Abstract
Objectives
To identify with children, parents and physicians the objectives to be used as parameters for algorithmic decision-making systems (ADMSs) adapting treatments in childhood asthma.
Methods
We first conducted a qualitative study based on semi-structured interviews to explore the objectives that children aged 8–17 years, their parents, and their physicians seek to achieve when taking/giving/prescribing a treatment for asthma. Following the grounded theory approach, each interview was independently coded by two researchers; reconciled codes were used to assess code frequency, categories were defined, and the main objectives identified. We then conducted a quantitative study based on questionnaires using these objectives to determine how children/parents/physicians ranked these objectives and whether their responses were aligned.
Results
We interviewed 71 participants (31 children, 30 parents and 10 physicians) in the qualitative study and identified seven objectives associated with treatment uptake and five objectives associated with treatment modalities. We included 291 participants (137 children, 137 parents, and 17 physicians) in the quantitative study. We found little correlation between child, parent, and physician scores for each of the objectives. Each child's asthma history influenced the choice of scores assigned to each objective by the child, parents, and physician.
Conclusion
The identified objectives are quantifiable and relevant to the management of asthma in the short and long term. They can therefore be incorporated as parameters for future ADMS. Shared decision-making seems essential to achieve consensus among children, parents, and physicians when choosing the weight to assign to each of these objectives.
Keywords: Decision-making: computer-assisted, algorithms, asthma/drug therapy, child, shared decision-making
Introduction
Children with chronic respiratory diseases such as asthma experience periods of disease symptoms interspersed with periods of quiescence, posing daily management challenges. Instead of medical consultations every 3–6 months, they would ideally benefit from having assistance to manage their disease in real time. Such care was not accessible until the digital revolution and the emergence of systems that combine continuous data collection and algorithmic decision-making systems (ADMSs). For instance, in cardiology, children at risk of heart rhythm disorders have access to implantable cardioverters-defibrillators that continuously analyze their heart rhythm and automatically administer an electric shock in case of anomalies. 1 In type 1 diabetes, artificial pancreas systems combine continuous glucose monitoring and ADMS to adjust insulin doses in real time. 2 In both cases, this has significantly improved the lives of these children1–4.
Such an approach would be particularly interesting in childhood asthma, the most common chronic disease affecting 10–20% of children in high-income countries.5,6 These children experience episodes of coughing, wheezing, and breathlessness, especially during exercise or at night, which can escalate into asthma attacks. 7 Despite current management, two-thirds of children still experience symptoms during sports, and one in eight has had an emergency department visit in the last 12 months. 8 Managing these children through a system that combines continuous data collection and ADMS for real-time treatment adaptation could change the daily lives of these children. 9 In a survey conducted in France, 93% of children and 69% of parents were willing to use such a system if it could reduce the risk of asthma attacks by 30%. 10
Regarding continuous data collection for childhood asthma, an increasing number of tools are available. For example, the use of smartwatches can record parameters associated with poor asthma control, such as activity, heart rate, and saturation. 11 Smartinhalers record the real-time use of the reliever treatment used to treat asthma symptoms and adherence to the controller treatment taken daily to prevent asthma symptoms and attacks. 12 Additionally, environmental databases can collect information on potential triggers of asthma attacks such as weather conditions, pollutants, and pollens. 9
The development of ADMS is more complex. Unlike type 1 diabetes in children, where normalization of a single parameter (blood glucose) by a single treatment (insulin) can prevent all the child's symptoms and long-term comorbidities, in asthma there are multiple pathophysiological pathways and different treatments with different impacts on symptoms, risk of asthma attack, long-term lung function, 7 and other objectives that may have not been identified to date. Therefore, it is important to define the objectives one is trying to achieve when letting an ADMS make the treatment choice. These objectives must be defined with the children, who bear the burden of their disease every day, but also the burden of their care including their daily treatment; with their parents, who are their legal representatives; and with their physicians, who have the medical knowledge and the long-term vision of care.
In this study, we aimed to assess the objectives of children with asthma, their parents, and their physicians when taking, giving, or prescribing treatment for asthma, so that future ADMS could incorporate these objectives. Our secondary objective was to determine whether children's, parents’, and physicians’ objectives were broadly aligned across a triad or, alternatively, quite different.
