Intervention: manual therapy plus exercise for six weeks following joint distension Comparison: sham ultrasound following joint distension | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham ultrasound following joint distension | Manual therapy plus exercise following joint distension | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Overall pain (change from baseline) 0‐10 visual analogue scale (lower scores = lower pain) Follow‐up: 6 weeks | Mean overall pain (change from baseline) in the control group was 3.4 | Mean overall pain (change from baseline) in the intervention group was 0 higher (0.69 lower‐0.69 higher) | 148 (1 study) | ⊕⊕⊕⊕ high | Absolute risk difference 0% (7% fewer to 7% more);
relative percentage change 0% (13% fewer to 13% more) NNTB not applicable |
|
| Function (change from baseline) SPADI 0‐100 (lower scores = better function) Follow‐up: 6 weeks | Mean function (change from baseline) in the control group was 38.5 | Mean function (change from baseline) in the intervention group was 0.5 lower (7.6 lower‐6.6 higher) | 148 (1 study) | ⊕⊕⊕⊕ high | Absolute risk difference 1% (8% fewer‐7% more);
relative percentage change 1% (12% fewer to 11% more) NNTB not applicable |
|
|
Active shoulder abduction (change from baseline) Degrees Follow‐up: 6 weeks |
Mean active range of abduction (change from baseline) in the control group was 36 |
Mean active range of abduction (change from baseline) in the intervention group was 13.1 higher (4.2‐22 higher) |
148 (1 study) |
⊕⊕⊕⊕ high | Absolute risk difference 7% (2% fewer to 12% more);
relative percentage change 19% (6% to 33% more). NNTB not calculated because there is no established minimal clinically important difference for this outcome. |
|
|
Quality of life (change from baseline) SF‐36 Physical Component Score (0‐100) (lower scores = worse quality of life) Follow‐up: 6 weeks |
Mean quality of life (change from baseline) in the control group was 8.3 |
Mean quality of life (change from baseline) in the intervention group was 0.5 lower (4.25 lower‐3.25 higher) |
148 (1 study) |
⊕⊕⊕⊕ high | Absolute risk difference 1% (4% fewer to 3% more);
relative percentage change 2% (14% fewer to 11% more) NNTB not applicable |
|
| Global assessment of treatment success "Success, much improved, and/or completely recovered" (self‐rated) Follow‐up: 6 weeks | Study populationa | RR 1.33 (1.04‐1.69) | 148 (1 study) | ⊕⊕⊕⊕ high | Absolute risk difference 19% (3% to 34% more);
relative percentage change 33% (4% to 69% more) NNTB = 6 (3 to 45) |
|
| 562 per 1000 | 747 per 1000 (584‐949) | |||||
| Adverse events | Study population | RR 0.99 (0.14‐6.82) | 149 (1 study) | ⊕⊕⊕⊕ high | Absolute risk difference 0% (5% fewer to 5% more);
relative percentage change 1% (86% fewer to 582% more) NNTH not applicable |
|
| 27 per 1000 | 27 per 1000 (4‐184) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aRisk of treatment success in the sham ultrasound group of Buchbinder 2007 used as the assumed control group risk.
Summary of findings 6. Combination of manual therapy, exercise, electrotherapy and oral NSAID compared with oral NSAID for adhesive capsulitis (frozen shoulder).
| Combination of manual therapy, exercise, electrotherapy and NSAID compared with NSAID for adhesive capsulitis (frozen shoulder) | ||||||
| Patient or population: patients with adhesive capsulitis (frozen shoulder) Settings: orthopaedic and rehabilitation clinic in low‐ to middle‐income countries Intervention: manual therapy plus exercise plus electrotherapy plus oral NSAID Comparison: oral NSAID | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral NSAID | Manual therapy plus exercise plus electrotherapy plus oral NSAID | |||||
| Participant‐reported pain relief ≥ 30% | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Overall pain | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Function (change from baseline) Shoulder Pain and Disability Questionnaire (SPADI, 0‐100) (lower scores = better function) Follow‐up: 3 weeks | Mean function (change from baseline) in the control group was 11.9 | Mean function (change from baseline) in the intervention group was 8.6 higher (3.28‐13.92 higher) | 119 (1 study) | ⊕⊕⊝⊝ lowa,b | Absolute risk difference 9% (3% to 14% more);
relative percentage change 17% (6% to 28% more) NNTB 4 (2 to 10) |
|
| Global assessment of treatment success "Disappearance of shoulder complaints or some pain/limitation which does not interfere with everyday life" Follow‐up: 6 weeks | Study population | RR 1.45 (0.99‐2.12) | 109 (1 study) | ⊕⊕⊝⊝ lowa | Absolute risk difference 19% (1% to 38% more);
relative percentage change 45% (1% fewer to 112% more) NNTH not applicable |
|
| 423 per 1000 | 613 per 1000 (419‐897) | |||||
| Active shoulder abduction | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | No studies reported this outcome |
| Adverse events Pain persisting longer than 2 hours after treatment (during the 3‐week treatment period) Follow‐up: 3 weeks | Study population | RR 8.85 (0.49‐160.87) | 119 (1 study) | ⊕⊕⊝⊝ lowa,c | Absolute risk difference 7% (1% fewer to 14% more);
relative percentage change 785% (51% fewer to 15987% more) NNTH not applicable |
|
| 0 per 1000 | 0 per 1000 (0‐0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; NSAID: non‐steroidal anti‐inflammatory drug; RR: risk ratio; SPADI: Shoulder Pain and Disability Index. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aParticipants were not blinded. b95% CI relatively wide, incorporating both a clinically insignificant difference and a minimal clinically important difference favouring the combined intervention group.
c95% CI very wide.
Background
Description of the condition
This review is one of a series of reviews seeking to gather evidence for benefits and harms of common interventions for shoulder pain. This series of reviews forms the update of an earlier Cochrane review of physical therapy for shoulder disorders (Green 2003). Since the time of our original review, many new clinical trials studying a diverse range of interventions have been performed. To improve usability of this series, we have subdivided the reviews by type of shoulder disorder, as patients within different diagnostic groupings may respond differently to interventions. This review focuses on manual therapies and exercise alone or in combination for adhesive capsulitis (frozen shoulder). Separate reviews of (1) electrotherapy modalities for adhesive capsulitis, (2) manual therapy, exercise and taping for rotator cuff disorders and (3) electrotherapy modalities for rotator cuff disorders are currently under way.
Adhesive capsulitis (also termed frozen shoulder, painful stiff shoulder or periarthritis) is a common condition characterised by spontaneous onset of pain, progressive restriction of movement of the shoulder and disability that restricts activities of daily living, work and leisure (Codman 1934; Neviaser 1945; Reeves 1975). Lack of specific diagnostic criteria for the condition has been acknowledged. Reviews of the diagnostic criteria used in clinical trials of adhesive capsulitis have found that all trialists reported that restricted movement must be present, but the amount of restriction, whether the restriction had to be active or passive or both and the direction of restriction were inconsistently defined (Green 1998; Schellingerhout 2008). The cumulative incidence of adhesive capsulitis has been reported as 2.4 per 1000 people per year (95% confidence interval (CI) 1.9 to 2.9) based on presentations to Dutch general practice (van der Windt 1995). Adhesive capsulitis has been reported to affect slightly more women than men (Tekavec 2012; Walker 2004). Most studies indicate that it is a self‐limiting condition lasting up to two to three years (Reeves 1975), although some people may have residual clinically detectable restriction of movement and disability beyond this time point (Binder 1984; Hazelman 1972). The largest case series (269 shoulders in 223 patients) found that at a mean follow‐up of 4.4 years (range two to 20 years), 41% had ongoing symptoms (Hand 2008).
Description of the intervention
Manual therapy and exercise, usually delivered together as components of a physical therapy intervention, are commonly used interventions for adhesive capsulitis (Hanchard 2011a). Manual therapy includes any clinician‐applied movement of the joints and other structures, for example, mobilisation (of which several types exist, e.g. Kaltenborn 1976; Maitland 1977) or manipulation. Exercise includes any purposeful movement of a joint, muscle contraction or prescribed activity. It may be performed under the supervision of a clinician or unsupervised at home. Examples include range of motion, stretching, strengthening, pendulum, pulley, "shoulder wheel" and "wall climbing" exercises. Manual therapy and exercises are delivered by various clinicians, including physiotherapists, physical therapists, chiropractors and osteopaths. The aims of both types of interventions are to relieve pain, promote healing, reduce muscle spasms, increase joint range, strengthen weakened muscles and improve biomechanics and function (Hanchard 2011b). In practice, patients with adhesive capsulitis seldom receive a single intervention in isolation (i.e. manual therapy or exercise alone). In addition, electrotherapy modalities (e.g. therapeutic ultrasound, laser therapy) may be delivered along with manual therapy and exercise as part of a physical therapy intervention (Hanchard 2011a).
How the intervention might work
Although previous systematic reviews have found limited evidence for the benefit of manual therapy and exercise when used in isolation to treat adhesive capsulitis (Green 1998; Green 2003), these interventions are hypothesised to produce a number of beneficial physiological and biomechanical effects. Restricted movement of the shoulder for an extended period of time can result in loss of strength, proprioception and coordination of the shoulder complex (Ballantyne 1993), along with contraction of muscles, tendons and ligaments around the shoulder (Mao 1997). Mobilisation is employed to reduce pain by stimulating peripheral mechanoreceptors and inhibiting nociceptors, and to increase joint mobility by enhancing exchange between synovial fluid and cartilage matrix (Frank 1984; Mangus 2002; Vermeulen 2006). Exercises aim to improve range of motion and muscle function by restoring shoulder mobility, proprioception and stability (Nicholson 1985).
Why it is important to do this review
The previous version of this review (Green 2003), which included four trials investigating the benefits and harms of manual therapy or exercise (or both) for adhesive capsulitis (Bulgen 1984; Dacre 1989; Nicholson 1985; van der Windt 1998), concluded that little evidence was available to support or refute the benefits or harms of these interventions for adhesive capsulitis. Other recently published systematic reviews of interventions for adhesive capsulitis (Blanchard 2010; Favejee 2011; Hanchard 2011b; Maund 2012) have identified several new trials. Therefore, this review of manual therapy and exercise for adhesive capsulitis needs to be updated.
Objectives
To synthesise available evidence regarding the benefits and harms of manual therapy and exercise, alone or in combination, for the treatment of patients with adhesive capsulitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any design (e.g. parallel, cross‐over, factorial) and controlled clinical trials using a quasi‐randomised method of allocation, such as by alternation or date of birth. Reports of trials were eligible regardless of the language or date of publication.
Types of participants
We included trials that enrolled adults (> 16 years of age) with adhesive capsulitis (as defined by trialists) for any duration. We included trials consisting of participants with various soft tissue disorders only if the results for participants with adhesive capsulitis were presented separately, or if 90% or more of participants in the trial had adhesive capsulitis. We excluded trials that enrolled participants with a history of significant trauma or systemic inflammatory conditions such as rheumatoid arthritis, osteoarthritis, hemiplegic shoulder and pain in the shoulder region as part of a complex myofascial neck/shoulder/arm pain condition.
Types of interventions
We included trials comparing any manual therapy or exercise intervention versus no treatment, placebo, a different type of manual therapy or exercise or any other intervention. Eligible interventions included mobilisation, manipulation and supervised or home exercise. Exercises could be land‐based or water‐based but had to consist of tailored shoulder exercises rather than just general activity (e.g. swimming). Trials primarily evaluating the effects of electrotherapy modalities such as therapeutic ultrasound, low‐level laser therapy, transcutaneous electrical nerve stimulation (TENS), pulsed electromagnetic field therapy, interferential current, phonophoresis, iontophoresis or continuous short‐wave diathermy were excluded and are included in a separate Cochrane review.
Types of outcome measures
We did not consider outcomes as part of the eligibility criteria.
Considerable variation has been noted in the outcome measures reported in clinical trials of interventions for pain. However, it is generally agreed that outcome measures of greatest importance to patients should be considered.
The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has published consensus recommendations for determining clinically important changes in outcome measures in clinical trials of interventions for chronic pain (Dworkin 2008). Reductions in pain intensity of ≥ 30% and ≥ 50% reflect moderate and substantial clinically important differences, respectively, and it is recommended that the proportion of participants who respond with these degrees of pain relief should be reported.
Continuous outcome measures in pain trials (such as mean change on a 100‐mm visual analogue scale (VAS)) may not follow a Gaussian distribution. Often, a bimodal distribution is seen instead, in which participants tend to report very good or very poor pain relief (Moore 2010). This creates difficulty in interpreting the meaning of average changes in continuous pain measures. For this reason, a dichotomous outcome measure (the proportion of participants reporting ≥ 30% pain relief) may be clinically relevant for trials of adhesive capsulitis.
The original review determined that no trials had included a dichotomous outcome for pain, in keeping with the recognition that it has been the practice in most trials of interventions for chronic pain to report continuous measures only. We therefore also included a continuous measure of overall pain.
A global rating of treatment success, such as the Patient Global Impression of Change scale (PGIC), which provides an outcome measure that integrates pain relief, changes in function and adverse events into a single, interpretable measure, is also recommended by IMMPACT and was included as a main outcome measure (Dworkin 2008).
Main outcomes
Participant‐reported pain relief of 30% or greater (a moderate clinically important difference).
Overall pain (mean or mean change measured by VAS, numerical or categorical rating scale).
Function. When trialists reported outcome data for more than one function scale, we extracted data on the scale that was highest on the following a priori defined list: (1) Shoulder Pain and Disability Index (SPADI); (2) Croft Shoulder Disability Questionnaire; (3) Constant Score; (4) Short Form (SF)‐36 Physical Component Score; (5) Health Assessment Questionnaire; and (6) any other function scale.
Global assessment of treatment success as defined by trialists (e.g. proportion of participants with significant overall improvement).
Active shoulder abduction (measured in degrees or other).
Quality of life as measured by generic measures (such as components of the SF‐36 or disease‐specific tools).
Number of participants experiencing any adverse events.
Other outcomes
Night pain measured by VAS, numerical or categorical rating scale.
Pain on motion measured by VAS, numerical or categorical rating scale.
Other measures of range of motion (ROM) (flexion, external rotation and internal rotation (measured in degrees or other, e.g. hand‐behind‐back distance in centimetres)). When trialists reported outcome data for both active and passive ROM measures, we extracted the data on active ROM only.
Work disability.
Requiring surgery (e.g. manipulation under anaesthesia, arthroscopy).
We extracted benefit outcome measures (e.g. overall pain or function) at the following time points.
Up to three weeks.
Longer than three weeks and up to six weeks (this was the main time point).
Longer than six weeks and up to six months.
Longer than six months.
If data were available in a trial at multiple time points within each of the above periods (e.g. at four, five and six weeks), we extracted only data at the latest possible time point of each period. We extracted adverse events reported at all time points.
We collated the main results of the review into summary of findings (SoF) tables, which provide key information on the quality of evidence and the magnitude and precision of the effects of interventions. We included the main outcomes (see above) in the SoF tables, with results at, or nearest, the main time point (six weeks) presented.
Search methods for identification of studies
Electronic searches
We searched the Central Register of Controlled Trials (CENTRAL) (to 2013, Issue 4), MEDLINE (January 1966 to May 2013), EMBASE (January 1980 to May 2013) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus (January 1937 to May 2013). The complete search strategies are presented in Appendix 1. Note that the search terms used included clinical terms relevant to rotator cuff disorders and electrotherapy interventions, as the current review and Cochrane reviews of (1) electrotherapy modalities for adhesive capsulitis, (2) manual therapy and exercise for rotator cuff disorders and (3) electrotherapy modalities for rotator cuff disorders were conducted simultaneously.
In May 2014, we reran the search of all four electronic bibliographic databases and screened the results for potentially eligible records, but we did not incorporate any studies identified in the updated search.
Searching other resources
We searched for ongoing trials and protocols of published trials in the clinical trials register that is maintained by the US National Institutes of Health (http://clinicaltrials.gov) and the Clinical Trial Register at the International Clinical Trials Registry Platform of the World Health Organization (http://www.who.int/ictrp/en/). We also reviewed the reference lists of included trials and of relevant review articles retrieved from the electronic searches to identify other potentially relevant trials.
Data collection and analysis
Selection of studies
Two review authors (MJP and BM) independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups.
Possibly relevant—trials that met the inclusion criteria and trials from which it was not possible to determine from their title or abstract whether they met the criteria.
Excluded—trials clearly not meeting the inclusion criteria.
If a title or abstract suggested that the trial was eligible for inclusion, or if we could not tell, we obtained a full‐text version of the article, and two review authors (MJP and BM) performed an independent assessment to determine whether it met the inclusion criteria. The review authors resolved discrepancies through discussion or through adjudication by a third review author (SG or RB).
Data extraction and management
Two review authors (MJP and SK, RJ or MC) independently extracted data using a standard data extraction form developed for this review. The review authors resolved discrepancies through discussion or adjudication by a third review author (SG or RB) until consensus was reached. We pilot‐tested the data extraction form and modified it as needed before use. In addition to items for assessing risk of bias and numerical outcome data, we recorded the following characteristics.
Trial characteristics, including type (e.g. parallel, cross‐over), country, source of funding and trial registration status (with registration number recorded if available).
Participant characteristics, including age, sex, duration of symptoms and inclusion/exclusion criteria.
Intervention characteristics, including type of manual therapy or exercise, duration of treatment and use of co‐interventions.
Outcomes reported, including the measurement instrument used and timing of outcome assessment.
One review author (MJP) compiled all comparisons and entered outcome data into Review Manager 5.2.
For a particular systematic review outcome, a multiplicity of results may be available in the trial reports (e.g. multiple scales, time points, analyses). To prevent selective inclusion of data based on the results (Page 2013), we used the following a priori defined decision rules to select data from trials.
When trialists reported both final values and change from baseline values for the same outcome, we extracted final values.
When trialists reported both unadjusted and adjusted values for the same outcome, we extracted unadjusted values.
When trialists reported data analysed on the basis of the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
For cross‐over RCTs, we extracted data from the first period only.
When trials did not include a measure of overall pain but included one or more other measures of pain, for the purpose of combining data for the primary analysis of overall pain, we combined overall pain with other types of pain in the following hierarchy: unspecified pain; pain with activity; daytime pain.
Assessment of risk of bias in included studies
Two review authors (MJP and SK, RJ or MC) independently assessed the risk of bias in included trials using the tool of The Cochrane Collaboration for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The following domains were assessed.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment (assessed separately for self‐reported and objectively assessed outcomes).
Incomplete outcome data.
Selective reporting.
Other sources of bias (specifically, baseline imbalance).
Each item was rated as at 'Low risk,' 'Unclear risk' or 'High risk' of bias. We resolved discrepancies through discussion or adjudication by a third review author (SG or RB).
Measures of treatment effect
We used the statistical software of The Cochrane Collaboration, Review Manager 5.2, to perform data analysis. We expressed dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) and continuous outcomes as mean differences (MDs) with 95% CIs if different trials used the same measurement instrument to measure the same outcome. Alternatively, we analysed continuous outcomes using the standardised mean difference (SMD) when trials measured the same outcome but employed different measurement instruments. To enhance interpretability of dichotomous outcomes, risk differences and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) were calculated. To enhance interpretability of continuous outcomes, pooled SMDs for overall pain and function were back‐transformed to an original SPADI (0 to 100) pain or disability score by multiplying SMDs and 95% CIs by a representative pooled standard deviation (SD) at baseline for one of the included trials.
Unit of analysis issues
The unit of analysis was the participant. Two trials included a small number of participants with bilateral adhesive capsulitis. For these trials, we analysed data based on the number of participants, not the number of shoulders, to produce conservative estimates of effect.
Dealing with missing data
When required, we contacted trialists via email (twice, separated by three weeks) to retrieve missing information about trial design, outcome data or attrition rates, such as dropouts, losses to follow‐up and postrandomisation exclusions in the included trials. For continuous outcomes with no SD reported, we calculated SDs from standard errors (SEs), 95% CIs or P values. If no measures of variation were reported and SDs could not be calculated, we planned to impute SDs from other trials in the same meta‐analysis, using the median of other available SDs (Ebrahim 2013). When data were imputed or calculated (e.g. SDs calculated from SEs, 95% CIs or P values, or imputed from graphs or from SDs in other trials), we reported this in the Characteristics of included studies tables.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across trials. We assessed statistical heterogeneity using the Chi2 statistic and the I2 statistic (Higgins 2002). We interpreted the I2 statistic by using the following as an approximate guide.
0% to 40% may not be important heterogeneity.
30% to 60% may represent moderate heterogeneity.
50% to 90% may represent substantial heterogeneity.
75% to 100% may represent considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess publication bias, we planned to generate funnel plots if at least 10 trials examining the same intervention comparison were included in the review and to comment on whether asymmetry in the funnel plot was due to publication bias or to methodological or clinical heterogeneity of the trials (Sterne 2011). To assess outcome reporting bias, we compared outcomes specified in trial protocols versus outcomes reported in corresponding trial publications; if trial protocols were unavailable, we compared outcomes reported in the methods and results sections of trial publications (Dwan 2011; Norris 2012). We generated an outcome reporting bias in trials (ORBIT) matrix (http://ctrc.liv.ac.uk/orbit/) using the ORBIT classification system (Kirkham 2010).
Data synthesis
For this review update, a large number of identified trials studied a diverse range of interventions. To define the most clinically important questions to be answered in the review, after data extraction was completed, one review author (MJP) sent the list of all possible trial comparisons to both of the original primary authors of this review (SG and RB). After reviewing the list of possible trial comparisons, both of these review authors discussed and drafted a list of clinically important review questions and categorised each trial comparison under the review question with which it fit best. This process was conducted iteratively until all trial comparisons were allocated to a single review question, and it was conducted without knowledge of the results of any outcomes. The following review questions were defined.
Is the combination of manual therapy and exercise (with or without electrotherapy) effective compared with placebo, no intervention or another active intervention (e.g. glucocorticoid injection, oral non‐steroidal anti‐inflammatory drug (NSAID), arthrographic joint distension)?
Is the combination of manual therapy and exercise (with or without electrotherapy) delivered in addition to another active intervention more effective than the other active intervention alone?
Is manual therapy (with or without electrotherapy) effective compared with placebo, no intervention or another active intervention?
Are supervised or home exercises (with or without electrotherapy) effective compared with placebo, no intervention or another active intervention?
Is one type of manual therapy or exercise (with or without electrotherapy) more effective than another (i.e. one type of manual therapy vs another type of manual therapy or one type of exercise vs another type of exercise)?
Is the combination of manual therapy and exercise (with or without electrotherapy) delivered in addition to another active intervention more effective than placebo or no treatment?
The first two of these were considered the main questions of the review, as these combination interventions are best reflective of clinical practice.
We combined results of trials with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of benefits and harms. When we could not combine data, we summarised effect estimates and 95% CIs of each trial narratively. We combined results using a random‐effects meta‐analysis model based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an impact on the results.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses.
Sensitivity analysis
We planned to perform a sensitivity analysis to investigate the robustness of the treatment effect (of main outcomes) to allocation concealment and participant blinding, by removing trials that reported inadequate or unclear allocation concealment and lack of participant blinding from the meta‐analysis, to see if this changed the overall treatment effect.
Summary of findings tables
We presented the results of the most important comparisons of the review in Summary of findings tables, which summarise the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on outcomes, as recommended by The Cochrane Collaboration (Schünemann 2011a). The Summary of findings tables include an overall grading of the evidence related to each of the main outcomes, using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) approach (Schünemann 2011b).
In the Comments column of the Summary of findings table, we report the absolute per cent difference, the relative per cent change from baseline and the number needed to treat for an additional beneficial outcome (NNTB) (the NNTB is provided only when the outcome shows a statistically significant difference).
For dichotomous outcomes (pain relief ≥ 30%, global assessment, adverse events), the absolute risk difference was calculated using the risk difference statistic in RevMan, and the result expressed as a percentage; the relative per cent change was calculated as the risk ratio ‐1 and was expressed as a percentage. For continuous outcomes (overall pain, function, active shoulder abduction, quality of life), the absolute risk difference was calculated as the improvement in the intervention group minus the improvement in the control group, expressed in the original units (i.e. mean difference from RevMan divided by units in the original scale), expressed as a percentage. The relative per cent change is calculated as the absolute change (or mean difference) divided by the baseline mean of the control group, expressed as a percentage.
In addition to the absolute and relative magnitude of effect provided in the SoF table, for dichotomous outcomes the NNTB or the number needed to treat for an additional harmful effect (NNTH) was calculated from the control group event rate and the risk ratio using the Visual Rx NNT calculator (Cates 2004). For continuous outcomes of overall pain and function, the NNTB was calculated using Wells calculator software, which is available at the Cochrane Musculoskeletal Group (CMSG) editorial office (http://musculoskeletal.cochrane.org). We assumed a minimal clinically important difference (MCID) of 1.5 points on a 10‐point scale for pain, and of 10 points on a 100‐point scale for function or disability, for input into the calculator.
Results
Description of studies
Results of the search
The search, which was conducted up to May 2013, yielded 3173 records across the four databases. Three additional records were identified by screening reference lists of previously published systematic reviews and included trials. After duplicates were removed, 2118 unique records remained. Of these, 290 were retrieved for full‐text screening on the basis of title and abstract. Thirty‐two trials were deemed eligible for inclusion (Buchbinder 2007; Bulgen 1984; Carette 2003; Celik 2010; Chan 2010; Chauhan 2011; Cheing 2008; Dacre 1989; Dundar 2009; Ghosh 2012; Guler‐Uysal 2004; Harsimran 2011; Johnson 2007; Ma 2006; Maricar 1999; Maryam 2012; Nellutla 2009; Nicholson 1985; Pajareya 2004; Rainbow 2008; Ryans 2005; Samnani 2004; Sharad 2011; Shrivastava 2011; Sirajuddin 2010; Tanaka 2010; van der Windt 1998; Vermeulen 2006; Wen 2009; Yan 2005; Yang 2007; Yang 2012). Two additional trials are available only as conference abstracts (Uddin 2012; Wies 2003); one is written in German and requires translation (Fink 2012). These three trials are awaiting classification (see Characteristics of studies awaiting classification table). Three ongoing trials were identified in clinical trials registries (see Characteristics of ongoing studies table). A flow diagram of the study selection process is presented in Figure 1.
1.
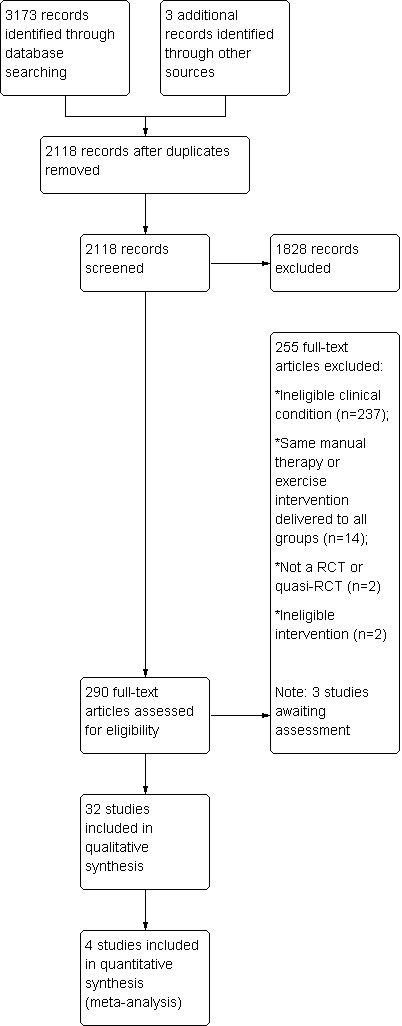
Study flow diagram.
The updated search in May 2014 yielded three studies deemed eligible for inclusion (Doner 2013; Ibrahim 2013; Russell 2014). These studies did not address the two main questions of the review (which focus on the effects of the combination of manual therapy and exercise) so were not incorporated into the review, but they are listed in the Characteristics of studies awaiting classification table. We will include these studies in a future update of the review.
Included studies
A full description of all included trials is provided in the Characteristics of included studies table. We contacted authors of 26 trials to retrieve (1) information about study design, participants, interventions and outcomes of the trial, (2) information required to complete the risk of bias assessments or (3) missing data for unreported or partially reported outcomes. We received replies from seven trialists (Carette 2003; Chan 2010; Maryam 2012; Pajareya 2004; Ryans 2005Yang 2007; Yang 2012).
Design
All trials except two were described as RCTs (Nicholson 1985 and Rainbow 2008 used a quasi‐random method of allocation). All trials except one used a parallel‐group design (Yang 2007 used a multiple‐treatment trial design, which involves the application of two or more treatments for a single participant and was used to assess differences among three interventions in two groups of participants). Twenty‐one trials included two intervention arms (Buchbinder 2007; Celik 2010; Chan 2010; Chauhan 2011; Dundar 2009; Guler‐Uysal 2004; Harsimran 2011; Johnson 2007; Maricar 1999; Nellutla 2009; Nicholson 1985; Pajareya 2004; Rainbow 2008; Samnani 2004; Sharad 2011; Shrivastava 2011; van der Windt 1998; Vermeulen 2006; Wen 2009; Yan 2005; Yang 2012), eight included three arms (Cheing 2008; Dacre 1989; Ghosh 2012; Ma 2006; Maryam 2012; Sirajuddin 2010; Tanaka 2010; Yang 2007) and three included four arms (Bulgen 1984; Carette 2003; Ryans 2005).
Participants
A total of 1836 participants were included in the 32 trials, and the number of participants per trial ranged from eight to 156. The median of the mean age of participants was 55 years, and the median of the mean duration of symptoms was six months. Fifty‐four per cent of participants were female. Diagnostic criteria for (or definitions of) adhesive capsulitis varied with regards to type, amount and direction of shoulder restriction, ranging from undefined (e.g. Samnani 2004) to very specific (e.g. ≥ 50% loss of passive movement of the shoulder joint relative to the non‐affected side, in one or more of three movement directions (i.e. abduction in the frontal plane, forward flexion or external rotation in 0° of abduction)) (Vermeulen 2006). Trials were conducted in India (N = 8); UK (N = 4); Turkey, USA and Taiwan (N = 3 each); China and The Netherlands (N = 2); and Australia, Canada, Hong Kong, Iran, Japan, Singapore and Thailand (N = 1 each).
Interventions
A detailed description of the interventions delivered in each trial is summarised in the Characteristics of included studies table. A summary of the manual therapy or exercise intervention components tested in each trial is presented in Table 7. The types of manual therapy and exercise delivered were very heterogeneous across trials; Maitland's mobilisation techniques were the most common type of manual therapy, and Codman's pendulum exercises, active and passive ROM exercises, pulley exercises and shoulder wheel exercises were the most common types of exercise. The median duration of manual therapy or exercise interventions was four weeks (range one to 18), with a median of three treatment sessions delivered per week (range one to seven) and a median of 12 treatment sessions provided in total across the treatment period (range five to 84).
1. Characteristics of manual therapy and exercise interventions.
| Study ID | Manual therapy component(s) | Exercise component(s) | Duration of session (minutes) | No. sessions (per week) | No. weeks treatment | Total no. sessions |
| Buchbinder 2007 | Both passive and self‐executed muscle stretching techniques to stretch muscles passing over the glenohumeral joint; cervical and thoracic spine mobilization, glenohumeral joint passive accessory glides; glenohumeral joint passive physiologic mobilization including rotation. | Supervised: strength and coordination exercises for rotator cuff and scapular stabilizers; proprioceptive challenge | 30 | 2 per week in first 2 weeks; 1 per week in next 4 weeks | 6 | 8 |
| Bulgen 1984 | Maitland's mobilisations (no other details provided) | NA | NR | 3 | 6 | 18 |
| Carette 2003 | Mobilisation techniques (no other details provided) | Supervised: active ROM exercises (for acute adhesive capsulitis); active and auto‐assisted ROM exercises and isometric strengthening exercises (for chronic adhesive capsulitis) | 60 | 3 | 4 | 12 |
| Celik 2010 | NA | Supervised: scapulothoracic strengthening (serratus anterior, middle and lower trapezius, latissimus dorsi), upper trapezius stretching, and postural exercises. Home: active assistive ROM exercises (flexion, scapular elevation, and internal and external rotation exercises), posterior and inferior capsule stretching exercises, and self‐stick exercises |
Dependent on participants pain and muscle strength | 5 | 6 | 30 |
| Chan 2010 | Passive mobilisation (Grade A and Grade B mobilisation techniques, as advocated by Cyriax for treatment of stage II capsulitis) | Home: active and active‐assisted ROM exercises, capsular stretching exercise, postural correction and scapular stabilising work | 30 | 1 | 6 | 6 |
| Chauhan 2011 | Deep transverse friction massage of the two tendon supraspinatus and subscapularis as laid by Cyriax 1983, followed by inferior capsular stretching. Deep friction was given transverse to the fiber direction | Supervised: passive ROM exercises. Home: Not specified | 60 | 3 | 2 | 6 |
| Cheing 2008 | NA | Home: exercises in four directions: (i) forward flexion; (ii) external rotation; (iii) horizontal adduction; and (iv) internal rotation | NR | 2 to 3 | 4 | 10 |
| Dacre 1989 | Mobilisation (no other details provided) | NA | NR | 1 | 4 to 6 | 4 to 6 |
| Dundar 2009 | NA | Supervised: active stretching and pendulum exercises | 60 | 5 | 4 | 20 |
| Ghosh 2012 | NA | Supervised: active and passive shoulder mobilisation exercises plus shoulder wheel and pulley exercise | NR | NR | NR | NR |
| Guler‐Uysal 2004 | Cyriax approach of deep friction massage and manipulation | NA | 60 | 3 | 2 | 6 |
| Harsimran 2011 | Either anterior or posterior glide mobilisation (Kaltenborn grade III) | NA | NR | 5 | 1 | 5 |
| Johnson 2007 | Either anterior or posterior glide mobilisation (Kaltenborn grade III) | NA | NR | 2 to 3 | 2 to 3 | 6 |
| Ma 2006 | Mobilisation (no other details provide) | Supervised: active exercises (not specified) | 20 | 5 | 4 | 20 |
| Maricar 1999 | Mobilisation of shoulder quadrant, shoulder capsular stretch, shoulder flexion, shoulder abduction, shoulder external and internal rotation using Maitland Grade III+ and IV | NA | NR | 1 | 8 | 8 |
| Maryam 2012 | NA | Supervised: active ROM exercises | NR | NR | NR | 10 |
| Nellutla 2009 | NA | Supervised: either proprioceptive neuromuscular facilitation (PNF) movement patterns for exercises or conventional free exercises, such as finger ladder exercises, Codman's pendulum exercises, and overhead shoulder pulley and shoulder wheel | NR | 5 | 3 | 15 |
| Nicholson 1985 | Passive mobilisation. Generally, in the early sessions gliding and distractive mobilisation techniques were performed with the joint near its neutral position, progressing in the later sessions to mobilisation towards the end of the range of motion | NA | NR | 2 to 3 | 4 | 8 to 12 |
| Pajareya 2004 | Passive mobilisation (no other details provided) | Supervised: passive glenohumeral joint stretching exercises up to the participant's tolerance, based on Cyriax. Home: pulley exercises (actively assisted exercises) and active non‐assisted exercises using a towel and wall |
NR | 3 | 3 | 9 |
| Rainbow 2008 | Either high‐velocity, low‐amplitude chiropractic manipulative therapy to the cervical and thoracic spine and glenohumeral joint or Grade 4 mobilisation of the glenohumeral joint according to the supine glenohumeral mobilisation technique | NA | NR | 2 | 6 | 12 |
| Ryans 2005 | Maitland mobilizations which were progressed as the condition improved, and proprioceptive neuromuscular facilitation | Supervised: active exercise therapy with gym equipment | NR | 2 | 4 | 8 |
| Samnani 2004 | NA | Passive ROM exercises | 15 | 6 | 6 | 36 |
| Sharad 2011 | End range mobilisation techniques. Initially a few minutes of warming up was given using mid‐range mobilization with the patient positioned supine, after which intensive end rage mobilisation techniques, Grades 3 and 4 as described by Maitland in all the movement planes were delivered, interspersed with accessory movements (glides) | NA | Dependent on participants presentation and tolerance | 5 | 3 | 15 |
| Shrivastava 2011 | Either Maitland's Graded Oscillations Technique or Mulligan's Mobilisation with Movement technique | NA | Dependent on participants symptoms | 6 | 2 | 12 |
| Sirajuddin 2010 | Either anterior or posterior glide mobilisation (Kaltenborn grade III) | NA | 15 | 2 | 3 | 6 |
| Tanaka 2010 | Mobilization techniques used by Vermeulen 2000, which are performed in the end‐ranges of limited joint mobility | NA | 40 | 2 | 18 | 36 |
| van der Windt 1998 | Passive mobilisation (no other details provided) | Supervised: not specified | 30 | 2 | 6 | 12 |
| Vermeulen 2006 | Either intensive passive mobilisation techniques in end‐range positions of the glenohumeral joint (applied with intensities according to Maitland grades III and IV) or assive mobilisation techniques within the pain‐free zone (applied with intensities according to Maitland grades I and II) | NA | 30 | 2 | 12 | 24 |
| Wen 2009 | NA | Supervised: hand exercises, Codman hanging and swinging movement, flexion in front of the body at 90 degrees, hanging the limb and swinging it backwards and forwards, inside and out, and in circles, and wall climbing the 'ladder' exercises (if duration of symptoms was 1‐2 months); passive range of motion exercises, active exercises with gym equipment, active exercises with a wooden stick, and shoulder wheel exercises (if duration of symptoms was 2‐3 months); pulley exercises (if durations of symptoms was 3‐6 months) | NR | 7 | 2 | 15 |
| Yan 2005 | NA | Supervised: either gymnastics training exercises with dumbbells weighing 2‐5 kg or bare‐handed exercises | 5 to 10 | 7 | 12 | 84 |
| Yang 2007 | Either end‐range mobilisation or mid‐range mobilisation or mobilisation with movement | NA | 30 | 2 | 3 | 6 |
| Yang 2012 | End‐range mobilisation and scapular mobilisation | NA | NR | 2 | 8 | 16 |
NA = Not applicable
NR = Not reported
Outcomes
An ORBIT matrix that presents outcomes measured and level of reporting for each outcome in each trial (rated as fully reported, partially reported, measured but not reported, unclear if measured or not measured) is presented in Table 8. Of the main outcomes, no trial measured "participant‐reported pain relief of 30% or greater." Twenty‐three trials measured overall pain (mean or mean change), 20 measured function, seven measured global assessment of treatment success, 10 measured active shoulder abduction, six measured quality of life and seven measured adverse events. Overall pain was most commonly measured using a zero to 10 or zero to 100 VAS. Function was most commonly measured using the SPADI, followed by the Constant Score and the Croft Shoulder Disability Questionnaire. Of the other outcomes, 28 trials measured other measures of ROM, eight measured night pain and seven measured pain on motion. No trials reported measuring work disability or requiring surgery. Partial reporting of outcomes occurred in 15 trials. We contacted authors of all 15 trials to retrieve missing outcome data, and we obtained data from two (Yang 2007; Yang 2012).
