Abstract
Risk assessment of pesticide impacts on remote ecosystems makes use of model-estimated degradation in air. Recent studies suggest these degradation rates to be overestimated, questioning current pesticide regulation. Here, we investigated the concentrations of 76 pesticides in Europe at 29 rural, coastal, mountain, and polar sites during the agricultural application season. Overall, 58 pesticides were observed in the European atmosphere. Low spatial variation of 7 pesticides suggests continental-scale atmospheric dispersal. Based on concentrations in free tropospheric air and at Arctic sites, 22 pesticides were identified to be prone to long-range atmospheric transport, which included 15 substances approved for agricultural use in Europe and 7 banned ones. Comparison between concentrations at remote sites and those found at pesticide source areas suggests long atmospheric lifetimes of atrazine, cyprodinil, spiroxamine, tebuconazole, terbuthylazine, and thiacloprid. In general, our findings suggest that atmospheric transport and persistence of pesticides have been underestimated and that their risk assessment needs to be improved.
Keywords: pesticides, atmosphere, transport, degradation, risk assessment
Short abstract
Atmospheric pesticide presence and levels in remote areas mirror those in continental Europe, calling into question the current risk assessment assumptions related to degradation in air.
Introduction
Pesticides are synthetic chemicals used for their toxic effects.1 Their agricultural use has significantly increased globally from 2.4 million tons in 1990 to 4.1 million tons in 2020.2,3 Chemicals authorized for pesticidal use vary widely in their chemical structures and physicochemical properties.4,5 Since the 1960s, growing environmental and health concerns have led to usage restrictions of the previously dominant organochlorine pesticides, to their substitution by more biodegradable ones, and, where possible, to less intensive application.6 Because of the toxicity of pesticides and their metabolites to nontarget organisms, environmental exposure to pesticides is a concern.7,8 Upon bioaccumulation and biomagnification, lipophilic pesticides (with an octanol–water partition coefficient KOW of > 105) may reach effect levels in top predators and humans.9−11
Most pesticides are semivolatile organic compounds (SVOCs).10 They enter the atmosphere upon application via direct emission from spray drift, through wind erosion of soil particles containing pesticides, and via volatilization from soil, vegetation, or water surfaces.3,12−14 Currently used pesticides have been found in rural air15−17 and ecosystems18,19 but have also been observed far from the sources, at high mountains,20 in the marine boundary layer,21,22 and in the Arctic.10,23,24 Such observations have been sporadic because unlike organochlorine pesticides, these pesticides are rarely included in air-monitoring programs (in Europe apart from France and Sweden25).
Evidence for long-range transport to remote areas is one of the criteria for a chemical to be considered a persistent organic pollutant (POP).26,27 Unlike organochlorine pesticides (e.g., DDT, lindane) which are classified as POPs, it has been largely considered that the new generation of pesticides, currently in use globally, are not prone to long-range atmospheric transport (LRAT) due to their short atmospheric half-lives (i.e., <2 days).27 The atmospheric lifetime of SVOCs is determined by gas/particle partitioning and their reactivity in the gaseous and particulate phases and, if degradation is resisted in Earth surface compartments, might be enhanced by multiple cycles of deposition and revolatilization (grasshopper effect).28,29 However, gas/particle partitioning of currently used pesticides is incompletely understood and experimental data for reactions with atmospheric oxidants are available for only few substances.30−35 Without such data, degradability in air is often assessed based on model-estimated reactivity with the hydroxyl (OH) radical in the homogeneous gas phase.36 However, recent findings have shown the presence of pesticides with theoretical persistence in air below the LRAT potential threshold also in remote areas.22−24 This questions the accuracy of the pesticide risk assessment in Europe to protect the atmospheric environment and remote ecosystems, which considers only the atmospheric half-life, for which only the modeled reactivity with the OH radical in the gas phase is considered.
Here, taking advantage of air-monitoring infrastructures, we present the continental-scale distributions of 76 pesticides in the atmosphere over Europe. To this end, 77 particulate and 17 gas-phase samples were collected during the main pesticide application period in spring 2020 at 29 rural, coastal, mountain, and polar sites across Europe and the European Arctic (Figure S1 and Table S1).
Materials and Methods
Sampling Sites
The contributing 29 sampling sites (Table S1) are observational platforms of the Aerosol, Clouds and Trace Gases Research Infrastructure (ACTRIS, www.actris.eu/) and/or the Co-operative Programme for Monitoring and Evaluation of the Long-range Transmission of Air Pollutants in Europe (EMEP, www.emep.int/) and have long-term expertise with atmospheric aerosol particle and trace gas sampling. These 29 sampling sites are located in 17 different European countries and in the European Arctic (Figure S1) and were classified as rural (n = 16), coastal (n = 4), mountain (n = 6), and polar (n = 3) based on their geographical characteristics and/or land use analysis. Indeed, the mountain and polar sites were defined based on their geographical characteristics with, respectively, an altitude of >2000 m a.s.l. and a latitude of >67°N. In the second step, the type of land use surrounding each sampling site (10 km radius) was characterized using the CORINE Land Cover 2018 for all sites except the Zeppelin Observatory for which the Global Land Cover 2000 was used (Table S2).37 To this end, the many categories available from these databases were grouped into more relevant ones considering the aim of this study (Table S3). The coastal sites were defined as those having >35% of their surrounding areas (10 km radius) as water bodies, while for rural sites, it was >60% of agricultural land, forest and shrub, and/or herbaceous vegetation associations. In addition, the rural sites were subcategorized as agricultural-adjacent (A.) or nonagricultural-adjacent (N.A.) if their share of agricultural land in their surrounding area was above or below 45%, respectively.
Sampling
Sampling took place simultaneously at all 29 sites in the main pesticide application season in spring 2020 during three 48 h sampling periods (namely, 28–30/04, 12–14/05, and 26–28/05). Sampling was performed with active air samplers (low or high volume, based on on-site availability, Table S1). Due to Covid-19 epidemic-related restrictions in some countries, only 22 sites collected samples during all three sampling periods, while 4 and 3 sites collected samples during 2 and 1 sampling period, respectively (Table S1).
All sites collected the particulate phase on quartz fiber filters (QFF, QM-A, Whatman, U.K.), preferentially (but not always) with a PM10 inlet, as CUPs have previously been found in both the fine and coarse particles.15 In addition, six sites (ADA, BKO, KOS, SBO, UFS, and ZPO) sampled also the gaseous phase on a sandwich sorbent (i.e., PUF/XAD2/PUF sandwich), consisting of a polyurethane foam plug (PUF, Molitan a.s., Czech Republic, density 0.030 g cm–3, 5 cm depth, diameter of 5.5/11 cm for the low/high-volume air sampler), a layer of XAD resin (Supelpak-2, Supelco), and another PUF plug, separated by cotton wool. This sandwich configuration has been shown to be the most efficient for the collection of gaseous pesticides.38 Prior to sampling, PUFs and XAD2 were precleaned via Soxhlet extraction with acetone for 8 h, followed by 8 h of extraction in methanol. All sampling media were provided by RECETOX and shipped to the sites. In total, 77 samples and 35 field blank samples were collected (i.e., 77 QFFs and 17 PUF/XAD2/PUF sandwiches) and kept in the freezer at −18 °C until extraction.