Methods
We conducted this study in two phases: first, we conducted a qualitative study to explore the objectives that children/parents/physicians seek to achieve when taking/giving/prescribing a daily controller treatment for asthma. Next, we conducted a quantitative study on a larger population to determine what the priorities of children/parents/physicians were based on the identified objectives and if these objectives were aligned across a triad (child/parent/physician). The study was approved by the institutional review board CPP Ile de France 2 (n° 21.00315.000005 RIPH 3HPS), and registered in ClinicalTrials.gov (NCT05064579). This research was considered by the institutional review board as non-interventional research. This research was considered by the institutional review board as non-interventional research. In this context, in accordance with the Jardé law in France, children and their legally authorized representatives provided oral consent and we recorded their non-objection to participate in this study in their health records. 13
Population
In both parts of this study, we included children aged 8 to 17 years with a physician diagnosis of asthma who were prescribed a controller treatment for their asthma, as well as their parents and physicians. Inability to write or speak French was the only exclusion criterion. All participants were informed that the aim of the study was to define the objectives sought by families (children with asthma and their parents) when using daily asthma treatment, as well as the objectives of doctors prescribing asthma treatment, in order to program ADMS in the future.
Identification of the objectives: qualitative study
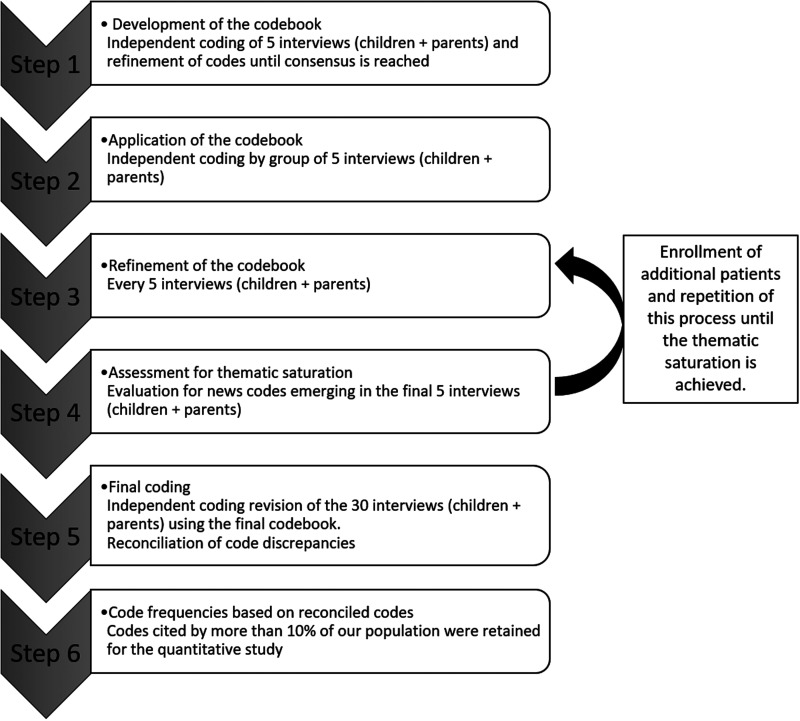
The qualitative study was based on semi-structured interviews conducted from October 2020 to January 2021, in the University Hospital Necker-Enfants Malades in Paris, France. The five-step process developed by Kallio et al. was used to develop the semi-structured interview guide (Supplemental Material S1). 14 All semi-structured interviews were conducted by the same author (J.P.). A grounded theory approach was used to develop the codebook (Figure 1) presented in Supplemental Material S2.
Figure 1.
Development of the codebook.
Two of the authors (J.P. and D.D.) independently coded the first five interviews from children and their parents, and codes were refined until consensus was reached. Then, J.P. and D.D. independently coded the subsequent interviews by a group of five children/parents/physicians, refining novel codes until consensus was reached. No new code emerged in the interviews n°25 to n°30 in all groups, achieving thematic saturation. Code frequencies were calculated based on reconciled codes.
In parallel, an automated textual analysis of the responses from all participants was also performed using Reinert's automatic textual analysis method by the software IRaMuTeQ 0.6 alpha 3. 15 This method corresponds to a statistical analysis of the vocabulary distribution in a text and involves four steps: the identification of lexical forms (nouns, adverbs, adjectives, verbs) contained in the text, the fragmentation of the text in units of 10 to 15 words delimited by punctuation, the creation of a two-dimensional table of lexical forms and units, and the analysis of this table using hierarchical cluster analysis leading to different categories of words frequently associated together.
At the end of this qualitative study, the codes corresponding to child/parent/physician objectives that were cited by more than 10% of these populations were retained for the quantitative study.
Concordance of objectives between children/parents/physicians: quantitative study
Based on the objectives identified by the qualitative study, we developed a constant-sum questionnaire to assess the priorities of children/parents/physicians when taking/giving/prescribing a controller treatment (Supplemental Material S3). We recruited participants from five pediatrician or pediatric pulmonology departments in four hospitals in the Ile de France region (Paris and suburbs) between January 2022 and January 2023.
