The cell cycle plays a crucial role in plant development. Organogenesis takes place throughout the lifetime and most cells maintain their ability to reenter and to regulate the cell cycle in response to a wide range of endogenous and external signals. In planta, phytohormones, particularly auxin and cytokinin, are essential for cell proliferation. New organs arise from local foci of proliferating cells, called meristems, which either persist or are de novo formed. In the organ primordium (initial stage of an organ before reaching maturity), cell differentiation starts with cell division arrest. These nondividing cells can exit the mitotic cycle with either complete loss or partial activity of the cell cycle. In the latter case, plant cells frequently enter an altered version of the cell cycle, known as endoreduplication cycles or endocycles, where the genome is duplicated without mitosis. Single or multiple rounds of endoreduplication cycles result in the formation of polyploid cells. The physiological role of ploidy is poorly understood.
Cell cycle progression is controlled by ordered action of cyclin-dependent kinases (CDKs), activated by defined cyclins, appearing for given periods in the cycle. When the function of a CDK-cyclin complex is accomplished, the associated cyclin partner becomes polyubiquitinated and destroyed by the ubiquitin-26S proteasome system (UPS). The vital importance of the UPS became evident during the last few years and its discovery was awarded by the Nobel Prize in Chemistry 2004 to Aaron Ciechanover, Avram Hershko, and Irwin Rose. The UPS is essential for many cellular processes including cell cycle, signal transduction and regulation of gene expression, circadian clocks, or phytohormone signaling pathways (Vierstra, 2003).
This Update focuses on the possible implications of the ubiquitin-mediated proteolysis in differential regulation of the cell cycle in plant development using nitrogen-fixing root nodules of Medicago truncatula as a model organ. Nodules develop on the roots of legume plants in symbiosis with Rhizobium soil bacteria. One can ask whether studies on this legume-specific symbiotic organ can provide general information on cell cycle and differentiation that is also valid for other plant organs. The answer is yes. The plant encodes nodule development, which resembles, in many respects, lateral root development. Moreover, nodules have several advantages over other plant organs. First, their development can be programmed by application of Rhizobium signal molecules, the Nod factors, which allows studying the mechanisms of cell cycle reactivation and meristem formation from the instant of addition of the morphogen signal. Second, Medicago nodules are indeterminate, which means that the meristem remains active and generates cells that constantly enter differentiation. Thus, different stages of development can be monitored even in a mature nitrogen-fixing nodule. Third, in the submeristematic cell layers, endoreduplication cycles occur permanently. Such a local concentration of endocycling cells is rare and ideal to elucidate the mechanisms that generate polyploid cells in plants. In the following, we concentrate on two critical steps of nodule development: (1) how cell cycle is activated; and (2) how proliferating cells exit the mitotic cycle and enter differentiation via endoreduplication cycles.
NITROGEN-FIXING NODULES: A PLANT ORGAN INDUCED BY BACTERIAL SIGNAL MOLECULES WITH RESEMBLANCE TO LATERAL ROOTS
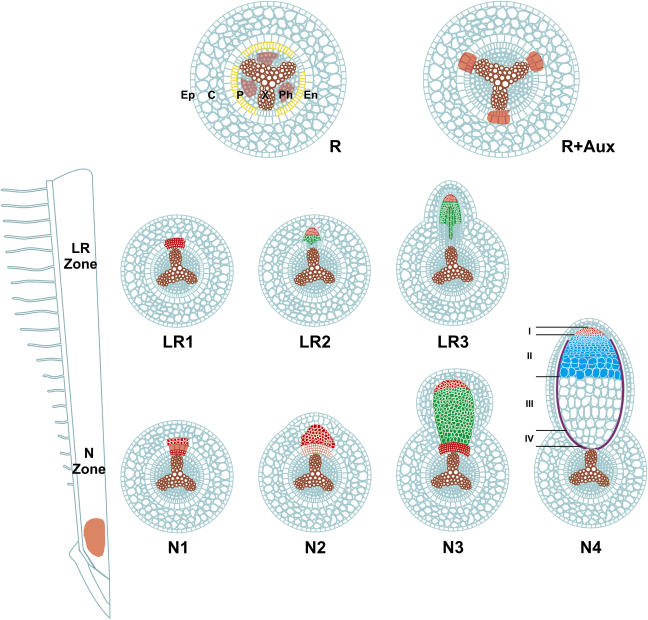
Nodule development requires active photosynthesis and limited nitrogen supply. There are two major nodule types: the indeterminate and determinate nodules with permanently or transiently active meristem that originate from the inner and outer cortex, respectively. Indeterminate nodule development (Fig. 1) has been studied mainly in the symbiosis of Medicago sativa/M. truncatula with Sinorhizobium meliloti and Pisum sativum/Trifolium repens with Rhizobium leguminosarum. In these plants, Nod factors elicit dedifferentiation and cell cycle reentry of the cortical cells in front of the protoxylem poles in the emerging root hair zone. Cell division in the inner cortex and in the pericycle below the activated cortical cells results in the formation of the nodule primordium and the vasculature, respectively (Yang et al., 1994). When the nodule primordium is formed and emerged from the root, it differentiates, generating a complex structure composed of different peripheral and central tissues (Vasse et al., 1990). The central region of a nitrogen-fixing nodule contains the persistent apical meristem zone I, the infection zone II, the nitrogen fixation zone III and, in old nodules, the proximal senescent zone IV (Fig. 1, N4). Infection of plant cells and differentiation of symbiotic cells take place in zone II. In this zone, the bacteria still produce Nod factors and, although the cells do not divide, they are able to undergo successive rounds of endoreduplication cycles. As a consequence, the nuclear DNA content increases from 2C up to 64C and, proportional to the genome size, the cells enlarge as they become older and more distant from the meristem (Cebolla et al., 1999). Zone III contains terminally differentiated giant plant cells hosting thousands of nitrogen-fixing bacteria, called bacteroids. The bacteroids stop Nod factor production and the expression of cell cycle genes is switched off. In contrast to zones I and II, the size of zone III increases during the lifetime of the nodule by continuous production of nitrogen-fixing cells.
