Abstract
In the realm of cancer immunotherapy, a profound evolution has ushered in sophisticated strategies that encompass both traditional cancer vaccines and emerging viral vaccines. This comprehensive Review offers an in-depth exploration of the methodologies, clinical applications, success stories, and future prospects of these approaches. Traditional cancer vaccines have undergone significant advancements utilizing diverse modalities such as proteins, peptides, and dendritic cells. More recent innovations have focused on the physiological mechanisms enabling the human body to recognize and combat precancerous and malignant cells, introducing specific markers like peptide-based anticancer vaccines targeting tumor-associated antigens. Moreover, cancer viral vaccines, leveraging engineered viruses to stimulate immune responses against specific antigens, exhibit substantial promise in inducing robust and enduring immunity. Integration with complementary therapeutic methods, including monoclonal antibodies, adjuvants, and radiation therapy, has not only improved survival rates but also deepened our understanding of viral virulence. Recent strides in vaccine design, encompassing oncolytic viruses, virus-like particles, and viral vectors, mark the frontier of innovation. While these advances hold immense potential, critical challenges must be addressed, such as strategies for immune evasion, potential off-target effects, and the optimization of viral genomes. In the landscape of immunotherapy, noteworthy innovations take the spotlight from the use of immunomodulatory agents for the enhancement of innate and adaptive immune collaboration. The emergence of proteolysis-targeting chimeras (PROTACs) as precision tools for cancer therapy is particularly exciting. With a focus on various cancers, from melanoma to formidable solid tumors, this Review critically assesses types of cancer vaccines, mechanisms, barriers in vaccine therapy, vaccine efficacy, safety profiles, and immune-related adverse events, providing a nuanced perspective on the underlying mechanisms involving cytotoxic T cells, natural killer cells, and dendritic cells. The Review also underscores the transformative potential of cutting-edge technologies such as clinical studies, molecular sequencing, and artificial intelligence in advancing the field of cancer vaccines. These tools not only expedite progress but also emphasize the multidimensional and rapidly evolving nature of this research, affirming its profound significance in the broader context of cancer therapy.
Unraveling the Complexity of Cancer Treatment: A Comprehensive Overview of Modern Innovations and Emerging Technologies
Cancer, a word synonymous with uncertainty, suffering, and complexity, has emerged as a multifaceted challenge that transcends biological understanding and societal boundaries. As the leading cause of mortality worldwide, it prompts an immediate yet intricate response from the medical and scientific communities. The panorama of cancer is vast, encompassing various types and stages, each with unique characteristics and demanding individualized therapeutic strategies.1 In the last century, the quest to understand and treat cancer has led to an exponential growth in knowledge, unearthing the underlying mechanisms that govern tumor formation, progression, and resistance. The initial breakthroughs in cytotoxic medications, surgical interventions, and radiotherapy offered a glimpse of hope; however, the realization soon dawned that conventional methodologies alone were insufficient.2 Early detection has, without a doubt, revolutionized patient outcomes, significantly impacting survival rates in cancers such as breast, prostate, skin, and cervical. The advent of personalized medicine and systemic inhibition of cancer cell proliferation has brought about nuanced approaches, integrating genetics, biochemistry, and immunology.3,4 The evolution of our understanding of cancer as a disease originating from the “self” through various immunodetectable processes heralded a transformative era.3 Research delving into the ways tumors manipulate their local microenvironments through cytokine secretion, extracellular matrix remodeling, and cellular signaling opened new vistas for therapeutic interventions.4 The recognition of the symbiotic relationship between tumors and their environment has spawned a plethora of research dedicated to immunotherapeutic strategies with a particular focus on cancer vaccines. The intricate journey from early experimentation to contemporary applications has spanned over a century, reflecting the relentless human endeavor to harness the body’s immune system against malignancy.5−7 From the design of vaccines based on tumor antigen peptides to the development of viral vectors and dendritic cell therapies, the scientific landscape of cancer vaccines has flourished.8 However, clinical success has been uneven, with many promising strategies falling short in large-scale trials, highlighting the nuanced challenges inherent in translating scientific discovery into medical practice.9
An exciting development in this dynamic field is the advent of Proteolysis-Targeting Chimeras (PROTACs). By manipulation of the ubiquitin–proteasome system to induce targeted protein degradation, PROTACs have ushered in a new wave of possibilities in drug discovery and cancer treatment. Their potential to target previously “undruggable” proteins represents an innovative approach that expands the horizon of therapeutic options.10,11
This review aspires to provide an all-encompassing examination of the current frontiers of cancer therapy. From the historical roots to the latest breakthroughs, including the novel and promising field of PROTACs, we embark on a detailed exploration that navigates the complex interplay among scientific innovation, clinical application, and ethical considerations. In addressing these multifaceted dimensions, our inquiry extends beyond the conventional scope, offering insights into the strategies that have shaped the present and are poised to define the future of cancer therapy (Figure 1). The novelty of this review lies not only in the depth of analysis but also in the integration of diverse approaches, thereby contributing a unique perspective to a field that continues to evolve, inspire, and challenge our understanding of life and disease.
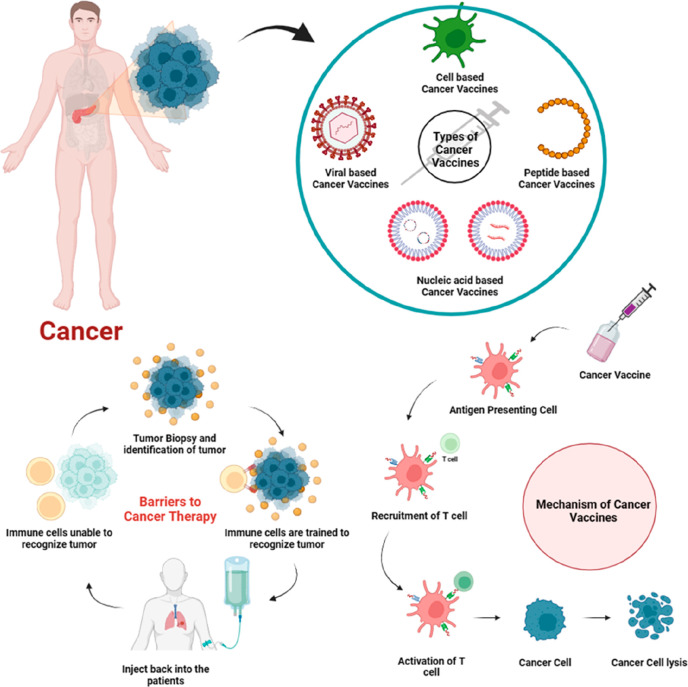
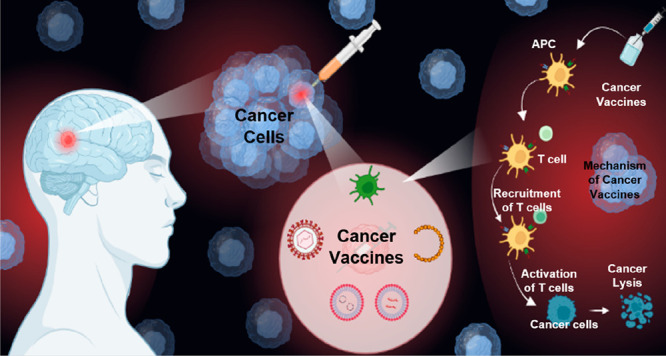
Figure 1.
Schematic representation of viral vaccines in cancer, their types, and mechanism.
Evolution of Vaccines in Cancer Therapy: Historical Perspectives, Modern Innovations, and Emerging Therapeutic Techniques
The art of vaccination has opened a plethora of opportunities in the prevention and treatment of infectious diseases, with its roots traced back to Edward Jenner’s groundbreaking discovery in 1796. Utilizing the cowpox vaccine, Jenner laid the foundation for immunization, offering protection against the dreadful smallpox infection.10 Over the years, the spectrum of vaccination has extended beyond infectious diseases to embrace the challenge of cancer.
The modern history of cancer vaccines is marked by milestones and significant contributions. In 1980, the inception of the first cancer vaccine developed from tumor cells and lysates heralded a new era in colorectal cancer treatment, using the patients’ own tumor cells.11 Extensive reviews provide insight into the evolution of cancer vaccines, focusing on the characteristics of the developed antigens such as whole tumors, tumor cells, proteins, peptides, RNA, or DNA. They also explore the adjuvants used for antigen introduction, such as carrier proteins, dendritic cells (DCs), the CD40 ligand (CD40L), and other biological or chemical substances.12
The late 19th century witnessed the pioneering work of German physician Paul Ehrlich and American surgeon William Bradley Coley, who first proposed cancer immunotherapy.13 Coley’s innovative approach, using streptococcal organisms and bacterial extracts known as “Coley’s toxins”, targeted a broader systemic immunity activation against the tumor. By the 1890s, this technique led to the specific induction of immune responses against distinct sarcoma antigens both therapeutically and preventatively. Several theories emerged from this method, revolving around incorrect antigen exposure and the influence of previous infections or inflammatory responses on cancer cells.
The late 1990s marked renewed enthusiasm for cancer vaccines, buoyed by conceptual advances like Polly Matzinger’s danger theory and the discovery of antigens preferentially expressed by malignant cells. Innovations such as the discovery of melanoma-associated antigen 1, the dendritic cell-based vaccine (Sipuleucel-T), and groundbreaking research in mRNA coding and plasmid DNA in the early 1990s brought excitement to the field.14−16 Today, chemotherapy, radiation, and biopsy stand as the three principal cancer treatment techniques targeting or eradicating cancer cells. Yet, the exploration of the human immune system in immunotherapies has not lagged. An increasing body of research emphasizes the intricate role of the tumor microenvironment (TME), consisting of cancer, stromal, and immune cells in interaction, leading to the reconsideration of cancer immunotherapies as the fourth therapeutic method.16−18 Vaccinations, as a form of cancer immunotherapy, have been employed both therapeutically and preventively. While still facing challenges, the development and testing of cancer vaccines have become long-term goals for many researchers.19 Immunotherapy has been incredibly successful in treating certain late-stage cancers, which previously had limited treatment options. For some patients, immunotherapy treatment allows for a longer-term remission, which can eventually be considered a cure. The outcomes can be unpredictable, and some patients may not respond to immunotherapy.20,21
The type of cancer plays a significant role. Some cancers are more responsive to immunotherapy than others. For example, immunotherapy has been particularly effective in certain types of skin cancer (melanoma), lung cancer, kidney cancer, and some types of lymphoma.22
Moreover, advancements such as the creation of antibodies against aberrant cell surface-associated mucin (MUC1) after mumps infection and the application of Bacillus Calmette-Guerin (BCG) in bladder cancer treatment have added to the repertoire of cancer vaccines.23,24 Preventive viral vaccines, like those against the human papillomavirus (HPV) and hepatitis B virus (HBV), offer a glimpse into the prevention of virus-induced cancers, although they remain limited in scope and global acceptance.25,26
In a nutshell, the landscape of cancer vaccines has evolved considerably over the years, revealing a tapestry of innovation, research, and clinical application. From early discoveries to cutting-edge therapeutic techniques, the ongoing advancements offer hope and insights for a future where vaccines may play a pivotal role in both the prevention and treatment of cancer. The integration of emerging technologies, such as PROTACs, and the novel methodologies reviewed here provide a roadmap for the continued evolution of cancer immunotherapy, emphasizing its significance in modern medicine.
PROTACs are a class of molecules designed for targeted protein degradation. They represent a revolutionary approach to drug development, particularly in the field of cancer therapy.
How PROTACs work:
-
1.
Recognition: PROTACs are engineered molecules designed to simultaneously bind to a specific protein of interest (the target) and an E3 ubiquitin ligase enzyme.
-
2.
Binding: Once bound to both the target protein and the E3 ligase, PROTACs create a proximity effect. This proximity allows the E3 ligase to recognize the target protein as a substrate for degradation.
-
3.
Ubiquitination: The E3 ligase adds ubiquitin molecules to the target protein. Ubiquitination is a natural cellular process that marks proteins for destruction.
-
4.
Degradation: The ubiquitinated target protein is then recognized by the cell’s proteasome, a cellular structure responsible for protein degradation. The proteasome breaks down the target protein into its constituent parts.