2. Outcome Reporting Bias In Trials (ORBIT) matrix.
| Study ID | Main outcomes | Other outcomes | ||||||||||
| Participant‐reported pain relief ≥30% | Overall pain | Function | Global assessment | Active shoulder abduction | QoL | Adverse events | Night pain | Pain on motion | Other ROM | Work disability | Requiring surgery | |
| Buchbinder 2007 | Not measured | Full | Full | Full | Full | Full | Full | Full | Full | Full | Not measured | Not measured |
| Bulgen 1984 | ? | Measured | ? | ? | ? | ? | ? | Measured | Measured | Partial | ? | ? |
| Carette 2003 | ? | Full | Full | ? | Measured | Full | ? | ? | ? | Full | ? | ? |
| Celik 2010 | ? | Full | Full | ? | ? | ? | ? | ? | ? | Full | ? | ? |
| Chan 2010 | ? | Partial | Partial | ? | Partial | ? | ? | ? | ? | Partial | ? | ? |
| Chauhan 2011 | ? | Partial | Partial | ? | ? | ? | ? | ? | ? | Partial | ? | ? |
| Cheing 2008 | ? | Partial | Partial | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Dacre 1989 | ? | Partial | ? | ? | ? | ? | Full | Measured | Partial | Partial | ? | ? |
| Dundar 2009 | ? | Full | Full | ? | ? | ? | Full | Full | Full | Full | ? | ? |
| Ghosh 2012 | ? | ? | ? | Full | ? | ? | ? | ? | ? | ? | ? | ? |
| Guler‐Uysal 2004 | ? | Full | ? | Full | ? | ? | ? | Full | Full | Full | ? | ? |
| Harsimran 2011 | ? | Partial | ? | ? | ? | ? | ? | ? | ? | Partial | ? | ? |
| Johnson 2007 | ? | Full | Partial | ? | ? | ? | ? | Partial | ? | ? | ? | ? |
| Ma 2006 | ? | Partial | ? | ? | Partial | Partial | ? | ? | Partial | Partial | ? | ? |
| Maricar 1999 | ? | ? | ? | ? | ? | ? | ? | ? | ? | Partial | ? | ? |
| Maryam 2012 | Not measured | Full | Full | Not measured | Not measured | Not measured | Not measured | Not measured | Not measured | Full | Not measured | Not measured |
| Nellutla 2009 | ? | ? | Full | ? | ? | ? | ? | ? | ? | Full | ? | ? |
| Nicholson 1985 | ? | Full | ? | ? | Full | ? | ? | ? | ? | Full | ? | ? |
| Pajareya 2004 | ? | ? | Full | Full | ? | ? | Full | ? | ? | Full | ? | ? |
| Rainbow 2008 | ? | ? | Full | ? | ? | ? | Full | ? | ? | ? | ? | ? |
| Ryans 2005 | ? | Full | Full | ? | Measured | Measured | ? | ? | ? | Full | ? | ? |
| Samnani 2004 | ? | ? | ? | ? | ? | ? | ? | ? | ? | Full | ? | ? |
| Sharad 2011 | ? | Partial | ? | ? | Partial | ? | ? | ? | ? | Partial | ? | ? |
| Shrivastava 2011 | ? | Partial | Partial | ? | ? | ? | Full | ? | ? | Partial | ? | ? |
| Sirajuddin 2010 | ? | Full | Partial | ? | Full | ? | ? | ? | ? | Full | ? | ? |
| Tanaka 2010 | ? | ? | ? | ? | Full | ? | ? | ? | ? | Full | ? | ? |
| van der Windt 1998 | ? | Full | Full | Full | ? | ? | Full | Full | ? | Full | ? | ? |
| Vermeulen 2006 | ? | Full | Full | Full | Full | Full | ? | Full | Full | Full | ? | ? |
| Wen 2009 | ? | Partial | Partial | ? | ? | ? | ? | ? | ? | Partial | ? | ? |
| Yan 2005 | ? | ? | ? | Full | ? | ? | ? | ? | ? | Full | ? | ? |
| Yang 2007 | ? | Measured | Partial | ? | ? | Measured | ? | ? | ? | Partial | ? | ? |
| Yang 2012 | ? | ? | Full | ? | ? | ? | ? | ? | ? | Full | ? | ? |
"Full"= sufficient data for inclusion in a meta‐analysis was reported (e.g. mean, standard deviation, and sample size per group for continuous outcomes)
"Partial" = insufficient data for inclusion in a meta‐analysis was reported (e.g. means only, with no measures of variation)
"Measured" = outcome was measured but no outcome data was reported
"Not measured" = outcome was not measured by the trialists
"?" = unclear whether the outcome was measured or not (as a trial protocol was unavailable)
Excluded studies
Of 290 full‐text articles retrieved for further scrutiny, most (n = 237) were excluded because they were studies or commentaries focused on shoulder pain due to conditions other than adhesive capsulitis (e.g. rotator cuff disorders). We have listed 18 adhesive capsulitis studies in the Characteristics of excluded studies table. Reasons for their exclusion were that the same manual therapy or exercise intervention was provided to all groups (n = 14), the study was not an RCT or a quasi‐RCT (n = 2) or the intervention was ineligible (n = 2).
Risk of bias in included studies
A summary of the risk of bias in included trials is presented in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Note that the white areas in "Blinding of outcome assessment (detection bias): self‐reported outcomes" and "Blinding of outcome assessment (detection bias): objective outcomes" indicate that the domain was not applicable to all trials because some trials did not measure any self‐reported or objective outcomes, respectively.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Note that white squares indicate that the domain was not applicable to the trial, because no self‐reported or objective outcomes, respectively, were measured in the trial.
Allocation
Eleven trials reported using an adequate method to generate a random allocation sequence (Buchbinder 2007; Carette 2003; Chan 2010; Johnson 2007; Pajareya 2004; Ryans 2005; Shrivastava 2011; van der Windt 1998; Vermeulen 2006; Yang 2007; Yang 2012), and only eight trials reported using an adequate method of allocation concealment (Buchbinder 2007; Carette 2003; Chan 2010; Pajareya 2004; Ryans 2005; van der Windt 1998; Yang 2007; Yang 2012). Two trials allocated participants using a quasi‐random sequence (Nicholson 1985; Rainbow 2008), and one trial used a method that did not conceal the allocation sequence (Johnson 2007), so all three were rated at high risk of selection bias. Nineteen trials did not report how the allocation sequence was generated, and 21 trials did not report how the allocation sequence was concealed. The risk of selection bias in these trials was therefore unclear.
Blinding
Three trials were rated at low risk of performance bias because of successful blinding of participants (Buchbinder 2007; Shrivastava 2011; Tanaka 2010). Seven trials were rated at unclear risk of performance bias because participants received different types of manual therapy or exercise, but it is unclear whether they were provided any information that would make them perceive the type of manual therapy or exercise they received as superior or inferior to the alternative type of manual therapy or exercise (Harsimran 2011; Johnson 2007; Nellutla 2009; Sirajuddin 2010; Vermeulen 2006; Yan 2005; Yang 2007). All remaining trials were rated at high risk of performance bias, as participants were not blinded and may have had different expectations about the benefits of each intervention. Of 29 trials assessing self‐reported outcomes, two were rated at low risk of detection bias because of clear participant blinding (Buchbinder 2007; Shrivastava 2011), seven were rated at unclear risk of detection bias because of unclear participant blinding (Harsimran 2011; Johnson 2007; Nellutla 2009; Sirajuddin 2010; Vermeulen 2006; Yan 2005; Yang 2007) and the remaining trials were rated at high risk of detection bias for self‐reported outcomes because of lack of participant blinding. Of 31 trials measuring objectively rated outcomes (e.g. ROM), 17 reported blinding of outcome assessors and thus were rated at low risk of detection bias for objective outcomes (Buchbinder 2007; Bulgen 1984; Carette 2003; Chan 2010; Cheing 2008; Dacre 1989; Guler‐Uysal 2004; Harsimran 2011; Nicholson 1985; Pajareya 2004; Ryans 2005; Shrivastava 2011; Tanaka 2010; van der Windt 1998; Vermeulen 2006; Yang 2007; Yang 2012). Three trials failed to blind assessors of objective outcomes, so the risk of detection bias for objective outcomes was high (Johnson 2007; Maryam 2012; Sharad 2011). Eleven trials did not report whether such blinding was done, so the risk of detection bias for objective outcomes in these trials was unclear.
Incomplete outcome data
Twenty‐four trials had no dropouts, losses to follow‐up or exclusions, or had a small quantity of incomplete data that was deemed unlikely to bias the results (Buchbinder 2007; Bulgen 1984; Celik 2010; Chan 2010; Cheing 2008; Dacre 1989; Dundar 2009; Ghosh 2012; Guler‐Uysal 2004; Harsimran 2011; Johnson 2007; Ma 2006; Nellutla 2009; Nicholson 1985; Rainbow 2008; Shrivastava 2011; Sirajuddin 2010; Tanaka 2010; van der Windt 1998; Vermeulen 2006; Wen 2009; Yan 2005; Yang 2007; Yang 2012). One trial reported differential dropout across groups, with reasons appearing to be related to the treatments received, and thus was rated at high risk of attrition bias (Ryans 2005). The remaining seven trials did not report the quantity of or reasons for incomplete outcome data and so had an unclear risk of attrition bias.
Selective reporting
Two trials were rated at low risk of selective reporting bias because all outcomes specified in the trial registry entry or the trial protocol were fully reported in the trial publication (Buchbinder 2007; Maryam 2012). Six trials were rated at high risk of selective reporting bias because some of the outcomes that were reported in the trial registry entry or the methods section of the publication were not reported in the results section at all (Bulgen 1984; Chauhan 2011; Cheing 2008; Dacre 1989; Ryans 2005; Yang 2007). The remaining 24 trials were rated at unclear risk of selective reporting bias because (1) outcome data were completely reported for all outcomes specified in the methods section of the publication, but none of these trials were registered in a trials registry or had an available trial protocol, so it is unclear whether other outcomes were measured but not reported based on the results, or (2) outcome data were incompletely reported (e.g. reporting means without measures of variation), but it was unclear whether data were incompletely reported based on the statistical significance or magnitude of the results.
Other potential sources of bias
All trials were rated as free from other potential sources of bias (specifically, baseline imbalance).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary data and effect estimates (with 95% CIs) of all outcomes are presented in the Data and analyses or Additional tables section. To enhance readability, we have reported in the following main text only effect estimates and 95% CIs for main outcomes at, or nearest, the main time point (six weeks) for comparisons addressing the two primary questions of the review (i.e. Is the combination of manual therapy and exercise (with or without electrotherapy) effective compared with placebo, no intervention or another active intervention? and Is the combination of manual therapy and exercise (with or without electrotherapy) delivered in addition to another active intervention more effective than the other active intervention alone?). Unless otherwise stated, differences between groups in overall pain and function reported as 'significant' mean that the effect estimate met our criteria for an MCID (i.e. 1.5 points on a 10‐point scale for pain, and 10 points on a 100‐point scale for function or disability) and were statistically significant (P value < 0.05).
As the result of heterogeneity of interventions, comparators and outcomes, we were able to conduct meta‐analyses to synthesise outcome data in only four trials. We synthesised results of two trials (Carette 2003; Ryans 2005) that addressed review questions 1 and 2. Both trials delivered an intervention lasting four weeks, which comprised mobilisation techniques, supervised exercise (active ROM exercises in Carette 2003 and active exercises with gym equipment in Ryans 2005), electrotherapy (TENS or ultrasound in Carette 2003 and interferential modality in Ryans 2005) and glucocorticoid or placebo injection. Both trials included participants with adhesive capsulitis for less than six months. To conduct meta‐analyses of overall pain, we combined SPADI pain data in Carette 2003 with VAS daytime rest pain data in Ryans 2005 using SMDs, and we back‐transformed the SMDs using the pooled baseline SD of SPADI pain scores reported in Carette 2003 (SD = 18). To conduct meta‐analyses of function, we combined SPADI disability data in Carette 2003 with Croft Shoulder Disability Questionnaire data in Ryans 2005 using SMDs, and we back‐transformed the SMDs using the pooled baseline SD of SPADI disability scores reported in Carette 2003 (SD = 18.6). We combined (1) six‐week data reported in both trials and (2) six‐month data reported in Carette 2003 with four‐month data provided in Ryans 2005.
We combined results of two trials (Johnson 2007; Sirajuddin 2010) that addressed review question 5 (Is one type of manual therapy or exercise (with or without electrotherapy) more effective than another?). Both trials compared anterior glide mobilisation plus ultrasound and exercise versus posterior glide mobilisation plus ultrasound and exercise, delivered twice a week for three weeks. Both trials measured overall pain using the same VAS (0 to 10) at three weeks.
Is the combination of manual therapy and exercise (with or without electrotherapy) more effective than placebo, no intervention or another active intervention (e.g. glucocorticoid injection, oral non‐steroidal anti‐inflammatory drug (NSAID), arthrographic joint distension)?
No trial compared a combination of manual therapy and exercise versus placebo or no intervention. Five trials compared a combination of manual therapy and exercise versus another active intervention (Carette 2003; Chauhan 2011; Ma 2006; Ryans 2005; van der Windt 1998). Outcome data from three trials were available for analysis (Carette 2003; Ryans 2005; van der Windt 1998).
Manual therapy plus exercise versus glucocorticoid injection
See Table 9; Table 1. One trial compared passive mobilisation and supervised exercise for six weeks versus glucocorticoid injection (van der Windt 1998). Given the inability to blind participants and personnel, the trial had a high risk of performance bias and detection bias for self‐reported outcomes.
3. van der Windt 1998: Passive mobilisation and supervised exercise (intervention) versus glucocorticoid injection (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐100) change from baseline to 3 weeks | 17 | 21 | 55 | 32 | 26 | 52 | ‐15.00 [‐23.99, ‐6.01] |
| Overall pain (VAS 0‐100) change from baseline to 7 weeks | 32 | 29 | 56 | 58 | 28 | 51 | ‐26.00 [‐36.80, ‐15.20] |
| Overall pain (VAS 0‐100) change from baseline to 6 months | 54 | 33 | 54 | 63 | 31 | 51 | ‐9.00 [‐21.24, 3.24] |
| Overall pain (VAS 0‐100) change from baseline to 12 months | 59 | 30 | 55 | 70 | 24 | 48 | ‐11.00 [‐21.44, ‐0.56] |
| Function (Shoulder Disability Questionnaire 0‐100) change from baseline to 3 weeks | 6 | 22 | 55 | 19 | 27 | 52 | ‐13.00 [‐22.36, ‐3.64] |
| Function (Shoulder Disability Questionnaire 0‐100) change from baseline to 7 weeks | 14 | 27 | 56 | 39 | 27 | 51 | ‐25.00 [‐35.24, ‐14.76] |
| Function (Shoulder Disability Questionnaire 0‐100) change from baseline to 6 months | 33 | 34 | 54 | 45 | 30 | 51 | ‐12.00 [‐24.25, 0.25] |
| Function (Shoulder Disability Questionnaire 0‐100) change from baseline to 12 months | 38 | 34 | 55 | 42 | 33 | 48 | ‐4.00 [‐16.96, 8.96] |
| Night pain (VAS 0‐100) change from baseline to 3 weeks | 9 | 23 | 55 | 21 | 26 | 52 | ‐12.00 [‐21.32, ‐2.68] |
| Night pain (VAS 0‐100) change from baseline to 7 weeks | 22 | 30 | 56 | 36 | 28 | 51 | ‐14.00 [‐24.99, ‐3.01] |
| Night pain (VAS 0‐100) change from baseline to 6 months | 33 | 41 | 54 | 34 | 36 | 51 | ‐1.00 [‐15.74, 13.74] |
| Night pain (VAS 0‐100) change from baseline to 12 months | 35 | 39 | 55 | 37 | 33 | 48 | ‐2.00 [‐15.91, 11.91] |
| Passive abduction (degrees) change from baseline to 3 weeks | ‐3 | 13 | 55 | 2 | 12 | 52 | ‐5.00 [‐9.74, ‐0.26] |
| Passive abduction (degrees) change from baseline to 7 weeks | ‐1 | 14 | 56 | 4 | 11 | 51 | ‐5.00 [‐9.75, ‐0.25] |
| Passive abduction (degrees) change from baseline to 6 months | 7 | 17 | 54 | 9 | 12 | 51 | ‐2.00 [‐7.60, 3.60] |
| Passive external rotation (degrees) change from baseline to 3 weeks | ‐3 | 12 | 55 | 6 | 14 | 52 | ‐9.00 [‐13.95, ‐4.05] |
| Passive external rotation (degrees) change from baseline to 7 weeks | ‐2 | 14 | 56 | 13 | 16 | 51 | ‐15.00 [‐20.72, ‐9.28] |
| Passive external rotation (degrees) change from baseline to 6 months | 7 | 21 | 54 | 16 | 18 | 51 | ‐9.00 [‐16.47, ‐1.53] |
| Events | Total | Events | Total | Risk ratio (95%CI) | |||
| Total adverse events | 32 | 57 | 30 | 57 | 1.07 [0.76, 1.49] | ||
| Adverse events: pain after treatment lasting <1 day | 17 | 57 | 9 | 57 | 1.89 [0.92, 3.88] | ||
| Adverse events: pain after treatment lasting >2 days | 13 | 57 | 16 | 57 | 0.81 [0.43, 1.53] | ||
| Adverse events: facial flushing | 1 | 57 | 9 | 57 | 0.11 [0.01, 0.85] | ||
| Adverse events: irregular menstrual bleeding | 0 | 57 | 6 | 57 | 0.08 [0.00, 1.33] | ||
| Adverse events: fever | 1 | 57 | 4 | 57 | 0.25 [0.03, 2.17] | ||
| Adverse events: skin irritation | 2 | 57 | 1 | 57 | 2.00 [0.19, 21.44] | ||
| Adverse events: any other adverse event | 4 | 57 | 6 | 57 | 0.67 [0.20, 2.24] | ||
| Global assessment of treatment success ("complete recovery or much improvement) at 7 weeks | 26 | 56 |
40 | 52 |
0.60 [0.44, 0.83] | ||
Main outcomes
A combination of passive mobilisation and supervised exercise for six weeks resulted in significantly less improvement than glucocorticoid injection in overall pain at three weeks, seven weeks (MD ‐26.00, 95% CI ‐36.80 to ‐15.20; 100‐point scale, 107 participants) and 12 months, and significantly less improvement in function at three weeks and seven weeks (MD ‐25.00, 95% CI ‐35.24 to ‐14.76; 100‐point scale, 107 participants). In addition, participants receiving passive mobilisation and supervised exercise were 40% less likely to rate themselves as having global treatment success at seven weeks (RR 0.60, 95% CI 0.44 to 0.83; 108 participants). However, 95% CIs for overall pain and function include both MCIDs and clinically insignificant differences as possible estimates of effect. Differences between groups in improvement in overall pain at six months, improvement in function at six months and 12 months and the number of participants with adverse events (RR 1.07, 95% CI 0.76 to 1.49; 114 participants) were not clinically or statistically significant.
Other outcomes
Participants receiving passive mobilisation and supervised exercise for six weeks had statistically significantly less improvement in night pain and passive abduction at three and seven weeks, and less improvement in passive external rotation at three weeks, seven weeks and six months.
Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection
See Table 2. Two trials compared a combination of mobilisation, supervised exercise and electrotherapy for four weeks and placebo injection versus glucocorticoid injection alone (Carette 2003; Ryans 2005). Both trials were unable to blind participants and personnel to the physical therapy component of the intervention, so they had a high risk of performance bias and detection bias for self‐reported outcomes. Ryans 2005 also had a high risk of attrition bias.
Main outcomes
The combination of mobilisation, supervised exercise and electrotherapy for four weeks and placebo injection was not significantly different from glucocorticoid injection alone in terms of improvement in overall pain at six weeks (SMD 0.21, 95% CI ‐0.65 to 1.07; I2 = 75%, 86 participants; this is equivalent to an MD of 3.78 (95% CI ‐11.7 to 19.26) on a 100‐point scale), six months or 12 months (12‐month data based on Carette 2003 only) (Analysis 1.1). Note that a large amount of statistical heterogeneity was noted in the meta‐analyses of overall pain, with the direction of effect differing between trials. Improvement in function was statistically (but not clinically) significantly lower in the "mobilisation, exercise, electrotherapy and placebo injection" group at six weeks (SMD 0.46, 95% CI 0.03 to 0.89; I2 = 0%, 86 participants; this is equivalent to an MD of 8.56 (95% CI 0.56 to 16.56) on a 100‐point scale) but not significantly different at six months or 12 months (12‐month data based on Carette 2003 only) (Analysis 1.2). Carette 2003 also found no significant differences between groups in improvement in quality of life at six weeks (SF‐36 Physical Component Score: MD ‐3.30, 95% CI ‐8.57 to 1.97; 100‐point scale, 86 participants; SF‐36 Mental Component Score: MD 0.50, 95% CI ‐5.60 to 6.60; 100‐point scale, 49 participants), six months and 12 months (Analysis 1.3).
1.1. Analysis.

Comparison 1 Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection, Outcome 1 Overall pain.
1.2. Analysis.

Comparison 1 Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection, Outcome 2 Function.
1.3. Analysis.
Comparison 1 Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection, Outcome 3 Quality of life.
Other outcomes
Carette 2003 found no significant differences between groups in active ROM at six weeks, six months and 12 months (Analysis 1.4; Analysis 1.5). Ryans 2005 found no significant differences between groups in passive external rotation at six weeks and four months (Analysis 1.6).
1.4. Analysis.
Comparison 1 Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection, Outcome 4 Active range of motion (degrees).
1.5. Analysis.
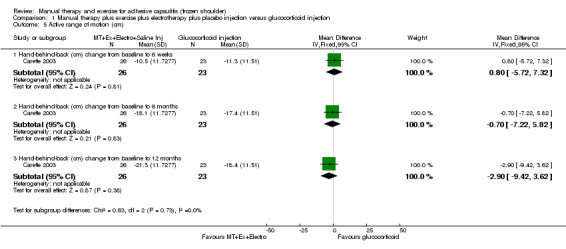
Comparison 1 Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection, Outcome 5 Active range of motion (cm).
1.6. Analysis.
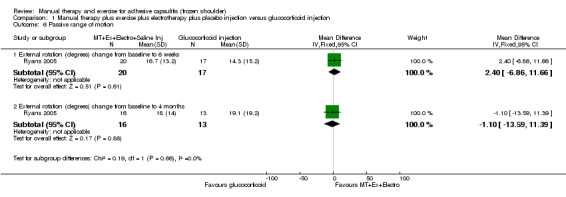
Comparison 1 Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection, Outcome 6 Passive range of motion.
Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection
See Table 3. The two trials above also compared the combination of mobilisation, supervised exercise and electrotherapy for four weeks and placebo injection versus placebo injection alone (Carette 2003; Ryans 2005).
Main outcomes
The combination of mobilisation, supervised exercise, and electrotherapy for four weeks and placebo injection was not significantly different from placebo injection alone in terms of improvement in overall pain at six weeks (SMD ‐0.24, 95% CI ‐0.67 to 0.18; I2 = 0%, 86 participants; this is equivalent to an MD of ‐4.32 (95% CI ‐12.06 to 3.24) on a 100‐point scale), six months or 12 months (12‐month data based on Carette 2003 only) (Analysis 2.1). No significant difference between groups was found in improvement in function at six weeks (SMD ‐0.09, 95% CI ‐0.52 to 0.33; I2 = 0%, 86 participants; this is equivalent to an MD of ‐1.67 (95% CI ‐9.67 to 6.14) on a 100‐point scale), six months or 12 months (12‐month data based on Carette 2003 only) (Analysis 2.2). Carette 2003 also found no significant differences between groups in improvement in quality of life at six weeks (SF‐36 Physical Component Score: MD ‐1.40, 95% CI ‐6.67 to 3.87; 100‐point scale, 49 participants; SF‐36 Mental Component Score: MD ‐0.60, 95% CI ‐6.70 to 5.50; 100‐point scale, 49 participants), six months and 12 months (Analysis 2.3).
2.1. Analysis.
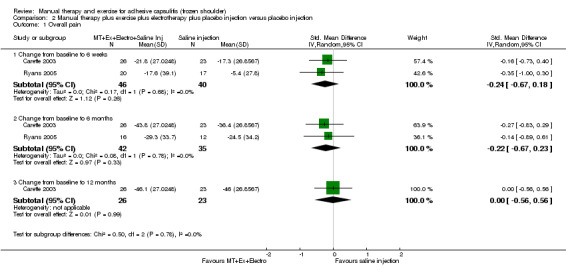
Comparison 2 Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection, Outcome 1 Overall pain.
2.2. Analysis.
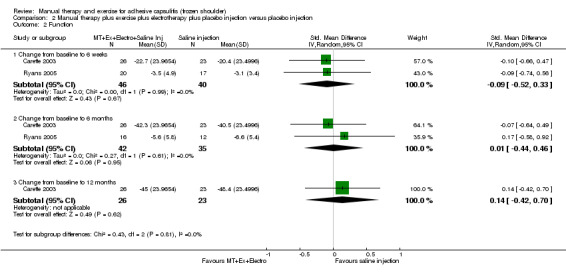
Comparison 2 Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection, Outcome 2 Function.
2.3. Analysis.
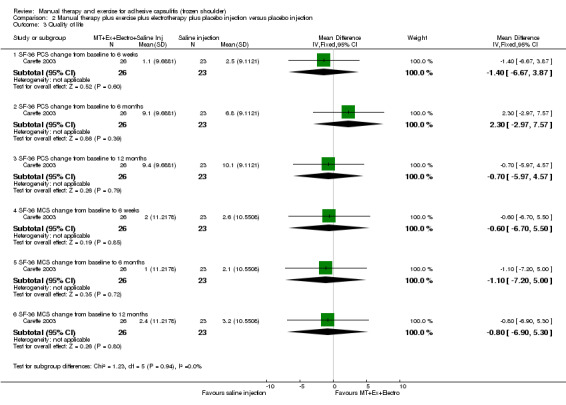
Comparison 2 Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection, Outcome 3 Quality of life.
Other outcomes
Carette 2003 found no significant differences between groups in active ROM at all time points (Analysis 2.4; Analysis 2.5). In contrast, Ryans 2005 reported statistically significantly greater improvement in passive external rotation in the multi‐component intervention group at six weeks, but not at four months (Analysis 2.6).
2.4. Analysis.

Comparison 2 Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection, Outcome 4 Active range of motion (degrees).
2.5. Analysis.
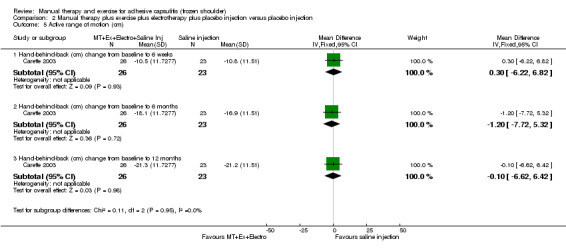
Comparison 2 Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection, Outcome 5 Active range of motion (cm).
2.6. Analysis.

Comparison 2 Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection, Outcome 6 Passive range of motion.
Is the combination of manual therapy and exercise (with or without electrotherapy) delivered in addition to another active intervention more effective than the other active intervention alone?
Five trials addressed this question (Buchbinder 2007; Carette 2003; Ma 2006; Pajareya 2004; Ryans 2005). Outcome data in four trials were available for analysis (Buchbinder 2007; Carette 2003; Pajareya 2004; Ryans 2005).
Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection
See Table 4. The two trials above also compared the combination of mobilisation, supervised exercise and electrotherapy for four weeks and glucocorticoid injection versus glucocorticoid injection alone (Carette 2003; Ryans 2005).
Main outcomes
The combination of mobilisation, supervised exercise and electrotherapy for four weeks and glucocorticoid injection was not significantly different from glucocorticoid injection alone in terms of improvement in overall pain at six weeks (SMD ‐0.32, 95% CI ‐0.77 to 0.13; I2 = 0%, 86 participants; this is equivalent to an MD of ‐5.76 (95% CI ‐13.86 to 2.34) on a 100‐point scale), six months or 12 months (12‐month data based on Carette 2003 only) (Analysis 3.1). In addition, improvements in function were not significantly different between groups at six weeks (SMD ‐0.35, 95% CI ‐0.80 to 0.10; I2 = 0%, 86 participants; this is equivalent to an MD of ‐6.51 (95% CI ‐14.88 to 1.86) on a 100‐point scale), six months or 12 months (12‐month data based on Carette 2003 only) (Analysis 3.2). Carette 2003 also found no significant differences between groups in improvement in quality of life at six weeks (SF‐36 Physical Component Score: MD 2.00, 95% CI ‐3.27 to 7.27; 100‐point scale, 44 participants; SF‐36 Mental Component Score: MD 4.20, 95% CI ‐2.04 to 10.44; 100‐point scale, 44 participants), six months and 12 months (Analysis 3.3).
3.1. Analysis.
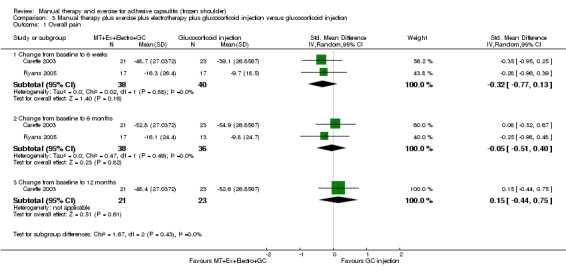
Comparison 3 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection, Outcome 1 Overall pain.
3.2. Analysis.
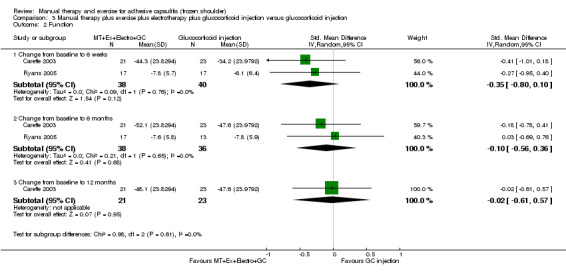
Comparison 3 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection, Outcome 2 Function.
3.3. Analysis.
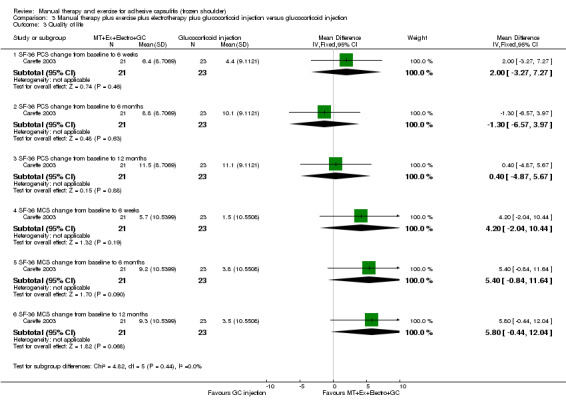
Comparison 3 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection, Outcome 3 Quality of life.
Other outcomes
Carette 2003 found that the combination of mobilisation, supervised exercise, electrotherapy and glucocorticoid injection resulted in statistically significantly greater improvement than glucocorticoid injection alone in active ROM at six weeks, but not at six months or 12 months (Analysis 3.4; Analysis 3.5). Ryans 2005 reported no significant differences between groups in improvement in passive external rotation at six weeks or at four months (Analysis 3.6).
3.4. Analysis.

Comparison 3 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection, Outcome 4 Active range of motion (degrees).
3.5. Analysis.
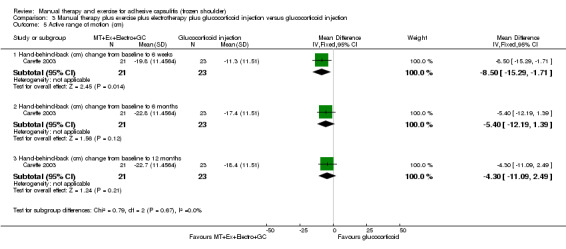
Comparison 3 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection, Outcome 5 Active range of motion (cm).
3.6. Analysis.

Comparison 3 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection, Outcome 6 Passive range of motion.
Manual therapy plus exercise following arthrographic joint distension versus sham ultrasound following arthrographic joint distension
See Table 10; Table 5. One trial, following arthrographic joint distension, compared passive mobilisation plus supervised exercise (stretching, strength and coordination) for six weeks versus sham ultrasound. All risk of bias domains were rated as low risk.