Figure 1.
Developmental stages of indeterminate-type nodule (N) and lateral root (LR) formation. The root zones competent for N or LR development are indicated on the longitudinal root section. Transverse section of the root (R) shows epidermis (Ep), cortex (C), endodermis (En), pericycle (P), phloem (Ph), and xylem (X). Colors indicate expression of cycA2 (red), 1-aminocyclopropane-1-carboxylate synthase (yellow), and ccs52A, the overlapping expression of cycA2 and ccs52A (green), and the position of protoxylem poles (brown). R + aux, auxin-treated root section.
Development of nodules and lateral roots displays common but also distinct features (Fig. 1). Both organs originate from de novo formed meristems initiated in front of the protoxylem/xylem poles. However, lateral roots develop from a more distal root zone than nodules and arise from division of pericycle cells. The lateral root primordium is smaller than the nodule primordium and it starts differentiation before its outgrowth from the root. Endoreduplication cycles also occur during lateral root development; however, only in a few cells and not exceeding the 8C ploidy level (Cebolla et al., 1999). In lateral roots, the vasculature is central, while in nodules the vascular bundles are branched and localized at the nodule periphery. Certain aberrant nodules display properties of both organs, such as the outgrowth of lateral roots from nodule-like structures where the nodule-specific meristem is overtaken by a lateral root meristem (Ferraioli et al., 2004).
CONCERTED ACTION OF NOD FACTORS AND PHYTOHORMONES IN CELL CYCLE ACTIVATION AND PRIMORDIUM FORMATION
Auxin is a key signal in plant development. The asymmetric distribution of auxin (termed auxin maxima) affects polarity and pattern formation and is required for embryonic, root, and shoot organogenic processes. Auxin is mobilized by auxin influx and efflux carriers, encoded by the AUX/LAX and PIN genes, respectively (Kramer, 2004). During lateral root development, division of pericycle founder cells and cell proliferation in the young lateral root primordium are auxin-dependent (Casimiro et al., 2003). At a later stage, the lateral root primordium becomes independent of externally applied auxin, indicating the existence of an internal auxin source.
Several studies suggest that Nod factors affect local distribution and concentration of auxin. The use of the auxin-sensitive GH3 promoter-reporter gene fusion indicated transient inhibition of auxin transport by rhizobia and Nod factors, leading to transient accumulation of auxin at the site where indeterminate root nodules initiate (Mathesius et al., 1998). In Lotus japonicus, which develops determinate nodules, a local up-regulation in auxin transport was detected in the root after inoculation with Nod factors and a strong GH3 promoter-β-glucuronidase expression was present in the dividing outer cortical cells leading to nodule primordium formation (Pacios-Bras et al., 2003). These results indicate that indeterminate and determinate type legumes might have different auxin distribution patterns that could lead to cell division either in the inner or outer cortex. In M. truncatula, expression studies on the AUX1-like genes suggest that auxin is required at two common stages of lateral root and nodule development, for the formation of primordia and differentiation of the vasculature (de Billy et al., 2001). Moreover, application of a polar auxin transport inhibitor resulted in the formation of pseudonodules (Hirsch et al., 1989). Cytokinin treatment of Medicago roots increased amyloplast accumulation and the number of cell division foci in the inner cortex recapitulating the responses to Nod factors (Bauer et al., 1996). Cytokinin has effects on the G1/S and G2/M transitions as well as on progression through S-phase (Dewitte and Murray, 2003). In L. japonicus, a legume forming determinate nodules, expression of the cytokinin-responsive ARR5 gene was absent in pericycle founder cells of lateral roots and at the initial divisions of cortical cells but was present in the nodule primordium (Lohar et al., 2004). This suggests that cytokinin is not needed for cell cycle reactivation, while it is necessary for maintaining cell proliferation. Or, local changes in cytokinin levels at the site of nodule initiation may alter auxin redistribution, thereby stimulating nodule organogenesis.