By harnessing this cellular machinery, PROTACs enable the specific and controlled degradation of disease-associated proteins, which can be advantageous in conditions like cancer. This approach offers several advantages over traditional drug inhibition, including the potential to target previously “undruggable” proteins and reduce the risk of drug resistance. As a result, PROTACs represent a promising avenue for the development of highly precise and effective therapies.27,28
Distinguishing between Viral Vaccines, Viruses Utilized for Gene Therapy, and Oncolytic Viruses: A Comprehensive Examination
A.1. Viral Vaccines: From Origins to Modern Applications
Traditional Vaccines
Live Attenuated Vaccines
Created by reducing the virulence of the pathogen but maintaining its viability, allowing for a robust immune response without disease manifestation. This strategy has been used in various vaccines including for measles and mumps.1,29
Inactivated Vaccines
Involve chemically treating the virus to eliminate infectivity while preserving antigenic structure and applied in vaccines like polio.2,30
Subunit and Conjugate Vaccines
Focus on utilizing specific viral components, coupled with adjuvants to enhance immunogenicity, exemplified in the Hepatitis B vaccine.3
Viral Vector-Based Vaccines
Adenoviral Vectors
These nonreplicating vectors are engineered to carry genes encoding specific antigens, resulting in transient expression in host cells.4
Lentiviral Vectors
Used for stable integration, allowing for sustained antigen expression. Used in various experimental vaccines.5
Challenges and Ethics
Addressing issues like potential recombination with wild-type viruses, host immune response to the vector, and societal acceptance.6
B.1. Gene Therapy Vectors: The Future of Precision Medicine
Choice of Vectors
Viral Vectors
Different viral vectors offer unique benefits and drawbacks, considering aspects like host range, integration, and immune response.7
Nonviral Vectors
Provide an alternative to viral vectors, often with reduced immunogenicity and increased flexibility in design.8
Gene Editing and Integration
CRISPR-Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats - CRISPR-Associated Protein 9)
CRISPR-Cas9 is an adaptive immune system in bacteria and archaea that has been harnessed for genome editing. It works by using a guide RNA to target specific DNA sequences and the Cas9 enzyme to introduce precise changes or modifications.31 It allows precise genome modification, holding the promise for treating genetic diseases, although ethical and safety concerns exist.32
TALENs (Transcription Activator-Like Effector Nucleases) and ZFNs (Zinc Finger Nucleases)
TALENs are artificial DNA-binding proteins fused with an endonuclease. They work by recognizing and binding to specific DNA sequences and then cleaving the DNA at the targeted site. ZFNs are engineered proteins that combine a zinc finger domain for DNA recognition with a nuclease for DNA cleavage. They work by binding to specific DNA sequences and inducing double-strand breaks in the DNA. They predate CRISPR, with applications in targeted gene addition and disruption.33,34
Regulation and Safety
Clinical Trials and Regulations
Ensuring patient safety and compliance with international laws and guidelines is paramount.35
C.1. Oncolytic Viruses: Targeted Cancer Therapy
Selectivity and Engineering
Virus Selection and Modification
Selection based on tumor selectivity and further genetic engineering to increase safety and efficacy.36
Enhanced Cytotoxicity and Immune Activation
Oncolytic viruses not only kill tumor cells but also stimulate antitumor immunity.37
Combination Therapies and Challenges
Combination with Other Therapies
Often used in conjunction with radiation or chemotherapy, leveraging synergistic effects.38
Challenges and Future Directions
Barriers like the immune response to the virus itself and the complexity of tumor microenvironments must be addressed.39
The diverse and intricate nature of viral vaccines, gene therapy vectors, and oncolytic viruses requires a comprehensive understanding spanning virology, genetics, immunology, oncology, bioethics, and regulatory affairs. The combined potential of these three domains represents the vanguard of contemporary medicine, with transformative implications for the future. The integration of interdisciplinary collaboration and technological innovation is vital to fully realize the promise within these realms, navigating challenges and crafting therapies that are effective, safe, and tailored to individual patient needs (Table 1). Further inquiry and development will not only continue to reshape our understanding of disease and treatment but also redefine paradigms of healthcare and patient care in the years to come.
Table 1. Viral Vectors and Cancer Immunotherapya.
| Viral Vector | Advantages | Disadvantages |
|---|---|---|
| Mammalian Poxviruses | • Easily manipulated in a laboratory setting | • Neutralizing antibodies develop with subsequent vaccinations; recipients of the vaccinia (smallpox) vaccine have pre-existing immunity to vector |
| • Vaccinia virus (VV) | • Accepts large gene inserts | • Replication-competent virus (VV), not appropriate for use in immunocompromised patients |
| • Modified virus Ankara (MVA) | • Naturally immunogenic | |
| • Cellular and humoral immune response to transgene | ||
| • Expresses transgenes in target cells, including DC | ||
| • No risk of insertional mutagenesis | ||
| • MVA strain is replication-incompetent | ||
| Avian Poxvirus | • Incomplete lifecycle in mammalian cells; no infectious viral particles can form | • Immune response is not as robust as the vaccinia virus |
| • Fowlpox | • Multiple vaccinations possible; no neutralizing antibodies develop | |
| • Canarypox (ALVAC) | ||
| Adenovirus (Ad) | • Easily manipulated in a laboratory setting | • Infection of target cells dependent on expression of an Ad receptor (e.g., CAR), which is not expressed on all cancer cells |
| • Cellular and humoral immune response to transgene | • Pre-existing host neutralizing antibodies to several Ad serotypes | |
| • High expression of transgene | • Limited capacity for gene inserts | |
| • Broad tropism, including DC | ||
| • No risk of insertion AL mutagenesis | ||
| • Many strains available | ||
| • Replication-deficient strains used, limiting pathogenicity | ||
| Alphavirus | • Naturally immunogenic | • Limited capacity for gene inserts |
| • High expression of transgene | • Limited duration of expression of transgene due to induction of apoptosis in an infected target cell | |
| • Replicon-competent vector | ||
| • No neutralizing antibodies develop against the nonpropagating vector | ||
| • Broad tropism | ||
| • Multiple vaccinations possible; no neutralizing antibodies develop | ||
| Measles Virus (MV) | • Specificity for tumor cells | • Contraindicated in severely immunocompromised patients |
| • Oncolytic virus | • Pre-existing immunity to MV | |
| • No risk of insertional mutagenesis | • Modest capacity for gene inserts | |
| • Vaccine strain nonpathogenic, noncontagious (nonpathogenic, does not cause a clinical syndrome associated with nonvaccine strain measles infection; noncontagious, no human-to-human transmission of MV) | • Viral transgene expression limited by lysis of a target cell | |
| Herpes Simplex Virus (HSV) | • Easily manipulated in a laboratory setting | • Neurotropsim of concern |
| • Broad tropism, including DC | • Viral transgene expression limited by lysis of a target cell | |
| • Oncolytic virus | ||
| • Large capacity for gene inserts | ||
| Vesicular Stomatitis Virus | • Broad tropism, including DC | • Neurotropsim of concern |
| • High efficacy of gene expression | • Modest capacity for gene inserts | |
| • No risk of insertional mutagenesis | • Viral transgene expression limited by lysis of a target cell | |
| • Vaccine strain nonpathogenic, noncontagious | ||
| • Oncolytic virus | ||
| • Enhances immune-mediated attack of tumor cells |
DC – Dendritic cells. CAR – Coxsackie and adenovirus receptor.
Types of Cancer Vaccines: A Comprehensive Overview
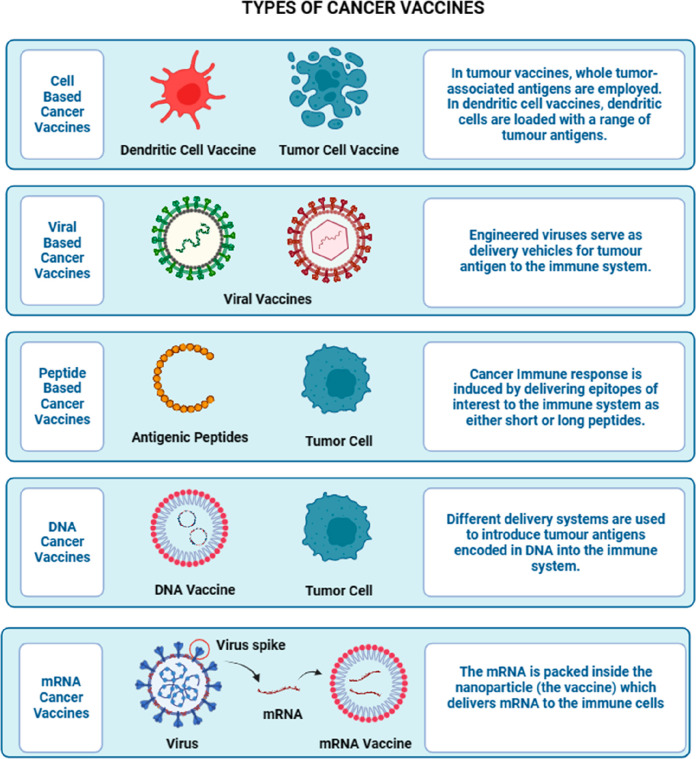
A.2. Cell-Based Vaccines
Cell-based cancer vaccines, often designed from entire cells or cell fragments mirroring tumor antigens, are devised to instigate a wide-ranging immune response. These vaccines predominantly utilize Dendritic Cell (DC) vaccines, known for their essential role. Dendritic cells are a specialized type of immune cell responsible for capturing, processing, and presenting antigens to other immune cells such as T cells. Their crucial role is to initiate and modulate immune responses, making them essential for the adaptive immune system’s functioning.40 Although clinical trials have revealed promising outcomes in combating tumors through personalized DC-based neoantigen vaccines, limitations in the scalability and cost-intensive nature of production persist.1 Examples include the Hepatitis B virus (HBV) and Human Papillomavirus (HPV) Vaccines.41
B.2. Viral-Based Vaccines
Viral-based cancer vaccines leverage the properties of viruses to stimulate the immune system and target cancer cells, holding promise as an innovative approach in cancer immunotherapy. Viral-based vaccines capitalize on the inherent immunogenicity of cancer antigens by utilizing genetically modified viruses, such as adenoviruses, to encode these antigens. Genetically modified viruses enhance the immune system’s recognition of cancer antigens by carrying these specific tumor proteins, prompting dendritic cells to present them to T cells. This activation leads to the cultivation of antitumor immunity, involving the targeting and destruction of cancer cells and the development of immunological memory for long-term defense.42 The process augments the immune system’s recognition of cancer antigens and cultivates antitumor immunity. Oncolytic viruses serve as essential vectors, with capabilities extending to tumor lysis and enhancing vaccination efficacy. However, the complexity in manufacturing vaccines with viral vectors remains a formidable challenge.2 Other examples are Herpes viruses, the Vesicular Stomatitis virus (VSV), and the Measles virus.43 These viruses are modified to be safe and effective carriers for cancer-specific antigens or to directly lyse cancer cells, thereby enhancing the immune system’s ability to recognize and combat cancer.
C.2. Peptide-Based Vaccines
Peptide-based subunit vaccines are a type of vaccine that employs short protein fragments, known as peptides, to stimulate an immune response against specific pathogens. These vaccines work by presenting these peptide fragments to the immune system, primarily T cells. Once exposed to the peptides, T cells recognize them as foreign or harmful, leading to the activation of immune responses, such as the production of antibodies and memory T cells. These immune responses help the body recognize and defend against a pathogen if it is encountered in the future. Peptide-based subunit vaccines are designed to be highly specific, safe, and well-tolerated, as they only contain the essential parts of the pathogen required to trigger an immune response.44 Peptide-based subunit vaccines comprise chemical and biosynthetic formulations of specific cancer antigens aimed at robustly stimulating immune responses. Employed prominently in the prevention and treatment of viral infections, these vaccines have proven their mettle in eliciting humoral immune reactions. Renowned vaccines against the Hepatitis B virus (HBV) and Human Papillomavirus (HPV) have employed this strategy. Vaccines for HBV and HPV have been remarkable success stories in cancer prevention. These vaccines have significantly reduced the incidence of liver cancer associated with HBV and have the potential to virtually eliminate certain HPV-related cancers such as cervical cancer in the long term. Furthermore, virus-like particle (VLP)-based subunit vaccines have recently demonstrated commendable anticancer efficacy. VLPs have shown great promise in cancer treatment by serving as a platform for developing cancer vaccines. VLP-based cancer vaccines can effectively stimulate the immune system to target cancer-specific antigens, offering a potentially innovative approach to combating various cancer types.3
D.2. Nucleic Acid-Based Vaccines
Nucleic acid vaccines represent an innovative approach to immunization by utilizing genetic material, such as DNA or RNA, to trigger an immune response against specific pathogens. These vaccines have gained attention for their flexibility, rapid development potential, and role in providing protection against infectious diseases including viral and bacterial infections. Their propensity to elicit strong CD8+ T cell responses and simultaneous delivery of multiple antigens has marked them as ideal platforms for cancer vaccines.4 Nucleic acid vaccines offer the benefit of eliciting robust CD8+ T cell responses, which are crucial for combating intracellular pathogens and providing long-lasting immunity.45 These vaccines are also lauded for their swift and straightforward production, making them suitable for tailored neo-antigen cancer vaccines.