Sample Preparation, Analysis, and QAQC
All samples underwent spiking with labeled standards (Table S4) before extraction. The extraction process involved using an automatic extractor (Büchi Extraction System, B-811, Switzerland) with 5 mM ammonium acetate in methanol. The extraction process consisted of 1 h of warm Soxhlet, followed by 1 h of solvent rinsing, and a concentration step to 1 mL using nitrogen. After centrifugation for 10 min (12,000 G, Z-36 HK, Hermle Labortechnik, Germany) within polypropylene tubes (Corning Costar Spin-X), the extracts were filtered (cellulose acetate membrane and 0.22 μm pore size) and further concentrated to 0.5 mL under nitrogen.
Postextraction, the samples were divided into three 100 μL aliquots, each undergoing a different analysis. The three analyses allowed the quantification of 76 pesticides (35 herbicides, 22 insecticides, and 19 fungicides) (Table S5). Among these pesticides, 40 are approved for agricultural use in Europe, 22 are among the most globally used pesticides, 34 are characterized as priority active ingredients to be monitored in France, 13 are highly hazardous pesticides, and 25 are high-risk pesticides.
To ensure quality assurance, field blanks were analyzed alongside air samples collected. Blank levels of most individual analytes were generally below or low (Tables S6 and S7). Procedural recoveries were assessed by spiking sampling media with native standards and their corresponding isotopic-labeled standards, followed by processing as per sample. Most procedural recoveries fell within the range of 60–120% with standard deviations below 20%, except for a few exceptions.
Detailed information about the procedures is provided in the Supporting Information.
Air Mass Origin
The Lagrangian particle dispersion model FLEXPART39 was used to identify the potential source regions by calculating the residence times in the surface layer of air sampled.
The meteorological data used (0.5 and 1° and 3 h horizontal and temporal resolutions, 137 vertical levels) were obtained from the European Centre for Medium-Range Weather Forecast (www.ecmwf.int, last access: 21/06/2022). For each simulation, 100,000 particles were continuously released from the sites at the ground level for the polar sites and at altitudes ranging 190–210 m agl for mountain sites and were followed for 30 and 10 days backward in time at polar sites and mountain sites, respectively (Figures S2–S5).
Advection to Mountain Sites
For the six high-mountain sites, the advection was characterized using various combinations of on-site tracer measurements and meteorological data and modeling. For all mountain sites, the conclusions on the planetary boundary layer (PBL) influence on the sampled air were based on the site-specific experiences (Table S8).
At CPK, regional chemistry-transport modeling data (ALADIN model, 2 km × 2 km horizontal resolution)40 were used to judge whether the sampled air was within the free troposphere (FT) or within the PBL. The CPK site was within the FT during most of the sampling time. However, due to short-term (<1 h) liftings of the inversion (which occurred 2, 10, and 2 times during the sampling periods 1, 2, and 3, respectively), PBL air was mixed into all three air samples.
The model terrain is ca. 500 m below the true altitude. Moreover, due to resolution limitations of the model, in particular, in complex terrain, upslope movement of air from valleys will be systematically underestimated. For HAC, the on-site gaseous (i.e., CO, CO2) and aerosol tracer measurements, meteorological parameters, and planetary boundary layer height (obtained from ECMWF) were used.41−43 Air collected during period 2 represented almost exclusively the FT. It was heavily affected by Sahara dust long-range transport. Sampling period 3 was characterized by relatively low and stable concentrations of specific tracers (i.e., aerosol absorption and particle number concentration), but the station was mostly in clouds, indicating conditions characteristic of the interface between FT and PBL. PBL influence on air collected cannot be excluded. No sample was collected during period 1. For JFJ, on-site Rn measurements44 and data from a ceilometer, obtained at the foot of the site (measured at Kleine Scheidegg, altitude difference of 1510 m; 6 km direct distance from JFJ),45 were used to judge free tropospheric vs boundary layer air. JFJ was above the planetary boundary layer during short periods in all three sampling periods. The samples represent mixed FT and PBL air. At PDM, the analysis of 222Rn measurements (α-detection) suggested mixed FT and PBL air during sampling period 3. No samples were collected during periods 1 and 2. For SBO, data from a ceilometer (Vaisala CL51)46 obtained at the foot of the site (measured at Kolm-Saigurn, altitude difference of 1466 m; 5 km direct distance from SBO) were used to judge on FT vs PBL air. Heights for measuring periods with signals under the detection limit, which occurred during nighttime, were interpolated. The derived heights of the PBL were below SBO during all three sampling periods. No sample was collected during period 1. At UFS, the 222Rn measurements (α-detection)47 suggested FT air with some PBL influence during the sampling periods 1 and 2 and almost exclusively FT air during sampling period 3. The attribution is supported by on-site measurements of humidity and other meteorological parameters.48 In addition, advection and possible collection of FT air were investigated for two other elevated sites, i.e., RIG (Switzerland, 1031 m a.s.l.) and ZPO (Svalbard, 474 m a.s.l.). In situ and ceilometer data,49 respectively, besides others, indicated that at both sites, the air collected during the three sampling periods was PBL air or PBL with little FT air mixed in.
Data Analysis
All the statistical analysis was performed using software GraphPad Prism (v9.0.0). For these analyses, when the concentration of a compound was lower than that of iLOD, iLOQ, or LOQb, these values were not taken into account. Substitutions of values below LOQ by LOQ/2 were used to determine the relative standard deviation (Table 1).
Table 1. Spatial Homogeneity of Distributions: Concentration Range (Expressed as log(cmax/cmin)), Relative Standard Deviation (RSD; %) at All Sites and Comparison of Mean Particulate Concentrations at Remote Sites, crs (Polar + Free Tropospheric Mountain Sites) and Other Sites, cos (Coastal + Nonfree Tropospheric Mountain + Rural Sites)a,b.
| crs (pg m–3) | cos (pg m–3) | log(cos/crs) | log(cmax/cmin) | RSD (%) | |
|---|---|---|---|---|---|
| 2,4-D | <LOQ | 4.46 | N.A.b | 2.1 | 159 |
| atrazine | 0.11 | 0.30 | 0.4 | 1.6 | 107 |
| cyprodinil | 26.5 | 215 | 0.9 | 5.7 | 602 |
| fenpropidin | 7.30 | 127 | 1.2 | 4.5 | 548 |
| fenpropimorph | 2.37 | 46.1 | 1.3 | 3.6 | 199 |
| metazachlor | <LOQ | 2.59 | N.A.b | 3.2 | 263 |
| S-metolachlor | 5.16 | 81.7 | 1.2 | 3.2 | 147 |
| spiroxamine | 11.9 | 78.3 | 0.8 | 4.0 | 378 |
| tebuconazole | 0.95 | 10.3 | 1.0 | 3.4 | 213 |
| terbuthylazine | 54.3 | 53.5 | 0.0 | 3.4 | 291 |
| thiacloprid | 0.24 | 1.14 | 0.7 | 1.7 | 103 |
Values < LOQ not included. Substances with a quantification frequency higher than 50% only.
N.A. = not applicable.
Detailed information about the procedures is provided in the Supporting Information.
