Abstract
Cancer continues to affect underserved populations disproportionately. Novel optical imaging technologies, which can provide rapid, non-invasive, and accurate cancer detection at the point of care, have great potential to improve global cancer care. This article reviews the recent technical innovations and clinical translation of low-cost optical imaging technologies, highlighting the advances in both hardware and software, especially the integration of artificial intelligence, to improve in vivo cancer detection in low-resource settings. Additionally, this article provides an overview of existing challenges and future perspectives of adapting optical imaging technologies into clinical practice, which can potentially contribute to novel insights and programs that effectively improve cancer detection in low-resource settings.
Keywords: In vivo cancer detection, Low-resource settings, Optical imaging, Deep learning
Introduction
Cancer presents a high global burden, disparately impacting those living in low- and middle-income countries (LMICs). The incidence rate of cancers in LMICs is anticipated to grow, increasing the need to expand cancer screening and early detection programs that enable early treatment with good patient prognoses [1,2]. Yet, inequities exist in access to cancer screening and early detection programs due to limited infrastructure and shortages of trained personnel, particularly in low-resource settings (LRS). As a result, patient care is compromised, leading to several challenges, including late-stage diagnosis, lack of targeted interventions, and lack of continuity of care (Figure 1). Optical imaging has emerged as a promising technology for in vivo cancer detection and diagnosis, which offers real-time imaging capabilities, noninvasiveness, automated diagnostic performance, and potential for cost-effectiveness [3]. Implementing optical imaging in LRS, however, usually presents several challenges, including high upfront cost, lack of infrastructure, trained personnel, maintenance and technical support, connectivity and data management, and difficulties in integration within existing healthcare systems. Addressing these challenges in LRS requires a multi-faceted approach, including financial investments, capacity building, infrastructure development, and collaboration with relevant stakeholders to ensure successful implementation and sustainable use of optical imaging technologies. In recent years, low-cost, high-quality, and easy-to-use optical imaging systems tailored for LRS have been developed. These tools aim to provide reliable and accurate cancer diagnosis in a simple format, while overcoming financial, infrastructure, and resource limitations. Furthermore, integrating artificial intelligence (AI) with optical imaging modalities opens new opportunities to improve global cancer detection by providing accurate computer-aided diagnosis in real time and reducing reliance on clinical expertise. For example, a colposcope is an expensive piece of equipment used for cervical examination that requires a trained colposcopist to interpret clinical images. This has partly been addressed in low-resource settings with the development of low-cost, battery powered alternatives that reduce cost and the need for infrastructure. In the future, the use of automated algorithms to interpret colposcopic images could reduce the need for expertise. This review explores recent advancements in low-cost optical imaging technologies for in vivo cancer detection in LRS, highlighting benefits, prospects, and remaining challenges to improve cancer care in underserved populations.
Figure 1.
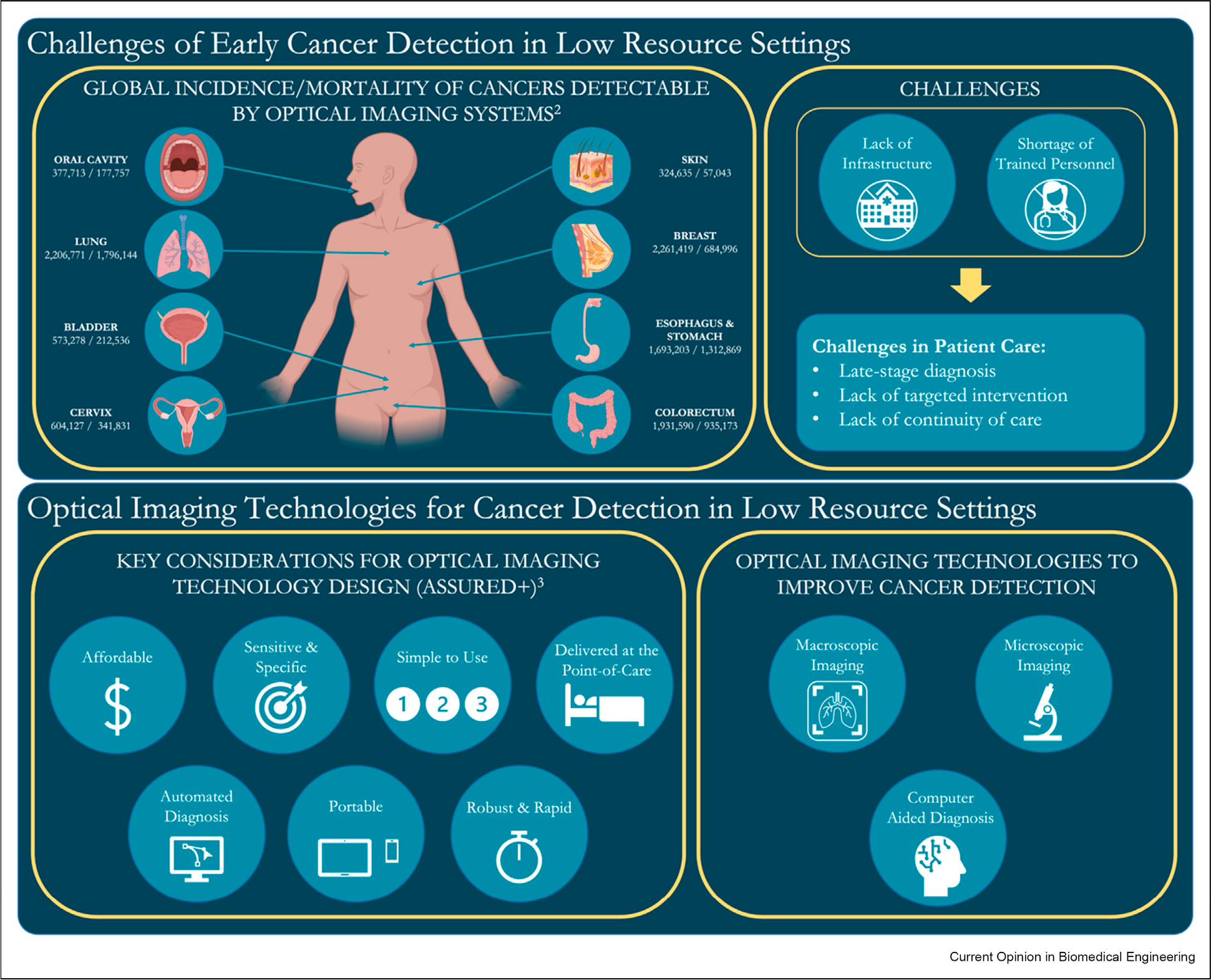
Enabling early cancer detection in low-resource settings (LRS) through in vivo optical imaging. The top panel illustrates the global burden of selected cancers that can potentially be addressed by optical imaging, and current challenges in early detection and its impact on patient care. The bottom panel shows important considerations when designing optical imaging technologies and the potential of these imaging systems, when combined with computer-aided diagnosis, for early cancer detection in LRS. Human anatomy figure created with BioRender.com.