E.2. Considerations in Vaccine Platform Selection
The choice of a vaccine platform necessitates a multifaceted analysis encompassing factors such as the preparation timeline, potential for tailored applications, and appropriateness for various metastatic conditions. Nucleic acid vaccines may offer a time-efficient solution, particularly for metastatic ailments.6 Different platforms may require distinct administration schedules, dosages, and potential interactions that must be carefully coordinated to optimize therapeutic outcomes and minimize adverse effects. Moreover, the integration of combination therapy, delivery methods, and the dosage regimen further complicates the selection process.
F.2. Antigen Optimization and Design
The intricacy of antigen design requires innovative approaches such as employing coupling binding vectors such as diphtheria toxoid and tetanus endotoxin to elevate immunogenicity. Enhancing immune reactions can be achieved through vaccines featuring VLPs or other antigens with refined protein structures.5 Innovative approaches for antigen design involve coupling binding vectors, such as antibodies or aptamers, with VLPs to create highly targeted and immunogenic vaccines. These strategies enable the presentation of specific antigens in a structured and repetitive manner, enhancing the immune system’s recognition and response, and have shown promise in the development of precision vaccines for various diseases.46 Cutting-edge bioinformatics and deep sequencing techniques are integral to guiding the vaccine creation process. This examination synergizes the latest optimization methodologies for four diverse cancer vaccines with particular emphasis on nucleic acid vaccines. The multifaceted landscape of cancer vaccines demands meticulous consideration of factors such as immunogenicity, manufacturing, customization, and ethical implications in research and application7−9 (Figure 2).
Figure 2.
Types of cancer vaccines.
Cell-Based Cancer Vaccines: A Comprehensive Insight
Immune Cell Vaccines: Dendritic Cells at the Helm
Dendritic cells (DCs), the professional antigen-presenting cells (APCs) of the immune system, play a pivotal role in the formation of immune cell vaccines. This innovative approach involves introducing tumor-associated antigens into DCs, thereby enabling them to activate T cells. The integration of monocyte-derived DCs (MoDCs) into this paradigm has led to novel methods, such as employing tumor cell lysate, which have shown encouraging tolerance and efficacy in immunotherapy trials.47
DCexos: The Emerging Frontier
DCexos, or inert membrane vesicles expressing costimulatory molecules like MHC I and MHC II, have emerged as an exciting development in DC-based vaccines.48 These biostable structures represent a new dimension in cancer treatment. Although their efficacy has been demonstrated in clinical studies, further research is needed to establish their definitive clinical benefit.49
Virus-Based Cancer Vaccines: A Multifaceted Approach
Virus-based vaccines are a class of vaccines that use a weakened or inactivated form of a virus to stimulate an immune response without causing the disease. These vaccines have been pivotal in preventing numerous viral infections, including polio, measles, and influenza, and they are a key tool in public health efforts worldwide.50
Classification Spectrum
The versatility of virus-based vaccines lies in their ability to foster a synergetic relationship between innate and adaptive immune systems. This results in powerful and lasting immune responses. The three categories are:
Oncolytic Viral Vaccines: Oncolytic viral vaccines are a cutting-edge approach to cancer treatment that harnesses genetically modified viruses, such as Talimogene laherparepvec (T-VEC), to selectively infect and destroy cancer cells while also stimulating the immune system to fight the tumor.51
Virus Vector Vaccines: Virus vector vaccines are a type of vaccine that uses harmless viruses, like the adenovirus or vesicular stomatitis virus, as delivery systems to transport genetic material encoding antigens into host cells, stimulating a protective immune response without causing disease. For example, the COVID-19 vaccines from AstraZeneca and Johnson & Johnson are based on adenovirus vector technology.52
Specialized Vaccines against Tumor-Causing Viruses: Vaccines against tumor-causing viruses are designed to prevent infections with viruses known to contribute to cancer development; a notable example is the HPV vaccine, which targets HPV strains associated with cervical and other cancers.53
Addressing Viral-Induced Cancers
With approximately 12% of cancer cases being linked to viral infections, the realm of virus-based vaccines is of significant interest. Notable viruses related to cancer include Epstein–Barr virus, Hepatitis B, and Hepatitis C.54 The success of vaccines against fully inactivated viruses such as Covid-19 and Ebola provides valuable insights into the treatment approach for virally induced cancers.55,56 Epstein–Barr virus (EBV) is associated with several types of cancer, particularly lymphomas and nasopharyngeal carcinoma. It does so by persisting in the body and causing genetic changes in infected cells, leading to uncontrolled cell growth.
Hepatitis B and C viruses are known to cause chronic liver infections, which over time can lead to liver cirrhosis and ultimately hepatocellular carcinoma (liver cancer). These viruses can directly damage liver cells, leading to inflammation, fibrosis, and an increased risk of genetic mutations that can result in cancerous growth. Preventive measures, including vaccinations for Hepatitis B and antiviral therapies for Hepatitis C, have significantly reduced the risk of virus-induced liver cancer.53,57
C. Oncolytic Viruses: Pioneering the Future
Oncolytic viruses, characterized by their specific targeting of cancer cells, have become a novel and promising treatment pathway. Their ability to stimulate the immune system via the release of reactive oxygen species (ROS) and cytokines, leading to oncolysis and release of tumor-associated antigens (TAAs), is particularly intriguing.58 ROS generated during viral replication can activate immune cells, while the viruses also induce the production of cytokines that further attract and activate immune responses, collectively enhancing the immune system’s ability to target and destroy cancer cells.59 Several clinical studies have showcased the efficacy of viruses such as herpes simplex, adenovirus, measles, vaccinia, and others in this context.60 An example of a clinical study involving oncolytic viruses is the use of T-VEC (Talimogene laherparepvec) in advanced melanoma.51 In clinical trials, T-VEC, an oncolytic herpes simplex virus, was injected directly into melanoma lesions.
D. Adenoviruses: A Versatile Tool
Adenoviruses stand out due to their broad host cell tropism, ability for swift mass production, and compatibility with various treatment modalities. Their role as nonreplicating vectors and oncolytic adenoviruses has been substantiated by their successful utilization in both preclinical and clinical trials.61 Adenoviruses share capabilities with vectors such as lentivirus and adeno-associated virus in expressing the transgene over an extended period, even in nondividing cells.62,63
The evolving landscape of cell- and virus-based cancer vaccines continues to pave the way for innovative and multifaceted therapeutic strategies. The synthesis of traditional approaches, modern technological advancements, and strategic application of immune mechanisms delineate a progressive path in oncology. Whether leveraging the potent capabilities of dendritic cells or harnessing the nuanced functionalities of various viral vectors, the field exemplifies a robust blend of creativity, scientific rigor, and the relentless pursuit of excellence. The journey from understanding the immune system’s complex orchestration to the application of customized solutions represents the future of personalized medicine in cancer therapy.
Peptide-Based Cancer Vaccines
The focus of cancer vaccines has shifted from entire, inactive, or attenuated viruses to subunit components. Peptide-based vaccines are composed of polypeptides made of known or expected cancer antigen epitopes.
Immunodominance
The immunogenicity of peptide-based vaccinations is adversely affected by the MHC polymorphism restriction and the small size of the antigen epitope itself. Strong immunological responses, which also lead to immune tolerance, are typically difficult to induce. Adjuvants are used in conjunction with peptide-based vaccines to enhance the immune response all around. Protein antigens differ in their ability to induce immune responses in B and T cells. In comparison to inactivated cancer cell vaccines, peptide-based vaccinations provide a more focused immune response against significant neutralization epitopes. This immunological benefit is known as immunodominance.64
CD8+ T cell Epitopes and CD4+ T Cell epitopes
For peptide-based cancer vaccines, CD8+ T and CD4+ T cell epitopes are typically required. While CD8+ T cell epitopes activate CTL tumor immunity via the antigen cross-presentation pathway, CD4+ T cells stimulate helper T cells to maintain the effectiveness of peptide-based cancer vaccines, which often require both CD8+ T cell epitopes and CD4+ T cell epitopes. CD8+ T cell epitopes activate CTL tumor immunity through the antigen cross-presentation route, while CD4+ T cells stimulate helper T cells in order to maintain CTL activity.65 The peptide chain’s length has a significant impact on the peptide vaccination’s efficacy. In most cases, the smallest possible CD8+ T cell epitope is a short peptide with a short in vivo half-life. It is not necessary to digest this peptide in specialist APCs; instead, it is immediately loaded into the MHC I molecules of APCs or other nucleus cells. Insufficient costimulatory molecules prevent optimal CD8+ T cell activation, which limits the production of CTLs (cytotoxic T lymphocytes). Because of this, short peptides frequently activate CTLs for a short period of time and even cause CTL tolerance.66
Peptide Chain Length
A restriction on short peptides is typically imposed by HLA types, as well. Longer peptides support motif recognition and binding, which boosts immunogenicity, and provide more HLA coverage than shorter peptides do. APCs must break down long peptides before they can be loaded onto MHC (major histocompatibility complex) molecules.67 The endosomal pathway then breaks down some of the long peptides that were previously internalized, loading them onto MHC-II molecules that are then identified by CD4+ T helper cells. As the other components pass through the cytoplasmic or vacuolar route, MHC-I molecules cross-present them to CD8+ T cells, activating them.68
Expression Platforms
Therefore, there is a higher likelihood that lengthy peptide vaccines may induce robust and long-lasting antitumor activity responses. Short peptides are often made through chemical synthesis, whereas long peptides are frequently made through protein expression systems. The immunogenicity of recombinant protein subunit vaccines varies depending on the expression platform. One of the expression platforms used for the creation of cancer vaccines is Escherichia coli (E. coli).69 Plants, yeasts, insect cells, and mammalian cells are also platforms.70 The most similar proteins to natural tumor antigens are expressed by mammalian cells. Baculovirus in insect cells is a promising strategy with low cost and certain postexpression protein modification.71
Nucleic Acid-Based Cancer Vaccines
Nucleic acid vaccines impart genetic information encoding cancer antigens to the host through regular physiological processes, causing the host to manufacture antigen proteins. After that, the produced tumor antigens might stimulate an immune response that would cause the death of cancer cells. DNA is more stable and persists in the human body for a longer amount of time than mRNA due to the extensive employment of the RNA enzyme and the structural differences between the two. As a result, the initial nucleic acid vaccines were mostly focused on DNA vaccinations.72 While DNA molecules have to reach the cell nucleus to start transcription, mRNA can directly translate and express antigens in the cytoplasm. Therefore, the production of mRNA antigens is efficient and rapid. Due to the additional step needed to access the cell nucleus, the immunological response to DNA vaccines is lower than it is to mRNA vaccines. The creation of various antigens compared to a single mRNA molecule occurs as a result of the ability of plasmid DNA to produce multiple copies of mRNA once it has entered the nucleus. Furthermore, while DNA vaccines may contain a risk of insertion mutations, mRNA carries no risk of insertion and integration into the genome.73
DNA Vaccine