Results and Discussion
European Distribution of Atmospheric Pesticides
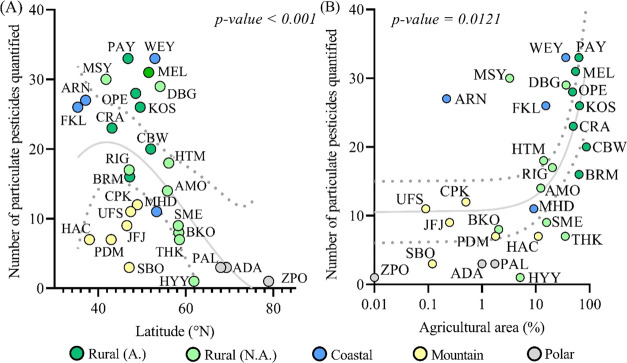
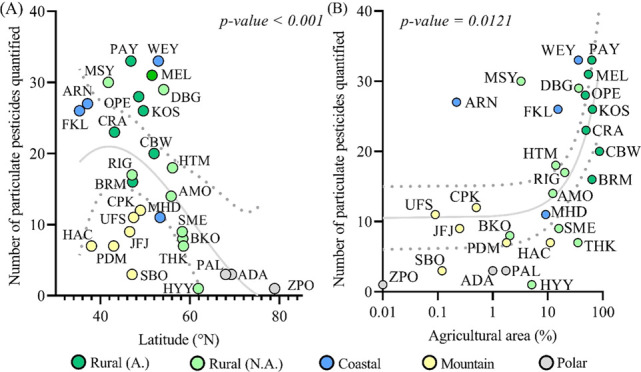
Out of the 76 pesticides targeted, 58 were found in the atmosphere, including the European Arctic. In the atmospheric particulate phase, 51, 38, 24, and 6 pesticides were found at rural, coastal, mountain, and polar sites, respectively (Figures S6–S8). Overall, the number of particulate pesticides decreases with latitude and increases with the proximity to agricultural fields (Figure 1). Among these 58 pesticides present in European air, about 50% were rarely found (1–5 sites), while around 20% were quantified in more than half of the sites investigated (Table S9).
Figure 1.
Number of pesticides quantified in the particulate phase: (A) latitudinal distribution (third-order polynomial regression) and (B) related to agriculture (area fraction within 10 km, in %). On each figure, the gray line represents the regression and the dotted lines represent the 95% confidence interval.
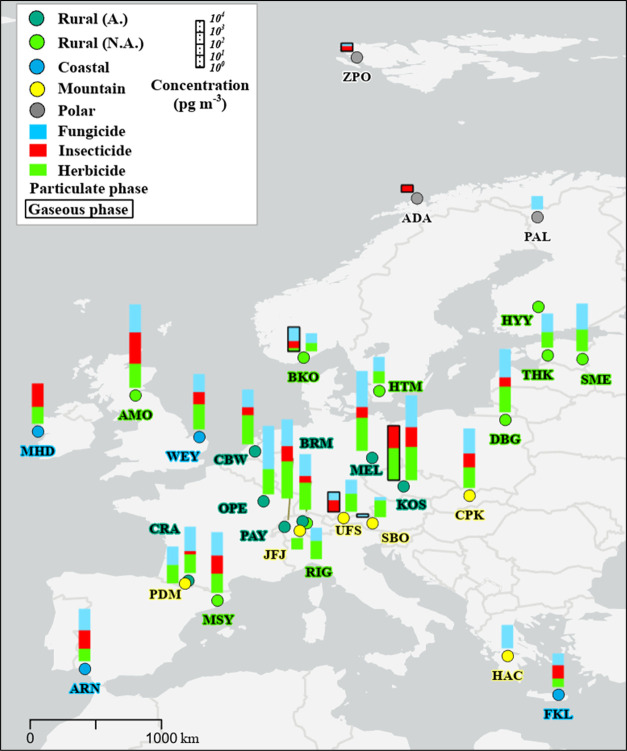
The concentrations of pesticides quantified on aerosol particles were minimal at ZPO (Svalbard) with 24.5 fg m–3 on average and ranged from 0.14 to 3.9 ng m–3 at agricultural sites (CRA and OPE sites, respectively) (Figure 2). On an individual substance point of view, cyprodinil, fenpropidin, prosulfocarb, and spiroxamine were the pesticides with the highest concentrations (i.e., up to 7.51, 3.53, 1.71, and 1.82 ng m–3, respectively), while for all other pesticides, their concentrations were mostly below 1 ng m–3.
Figure 2.
Pesticide mean concentrations quantified at each site in the particulate (all sites) and gaseous phases (6 sites).
8 out of 11 pesticides exhibiting a quantification frequency exceeding 50% (Table S9) (i.e., cyprodinil, fenpropidin, fenpropimorph, metazachlor, S-metolachlor, spiroxamine, tebuconazole, and terbuthylazine) had their particulate concentrations spanning 3 to 6 orders of magnitude (log(cmax/cmin) = 3–6), while the other three (i.e., 2,4-D, atrazine, and thiacloprid) exhibited a more uniform distribution across the continent (log(cmax/cmin) ≈ 2 and RSD < 200%) (Table 1 and Figures S9 and S10). Moreover, for atrazine and thiacloprid, the concentration gradient from the source areas was particularly small: The average concentrations at remote sites (free tropospheric mountain and Arctic) were within an order of magnitude of the average concentrations at other sites (rural + coastal + mountain) (log(cos/crs) < 1; Table 1). The same was found for cyprodinil, spiroxamine, tebuconazole, and terbuthylazine. Such uniform distributions suggest that the atmospheric lifetime of the compounds is similarly long or its source distribution is similarly wide as that of atrazine. Atrazine, an herbicide banned since 2004,50 continues to be present in European air, likely a consequence of its persistence and long-range transport from regions where it is still used.3,51−53 Because of its persistence, atrazine keeps cycling in air, even 25 years after its ban in Europe. It is re-emitted from secondary sources, but primary sources outside Europe may also contribute.54
Among these frequently observed pesticides, 8 are approved for agricultural use in Europe, except for atrazine, fenpropimorph, and thiacloprid (Tables S5). Fenpropimorph is a fungicide banned shortly before our sampling campaign but remained authorized for use as a biocide until 2021.55 Thiacloprid, banned for agricultural use in May 2020, is another exception. Among the less frequently found pesticides, acetochlor, carbaryl, and simazine, their European approvals had lapsed more than 9 years before our sampling. Interestingly, concentrations of these substances at remote sites did not significantly differ from those at rural sites by more than an order of magnitude (Table 1), indicating their persistence and long-range transport from regions where they are still in use, such as North America and Africa.
By hierarchical cluster analysis, we find a high degree of similarity in particulate pesticide substance patterns between sites far from application, i.e., polar and mountain sites, particularly evident during the third sampling period (Figures S11–S13).
