Abstract
Background and aims
Sepsis is a serious and life-threatening condition caused by a dysregulated immune response to an infection. Recent guidance issued in the UK gave recommendations around recognition and antibiotic treatment of sepsis, but did not consider factors relating to health inequalities. The aim of this study was to summarise the literature investigating associations between health inequalities and sepsis.
Methods
Searches were conducted in Embase for peer-reviewed articles published since 2010 that included sepsis in combination with one of the following five areas: socioeconomic status, race/ethnicity, community factors, medical needs and pregnancy/maternity.
Results
Five searches identified 1,402 studies, with 50 unique studies included in the review after screening (13 sociodemographic, 14 race/ethnicity, 3 community, 3 care/medical needs and 20 pregnancy/maternity; 3 papers examined multiple health inequalities). Most of the studies were conducted in the USA (31/50), with only four studies using UK data (all pregnancy related). Socioeconomic factors associated with increased sepsis incidence included lower socioeconomic status, unemployment and lower education level, although findings were not consistent across studies. For ethnicity, mixed results were reported. Living in a medically underserved area or being resident in a nursing home increased risk of sepsis. Mortality rates after sepsis were found to be higher in people living in rural areas or in those discharged to skilled nursing facilities while associations with ethnicity were mixed. Complications during delivery, caesarean-section delivery, increased deprivation and black and other ethnic minority race were associated with post-partum sepsis.
Conclusion
There are clear correlations between sepsis morbidity and mortality and the presence of factors associated with health inequalities. To inform local guidance and drive public health measures, there is a need for studies conducted across more diverse setting and countries.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12939-024-02114-6.
Keywords: Sepsis, Antimicrobial resistance, Health inequalities, Socioeconomic status, Deprivation, Ethnicity, Communities, Maternal
Introduction
Sepsis is “life-threatening organ dysfunction caused by a dysregulated host immune response to an infection” [1]. A 2015 study estimated the in-hospital mortality rate for sepsis in UK hospitals to be around 30% [2]. As well as high mortality rates, sepsis survivors often experience longer-term mental and physical health problems and are at high risk of post-discharge hospital readmission or death [3–5]. Risk factors for developing sepsis include frailty, immunocompromised status, recent surgical procedures, and comorbidities such as cancer, kidney disease, lung disease and diabetes [6–8]. The risk of contracting sepsis increases with age, with many sepsis cases occurring in people over the age of 65 [9]. Additionally, there is a higher risk of sepsis in neonates and women who are pregnant or have recently given birth.
Bacterial infections are the most common cause of sepsis and therefore antibiotics are widely used for treatment. A 2022 report published by the United Kingdom (UK) Academy of Medical Royal Colleges (AMRC) outlined recommendations for the recognition and early management of sepsis [10]. An aspect the report did not address, however, was the impact of health inequalities on sepsis recognition, management and outcomes. Inequalities can impact life expectancy, access to healthcare and general health status. Factors that are associated with these disparities include level of deprivation, ethnicity and belonging to more vulnerable groups within society, for example people experiencing homelessness [11]. In 2021, the National Healthcare Inequalities Programme was set up and developed the Core20PLUS5 approach, with the aim of supporting the National Health Service and local authorities in reducing health inequalities [11]. Core20 refers to populations living in the most deprived 20% of areas according to the Index of Multiple Deprivation (IMD). PLUS refers to population groups identified at local level that could include ethnic minority groups, coastal communities, populations defined as having a protected characteristic under the Equality Act 2010 or belonging to an inclusion health group, amongst others. ‘5’ refers to five clinical areas of importance, which are maternity, severe mental illness, chronic respiratory disease, early cancer diagnosis, and hypertension case-finding.
Variations in rates of antimicrobial resistant infections and microorganisms (associated with higher mortality rates in sepsis [12]) have been reported in the UK amongst different ethnic groups and levels of deprivation [13]. A recent study reported increased odds of non-COVID 19 related sepsis and increased mortality in more socioeconomically deprived people during the pandemic [14]. In the face of increasing resistance to antimicrobials globally, knowing who is at greatest risk of developing sepsis may not only improve patient outcomes but help target the use of antimicrobials more effectively.
A 2019 systematic review assessed the link between race and socioeconomic status and sepsis outcomes. However, they only included studies conducted in the USA [15]. The purpose of this review, therefore, was to identify studies from all high- income countries that have assessed additional factors associated with health inequality. The aims of this rapid review were (i) to summarise the literature that investigated health inequalities and sepsis incidence and mortality outcome and (ii) to provide an evidence base for public health advice to reduce the impact of health inequalities with sepsis.
Methods
Eligibility criteria
Peer-reviewed journal articles published between 01/01/2010 and 31/01/2023, written in English, were eligible for inclusion. Included studies had to be observational in design where the main outcome was either incidence or risk of sepsis (in the general population or hospital admissions) or sepsis-associated mortality. We included studies where the aim was assessing the impact of one of the following health inequality factors: socioeconomic, race/ethnicity, community, medical vulnerability, or pregnancy. Studies were excluded if they were conducted in a low- or middle-income country (LMIC) (according to the World Bank, to minimise differences in healthcare systems), were not observational in design (intervention studies or qualitative studies), full text was not available, or abstract was published in conference proceedings.
Study selection
The database Embase (accessed through Ovid, last searched 25/03/3023) was used to search for relevant articles. Separate searches were carried out using the following terms in the titles of articles: sepsis OR septic in combination with one of the following groups of terms:
Socioeconomic factors – depriv* or socioeconomic or socio-economic or socio or social or SES or IMD or income or occupation or education.
Race/ethnicity factors – race or racial or ethnic* or minorit*.
Community factors – urban* or rural or coast*.
Medical vulnerability factors – residen* or care home or nursing home or care facility or living or social care or drug* or alcohol or disabil* or vulnerab*.
Pregnancy – pregnan* or matern* or “post-partum” or “postpartum”.
Duplicated articles were removed. All articles identified in the search went through a title and abstract screening to exclude ineligible articles. A full article review was then performed on the remaining papers, with any ineligible articles identified during the full paper review being excluded. In accordance with the PRISMA guidelines, the reasons for exclusion at this stage were recorded (see Fig. 1). Any further duplicates (studies that appeared in multiple searches) were also removed. The searches were performed in Ovid and the results were downloaded to Mendeley Reference Manager to apply the inclusion/exclusion criteria. Data extracted from the eligible articles were stored in Microsoft Excel. The following information was extracted from each included paper: title, authors, year published, study design, country where study was conducted, data source, sepsis identification method, number of patients in sepsis cohort, factors associated with inequality used in study and how they are measured, outcome(s) assessed in the study and key findings of associations between the factors and outcomes. For reporting we referred to the PRISMA guidelines [16] for systematic reviews, however, as this is a rapid review not all items are relevant. Further details of the search strategy can be found in the supplementary information.
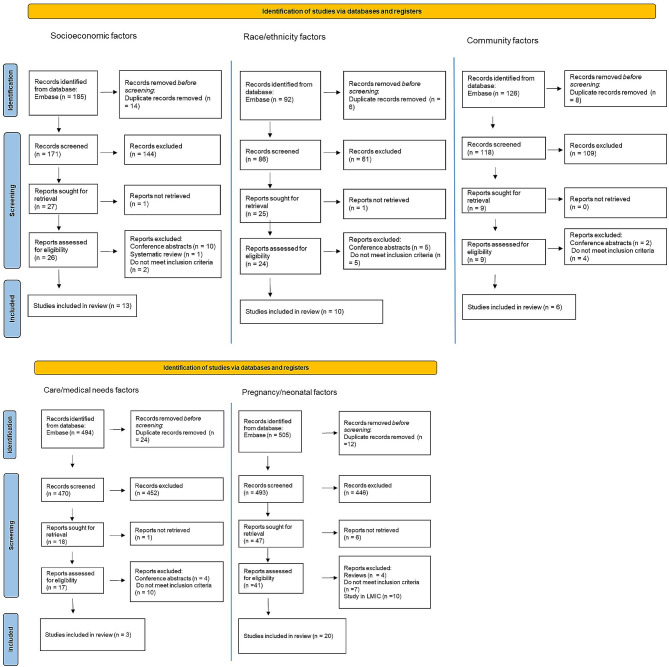
Fig. 1.