Cancer DNA vaccines are based on bacterial plasmids which encode one or more oncology antigens and stimulate the activation of both the innate and adaptive immune systems.74 In 1990, Wolff et al. directly injected naked DNA into mouse muscle, where they discovered the creation of matching proteins for the first time.75 Trials of the human Immunodeficiency virus type 1 (HIV) DNA vaccine were first reported in 1998.76 There are still very few results from our considerable study of DNA vaccinations. India had already approved ZycoV-D, a COVID-19 DNA vaccine. The introduction of DNA vaccinations for a number of diseases was heralded by ZycoVD, the first human DNA vaccine to receive approval. DNA vaccines stimulate the humoral and cellular immune responses. DNA vaccinations need to penetrate the nucleus in order to convert them into the cytoplasmic antigens that are then transcribed. To elicit specific immunological reactions, MHC-I and MHC-II molecules are preparing and presenting the antigen to CD8+ T and CD4+ T cells. The following are the three types of DNA vaccine action modalities.77 A somatic cell, such as a muscle cell, quickly absorbs DNA. Following translation, MHC-1 molecules carry the DNA-encoded antigens straight to cytotoxic CD8+ T lymphocytes. The antigen encoded by DNA in somatic cells is released by secreting apoptotic bodies in the second step. These peptides are phagocytosed, digested, and cross-presented to CD4+ T lymphocytes by APCs via MHC-II molecules. The third technique involves the introduction of DNA directly into APCs. MHC-I and MHC-II, respectively, process and present the endogenous antigens that APCs create in CD8+ T and CD4+ T cells. CD4+ T cell activation results in an innate immunity. CD8+ T cells are transformed to CTLs to enhance cellular immunity. The intradermal delivery of DNA plasmids instantly into APCs is regarded to be the most important method for DNA cancer vaccines.78 The activation of innate immune responses is also facilitated by CpG patterns in plasmid DNA. CpG motifs can cause TLR9 to react in a cautionary manner. Inflammatory cytokines and chemokines are released as a result of the signaling cascade that TLR9 starts by activating NF-B and IRAK.79 The double-stranded nature of DNA also activates the STING signaling system. Due to the fact that STING is a prominent DNA sensor that controls the cascade of cytoplasmic DNA signals independently of TLR, DNA vaccines failed to significantly elicit an adaptive immune response in STING deficient mice.80 DNA vaccines can have large or many antigens. DNA vaccines are very specific, safe, and easy to transport, in addition to having low production costs. In contrast to spontaneous mutations, DNA vaccines have a reduced rate of insertional mutations, and the DNA rarely binds to host chromosomes.81,82 Furthermore, the tumor antigens in DNA cancer vaccines have the same species alteration as their natural counterparts. Cancer DNA vaccines offer distinct advantages, and enhanced DNA vaccines have successfully prevented cancer in preclinical animals.83 However, DNA vaccines have not advanced very far in clinical trials because of their weak immunogenicity.84
mRNA Vaccines
The FDA recently approved two COVID-19 mRNA vaccines (Moderna’s Spikevax and Pfizer’s BNT162b2). The FDA has additionally approved BNT162b2 as the first mRNA vaccine for marketing. The market value of mRNA vaccines has significantly increased as a result of their capacity to offer prompt protection against the COVID-19 global epidemics. CureVac, BioNTech, and Moderna are now working on in vitro transcription (IVT) mRNA vaccines. The mRNA vaccine, which inserts exogenous synthesized mRNA into cells to act as templates for the synthesis of antigens, is a promising cancer vaccine method. To produce antitumor immunity, MHC molecules transmit expressed antigens to the surface of APCs.85,86 Researchers successfully produced the enzymes luciferase, beta-galactosidase, and chloramphenicol acetyltransferase in vivo in 1990 to show the survivability of the mRNA vaccine.87 In 1992, Jirikowski found that diabetes insipidus rats treated with injection of oxytocin and vasopressin mRNAs might experience a temporary remission of their disease within hours of the treatment.88 In the treatment of a number of solid tumors, mRNA cancer vaccines have recently demonstrated some encouraging clinical findings.89 The effectiveness of other treatments could also be improved by the mRNA vaccination, it was discovered. For instance, it has been demonstrated that mRNA vaccines producing chimeric receptors directed toward CLDN6, a target expressed on several solid cancers, increase the effectiveness of claudin-CAR-T cells against solid tumors.90 The bulk of mRNA vaccines on the market are self-amplifying RNA (SAM) and nonreplicating mRNA vaccines. The 7-methylguanosine (m7G) 5′ cap, the 5′-UTR, the open reading frame (OFR), the 3′-UTR, and the 3′ poly (A) tail are all parts of nonreplacing mRNA. Both the durability of mRNA and the recruitment of transcription factors, which in turn affect how well proteins are translated, are significantly influenced by these elements. After the initial IVT, an enzyme can insert the 5′ cap and 3′ poly (A) tail. SAM has two OFRs in contrast with nonreplacing mRNA. Two genes, one encoding an objective antigen and the other a viral replication component, enable long-lasting RNA amplification in cells. SAM is an alphavirus derivative that grows and replicates within the body to elicit a powerful and enduring immune response. SAM makes it possible for the antigen to be produced in large quantities over time from modest vaccination doses.91 SAM needs additional study because it is currently in the preclinical phases of development as a cancer vaccine.92 The majority of cancer mRNA vaccines do not reproduce. Therefore, our focus is mostly on mRNA that does not replicate. mRNA vaccines offer a variety of advantages. mRNA vaccines can simultaneously encode several antigens along with full-length cancer antigens. With the generation of increased humoral and cellular immunity via multiple antigen encoding, the possibility of overriding cancer vaccine resistance rises. APCs that show a variety of HLA epitopes and subsequently encode full-field tumor antigens may result in broader T-cell responses.93 MRNA vaccines can also be developed quickly, adaptably, and effectively. Therefore, mRNA is the ideal starting point for developing tailored neoantigen vaccines.94 IVT uses a template of linearized DNA and bacteriophage RNA polymerase to generate mRNA in vitro. Because IVT is independent of cells and their complex regulations, mRNA production is made simpler, quicker, and clearer. Additionally, mRNA vaccinations are relatively safe since they cannot be incorporated into the host DNA. The mRNA vaccination-induced MHC-I-mediated CD8+ T cell responses are beneficial for the treatment of cancer. Whereas mRNA vaccines have a lot of advantages, their potential is limited by their instability, intrinsic immunogenicity, and inefficient in vivo delivery.95−97 mRNA vaccines offer the advantage of encoding multiple antigens simultaneously, allowing for the development of personalized cancer vaccines. For example, in cancer immunotherapy, mRNA vaccines can be tailored to include antigens specific to an individual’s tumor, enhancing the immune response against the unique characteristics of the cancer cells and potentially improving treatment outcomes.59,98
Examples of Vaccines Used in Cancer Treatment
-
1.
Sipuleucel-T (Provenge): Sipuleucel-T is a therapeutic cancer vaccine used in the treatment of metastatic prostate cancer. It is an autologous cellular immunotherapy, meaning it is made from a patient’s own immune cells. These cells are collected, exposed to a prostate cancer antigen, and then reinfused into the patient to stimulate an immune response against the cancer.99
-
2.
Talimogene laherparepvec (T-VEC, Imlygic): T-VEC is an oncolytic virus-based therapy approved for the treatment of melanoma. It is injected directly into melanoma lesions, where it can destroy cancer cells and stimulate an immune response.51
-
3.
Nivolumab (Opdivo) and Pembrolizumab (Keytruda): While not traditional vaccines, these immune checkpoint inhibitors are used in cancer treatment to help the immune system recognize and attack cancer cells. They have shown remarkable success in various types of cancer, including melanoma, lung cancer, and others.100
-
4.
HPV Vaccines: Vaccines like Gardasil and Cervarix are prophylactic vaccines used to prevent Human Papllomavirus (HPV) infection. HPV is a known cause of cervical and other cancers, so these vaccines indirectly reduce cancer risk.101
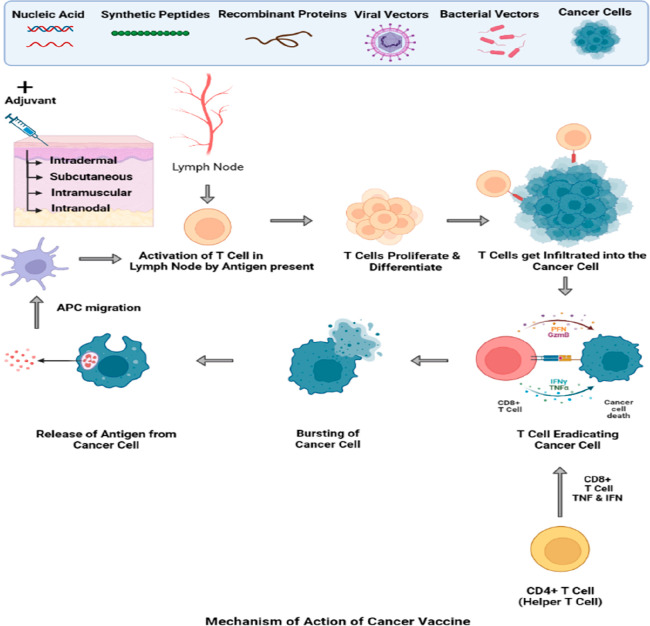
Mechanism of Action of Cancer Vaccine
Antigen-presenting cells (APCs), such as dendritic cells (DCs), first come into contact with the antigen at the injection site. The principal site of T cell priming is the draining lymph nodes, where the antigen-loaded APCs go through the lymphatic system.102 The costimulatory signals present at the time of antigen identification are what drives the immunological response at the T cell level. Antigen-presenting cells (APCs) react to specific inflammatory cytokines.
Basically, there are two different types of lymphocytes in the immune system: killer T lymphocytes, which destroy tumors or virally infected cells, and helper T lymphocytes, which aid in the activation of killer T lymphocytes. T cells are essential in the fight against infection and cancer. Cytotoxic T cells that have been activated grow, proliferate, and circulate throughout the body.
By controlling and priming antigen-specific CD8+ T cells (boosting function, magnitude, quality, persistence, and memory), CD4+ T cells play a complex and crucial helper role in orchestrating the immune response against cancer.
In addition, these cells offer protective immunity through effector function, cytokine secretion, and activation of tumoricidal macrophages.103
Immune attack against tumors depends on both CD4+ and CD8+ T cells, as the release of interferon (IFN)-g by CD4+ T cells is necessary for tumor cell eradication, and the tumor necrosis factor (TNF) is necessary for cell necrosis and also strengthens the person’s immune system.104 The lysed cancer cells then discharge tumor antigens that can once more be collected, processed, and presented by APCs to trigger polyclonal T cell responses, hence broadening the antigenic scope of the immune response against the tumor and initiating the epitope spreading process (Figure 3). Acid-based vaccines work by leveraging acidic conditions in the tumor microenvironment to trigger the release and activation of therapeutic agents. This acidic environment activates the vaccine, leading to the targeted destruction of tumor cells and the stimulation of an immune response against the cancer.105
Figure 3.
Mechanism of action of cancer vaccines.
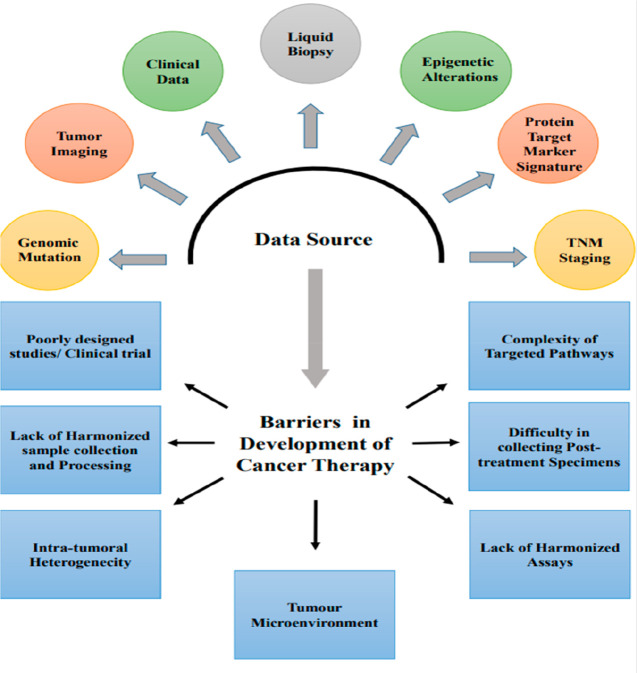
Barriers in Cancer Therapy
Despite vaccination’s effectiveness in significantly reducing or eliminating the threat of diseases brought on by infections, there are still several well-known diseases and new pathogens for which the creation of effective vaccines against them is intrinsically challenging (Figure 4). The development of vaccines for people with weakened immune systems along with other pre-existing medical disorders has also continued to be a significant difficulty. In addition to the conventional inactivated or live attenuated, virus-vectored, and subunit vaccines, emerging nonviral vaccine technologies, like viral-like particle and nanoparticle vaccines, DNA/RNA vaccines, and rational vaccine design, offer creative solutions to current challenges in vaccine development.106 An immunosuppressive network is formed by the interactions of cancer cells with the stroma and immune cells that surround them, preventing the development of antitumor immunity and promoting the growth of cancer cells.107 External mechanisms involving elements of the tumor matrix and intrinsic mechanisms based on the features of the tumor cells make up tumor-immune escape. Cancer vaccine effectiveness is determined by these two processes. The lack of tumor antigen expression or down regulation, changed antigen processing pathways, or altered human leukocyte antigen expression are examples of intrinsic variables that contribute to the resistance to cancer vaccines. In particular, it might lead to T cells’ ineffective identification of tumor cells.108 The effectiveness of cancer vaccines can vary widely depending on several factors, including the type of cancer, the specific vaccine, and the patient’s individual characteristics. Cancer vaccines have shown varying degrees of success in clinical trials, but their overall effectiveness is not uniform across all cancer types.109
Figure 4.
Barriers in the development of cancer therapy.
For example, the therapeutic cancer vaccine Sipuleucel-T (Provenge) has been used in the treatment of metastatic prostate cancer.110 Clinical trials demonstrated an increase in overall survival of approximately 4.1 months compared to the placebo group. However, this vaccine is specific to prostate cancer and may not be effective for other cancer types.