Children, parents, and physicians were given 20 points to distribute between the different “objectives” presented on the paper questionnaire, which was composed of two parts: one part on objectives related to taking the treatment (why would I want to take the treatment?) and one part on objectives related to the treatment modality (how would I want to take the treatment?). The objectives were ranked according to their importance for each population category. Participants were also asked to answer questions about potential explanatory variables for their preference for each objective.
Our primary outcome was the median score assigned to each objective for each population. Our secondary outcome was the correlation of scores between children, parents, and physicians for each objective.
Statistical analysis
We reported categorical variables as proportions and percentages, and continuous variables as medians and interquartile ranges. We compared children's, parents’, and physicians’ scores for each objective by a Kruskall–Wallis test, with a Bonferroni post hoc test if statistically significant to compare pairs of scores (children/parents, parents/physicians, children/physicians). We assessed the correlation between the scores assigned by each child/parent/physician triad, overall and for each objective, by the Spearman coefficient. If a questionnaire was invalid or missing within a triad, we still assessed the correlation between the two remaining questionnaires. We used multiple linear regression to identify factors associated with the scores assigned to each objective by children and parents. These potential explanatory variables included factors related to the child's asthma (duration of asthma, severity of asthma according to the GINA classification, asthma control according to the Asthma Control Test (ACT) for 12–17 year olds and the Childhood ACT (c-ACT) for 8–11 year olds, history of asthma attack, hospital admission, abnormal lung function test, medication-related side effect); for children, we added their age and sex, and for parents, their age, sex, and education level. Unlike children and parents, who each completed a questionnaire, physicians completed questionnaires for multiple children. Therefore, we used mixed models with the physician as a random variable to identify factors associated with their scores for each objective, including described factors related to the child's asthma, along with the physician's sex, pediatric pulmonary experience, number of asthma patients seen per month, and length of follow-up of the child, i.e. how long the physician has been caring for each child patient. Analyses were performed with R software (4.1.2).
Results
Identification of the objectives: qualitative study
After being informed about the study, five parent–child dyads refused to participate. Thirty-one children, their 30 parents, and 10 physicians were interviewed, for a total of 71 participants. The characteristics of the children are presented in Supplemental Material S4.
Manual qualitative content analysis and automatic textual analysis provided consistent results and allowed us to classify objectives into four categories: (a) to reduce the burden of asthma on health, (b) to reduce the burden of asthma on quality of life, (c) to decrease asthma-related treatments, (d) to optimize asthma-related treatments. Codes related to the first two categories, i.e. the burden of asthma are presented in Supplemental Material S5. Codes related to the last two categories, i.e. asthma treatments are presented in Supplemental Material S6.
Manual content qualitative analysis
In total, there were 662 text units coded among 530 different codes. The inter-rater reliability between the two coders (J.P. and D.D.) was 90.5%. The mean number (SD) of different codes per interview was 7.5 (2.8). It was 6.3 (2.6) for children, 8.7 (3) for parents, and 7.5 (1.4) for doctors.
The first category of objectives was to reduce the burden of asthma on health. Beside the ideal objective of “being cured of asthma” forever, reported by more than 40% of children and their parents, most participants reported that their objectives were to control asthma symptoms, prevent asthma exacerbations, and/or preserve lung function. Regarding the control of asthma symptoms, the most-cited item by children and parents was exercise-related symptoms (90% and 70%, respectively), whereas the prevention of night-time symptoms was mostly cited by doctors (60%). The preservation of lung function was an objective that could be identified from the interviews of eight doctors (80%) and one parent. No child raised this objective.
The second category of objectives was to reduce the burden of asthma on daily life. Participants considered asthma as a limitation to perform several activities, regarding social life (sleeping in a friend's house), sports and leisure (swimming pool, scuba diving), and future jobs (baker). Restrictions in the possibility to adopt a pet were also raised by 7 (23%) children. Reducing school absenteeism, face-to-face follow-up consultations, and active monitoring of the environment (i.e. looking for situations at risk of asthma attacks) were objectives mainly reported by parents, whereas improving the child's autonomy and quality of life were reported mainly by doctors.
Regarding the psychological impact of asthma, several children and their parents were anxious about asthma attacks, fearing the occurrence of an asthma attack, the symptoms themselves, and/or their ability to manage it properly. One child and one parent were concerned about what others think of the child's asthma. Improving the understanding of the pathology was a mean to better manage asthma for six participants including four parents.
