Abstract
In rats, eating obesogenic diets increases calcium-permeable AMPA receptor (CP-AMPAR) transmission in the nucleus accumbens (NAc) core, and enhances food-motivated behavior. Interestingly, these diet-induced alterations in NAc transmission are pronounced and sustained in obesity-prone (OP) male rats and absent in obesity-resistant (OR) populations. However, effects of diet manipulation on food motivation, and the mechanisms underlying this NAc plasticity in OPs is unknown. Using male selectively-bred OP and OR rats, we assessed food-motivated behavior following ad lib access to chow (CH), junk-food (JF), or 10d of JF followed by a return to chow diet (JF-Dep). Motivation for food was greater in OP than OR rats, as expected. However, JF-Dep only produced enhancements in food-seeking in OP groups, while continuous JF access reduced food-seeking in both OPs and ORs. Additionally, optogenetic, chemogenetic, and pharmacological approaches were used to examine NAc CP-AMPAR recruitment following diet manipulation and ex vivo treatment of brain slices. Reducing excitatory transmission in the NAc was sufficient to recruit CP-AMPARs to synapses in OPs, but not ORs. In OPs, JF-induced increases in CP-AMPARs occurred in mPFC-, but not BLA-to-NAc inputs. Together results show that diet differentially affects behavioral and neural plasticity in obesity susceptible populations. We also identify conditions for acute recruitment of NAc CP-AMPARs; these results suggest that synaptic scaling mechanisms contribute to NAc CP-AMPAR recruitment. Overall, this work helps elucidate how diet interacts with obesity susceptibility to influence food-motivated behavior and extends our fundamental understanding of NAc CP-AMPAR recruitment.
Keywords: Obesity, AMPAR, Synaptic scaling, High-fat diet, Striatum, Motivation
1. Introduction
As obesity rates continue to rise globally (Swinburn et al., 2011), it is increasingly important to understand the neural and behavioral consequences of over-consuming calorie-dense palatable foods. In humans, obesogenic diets (i.e., calorie dense, high-fat/high-sugar) alter the function of brain reward centers including the Nucleus Accumbens (NAc; Horstmann et al., 2011; Small, 2009; Stice et al., 2013; Volkow et al., 2013). Furthermore, greater activations in the NAc in response to food cues are observed in obesity-susceptible people before weight gain (Demos et al., 2012; Murdaugh et al., 2012). These data highlight interactions between diet and obesity-susceptibility that contribute to over-eating and weight gain. However, our understanding of neurobehavioral differences in obesity-prone and -resistant populations is limited, and relatively little is known about how obesogenic diets affect NAc function and food-seeking behaviors.
In rodents, consuming obesogenic diets produces a number of alterations in neuronal function (Ferrario et al., 2016), including enhancements in NAc dendritic spine density and excitatory transmission (Counotte et al., 2014; Dingess et al., 2017; Tukey et al., 2013), and decreases in dendritic spine density in the prefrontal cortex (PFC; Dingess et al., 2017). Furthermore, obesity-susceptible rats show enhanced diet-induced NAc glutamatergic plasticity, including potentiation of NAc glutamate synapses (Brown et al., 2017) and increased calcium-permeable AMPA receptor (CP-AMPAR) transmission (Alonso-Caraballo et al., 2021; Nieto et al., 2023; Oginsky et al., 2016). This suggests that food-seeking and feeding behaviors that rely on the NAc may also be altered by obesogenic diets.
Consistent with this, consumption of obesogenic foods enhances cue-triggered food-seeking (Derman and Ferrario, 2018b; Dingess et al., 2017). Activity of NAc CP-AMPARs is required for the expression of cue-triggered food-seekingand increases in NAc CP-AMPAR expression are associated with enhancements in this behavior in obesity-prone, but not obesity-resistant rats (Crombag et al., 2008; Derman and Ferrario, 2018a). However, results describing how eating obesogenic foods alter the willingness to work for food are mixed, with both enhancements and reductions reported (e.g., Finger et al., 2012; Matikainen-Ankney et al., 2023). Thus, there is a need to further tease apart factors that influence food motivated behavior in states of over-abundance and obesity. In addition, despite established differences in NAc function and incentive-motivation in obesity-prone vs -resistant populations, how obesity susceptibility interacts with diet to alter food motivation and NAc glutamatergic plasticity is unclear.
Here we examined how eating a sugary, fatty “junk-food” (JF) diet affects several key aspects of food-seeking and feeding behavior in male rats. This was done both while rats were maintained on this diet, and after a brief JF “deprivation” (JF-Dep) period during which rats only had access to ad lib standard lab chow. Additionally, to understand the mechanisms underlying CP-AMPAR recruitment during JF-Dep, we conducted ex vivo pharmacological studies to identify conditions that induce increases in NAc CP-AMPAR transmission. Finally, given that the medial PFC (mPFC) and basolateral amygdala (BLA) send direct glutamatergic projections to the NAc and are both involved in food-seeking behavior (Cardinal et al., 2002; Christoffel et al., 2021; Holland and Petrovich, 2005), we also determined the input specificity of CP-AMPAR upregulation. Only males were studied here because junk-food deprivation results in long-lasting synaptic increases in CP-AMPARs in males, but not females (see Nieto et al. (2023) for additional discussion).
2. Materials and methods
2.1. Subjects
Male obesity-prone (OP) and obesity-resistant (OR) rats bred in house (50–60 days old at the start of each study) were used (Levin et al., 1997). Rats were singly housed for behavior experiments and pair-housed for electrophysiology experiments (reverse 12-h light/dark cycle). Procedures were approved by The University of Michigan Committee on the Use and Care of Animals in accordance with AAALAC guidelines.
2.2. Food and weight
Rats were weighed daily unless otherwise specified and home cage food intake was measured throughout. Chow (CH) controls were maintained on standard lab chow (Lab Diet 5L0D: 4 kcal/g; 13.6% fat, 28.9% protein, 57.5% carbohydrates; % of caloric content), while experimental junk-food (JF) groups were given free access to JF made in house ((Oginsky et al., 2016; Robinson et al., 2015); 4.5 kcal/g; 19.6% fat, 14% protein, and 48% carbohydrates). This JF diet was a mash of Ruffle potato chips (40g), Chips Ahoy (130g), Nesquik (130g), Jiff peanut butter (130g), powdered Lab Diet 5L0D (200g) and 180 mL of water. JF Deprivation (JF-Dep) consisted of removing JF from the home cage and returning rats to free-access standard lab chow.
2.3. Behavioral procedures
All behavioral testing was conducted in standard operant boxes within sound attenuating chambers (Med Associates; one session/day). The same type of food pellet reinforcer was used for all behavioral studies (45 mg, Bioserv, #F0059). A general description of each experiment is given first followed by details of each behavioral approach.
2.3.1. Experiment 1: effects of junk-food and junk-food deprivation in OP rats
OPs were food restricted (85–90% free-feeding bodyweight) and given 12 Pavlovian Conditioning sessions. Rats were then assigned to chow, JF and JF-Dep groups counterbalanced by behavior during the final three conditioning sessions; they had ad lib access to food for the remainder of the study. Next, rats underwent a single conditioned reinforcement test session followed by instrumental training and testing. Finally, rats underwent free-consumption testing in an ad lib or acutely food restricted state (4.5h without food in the home cage prior to testing; see timeline Fig. 1A).