In the indeterminate legumes, ethylene provides positional information on cortical cell division. Expression of 1-aminocyclopropane-1-carboxylate synthase, encoding the last enzyme in ethylene biosynthesis, is localized to pericycle cells opposite to the phloem poles (Fig. 1, R; Heidstra et al., 1997). As ethylene inhibits division of cortical cells and nodule primordium formation, the absence of ethylene production in front of the xylem poles may explain why nodules or even lateral roots develop at the xylem but not at the phloem poles.
CDK-CENTRIC VIEW OF THE CELL CYCLE
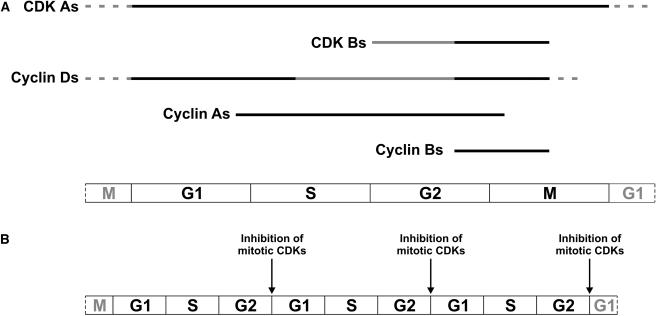
In eukaryotes, regulation of cell cycle has been attributed to the sequential activation of CDKs by cyclins. In Arabidopsis, 30 to 43 cyclins are predicted and the CDK family is composed of 12 proteins grouped in 6 types, from A to F (Vandepoele et al., 2002; Wang et al., 2004). The genome sequence of M. truncatula has not been completed yet; therefore, the exact numbers of cyclins and CDKs are not known but based on the identification of the six CDK types in Medicago (Magyar et al., 1997), similar complexity of the CDK-cyclin network is expected. The CDKs with the hallmark of PSTAIRE motif in the cyclin binding site are conserved in all eukaryotes. In plants, these are the A-type CDKs that express throughout the cell cycle and control both the G1/S and G2/M transitions, while the B-type CDKs are mitotic and plant specific (Fig. 2A). The C-type CDKs are involved in the regulation of transcription, whereas the D- and F-type CDKs are CDK-activating kinases.
Figure 2.
A, Plant CDKs and cyclins control different phases of the mitotic cycle. B, Inhibition of mitotic CDKs converts the mitotic cycle to endocycle.
In the cell cycle, specific cyclins are associated with G1 (cyclin D), S-phase (cyclin E and cyclin A), and mitosis (cyclin A and cyclin B). Cyclin E is missing from plants, while other cyclin types are present and represented by multiple members. In Arabidopsis (Arabidopsis thaliana), there are 9 or 10 D-type cyclins, 10 A-type, and 9 B-type cyclins (Vandepoele et al., 2002; Wang et al., 2004). With the exception of a few plant cyclins, it is unknown when and where they are expressed and what their functions are.
By responding to nutrient and other signals, D-type cyclins are believed to have primary roles during G1 and G1-S transition. In Arabidopsis, cycD2 and cycD4 respond to sugar availability, while D3-type cyclins to cytokinin and brassinosteroid (Riou-Khamlichi et al., 1999, 2000). In Medicago roots, cycD3;1 was transiently induced in the reactivated cortical cells in response to Nod factors, while cycD3;2 expression was linked to endocycles in nodule zone II (Foucher and Kondorosi, 2000), indicating that different sets of CycDs may operate in mitotic and endocycles. Unlike in animals, specific CycDs in association with CDKBs act in G2-M (Kono et al., 2003).
