Abstract

The compositional engineering of lead-halide perovskite nanocrystals (NCs) via the A-site cation represents a lever to fine-tune their structural and electronic properties. However, the presently available chemical space remains minimal since, thus far, only three A-site cations have been reported to favor the formation of stable lead-halide perovskite NCs, i.e., Cs+, formamidinium (FA), and methylammonium (MA). Inspired by recent reports on bulk single crystals with aziridinium (AZ) as the A-site cation, we present a facile colloidal synthesis of AZPbBr3 NCs with a narrow size distribution and size tunability down to 4 nm, producing quantum dots (QDs) in the regime of strong quantum confinement. NMR and Raman spectroscopies confirm the stabilization of the AZ cations in the locally distorted cubic structure. AZPbBr3 QDs exhibit bright photoluminescence with quantum efficiencies of up to 80%. Stabilized with cationic and zwitterionic capping ligands, single AZPbBr3 QDs exhibit stable single-photon emission, which is another essential attribute of QDs. In particular, didodecyldimethylammonium bromide and 2-octyldodecyl-phosphoethanolamine ligands afford AZPbBr3 QDs with high spectral stability at both room and cryogenic temperatures, reduced blinking with a characteristic ON fraction larger than 85%, and high single-photon purity (g(2)(0) = 0.1), all comparable to the best-reported values for MAPbBr3 and FAPbBr3 QDs of the same size.
Keywords: nanocrystals, quantum dots, aziridinium, perovskite, ligands, photoluminescence
The past decade has seen the discovery and the rapid development of colloidal lead halide perovskite nanocrystals (NCs) of APbX3 stoichiometry, where A represents the Cs cation or an organic cation, and X indicates a halogen anion.1−3 These materials have captured broad interest due to their straightforward synthesis and outstanding optical properties, foremost narrowband photoluminescence (PL) with near-unity quantum yield (QY) and wide spectral tunability (410–750 nm), high absorption coefficients and, at cryogenic temperatures, high radiative rates, and long excitonic coherence times.4−7 A rather stringent structural requirement for forming the perovskite lattice (i.e., a three-dimensional (3D) network of corner-shared lead-halide octahedra), usually expressed via the Goldschmidt tolerance factor, is the insertion of A-site cations of an appropriate size. Cs is the only sufficiently large inorganic cation, whereas suitably small and yet chemically robust organic cations include methylammonium (MA) and formamidinium (FA), both extensively used in perovskites as optoelectronic materials. Judicious choices of these three ions in either single- or mixed-cation compositions enable fine-tuning of electronic properties via, e.g., the octahedral tilt angles, as well as improving materials phase stability and chemical durability in bulk, thin-film, and nanocrystalline forms.8−12 Further expanding the thus-far-limited choice for the A-site cation may unlock additional opportunities in the precision engineering of the structural and electronic properties of lead-halide perovskites.
Very recently, the aziridinium cation [(CH2)2NH2+, labeled hereafter as AZ], a highly reactive and labile triangular heterocycle, was reported to form stable AZPbX3 compounds.13−15 Seemingly in contradiction to the latter, computational assessments of the ring opening within the formed perovskite A-site voids had yielded an enthalpy difference of 0.7 eV per formula unit (f.u.) in favor of ring opening in AZPbI3.16 However, this value is smaller than the computed and experimental ring-strain energies of ca. 27 kcal/mol (1.16 eV/f.u.),17,18 suggesting an overall stabilizing effect by the Pb-halide cage as a “counter-strain” due to the A-site being too small for the opened ring. Furthermore, several favorable formation conditions exist. First, the size of the three-membered AZ ring cation is similar to that of the commonly used MA cation, and the Goldschmidt tolerance factor for AZPbBr3 is 0.95 (the used ionic radii are rBr = 196 pm, rPb = 119 pm, rAZ = 227 pm),19 well within the perovskite formability limits (Figure 1a). Second, the thermodynamic stability of AZPbX3 (as well as that of Sn analogues) has been anticipated in first-principles calculations focused on the ionization energy of the A-site as a stability predictor.19−22 Since the AZ moiety, in its neutral form, exhibits a lower ionization energy than (neutral) MA, with a value closely resembling that of atomic Cs, AZPbX3 phases were expected to be more stable than MAPbX3 with thermochemical calculations further supporting the formation of stable perovskite phases. A remaining concern is the reactivity of AZ (polymerization, nucleophilic attack on the ring, etc.), which also breaks its cyclic nature. However, here again, the perovskite cage apparently guards the AZ cations, as seen from the air stability of the reported AZPbX3 compounds.13−15 Additionally, strong steric requirements provided by the size and shape of the “reaction cavity” within the crystal lattice may increase the actual activation energy for any thermodynamically allowed transformation to a threshold not easily achievable if no additional power is provided (in the form of heat or light). Indeed, when the space surrounding the molecules becomes restricted, their reactivity and related behavior can be significantly altered.23
Figure 1.
(a) Calculated Goldschmidt tolerance factors for different cations (cesium, methylammonium, aziridinium, and formamidinium) in the APbBr3 perovskite lattice. (b) Top panel: a reaction scheme; bottom panel: an overview of carboxylic and phosphonic acids as well as ligands used in the synthesis. (c) Optical absorption and (d) PL spectra of purified AZPbBr3 NCs ranging from 4.5 to 14 nm in size (for visualization purposes, a cumulative vertical offset was applied to each subsequent spectra). (e) A high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) image of purified 8 nm NCs with a high-resolution HAADF-STEM image of few single NC in the inset. (f) Size-dependent (absorption) band gap in AZPbBr3 NCs for sizes obtained via TEM (green) and SAXS (gray); the experimental data sets (open squares, with error bars denoting the standard deviation) were fitted by a semiempirical sizing curve (solid line), with the bulk band gap (dashed lines) as one of the fit parameters.
In this work, we explored the prospects of AZ-based perovskite NCs as a potentially powerful addition to the family of highly luminescent MA/FA/Cs lead halide NCs. We focused on the AZPbBr3 composition and devised the synthesis of monodisperse and size-tunable NCs in strong and weak quantum-confinement regimes, e.g., QDs. The preparation of AZPbBr3 NCs leveraged the recently reported room-temperature synthesis platform based on PbBr2/trioctylphosphine oxide (TOPO) molecular adducts as the precursor,24 wherein the formation of NCs is precisely adjustable and traceable in situ, due to slower reaction kinetics, compared to more traditional hot-injection or solvent-assisted reprecipitation methods. Furthermore, this PbBr2/TOPO synthesis benefits from the decoupling of the NC formation and subsequent coating with the capping ligand of choice before the purification and isolation of NCs. We tested several state-of-the-art capping ligands, cationic and zwitterionic, and found that didodecyldimethylammonium bromide (DDAB) and 2-octyldodecyl-phosphoethanolamine (C8C12–PEA) ligands render AZPbBr3 NCs robust for both ensemble and single-particle studies. Their photostability, high ON-fraction (>85%) in blinking studies, and high single-photon purity (g(2)(0) as low as 0.1) render AZPbBr3 NCs comparable to the best reported MAPbBr3 and FAPbBr3 NCs.25
Results and Discussion
Synthesis of AZPbBr3 NCs
The room-temperature synthesis of AZPbBr3 QDs proceeds through rapid injection of an aziridine solution in chloroform or dibromomethane (see further details in Figure 1b and the Supporting Information) into a precursor solution comprising PbBr2/TOPO adduct, diisooctylphosphinic (DOPA), and alk(en)ylcarboxylic acids (2-ethylhexanoic acid, oleic acid, or erucic acid) in hexane, with the optional addition of alkylphosphonic acids (for obtaining the smallest NCs; see the subsequent discussion). We note that AZ cations cannot be formed ex situ for use as a stable precursor, due to their high ring instability in common solvents. Instead, AZ cations form in situ, because of the high acidity of the PbBr2/TOPO precursor solution (aziridine:DOPA:carboxylic acids molar ratios = 1:8.5:17). TOPO, DOPA, and alkyl carboxylic acids are known as weakly binding ligands for perovskite NCs.24 They can be readily displaced by more strongly binding alternatives such as didodecyldimethylammonium bromide (DDAB),26,27 custom-engineered zwitterionic phospholipid ligand [2-octyldodecylphosphoethanolamine (C8C12–PEA)],25 or the commercially available natural phospholipid lecithin,28 followed by purification and isolation steps. This procedure yields stable colloids of highly monodispersed cuboidal AZPbBr3 NCs exhibiting bright green emission and high stability over long-term storage in air (Figure S1).
The size of NCs was adjusted between 4.5 and 14 nm in diameter, resulting in tunable absorption and PL, with PL peaks in the range from 498 to 530 nm (see Figures 1c and 1d, as well as Figures S2 and S3 and Table S1), by manipulating the reaction time (the time delay between the injection of the aziridine solution and the injection of the ligand solution was typically 10–240 s) as well as by introducing various amounts of alkylphosphonic acids (hexyl-, octyl-, decyl-, or dodecylphosphonic acid) into the PbBr2/TOPO precursor solution. Phosphonic acids slow the reaction kinetics, facilitating the preparation of strongly confined AZPbBr3 NCs (down to 4.5 nm, Figure S2d). Conversely, utilizing mesitylene as a reaction solvent and a longer reaction time yields NCs larger than 10 nm, with PL peaks from 525 to 530 nm. The overall dilution of precursors does not significantly alter the PL peak of AZPbBr3 (Figure S2c), unlike in the synthesis of CsPbBr3 NCs.24 Importantly, a narrow size dispersion of AZPbBr3 NCs can be reached only under a high Pb-precursor excess (aziridine:Pb molar ratio = 1:4). The minute-scale formation kinetics of AZPbBr3 NCs allow in situ optical monitoring with ultraviolet-visible light (UV-vis) absorption, as exemplified for 6.5 nm samples (Figure S4).