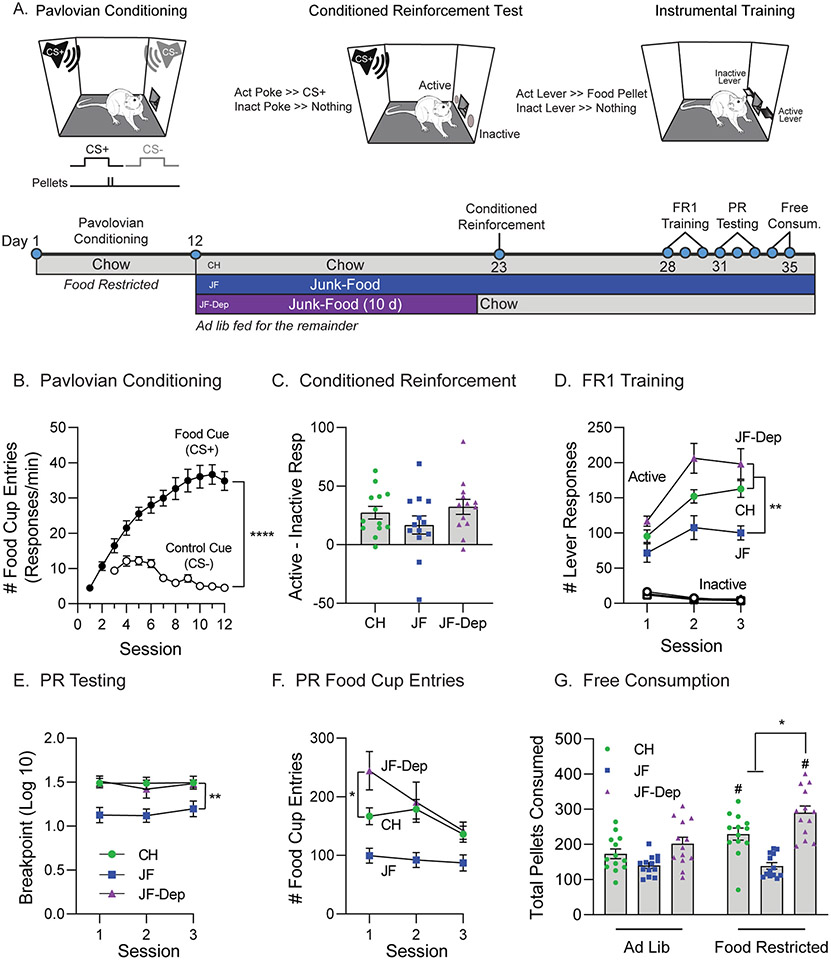
Fig. 1.
Effects of junk-food and junk-food deprivation on behavior in obesity-prone rats. A) Cartoons of behavioral conditioning, testing and experimental timeline. Obesity-prone rats (N = 39) first received Pavlovian conditioning and were then divided into chow (CH), junk-food (JF), and junk-food deprivation (JF-Dep) groups (N = 13 rats/group) before undergoing behavioral testing. B) Pavlovian conditioning. All rats learned to discriminate between the Food Cue (CS+) and Control Cue (CS−; ****p < 0.0001, main effect of cue). C) The magnitude of conditioned reinforcement (active responses - inactive responses) did not differ between groups. D) Number of lever responses during each of three fixed ratio 1 (FR1) training sessions. All rats preferentially responded on the active vs inactive lever. Rats consuming JF showed lower active lever responding compared to rats that underwent JF-Dep or those that remained on CH (**p < 0.01, Sidak’s post-test). E) Breakpoint during each of three progressive ratio (PR) tests was similar across repeated testing. Break point was reduced in JF vs CH and JF-Dep groups, but similar between CH and JF-Dep groups (**p < 0.01, Sidak’s post-test). F) Number of food cup entries during each PR test. Food cup entries were elevated during the first session in the JF-Dep group compared to CH (*p < 0.05, Sidak’s post-test). G) Pellets consumed during free consumption testing conducted when rats were fed ad lib or food restricted (4.5h) prior to the test. Pellet consumption did not differ across diet groups when rats were tested in the ad lib state (left bars). Thus, lower responding during FR1 and PR sessions in the JF group is not likely due to reductions in value of the food pellets per se. Food restriction increased pellet consumption in CH and JF-Dep groups (#p < 0.05, Sidak’s post-test ad lib vs. restricted), but had no effect on consumption in the JF group. Furthermore, following food restriction the JF-Dep group consumed more pellets than either CH or JF groups (*p < 0.05, Sidak’s post-test). All data are presented as mean ± SEM.
2.3.2. Experiment 2: effects of junk-food and junk-food deprivation in OP vs OR rats
OPs and ORs were assigned to diet groups counterbalanced by starting weight within strain and had ad lib access to food throughout. Following diet manipulation, rats underwent instrumental training and testing (see timeline Fig. 2A).
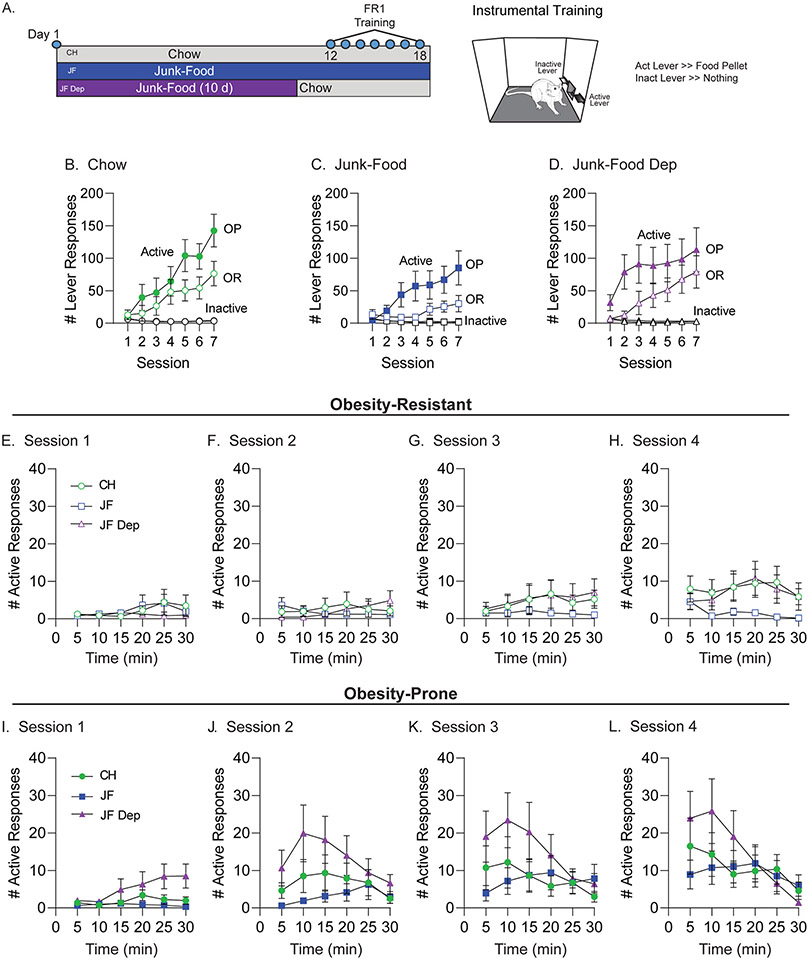
Fig. 2.
Effects of junk-food and junk-food deprivation on instrumental responding in obesity-prone and obesity-resistant rats. A) Cartoon of instrumental training, testing, and experimental timeline. Obesity-prone (OP) and obesity-resistant (OR) rats were first assigned to chow (CH), junk-food (JF), and junk-food deprivation (JF-Dep) groups (N = 12/group) before undergoing instrumental training (FR1). B-D) Number of active and inactive lever responses during each training session organized by diet group. All groups responded preferentially on the active lever, and inactive lever responses remained low and stable across sessions. For all conditions, OP groups showed greater active lever responses than OR groups. In addition, free access to JF in the home cage reduced active lever responses in both OPs and ORs compared to their chow controls. JF-Dep (D) resulted in enhanced active lever responses early in training (sessions 1–4) in OP rats as compared to OR rats (see results for statistics). E-H) Number of active lever responses per 5 min during training sessions 1–4 in OR groups. Active responding was low and similar in CH and JF-Dep groups across these sessions, and begins to increase compared to the JF group by session 4 (H). I-L) Number of active lever responses per 5 min during training sessions 1–4 in OP groups. In contrast to all other groups, increases in active lever responses emerged earlier during training in the JF-Dep group. In addition, there was a shift in activity, such that by the third and fourth sessions (K,L) responding was highest during the first half of the session and then declined across time in the OP-JF-Dep group. This is contrasted by a slower emergence of increased responding across sessions and stable responding through the entire 30 min session in OP-CH and OP-JF groups.
2.3.3. Pavlovian Conditioning
Pavlovian conditioning procedures were adapted from Holland et al. (2002) and Petrovich et al. (2002). A white noise and a tone were used, counter-balanced across CS+/CS− assignment. Rats first underwent two sessions where presentation of one cue (CS+, 10s, 8 presentations/session) was followed by delivery of two food pellets (45 mg, Bioserv, #F0059, banana; 6.3% fat, 20.2% protein, 52% carbohydrates; % of caloric content). Next, rats were given ten discrimination sessions where presentation of a second cue (CS−, 10s) that was never paired with food pellets was interspersed with CS+ presentations (4 CS+, 4 CS−, 8 presentations total; inter-trial-interval [ITI] 2–6 min, 32 min/session). Entries into the food cup during CS presentations and the ITI were recorded throughout.