The diversity of A-type cyclins is plant specific. In contrast to a single cyclin A in animal cells, plants have three groups of A-type cyclins with multiple members in each. A-type cyclins function from S- to M-phase, but some of them may control S-phase entry (Roudier et al., 2000; Menges et al., 2005). Expression of B-type cyclins is confined to G2-M and cyclin-B associated CDKs are required for mitosis. In the absence of mitotic CDK activities, cells stop to divide and either exit the mitotic cycle and become quiescent or enter endoreduplication cycle(s), which operates with G1-S-G2 activities of the cell cycle (Fig. 2B). If cell cycle activity is maintained for DNA replication and mitotic CDKs are inhibited, endoreduplication cycles can be repeated in multiple rounds leading to the formation of polyploid cells.
The presented CDK-cyclin centric view on cell cycle control is an extreme oversimplification. Many important components such as the Rb-E2F pathway or CDK inhibitors have not been discussed here, as this minimal information is sufficient to discuss nodule primordium formation and differentiation.
CELL CYCLE CONTROL AND PHYTOHORMONE SIGNALING PATHWAYS DEPEND ON TARGETED DEGRADATION OF PROTEINS BY THE UBIQUITIN-PROTEASOME SYSTEM
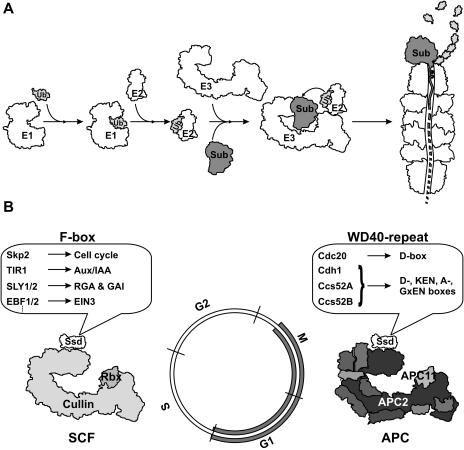
UPS is the primary mechanism in eukaryotic cells for degrading unwanted and misfolded proteins (Fig. 3A; Ciechanover et al., 2000). Through the cascade of the E1 ubiquitin activating, E2 ubiquitin conjugating, and E3 ubiquitin ligase enzymes, ubiquitin monomers are attached sequentially to the target proteins. The polyubiquitinated proteins are then recognized by the 26S proteasome, a large ATP-dependent multicatalytic protease, which removes the ubiquitin chain and degrades the proteins to short peptides. The UPS appears to be the most elaborate regulatory mechanism in plants as 5% of their genome encodes core components of UPS and more than 1,000 E3 ubiquitin ligases are predicted (for review, see Vierstra, 2003; Schwechheimer and Villalobos, 2004).
Figure 3.
A, The ubiquitin proteasome system. The ubiquitin (Ub) is covalently attached to substrate proteins (Sub) through sequential action of the E1, E2, and E3 enzymes. The polyubiquitinated proteins are recognized by the 26S proteasome, which degrades the substrate proteins and recycles the ubiquitin. The E3 enzymes achieve the specificity of ubiquitin-dependent proteolysis. B, The SCF and the APC E3 enzymes are dedicated to basic cell-cycle control. Rbx/APC11 and Cullin/APC2 are related subunits. SCF is constitutively active, while the WD40-repeat proteins Cdc20 and Cdh1/Ccs52A,B activate APC from M-phase to S-phase. The cullin/APC2 and the Rbx/APC11 subunits are related. The substrate-specificity determinants (Ssd) are the F-box proteins in the SCF and Cdc20 and Cdh1/Ccs52A,B in the APC. In addition to polyubiquitination of cell cycle proteins, the SCF and the APC are also active in nonproliferating cells mediating protein degradation in various cellular processes. The SCF via different F-box proteins is involved in phytohormone signaling pathways.
The selection and specific timing of polyubiquitination of the target proteins are conferred by different E3 ubiquitin ligases. In the cell cycle, two structurally related multicomponent ubiquitin ligases, the anaphase-promoting complex (APC) and the Skp1/Cul1/F-box protein (SCF) complexes (Fig. 3B) have essential and complementary functions by temporally controlled degradation of various cell cycle proteins (Peters, 2002; Vodermaier, 2004).
Different F-box proteins provide the substrate-specificity of SCF. In Arabidopsis, the presence of almost 700 F-box proteins indicates the involvement of SCF in a wide range of cellular processes including various hormone responses. Auxin signaling is mediated by auxin-induced degradation of the Aux/IAA proteins by SCFTIR1, where TIR1 is an F-box protein (Gray et al., 1999, 2001). Elimination of the Aux/IAA proteins leads to the release of the interacting ARF transcription factors that regulate the expression of auxin responsive genes. In a similar way, in response to GA3, the SCFSLY1/2 mediates degradation of the putative transcription factors RGA and GAI (Dill et al., 2004; Fu et al., 2004), while SCFEBF1/2 is involved in ethylene signaling by degrading EIN3 in the absence of ethylene (Potuschak et al., 2003).