Similarly, Talimogene laherparepvec (T-VEC) is approved for melanoma treatment.111 In clinical trials, it showed a durable response rate of around 16%, indicating that a subset of melanoma patients benefited significantly. Due to complex physiological and immunological barriers, including physical impediments to immune infiltration, an immunosuppressive tumor microenvironment, an upregulation of immunosuppressive pathways, and metabolic restriction, the therapeutic efficacy of cell-based methods of delivery for immunotherapy is still limited.112 Over the past two decades, a great deal of research has been done on cancer metabolism, and it is now generally acknowledged that oncogenic transformation can enable cancer cells to adopt a well-characterized metabolic phenotype that may have a significant impact on the tumor microenvironment (TME). The TME is made up of several populations of cells in a complex matrix that frequently has inadequate or poorly defined vasculature, leading to inefficiencies in the delivery of nutrients or oxygen as well as in the disposal of waste. While the bioenergetic demands of rapidly expanding cancer and immune cells compete for resources essential to carry out an antitumor defense, this inadequate vascular exchange can lead to nutritional constraint in the TME.113 A previously unheard-of prospect for the treatment of notoriously refractory malignancies has surfaced in immunotherapy. Numerous immunotherapies and experimental medicines, including combination regimens, are being studied in both preclinical and clinical settings. However, due to the existence of several different mechanisms of resistance, a relatively small patient subgroup across various cancer types responds to the therapy. Physical stromal barriers are one of the many sources of resistance that prevent natural and modified immune cells as well as immunotherapeutic drug molecules from reaching cancerous tissues and cells. Abnormal tumor vasculatures and extensive extracellular matrix buildups that prevent extravasation and infiltration of molecular and cellular immunotherapeutic agents into tumor tissues are two key stromal barriers that contribute to the resistance.114 The majority of solid tumors are epithelial in origin, and although being dedifferentiated, malignant cells nevertheless possess intercellular connections, a crucial characteristic of epithelial cells, in both the primary tumor and in metastatic lesions. These intercellular connections serve as physical barriers that impede intratumorally penetration and spread of cancer therapies and serve as a defense mechanism against the attacks by the host’s immune system.115 Therapeutic strategies based on RNA interference (RNAi) are being closely examined in an effort to treat cancer. By focusing on mRNA expression, siRNA blocks the expression of cancer-causing genes. However, obstacles include inadequate cellular uptake, instability under physiological settings, off-target effects, and potential immunogenicity limit in vivo systemic siRNA therapy.116
Barriers in Cancer Therapy and Their Role
Cancer therapy is impeded by several complex and multifaceted barriers that can significantly affect its effectiveness and accessibility. Among these challenges is the issue of late diagnosis, where early detection is crucial, but barriers such as limited healthcare access and a lack of awareness often lead to late-stage diagnosis. Furthermore, tumor heterogeneity complicates the development of effective targeted therapies, as cancer cells exhibit diverse genetic and molecular characteristics. Resistance to treatments, whether inherent or acquired, presents a substantial obstacle, necessitating the search for alternative therapies. The financial burden of cancer treatment, encompassing the costs of therapy, medications, and support care, can be overwhelming for patients. Additionally, cancer therapy’s often debilitating side effects and disparities in care, particularly among underserved populations, further compound these challenges. Psychological and emotional impacts, difficulties in clinical trial participation, and the lengthy process of drug development and approval also contribute to the barriers. Overcoming these obstacles in cancer therapy necessitates a comprehensive approach including improved access to healthcare, early detection efforts, research funding, and strategies to reduce healthcare disparities. The ongoing progress in cancer research and treatment modalities provides optimism for surmounting these barriers and enhancing cancer patient outcomes.117−119
Optimization Strategy for Virus-Based Cancer Vaccines
Other potential approaches, in addition to combining with checkpoint inhibitors, should raise caution. It was discovered that the extensively investigated protein YAP, a coactivator of the Hippo pathway, has to do with regulation of the important immunosuppressive cell known as the Treg. Treg dysfunction would result from an YAP deficit. Therefore, by removing the immunosuppressive environment created by Tregs, targeting YAP may increase the antitumor effectiveness of the cancer vaccine.120 It was found that the protein YAP, a significant coactivator of the Hippo pathway, regulates Treg, a crucial immunosuppressive cell. Treg dysfunction would occur from a YAP deficiency. Inhibiting the Treg-mediated immunosuppressive TME while additionally inactivating YAP may thereby enhance the anticancer effectiveness of the viral vaccination. Since TGF has been shown to affect a variety of immune cells, including T-cells and NK cells, it can cause immunological suppression.121 Viral agents that express specific antigens and immunomodulatory molecules have been developed to disturb the TME.122 In different yet related ways, SPD-L1 and TGF regulate immunological suppression in the TME. It is feasible that simultaneous targeting of the PD-L1 and TGF-negative regulatory pathways would improve the antitumor effectiveness of M7824 when combined with a virus-based cancer vaccination and in various murine tumor models.123 A very effective post-translational processing of the introduced genes within the host cell cytoplasm is another benefit of this vector. In numerous tumor model systems, recombinant vaccinia viruses were found to induce strong cellular and humoral immune responses.124 Because viral infection frequently causes the presentation of MHC class I/II restricted, virus-specific peptides on infected cells, viruses are frequently used as immunization vehicles. TAAs or TAAs paired with immunomodulating compounds are designed into viral vectors with low disease-causing potential and low inherent immunogenicity.125
Current Status of Cancer Vaccines
The landscape of cancer treatment has traditionally been dominated by surgery, chemotherapy, and radiotherapy. While these treatments have saved countless lives and effectively eradicated primary tumors, their limitations are becoming increasingly apparent. The recurrence of disease and the resistance of leftover malignant cells and/or tumor metastases to conventional treatments are persistent challenges that highlight an unmet need for alternative therapeutic approaches.126 Cancer immunotherapy is emerging as a promising and fascinating treatment option. This approach leverages the body’s immune system to identify and eradicate cancer cells, offering a novel avenue for battling malignancies. In recent years, various vaccine platforms have been rigorously assessed in Phase II and III clinical trials. These include the use of proteins or peptides as adjuvants, the injection of genetically engineered viruses or microorganisms, and the delivery of killed tumor cells or activated dendritic cells (DCs) to patients.127 The specific area of cancer vaccines aims to stimulate an immune response against cancer-specific antigens. While decades of study have culminated in the approval and licensing of a few cancer vaccines, many others are still undergoing clinical trials (Table 2). The complexity of the human immune system, variations in the tumor microenvironment, and challenges in identifying effective antigens contribute to the difficulty in developing universally effective vaccines.128
Table 2. Selected Ongoing Clinical Application of Virus-Based Cancer Vaccinesa.
| Sl. No. | Virus | Condition | Phases | Status | Route |
|---|---|---|---|---|---|
| 1. | Recombinant Vaccinia Virus | Prostate cancer | I | Completed | Subcutaneous |
| Prostatic neoplasm | I | Completed | Subcutaneous | ||
| 2. | Trivalent Baculovirus expressed Influenza HA | Lymphoma | II | Completed | Intramuscular |
| 3. | DNX2401 | Glioblastoma | I | Recruiting | Intratumorem |
| 4. | Semliki Forest Virus | Cervical cancer | I | Completed | Intramuscular |
| 5. | Pembrolizumab Virsus & BNT113 | Unresectable head and neck squamous cell carcinoma metastatic head and neck cancer and recurrent head and neck cancer | II | Recruiting | BNT113: IV injection |
| Pembrolizumab: IV infusion | |||||
| 6. | Zoster vaccine recombinant, adjuvanted | Chronic lymphocytic leukemia | II | Not yet recruiting | Intramuscular |
| Small lymphocytic lymphoma | |||||
| 7. | Human Papillomavirus Virus | Cervical cancer | I | Completed | Intramuscular |
| 8. | Human Papillomavirus Virus | Cervical intraepithelial neoplasia | II | Completed | Intramuscular |
| Cervical cancer | |||||
| 9. | ColoAd1 | Ovarian carcinoma | I/II | Not yet recruiting | Intraperitoneum |
| 10. | Epstein–Barr Virus | Gastric cancer | I | Completed | Intradermal |
| Head and neck | |||||
| Cancer lymphoma | |||||
| 11. | ColoAd1 | Solid tumors | I | Not yet recruiting | Intravenam |
| 12. | S03-adjuvanted H1N1 influenza | Lymphoma | III | Completed | Intramuscular |
| Multiple myeloma | |||||
| 13. | rV-DF3/MUC1 | Metastatic breast cancer | I | Completed | Intradermal |
| 14. | Ad/MAGEA3, MG1-MAGEA3 and pembrolizumab | Nonsmall cell lung cancer | I/II | Completed | Ad-MAGEA3: Intramuscular injection |
| MG1-MAGEA3: Intravenous infusion | |||||
| 15. | ICOVIR-5 | Melanoma | I | Recruiting | Intravenam |
The efficacy of cancer vaccines is further influenced by factors such as the type and quality of antigens used, the administration method, and the patient’s immune response. Emerging research into individualized therapies and combination treatments may provide new insights into overcoming these challenges. Recent advancements have provided a deeper understanding of the physiological processes through which the body can recognize and eliminate precancerous and malignant cells. In peptide-based anticancer vaccines, markers have been employed to create targeted responses against specific tumor-associated antigens (TAAs). This has led to the development of innovative therapies, including the use of vaccine adjuvants, precision delivery based on biomarkers, and immunomodulatory monoclonal antibodies (mAbs) targeting key immunological pathways.129
PROTACs represent cutting-edge innovations in cancer therapy. By enabling targeted degradation of specific proteins, PROTACs allow unprecedented control over cellular protein levels. The ability to selectively degrade proteins related to cancer growth has vast potential for creating a new class of potent anticancer therapeutics The field of cancer vaccines is rapidly evolving, with research extending into areas such as neoantigen vaccines, RNA-based therapies, and oncolytic viruses. Collaboration between researchers, clinicians, and industry stakeholders will be vital in translating scientific advancements into life-saving therapies. The integration of computational techniques, next-generation sequencing, and personalized medicine is expected to further propel the field, allowing for the development of highly tailored and effective cancer treatments. Additionally, the continued study of immune modulation and the tumor microenvironment may uncover novel targets and mechanisms for intervention. The current status of cancer vaccines represents an exciting convergence of scientific discovery, technological innovation, and clinical application. While there are still significant hurdles to overcome, the potential for cancer vaccines to transform cancer treatment is immense. The ongoing collaboration and determination within the scientific and medical communities continue to drive progress with the goal of providing more effective, less toxic, and individualized treatment options for patients around the world.
Clinical Aspects of Cancer Vaccines
The clinical landscape of cancer vaccines is a dynamic and highly nuanced field that requires a synergistic approach involving scientific innovation, robust clinical trials, regulatory acumen, and patient-centered care. Beginning with the identification of suitable antigens, clinical researchers are tasked with selecting tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs), which are often personalized to match the unique immunological landscape of individual patients. This precision-based approach demands an in-depth understanding of oncology, immunology, genomics, and bioinformatics. In the arena of clinical trials, the design, execution, and interpretation of results require meticulous planning and adherence to ethical principles. Early phase studies evaluate the safety and biological activity of the vaccines, employing immunomonitoring to assess the induced immune responses. Phase II and III trials, conducted on a broader scale, focus on therapeutic efficacy, often in combination with existing therapies such as chemotherapy, radiotherapy, or immune checkpoint inhibitors.126,127
The successful translation of cancer vaccines from the bench to bedside faces inherent challenges, including manufacturing complexities, varying immunogenicity among patients, and potential adverse effects. Optimizing the adjuvant components and delivery methods for individual vaccines to enhance their immunogenicity and reduce unwanted side effects remains a vital area of research and development. Also, considering the heterogeneous nature of tumors, the integration of cancer vaccines with targeted therapies like PROTACs may open new avenues for synergistic anticancer effects. Regulatory considerations add another layer of complexity, with agencies requiring comprehensive data on the quality, safety, and efficacy of the vaccines. The collaboration among pharmaceutical companies, academic research institutions, clinicians, and regulatory authorities is essential to navigate these multifaceted challenges. Moreover, health economics plays a critical role in the clinical implementation of cancer vaccines. The cost-effectiveness, reimbursement strategies, and accessibility to patients across diverse socioeconomic strata require careful evaluation and strategic planning.128,129 The clinical aspects of cancer vaccines encompass a complex interplay of scientific innovation, clinical trials, manufacturing, regulation, and health economics. The field continues to evolve, driven by interdisciplinary collaboration and the relentless pursuit of excellence in cancer care. The potential to transform oncological treatment paradigms is immense; yet realizing this potential requires overcoming multifaceted challenges and leveraging opportunities through continuous innovation and collaboration.