Long-Range Atmospheric Transport (LRAT) of Pesticides
The substances prone to long-range atmospheric transport were identified using the samples collected at the polar sites as well as those collected at high mountain sites from free tropospheric air (see the Supporting Information for the determination of free tropospheric conditions at each site). Indeed, in the free troposphere, zonal and meridional transport is more efficient due to higher wind speeds and longer depositional lifetime.56
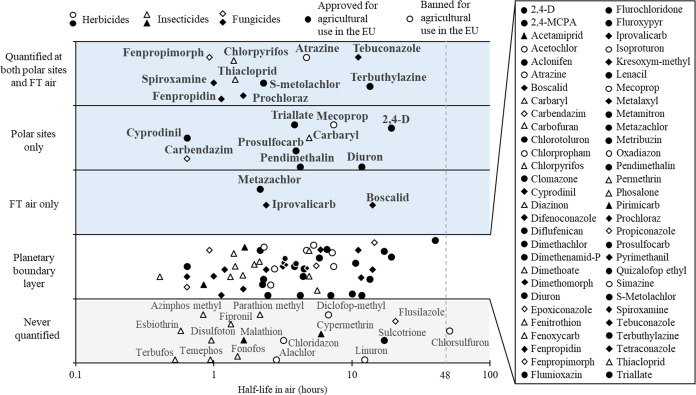
In the polar atmosphere, 19 pesticides were quantified, including 15 for which this is the first evidence of their potential to reach the Arctic. Out of these 19 pesticides, 12 were approved for agricultural use in Europe in 2020. In particular, most of these pesticides were quantified in the gas phase at the ZPO site, Svalbard (78.9°N). 13 pesticides were found in the four samples at mountain sites that were confirmed to have been collected exclusively in free tropospheric air (Table S8). This included 10 approved for agricultural use and 10 that were also found at the polar sites. Therefore, by combining the results from the polar and free tropospheric air samples, 22 pesticides were identified to be prone to LRAT (blue area in Figure 3), which included 15 approved for agricultural use in Europe.
Figure 3.
Pesticides identified as prone to long-range atmospheric transport (blue area, FT = free tropospheric), pesticides quantified in the planetary boundary layer (white area), and pesticides never observed (gray area) ordered along model estimate of half-life in air.36 Not pictured is one pesticide never observed, i.e., fluazinam, with a model-estimated half-life of 1956 h. Annual mean OH concentration of 7.5 × 105 cm−3 used for calculation of half-life in air.
Source regions, based on 30-day footprint analyses by the FLEXPART model, for the air collected from 28 to 30/04/2020 and 26–28/05/2020 at ZPO, Svalbard, are located in the North Atlantic and the inner Arctic (Figure S2) and contained the lowest number and concentrations of pesticides. Higher levels were quantified in the air collected from 12 to 14/05/2020 at ZPO, which originated from North America and the northernmost agricultural regions of Russia (Figure S2). Similar behavior has also been found for the two other polar sites, where higher numbers of pesticides were encountered in the samples, where air masses were influenced by Scandinavia and the British Isles (Figures S3 and S4). The origins of free tropospheric air collected at mountain sites were all from continental Europe (Figure S5).
Previous observations of more than 20 currently used pesticides in the polar regions in recent years have highlighted the LRAT potential of currently used pesticides10,22−24 (Table S10). However, these studies have mainly focused on a limited number of currently used pesticides. In this study, 19 pesticides were found to be associated with LRAT at the polar sites. Out of these, four substances, atrazine, chlorpyrifos, pendimethalin, and triallate, had previously been reported in polar regions, whereas we are establishing the first evidence of LRAT for 15 pesticides that have previously not been encountered in these regions (Figure 3). Three of these substances, atrazine, chlorpyrifos, and diuron, are water policy-prioritized in the EU. The potential risk for the environment and human health is also evidenced by the fact that among the pesticides identified as prone to LRAT, 2 are carcinogens, 6 are genotoxic, 10 are endocrine disruptors, and 9 are reprotoxic.10,57 In addition, the 22 long-range transported pesticides belong to 16 different chemical classes57 and their physicochemical properties range widely, i.e., saturation vapor pressure ranging from 9.2 × 10–9 to 0.67 Pa with log KOW from 1.55 to 6.52 (Table S11), with chlorpyrifos, fenpropidin, fenpropimorph, pendimethalin, S-metolachlor, and spiroxamine potentially (logKow > 5) known to be bioaccumulative.19
There was a considerable shift of the pesticides’ gas/particle partitioning from the continent to the Arctic, with a higher gaseous fraction for 14 out of 15 pesticides observed at the polar sites than at midlatitude sites. This trend was most prominent for terbuthylazine, which was on average 99% in the particulate phase at rural sites but >98% in the gaseous phase at polar sites. The exception was triallate, which at one polar site was quantified in the particulate phase only (>87%, Table S12). Our observations suggest that pesticides which are predominantly partitioning to the particulate phase in aerosols over Europe tend to be found in the gaseous phase in the Arctic (Figure 2). This pattern is consistent with previous studies, with pesticides such as 2,6-dichlorobenzonitrile, chloroneb, dicofol, nitrapyrin, and triallate observed only in the gas phase of Arctic air22,24 (Table S10), while in in this study, pesticides in continental Europe preferentially partitioned to the particulate phase, as suggested by the results from mountains and rural sites where the gas phase was also collected.15
A preference for partitioning to the gas phase at polar sites can be explained by low aerosol mass and surface concentrations and the prevalence of hydrophilic particulate matter (PM) components in particular seasalt.58 For example, at the central European rural site KOS (Czech Republic), the concentration of PM10 was 12.8 μg m–3 on average during the study, while 4.3 μg m–3 was measured at the polar site ZPO, Svalbard.59 Organic matter is a key constituent influencing the partitioning of chemicals onto PM.60 In continental Europe, the fraction of organic matter in PM is generally higher than that in the Arctic. At the rural sites adjacent to agriculture, the particulate organic carbon averaged 1.53 μg m–3 (≈12%), whereas at polar sites, it was more than 10-fold lower, 0.11 μg m–3 (≈3%) on average. No in-depth analysis of gas/particle partitioning is possible because of the lack of particulate phase chemical composition data for most of the sites. Another possible explanation could be volatilization of CUPs from melting snow, mixed into sample air shortly before collection, i.e., before complete relaxation to phase equilibrium. Volatilization from melting snow is expected for organic substances, which within snowpacks would partition significantly to the pore space,61 however, this does not apply for the CUPs, which partitioning apparently shifted to the gas phase (Table S12).
The three polar sites included in this study had similar environmental conditions (temperature, PM concentration) but are distanced from each other by >1000 km and were influenced by different air masses (Figures S1–S5). Still, out of the 19 pesticides encountered at these sites, five were observed at more than one polar site, which provides independent evidence of long-range atmospheric transport. Moreover, we quantified 13 pesticides in free tropospheric air collected at three high mountain sites, indicating that they are prone to LRAT. 11 pesticides identified at those mountain sites were also identified at polar sites, thus providing strong evidence of their LRAT potential.
Current Limitations Regarding Pesticide Environmental Risk Assessments
Pesticide authorization in Europe presupposes environmental risk assessment with criteria for persistence, bioaccumulation potential, long-range transport potential, and toxicity for soil and water. However, there is no threshold value to consider a pesticide as persistent in the atmosphere.27 The only parameter through which the atmosphere is included in the risk assessment procedure is the potential for long-range atmospheric transport. To assess this potential, the atmospheric half-life is used as a proxy. For this, only the reactivity with the OH radical in the gas phase is considered, using global and annual mean OH radical concentrations.62 A pesticide exhibiting a half-life higher than 2 days is considered as prone to LRAT.