The APC is composed of 11 to 13 subunits in human and yeast (Saccharomyces cerevisiae) and homologous APC subunits have also been found in plants (Capron et al., 2003). Except for APC2 and APC11, relatively little is known about the role of the other APC subunits or the assembly of the complex. APC functions both in mitotic and nondividing postmitotic cells. Binding of the APC substrates and activation of the APC are controlled by 2 WD40-repeat activator proteins, Cdc20 and Cdh1. They determine stage-specific activation of the APC from metaphase until S-phase and degradation of various cell cycle proteins during the cell cycle (Harper et al., 2002; Peters, 2002). Cdc20 appears to be active only in proliferating cells. In contrast, Cdh1 functions in both mitotic and differentiating cells. In plants, there are multiple cdc20 genes and Cdh1-type activators, identified as cell cycle switch Ccs52 proteins (Cebolla et al., 1999). The latter form 2 classes: Ccs52A, representing a plant ortholog of the yeast and animal Cdh1 proteins; and Ccs52B, which is plant specific (Tarayre et al., 2004). These proteins differ in their expression pattern during the cell cycle and plant development (Tarayre et al., 2004) and interact with distinct sets of APC substrate proteins (Z. Kelemen, G. Horvath, and E. Kondorosi, unpublished data).
Mitotic cyclins that contain a destruction or D-box in their N terminus were the first identified substrates of the APC. Both the Cdc20 and the Cdh1/Ccs52 proteins can mediate the degradation of mitotic cyclins; however, at different phases of the cell cycle. A-type cyclins are not only substrates but also regulators of the APC as phosphorylation of Cdh1 by cyclin A-associated CDK inactivates Cdh1 and abolishes its binding to the core APC. Similarly, phosphomimetic amino acid replacements in the Medicago Ccs52A protein inhibit the interaction of Ccs52A with the APC (Tarayre et al., 2004). The Cdh1/Ccs52 proteins interact with many different proteins containing D-, KEN, A-, or GxEN boxes or other, yet unidentified degradation motifs and activate the APC both in and outside the cell cycle with essential roles in the differentiation of specific cell types. In plants, the number of APC substrates can be estimated from a few hundred up to a few thousand.
CELL CYCLE ACTIVATION AND NODULE MERISTEM FORMATION REQUIRE AN AUXIN-REGULATED A2-TYPE CYCLIN
Previous studies showed that Nod factors trigger reactivation of G0-arrested cells (Savouré et al., 1994; Yang et al., 1994). Surprisingly, one of the earliest Nod factor induced cell cycle genes was a cyclin that, based on its structure, was classified as mitotic A2-type cyclin, cycA2 (Foucher and Kondorosi, 2000; Roudier et al., 2003). In the nodulation competent root zone, activation of cycA2 coincided with the induction of G1-S regulators (such as cycD3;1). Unlike other mitotic cyclins, the level of cycA2 mRNA and the protein did not display marked oscillation from late G1 until prometaphase where the CycA2 protein was abruptly degraded (Roudier et al., 2000). Expression of cycA2 in late G1 as well as its activation by the Nod factors suggested that CycA2 might be involved in the cell cycle reentry. This was studied in roots, lateral roots and nodules, and in galls, abnormal swellings of roots, where endoparasitic root-knot nematodes trigger division of cortical cells and formation of polyploid feeding cells (Roudier et al., 2003).
In the primary root, cycA2 expression was observed in the root apical meristem and faintly in the phloem cells (Fig. 1, R). cycA2 was induced at the onset of lateral root development, in the dividing cells and the lateral root primordium (Fig. 1, LR1 and 2). By differentiation of the primordium, cycA2 expression becomes restricted to the meristem (Fig. 1, LR3). During nodule organogenesis, cycA2 was induced 5 h after Nod factor treatment and the expression was maintained in the dividing cortical cells and in the nodule primordium (Fig. 1, N1–3). In nitrogen-fixing nodules, cycA2 was expressed only in the nodule meristem (Fig. 1, N4). In galls, expression of cycA2 was undetectable. Therefore, it is possible that the cycA2 function is linked to mitotic cycles, which lead to the formation of secondary meristems, but it is dispensable or even incompatible with endoreduplication cycles (Roudier et al., 2003).