Flowchart showing search results and screening of studies. Five separate searches were conducted. After screening 53 papers were included
Results
Selection of sources of evidence
The five searches returned a total of 1,402 results (185 socioeconomic, 92 race/ethnicity, 126 community, 494 medical/care needs, 505 pregnancy). After deleting duplicates, 1,338 papers were screened on title and abstract, with 1,254 excluded. 108 papers underwent full article screening, after which 53 were eligible. Of these, there were 13 articles assessing socioeconomic factors, 14 race or ethnicity, 3 assessing community, 3 care/medical needs, and 20 assessing pregnancy and post-partum factors. As the searches and selection were conducted separately there were 3 papers duplicated between the searches, resulting in 50 unique papers to include. Flowcharts showing the selection process for each search are shown in Fig. 1.
Characteristics of sources of evidence
Table 1 displays the characteristics of studies included in the review. The majority of the included studies (31 out of 50) used data collected in the United States of America (USA) [17–47], with four in the UK [48–51], three in Israel [41, 52, 53], two in Canada [54, 55], one in Australia [56] and the others in Europe [57–63] or not specified [64, 65].Most of the studies used hospital or ICU discharge databases [17, 21, 23–25, 28–31, 36–39, 41–45, 47, 50, 60], other sources included national birth or obstetric registries [48, 49, 51, 62, 63], death registry datasets [27, 35], secondary analysis on data collected for other cohort studies [18, 26, 61], and a US cities public health dataset [22]. Six identified sepsis in neonatal patients or infants [21, 47, 53, 55, 56, 63] and another only included children aged between 0 and 20 years [28]. The rest either specified adults only or did not specify any age restrictions for the cohorts.
Table 1.
Characteristics of all included studies in the review
| Title | Authors | Year | Country | Data source | Population | Sepsis Identification | Factors associated with inequality | Outcome (s) | Sepsis cohort size |
|---|---|---|---|---|---|---|---|---|---|
| Association between sepsis incidence and regional socioeconomic deprivation and health care capacity in Germany - an ecological study | Rose N; Matthaus-Kramer C; Schwarzkopf D; Scherag A; Born S; Reinhart K, Fleischmann-Struzek C | 2021 | Germany | Inpatient database (DRG) covering all acute-care hospitals in Germany (except prison hospitals and psychiatric facilities) | Inpatient admissions in 2016, do not specify age restrictions | Explicit ICD-10 codes and Angus ICD-10 code criteria | Socioeconomic | Crude and age-standardised incidence of sepsis per district in 2016 | 146,985 |
| Association of neighbourhood socioeconomic status with risk of infection and sepsis | Donnelly JP; Lakkr S; Judd SE; Levitan EB; Griffin R; Howard G; Safford MM; Wang HE | 2018 | USA | Reasons for Geographic and Racial Differences in Stroke (REGARDS) study | Hospital admissions between 2003–2012, adults aged over 45 | Chart review using Sepsis-3 criteria | Socioeconomic | Hospital admissions and ED visits for serious infection/sepsis | 964 |
| Black-white racial disparities in sepsis: a prospective analysis of the Reasons for Geographic And Racial Differences in Stroke (REGARDS) cohort | Moore J; Donnelly J; Griffin R; Safford M; Howard G; Baddley J; Wang H | 2015 | USA | Reasons for Geographic and Racial Differences in Stroke (REGARDS) study | Hospitalisations for adults over 45 between 2003–2012 | SIRS criteria | Race/ethnicity | Rates of sepsis | 1,526 |
| Socioeconomic status and risk of intensive care unit admission with sepsis | Storm, L; Schegelsberg, A; Andersen, LW; Jessen, MK; Kirkegaard, H | 2018 | Denmark | Tertiary ICU records | Tertiary ICU admissions with, 2008–2010, adults over 18 years old. Matched to up to 10 controls from background population on age, sex & zip code | severe sepsis or septic shock, not stated how identified | Socioeconomic | Admission to ICU with sepsis | 383 |
| Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis | Mayr F; Yende S; Linde-Zwirble W; Peck-Palmer O; Barnato A; Weissfeld L; Angus D | 2010 | USA | Hospital discharge databases of 7 US states | Admissions for all ages with severe sepsis in 2005 | ICD-9 codes | Race/ethnicity | Incidence rates of severe sepsis hospitalisations. | 381,787 |
| Direct and indirect effects of socioeconomic status on sepsis risk and mortality: a mediation analysis of the HUNT study | Stensrud VH; Gustad LT; Damas JK; Solligard E; Krokstad S; Nilsen TIL | 2023 | Norway | Population-based HUNT studies | Adults aged between 20 and 70 admitted to hospital, 1995–1997 and 2006–2008 | ICD-9 & ICD-10 codes, both explicit and implicit list | Socioeconomic | Sepsis and sepsis-attributable mortality | 4,200 |
| Where you live matters: the place of residence on severe sepsis incidence and mortality | Goodwin AJ; Nadig NR; McElligot JT; Simpson KN; Ford DW | 2016 | USA | Hospital discharge database in South Carolina | Adults (> 20 years of age) hospitalised with severe sepsis or septic shock | ICD-9 codes including Martin criteria | Care/medical | Age-adjusted severe sepsis incidence and in-hospital mortality rates | 24,395 |
| Socio-demographic characteristics associated with hospitalisation for sepsis among adults in Canada: a Census-linked cohort study | Hennessy DA; Soo A; Niven DJ; Jolley RJ; Posadas-Calleja J; Stelfox HT; Doig CJ | 2020 | Canada | National hospital discharge database linked to Canadian census | Adults over 18 years admitted with sepsis and severe sepsis, 2006–2009 | ICD-10 codes | Socioeconomic | Hospital admission with a diagnosis of sepsis, secondary in-hospital death, admission to special care unit & LOS, hospital LOS, discharge disposition. | 10,400 |
| Racial differences in sepsis mortality at US academic medical center-affiliated hospitals | Chaudhary N; Donnelly J; Wang H | 2018 | USA | Hospital discharge data from Vizient, covers 120 medical centers and 300 hospitas in US. | Adults > 18 years old hospitalised with sepsis 2012–2014, | Angus ICD-9 codes | Race/ethnicity | Sepsis hospitalisation and hospital mortality. | 1,114,386 |
| Association of household income level and in-hospital mortality in patients with sepsis: a nationwide retrospective cohort analysis | Rush B; Wiskar K; Celi LA; Walley KR; Russell JA; McDermid RC; Boyd, JH | 2018 | USA | Nationwide Inpatient Sample (NIS), covers 20% of national hospital admissions | Identified patients over 18 years with sepsis admission in 2011 | Angus ICD-10 code criteria | Socioeconomic | In-hospital mortality | 671,858 |
| Impact of socioeconmic status on mortality and unplanned readmission in septic intensive care unit patients | Schnegelsberg, A; Mackenhauer, J; Nibro, HL; Dreyer, P; Koch, K; Kirkegaard, H | 2016 | Denmark | Single ICU records | Tertiary ICU admissions with, 2008–2010, adults over 18 years old | severe sepsis or septic shock, not stated how identified | Socioeconomic | 30-day mortality after ICU admission; 180-day mortality after hospital discharge, 180-day unplanned readmission after discharge | 387 |
| Lower socioeconomic factors are associated with higher mortality in patients with septic shock | Hidalgo DC; Tapaskar N; Rao S; Masic D; Su A; Portillo J; Rech M | 2021 | USA | Two hospitals dicharge records | Hospital admissions between 2017–2019, adults aged over 18 | Sepsis-3 criteria for septic shock | Socioeconomic | 30-day mortality, ICU length of stay, hospital length of stay | 362 |
| Race does not impact sepsis outcomes when considering socioeconomic factors in multilevel modeling | Vazquez Guillamet MC; Dodda S; Liu L; Kollef MH; Micek ST | 2022 | USA | Single centre records | Admissions with sepsis or septic shock, all adults (age not specified) 2010–2017 | ICD-9 and ICD-10 codes | Socioeconomic; race/ethnicity | In-hospital mortality, hospital LOS, use of vasopressors, use of mechanical ventilation | 11,432 |
| Race, income and insurance status affect neonatal sepsis mortality and healthcare resource utilisation | Bohanon FJ;. Lopez ON; Adhikari D; Mehta HB; Rojas-Khalil Y; Bowen-Jallow, KA; Radhakrishnan, RS | 2018 | USA | Healthcare Cost and Utilisation Project’s (HCUP) Kids Inpatient Database (KID) | Sepsis admissions in neonates (< 28 days old) in 2006, 2009 & 2012. | ICD-9 codes | Socioeconomic; race/ethnicity | In-hospital mortality, LOS and total costs | 116,882 |