Low-cost optical imaging technologies for low-resource settings
Macroscopic imaging
Macroscopic optical imaging utilizes light at various wavelengths to illuminate a target area that usually spans more than several centimeters. One advantage of macroscopic optical imaging is its ability to provide real-time, non-invasive visualization of tissue structures over a wide area and therefore provide a comprehensive view of the suspicious area [4]. Key innovations in macroscopic imaging have significantly advanced the field. Two notable areas of recent progress are 1) affordable and accessible imaging instrumentation coupled with deep learning (DL) methods and 2) integration of multispectral and multimodal widefield imaging techniques. Macroscopic imaging modalities have become more accessible with enhanced imaging capabilities, potentially enabling more cost-effective imaging strategies. Additionally, DL methods have been applied to interpret macroscopic images, which holds promise to improve diagnostic performance and reduce the dependence on expert interpretation [5]. The integration of multimodal macroscopic imaging has expanded imaging capabilities and enhanced diagnostic accuracy. By combining different imaging modalities, such as autofluorescence imaging (AFI), multispectral imaging (MSI), or narrow-band imaging (NBI), more comprehensive assessments of tissue can be achieved [4]. Furthermore, macroscopic imaging clinical validation studies have demonstrated its effectiveness in various resource-limited settings. The following section focuses on various macroscopic imaging modalities including white light and multispectral imaging, and highlights key technological advancements, real-time image analysis, AI integration, and multimodal imaging approaches.
White light imaging: integrating low-cost instrumentation with deep learning
White light imaging (WLI) is increasingly affordable and accessible with advances in optics, sensors, and smartphone cameras that provide improved image quality from cost-effective imaging equipment. This is exemplified by digital colposcopy. Historically, standard-of-care examinations were conducted with visual inspection by trained professionals using colposcopes and, at most, captured film cervicograms. More recently, advances in cost-effective and miniaturized optics and camera sensors gave rise to the development of various low-cost, digital, portable colposcopes. For example, in a study of 129 patients in Peru, Mueller et al. showed that the Pocket Colposcope achieves comparable performance for identifying precancerous and cancerous cervical lesions when compared to the standard of care [6].
The capability to acquire high-quality digital images opens new opportunities for image interpretation via telemedicine or machine learning, a critical feature for technology dissemination in LRS. For example, the validation of a portable colposcope, Gynocular, with scoring in a clinical study in India demonstrated reliable detection of precancerous lesions. A total of 495 images from 94 patients were examined and assessed by clinicians in real-time and also separately by remote specialists/clinicians [7]. Notably, this still relies on time and availability of expert clinicians that might not always be feasible in LRS.
Digital image acquisition has enabled image capture for real-time analysis, specifically using point-of-care-friendly platforms that also utilize DL. Although macroscopic WLI has proven to be useful for cancer detection, these images still require trained experts for accurate interpretation. DL can play a role to reduce the need for clinical expertise. For example, an automated algorithm developed on digitized cervicograms and digital colposcopy images collected with a smartphone colposcope, MobileODT, was trained, validated, and tested on a combined dataset of 17,013 images from 9462 women across five different studies [8]. The best-performing algorithm showed promise in classifying cervical images in a repeatable and reliable manner for the intended test population. Similarly, a smartphone endoscope and a DL algorithm were developed to distinguish abnormal cervical lesions from normal tissue [9]; results from 20 patients showed good agreement with histology, suggesting the potential to use a simple smartphone endoscope for cervical cancer screening in low-resource settings.
Also of key importance is the integration of various forms of clinical data, such as multimodal and temporal data to develop DL algorithms that approximate clinical decision-making and provide a more comprehensive approach to diagnose diseases with available data. This is illustrated by development of diagnostic algorithms using relevant datasets collected with digital colposcopes that combine multimodal and multi-contrast images [10], utilize time series acetic acid images, green filter images, and Lugol’s iodine images [11], and combine cross-modal information [12].
With increased use of DL, there need to be effective methods to evaluate the accuracy of these diagnostic algorithms. DL is a rapid driver of innovation in macroscopic imaging that lacks substantial oversight and requires comprehensive methods of evaluation at all points in data collection and algorithm development. Development oversight becomes increasingly important in the context of deployment of algorithms to LRS.