The third category of objectives was to reduce asthma-related treatments. Decreasing asthma treatments was frequently reported, mainly by parents (73%). Besides the currently unrealistic objective to definitively stop all treatments reported by 35% of children and 60% of parents, the expectations identified were to reduce the frequency of treatments, to switch for non-daily treatments, to use combined treatments, and for those on biologics, to receive less injections. The idea behind the reduction of asthma treatments was also to decrease the risk of side effects, expressed more by doctors (40%) rather than parents (13%) and children (6%).
The fourth category of objectives was to optimize asthma-related treatments. The main objectives of parents, followed by their children, were to be provided treatments easier and faster to take, without the need for parental supervision. Five parents (17%) identified reminders and automated feedback on the inhalation technique as desirable characteristics for their children's treatments. Two parents (7%) suggested a treatment to be taken passively, such as a patch.
Automated textual analysis
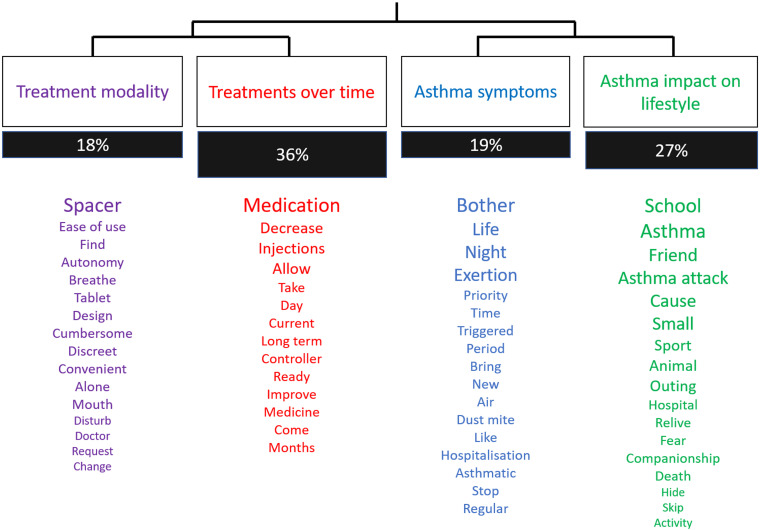
After descendant hierarchical classification, we obtained four classes (Figure 2).
Figure 2.
Cluster of words obtained from the automated textual analysis. Words association automatically identified by the Software IRaMuTeQ. The size of each word is proportionate to the frequency of its use during the interviews.
These classes confirmed the orientations chosen during the manual analysis. Two of these classes (groups 1 and 2) corresponded to the optimization of treatment modalities (spacer, ease of use, galenic form) and expectations regarding asthma treatments over time (decrease, allow, take, improve, long term, day, months), These two classes represented 18% and 36% of the corpus, respectively. The other two classes (groups 3 and 4) were related to asthma symptoms (bother, night, exertion) and the consequences of asthma on lifestyle (school, friend, asthma attack, sport, animal), for 19% and 27% of the corpus, respectively.
Development of a list of objectives to be used in algorithmic decision-making systems for childhood asthma
Seventeen codes were cited by more than 10% of our overall population. We retained 13 codes that can be used as a list of objectives when setting up ADMSs for childhood asthma. Four others were excluded because considered unrealistic (“be cured of asthma”), potentially dangerous (“definitely stop asthma treatments”), too vague (“reduce symptoms – unspecified” and “Decrease treatments – unspecified”), or not concerning all families (“limitation of domestic animals”). The objectives retained were integrated in the questionnaire developed for the quantitative study (Supplemental Material S3).
Concordance of objectives between children/parents/physicians: quantitative study
For this part of the study, we recruited a total of 291 participants: 137 children, their 137 parents, and their 17 physicians. The characteristics of the participants are presented in Table 1.
Table 1.
Participant characteristics.