2.3.4. Conditioned reinforcement test
Conditioned reinforcement for the CS+ was assessed in a single session (30 min, Robinson et al., 2015). Responses in one nosepoke port (active) resulted in CS+ presentation (3s), but no food pellets. Responses in the other port (inactive) produced no outcome. Active and inactive responses were recorded throughout.
2.3.5. Instrumental training and testing
Rats were trained to press one lever (active) to obtain the same food pellet used above (fixed ratio 1; FR1, 30 min/session). A second lever (inactive) was present but had no consequences. Rats had three FR1 training sessions followed by three progressive ratio (PR) testing sessions. During each PR session the number of active lever presses required to obtain each subsequent food pellet delivery increased exponentially (5e(delivery#X0.2)-5; (Richardson and Roberts, 1996)). The PR session ended when rats did not meet the next ratio requirement within 30 min (i.e., breakpoint).
2.3.6. Free pellet consumption
Rats underwent two free pellet consumption tests (30 min/session). During each test, rats were placed in the operant box and allowed to freely consume food pellets that had been placed in the food cup. The first test occurred under ad lib fed conditions, whereas the second session occurred following a 4.5h home-cage food restriction period (no food in cage). The total number of pellets consumed during each session was measured.
2.4. Electrophysiology and ex vivo CP-AMPAR recruitment
2.4.1. Surgeries and viral microinjection
Rats were anesthetized with isoflurane and injected intracranially with AAV constructs expressing either Chronos or hM4D(Gi)-DREADD (500 nL/hemisphere; 1 μL/min). The analgesic carprofen (2.5 mg/kg, i.p.) was administered at the time of surgery and once every 24h for 48h following surgery. For optical stimulation experiments, pAAV-Syn-Chronos-GFP (Addgene viral prep # 59170-AAV1; http://n2t.net/addgene:59170; RRID:Addgene_59170; gifted by E. Boyden) was infused into either the mPFC or BLA (mPFC: AP +3.00, ML ± 0.60, DV −3.0; BLA: AP −2.28, ML ± 5.00, DV −7.2). For DREADD experiments, pAAV-Syn-hM4D(Gi)-mCherry (Addgene viral prep # 50475-AAV8; http://n2t.net/addgene:50475; RRID:Addgene_50475; gifted by B. Roth) was infused into the mPFC. DREADD controls were injected with either the pAAV-Syn-Chronos-GFP or pAAV-Syn-mCherry. Electrophysiological recordings were made from these rats 3–4 weeks after surgery. Rats with viral expression extending outside the mPFC or BLA were excluded.
2.4.2. Whole-cell, voltage-clamp recordings
All reagents were purchased from Millipore Sigma unless otherwise noted. Brain slices containing the NAc (bregma +0.96–2.52) were prepared as previously described (Fetterly et al., 2021; Oginsky et al., 2016). Briefly, coronal sections (300 μm) were prepared using a vibratome (Lecia Biosystems). Slices recovered in oxygenated artificial cerebrospinal fluid (aCSF; in mM: 122.5 NaCl, 25 NaHCO3, 12.5 Glucose, 1 NaH2PO4, 1 l-ascorbic acid, 2.5 KCl, 2.5 CaCl2, 1 MgCl2; 295–305 mOsm, pH 7.45) at 37 °C for 30 min.
Established whole-cell patch-clamping approaches were used (Alonso-Caraballo et al., 2021; Fetterly et al., 2021; Oginsky et al., 2016). All recordings were made using Clampex 10.7–11.1 and analyzed using Clampfit 10.7–11.1 (Molecular Devices). Patch pipettes were pulled from 1.5 mm borosilicate glass capillaries (WPI; 3–7 MΩ resistance) and filled with a solution containing (in mM): 140 CsCl, 10 HEPES, 2 MgCL2, 5 Na+-ATP, 0.6, Na+-GTP, 2 QX314; 285 mOsm; pH 7.3. Whole-cell voltage-clamp recordings of AMPA receptor-mediated EPSCs were made at −70 mV in the presence of picrotoxin (50 μM). Electrically evoked EPSCs (eEPSCs) were elicited by local stimulation (0.05–0.30 mA square pulses, 0.1 ms, delivered every 20s) using a bi-polar electrode placed ~300 μm lateral to recorded neurons. Optically evoked EPSCs (oEPSCs) were elicited under the control of a CoolLED pE-300ultra and passed through a LED-FITC-A-OMF filter cube to produce blue wavelength light (0.2 ms, delivered every 20s). Epifluorescent images were used to confirm injection sites prior to recording (Olympus, BX43 with X-Cite LED). The minimum amount of current or light intensity needed to elicit a synaptic response with <15% variability in amplitude was used. Cells for which access resistance changed by >20% across the recording were discarded. EPSCs were recorded before and after application of the CP-AMPAR selective antagonist Naspm (200 μM; as in Conrad et al., 2008; Oginsky et al., 2016) to measure CP-AMPAR mediated transmission, or Clozapine-N-Oxide (CNO; 10 μm; as in (Fetterly et al., 2019)) to activate DREADDs. All stock drug solutions were diluted in ddH2O, except for picrotoxin and CNO (diluted in DMSO; final DMSO concentration: 0.05% (picrotoxin) and 1% (CNO)).
2.4.3. Ex vivo CP-AMPAR recruitment
For ex vivo studies of CP-AMPAR recruitment, slices were incubated in oxygenated aCSF containing either CNO (10 μM) or the NMDAR antagonist (2R)-amino-5-phosphonovaleric acid (APV; 50 μM) for at least 2 h before recordings began. For these studies, slices from one hemisphere were treated with drug while slices from the other hemisphere of the same rat were treated with CNO or APV. In addition, because the synaptic incorporation of CP-AMPARs relies on phosphorylation of S845 on the GluA1 subunit (Clem and Huganir, 2010; Hanley, 2014), we also evaluated protein expression of total GluA1 and GluA1 S845 phosphorylation (pS845) after ex vivo APV slice treatment using standard SDS-PAGE and immunoblotting procedures. Specifically, a separate set of slices were prepared and treated with APV or control aCSF for 2 h as described above. Tissue from the NAc was then dissected from slices using the anterior comissure as the primary landmark and homogenized in standard lysis buffer (200μl/hemisphere; in mM: 25 HEPES; 500 NaCl, 2 EDTA, 1 DTT, 1 phenylmethyl sulfonyl fluoride, 20 NaF, 1:100 protease inhibitor cocktail set I [Calbiochem, San Diego, CA], and 0.1% Nonidet P-40 [v/v]) and stored at −80 °C. Protein concentration was determined by BCA assay and samples were then used for SDS-PAGE and immunoblotting using standard procedures as in Ferrario et al. (2011). Briefly, samples were then heated (70 °C, 10 min) in Laemmli sample treatment buffer with 5% β-mercaptoethanol, loaded (20 μg protein) and electrophoresed on 4–15% Bis-Tris gradient gels under reducing conditions. Proteins were transferred onto PVDF membranes (Amersham, Hybond 0.45 PVDF). Membranes were rinsed, blocked (1hr, RT, 5% [w/v] nonfat dry milk in TBS-Tween 20 [TBS-T; 0.05% Tween 20, v/v]), and incubated overnight (4 °C) with the primary antibody to pS845 (Phosphosolutions p1160-845, lot ks422b; 1:500 in TBS-T containing 5% nonfat dry milk and 1% normal goat serum). Membranes were washed in TBS-T, incubated with HRP-conjugated secondary (Invitrogen by Thermo Fisher Scientific; 1hr, RT; 1:10,000 in TBS-T), washed, and immersed in chemiluminescence detecting substrate (Amersham, Global Life Scinces Solutions Operatins, UK). Images were acquired on film (Amersham, Hyperfilm, Cytiva Sweden). Total GluA1 protein was determined after stripping the membrane to remove signal from pS845 primary. Briefly, the membrane was incubated in stripping buffer (Thermo Scientific, Resotre PLUS Western Blot Stripping Buffer; 15 min RT), washed (3 × 5 min TBS-T, 2 × 5 min TBS), blocked (1hr, RT, 5% nonfat dry milk in TBS-T), and incubated in primary antibody to GluA1 (Millipore Sigma; MAB2263 clone RH95; 1:5000 in TBS-T containing 5% nonfat dry milk and 1% normal goat serum). Membranes were then washed again and imaged as described above. Amido Black stain was used to determine total protein (Goldman et al., 2016). In addition, removal of signal from the primary antibody was confirmed by re-exposing the membrane to the secondary corresponding to the first primary antibody and confirming the absence of any chemiluminescent signal before re-blocking the membrane. Bands of interest were quantified using Image J (NIH).