4. Buchbinder 2007: Passive mobilisation and supervised strength, stretching and coordination exercises following arthrographic joint distension (intervention) versus sham ultrasound following arthrographic joint distension (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐10) change from baseline to 6 weeks | ‐3.4 | 2.1 | 75 | ‐3.4 | 2.2 | 73 | 0.00 [‐0.69, 0.69] |
| Overall pain (VAS 0‐10) change from baseline to 6 months | ‐3.5 | 2.5 | 74 | ‐3.6 | 2.6 | 70 | 0.10 [‐0.73, 0.93] |
| Function (SPADI 0‐100) change from baseline to 6 weeks | 38 | 20.4 | 75 | 38.5 | 23.5 | 73 | ‐0.50 [‐7.60, 6.60] |
| Function (SPADI 0‐100) change from baseline to 6 months | 40 | 21.8 | 74 | 42.4 | 22.8 | 70 | ‐2.40 [‐9.69, 4.89] |
| Night pain (VAS 0‐10) change from baseline to 6 weeks | ‐3.7 | 2.9 | 75 | ‐3.6 | 2.5 | 73 | ‐0.10 [‐0.97, 0.77] |
| Night pain (VAS 0‐10) change from baseline to 6 months | ‐3.9 | 3.2 | 74 | ‐3.6 | 2.5 | 70 | ‐0.30 [‐1.24, 0.64] |
| Pain on motion (VAS 0‐10) change from baseline to 6 weeks | ‐4.2 | 2.3 | 75 | ‐4.4 | 2.7 | 73 | 0.20 [‐0.61, 1.01] |
| Pain on motion (VAS 0‐10) change from baseline to 6 months | ‐4.4 | 3 | 74 | ‐4.5 | 2.9 | 70 | 0.10 [‐0.86, 1.06] |
| Active hand behind back distance (cm) change from baseline to 6 weeks | ‐18.2 | 9.5 | 75 | ‐13.1 | 9 | 73 | ‐5.10 [‐8.08, ‐2.12] |
| Active hand behind back distance (cm) change from baseline to 6 months | ‐22.8 | 11.6 | 74 | ‐17.4 | 11.9 | 70 | ‐5.40 [‐9.24, ‐1.56] |
| Active abduction (degrees) change from baseline to 6 weeks | 49.1 | 29 | 75 | 36 | 26.2 | 73 | 13.10 [4.20, 22.00] |
| Active abduction (degrees) change from baseline to 6 months | 55.9 | 31.1 | 74 | 48.6 | 32.3 | 70 | 7.30 [‐3.07, 17.67] |
| Active flexion (degrees) change from baseline to 6 weeks | 37.2 | 19.9 | 75 | 28.1 | 19.3 | 73 | 9.10 [2.78, 15.42] |
| Active flexion (degrees) change from baseline to 6 months | 41.8 | 23.4 | 74 | 36.2 | 26.7 | 70 | 5.60 [‐2.62, 13.82] |
| Active external rotation (degrees) change from baseline to 6 weeks | 25.3 | 16.7 | 75 | 16.2 | 15.8 | 73 | 9.10 [3.86, 14.34] |
| Active external rotation (degrees) change from baseline to 6 months | 31 | 16.9 | 74 | 25.9 | 17.9 | 70 | 5.10 [‐0.59, 10.79] |
| Quality of life (SF‐36 PCS 0‐100) change from baseline to 6 weeks | 7.8 | 10.9 | 75 | 8.3 | 12.3 | 73 | ‐0.50 [‐4.25, 3.25] |
| Quality of life (SF‐36 PCS 0‐100) change from baseline to 6 months | 9.4 | 12.4 | 74 | 9.4 | 11.5 | 70 | 0.00 [‐3.90, 3.90] |
| Quality of life (SF‐36 MCS 0‐100) change from baseline to 6 weeks | 12.4 | 11.6 | 75 | 13.2 | 12.8 | 73 | ‐0.80 [‐4.74, 3.14] |
| Quality of life (SF‐36 MCS 0‐100) change from baseline to 6 months | 13.3 | 12.2 | 74 | 13.7 | 11.2 | 70 | ‐0.40 [‐4.22, 3.42] |
| Events | Total | Events | Total | Risk ratio (95%CI) | |||
| Total adverse events | 2 | 75 | 2 | 74 | 0.99 [0.14, 6.82] | ||
| Global assessment of treatment success ("Success/much improved, and/or completely recovered") at 6 weeks | 56 | 75 | 41 | 73 | 1.33 [1.04, 1.69] | ||
| Global assessment of treatment success ("Success/much improved, and/or completely recovered") at 6 months | 58 | 74 | 43 | 70 | 1.28 [1.02, 1.59] | ||
Main outcomes
Following arthrographic joint distension, no significant differences were found between passive mobilisation plus supervised exercise for six weeks and sham ultrasound for six weeks, in terms of improvement in overall pain at six weeks (MD 0.00, 95% CI ‐0.69 to 0.69; 10‐point scale, 148 participants) and six months, improvement in function at six weeks (MD ‐0.50, 95% CI ‐7.60 to 6.60; 100‐point scale, 148 participants) and six months, and the number of participants with adverse events (RR 0.99, 95% CI 0.14 to 6.82; 149 participants). Participants receiving passive mobilisation and supervised exercise for six weeks were statistically significantly more likely to self‐report having global treatment success at six weeks (RR 1.33, 95% CI 1.04 to 1.69; 148 participants) and six months, and had greater improvement in active shoulder abduction at six weeks (MD 13.10, 95% CI 4.2 to 22; 148 participants), but not at six months, compared with participants receiving sham ultrasound. Differences between groups in quality of life were small and not significant at six weeks (SF‐36 Physical Component Score: MD ‐0.50, 95% CI ‐4.25 to 3.25; 100‐point scale, 148 participants; SF‐36 Mental Component Score: MD ‐0.80, 95% CI ‐4.74 to 3.14; 100‐point scale, 148 participants) and six months.
Other outcomes
Participants receiving passive mobilisation and supervised exercise for six weeks had greater improvement in active hand‐behind‐back distance at six weeks and six months, and had greater improvement in active flexion and external rotation at six weeks, compared with participants receiving sham ultrasound. However, no significant differences between groups were found in night pain nor pain on motion at either time point.
Manual therapy plus exercise plus electrotherapy plus oral NSAID versus oral NSAID
See Table 11; Table 6. One trial compared a combination of passive mobilisation, supervised stretching and pulley exercises, electrotherapy and oral NSAID for three weeks versus oral NSAID alone. Given the inability to blind participants and personnel, this trial had a high risk of performance bias and detection bias for self‐reported outcomes.
5. Pajareya 2004: Passive mobilisation, supervised stretching and pulley exercises, electrotherapy and oral glucocorticoid (intervention) versus oral glucocorticoid (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Function (SPADI 0‐100) change from baseline to 3 weeks | 20.5 | 15.4 | 60 | 11.9 | 14.2 | 59 | 8.60 [3.28, 13.92] |
| Passive internal rotation (cm) change from baseline to 3 weeks | 6.3 | 7.7 | 60 | 3 | 7 | 59 | 3.30 [0.66, 5.94] |
| Passive external rotation (degrees) change from baseline to 3 weeks | 21.3 | 15.3 | 60 | 18.3 | 15.4 | 59 | 3.00 [‐2.52, 8.52] |
| Passive abduction (degrees) change from baseline to 3 weeks | 21.9 | 21 | 60 | 14.7 | 18.1 | 59 | 7.20 [0.16, 14.24] |
| Events | Total | Events | Total | Risk ratio (95%CI) | |||
| Adverse events: pain persisting more than 2 hours after treatment | 4 | 60 | 0 | 59 | 8.85 [0.49, 160.87] | ||
| Global assessment of treatment success ("disappearance of shoulder complaints or some pain/limitation which does not interfere with everyday life") at 3 weeks | 21 | 60 | 11 | 59 | 1.88 [0.99, 3.54] | ||
| Global assessment of treatment success ("disappearance of shoulder complaints or some pain/limitation which does not interfere with everyday life") at 6 weeks | 35 | 57 | 22 | 52 | 1.45 [0.99, 2.12] | ||
| Global assessment of treatment success ("disappearance of shoulder complaints or some pain/limitation which does not interfere with everyday life") at 6 months | 45 | 56 | 42 | 51 | 0.98 [0.81, 1.17] | ||
Main outcomes
The combination of passive mobilisation, supervised stretching and pulley exercises, electrotherapy and oral NSAID for three weeks resulted in statistically significantly greater improvement in function at three weeks than oral NSAID alone (MD 8.60, 95% CI 3.28 to 13.92; 100‐point scale, 119 participants). However this result did not reach our criterion for a minimal clinically important difference. The number of participants with global treatment success did not significantly differ between groups at three weeks, six weeks (RR 1.45, 95% CI 0.99 to 2.12; 109 participants) or six months. A slightly greater number of participants receiving the multi‐component intervention reported the adverse event of pain persisting for longer than two hours after treatment, but the 95% CI of the RR was very wide and includes no association and decreased risk as plausible estimates of association (RR 8.85, 95% CI 0.49 to 160.87; 119 participants).
Other outcomes
The multi‐component intervention resulted in greater improvement in passive abduction and internal rotation at three weeks than with oral NSAID alone, but improvement in passive external rotation at three weeks did not significantly differ.
Is manual therapy (with or without electrotherapy) more effective than placebo, no intervention or another active intervention?
One trial compared manual therapy versus no treatment but reported no useable outcome data (Bulgen 1984). Nine trials compared manual therapy (with or without electrotherapy) versus another active intervention (Bulgen 1984; Chan 2010; Dacre 1989; Guler‐Uysal 2004; Maricar 1999; Nicholson 1985; Sharad 2011; Sirajuddin 2010; Yang 2012). Given the nature of the interventions, all trials had a high risk of performance bias and detection bias for self‐reported outcomes. Outcome data in four trials were available for analysis.
Table 12 presents results of Guler‐Uysal 2004 (40 participants), which compared deep friction massage (Cyriax approach) and active stretching and pendulum exercises versus short‐wave diathermy, hot pack and active stretching and pendulum exercises.
Table 13 presents results of Nicholson 1985 (20 participants), which compared passive mobilisation and supervised exercise versus supervised exercise alone.
Table 14 and Table 15 present results of Sirajuddin 2010 (45 participants), which compared (1) anterior glide mobilisation, exercise and electrotherapy and (2) posterior glide mobilisation, exercise and electrotherapy versus exercise and electrotherapy alone.
Table 16 presents results of Yang 2012 (23 participants), which compared end range and scapular mobilisation, exercise and electrotherapy versus exercise and electrotherapy alone.
6. Guler‐Uysal 2004: Cyriax deep friction massage and manipulation (intervention) versus short wave diathermy and hot pack (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐100) at 2 weeks | 15.2 | 18.5 | 20 | 21.2 | 17.9 | 20 | ‐6.00 [‐17.28, 5.28] |
| Night pain (VAS 0‐100) at 2 weeks | 39.1 | 28.1 | 20 | 42 | 25.6 | 20 | ‐2.90 [‐19.56, 13.76] |
| Pain on motion (VAS 0‐100) at 2 weeks | 50.4 | 24.5 | 20 | 62.5 | 12.6 | 20 | ‐12.10 [‐24.17, ‐0.03] |
| Passive internal rotation (degrees) at 2 weeks | 66.7 | 10 | 20 | 56.1 | 14.7 | 20 | 10.60 [2.81, 18.39] |
| Passive external rotation (degrees) at 2 weeks | 74.4 | 14.2 | 20 | 52.8 | 24.3 | 20 | 21.60 [9.27, 33.93] |
| Passive abduction (degrees) at 2 weeks | 157.7 | 21.6 | 20 | 145.3 | 28.5 | 20 | 12.40 [‐3.27, 28.07] |
| Passive flexion (degrees) at 2 weeks | 155.5 | 14.2 | 20 | 146.4 | 22.7 | 20 | 9.10 [‐2.63, 20.83] |
| Events | Total | Events | Total | Risk ratio (95%CI) | |||
| Global assessment of treatment success (reaching 80% of normal ROM) at 2 weeks | 19 | 20 | 13 | 20 | 1.46 [1.04, 2.05] | ||
7. Nicholson 1985: Passive mobilisation and supervised exercise (intervention) versus supervised exercise (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (no scale units) change from baseline to 4 weeks | ‐5.1 | 4.56 | 10 | ‐2.9 | 4.41 | 10 | ‐2.20 [‐6.13, 1.73] |
| Active internal rotation (degrees) change from baseline to 4 weeks | 11.04 | 6.12 | 10 | 4.96 | 7.55 | 10 | 6.08 [0.06, 12.10] |
| Active external rotation (degrees) change from baseline to 4 weeks | 17.62 | 13.23 | 10 | 13.22 | 17.14 | 10 | 4.40 [‐9.02, 17.82] |
| Active abduction (degrees) change from baseline to 4 weeks | 25.04 | 19.94 | 10 | 14.18 | 12.42 | 10 | 10.86 [‐3.70, 25.42] |
8. Sirajuddin 2010: Anterior glide mobilisation, exercise and electrotherapy (intervention) versus exercise and electrotherapy (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐10) at 3 weeks | 1.26 | 0.97 | 15 | 2.86 | 1.07 | 15 | ‐1.60 [‐2.33, ‐0.87] |
| Active internal rotation (degrees) at 3 weeks | 76.66 | 3.45 | 15 | 57.93 | 7.33 | 15 | 18.73 [14.63, 22.83] |
9. Sirajuddin 2010: Posterior glide mobilisation, exercise and electrotherapy (intervention) versus exercise and electrotherapy (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐10) at 3 weeks | 1.3 | 1.03 | 15 | 2.86 | 1.07 | 15 | ‐1.56 [‐2.31, ‐0.81] |
| Active internal rotation (degrees) at 3 weeks | 68.4 | 6.4 | 15 | 57.93 | 7.33 | 15 | 10.47 [5.55, 15.39] |
10. Yang 2012: End‐range and scapular mobilisation, exercise and electrotherapy (intervention) versus exercise and electrotherapy (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Function (Flexilevel Scale of Shoulder Function (FLEX‐SF, 1‐50 scale) at 4 weeks | 32.7 | 1.7 | 11 | 31.8 | 1.5 | 12 | 0.90 [‐0.42, 2.22] |
| Function (Flexilevel Scale of Shoulder Function (FLEX‐SF, 1‐50 scale) at 8 weeks | 39.9 | 1.8 | 11 | 32.4 | 1.6 | 12 | 7.50 [6.10, 8.90] |
| Passive internal rotation (degrees) at 4 weeks | 41.3 | 15.59 | 11 | 39.3 | 14.55 | 12 | 2.00 [‐10.35, 14.35] |
| Passive internal rotation (degrees) at 8 weeks | 45.6 | 20.89 | 11 | 42.9 | 19.75 | 12 | 2.70 [‐13.95, 19.35] |
| Passive external rotation (degrees) at 4 weeks | 41.5 | 15.59 | 11 | 38 | 14.90 | 12 | 3.50 [‐8.99, 15.99] |
| Passive external rotation (degrees) at 8 weeks | 60.1 | 16.58 | 11 | 37.7 | 15.59 | 12 | 22.40 [9.22, 35.58] |
The magnitude, direction of effect and statistical significance of between‐group differences in outcomes varied across trials. In terms of the main benefit outcomes, statistically significant differences favouring the manual therapy group were reported only in Guler‐Uysal 2004 (for global treatment success, pain on motion and passive internal and external rotation at two weeks), Sirajuddin 2010 (for overall pain at three weeks) and Yang 2012 (for function at eight weeks). However, 95% CIs included estimates of effect that were not clinically important. Only one trial (66 participants) measured adverse events, and none were recorded in either group (Dacre 1989).
Is supervised or home exercise (with or without electrotherapy) more effective than placebo, no intervention or another active intervention?
One trial compared home exercise (with electrotherapy) versus no intervention but reported no useable outcome data (Cheing 2008). Eight trials compared supervised or home exercise (with or without electrotherapy) versus another active intervention (Bulgen 1984; Celik 2010; Cheing 2008; Dundar 2009; Ghosh 2012; Maryam 2012; Samnani 2004; Wen 2009). Given the nature of the interventions, all trials had a high risk of performance bias and detection bias for self‐reported outcomes. Outcome data for analysis were available in four trials.
Table 17 presents results of Celik 2010 (29 participants), which compared scapulothoracic exercises, ROM exercises, electrotherapy, cold pack and NSAID versus the same intervention without scapulothoracic exercises.
Table 18 presents results of Dundar 2009 (57 participants), which compared supervised stretching and pendulum exercises versus continuous passive motion.
Table 19 and Table 20 present results of Ghosh 2012 (72 participants), which compared supervised active and passive mobilisation exercises and electrotherapy versus (1) manipulation under anaesthesia, and (2) glucocorticoid injection.
Table 21 and Table 22 presents results of Maryam 2012 (87 participants), which compared glucocorticoid injection versus (1) supervised active ROM exercises, electrotherapy and ice, and (2) supervised active ROM exercises, electrotherapy, ice and glucocorticoid injection.
11. Celik 2010: Scapulothoracic exercises, ROM exercises, electrotherapy, cold pack and NSAID (intervention) versus ROM exercises, electrotherapy, cold pack and NSAID (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐10) at 6 weeks | 0 | 1.6 | 15 | 1.5 | 1.4 | 14 | ‐1.50 [‐2.59, ‐0.41] |
| Overall pain (VAS 0‐10) at 12 weeks | 0 | 0.7 | 15 | 1 | 0.9 | 14 | ‐1.00 [‐1.59, ‐0.41] |
| Function (Constant score 0‐100) at 6 weeks | 60 | 13.3 | 15 | 53 | 7.9 | 14 | 7.00 [‐0.90, 14.90] |
| Function (Constant score 0‐100) at 12 weeks | 68 | 10.7 | 15 | 59 | 5.9 | 14 | 9.00 [2.77, 15.23] |
| Passive internal rotation (degrees) at 6 weeks | 70 | 9.2 | 15 | 70 | 3.3 | 14 | 0.00 [‐4.97, 4.97] |
| Passive internal rotation (degrees) at 12 weeks | 78 | 8.4 | 15 | 78 | 3.9 | 14 | 0.00 [‐4.72, 4.72] |
| Passive external rotation (degrees) at 6 weeks | 60 | 14.7 | 15 | 61.5 | 7.9 | 14 | ‐1.50 [‐10.01, 7.01] |
| Passive external rotation (degrees) at 12 weeks | 70 | 12.5 | 15 | 67.5 | 5.6 | 14 | 2.50 [‐4.47, 9.47] |
| Passive flexion (degrees) at 6 weeks | 160.33 | 14.7 | 15 | 153.07 | 13 | 14 | 7.26 [‐2.83, 17.35] |
| Passive flexion (degrees) at 12 weeks | 172.13 | 7.4 | 15 | 159.92 | 13.1 | 14 | 12.21 [4.39, 20.03] |
12. Dundar 2009: Supervised stretching and pendulum exercises (intervention) versus continuous passive motion (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (SPADI pain scale 0‐10) at 4 weeks | 4.58 | 1.28 | 28 | 4.01 | 2.1 | 29 | 0.57 [‐0.33, 1.47] |
| Overall pain (SPADI pain scale 0‐10) at 12 weeks | 4.39 | 1.82 | 28 | 3.79 | 2.01 | 29 | 0.60 [‐0.39, 1.59] |
| Function (SPADI disability scale 0‐10) at 4 weeks | 4.29 | 1.91 | 28 | 4.03 | 1.58 | 29 | 0.26 [‐0.65, 1.17] |
| Function (SPADI disability scale 0‐10) at 12 weeks | 3.99 | 1.84 | 28 | 3.82 | 1.61 | 29 | 0.17 [‐0.73, 1.07] |
| Night pain (VAS 0‐10) at 4 weeks | 4.84 | 1.66 | 28 | 3.91 | 2.61 | 29 | 0.93 [‐0.20, 2.06] |
| Night pain (VAS 0‐10) at 12 weeks | 4.64 | 1.77 | 28 | 3.74 | 2.14 | 29 | 0.90 [‐0.12, 1.92] |
| Pain on motion (VAS 0‐10) at 4 weeks | 4.93 | 1.87 | 28 | 4.06 | 2.13 | 29 | 0.87 [‐0.17, 1.91] |
| Pain on motion (VAS 0‐10) at 12 weeks | 4.65 | 1.65 | 28 | 3.75 | 1.92 | 29 | 0.90 [‐0.03, 1.83] |
| Passive internal rotation (degrees) at 4 weeks | 64.45 | 17.8 | 28 | 62.89 | 19.96 | 29 | 1.56 [‐8.25, 11.37] |
| Passive internal rotation (degrees) at 12 weeks | 67.19 | 18.47 | 28 | 66.27 | 17.14 | 29 | 0.92 [‐8.34, 10.18] |
| Passive external rotation (degrees) at 4 weeks | 64.9 | 21.52 | 28 | 65.82 | 17.54 | 29 | ‐0.92 [‐11.13, 9.29] |
| Passive external rotation (degrees) at 12 weeks | 68.98 | 14.22 | 28 | 68.22 | 17.11 | 29 | 0.76 [‐7.40, 8.92] |
| Passive abduction (degrees) at 4 weeks | 127.67 | 26.66 | 28 | 137.96 | 16.26 | 29 | ‐10.29 [‐21.80, 1.22] |
| Passive abduction (degrees) at 12 weeks | 137.33 | 15.31 | 28 | 141.75 | 13.11 | 29 | ‐4.42 [‐11.83, 2.99] |
| Passive flexion (degrees) at 4 weeks | 132.78 | 15.96 | 28 | 133.96 | 10.09 | 29 | ‐1.18 [‐8.14, 5.78] |
| Passive flexion (degrees) at 12 weeks | 138.75 | 14.21 | 28 | 139.26 | 11.19 | 29 | ‐0.51 [‐7.17, 6.15] |
13. Ghosh 2012: Supervised active and passive mobilisation exercises and electrotherapy (intervention) versus manipulation under anaesthesia (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||
| Events | Total | Events | Total | Risk ratio (95%CI) | |
| Global assessment of treatment success (clinical improvement rated as "good") at 6 months | 16 | 24 | 19 | 24 | 0.84 [0.59, 1.19] |
14. Ghosh 2012: Supervised active and passive mobilisation exercises and electrotherapy (intervention) versus glucocorticoid injection (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||
| Events | Total | Events | Total | Risk ratio (95%CI) | |
| Global assessment of treatment success (clinical improvement rated as "good") at 6 months | 16 | 24 | 19 | 23 | 0.81 [0.57, 1.13] |
15. Maryam 2012: Supervised active ROM exercises, electrotherapy and ice (intervention) versus glucocorticoid injection (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐100) at 6 weeks | 19.94 | 11.75 | 19 | 12.61 | 13.69 | 28 | 7.33 [0.01, 14.65] |
| Overall pain (VAS 0‐100) at 6 months | 8.62 | 4.2 | 8 | 12 | 14.18 | 14 | ‐3.38 [‐11.36, 4.60] |
| Function (SPADI 0‐100) at 6 weeks | 33.1 | 16.08 | 19 | 22.03 | 21.45 | 28 | 11.07 [0.33, 21.81] |
| Function (SPADI 0‐100) at 6 months | 19.87 | 16.23 | 8 | 19.92 | 15.62 | 14 | ‐0.05 [‐13.96, 13.86] |
| Passive flexion (degrees) change from baseline to 6 weeks | 13.75 | 19.7 | 19 | 14.73 | 18.6 | 28 | ‐0.98 [‐12.20, 10.24] |
| Passive flexion (degrees) change from baseline to 6 months | 6.66 | 34.3 | 8 | 15 | 14.4 | 14 | ‐8.34 [‐33.28, 16.60] |
| Passive abduction (degrees) change from baseline to 6 weeks | 18.43 | 19.2 | 19 | 20.26 | 22.1 | 28 | ‐1.83 [‐13.73, 10.07] |
| Passive abduction (degrees) change from baseline to 6 months | 12.5 | 22.3 | 8 | 18.57 | 20.7 | 14 | ‐6.07 [‐24.95, 12.81] |
| Passive external rotation (degrees) change from baseline to 6 weeks | 1.56 | 16.9 | 19 | 9.8 | 10.7 | 28 | ‐8.24 [‐16.81, 0.33] |
| Passive external rotation (degrees) change from baseline to 6 months | 5.62 | 5.6 | 8 | 9.61 | 17.6 | 14 | ‐3.99 [‐13.99, 6.01] |
| Passive hand behind back distance (cm) change from baseline to 6 weeks | ‐7.31 | 9.25 | 19 | ‐5.07 | 5.95 | 28 | ‐2.24 [‐6.95, 2.47] |
| Passive hand behind back distance (cm) change from baseline to 6 months | ‐9.75 | 11.31 | 8 | ‐6.64 | 7.22 | 14 | ‐3.11 [‐11.81, 5.59] |
16. Maryam 2012: Supervised active ROM exercises, electrotherapy, ice and glucocorticoid injection (intervention) versus glucocorticoid injection (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐100) at 6 weeks | 16.4 | 13.93 | 22 | 12.61 | 13.69 | 28 | 3.79 [‐3.93, 11.51] |
| Overall pain (VAS 0‐100) at 6 months | 17 | 15.48 | 14 | 12 | 14.18 | 14 | 5.00 [‐6.00, 16.00] |
| Function (SPADI 0‐100) at 6 weeks | 14.59 | 13.66 | 22 | 22.03 | 21.45 | 28 | ‐7.44 [‐17.22, 2.34] |
| Function (SPADI 0‐100) at 6 months | 18.57 | 22.35 | 14 | 19.92 | 15.62 | 14 | ‐1.35 [‐15.63, 12.93] |
| Passive flexion (degrees) change from baseline to 6 weeks | 14.52 | 18.5 | 22 | 14.73 | 18.6 | 28 | ‐0.21 [‐10.56, 10.14] |
| Passive flexion (degrees) change from baseline to 6 months | 11.07 | 29.5 | 14 | 15 | 14.4 | 14 | ‐3.93 [‐21.13, 13.27] |
| Passive abduction (degrees) change from baseline to 6 weeks | 19.28 | 28.2 | 22 | 20.26 | 22.1 | 28 | ‐0.98 [‐15.33, 13.37] |
| Passive abduction (degrees) change from baseline to 6 months | 7.5 | 18.4 | 14 | 18.57 | 20.7 | 14 | ‐11.07 [‐25.58, 3.44] |
| Passive external rotation (degrees) change from baseline to 6 weeks | 3.8 | 12.3 | 22 | 9.8 | 10.7 | 28 | ‐6.00 [‐12.49, 0.49] |
| Passive external rotation (degrees) change from baseline to 6 months | 5 | 11 | 14 | 9.61 | 17.6 | 14 | ‐4.61 [‐15.48, 6.26] |
| Passive hand behind back distance (cm) change from baseline to 6 weeks | ‐7.57 | 6.59 | 22 | ‐5.07 | 5.95 | 28 | ‐2.50 [‐6.03, 1.03] |
| Passive hand behind back distance (cm) change from baseline to 6 months | ‐4.21 | 9.55 | 14 | ‐6.64 | 7.22 | 14 | 2.43 [‐3.84, 8.70] |
Nearly all between‐group differences in outcomes were not statistically significant. In terms of the main benefit outcomes, statistically significant differences favouring the exercise group were reported only in Celik 2010 (for overall pain at six weeks and 12 weeks); however 95% CIs included estimates of effect that were not clinically important. Only one trial measured adverse events, and none were recorded in either group (Dundar 2009).
Is one type of manual therapy or exercise (with or without electrotherapy) more effective than another?
Ten trials compared one type of manual therapy or exercise versus another (with or without electrotherapy) (Harsimran 2011; Johnson 2007; Nellutla 2009; Rainbow 2008; Shrivastava 2011; Sirajuddin 2010; Tanaka 2010; Vermeulen 2006; Yan 2005; Yang 2007). Given the nature of the interventions, all trials except two (Shrivastava 2011; Tanaka 2010) had a high risk of performance bias and detection bias for self‐reported outcomes. Outcome data for analysis were available in eight trials.
Analysis 4.1 and Analysis 4.2 present results of Johnson 2007 and Sirajuddin 2010 (48 participants), both of which compared anterior versus posterior glide mobilisation, each with exercise and electrotherapy.
Table 23 presents results of Nellutla 2009 (40 participants), which compared proprioceptive neuromuscular facilitation (PNF) exercises versus conventional free exercises including finger ladder, Codman's pendulum, overhead shoulder pulley and shoulder wheel exercises.
Table 24 presents results of Rainbow 2008 (eight participants), which compared high‐velocity, low‐amplitude chiropractic manipulative therapy to the cervical and thoracic spine along with home exercise versus grade IV mobilisation and home exercise.
Table 25, Table 26 and Table 27 present results of Tanaka 2010 (120 participants), which compared high‐frequency (more often than twice a week) versus moderate‐frequency (once a week) and low‐frequency (less often than once a week) end range mobilisation.
Table 28 presents results of Vermeulen 2006 (100 participants), which compared high‐grade mobilisation (intensive passive end range mobilisation) versus low‐grade mobilisation (passive mobilisation within the pain‐free zone).
Table 29 presents results of Yan 2005 (54 participants), which compared dumbbell exercises versus bare‐handed exercises
Table 30 presents results of Yang 2007 (30 participants), which compared end range mobilisation following midrange mobilisation versus mobilisation with movement following midrange mobilisation.
4.1. Analysis.

Comparison 4 Anterior glide mobilisation plus ultrasound plus exercises versus posterior glide mobilisation plus ultrasound plus exercises, Outcome 1 Overall pain (VAS 0‐10).
4.2. Analysis.
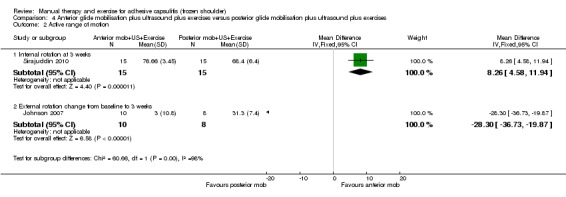
Comparison 4 Anterior glide mobilisation plus ultrasound plus exercises versus posterior glide mobilisation plus ultrasound plus exercises, Outcome 2 Active range of motion.
17. Nellutla 2009: Proprioceptive neuromuscular facilitation exercises (intervention) versus conventional free exercises (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Function (Constant score 0‐100) at 3 weeks | 85.2 | 4.65 | 20 | 83.8 | 4.75 | 20 | 1.40 [‐1.51, 4.31] |
| Internal rotation (degrees) at 3 weeks (unclear if active or passive) | 57 | 6.69 | 20 | 59.05 | 4.76 | 20 | ‐2.05 [‐5.65, 1.55] |
| External rotation (degrees) at 3 weeks (unclear if active or passive) | 63.55 | 11.15 | 20 | 53.35 | 5.88 | 20 | 10.20 [4.68, 15.72] |
| Abduction (degrees) at 3 weeks (unclear if active or passive) | 140.65 | 10.24 | 20 | 135.55 | 9.26 | 20 | 5.10 [‐0.95, 11.15] |
| Flexion (degrees) at 3 weeks (unclear if active or passive) | 146.75 | 10.59 | 20 | 144.6 | 9.06 | 20 | 2.15 [‐3.96, 8.26] |
18. Rainbow 2008: High‐velocity, low‐amplitude chiropractic manipulative therapy to the cervical and thoracic spine and home exercises (intervention) versus grade IV mobilisation and home exercises (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Function (SPADI 0‐100) at 3 weeks | 35.25 | 27.77 | 4 | 52.83 | 16.2 | 4 | ‐17.58 [‐49.09, 13.93] |
| Function (SPADI 0‐100) at 6 weeks | 14.1 | 4.15 | 4 | 35.08 | 15.16 | 4 | ‐20.98 [‐36.38, ‐5.58] |
19. Tanaka 2010: High‐frequency (>twice a week) end‐range mobilisation (intervention) versus moderate‐frequency (once a week) end‐range mobilisation (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Active abduction (degrees) change from baseline to 6 months | 56.4 | 24 | 39 | 49.9 | 24.2 | 35 | 6.50 [‐4.50, 17.50] |
20. Tanaka 2010: High‐frequency (>twice a week) end‐range mobilisation (intervention) versus low‐frequency (<once a week) end‐range mobilisation (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Active abduction (degrees) change from baseline to 6 months | 56.4 | 24 | 39 | 49.3 | 28.1 | 36 | 7.10 [‐4.77, 18.97] |
21. Tanaka 2010: Moderate‐frequency (once a week) end‐range mobilisation (intervention) versus low‐frequency (<once a week) end‐range mobilisation (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Active abduction (degrees) change from baseline to 6 months | 49.9 | 24.2 | 35 | 49.3 | 28.1 | 36 | 0.60 [‐11.59, 12.79] |
22. Vermeulen 2006: High‐grade mobilisation (intervention) versus low‐grade mobilisation (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Overall pain (VAS 0‐100) change from baseline to 6 months | ‐22.3 | 34.1 | 49 | ‐24.3 | 24.9 | 51 | 2.00 [‐9.74, 13.74] |
| Overall pain (VAS 0‐100) change from baseline to 12 months | ‐23.9 | 27.2 | 49 | ‐23 | 27.7 | 51 | ‐0.90 [‐11.66, 9.86] |
| Function (Shoulder Disability Questionnaire 0‐100) change from baseline to 6 months | ‐38.9 | 35.5 | 49 | ‐33.2 | 27.7 | 51 | ‐5.70 [‐18.22, 6.82] |
| Function (Shoulder Disability Questionnaire 0‐100) change from baseline to 12 months | ‐50 | 30.3 | 49 | ‐38.8 | 27.4 | 51 | ‐11.20 [‐22.53, 0.13] |
| Night pain (VAS 0‐100) change from baseline to 6 months | ‐38.3 | 43.5 | 49 | ‐31.7 | 29.9 | 51 | ‐6.60 [‐21.29, 8.09] |
| Night pain (VAS 0‐100) change from baseline to 12 months | ‐43.7 | 34.5 | 49 | ‐35.9 | 30.2 | 51 | ‐7.80 [‐20.53, 4.93] |
| Pain on motion (VAS 0‐100) change from baseline to 6 months | ‐31.4 | 27.9 | 49 | ‐31.9 | 26.0 | 51 | 0.50 [‐10.06, 11.06] |
| Pain on motion (VAS 0‐100) change from baseline to 12 months | ‐39.2 | 27.9 | 49 | ‐32.6 | 31.3 | 51 | ‐6.60 [‐18.20, 5.00] |
| Active abduction (degrees) change from baseline to 6 months | 55.8 | 37.3 | 49 | 46.9 | 32.0 | 51 | 8.90 [‐4.74, 22.54] |
| Active abduction (degrees) change from baseline to 12 months | 72.9 | 31.7 | 49 | 60.3 | 32.7 | 51 | 12.60 [‐0.02, 25.22] |
| Active flexion (degrees) change from baseline to 6 months | 34.2 | 23.3 | 49 | 33.6 | 16.7 | 51 | 0.60 [‐7.38, 8.58] |
| Active flexion (degrees) change from baseline to 12 months | 47 | 24.4 | 49 | 42.9 | 19.9 | 51 | 4.10 [‐4.64, 12.84] |
| Active external rotation (degrees) change from baseline to 6 months | 15.9 | 12.9 | 49 | 13.2 | 13.2 | 51 | 2.70 [‐2.40, 7.80] |
| Active external rotation (degrees) change from baseline to 12 months | 20.8 | 11.8 | 49 | 15.9 | 16.0 | 51 | 4.90 [‐0.60, 10.40] |
| Quality of life (SF‐36 PCS 0‐100) change from baseline to 6 months | 19.2 | 18.8 | 49 | 17.1 | 17.8 | 51 | 2.10 [‐5.08, 9.28] |
| Quality of life (SF‐36 PCS 0‐100) change from baseline to 12 months | 23.2 | 21.9 | 49 | 22.8 | 19.9 | 51 | 0.40 [‐7.82, 8.62] |
| Quality of life (SF‐36 MCS 0‐100) change from baseline to 6 months | 8.2 | 20.2 | 49 | 7.9 | 21.7 | 51 | 0.30 [‐7.91, 8.51] |
| Quality of life (SF‐36 MCS 0‐100) change from baseline to 12 months | 7.7 | 20.5 | 49 | 10.2 | 22.4 | 51 | ‐2.50 [‐10.92, 5.92] |
| Events | Total | Events | Total | Risk ratio (95%CI) | |||
| Global assessment of treatment success ("better or much better") at 6 months | 40 | 49 | 43 | 51 | 0.97 [0.81, 1.16] | ||
| Global assessment of treatment success ("better or much better") at 12 months | 43 | 49 |
40 | 51 |
1.12 [0.94, 1.34] | ||
23. Yan 2005: Dumbbell exercises (intervention) versus bare‐handed exercises (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Events | Total | Events | Total | Risk ratio (95%CI) | |||
| Global assessment of treatment success ("excellent" overall rating) at 3 months | 24 | 26 | 0 | 28 | 52.63 [3.36, 823.64] | ||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Flexion (degrees) at 3 months (unclear if active or passive) | 92.46 | 17.14 | 26 | 85.41 | 13.34 | 28 | 7.05 [‐1.19, 15.29] |
| Abduction (degrees) at 3 months (unclear if active or passive) | 96.63 | 24.49 | 26 | 89.81 | 20.53 | 28 | 6.82 [‐5.28, 18.92] |
24. Yang 2007: End‐range mobilisation following mid‐range mobilisation (intervention) versus mobilisation with movement following mid‐range mobilisation (control).
| OUTCOME | INTERVENTION | CONTROL | EFFECT ESTIMATE | ||||
| Mean | SD | n | Mean | SD | n | Mean difference (95%CI) | |
| Function (Flexilevel Scale of Shoulder Function (FLEX‐SF, 1‐50 scale) percentage change from baseline to 6 weeks | 19.90% | 8.10% | 15 | 17.25% | 12.20% | 15 | Not estimable |
Most between‐group differences in outcomes were not statistically significant. In terms of the main benefit outcomes, statistically significant differences favouring one type of manual therapy or exercise over another were reported only in Rainbow 2008 (for overall pain at six weeks, which was less in the chiropractic manipulative therapy group) and Yan 2005 (for global assessment of treatment success, which was more likely in the dumbbell exercises group). Only two trials measured adverse events, and none were recorded in either group (Rainbow 2008; Shrivastava 2011).