DDAB-capped AZPbBr3 NCs are sharp cuboids, in agreement with the known tendency of cationic ligands to stabilize the set of (100) facets.26,29,30 C8C12–PEA-coated NCs are rather truncated, presumably due to surface reconstruction or etching (see Figure S3 and Table S1). Lower colloidal robustness was observed when employing the recently reported dicationic ligands (propanediyl-1,3-N,N-bis(didodecylmethylammonium bromide), C3-4C12AB)31 or lecithin. The AZPbBr3 NCs capped with DDAB or C8C12-PEA exhibit an average PL QY of 80% ± 2% for NC sizes between 8 and 10 nm, and the PL QY increases up to 90% ± 4% for samples prepared with the addition of phosphonic acids (4.5–8 nm).
Electron Microscopy and SAXS Studies
The high-angle annular dark field-STEM (HAADF-STEM) and high-resolution HAADF-STEM images evidence the (100) termination of the DDAB-capped NC surfaces (Figure 1e; synthesis without phosphonic acids, 20 s reaction labeled as “standard” in Table S1). Shape retrieval from small-angle X-ray scattering (SAXS, Figure 2a) yields a slightly prolate cuboid shape (aspect ratio of ca. 1.04) with the lengths of the three NC edges being 8.27 ± 0.12, 8.28 ± 0.93, and 8.60 ± 0.48 nm (that is, substantially isotropic; see Figure S5 and Table S2 in the Supporting Information for SAXS data of an extended NC size series).
Figure 2.
(a) The fit of experimental SAXS data from an AZPbBr3 NC colloidal suspension (gray line) via an analytical model (black line) yields cuboids with edge lengths of 8.27 ± 0.12, 8.28 ± 0.93, and 8.60 ± 0.48 nm, respectively. (b) Solvent-subtracted synchrotron WAXTS data (black line) and the best fit (green line) of AZPbBr3 NCs using the split cubic model with locally tilted/disordered PbBr6 octahedra (for a more extended discussion, see the Supporting Information); the inset shows a 2D map of the refined (number-based) log-normal size distribution function (Dab: the diameter of the circle of area equivalent to the prisms basal plane and Lc, the height of the prismatic clusters). (c) The split cubic structural model of AZPbBr3 NCs (the four Br of each PbBr6 octahedra, shown in green, have 1/4 site occupancy factor each, i.e., only one out of four is stochastically present in each site) and the model of AZ cation formed by disordering in 12 symmetry-equivalent orientations (4 equivalent geometrical orientations × 3 “elemental” C–C/N dispositions, H atoms were omitted, similar to bulk AZPbBr3). (d) The temperature dependence of the tetragonal unit cell parameters a′ = a/√2 and c for a weakly distorted (ca. 1%) cubic lattice, plotted in the entire 11–290 K range, showing an anomaly below 90 K. (e) The temperature dependence of the fwhm0 parameter (the θ-dependence of the peak width is described according to the relation fwhm(θ) = fwhm0/cos θ), which suggests additional peak broadening (hidden splitting) below 90 K.
Sizing Curve
Figure 1f presents the size dependence of the band gap energies in AZPbBr3, using the NC size determined via either TEM or SAXS, and the band gap estimated from the lowest-energy minimum of the second derivative of the absorbance (see the Supporting Information for details). Such a “sizing curve” is of great practical utility as an express method for obtaining the approximate NC size using only a standard UV-vis spectrometer. Furthermore, the size dependence associates with the basic electronic structure of the underlying bulk material. Within the semiempirical expression derived in Aubert et al.,32 (see the Supporting Information for the equation and further details), the functional form of the size dependence for a wide range of semiconductors is given by only three bulk parameters: the bulk band gap (E0), the reduced mass (μ) of the exciton, and the dielectric constant (ϵ∞) at the optical frequency (see the Supporting Information for further details). In Figure1f, we have tested the inverse of this idea by fitting our experimental AZPbBr3 size-dependent band gap (open squares) with the semiempirical expression (solid line) given by Aubert et al.,32 hereby yielding approximate estimates for the thus far still ill-defined electronic parameters for bulk AZPbBr3. To limit the parameter space and stabilize the fit, we keep the bulk band gap E0 and the reduced mass μ as free fit parameters and estimate the dielectric constant ϵ∞ with the help of DFT calculations (see Table S5 and associated details in the Supporting Information). For the SAXS dataset, the best fit is obtained for the following parameters: E0 = 2.35 eV (fitted), μ = 0.17 (fitted), and ϵ∞ = 8.25 (fixed; based on our DFT calculations 13% higher than for CsPbBr333), translating into a Bohr diameter d0 of about 5.0 nm. Similar fit parameters are also obtained for the TEM dataset, albeit with a slightly higher estimate for the bulk band gap. Overall, we conclude that the electron and hole effective masses, dielectric constant, and, thus, the exciton Bohr diameter should be comparable in AZPbBr3 and CsPbBr3 (see also Tables S4–S6),32,34 while the bulk band gap of AZPbBr3 appears few tens of millielectronvolts lower than in CsPbBr3 (E0 = 2.38 eV in CsPbBr3).35 We also note that a previous estimate for the bulk AZPbBr3 band gap by Petrosova et al.13 found an even lower value (2.27 eV); however, their different definition for the band gap (via the zero-crossing in a Tauc plot) precludes a direct comparison to the values found by us for AZPbBr3 and by Mannino et al.35 for CsPbBr3 (in both cases defined via second derivatives).
Crystal Structure
The crystal structure of AZPbBr3 NCs suspended in cycloheptane was investigated with synchrotron X-ray total scattering methods. Bulk AZPbBr3 was previously reported to crystallize in the cubic lattice with a Pm3̅m space group symmetry and ordered Br atoms with a straight Pb–Br–Pb bond angle of 180°.13 However, evidence of local and dynamic symmetry breaking has been found in various lead-halide perovskites that exhibit a long-range cubic structure.36−40 We account for local symmetry breaking with a split-cubic perovskite model, for a disordered AZ cation with a cuboctahedral cluster, and for disorder of Br anions into four equivalent positions, as well as a cuboidal NC morphology with the Debye scattering equation. The resulting fit to the wide-angle X-ray total scattering (WAXTS) data is shown in Figure 2b, together with a 2D map of the refined bivariate log-normal size-distribution function. Figure 2c displays the obtained structure, with Pb–Br–Pb bond angles deviating by 13° from the 180° angle expected for an ideal cubic structure. Deviations of similar magnitude are also found in FAPbBr3, FAPbI3, and FASnI3 NCs,8,12,41−43 corroborating that AZPbBr3 NCs share the locally broken crystal symmetry. Furthermore, AZPbBr3 NCs exhibit a slight lattice expansion of ∼0.10%–0.15% with respect to the bulk value, similar to many other nanosized samples of lead-halide perovskites (Table S3).44−46 We further uncover signs of a noncubic long-range structure. A small deviation in lattice parameters from a cubic cell (with the c axis about 1% larger than the a = b axis) suggests that the average cell is tetragonal (see Figure S6 and the pertinent discussion in the Supporting Information).
To further understand the crystal symmetry, we performed variable-temperature WAXTS measurements of dried NCs loaded into a glass capillary and collected 93 WAXTS scattering patterns (at 3 K steps, from 11 K to room temperature; see the Supporting Information). Similar to the RT WAXTS data of the solution-phase NCs, also the RT crystal metrics of the dried NCs suggest a distortion from the cubic lattice when analyzed according to the structureless Le Bail method (which avoids the occurrence of the nonrandom orientation distribution function of the NCs and is fully unbiased by errors in the structural model). With decreasing temperature, the crystal unit cell volume monotonously decreases. More importantly, an additional symmetry change is found below 90 K (Figure 2d). From 90 to 11 K, the peak widths progressively increase (Figure 2e), suggesting an additional peak splitting (partially hidden under the broad Bragg peaks), which is consistent with a symmetry-lowering transition to an orthorhombic metric, as observed also in other 3D lead-halide perovskites.
Computational Study
Having elucidated the experimentally observed crystal structure of the synthesized AZPbBr3 NCs, we further consider the crystal stability via a computational approach. The Goldschmidt tolerance factor (t)47 is used extensively to predict the formation and stability of the perovskite structure. However, some studies suggested that a revision of the Goldschmidt tolerance factors may be required.48 Recently, Bartel et al. introduced a different tolerance factor49
| 1 |
with nAZ representing the oxidation state of the AZ-cation and a value of τ < 4.18 suggests a perovskite structure. We estimated τ = 3.30 for AZPbBr3, further supporting the perovskite structure formation (the ionic radii used in the present work were rBr = 196 pm, rPb = 119 pm, rAZ = 227 pm, and nAZ = 1).
To investigate the stability of AZPbBr3, we considered the formation reaction equation (CH2)2NH2Br + PbBr2 → (CH2)2NH2PbBr3 and the corresponding reaction enthalpy:19
| 2 |
A negative reaction enthalpy would indicate a stable perovskite structure. To obtain the total energy (Etot) of the reactant and products, we performed density functional theory (DFT) calculations, employing the PBE exchange-correlation functional with van der Waals (vdW) corrections. We further considered an orthorhombic AZPbBr3 crystal structure, the most stable phase at 0 K calculations, in coarse agreement with the lower-than-tetragonal symmetry in our low-temperature X-ray total scattering data (see Supporting Information for further details, including a discussion of the likelihood of polymorphism). We then obtained negative ΔHr values of −0.362 eV and −0.373 eV with and without spin–orbit coupling, respectively, affirming the stability of AZPbBr3. The respective electronic-structure calculations show a direct band gap of AZPbBr3. The conduction band originates from Pb p orbitals, while the valence band is predominantly of Br p character, consistent with other lead halide perovskites (see the Supporting Information for details (Figures S7 and S8, and Tables S7 and S8).