There is an important need to develop standardized guidelines for evaluating datasets and models and to standardize the metadata collected for datasets, especially publicly available datasets. Datasets should be collected with validated endpoints and include the methods used to collect them - such as instrumentation used. They should reflect the diversity of the population for its intended use. Although transformative progress has been made in DL for cancer detection, this progress often comes at the cost of biased and less accurate results for underrepresented populations. Dataset and algorithm bias can be present throughout the development process and should be evaluated and identified at each stage of development [13]. Researchers must examine the effort devoted to the evaluation of algorithms and standardization of datasets to ensure development of reliable algorithms that are generalizable in their intended use-case setting. For example, overfitting on training data is a significant problem that can lead to poor performance in test settings and, as such, should be identified before deployment using evaluation metrics such as repeatability [8]. Several tools and models currently exist in the Medical Imaging and Data Resource Center, Microsoft’s Datasheets for Datasets, and a skin cancer specific evaluation checklist [14,15], but field-specific evaluation metrics for other applications should also be established. The World Health Organization (WHO) guidelines for AI also highlight the need for a systematic and standardized approach to evidence generation for AI-based medical devices. It provides a framework outlining key considerations and steps to ensure development, validation, and evaluation of safe and effective AI technologies in healthcare [16].
Multispectral and multimodal macroscopic imaging
MSI, AFI, and NBI have shown significantly enhanced imaging capabilities compared to WLI by providing additional information about tissue characteristics and highlighting specific features of interest, such as increased optical scattering, loss of autofluorescence, and increased vascularization. By combining data from these different imaging modalities, a more comprehensive assessment of an area of interest can be achieved, leading to improved clinical diagnosis and decision-making. This multimodal approach holds great potential for enhancing cancer detection, biopsy guidance, and informing treatment decisions in various clinical settings.
MSI often involves bulky instrumentation and requires specialized optics that allow for spectral scanning processes, such as filters and diffraction grating elements. Advancements in optics and electronics, such as the rapid development of smartphone technologies, have enabled a move toward integration into point-of-care-friendly platforms. MSI has been implemented in various smartphone platforms, which demonstrate the potential for low-resource applications [17–20]. A low-cost commercial instrument called OralScan implements MSI and was tested in India for screening and detection of oral cancers. The evaluation of this MSI device demonstrates the potential for such a device to be used as an adjunct in LRS for lesion assessment and biopsy guidance [21].
While recent efforts have translated MSI to point-of-care friendly platforms, low-cost NBI remains to be implemented with great potential for being adapted for LRS. Advances in DL for NBI have enabled its use for detection, classification, and real-time analysis of precancers and cancers in the gastrointestinal tract, which could be translated to the point of care [22]. For example, an algorithm developed in a large-scale study with 1777 narrowband images from 295 cases collected across three centers showed comparable performance to senior endoscopists and improved performance to junior endoscopists in detection of early gastric cancer, demonstrating the potential for this tool to aid in cancer diagnosis [23]. While the diagnostic performance of the algorithm was assessed on still images, it has the potential to be applied to video frames collected during the endoscopic imaging session.
Integration of multiple imaging modalities such as WLI, AFI, and MSI has proven to be beneficial in improving the sensitivity and specificity of cancer detection. For example, a smartphone-based device with two imaging modalities (WLI and AFI) and a DL algorithm was tested in a large cohort in India [24,25]. Here, the potential for the algorithm to be used by non-expert clinicians was demonstrated. In another case, a bimodal system integrating AFI and MSI with a cloud-based machine learning algorithm demonstrated potential for real-time screening and detection of potentially malignant oral lesions and for providing biopsy guidance [26]. A dermascope that combines WLI and laser speckle contrast imaging for blood flow imaging showed distinct profiles for two different skin lesion types [27]. Overall, the combination of various imaging modalities shows improved diagnostic performance, and the integration of computer-aided diagnosis shows the potential to reduce reliance on expertise.
Microscopic imaging
Besides in vivo macroscopic imaging, promising technical advances have been achieved in developing in vivo microscopic imaging techniques for early cancer detection. The integration of in vivo microscopy (IVM) and computer-aided algorithms has shown potential to enable real-time, non-invasive, accurate point-of-care diagnosis. This approach can potentially address several challenges associated with standard-of-care histopathology, which are particularly significant in LRS, including increased cost, patient risk resulting from invasive biopsies, inaccurate diagnosis due to examining limited areas of suspicious lesions, and extensive needs for infrastructure and expertise [28]. Recent efforts to promote the implementation of IVM in LRS are highlighted in three aspects: 1) advancements in technical design to enhance system portability and reduce cost, as well as field implementation for clinical performance evaluation in LRS; 2) leveraging computer-aided algorithms to enhance imaging capabilities and facilitate clinical data interpretation; 3) integration of multiscale, multimodal imaging for improved diagnostic capability. In this section, we review and discuss key innovations in these aspects of in vivo microscopic imaging that aim to improve cancer screening, diagnosis, and treatment in LRS. While this review primarily focuses on in vivo imaging techniques, it is important to acknowledge recent advances in ex vivo imaging techniques for histopathology, such as digital pathology and AI-based analysis [29]. The clinical adaptation of both novel ex vivo and in vivo imaging techniques in LRS can improve the screening, diagnosis, and treatment process, thus addressing the global health challenges in cancer prevention.