However, all 22 pesticides identified as prone to LRAT in this study have been estimated to have atmospheric half-lives shorter than 2 days based on the model currently used in the risk assessment36 (Table S11). But OH concentrations can be much lower seasonally, at high latitudes, as well as during nighttime or in the polar night.63 In these cases, degradation processes are substantially reduced, with correspondingly longer effective atmospheric half-lives and travel distances.24 For instance, the atmospheric and total environmental lifetimes of atrazine are almost 1 order of magnitude longer for midlatitude winter than in summer64 and the characteristic travel distance (CTD) of chlorpyrifos increases from 30 to 290 km with a 10-fold reduced OH concentration.52 CTD is an indicator of a chemical’s long-range transport potential in a generic multicompartment environment under steady-state conditions and is influenced by also lifetime in soil and water.52,65 It is defined as the distance from the source region at which the concentration is reduced by 63%.66 Note that because of the generic nature of the underlying multimedia model, CTD is not suitable to test substance fate or should not be interpreted in absolute terms (km). CTDs of the targeted pesticides are estimated mostly below 100 km (median is 92 km) and are estimated even somewhat lower with a median of 68 km for the substances identified as prone to LRAT, suggesting that these chemicals are unlikely to reach remote locations. The CTD values or their ranking do not correspond with substances suggested from this or earlier studies to have high long-range transport potential, with only one exception, i.e., thiacloprid (Figure S14 and Table S11).
Moreover, for pesticides transported in the particulate phase, degradation is expected to be slower than in the gas phase due to diffusion limitation in low-viscosity aerosol particles.33,67 Experiments on degradation rate coefficients with OH in the particulate phase suggest atmospheric half-lives of weeks (given global mean OH radical concentration).33,34 Oxidation is particularly slow in aerosols transported at high altitudes or to high latitudes and expectedly most relevant for moderately polar pesticides such as carbamates, triazines, thiophosphoric acid esters, phenols, and anilines.34,67 As confirmed in this study, pesticides tend to have higher particle-bound mass fractions in continental air than in polar air, and their persistence in air is therefore likely underestimated. This aspect is neglected by the current risk assessment practice.
Furthermore, the effective atmospheric lifetime of semivolatiles resisting degradation in soils and surface water can be much longer than the residence time in air based on degradation kinetics because of several cycles of revolatilization and deposition enhancing the LRAT potential (multihopping).28
In addition to the lack of information about active ingredients, the influence of application methods, formulants, and adjuvants on CUP emissions is not well known. While aerial application is banned in Europe, spraying of soil and plant,12 volatilization,68,69 and also pellet application to soil and seed treatment contribute to pesticide emissions.70 Formulants and adjuvants are used to improve the effectiveness of application but can modify their effective vapor pressure and atmospheric half-lives.71 A recent experimental study suggested that the reactivity of the chlorpyrifos with OH radicals was different in a commercial formulation than that of the substance alone.35
This study shows the limits of the risk assessment in place in the regulatory process on the atmospheric fate of pesticides and, in particular, their potential for LRAT, by providing empirical evidence in direct contrast to current model predictions. There is a real need to revise the current methods used for environmentally relevant conditions (different temperature and/or OH concentrations) as well as to obtain more experimental data on atmospheric degradability of pesticides including pesticide formulations and preparations, in addition to data from monitoring studies. Currently, the framework does not consider partitioning into the particulate phase or slowed degradation in soil/water during the multihopping. More realistic modeling is extremely important if we want to ensure that the pesticides authorized for agricultural use in Europe (and elsewhere) do not contaminate the environment and pose health risks hundreds of thousands of kilometers away from the source areas.
Acknowledgments
The authors thank Yannick Bezombes, Jean-Marc Fort, Achim Grüner, Hilja Iher, Marcin Jackowicz-Korczyński, Olav Lien, Reidar Lyngra, Helge T. Markussen, Eric Pique, René Rabe, and Tomáš Ištok for on-site help, Cecilia Akselsson, Jenny Kreuger, and Cornelius Zetzsch for discussion, and Franz Conen, Gabriele Frank, Radovan Krejci, Thomas Werner, Paul Zieger, and MeteoSwiss for supporting field data. The study received support from the National Facilities of the ACTRIS (Aerosol, Clouds and Trace gases Research Infrastructure) and EMEP (Co-operative Programme for Monitoring and Evaluation of the Long-range Transmission of Air Pollutants in Europe) network of platforms. The ARN and MSY sites were supported by the European Regional Development Fund in Spain related to LifeWatch ERIC (INDALO, LIFEWATCH-2019-04-AMA-01). The HTM site was supported by the Swedish Environmental Protection Agency, Lund University, and the Swedish Research Council through ICOS Sweden. The MEL site was supported by the H2020 RIs ACTRIS 262254 and ACTRIS-2 654109. The MHD site was supported by EPA Ireland through the Atmosphere Chemistry and Climate Change (AC3) network. The PDM site received technical support from the UMS 831 Pic du Midi observatory team and the Pyrenean Platform for Atmospheric Observations (P2OA), funded by CNRS-INSU. The SME and THK sites were supported by the Estonian Environmental Observatory (KKOBS 2014-2020.4.01.20-0281) and the Estonian Environmental Agency and ACTRIS IMP (871115). The WEY site was supported by the Natural Environmental Research Council’s Atmospheric Measurement and Observation Facility (AMOF, AMF_20022020084418).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c08488.
Materials and methods, map of the 29 sites, 30-days footprint sensitivities and 10-day backward trajectories (FLEXPART model), CUPs physico-chemical parameters, detailed site results, gas-particle partitioning, hierarchical cluster analysis, sampling methodology, land-use analysis, internal standards and chemical analyses information, recoveries, information on previous CUPs found in Arctic and high-mountain sites (PDF)
Author Present Address
⊥⊥⊥⊥ Céline Degrendele; now unemployed
Author Contributions
#### L.M. and C.D. contributed equally to this work. Conceptualization and methodology: C.D. and G.L. Validation: L.M., P.Š., A.Du., and C.D. Formal analysis: L.M., P.Š., P.K., A.Du., S.Ra., K.B.-S., S.E., F.G., S.H., and J.M. Investigation: L.M., J.Ko., P.Š., P.K., P.P., S.Ra., A.Du., G.L., C.D., K.B.-S., S.E., S.H., F.G., J.E.S., J.M., P.B.-N., C.C., DC. S.C., A.De., G.L.F., M.I.G., V.V., K.E., U.H., H.J., A.K., P.L., E.L., U.M., V.M., L.P., M.S., and U.B. Writing—original draft: L.M., C.D., and G.L. Writing—review and editing: L.M., G.L., L.E.M., and U.B. All visualization: L.M. and S.E. Supervision: G.L. and C.D. Project administration: G.L., J.Kl., and C.D. Funding acquisition: J.Kl., G.L., P.P., and H.W. The article was written through contributions of all authors. All authors have given approval to the final version of the article.
This work was supported by the European Union’s Horizon 2020 research and innovation program (857560); the Czech Ministry of Education, Youth and Sports through RECETOX Research Infrastructure (LM2023069), ACTRIS-CZ RI (LM2023030) and CETOCOEN EXCELLENCE (CZ.02.1.01/0.0/0.0/17_043/0009632); the Brno City Municipality and the South Moravian Centre for International Mobility (JCMM, fellowship award to L.M.); the Czech Science Foundation (20–07117S); the Estonian Research Council (PRG714, PRG1674); the General Secretariat of Research and Innovation of the Greek Ministry of Development and Investments, Public Investment Program, through the National Network on Climate Change and Its Impacts; the Norwegian Fram Center (CLEAN-FRAM) and COPE (NFR #28114); and the Swedish Research Council (ACTRIS_SE RI 2021–00177). This publication reflects only the author’s view and the European Commission is not responsible for any use that may be made of the information it contains.