| Association between race and case fatality rate in hospitlisations for sepsis | Sandoval E; Chang DW | 2016 | USA | Healthcare Cost and Utilization Project (HCUP) State Inpatient Database (SID) and California OSHPD Patient Discharge Pivot Database | Adults over 18 years admitted in 2011 | ICD-9 codes Martin criteria | Race/ethnicity | Case-fatality rate | 131,831 |
| Association of gender, age and race on renal outcomes and mortality in patients with severe sepsis and septic shock | Cerceo E; Rachoin JS; Gaughan J; Weisberg L | 2021 | USA | National Inpatient Sample (NIS) | Patients discharged between 2005–2014 after admission for severe sepsis or septic shock | ICD-9 codes for severe sepsis or septic shock | Race/ethnicity | In-hospital mortality | 1,064,790 |
| Ethnicity and sepsis characteristics and outcomes. Population based study | Karp G; Perl Y; Fuchs L; Almog Y; Klein M; Vodonos A; Drieher J: Talmor D; Codish S; Novack V | 2013 | Israel | Single ICU records, sepsis id’d with ICD-9 codes, between 2002–2008 | All adults aged over 20 with sepsis admission, 2002–2008 | ICD-9 codes | Race/ethnicity | In-hospital/28 day mortality | 1,542 |
| Impact of older age and nursing home residence on clinical outcomes of US emergency department visits for severe sepsis | Ginde A; Moss M; Shapiro N; Schwartz R | 2013 | USA | National Hospital Ambulatory Medical Care Survey (NHAMCS) | Adults over 18 years with sepsis ED visits, 2005–2009 | Angus ICD-9 codes | Care/medical | In-hospital mortality, hospital LOS, admission to ICU | 350,000 (estimate) |
| Hospital outcomes for children with severe sepsis in the USA by race or ethnicity and insurance status: a population-based, retrospective cohort study | Mitchell H; Reddy A; Montoya-Williams D; Harhay M; Fowler J; Yehya N | 2020 | USA | Healthcare Cost and Utilization Project (HCUP) Kids Inpatient Database (KID), covering 4200 US hospitals | Patients aged 0–20 years with severe sepsis in 2016 | ICD-10 codes | Race/ethnicity | In-hospital mortality, hospital LOS (death as competing risk, censored at 30 days) | 9,816 |
| Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004–2013 | Jones J; Fingar K; Miller M; Coffey R; Barrett M; Flottemesch T; Heslin K; Gray D; Moy E | 2017 | USA | Healthcare Cost and Utilization Project (HCUP) State Inpatient Database (SID) | Sepsis admissions, no age restrictions | Angus ICD-9 codes | Race/ethnicity | In-hospital mortality rates per 1,000 total sepsis hospitalisations | 16,779,820 |
| Treatment in disproportionately minority hospitals is associated with increased risk of mortality in sepsis: a national analysis | Rush B; Danziger J; Walley KR; Kumar A; Celi LA | 2020 | USA | National Inpatient Sample (NIS) | Sepsis admissions between 2008–2014, adults over 18 years | Angus ICD codes (doesn’t specify version) | Race/ethnicity | In-hospital mortality | 4,221,221 |
| Temporal trends in rural vs. urban sepsis-related mortality in the United States, 2010–2019 | Oud L; Garza J | 2022 | USA | CDC and Prevention Wide Ranging Online Data for Epidemiological Research Multiple Cause of Death dataset | Sepsis-related deaths in US from 2010–2019 | not stated | Community | Age-adjusted mortality rates per 100,000 population | |
| The effect of community socioeconomic status on sepsis-attributable mortality | Galiatsatos P; Brigham EP; Pietri J; Littleton K; Hwang S; Grant MC; Hansel NN; Chen ES | 2018 | USA | Neighborhood Health Profiles for Baltimore City, includes demographic & outcome data for 55 community statistical areas. | 2017 | not stated | Socioeconomic | Sepsis-attributable mortality | |
| Disparities in sepsis mortality by region, urbanisation, and race in the USA, a multiple cause of death analysis | Ogundipe F; Kodadhala V; Ogundipe T; Mehari A; Gillum R | 2019 | USA | CDC multiple cause of death data | Sepsis deaths in people aged 15 or over, between 2013 and 2016 | ICD-10 codes | Race/ethnicity; community | Age-adjusted sepsis death rates | 746,725 |
| Rural patients with severe sepsis or septic shock who bypass rural; hospitals have increased mortality: an instrumental variables approach | Mohr N; Harland K; Shane D; Ahmed A; Fuller B; Ward M; Torner J | 2017 | USA | Administrative claims from emergency departments of midwestern state | All adults over 18 between 2005 and 2014 | ICD-9 codes | Community | Hospital mortality, subsequent interhospital transfer, hospital LOS | 13,461 |
| Sepsis survivors admitted to nursing facilities: cognitive impairment, activities of daily living dependence, and survival | Ehlenbach W; Gilmore-Bykovski A; Repplinger M; Westergaard R; Jacobs E; Kind A; Smith M | 2018 | USA | Centers for Medicare and Medicaid Services (CMS) Chronic Conditions Data Warehouse (CCW) | People aged > 65 with severe sepsis episode, 2005–2009 | Angus ICD-9 codes | Care/medical | 1- year mortality, cognitive impairment, ADL | 175,755 |
| Race and sex based disparities in sepsis | Engoren M; Arslanian-Engoren C | 2022 | USA | Michigan hospital administrative dataset | First episode sepsis between 2009–2019, adults over 18 years | Sepsis-3 criteria | Race/ethnicity | 90-day mortality, mechanical ventilation, RRT, time to initial antibiotic, post-sepsis hospital stay | 34,999 |
| Racial disparities in readmissions following initial hospitalisation for sepsis | Lizza B; Betthauser K; Juang P; Hampton N; Lyons P; Kollef M; Micek S | 2021 | USA | Tertiary care referral center | Patients hospitalised for severe sepsis and septic shock (and survived) between 2010–2017 | ICD-9 and ICD-10 codes for severe sepsis and septic shock. | Race/ethnicity | All-cause readmission, sepsis readmission, postdischarge death. | 3,390 |
| The association between neighborhood socioeconomic disadvantage and readmissions for patients hospitalised with sepsis | Galiatsatos P; Follun A; Alghanim F; Sherry M; Sylvester C; Daniel Y; Chanmugam A; Townsend J; Saria S; Kind AJ; Chen E | 2020 | USA | Single hospital records | Sepsis admissions who survived to discharge, adults over 18 years in 2017 | ICD-10 codes then reviewed charts for Sepsis-3 criteria | Socioeconomic | 30-day readmission | 531 |
| Inclusion of social determinants of health improves sepsis readmission prediction models | Amrollahi F; Shashikumar S; Meier A; Ohno-Machado L; Nemati S; Wardi G | 2022 | USA | AllofUs study covering 35 hospitals | Patients over 18 years old admitted to hospital between 2017 and 2021 | Sepsis-3 criteria | Socioeconomic | 30-day unplanned readmission in sepsis patients | 8,935 |
| Risk factors, etiologies, and screening tools for sepsis in pregnant women: a multicenter case-control study | Bauer M; Housey M; Bauer S; Behrmann S; Chau A; Clancy C; Clark E; Einav S; Langen E; Leffert L; Lin S; Madapu M; Maile M; Mcquaid-Hanson E; Priessnitz K; Sela H; Shah A; Sobolewski P; Toledo P; Tsen L; Bateman B | 2019 | USA/Israel | Admissions data from 7 hospitals | Delivery admissions between 1994–2012 (depending on centre), matched 1:4 to non-sepsis controls | ICD-9 codes for sepsis, severe sepsis or septic shock, with manual chart review | Pregnancy | Risk factors | 82 |
| Severe sepsis in women with group B Streptococcus in pregnancy: An exploratory UK national case-control study | Kalin A; Acosta C; Kurinczuk J; Brocklehurst P; Knight M | 2015 | UK | UK Obstetric Surveillance System | All cases of severe maternal sepsis caused by Group B Streptococcus (GBS), 2011–2012, matched with controls who were non-sepsis deliveries | Prospective chart review, severe sepsis with laboratory confirmation of GBS | Pregnancy | Incidence of GBS sepsis, risk factors | 30 |
| The continuum of maternal sepsis severity: incidence and risk factors in a population-based cohort study | Acosta C; Knight M; Lee H; Kurinczuk J; Gould J; Lyndon A | 2013 | USA | California Vital Statistics records linked with statewide hospital discharge data | Maternal sepsis cases among all in-hospital live births, 2005–2007 | ICD-9 codes | Pregnancy | Incidence of sepsis and severe sepsis, risk factors | 1,598 |