Challenges for the Future Development of Cancer Viral Vaccines
The development of cancer viral vaccines presents a multifaceted and complex landscape that integrates insights and approaches from immunology, virology, oncology, and translational medicine. A key challenge lies in the viral genome optimization, requiring intricate genetic engineering, selection of strains, and attenuation processes to yield precise immune responses.130 Coupled with this is the need for the exploration and validation of immunomodulatory agents and combinations. Researchers must identify synergistic effects and the proper timing for integration with other therapies like radiation or chemotherapy, all while considering patient safety and efficacy.131 The role of viral infections such as Epstein–Barr, HBV, Hepatitis C, and HPV in 12% of cancer cases adds another layer of complexity and necessitates both preventive and therapeutic approaches.132 Striking a delicate balance between viral virulence, replication competence, and immunogenicity is paramount, as findings in animal studies may not always translate to humans.133 Regulatory and manufacturing challenges, such as scaling production, adhering to quality control standards, and navigating regulatory pathways, further complicate the development process. The heterogeneity of tumors calls for a personalized approach, including biomarker-driven patient selection and tailored monitoring. Finally, economic and accessibility considerations must be strategically addressed to ensure that innovative therapies reach all who could benefit from them. In summary, the path to successful cancer viral vaccines is laden with scientific, clinical, regulatory, and socioeconomic challenges, demanding a coordinated and relentless pursuit of excellence across various domains of science and medicine.
Vaccinology, while highly successful, faces several challenges and limitations. The emergence of new infectious diseases demands a faster response, and vaccine hesitancy fueled by misinformation is a growing concern.134 The need for ultracold storage for some vaccines complicates distribution, and global access disparities persist. Ensuring vaccine safety and addressing rare side effects are ongoing challenges. Pathogens with high antigenic variation make long-lasting vaccines difficult. Aging populations and the need for cross-protection add complexity. Moreover, the threat of antibiotic resistance requires effective bacterial vaccines. Vaccine development costs, rapid scale-up capacity, and high-risk group efficacy are persistent issues. Tackling these challenges demands multidisciplinary collaboration and sustained investment in research and healthcare infrastructure.
While exosomes and PROTACs hold promise in various aspects of medical research and drug development, they also face limitations and challenges within the field of vaccinology, which includes the development and deployment of vaccines:
-
1.
Exosomes in Vaccine Delivery: Exosomes, as promising carriers for vaccine delivery due to their natural cargo transportation ability, face challenges in translating preclinical success to clinical applications. Ensuring the safety, scalability, and efficacy of exosome-based vaccines in humans is an ongoing concern.135
-
2.
PROTACs in Vaccine Development: PROTACs are primarily used in the targeted degradation of specific proteins within cells, a concept that is not commonly associated with vaccine development.136 Their utilization in vaccines would require innovative approaches and may not be a conventional solution to common vaccine challenges.
-
3.
Common Limitations and Challenges: Both exosome-based vaccines and PROTACs must ensure safety and minimal immunogenicity when administered to patients. Unwanted immune responses could hinder their effectiveness or cause adverse effects.The regulatory approval process for novel vaccine technologies can be lengthy and rigorous. Both exosome-based vaccines and PROTACs would need to navigate this process to gain acceptance for clinical use. Developing and producing vaccines, regardless of the technology, can be costly. Ensuring scalability and affordability is essential, particularly in the context of global vaccine distribution. Any new technology introduced into the field of vaccinology may face public acceptance challenges.137 Ensuring transparent communication about their safety and efficacy is vital.
Conclusion and Future Prospectives
The past decade has marked a transformative era in cancer immunotherapy, driven by deep insights into cancer biology, immune escape mechanisms, and breakthroughs in treatments, including cancer vaccines. This evolution has been enriched by the development of antibody-inducing vaccines and the proliferation of licensed monoclonal antibodies in cancer therapy. The cumulative experience in therapeutic cancer vaccines and fundamental advancements in cancer immunobiology have laid a roadmap for future vaccine development, yet challenges persist. One such challenge is the identification of robust antigens and vaccine vectors that can trigger widespread T cell responses, requiring a detailed understanding of host–tumor interactions and tumor immune escape mechanisms. Customizing vaccine designs for optimal antigen presentation by expert APCs and locating combination partners using complementary mechanisms of action further add complexity to the task. The discovery of distinct tumor genes or protein products responsible for malignant transformation will open new vistas for targeted vaccination therapy.
The integration of PROTACs, molecules that promote targeted protein degradation, offers a promising frontier in the battle against cancer. These chimeric molecules could be tailored to address specific cancer mutations, enhance the overall effectiveness of vaccines, and offer a new layer of precision in immunotherapies. Collaboration with vaccine strategies will potentially lead to innovative therapeutics that can selectively modulate or eliminate undesired proteins within cancer cells. The future of anticancer vaccines seems promising, yet laden with intricate scientific endeavors. “Immune signatures” will need to be refined to identify patients most likely to respond favorably to vaccination therapy. Further enhancement of clinical outcomes might be derived from intelligent combinations of vaccine strategies with additional drugs or interventions that synergistically elevate antitumor immunity. The rise of modern technologies such as molecular sequencing, artificial intelligence, and cellular engineering further emboldens the prospects of faster, cheaper, and more effective cancer vaccines. The landscape of cancer vaccine development is at an exciting yet demanding juncture. The relentless pursuit of understanding, innovation, and collaboration across the disciplines of immunology, oncology, genomics, and pharmaceuticals is vital. It will help to not only overcome current challenges but also enable the creation of more targeted and effective therapies, including the utilization of PROTACs.
CAR-T cell therapy or Chimeric Antigen Receptor T-cell therapy is a revolutionary approach to cancer treatment. This innovative technique begins with the extraction of a patient’s T cells, a vital component of the immune system. These T cells are then genetically engineered to express chimeric antigen receptors (CARs) on their surface, which are designed to recognize specific antigens found on the surface of cancer cells. After this modification, the CAR-T cells are cultured and expanded in the laboratory, creating a substantial population of these specialized immune cells. Once infused back into the patient’s body, the CAR-T cells actively circulate through the bloodstream, homing in on cancer cells that exhibit the target antigen. Upon engagement, these CAR-T cells trigger a potent immune response, resulting in the destruction of cancer cells. This highly targeted approach minimizes damage to healthy cells and reduces the risk of side effects, making CAR-T cell therapy a promising and personalized method in the fight against cancer.138,139
The future of CAR-T cell therapy holds exciting prospects, especially when combined with emerging technologies such as exosomes and PROTACs. Exosomes, as natural carriers of bioactive molecules, could enhance the delivery and precision of CAR-T cell therapy, potentially improving their efficacy and reducing side effects. Additionally, PROTACs, originally designed for targeted protein degradation, may be adapted to regulate CAR-T cell activity, allowing for more precise control and safety. The convergence of these technologies presents an avenue for even more refined and effective cancer therapies, unlocking new possibilities in the realm of personalized and innovative cancer treatment. The synergy of these efforts holds the potential to revolutionize cancer treatment, delivering on the promise of personalized medicine and changing the narrative of cancer from a fatal disease to one that is preventable, treatable, and potentially curable.
In the evolving landscape of cancer vaccine research, researchers and clinicians have several avenues for contributing to the ongoing progress. This includes the identification of new tumor-specific antigens, which are essential for formulating effective vaccines. Personalized cancer vaccines, tailored to an individual’s unique tumor antigens, hold significant promise and require optimization. Researchers should explore combination therapies that integrate cancer vaccines with immunotherapies, enhancing the overall immune response and improving tumor control. Overcoming tumor-induced immune suppression within the tumor microenvironment is a critical area for investigation. Additionally, expanding the applicability of cancer vaccines beyond specific cancer types, refining adjuvants for optimal immune stimulation, and enhancing antigen presentation by dendritic cells are key directions. Rigorous clinical trials focusing on specific patient populations and tumor types are crucial for providing valuable data to refine vaccine approaches. Ensuring vaccine safety, managing side effects, and working on cost-effective production and global access are equally essential. Public awareness and education, as well as streamlined regulatory pathways, are essential elements in driving the adoption of cancer vaccines. Collaborative efforts across these domains will advance the field and offer innovative treatment options for cancer patients.
Acknowledgments
All authors would like to acknowledge their respective departments for the conduct of the study.
Author Contributions
▽ Equally contributing first authors.
The GGPM grant from Universiti Kebangsaan Malaysia (GGPM-2023–020) funded this research. The authors would like to express their gratitude to Universiti Kebangsaan Malaysia for providing the approved funding necessary to conduct this study.
The authors declare no competing financial interest.
References
- Harrop R.; John J.; Carroll M. W. Recombinant viral vectors: Cancer vaccines. Adv. Drug Deliv Rev. 2006, 58 (8), 931–47. 10.1016/j.addr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Xiang S. D.; Scalzo-Inguanti K.; Minigo G.; Park A.; Hardy C. L.; Plebanski M. Promising particle-based vaccines in cancer therapy. Expert Rev. Vaccines. 2008, 7 (7), 1103–19. 10.1586/14760584.7.7.1103. [DOI] [PubMed] [Google Scholar]
- Cawood R.; Hills T.; Wong S. L.; Alamoudi A. A.; Beadle S.; Fisher K. D. Recombinant viral vaccines for cancer. Trends Mol. Med. 2012, 18 (9), 564–74. 10.1016/j.molmed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Kroll A. V.; Jiang Y.; Zhou J.; Holay M.; Fang R. H.; Zhang L. Biomimetic Nanoparticle Vaccines for Cancer Therapy. Adv. Biosyst. 2019, 3, 1800219. 10.1002/adbi.201800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawood R.; Hills T.; Wong S. L.; Alamoudi A. A.; Beadle S.; Fisher K. D. Recombinant viral vaccines for cancer. Trends Mol. Med. 2012, 18 (9), 564–74. 10.1016/j.molmed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Cawood R.; Hills T.; Wong S. L.; Alamoudi A. A.; Beadle S.; Fisher K. D. Recombinant viral vaccines for cancer. Trends Mol. Med. 2012, 18 (9), 564–74. 10.1016/j.molmed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Thomas S.; Prendergast G. C. Cancer Vaccines: A Brief Overview. Vaccine Design 2016, 1403, 755–761. 10.1007/978-1-4939-3387-7_43. [DOI] [PubMed] [Google Scholar]
- Horton H. M.; Parker S. E.; Wloch M. K.; Norman J. A. DNA vaccines for cancer therapy. Expert Opin Investig Drugs. 1999, 8 (12), 2017–26. 10.1517/13543784.8.12.2017. [DOI] [PubMed] [Google Scholar]
- Itoh K.; Yamada A.; Mine T.; Noguchi M. Recent Advances in Cancer Vaccines: An Overview. Jpn. J. Clin Oncol. 2008, 39 (2), 73–80. 10.1093/jjco/hyn132. [DOI] [PubMed] [Google Scholar]
- Mukerjee N.; Ghosh A. Revolutionizing viral disease treatment: PROTACs therapy could be the ultimate weapon of the future. J. Med. Virol. 2023, 95 (8), 28981. 10.1002/jmv.28981. [DOI] [PubMed] [Google Scholar]
- Mukerjee N.; Maitra S.; Ghosh A.; Subramaniyan V.; Sharma R. Exosome-mediated PROTACs delivery to target viral infections. Drug Dev Res. 2023, 84 (6), 1031–6. 10.1002/ddr.22091. [DOI] [PubMed] [Google Scholar]
- Lin M. J.; Svensson-Arvelund J.; Lubitz G. S.; Marabelle A.; Melero I.; Brown B. D. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer. 2022, 3 (8), 911–26. 10.1038/s43018-022-00418-6. [DOI] [PubMed] [Google Scholar]