2.5. Statistical analysis
One-tailed or two-tailed t-tests, one-way, two-way, or three-way ANOVAs were used. Sidak’s multiple comparisons were used for post-hoc analysis (GraphPad Prism 9). Comparisons were made between OP, OR and diet groups within each experiment. All Ns are given in the figure captions.
3. Results
3.1. Experiment 1: effects of junk-food and junk-food deprivation in OP rats
After Pavlovian conditioning rats were separated into chow (CH), junk-food (JF), and JF-deprivation (JF-Dep) groups (Fig. 1A). Rats discriminated between the CS+ and CS−, making more entries into the food cup during CS + vs CS− presentations (Fig. 1B; main effect of cue, F(1,76) = 102.4, p < 0.0001; session × cue interaction, F(9,684) = 25.06, p < 0.0001). Rats were then given a single conditioned reinforcement test after 10 days of JF, or 10 days of JF plus 24h of JF-Dep; controls remained on chow throughout (Fig. 1A). All groups showed conditioned reinforcement, responding more in the active vs inactive port to receive a presentation of the CS+ (data not shown: main effect of port, F(1,72) = 38.72, p < 0.0001). To simplify comparisons, the magnitude of conditioned reinforcement (active responses - inactive responses) was compared across groups (Fig. 1C). All groups showed similar motivation to work for presentations of the CS + alone (no effect of diet group, F(2,36) = 1.46, p = 0.24).
We next evaluated instrumental responding for banana flavored food pellets (Bioserv, #F0059; see also methods) in these same rats (Fig. 1D). During training, all groups responded preferentially on the active vs inactive lever and inactive responses were low and stable throughout (Fig. 1D; main effect of lever, CH: F(1,24) = 247.9, p < 0.0001, JF: F(1,24) = 83.67, p < 0.0001, JF-Dep: F(1,24) = 111.3, p < 0.0001). In addition, the magnitude of active responding differed across groups, with JF rats responding the least and JF-Dep responding the most across all three sessions (Fig. 1D; active responses: main effect of diet group, F(2,36) = 12.44, p < 0.0001). Motivation to work for the food pellets was assessed in three PR testing sessions. Behavior was stable across these tests (Fig. 1E; no effect of session, F(1.724,62.06) = 0.99, p = 0.36). Similar to FR1 training, breakpoint was reduced in JF vs both CH and JF-Dep groups (Fig. 1E; main effect of diet group, F(2,36) = 8.57, p = 0.0009; no group × session interaction, F(4,72) = 0.59, p = 0.67). Interestingly, during the first PR session the number of food cup entries was greater in the JF-Dep group compared to the chow group (Fig. 1F; main effect of diet group, F(2,36) = 10.95, p = 0.0002; post-test: CH vs JF-Dep, p = 0.05), even though breakpoints were similar across these groups. Thus, rats in the JF-Dep group show greater instrumental responding during FR1 testing, and increased food-seeking (i.e., food cup entries) during the first PR session.
Fig. 1G shows pellet consumption during free access testing when rats were fed ad lib or following a 4.5hr food restriction period. Pellet consumption did not robustly differ across diet groups when rats were tested in the ad lib state. Thus, lower responding during FR1 and PR sessions in the JF group is not likely due to reductions in value of the food pellets per se. Food restriction increased pellet consumption in CH and JF-Dep groups (Fig. 1G; main effect of state, F(1,36) = 32.53, p < 0.0001; diet group × state interaction, F(2,36) = 9.78, p = 0.0004; post-test ad lib vs. restricted: CH, p = 0.0013; JF-Dep, p < 0.0001). However, no such effect of food restriction was found in the JF group (post-test ad lib vs fasted: p = 0.99). In addition, the JF-Dep group consumed significantly more pellets than either the CH or JF-Dep group when tested following food restriction (post-test: CH vs JF-Dep, p = 0.015; JF vs JF-Dep, p < 0.0001). These results are consistent with greater instrumental responding in the JF-Dep group when effort is relatively low (Fig. 1D) and with enhanced food cup entries during the first PR test (Fig. 1F).
3.2. Experiment 2: effects of junk-food and junk-food deprivation in OP vs OR rats
We next compared effects of JF and JF-Dep on instrumental responding in OP and OR rats. After two days of JF-Dep, all rats began instrumental training (Fig. 2A). All groups responded preferentially on the active vs inactive lever and inactive responses were low and stable throughout (Fig. 2B-D; main effect of lever, CH: F(1,22) = 25.42, p < 0.0001, JF: F(1,22) = 13.44, p = 0.0014, JF-Dep: F(1,22) = 16.65, p = 0.0005). The OP-CH group made more active responses than OR-CH (Fig. 2B; session × strain interaction, F(6,132) = 2.43, p = 0.029). Although active responding was generally lower in JF groups, the pattern of greater responding in OP vs OR groups persisted (Fig. 2C: session × strain interaction, F(6,132) = 3.03, p = 0.0083). This pattern was altered by JF-Dep, as seen by greater active responding in OPs vs ORs during sessions 1–3, the emergence of discrimination between active and inactive levers in OPs earlier in training than ORs, and more overlap in the variance of active responding of OP and OR groups later in training (Fig. 2D). Overall, greater active responding was maintained in OP vs OR groups in all conditions, with continued access to junk-food producing similar reductions in active responding in both groups. In addition, OP rats in the JF-Dep group learned the instrumental task more quickly than other groups, showing strong discrimination between active and inactive levers on the first training session.
We next examined within session active responses during the initial acquisition period for OR (Fig. 2E-H) and OP (Fig. 2I-L) groups. For OR rats, elevations in active responding begin to emerge by the fourth training session, with a slight trend towards greater responding in OR-CH and OR-JF-Dep groups as compared to OR-JF (Fig. 2H: main effect of diet F(2,33) = 1.83, p = 0.18, main effect of time F(3.776,124.6) = 2.01, p = 0.10). In contrast, increased responding in the OP-JF-Dep group was present even during the first session compared to OP-CH and OP-JF groups (Fig. 2I: main effect of diet F(2,33) = 3.45, p = 0.044, main effect of time, F(2.757,90.98) = 3.26, p = 0.028, time × diet interaction, F(10,165) = 2.22, p = 0.019). This same general pattern is seen in sessions two and three (Fig. 2J and K). In addition, active responding in the OP-JF-Dep group was greater early vs late within training sessions two through four. Indeed, by session four, responding is fairly consistent across time in OP-CH and OP-JF groups (similar to the OR rats), while responding in the OP-JF-Dep group was significantly greater early in the session, and declined across time (Fig. 2L: main effect of time, F(2.08,68.65) = 7.63, p = 0.00009, time × diet interaction, F(10,165) = 2.33, p = 0.013). Food intake and weight gain were tracked throughout both behavioral experiments and did not vary systematically with behavioral differences described above (Fig. S1). Overall, OPs with a history of JF-Dep showed greater active lever responding than OP-CH and OP-JF groups, an effect that was not seen in the ORs.