| Maternal obesity, obstetric interventions and post-partum anaemia increase the risk of post-partum sepsis: a population-based cohort study based on Swedish medical health registers | Axelsson D; Blomberg M | 2017 | Sweden | Medical Birth Registry, National Patient Register and Prescribed Drug Register | Maternal sepsis in women who gave birth between 1997–2012 | ICD-10 codes | Pregnancy | Incidence and risk factors for post-partum sepsis | 376 |
| Severe maternal sepsis in the UK, 2011–2012: A National Case-Control Study | Acosta C; Kurinczuk J; Lucas D; Tuffnell D; Sellers S; Knight M | 2014 | UK | UK Obstetric Surveillance System | Maternal severe sepsis or septic shock, 2011–2012, matched to non-sepsis controls who delivered immediately before in same hospital | Prospective chart review | Pregnancy | Incidence and risk factors for severe maternal sepsis | 365 |
| Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study | Knowles S; O’Sullivan N; Meenan A; Hanniffy R; Robson M | 2015 | Ireland | Hospital records from two maternity hospitals | Pregnant and postpartum women with maternal sepsis between 2005–2012 | Prospective chart review identifying primary or secondary BSI | Pregnancy | Incidence, maternal mortality and neonatal mortality | 272 |
| Case fatality and adverse outcomes are reduced in pregnant women with severe sepsis or septic shock compared with age-matched comorbid-matched nonpregnant women | Kidson K; Henderson W; Hutcheon J | 2018 | USA | National Inpatient Sample | Severe sepsis or septic shock in pregnancy admissions, women aged between 15–44 years, between 1998–2012. Compared with severe sepsis in non-pregnant women of the same age | ICD-9 codes | Pregnancy | Case fatality rates in pregnancy associated severe sepsis (PASS) and non-PASS (NPSS), hospital LOS | 5,968 |
| Maternal morbiditiy and mortality from severe sepsis: a national cohort study | Acosta C; Harrison D; Rowan K; Lucas D; Kurinczuk J; Knight M | 2016 | UK (excluding Scotland) | Intensive Care National Audit & Research Centre (ICNARC) Case Mix Programme | Severe sepsis in pregnant or recently pregnant women, admitted to critical care, between 2008–2010 | Infection, at least 3 SIRS criteria and at least one organ dysfunction | Pregnancy | Maternal critical care admission rates, risk factors and death rates | 646 |
| Contemporary trends of reported sepsis among maternal decedents in Texas: a population-based study | Oud L | 2015 | USA | Texas Inpatient Public Use Data File (TIPUDF), covers all state-licensed hospitals | Pregnancy associated hospitalizations between 2001–2010 | ICD-9 codes, for septic shock or infection (SIRS) in combination with organ failure | Pregnancy | Annual rate of sepsis among maternal decedents | 131 |
| Mortality associated with severe sepsis among age-similar women with and without pregnancy-associated hospitalization in Texas: a population-based study | Oud L | 2016 | USA | Texas Inpatient Public Use Data File (TIPUDF), covers all state-licensed hospitals | Pregnancy associated hospitalizations between 2001–2010 in women aged 20–34, compared with non pregnancy-associated hospitalizations | ICD-9 codes, for septic shock or infection (SIRS) in combination with organ failure | Pregnancy | Hospital mortality, number and type of failing organs | 449 |
| Progression from severe sepsis in pregnancy to death: a UK population-based case-control analysis | Mohamed-Ahmed O; Nair M; Acosta C; Kurinczuk J; Knight M | 2015 | UK | UK Obstetric Surveillance System and Confidential Enquiry into Maternal Death (CEMD) | Maternal deaths from non-influenza sepsis, compared with women who survived, between 2009–2012 | Prospective chart review | Pregnancy | Risk factors associated with sepsis death | 401 |
| The sepsis in obstetrics score: a model to identify risk of morbidity from sepsis in pregnancy | Albright C; Ali T; Lopes V; Rouse D; Anderson B | 2014 | Discharge records from single centre | Pregnant and postpartum women presenting at ED with suspected SIRS or sepsis, between 2009 to 2011 | SIRS with swab confirmation | Pregnancy | Morbidity - ICU admission within 48 h of presentation to ED, mortality, adverse perinatal outcome | 850 | |
| Severe sepsis and septic shock in pregnancy: indications for delivery and maternal and perinatal outcomes | Snyder C; Barton J; Habli M; Sibai B | 2013 | Hospital records from two hospitals | Obstetric patients treated in ICU between 1995 to 2010 with severe sepsis or septic shock | ICD-9 codes confirmed with chart review | Pregnancy | Mortality, morbidity | 30 | |
| Perinatal outcomes among patients with sepsis during pregnancy | Blauvelt C; Nguyen K; Cassidy A; Gaw S | 2021 | USA | Hospital records from single center | Patients who delivered at 20 weeks gestation or later, with antepartum clinical concern for sepsis discharged before delivery, 2012–2018 | ICD-9 and ICD-10 codes | Pregnancy | Composite perinatal outcome of 1 or more of the following: fetal growth restriction, oligohydramnios, hypertensive disease of pregnancy, cesarean delivery, infant born small for gestational age, or stillbirth | 59 |
| Correlation of bacterial type and antibiotic sensitivity with antibiotic exposure in early-onset neonatal sepsis | Bromiker R; Ernest N; Meir M; Kaplan M; Hammerman C; Schimmel M; Schlesinger Y | 2013 | Israel | Hospital records from single center | Infants born betweem 1997–2007 with early onset neonatal sepsis | Culture confirmed infection | Pregnancy | Isolates with antibiotic resistance, Gram-negative isolates | 94 |
| Is peri-partum maternal fever alone a reliable predictor of neonatal sepsis? A single-centre, retrospective cohort study | Gupta S; Forbes-Coe A; Rudd D; Kandasamy Y | 2021 | Australia | Hospital records from single center | Term infants admitted to neonatal ICU with sepsis, between 2015 to 2020 | Culture-proven neonatal bacteraemia and sepsis | Pregnancy | Predictors of EONS | 14 |
| Early-onset sepsis: a predictive model based on maternal risk factors | Puopolo K; Escobar G | 2013 | USA | Hospital records from multiple centers | Live births > = 34 weeks gestation with EONS from 1995–2007, matched to controls | Pregnancy | Predictors of EONS | 350 | |
| Maternal obesity and risk of early onset neonatal bacterial sepsis: nationwide cohort and sibling-controlled studies | Villamor E; Norman M; Johansson S; Cnattingius S | 2020 | Sweden | Medical Birth Register | Live births > = 22 weeks, between 1997–2016 with EOS admission to neonatal unit within 72 h of birth | ICD-10 codes | Pregnancy | Incidence and risk factors of EONS | 2,913 |
| Maternal antibiotic exposure and risk of antibiotic resistance in neonatal early-onset sepsus: a case-cohort study | Wright A; Unger S; Coleman B; Lam P; McGeer A | 2012 | Canada | Hospital records from single center | Admissions to neonatal ICU within 24 h after birth, between 2008–2010 | Confirmed serious bacterial infection | Pregnancy | Antibiotic resistance in EONS | 60 |
ED – emergency department, LOS – length of stay, RRT – renal replacement therapy, EONS – early onset neonatal sepsis, ICU – intensive care unit, ADL – activities of daily living
The most common method of identifying sepsis in the studies was based on ICD codes. ICD-10 codes were used in twelve studies [17, 20, 27, 28, 32, 40, 46, 54, 60–63], ICD-9 codes were used in twenty-one studies [20, 21, 24, 25, 29, 31–33, 36–39, 41–46, 52, 61, 65] and one study did not specify which ICD version [34]. Of these studies, some used specific codes for sepsis, severe sepsis or septic shock whilst others used more comprehensive sets of codes including the Angus criteria [66] or Martin criteria [67]. Both the Angus and Martin methods include the sepsis specific codes and non-specific ICD codes for infection in combination with a code for organ dysfunction. Five studies [18, 19, 22, 23, 30] used the 2016 International Consensus definition for sepsis, otherwise known as the Sepsis-3 criteria [1]. Other studies used Systemic Inflammatory Response Syndrome with [50] and without criteria for organ dysfunction [26, 64], medical chart review [48, 49, 51, 53, 55, 56, 59] or did not specify [22, 35, 47, 57, 58]. The size of the sepsis patient cohorts varied from 14 [56] to 16,779,820 [[33] with a median cohort size of 2,913.