If cycA2 is involved in cell cycle reentry during lateral root and nodule initiation, it is expected that its expression is regulated by auxin. The cycA2 promoter contains two auxin-response-like elements. Treatment of M. truncatula roots with auxin or with a polar auxin transport inhibitor demonstrated that cycA2 is indeed auxin regulated (Roudier et al., 2003). Auxin-treatment resulted not only in the up-regulation of cycA2 but affected also the spatial expression pattern. Instead of phloem-associated expression, auxin induced de novo transcription of cycA2 in front of the xylem poles, where both lateral roots and nodules initiate (Fig. 1, R and R + aux). This was also consistent with the Nod factor-triggered expression of the auxin-responsive GH3 promoter at the inner cortex prior to nodule initiation (Mathesius et al., 1998). It is still unknown, however, how this auxin response emerges on cycA2, which IAA protein(s) are degraded, and whether only the SCFTIR1 system or other E3 ubiquitin ligases are involved in auxin signaling in Medicago roots.
ENDOREDUPLICATION CYCLES MEDIATED BY THE APC ACTIVATOR CCS52A ARE INDISPENSABLE FOR SYMBIOTIC CELL DIFFERENTIATION
After the formation of the nodule primordium, the next critical step is nodule differentiation that involves cell cycle arrest in the various nodule cell types but modified regulation of the cell cycle in the symbiotic cells. This raises the questions of how cell proliferation is arrested, how endocycles are triggered, and whether genome amplification has any biological meaning. Endoreduplication cycles result in periodic replication of the genome. This is achieved by the loss of M-phase and oscillations in the activity of S-phase cyclin-dependent kinase.
In nodule zone II, expression of CDKA, G1-, and S-phase specific marker genes indicates that cell cycle activities for DNA replication and endoreduplication cycles are present (Foucher and Kondorosi, 2000). On the other hand, mitotic B-type cyclins are also expressed in the infected cells, albeit formation of mitotic cyclin-CDK complexes should be avoided during endocycles (Cebolla et al., 1999). How are the mitotic CDKs inactivated? Amongst many possible mechanisms, such as inhibitory phosphorylation of CDKs or binding of CDK inhibitors, mitotic CDKs can also be inactivated by destruction of the cyclin partner. This latter mechanism operates in nodules and involves the cell cycle switch gene ccs52A. Ccs52A binds and targets mitotic cyclins to the APC, resulting in their polyubiquitination and degradation (S. Tarayre, Z. Kelemen, and E. Kondorosi, unpublished data; Cebolla et al., 1999). In the absence of mitotic cyclins, the mitotic CDKs are inactive and M-phase progression as well as cell division is inhibited. If the cell cycle is otherwise active, the cells switch to endocycles entering directly G1-phase instead of M and synthesize the DNA in S-phase. If M-phase is blocked again by degradation of mitotic cyclins by APCCcs52A, the cells can enter a second or on a similar way repeated endoreduplication cycles (Fig. 2B).
The ccs52A gene is not expressed in the dividing cortical cells and in the growing nodule primordium. Ccs52A becomes activated in the fully grown primordium before differentiation and expresses in zones I and II of nitrogen-fixing nodules (Fig. 1, N3 and N4; Vinardell et al., 2003). The ccs52A mRNA and the Ccs52A protein are present in all endoreduplicating cells in zone II but absent in zone III (Cebolla et al., 1999; Vinardell et al., 2003). Inversely, ccs52B expression is present in the root and declines with the formation of nodule primordium.
As it was discussed before, phosphorylation by cyclin A-CDK inactivates the Cdh1-type APC activators. In human cells, depletion of cyclin A provoked a nonperiodic APC activity and endoreduplication cycles (Sorensen et al., 2000). It is unknown which cyclin A associated CDK regulates the activity of CCS52A in Medicago. It is tempting to speculate that it is CycA2. If this cyclin were a negative regulator of Ccs52A, its absence in nodule zone II, could lead to permanently unphosphorylated state of Ccs52A, providing constitutive APCCCS52A activity. Therefore, mitotic cyclins produced in zone II could be degraded at the instant of their production, generating multiple rounds of endocycles. This would also mean that CycA2 is dispensable for DNA replication and S-phase functions in the endoreduplicating cells, which are ensured by other A-type Medicago cyclins.
But why are the nodule cells polyploid? Will a nodule be functional without endocycles? This was tested in ccs52A antisense plants where reduction of the ccs52A transcript level did not affect the formation of nodule primordia but aborted nodule development (Vinardell et al., 2003). These nodules displayed significantly a lower degree of ploidy, and the nodule cells were smaller and poorly or not infected and contained no nitrogen-fixing cells. These results demonstrated that repeated endoreduplication cycles controlled by Ccs52A are indispensable for nitrogen-fixing nodule development (Vinardell et al., 2003). In the determinate nodules, the symbiotic cells are also big and polyploid. Ccs52A is highly conserved in legumes and present in nodules; therefore, Ccs52A likely mediates nodule ploidy in all nodule types.