NMR Study
The ligand chemistry of C8C12–PEA and DDAB-capped AZPbBr3 NCs was elucidated with NMR experiments performed in solution and the solid state. 1H solution NMR spectra for both C8C12–PEA and DDAB-capped AZPbBr3 NCs confirm the presence of the corresponding capping ligand (Figure 3a). DDAB-capped NCs do not sustain more than one washing cycle, after which a free alkyl carboxylic acid (in this case, oleic acid) is still detected in the DDAB-sample (as alkenyl protons at 5.4 ppm). We thus inspected the C8C12–PEA-capped NCs in greater detail, since they can be purified at least three times without a notable loss of NC dispersibility. Already twice-washed samples lack any oleate-related signal. 31P NMR spectra evidence the surface-bound C8C12–PEA ligands in solution (Figure 3b) and in the solid state (Figure S10b). The twice-washed C8C12–PEA–AZPbBr3 sample shows only a broad signal centered at approximately −1 ppm from bound C8C12–PEA (Figure 3b, top). No other signals were detected, excluding free or surface-bound TOPO and DOPA. We also analyzed the NCs synthesized with the addition of alkyl phosphonic acids (Figure 3b, middle), whose 31P NMR signal is expected at 25–28 ppm (see the octylphosphonic acid spectrum in Figure S9 and ref (50)). Colloids washed only once exhibit a broad surface-bound C8C12–PEA signal at −1 ppm and three narrow peaks in the range of 40–60 ppm (absent in twice-washed NC samples), originating from the residual TOPO (49 ppm) and DOPA (57 and 43 ppm for its main impurity). These NC cores were then digested by the addition of DMSO-d6, liberating surface-bound species, leading to a narrow signal from the free C8C12–PEA ligand (Figure 3b, bottom) and solvent-related shifts from the TOPO/DOPA species but still no signatures of phosphonic acids. We thus conclude that the small quantities of alkylphosphonic acids used in the synthesis are fully removed upon washing and do not bind to the NC surfaces.
Figure 3.
(a) Solution 1H NMR spectra of C8C12–PEA (black line) and DDAB-capped (blue line) AZPbBr3 NCs in cyclohexane-d12. (b) Solution 31P NMR spectra of C8C12–PEA-capped AZPbBr3 NCs synthesized without (top) and with (middle) addition of alkylphosphonic acid (for instance, octylphosphonic acid-C8PAc) during the synthesis and after their further digestion with DMSO-d6 (bottom). (c) Solid-state 207Pb NMR spectrum of C8C12–PEA-capped AZPbBr3 NCs.
The presence and integrity of the AZ cation in AZPbBr3 NCs were characterized with 1H solid-state NMR spectra (Figure S11a). The bulk material features two main peaks at 4 and 6 ppm. Additional minor species are resolved at 5 and 8 ppm, although the material was phase-pure according to powder XRD (Figure S12). No NMR signal from possible ring-opened alkyl ammonium was detected. The observed species were also found in the AZPbBr3 NCs in solution and solid-state 1H measurements, with the addition of the alkyl protons from the ligands at 2 ppm (Figure 3a and Figure S10a). 207Pb solid-state NMR is highly sensitive to deviations from a cubic crystal structure in lead-halide perovskites. Experiments on bulk AZPbBr3 show a single signal centered at ∼485 ppm with a full width at half-maximum (fwhm) of 13.1 kHz, similar to the cubic FAPbBr3 signal (Figure S11b).51 The 207Pb solid-state NMR signal for C8C12–PEA-capped AZPbBr3 NCs fits very well with the bulk reference, exhibiting a single signal at ∼505 ppm with a fwhm of 16.5 kHz (Figure 3c). Broader peaks in NCs, compared to bulk, have previously been also reported for CsPbBr3, likely caused by the increased disorder and higher ion mobility.51
Raman Spectroscopy
Raman spectra of AZPbBr3 NCs and AZPbBr3 bulk powders confirmed the presence of the AZ cation within the Pb–Br perovskite cage. Beyond the dense and almost featureless spectrum of bands below 150 cm–1, characteristic for the Pb–Br framework in 3D lead-bromide perovskites,52 both NCs and bulk exhibit a band at ∼308 cm–1, a doublet at 807 and 871 cm–1, and a band at 1227 cm–1, previously assigned to the AZ-cage mode, ring deformation, and ring stretching, respectively.15 Only a limited amount of degradation products related to AZ ring opening was detected (Figure S13 and Table S9). Note that some bands remain challenging to assign due to the lack of Raman measurements conducted explicitly on the AZ cation, which is unstable outside the perovskite framework. Notwithstanding these uncertainties, Raman spectroscopy also evidences the AZ cations incorporated in the Pb–Br framework, both in AZPbBr3 NCs and bulk.
Single-QD Spectroscopy
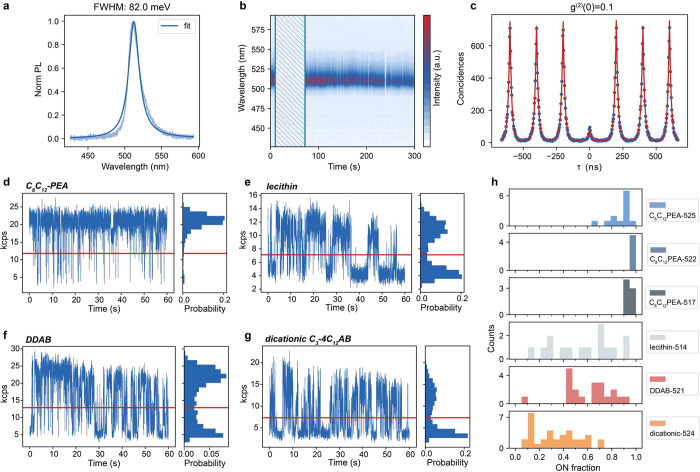
Room-temperature optical properties of AZPbBr3 NCs, also referenced here as QDs, due to their quantum-light emission capabilities (vide infra), were examined via single-particle PL spectroscopy in a home-built inverted oil-immersion microscope (see details in the Supporting Information). Such PL studies at the single-QD level unveil basic structure–property relationships,53 as well as sample heterogeneities54 and temporal fluctuations55,56 of emitters, which are otherwise unresolved in the ensemble spectra. Figure 4a shows a single-particle PL spectrum of an AZPbBr3 QD capped by C8C12–PEA ligands. Fitting a Lorentz function to the experimental data returns an emission peak centered at 512 nm and an fwhm of 82 meV, demonstrating the spectrally narrow emission of the individual AZPbBr3 QDs. Measurements were performed in a nitrogen atmosphere for extended spectral photostability, as evidenced in the PL spectra series in Figure 4b. AZPbBr3 QDs exhibited high single-photon purity, as confirmed by a strongly suppressed peak at a zero delay time in the second-order photon–photon correlation function g(2)(t) (Figure 4c). The high single-photon purity with g(2)(0) = 0.1 is on par with FAPbBr3 and MAPbBr3 QDs capped by the same C8C12–PEA ligand.25
Figure 4.
Room-temperature PL of single AZPbBr3 QDs with various surface capping ligands. (a) PL spectrum of a single C8C12–PEA-capped AZPbBr3 QD displaying narrow-band emission with a fwhm of 82 meV. (b) Spectra series of a C8C12–PEA-capped QD exhibiting spectrally stable PL and small intensity variations. The highlighted time period (shaded area without PL spectral detection) corresponds to the acquisition of the second-order correlation function (g(2)(t), shown in panel (c)) and blinking trace by a Hanbury–Brown and Twiss experiment. (c) Second-order photon–photon correlation of a C8C12–PEA-capped AZPbBr3 QD displaying high single-photon purity (g(2)(0) = 0.1). (d–g) Representative PL blinking traces (10 ms binning time) of a QD capped with branched C8C12–PEA ligands (panel (d)), lecithin (panel (e)), DDAB (panel (f)), and dicationic amine C3–4C12AB (panel (g)). (h) Histograms of the fraction of time that single QDs spend in their bright (ON) state. The numbers after the ligand name indicate the ensemble PL central wavelength of the respective sample.
Furthermore, we quantify the PL intensity fluctuations, termed “PL blinking”, i.e., the stochastic switching between a bright (ON) and a dimmed (OFF) state. PL blinking roots in a photoinduced charge trapping at surface defects, possibly mediated by efficient Auger–Meitner recombination, and could be strongly affected by the QD surface passivation.57 The fraction of time spent in the ON state (ON fraction) is therefore a suitable metric of the surface quality for the nanomaterial under study at the single-QD level. Recently, we demonstrated that the C8C12–PEA ligands stabilize hybrid organic–inorganic lead-halide perovskite QDs and enable emission at the single-particle level with >90% ON fraction.25 A blinking trace from a single C8C12–PEA-capped AZPbBr3 QD (Figure 4d) also exhibits a high ON fraction (∼95%), representative of the high ON fraction (typically >85%) for AZPbBr3 QDs with this ligand capping (Figure 4h). We then surveyed the blinking behavior of QDs capped by various alternative and post-synthetically attached ligands, i.e., zwitterion lecithin (Figures 4e), monocationic DDAB (Figure 4f), and dicationic C3–4C12AB (Figure 4g). Although C8C12–PEA and lecithin are both zwitterionic phospholipids, lecithin-capped QDs exhibit longer OFF events and a significantly smaller ON fraction than those of C8C12–PEA-capped QDs, symptomatic for the compromised surface passivation of the former. We attribute the superior performance of C8C12–PEA to the better fit of its primary ammonium binding group into the A-cation site at the QD surface.25,30 Likewise, blinking traces of QDs capped by one of the two quaternary ammonium ligands, DDAB or C3–4C12–AB, displayed ON fractions smaller than those of the C8C12–PEA-capped QDs. We further observed that all samples, except C8C12–PEA-capped QDs, feature a large QD-to-QD variation in the ON fraction (Figure 4h). This could be assigned to the sample preparation procedure needed for single-QD spectroscopy, which requires a strong dilution (by a factor of ∼50 000) of the colloids.