Low-cost system development and clinical validation
Recent progress has been made in the development of portable, affordable, and easy-to-use in vivo microscopes. Several systems have shown promising preliminary results in pilot studies. Currently, IVM reveals microscopic features based on endogenous contrast, such as autofluorescence and reflectance, or exogenous contrast agents, such as fluorescein. Microscopic autofluorescence imaging can be performed using several modalities, like multiphoton microscopy [30] and light-sheet microscopy [31]. However, the effectiveness of using these systems for in vivo cancer detection still requires further clinical evaluation. Although there are some efforts to improve usability, such as development of a probe-based two-photon microscope [32], most existing systems are built in a large form factor with a high cost. Future work can focus on clinical validation and adapting the technology for application in LRS.
Reflectance, another endogenous source of contrast, is more commonly used in clinical settings. Reflectance confocal microscopy (RCM) is an established, commercially available imaging technique. Its clinical performance has been validated in clinical studies over decades, especially for skin cancer detection [33]. However, the large size and high cost of RCM hinder its application in LRS. Recent progress in eliminating the need for expensive mechanical scanning components in RCM, such as the use of chromatic dispersion-based techniques to enable lateral scanning [34] or depth scanning [35], opens new opportunities for designing low-cost, portable RCM systems.
Similar to RCM, optical coherence tomography (OCT) is a costly, commercially available technique and has been extensively studied for in vivo cancer detection. Portable, lower-cost OCT systems have been developed recently [36,37]. Despite advances in system development, only a limited number of clinical studies have been performed to evaluate the diagnostic performance. In a study of 232 patients in India, a low-cost OCT system delineated oral cancer with a sensitivity of 93% and a specificity of 74% when incorporating an automated image processing algorithm [37]. To facilitate the clinical translation and improve cancer diagnosis in LRS, future studies are necessary to further validate clinical performance of these systems.
In vivo microscopic imaging with exogenous contrast agents addresses several challenges associated with imaging with endogenous contrast, including low signal-to-noise ratio and limited resolving capability for cellular structures. Probe-based IVM using exogenous contrast agents have been developed to enable real-time lesion assessment at the point of care. These systems include commercially available options such as confocal laser endomicroscopy (CLE) [38], and other research prototypes with improved imaging capabilities, such as higher imaging speed [39,40]. Despite the capability to resolve subcellular features in real-time, the high cost of CLE (>$100,000) hinders its broad applicability in LRS. As an alternative, a portable and low-cost fluorescence fiber-optic high-resolution microendoscope (HRME) has been developed for in vivo cancer detection and validated in LRS. In a recent study conducted in Brazil, HRME was shown to detect high-grade cervical precancerous lesions with a sensitivity of 92% and a specificity of 60% in 1486 patients when integrated with computer-aided diagnosis, which is comparable to colposcopy interpreted by expert clinicians [41]. Besides system development, an additional challenge of implementing IVM with exogenous agents is that only a limited number of contrast agents have been approved for human use. Future advances in development of novel contrast agents can promote clinical application of IVM in different clinical settings.
Algorithm development to facilitate clinical applications
The rapid advances in development of novel algorithms, especially DL-based algorithms, facilitate the application of IVM in LRS in various aspects. First, recent algorithms enable cost-efficient ways to address the limitations of IVM and improve imaging capabilities. With enhanced computational power and high imaging speed, sophisticated mosaicking algorithms have been developed to significantly enlarge the field of view of IVM while preserving cellular resolution [40]. DL algorithms are also implemented to overcome the intrinsic tradeoffs among different imaging specifications. For example, a DL-based denoising algorithm has been employed in a low-cost RCM system to increase the signal-to-noise ratio at a high imaging speed [42]. Although not yet demonstrated in IVM or cancer detection, a novel DL algorithm can enhance the resolution even beyond the diffraction limitation in multiple fluorescence microscopy modalities [43]. In another non-IVM application, the DL algorithm enables virtual three-dimensional refocusing in a single two-dimensional widefield image without physical scanning, thus digitally increasing the depth of field in widefield fluorescence microscopy [44]. Similar concepts can be applied to enhance the imaging performance of IVM and diagnostic capability for cancer detection without adding additional cost, which is particularly advantageous for potential implementation in LRS.
Second, novel optical system designs can be provided by leveraging AI algorithms at the system development stage. This hardware-software co-design concept has derived design insights to improve imaging capabilities in cost-efficient ways. For example, a microscope with an extended depth of field has been developed by adding an inexpensive phase mask into the optical path and incorporating a DL reconstruction algorithm [45]. In another example, the point-spread-function modulation was designed and optimized with a neural network to enable three-dimensional reconstruction in the microscopy system [46]. These strategies represent promising directions for the development of the next generation of IVM systems for cancer detection that enhance imaging performance while maintaining cost-effectiveness and adaptability for LRS.
Finally, AI-based diagnostic algorithms effectively facilitate the field implementation of IVM in LRS. AI-based algorithms have demonstrated great potential in providing rapid, accurate, and automatic point-of-care diagnosis by analyzing high-resolution imaging data. This alleviates challenges associated with translating novel imaging techniques into clinical practice and ultimately reduces the need for clinical expertise in LRS. AI-based diagnostic algorithms have been incorporated with several low-cost IVM systems and shown promising results in cancer detection [37,41]. AI-based diagnosis will continue to play an important role in increasing accessibility of high-quality health care and improving cancer diagnosis globally. While promising results have been reported, the sample sizes of most studies are limited, and whether the performance will generalize in the absence of expert practitioners remains to be answered. As a result, the performance of AI algorithms needs to be further evaluated prospectively, especially in varied patient populations and clinical settings.