3.3. Experiment 3: recruitment of NAc CP-AMPARs and input specificity
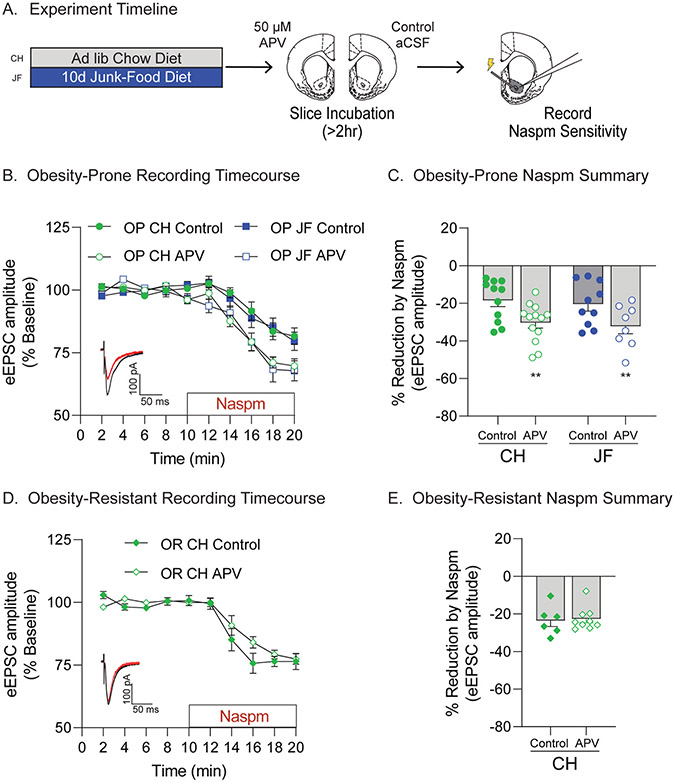
The same JF-Dep manipulation used above results in increases in NAc CP-AMPAR transmission in OP but not OR rats, and a JF deprivation period is necessary for this increase (Alonso-Caraballo et al., 2021; Oginsky et al., 2016). Synaptic recruitment of CP-AMPARs can be induced by prolonged reductions in excitatory transmission via synaptic scaling mechanisms in other neuronal systems (Ju et al., 2004; Sutton et al., 2006; Turrigiano et al., 1998). Therefore, we next determined whether incubating slices containing the NAc in the NMDAR antagonist APV was sufficient to recruit CP-AMPARs to synapses (Fig. 3A). In slices from OPs, APV incubation increased the Naspm sensitivity in both chow and JF groups (Fig. 3B and C: main effect of slice treatment, F(1,38) = 11.76, p = 0.0015; no main effect of group, F(1,38) = 0.33, p = 0.57; no group × treatment interaction, F(1,38) = 0.00024, p = 0.99). In contrast, APV incubation of slices from ORs did not alter Naspm sensitivity (Fig. 3D and E: t(13) = 0.26, p = 0.79). Thus, dampening excitatory transmission was sufficient to increase NAc synaptic CP-AMPAR transmission in OP but not in ORs; this effect was similar in slices from OP-CH and OP-JF groups. To corroborate this effect, we measured NAc protein expression of GluA1 S845 phosphorylation (pS845) and total GluA1 following APV or control treatment in slices from additional OP-CH rats (Fig. S2). As expected, total GluA1 expression was not strongly affected by 2 h APV treatment (Fig. S2B, two-tailed paired t-test, t(7) = 2.09, p = 0.07), while pS845 (Fig. S2C) and pS845/total GluA1 (Fig. S2D) were increased (pS845: one-tailed paired t-test, t(7) = 1.87, p = 0.05, pS845/GluA1: one-tailed paired t-test, t(7) = 1.67, p = 0.07). Together these data suggest that CP-AMPAR increases may be secondary to reductions in excitatory input to the NAc during JF-deprivation.
Fig. 3.
Ex vivo NMDA receptor blockade is sufficient to recruit NAc synaptic CP-AMPARs in obesity-prone, but not obesity-resistant rats. A) Schematic of experimental timeline. After 10 days of free access to junk-food (JF) or chow (CH), slices containing the NAc were prepared for electrophysiology. All slices were immediately incubated in either aCSF (Control) or aCSF with APV (50 μM) for at least 2 h prior to recording. B) Timecourse showing effects of bath application of the CP-AMPAR antagonist Naspm (200 μM) on eEPSC amplitude in recordings made from obesity-prone (OP) groups (N = # of rats, # of cells; CH: Control N = 10,11 APV N = 11,13; JF: Control N = 9,10 APV N = 8,8). Inset: Example eEPSC traces before (black) and after (red) Naspm application. C) Percent reduction in eEPSC amplitude following Naspm application (avg. of last 2 min of drug wash-on from B). APV incubation increased Naspm sensitivity (i.e., CP-AMPAR-mediated transmission) in OP-CH and OP-JF groups (**p < 0.01, main effect of slice treatment). D) Timecourse showing effects of bath application of Naspm on oEPSC amplitude in recordings made from obesity-resistant (OR) groups (CH: Control N = 6,6 APV N = 9,9). Inset: Example eEPSC traces before (black) and after (red) Naspm application. E) Percent reduction in eEPSC amplitude following Naspm application (avg. of last 2 min of drug wash-on from D). APV incubation did not alter CP-AMPAR-mediated transmission in ORs.
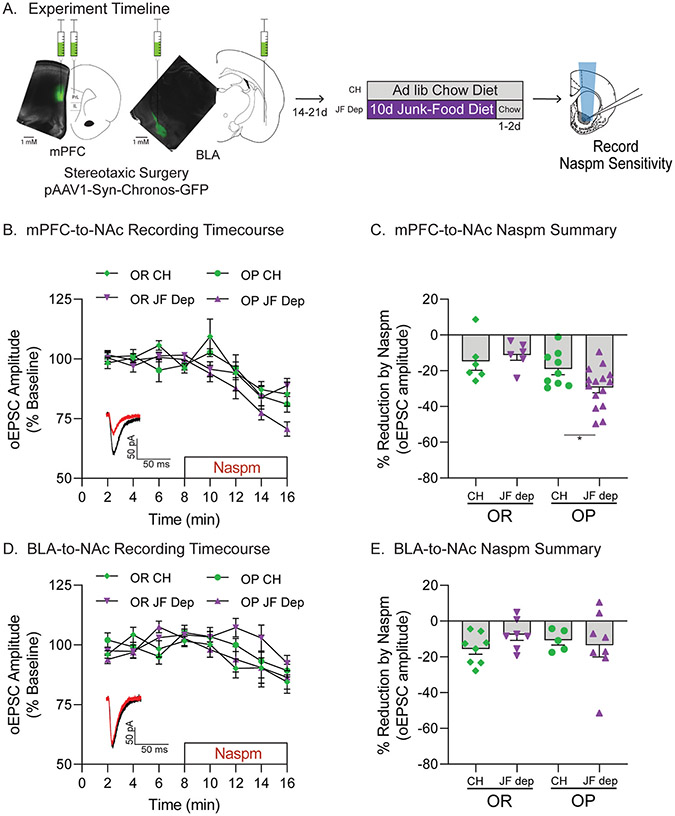
As a step towards addressing this possibility, we first identified in which synaptic inputs CP-AMPAR transmission is enhanced by comparing the effects of JF-Dep in mPFC or BLA inputs to the NAc using optogenetics combined with Naspm sensitivity (Fig. 4A). Fig. 4A shows an example of viral expression within mPFC and BLA. Fig. 4B and C show data from oEPSCs in mPFC-to-NAc inputs. Naspm sensitivity of mPFC-to-NAc inputs was increased only in OP-JF-Dep group (Fig. 4B and C: main effect of strain, F(1,32) = 8.64, p = 0.0061; OP-CH vs OP-JF-Dep: t(22) = 2.25, p = 0.017, OR-CH vs OR-JF-Dep: t(10) = 0.61, p = 0.28, based on a priori planned comparisons). Fig. 4D and E show data from oEPSCs in BLA-to-NAc inputs. Interestingly, no effects of JF-Dep were found in BLA-to-NAc inputs (Fig. 4E: no effect of strain, F(1,24) = 0.012, p = 0.91, no effect of diet group, F(1,24) = 0.31, p = 0.58, no group × strain interaction, F(1,24) = 1.30, p = 0.27).
Fig. 4.
Junk-food deprivation increases CP-AMPAR transmission in mPFC-to-NAc, but not BLA-to-NAc, synapses. A) Schematic of experimental timeline. pAAV-Syn-Chronos-GFP was bilaterally infused into either the mPFC or BLA of obesity-prone (OP) and obesity-resistant (OR) rats. Rats were then given 2–3 weeks to recover and allow for viral expression before diet manipulation (Chow or JF-Dep). Slices were then prepared for electrophysiology using optical stimulation of mPFC or BLA inputs to the NAc core. B) Timecourse showing effects of bath application of the CP-AMPAR antagonist Naspm (200 μM) on oEPSC amplitude in mPFC-to-NAc synapses (OR: CH N = 5,6 JF-Dep N = 5,6; OP: CH N = 6,9 JF-Dep OP N = 9,15). Inset: Example oEPSC traces before (black) and after (red) Naspm application. C) Percent reduction in oEPSC amplitude following Naspm application (avg. of last 2 min of drug wash-on from B). JF-Dep increased Naspm sensitivity in mPFC-to-NAc synapses of OPs but not ORs (*p < 0.05, OP-CH vs OP-JF-Dep: one-tailed t-test based on a priori planned comparisons). D) Timecourse showing effects of bath application of Naspm on oEPSC amplitude in BLA-to-NAc inputs (OR: CH N = 4,8 JF-Dep OR N = 4,7; OP: CH N = 4,5 JF-Dep N = 7,8). Inset: Example oEPSC traces before (black) and after (red) Naspm application. E) Percent reduction in oEPSC amplitude following Naspm application in BLA-to-NAc inputs (avg. of last 2 min of drug wash-on from D). JF-Dep did not alter CP-AMPAR transmission in BLA-to-NAc inputs of OPs or ORs.