Regarding outcomes, 15 studies assessed the incidence or risk of sepsis [18, 26, 29, 31, 37, 41, 42, 48–50, 54, 57, 59–62], 23 studies looked at in-hospital (or short-term) mortality [17, 19–21, 24, 25, 28, 31, 33, 34, 36, 37, 39, 43–45, 50–52, 54, 58, 59, 65], four studies assessed mortality after hospital discharge [30, 32, 38, 58], five studies assessed hospital readmission rates after discharge [23, 30, 32, 40, 58] and four studies calculated population-level sepsis mortality rates [22, 27, 35, 61]. Seven of the studies relating to pregnancy assessed adverse perinatal outcomes and incidence of sepsis in neonates [46, 47, 53, 55, 56, 63, 64].
Results of individual sources of evidence
Socioeconomic factors
The most common socioeconomic factors were income, level of education, employment status, unemployment rate and poverty rate. Others included were insurance status, occupation, cohabitation status and access to healthcare. There was variation in whether these were recorded at an individual level or matched to local data based on small area geographic identifiers (ZIP/postcode), and whether they were summarised into an overall score or included as individual covariates.
Five studies assessed the impact of socioeconomic factors on sepsis incidence or risk of developing sepsis. Factors found to be associated with increased risk of sepsis included low income [54, 60], low education level [57, 60, 61], lower socioeconomic status [18], marital/living status [54, 57] not being in work [54, 57], lower class of occupation and those who receive social benefits [61]. Three studies assessed 30-day or in-hospital mortality, which all found lower income was associated with increased risk of mortality when compared to the highest income groups. Another study reported decreased odds for highest household income quartile compared to the lowest quartile [20]. Hidalgo et al. [19] reported that unemployment and a neighbourhood poverty rate > 10% were all predictive of greater 30-day mortality. One study calculated population sepsis mortality rates per 10,000 persons and compared between income and poverty levels. Low-income neighbourhoods had a death rate of 3.65 (inter-quartile range (IQR) 2.78–4.40) versus high income neighbourhoods 2.80 (IQR 2.05–3.55) and high poverty neighbourhoods 4.20 (IQR 2.90–5.30) versus low poverty neighbourhoods 2.90 (IQR 2.00-3.60) [22]. For longer-term outcomes, two studies assessed the impact of socioeconomic factors on 30-day readmission after discharge. Lower income, lack of health insurance [23] and being more socioeconomically disadvantaged [23] were found to be associated with increased risk.
Race & ethnicity
Of the 14 studies assessing the impact of race or ethnicity on sepsis, 13 were based in the USA. Five of these studies only included race categorised as white or black/African-American [25,26,29,31,32], whilst other studies included categories for Hispanic [21, 24, 27, 28, 33, 34], Asian-American [24, 30], Asian/Pacific Islander (API) or Native American [21, 28, 33] and other/unknown. The study by Rush et al. [34] used a different approach by classifying hospitals as non-minority or minority, if the patient population of the hospital was more than twice the geographical census division mean.
The studies by Chaudhary et al. [31], Mayr et al. [29] and Moore et al. [26] compared rates of sepsis amongst either black or white populations only. Chaudhary et al. reported a higher sepsis rate for white patients compared to black patients with 109.4 cases (95% CI 109.2-109.6) per 1,000 hospitalisations versus 106.7 cases (95% CI 106.3-107.1). Moore et al. also reported a higher incidence of sepsis in white patients (9.10 per 1,000 person years) compared to black patients (6.93 per 1,000 person years). Contrary to these, Mayr et al. found a 67% higher severe sepsis hospitalisation rate in black patients (9.4 per 1,000 population) than white (5.6 per 1,000 population). All three studies covered hospital admissions in multiple US states, but they did differ in the age of patients included and severity of sepsis.
Eight studies considered the impact of race on in-hospital mortality in sepsis patients, with mixed results. Three studies reported higher mortality rates or increased risk of mortality in black or African-American patients than white patients [25, 28, 33] whilst Sandoval et al. [24] reported higher case fatality rates in white patients (15.1%) compared to black (14.0%), Hispanic (13.8%) or Asian patients (16.2%). One study [33] reported increased mortality rates in Hispanic patients compared to white patients, but two other studies did not find significant differences [21, 28]. Rush et al. reported unadjusted mortality rates at non-minority hospitals of 11.1%, compared with 12.3% (p < 0.001) at minority black hospitals and 12.7% (p < 0.001) at minority Hispanic hospitals. The only non-USA based study was based in Israel. Karp et al. [52] found that differences in risk of in-hospital mortality between Bedouin Arabs and the Jewish population could be explained by differences in age and Charlson comorbidity score.
For longer-term outcomes, one study [30] reported small differences in 90-day mortality rates between African American (18%), Asian-American (19%) and white (22%) patients. Lizza et al. [32] reported black patients had significantly higher rates of all-cause readmission (71.1% vs. 60.8%, p < 0.001) and sepsis readmission (19.8% vs. 14.0%, p < 0.001) than white patients. However, rates of post-discharge death were similar (white patients 36.5% vs. black patients 36.7%, p = 0.876). Ogundipe et al. [27] calculated age-adjusted sepsis death rates in non-Hispanic black, non-Hispanic white and Hispanic populations and reported lower death rates in Hispanic populations than non-Hispanic populations.
Community factors
The three papers included in the review that looked at community factors were conducted in the USA and used different ways of measuring urbanicity or rurality. Oud et al. [35] compared age-adjusted sepsis mortality rates between rural and urban communities from 2010 to 2019. The study reported in 2019 the overall rural rate was 57.9 deaths per 100,000, but in urban areas it was 48.3 deaths per 100,000 population. This was not a consistent pattern when adjusting for race. For example, in non-Hispanic blacks the urban mortality rates were higher than the rural rates. Ogundipe et al. [27] found the highest age-adjusted sepsis death rates were in non-metropolitan areas for both non-Hispanic black (micropolitan area 120.4 per 100,000 population, non-core area 109.4 per 100,000) and non-Hispanic white populations (micropolitan area 67.6 per 100,000, non-core area 66.4 per 100,000). Mohr et al. [36] assessed whether there were differences in patients in rural areas who attended their local emergency department or who bypassed their local hospital and travelled further to present to a hospital of top-decile inpatient sepsis volume. Sepsis patients who bypassed their local hospital had increased odds of mortality, with an OR of 1.26 (95% CI 1.03–1.53).