Multiscale and multimodal imaging
Integrating macroscopic and microscopic imaging techniques for multiscale, multimodal imaging (MMI) creates new possibilities to further improve diagnostic capabilities. First, MMI provides an intuitive and accurate method for real-time clinical guidance. A significant barrier to implement IVM in clinical practice is the correlation of microscopic assessment with corresponding lesion location, especially when a substantial amount of microscopic imaging data is acquired over a large tissue area. Introducing physical landmarks onto tissue surfaces can facilitate the placement and position tracking of the microscopic probe for improved correlation [47], but this approach is limited by the accessibility of anatomic sites. Enabled by the multiscale imaging approach, microscopic images are correlated to anatomic locations by tracking the microscopic imaging probe via macroscopic imaging. This provides the spatial context necessary for assessment of suspicious lesions over a large field of view with co-registered microscopic information. Preliminary ex vivo studies have demonstrated promising results in utilizing multiscale imaging for real-time tumor margin delineation in skin [48]. In a recent pilot study, a low-cost multiscale and multimodal mobile colposcope was developed, which integrates a commercially available low-cost colposcope and a high-resolution fiber-optic microendoscope [49]. By implementing algorithms for real-time diagnosis, probe tracking, and image co-registration, the mobile colposcope correlates diagnostic scores to anatomic location and shows potential for improving cervical cancer detection in LRS.
Second, MMI can potentially enable the entire screening, diagnosis, and treatment procedure in a single visit in LRS. Macroscopic imaging provides a rapid examination of suspicious lesions at low resolution, which then guides microscopic investigations of specific regions of concern at high resolution for diagnosis. Multiscale and multimodal algorithms can be developed to provide automatic lesion detection and treatment guidance at the point of care, thereby significantly mitigating reliance on clinical expertise in LRS. Finally, additional diagnostic information provided by different imaging modalities in different scales can be potentially integrated to further improve diagnostic performance. In a pilot study, an MMI system that integrates macroscopic autofluorescence imaging and high-resolution imaging shows improved sensitivity of high-grade oral dysplasia compared to clinical evaluation by an expert [50]. Despite the potential benefits and promising preliminary results, future clinical studies are necessary to further validate the diagnostic capability of MMI systems in LRS.
Discussion and outlook
Recent advances in the development of low-cost optical imaging systems, along with computational algorithms, show significant promise in addressing the global health challenge of cancer prevention in LRS. The rapid progress in optical technology has enabled development of several portable, easy-to-use imaging systems that offer high-quality imaging at a much lower cost. Some of these innovative imaging tools have been validated in clinical studies, demonstrating their adaptability and ability to overcome infrastructure limitations in LRS. Development of algorithms for computer-aided diagnosis, especially the rapid advent of DL-based approaches, holds great potential for providing accurate, real-time, diagnostic guidance, potentially reducing reliance on clinical expertise. Furthermore, integration of macroscopic and microscopic imaging techniques opens new opportunities to improve cancer detection capabilities and enable screening-diagnosis-treatment programs in a single visit in LRS. Table 1 summarizes the low-cost optical imaging technologies discussed in this review, highlighting hallmarks of cancer [51] that can potentially be detected, stage of clinical validation, and clinical applicability in LRS for each imaging modality.
Table 1.
Low-cost optical imaging technologies for in vivo cancer detection in low-resource settings.
| Imaging modality | Macroscopic imaging | Microscopic imaging | MMI | ||||
|---|---|---|---|---|---|---|---|
| WLI | AFI | MSI | RCM | OCT | HRME | ||
| Hallmarks of cancer [51] that can potentially be detected with the modality | • Inducing angiogenesis • Tumor-promoting inflammation |
• Activating invasion and metastasis • Deregulating cellular energetics |
• Inducing angiogenesis • Tumor-promoting inflammation |
• Sustaining proliferative signaling • Evading growth suppressors • Resisting cell death • Enabling replicative immortality |
• Sustaining proliferative signaling • Evading growth suppressors • Resisting cell death • Enabling replicative immortality • Activating invasion and metastasis • Tumor-promoting inflammation |
• Sustaining proliferative signaling • Evading growth suppressors • Resisting cell death • Enabling replicative immortality |
• Sustaining proliferative signaling • Evading growth suppressors • Resisting cell death • Enabling replicative immortality • Activating invasion and metastasis • Deregulating cellular energetics • Inducing angiogenesis • Tumor-promoting inflammation |
| Stage of clinical validation | Clinically evaluated in large studies | Clinically evaluated in large studies | Tested in pilot studies | Clinically evaluated in large studies | Clinically evaluated in large studies | Clinically evaluated in large studies | Tested in pilot studies |
| Comments on clinical applicability in LRS |
• Low-cost FDA cleared devices available • DL algorithms in development for diagnosis • Easy to obtain high quality images with simple devices |
• FDA cleared devices available • DL algorithms in development for diagnosis • Improved sensitivity, but reduced specificity • Must be performed in a dark environment |
• Provides additional spectral characteristics for cancer detection • Requires further clinical evaluation |
• High-cost FDA cleared devices available • Low-cost systems in development • No contrast agent needed |
• High-cost FDA cleared devices available • Low-cost systems in development • No contrast agent needed |
• Low-cost systems in development • DL algorithms in development for diagnosis • Contrast agent needed |
• Facilitate real-time clinical guidance • Potentially enable the entire screening, diagnosis, and treatment procedure in a single visit • Relatively higher system complexity |
WLI: white light imaging; AFI: autofluorescence imaging; MSI: multispectral imaging; RCM: reflectance confocal microscopy; OCT: optical coherence tomography; HRME: high-resolution microendoscope; MMI: multiscale, multimodal imaging; LRS: low-resource settings; DL: deep learning.