Is the combination of manual therapy and exercise (with or without electrotherapy) delivered in addition to another active intervention more effective than placebo or no treatment?
The two trials above (total of 78 participants) compared the combination of mobilisation, supervised exercise and electrotherapy for four weeks and glucocorticoid injection versus placebo injection alone (Carette 2003; Ryans 2005). Both trials were unable to blind participants and personnel to the physical therapy component of the intervention, so they had a high risk of performance bias and detection bias for self‐reported outcomes. Ryans 2005 also had a high risk of attrition bias. The multi‐component intervention resulted in significantly more improvement in overall pain (Analysis 5.1) and function (Analysis 5.2) than placebo injection alone at six weeks, but not at six months or 12 months. In terms of secondary outcomes, the multi‐component intervention group had statistically significantly greater improvement in quality of life (Analysis 5.3) and ROM at some, but not all, time points (Analysis 5.4; Analysis 5.5; Analysis 5.6).
5.1. Analysis.
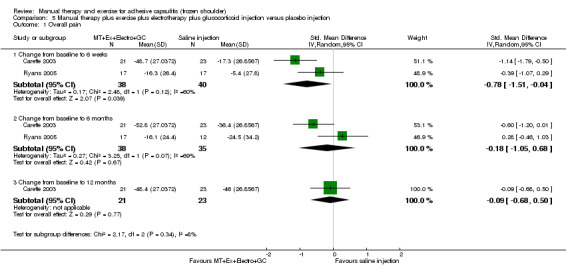
Comparison 5 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus placebo injection, Outcome 1 Overall pain.
5.2. Analysis.
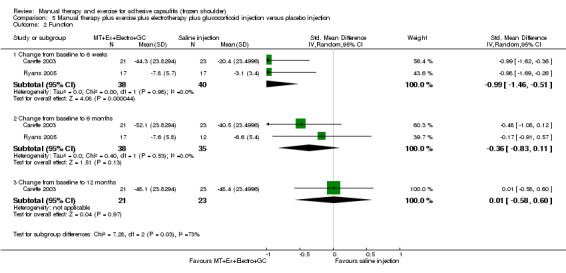
Comparison 5 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus placebo injection, Outcome 2 Function.
5.3. Analysis.

Comparison 5 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus placebo injection, Outcome 3 Quality of life.
5.4. Analysis.
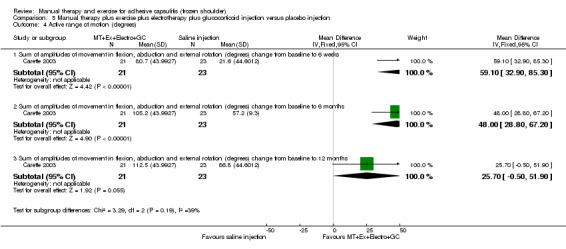
Comparison 5 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus placebo injection, Outcome 4 Active range of motion (degrees).
5.5. Analysis.
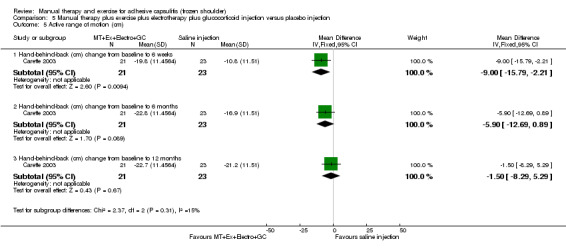
Comparison 5 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus placebo injection, Outcome 5 Active range of motion (cm).
5.6. Analysis.
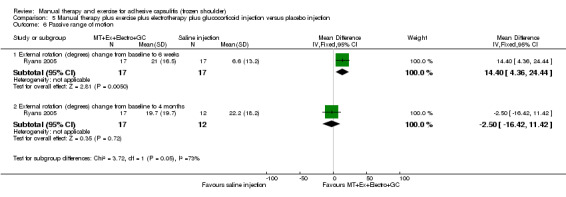
Comparison 5 Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus placebo injection, Outcome 6 Passive range of motion.
Other outcome data
Partially reported outcome data (e.g. where trialists did not report SDs or any other measures of variance) in Chan 2010, Dacre 1989, Harsimran 2011, Johnson 2007, Ma 2006, Maricar 1999, Samnani 2004, Sharad 2011 and Shrivastava 2011 are presented in Table 31.
25. Additional data reported in trials (partially reported).
| Ma 2006: Mobilisation plus exercise (intervention) versus acupuncture (control) | ||
| Outcome | Intervention | Control |
| Mean; SD not reported | ||
| Static pain (VAS 0‐10) at 4 weeks | 0.4 | 0.7 |
| Dynamic pain (VAS 0‐10) at 4 weeks | 3.7 | 4.3 |
| Active flexion (degrees) at 4 weeks | 129.9 | 129.3 |
| Active abduction (degrees) at 4 weeks | 98.3 | 115.7 |
| Active internal rotation (degrees) at 4 weeks | 43.9 | 51.7 |
| Ma 2006: Mobilisation plus exercise plus acupuncture (intervention) versus acupuncture (control) | ||
| Outcome | Intervention | Control |
| Mean; SD not reported | ||
| Static pain (VAS 0‐10) at 4 weeks | 0.7 | 0.7 |
| Dynamic pain (VAS 0‐10) at 4 weeks | 3.3 | 4.3 |
| Active flexion (degrees) at 4 weeks | 137.2 | 129.3 |
| Active abduction (degrees) at 4 weeks | 110.1 | 115.7 |
| Active internal rotation (degrees) at 4 weeks | 54.4 | 51.7 |
| Chan 2010: Passive mobilisation plus home care plus glucocorticoid injection (intervention) versus home care programme plus glucocorticoid injection (control) | ||
| Outcome | Intervention | Control |
| Mean change; SD not reported | ||
| Overall pain (VAS 0‐10) change from baseline to 10 weeks | 5.7 | 6.2 |
| Active abduction (degrees) change from baseline to 10 weeks | 40.7 | 80 |
| Active internal rotation (cm) change from baseline to 10 weeks | 28.4 | 38.9 |
| Dacre 1989: Mobilisation (intervention) versus glucocorticoid injection (control) | ||
| Outcome | Intervention | Control |
| Mean extracted from Figures; SD not reported | ||
| Day pain (VAS 0‐100) at 6 weeks | 23.9 | 25.9 |
| Day pain (VAS 0‐100) at 6 months | 18.7 | 17.2 |
| Pain on motion (VAS 0‐100) at 6 weeks | 35.4 | 35 |
| Pain on motion (VAS 0‐100) at 6 months | 21.5 | 28.5 |
| Passive abduction (degrees) at 6 weeks | 128.4 | 127.2 |
| Passive abduction (degrees) at 6 months | 133.1 | 140.5 |
| Passive internal rotation (mm) at 6 weeks | 259.6 | 290.8 |
| Passive internal rotation (mm) at 6 months | 247.5 | 235.4 |
| Dacre 1989: Mobilisation plus glucocorticoid injection (intervention) versus glucocorticoid injection (control) | ||
| Outcome | Intervention | Control |
| Mean extracted from Figures; SD not reported | ||
| Day pain (VAS 0‐100) at 6 weeks | 21.7 | 25.9 |
| Day pain (VAS 0‐100) at 6 months | 22.6 | 17.2 |
| Pain on motion (VAS 0‐100) at 6 weeks | 34.2 | 35 |
| Pain on motion (VAS 0‐100) at 6 months | 18.1 | 28.5 |
| Passive abduction (degrees) at 6 weeks | 124.8 | 127.2 |
| Passive abduction (degrees) at 6 months | 138.6 | 140.5 |
| Passive internal rotation (mm) at 6 weeks | 276.9 | 290.8 |
| Passive internal rotation (mm) at 6 months | 240.6 | 235.4 |
| Maricar 1999: Manual therapy plus exercise (intervention) versus exercise (control) | ||
| Outcome | Intervention | Control |
| Mean; SD not reported | ||
| Active flexion (degrees) at 3 weeks | 151.8 | 146.2 |
| Active flexion (degrees) at 5 weeks | 159.5 | 155.6 |
| Active flexion (degrees) at 8 weeks | 170.9 | 164 |
| Active internal rotation (degrees) at 3 weeks | 56.1 | 50 |
| Active internal rotation (degrees) at 5 weeks | 67.4 | 58.9 |
| Active internal rotation (degrees) at 8 weeks | 78.3 | 70.3 |
| Samnani 2004: Passve ROM exercises and therapeutic activity program (intervention) versus therapeutic activity program (control) | ||
| Outcome | Intervention | Control |
| Mean (SD); n not reported | ||
| Active functional‐hand‐to‐back score (0‐10 scale, higher scores=increased ROM) at 6 weeks | 5.8 (1.9) | 2.6 (0.9) |
| Sharad 2011: End‐range mobilisation plus ultrasound plus exercises (intervention) versus ultrasound plus exercises (control) | ||
| Outcome | Intervention | Control |
| Mean; SD not reported | ||
| Overall pain (VAS 0‐10) at 3 weeks | 0.9 | 1.09 |
| Active abduction (degrees) at 3 weeks | 40.26 | 22 |
| Active flexion (degrees) at 3 weeks | 36.82 | 21.18 |
| Harsimran 2011: Anterior glide mobilization (intervention) versus posterior glide mobilisation (control) | ||
| Outcome | Intervention | Control |
| Median change; SD not reported | ||
| Overall pain (VAS 0‐10) change from baseline to 5 days | 2.5 | 3 |
| Johnson 2007: Anterior glide mobilization (intervention) versus posterior glide mobilisation (control) | ||
| Outcome | Intervention | Control |
| Median (range); SD not reported | ||
| Night pain (0‐4 ordinal scale) at 3 weeks | 3 (1‐4) | 4 (1‐4) |
| Overall function (0‐4 ordinal scale) at 3 weeks | 2.5 (1‐3) | 2 (0‐3) |
| Shrivastava 2011: Maitland's mobilisation (intervention) versus Mulligan's mobilisation with movement (control) | ||
| Outcome | Intervention | Control |
| Mean; SD not reported | ||
| Overal pain (VAS 0‐10) at 4 weeks | 4.05 | 3.6 |
| Function (SPADI 0‐100) at 4 weeks | 40 | 42 |
| Flexion (degrees) at 4 weeks (unclear if active or passive) | 121.25 | 122 |
| Abduction (degrees) at 4 weeks (unclear if active or passive) | 91.25 | 99.5 |
| Internal rotation (degrees) at 4 weeks (unclear if active or passive) | 46.5 | 41.25 |
Subgroup and sensitivity analyses, and assessment of publication bias
Because of the limited opportunity for meta‐analysis, no subgroup or sensitivity analyses were undertaken. We were unable to formally assess publication bias using funnel plots because of the small number of trials included in each meta‐analysis.
Discussion
Summary of main results
Overall, based on the results of 32 trials involving 1836 participants, limited evidence is available from which firm conclusions can be drawn about the benefits or harms of (1) the combination of manual therapy and exercise compared with another active intervention (e.g. glucocorticoid injection), (2) the combination of manual therapy, exercise and another active intervention compared with the other active intervention alone, (3) manual therapy compared with another active intervention, (4) exercise compared with another active intervention or (5) one type of manual therapy or exercise versus another, in terms of patient‐relevant outcomes such as pain, function, global assessment of treatment success, ROM and quality of life. Only seven trials measured adverse events, with three reporting marginal differences between groups (Buchbinder 2007; Pajareya 2004; van der Windt 1998), and four reporting no adverse events in any group (Dacre 1989; Dundar 2009; Rainbow 2008; Shrivastava 2011).
The two main questions of the review (which focus on the effects of the combination of manual therapy and exercise) were investigated in only seven trials (Buchbinder 2007; Carette 2003; Chauhan 2011; Ma 2006; Pajareya 2004; Ryans 2005; van der Windt 1998). The overall impression gained from these trials is that the few outcome differences between interventions that were clinically important were detected only at short‐term follow‐up (i.e. up to seven weeks). Based on one trial that did not blind participants, glucocorticoid injection was superior to the combination of manual therapy and exercise for six weeks in terms of overall pain, function, global treatment success, night pain and passive range of abduction at three weeks and seven weeks, but differences were small at six months and 12 months (van der Windt 1998) (Table 1). Meta‐analysis of two trials that did not blind participants (one of which had a high risk of attrition bias) suggested no clinically important differences between a combination of manual therapy, exercise, and electrotherapy for four weeks and placebo injection compared with glucocorticoid injection alone or placebo injection alone in terms of overall pain, function, active range of motion and quality of life at six weeks, six months and 12 months (though the 95% CI suggested function may be better with glucocorticoid injection at six weeks) (Carette 2003; Ryans 2005) (Table 2; Table 3). The same two trials found that adding a combination of manual therapy, exercise and electrotherapy for four weeks to glucocorticoid injection did not confer clinically important benefits over glucocorticoid injection alone at each time point (Table 4). The only trial rated at low risk of bias on each domain of the Cochrane risk of bias tool found that following arthrographic joint distension, the combination of manual therapy and supervised exercise for six weeks was superior to sham ultrasound in terms of global treatment success and active shoulder abduction at six weeks, but conferred similar effects in terms of overall pain, function, adverse events, night pain, pain on motion and quality of life at six weeks and at six months (Buchbinder 2007) (Table 5). Finally, one trial that did not blind participants (Pajareya 2004) found that a combination of manual therapy, exercise, electrotherapy and oral non‐steroidal anti‐inflammatory drug (NSAID) for three weeks did not confer clinically important benefits over oral NSAID alone in terms of function, patient‐reported treatment success and passive ROM at three weeks (Table 6). Two trials (Chauhan 2011; Ma 2006) reported no or partial outcome data, thus preventing their inclusion in this summary analysis.
that a combination of manual therapy, exercise, electrotherapy and oral non‐steroidal anti‐inflammatory drug (NSAID) for three weeks did not confer clinically important benefits over oral NSAID alone in terms of function and patient‐reported treatment success at three weeks.
For all other comparisons, most of the differences between groups in both primary and secondary outcomes were small and were not statistically significant. Any statistically significant differences that were detected in these trials are likely to be exaggerated because of the high risk of performance and detection bias resulting from non‐blinding of participants and personnel.
Overall completeness and applicability of evidence
Similar to what has been found in previous reviews (Green 1998; Schellingerhout 2008), the diagnostic criteria for (or definitions of) adhesive capsulitis varied across trials with regard to type, extent and direction of shoulder restriction. Despite this, the study populations in all trials appeared to be representative of patients seen in routine care, and age, gender ratio and symptom duration were similar across trials. Also, trials were conducted in a range of high‐ and low‐ to middle‐income countries. The median duration of the manual therapy or exercise intervention was four weeks (range one to 18), with a median of three treatment sessions delivered per week (range one to seven). Of major clinical concern is that few of the identified trials tested multi‐component interventions (manual therapy combined with exercise or manual therapy and exercise combined with a non‐physical therapy intervention (e.g. glucocorticoid injection)), although this is the most common way in which adhesive capsulitis is treated in practice (Hanchard 2011a). Of our six review questions, 'Is one type of manual therapy or exercise (with or without electrotherapy) more effective than another?' was the one addressed by the largest number of trials (n = 10). This failure of trials to reflect actual practice in their tested interventions needs to be considered not only in interpreting available evidence for the management of adhesive capsulitis, but also in planning future research. Trials should consider testing standardised delivery methods for combinations of manual therapy and exercise. Several of the identified trials delivered an electrotherapy modality (e.g. ultrasound, TENS) to the group receiving manual therapy or exercise, so the effect of manual therapy or exercise cannot be isolated from that of electrotherapy in these trials. This issue applies to three of the seven trials included under our two primary review questions (Carette 2003; Pajareya 2004; Ryans 2005). However, this is not necessarily problematic, as delivery of electrotherapy modalities along with manual therapy and exercise is reflective of many types of physical therapy practice.
An issue of greater concern is the variable choice of outcomes assessed in the trials. Various measures of ROM were measured in nearly all trials, but no trial measured pain using a dichotomous measure, as recommended by IMMPACT (Dworkin 2008). The proportion of trials measuring other main outcomes of the review were as follows: overall pain measured by visual analogue scale, numerical or categorical rating scale (72%); function (63%); global assessment of treatment success (22%); active shoulder abduction (31%); quality of life (19%); and adverse events (22%). Development of a core set of outcomes for trials of adhesive capsulitis and other shoulder disorders would improve our ability to synthesise the evidence.
Quality of the evidence
The overall quality of evidence for most comparisons was low according to the GRADE approach (Schünemann 2011b). Although we presented only SoF tables for trials addressing the two primary questions of the review, we used the GRADE approach to assess the quality of all included trials. Most trials were of low or very low quality, with the evidence downgraded for three reasons: (1) Risk of performance and detection bias for self‐reported outcomes was high, (2) risk of selection bias was unclear because trialists did not report whether the allocation sequence was concealed and (3) some imprecision surrounded the effect estimate. Few trials were rated at low risk of performance bias and detection bias for self‐reported outcomes because of lack of participant and personnel blinding. Blinding of participants and personnel is difficult to achieve in procedural trials; therefore performance bias and detection bias are often difficult to minimise. However, this is problematic because trials with unblinded assessment of subjective outcomes (such as pain and function) are estimated to exaggerate the treatment benefit by 22% on average (ratio of odds ratios 0.78, 95% credible interval 0.65 to 0.92) (Savovic 2012). In addition, trials with inadequate or unclear allocation concealment have been found to exaggerate treatment effect by 7% on average (ratio of odds ratios 0.93, 95% credible interval 0.87 to 0.99) (Savovic 2012). Overall, for most comparisons and outcomes in our review, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Only two trials were not rated as of low or very low quality: van der Windt 1998 was a moderate‐quality trial that was downgraded only for lack of participant blinding, and Buchbinder 2007 was a high‐quality trial.
Potential biases in the review process
Upon completion of a thorough search of all major databases with no language restrictions, we believe that all relevant trials were identified. Two review authors independently assessed the trials for inclusion in this review, extracted data and assessed the risk of bias, and a third review author adjudicated when any discrepancy arose. Two of the review authors (SG and RB) are authors of one of the trials included in this review (Buchbinder 2007). To avoid bias, the paper was sent to an independent review author for assessment of whether it met the inclusion criteria for this review. Neither review author was involved in data extraction or assessment of risk of bias for this trial. Review questions of interest were defined with full knowledge of comparisons reported in the trials, but with no knowledge of the results. We used a priori defined decision rules to select data from trials when multiple measurement scales, time points and analyses were reported, to prevent selective inclusion of results (Page 2013). The biggest limitation of the review process was that many trials did not report sufficient outcome data or did not present data in a form that would allow them to be extracted for meta‐analysis, and attempts to obtain data from trialists were often unsuccessful. In addition, measures of both pain and function varied across trials. For overall pain—one of our prespecified primary outcomes—we elected to combine results for overall pain severity and daytime rest pain, each measured on different scales. For function, we also combined different measures that may not necessarily be measuring the exact same concept.
Agreements and disagreements with other studies or reviews
We are aware of four other relevant systematic reviews of interventions for adhesive capsulitis published within the past five years (Blanchard 2010; Favejee 2011; Hanchard 2011b; Maund 2012). All examined a range of conservative and surgical interventions, except for Blanchard 2010, which included only trials comparing physical therapy versus glucocorticoid injections. Some differences were noted in the meta‐analysis methods used across the reviews. For example, the results of our meta‐analyses of data in Carette 2003 and Ryans 2005 are slightly different from those reported by Maund 2012, who, rather than including the change from baseline data reported in both trials, as we did, calculated final values and imputed SDs of final values. Maund 2012 also combined data at different time points from ours. Blanchard 2010 chose to combine data from Carette 2003 and Ryans 2005 with those from van der Windt 1998; this was not done in our review or in the reviews by Hanchard 2011b and Maund 2012, as the intervention in van der Windt 1998 was not deemed sufficiently similar to that in Carette 2003 and Ryans 2005. Despite this fact, we reached a similar conclusion to that of Blanchard 2010, Hanchard 2011b and Maund 2012—that a multi‐component intervention comprising manual therapy and exercise is potentially less effective than glucocorticoid injection in the short term but no different in the long term, and that this result should be interpreted with caution because of the high risk of performance bias.
Favejee 2011 concluded that moderate evidence favours mobilisation techniques, which is a stronger conclusion than ours and that of Hanchard 2011b and Maund 2012. However, this conclusion appears to be based on the statistically significant effects of mobilisation on particular measures of ROM, as reported in Johnson 2007, Nicholson 1985, Vermeulen 2006 and Yang 2007, and does not take into consideration the finding that effects on patient‐relevant outcomes like pain and function were not clinically or statistically important.
Authors' conclusions
Implications for practice.
No trials have investigated the effect of the combination of manual therapy and exercise compared with placebo or no treatment for adhesive capsulitis. The best currently available data indicate that the combination of manual therapy and exercise may not be as effective as glucocorticoid injection in the short term. It is unclear whether the combination of manual therapy, exercise and electrotherapy is an effective adjunct to glucocorticoid injection or oral NSAIDs. Following arthrographic joint distension with glucocorticoid and saline, manual therapy and exercise may confer similar effects to those of sham ultrasound in terms of overall pain, function and quality of life, but may provide greater patient‐reported treatment success and active range of motion.
Implications for research.
High‐quality randomised controlled trials are needed to investigate whether the combination of manual therapy and exercise is more effective than placebo or no treatment for adhesive capsulitis. Additional high‐quality randomised controlled trials are needed to establish the benefits and harms of manual therapy and exercise interventions that reflect actual practice, compared with active interventions with evidence of benefit (e.g. glucocorticoid injection). Future trials should include strategies designed to minimise the potential for bias, including adequate allocation concealment and blinding of participants and outcome assessors. Development of a core set of outcomes for trials of adhesive capsulitis and other shoulder disorders would enhance this endeavour and improve our ability to synthesise the evidence.
History
Review first published: Issue 8, 2014
| Date | Event | Description |
|---|---|---|
| 1 May 2008 | Amended | Converted to RM5. CMSG ID C067‐R |
| 24 February 2003 | New citation required and conclusions have changed | Substantive amendment |
| 24 February 2003 | Amended | This review is based on the original review, 'Interventions for shoulder pain.' Please see published notes for further details |
Notes
The original review, 'Physiotherapy interventions for shoulder pain,' was split into four reviews upon updating: 'Manual therapy and exercise for adhesive capsulitis (frozen shoulder),' 'Electrotherapy modalities for adhesive capsulitis (frozen shoulder),' 'Manual therapy, exercise and taping for rotator cuff disorders' and 'Electrotherapy modalities for rotator cuff disorders.' This review has also been broadened by inclusion of all randomised and quasi‐randomised clinical trials, regardless of whether outcome assessment was blinded.
Acknowledgements
We are grateful to Steve McDonald from the Australasian Cochrane Centre for help provided with the search strategy. We thank Simon Carette, Sharon Chan, Jiu‐jenq Lin, Mobini Maryam, Kingkaew Pajareya and Ian Ryans for providing us with unpublished trial data.
Appendices
Appendix 1. Search strategies
Search strategy for CENTRAL:
MeSH descriptor: [Shoulder Pain] explode all trees
MeSH descriptor: [Shoulder Impingement Syndrome] explode all trees
MeSH descriptor: [Rotator Cuff] explode all trees
MeSH descriptor: [Bursitis] explode all trees
((shoulder* in All Text or rotator* in All Text) and (bursitis in All Text or frozen in All Text or impinge* in All Text or tendonitis in All Text or tendonitis in All Text or tendinopathy in All Text or pain* in All Text))
"rotator cuff" in All Text
"adhesive capsulitis" in All Text
#1 or #2 or #3 or #4 or #5 or #6 or #7
MeSH descriptor: [Rehabilitation] explode all trees
MeSH descriptor: [Physical Therapy Modalities] explode all trees
MeSH descriptor: [Exercise Movement Techniques] explode all trees
MeSH descriptor: [Ultrasonography, Interventional] explode all trees
rehabilitat* in All Text or physiotherapy* in All Text or "physical therap*" in All Text or "manual therap*" in All Text or exercis* in All Text
(ultrasound in All Text or ultrasonograph* in All Text or tns in All Text or tens in All Text or shockwave in All Text or electrotherap* in All Text or mobili* in All Text)
#9 or #10 or #11 or #12 or #13 or #14
#8 and #15
Search strategy for MEDLINE:
shoulder pain/
shoulder impingement syndrome/
rotator cuff/
exp bursitis/
((shoulder$ or rotator cuff) adj5 (bursitis or frozen or impinge$ or tendinitis or tendonitis or tendinopathy or pain$)).mp.
rotator cuff.mp.
adhesive capsulitis.mp.
or/1‐7
exp rehabilitation/
exp physical therapy techniques/
exp musculoskeletal manipulations/
exp exercise movement techniques/
exp ultrasonography, interventional/
(rehabilitat$ or physiotherap$ or physical therap$ or manual therap$ or exercis$ or ultrasound or ultrasonograph$ or TNS or TENS or shockwave or electrotherap$ or mobili$). mp.
or/9‐14
clinical trial.pt
random$.mp.
((single or double) adj (blind$ or mask$)).mp.
placebo$.mp.
or/16‐19
8 and 15 and 20
Search strategy for EMBASE:
‘shoulder pain’/exp
‘shoulder impingement syndrome’/exp
‘rotator cuff’/exp
‘bursitis’/exp
((shoulder* OR rotator*) AND (‘bursitis’/de OR frozen OR impinge* OR ‘tendonitis’/de OR ‘tendinitis’/de OR ‘tendinopathy’/de OR pain*))
‘rotator cuff’
‘adhesive capsulitis’
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
‘rehabilitation’/exp
‘physiotherapy’/exp
‘kinesiotherapy’/exp
‘endoscopic echography’/exp
rehabilitat* OR physiotherapy* OR ‘physical therapy’ OR ‘manual therapy’ OR kinesiotherap* OR exercis*
‘ultrasound’/de OR ultrasonograph* OR ‘transcutaneous nerve stimulation’ OR ‘transcutaneous electrical nerve stimulation’ OR shockwave OR electrotherap* OR mobili*
#9 OR #10 OR #11 OR #12 OR #13 OR #14
‘randomized controlled trial’/exp
#8 AND #15 AND #16
Search strategy for CINAHL Plus:
S1 MH “shoulder pain”
S2 MH “shoulder impingement syndrome”
S3 MH “rotator cuff”
S4 MH bursitis+
S5 TX (shoulder* N5 bursitis) or TX(shoulder* N5 frozen) or TX(shoulder* N5 impinge*) or TX(shoulder* N5 tend?nitis) or TX(shoulder* N5 tendinopathy) or TX(shoulder* N5 pain*)
S6 TX (rotator cuff N5 bursitis) or TX(rotator cuff N5 frozen) or TX(rotator cuff N5 impinge*) or TX(rotator cuff N5 tend?nitis) or TX(rotator cuff N5 tendinopathy) or TX(rotator cuff N5 pain*)
S7 TX rotator cuff
S8 TX adhesive capsulitis
S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8
S10 MH Rehabilitation+
S11 MH physical therapy+
S12 MH Manual Therapy+
S13 MH Therapeutic Exercise+
S14 MHUltrasonography+
S15 TX rehabilitat* or physiotherapy* or physical therap* or manual therap* or exercise* or ultrasound or ultrasonograph* or TNS or TENS or shockwave or electrotherapy* or mobili*
S16 S10 or S11 or S12 or S13 or S14 or S15
S17 PT clinical trial
S18 TX random*
S19 TX(single blind*) or TX(single mask*)
S20 TX(double blind*) or TX(double mask*)
S21 placebo*
S22 S17 or S18 or S19 or S20 or S21
S23 S9 and S16 and S22
Data and analyses
Comparison 1. Manual therapy plus exercise plus electrotherapy plus placebo injection versus glucocorticoid injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall pain | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Change from baseline to 6 weeks | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.65, 1.07] |
| 1.2 Change from baseline to 6 months | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐1.10, 0.93] |
| 1.3 Change from baseline to 12 months | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.33, 0.80] |
| 2 Function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Change from baseline to 6 weeks | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.03, 0.89] |
| 2.2 Change from baseline to 6 months | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.18, 0.72] |
| 2.3 Change from baseline to 12 months | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.45, 0.67] |
| 3 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 SF‐36 PCS change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐8.57, 1.97] |
| 3.2 SF‐36 PCS change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.27, 4.27] |
| 3.3 SF‐36 PCS change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.97, 3.57] |
| 3.4 SF‐36 MCS change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐5.60, 6.60] |
| 3.5 SF‐36 MCS change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐2.8 [‐8.90, 3.30] |
| 3.6 SF‐36 MCS change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐7.20, 5.00] |
| 4 Active range of motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐37.09, 13.09] |
| 4.2 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐10.20 [‐35.29, 14.89] |
| 4.3 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐22.59, 27.59] |
| 5 Active range of motion (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hand‐behind‐back (cm) change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐5.72, 7.32] |
| 5.2 Hand‐behind‐back (cm) change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐7.22, 5.82] |
| 5.3 Hand‐behind‐back (cm) change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐9.42, 3.62] |
| 6 Passive range of motion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 External rotation (degrees) change from baseline to 6 weeks | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐6.86, 11.66] |
| 6.2 External rotation (degrees) change from baseline to 4 months | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐13.59, 11.39] |
Comparison 2. Manual therapy plus exercise plus electrotherapy plus placebo injection versus placebo injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall pain | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Change from baseline to 6 weeks | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.67, 0.18] |
| 1.2 Change from baseline to 6 months | 2 | 77 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.67, 0.23] |
| 1.3 Change from baseline to 12 months | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.56, 0.56] |
| 2 Function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Change from baseline to 6 weeks | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.52, 0.33] |
| 2.2 Change from baseline to 6 months | 2 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.44, 0.46] |
| 2.3 Change from baseline to 12 months | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.42, 0.70] |
| 3 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 SF‐36 PCS change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐6.67, 3.87] |
| 3.2 SF‐36 PCS change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 2.3 [‐2.97, 7.57] |
| 3.3 SF‐36 PCS change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐5.97, 4.57] |
| 3.4 SF‐36 MCS change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐6.70, 5.50] |
| 3.5 SF‐36 MCS change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐7.20, 5.00] |
| 3.6 SF‐36 MCS change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐6.90, 5.30] |
| 4 Active range of motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 15.6 [‐9.49, 40.69] |
| 4.2 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 16.80 [‐0.86, 34.46] |
| 4.3 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐22.59, 27.59] |
| 5 Active range of motion (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hand‐behind‐back (cm) change from baseline to 6 weeks | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐6.22, 6.82] |
| 5.2 Hand‐behind‐back (cm) change from baseline to 6 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐7.72, 5.32] |
| 5.3 Hand‐behind‐back (cm) change from baseline to 12 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐6.62, 6.42] |
| 6 Passive range of motion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 External rotation (degrees) change from baseline to 6 weeks | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 10.1 [1.57, 18.63] |
| 6.2 External rotation (degrees) change from baseline to 4 months | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐16.57, 8.17] |
Comparison 3. Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus glucocorticoid injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall pain | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Change from baseline to 6 weeks | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.77, 0.13] |
| 1.2 Change from baseline to 6 months | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.51, 0.40] |
| 1.3 Change from baseline to 12 months | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.44, 0.75] |
| 2 Function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Change from baseline to 6 weeks | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.80, 0.10] |
| 2.2 Change from baseline to 6 months | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.56, 0.36] |
| 2.3 Change from baseline to 12 months | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.61, 0.57] |
| 3 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 SF‐36 PCS change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐3.27, 7.27] |
| 3.2 SF‐36 PCS change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐6.57, 3.97] |
| 3.3 SF‐36 PCS change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐4.87, 5.67] |
| 3.4 SF‐36 MCS change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 4.2 [‐2.04, 10.44] |
| 3.5 SF‐36 MCS change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [‐0.84, 11.64] |
| 3.6 SF‐36 MCS change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 5.80 [‐0.44, 12.04] |
| 4 Active range of motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 31.5 [5.30, 57.70] |
| 4.2 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 21.0 [‐5.20, 47.20] |
| 4.3 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 25.70 [‐0.50, 51.90] |
| 5 Active range of motion (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hand‐behind‐back (cm) change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐8.5 [‐15.29, ‐1.71] |
| 5.2 Hand‐behind‐back (cm) change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐5.40 [‐12.19, 1.39] |
| 5.3 Hand‐behind‐back (cm) change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐11.09, 2.49] |
| 6 Passive range of motion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 External rotation (degrees) change from baseline to 6 weeks | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [‐3.96, 17.36] |
| 6.2 External rotation (degrees) change from baseline to 4 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐13.42, 14.62] |
Comparison 4. Anterior glide mobilisation plus ultrasound plus exercises versus posterior glide mobilisation plus ultrasound plus exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall pain (VAS 0‐10) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 3 weeks | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐1.00, 1.46] |
| 2 Active range of motion | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Internal rotation at 3 weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 8.26 [4.58, 11.94] |
| 2.2 External rotation change from baseline to 3 weeks | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐28.3 [‐36.73, ‐19.87] |
Comparison 5. Manual therapy plus exercise plus electrotherapy plus glucocorticoid injection versus placebo injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall pain | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Change from baseline to 6 weeks | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.51, ‐0.04] |
| 1.2 Change from baseline to 6 months | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐1.05, 0.68] |
| 1.3 Change from baseline to 12 months | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.68, 0.50] |
| 2 Function | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Change from baseline to 6 weeks | 2 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.46, ‐0.51] |
| 2.2 Change from baseline to 6 months | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.83, 0.11] |
| 2.3 Change from baseline to 12 months | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.58, 0.60] |
| 3 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 SF‐36 PCS change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 3.90 [‐1.37, 9.17] |
| 3.2 SF‐36 PCS change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐3.27, 7.27] |
| 3.3 SF‐36 PCS change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐3.87, 6.67] |
| 3.4 SF‐36 MCS change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 3.1 [‐3.14, 9.34] |
| 3.5 SF‐36 MCS change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 7.1 [0.86, 13.34] |
| 3.6 SF‐36 MCS change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [‐0.14, 12.34] |
| 4 Active range of motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 59.1 [32.90, 85.30] |
| 4.2 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 48.00 [28.80, 67.20] |
| 4.3 Sum of amplitudes of movement in flexion, abduction and external rotation (degrees) change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 25.70 [‐0.50, 51.90] |
| 5 Active range of motion (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hand‐behind‐back (cm) change from baseline to 6 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐15.79, ‐2.21] |
| 5.2 Hand‐behind‐back (cm) change from baseline to 6 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐5.90 [‐12.69, 0.89] |
| 5.3 Hand‐behind‐back (cm) change from baseline to 12 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐8.29, 5.29] |
| 6 Passive range of motion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 External rotation (degrees) change from baseline to 6 weeks | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 14.40 [4.36, 24.44] |
| 6.2 External rotation (degrees) change from baseline to 4 months | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐16.42, 11.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Buchbinder 2007.