CONCLUSIONS AND FUTURE DIRECTIONS
During the past few years, enormous progress on the UPS highlighted the vital importance of this regulatory mechanism that turns off protein functions in the right place and at the right moment. Most knowledge on UPS arises from cell cycle studies where ordered destruction of proteins by the APC and SCF ensures unidirectional progression of the cycle. In plants, the discovery of the APC- and SCF-controlled processes is still at the elementary stage and out of hundreds or much more potential candidates, only a few are known as APC or SCF substrates. Future studies on the identification of novel targets and the APC- and SCF-regulated pathways will likely result in significant breakthroughs in understanding plant development. Data on degradation of plant cell cycle proteins are rather limited and it is unknown how hormone-signaling pathways communicate with the cell cycle. In M. truncatula, lateral root and nodule initiation depends on auxin maxima, formed de novo in front of the xylem/protoxylem poles and associated with the induction of an auxin-responsive cell cycle gene.
Studies on nodule organogenesis have led to the identification of the plant APC activators, Ccs52A and Ccs52B, as well as to the discovery of APCCcs52A-mediated degradation of mitotic cyclins as a key regulatory mechanism inducing and driving endoreduplication cycles. Though endoreduplication is widespread in plants, until recently its mechanism and biological significance of polyploidy were poorly understood. Are the endocycles the cause or the consequence of differentiation? Several studies suggest that increased genome size may control cell size and may be required for faster cell growth and for increasing the storing capacity of cells. In addition, the multiple gene copies and the lack of chromosome condensation may enhance transcriptional and metabolic activities in polyploid cells. In the case of nodules, the polyploid genome is essential for the development of nitrogen-fixing symbiotic cells. This is likely needed for extreme cell enlargement to host a vast quantity of bacteroids as well as to modify nodule metabolism for symbiotic nitrogen fixation. In the coming years, the genome sequence of M. truncatula, transcriptome, proteome, and metabolome analyses of polyploid cells are expected to shed light on the physiological roles of endocycles.
This work was supported by the Spanish Ministerio de Educación y Ciencia Progam Becas Postdoctorales en España y en el extranjero 2003 (to M.R.-N.).
References
- Bauer P, Ratet P, Crespi MD, Schultze M, Kondorosi A (1996) Nod factors and cytokinins induce similar cortical cell division, amyloplast deposition and MsEnod12A expression patterns in alfalfa roots. Plant J 10: 91–105 [Google Scholar]
- Capron A, Serralbo O, Fulop K, Frugier F, Parmentier Y, Dong A, Lecureuil A, Guerche P, Kondorosi E, Scheres B, et al (2003) The Arabidopsis anaphase-promoting complex or cyclosome: molecular and genetic characterization of the APC2 subunit. Plant Cell 15: 2370–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Cebolla A, Vinardell J, Kiss E, Oláh B, Roudier F, Kondorosi A, Kondorosi E (1999) The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J 18: 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A, Orian A, Schwartz AL (2000) Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22: 442–451 [DOI] [PubMed] [Google Scholar]
- de Billy F, Grosjean C, May S, Bennett M, Cullimore JV (2001) Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol Plant Microbe Interact 14: 267–277 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JAH (2003) The plant cell cycle. Annu Rev Plant Biol 54: 235–264 [DOI] [PubMed] [Google Scholar]
- Dill A, Thomas SG, Hu J, Steber CM, Sun TP (2004) The Arabidopsis F-box protein SLEEPY1 targets gibberellin signalling repressors for gibberellin-induced degradation. Plant Cell 16: 1392–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraioli S, Tate R, Rogato A, Chiurazzi M, Patriarca EJ (2004) Development of ectopic roots from abortive nodule primordia. Mol Plant Microbe Interact 17: 1043–1050 [DOI] [PubMed] [Google Scholar]
- Foucher F, Kondorosi E (2000) Cell cycle regulation in the course of nodule organogenesis in Medicago. Plant Mol Biol 43: 773–786 [DOI] [PubMed] [Google Scholar]
- Fu X, Richards DE, Fleck B, Xie D, Burton N, Harberd NP (2004) The Arabidopsis mutant sleepy1gar2-1 protein promotes plant growth by increasing the affinity of the SCFSLY1 E3 ubiquitin ligase for DELLA protein substrates. Plant Cell 16: 1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M (1999) Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13: 1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Harper JW, Burton JL, Solomon MJ (2002) The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev 16: 2179–2206 [DOI] [PubMed] [Google Scholar]