The authors declare no competing financial interest.
Supplementary Material
References
- Alexandratos N.; Bruinsma J.. World agriculture towards 2030/2050: The 2012 revision. ESA Working Paper 12–03; Rome. www.fao.org/economic/esa (accessed April 20, 2022).
- FAOSTAT. FAOSTAT: Pesticides use. In FAO.org. www.fao.org/faostat/en/#data/RP (accessed March 21, 2022).
- Sharma A.; Kumar V.; Shahzad B.; Tanveer M.; Sidhu G. P. S.; Handa N.; Kohli S. K.; Yadav P.; Bali A. S.; Parihar R. D.; Dar O. I.; Singh K.; Jasrotia S.; Bakshi P.; Ramakrishnan M.; Kumar S.; Bhardwaj R.; Thukral A. K. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446 10.1007/s42452-019-1485-1. [DOI] [Google Scholar]
- Franklin J.; Atkinson R.; Howard P. H.; Orlando J. J.; Seigneur C.; Wallington T. J.; Zetzsch C.. Quantitative Determination of Persistence in Air. In Criteria for Persistence and Long-Range Transport of Chemicals in the Environment; Klecka G.; Boethling B.; Franklin J.; Grady L.; Graham D.; Howard P. H.; Kannan K.; Larson R. J.; Mackay D.; Muir D.; van de Meent D., Eds.; SETAC press: Pensacola, 2000; pp 7–62. [Google Scholar]
- British Crop Protection Council . The Pesticide Manual: A World Compendium, 16th ed.; MacBean C., Ed.;BCPC Publications: Alton, UK, 2012. [Google Scholar]
- Carvalho F. P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. 10.1002/fes3.108. [DOI] [Google Scholar]
- Rico A.; Van den Brink P. J. Evaluating aquatic invertebrate vulnerability to insecticides based on intrinsic sensitivity, biological traits, and toxic mode of action. Environ. Toxicol. Chem. 2015, 34, 1907–1917. 10.1002/etc.3008. [DOI] [PubMed] [Google Scholar]
- Sánchez-Bayo F.; Wyckhuys K. A. G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. 10.1016/j.biocon.2019.01.020. [DOI] [Google Scholar]
- Vorkamp K.; Rigét F. F. A review of new and current-use contaminants in the Arctic environment: evidence of long-range transport and indications of bioaccumulation. Chemosphere 2014, 111, 379–395. 10.1016/j.chemosphere.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Arctic Monitoring and Assessment Programme (AMAP) . AMAP Assessment 2016: Chemicals of Emerging Arctic Concern; Oslo, Norway. www.amap.no (accessed March 19, 2023).
- Mostafalou S.; Abdollahi M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91 (2), 549–599. 10.1007/s00204-016-1849-x. [DOI] [PubMed] [Google Scholar]
- van den Berg F.; Kubiak R.; Benjey W. G.; Majewski M. S.; Yates S. R.; Reeves G. L.; Smelt J. H.; van der Linden A. M. A. Emission of pesticides into the air. Water Air Soil Pollut. 1999, 115, 195–218. 10.1023/A:1005234329622. [DOI] [Google Scholar]
- Glotfelty D. E.; Leech M. M.; Jersey J.; Taylor A. W. Volatilization and wind erosion of soil surface applied atrazine, simazine, alachlor and toxaphene. J. Agric. Food Chem. 1989, 37, 546–551. 10.1021/jf00086a059. [DOI] [Google Scholar]
- Davie-Martin C. L.; Hageman K. J.; Chin Y. P.; Rougé V.; Fujita Y. Influence of temperature, relative humidity, and soil properties on the soil-air partitioning of semivolatile pesticides: laboratory measurements and predictive models. Environ. Sci. Technol. 2015, 49 (17), 10431–10439. 10.1021/acs.est.5b02525. [DOI] [PubMed] [Google Scholar]
- Degrendele C.; Okonski K.; Melymuk L.; Landlová L.; Kukučka P.; Audy O.; Kohoutek J.; Čupr P.; Klánová J. Pesticides in the atmosphere: a comparison of gas-particle partitioning and particle size distribution of legacy and current-use pesticides. Atmos. Chem. Phys. 2016, 16 (3), 1531–1544. 10.5194/acp-16-1531-2016. [DOI] [Google Scholar]
- Désert M.; Ravier S.; Gille G.; Quinapallo A.; Armengaud A.; Pochet G.; Savelli J. L.; Wortham H.; Quivet E. Spatial and temporal distribution of current-use pesticides in ambient air of Provence-Alpes-Côte-d’Azur Region and Corsica, France. Atmos. Environ. 2018, 192, 241–256. 10.1016/j.atmosenv.2018.08.054. [DOI] [Google Scholar]
- López A.; Yusà V.; Muñoz A.; Vera T.; Borràs E.; Ródenas M.; Coscollà C. Risk assessment of airborne pesticides in a Mediterranean region of Spain. Sci. Total Environ. 2017, 574, 724–734. 10.1016/j.scitotenv.2016.08.149. [DOI] [PubMed] [Google Scholar]
- Klöppel H.; Kördel W. Pesticides volatilisation and exposure of terrestrial ecosystems. Chemosphere 1997, 35 (6), 1271–1289. 10.1016/S0045-6535(97)00213-0. [DOI] [Google Scholar]
- Gkotsis G.; Nika M. C.; Nikolopoulou V.; Alygizakis N.; Bizani E.; Aalizadeh R.; Badry A.; Chadwick E.; Cincinelli A.; Claßen D.; Danielsson S.; Dekker R.; Duke G.; Drost W.; Glowacka N.; Göckener B.; Jansman H. A. H.; Juergens M.; Knopf B.; Koschorreck J.; Krone O.; Martellini T.; Movalli P.; Persson S.; Potter E. D.; Rohner S.; Roos A.; O’ Rourke E.; Siebert U.; Treu G.; van den Brink N. W.; Walker L. A.; Williams R.; Slobodnik J.; Thomaidis N. S. Assessment of Contaminants of Emerging Concern in European apex predators and their prey by LC-QToF MS wide-scope target analysis. Environ. Int. 2022, 170, 107623 10.1016/j.envint.2022.107623. [DOI] [PubMed] [Google Scholar]
- Feltracco M.; Barbaro E.; Maule F.; Bortolini M.; Gabrieli J.; de Blasi F.; Cairns W. R.; Dallo F.; Zangrando R.; Barbante C.; Gambaro A. Airborne polar pesticides in rural and mountain sites of north-eastern Italy: an emerging air quality issue. Environ. Pollut. 2022, 308, 119657 10.1016/j.envpol.2022.119657. [DOI] [PubMed] [Google Scholar]
- Mai C.; Theobald N.; Lammel G.; Hühnerfuss H. Spatial, Seasonal and vertical distributions of currently-used pesticides in the marine boundary layer of the North Sea. Atmos. Environ. 2013, 75, 92–102. 10.1016/j.atmosenv.2013.04.027. [DOI] [Google Scholar]
- Gao Y.; Zheng H.; Xia Y.; Chen M.; Meng X. Z.; Cai M. Spatial Distributions and seasonal changes of current-use pesticides from the North Pacific to the Arctic Oceans. J. Geophys. Res.: Atmos. 2019, 124 (16), 9716–9729. 10.1029/2018JD030186. [DOI] [Google Scholar]
- Balmer J. E.; Morris A. D.; Hung H.; Jantunen L.; Vorkamp K.; Rigét F.; Evans M.; Houde M.; Muir D. C. G. Levels and trends of current-use pesticides (CUPs) in the Arctic: An updated review, 2010–2018. Emerging Contam. 2019, 5, 70–88. 10.1016/j.emcon.2019.02.002. [DOI] [Google Scholar]
- Röhler L.; Schlabach M.; Haglund P.; Breivik K.; Kallenborn R.; Bohlin-Nizzetto P. Non-target and suspect characterisation of organic contaminants in Arctic air - Part 2: Application of a new tool for identification and prioritisation of Chemicals of Emerging Arctic Concern in air. Atmos. Chem. Phys. 2020, 20 (14), 9031–9049. 10.5194/acp-20-9031-2020. [DOI] [Google Scholar]
- IVL, Nationell Luftövervakning 2019, Report No. C584 Swedish Environmental Research Institute: Stockholm: 2021.