The dilution step can also alter the QD morphology58 and induce a blue shift of the PL energy (Figure S14). Occurring under water-free and inert conditions, structural and optical alterations upon dilution are rationalized considering ligand desorption enhanced by the dynamic ligand binding observed in ionic perovskite QDs.59 Dilution-induced alterations can also explain the deviation of ON fractions for the different ligands, despite comparably high PL QYs in the undiluted ensemble (Figure S14). Single-particle spectroscopy thus acts as a stress test for ionic QDs with inherently dynamic ligand binding that is better endured by C8C12–PEA-capping. The reduced blinking for such a ligand formulation could be exploited for the realization of single-photon sources without the loss in the single-photon purity.60
While room-temperature single-QD experiments unveiled the optimal ligand choice, the intrinsic electronic properties of the semiconductor core are probed at cryogenic temperatures. At 4 K, the perturbation by phonons via exciton–phonon coupling is highly suppressed, enabling observation of the exciton fine structure arising from electron–hole exchange interaction as well as emission from exciton complexes. We studied single AZPbBr3 QDs capped with C8C12–PEA, DDAB, or lecithin ligands. Single QDs with ensemble QD sizes from ∼7–9 nm exhibit exciton PL bands in the range of 2.31–2.20 eV (537–564 nm), with a fwhm ranging from 0.2 to 0.8 meV (where 0.2 meV is our setup resolution). Among the studied ligand systems, only DDAB- and C8C12–PEA-capped single QDs exhibit a spectrally stable multiline exciton spectrum with sub-meV spectral diffusion (Figure 5a). The multiline spectrum is ascribed to the bright triplet character of excitonic emission in lead-halide perovskites.7,61 By introducing a linear polarizer in the collection path and recording the angle-dependent exciton intensities, we obtained the polarization profile typical for a bright triplet exciton: individual exciton sublevels are highly linearly polarized and orthogonal to each other, as in the example displayed in Figure 5b. Across various single QDs, we observed both doublet and triplet exciton fine structures (see insets of Figures 5c and 5d). Splitting energies of doublet (Δ) and triplet (Δ1 and Δ2) exciton fine structures are plotted as a function of the exciton energy in Figures 5c and 5d, respectively. Splitting energies vary from 0.2 to 1.4 meV for QDs with PL peak ranging from 2.21 to 2.31 eV. In addition to a systematic trend of increasing splitting energies with increasing exciton energy, large variations in splitting energy are observed for a given exciton energy. This variation was also reported in other lead halide perovskite QDs,6,7,61−65 and suggests that the exciton fine-structure splitting is sensitive to the shape anisotropy of the studied QDs. In general, exciton properties of AZPbBr3 QDs are comparable to CsPbBr37,66,67 and FAPbBr3 QDs.61,68 Compared to the C8C12–PEA- or DDAB-capped single QDs, lecithin-capped QDs exhibit stronger spectral diffusion (∼10 meV, see Figure S15), consistent with their more-pronounced PL blinking and lower ON fraction at room temperature.
Figure 5.
Exciton fine-structure of single AZPbBr3 QDs at 4 K. (a) Time series of a single QD (1 s integration time and 1800 g/mm grating); sub-meV spectral diffusion allows to resolve the exciton fine structure. This QD exhibits a doublet fine structure with peaks denoted as FS1 and FS2. The inset shows an associated histogram of the peak energies (bars), fitted with a Gaussian function (lines). (b) The PL spectra of one single QD measured at two different angles of a linear polarizer in the detection path: 45° (blue) and 135° (gray); this QD has a doublet exciton fine structure. The inset shows a polar plot with the respective PL intensities, as a function of the polarizer angle; both doublet sublevels exhibit highly linear polarization, oriented perpendicular to each other; (c, d) Fine-structure splitting energy (FSS) for all of the single QDs (capped with DDAB or C8C12–PEA ligands) exhibit doublet sublevels (panel (c)) or triplet sublevels (panel (d)). The insets in panels (c) and (d) show representative spectra for doublet and triplet exciton fine structures, respectively. (e) Second-order correlation function g(2)(τ) of a single AZPbBr3 QD under 0.3 μJ/cm2 before (dark gray line) and after (blue line) spectrally filtering out the biexciton emission by a tunable short-pass filter.
To quantify the quantum correlations among photogenerated exciton complexes, we drive the single QDs under high excitation fluence, hereby obtaining emission also from trion (X*) and biexciton (XX); see one example in Figure S16a. As the excitation fluence increases from 0.003 μJ/cm2 to 0.09 μJ/cm2, two additional peaks emerge on the lower-energy side of the exciton emission spectrum, which are assigned to the trion (red-shifted from Ex by 25 meV) and biexciton (red-shifted from Ex by 40 meV). Summarizing the results from all studied single QDs, binding energies of trion (ΔX* = EX – EX*) and biexciton (ΔXX = EX–EXX) are plotted as a function of the exciton energies (see Figure S16b). ΔX* increases from 10 to 25 meV for exciton energies increasing from 2.20 to 2.30 eV, and ΔXX increases from 30 to 40 meV for exciton energies increasing from 2.24 to 2.30 eV. Qualitatively, the observed trend of increasing ΔX* or ΔXX with increasing exciton energy is universal in semiconductor QDs69,70 as the Coulomb interaction among photogenerated charge carriers is steadily enhanced with decreasing QD size. Quantitatively, the size-dependent trends of ΔX* and ΔXX are similar to those reported for FAPbBr3 QDs.71 Utilizing the obtained knowledge of ΔXX in AZPbBr3 QDs, we performed single-QD antibunching experiments under high excitation fluence (0.3 μJ/cm2) with and without filtering the biexciton emission. When the spectrum is unfiltered (Figure 5e, dark gray line), no antibunched emission was observed, i.e., g(2)(0) ≈ 1, suggesting very efficient biexciton emission at 4 K. When the biexciton emission is discarded by spectral filtering (Figure 5e, blue line), emission is characterized by a regular stream of single photons (g(2)(0) ≈ 0, within the noise floor of ∼0.1). In addition, all the studied individual AZPbBr3 QDs feature a monoexponential PL decay with lifetimes between 400 and 1600 ps, as shown in Figure S17. These lifetimes are much longer than reported in CsPbBr3 QDs, suggesting the absence of a pronounced giant oscillator strength,7 probably related to the softer and more dynamic lattice of the AZPbBr3 QDs. The room-temperature PL decay curves (Figure S18) reveal a reduction in the radiative rates with decreasing NC size. For CsPbBr3 NCs, such dependence was previously explained by the size-dependent thermal mixing with optically forbidden excitonic transitions.72
Conclusions
In summary, we have presented a room-temperature colloidal synthesis of monodisperse and quantum-confined AZPbBr3 NCs with bright emission, size-tunable PL peaks from 498 to 530 nm, and a locally distorted cubic crystal. The stabilization of the AZ cation is confirmed by NMR and Raman spectroscopy. At the single-particle level, AZPbBr3 QDs capped with DDAB or C8C12–PEA are as robust as analogously synthesized MAPbBr3 and FAPbBr3 QDs reaching high single-photon purity and suppressed blinking behavior at room temperature, and exhibiting bright triplet exciton character of the single-exciton emission at 4 K. This report on the synthesis of colloidal AZPbBr3 NCs can inspire follow-up investigations of such NCs, for example, further exploring potentials for compositional engineering (mixed cations or anions) and integration into devices such as color enhancers or blue-to-green down-converters.
Methods
Safety Statement
No unexpected or unusually high safety hazards were encountered.
Synthesis of AZPbBr3 NCs
Stock Solutions
Aziridine stock solution (0.15 M) was prepared as follows: 15.5 μL of aziridine was dissolved in 2 mL of anhydrous chloroform and stored in a refrigerator. For PbBr2-TOPO stock solution (0.04 M), PbBr2 (1 mmol, 376 mg) and TOPO (5 mmol, 2.15 g) were dissolved in octane (5 mL) at 100 °C, followed by dilution with hexane (20 mL) and filtering through a 0.2 μL PTFE filter before use. DOPA stock solution (0.57 M) was prepared by dissolving 0.8 mL of DOPA in 3.2 mL hexane. OA stock solution (0.515 M) was prepared by dissolving 0.8 mL of OAc in 3.6 mL of hexane. EtHAc stock solution (0.31 M) was prepared by dissolving 0.2 mL of EtHAc in 3.8 mL of hexane. Erucic acid stock solution (0.56 M) was prepared by dissolving 383 mg of EAc in 2 mL hexane. Alkylphosphonic acid stock solution (alkylPAc, 0.257 M) was prepared by dissolving alkyl phosphonic acid in anhydrous toluene (hexyl-, octyl-, decyl-, or dodecylphosphonic acid). C10PAc and C12PAc dissolve in toluene only upon heating to 80 °C, and the stock solution should be preheated before use. DDAB stock solution (100 mg/mL, 0.215 M) was prepared by dissolving 300 mg of DDAB in 3 mL of anhydrous toluene. C8C12-PEA stock solution (50 mg/mL) was prepared by dissolving 100 mg of C8C12–PEA in 2 mL of distilled mesitylene. Lecithin stock solution (50 mg/mL) was prepared by dissolving 200 mg of lecithin in 4 mL hexane. C3-4C12AB stock solution (100 mg/mL) was prepared by dissolving 100 mg C3-4C12AB in 1 mL of mesitylene.
Synthesis
In a 25 mL one-neck flask, PbBr2-TOPO stock solution was combined with additional hexane. Then, a desired volume of DOPA, alkyl-PAc, or OAc (or other carboxylic acids) stock solution was added. Under vigorous stirring (1100 rpm), aziridine in chloroform was swiftly injected into the reaction mixture. After 20 s to 3 min, a stock solution of ligands (DDAB or C8C12–PEA, lecithin, or C3-4C12AB) is added to initiate the ligand exchange on the NC surface. Within 2–4 min after the addition of ligands, the crude solution was concentrated by evaporating hexane on a rotary evaporator down to <0.5 mL of residual solvent. The NCs were precipitated from the concentrated colloid by adding nonsolvent. Specific volumes of stock solutions are indicated in Table S1 for all reactions presented in this work.