- Heidstra R, Yang WC, Yalcin Y, Peck S, Emons AM, van Kammen A, Bisseling T (1997) Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 124: 1781–1787 [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Bhuvaneswari JG, Torrey JG, Bisseling T (1989) Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci USA 86: 1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono A, Umeda-Hara C, Lee J, Ito M, Uchimiya H, Umeda M (2003) Arabidopsis D-type cyclin CYCD4;1 is a novel cyclin partner of B2-type cyclin-dependent kinase. Plant Physiol 132: 1315–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM (2004) PIN and AUX/LAX proteins: their role in auxin accumulation. Trends Plant Sci 9: 578–582 [DOI] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM (2004) Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. Plant J 38: 203–214 [DOI] [PubMed] [Google Scholar]
- Magyar Z, Mészaros T, Miskolczi P, Deak M, Fehér A, Brown S, Kondorosi E, Athanasiadis A, Pongor S, Bilgin M, et al (1997) Cell cycle phase specificity of novel cyclin-dependent kinase variants in synchronized alfalfa cells. Plant Cell 9: 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14: 23–34 [DOI] [PubMed] [Google Scholar]
- Menges M, de Jager SM, Gruissem W, Murray JA (2005) Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and high specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J 41: 546–566 [DOI] [PubMed] [Google Scholar]
- Pacios-Bras C, Schlaman HRM, Boot K, Admiraal P, Langerak JM, Stougaard J, Spaink HP (2003) Auxin distribution in Lotus japonicus during root nodule development. Plant Mol Biol 52: 1169–1180 [DOI] [PubMed] [Google Scholar]
- Peters JM (2002) The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell 9: 931–943 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P (2003) EIN3-dependent regulation of plant ethylene hormone signalling by two Arabidopsis F-box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy JM, Murray JA (2000) Sugar control of the plant cell cycle: differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol 20: 4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier F, Fedorova E, Györgyey J, Fehér A, Brown S, Kondorosi A, Kondorosi E (2000) Cell cycle function of a Medicago sativa A2-type cyclin interacting with a PSTAIRE-type cyclin-dependent kinase and a retinoblastoma protein. Plant J 23: 73–83 [DOI] [PubMed] [Google Scholar]
- Roudier F, Fedorova E, Lebris M, Lecomte P, Györgyey J, Vaubert D, Horvath G, Abad P, Kondorosi A, Kondorosi E (2003) The Medicago species A2-type cyclin is auxin regulated and involved in meristem formation but dispensable for endoreduplication-associated developmental programs. Plant Physiol 131: 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savouré A, Magyar Z, Pierre M, Brown S, Schultze M, Dudits D, Kondorosi A, Kondorosi E (1994) Activation of the cell cycle machinery and the isoflavonoid biosynthesis pathway by active Rhizobium meliloti Nod signal molecules in Medicago microcallus suspensions. EMBO J 13: 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Villalobos LI (2004) Cullin-containing E3 ubiquitin ligases in plant development. Curr Opin Plant Biol 7: 677–686 [DOI] [PubMed] [Google Scholar]
- Sorensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J (2000) Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol Cell Biol 20: 7613–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarayre S, Vinardell JM, Cebolla A, Kondorosi A, Kondorosi E (2004) Two classes of the Cdh1-type activators of the anaphase-promoting complex in plants: novel functional domains and distinct regulation. Plant Cell 16: 422–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D (2002) Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14: 903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G (1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172: 4295–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodermaier HC (2004) APC/C and SCF: controlling each other and the cell cycle. Curr Biol 14: R787–R796 [DOI] [PubMed] [Google Scholar]
- Vierstra RD (2003) The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci 8: 135–142 [DOI] [PubMed] [Google Scholar]
- Vinardell JM, Fedorova E, Cebolla A, Kevei Z, Horvath G, Kelemen Z, Tarayre S, Roudier F, Mergaert P, Kondorosi A, et al (2003) Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15: 2093–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Kong H, Sun Y, Zhang X, Zhang W, Altman N, DePamphilis CW, Ma H (2004) Genome-wide analysis of the cyclin family in Arabidopsis and comparative phylogenetic analysis of plant cyclin-like proteins. Plant Physiol 135: 1084–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WC, de Blank C, Meskiene I, Hirt H, Bakker J, van Kammen A, Franssen H, Bisseling T (1994) Rhizobium Nod factors reactivate the cell cycle during infection and nodule primordium formation, but the cycle is only completed in primordium formation. Plant Cell 6: 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]