Medical needs
The three studies that considered factors relating to additional medical needs each used different measures. Goodwin et al. [37] identified patients living in medically underserved areas (MUA’s) based on the ratio of primary care physicians per 1,000 population, infant mortality rate, the proportion of the population with income below the poverty level and the proportion of the population over 65 years of age. The study reported higher incidence of sepsis (8.6 vs. 6.8 admissions per 1,000 people, p < 0.01) and mortality rates (15.5 versus 11.9 deaths per 10,000, p < 0.01) in MUA residents compared to non-MUA. Ginde et al. [39] included residence in a nursing home prior to an emergency department visit for sepsis, and reported increased risk of mortality for nursing home residents (OR 3.1, 95% CI 1.2–7.8). The study by Ehlenbach et al. [38] found that sepsis patients not discharged to a skilled nursing facility (SNF) had a mortality rate of 35.6%, while those discharged to a SNF but whom had not been resident in an SNF prior to sepsis had a mortality rate of 43.2% and patients who had been in a SNF before and after sepsis had a mortality rate of 52.8%.
Pregnancy/maternity
Studies assessing incidence of maternal sepsis reported rates of severe sepsis of 1.00 per 100,000 [48] maternities, 4.7 per 10,000 [49] maternities and 4.9 per 10,000 live births [42]. Estimates of non-severe sepsis included 198.69 per 100,000 [59] maternities, 2.4 per 10,000 women [62] and 10 per 10,000 live births [42]. Acosta et al. [50] estimated the absolute risk of maternal critical care unit admission with severe sepsis was 4.1 per 10,000 maternities (95% CI 2.9–5.6). Factors including increased BMI [41, 50, 62], older age [42, 50, 62], black and other ethnic minority race [49], increased levels of deprivation [50], African American race [41], pre-existing medical conditions [41, 49], complications of delivery and delivery via caesarean Sects [41, 49, 50, 59, 62] were found to be associated with an increased risk of developing maternal sepsis.
Two studies [43, 44] assessing mortality in maternity patients reported lower case-fatality rates in pregnancy associated severe sepsis (PASS) compared to non-pregnancy associated severe sepsis (NPSS). Maternal mortality rates in other studies varied, with reported rates of 10% [65], 10.7% [51], 1.8/100,000 maternities [50] and no deaths in one study [59]. Increased BMI [50], being in the most deprived two IMD quintiles [50], pre-existing medical conditions [51] and being multi-parous [51] was found to be associated with increased maternal mortality. Antepartum sepsis was found to be associated with increased risk of placental dysfunction and maternal ICU admission during delivery hospitalization [46]. Five studies considered outcomes relating to early onset neonatal sepsis (EONS), with reported rates of 1.03 cases per 1,000 live births [53] and 1.48 per 1,000 live births [63]. Risk factors associated with EONS were maternal exposure to antibiotics [47, 53, 55], maternal BMI [63], caesarean section delivery [63] and gestational age [47].
Supplementary Tables 1 and 2 show the findings from all included studies and can be found in additional file 1.
Discussion
Summary of evidence
Socioeconomic factors associated with increased incidence of sepsis included lower socioeconomic status, unemployment, and lower education level, although findings were not consistent across studies. Studies assessing the association between ethnicity and sepsis rates reported mixed results, with two studies finding increased sepsis rates in white populations compared to black populations and another showing higher rates in black populations than white. Living in a medically underserved area or being resident in a nursing home was also shown to increase risk of sepsis. In terms of mortality, lower income, unemployment, and poverty levels were all associated with increased in-hospital mortality. In studies considering effects of ethnicity on in-hospital and longer-term mortality the results were mixed, with some studies finding no significant associations, some reporting increased odds of mortality in non-white populations and others reporting increased mortality in white populations. Sepsis mortality rates were also found to be higher in people living in rural areas and those who were resident in a skilled nursing facility.
It is notable that the literature is dominated by research conducted in the USA and none of the studies identified under the non-pregnancy related searches used UK data. This is an important consideration for healthcare and public health professionals outside of the USA as differences in structural inequalities between the USA and other high-income countries may make the results less generalisable. The majority of studies focused on in-hospital mortality as the primary outcome, so there also needs to be more focus on the risks of developing sepsis and longer-term outcomes such as healthcare utilisation.
The sources of data varied between the studies, as did the methods of identifying sepsis. Differences in sepsis definitions leads to different reported prevalence/cohort sizes [68]. Some of the studies were based in single centres and only included a few hundred patients, whilst others represented national populations and included millions of patients. Many of the studies used data from secondary care only and none used primary care data, even though the majority of cases of sepsis develop in the community rather than the hospital. Additionally, there was a lot of variation in measures used in the analyses, particularly in the studies assessing socioeconomic factors, where there was no standardised definition of socioeconomic status and therefore results varied. There were some studies who assessed a combination of socioeconomic, community and race factors, however, some only focused on one area related to health inequality. This is important as there is overlap between the different areas. The paper by Vazquez Guillamet et al. [20] concluded that race did not have a significant effect on sepsis mortality when accounting for socioeconomic variables. A commentary piece published in 2018 by Shankar-Hari and Rubenfeld [69] titled “Race, ethnicity and sepsis: beyond adjusted odds ratios” suggested that there needs to be more research into the underlying causes of race/ethnicity disparities not just in sepsis but in wider health areas. Future studies should take into account not just socioeconomic status and population demographics, which will likely vary between ethnic groups, but also consider the intersectionality between these and other factors such as comorbidity levels and health behaviours e.g. smoking, alcohol use or exercise.
Limitations
Due to the rapid nature of the review the scope was limited and the search strategy not as comprehensive as for systematic reviews. We searched for the key terms in the titles only, searched a single database (Embase) and only included studies published from 2010 onwards. We also acknowledge that pathogen specific publications which do not specifically include the word sepsis may have been screened out. Examples include those that report on invasive group A and B streptococcal disease [67]. Studies conducted in LMICs were excluded as the results will be less generalisable to the UK population. Whilst the burden of sepsis is highest in LMICs there is a lack of good quality data from these countries [70]. The challenges in recognising and managing sepsis within LMICs, such as lack of access to healthcare, malnutrition and infrastructure [71], are not as applicable in higher- income countries. Some aspects of the Core20PLUS5 approach to addressing health inequalities were not included in this rapid review. These mainly related to inclusion health groups, including people with multi-morbidities, vulnerable migrants, Gypsy, Roma and Traveller communities, sex workers, people in contact with the justice system and victims of modern slavery. Additionally, the 4 comorbidities/conditions within the ‘5’ component other than maternity were not areas of focus (severe mental illness, COPD, cancer & hypertension). The five included areas were chosen as they are the factors that cover the largest groups in the population and were identified as the most important. Although we did not include the other areas in our review it is still vital that future studies consider these aspects in order to address all potential influences on sepsis risks and outcomes. The bias of the included studies was not assessed, nor did we critically appraise them.
Future work
From the studies identified, there are clear correlations between sepsis morbidity and mortality and the presence of factors associated with health inequalities. There is a need for UK based studies, using nationally representative data, to better understand how factors associated with health inequalities affect sepsis incidence and mortality in the UK population. With the availability of electronic health record data for research there are increasing opportunities to disaggregate the data and stratify risk by patient demographic. For example, in the UK the Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES) data provide nationally representative primary and secondary care records with linkage available to deprivation and socioeconomic scores. Once this is better understood, healthcare and public health professionals can be empowered to close the health gap and reduce inequalities through targeted recommendations for the recognition and early management of sepsis. Recent guidance in the UK highlighted the importance of early intervention in sepsis whilst balancing that with the need to use antibiotics more appropriately. Understanding which patients are at greater risk of sepsis mortality and morbidity, in terms of the factors associated with inequalities discussed in this review and other known risk factors, may help clinicians target antibiotic use more effectively. Given the lack of evidence from outside the USA, there is not sufficient information available to inform policy, at either a global level or an individual country level (except the USA). Although some of the findings from USA studies may be generalisable to other settings there needs to be further exploration of the similarities and differences in inequality factors in different populations. Critical to the above is improved coding in electronic health records alongside appropriate data linkage.