- Pol J.; Bloy N.; Obrist F.; Eggermont A.; Galon J.; Hervé Fridman W. Trial Watch. Oncoimmunology. 2014, 3 (4), e28185. 10.4161/onci.28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T.; Elzey B.; Hahn N. M. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother. 2012, 8 (4), 534–9. 10.4161/hv.19795. [DOI] [PubMed] [Google Scholar]
- Kreiter S.; Diken M.; Selmi A.; Türeci Ö; Sahin U. Tumor vaccination using messenger RNA: prospects of a future therapy. Curr. Opin Immunol. 2011, 23 (3), 399–406. 10.1016/j.coi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Ferraro B.; Morrow M. P.; Hutnick N. A.; Shin T. H.; Lucke C. E.; Weiner D. B. Clinical Applications of DNA Vaccines: Current Progress. Clinical Infectious Diseases. 2011, 53 (3), 296–302. 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A. D.; Fritz J. M.; Lenardo M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20 (11), 651–68. 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani K.; Roudaia L.; Buhlaiga N.; Del Rincon S. V.; Papneja N.; Miller W. H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Current Oncology. 2020, 27 (12), 87–97. 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A.; Eidinger D.; Bruce A. W. Intracavitary Bacillus Calmette-guerin in the Treatment of Superficial Bladder Tumors. Journal of Urology. 1976, 116 (2), 180–2. 10.1016/S0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- Golshani G.; Zhang Y. Advances in immunotherapy for colorectal cancer: a review. Therap Adv. Gastroenterol. 2020, 13, 175628482091752. 10.1177/1756284820917527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C. L. Cancer Immunotherapy, Part 2: Efficacy, Safety, and Other Clinical Considerations. P. T. 2017, 42 (7), 452–463. [PMC free article] [PubMed] [Google Scholar]
- Aborode A. T.; Fajemisin E. A.; Obadawo B.; Alexiou A.; Mukerjee N.; Ghosh S.; Ghosh A.; Sharma R. Advancements in cutting-edge cancer treatments using nanotechnology. International Journal of Surgery (London, England) 2023, 10.1097/JS9.0000000000000079. [DOI] [PubMed] [Google Scholar]
- Farhood B.; Najafi M.; Mortezaee K. CD8 + cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234 (6), 8509–21. 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- Le D. T.; Pardoll D. M.; Jaffee E. M. Cellular Vaccine Approaches. Cancer Journal. 2010, 16 (4), 304–10. 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuglia A. R.; Lapa D.; Sias C.; Capobianchi M. R.; Del Porto P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front Immunol. 2020, 11, 11. 10.3389/fimmu.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. H.; Wong G.; Gane E.; Kao J. H.; Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020, 10.1128/CMR.00046-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Hu M.; Yang Y.; Du C.; Zhou H.; Liu C. An overview of PROTACs: a promising drug discovery paradigm. Molecular Biomedicine. 2022, 3 (1), 46. 10.1186/s43556-022-00112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S. M.; Dong J.; Xu Z. Y.; Cheng X. D.; Zhang W. D.; Qin J. J. PROTAC: An Effective Targeted Protein Degradation Strategy for Cancer Therapy. Front Pharmacol. 2021, 12, 12. 10.3389/fphar.2021.692574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D. K.; Yadav N.; Khurana S. M. P.. Vaccines. In: Animal Biotechnology; Elsevier; 2014; pp 491–508. [Google Scholar]
- Rachal L.Immunizations. In: Encyclopedia of Child and Adolescent Health; Elsevier; 2023; pp 378–97. [Google Scholar]
- Kato-Inui T.; Takahashi G.; Hsu S.; Miyaoka Y. Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 with improved proof-reading enhances homology-directed repair. Nucleic Acids Res. 2018, 46 (9), 4677–88. 10.1093/nar/gky264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K.; Yamada A.; Mine T.; Noguchi M. Recent Advances in Cancer Vaccines: An Overview. Jpn. J. Clin Oncol. 2008, 39 (2), 73–80. 10.1093/jjco/hyn132. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P.; Traversari C.; Chomez P.; Lurquin C.; De Plaen E.; Van den Eynde B. A Gene Encoding an Antigen Recognized by Cytolytic T Lymphocytes on a Human Melanoma. Science (1979) 1991, 254 (5038), 1643–7. 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- Gaj T.; Gersbach C. A.; Barbas C. F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31 (7), 397–405. 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T.; Elzey B.; Hahn N. M. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother. 2012, 8 (4), 534–9. 10.4161/hv.19795. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.; Dhar R.; Jonnalagadda S.; Gorai S.; Nag S.; Kar R.; Mukerjee N.; Mukherjee D.; Vatsa R.; Arikketh D.; Krishnan A. Exosomal miRNAs and breast cancer: A complex theranostics interlink with clinical significance. Biomarker 2023, 28, 502–518. 10.1080/1354750X.2023.2229537. [DOI] [PubMed] [Google Scholar]
- Saxena M.; van der Burg S. H.; Melief C. J. M.; Bhardwaj N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021, 21 (6), 360–78. 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- Ward E. M.; Flowers C. R.; Gansler T.; Omer S. B.; Bednarczyk R. A. The importance of immunization in cancer prevention, treatment, and survivorship. CA Cancer J. Clin. 2017, 67 (5), 398–410. 10.3322/caac.21407. [DOI] [PubMed] [Google Scholar]
- Dallal R. M.; Lotze M. T. The dendritic cell and human cancer vaccines. Curr. Opin Immunol. 2000, 12 (5), 583–8. 10.1016/S0952-7915(00)00146-1. [DOI] [PubMed] [Google Scholar]
- Soto J. A.; Galvez N. M. S.; Andrade C. A.; Pacheco G. A.; Bohmwald K.; Berrios R. V.; Bueno S. M.; Kalergis A. M. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front Immunol. 2020, 11, 11. 10.3389/fimmu.2020.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M. Tumour virus vaccines: hepatitis B virus and human papillomavirus. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017, 372 (1732), 20160268. 10.1098/rstb.2016.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn O. J. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Annals of Oncology 2012, 23, viii6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobleigh M. A.; Bradfield C.; Liu Y.; Mehta A.; Robek M. D. The Immune Response to a Vesicular Stomatitis Virus Vaccine Vector Is Independent of Particulate Antigen Secretion and Protein Turnover Rate. J. Virol. 2012, 86 (8), 4253–61. 10.1128/JVI.05991-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonis R. J.; Lai J. R.; Vergnolle O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120 (6), 3210–29. 10.1021/acs.chemrev.9b00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gascon A.; del Pozo-Rodríguez A.; Solinís M. A. Development of nucleic acid vaccines: use of self-amplifying RNA in lipid nanoparticles. Int. J. Nanomedicine. 2014, 1833. 10.2147/IJN.S39810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirbaghaee Z.; Bolhassani A. Different applications of virus-like particles in biology and medicine: Vaccination and delivery systems. Biopolymers. 2016, 105 (3), 113–32. 10.1002/bip.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.; Zhou L.; Mi Q. S.; Jiang A. DC-Based Vaccines for Cancer Immunotherapy. Vaccines (Basel). 2020, 8 (4), 706. 10.3390/vaccines8040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y.; Fu C.; Zhou L.; Mi Q. S.; Jiang A. DC-Derived Exosomes for Cancer Immunotherapy. Cancers (Basel). 2021, 13 (15), 3667. 10.3390/cancers13153667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar R.; Dhar R.; Mukherjee S.; Nag S.; Gorai S.; Mukerjee N. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater Sci. Eng. 2023, 9 (2), 577–94. 10.1021/acsbiomaterials.2c01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornesello A. L.; Tagliamonte M.; Buonaguro F. M.; Tornesello M. L.; Buonaguro L. Virus-like Particles as Preventive and Therapeutic Cancer Vaccines. Vaccines (Basel). 2022, 10 (2), 227. 10.3390/vaccines10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci P. F.; Pala L.; Conforti F.; Cocorocchio E. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers (Basel). 2021, 13 (6), 1383. 10.3390/cancers13061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda V. P.; Bezbaruah R.; Valu D.; Patel B.; Kumar A.; Prasad S. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines (Basel). 2023, 11 (2), 432. 10.3390/vaccines11020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T.; Lowy D. R. Vaccines to Prevent Infections by Oncoviruses. Annu. Rev. Microbiol. 2010, 64 (1), 23–41. 10.1146/annurev.micro.112408.134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro H.; Brenner M. K. Immunotherapy against cancer-related viruses. Cell Res. 2017, 27 (1), 59–73. 10.1038/cr.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzani O. T.; Hitchings M. D. T.; Dorion M.; D’Agostini T. L.; de Paula R. C.; de Paula O. F. P. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021, n2015. 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Mukerjee N.; Mukherjee D.; Devi A.; Jha S. K.; Gorai S. Plant-derived exosomes: A new dimension in cancer therapy. Phytotherapy Research. 2023, 10.1002/ptr.7828. [DOI] [PubMed] [Google Scholar]
- Patel P. D.; Alghareeb R.; Hussain A.; Maheshwari M. V.; Khalid N. The Association of Epstein-Barr Virus With Cancer. Cureus 2022, 10.7759/cureus.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman H. L.; Kohlhapp F. J.; Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discovery 2015, 14 (9), 642–62. 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M.; Siddiqui M. R.; Tran K.; Reddy S. P.; Malik A. B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal. 2014, 20 (7), 1126–67. 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja J.; Ludwig J. M.; Gettinger S. N.; Schalper K. A.; Kim H. S. Oncolytic virus immunotherapy: future prospects for oncology. J. Immunother Cancer. 2018, 6 (1), 140. 10.1186/s40425-018-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhen D.; Calderon H.; Chandra N.; Fajardo C. A.; Rajan A.; Alemany R. Adenovirus-Based Vaccines for Fighting Infectious Diseases and Cancer: Progress in the Field. Hum. Gene Ther. 2014, 25 (4), 301–17. 10.1089/hum.2013.235. [DOI] [PubMed] [Google Scholar]
- Morante V.; Borghi M.; Farina I.; Michelini Z.; Grasso F.; Gallinaro A. Integrase-Defective Lentiviral Vector Is an Efficient Vaccine Platform for Cancer Immunotherapy. Viruses. 2021, 13 (2), 355. 10.3390/v13020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel J. C.; Di Pasquale G.; Ramlogan C. A.; Patel V.; Chiorini J. A.; Morris J. C. Oral Vaccination With Adeno-associated Virus Vectors Expressing the Neu Oncogene Inhibits the Growth of Murine Breast Cancer. Molecular Therapy. 2013, 21 (3), 680–7. 10.1038/mt.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. B.; Luo C.; O’Connell D. J.; Lefkovith A.; Brown E. M.; Yassour M. Antigen discovery and specification of immunodominance hierarchies for MHCII-restricted epitopes. Nat. Med. 2018, 24 (11), 1762–72. 10.1038/s41591-018-0203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay R. E.; Richardson E. K.; Toh H. C. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 2021, 28 (1–2), 5–17. 10.1038/s41417-020-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijker M. S.; van den Eeden S. J. F.; Franken K. L.; Melief C. J. M.; Offringa R.; van der Burg S. H. CD8+ CTL Priming by Exact Peptide Epitopes in Incomplete Freund’s Adjuvant Induces a Vanishing CTL Response, whereas Long Peptides Induce Sustained CTL Reactivity. Journal of Immunology. 2007, 179 (8), 5033–40. 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- Kaufman H. L.; Kohlhapp F. J.; Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discovery 2015, 14 (9), 642–62. 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja J.; Ludwig J. M.; Gettinger S. N.; Schalper K. A.; Kim H. S. Oncolytic virus immunotherapy: future prospects for oncology. J. Immunother Cancer. 2018, 6 (1), 140. 10.1186/s40425-018-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhunjhunwala S.; Hammer C.; Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer. 2021, 21 (5), 298–312. 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- Mello C. F. Vacinação contra papilomavírus humano. Einstein (São Paulo). 2013, 11 (4), 547–9. 10.1590/S1679-45082013000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet J.; Chen W. H.; Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv Rev. 2021, 170, 71–82. 10.1016/j.addr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Fu M.; Wang M.; Wan D.; Wei Y.; Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J. Hematol Oncol. 2022, 15 (1), 28. 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanafrooz Z.; Baradaran B.; Mosafer J.; Hashemzaei M.; Rezaei T.; Mokhtarzadeh A. Comparison of DNA and mRNA vaccines against cancer. Drug Discov Today. 2020, 25 (3), 552–60. 10.1016/j.drudis.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. A. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011, 239 (1), 62–84. 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Wolff J. A.; Malone R. W.; Williams P.; Chong W.; Acsadi G.; Jani A. Direct Gene Transfer into Mouse Muscle in Vivo. Science (1979) 1990, 247 (4949), 1465–8. 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R.; Boyer J. D.; Ugen K. E.; Lacy K. E.; Gluckman S. J.; Bagarazzi M. L. First Human Trial of a DNA-Based Vaccine for Treatment of Human Immunodeficiency Virus Type 1 Infection: Safety and Host Response. Journal of Infectious Diseases. 1998, 178 (1), 92–100. 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- Tiptiri-Kourpeti A.; Spyridopoulou K.; Pappa A.; Chlichlia K. DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy. Pharmacol Ther. 2016, 165, 32–49. 10.1016/j.pharmthera.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Rezaei T.; Davoudian E.; Khalili S.; Amini M.; Hejazi M.; de la Guardia M.; Mokhtarzadeh A. Strategies in DNA vaccine for melanoma cancer. Pigment Cell Melanoma Res. 2021, 34 (5), 869–91. 10.1111/pcmr.12933. [DOI] [PubMed] [Google Scholar]