| Methods |
Design: parallel‐group, 2‐arm, single‐blind randomised controlled trial (Australia) Interventions: following arthrographic joint distension, manual therapy plus directed exercises or sham ultrasound Sample size calculation: 78 participants per group were estimated to be needed on the basis of detection of a clinically relevant difference of 10 points in the Shoulder Pain and Disability Index (SPADI) (SD = 23.9) at 3 months at the 5% level of statistical significance with 80% power, including a 10% rate of loss at follow‐up Analysis: intention‐to‐treat analysis using all randomly assigned participants who provided any postbaseline data planned Source of funding: Australian National Health and Medical Research Council Project Grant (non‐industry) |
|
| Participants |
Number of participants: 156 (78 per group) Baseline characteristics Group receiving arthrographic joint distension followed by manual therapy plus directed exercises Mean (SD) age = 55 (9.3) years; male:female = 24:51 Median (range) duration of symptoms: 6 (3‐60) months Group receiving arthrographic joint distension followed by sham ultrasound Mean (SD) age = 55.3 (7.7) years; male:female = 31:43 Median (range) duration of symptoms: 6 (3‐57) months Inclusion criteria
Exclusion criteria
|
|
| Interventions | Before active physiotherapy or placebo interventions, all participants received arthrographic distension of the glenohumeral joint with glucocorticoid and normal saline performed under radiological guidance. Both groups were permitted to use analgesia and non‐steroidal anti‐inflammatory drugs during the study period. At the end of 6 weeks' treatment, participants were instructed to maintain a 10‐minute daily exercise programme Arthrographic joint distension followed by manual therapy plus directed exercises (N = 78) Components of intervention
Dosage: 30 minutes each session Frequency of administration: Twice per week for 2 weeks, then once per week for 4 weeks (8 sessions) Provider: physiotherapist Arthrographic joint distension followed by sham ultrasound (N = 78) Components of intervention: sham ultrasound and application of a non‐therapeutic gel. Participants received no instruction in exercise techniques and no manual therapy Dosage: 30 minutes each session Frequency of administration: Twice per week for 2 weeks, then once per week for 4 weeks (8 sessions) |
|
| Outcomes | Outcomes assessed at the end of 6 weeks' treatment, at 12 weeks (post randomisation) and 26 weeks (post randomisation) Primary outcome
Secondary outcomes
|
|
| Notes | Trial registered in ANZCTR (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12605000685617) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Consenting, eligible participants were randomized in permuted blocks of 4 and 6, stratified by treatment center, to receive either active or placebo regimens according to a computer‐generated table of random numbers created by the study biostatistician" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure treatment allocation concealment, just prior to commencement of treatment, study centers telephoned a central number for the treatment allocation according to the participant’s identification number. Only the telephone receptionist had access to the allocation schedule (and no other role in the trial)" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Thirty‐nine participants (53%) in the active group correctly identified their treatment group compared with 35 participants (50%) in the placebo group; 31 participants (42%) in the active group were uncertain which treatment they had received compared with 23 participants (33%) in the placebo group. Blinding index was 0.49 (bootstrap 95% CI 0.40, 0.56), interpreted as moderate success of blinding" Comment: Given the nature of the interventions, personnel could not be blinded to treatment. However, participants were not informed which intervention was expected to be superior |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Quote: "Between March 2002 and April 2005, we performed a randomized, placebo‐controlled, participant and single assessor blinded trial in participants with adhesive capsulitis..." Quote: "All participants were evaluated by the same blinded outcome assessor (JMY) at baseline (just prior to arthrographic joint distension), 6 weeks (at the conclusion of the physiotherapy or placebo program), 12 weeks, and 26 weeks" Comment: Participants who completed self‐reported outcome measures were blind to treatment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Between March 2002 and April 2005, we performed a randomized, placebo‐controlled, participant and single assessor blinded trial in participants with adhesive capsulitis..." Quote: "All participants were evaluated by the same blinded outcome assessor (JMY) at baseline (just prior to arthrographic joint distension), 6 weeks (at the conclusion of the physiotherapy or placebo program), 12 weeks, and 26 weeks" Comment: An assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "We recruited 156 study participants (78 in both groups), and 144 (74 active, 70 placebo; 92.3%) completed the 26‐week trial. Participants moved through the trial as outlined in Figure 2. Seven participants (3 active, 4 placebo) withdrew from the trial prior to completing the allocated intervention. Because there were no postbaseline followup data for these participants, they were excluded from the efficacy analysis. Three participants withdrew prior to the 12‐week followup (1 active, 2 placebo) and 2 participants (2 placebo) withdrew prior to the 26‐week followup. These participants were excluded from the 12‐week and 26‐week efficacy analyses, respectively. Six participants (2 active, 4 placebo) had a second arthrographic joint distension during the trial period but remained in the efficacy analysis. Overall, of those who completed their allocated treatment, 3 (4%) of 75 in the active group and 8 (10.8%) of 74 in the placebo group had further treatment during the 26‐week trial (P = 0.11)" Quote: "Characteristics of the 7 participants with no followup data are also provided in Table 1. Although comparisons with the 149 participants are limited, these 7 participants appeared somewhat younger and had worse symptom severity" Quote: "To assess the sensitivity of the results to the exclusion of the 7 participants who provided no postbaseline data and to the missing data from participants lost to followup during the trial period, we performed a single imputation of these participants’ 3‐month postbaseline values using regression modeling. These models predicted the 12‐week data for these participants based on the relationship between the 12‐week data, baseline characteristics, and randomized treatment arm among the 149 participants who did have postbaseline data. All analyses in Table 3 corresponding to the 12‐week time point were then repeated using the complete data set with these imputed values and the results differed very minimally" Comment: The numbers and reasons for dropouts and losses to follow‐up were relatively small and balanced between groups, so they are unlikely to have biased the results |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes reported in the trial protocol of the publication |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Bulgen 1984.
| Methods |
Design: Parallel‐group, 4‐arm randomised controlled trial (United Kingdom) Interventions: Mobilisation 3 times per week for 6 weeks or intra‐articular glucocorticoid injection every week for 3 weeks or ice therapy plus proprioceptive neuromuscular facilitation (PNF) 3 times per week for 6 weeks or no treatment (all were taught pendular exercises) Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: Arthritis and Rheumatism Council (non‐industry) |
|
| Participants |
Number of participants: 42 (11, 11, 12 and 8 in each respective group) Baseline characteristics: Baseline characteristics were not reported by group Mean (range) age: 55.8 (44‐74) years; male:female = 14:28 Mean (range) duration of symptoms: 4.8 (1‐12) months Inclusion criteria
Exclusion criteria
|
|
| Interventions | All participants were taught pendular exercises and were advised to do them for 2 to 3 minutes every hour. Non‐salicylate analgesics and diazepam 5 mg at night were available as required Mobilisation (N = 11) Components of intervention
Dosage: not reported Frequency of administration: 3 times weekly for 6 weeks (18 sessions) Provider: physiotherapist Glucocorticoid injection (N = 11) Components of intervention: methyl prednisolone acetate 20 mg and 1% lignocaine hydrochloride 0‐5 mL injected into the subacromial bursa and a similar amount into the shoulder joint by the anterior route Frequency of administration: weekly, for 3 weeks Provider: rheumatologist Ice therapy plus proprioceptive neuromuscular facilitation (N = 12) Components of intervention: ice packs followed by proprioceptive neuromuscular facilitation (PNF) Frequency of administration: 3 times weekly for 6 weeks (18 sessions) Provider: physiotherapist No treatment (N = 8) |
|
| Outcomes | Outcomes assessed at baseline, weekly for 6 weeks, then monthly for 6 months. No primary outcome was stated by trialists
|
|
| Notes | No outcome data were reported in a way that was suitable for inclusion in a meta‐analysis. In the 2003 version of this review, study authors were contacted, but this attempt was unsuccessful. The trial report states, "All patients reported an improvement in pain during the study, but 17 still had residual pain at the end of it, usually mild, but moderate in four. Residual pain was commoner in the ice group, affecting half the patients, but was equally distributed in the other groups. All types of pain were still experienced—rest pain, night pain, and pain on movement. The maximum improvement in pain was achieved by the fourth week of treatment and then continued slowly after that. This was most obvious in the steroid group, but did not reach statistical significance" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to one of four treatment groups" Comment: No information was reported about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported about how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported some outcomes (e.g. pain) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Clinical assessment was performed before treatment, weekly for 6 weeks and monthly for a further 6 months by an independent observer who was not aware of the treatment given" Comment: Outcome assessor of objective outcomes was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The patients were randomly allocated to one of four treatment groups: (1) steroid group (11 patients)…; (2) Mobilization group (11 patients)…; (3) Ice group (12 patients)…; (4) Non‐treatment group (8 patients)" Quote: "Forty‐two patients, 28 females and 14 males, with previously untreated frozen shoulder completed this study" Comment: All randomly assigned participants completed the study |
| Selective reporting (reporting bias) | High risk | Comment: No outcome data were reported in a way that was suitable for inclusion in a meta‐analysis. Trialists did not report any numerical data for pain outcomes and presented only mean scores (with no measures of variation) for total flexion, total abduction, external rotation and total rotation in figure format. No results for glenohumeral flexion or glenohumeral abduction were reported |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Carette 2003.
| Methods |
Design: parallel‐group, 4‐arm, single‐blind randomised controlled trial (Canada) Interventions: 12 sessions of supervised physiotherapy plus glucocorticoid injection (triamcinolone hexacetonide 40 mg) or glucocorticoid injection alone or supervised physiotherapy plus saline injection or saline injection alone Sample size calculation: 36 participants per group were estimated to be needed on the basis of detection of a clinically relevant difference ≥ 10 points in the Shoulder Pain and Disability Index (SPADI) (SD ≤ 15) at the 5% level of statistical significance with 80% power Analysis: intention‐to‐treat analysis (analysing all participants randomly assigned, using a last observation carried forward analysis) Source of funding: Arthritis Society of Canada (non‐industry) |
|
| Participants |
Number of participants: 93 (21, 23, 26 and 23 in each respective group) Baseline characteristics Group receiving supervised physiotherapy plus glucocorticoid injection: Mean (SD) age = 54.9 (10.5) years; male:female = 7:14 Mean (SD) duration of symptoms: 22.1 (14.9) weeks Group receiving glucocorticoid injection alone Mean (SD) age = 55.4 (10) years; male:female = 8:15 Mean (SD) duration of symptoms: 21.2 (11) weeks Group receiving supervised physiotherapy plus saline injection Mean (SD) age = 54.2 (8.3) years; male:female = 14:12 Mean (SD) duration of symptoms: 20.8 (11.2) weeks Group receiving saline injection alone Mean (SD) age = 56.5 (9.4) years; male:female = 9:14 Mean (SD) duration of symptoms: 20.3 (7.3) weeks Inclusion criteria
Exclusion criteria
|
|
| Interventions | All participants were taught a 10‐minute exercise programme consisting of active and auto‐assisted ROM exercises in the planes of flexion, abduction, external rotation and internal rotation (hand behind back) to be done at home twice daily for 3 months Supervised physiotherapy plus glucocorticoid injection (N = 21) Components of physiotherapy intervention
Dosage: 1 hour overall Frequency of administration: 3 times a week for 4 weeks (12 sessions) Provider: physiotherapist Components of glucocorticoid injection: Under fluoroscopic guidance, a 21‐gauge needle, 2.5–3" long, was directed into the shoulder joint space. Aqueous contrast material (Omnipaque; Sanofi‐Winthrop, Markham, Ontario, Canada) was injected to confirm the correct location of the needle in the joint. This was followed by injection of 40 mg triamcinolone hexacetonide (2 mL) Glucocorticoid injection alone (N = 23) The same injection method as described above was delivered Supervised physiotherapy plus placebo injection (N = 26) The same injection and supervised physiotherapy methods as described above were delivered, except that isotonic saline (2 mL) was injected into the shoulder joint space Placebo injection alone (N = 23) The same injection method as described above was delivered, except that isotonic saline (2 mL) was injected into the shoulder joint space |
|
| Outcomes | Outcomes assessed at 6 weeks, 3 months, 6 months and 1 year post randomisation Primary outcome
Secondary outcomes
|
|
| Notes | Trialists reported the following protocol violation: "Five patients (2 in the combination group and 1 in each of the other groups) received, in addition to their assigned injection, a glucocorticoid injection (triamcinolone hexacetonide, 20 mg) after randomization, and 1 patient in the saline group underwent rotator cuff repair 8 months after enrolment. All of these injections were prescribed by study investigators who were blinded to the original treatment assignment, and all were done under fluoroscopic guidance. The patient in the placebo group and the patient in the physiotherapy group each received the injection after the 6‐week visit; the 3 patients in the corticosteroid and combination group received it after the 3‐month or 6‐month visits" Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The assignment scheme was generated from a table of random numbers. Random assignments to the treatment groups were stratified according to study center and balanced after every 12 assignments" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The opaque prenumbered envelopes containing the assignments were kept by the hospital pharmacist at each center" Comment: An adequate method was probably used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The syringes containing the triamcinolone hexacetonide or saline were prepared by the hospital pharmacist and covered with aluminum foil so the radiologist administering the injections and the patient were not aware of the treatment" Comment: Participants and personnel were blind to the injection component of the intervention, but not the physiotherapy component. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Participants self‐reported their SPADI and general health scores and were not blind to whether they had received physiotherapy. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Each subject was assessed by the same physiotherapist throughout the trial, with a few exceptions. The physiotherapists involved in these assessments were unaware of the treatment allocation and did not normally work in the clinics where the physiotherapy was administered" Comment: Outcome assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The primary analysis was based on an intent‐to‐treat principle, and all subjects were included in the analysis. In the case of subjects lost to followup, the data from the last available assessment were imputed to all subsequent evaluations" Quote: "Of the remaining 93 patients, 2 in the combination group, 9 in the corticosteroid group, 4 in the physiotherapy group, and 1 in the placebo group did not return for all visits" Comment: Greater loss to follow‐up was seen in the glucocorticoid injection group compared with the other 3 groups, but it is unclear whether the reasons for loss to follow‐up were related to treatment received (or whether they were balanced across groups) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Celik 2010.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (Turkey) Interventions: scapulothoracic exercises added or not added to glenohumeral ROM exercises, TENS, cold pack, home exercise and NSAIDs Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 29 (14 and 15 in each respective group) Baseline characteristics Group receiving scapulothoracic exercises plus glenohumeral ROM exercises, TENS, cold pack, home exercise and NSAIDs Mean (range) age = 49.6 (38‐62) years; male:female = 2:13 Duration of symptoms not reported Group receiving glenohumeral ROM exercises, TENS, cold pack, home exercise and NSAIDs Mean (range) age = 54.78 (42‐65) years; male:female = 5:9 Duration of symptoms not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Scapulothoracic exercises plus glenohumeral ROM exercises, TENS, cold pack, home exercise and NSAIDs (N = 15) Components of intervention:
Dosage: Supervised exercises gradually increased according to the pain and muscle strength of participants. TENS delivered for 20 minutes. During the fifth and sixth weeks of treatment, the dose of scapulothoracic and glenohumeral stretching exercises was increased (considering pain levels), TENS and NSAIDs were terminated and cold pack was applied only when the participant had pain Frequency of administration: 5 times a week for 6 weeks (30 sessions) for supervised exercises, twice a day for home exercise Provider: physiotherapist Glenohumeral ROM exercises, TENS, cold pack, home exercise and NSAIDs (N = 14) The same intervention as described above was delivered, except that scapulothoracic exercises were not undertaken |
|
| Outcomes | Outcomes assessed at the end of 6 weeks' treatment and at 12 weeks from baseline. No primary outcome was reported by trialists
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were divided randomly into two groups" Comment: No information was reported about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported about how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (the same multi‐component physical therapy intervention with or without additional exercises), participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention they received self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: Blinding of outcome assessors was not reported, although this could have been done for the objectively assessed outcome—range of motion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, and analyses are reported as based on the number of randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Chan 2010.
| Methods |
Design: parallel‐group, 2‐arm, single‐blind randomised controlled trial (United Kingdom) Interventions: passive mobilisation plus home care programme or home care programme alone, 2 weeks after receiving a glucocorticoid injection Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 14 (7 per group) Baseline characteristics Group receiving passive mobilisation plus home care programme Mean (range) age = 50.9 (48‐76) years; male:female = 2:5 Mean duration of symptoms = 2.5 months Group receiving home care programme alone Mean (range) age = 56.7 (39‐59) years; male:female = 6:1 Mean duration of symptoms = 2.4 months Inclusion criteria
Exclusion criteria
|
|
| Interventions | Before randomisation, all participants received a glucocorticoid injection given by the posterior approach, containing 30 mg triamcinolone acetonide (Kenalog) and 3.25 mL 1% lidocaine Participants were randomly allocated to the intervention or control group 2 weeks later. Passive mobilisation plus home care programme (N = 7) Components of intervention:
Dosage: 30 minutes (for manual therapy); 10 repetitions and a 15‐second hold in all capsular stretches (for home exercise) Frequency of administration: 6 weekly sessions of manual therapy over period of 10 weeks (6 sessions); home exercise completed 3 times daily Provider: physiotherapist Home care programme alone (N = 7) The same home care programme as described above was delivered |
|
| Outcomes | Outcomes assessed at weeks 2, 4, 7 and 10 (end of treatment). No primary outcome was reported by the trialists
|
|
| Notes | Mean scores for each outcome were presented in figure format at each time point, but with no measures of variation. For 3 of the 5 outcomes, trialists reported (numerically) the mean change from baseline to the end of 10 weeks' treatment (with no measure of variation); for the other 2 outcomes, only percentage improvement data were reported; this is not suitable for inclusion in a meta‐analysis. Attempts to retrieve non‐reported outcome data from trialists were unsuccessful (although other information about the trial was provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random assignments were placed into a sealed, opaque, prenumbered envelope. The 14 subjects were randomized 1:1 into two treatment groups, based on a computer‐generated randomisation list prepared and held by staff not involved in the research process, and the sealed envelope was only opened at this point to ensure allocation concealment" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The random assignments were placed into a sealed, opaque, prenumbered envelope. The 14 subjects were randomized 1:1 into two treatment groups, based on a computer‐generated randomisation list prepared and held by staff not involved in the research process, and the sealed envelope was only opened at this point to ensure allocation concealment" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A single‐blinded procedure was used, aiming to reduce the bias from the experimenter’s expectations and predictions. The independent observers, who collected data, were unaware to which intervention the subjects had been allocated. The subject was also instructed not to reveal any information about their treatment. This blinding method was used in previous shoulder studies" Quote: "All subjects were treated by the senior author (SC): because she was not blind to the hypothesis being tested, any personal pre‐conceptions regarding treatment outcome could influence the results. Double‐blinding of the passive mobilisation was not considered practical" Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "A single‐blinded procedure was used, aiming to reduce the bias from the experimenter’s expectations and predictions. The independent observers, who collected data, were unaware to which intervention the subjects had been allocated. The subject was also instructed not to reveal any information about their treatment. This blinding method was used in previous shoulder studies" Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported some outcomes (pain, SPADI) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "A single‐blinded procedure was used, aiming to reduce the bias from the experimenter’s expectations and predictions. The independent observers, who collected data, were unaware to which intervention the subjects had been allocated. The subject was also instructed not to reveal any information about their treatment. This blinding method was used in previous shoulder studies" Comment: Assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All subjects entering the study fully attended their sessions and were followed up to week 10" Comment: No dropouts, losses to follow‐up or exclusions occurred |
| Selective reporting (reporting bias) | Unclear risk | Comment: Mean scores were presented in figure format at each time point, but with no measures of variation. For 3 of the 5 outcomes, trialists reported (numerically) the mean change from baseline to the end of 10 weeks' treatment (with no measure of variation); for the other 2 outcomes, only percentage improvement data were reported; this is not suitable for inclusion in a meta‐analysis. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Chauhan 2011.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (India) Interventions: deep transverse friction massage of the 2 tendon supraspinatus and subscapularis as laid by Cyriax, followed by inferior capsular stretching, passive ROM exercises, hot packs and home exercise programme or only hot packs and home exercise programme Sample size calculation: not reported Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 30 (14 and 16 in each respective group) Baseline characteristics: Age by group was not reported, and duration of symptoms was not reported at all Age range: 40‐60 years Group receiving deep transverse friction massage, capsular stretching, passive ROM exercises, hot packs and home exercise programme Male:female = 6:7 Group receiving hot packs and home exercise programme Male:female = 5:8 Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Deep transverse friction massage, capsular stretching, passive ROM exercises, hot packs and home exercise programme (N = 14) Components of intervention:
Dosage: 1 hour Frequency of administration: 3 times a week for 2 weeks (6 sessions) Provider: physiotherapist Hot packs and home exercise programme (N = 16) Hot pack and home exercise programme 3 times a week for 2 weeks |
|
| Outcomes | Outcomes assessed at end of first session (day 1), second session (day 3), third session (day 5), fourth session (day 7), fifth session (day 9) and sixth session (day 11). No primary outcome reported by trialists
|
|
| Notes | Mean scores with no measures of variation are reported in figure format for all outcomes at days 1, 3, 5, 7, 9 and 11 (except for SPADI score, which was reported only at days 5 and 11) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects recruited through screening, were then randomly assigned to one of the two treatment groups—Experimental group and Control group via simple randomization method" Comment: No information was reported about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported about how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Neither the subjects nor the therapist were blinded to group assignment" Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported some outcomes (pain, SPADI) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: Blinding of outcome assessors was not reported, although this could have been done for the objectively assessed outcome of range of motion |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Total thirty subjects were recruited and were randomly assigned to 2 groups. The experimental group consisted of 6 males and 7 females whereas control group consisted of 5 males and 8 females as in Experimental group 1 and in control group 3 subjects were unable to continue the treatment" Comment: A slightly larger number of dropouts were reported in the control group, although the reason provided is ambiguous (i.e. not clear whether participants were "unable to continue treatment" because they moved cities, or because of adverse effects of treatment) |
| Selective reporting (reporting bias) | High risk | Comment: Trialists reported mean scores with no measures of variation for all outcomes at days 1, 3, 5, 7, 9 and 11 (except for SPADI score, which was reported only at days 5 and 11) in figure format. Exact P values for differences between groups were reported only when statistically significant. Trialists also reported the proportion of participants with "improvement" in each outcome (although multiplying these percentages by the number of participants randomly assigned or by the number who completed the study did not produce whole numbers, or values close to whole numbers, so it is not clear how these percentages were calculated by the trialists). Also, the definition of "improvement" for all of these outcomes was not reported. Finally, it is not clear whether other range of motion measures were collected and not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Cheing 2008.
| Methods |
Design: parallel‐group, 3‐arm, single‐blind randomised controlled trial (Hong Kong) Interventions: home exercise plus electroacupuncture or home exercise plus interferential electrotherapy or no treatment Sample size calculation: not reported Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 74 (25, 24 and 25 in each respective group) Baseline characteristics: Sex of participants was reported as 22 males and 48 females. Age range for all participants was reported as 33‐90 years Group receiving electroacupuncture plus home exercise Mean (SD) duration of treatment = 6.71 (6.5) months Group receiving interferential electrotherapy plus home exercise Mean (SD) duration of treatment = 6.7 (6.05) months Group receiving no treatment Mean (SD) duration of treatment = 8.26 (7.94) months Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Home exercise plus electroacupuncture (N = 25) Components of intervention
Dosage
Frequency of administration
Provider: physiotherapist Home exercise plus interferential electrotherapy (N = 25) Components of intervention
Dosage
Frequency of administration
Provider: physiotherapist No treatment (N = 25) |
|
| Outcomes | Outcomes assessed at the end of 4 weeks' treatment and at 1, 3 and 6 months' follow‐up for Groups 1 and 2, but only at the end of 4 weeks' treatment for Group 3. No primary outcome was reported by the trialists
|
|
| Notes | No outcome data from this trial could be analysed, as no outcome data were reported for the comparison group of interest to this review (no treatment group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly allocated into: (i) the EA group (n = 24); (ii) IFE group (n = 23); or (iii) control group (n = 23)" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study was a double‐blind, randomized, controlled clinical trial. An independent assessor was blind to the group allocation" Comment: Despite reporting this trial as "double‐blind," given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain and some components of the Constant score |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: The study was a double‐blind, randomized, controlled clinical trial. An independent assessor was blind to the group allocation" Comment: Outcome assessors of some components of the Constant score were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One participant dropped out of each of the electroacupuncture group and interferential electrotherapy group, both because of time conflict, and two participants dropped out of the no treatment group because they experienced no improvement" Comment: Although dropout is related to treatment in the no treatment group, the number of dropouts is small and is unlikely to affect function and pain outcomes |
| Selective reporting (reporting bias) | High risk | Comment: The trialists reported mean (SD) scores for the Constant Murley Assessment scale and VAS pain at the end of 4 weeks' treatment for the electroacupuncture and interferential electrotherapy groups, but not for the no treatment group, because the no treatment group did not have a statistically significant improvement from baseline. Also, without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Dacre 1989.
| Methods |
Design: parallel‐group, 3‐arm, single‐blind randomised controlled trial (United Kingdom) Interventions: physiotherapy (mobilisation) or local glucocorticoid injection or physiotherapy (mobilisation) plus local glucocorticoid injection for 6 weeks Sample size calculation: not reported Analysis: per‐protocol analysis Source of funding: Arthritis and Rheumatism Council (non‐industry) |
|
| Participants |
Number of participants: 66 (20, 22 and 20 in each respective group) Baseline characteristics: duration of symptoms not reported Group receiving physiotherapy (mobilisation) Mean age = 53 years; male:female = 9:11 Group receiving local glucocorticoid injection Mean age = 55.8 years; male:female = 11:11 Group receiving physiotherapy (mobilisation) plus local glucocorticoid injection Mean age = 58.8 years; male:female = 8:12 Inclusion criteria
Exclusion criterion
|
|
| Interventions |
Physiotherapy (mobilisation) (N = 20) Components of intervention
Dosage: not reported Frequency of administration: 4‐6 weeks (4‐6 sessions) Provider: physiotherapist Local glucocorticoid injection (N = 22) Components of intervention: 20 mg triamcinolone with 1 mL 2% lignocaine injected anteriorly around the shoulder joint Dosage: See above Frequency of administration: once Provider: rheumatologist Physiotherapy (mobilisation) plus local glucocortcoid injection (N = 20) Combination of both interventions described above |
|
| Outcomes | Outcomes assessed at 6 weeks (end of physiotherapy treatment) and at 6 months. No primary outcome reported by the trialists
|
|
| Notes | Trialists reported means (standard errors) for each group in figure format for day pain, pain on active movement, total abduction and internal rotation, but no data for night pain, pain on passive movement, glenohumeral abduction and external rotation. No numerical data were reported for any outcome. The study authors were contacted in an attempt to access additional data, but attempts have been unsuccessful. DigitizeIt was used to extract data from figures Trialists reported in the Discussion section, "No treatment gave complications" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to receive physiotherapy alone, local steroid injection alone, or a combination of the two" Comment: No information was reported about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported about how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Patients were assessed initially, at six weeks, and at six months by an independent observer unaware of the treatment given" Comment: Assessors of the outcome, passive range of motion, were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Four patients dropped out of the study before completion. They failed to attend for review at six weeks or six months. The analysis was based on 62 patients (34 female, 28 male)" Comment: The number of participants randomly assigned to each group and the number who dropped out of each group were not reported. Also, it is unclear whether failure of participants to attend for review was related to the treatment they received. Despite this, the relatively low dropout rate is unlikely to have biased the results |
| Selective reporting (reporting bias) | High risk | Comment: Trialists reported means (standard errors) for each group in figure format for day pain, pain on active movement, total abduction and internal rotation, but no data for night pain, pain on passive movement, glenohumeral abduction and external rotation |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Dundar 2009.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (Turkey) Interventions: active stretching and pendulum exercises plus home exercise or continuous passive motion plus home exercise Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: none |
|
| Participants |
Number of participants: 57 (29 and 28 in each respective group) Baseline characteristics Group receiving continuous passive motion Mean (SD) age = 56.3 (7.8) years; male:female = 9:20 Median (SD) duration of symptoms: 6.3 (4.2) months Group receiving active stretching and pendulum exercises Mean (SD) age = 57.1 (8.3) years; male:female = 9:19 Median (SD) duration of symptoms: 5.9 (4) months Inclusion criterion
Exclusion criteria
|
|
| Interventions | All participants were instructed in a standardised home exercise programme consisting of passive ROM and pendulum exercises to be performed every day until week 12. Home exercise was demonstrated by a physiotherapist on 1 occasion, and participants then were given written advice Active stretching and pendulum exercises (N = 28) Components of intervention: supervised active stretching and pendulum exercises Dosage: 1 hour Frequency of administration: once a day for 20 days during a period of 4 weeks (i.e. 5 days per week (20 sessions)) Provider: physiotherapist Continuous passive motion (N = 29) Components of intervention: continuous passive motion Dosage: gradual increase in motion for 1 hour Frequency of administration: once a day for 20 days during a period of 4 weeks (i.e. 5 days per week (20 sessions)) Provider: physiotherapist |
|
| Outcomes | Outcomes assessed at the end of 4 weeks' treatment and at week 12 (12 weeks from baseline). No primary outcome was reported by trialists
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were assigned randomly to receive daily CPM treatments or CPT protocol" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain, the SPADI and the Constant score |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported on assessors of objective outcomes (range of motion), and this could have been done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed the study" Comment: No dropouts, losses to follow‐up or exclusions from this study |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Ghosh 2012.
| Methods |
Design: parallel‐group, 3‐arm randomised controlled trial (India) Interventions: active and passive mobilisation exercises plus shoulder wheel and pulley exercises plus ultrasound or manipulation under anaesthesia or glucocorticoid injection (all received home exercise) Sample size calculation: not reported Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 72 (24 per group) Baseline characteristics: Baseline characteristics by group were not reported. Sex was not reported Age range: 40‐73 years Duration of symptoms: 0‐2 months (n = 33), 2‐4 months (n = 23), 4‐6 months (n = 16) Inclusion criteria
Exclusion criteria
|
|
| Interventions | All participants were advised to perform active shoulder mobilisation exercises at home Mobilisation exercises plus shoulder wheel and pulley exercises plus ultrasound (N = 24) Components of intervention
Dosage: not reported Frequency of administration: for 6 months (number of sessions per week not reported) Provider: physiotherapist Manipulation under anaesthesia (N = 24) Components of intervention: After general anaesthesia, manipulations were done in the sequence of flexion, extension, abduction, adduction, external rotation and internal rotation. Analgesics were given post manipulation period for 2 to 3 days, and shoulder mobilisation exercises started 3 to 4 days after manipulation, which was taught previously Dosage: not reported Frequency of administration: once Provider: not reported Glucocorticoid injection (N = 24) Components of intervention: An injection of methylprednisolone in 40‐mg dosage was given intra‐articularly by the anterior approach under strict aseptic preparation Dosage: See above Frequency of administration: Average of 3 doses at 3‐week intervals Provider: not reported |
|
| Outcomes | Outcome assessed at the end of 6 months' treatment
|
|
| Notes | To analyse the "treatment success" outcome, we dichotomised participants into those who had a clinical improvement rating of "Good" versus those who had a rating of "Fair" or "Poor." Trialists reported that participants in the study had "almost equal right and left sided affection with one having bilateral affection." However, the group that the bilaterally affected participant was allocated to was not reported, nor was any mention made of controlling for the correlation between shoulders (but this is unlikely to have affected the results substantially, given the dichotomous 'clinical improvement' outcome used) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "These patients were randomly allocated in 3 groups" Comment: No information was reported about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported about how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain and tenderness |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported about whether assessors of muscle wasting and range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Only 1 participant (in the glucocorticoid injection group) was lost to follow‐up. This is unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Results of the single outcome reported in the methods section of the publication (treatment success) were fully reported, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Guler‐Uysal 2004.