Although these efforts hold promise, several major challenges remain to be addressed for improved cancer diagnosis worldwide. Currently, only a limited number of low-cost imaging technologies have been evaluated in clinical studies conducted in LRS. Continued efforts should be made to facilitate technological advancements for LRS. Clinical barriers to technology adoption should be considered during development stages. While AI-based diagnostic algorithms are widely implemented in clinical practice, new challenges have also been introduced, including standardization of training datasets, interpretation of training outcomes and potential artifacts, and ensuring algorithm robustness. Nevertheless, with ongoing efforts to address these challenges, AI-based algorithms will continue to play an increasingly important role in cancer detection. Rapid development of powerful, low-cost optical imaging tools and computer-aided diagnostic algorithms presents opportunities to enable accurate point-of-care cancer diagnosis, thus significantly improving detection of precancer and cancer at early stages globally.
Acknowledgments
This research was supported in part by the National Cancer Institute of the National Institutes of Health under award numbers R01CA251911 and R01CA252245, the National Institute of Dental & Craniofacial Research of the NIH under award number R21DE030532, and through National Academy of Sciences, United States Agency for International Development (Partnerships for Enhanced Engagement in Research, Cooperative Agreement AID-OAA-A-11–00012).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This review comes from a themed issue on Biomedical Imaging: Low-cost and portable imaging systems for rural and low-cost environments
Edited by Hatice Ceylan Koydemir and Aydogan Ozcan
For complete overview of the section, please refer the article collection - Biomedical Imaging: Low-cost and portable imaging systems for rural and low-cost environments
Data availability
No data was used for the research described in the article.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.dos-Santos-Silva I, Gupta S, Orem J, Shulman LN: Global disparities in access to cancer care. Commun Med 2022, 2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2021, 71:209–249. [DOI] [PubMed] [Google Scholar]
- 3.Land KJ, Boeras DI, Chen X-S, Ramsay AR, Peeling RW: REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 2019, 4:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L, Jiang P, Bao Z, Pang W, Ding S, Yin M-J, Li P, Gu B: Fundamentals of optical imaging. In Optical imaging in human disease and biological research. Edited by Wei X, Gu B, Springer; 2021:1–22. [DOI] [PubMed] [Google Scholar]
- 5.Williams D, Hornung H, Nadimpalli A, Peery A: Deep learning and its application for healthcare delivery in low and middle income countries. Frontiers in Artificial Intelligence 2021:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller J, Lam C, Dahl D, Asiedu M, Krieger M, Bellido-Fuentes Y, Kellish M, Peters J, Erkanli A, Ortiz E, et al. : Portable Pocket colposcopy performs comparably to standard-of-care clinical colposcopy using acetic acid and Lugol’s iodine as contrast mediators: an investigational study in Peru. BJOG An Int J Obstet Gynaecol 2018, 125:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taghavi K, Banerjee D, Mandal R, Kallner HK, Thorsell M, Friis T, Kocoska-Maras L, Strander B, Singer A, Wikström E: Colposcopy telemedicine: live versus static swede score and accuracy in detecting CIN2+, a cross-sectional pilot study. BMC Wom Health 2018, 18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed Syed Rakin Befano Brian, Andreanne Lemay, Didem Egemen, Rodriguez Ana Cecilia Angara Sandeep, Kanan Desai, Jeronimo Jose, Sameer Antani, Nicole Campos, et al. : Reproducible and clinically translatable deep neural networks for cervical screening. medRxiv 2022. 10.1101/2022.12.17.22282984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae JK, Roh H-J, You JS, Kim K, Ahn Y, Askaruly S, Park K, Yang H, Jang G-J, Moon KH, et al. : Quantitative screening of cervical cancers for low-resource settings: pilot study of smartphone-based endoscopic visual inspection after acetic acid using machine learning techniques. JMIR Mhealth Uhealth 2020, 8, e16467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skerrett E, Miao Z, Asiedu MN, Richards M, Crouch B, Sapiro G, Qiu Q, Ramanujam N: Multicontrast Pocket colposcopy cervical cancer diagnostic algorithm for referral populations. BME Front 2022, 2022. 2022/9823184. * * Authors develop deep learning models to classify cervical lesions based on images collected by a low-cost colposcope. The results show that this method can provide accurate detection of early cervical cancer.
- 11.Yue Z, Ding S, Zhao W, Wang H, Ma J, Zhang Y, Zhang Y: Automatic CIN grades prediction of sequential cervigram image using LSTM with multistate CNN features. IEEE J Biomed Health Inform 2020, 24:844–854. [DOI] [PubMed] [Google Scholar]
- 12.Fu L, Xia W, Shi W, Cao G, Ruan Y, Zhao X, Liu M, Niu S, Li F, Gao X: Deep learning based cervical screening by the cross-modal integration of colposcopy, cytology, and HPV test. Int J Med Inf 2022, 159:104675. [DOI] [PubMed] [Google Scholar]
- 13. Drukker K, Chen W, Gichoya JW, Gruszauskas NP, Kalpathy-Cramer J, Koyejo S, Myers KJ, Sá RC, Sahiner B, Whitney HM, et al. : Toward fairness in artificial intelligence for medical image analysis: identification and mitigation of potential biases in the roadmap from data collection to model deployment. JMI 2023, 10:61104. * Authors detail various biases that may arise in the development of machine learning for medical imaging applications. The recommendations provide a clear outline of biases to be aware of and how these biases may be mitigated and serve as a useful tool for those in the field.