- Nigar S.; Shimosato T. Cooperation of Oligodeoxynucleotides and Synthetic Molecules as Enhanced Immune Modulators. Front Nutr. 2019, 6, 6. 10.3389/fnut.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigar S.; Shimosato T. Cooperation of Oligodeoxynucleotides and Synthetic Molecules as Enhanced Immune Modulators. Front Nutr. 2019, 6, 6. 10.3389/fnut.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurez F.; Dory D.; Le Moigne V.; Gravier R.; Jestin A. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine. 2010, 28 (23), 3888–95. 10.1016/j.vaccine.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Troilo P. J.; Wang X.; Griffiths T. G.; Pacchione S. J.; Barnum A. B. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11 (8), 711–21. 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- Lopes A.; Vanvarenberg K.; Préat V.; Vandermeulen G. Codon-Optimized P1A-Encoding DNA Vaccine: Toward a Therapeutic Vaccination against P815 Mastocytoma. Mol. Ther Nucleic Acids. 2017, 8, 404–15. 10.1016/j.omtn.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschak J. J.; Williams J. A.; Schmaljohn C. S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccin Immunother. 2017, 13 (12), 2837–48. 10.1080/21645515.2017.1330236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard C.; De Koker S.; Saelens X.; Vanham G.; Grooten J. Challenges and advances towards the rational design of mRNA vaccines. Trends Mol. Med. 2013, 19 (12), 705–13. 10.1016/j.molmed.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Pardi N.; Hogan M. J.; Porter F. W.; Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discovery 2018, 17 (4), 261–79. 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A.; Malone R. W.; Williams P.; Chong W.; Acsadi G.; Jani A. Direct Gene Transfer into Mouse Muscle in Vivo. Science (1979) 1990, 247 (4949), 1465–8. 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Jirikowski G. F.; Sanna P. P.; Maciejewski-Lenoir D.; Bloom F. E. Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA. Science (1979) 1992, 255 (5047), 996–8. 10.1126/science.1546298. [DOI] [PubMed] [Google Scholar]
- Papachristofilou A.; Hipp M. M.; Klinkhardt U.; Früh M.; Sebastian M.; Weiss C. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother Cancer. 2019, 7 (1), 38. 10.1186/s40425-019-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard K.; Rengstl B.; Oehm P.; Michel K.; Billmeier A.; Hayduk N. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science (1979) 2020, 367 (6476), 446–53. 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- Bloom K.; van den Berg F.; Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28 (3–4), 117–29. 10.1038/s41434-020-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghfuri E.; Pourfarzi F.; Faghfouri A. H.; Abdoli Shadbad M.; Hajiasgharzadeh K.; Baradaran B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin Biol. Ther. 2021, 21 (2), 201–18. 10.1080/14712598.2020.1815704. [DOI] [PubMed] [Google Scholar]
- Van Nuffel A. M. T.; Wilgenhof S.; Thielemans K.; Bonehill A. Overcoming HLA restriction in clinical trials. Oncoimmunology. 2012, 1 (8), 1392–4. 10.4161/onci.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. D.; Reidenbach D.; Salomon N.; Sahin U.; Tureci O.; Vormehr M.; Kranz L. M. mRNA therapeutics in cancer immunotherapy. Mol. Cancer. 2021, 20 (1), 69. 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N.; Hogan M. J.; Weissman D. Recent advances in mRNA vaccine technology. Curr. Opin Immunol. 2020, 65, 14–20. 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Naik R.; Peden K. Regulatory Considerations on the Development of mRNA Vaccines. mRNA Vaccines 2020, 440, 187–205. 10.1007/82_2020_220. [DOI] [PubMed] [Google Scholar]
- Brito L. A.; Kommareddy S.; Maione D.; Uematsu Y.; Giovani C.; Berlanda Scorza F. Self-Amplifying mRNA Vaccines. Nonviral Vectors for Gene Therapy - Physical Methods and Medical Translation 2015, 89, 179–233. 10.1016/bs.adgen.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Maruggi G.; Shan H.; Li J. Advances in mRNA Vaccines for Infectious Diseases. Front Immunol. 2019, 10, 10. 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anassi E.; Ndefo U. A. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T. 2011, 36 (4), 197–202. [PMC free article] [PubMed] [Google Scholar]
- Shiravand Y.; Khodadadi F.; Kashani S. M. A.; Hosseini-Fard S. R.; Hosseini S.; Sadeghirad H. Immune Checkpoint Inhibitors in Cancer Therapy. Current Oncology. 2022, 29 (5), 3044–60. 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cancerresearch.org/treatment-types/cancer-vaccines. (Accessed on Sept 15, 2023)
- Reyes N. J.; O’Koren E. G.; Saban D. R. New insights into mononuclear phagocyte biology from the visual system. Nat. Rev. Immunol. 2017, 17 (5), 322–32. 10.1038/nri.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh C. S.; Hsu J. C.; Hosseini I.; Shen B. Q.; Rotte A.; Twomey P. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Molecular Therapy. 2021, 29 (2), 555–70. 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh C. S.; Hsu J. C.; Hosseini I.; Shen B. Q.; Rotte A.; Twomey P. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Molecular Therapy. 2021, 29 (2), 555–70. 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gote V.; Bolla P. K.; Kommineni N.; Butreddy A.; Nukala P. K.; Palakurthi S. S. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24 (3), 2700. 10.3390/ijms24032700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisse M.; Vrba S. M.; Kirk N.; Liang Y.; Ly H. Emerging Concepts and Technologies in Vaccine Development. Front Immunol. 2020, 11, 11. 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E.; Dobrovolskaia M. A. Addressing barriers to effective cancer immunotherapy with nanotechnology: achievements, challenges, and roadmap to the next generation of nanoimmunotherapeutics. Adv. Drug Deliv Rev. 2019, 141, 3–22. 10.1016/j.addr.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Liu J.; Fu M.; Wang M.; Wan D.; Wei Y.; Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J. Hematol Oncol. 2022, 15 (1), 28. 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donninger H.; Li C.; Eaton J. W.; Yaddanapudi K. Cancer Vaccines: Promising Therapeutics or an Unattainable Dream. Vaccines (Basel). 2021, 9 (6), 668. 10.3390/vaccines9060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T.; Elzey B.; Hahn N. M. Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother. 2012, 8 (4), 534–9. 10.4161/hv.19795. [DOI] [PubMed] [Google Scholar]
- Ferrucci P. F.; Pala L.; Conforti F.; Cocorocchio E. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers (Basel). 2021, 13 (6), 1383. 10.3390/cancers13061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Wang Y.; Hu Q. Recent advances in overcoming barriers to cell-based delivery systems for cancer immunotherapy. Exploration. 2022, 2 (3), 20210106. 10.1002/EXP.20210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. E.; Voss K.; Rathmell J. C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell 2020, 78 (6), 1019–33. 10.1016/j.molcel.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. W.; Xie Y.; Suk J. S. Overcoming physical stromal barriers to cancer immunotherapy. Drug Deliv Transl Res. 2021, 11 (6), 2430–47. 10.1007/s13346-021-01036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer I.; van Rensburg R.; Lieber A. Overcoming physical barriers in cancer therapy. Tissue Barriers. 2013, 1 (1), e23647. 10.4161/tisb.23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Trivedi P.; Jain N. K. Advances in siRNA delivery in cancer therapy. Artif Cells Nanomed Biotechnol. 2018, 46 (2), 274–83. 10.1080/21691401.2017.1307210. [DOI] [PubMed] [Google Scholar]
- Hendren S.; Chin N.; Fisher S.; Winters P.; Griggs J.; Mohile S. Patients’ Barriers to Receipt of Cancer Care, and Factors Associated With Needing More Assistance From a Patient Navigator. J. Natl. Med. Assoc. 2011, 103 (8), 701–10. 10.1016/S0027-9684(15)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filteau C.; Simeone A.; Ravot C.; Dayde D.; Falandry C. Cultural and Ethical Barriers to Cancer Treatment in Nursing Homes and Educational Strategies: A Scoping Review. Cancers (Basel). 2021, 13 (14), 3514. 10.3390/cancers13143514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzero S.; Ziemys A.; Ferrari M. Transport Barriers and Oncophysics in Cancer Treatment. Trends Cancer. 2018, 4 (4), 277–80. 10.1016/j.trecan.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Fu M.; Wang M.; Wan D.; Wei Y.; Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J. Hematol Oncol. 2022, 15 (1), 28. 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M.; Wahi A.; Sharma P.; Nagpal R.; Raina N.; Kaurav M. Recent Advances in Cancer Vaccines: Challenges, Achievements, and Futuristic Prospects. Vaccines (Basel). 2022, 10 (12), 2011. 10.3390/vaccines10122011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper S. J.; Heeney J. L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010, 8 (1), 62–73. 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- Knudson K. M.; Hicks K. C.; Luo X.; Chen J. Q.; Schlom J.; Gameiro S. R. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018, 7 (5), e1426519. 10.1080/2162402X.2018.1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minev B. R.; Chavez F. L.; Mitchell M. S. Cancer Vaccines. Pharmacol Ther. 1999, 81 (2), 121–39. 10.1016/S0163-7258(98)00039-4. [DOI] [PubMed] [Google Scholar]
- Guo C.; Manjili M. H.; Subjeck J. R.; Sarkar D.; Fisher P. B.; Wang X. Y. Therapeutic Cancer Vaccines. Advances in Cancer Research 2013, 119, 421–475. 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingluff C. L.; Speiser D. E. Progress and controversies in developing cancer vaccines. J. Transl Med. 2005, 3 (1), 18. 10.1186/1479-5876-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J. Therapeutic Cancer Vaccines: Current Status and Moving Forward. JNCI Journal of the National Cancer Institute. 2012, 104 (8), 599–613. 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmett E.; Al-Share B.; Alkassab M. B.; Zhou R. W.; Desai A.; Rahim M. M. A.; Woldie I. Cancer vaccines: past, present and future; a review article. Discover Oncology. 2022, 13 (1), 31. 10.1007/s12672-022-00491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M.; Wahi A.; Sharma P.; Nagpal R.; Raina N.; Kaurav M. Recent Advances in Cancer Vaccines: Challenges, Achievements, and Futuristic Prospects. Vaccines (Basel). 2022, 10 (12), 2011. 10.3390/vaccines10122011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger A.; Elliott B. M.; Kaufman H. L. Viral Vaccines for Cancer Immunotherapy. Hematol Oncol Clin North Am. 2006, 20 (3), 661–87. 10.1016/j.hoc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Le I.; Dhandayuthapani S.; Chacon J.; Eiring A. M.; Gadad S. S. Harnessing the Immune System with Cancer Vaccines: From Prevention to Therapeutics. Vaccines (Basel). 2022, 10 (5), 816. 10.3390/vaccines10050816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Fu M.; Wang M.; Wan D.; Wei Y.; Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J. Hematol Oncol. 2022, 15 (1), 28. 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper S. J.; Heeney J. L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010, 8 (1), 62–73. 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- Emens L. A. Cancer vaccines: on the threshold of success. Expert Opin Emerg Drugs. 2008, 13 (2), 295–308. 10.1517/14728214.13.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negahdaripour M.; Vakili B.; Nezafat N. Exosome-based vaccines and their position in next generation vaccines. Int. Immunopharmacol. 2022, 113, 109265. 10.1016/j.intimp.2022.109265. [DOI] [PubMed] [Google Scholar]
- Mukerjee N.; Maitra S.; Gorai S.; Ghosh A.; Alexiou A.; Thorat N. D. Revolutionizing Human papillomavirus (HPV)-related cancer therapies: Unveiling the promise of Proteolysis Targeting Chimeras (PROTACs) and Proteolysis Targeting Antibodies (PROTABs) in cancer nano-vaccines. J. Med. Virol. 2023, 95 (10), e29135. 10.1002/jmv.29135. [DOI] [PubMed] [Google Scholar]
- Gao H.; Sun X.; Rao Y. PROTAC Technology: Opportunities and Challenges. ACS Med. Chem. Lett. 2020, 11 (3), 237–40. 10.1021/acsmedchemlett.9b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee N.; Maitra S.; Ghosh A.; Sharma R. Impact of CAR-T cell therapy on treating viral infections: unlocking the door to recovery. Human Cell. 2023, 36, 1839. 10.1007/s13577-023-00942-2. [DOI] [PubMed] [Google Scholar]
- Levine B. L.; Miskin J.; Wonnacott K.; Keir C. Global manufacturing of CAR T cell therapy. Mol. Ther Methods Clin Dev. 2017, 4, 92–101. 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]