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs) 2001http://chm.pops.int/tabid/208/Default.aspx (accessed March 20, 2023).
- European Commission, Regulation No. 107/, Regulation of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing council directives 79/117/EEC and 91/414/EEC2009L3091, 2009.
- Semeena V. S.; Lammel G. The significance of the grasshopper effect on the atmospheric distribution of persistent organic substances. Geophys. Res. Lett. 2005, 32 (7), L07804 10.1029/2004GL022229. [DOI] [Google Scholar]
- Bidleman T. F. Atmospheric processes. Environ. Sci. Technol. 1988, 22 (4), 361–367. 10.1021/es00169a002. [DOI] [PubMed] [Google Scholar]
- Borrás E.; Ródenas M.; Vázquez M.; Vera T.; Muñoz A. Particulate and gas-phase products from the atmospheric degradation of chlorpyrifos and chlorpyrifos-oxon. Atmos. Environ. 2015, 123, 112–120. 10.1016/j.atmosenv.2015.10.049. [DOI] [Google Scholar]
- Murschell T.; Farmer D. K. Atmospheric OH oxidation of three chlorinated aromatic herbicides. Environ. Sci. Technol. 2018, 52 (8), 4583–4591. 10.1021/acs.est.7b06025. [DOI] [PubMed] [Google Scholar]
- Murschell T.; Farmer D. K. Atmospheric OH oxidation chemistry of trifluralin and acetochlor. Environ. Sci. Process Impacts 2019, 21 (4), 650–658. 10.1039/C8EM00507A. [DOI] [PubMed] [Google Scholar]
- Socorro J.; Durand A.; Temime-Roussel B.; Gligorovski S.; Wortham H.; Quivet E. The persistence of pesticides in atmospheric particulate phase: an emerging air quality issue. Sci. Rep. 2016, 6 (1), 33456 10.1038/srep33456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socorro J.; Lakey P. S. J.; Han L.; Berkemeier T.; Lammel G.; Zetzsch C.; Pöschl U.; Shiraiwa M. Heterogeneous OH oxidation, shielding effects, and implications for the atmospheric fate of terbuthylazine and other pesticides. Environ. Sci. Technol. 2017, 51 (23), 13749–13754. 10.1021/acs.est.7b04307. [DOI] [PubMed] [Google Scholar]
- Muñoz A.; Borrás E.; Vera T.; Colmenar I.; Ródenas M.; Gimeno C.; Fuentes E.; Coscollá C.; Calvete-Sogo H. Atmospheric degradation of two pesticides mixed with volatile organic compounds emitted by citrus trees. Ozone and secondary organic aerosol production. Atmos. Environ. 2023, 295, 119541 10.1016/j.atmosenv.2022.119541. [DOI] [Google Scholar]
- US EPA . Estimation Programs Interface SuiteTM for Microsoft Windows. United States Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- CORINE Land Cover 2018, European Environment Agency (EEA). © European Union, Copernicus Land Monitoring Service. 2018.
- López A.; Coscollà C.; Yusà V. Evaluation of sampling adsorbents and validation of a LC-HRMS method for determination of 28 airborne pesticides. Talanta 2018, 189, 211–219. 10.1016/j.talanta.2018.06.078. [DOI] [PubMed] [Google Scholar]
- Pisso I.; Sollum E.; Grythe H.; Kristiansen N. I.; Cassiani M.; Eckhardt S.; Arnold D.; Morton D.; Thompson R. L.; Groot Zwaaftink C. D.; Evangeliou N.; Sodemann H.; Haimberger L.; Henne S.; Brunner D.; Burkhart J. F.; Fouilloux A.; Brioude J.; Philipp A.; Seibert P.; Stohl A. The Lagrangian particle dispersion model FLEXPART Version 10.4. Geosci. Model Dev. 2019, 12 (12), 4955–4997. 10.5194/gmd-12-4955-2019. [DOI] [Google Scholar]
- Simon A.; Belluš M.; Čatlošová K.; Derková M.; Dian M.; Imrišek M.; Kaňák J.; Méri L.; Neštiak M.; Vivoda J. Numerical simulations of June 7, 2020 convective precipitation over Slovakia using deterministic, probabilistic, and convection-permitting approaches. Idöjaras 2021, 125 (4), 571–607. 10.28974/idojaras.2021.4.3. [DOI] [Google Scholar]
- Collaud Coen M.; Andrews E.; Aliaga D.; Andrade M.; Angelov H.; Bukowiecki N.; Ealo M.; Fialho P.; Flentje H.; Hallar A. G.; Hooda R.; Kalapov I.; Krejci R.; Lin N. H.; Marinoni A.; Ming J.; Nguyen N. A.; Pandolfi M.; Pont V.; Ries L.; Rodríguez S.; Schauer G.; Sellegri K.; Sharma S.; Sun J.; Tunved P.; Velasquez P.; Ruffieux D. Identification of topographic features influencing aerosol observations at high altitude stations. Atmos. Chem. Phys. 2018, 18 (16), 12289–12313. 10.5194/acp-18-12289-2018. [DOI] [Google Scholar]
- McClure C. D.; Jaffe D. A.; Gao H. Carbon Dioxide in the free troposphere and boundary layer at the Mt. Bachelor Observatory. Aerosol Air Qual. Res. 2016, 16 (3), 717–728. 10.4209/aaqr.2015.05.0323. [DOI] [Google Scholar]
- Andrews E.; Ogren J. A.; Bonasoni P.; Marinoni A.; Cuevas E.; Rodríguez S.; Sun J. Y.; Jaffe D. A.; Fischer E. V.; Baltensperger U.; Weingartner E.; Coen M. C.; Sharma S.; Macdonald A. M.; Leaitch W. R.; Lin N. H.; Laj P.; Arsov T.; Kalapov I.; Jefferson A.; Sheridan P. Climatology of aerosol radiative properties in the free troposphere. Atmos. Res. 2011, 102, 365–393. 10.1016/j.atmosres.2011.08.017. [DOI] [Google Scholar]
- Herrmann E.; Weingartner E.; Henne S.; Vuilleumier L.; Bukowiecki N.; Steinbacher M.; Conen F.; Coen M. C.; Hammer E.; Jurányi Z.; Baltensperger U.; Gysel M. Analysis of long-term aerosol size distribution data from Jungfraujoch with emphasis on free tropospheric conditions, cloud influence, and air mass transport. J. Geophys. Res. 2015, 120 (18), 9459–9480. 10.1002/2015JD023660. [DOI] [Google Scholar]
- Poltera Y.; Martucci G.; Collaud Coen M.; Hervo M.; Emmenegger L.; Henne S.; Brunner D.; Haefele A. PathfinderTURB: An automatic boundary layer algorithm. Development, validation and application to study the impact on in situ measurements at the Jungfraujoch. Atmos. Chem. Phys. 2017, 17 (16), 10051–10070. 10.5194/acp-17-10051-2017. [DOI] [Google Scholar]
- Lotteraner C.; Piringer M. Mixing-height time series from operational ceilometer aerosol-layer heights. Boundary Layer Meteorol. 2016, 161 (2), 265–287. 10.1007/s10546-016-0169-2. [DOI] [Google Scholar]
- Frank G.; Salvamoser J.; Steinkopff T.. Radon-222 and Beryllium-7 as natural tracer, 2019https://www.dach2019.de/DACH2019-abstracts.pdf (accessed April 02, 2023).