- 14. Daneshjou R, Barata C, Betz-Stablein B, Celebi ME, Codella N, Combalia M, Guitera P, Gutman D, Halpern A, Helba B, et al. : Checklist for evaluation of image-based artificial intelligence reports in dermatology: CLEAR derm consensus guidelines from the international skin imaging collaboration artificial intelligence working group. JAMA Dermatology 2022, 158:90–96. * Authors create a field specific checklist for assessing machine learning methods in dermatology applications. These findings provide a formalized evaluation of the development and validation of image-based artificial intelligence that will enable fair and trustworthy results in deployment.
- 15.Gebru T, Morgenstern J, Vecchione B, Vaughan JW, Wallach H, Daumé III H, Crawford K: Datasheets for datasets. 2020. arXiv: 180309010 [cs]. [Google Scholar]
- 16. Geneva: World Health Organization: Generating evidence for artificial intelligence based medical devices: a framework for training validation and evaluation. 2021. * WHO’s guidelines on artificial intelligence provide a framework for assessing machine learning methods in a standardized format to ensure proper safety and efficacy in deployment. These guidelines provide a comprehensive outlook on evaluation of artificial intelligence at all points in the development, validation, and deployment process.
- 17.Uthoff RD, Song B, Maarouf M, Shi V, Liang R: Point-of-care, multispectral, smartphone-based dermascopes for dermal lesion screening and erythema monitoring. J Biomed Opt 2020, 25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Q, Wang R: Hyperspectral imaging enabled by an unmodified smartphone for analyzing skin morphological features and monitoring hemodynamics. Biomed Opt Express 2020, 11:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzmina I, Oshina I, Dambite L, Lukinsone V, Maslobojeva A, Berzina A, Spigulis J: Skin chromophore mapping by smartphone RGB camera under spectral band and spectral line illumination. J Biomed Opt 2022:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding H, Chen C, Zhao H, Yue Y, Han C: Smartphone based multispectral imager and its potential for point-of-care testing. Analyst 2019, 144:4380–4385. [DOI] [PubMed] [Google Scholar]
- 21.Prasanna R, Nair SP, Baby A, Unni DAS: Non-invasive detection of oral potentially malignant and malignant lesions using an optical multispectral screening device. Photodiagnosis Photodyn Ther 2023, 42:103300. [DOI] [PubMed] [Google Scholar]
- 22.Pannala R, Krishnan K, Melson J, Parsi MA, Schulman AR, Sullivan S, Trikudanathan G, Trindade AJ, Watson RR, Maple JT, et al. : Artificial intelligence in gastrointestinal endoscopy. VideoGIE 2020, 5:598–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu H, Gong L, Dong D, Zhu L, Wang M, He J, Shu L, Cai Y, Cai S, Su W, et al. : Identifying early gastric cancer under magnifying narrow-band images with deep learning: a multicenter study. Gastrointest Endosc 2021, 93:1333–1341.e3. [DOI] [PubMed] [Google Scholar]
- 24.Uthoff RD, Song B, Sunny S, Patrick S, Suresh A, Kolur T, Gurushanth K, Wooten K, Gupta V, Platek ME, et al. : Small form factor, flexible, dual-modality handheld probe for smartphone-based, point-of-care oral and oropharyngeal cancer screening. J Biomed Opt 2019, 24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birur NP, Song B, Sunny SP, K G, Mendonca P, Mukhia N, Li S, Patrick S, S G, S AR, et al. : Field validation of deep learning based Point-of-Care device for early detection of oral malignant and potentially malignant disorders. Sci Rep 2022, 12:14283. * * The study evaluates the clinical performance of a low-cost, portable, and multimodal imaging system equipped with deep learning algorithms for oral cancer detection. By screening 5025 patients in India, the results demonstrate the potential of the system as a powerful triaging tool that can be used in low-resource settings.
- 26.Narayanan S, Anand S, Prasanna R, Managoli SP, Suvarnadas R, Shyamsundar V, Nagarajan K, Mishra SK, Johnson M, Dathurao Ramanand M, et al. : Bimodal multispectral imaging system with cloud-based machine learning algorithm for real-time screening and detection of oral potentially malignant lesions and biopsy guidance. J Biomed Opt 2021:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White SM, Valdebran M, Kelly KM, Choi B: Simultaneous blood flow measurement and dermoscopy of skin lesions using dual-mode dermascope. Sci Rep 2018, 8:16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bishop KW, Maitland KC, Rajadhyaksha M, Liu JTC: In vivo microscopy as an adjunctive tool to guide detection, diagnosis, and treatment. J Biomed Opt 2022:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxi V, Edwards R, Montalto M, Saha S: Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod Pathol 2022, 35:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lentsch G, Valdebran M, Saknite I, Smith J, Linden KG, König K, Barr RJ, Harris RM, Tromberg BJ, Ganesan AK, et al. : Non-invasive optical biopsy by multiphoton microscopy identifies the live morphology of common melanocytic nevi. Pigment Cell Melanoma Res 2020, 33:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel KB, Liang W, Casper MJ, Voleti V, Li W, Yagielski AJ, Zhao HT, Perez Campos C, Lee GS, Liu JM, et al. : High-speed light-sheet microscopy for the in-situ acquisition of volumetric histological images of living tissue. Nat Biomed Eng 2022, 6:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li A, Hall G, Chen D, Liang W, Ning B, Guan H, Li X: A biopsy-needle compatible varifocal multiphoton rigid probe for depth-resolved optical biopsy. J Biophot 2019, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rey-Barroso L, Peña-Gutiérrez S, Yáñez C, Burgos-Fernández FJ, Vilaseca M, Royo S: Optical technologies for the improvement of skin cancer diagnosis: a review. Sensors 2021, 21:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong C, Kulkarni N, Zhu W, Nguyen CD, Curiel-Lewandrowski C, Kang D: Low-cost, high-speed near infrared reflectance confocal microscope. Biomed Opt Express 2019, 10:3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kulkarni N, Masciola A, Nishant A, Kim K-J, Choi H, Gmitro A, Freeman EE, Semeere A, Nakalembe M, Kang D: Low-cost, chromatic confocal endomicroscope for cellular imaging in vivo. Biomed Opt Express 2021, 12:5629. * Authors develop a low-cost, chromatic confocal endomicroscope for cross-sectional reflectance imaging at cellular resolution. The study shows a promising way to enable confocal depth scanning at a significantly low cost.