| Characteristics | Total |
|---|---|
| Children | n = 137 |
| Age (years), median (IQR) | 12.0 (10.0–14.0) |
| ▪ 8–11 years old, n (%) | 56 (41) |
| ▪ 12–17 years old, n (%) | 81 (59) |
| Sex, n (%) | |
| ▪ Male | 80 (59) |
| ▪ Female | 57 (41) |
| Asthma severity – GINA, n (%) | |
| ▪ Step 2 | 2 (2) |
| ▪ Step 3 | 44 (32) |
| ▪ Step 4 | 65 (47) |
| ▪ Step 5 | 26 (19) |
| Age of onset of asthma (years), median (IQR) | 2 (1–5) |
| Asthma attack in the last 12 months, n (%) | |
| ▪ 0 | 41 (34) |
| ▪ 1 | 23 (19) |
| ▪ 2 | 15 (12) |
| ▪ 3 | 15 (12) |
| ▪ 4 and more | 27 (23) |
| Emergency department visit or hospital admission in the last 12 months, n (%) | 38 (29) |
| History of abnormal lung function tests, n (%) | 56 (45) |
| Frequency of administration of asthma treatments, n (%) | |
| ▪ Once a day | 26 (19) |
| ▪ Twice a day | 111 (81) |
| Treatment type, n (%) | |
| ▪ ICS + LABA | 102 (74) |
| ▪ ICS | 15 (11) |
| ▪ ICS + LABA + LTRA | 11 (8) |
| ▪ ICS + LABA + Biologics | 5 (4) |
| ▪ ICS + Biologics | 2 (1.5) |
| ▪ ICS + LTRA + Biologics | 1 (0.75) |
| ▪ LTRA + Biologics | 1 (0.75) |
| History of treatment side effect, n (%) | 24 (18) |
| Symptom control score, median (IQR) | |
| ▪ ACT score (12–17 years old) | 20.5 (18.2–23.8) |
| ▪ cACT score (8–11 years old) | 21.0 (18.0–24.0) |
| Parents | n = 137 |
| Age (years) – median (IQR) | 43 (39–50) |
| Sex, n (%) | |
| ▪ Male | 23(17) |
| ▪ Female | 111(83) |
| Level of educationa – n (%) | |
| • Middle school | 29 (22) |
| • High school | 30 (23) |
| • Graduate or undergraduate degree | 73 (55) |
| Physicians | n = 17 |
| Sex – n (%) | |
| ▪ Male | 6 (35) |
| ▪ Female | 11 (65) |
| Experience in pediatric pulmonology (years) – median (IQR) | 7 (3.0–13.5) |
| Number of asthma patients seen by month – median (IQR) | 30 (22–48) |
ACT: asthma control test; c-ACT: childhood asthma control test; GINA: Global initiative for asthma classification; ICS: inhaled corticosteroids; IQR: interquartile range; LTRA: Leukotriene receptor antagonist; LABA: long-acting beta agonist: LAMA: long-acting muscarinic antagonist. a5 missing values
Most children had moderate (79%) or severe (19%) asthma according to GINA guidelines. This was reflected in their morbidity, with 66% having had at least one asthma attack and 29% having had an emergency room visit or hospital admission in the past 12 months, and 47% having a history of abnormal lung function tests.
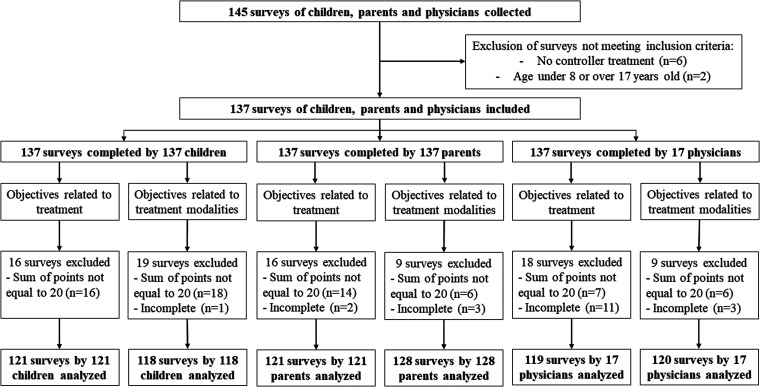
Among the collected questionnaires, the sum of the scores for the objectives of the treatment (why would I want to take the treatment?) was incorrect (total unequal to 20) for 16, 14, and 7 questionnaires completed by children, their parents, and their physicians, respectively (Figure 3). Adding the removal of incomplete questionnaires, we were able to analyze 121, 121, and 101 questionnaires completed by children, their parents, and their physicians for these objectives, respectively. For the objectives related to treatment modalities (how would I like to take the treatment), we were able to analyze 118, 128, and 120 questionnaires completed by children, parents, and physicians, respectively.
Figure 3.
Flow chart of the surveys analyzed in the quantitative study.
When asked about their objectives when taking/giving/prescribing a controller treatment for asthma, children scored highest for prevention of symptoms during exercise, parents for preservation of lung function, and physicians for prevention of asthma attacks (Table 2).
Table 2.