| Methods |
Design: parallel‐group, 2‐arm, single‐blind randomised controlled trial (Turkey) Interventions: Cyriax approach of deep friction massage and manipulation, active stretching exercises and home exercise or short wave diathermy application, hot pack, stretching exercises and home exercise Sample size calculation: 20 participants per group were estimated to be needed based upon detection of a 40% increase in the number of participants treated successfully in the Cyriax group at the 5% level of statistical significance with 80% power Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 42 (21 per group) Baseline characteristics Group receiving Cyriax approach of deep friction massage and manipulation Mean (SD; range) age = 53.6 (6.9; 43‐70) years; male:female = 5:15 Median (SD; range) duration of symptoms: 7.6 (3.9; 2‐12) months Group receiving hot pack and short‐wave diathermy application Mean (SD; range) age = 58.4 (9.7; 44‐82) years; male:female = 7:13 Median (SD; range) duration of symptoms: 5.6 (3.9; 2‐12) months Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups received active stretching and pendulum exercises at the end of each treatment session and were instructed in a standardised home exercise programme consisting of passive ROM and pendulum exercises to be performed every day. Cyriax approach of deep friction massage and manipulation (N = 21) Components of intervention
Dosage: 1 hour Frequency of administration: 3 times per week for 2 weeks (6 sessions) Provider: physiotherapist Short‐wave diathermy application and hot pack (N = 21) Components of intervention
Dosage
Frequency of administration: every day except weekends for 2 weeks (10 sessions) Provider: physiotherapist |
|
| Outcomes | Outcomes assessed at the end of the first and second weeks of treatment Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "42 patients were randomised for enrolment in the study. The patients were numbered sequentially and allocated to two groups (the Cyriax group and the physical therapy group)" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The pre‐treatment evaluation of shoulder pain and ROM was carried out by a blinded observer at the beginning of the study" Comment: Outcome assessors of range of motion were probably blind to treatment (although it is unclear how blinding of pain was achieved, given that it was self‐reported by unblinded participants) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in the CYR group were excluded from the study due to poor compliance and one from the PT group discontinued the intervention due to attacks of unstable hypertension in the first week" Comment: The number of dropouts or exclusions was low and equal between groups, and reasons are unlikely to influence the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Harsimran 2011.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (India) Interventions: anteroposterior glide mobilisation (Kaltenborn grade III) plus supervised and home exercise or posteroanterior glide mobilisation (Kaltenborn grade III) plus supervised and home exercise Sample size calculation: not reported Analysis: intention‐to‐treat analysis (imputed median values for participants with missing data) Source of funding: not reported |
|
| Participants |
Number of participants: 15 (8 and 7 in each respective group) Baseline characteristics Group receiving anteroposterior glide mobilisation Median (interquartile range) age = 52 (50‐57.8) years; male:female = 5:3 Median (interquartile range) duration of symptoms: 3 (3‐3.75) months Group receiving posteroanterior glide mobilisation Median (interquartile range) age = 56 (49‐62) years; male:female = 4:3 Median (interquartile range) duration of symptoms: 1.5 (1‐7) months Inclusion criteria
Exclusion criterion
|
|
| Interventions | Both groups received moist heat for 15 minutes, followed by Codman's exercises and finger ladder exercises following mobilisation. Participants were then advised to continue the same exercises at home Anteroposterior glide mobilisation (N = 8) Components of intervention: anteroposterior glide mobilisation (Kaltenborn grade III). Participants were positioned appropriately on the treatment table in supine position. The affected limb was taken to the available abduction range of motion, and mobilisations were provided for 30 seconds. This technique was repeated 5 times in a single treatment session. Physiological movements of the affected extremity were provided for 1 minute after every 30 seconds of the mobilisation procedure Dosage: See above Frequency of administration: 1 session per day for 5 consecutive days (5 sessions) Provider: physiotherapist Posteroanterior glide mobilisation (N = 7) Components of intervention: posteroanterior glide mobilisation (Kaltenborn grade III). Participants were positioned appropriately on the treatment table in prone position. The affected limb was taken to the available abduction range of motion, and mobilisations were provided for 30 seconds. This technique was repeated 5 times in a single treatment session. Physiological movements of the affected extremity were provided for 1 minute after every 30 seconds of the mobilisation procedure Dosage: See above Frequency of administration: 1 session per day for 5 consecutive days (5 sessions) Provider: physiotherapist |
|
| Outcomes | Outcomes assessed at the end of 5 days' treatment Primary outcome
Secondary outcomes
|
|
| Notes | Trialists reported only medians with no measures of variation in figure format for all outcomes and reported that no statistical significance testing was undertaken because this RCT was only a pilot RCT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were then randomized in 2 treatment groups by block randomization, group AP (antero‐posterior) & PA (postero‐anterior). During Randomization 3 blocks were used, with each block consisting of 6 units (3 AP & 3 PA). Two blocks out of 3 were utilized completely & from the 3rd block only 3 units were used. After allocation, group AP consisted of 8 & group PA consisted of 7 subjects" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Primary investigator performed the mobilization technique and second investigator was blinded to the group allocation of the participants and measured range of motion before and after every treatment session" Comment: Participants received slightly different types of mobilisation, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Primary investigator performed the mobilization technique and second investigator was blinded to the group allocation of the participants and measured range of motion before and after every treatment session" Comment: Assessors of range of motion were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Five subjects out of fifteen were lost to follow up. Three were from AP group and two from PA group. Two subjects from AP group underwent Manipulation under anaesthesia and other three subjects could not be followed due to personal constraints. Data of these five subjects was analyzed for intention to treat analysis" Comment: The number of participants lost to follow‐up in each group and reasons for this were reported and do not appear to be related to the result. Intention‐to‐treat analysis was conducted |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trialists reported only medians with no measures of variation in figure format for all outcomes (and the figure appears to be missing the median values for internal rotation at 45 degrees of abduction range of motion). However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Johnson 2007.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (USA) Interventions: anterior or posterior glenohumeral joint mobilisation, both with therapeutic ultrasound and upper extremity exercises using the upper body ergometer Sample size calculation: not reported Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 20 (10 per group) Baseline characteristics Group receiving anterior glenohumeral joint mobilisation Mean (SD) age = 54.7 (8) years; male:female = 2:8 Median (range) duration of symptoms: 8.4 (2‐12) months Group receiving posterior glenohumeral joint mobilisation Mean (SD) age = 50.4 (6.9) years; male:female = 2:6 (sex reported only for participants completing treatment) Median (range) duration of symptoms: 10.9 (4‐60) months Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups received ultrasound and upper extremity exercises using the upper body ergometer. All ultrasound treatments were applied at 1.5 W/cm2 continuously for 10 minutes, using a Sonicator Ultrasound Generator (ME 730; Mettler Electronics Corporation, Anaheim, CA). A coupling gel (Tyco Health Care Group LP, Mansfield, MA) was used and the sound head was moved in a circular pattern at the rate of approximately 4 cm/s. The area covered by the ultrasound head was about twice the size of the sound head. The upper body ergometer was used for 3 minutes in the forward direction only, at an arm position height that allowed pain‐free movement Anterior glenohumeral joint mobilisation (N = 10) Components of intervention
Dosage: not reported Frequency of administration: 2‐3 sessions per week for 2‐3 weeks (6 sessions in total) Provider: physiotherapist Posterior glenohumeral joint mobilisation (N = 10) Components of intervention
Dosage: not reported Frequency of administration: 2‐3 sessions per week for 2‐3 weeks (6 sessions in total) Provider: physiotherapist |
|
| Outcomes | Outcomes assessed at the end of 6 treatment sessions (i.e. 2‐3 weeks) Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was predetermined by using a random‐numbers table" Comment: An adequate method was used to generated the allocation sequence |
| Allocation concealment (selection bias) | High risk | Quote: "The randomization was predetermined by using a random‐numbers table. Folders labeled with the group name and subject number were made ahead of time and used sequentially as the subjects joined the study" Comment: Folders had the intervention name visible to the person responsible for allocating participants (and thus whether the allocation sequence was not concealed) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received slightly different types of mobilisation, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain, overall function and ability to do specific activities, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "The physical therapist assistant measured the first 14 subjects who participated in the study (13 of which remained in the study). This measurer was blinded to treatment and group placement of these subjects. Due to taking a position at another facility, measurements for the final 6 subjects (5 of which remained in the study) were taken by the primary investigator (A.J.). This measurer was therefore not blinded to the treatment or group placement of these subjects" Comment: Most, but not all, assessments of range of motion were conducted by a blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two subjects, both in the PM group, left the study. One subject, after the third treatment session, for personal reasons, requested arthroscopic surgery to obtain a definitive diagnosis of her condition. The presence of adhesive capsulitis was confirmed during surgery and she received manipulation under anesthesia. The second subject left the study after the fourth treatment session as a result of a fall that injured her affected shoulder" Quote: "One subject left the PM group to have arthroscopic surgery and manipulation. However, the external rotation ranges for the first 3 treatment sessions that the subject completed show her gaining 15°. Including this subject in an intention‐to‐treat analysis would not have been a true representation of the effects of the mobilization procedure, so it was decided to not include her data" Comment: The number of dropouts, and reasons for this, were reported and are not related to the treatment received (so are unlikely to bias the results) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Ma 2006.
| Methods |
Design: parallel‐group, 3‐arm randomised controlled trial (Taiwan) Interventions: physical therapy (hot pack, joint mobilisation and active shoulder exercises) only or acupuncture only or physical therapy plus acupuncture Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 75 (30, 30 and 15 in each group, respectively) Baseline characteristics: Sex and duration of symptoms were not reported by group Male:female = 36:39 Mean duration of symptoms: 25.8 weeks Group receiving physical therapy only Mean age: 54.1 years Group receiving acupuncture only Mean age: 56.4 years Group receiving physical therapy plus acupuncture Mean age: 52.8 years Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Physical therapy (N = 30) Components of intervention
Dosage:
Frequency of administration: 5 times a week for 4 weeks (20 sessions) Provider: physical therapist Acupuncture (N = 30) Components of intervention: acupuncture. The therapeutic protocol included (1) therapeutic principles in promoting flow of qi and blood, driving out the wind and cold, removing dampness and activating meridians; (2) therapeutic methods on 3 yang meridians of the hand and (3) prescriptions with jianjiao, jianyu, fengchi, hegu and yanglingquan Dosage: 15 minutes Frequency of administration: twice a week for 4 weeks (8 sessions) Provider: acupuncturist Physical therapy plus acupuncture (N = 15) Combination of interventions described above |
|
| Outcomes | Outcomes assessed at the end of the second and fourth (final) weeks of treatment. No primary outcome was reported by the trialists
|
|
| Notes | Trialists reported only final value and change from baseline means (with no measures of variation) of outcomes measured before and after the intervention was delivered at the end of 2 and 4 weeks' treatment. MP extracted the data only after intervention was delivered at 4 weeks, as this was the final measurement (and measures of variation need to be retrieved for this and all other time points) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Those subjects were randomly assigned to one of three groups" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Those subjects were randomly assigned to one of three groups" Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain and health‐related quality of life |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported about whether assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, and the number of participants randomly assigned was reported as the number of participants analysed |
| Selective reporting (reporting bias) | Unclear risk | Comment: Mean scores with no measures of variation were reported for all outcomes specified in the methods section of the publication. However, the incompletely reported data do not appear to have been incompletely reported based on the statistical significance or magnitude of the results, although without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Maricar 1999.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (Singapore) Interventions: manual therapy plus exercise or exercise alone Sample size calculation: not reported Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 54 (number randomly assigned not reported; 16 per group completed treatment) Baseline characteristics: Duration of symptoms was not reported by group Mean duration of symptoms: 3 months Group receiving manual therapy plus exercises Mean (SD) age = 57.9 (9.5) years; male:female = 9:7 Group receiving exercises alone Mean (SD) age = 54.9 (5.4) years; male:female = 10:6 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups received exercises for 15 minutes once a week for 8 weeks. The strengthening regime for the rotator cuff muscles was included in the exercise programme from week 5 onwards. Home exercises were also assigned to both groups Manual therapy plus exercises (N = 16 completers) Components of intervention
Dosage: not reported Frequency of administration: once a week for 8 weeks Provider: physiotherapist Exercises only (N = 16 completers) See above |
|
| Outcomes | Outcomes assessed at weeks 3, 5, 7 and 8 (end of treatment). No primary outcome was reported by trialists
|
|
| Notes | Means and 95% CIs of the 5 measures of active range of motion were presented in figure format only (i.e. no numerical data reported). DigitizeIt was used to extract mean values from the figures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thirty‐two subjects were randomly assigned to either Group A having exercise and manual therapy or to Group B having exercises only" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Thirty‐two subjects were randomly assigned to either Group A having exercise and manual therapy or to Group B having exercises only" Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (the same physical therapy intervention with or without additional manual therapy), participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported about whether assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Fifty‐four subjects were recruited for this study. However, only 32 subjects, 19 men and 13 women, were used in the analysis of the results. The attrition was due to poor compliance to attendance and home exercise regime" Comment: The number of participants randomly assigned to, and who dropped out of, each group, was not reported, so it is unclear whether dropout was related to the interventions received |
| Selective reporting (reporting bias) | Unclear risk | Comment: Means and 95% CIs for each measure of active range of motion were presented in figure format only (i.e. no numerical data reported). However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Maryam 2012.
| Methods |
Design: parallel‐group, 3‐arm, single‐blind randomised controlled trial (Iran) Interventions: physiotherapy (including TENS, active range of motion exercises and ice application in 10 sessions) or glucocorticoid injection or physiotherapy plus glucocorticoid injection Sample size calculation: 35 participants per group were estimated to be needed based upon detection of a clinically relevant difference at the 5% level of statistical significance with 80% power (outcome used in power calculation not reported) Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 87 (27, 31 and 29 in each respective group) Baseline characteristics Group receiving physiotherapy Mean (SD) age = 53.73 (7.49) years; male:female = 1:26 Mean (SD) duration of symptoms: 4.48 (3.37) months Group receiving glucocorticoid injection Mean (SD) age = 53.33 (7.49) years; male:female = 4:25 Mean (SD) duration of symptoms: 6.83 (3.75) months Group receiving physiotherapy plus glucocorticoid injection Mean (SD) age = 53.71 (6.69) years; male:female = 4:27 Mean (SD) duration of symptoms: 6.21 (3.95) months Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Physiotherapy (N = 27) Components of intervention
Dosage: not reported Frequency of administration: 10 sessions (number of sessions per week not reported) Provider: physiotherapist Glucocorticoid injection (N = 31) Components of intervention: corticosteroid injection included as 60 mg triamcinolone acetonide and 3 cc lidocaine in shoulder joint with posterior approach and 20 mg triamcinolone acetonide and 1.5 cc lidocaine in subacromial bursa Dosage: See above Frequency of administration: once Provider: rheumatologist Physiotherapy plus glucocorticoid injection (N = 29) Physiotherapy (as above) 1 week after glucocorticoid injection (as above) |
|
| Outcomes | Outcomes assessed at 6 weeks and 6 months. No primary outcome was reported by trialists
|
|
| Notes | Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request Trial registered in the Iranian Registry of Clinical Trials (http://www.irct.ir/searchresult.php?id=1828&number=1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After taking written informed consent, the patients were randomized to 1 of the following 3 groups" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After taking written informed consent, the patients were randomized to 1 of the following 3 groups" Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "Evaluations of SPADI score were done by an observer blind to treatment allocation" Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported some components of the SPADI |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Comment: Trialists confirmed via personal communication that the assessor of range of motion was not blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Eight patients in physiotherapy group, 7 in combination therapy group and 3 in injection group did not continue, so statistical analysis was done on 69 remaining patients" Quote: "About 36 patients have been reevaluated in 24 weeks (Table‐III). However we cannot consider this stage of study because of a high number of missed patients, but we can see a more subjective improvement during 6 months in physiotherapy group" Comment: Trialists did not report the reasons for participants not continuing (and did not provide this information when requested), so it is unclear whether the reasons were balanced between groups and related to the treatment received |
| Selective reporting (reporting bias) | Low risk | Comment: Outcome data were fully reported for all outcomes specified in the trial registry entry |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Nellutla 2009.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (India) Interventions: proprioceptive neuromuscular facilitation (PNF) movement patterns for exercises plus mobilisation and ultrasound or conventional free exercises plus mobilisation and ultrasound Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 40 (20 per group) Baseline characteristics: Baseline characteristics were not reported by group Mean (SD) age = 56.15 (8.71) years; male:female = 24:16 Duration of symptoms not reported Inclusion criteria
Exclusion criteria Not reported |
|
| Interventions | Both groups received ultrasound at each session, with dosage of 0.8 W/cm2 and a pulse ratio of 1:2 for 8 minutes. Both groups also received mobilisation techniques using grade 3 oscillations. For the glenohumeral joint, caudal glide, posterior glide and anterior glide were provided. For the acromioclavicular joint, anterior glide was provided. For the sternoclavicular joint, posterior glide, anterior glide, inferior glide and superior glide were provided Proprioceptive neuromuscular facilitation (PNF) movement patterns for exercises (N = 20) Components of intervention: proprioceptive neuromuscular facilitation (PNF) movement patterns for exercises. 2 types of patterns were applied, with the shoulder starting from one position and ending in other position according to PNF movement patterns: (1) shoulder flexion, abduction and external rotation started at shoulder extension, adduction and internal rotation; and (2) shoulder flexion, adduction and external rotation started at shoulder extension, abduction and internal rotation. This was performed until the participant understood properly about how he or she should be performing the patterns within the available range. Participants were made to perform in front of a mirror for feedback and to show the performance to the therapist. The participant was advised to perform these patterns thrice daily 10 times for each set and 2 sets for each session Dosage: not reported Frequency of administration: 5 times a week for 3 weeks (15 sessions) Provider: physiotherapist Conventional free exercises (N = 20) Components of intervention: conventional free exercises, such as finger ladder exercises, Codman's pendulum exercises and overhead shoulder pulley and shoulder wheel, 5 times a week for 3 weeks. Each exercise was done with 10 repetitions in a set of movements and with total of 2 sets for each movement. Apart from all these, home exercises were taught to the participant, such as simple Codman's exercises with an iron box in hand, finger wall exercises and all active movements around the shoulder. Participants were told to repeat each movement 10 times and were advised to perform these home exercises twice daily—once in the early morning before coming for the treatment and once in the evening Dosage: not reported Frequency of administration: 5 times a week for 3 weeks (15 sessions) Provider: physiotherapist |
|
| Outcomes | Outcomes assessed at the end of 3 weeks' treatment. No primary outcome was reported by the trialists
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were then randomly allocated into two groups, one control group and one experimental group" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were then randomly allocated into two groups, one control group and one experimental group" Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different types of exercise, but it is unclear whether they were provided any information that would make them perceive the type of exercise they received as superior or inferior to the alternative type of exercise |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported both measures of function, but it is unclear whether they were provided any information that would make them perceive the type of exercise they received as superior or inferior to the alternative type of exercise |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported about whether assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, and the analyses are reported as based on the number of randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Nicholson 1985.
| Methods |
Design: parallel‐group, 2‐arm, single‐blind randomised controlled trial (USA) Interventions: passive mobilisation and active exercises or active exercises alone Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 20 (10 per group) Baseline characteristics Group receiving passive mobilisation plus active exercises Mean (SD; range) age = 51 (12.16; 31‐70) years; male:female = 4:6 Mean (SD; range) duration of symptoms: 27.6 (33.41; 1‐104) weeks Group receiving active exercises alone Mean (SD; range) age = 55 (16.43; 20‐77) years; male:female = 6:4 Mean (SD; range) duration of symptoms: 30.8 (31.28; 3‐104) weeks Inclusion criterion
Exclusion criteria
|
|
| Interventions | Both groups received active exercises in those ranges found to be restricted and additional resistive exercises if weaknesses were present, in 2‐3 physiotherapy sessions a week for 4 weeks, and also 3 times per day independently Passive mobilisation plus active exercises (N = 10) Components of intervention: passive mobilisation. Generally, in the early sessions, gliding and distractive mobilisation techniques were performed with the joint near its neutral position, progressing during later sessions to mobilisation towards the end of the range of motion. The decision to progress to mobilisation at the end of the range of motion was based on the participant’s satisfactory tolerance of mobilisation within the range and "levelling off" of progress made by less vigorous techniques. The force and amplitude of treatment movements varied, but eventually all participants were able to tolerate grade IV oscillations (small amplitude motions at the end of the range of motion) without significant discomfort Dosage: not reported Frequency of administration: 2‐3 physiotherapy sessions a week for 4 weeks (8‐12 sessions) Provider: physiotherapist Active exercises (N = 10) See above |
|
| Outcomes | Outcomes assessed weekly for 4 weeks. No primary outcome was reported by the trialists
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "…the subjects were assigned to either the experimental or control group, using the toss of a coin, i.e., when the first subject consented, a coin toss determined the group assignment and the next successive subject was assigned to the opposite group. The coin toss was repeated for each odd numbered subject" Comment: A quasi‐random method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | High risk | Quote: "…the subjects were assigned to either the experimental or control group, using the toss of a coin, i.e., when the first subject consented, a coin toss determined the group assignment and the next successive subject was assigned to the opposite group. The coin toss was repeated for each odd numbered subject". Comment: Using this method, the allocation sequence is unlikely to have been concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (the same physical therapy intervention either with or without additional mobilisation), participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants who may have had different expectations about the benefits of the intervention received self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All mobility measurements were visualised and recorded by an assistant who was unaware of the patient's group designation" Comment: Assessors of range of motion were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, and outcome data are reported as based on the number of randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Pajareya 2004.
| Methods |
Design: parallel‐group, 2‐arm, single blind randomised controlled trial (Thailand) Interventions: physical therapy (mobilisation, passive glenohumeral joint‐stretching exercises and short‐wave diathermy) plus ibuprofen or ibuprofen alone Sample size calculation: 60 participants per group were estimated to be needed based upon detection of a difference in success rate (measured by improvement in global pain and disability index) of 25% at the 5% level of statistical significance with 80% power Analysis: per‐protocol analysis (reported that intention‐to‐treat analysis was used to test statistical significance, but outcome data presented in tables was reported as based on the number of participants completing assessments at each week) Source of funding: Department of Research Promotion, Faculty of Medicine, Siriraj Hospital, Mahidol University and partially supported by Thailand Research Fund (non‐industry) |
|
| Participants |
Number of participants: 122 (61 per group) Baseline characteristics: baseline characteristics reported for participants who completed the week 3 assessment (n = 119) Group receiving physical therapy plus ibuprofen Mean (SD) age = 56.3 (10.6) years; male:female = 14:45 Duration of symptoms: number of participants with duration < 6 weeks (n = 6), between 6 and 11 weeks (n = 20) and 12 or more weeks (n = 33) Group receiving ibuprofen alone Mean (SD) age = 57.7 (10) years; male:female = 24:36 Duration of symptoms: number of participants with duration < 6 weeks (n = 13), between 6 and 11 weeks (n = 20) and 12 or more weeks (n = 27) Inclusion criterion
Exclusion criteria
|
|
| Interventions | Both groups received ibuprofen 400 mg 3 times daily for 3 weeks, and general advice (an information sheet containing advice on protection of the shoulder from vigorous activities such as pushing and pulling, and encouragement to use the arms in a normal fashion for reaching and other activities of daily life) Physical therapy plus ibuprofen (N = 61) Components of intervention
Dosage
Frequency of administration
Provider: physical therapist Ibuprofen (N = 61) See above |
|
| Outcomes | All outcomes assessed at the end of 3 weeks' treatment (except for "success," which was also assessed at 6, 12 and 24 weeks) Primary outcome
Secondary outcomes
|
|
| Notes | Adverse events due to ibuprofen were not reported separately per group: "During the 3‐week period, the patients in the study group reported a total of 10 episodes of pain that persisted more than 2 hours after treatment from 4 subjects.There were no other complications recorded. Regarding NSAIDs, 15 subjects (12.6%) had gastrointestinal side effects; the number of those who had severe dyspepsia and had to stop NSAIDs was 6 (4.2%). There were 2 reports of severe oedema and 1 case with a severe headache, which rapidly subsided after the drug was discontinued" (pg 477 of trial publication) Unpublished data regarding study design (required for risk of bias assessment) provided by trialist on request |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients who gave informed written consent were randomly allocated to a 3‐week treatment protocol by simple randomisation using a random numbers table and allocation concealed within an opaque envelope" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients who gave informed written consent were randomly allocated to a 3‐week treatment protocol by simple randomisation using a random numbers table and allocation concealed within an opaque envelope" Personal communication: "I prepared opaque envelopes before hand. Within each envelope, I put the letter 'I' or 'C.' The series of 'I' and 'C' came from the random number table. I didn't remember any part of the series" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported global pain and disability index and the SPADI |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Moreover, at each follow‐up, an investigator, blinded to treatment modality asked all patients 'Have the trial drugs and/or treatment program upset you in any way?' and examined the patient for any signs of ecchymosis or burn during range of motion evaluation" Quote: "Range of shoulder motion measured...by a investigator blinded to the type of treatment" Personal communication: "The range of motion assessor was blinded. I had told all of the participants that 'Please don't tell the assessor about the treatment you have'" Comment: Assessors of adverse events and range of motion were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "At the end of the 3rd week, 2 subjects dropped out from the study; 1 from the control group and 1 from the study group. The total number of cases included in the analysis was 59 in the control and 60 in the study group. By the end of the 24th week, a total of 12 cases (10.1%) had withdrawn from the study (Fig. 1). All of them lost to follow‐up for unknown reasons and the investigators could not contact them" Quote: "The results were analysed by intention to treat analysis even though the treatments actually received were modified from the protocol, because it was found that the reasons for modifying the treatment were strongly related to the results of allocated interventions" Comment: It is unclear whether reasons for losses to follow‐up were related to the interventions received |
| Selective reporting (reporting bias) | Unclear risk | Quote: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Quote: "“About three‐quarters of the subjects of both groups received NSAIDs as prescribed. The reasons why some patients received fewer NSAIDs than the others was due to gastrointestinal discomfort, forgetting to take them or a misunderstanding about the schedule. In the study group, 7 cases (11.7%) received fewer than 6 sessions of hospital‐based PT, 5 cases (8.3%) performed the home programme exercises fewer than 6 sessions. Two cases from the control group reported that they had additional treatment; 1 had Chinese herbal medicine and 1 received analgesics from a private clinic. No patient in the control group had hospital‐based PT or home exercise therapy for their shoulder" Quote: "The deviation from the protocol in the present study might not reverse the results. On the contrary, the differences of the outcomes at the end of the study should be elicited more easily if there was no protocol deviation. Because the patients in the study group received fewer treatments than the schedule determined (six cases had fewer than 6 sessions of hospital‐based PT and 6 cases performed home exercise fewer than 6 sessions), while the subjects in the control group received more treatment than the schedule (one case had Chinese herbal medicine and 1 case had analgesics from a private clinic)" Comment: Protocol violations are unlikely to have influenced the results |
Rainbow 2008.
| Methods |
Design: parallel‐group, 2‐arm quasi‐randomised controlled trial (USA) Interventions: high‐velocity, low‐amplitude chiropractic manipulative therapy to the cervical and thoracic spine and glenohumeral joint plus home exercise therapy, or grade 4 mobilisation of the glenohumeral joint plus home exercise therapy Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: funded and completed as part of a Master’s in Science (chiropractic) degree for Dr Daniel Rainbow and supervised by Dr Paul Weston at the Division of Health and Social Care (formerly the European Institute of Health and Medical Sciences), University of Surrey, Guilford, United Kingdom |
|
| Participants |
Number of participants: 8 (4 per group) Baseline characteristics: Baseline characteristics were not reported by group Age range: 35‐60 years Male:female = 2:6 Duration of symptoms not reported Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups performed home exercises including Codman's pendulum exercise and simple wall walking. Participants were provided with verbal and written home exercise (with technical instructions and fully illustrated for later reference) Chiropractic manipulative therapy (N = 4) Components of intervention: high‐velocity, low‐amplitude chiropractic manipulative therapy to the cervical and thoracic spine and glenohumeral joint. No more than 2 spinal adjustive procedures and 2 glenohumeral joint adjustive procedures were performed per region (a maximum of 4) per visit. Selected methods for motion palpation and adjustive techniques were left to the discretion of the treating doctor Dosage: not reported Frequency of administration: twice a week for 6 weeks (12 sessions) Provider: chiropractor Mobilisation (N = 4) Components of intervention: grade 4 mobilisation of the glenohumeral joint for 3 minutes according to the supine glenohumeral mobilisation technique. Participant is supine with the arm outstretched and in slight abduction. The chiropractor grasps either side of the proximal humerus with both hands, and the participant's extended forearm is held against the chiropractor's thoracic cage. The chiropractor lightly distracts the shoulder inferiorly and produces mild distraction, circumduction and movement of the shoulder in all directions Dosage: 3 minutes Frequency of administration: twice a week for 6 weeks (12 sessions) Provider: chiropractor |
|
| Outcomes | Outcome assessed at the second treatment session each week for 6 weeks Primary outcome
Secondary outcome
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Once selected, subjects were allocated to 1 of 2 treatment groups by systematic assignment. Group allocation was determined by the order in which patients qualified and entered the trial. For example, the 1st subject accepted into the trial was allocated randomly to Group 1, accomplished by folding 2 sheets of completely obscured paper (one marked Group 1, the other marked Group 2) and placed in an envelope. One sheet was picked from the envelope and revealed assignment to Group 1. Further quasi‐randomization occurred as the next patient was automatically allocated to Group 2, the 3rd to Group 1, etc. Subjects were unaware of this allocation process prior to presentation" Comment: A non‐random (predictable) sequence was used to allocate participants |
| Allocation concealment (selection bias) | High risk | Comment: A non‐random (predictable) sequence was used to allocate participants (see above quote) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Quote: "The SPADI was selected as the primary and singular outcome measure" Quote: "At each treatment session, patients' subjective symptoms were briefly noted by the primary researcher. The treating doctor (a registered/licensed doctor of chiropractic) was then taken into the room by the primary researcher, and the assigned treatment was administered. This procedure was followed for 12 treatments, with the SPADI administered on the 2nd treatment visit of each week immediately prior to the application of any treatment. All treating practitioners remained blinded to the results generated from the outcome measures throughout the duration of the trial" Quote: "These findings must be interpreted cautiously, however, secondary to...the absence of blinded assessors" Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported the SPADI |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 8 subjects who entered the trial completed the trial with no reported complications, side effects, or adverse reactions" Quote: "Because no subjects dropped out of the study, intention‐to‐treat analysis was not necessary to calculate" Comment: No losses to follow‐up, drop‐outs or exclusions occurred |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Ryans 2005.
| Methods |
Design: parallel‐group, 4‐arm, single‐blind randomised controlled trial (United Kingdom) Interventions: physiotherapy (proprioceptive neuromuscular facilitation, Maitland mobilisations and active exercise, interferential modality) plus glucocorticoid injection or glucocorticoid injection alone or physiotherapy plus placebo injection or placebo injection alone Sample size calculation: 20 participants per group were estimated to be needed based upon detection of a difference of 1.04 points on a 5‐point pain scale (SD = 1.6) at 4 weeks at the 5% level of statistical significance with 82% power Analysis: per‐protocol analysis Source of funding: Arthritis Research Campaign (non‐industry) |
|
| Participants |
Number of participants: 80 (20 per group) Baseline characteristics Group receiving physiotherapy plus glucocorticoid injection Mean (SD) age = 56.3 (6.4) years; male:female = 11:9 Mean (SD) duration of symptoms: 14.2 (4.4) weeks Group receiving glucocorticoid injection alone Mean (SD) age = 52.3 (9.3) years; male:female = 6:13 Mean (SD) duration of symptoms: 12.2 (5.3) weeks Group receiving physiotherapy plus placebo injection Mean (SD) age = 52.6 (7.7) years; male:female = 6:14 Mean (SD) duration of symptoms: 14.4 (4.4) weeks Group receiving placebo injection alone Mean (SD) age = 55.2 (9.4) years; male:female = 9:10 Mean (SD) duration of symptoms: 14.9 (3.7) weeks Inclusion criteria
Exclusion criteria
|
|
| Interventions | All participants were provided with 50 × 500 mg paracetamol tablets with suggestions to take 1 or 2 tablets 4‐ to 6‐hourly as required for pain, taking no more than a maximum of 8 tablets daily. All participants were also instructed by a physiotherapist in an identical home exercise programme using a video and home exercise instruction sheet Physiotherapy plus glucocorticoid injection (N = 20) Components of physiotherapy intervention
Dosage: not reported Frequency of administration: twice a week for 4 weeks (8 sessions) Provider: physiotherapist Components of glucocorticoid injection: injections of triamcinolone 20 mg (1 mL) and normal saline 2 mL plus physiotherapy for 4 weeks. Injections were given (without imaging guidance) by a combined approach to the shoulder: Half the solution (1.5 mL) was injected by an anterior approach and half (1.5 mL) by a lateral approach Glucocorticoid injection alone (N = 20) The same injection method as described above was delivered Physiotherapy plus placebo injection (N = 20) The same injection and physiotherapy method as described above was delivered, except that normal saline 3 mL was injected into the shoulder Placebo injection alone (N = 20) The same injection method as described above was delivered, except that normal saline 3 mL was injected into the shoulder |
|
| Outcomes | Outcomes assessed at 6 and 16 weeks' post randomisation Primary outcome
Secondary outcomes
|
|
| Notes | *Outcome data fully reported only for these outcomes. No outcome data reported for other outcomes Unpublished data regarding study design provided by trialist on request Trial registered in ISRCTN, but outcomes not provided at time of registration (http://www.controlled‐trials.com/ISRCTN25152388) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly allocated in permuted blocks of four using random number tables to one of four treatments. The randomization process took place in the hospital pharmacy department. Allocations were placed in sealed envelopes which were opened by the physiotherapist teaching the home exercise programme" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Comment: See quote above. An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Injections were provided in opaque syringes, and the investigator measuring outcomes (IR) was not present at the time of randomization or injection and was blinded to all study interventions. Both patients and the physiotherapist were blinded to the nature of the injection. Clearly, it was impossible to blind subjects regarding physiotherapy but subjects were asked not to reveal if they were having physiotherapy treatment" Comment: Participants and personnel were blind to the injection component of the intervention, but not to the physiotherapy component. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Participants self‐reported pain, general health status and function, and were not blind to whether they had received physiotherapy. Participants may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Injections were provided in opaque syringes, and the investigator measuring outcomes (IR) was not present at the time of randomization or injection and was blinded to all study interventions. Both patients and the physiotherapist were blinded to the nature of the injection. Clearly, it was impossible to blind subjects regarding physiotherapy but subjects were asked not to reveal if they were having physiotherapy treatment" Comment: Assessors of objective outcomes were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Eighty subjects were recruited and randomly assigned to four groups. One subject was randomized twice and another failed to attend for intervention after randomization; 78 subjects were therefore available for analysis. Twenty subjects were enrolled in Group A (steroid injection and physiotherapy), 19 in Group B (steroid injection and no physiotherapy), 20 in Group C (placebo injection and physiotherapy) and 19 in Group D (placebo injection and no physiotherapy). Six subjects did not return for all follow‐up visits: three in Group A, one in Group B, one in Group C and one in Group D. Fifteen subjects withdrew from the study due to failure of the study treatment. Six patients withdrew from Group B, three from Group C and six from Group D" Quote: "We also looked to see if there were significant differences in numbers dropping out in each group due to failure of treatment. Significantly more patients dropped out in Group D (placebo injection and no physiotherapy) and in Group B (steroid injection and no physiotherapy (Pearson chi‐square=8.72, P=0.033). No subjects dropped out of Group A (steroid injection and physiotherapy)" Comment: The was differential dropout across the groups, and the reasons appear to be related to the treatments received |
| Selective reporting (reporting bias) | High risk | Quote: "Secondary outcome measures were...range of movement as measured by passive external rotation. External rotation was chosen as the indicator range of movement as restriction in this range has been described as the most severely restricted plane of movement in shoulder capsulitis" Quote: "Analysis of improvement in the range of movement in abduction and internal rotation (thumb–C7 distance) revealed no significant association with either steroid injection or physiotherapy" Comment: Trialists reported measuring passive and active range of motion (forward flexion, abduction, external rotation, internal rotation) using a goniometer. However, outcome data were reported only for passive external rotation. The decision not to report outcome data for the other measures of range of motion appears to be related to the statistical significance of the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Samnani 2004.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (India) Interventions: passive range of motion exercises plus therapeutic activity programme and active exercises or therapeutic activity programme and active exercises alone Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 20 (10 per group) Baseline characteristics: duration of symptoms not reported Group receiving passive range of motion exercises plus therapeutic activity programme and active exercises Mean (SD) age = 42.9 (6.5) years; male:female = 3:7 Group receiving therapeutic activity programme and active exercises Mean (SD) age = 42.7 (9) years; male:female = 6:4 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups received a therapeutic activity programme and active exercises for a period of 45 minutes, 6 times a week for 6 weeks, consisting of (1) Codman's pendulum exercises; (2) pulley exercises; (3) shoulder wheel; (4) active range of motion exercises using a towel; (5) finger stepping and corner stretch and (6) reaching out tasks Passive range of motion exercises plus therapeutic activity programme and active exercises (N = 10) Components of intervention: passive range of motion exercises consisting of (1) participant in supine: passive range of motion to the shoulder by the therapist from neutral position to maximal flexion available; (2) participant in supine: passive range of motion to the shoulder by the therapist from neutral position to maximal adduction available; (3) shoulder abducted to less than or equal to 90 degree, elbow flexed 90 degrees: passive range of motion to the shoulder by the therapist from this initial position to maximal internal rotation available; (4) shoulder abducted to less than or equal to 90 degrees, elbow flexed 90 degrees: passive range of motion to the shoulder by the therapist from this initial position to maximal external rotation available; (5) shoulder flexed to less than or equal to 90 degrees, elbow flexed 90 degrees: passive circumductory range of motion to the shoulder; and (6) supraspinatus stretching Dosage: 15 minutes Frequency of administration: 6 times a week for 6 weeks (36 sessions) Provider: occupational therapist Therapeutic activity programme and active exercises (N = 10) See above |
|
| Outcomes | Outcome assessed at the end of 6 weeks' treatment Primary outcome
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly allocated to one of the two groups, A & B of 10 each" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The subjects were randomly allocated to one of the two groups, A & B of 10 each" Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (the same multi‐component physical therapy intervention with or without additional exercises), participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information on whether assessors were blind to treatment was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, but it was unclear whether the outcome data reported were based on the total number of randomly assigned participants (as sample sizes were not reported in data tables) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Sharad 2011.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (India) Interventions: end range mobilisation techniques plus ultrasound therapy plus active glenohumeral exercises plus home exercise or ultrasound therapy plus active glenohumeral exercises plus home exercise Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 22 (11 per group) Baseline characteristics Group receiving end range mobilisation techniques plus ultrasound therapy plus active glenohumeral exercises plus home exercise Mean (SD; range) age = 46.5 (4.44; 41‐55) years; male:female = 5:6 Mean (SD; range) duration of symptoms: 4.9 (1.17; 3‐7) months Group receiving ultrasound therapy plus active glenohumeral exercises plus home exercise Mean (SD; range) age = 47.45 (5.49; 40‐56) years; male:female = 4:7 Mean (SD; range) duration of symptoms: 4.63 (1.05; 3‐6) months Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups received ultrasound therapy plus active glenohumeral exercises plus home exercise 5 days a week for 3 weeks. Ultrasound was delivered for 10 minutes to the glenohumeral joint anteriorly, posterior and inferiorly with the arm abducted. A machine with 1 MHz frequency and an output of 0 to 3.5 Watt/cm2 with head size 2.5 cm2 was used. Active glenohumeral exercises consisted of self‐stretching exercises preceded by warm‐up exercises and ending with a cool‐down phase, done under therapist’s supervision and guidance. No mechanical exercises were given. Home exercises comprised simple stretching exercises done at home once daily. The exercises were progressed and modified as per the participant response. End range mobilisation techniques plus ultrasound therapy plus active glenohumeral exercises plus home exercise (N = 11) Components of intervention: End range mobilisation techniques were carried out immediately following the application of ultrasound. Initially a few minutes of warming up was given using midrange mobilisation with the participant positioned supine, after which intensive end range mobilisation techniques, grades 3 and 4, as described by Maitland in all the movement planes were delivered, interspersed with accessory movements (glides). The effort in each direction had ten to fifteen repetitions. The rhythm speed and duration were varied in accordance with participant presentation and tolerance Dosage: varied in accordance with participant presentation and tolerance Frequency of administration: 5 days a week for 3 weeks (15 sessions) Provider: physiotherapist Ultrasound therapy plus active glenohumeral exercises plus home exercise (N = 11) See description of intervention above |
|
| Outcomes | Outcomes assessed at the end of 3 weeks treatment. No primary outcome was reported by the trialists. 1. Active and passive range of motion in external rotation, abduction, flexion using a goniometer 2. Pain using a visual analogue scale (scale units not reported but assumed to be 0‐10 based on the outcome data reported) |
|
| Notes | No measures of variation were reported for any of the outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This randomised control trial was conducted to assess the effectiveness of End Range Mobilization Techniques in the treatment of chronic adhesive capsulitis" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "This randomised control trial was conducted to assess the effectiveness of End Range Mobilization Techniques in the treatment of chronic adhesive capsulitis" Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (the same multi‐component physical therapy intervention with or without additional mobilisation), participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Quote: "...no blinding was done this could have biased the results" Comment: Outcome assessors were not blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, but it was unclear whether the outcome data reported were based on the total number of randomly assigned participants (as sample sizes were not reported in data tables) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Only means with no measures of variation were reported for all outcomes specified in the methods section of the publication. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Shrivastava 2011.