Purification
For DDAB ligands, NCs were purified using acetone (crude solution:nonsolvent 1:2 (v/v)), followed by solubilization of the obtained NCs in cyclohexane. For C8C12-PEA ligands, ethyl acetate and acetonitrile mixture (1:1) were used (crude solution:nonsolvent, 1:1 (v/v)), followed by solubilization of the obtained NCs in cyclohexane. For lecithin ligands, ethyl acetate and acetonitrile mixture (1:1) was used (crude solution: nonsolvent, 1:1 (v/v)), followed by solubilization of the obtained NCs in anhydrous toluene. For dicationic C3-4C12AB ligands, acetone was used as a nonsolvent. In the case of introducing phosphonic acids in the synthesis, first, the crude solution was centrifuged, then a white precipitate was discarded, and the obtained supernatant was concentrated by evaporating on a rotary evaporator down to <0.5 mL of residual solvent, followed by purification with an ethyl acetate/acetone mixture (1:1), and the obtained NCs were dissolved in toluene. Both DDAB and C8C12–PEA-capped AZPbBr3 NCs are stable for several months in darkness under ambient conditions. The described synthesis is sensitive to the value of room temperature. Deviation in room temperature (in our case, 21 °C) by 2–3 °C leads to the shift of the PL maximum by 2–4 nm.
Ex-Situ Absorption and PL Spectroscopy
UV-vis absorption spectra were collected using a Jasco Model V770 spectrometer operated in transmission mode. A spectrofluorimeter (Horiba Jobin Yvon, Model Fluoromax 4) that was equipped with a PMT detector was used to acquire steady-state PL spectra from solutions. The excitation wavelength was 400 nm, provided by a 150 W xenon lamp dispersed with a monochromator. Measured intensities were corrected to consider the detector’s spectral response. The QY of the solutions was measured with a quantum yield spectrometer (Hamamatsu, Model Quantaurus-QY Absolute PL) that was equipped with an integrating sphere. Time-resolved PL traces were acquired in solution by using a FluoTime300 spectrometer from PicoQuant. Samples were excited using a 355 nm detector; the detection was set at the PL peak maximum for each sample.
In-Situ Absorption Setup
The reactions were carried out in a modified commercial cuvette holder (CVH100; Thorlabs). The absorption spectra were recorded in transmission mode with a deuterium-tungsten light source (Ocean Optics, Model DH-2000-BAL-TTL-24 V) and a broad-band spectrometer (OceanInsight, Model HDX-XR). The time resolution in these measurements was set to 50 ms. Custom-developed batch analysis scripts (written in Python) were employed for the data analysis and fitting.
Electron Microscopy Characterization
Transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) images were collected by using a JEOL Model JEM-2200FS microscope operated at 200 kV. HR HAADF-STEM was carried out using a probe-aberration-corrected FEI Titan Themis system that was operated at 300 kV, using a beam current of ∼1 pA. High-resolution images were obtained by summing up 3–5 frames.
SAXS Measurements
SAXS experiments were carried out on a benchtop Bruker Nanostar (Bruker AXS GmbH, Karlsruhe, Germany) using the Kα-line of a microfocused X-ray Cu source with a wavelength of 1.5406 Å. The beam was collimated using a 0.3 mm pinhole, leading to a beam diameter of ∼0.4 mm at the sample position. The sample–detector distance was set to 107 cm and further calibrated with silver behenate, achieving a resolvable q-range of 0.07 nm–1 ≤ q ≤ 2.3 nm–1. For selected samples, an additional measurement was performed at a sample-to-detector distance of 27 cm, further calibrated with silver behenate, and combined with previous measurements to obtain an extended q-range of 0.07 nm–1 ≤ q ≤ 10 nm–1. The length of the scattering vector q⃗ is defined as q = 4π sin 2θ/λ, where 2θ as the scattering angle and λ the wavelength of the X-ray source; the scattered intensity was recorded on a gaseous avalanche-based detector (VÅNTEC-2000, Bruker AXS) with 2048 × 2048 pixels and a pixel size of 68 μm × 68 μm. The scattering patterns were recorded at room temperature under moderate vacuum conditions (10–2 mbar) to limit air scattering.
Calibration of the scattering vector length q and estimation of the instrumental resolution Δq = 0.25 nm–1 were done by measuring the first diffraction peak of a silver behenate sample and calculating its width. Each sample was sealed in a quartz capillary and mounted in the sample chamber. The scattering intensity was recorded for 700 s for each sample. The intensity of the semitransparent beamstop from empty beam scans was used for transmission calibration. The scattered intensity was extracted, azimuthally averaged, and integrated over each q-value using the Bruker software DIFFRAC.EVA (Bruker AXS, version 4.1). The 1D data was transmission-corrected and background-subtracted from the scattering of the respective solvent, polymers, and the empty quartz capillary using an in-house data pipeline operating under Matlab 2022.
The final scattering pattern was then fitted using the parallelepiped model. For more details, we refer to the section “Small-Angle X-ray Scattering (SAXS) Measurements” in the Supporting Information.
Synchrotron WAXTS Measurements
X-ray total scattering measurements on AZPbBr3 NCs were performed at the X04SA-MS beamline of the Swiss Light Source (Paul Scherrer Institute, Villigen, Switzerland)73 and at the Swiss–Norwegian beamline (BM01) of the European Synchrotron Radiation Facility (ESRF, Grenoble, France). For more details, we refer to the section “Synchrotron X-ray Total Scattering Measurements” in the Supporting Information.
NMR Measurements
Solid-State Magic Angle Spinning Nuclear Magnetic Resonance (MAS NMR)
NMR experiments were performed under ambient conditions on a 16.4T Bruker Avance IIIHD spectrometer (Bruker Biospin, Fällanden, Switzerland), that was equipped with a 2.5-mm trippel-channel solid-state probe head. All samples were ground into a fine powder and densely packed into ZrO2 rotors. The spinning frequency was set to 20 kHz. 1H, 31P, and 207Pb NMR chemical shifts were externally referenced to TMS, 85% H3PO4 in H2O, and PbMe4. The 1H excitation pulse was set to 2.5 μs. The 31P excitation pulse was set to 5.6 μs, and proton decoupling with a Spinal64 sequence was performed during the detection. For 207Pb, a Hahn echo pulse-sequence was used for all measurements with an echo delay of 0.9 ms. The radio-frequency (rf) field of the echo pulses was set to 19.8 kHz.
Solution NMR
Solution 1H and 31P NMR spectra were recorded on a 11.7 T Bruker Avance IIIHD spectrometer (Bruker Biospin, Fällanden, Switzerland). The instrument was equipped with a BBO cryogenic probe. The sample temperature was set to 298 K. 1H spectra were acquired using a one-pulse sequence with a 4 μs excitation pulse and a 1 s recycle delay. 31P spectra were acquired using a one-pulse excitation pulse (14 μs) and proton decoupling with a 2 s recycle delay. All spectra were referenced externally to TMS (1H) and 85% H3PO4 in H2O (31P).
Powder X-ray Diffraction
Powder X-ray diffraction (XRD) patterns were collected in transmission mode with a STADI P diffractometer (STOE&Cie GmbH) that was equipped with a curved Ge (111) monochromator (Cu Kα1 = 1.54056 Å) and a silicon strip MYTHEN 1K Detector (Fa. DECTRIS). For the measurement, the ground powder was placed between the adhesive tape.
Raman Spectroscopy
Raman spectra were acquired using a Horiba LabRAM HR Evolution confocal microscope. An objective lens (100× magnification, 0.9 NA) was used to induce and collect Raman-scattered light from micrometer-scale regions of the samples, using laser excitation at 785 nm (continuous wave, 30 mW). No sample degradation was observed during or after the typical acquisition time of ∼25 s. For more details, we refer to the section “Raman Spectroscopy” in the Supporting Information.
Single-QD Spectroscopy
Sample Preparation (Room Temperature)
The following steps were performed in a glovebox that is kept under a nitrogen atmosphere and employing dry and filtered octane (Acros Organics, 99+% extra dry), cyclohexane (Acros Organics, 99.5% extra dry), and toluene (Acros Organics, 99.85% extra dry over molecular sieve). AzPbBr3 NCs were diluted by a factor of 1500–40 000, depending on initial concentration and solvent. Samples were diluted in octane (in the case of C3–4C12AB-capped NCs), cyclohexane (in the case of DDAB-capped NCs), or toluene (C8C12PEA or lecithin-capped NCs). Subsequently, 100 μL of this solution was spin-coated onto a cover glass (Thorlabs, 170 ± 5 μm thickness and 25 mm diameter) at 150 rps for one min or 50 rps for 80 s. The samples were then placed in a home-built sample holder filled with nitrogen to preclude moisture-induced (water and/or oxygen) degradation during the measurements for room temperature experiments.
Room-Temperature Measurements
Room-temperature single-particle fluorescence measurements were performed on a home-built uPL inverted microscope. The setup consists of a 405-nm pulsed laser (PicoQuant, 10 MHz repetition rate, <50 ps pulse width, <100 W/cm2) focused (1/e2 = 1 μm) using an oil immersion objective (1.3 NA) onto the sample mounted on a XYZ-stage (SmarAct). The emitted light is collected by the same objective and passed through a dichroic mirror and a long-pass filter (both 450-nm cut-on wavelength). To record the spectrum, the emitted light is sent to a monochromator coupled to an EMCCD (Princeton Instruments, one frame per second). PL intensity time traces and second-order photon–photon correlations are obtained by sending the emitted light to a Hanbury-Brown and Twiss (HBT) setup consisting of a 50/50 beam splitter, two APDs (Excelitas, 250 ps time resolution), and a TCSPC module (PicoQuant, HydraHarp).