Scores assigned to each treatment objective by children, parents, and physicians.
| Objective | Children (121 questionnaires) | Parents (121 questionnaires) | Physicians (119 questionnaires) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rank | Median (IQR) [Range] | Mean (SD) | Rank | Median (IQR) [Range] | Mean (SD) | Rank | Median (IQR) [Range] | Mean (SD) | |
| Prevent symptoms during exercise | 1 | 4 (2–6) [0–20] | 4.25 (3.17) | 3 | 3 (1–4) [0–10] | 2.92 (2.30) | 3 | 3 (2–5) [0–15] | 3.75 (2.68) |
| Prevent night-time symptoms | 5 | 2 (0–4) [0–9] | 2.23 (2.01) | 7 | 2 (0–3) [0–10] | 2.36 (2.11) | 6 | 2 (0.5–3) [0–8] | 1.87 (1.59) |
| Prevent reliever treatment use | 6 | 2 (0–3) [0–10] | 2.24 (2.13) | 6 | 2 (1–3) [0–10] | 2.39 (2.24) | 5 | 2 (1–4) [0–10] | 2.50 (1.78) |
| Prevent asthma attacks | 2 | 3 (2–5) [0–15] | 3.51 (2.74) | 2 | 3 (1–5) [0–11] | 3.13 (2.48) | 1 | 4 (3–5) [0–12] | 4.09 (2.15) |
| Prevent hospital admissions | 4 | 2 (1–4) [0–15] | 2.88 (2.77) | 4 | 2 (1–4) [0–10] | 2.50 (1.99) | 2 | 4 (2–5) [0–10] | 3.43 (2.05) |
| Preserve lung function | 3 | 3 (1–5) [0–14] | 3.21 (2.51) | 1 | 4 (2–6) [0–15] | 4.26 (3.12) | 4 | 3 (2–4) [0–10] | 3.07 (2.18) |
| Prevent treatment side effects | 7 | 1 (0–3) [0–6] | 1.67 (1.63) | 5 | 2 (1–3) [0–10] | 2.45 (2.31) | 7 | 1 (0–2) [0–5] | 1.29 (1.32) |
IQR: interquartile range.
Overall, prevention of symptoms during exercise and prevention of asthma attacks were in the top three priorities for all categories of participants. Similarly, prevention of night-time symptoms, reliever treatment use, and treatment side effects were the lowest priorities of all categories.
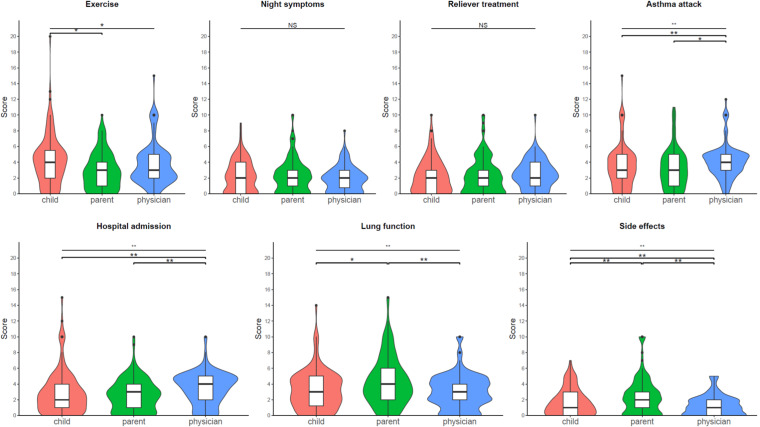
We then compared the scores assigned by each population to each objective (Figure 4). Scores on prevention of night-time symptoms and use of reliever treatments were not different between the three groups. Children scored higher than their parents on preventing symptoms during exercise, parents scored higher than children and physicians on preserving lung function and preventing side effects, and physicians scored higher than children and parents on preventing asthma attacks, emergency department visits, and hospital admissions (Figure 4).
Figure 4.
Comparison of scores assigned to each treatment objective by children, parents, and physicians. Violins plots are presented: the length of the colored area illustrates the distribution of scores, with wider sections indicating a greater proportion of participants giving that score. In each violin diagram, the box plot with median and interquartile values is presented. The child's responses are shown in red, those of the parents in green and those of the doctor in blue. NS: not statistically significant; *p < .05; **p < .01.