| Methods |
Design: parallel‐group, 2‐arm, double‐blind randomised controlled trial (India) Interventions: Maitland’s or Mulligan’s mobilisation technique for 2 weeks followed by home exercise programme for 2 weeks Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 40 (20 per group) Baseline characteristics: duration of symptoms not reported Group receiving Maitland's mobilisation technique plus home exercise programme Mean (SD) age = 59.2 (7.18) years; male:female = 7:13 Group receiving Mulligan's mobilisation technique plus home exercise programme Mean (SD) age = 51.15 (8.53) years; male:female = 12:8 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups received hot fomentation for 10 minutes, along with a 2‐week home exercise programme consisting of Codman's pendulum exercises, scapular setting exercises, finger ladder, wand exercises and stretching of the tightened muscles of the shoulder girdle. The 2‐week home exercise programme was delivered after the 2‐week mobilisation programme Maitland's mobilisation technique (N = 20) Components of intervention: Maitland's graded oscillations technique. The grade of glide was decided during treatment depending on the participant's symptoms. Individual glides delivered were reported as follows: (1) posterior glide: With participant in supine position, the therapist holds his/her arm proximally, applies a distraction force and glides the humeral head posteriorly; (2) inferior glide: Participant lies supine, while the therapist stands at the head end of the participant facing his/her feet. Holding the proximal arm of the participant, the therapist gives a distraction force to the glenohumeral joint and glides the humeral head inferiorly; and (3) the anterior glide: Participant is positioned prone, and the therapist holds the distal arm above the epicondyles with 1 hand for distraction; with the other hand, the therapist applies anterior glide to the humeral head Dosage: dependent on participants' symptoms Frequency of administration: 6 times a week for 2 weeks (12 sessions) Provider: physiotherapist Mulligan's mobilisation technique (N = 20) Components of intervention: Mulligan's mobilisation with movement technique. Passive overpressure was applied in the end of range, and 3 sets of 10 repetitions were given for each mobilisation. Individual glides delivered were reported as follows: (1) flexion and abduction: Participant sits with the therapist posterolateral to him/her. Therapist places the Mulligan belt across the humeral head and to his waist. Leaning backward, he applies a posterolateral glide to the shoulder joint and then asks the participant to perform the painful/restricted movement of shoulder flexion or abduction, which would be pain free; (2) internal rotation: Participant sits or stands with the therapist by his/her side. The therapist applies an inferior glide to the humerus head with the participant’s shoulder in available degrees of abduction. With the glide maintained, the participant actively rotates the shoulder internally without pain; (3) external rotation: Participant lies supine with his/her shoulder horizontally flexed till 90 degrees. The therapist places the belt at the humeral head, applying a lateral distraction to the joint the participant was asked to rotate the shoulder externally Dosage: dependent on participants' symptoms Frequency of administration: 6 times a week for 2 weeks (12 sessions) Provider: physiotherapist |
|
| Outcomes | Outcomes assessed at the end of 4 weeks' treatment. No primary outcome was reported by trialists
|
|
| Notes | No measures of variation were reported for any of the outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly allotted to the two groups, Maitland group and Mulligan group by computerised random sequence generator" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blinding was done with the assessment therapist and the patient both being blinded with respect to treatment protocol followed" Comment: Participants received different types of mobilisation but were not provided information that would make them perceive the intervention they received as superior or inferior to the alternative intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Low risk | Quote: "Double blinding was done with the assessment therapist and the patient both being blinded with respect to treatment protocol followed" Comment: Blinded participants self‐reported pain, function and adverse events |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Double blinding was done with the assessment therapist and the patient both being blinded with respect to treatment protocol followed" Comment: Assessors of range of motion were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "...no patient dropped out of the study due to problems in therapy" Comment: No dropouts, losses to follow‐up or exclusions were reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Only means with no measures of variation were reported for all outcomes specified in the methods section of the publication. However, it is not clear whether data were incompletely reported based on the statistical significance or magnitude of the results. Also, without a trial protocol, it is unclear whether other outcomes were assessed but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Sirajuddin 2010.
| Methods |
Design: parallel‐group, 3‐arm randomised controlled trial (India) Interventions: anterior glide mobilisation plus ultrasound therapy plus exercises or posterior glide mobilisation plus ultrasound therapy plus exercises or no mobilisation plus ultrasound therapy plus exercises Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 45 (15 per group) Baseline characteristics: Baseline characteristics were not reported by group Age range 40‐60 years; male:female = 17:28 Duration of symptoms: longer than 3 months Inclusion criteria
Exclusion criteria
|
|
| Interventions | All groups received ultrasound therapy and exercises. Ultrasound therapy was delivered at a frequency of 1 Mhz and an intensity of 1.0 W/cm2 for 8 minutes. To reduce postmobilisation soreness, each participant was given moist heat to the mobilised shoulder for 20 minutes. The exercise programme comprised Codman's pendular exercises for 2 minutes, wall ladder in abduction and flexion (5 repetitions 3 times daily) and wand‐assisted exercises for the shoulder (5 repetitions 3 times daily) Anterior glide mobilisation (N = 15) Components of intervention: anterior glide mobilisation. Participant was made prone and lateral humeral distraction was maintained in its midrange position, while the anterior stretch mobilisation was performed to end range, at the end range of abduction and internal rotation. If the participant was able to tolerate a stronger stretching force, body weight and gravity were used to generate the mobilisation force in a similar combined fashion of distraction to midrange and anterior glide to end range. Kaltenborn stretch mobilisation (which loads the restricting tissue at the end of the available range of motion) and Kaltenborn grade 3 mobilisation (which applies force after the slack of the joint has been taken up, to stretch tissues crossing the joint) were used. The end range positions of mobilisation were held for at least 1 minute. Fifteen minutes of sustained stretch was performed at each treatment session. During joint mobilisation, participants were instructed to describe their sensation, so that the therapist could modify the force or position to maintain a moderate stretch on the targeted tissue (however, each participant was encouraged to tolerate the pain to allow a moderate stretch sensation) Dosage: 15 minutes of sustained stretch Frequency of administration: twice a week for 3 weeks (6 sessions) Provider: physiotherapist Posterior glide mobilisation (N = 15) Components of intervention: posterior glide mobilisation. The posterior glide to the shoulder joint was given in the supine position. In this position, lateral humeral distraction was maintained in its midrange position, while posterior stretch mobilisation was performed to the end range of abduction and internal rotation. Kaltenborn stretch mobilisation (which loads the restricting tissue at the end of the available range of motion) and Kaltenborn grade 3 mobilisation (which applies force after the slack of the joint has been taken up, to stretch tissues crossing the joint) were used. The end range positions of mobilisation were held for at least 1 minute. Fifteen minutes of sustained stretch was performed at each treatment session. During joint mobilisation, participants were instructed to describe their sensation, so that the therapist could modify the force or position to maintain a moderate stretch on the targeted tissue (however, each participant was encouraged to tolerate the pain to allow a moderate stretch sensation) Dosage: 15 minutes of sustained stretch Frequency of administration: twice a week for 3 weeks (6 sessions) Provider: physiotherapist No mobilisation (N = 15) Only ultrasound therapy and exercises (see above) were delivered |
|
| Outcomes | Outcomes assessed at the sixth and last (i.e. end of 3 weeks' treatment) sessions. No primary outcome was reported by the trialists
|
|
| Notes | Numerical outcome data were fully reported for VAS pain and active range of motion in internal rotation, but data for active range of motion in external rotation and abduction and for the Shoulder Rating Questionnaire were presented in figures with unlabelled error bars only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After screening for inclusion and exclusion criteria the subjects were randomly assigned into either of 3 groups with 15 subjects in each group" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received slightly different types of mobilisation, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain and function, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported on whether assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, and outcome data were reported as based on the total number of randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Numerical outcome data were fully reported for VAS pain and active range of motion in internal rotation, but data for active range of motion in external rotation and abduction and for the Shoulder Rating Questionnaire were presented in figures with unlabelled error bars only. However, the incompletely reported data do not appear to have been incompletely reported on the basis of statistical significance or magnitude of results, although without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Tanaka 2010.
| Methods |
Design: parallel‐group, 3‐arm, double‐blind randomised controlled trial (Japan) Interventions: high‐frequency (more than 2 times a week), moderate‐frequency (once a week) or low‐frequency (less than once a week) joint mobilisation and home self‐exercises Sample size calculation: not reported Analysis: per‐protocol analysis Source of funding: not reported |
|
| Participants |
Number of participants: 120 (40 per group) Baseline characteristics: Age and duration of symptoms were not reported by group Mean (SD) age: 63.7 (9.1) years Duration of symptoms: 37 had condition for less than 1 month, 39 for less than 3 months, 21 for less than 6 months and 13 for longer than 7 months Group receiving high‐frequency joint mobilisation Male:female = 18:21 Group receiving moderate‐frequency joint mobilisation Male:female = 17:18 Group receiving low‐frequency joint mobilisation Male:female = 17:19 Inclusion criteria
Exclusion criterion
|
|
| Interventions | All groups received home exercise performed 2 to 3 times a day, including Codman's or pendulum exercises (circumduction) and passive stretching exercises such as "climbing the wall exercise" (i.e. facing a wall about three quarters of an arm's length away and raising the affected arm up to the shoulder level using only one's fingers without using shoulder muscles). Mean (SD) duration of treatment was 4.6 (1.2) months High‐frequency joint mobilisation (N = 40) Components of intervention: Mobilisation techniques used were those described by Vermeulen 2000, which are performed in the end ranges of limited joint mobility Dosage: 40 minutes Frequency of administration: More than twice a week for a mean (SD) duration of treatment of 4.6 (1.2) months Provider: physical therapist Moderate‐frequency joint mobilisation (N = 40) Components of intervention: Mobilisation techniques used were those described by Vermeulen 2000, which are performed in the end ranges of limited joint mobility Dosage: 40 minutes Frequency of administration: once a week for a mean (SD) duration of treatment of 4.6 (1.2) months Provider: physical therapist Low‐frequency joint mobilisation (N = 40) Components of intervention: Mobilisation techniques used were those described by Vermeulen 2000, which are performed in the end ranges of limited joint mobility Dosage: 40 minutes Frequency of administration: less than once a week for a mean (SD) duration of treatment of 4.6 (1.2) months Provider: physical therapist |
|
| Outcomes | Outcomes assessed until the time required to reach the range of motion plateau point (see below). Average length of therapy was 4.6 ± 1.2 months, and follow‐up time was 5.9 ± 1.3 months. No primary outcome was reported by the trialists
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "From the frequency of sessions for joint mobilization by physical therapists in the hospital setting, the patients were divided randomly into the high‐frequency session group (HF group, more than two times a week), moderate‐frequency session group (MF group, once a week), and low‐frequency session group (LF group, less than once a week)" Comment: No information was reported on how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We performed a randomized, placebo‐controlled, participant and single assessor‐blinded trial" Comment: Participants, but not personnel, were probably blind to treatment (i.e. probably did not know how the length of their intervention compared with that of participants in other groups) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | – |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "ROM was measured every week by one examiner who was not informed of the group designation of patients" Comment: Objectively assessed outcomes were probably assessed by an assessor who was blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The results of ten patients (men, 7; women, 3) were excluded from the data analysis because they received corticosteroidal injections during this study" Comment: One participant in the high‐frequency group, 5 participants in the moderate‐frequency group and 4 participants in the low‐frequency group were excluded from the analysis because they received corticosteroid injections during the study. As the reason for exclusions was the same for each group, the results are unlikely to be biased because of these exclusions |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
van der Windt 1998.
| Methods |
Design: parallel‐group, 2‐arm, single‐blind randomised controlled trial (The Netherlands) Interventions: physiotherapy (passive joint mobilisation and exercise treatment) or glucocorticoid injection Sample size calculation: 60 participants per group were estimated to be needed based upon detection of a clinically relevant difference of 25% in success rate at the 5% level of statistical significance with 80% power Analysis: For the primary outcome of "improvement," modified intention‐to‐treat analysis excluding 1 participant who withdrew from the injection group immediately post randomisation was used; for all other outcomes, modified intention‐to‐treat analysis, where only participants with missing data were excluded from the analysis, regardless of what treatments they actually received, was used Source of funding: Netherlands Organisation for Scientific Research and the Fund for Investigative Medicine of the Health Insurance Council (non‐industry) |
|
| Participants |
Number of participants: 109 (56 and 53 participants in each group, respectively) Baseline characteristics Group receiving physiotherapy Mean (SD) age = 60.2 (10.7) years; male:female = 23:33 Duration of symptoms: 6 (11%) less than 1 month; 26 (46%) ≥ 1‐3 months; 9 (16%) > 3‐6 months; 9 (16%) > 6‐12 months; 6 (11%) > 12 months Group receiving glucocorticoid injection Mean (SD) age = 57.3 (10.2) years; male:female = 28:25 Duration of symptoms: 7 (13%) less than 1 month; 21 (40%) ≥ 1‐3 months; 13 (24%) > 3‐6 months; 8 (15%) > 6‐12 months; 4 (8%) > 12 months Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Physiotherapy (N = 56) Components of intervention:
Dosage: 30 minutes Frequency of administration: twice a week for 6 weeks (12 sessions) Provider: physiotherapist Glucocorticoid injection (N = 53) Components of intervention: Intraarticular injections of 40 mg triamcinolone acetonide were given by general practitioners using the posterior route. Nearly all of the general practitioners had attended training in this technique before the study began, although most had had previous experience with the technique Dosage: 40 mg triamcinolone acetonide Frequency of administration: No more than 3 injections were given during the 6 weeks Provider: general practitioner |
|
| Outcomes | Outcomes assessed at weeks 3, 7, 13, 26 and 52 Primary outcomes
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence of the blocks was generated using random number tables" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Numbered, opaque, sealed envelopes containing the treatment allocation were prepared before the trial. After selection and baseline assessment an administrative assistant opened the next envelope in the appropriate stratum" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The independent observer did not know to which intervention a patient had been allocated. To optimise blinding the patient was instructed by the administrative assistant not to reveal any information about their treatment. In all patients the actual or potential injection site was covered with gauze. Immediately after each examination the observer was asked to guess to which intervention the patient had been assigned" Comment: Given the nature of the interventions, participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported "improvement" (i.e. global assessment of treatment success), pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The independent observer did not know to which intervention a patient had been allocated. To optimise blinding the patient was instructed by the administrative assistant not to reveal any information about their treatment. In all patients the actual or potential injection site was covered with gauze. Immediately after each examination the observer was asked to guess to which intervention the patient had been assigned" Quote: "The observer correctly guessed the allocated treatment for 65 (60%) out of 108 patients after 7 weeks and for 51 (48%) out of 105 after 26 weeks. The frequency of correct guesses was similar in both groups (30/52 (58%) for patients having injections and 35/56 (63%) for those having physiotherapy at 7 weeks)" Comment: Assessors of objective outcomes were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient withdrew from the study immediately after randomisation, refusing to have any injections. A total of six patients (5.5%) withdrew from the study, four of whom reported complete recovery before withdrawal. All patients who withdrew from the study were included in the statistical analysis until withdrawal" Quote: "At 3 and 13 weeks there is one missing value in each group. At 7 weeks there is one missing value in the injection group. At 26 weeks there are one missing value in the injection group and two in the physiotherapy group. At 52 weeks there are four missing values in the injection group and one in the physiotherapy group" Comment: 2 participants dropped out of the physiotherapy group and 4 participants dropped out of the control group. Reasons were balanced between groups, so the results are unlikely to be biased for these reasons |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Vermeulen 2006.
| Methods |
Design: parallel‐group, 2‐arm, single blind randomised controlled trial (The Netherlands) Interventions: intensive passive mobilisation techniques in end range positions of the glenohumeral joint (high‐grade mobilisation) or passive mobilisation techniques within the pain‐free zone (low‐grade mobilisation) Sample size calculation: 45 participants per group were estimated to be needed based upon detection of a difference of 18.6 degrees in active range of motion in abduction at the 5% level of statistical significance with 90% power, including a 15% rate of loss at follow‐up Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 100 (49 and 51 participants in each respective group) Baseline characteristics Group receiving high‐grade mobilisation Mean (SD) age = 51.6 (7.6) years; male:female = 17:32 Mean (range) duration of symptoms: 8 (5‐14.5) months Group receiving low‐grade mobilisation Mean (SD) age = 51.6 (8.6) years; male:female = 17:34 Mean (range) duration of symptoms: 8 (6‐14) months Inclusion criteria
Exclusion criteria
|
|
| Interventions |
High‐grade mobilisation (N = 49) Components of intervention: intensive passive mobilisation techniques in end range positions of the glenohumeral joint (high‐grade mobilisation). Mobilisation techniques were applied with intensities according to Maitland grades III and IV. The duration of prolonged stress on the shoulder capsule in the end range position varied according to the participant's tolerance Dosage: 30 minutes Frequency of administration: twice a week for 12 weeks (24 sessions) Provider: physiotherapist Low‐grade mobilisation (N = 51) Components of intervention: passive mobilisation techniques within the pain‐free zone (low‐grade mobilisation). Participants were explicitly informed that all techniques should be performed without causing pain in the shoulder. Mobilisation techniques commenced in the basic starting positions, with translation and distraction techniques performed with the joint near its neutral position (grade I). Reflex muscle activity was carefully monitored because it can be a first indication of joint pain. If joint mobility increased, mobilisation techniques were adjusted and the amplitude of movements was increased without reaching the limits of ROM (grade II). During the last 3 minutes of each treatment session, passive PNF patterns within the pain‐free zone in the supine position were applied. In addition, Codman's pendular exercises were performed for 2 minutes in a prone position to move the shoulder joint in more than 1 direction at a time and to obtain maximal relaxation of the shoulder muscles Dosage: 30 minutes Frequency of administration: twice a week for 12 weeks (24 sessions) Provider: physiotherapist |
|
| Outcomes | Outcomes assessed at the end of 3 months' treatment and at 6 months and 12 months (post randomisation) Primary outcome
Secondary outcomes
|
|
| Notes | Pain at rest was used as a measure of overall pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by a random‐number generator with permuted blocks of 4 and stratification for the presence of diabetes mellitus and for joint capacity as measured by arthrography (≤15 or >15 cm). The latter stratification was done because joint capacity may vary in people with adhesive capsulitis, and its potential influence on the recovery process remains unknown. After the baseline assessments were carried out, an administrative assistant assigned the subjects to the intervention groups according to the randomization scheme" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported on how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different types of mobilisation, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported several outcomes (e.g. pain, disability, general health status), but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All examinations were carried out by a masked assessor who is a trained physical therapist and manual therapist (HMV). Subjects were instructed not to reveal any details about the treatment or therapist to the assessor" Comment: Assessors of objective outcomes were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Thus, 100 subjects entered the study and were randomly assigned to either the HGMT group (n=49) or the LGMT group (n=51) (Figure), In both groups, 2 subjects withdrew from the trial in the first 3 months and were lost to follow‐up. One violation of the protocol occurred in the HGMT group after 3 weeks, as the subject did not want to be treated in either of the treatment groups anymore. She was further treated for 3 months by a physical therapist who was not involved in this study, but she did return for the follow‐up visits" Quote: "The outcome analysis was based on an intention‐to‐treat principle, and all subjects were included in the analysis. For subjects lost to follow‐up, all of the available data were used" Comment: Numbers of dropouts and losses to follow‐up were low, and the reasons for this are unlikely to have biased the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified. |
Wen 2009.
| Methods |
Design: parallel, 2‐arm randomised controlled trial (China) Interventions: functional exercises plus shoulder traction plus intermediate frequency electrical stimulation or shoulder traction plus intermediate frequency electrical stimulation Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 72 (36 per group) Baseline characteristics: Baseline characteristics were not reported by group Mean (range) age: 52 (44‐63) years Male:female = 19:53 Mean (range) duration of symptoms: 4 (2‐7) months Inclusion criterion
Exclusion criteria
|
|
| Interventions | Both groups received shoulder traction plus intermediate frequency electrical stimulation once a day for 15 days. Shoulder traction is translated as follows: Using a traction grid, the participant is in a sitting or lying position. A strap, attached to a rope pulley suspension and weight, is placed on the participant's wrist. The angle and weight are selected appropriately according to the degree of pain and functional limitation. The participant performs shoulder flexion, abduction, extension, adduction, etc, at different traction angles. Each angle is continued for 20‐30 minutes, with a rest period in the middle. An appropriate session has no more than 2 body positions. If the weight of the suspension does not cause obvious pain to the participant, the traction angle and weight are gradually increased according to the participant's improvement progress, to the point where recovery is that of normal range of motion. Intermediate‐frequency electrical stimulation is translated as follows: Using the J48A model computer with diathermy instrument, the electrode plate is placed on the shoulder at the prominent pain site, with a frequency of 4‐5 KHz, modulating the sine and square waveform, and is adjusted to the participant's tolerance and at a comfortable heat. Participants received 1 session per day (lasting 20 minutes) for 15 days Functional exercises plus shoulder traction plus intermediate‐frequency electrical stimulation (N = 36) Components of intervention: supervised functional exercises. Different exercises were performed depending on the duration of the participant's symptoms. If duration of symptoms was 1‐2 months, the following exercises were performed: hand exercises, Codman's hanging and swinging movement, flexion in front of the body at 90 degrees, hanging the limb and swinging it backward and forward, inside and out and in circles and wall climbing the 'ladder' exercises. If duration of symptoms was 2‐3 months, the following exercises were performed: passive range of motion exercises, active exercises with gym equipment, active exercises with a wooden stick and shoulder wheel exercises. If duration of symptoms was 3‐6 months, pulley exercises were performed Dosage: not reported Frequency of administration: once a day for 15 days (15 sessions) Provider: physical therapist Shoulder traction plus intermediate‐frequency electrical stimulation (N = 36) See above |
|
| Outcomes | Outcomes assessed at the end of 15 days' treatment. No primary outcome was reported by the trialists
|
|
| Notes | This article was written in Chinese and was translated by MC. As the direction of the scale was unclear for all outcomes, and no information about how range of motion was assessed on a 25‐point scale was reported, no outcome data were extracted from this trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: [Translated] "Patients were randomly divided into treatment and control group, with each group having 36 patients" Comment: No information was reported about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported about how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (the same multi‐component physical therapy intervention with or without additional exercises), participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported pain and function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported about whether assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No dropouts, losses to follow‐up or exclusions were reported, and the analyses are reported as based on the number of randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Yan 2005.
| Methods |
Design: parallel‐group, 2‐arm randomised controlled trial (China) Interventions: dumbbell gymnastics training exercises or bare‐handed exercises Sample size calculation: not reported Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 54 (26 and 28 in each respective group) Baseline characteristics Group receiving dumbbell gymnastics training exercises Mean (SD) age = 56.6 (12.4) years; male:female = 23:3 Mean duration of symptoms: 6.8 years Group receiving bare‐handed exercises Mean (SD) age = 54.2 (11.6) years; male:female = 20:8 Mean duration of symptoms: 5.8 years Inclusion criterion
Exclusion criterion
|
|
| Interventions |
Dumbbell gymnastics training exercises (N = 26) Components of intervention: Gymnastics training exercises with dumbbells weighing about 2‐5 kg, corresponding to the participant's circumstances. In a preparatory standing position with both hands holding dumbbells down on the side of the body, the following specific exercises were performed: (1) elbow facing forward: hands facing forward and bending the elbows 10 times; (2) abduction‐adduction: both shoulders in abduction, hands facing inwards, bending the elbow towards the chest 10 times; (3) swinging the arm back and forth: both arms holding the dumbbells swinging the arm back and forth 10 times or bending hips forward 90°, hanging the dumbbells down, with both arms abducted 90°, adducting and bending the elbows towards the chest 10 times; (4) lifting the dumbbells up: standing position, with both arms lifting up 10 times or both arms alternatively crossing each other 10 times; (5) extending and flexing forwards: both arms straight and extending forward, with hands facing inwards, extending and flexing the shoulder and elbow joints 10 times; (6) abduction, pushing and lifting: standing position, with both hands holding the dumbbells, both arms abducted 90°, hands facing forward, extending and flexing the elbow joint 10 times; (7) extending shoulder backwards: bending forward, with both hands holding the dumbbells, swinging both arms backward and back to the ankle area 10 times; (8) abduction of the elbows: both hands holding the dumbbells, with face left, right arm straight and extending both arms 90°, hands facing upwards, bending the elbow 10 times; (9) extending and flexing the elbows forwards: both hands holding the dumbbells, facing forward extending and straightening both arms 90°, with hands facing upwards, bending the elbows 10 times; (10) integrated movements: both hands holding the dumbbells, pushing forward horizontally, moving back to the shoulder, elbow, and then moving left/right, with both sides extending straight both arms, moving back and lifting up both arms, repeating 10 times Dosage: 5‐10 minutes Frequency of administration: 2‐3 times a day for 3 months (84 sessions) Provider: physical therapist Bare‐handed exercises (N = 28) Components of intervention: Specific exercises included (1) swinging back and forth: standing position, with both arms hanging down the side of the body, swinging both arms back and forth 10 times; (2) rotation method: standing position, with both arms moving in a clockwise direction and rotating the shoulder joints 10 times, then rotating the shoulder joint in an anticlockwise direction 10 times; (3) facing and climbing the wall method: standing position, facing the wall, affected limb straightened and extended, with fingers touching the wall and moving up along the wall 10 times; (4) climbing the wall on the side method: standing position, with the affected arm facing the wall, abduction upwards, the fingers touching the wall, during training, the body needs to be straight, not curved; (5) extending and touching the shoulder: affected limb extending to the back, rotating the elbow inwards, with backs of the fingers touching the opposite side of the shoulder area and using the healthy hand supporting the affected wrist and touching upwards 10 times; (6) flexing, bending and touching the shoulder: participants with limited adduction, using the affected hand, bending the elbow forward and touching the healthy, opposite side shoulder 10 times Dosage: 5‐10 minutes Frequency of administration: 2‐3 times a day for 3 months (84 sessions) Provider: physical therapist |
|
| Outcomes | Outcomes assessed at the end of 3 months' treatment. No primary outcome was reported by the trialists
|
|
| Notes | To analyse the "treatment success" outcome, we dichotomised participants into those who had a clinical improvement rating of "Excellent" and those who had a rating of "Good," "Acceptable" or "Bad" Trialists reported that 35 participants had a left‐affected shoulder, 16 had a right‐affected shoulder and 3 had bilateral periarthritis. However, the group to which bilaterally affected participants were allocated was not reported, nor was mention made of controlling for the correlation between shoulders. We analysed data on the basis of number of participants, not number of shoulders, to produce conservative estimates of effect |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: [Translated] "Randomised controlled observations of frozen shoulder patients" Comment: No information was reported about how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported about how the allocation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received different types of exercises, but it is unclear whether they were provided any information that would make them perceive the type of exercises they received as superior or inferior to the alternative types of exercise |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported pain, but it is unclear whether they were provided any information that would make them perceive the type of exercises they received as superior or inferior to the alternative type of exercises |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: No information was reported about whether assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: [Translated] "According to intention‐to‐treat analysis, 54 patients with frozen shoulder all were analysed, there were no patients drop‐out" Comment: No dropouts, losses to follow‐up or exclusions were reported, and outcome data were reported as analysed on the basis of the number of randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcome data were fully reported for all outcomes reported in the methods section of the publication, but without a trial protocol, it is unclear whether other outcomes were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Yang 2007.
| Methods |
Design: multiple‐treatment, 2‐group, 3‐arm randomised controlled trial (Taiwan) Interventions: end range mobilisation or midrange mobilisation or mobilisation with movement Sample size calculation: 15 participants per group were estimated to be needed based upon detection of a difference of 5 degrees of ROM at the 5% level of statistical significance with 80% power Analysis: per‐protocol analysed data reported (but intention‐to‐treat analysis was also performed, and results were similar to those of the per‐protocol analysis) Source of funding: National Science Council, Taiwan (non‐industry) |
|
| Participants |
Number of participants: 30 (15 per group) Baseline characteristics Group receiving midrange mobilisation, then end range mobilisation, then midrange mobilisation, then mobilisation with movement Mean (SD) age = 53.3 (6.5) years; male:female = 1:13 Mean (SD) duration of symptoms: 18.8 (8) weeks Group receiving midrange mobilisation, then mobilisation with movement, then midrange mobilisation, then end range mobilisation Mean (SD) age = 58 (10.1) years; male:female = 3:11 Mean (SD) duration of symptoms: 22 (10) weeks Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Midrange mobilisation, then end range mobilisation, then midrange mobilisation, then mobilisation with movement (N = 15) Components of intervention
Dosage: 30 minutes Frequency of administration: Each treatment phase lasted twice a week for 3 weeks Provider: physical therapist Midrange mobilisation, then mobilisation with movement, then midrange mobilisation, then end range mobilisation (N = 15) See above (the only difference is the order in which each phase of mobilisation was provided) |
|
| Outcomes | Outcomes assessed at 3, 6, 9 and 12 weeks (i.e. end of each 3‐week treatment phase): No primary outcome was reported by the trialists
|
|
| Notes | Unpublished outcome data provided by trialist on request Trial was registered at ClinicalTrials.gov (http://clinicaltrials.gov/show/NCT00172601) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Consenting subjects were randomly assigned by computer‐generated permuted block randomization of 5 by sequentially numbered, sealed, opaque envelopes to receive different mobilization treatments. In group 1, an A‐B‐A‐C (A=MRM,B=ERM, and C=MWM) multiple treatment design was used. In group 2, an A‐C‐A‐B multiple‐treatment design was used. The 2 groups used here were intended to counterbalance the order effects of treatments" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Consenting subjects were randomly assigned by computer‐generated permuted block randomization of 5 by sequentially numbered, sealed, opaque envelopes to receive different mobilization treatments. In group 1, an A‐B‐A‐C (A=MRM,B=ERM, and C=MWM) multiple treatment design was used. In group 2, an A‐C‐A‐B multiple‐treatment design was used. The 2 groups used here were intended to counterbalance the order effects of treatments" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participants received slightly different types of mobilisation, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | Unclear risk | Comment: Participants self‐reported function, but it is unclear whether they were provided any information that would make them perceive the type of mobilisation they received as superior or inferior to the alternative type of mobilisation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "To minimize bias, an independent trained outcome assessor, masked to treatment allocation, evaluated the participants at baseline and at 3‐week intervals for 12 weeks" Comment: Assessors of shoulder complex kinematics were probably blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Thirty subjects were recruited and randomly assigned to 2 groups (Tab. 1). Two subjects failed to attend the treatment. In addition, 3 subjects in the A‐B‐A‐C group were lost to follow‐up because there was no improvement during MRM treatment at 9 weeks. In the A‐C‐A‐B group, 2 subjects were lost to follow‐up because there was no improvement during MRM treatments at 3 weeks and 9 weeks" Quote: "For the analysis, dropout data were excluded. Additionally, intention‐to‐treat analysis was performed by including the dropout data (carrying the last data point forward into analysis)" Quote: "Similar results were found between exclusion of dropout data and intention‐to‐treat analysis (inclusion of dropout data)" Quote: "No benefit was shown during MRM treatment, but different missing data due to subjects dropping out due to lack of improvement at 3 and 9 weeks between the 2 groups makes interpretation difficult. We addressed this by secondary analysis (i.e. analysis of dropping out between 2 groups and survival analysis). There were no differences in numbers of subjects dropping out and no significant differences in the survival experiences of the 2 groups. These findings suggest that the multiple treatment trial on our 2 groups was balanced. It may be, however, that subjects continued in the treatment for reasons other than treatment effectiveness" Comment: The numbers of dropouts (and reasons for this) were similar between groups, and results of intention‐to‐treat and per‐protocol analysis were similar |
| Selective reporting (reporting bias) | High risk | Comment: The following outcomes were specified in the trial registry entry but were not reported in the publication: Disabilities of the Arm, Shoulder and Hand (DASH), disability assessment by self‐reports, pain perception and SF‐36 health survey |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Yang 2012.
| Methods |
Design: parallel‐group, 2‐arm, single blind randomised controlled trial (Taiwan) Interventions: end range mobilisation and scapular mobilisation plus standardised physiotherapy (passive midrange mobilisation, flexion and abduction stretching techniques, physical modalities, i.e. ultrasound, short‐wave diathermy and/or electrotherapy and active exercises) or standardised physiotherapy alone Sample size calculation: 10 participants per group were estimated to be needed based upon detection of a clinically relevant difference of 5 degrees range of motion or a score of 4 on the Flexilevel Scale of Shoulder Function (FLEX‐SF) at the 5% level of statistical significance with 80% power Analysis: intention‐to‐treat analysis Source of funding: not reported |
|
| Participants |
Number of participants: 23 (11 and 12 participants in each respective group) Baseline characteristics Group receiving end range mobilisation and scapular mobilisation plus standardised physiotherapy Mean (SD) age = 56.8 (7.2) years; male:female = 3:7 Mean (SD) duration of symptoms: 19.6 (12.8) weeks Group receiving standardised physiotherapy Mean (SD) age = 54.9 (10.3) years; male:female = 2:10 Mean (SD) duration of symptoms: 22.4 (9.2) weeks Inclusion criteria
Exclusion criteria
|
|
| Interventions | Both groups received standardised physiotherapy comprising passive midrange mobilisation, flexion and abduction stretching techniques, physical modalities (i.e. ultrasound, short‐wave diathermy and/or electrotherapy) and active exercises, twice a week for 8 weeks. End range mobilisation and scapular mobilisation plus standardised physiotherapy (N = 11) Components of intervention
Dosage: not reported Frequency of administration: twice a week for 8 weeks (16 sessions) Provider: physiotherapist Standardised physiotherapy (N = 12) See above |
|
| Outcomes | Outcomes assessed at weeks 4 and 8 (end of treatment). No primary outcome was reported by the trialists
|
|
| Notes | Another group of control participants was included in this study, but as they were not randomly assigned to any intervention, their data were not included in the review Unpublished outcome data were provided by trialist on request |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects who had less shoulder kinematics in at least one of the 3 criteria were randomized by computer generated permuted block randomizations of 5 by sequentially numbered, sealed, opaque envelopes to 2 groups: the criteria‐control group and criteria‐intervention group" Comment: An adequate method was used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects who had less shoulder kinematics in at least one of the 3 criteria were randomized by computer generated permuted block randomizations of 5 by sequentially numbered, sealed, opaque envelopes to 2 groups: the criteria‐control group and criteria‐intervention group" Comment: An adequate method was used to conceal the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Because of our treatment procedures, the patient was not blinded to the intervention" Comment: Given the nature of the interventions (the same multi‐component physical therapy intervention with or without additional mobilisation), participants were not blind to treatment and may have had different expectations about the benefits of each intervention |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Comment: Unblinded participants, who may have had different expectations about the benefits of the intervention they received, self‐reported function |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "To minimize bias, an independent trained outcome assessor, blinded to treatment allocation, evaluated the participants at baseline and at 4‐week intervals for 8 weeks" Comment: Assessors of range of motion were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention‐to‐treat analysis was performed by including the drop‐out data, carrying the last data point forward into analysis" Quote: "In the criteria‐intervention group, one subject was lost to follow‐up due to personal factors" Quote: "Additionally, similar results were found between exclusion of dropout and intention‐to‐treat analysis (inclusion of drop‐out data)" Comment: Only 1 participant (in the intervention group) was lost to follow‐up, for personal reasons. This is unlikely to bias the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trialists presented means and unlabelled error bars for all outcomes in figure format and reported numerical mean differences and 95% CIs in text only for outcomes and times points that were statistically significant. Means and standard deviations for all outcomes at all time points were provided on request. However, without a trial protocol, it is unclear whether other outcomes (e.g. pain) were measured but not reported based on the results |
| Other bias | Low risk | Comment: No other sources of bias were identified |
Abbreviations:
CI: confidence interval.