Cryogenic Measurements (at 4 K)
For single-QD spectroscopy at 4 K, a home-built micro-PL setup is used. The samples were mounted on XYZ nanopositioning stages inside an evacuated liquid-helium closed-loop cryostat (Montana Instruments) and cooled to a targeted temperature of 4 K. Single NCs were excited using a fiber-coupled excitation laser at an energy of 2.585 eV (480 nm) with a repetition rate of 80 MHz (Toptica, <200 fs pulses), which is focused (1/e2 diameter = 2.4 μm) on the sample by a microscope objective (0.8 NA, 100× magnification). Typical energy densities used to excite single NCs were 0.003–0.008 μJ/cm2. The emitted light is collected by the same objective and passed through a dichroic mirror (long-pass, cut-on wavelength of 488 nm) and a long-pass filter at 500 nm. A monochromator (Princeton Instruments, 0.75 m) coupled to a back-illuminated CCD camera is used for spectra measurements. Spectra are measured with a grating featuring 1800 (or 300) lines/mm, blaze 500 nm, resulting in a 0.2 (or 1) meV spectral resolution.
Computational Methods
For details of the computational study, we refer to the section “Computational Methodology” in the Supporting Information.
Acknowledgments
This work was supported by the Swiss National Science Foundation (Grant No. 200021_192308, project Q-Light) and, in part, by the European Union through Horizon 2020 Research and Innovation Programme (ERC CoG Grant, Grant Agreement No. 819740, Project No. SCALE-HALO) and by the Air Force Office of Scientific Research (under Award No. FA8655-21-1-7013). A.G. acknowledges partial funding from project PE0000021, “Network 4 Energy Sustainable Transition-NEST”, Spoke 1, funded by European Union-NextGenerationEU under NRRP, Mission 4, Component 2, Investment 1.3-Call for tender No. 1561 of Ministero dell'Università e della Ricerca (MUR). Antonio Cervellino and the technical staff of the MS-X04SA beamline of the Swiss Light Source (Paul Scherrer Institute, Switzerland) are acknowledged for the room-temperature synchrotron measurements on AZPbBr3, as colloidal suspensions of NCs and bulk powder. Dmitry Chernyshov and the technical staff of the Swiss-Norwegian Beamline (BM01) of the European Synchrotron Radiation Facility (ESRF, France) are acknowledged for the technical assistance during the RT and temperature-dependent experiment on AZPbBr3 NCs. Amrutha Rajan is acknowledged for assistance with PLQY measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.3c11579.
Additional details on materials and methods including complete description of the synthetic procedure, TEM and STEM images, Raman spectroscopy measurements, NMR, additional SAXS, WAXTS and single-dot spectroscopy data, and the computational study (PDF)
This manuscript has been submitted to a preprint server: Bodnarchuk, M. I.; Feld, L. G.; Zhu, C.; et al. Colloidal aziridinium lead bromide quantum dots. Research Square, 2023, 10.21203/rs.3.rs-3671642/v1 (accessed November 29, 2023).
The authors declare no competing financial interest.
Figure 1 was corrected on February 8, 2024.
Supplementary Material
References
- Dey A.; Ye J.; De A.; Debroye E.; Ha S. K.; Bladt E.; Kshirsagar A. S.; Wang Z.; Yin J.; Wang Y.; et al. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775–10981. 10.1021/acsnano.0c08903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman Q. A.; Rainò G.; Kovalenko M. V.; Manna L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. 10.1038/s41563-018-0018-4. [DOI] [PubMed] [Google Scholar]
- Kovalenko M. V.; Protesescu L.; Bodnarchuk M. I. Properties and Potential optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. 10.1126/science.aam7093. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainò G.; Nedelcu G.; Protesescu L.; Bodnarchuk M. I.; Kovalenko M. V.; Mahrt R. F.; Stöferle T. Single Cesium Lead Halide Perovskite Nanocrystals at Low Temperature: Fast Single-Photon Emission, Reduced Blinking, and Exciton Fine Structure. ACS Nano 2016, 10, 2485–2490. 10.1021/acsnano.5b07328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzat H.; Sun W.; Kaplan A. E. K.; Krieg F.; Ginterseder M.; Spokoyny B.; Klein N. D.; Shulenberger K. E.; Perkinson C. F.; Kovalenko M. V.; Bawendi M. G. Coherent Single-Photon Emission from Colloidal Lead Halide Perovskite Quantum Dots. Science 2019, 363, 1068–1072. 10.1126/science.aau7392. [DOI] [PubMed] [Google Scholar]
- Becker M. A.; Vaxenburg R.; Nedelcu G.; Sercel P. C.; Shabaev A.; Mehl M. J.; Michopoulos J. G.; Lambrakos S. G.; Bernstein N.; Lyons J. L.; Stöferle T.; Mahrt R. F.; Kovalenko M. V.; Norris D. J.; Rainò G.; Efros A. L. Bright Triplet Excitons in Caesium Lead Halide Perovskites. Nature 2018, 553, 189–193. 10.1038/nature25147. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Yakunin S.; Kumar S.; Bär J.; Bertolotti F.; Masciocchi N.; Guagliardi A.; Grotevent M.; Shorubalko I.; Bodnarchuk M. I.; Shih C.-J.; Kovalenko M. V. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium–Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. 10.1021/acsnano.7b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba M.; Matsui T.; Seo J.-Y.; Domanski K.; Correa-Baena J.-P.; Nazeeruddin M. K.; Zakeeruddin S. M.; Tress W.; Abate A.; Hagfeldt A.; Grätzel M. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. 10.1039/C5EE03874J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Zhang Y.; Zhu X.; Yang Z.; Ke W.; Feng J.; Ren X.; Zhao K.; Liu M.; Kanatzidis M. G.; Liu S. Inch-Sized High-Quality Perovskite Single Crystals by Suppressing Phase Segregation for Light-Powered Integrated Circuits. Sci. Adv. 2021, 7, eabc8844. 10.1126/sciadv.abc8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Martínez C.; Imran M.; Schrenker N. J.; Ye J.; Ji K.; Rao A.; Stranks S. D.; Hoye R. L. Z.; Bals S.; Manna L.; Pérez-Juste J.; Polavarapu L. Fast A-Site Cation Cross-Exchange at Room Temperature: Single-to Double- and Triple-Cation Halide Perovskite Nanocrystals. Angew. Chem., Int. Ed. 2022, 61, e202205617. 10.1002/anie.202205617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lignos I.; Morad V.; Shynkarenko Y.; Bernasconi C.; Maceiczyk R. M.; Protesescu L.; Bertolotti F.; Kumar S.; Ochsenbein S. T.; Masciocchi N.; Guagliardi A.; Shih C.-J.; Bodnarchuk M. I.; deMello A. J.; Kovalenko M. V. Exploration of Near-Infrared-Emissive Colloidal Multinary Lead Halide Perovskite Nanocrystals Using an Automated Microfluidic Platform. ACS Nano 2018, 12, 5504–5517. 10.1021/acsnano.8b01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosova H. R.; Kucheriv O. I.; Shova S.; Gural’skiy I. A. Aziridinium Cation Templating 3D Lead Halide Hybrid Perovskites. Chem. Commun. 2022, 58, 5745–5748. 10.1039/D2CC01364A. [DOI] [PubMed] [Google Scholar]
- Semenikhin O. A.; Kucheriv O. I.; Sacarescu L.; Shova S.; Gural’skiy I. A. Quantum Dots Assembled from an Aziridinium Based Hybrid Perovskite Displaying Tunable Luminescence. Chem. Commun. 2023, 59, 3566–3569. 10.1039/D2CC06791A. [DOI] [PubMed] [Google Scholar]
- Stefańska D.; Ptak M.; Ma̧czka M. Synthesis, Photoluminescence and Vibrational Properties of Aziridinium Lead Halide Perovskites. Molecules 2022, 27, 7949. 10.3390/molecules27227949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K.; Zheng C.; Brook M. A.; Rubel O.. Stability of Aziridinium Lead Iodide Perovskite: Ring Strain and Water Vulnerability. arXiv 2019, arXiv:1908.10462.