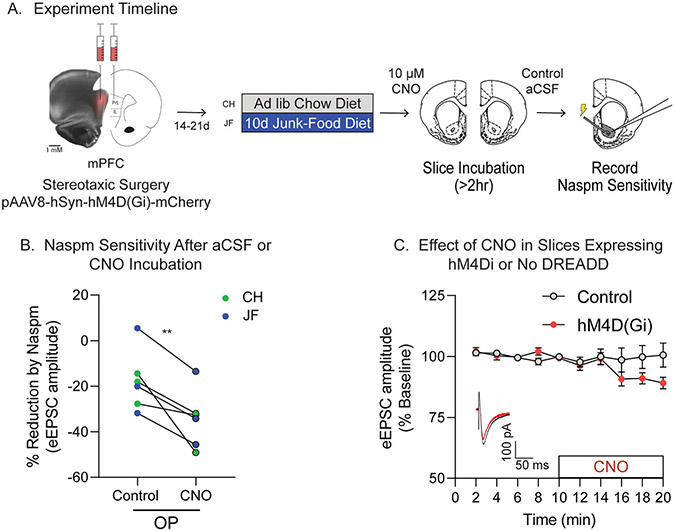
Given that CP-AMPAR increases occur in mPFC-to-NAc synapses following JF-Dep in OPs, and that reducing excitatory transmission was sufficient to increase NAc CP-AMPAR transmission, we next determined whether reducing activity of mPFC terminals within the NAc was sufficient to recruit CP-AMPARs. This was accomplished by expressing a Gi-coupled DREADD (hM4D(Gi)) in the mPFC and bath applying the agonist CNO to coronal slices containing the NAc and mPFC terminals. Fig. 5A shows an example of viral expression within mPFC. Slices from one hemisphere were incubated in CNO while slices from the opposite hemisphere of the same rat were incubated in vehicle aCSF. This allowed for comparison of CNO effects within the same set of slices (Fig. 5A). CNO incubation increased Naspm sensitivity of the eEPSC amplitude compared to vehicle aCSF (Fig. 5B: t(5) = 4.14, p = 0.0045), suggesting that reducing activity of mPFC terminals within the NAc is sufficient to increase synaptic CP-AMPAR transmission. To verify that CNO does not alter eEPSC amplitude on its own, and that activating hM4D(Gi) receptors on mPFC terminals in NAc slices decreases synaptic activity, control recordings in slices with and without hM4D(Gi)-expression were conducted. Acute CNO wash-on produced small, but significant reductions in eEPSC amplitude in slices expressing hM4D(Gi), but not in control slices (Fig. 5C: main effect of time, F(3.648,47.42) = 2.72, p = 0.045, time x viral injection interaction, F(9,117) = 2.43, p = 0.014).
Fig. 5.
Reducing mPFC-to-NAc activity via activation of hM4D(Gi) receptors ex vivo is sufficient to recruit synaptic NAc CP-AMPARs in obesity-prone rats. A) Schematic of experimental timeline. pAAV-Syn-hM4D(Gi)-mCherry was bilateral infused into the mPFC of obesity-prone (OP) rats. Rats were then given 2–3 weeks to recover and allow for viral expression before starting the diet manipulation (Chow or JF). Slices containing the NAc were then prepared for electrophysiology. All slices were immediately incubated in either aCSF plus vehicle (0.1% DMSO; Control) or aCSF containing CNO (10 μM) for at least 2 h prior to recording. B) Summary graph showing effect of CP-AMPAR antagonist Naspm (200 μM) on eEPSC amplitude in cells from OP-CH (blue) and OP-JF (green) groups. Pairs of cells indicate recordings from slices obtained from the same rat (e.g., left hemisphere incubated in CNO, right incubated in control aCSF). CNO incubation significantly increased Naspm sensitivity in OP-CH and OP-JF groups (N = 6,6/condition; **p < 0.01, one-tailed t-test). C) Timecourse of control recordings in slices from additional chow-fed control rats with and without hM4D(Gi) expression. There was no effect of CNO on eEPSC amplitude when hM4D(Gi) was not expressed, while CNO decreased eEPSC amplitude when hM4D(Gi) was expressed in mPFC-to-NAc synapses. (Control: N = 6,6; hM4D(Gi): N = 9,9). Inset: Example eEPSC traces before (black) and after (red) CNO application.
4. Discussion
We examined interactions between susceptibility to obesity and diet-induced neuronal and behavioral plasticity using established OP and OR models. Continuous junk-food access reduced food-seeking in both OPs and ORs, whereas consumption of junk-food followed by a period of deprivation (JF-Dep) enhanced food-seeking only in OPs. The persistence of behavioral effects beyond the removal of junk-food is consistent with long-lasting diet-induced neuroplasticity. Furthermore, we found that NAc CP-AMPAR increases induced by JF-Dep occur in mPFC, but not BLA, inputs to the NAc. Additionally, decreasing excitatory transmission by blocking NMDARs, or reducing activity of mPFC-to-NAc inputs ex vivo was sufficient to increase NAc CP-AMPAR transmission in slices from OPs. These data highlight behavioral and physiological changes that may contribute to diet-induced obesity and provide insights into the mechanism of NAc CP-AMPAR recruitment.
4.1. Effects of junk-food consumption on behavior
In OP rats, consumption of JF did not alter willingness to respond for the presentation of a food cue (Fig. 1C) compared to CH controls. Yet in these same rats, instrumental responding for food pellets was reduced compared to CH controls (Fig. 1D and E). This dichotomy of sustained responsivity to the food cue itself despite decreased motivation to work for food is consistent with results from outbred male rats where junk-food consumption enhances conditioned approach, without altering cue potentiated feeding (Derman and Ferrario, 2018b). Furthermore, junk-food-induced reductions in motivation for food in OPs was not related to lower motivational value of food pellets per se, as rats in the junk-food group consumed similar amounts of these pellets as CH controls during free consumption testing (Fig. 1G, ad lib). When OP and OR groups were studied side-by-side (Fig. 2A), active lever responding was generally greater in OPs than ORs, and was reduced by junk-food diet in both groups (Fig. 2B-D). Thus, despite a general enhancement in motivation in OPs compared to ORs, both groups were able to adjust their behavior in response to their dietary environment, working less for food pellets when JF was freely available in the home cage.
4.2. Effects of junk-food deprivation on behavior
In Experiment 1, active lever responding was higher in OPs with a history of JF-Dep compared to OP-CH controls (Fig. 1D). In addition, despite reaching equivalent breakpoints to the OP-CH group during PR testing, the OP-JF-Dep group had more entries into the food cup during the first PR session (Fig. 1F). This enhanced ‘checking’ behavior is suggestive of enhancements in motivation, although this difference did not persist during subsequent PR testing. Furthermore, when OP and ORs were tested side-by-side, we again found evidence for enhancements in motivation following JF-Dep only in OPs. Specifically, throughout FR1 training, active lever responding was indistinguishable between OR-JF-Dep and OR-CH controls (Fig. 2E-H). In contrast, the OP-JF-Dep group showed elevated active lever responding during FR1 training that was largely due to the emergence of increased active responding early in training compared to all other groups (Fig. 2I-L). Thus, when the overall effort to obtain food was relatively low, OPs that underwent JF-Dep showed more rapid acquisition of instrumental responding than their CH counterparts. Finally, when free pellet consumption was measured after food restriction, the OP-JF-Dep group also showed the greatest increase, consuming even more pellets than OPs maintained on chow (Fig. 1G). This difference cannot be attributed to weight gain, as both the OP-CH and OP-JF-Dep groups gained a similar amount of weight across the study (Fig. S1). In addition, these increases in motivation and food intake occurred when home cage food access during the deprivation period was unrestricted and was similar to food intake of CH controls (Fig. S1). These aspects, in combination with effects of JF-Dep on neural function (discussed below), point to potential antecedents of obesity and have implications for the relatively poor long-term outcomes of current behavioral weight-loss strategies.