Conclusion
Factors relating to health inequalities such as deprivation and ethnicity have been shown to be associated with poorer outcomes in COVID-19 and increased rates of antimicrobial resistance. In order to inform local guidance and drive public health measures, there is a need for studies conducted across more diverse setting and countries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1: Table 1. Findings of studies assessing the impact of factors of health inequality on sepsis incidence or risk. Table 2. Findings of studies assessing the impact of factors of health inequality on sepsis mortality and other outcomes.
Additional file 2. Search strategy including PICO criteria and exact search terms.
Acknowledgements
none.
Author contributions
D.A.O., N.C., M.M., C.B, and E.G. devised the study. D.A.O., N.C., E.G., T.vS, S.B., X.Z, V.P., A.P., G.M. and S.B. devised search terms and inclusion/exclusion criteria. S.B. conducted the searches, extracted the data and wrote the manuscript. All authors reviewed and edited the manuscript.
Funding
This study was supported by funding from the UK Health Security Agency.
Data availability
All data generated or analysed during this study are included in this published article and supplementary materials.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth. 2017;119:626–36. doi: 10.1093/bja/aex234. [DOI] [PubMed] [Google Scholar]
- 3.Shankar-Hari M, Rubenfeld GD. Understanding long-term outcomes following Sepsis: implications and challenges. Curr Infect Dis Rep 18, (2016). [DOI] [PMC free article] [PubMed]
- 4.Shankar-Hari M, Rubenfeld GD, Ferrando-Vivas P, Harrison DA, Rowan K. Development, Validation, and clinical Utility Assessment of a Prognostic score for 1-Year unplanned rehospitalization or death of adult Sepsis survivors. JAMA Netw Open. 2020;3:e2013580. doi: 10.1001/jamanetworkopen.2020.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ 353, i2375 (2016). [DOI] [PMC free article] [PubMed]
- 6.Wang HE et al. Chronic medical conditions and risk of sepsis. PLoS ONE 7, (2012). [DOI] [PMC free article] [PubMed]
- 7.Wang HE, et al. Derivation of novel risk prediction scores for community-acquired sepsis and severe sepsis. Crit Care Med. 2016;44:1285–94. doi: 10.1097/CCM.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henriksen DP, et al. Risk factors for hospitalization due to Community-Acquired Sepsis – A Population-based case-control study. PLoS ONE. 2015;10:e0124838. doi: 10.1371/journal.pone.0124838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.CCM.0000194535.82812.BA. [DOI] [PubMed] [Google Scholar]
- 10.Academy of Medical Royal Colleges. Initial antimicrobial treatment of sepsis. https://www.aomrc.org.uk/reports-guidance/statement-on-the-initial-antimicrobial-treatment-of-sepsis-v2-0/ (2022).
- 11.NHS National Healthcare Inequalities Programme. Core20PLUS5. An approach to reducing health inequalities. (2021).
- 12.Capsoni N et al. Prevalence, risk factors and outcomes of patients coming from the community with sepsis due to multidrug resistant bacteria. Multidiscip Respir Med 14, (2019). [DOI] [PMC free article] [PubMed]
- 13.UK Health Security Agency. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report 2021 to 2022. (2022).
- 14.Zhong X, et al. Clinical and health inequality risk factors for non-COVID-related sepsis during the global COVID-19 pandemic: a national case-control and cohort study. EClinicalMedicine. 2023;102321. 10.1016/j.eclinm.2023.102321. [DOI] [PMC free article] [PubMed]
- 15.Galiatsatos P, Sun J, Welsh J, Suffredini A. Health disparities and Sepsis: a systematic review and Meta-analysis on the influence of race on Sepsis-related mortality. J Racial Ethn Health Disparities. 2019;6:900–8. doi: 10.1007/s40615-019-00590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ vol. 372 Preprint at 10.1136/bmj.n71 (2021). [DOI] [PMC free article] [PubMed]
- 17.Rush B, et al. Association of household income level and in-hospital mortality in patients with sepsis: a nationwide retrospective cohort analysis. J Intensive Care Med. 2018;33:551–6. doi: 10.1177/0885066617703338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly JP, et al. Association of neighborhood socioeconomic status with risk of infection and sepsis. Clin Infect Dis. 2018;66:1940–7. doi: 10.1093/cid/cix1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidalgo D, et al. Lower socioeconomic factors are associated with higher mortality in patients with septic shock. Heart Lung. 2021;50:477–80. doi: 10.1016/j.hrtlng.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Vazquez Guillamet M, Dodda S, Liu L, Kollef MH, Micek ST. Race does not impact sepsis outcomes when considering socioeconomic factors in multilevel modeling. Crit Care Med. 2022;50:410–7. doi: 10.1097/CCM.0000000000005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohanon FJ, et al. Race, income and insurance status affect neonatal sepsis mortality and healthcare resource utilization. Pediatr Infect Disease J. 2018;37:E178–84. doi: 10.1097/INF.0000000000001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galiatsatos P, et al. The effect of community socioeconomic status on sepsis-attributable mortality. J Crit Care. 2018;46:129–33. doi: 10.1016/j.jcrc.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amrollahi F, et al. Inclusion of social determinants of health improves sepsis readmission prediction models. J Am Med Inform Assoc. 2022;29:1263–70. doi: 10.1093/jamia/ocac060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandoval E, Chang DW. Association between Race and Case Fatality Rate in Hospitalizations for Sepsis. J Racial Ethn Health Disparities. 2016;3:625–34. doi: 10.1007/s40615-015-0181-0. [DOI] [PubMed] [Google Scholar]
- 25.Cerceo E, Rachoin J, Gaughan J, Weisberg L. Association of gender, age, and race on renal outcomes and mortality in patients with severe sepsis and septic shock. J Crit Care. 2021;61:52–6. doi: 10.1016/j.jcrc.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Moore JX, et al. Black-white racial disparities in sepsis: a prospective analysis of the REasons for Geographic and racial differences in stroke (REGARDS) cohort. Crit Care. 2015;19:279. doi: 10.1186/s13054-015-0992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogundipe F, Kodadhala V, Ogundipe T, Mehari A, Gillum R. Disparities in Sepsis Mortality by Region, Urbanization, and race in the USA: a multiple cause of death analysis. J Racial Ethn Health Disparities. 2019;6:546–51. doi: 10.1007/s40615-018-00553-w. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell HK, et al. Hospital outcomes for children with severe sepsis in the USA by race or ethnicity and insurance status: a population-based, retrospective cohort study. Lancet Child Adolesc Health. 2020;5:103–12. doi: 10.1016/S2352-4642(20)30341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayr FB, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303:2495–503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engoren M, Arslanian-Engoren C. Race and sex based disparities in sepsis. Heart Lung. 2022;52:37–41. doi: 10.1016/j.hrtlng.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at U.S. academic medical center-affiliated hospitals. Crit Care Med. 2018;46:878–83. doi: 10.1097/CCM.0000000000003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lizza BD, et al. Racial disparities in readmissions following initial hospitalization for Sepsis. Crit Care Med. 2021;E258–E268. 10.1097/CCM.0000000000004809. [DOI] [PubMed]
- 33.Jones JM, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004–2013. Crit Care Med. 2017;45:e1209–17. doi: 10.1097/CCM.0000000000002699. [DOI] [PubMed] [Google Scholar]