- 36.Dsouza R, Won J, Monroy GL, Spillman DR, Boppart SA: Economical and compact briefcase spectral-domain optical coherence tomography system for primary care and point-of-care applications. J Biomed Opt 2018, 23:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. James BL, Sunny SP, Heidari AE, Ramanjinappa RD, Lam T, Tran AV, Kankanala S, Sil S, Tiwari V, Patrick S, et al. : Validation of a point-of-care optical coherence tomography device with machine learning algorithm for detection of oral potentially malignant and malignant lesions. Cancers 2021, 13:3583. * * The study implemented a low-cost optical coherence tomography system for oral cancer detection in 232 patients in India. This is a representative study of evaluating the clinical performance of optical coherence tomography systems in low-resource settings.
- 38.Villard A, Breuskin I, Casiraghi O, Asmandar S, Laplace-Builhe C, Abbaci M, Moya Plana A: Confocal laser endomicroscopy and confocal microscopy for head and neck cancer imaging: recent updates and future perspectives. Oral Oncol 2022, 127:105826. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Vyas K, Yang G-Z: Line scanning, fiber bundle fluorescence HiLo endomicroscopy with confocal slit detection. J Biomed Opt 2019, 24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita Y, Wei L, Cimino PJ, Liu JTC, Sanai N: Video-mosaicked handheld dual-Axis confocal microscopy of gliomas: an ex vivo feasibility study in humans. Front Oncol 2020, 10:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hunt B, Fregnani JHTG, Brenes D, Schwarz RA, Salcedo MP, Possati-Resende JC, Antoniazzi M, de Oliveira Fonseca B, Santana IVV, de Macêdo Matsushita G, et al. : Cervical lesion assessment using real-time microendoscopy image analysis in Brazil: the CLARA study. Int J Cancer 2021, 149:431–441. * * The paper reports the clinical evaluation of the high-resolution microendoscope for cervical cancer detection in 1486 patients in Brazil. This is a representative study of implementing low-cost optical microscopic imaging and computer-aided diagnosis in low-resource settings.
- 42.Zhao J, Jain M, Harris UG, Kose K, Curiel-Lewandrowski C, Kang D: Deep learning-based denoising in high-speed portable reflectance confocal microscopy. Laser Surg Med 2021, 53:880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Rivenson Y, Jin Y, Wei Z, Gao R, Günaydõn H, Bentolila LA, Kural C, Ozcan A: Deep learning enables cross-modality super-resolution in fluorescence microscopy. Nat Methods 2019, 16:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Rivenson Y, Wang H, Luo Y, Ben-David E, Bentolila LA, Pritz C, Ozcan A: Three-dimensional virtual refocusing of fluorescence microscopy images using deep learning. Nat Methods 2019, 16:1323–1331. [DOI] [PubMed] [Google Scholar]
- 45.Jin L, Tang Y, Wu Y, Coole JB, Tan MT, Zhao X, Badaoui H, Robinson JT, Williams MD, Gillenwater AM, et al. : Deep learning extended depth-of-field microscope for fast and slide-free histology. Proc Natl Acad Sci USA 2020, 117:33051–33060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nehme E, Freedman D, Gordon R, Ferdman B, Weiss LE, Alalouf O, Naor T, Orange R, Michaeli T, Shechtman Y: Deep-STORM3D: dense 3D localization microscopy and PSF design by deep learning. Nat Methods 2020, 17:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahu A, Oh Y, Peterson G, Cordova M, Navarrete-Dechent C, Gill M, Alessi-Fox C, Gonzalez S, Phillips W, Wilson S, et al. : In vivo optical imaging-guided targeted sampling for precise diagnosis and molecular pathology. Sci Rep 2021, 11:23124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horgan CC, Bergholt MS, Thin MZ, Nagelkerke A, Kennedy R, Kalber TL, Stuckey DJ, Stevens MM: Image-guided Raman spectroscopy probe-tracking for tumor margin delineation. J Biomed Opt 2021:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coole JB, Brenes D, Possati-Resende JC, Antoniazzi M, Fonseca B de O, Maker Y, Kortum A, Vohra IS, Schwarz RA, Carns J, et al. : Development of a multimodal mobile colposcope for real-time cervical cancer detection. Biomed Opt Express 2022, 13:5116. * Authors demonstrate a multimodal mobile colposcope for cervical cancer detection in low-resource settings which combines a low-cost colposcope and a high-resolution microendoscope. This is the first pilot study to show the clinical potential of the multiscale, multimodal imaging techniques to improve cancer detection.
- 50.Yang EC, Vohra IS, Badaoui H, Schwarz RA, Cherry KD, Jacob J, Rodriguez J, Williams MD, Vigneswaran N, Gillenwater AM, et al. : Prospective evaluation of oral premalignant lesions using a multimodal imaging system: a pilot study. Head Neck 2020, 42:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanahan D: Hallmarks of cancer: new dimensions. Cancer Discov 2022, 12:31–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