- Banks H. D. The Profound Effect of Fluorine Substitution on the Reactivity and Regioselectivity of Nucleophilic Substitution Reactions of Strained Heterocycles. A Study of Aziridine and Its Derivatives. J. Org. Chem. 2006, 71, 8089–8097. 10.1021/jo061255j. [DOI] [PubMed] [Google Scholar]
- Cox J. D.; Pilcher G.. Thermochemistry of Organic and Organometallic Compounds, Vol. 74; Academic Press: London and New York, 1970; 727 pp. [Google Scholar]
- Zheng C.; Rubel O. Aziridinium Lead Iodide: a Stable, Low-Band-Gap Hybrid Halide Perovskite for Photovoltaics. J. Phys. Chem. Lett. 2018, 9, 874–880. 10.1021/acs.jpclett.7b03114. [DOI] [PubMed] [Google Scholar]
- Zheng C.; Rubel O. Ionization Energy as a Stability Criterion for Halide Perovskites. J. Phys. Chem. C 2017, 121, 11977–11984. 10.1021/acs.jpcc.7b00333. [DOI] [Google Scholar]
- Teng Q.; Shi T.; Zhao Y.-J. First-Principles Study of Aziridinium Lead Iodide Perovskite for Photovoltaics. ChemPhysChem 2019, 20, 602–607. 10.1002/cphc.201801033. [DOI] [PubMed] [Google Scholar]
- Teng Q.; Shi T.; Liao C.; Zhao Y.-J. First-Principles Study of Aziridinium Tin Iodide Perovskites for Photovoltaics. J. Mater. Chem. C 2021, 9, 982–990. 10.1039/D0TC03902K. [DOI] [PubMed] [Google Scholar]
- Weckhuysen B. M.; Kitagawa S.; Tsapatsis M. Reactions in Confined Spaces. ChemPhysChem 2018, 19, 339–340. 10.1002/cphc.201800058. [DOI] [PubMed] [Google Scholar]
- Akkerman Q. A.; Nguyen T. P. T.; Boehme S. C.; Montanarella F.; Dirin D. N.; Wechsler P.; Beiglböck F.; Rainò G.; Erni R.; Katan C.; Even J.; Kovalenko M. V. Controlling the Nucleation and Growth Kinetics of Lead Halide Perovskite Quantum Dots. Science 2022, 377, 1406–1412. 10.1126/science.abq3616. [DOI] [PubMed] [Google Scholar]
- Morad V.; Stelmakh A.; Svyrydenko M.; et al. Designer Phospholipid Capping Ligands for Soft Metal Halide Nanocrystals. Nature 2023, 10.1038/s41586-023-06932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarchuk M. I.; Boehme S. C.; ten Brinck S.; Bernasconi C.; Shynkarenko Y.; Krieg F.; Widmer R.; Aeschlimann B.; Günther D.; Kovalenko M. V.; Infante I. Rationalizing and Controlling the Surface Structure and Electronic Passivation of Cesium Lead Halide Nanocrystals. ACS Energy Lett. 2019, 4, 63–74. 10.1021/acsenergylett.8b01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J.; Quan L. N.; Zhao Y.; Peng W.; Murali B.; Sarmah S. P.; Yuan M.; Sinatra L.; Alyami N. M.; Liu J.; Yassitepe E.; Yang Z.; Voznyy O.; Comin R.; Hedhili M. N.; Mohammed O. F.; Lu Z. H.; Kim D. H.; Sargent E. H.; Bakr O. M. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. 10.1002/adma.201600784. [DOI] [PubMed] [Google Scholar]
- Krieg F.; Ong Q. K.; Burian M.; Rainò G.; Naumenko D.; Amenitsch H.; Süess A.; Grotevent M. J.; Krumeich F.; Bodnarchuk M. I.; Shorubalko I.; Stellacci F.; Kovalenko M. V. Stable Ultraconcentrated and Ultradilute Colloids of CsPbX3 (X = Cl, Br) Nanocrystals Using Natural Lecithin as a Capping Ligand. J. Am. Chem. Soc. 2019, 141, 19839–19849. 10.1021/jacs.9b09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M.; Ijaz P.; Goldoni L.; Maggioni D.; Petralanda U.; Prato M.; Almeida G.; Infante I.; Manna L. Simultaneous Cationic and Anionic Ligand Exchange for Colloidally Stable CsPbBr3 Nanocrystals. ACS Energy Lett. 2019, 4, 819–824. 10.1021/acsenergylett.9b00140. [DOI] [Google Scholar]
- Stelmakh A.; Aebli M.; Baumketner A.; Kovalenko M. V. On the Mechanism of Alkylammonium Ligands Binding to the Surface of CsPbBr3 Nanocrystals. Chem. Mater. 2021, 33, 5962–5973. 10.1021/acs.chemmater.1c01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginterseder M.; Sun W.; Shcherbakov-Wu W.; McIsaac A. R.; Berkinsky D. B.; Kaplan A. E. K.; Wang L.; Krajewska C.; Šverko T.; Perkinson C. F.; Utzat H.; Tisdale W. A.; Van Voorhis T.; Bawendi M. G. Lead Halide Perovskite Nanocrystals with Low Inhomogeneous Broadening and High Coherent Fraction Through Dicationic Ligand Engineering. Nano Lett. 2023, 23, 1128–1134. 10.1021/acs.nanolett.2c03354. [DOI] [PubMed] [Google Scholar]
- Aubert T.; Golovatenko A. A.; Samoli M.; Lermusiaux L.; Zinn T.; Abécassis B.; Rodina A. V.; Hens Z. General Expression for the Size-Dependent Optical Properties of Quantum Dots. Nano Lett. 2022, 22, 1778–1785. 10.1021/acs.nanolett.2c00056. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Surrente A.; Galkowski K.; Miyata A.; Portugall O.; Sutton R. J.; Haghighirad A. A.; Snaith H. J.; Maude D. K.; Plochocka P.; Nicholas R. J. Impact of the Halide Cage on the Electronic Properties of Fully Inorganic Cesium Lead Halide Perovskites. ACS Energy Lett. 2017, 2, 1621–1627. 10.1021/acsenergylett.7b00416. [DOI] [Google Scholar]
- Bertolotti F.; Dengo N.; Cervellino A.; Bodnarchuk M. I.; Bernasconi C.; Cherniukh I.; Berezovska Y.; Boehme S. C.; Kovalenko M. V.; Masciocchi N.; Guagliardi A. Size- and Temperature-Dependent Lattice Anisotropy and Structural Distortion in CsPbBr3 Quantum Dots by Reciprocal Space X-ray Total Scattering Analysis. Small Struct. 2023, 2300264. 10.1002/sstr.202300264. [DOI] [Google Scholar]
- Mannino G.; Deretzis I.; Smecca E.; La Magna A.; Alberti A.; Ceratti D.; Cahen D. Temperature-Dependent Optical Band Gap in CsPbBr3, MAPbBr3, and FAPbBr3 Single Crystals. J. Phys. Chem. Lett. 2020, 11, 2490–2496. 10.1021/acs.jpclett.0c00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni G.; Diskin-Posner Y.; Gehrmann C.; Godse S.; Gkikas G. G.; Buchine I.; Aharon S.; Korobko R.; Stoumpos C. C.; Egger D. A.; Yaffe O. Static and Dynamic Disorder in Formamidinium Lead Bromide Single Crystals. J. Phys. Chem. Lett. 2023, 14, 1288–1293. 10.1021/acs.jpclett.2c03337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weadock N. J.; Sterling T. C.; Vigil J. A.; Gold-Parker A.; Smith I. C.; Ahammed B.; Krogstad M. J.; Ye F.; Voneshen D.; Gehring P. M.; Rappe A. M.; Steinrück H.-G.; Ertekin E.; Karunadasa H. I.; Reznik D.; Toney M. F. The Nature of Dynamic Local Order in CH3NH3PbI3 and CH3NH3PbBr3. Joule 2023, 7, 1051–1066. 10.1016/j.joule.2023.03.017. [DOI] [Google Scholar]
- Comin R.; Crawford M. K.; Said A. H.; Herron N.; Guise W. E.; Wang X.; Whitfield P. S.; Jain A.; Gong X.; McGaughey A. J. H.; Sargent E. H. Lattice Dynamics and the Nature of Structural Transitions in Organolead Halide Perovskites. Phys. Rev. B 2016, 94, 094301. 10.1103/PhysRevB.94.094301. [DOI] [Google Scholar]
- Carignano M. A.; Aravindh S. A.; Roqan I. S.; Even J.; Katan C. Critical Fluctuations and Anharmonicity in Lead Iodide Perovskites from Molecular Dynamics Supercell Simulations. J. Phys. Chem. C 2017, 121, 20729–20738. 10.1021/acs.jpcc.7b08220. [DOI] [Google Scholar]
- Beecher A. N.; Semonin O. E.; Skelton J. M.; Frost J. M.; Terban M. W.; Zhai H.; Alatas A.; Owen J. S.; Walsh A.; Billinge S. J. L. Direct Observation of Dynamic Symmetry Breaking Above Room Temperature in Methylammonium Lead Iodide Perovskite. ACS Energy Lett. 2016, 1, 880–887. 10.1021/acsenergylett.6b00381. [DOI] [Google Scholar]
- Hanusch F. C.; Wiesenmayer E.; Mankel E.; Binek A.; Angloher P.; Fraunhofer C.; Giesbrecht N.; Feckl J. M.; Jaegermann W.; Johrendt D.; Bein T.; Docampo P. Efficient Planar Heterojunction Perovskite Solar Cells Based on Formamidinium Lead Bromide. J. Phys. Chem. Lett. 2014, 5, 2791–2795. 10.1021/jz501237m. [DOI] [PubMed] [Google Scholar]
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Bertolotti F.; Masciocchi N.; Guagliardi A.; Kovalenko M. V. Monodisperse Formamidinium Lead Bromide Nanocrystals with Bright and Stable Green Photoluminescence. J. Am. Chem. Soc. 2016, 138, 14202–14205. 10.1021/jacs.6b08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirin D. N.; Vivani A.; Zacharias M.; Sekh T. V.; Cherniukh I.; Yakunin S.; Bertolotti F.; Aebli M.; Schaller R. D.; Wieczorek A.; Siol S.; Cancellieri C.; Jeurgens L. P. H.; Masciocchi N.; Guagliardi A.; Pedesseau L.; Even J.; Kovalenko M. V.; Bodnarchuk M. I. Intrinsic Formamidinium tin Iodide Nanocrystals by Suppressing the Sn(IV) Impurities. Nano Lett. 2023, 23, 1914–1923. 10.1021/acs.nanolett.2c04927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti F.; Protesescu L.; Kovalenko M. V.; Yakunin S.; Cervellino A.; Billinge S. J. L.; Terban M. W.; Pedersen J. S.; Masciocchi N.; Guagliardi A. Coherent nanotwins and dynamic disorder in cesium lead halide perovskite nanocrystals. ACS Nano 2017, 11, 3819–3831. 10.1021/acsnano.7b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S. C.; Bodnarchuk M. I.; Burian M.; Bertolotti F.; Cherniukh I.; Bernasconi C.; Zhu C.; Erni R.; Amenitsch H.; Naumenko D.; Andrusiv H.; Semkiv N.; John R. A.; Baldwin A.; Galkowski K.; Masciocchi N.; Stranks S. D.; Rainò G.; Guagliardi A.; Kovalenko M. V. Strongly Confined CsPbBr3 Quantum Dots as Quantum Emitters and Building Blocks for Rhombic Superlattices. ACS Nano 2023, 17, 2089–2100. 10.1021/acsnano.2c07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.; Hazarika A.; Schelhas L. T.; Liu J.; Gaulding E. A.; Li G.; Zhang M.; Toney M. F.; Sercel P. C.; Luther J. M. Size-Dependent Lattice Structure and Confinement Properties in CsPbI3 perovskite Nanocrystals: Negative Surface Energy for Stabilization. ACS Energy Lett. 2020, 5, 238–247. 10.1021/acsenergylett.9b02395. [DOI] [Google Scholar]