4.3. Recruitment of NAc CP-AMPARs
CP-AMPARs have largely been studied in the context of drugs of abuse, fear conditioning, and neurodegeneration (Clem and Huganir, 2010; Dong et al., 2017; Guo and Ma, 2021; Wolf and Tseng, 2012), and have roles in both Hebbian and homeostatic plasticity (Man, 2011). For example, activity of NAc CP-AMPARs is required for cue-triggered food- and drug-seeking (Conrad et al., 2008; Derman and Ferrario, 2018a). Yet to date, mechanisms specific to the recruitment of NAc CP-AMPARs (which lack the GluA2 subunit) vs ‘traditional’ calcium-impermeable-AMPARs (CI-AMPARs), which contain the GluA2 subunit, have not been identified (Hanley, 2014; Werner et al., 2017), although a role for synaptic scaling in the recruitment of CP-AMPARs following cocaine withdrawal has been proposed (Conrad et al., 2008). Here, we found that decreasing synaptic activity via inhibition of NMDARs (>2h APV treatment) was sufficient to recruit CP-AMPARs in the NAc of OPs, but not ORs (Fig. 3), an effect that was corroborated by enhanced phosphorylation of GluA1 at S845 (Fig. S2). This is reminiscent of homeostatic plasticity in cultured neurons that involves the “scaling up” of AMPARs in response to prolonged decreases in excitatory transmission (i.e., synaptic scaling), discussed further below.
With this in mind, we hypothesized that NAc core CP-AMPAR increases that occur following a necessary period of JF deprivation (Alonso-Caraballo et al., 2021; Nieto et al., 2023) may result from reductions in excitatory input to the NAc. To pursue this idea, we first identified which synaptic inputs CP-AMPAR transmission is enhanced in using an optogenetic approach. We focused on mPFC-to-NAc and BLA-to-NAc inputs because these provide direct inputs to the NAc core and because these regions influence food-motivated behavior via interactions with the NAc (Cardinal et al., 2002; Christoffel et al., 2021; Domingo-Rodriguez et al., 2020; Holland and Petrovich, 2005). CP-AMPAR increases following JF-Dep were found at mPFC-to-NAc, but not BLA-to-NAc, synapses (Fig. 4). This is consistent with diet-induced plasticity in the mPFC (Dingess et al., 2017; Reichelt et al., 2019). We next expressed a Gi-coupled DREADD in the mPFC and activated these receptors located within mPFC terminals in the NAc by incubating slices from OPs in CNO (Fig. 5A; ORs were not included because neither JF-Dep nor APV treatment altered their NAc CP-AMPAR transmission). We found that CNO treatment increased CP-AMPAR transmission compared to treatment of slices from the same rat with vehicle (Fig. 5B). Control recordings performed by washing CNO onto slices with and without DREADD expression confirmed that DREADD activation results in a small, but significant decrease in AMPAR-mediated transmission. Thus, a relatively brief reduction in activity of mPFC-to-NAc inputs was sufficient to support synaptic “scaling-up” of CP-AMPARs. It is worth-while to note that the degree of Naspm sensitivity in controls found here (~20% reduction) is consistent with recent reports from our lab (Alonso-Caraballo et al., 2021; Catalfio et al., 2023), but higher than earlier reports from our own group (Oginsky et al., 2016) and others studying striatal CP-AMPARs (Conrad et al., 2008; Kawa et al., 2022), where Naspm sensitivity ranged from 3 to 10%; it is unclear what is responsible for this difference.
Synaptic scaling in the NAc has been demonstrated using an NAc/mPFC neuronal co-culture system, however this involves increases in CI-AMPARs, not CP-AMPARs, induced by prolonged treatment (24–72h) with an AMPAR antagonist or by action potential blockade using TTX (with or without APV), but not APV alone (Sun and Wolf, 2009; Werner et al., 2017). These results may differ from those found here because of differences in duration of treatment, or as a result of differences in excitatory tone in the NAc/mPFC co-culture system as compared to ex vivo brain slices or hippocampal cultures (see Sun and Wolf, 2009; Werner et al., 2017 for additional discussion). In hippocampal cultures, NMDAR blockade combined with action potential blockade is sufficient to recruit CP-AMPARs to synapses (Ju et al., 2004; Sutton et al., 2006), with some evidence that NMDAR blockade alone is sufficient (Sutton et al., 2006). To our knowledge, results here are the first demonstration of a similar acute effect in adult brain slices or within the NAc.
Increases in CP-AMPAR transmission here occurred within 2h of NMDAR blockade, consistent with the scaling observed in culture (Sutton et al., 2006; Sutton and Schuman, 2006; Turrigiano et al., 1998). While this is a relatively rapid effect, the timescale is long enough that recruitment of existing receptors or the addition of newly synthesized receptors into synapse could contribute. Of course, these two possibilities are not mutually exclusive. In favor of new protein synthesis, CP-AMPAR recruitment in cultured hippocampal neurons requires protein synthesis (Sutton et al., 2006), as does the maintenance of CP-AMPARs at synapses in adult NAc slices (Scheyer et al., 2014) (see below for further discussion). In favor of recruitment of existing CP-AMPARs, we did not see any increase in total GluA1 expression following APV treatment, but were able to detect increases in GluA1 S845 phosphorylation (Fig. S2). However, these measures were made in whole cell lysates, so both intracellular and surface “pools” of GluA1 contribute to the signal, limiting our ability to detect potential increases in protein expression.
The magnitude of APV-induced increases in CP-AMPAR transmission here was similar in OP-CH and OP-JF groups. This was unexpected, as we’ve previously found evidence for the extra-synaptic accumulation of CP-AMPARs in OPs maintained on junk-food (10d) vs chow. Thus, we predicted that the magnitude of CP-AMPAR increases would be greater in OP-JF vs OP-CH groups here. It is possible that NMDAR blockade resulted in a maximal recruitment of CP-AMPARs, masking group differences, or conversely that more time may be needed to observe group differences in synaptic CP-AMPAR recruitment. Regardless, the effect seen in the OP-CH group in light of the lack of recruitment seen in ORs suggest inherent differences in the plasticity window between these populations. This is consistent with absence of diet-induced NAc AMPAR plasticity generally observed in ORs (Ferrario, 2020; Oginsky et al., 2016).
We do not know why CP-AMPAR plasticity occurs more readily in OPs than ORs. To date, no evidence for basal differences in NAc CP-AMPAR synaptic transmission between these lines has been found (e. g., Figs. 4-5, in Oginsky et al., 2016), although floor effects could mask differences. However, basal NAc GluA1, but not GluA2, surface protein expression is lower in male OPs vs ORs (Alonso-Caraballo et al., 2021; Derman and Ferrario, 2018a) and basal intrinsic excitability of NAc medium spiny neurons is enhanced in OPs vs ORs (Oginsky and Ferrario, 2019; Oginsky et al., 2016). This combination may render OPs more sensitive to experience-induced plasticity. In addition, it is possible that differences in basal activity of excitatory inputs between OPs and ORs could contribute to the enhanced ability to recruit CP-AMPARs in OPs. Consistent with this idea, extracellular glutamate levels within the NAc are greater in OPs vs ORs (Vollbrecht et al., 2023). Finally, it’s worth-while to note that JF-Dep also enhances NAc CP-AMPAR transmission in female OPs but not ORs. However, this increase is transient, in contrast to persistent effects observed in males (Alonso-Caraballo et al., 2021; Nieto et al., 2023; Oginsky et al., 2016), suggesting differential regulation in females that will be important to examine in the future.
4.4. Conclusions
Overall, we find that a history of junk-food consumption and obesity-susceptibility interact to enhance food-motivated behavior and recruit NAc CP-AMPARs. These data provide further evidence that interactions between predisposition and diet-induced neurobehavioral plasticity likely contribute to weight gain and the maintenance of obesity. In light of modern diet culture, these data also emphasize the importance of understanding lasting changes that occur after stopping a sugary, fatty diet and set the stage for future studies linking these synaptic changes to behavioral outcomes. Finally, data here demonstrate for the first time that reducing excitatory transmission can recruit synaptic CP-AMPARs in adult brain slices and the NAc. Thus, these data reveal novel insights into the mechanisms underlying CP-AMPAR recruitment in the NAc that likely involve synaptic scaling mechanisms. This has important implications for both cue-triggered food- and potentially drug-seeking behaviors.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grants R01DA044204, R01DK106188, R01DK115526, and R01DK130246 to CRF. TLF was supported by NIH grant T32DA007268. AMC was supported by NIH grant T32DA007281. We thank reviewer 2 for their helpful comments, particularly the suggestion for figure titles in Fig. 5B and C.
Footnotes
CRediT authorship contribution statement
Tracy L. Fetterly: Conceptualization, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Amanda M. Catalfio: Formal analysis, Investigation, Writing – review & editing. Carrie R. Ferrario: Conceptualization, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2023.109772.
Data availability
Data will be made available on request.