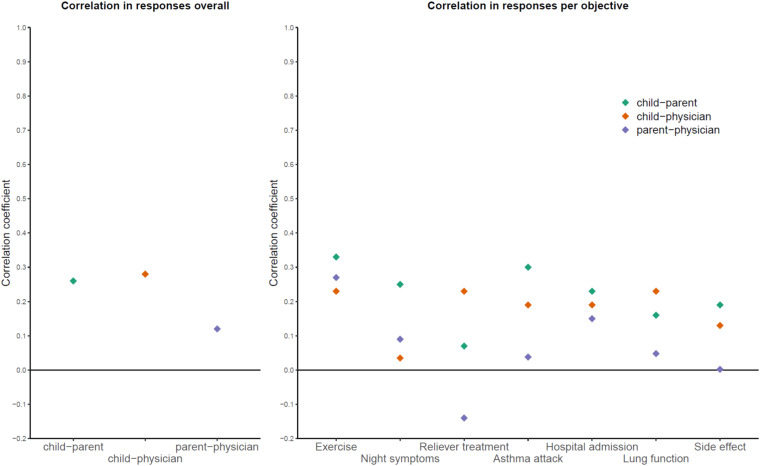
Within each child–parent–physician triad, we examined the correlation of scores assigned by each participant (Table 3 and Figure 5). Overall, the Spearman rank correlation coefficients (95% CI) were 0.26 (0.19–0.32), 0.28 (0.21–0.35), and 0.12 (0.05–0.19) for scores assigned by child–parent, child–physician, and parent–physician pairs, respectively. Thus, the correlation between the scores assigned by each population was weak. When assessed for each objective, the correlation coefficients ranged from −0.14 to 0.33, again indicating negligible or weak correlation (Table 3).
Table 3.
Correlations of scores assigned to each objective by children, their parents, and their physicians.
| Objective | Children–parents | Children–physicians | Parents–physicians | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Correlation coefficient | Lower 95% CI | Upper 95% CI | Correlation coefficient | Lower 95% CI | Upper 95% CI | Correlation coefficient | Lower 95% CI | Upper 95% CI | |
| Prevent symptoms during exercise | 0.33 | 0.15 | 0.49 | 0.23 | 0.04 | 0.40 | 0.27 | 0.08 | 0.44 |
| Prevent night-time symptoms | 0.25 | 0.06 | 0.42 | 0.04 | −0.16 | 0.23 | 0.09 | −0.10 | 0.28 |
| Prevent reliever treatment use | 0.07 | −0.12 | 0.25 | 0.23 | 0.04 | 0.41 | −0.14 | −0.32 | 0.05 |
| Prevent asthma attacks | 0.30 | 0.12 | 0.46 | 0.19 | 0.00 | 0.37 | 0.04 | −0.15 | 0.23 |
| Prevent hospital admissions | 0.23 | 0.04 | 0.40 | 0.19 | −0.01 | 0.36 | 0.15 | −0.05 | 0.33 |
| Preserve lung function | 0.16 | −0.03 | 0.34 | 0.23 | 0.04 | 0.40 | 0.05 | −0.14 | 0.24 |
| Prevent treatment side effects | 0.19 | 0.00 | 0.37 | 0.13 | −0.06 | 0.31 | 0.00 | −0.19 | 0.19 |
Figure 5.
Correlations of scores assigned overall and to each objective by children, their parents, and their physicians.
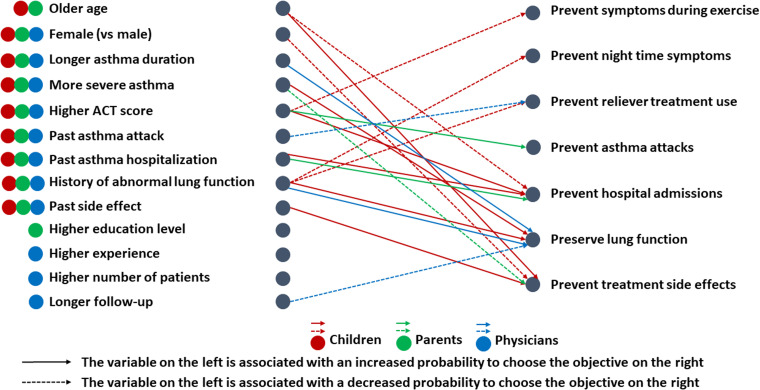
Finally, we examined the factors associated with the scores assigned to each objective (Figure 6 and Supplemental Material S7–S9). For children, being older was associated with a lower score for the objective “prevent asthma hospitalizations” and a higher score for the objective “prevent treatment side effects”; being female was associated with a lower score for the objective “prevent treatment side effects”; having moderate to severe asthma (GINA step 4) was associated with a higher score for the objective “preserve lung function” compared with moderate asthma (GINA step 3); better asthma control (higher ACT/c-ACT score) was associated with a lower score for “prevent of asthma symptoms during exercise” and a higher score for “prevent asthma hospitalizations”; a history of hospitalization was associated with a higher score for “prevent asthma hospitalizations” ; a history of lung function abnormalities was associated with a higher score for “preserve lung function” and a lower score for “prevent night time symptoms”; finally, a history of side effects was associated with a higher score for “prevent side effects of treatment”. For parents, better asthma control in their child was associated with a higher score for “prevent asthma attacks”; a history of hospitalization was associated with a higher score for “prevent asthma hospitalizations”; and higher asthma severity was associated with a lower score for “prevent treatment side effects.” For physicians, a history of asthma attacks was associated with a lower score for “prevent reliever treatment use” item; longer duration of asthma and a history of abnormal lung function were associated with higher scores on the “preserve lung function” item, whereas follow-up between 1 and 5 years was associated with a lower score on the same item compared with shorter follow-up.