- Goldschmidt V. M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. 10.1007/BF01507527. [DOI] [Google Scholar]
- Li W.; Wang Z.; Deschler F.; Gao S.; Friend R. H.; Cheetham A. K. Chemically Diverse and Multifunctional Hybrid Organic–Inorganic Perovskites. Nat. Rev. Mater. 2017, 2, 16099. 10.1038/natrevmats.2016.99. [DOI] [Google Scholar]
- Bartel C. J.; Sutton C.; Goldsmith B. R.; Ouyang R.; Musgrave C. B.; Ghiringhelli L. M.; Scheffler M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. 10.1126/sciadv.aav0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Tang R.; Kao J. L. F.; Dingman S. D.; Buhro W. E. Spectroscopic Identification of Tri-n-Octylphosphine Oxide (TOPO) Impurities and Elucidation of Their Roles in Cadmium Selenide Quantum-Wire Growth. J. Am. Chem. Soc. 2009, 131, 4983–4994. 10.1021/ja900191n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebli M.; Piveteau L.; Nazarenko O.; Benin B. M.; Krieg F.; Verel R.; Kovalenko M. V. Lead-Halide Scalar Couplings in 207Pb NMR of APbX3 Perovskites (A = Cs, Methylammonium, Formamidinium; X = Cl, Br, I). Sci. Rep. 2020, 10, 8229. 10.1038/s41598-020-65071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos A. G.; Manolis G. K.; Kaltzoglou A.; Palles D.; Kamitsos E. I.; Kanatzidis M. G.; Falaras P. Halogen–NH2+ Interaction, Temperature-Induced Phase Transition, and Ordering in (NH2CHNH2)PbX3 (X = Cl, Br, I) Hybrid Perovskite. J. Phys. Chem. C 2020, 124, 8479–8487. 10.1021/acs.jpcc.9b11334. [DOI] [Google Scholar]
- Zhou J.; Chizhik A. I.; Chu S.; Jin D. Single-Particle Spectroscopy for Functional Nanomaterials. Nature 2020, 579, 41–50. 10.1038/s41586-020-2048-8. [DOI] [PubMed] [Google Scholar]
- Galland C.; Ghosh Y.; Steinbrück A.; Sykora M.; Hollingsworth J. A.; Klimov V. I.; Htoon H. Two Types of Luminescence Blinking Revealed by Spectroelectrochemistry of Single Quantum Dots. Nature 2011, 479, 203–207. 10.1038/nature10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmal M.; Dabbousi B. O.; Bawendi M. G.; Macklin J. J.; Trautman J. K.; Harris T. D.; Brus L. E. Fluorescence Intermittency in Single Cadmium Selenide Nanocrystals. Nature 1996, 383, 802–804. 10.1038/383802a0. [DOI] [Google Scholar]
- Basché T.; Moerner W. E.; Orrit M.; Talon H. Photon Antibunching in the Fluorescence of a Single Dye Molecule Trapped in a Solid. Phys. Rev. Lett. 1992, 69, 1516–1519. 10.1103/PhysRevLett.69.1516. [DOI] [PubMed] [Google Scholar]
- Chen O.; Zhao J.; Chauhan V. P.; Cui J.; Wong C.; Harris D. K.; Wei H.; Han H.-S.; Fukumura D.; Jain R. K.; Bawendi M. G. Compact High-Quality CdSe–CdS Core–Shell Nanocrystals with Narrow Emission Linewidths and Suppressed Blinking. Nat. Mater. 2013, 12, 445–451. 10.1038/nmat3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainò G.; Yazdani N.; Boehme S. C.; Kober-Czerny M.; Zhu C.; Krieg F.; Rossell M. D.; Erni R.; Wood V.; Infante I.; Kovalenko M. V. Ultra-Narrow Room-Temperature Emission from Single CsPbBr3 Perovskite Quantum Dots. Nat. Commun. 2022, 13, 2587. 10.1038/s41467-022-30016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo J.; Ibáñez M.; Geiregat P.; Nedelcu G.; Walravens W.; Maes J.; Martins J. C.; Van Driessche I.; Kovalenko M. V.; Hens Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. 10.1021/acsnano.5b06295. [DOI] [PubMed] [Google Scholar]
- Park Y.-S.; Lim J.; Klimov V. I. Asymmetrically Strained Quantum Dots with Non-Fluctuating Single-Dot Emission Spectra and Subthermal Room-Temperature Linewidths. Nat. Mater. 2019, 18, 249–255. 10.1038/s41563-018-0254-7. [DOI] [PubMed] [Google Scholar]
- Tamarat P.; Bodnarchuk M. I.; Trebbia J.-B.; Erni R.; Kovalenko M. V.; Even J.; Lounis B. The Ground Exciton State of Formamidinium Lead Bromide Perovskite Nanocrystals is a Singlet Dark State. Nat. Mater. 2019, 18, 717–724. 10.1038/s41563-019-0364-x. [DOI] [PubMed] [Google Scholar]
- Tamarat P.; Hou L.; Trebbia J.-B.; Swarnkar A.; Biadala L.; Louyer Y.; Bodnarchuk M. I.; Kovalenko M. V.; Even J.; Lounis B. The Dark Exciton Ground State Promotes Photon-Pair Emission in Individual Perovskite Nanocrystals. Nat. Commun. 2020, 11, 6001. 10.1038/s41467-020-19740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C.; Chen L.; Song N.; Lv Y.; Hu F.; Sun C.; Yu W. W.; Zhang C.; Wang X.; Zhang Y.; Xiao M. Bright-Exciton Fine-Structure Splittings in Single Perovskite Nanocrystals. Phys. Rev. Lett. 2017, 119, 026401. 10.1103/PhysRevLett.119.026401. [DOI] [PubMed] [Google Scholar]
- Cho K.; Tahara H.; Yamada T.; Suzuura H.; Tadano T.; Sato R.; Saruyama M.; Hirori H.; Teranishi T.; Kanemitsu Y. Exciton–Phonon and Trion–Phonon Couplings Revealed by Photoluminescence Spectroscopy of Single CsPbBr3 Perovskite Nanocrystals. Nano Lett. 2022, 22, 7674–7681. 10.1021/acs.nanolett.2c02970. [DOI] [PubMed] [Google Scholar]
- Amara M.-R.; Said Z.; Huo C.; Pierret A.; Voisin C.; Gao W.; Xiong Q.; Diederichs C. Spectral Fingerprint of Quantum Confinement in Single CsPbBr3 Nanocrystals. Nano Lett. 2023, 23, 3607–3613. 10.1021/acs.nanolett.3c00793. [DOI] [PubMed] [Google Scholar]
- Fu M.; Tamarat P.; Huang H.; Even J.; Rogach A. L.; Lounis B. Neutral and Charged Exciton Fine Structure in Single Lead Halide Perovskite Nanocrystals Revealed by Magneto-Optical Spectroscopy. Nano Lett. 2017, 17, 2895–2901. 10.1021/acs.nanolett.7b00064. [DOI] [PubMed] [Google Scholar]
- Guilloux V.; Ghribi A.; Majrab S.; Margaillan F.; Bernard M.; Bernardot F.; Legrand L.; Lhuillier E.; Boujdaria K.; Chamarro M.; Testelin C.; Barisien T. Exciton Fine Structure of CsPbCl3 Nanocrystals: an Interplay of Electron–Hole Exchange Interaction, Crystal Structure, Shape Anisotropy, and Dielectric Mismatch. ACS Nano 2023, 17, 12266–12277. 10.1021/acsnano.3c00772. [DOI] [PubMed] [Google Scholar]
- Pfingsten O.; Klein J.; Protesescu L.; Bodnarchuk M. I.; Kovalenko M. V.; Bacher G. Phonon Interaction and Phase Transition in Single Formamidinium Lead Bromide Quantum Dots. Nano Lett. 2018, 18, 4440–4446. 10.1021/acs.nanolett.8b01523. [DOI] [PubMed] [Google Scholar]
- Zhu C.; Nguyen T.; Boehme S. C.; Moskalenko A.; Dirin D. N.; Bodnarchuk M. I.; Katan C.; Even J.; Rainò G.; Kovalenko M. V. Many-Body Correlations and Exciton Complexes in CsPbBr3 Quantum Dots. Adv. Mater. 2023, 35, 2208354. 10.1002/adma.202208354. [DOI] [PubMed] [Google Scholar]
- Tamarat P.; Prin E.; Berezovska Y.; Moskalenko A.; Nguyen T. P. T.; Xia C.; Hou L.; Trebbia J.-B.; Zacharias M.; Pedesseau L.; et al. Universal Scaling Laws for Charge-Carrier interactions with Quantum Confinement in lead-Halide Perovskites. Nat. Commun. 2023, 14, 229. 10.1038/s41467-023-35842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.; Yamada T.; Tahara H.; Tadano T.; Suzuura H.; Saruyama M.; Sato R.; Teranishi T.; Kanemitsu Y. Luminescence Fine Structures in Single Lead Halide Perovskite Nanocrystals: Size Dependence of the Exciton–Phonon Coupling. Nano Lett. 2021, 21, 7206–7212. 10.1021/acs.nanolett.1c02122. [DOI] [PubMed] [Google Scholar]
- Krieg F.; Sercel P. C.; Burian M.; Andrusiv H.; Bodnarchuk M. I.; Stöferle T.; Mahrt R. F.; Naumenko D.; Amenitsch H.; Rainò G.; Kovalenko M. V. Monodisperse Long-Chain Sulfobetaine-Capped CsPbBr3 Nanocrystals and Their Superfluorescent Assemblies. ACS Cent. Sci. 2021, 7, 135–144. 10.1021/acscentsci.0c01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott P. R.; Meister D.; Leake S. J.; Lange M.; Bergamaschi A.; Boge M.; Calvi M.; Cancellieri C.; Casati N.; Cervellino A. The Materials Science Beamline Upgrade at the Swiss Light Source. J. Synchrotron Rad. 2013, 20, 667–682. 10.1107/S0909049513018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.