- Thomas W.Personal Communication DWD: Hohenpeißenberg, Germany; 2022.
- Maturilli M.Ceilometer cloud base height from station Ny-Ålesund (2017–08 et Seq); Alfred Wegener Institute- Research Unit Potsdam, Germany, PANGAEA, 2022.
- 2004/248/EC Commission Decision of 10 March 2004 Concerning the Non-Inclusion of Atrazine in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing this Active Substance, 2004.
- Chernyak S. M.; Rice C. P.; Mcconnell L. L. Evidence of currently-used pesticides in air, ice, fog, seawater and surface microlayer in the Bering and Chukchi Seas. Mar. Pollut. Bull. 1996, 32 (5), 410–419. 10.1016/0025-326X(95)00216-A. [DOI] [Google Scholar]
- Muir D. C. G.; Teixeira C.; Wania F. Empirical and modeling evidence of regional atmospheric transport of current-use pesticides. Environ. Toxicol. Chem. 2004, 23 (10), 2421–2432. 10.1897/03-457. [DOI] [PubMed] [Google Scholar]
- Fuhrimann S.; Klánová J.; Přibylová P.; Kohoutek J.; Dalvie M. A.; Röösli M.; Degrendele C. Qualitative Assessment of 27 current-use pesticides in air at 20 sampling sites across Africa. Chemosphere 2020, 258, 127333 10.1016/j.chemosphere.2020.127333. [DOI] [PubMed] [Google Scholar]
- Jablonowski N. D.; Schäffer A.; Burauel P. Still present after all these years: persistence plus potential toxicity raise questions about the use of atrazine. Environ. Sci. Pollut. Res. 2011, 18 (2), 328–331. 10.1007/s11356-010-0431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Information on Biocides - ECHA. https://echa.europa.eu/information-on-chemicals/biocidal-active-substances (accessed Jan 11, 2023).
- Igel A. L.; Ekman A. M. L.; Leck C.; Tjernström M.; Savre J.; Sedlar J. The Free Troposphere as a potential source of Arctic boundary layer aerosol particles. Geophys. Res. Lett. 2017, 44 (13), 7053–7060. 10.1002/2017GL073808. [DOI] [Google Scholar]
- Lewis K. A.; Tzilivakis J.; Warner D. J.; Green A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess.: Int. J. 2016, 22 (4), 1050–1064. 10.1080/10807039.2015.1133242. [DOI] [Google Scholar]
- Götz C. W.; Scheringer M.; Macleod M.; Roth C. M.; Hungerbühler K. Alternative approaches for modeling gas-particle partitioning of semivolatile organic chemicals: model development and comparison. Environ. Sci. Technol. 2007, 41 (4), 1272–1278. 10.1021/es060583y. [DOI] [PubMed] [Google Scholar]
- Krejci R.; Zieger P.. Personal Communication; University of Stockholm, 2023. [Google Scholar]
- Harner T.; Bidleman T. F. Octanol-air partition coefficient for describing particle/gas partitioning of aromatic compounds in urban air. Environ. Sci. Technol. 1998, 32, 1494–1502. 10.1021/es970890r. [DOI] [Google Scholar]
- Meyer T.; Lei Y. D.; Muradi I.; Wania F. Organic contaminant release from melting snow. 1. Influence of chemical partitioning. Environ. Sci. Technol. 2009, 43 (3), 657–662. 10.1021/es8020217. [DOI] [PubMed] [Google Scholar]
- Atkinson R. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem. Rev. 1985, 85 (1), 69–201. 10.1021/cr00071a004. [DOI] [Google Scholar]
- Spivakovsky C. M.; Logan J. A.; Montzka S. A.; Balkanski Y. J.; Foreman-Fowler M.; Jones D. B. A.; Horowitz L. W.; Fusco A. C.; Brenninkmeijer C. A. M.; Prather M. J.; Wofsy S. C.; McElroy M. B. Three-Dimensional Climatological Distribution of Tropospheric OH: Update and evaluation. J. Geophys. Res.: Atmos. 2000, 105 (D7), 8931–8980. 10.1029/1999JD901006. [DOI] [Google Scholar]
- Lammel G. Effects of time-averaging climate parameters on predicted multicompartmental fate of pesticides and POPs. Environ. Pollut. 2004, 128 (1–2), 291–302. 10.1016/j.envpol.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Beyer A.; Mackay D.; Matthies M.; Wania F.; Webster E. Assessing long-range transport potential of persistent organic pollutants. Environ. Sci. Technol. 2000, 34 (4), 699–703. 10.1021/es990207w. [DOI] [Google Scholar]
- Bennett D. H.; McKone T. E.; Matthies M.; Kastenberg W. E. General formulation of characteristic travel distance for semivolatile organic chemicals in a multimedia environment. Environ. Sci. Technol. 1998, 32 (24), 4023–4030. 10.1021/es980328g. [DOI] [Google Scholar]
- Mattei C.; Wortham H.; Quivet E. Heterogeneous degradation of Pesticides by OH radicals in the atmosphere: influence of humidity and particle type on the kinetics. Sci. Total Environ. 2019, 664, 1084–1094. 10.1016/j.scitotenv.2019.02.038. [DOI] [PubMed] [Google Scholar]
- Smit A. A. M. F. R.; van den Berg F.; Leistra M.. Estimation Method for the Volatilisation of Pesticides from Fallow Soil, Technical Report 2, Environmental Planning Bureau Series DLO Winand Staring Centre: Wageningen, the Netherlands; 1997.
- Smit A. A. M. F. R.; Leistra M.; van den Berg F.. Estimation Method for the Volatilisation of Pesticides from Plants, Technical Report 2, Environmental Planning Bureau Series; DLO Winand: Wageningen, the Netherlands, 1998.
- Nuyttens D.; Devarrewaere W.; Verboven P.; Foqué D. Pesticide-laden dust emission and drift from treated seeds during seed drilling: a review. Pest. Manag. Sci. 2013, 69 (5), 564–575. 10.1002/ps.3485. [DOI] [PubMed] [Google Scholar]
- Das S.; Hageman K. J. Influence of adjuvants on pesticide soil-air partition coefficients: laboratory measurements and predicted effects on volatilization. Environ. Sci. Technol. 2020, 54 (12), 7302–7308. 10.1021/acs.est.0c00964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.