References
- Alonso-Caraballo Y, Fetterly TL, Jorgensen ET, Nieto AM, Brown TE, Ferrario CR, 2021. Sex specific effects of "junk-food" diet on calcium permeable AMPA receptors and silent synapses in the nucleus accumbens core. Neuropsychopharmacology 46, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Kupchik YM, Spencer S, Garcia-Keller C, Spanswick DC, Lawrence AJ, Simonds SE, Schwartz DJ, Jordan KA, Jhou TC, Kalivas PW, 2017. Addiction-like synaptic impairments in diet-induced obesity. Biol. Psychiatr 81, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ, 2002. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Catalfio AM, Fetterly TL, Nieto AM, Robinson TE, Ferrario CR, 2023. Cocaine-induced sensitization and glutamate plasticity in the nucleus accumbens core: effects of sex. Biol. Sex Differ 14, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Walsh JJ, Heifets BD, Hoerbelt P, Neuner S, Sun G, Ravikumar VK, Wu H, Halpern CH, Malenka RC, 2021. Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun 12, 2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL, 2010. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330, 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME, 2008. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Schiefer C, Shaham Y, O’Donnell P, 2014. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology (Berl) 231, 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Holland PC, Gallagher M, Huganir RL, 2008. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur. J. Neurosci 27, 3284–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM, 2012. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci 32, 5549–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman RC, Ferrario CR, 2018a. Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs. Neuropharmacology 131, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman RC, Ferrario CR, 2018b. Junk-food enhances conditioned food cup approach to a previously established food cue, but does not alter cue potentiated feeding; implications for the effects of palatable diets on incentive motivation. Physiol. Behav 192, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingess PM, Darling RA, Derman RC, Wulff SS, Hunter ML, Ferrario CR, Brown TE, 2017. Structural and functional plasticity within the nucleus accumbens and prefrontal cortex associated with time-dependent increases in food cue-seeking behavior. Neuropsychopharmacology 42, 2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Rodriguez L, Ruiz de Azua I, Dominguez E, Senabre E, Serra I, Kummer S, Navandar M, Baddenhausen S, Hofmann C, Andero R, Gerber S, Navarrete M, Dierssen M, Lutz B, Martín-García E, Maldonado R, 2020. A specific prelimbic-nucleus accumbens pathway controls resilience versus vulnerability to food addiction. Nat. Commun 11, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Taylor JR, Wolf ME, Shaham Y, 2017. Circuit and synaptic plasticity mechanisms of drug relapse. J. Neurosci 37, 10867–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, 2020. Why did I eat that? Contributions of individual differences in incentive motivation and nucleus accumbens plasticity to obesity. Physiol. Behav 227, 113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouébe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC, 2016. Homeostasis meets motivation in the battle to control food intake. J. Neurosci 36, 11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME, 2011. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 61, 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterly TL, Basu A, Nabit BP, Awad E, Williford KM, Centanni SW, Matthews RT, Silberman Y, Winder DG, 2019. α(2A)-Adrenergic receptor activation decreases parabrachial nucleus excitatory drive onto BNST CRF neurons and reduces their activity in vivo. J. Neurosci 39, 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterly TL, Oginsky MF, Nieto AM, Alonso-Caraballo Y, Santana-Rodriguez Z, Ferrario CR, 2021. Insulin bidirectionally alters NAc glutamatergic transmission: interactions between insulin receptor activation, endogenous opioids, and glutamate release. J. Neurosci 41, 2360–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF, 2012. Diet-induced obesity blunts the behavioural effects of ghrelin: studies in a mouse-progressive ratio task. Psychopharmacology (Berl) 220, 173–181. [DOI] [PubMed] [Google Scholar]
- Goldman A, Harper S, Speicher DW, 2016. Detection of proteins on Blot membranes. Curr. Protoc. Protein Sci 86, 10, 18.11–10.18.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Ma YY, 2021. Calcium permeable-AMPA receptors and excitotoxicity in neurological disorders. Front. Neural Circ 15, 711564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JG, 2014. Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca(2+)-permeable AMPA receptors. Semin. Cell Dev. Biol 27, 14–22. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, 2005. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol. Behav 86, 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M, 2002. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol. Behav 76, 117–129. [DOI] [PubMed] [Google Scholar]
- Horstmann A, Busse FP, Mathar D, Müller K, Lepsien J, Schlögl H, Kabisch S, Kratzsch J, Neumann J, Stumvoll M, Villringer A, Pleger B, 2011. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front. Hum. Neurosci 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC, 2004. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci 7, 244–253. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Hwang EK, Funke JR, Zhou H, Costa-Mattioli M, Wolf ME, 2022. Positive allosteric modulation of mGlu(1) reverses cocaine-induced behavioral and synaptic plasticity through the integrated stress response and oligophrenin-1. Biol. Psychiatr 92, 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE, 1997. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol 273, R725–R730. [DOI] [PubMed] [Google Scholar]
- Man HY, 2011. GluA2-lacking, calcium-permeable AMPA receptors–inducers of plasticity? Curr. Opin. Neurobiol 21, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen-Ankney BA, Legaria AA, Pan Y, Vachez YM, Murphy CA, Schaefer RF, McGrath QJ, Wang JG, Bluitt MN, Ankney KC, Norris AJ, Creed MC, Kravitz AV, 2023. Nucleus accumbens D(1) receptor-expressing spiny projection neurons control food motivation and obesity. Biol. Psychiatr 93, 512–523. [DOI] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE, 2012. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 59, 2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto AM, Catalfio AM, Papacostas Quintanilla H, Alonso-Caraballo Y, Ferrario CR, 2023. Transient effects of junk food on NAc core MSN excitability and glutamatergic transmission in obesity-prone female rats. Obesity (Silver Spring) 31, 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Ferrario CR, 2019. Eating "junk food" has opposite effects on intrinsic excitability of nucleus accumbens core neurons in obesity-susceptible versus-resistant rats. J. Neurophysiol 122, 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR, 2016. Eating ’junk-food’ produces rapid and long-lasting increases in NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacology 41, 2977–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M, 2002. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J. Neurosci 22, 8748–8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, Gibson GD, Abbott KN, Hare DJ, 2019. A high-fat high-sugar diet in adolescent rats impairs social memory and alters chemical markers characteristic of atypical neuroplasticity and parvalbumin interneuron depletion in the medial prefrontal cortex. Food Funct. 10, 1985–1998. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, Ferrario CR, 2015. Individual differences in cue-induced motivation and striatal systems in rats susceptible to diet-induced obesity. Neuropsychopharmacology 40, 2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Wolf ME, Tseng KY, 2014. A protein synthesis-dependent mechanism sustains calcium-permeable AMPA receptor transmission in nucleus accumbens synapses during withdrawal from cocaine self-administration. J. Neurosci 34, 3095–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, 2009. Individual differences in the neurophysiology of reward and the obesity epidemic. Int. J. Obes 33 (Suppl. 2), S44–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE, 2013. The contribution of brain reward circuits to the obesity epidemic. Neurosci. Biobehav. Rev 37, 2047–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wolf ME, 2009. Nucleus accumbens neurons exhibit synaptic scaling that is occluded by repeated dopamine pre-exposure. Eur. J. Neurosci 30, 539–550. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM, 2006. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125, 785–799. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM, 2006. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127, 49–58. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL, 2011. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378, 804–814. [DOI] [PubMed] [Google Scholar]
- Tukey DS, Ferreira JM, Antoine SO, D’Amour J A, Ninan I, Cabeza de Vaca S, Incontro S, Wincott C, Horwitz JK, Hartner DT, Guarini CB, Khatri L, Goffer Y, Xu D, Titcombe RF, Khatri M, Marzan DS, Mahajan SS, Wang J, Froemke RC, Carr KD, Aoki C, Ziff EB, 2013. Sucrose ingestion induces rapid AMPA receptor trafficking. J. Neurosci 33, 6123–6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB, 1998. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD, 2013. Obesity and addiction: neurobiological overlaps. Obes. Rev 14, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht PJ, Nesbitt KM, Addis VM, Boulnemour KM, Micheli DA, Smith KB, Sandoval DA, Kennedy RT, Ferrario CR, 2023. Differential regulation of nucleus accumbens glutamate and GABA in obesity-prone and obesity-resistant rats. J. Neurochem 164, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner CT, Murray CH, Reimers JM, Chauhan NM, Woo KK, Molla HM, Loweth JA, Wolf ME, 2017. Trafficking of calcium-permeable and calcium-impermeable AMPA receptors in nucleus accumbens medium spiny neurons co-cultured with prefrontal cortex neurons. Neuropharmacology 116, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY, 2012. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front. Mol. Neurosci 5, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.