- 34.Rush B, Danziger J, Walley KR, Kumar A, Celi LA. Treatment in disproportionately minority hospitals is associated with increased risk of mortality in sepsis: a national analysis*. Crit Care Med. 2020;48:962–7. doi: 10.1097/CCM.0000000000004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oud L, Garza J. Temporal trends in Rural vs Urban Sepsis-related mortality in the United States, 2010–2019. Chest. 2022;162:132–5. doi: 10.1016/j.chest.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Mohr NM, et al. Rural patients with severe Sepsis or septic shock who Bypass Rural hospitals have increased mortality: an instrumental variables Approach. Crit Care Med. 2017;45:85–93. doi: 10.1097/CCM.0000000000002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin AJ, Nadig NR, McElligott JT, Simpson KN, Ford DW. Where you live matters: the impact of place of Residence on severe Sepsis incidence and mortality. Chest. 2016;150:829–36. doi: 10.1016/j.chest.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehlenbach WJ, et al. Sepsis survivors admitted to skilled nursing facilities: cognitive impairment, activities of Daily Living Dependence, and Survival. Crit Care Med. 2018;46:37–44. doi: 10.1097/CCM.0000000000002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginde AA, Moss M, Shapiro NI, Schwartz RS. Impact of older age and nursing home residence on clinical outcomes of US emergency department visits for severe sepsis. J Crit Care. 2013;28:606–11. doi: 10.1016/j.jcrc.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galiatsatos P, et al. The association between neighborhood socioeconomic disadvantage and readmissions for patients hospitalized with sepsis. Crit Care Med. 2020;48:808–14. doi: 10.1097/CCM.0000000000004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauer ME, et al. Risk factors, etiologies, and screening tools for Sepsis in pregnant women: a Multicenter Case-Control Study. Anesth Analg. 2019;129:1613–20. doi: 10.1213/ANE.0000000000003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acosta CD, et al. The continuum of maternal Sepsis severity: incidence and risk factors in a Population-based Cohort Study. PLoS ONE. 2013;8:e67175. doi: 10.1371/journal.pone.0067175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidson KM, Henderson WR, Hutcheon JA. Case fatality and adverse outcomes are reduced in pregnant women with severe sepsis or septic shock compared with age-matched comorbid-matched nonpregnant women. Crit Care Med. 2018;46:1775–82. doi: 10.1097/CCM.0000000000003348. [DOI] [PubMed] [Google Scholar]
- 44.Oud L. Mortality associated with severe sepsis among age-similar women with and without pregnancy-associated hospitalization in Texas: a population-based study. Med Sci Monit. 2016;22:1976–86. doi: 10.12659/MSM.896547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oud L. Contemporary trends of reported Sepsis among maternal decedents in Texas: a Population-based study. Infect Dis Ther. 2015;4:321–35. doi: 10.1007/s40121-015-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blauvelt CA, Nguyen KC, Cassidy AG, Gaw SL. Perinatal outcomes among patients with Sepsis during pregnancy. JAMA Netw Open. 2021;4:e2124109. doi: 10.1001/jamanetworkopen.2021.24109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puopolo KM, Escobar GJ. Early-onset sepsis: a predictive model based on maternal risk factors. Curr Opin Pediatr. 2013;25:161–6. doi: 10.1097/MOP.0b013e32835e1f96. [DOI] [PubMed] [Google Scholar]
- 48.Kalin A, Acosta C, Kurinczuk JJ, Brocklehurst P, Knight M. Severe sepsis in women with group B Streptococcus in pregnancy: an exploratory UK national case-control study. BMJ Open. 2015;5:e007976. doi: 10.1136/bmjopen-2015-007976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acosta CD, et al. Severe maternal Sepsis in the UK, 2011–2012: a National Case-Control Study. PLoS Med. 2014;11:e1001672. doi: 10.1371/journal.pmed.1001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acosta CD et al. Maternal morbidity and mortality from severe sepsis: a national cohort study. BMJ Open 6, (2016). [DOI] [PMC free article] [PubMed]
- 51.Mohamed-Ahmed O, Nair M, Acosta C, Kurinczuk JJ, Knight M. Progression from severe sepsis in pregnancy to death: a UK population-based case-control analysis. BJOG. 2015;122:1506–15. doi: 10.1111/1471-0528.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karp G, et al. Ethnicity and sepsis characteristics and outcomes. Population based study. Eur J Intern Med. 2013;24:34–9. doi: 10.1016/j.ejim.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Bromiker R, et al. Correlation of bacterial type and antibiotic sensitivity with maternal antibiotic exposure in early-onset neonatal sepsis. Neonatology. 2013;103:48–53. doi: 10.1159/000342215. [DOI] [PubMed] [Google Scholar]
- 54.Hennessy D, et al. Socio-demographic characteristics associated with hospitalization for sepsis among adults in Canada: a Census-linked cohort study. Can J Anesth. 2020;67:408–20. doi: 10.1007/s12630-019-01536-z. [DOI] [PubMed] [Google Scholar]
- 55.Wright AJ, Unger S, Coleman BL, McGeer AJ. Maternal antibiotic exposure and risk of antibiotic resistance in neonatal early-onset sepsis: a case-cohort study. Pediatr Infect Disease J. 2012;31:1206–8. doi: 10.1097/INF.0b013e31826eb4f9. [DOI] [PubMed] [Google Scholar]
- 56.Gupta S, Forbes-Coe A, Rudd D, Kandasamy Y. Is peripartum maternal fever alone a reliable predictor of neonatal sepsis? A single-centre, retrospective cohort study. J Paediatr Child Health. 2021;57:1420–5. doi: 10.1111/jpc.15492. [DOI] [PubMed] [Google Scholar]
- 57.Storm L, et al. Socioeconomic status and risk of intensive care unit admission with sepsis. Acta Anaesthesiol Scand. 2018;62:983–92. doi: 10.1111/aas.13114. [DOI] [PubMed] [Google Scholar]
- 58.Schnegelsberg A, et al. Impact of socioeconomic status on mortality and unplanned readmission in septic intensive care unit patients. Acta Anaesthesiol Scand. 2016;60:465–75. doi: 10.1111/aas.12644. [DOI] [PubMed] [Google Scholar]
- 59.Knowles SJ, O’Sullivan NP, Meenan AM, Hanniffy R, Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG. 2015;122:663–71. doi: 10.1111/1471-0528.12892. [DOI] [PubMed] [Google Scholar]
- 60.Rose N, et al. Association between sepsis incidence and regional socioeconomic deprivation and health care capacity in Germany - an ecological study. BMC Public Health. 2021;21:1636. doi: 10.1186/s12889-021-11629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stensrud V, et al. Direct and indirect effects of socioeconomic status on sepsis risk and mortality: a mediation analysis of the HUNT study. J Epidemiol Community Health (1978) 2023;0:1–7. doi: 10.1136/jech-2022-219825. [DOI] [PubMed] [Google Scholar]
- 62.Axelsson D, Blomberg M. Maternal obesity, obstetric interventions and post-partum anaemia increase the risk of post-partum sepsis: a population-based cohort study based on Swedish medical health registers. Infect Dis. 2017;49:765–71. doi: 10.1080/23744235.2017.1341055. [DOI] [PubMed] [Google Scholar]
- 63.Villamor E, Norman M, Johansson S, Cnattingius S. Maternal obesity and risk of early-onset neonatal bacterial Sepsis: Nationwide Cohort and Sibling-controlled studies. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albright CM, Ali TN, Lopes V, Rouse DJ, Anderson BL. The Sepsis in Obstetrics score: a model to identify risk of morbidity from sepsis in pregnancy. Am J Obstet Gynecol. 2014;211:e1–39. doi: 10.1016/j.ajog.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Snyder CC, Barton JR, Habli M, Sibai BM. Severe sepsis and septic shock in pregnancy: indications for delivery and maternal and perinatal outcomes. J Maternal-Fetal Neonatal Med. 2013;26:503–6. doi: 10.3109/14767058.2012.739221. [DOI] [PubMed] [Google Scholar]
- 66.Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of Sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 68.Mariansdatter SE, Eiset AH, Søgaard KK, Christiansen CF. Differences in reported sepsis incidence according to study design: a literature review. BMC Med Res Methodol. 2016;16:1–13. doi: 10.1186/s12874-016-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shankar-Har M, Rubenfeld GD. Race, ethnicity, and Sepsis: Beyond Adjusted odds Ratios. Crit Care Med. 2018;46:1009–10. doi: 10.1097/CCM.0000000000003060. [DOI] [PubMed] [Google Scholar]
- 70.Fleischmann-Struzek C, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46:1552–62. doi: 10.1007/s00134-020-06151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rudd KE, et al. The global burden of sepsis: barriers and potential solutions. Crit Care. 2018;22:232. doi: 10.1186/s13054-018-2157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. Findings of studies assessing the impact of factors of health inequality on sepsis incidence or risk. Table 2. Findings of studies assessing the impact of factors of health inequality on sepsis mortality and other outcomes.
Additional file 2. Search strategy including PICO criteria and exact search terms.
Data Availability Statement
All data generated or analysed during this study are included in this published article and supplementary materials.