FLEX‐SF: Flexi‐Level Scale of Shoulder Function.
MRI: magnetic resonance imaging.
NSAIDs: non‐steroidal anti‐inflammatory drugs.
PNF: proprioceptive neuromuscular facilitation.
RCT: randomised controlled trial.
ROM: range of motion.
SD: standard deviation.
SF‐36: Short Form‐36.
SPADI: Shoulder Pain and Disability Index.
TENS: transcutaneous electrical nerve stimulation.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelshafi 2011 | All 3 groups received the same manual therapy and exercise intervention with or without continuous suprascapular nerve block under ultrasound guidance or intra‐articular steroid injection |
| Alicicco 2000 | Mobilisation was provided to all groups (with or without an electrotherapy modality) |
| Amir‐us‐Saqlain 2007 | Both groups received the same manual therapy intervention (manipulation) with or without steroid injection under anaesthesia |
| Arslan 2001 | Ineligible intervention: randomised controlled trial of glucocorticoid injection versus physical therapy plus non‐steroidal anti‐inflammatory drug. Not able to separate out the effects of physical therapy. Included in Cochrane review of corticosteroid injection for shoulder disorders |
| Bumin 2001 | Exercises were provided to all groups (with or without an electrotherapy modality) |
| Calis 2006 | Stretching and Codman exercises were provided to all groups (along with an electrotherapy modality) |
| Diercks 2004 | Not a randomised controlled trial |
| Khan 2005 | Participants received physical therapies alone versus physical therapies and shoulder arthrography with intra‐articular steroid |
| Kivimaki 2007 | The same manual therapy intervention was delivered to both treatment groups |
| Koh 2013 | Manual therapy and transcutaneous electrical nerve stimulation (TENS) were provided to all groups (with or without bee venom acupuncture) |
| Leclaire 1991 | Manual stretching and pulley exercises were provided to all groups (with or without an electrotherapy modality) |
| Lee 1973 | Exercises were provided to all groups (with or without an electrotherapy modality) |
| Leung 2008 | Stretching exercises were provided to all groups (with or without an electrotherapy modality) |
| Longbottom 2009 | Not a randomised controlled trial |
| Ma 2013 | Passive joint mobilisation was provided to all groups (with or without cryotherapy) |
| Rizk 1991 | The same manual therapy intervention was delivered to all 4 treatment groups |
| Srour 2008 | Conventional physiotherapy comprising passive mobilisation, specific mobilisations, stretches and muscle tension raises was provided to all groups; this RCT primarily tested the effect of cold pack versus cold air ventilation versus no cold intervention |
| Zhu 2004 | Ineligible intervention: trial comparing exercises plus Chinese medicine iontophoresis versus pain block therapy. A manual therapist/physical therapist/physiotherapist would be unable to deliver the Chinese medicine components |
Characteristics of studies awaiting assessment [ordered by study ID]
Doner 2013.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes |
Fink 2012.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Requires translation (in German) |
Ibrahim 2013.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes |
Russell 2014.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes |
Uddin 2012.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Currently available only as a conference abstract |
Wies 2003.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Currently available only as a conference abstract |
Characteristics of ongoing studies [ordered by study ID]
NCT00873158.
| Trial name or title | Outcomes Following Dynamic Splinting and/or Physical Therapy for Patients With Adhesive Capsulitis |
| Methods | Parallel‐group, 2‐arm randomised controlled trial (United States) |
| Participants |
Inclusion criteria
|
| Interventions |
Dynasplint Along with standard manual physical therapy, participants will use a stretching device (Dynasplint) in rehabilitation to regain ROM in stiff joints. Participants will use this device 20 to 30 minutes 2 times per day at home. Dynamic splinting utilises the protocols of low‐load prolonged stretch (LLPS) with calibrated adjustable tension to increase total end range time (TERT) to reduce contracture. The Dynasplint or "experimental" group will add this therapy to its standard of care regimen Physical therapy Participants in the physical therapy group will receive standard manual treatments during their usual physical therapy visits with no additional intervention |
| Outcomes |
Primary outcome measures Number of physical therapy treatments required Weeks of Dynasplint treatment Secondary outcome measures Shoulder range of motion Sharp FAS neck and shoulder Disabilities of Arm, Hand and Shoulder Questionnaire |
| Starting date | January 2006 |
| Contact information | Paul Gaspar, DPT, gasparPT@hotmail.com |
| Notes | http://clinicaltrials.gov/show/NCT00873158 |
NCT01249040.
| Trial name or title | The Study of Different Treatment Programs for Patients With Frozen Shoulder |
| Methods | Parallel‐group, 2‐arm randomised controlled trial (Taiwan) |
| Participants |
Inclusion criteria
Exclusion criterion
|
| Interventions | Group 1: Physical modalities + therapeutic exercise Group 2: Physical modalities + therapeutic exercise + intra‐articular injection of steroid Group 3: Therapeutic exercise |
| Outcomes |
Primary outcome measures Pain scale and range of motion within 6 weeks |
| Starting date | December 2010 |
| Contact information | Sui‐Foon Lo, MD, d4659@www.cmuh.org.tw |
| Notes | http://clinicaltrials.gov/show/NCT01249040 |
NCT01483963.
| Trial name or title | AA4500 for the Treatment of Adhesive Capsulitis of the Shoulder |
| Methods | Parallel group, 2‐arm randomised controlled trial (United States) |
| Participants |
Inclusion criteria
|
| Interventions | Group 1: Collagenase Clostridium histolyticum(AA4500) Group 2: Shoulder exercises |
| Outcomes |
Primary outcome measure Change (degrees) from baseline in active forward flexion in affected shoulder Secondary outcome measures Change (degrees) from baseline in passive forward flexion in affected shoulder Change (degrees) from baseline in abduction (active and passive) Change (degrees) from baseline in external rotation at 90° abduction (active and passive) Change from baseline in internal rotation with the elbow at 90° abduction (active and passive) |
| Starting date | November 2011 |
| Contact information | Diane McCaul, dmccaul@auxilium.com |
| Notes | http://clinicaltrials.gov/show/NCT01483963 |
Differences between protocol and review
The original review outcomes were pain, range of motion (active and passive), function/disability and quality of life, strength, return to work, participants' perception of overall effect, global preference, physicians' preference and adverse effects. The outcomes reported in this review have been modified from those of the original review to make them as consistent as possible with other Cochrane reviews on shoulder disorders and other chronic pain conditions. To improve succinctness of the review, we included only one measurement instrument per outcome domain. We assessed study risk of bias using the 'Risk of bias' tool of The Cochrane Collaboration in this update of the review. We have included a 'Summary of findings' table and an ORBIT outcome matrix.
Contributions of authors
MJP was responsible for writing the review, performing the searches, selecting trials, performing risk of bias assessment, extracting data, analysing data and interpreting the results of the updated review. SG was responsible for performing the searches, selecting trials and performing data extraction and quality assessment for the original review, defining the review comparisons and outcomes of interest of the original and updated reviews, analysing and interpreting the results and contributing to writing both original and updated reviews. SK was responsible for performing risk of bias assessment, extracting data and contributing to writing the manuscript for the updated review. RJ was responsible for performing risk of bias assessment, extracting data and contributing to writing the manuscript for the updated review. BM was responsible for selecting trials and contributing to writing the manuscript for the updated review. MC was responsible for performing risk of bias assessment, extracting data from three included trials written in Chinese and contributing to writing the manuscript for the updated review. RB was responsible for performing data extraction and quality assessment for the original review, defining the review comparisons and outcomes of interest for both original and updated reviews, analysing and interpreting the results and contributing to writing both original and updated reviews.
Sources of support
Internal sources
Australasian Cochrane Centre, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Monash Department of Clinical Epidemiology at Cabrini Hospital, Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
External sources
No sources of support supplied
Declarations of interest
SG and RB are authors of one of the trials included in this review (Buchbinder 2007). To avoid bias, this paper was sent to an independent review author for assessment of whether it met the inclusion criteria for this review. Neither review author was involved in data extraction or risk of bias assessment for this trial. RB is Joint Co‐ordinating Editor and RJ is Managing Editor of the Cochrane Musculoskeletal Group. To avoid bias, they excluded themselves from the editorial and publication processes for this review. SG and BMcB are practicing physiotherapists in part‐time private physiotherapy practice (self employed) and in this role receive remuneration for the delivery of physiotherapy interventions.
New
References
References to studies included in this review
Buchbinder 2007 {published data only}
- Buchbinder R, Youd JM, Green S, Stein A, Forbes A, Harris A, et al. Efficacy and cost‐effectiveness of physiotherapy following glenohumeral joint distension for adhesive capsulitis: a randomized trial. Arthritis and Rheumatism 2007;57:1027‐37. [DOI] [PubMed] [Google Scholar]
Bulgen 1984 {published data only}
- Bulgen DY, Binder AI, Hazleman Bl, Dutton J, Roberts S. Frozen shoulder: prospective clinical study with an evaluation of three treatment regimens. Annals of the Rheumatic Diseases 1984;43:353‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carette 2003 {published data only}
- Carette S, Moffet H, Tardif J, Bessette L, Morin F, Frémont P, et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo‐controlled trial. Arthritis and Rheumatism 2003;48:829‐38. [DOI] [PubMed] [Google Scholar]
Celik 2010 {published data only}
- Celik D. Comparison of the outcomes of two different exercise programs on frozen shoulder. Acta Orthopaedica et Traumatologica Turcica 2010;44:285‐92. [DOI] [PubMed] [Google Scholar]
Chan 2010 {published data only}
- Chan S, Hill R, Kerr J. Passive joint mobilisation: does it enhance outcome of adhesive capsulitis of the shoulder following corticosteroid injection?. International Musculoskeletal Medicine 2010;32:58‐67. [Google Scholar]
Chauhan 2011 {published data only}
- Chauhan V, Saxena S, Grover S. Effect of deep transverse friction massage and capsular stretching in idiopathic adhesive capsulitis. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:185‐8. [Google Scholar]
Cheing 2008 {published data only}
- Cheing GLY, So EML, Chao CYL. Effectiveness of electroacupuncture and interferential electrotherapy in the management of frozen shoulder. Journal of Rehabilitation Medicine 2008;40:166‐70. [DOI] [PubMed] [Google Scholar]
Dacre 1989 {published data only}
- Dacre JE, Beeney N, Scott DL. Injections and physiotherapy for the painful stiff shoulder. Annals of the Rheumatic Diseases 1989;48:322‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dundar 2009 {published data only}
- Dundar U, Toktas H, Cakir T, Evcik D, Kavuncu V. Continuous passive motion provides good pain control in patients with adhesive capsulitis. International Journal of Rehabilitation Research 2009;32:193‐8. [DOI] [PubMed] [Google Scholar]
Ghosh 2012 {published data only}
- Ghosh TK, Bera AK, Hossain ME, Sarkar PS. Comparison of results of three different methods of treatment for adhesive capsulitis of shoulder. Journal of the Indian Medical Association 2012;110(11):827‐8. [PubMed] [Google Scholar]
Guler‐Uysal 2004 {published data only}
- Guler‐Uysal F, Kozanoglu E. Comparison of the early response to two methods of rehabilitation in adhesive capsulitis. Swiss Medical Weekly 2004;134:353‐8. [DOI] [PubMed] [Google Scholar]
Harsimran 2011 {published data only}
- Harsimran K, Ranganath G, Ravi SR. Comparing effectiveness of antero‐posterior and postero‐anterior glides on shoulder range of motion in adhesive capsulitis—a pilot study. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:69‐72. [Google Scholar]
Johnson 2007 {published data only}
- Johnson AJ, Godges JJ, Zimmerman GJ, Ounanian LL. The effect of anterior versus posterior glide joint mobilization on external rotation range of motion in patients with shoulder adhesive capsulitis. Journal of Orthopaedic and Sports Physical Therapy 2007;37:88‐99. [DOI] [PubMed] [Google Scholar]
Ma 2006 {published data only}
- Ma T, Kao MJ, Lin IH, Chiu YL, Chien C, Ho TJ, et al. A study on the clinical effects of physical therapy and acupuncture to treat spontaneous frozen shoulder. The American Journal of Chinese Medicine 2006;34:759‐75. [DOI] [PubMed] [Google Scholar]
Maricar 1999 {published data only}
- Maricar NN, Chok B. A comparison of the effect of manual therapy with exercise therapy and exercise therapy alone for stiff shoulders. Physiotherapy Singapore 1999;2(3):99‐104. [Google Scholar]
Maryam 2012 {published data only}
- Maryam M, Zahra K, Adeleh B, Morteza Y. Comparison of corticosteroid injections, physiotherapy, and combination therapy in treatment of frozen shoulder. Pakistan Journal of Medical Sciences 2012;28:648‐51. [Google Scholar]
Nellutla 2009 {published data only}
- Nellutla M, Gin P. Comparative study between efficacy of PNF movement patterns versus conventional free exercises on functional activities among patients with chronic peri‐arthritis of shoulder. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:62‐7. [Google Scholar]
- Nellutla M, Giri P, M'Kumbuzi VRP, Patel HC. PNF movement patterns compared to the use of conventional free exercises to improve joint ROM in chronic peri‐arthritis of the shoulder. Indian Journal of Physiotherapy & Occupational Therapy 2009;3:31‐4. [Google Scholar]
Nicholson 1985 {published data only}
- Nicholson GG. The effects of passive joint mobilization on pain and hypomobility associated with adhesive capsulitis of the shoulder. Journal of Orthopaedic and Sports Physical Therapy 1985;6:238‐46. [DOI] [PubMed] [Google Scholar]
Pajareya 2004 {published data only}
- Pajareya K, Chadchavalpanichaya N, Painmanakit S, Kaidwan C, Puttaruksa P, Wongsaranuchit Y. Effectiveness of physical therapy for patients with adhesive capsulitis: a randomized controlled trial. Journal of the Medical Association of Thailand 2004;87:473‐80. [PubMed] [Google Scholar]
Rainbow 2008 {published data only}
- Rainbow DM, Weston JP, Brantingham JW, Globe G, Lee F. A prospective clinical trial comparing chiropractic manipulation and exercise therapy vs. chiropractic mobilization and exercise therapy for treatment of patients suffering from adhesive capsulitis/frozen shoulder. Journal of the American Chiropractic Association 2008;45:12‐28. [Google Scholar]
Ryans 2005 {published data only}
- Ryans I, Montgomery A, Galway R, Kernohan WG, McKane R. A randomized controlled trial of intra‐articular triamcinolone and/or physiotherapy in shoulder capsulitis. Rheumatology 2005;44:529‐35. [DOI] [PubMed] [Google Scholar]
Samnani 2004 {published data only}
- Samnani M. Passive exercises coupled with therapeutic activities—a comparative study in the management of frozen shoulder. Indian Journal of Occupational Therapy 2004;36:37‐40. [Google Scholar]
Sharad 2011 {published data only}
- Sharad KS. A Comparative study on the efficacy of end range mobilization techniques in treatment of adhesive capsulitis of shoulder. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:28‐31. [Google Scholar]
Shrivastava 2011 {published data only}
- Shrivastava A, Shyam AK, Sabnis S, Sancheti P. Randomised controlled study of Mulligan's vs. Maitland's mobilization technique in adhesive capsulitis of shoulder joint. Indian Journal of Physiotherapy & Occupational Therapy 2011;5:12‐5. [Google Scholar]
Sirajuddin 2010 {published data only}
- Sirajuddin M, Quddus N, Grover D. Comparison of anterior versus posterior glide mobilisation techniques for improving internal rotation range of motion in shoulder adhesive capsulitis. Indian Journal of Physiotherapy & Occupational Therapy 2010;4:152‐7. [Google Scholar]
Tanaka 2010 {published data only}
- Tanaka K, Saura R, Takahashi N, Hiura Y, Hashimoto R. Joint mobilization versus self‐exercises for limited glenohumeral joint mobility: randomized controlled study of management of rehabilitation. Clinical Rheumatology 2010;29:1439‐44. [DOI] [PubMed] [Google Scholar]
van der Windt 1998 {published data only}
- Windt D, Koes BW, Deville W, Boeke AJP, Jong BA, Bouter LM. Effectiveness of corticosteroid injections versus physiotherapy for treatment of painful stiff shoulder in primary care: randomised trial. British Medical Journal 1998;317:1292‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 2006 {published data only}
- Vermeulen HM, Rozing PM, Obermann WR, Cessie S, Vliet Vlieland TP. Comparison of high‐grade and low‐grade mobilization techniques in the management of adhesive capsulitis of the shoulder: randomized controlled trial. Physical Therapy 2006;86:355‐68. [PubMed] [Google Scholar]
- Hout WB, Vermeulen HM, Rozing PM, Vliet Vlieland TP. Impact of adhesive capsulitis and economic evaluation of high‐grade and low‐grade mobilisation techniques. Australian Journal of Physiotherapy 2005;51:141‐9. [DOI] [PubMed] [Google Scholar]
Wen 2009 {published data only}
- Wen L. Analysis on effect of functional exercises to promote rehabilitation of patients with periarthritis of shoulder. Chinese Nursing Research 2009;23:1925‐6. [Google Scholar]
Yan 2005 {published data only}
- Yan F. Comparison of dumbbell gymnastics and bare‐handed exercise in ameliorating the symptoms of shoulder periarthritis. Chinese Journal of Clinical Rehabilitation 2005;7:187‐9. [Google Scholar]
Yang 2007 {published data only}
- Yang JL, Chang CW, Chen SY, Wang SF, Lin JJ. Mobilization techniques in subjects with frozen shoulder syndrome: randomized multiple‐treatment trial. Physical Therapy 2007;87:1307‐15. [DOI] [PubMed] [Google Scholar]
Yang 2012 {published data only}
- Yang JL, Jan MH, Chang CW, Lin JJ. Effectiveness of the end‐range mobilization and scapular mobilization approach in a subgroup of subjects with frozen shoulder syndrome: a randomized control trial. Manual Therapy 2012;17:47‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelshafi 2011 {published data only}
- Abdelshafi ME, Yosry M, Elmulla AF, Al‐Shahawy EA, Adou Aly M, Eliewa EA. Relief of chronic shoulder pain: a comparative study of three approaches. Middle East Journal of Anesthesiology 2011;21:83‐92. [PubMed] [Google Scholar]
Alicicco 2000 {published data only}
- Alicicco E, Chinni LM, Castellano V, Aparo UL. Hyperthermia in scapulohumeral periarthritis due to overuse in athletes. Journal of Sports Sciences 2000;18:489. [Google Scholar]
Amir‐us‐Saqlain 2007 {published data only}
- Amir‐us‐Saqlain H, Zubairi A, Taufiq I. Functional outcome of frozen shoulder after manipulation under anaesthesia. Journal of Pakistan Medical Association 2007;57:181‐5. [PubMed] [Google Scholar]
Arslan 2001 {published data only}
- Arslan S, Celiker R. Comparison of the efficacy of local corticosteroid injections and physiotherapy for the treatment of adhesive capsulitis. Rheumatology International 2001;21:20‐3. [DOI] [PubMed] [Google Scholar]
Bumin 2001 {published data only}
- Bumin G, Can F. Effects of iontophoresis and phonophoresis methods on pain in cases with shoulder periarthritis. Pain Clinic 2001;13:159‐62. [Google Scholar]
Calis 2006 {published data only}
- Calis M, Demir H, Ulker S, Kirnap M, Duygulu F, Calis HT. Is intraarticular sodium hyaluronate injection an alternative treatment in patients with adhesive capsulitis?. Rheumatology International 2006;26:536‐40. [DOI] [PubMed] [Google Scholar]
Diercks 2004 {published data only}
- Diercks RL, Stevens M. Gentle thawing of the frozen shoulder: a prospective study of supervised neglect versus intensive physical therapy in seventy‐seven patients with frozen shoulder syndrome followed up for two years. Journal of Shoulder and Elbow Surgery 2004;13:499‐502. [DOI] [PubMed] [Google Scholar]
Khan 2005 {published data only}
- Khan AA, Mowla A, Shakoor MA, Rahman MR. Arthrographic distension of the shoulder joint in the management of frozen shoulder. Mymensingh Medical Journal 2005;14:67‐70. [PubMed] [Google Scholar]
Kivimaki 2007 {published data only}
- Kivimaki J, Pohjolainen T, Malmivaara A, Kannisto M, Guillaume J, Seitsalo S, et al. Manipulation under anesthesia with home exercises versus home exercises alone in the treatment of frozen shoulder: a randomized, controlled trial with 125 patients. Journal of Shoulder and Elbow Surgery 2007;16:722‐6. [DOI] [PubMed] [Google Scholar]
Koh 2013 {published data only}
- Koh PS, Seo BK, Cho NS, Park HS, Park DS, Baek YH. Clinical effectiveness of bee venom acupuncture and physiotherapy in the treatment of adhesive capsulitis: a randomized controlled trial. Journal of Shoulder and Elbow Surgery 2013;22(8):1053‐62. [DOI] [PubMed] [Google Scholar]
Leclaire 1991 {published data only}
- Leclaire R, Bourgouin J. Electromagnetic treatment of shoulder periarthritis: a randomized controlled trial of the efficiency and tolerance of magnetotherapy. Archives of Physical Medicine and Rehabilitation 1991;72:284‐7. [PubMed] [Google Scholar]
Lee 1973 {published data only}
- Lee M, Haq AM, Wright V, Longton EB. Periarthritis of the shoulder: a controlled trial of physiotherapy. Physiotherapy 1973;59:312‐5. [PubMed] [Google Scholar]
Leung 2008 {published data only}
- Leung MS, Cheing GL. Effects of deep and superficial heating in the management of frozen shoulder. Journal of Rehabilitation Medicine 2008;40:145‐50. [DOI] [PubMed] [Google Scholar]
Longbottom 2009 {published data only}
- Longbottom J, Green A. Effectiveness of single‐point acupuncture to Stomach 38 (Tiaokou) on pain and disability in subjects with frozen shoulder. Journal of the Acupuncture Association of Chartered Physiotherapists 2009:37‐46. [Google Scholar]
Ma 2013 {published data only}
- Ma S‐Y, Je HD, Jeong JH, Kim H‐Y, Kim H‐D. Effects of whole‐body cryotherapy in the management of adhesive capsulitis of the shoulder. Archives of Physical Medicine and Rehabilitation 2013;94(1):9‐16. [DOI] [PubMed] [Google Scholar]
Rizk 1991 {published data only}
- Rizk TE, Pinals RS, Talaiver AS. Corticosteroid injections in adhesive capsulitis: investigation of their value and site. Archives of Physical Medicine and Rehabilitation 1991;72:20‐2. [PubMed] [Google Scholar]
Srour 2008 {published data only}
- Srour F. Use of cold for the management of acute retractile capsulitis of the shoulder joint. Kinesitherapie Revue 2008, (83):29‐33. [Google Scholar]
Zhu 2004 {published data only}
- Zhu ZZ, Liu CM, Feng WX. Analgesic effects of traditional Chinese medicine iontophoresis and rehabilitation training on periarthritis of shoulder: a randomized controlled study. Chinese Journal of Clinical Rehabilitation 2004;8:2698‐9. [Google Scholar]
References to studies awaiting assessment
Doner 2013 {published data only}
- Doner G, Guven Z, Atalay A, Celiker R. Evalution of Mulligan's technique for adhesive capsulitis of the shoulder. Journal of Rehabilitation Medicine 2013;45(1):87‐91. [DOI] [PubMed] [Google Scholar]
Fink 2012 {published data only}
- Fink M, Schiller J, Buhck H. Efficacy of a manual treatment method according to the fascial distortion model in the management of contracted ("frozen") shoulder. Zeitschrift für Orthopädie und Unfallchirurgie 2012;150(4):420‐7. [DOI] [PubMed] [Google Scholar]
Ibrahim 2013 {published data only}
- Ibrahim M, Donatelli R, Hellman M, Echternach J. Efficacy of a static progressive stretch device as an adjunct to physical therapy in treating adhesive capsulitis of the shoulder: a prospective, randomised study. Physiotherapy 2013:pii:S0031‐9406(13)00085‐0. doi: 10.1016/j.physio.2013.08.006. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Russell 2014 {published data only}
- Russell S, Jariwala A, Conlon R, Selfe J, Richards J, Walton M. A blinded, randomized, controlled trial assessing conservative management strategies for frozen shoulder. Journal of Shoulder and Elbow Surgery 2014;23(4):500‐7. [DOI] [PubMed] [Google Scholar]
Uddin 2012 {published data only}
- Uddin T, Raihan H, Bashar MS, Rahman S. Effectiveness of supervised physiotherapy on patients with adhesive capsulitis. Journal of Rehabilitation Medicine 2012;44(Suppl 52):65‐66. [Google Scholar]
Wies 2003 {published data only}
- Wies J. A pilot randomised placebo controlled trial of osteopathic and physiotherapy treatment for frozen shoulder. Journal of Osteopathic Medicine 2003;6:40. [Google Scholar]
References to ongoing studies
NCT00873158 {published data only}
- http://clinicaltrials.gov/show/NCT00873158. Outcomes following dynamic splinting and/or physical therapy for patients with adhesive capsulitis.
NCT01249040 {published data only}
- http://clinicaltrials.gov/show/NCT01249040. The study of different treatment programs for patients with frozen shoulder.
NCT01483963 {published data only}
- http://clinicaltrials.gov/show/NCT01483963. A Phase 2a, open‐label, dose‐ranging study of the safety and effectiveness of AA4500 for the treatment of adhesive capsulitis of the shoulder.
Additional references
Ballantyne 1993
- Ballantyne BT, O'Hare SJ, Paschall JL, Pavia‐Smith MM, Pitz AM, Gillon JF, et al. Electromyographic activity of selected shoulder muscles in commonly used therapeutic exercises. Physical Therapy 1993;73:668‐77. [DOI] [PubMed] [Google Scholar]
Binder 1984
- Binder AI, Bulgen DY, Hazelman BL, Roberts S. Frozen shoulder: a long‐term prospective study. Annals of the Rheumatic Diseases 1984;43:361‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blanchard 2010
- Blanchard V, Barr S, Cerisola FL. The effectiveness of corticosteroid injections compared with physiotherapeutic interventions for adhesive capsulitis: a systematic review. Physiotherapy 2010;96:95‐107. [DOI] [PubMed] [Google Scholar]
Cates 2004 [Computer program]
- Dr Chris Cates EBM website. http://www.nntonline.net/. Visual Rx NNT Calculator. Dr Chris Cates EBM website. http://www.nntonline.net/, 2004.
Codman 1934
- Codman EA. The Shoulder. Boston: Thomas Toddog, 1934. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:www.cochrane‐handbook.org. [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Ebrahim 2013
- Ebrahim S, Akl EA, Mustafa RA, Sun X, Walter SD, Heels‐Ansdell D, et al. Addressing continuous data for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2013;66(9):1014‐21. [DOI] [PubMed] [Google Scholar]
Favejee 2011
- Favejee MM, Huisstede BMA, Koes BW. Frozen shoulder: the effectiveness of conservative and surgical interventions—systematic review. British Journal of Sports Medicine 2011;45:49‐56. [DOI] [PubMed] [Google Scholar]
Frank 1984
- Frank C, Akeson WH, Woo SL, Amiel D, Couts RD. Physiology and therapeutic value of passive joint motion. Clinical Orthopaedics and Related Research 1984;185:113‐25. [PubMed] [Google Scholar]
Hanchard 2011a
- Hanchard NCA, Goodchild L, Thompson J, O'Brien T, Davison D, Richardson C. A questionnaire survey of UK physiotherapists on the diagnosis and management of contracted (frozen) shoulder. Physiotherapy 2011;97:115‐25. [DOI] [PubMed] [Google Scholar]
Hanchard 2011b
- Hanchard N, Goodchild L, Thompson J, O’Brien T, Richardson C, Davison D, et al. Evidence‐based clinical guidelines for the diagnosis, assessment and physiotherapy management of contracted (frozen) shoulder, v. 1.7, ‘standard’ physiotherapy. Endorsed by the Chartered Society of Physiotherapy, www.csp.org.uk/skipp, 2011. [DOI] [PubMed] [Google Scholar]
Hand 2008
- Hand C, Clipsham K, Rees JL, Carr AJ. Long‐term outcome of frozen shoulder. Journal of Shoulder and Elbow Surgery 2008;17:231‐6. [DOI] [PubMed] [Google Scholar]
Hazelman 1972
- Hazleman BL. The painful stiff shoulder. Rheumatology and Physical Medicine 1972;11(8):413‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, www.cochrane‐handbook.org. [Google Scholar]
Kaltenborn 1976
- Kaltenborn FM. Manual Therapy for the Extremity Joints. Oslo, Norway: Olaf Norlis Bokhandel, 1976. [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Maitland 1977
- Maitland GD. Peripheral manipulation. London, United Kingdom: Butterworths, 1977. [Google Scholar]
Mangus 2002
- Mangus BC, Hoffman LA, Hoffman MA, Altenburger P. Basic principles of extremity joint mobilization using a Kaltenborn approach. Journal of Sport Rehabilitation 2002;11:235‐50. [Google Scholar]
Mao 1997
- Mao CY, Jaw WC, Cheng HC. Frozen shoulder: correlation between the response to physical therapy and follow‐up shoulder arthrography. Archives of Physical Medicine and Rehabilitation 1997;78:857‐9. [DOI] [PubMed] [Google Scholar]
Maund 2012
- Maund E, Craig D, Suekarran S, Neilson Ar, Wright K, Brealey S, et al. Management of frozen shoulder: a systematic review and cost‐effectiveness analysis. Health Technology Assessment 2012;16:1‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain—establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Neviaser 1945
- Neviaser JS. Adhesive capsulitis of the shoulder: a study of the pathological findings in periarthritis of the shoulder. Journal of Bone and Joint Surgery 1945;27:211‐22. [Google Scholar]
Norris 2012
- Norris SL, Holmer HK, Ogden LA, Fu R, Abou‐Setta AM, Viswanathan MS, et al. Selective outcome reporting as a source of bias in reviews of comparative effectiveness. (Prepared by the Oregon Evidence‐based Practice Center under Contract No. 290‐2007‐10057‐I.) AHRQ Publication No. 12‐EHC110‐EF. Rockville, MD: Agency for Healthcare Research and Quality, August 2012. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
Page 2013
- Page MJ, McKenzie JE, Forbes A. Many scenarios exist for selective inclusion and reporting of results in randomized trials and systematic reviews. Journal of Clinical Epidemiology 2013;66(5):524‐37. [DOI] [PubMed] [Google Scholar]
Reeves 1975
- Reeves B. The natural history of the frozen shoulder syndrome. Scandinavian Journal of Rheumatology 1975;4:193‐6. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schellingerhout 2008
- Schellingerhout JM, Verhagen AP, Thomas S, Koes BW. Lack of uniformity in diagnostic labeling of shoulder pain: time for a different approach. Manual Therapy 2008;13:478‐83. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:www.cochrane‐handbook.org. [Google Scholar]
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:www.cochrane‐handbook.org. [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:www.cochrane‐handbook.org. [Google Scholar]
Tekavec 2012
- Tekavec E, Joud A, Rittner R, Mikoczy Z, Nordander C, Petersson IF, et al. Population‐based consultation patterns in patients with shoulder pain diagnoses. BMC Musculoskeletal Disorders 2012;13:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Windt 1995
- Windt DA, Koes BW, Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Annals of Rheumatic Disease 1995;54:959‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Windt 1998b
- Windt DAWM, Heijden GJMG, Koes BW, Winter AF, Devillé W, Bouter LM. The responsiveness of the shoulder disability questionnaire. Annals of Rheumatic Disease 1998;57:82‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 2000
- Vermeulen HM, Obermann WR, Burger BJ, Kok GJ, Rozing PM, Ende CH. End‐range mobilization techniques in adhesive capsulitis of the shoulder joint: a multiple‐subject case report. Physical Therapy 2000;80:1204–13. [PubMed] [Google Scholar]
Walker 2004
- Walker‐Bone K, Palmer KT, Reading I, Coggon D, Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Care & Research 2004;51:642‐51. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Green 1998
- Green S, Buchbinder R, Glazier R, Forbes A. Systematic review of randomised controlled trials of interventions for painful shoulder: selection criteria, outcome assessment and efficacy. British Medical Journal 1998;316:354‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2003
- Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004258] [DOI] [PMC free article] [PubMed] [Google Scholar]