Figure 6.
Factors associated with the choice of each objective for children (in red), parents (in green), and physicians (in blue). ACT: asthma control test.
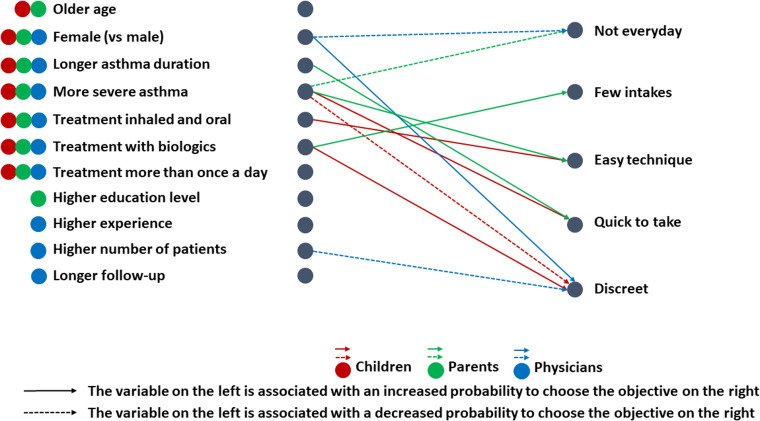
We repeated the same steps to analyze the treatment modality objectives, including describing the rank of each objective by group, comparing the scores of each objective across groups, examining correlation coefficients between groups overall and for each objective, and examining factors associated with each group's scores (Figure 7 and Supplemental Material S10–S14). Briefly, the “few intakes” and “easy technique” items had the highest scores, whereas the “quick to take” and “discreet” items had the lowest scores. Physicians scored higher on the “quick to take” item than did parents and children, whereas children scored higher on the “not every day” item than did physicians. Once again, the correlation between the scores assigned within each triad was low, with correlation coefficients less than 0.30 overall.
Figure 7.
Factors associated with the choice of each treatment modality objective for children (in red), parents (in green), and physicians (in blue).
Discussion
To our knowledge, this study is the first to identify quantifiable objectives with children, parents, and physicians that could be incorporated into ADMS to adapt the controller treatment of children with asthma. We identified seven objectives associated with treatment uptake and five objectives associated with treatment modalities. We then showed that there was little or no correlation between child, parent, and physician scores for each of the objectives. Finally, we found that each child's asthma history influenced the choice of scores assigned to each objective by the child, parents, and physician.
Unlike diabetes, where the focus is often on the objective of normalizing blood sugar levels because of its effectiveness in preventing symptoms and preventing short- and long-term consequences, asthma presents a more complex scenario with multiple potential objectives that may conflict with one another. Indeed, in asthma, it is important to avoid symptoms and asthma attacks, to preserve long-term lung function and to avoid treatment side effects, as recommended by the international experts from the Global Initiative for Asthma. 7 Thus, outcomes used in research studies evaluating a treatment for asthma include symptom scores, data on healthcare utilization, measures of lung function, and monitoring of potential side effects. 16 The objectives reported by children, parents, and physicians in the qualitative part of this study reflect the same concerns, and future ADMS should therefore incorporate these different objectives.
We showed that the age, sex of the child, and the history of asthma changed the perception of the objectives by children, their parents and their physicians. For example, a history of hospitalization, abnormal lung function, or side effects were associated with an increase in the score attributed to the prevention of these situations in children. This highlights the need for a personalized approach to asthma management, taking into account the history of patients and their families. The use of ADMS allowing a choice between different objectives in asthma management would support this personalized management by requiring children, parents, and doctors to think about and discuss together the most appropriate objectives. However, the lack of alignment in priorities between children, parents, and physicians found in our results indicates that reaching consensus on objectives for ADMS will be difficult. This underlines the need for explicitly facilitated shared decision-making during medical consultations to reach consensus. In practice, the child, parent, and physician would need to openly discuss each of their priorities and perspectives at length. Physicians should explain the importance of preserving lung function, which is a long-term goal in the management of asthma that families may be less familiar with. Through extensive dialogue, they can work through areas of discordance to eventually align on a customized set of priorities for that individual child. This set of priorities will need to be re-assessed regularly, as children's priorities may evolve as they mature and gain greater understanding of their disease.
We would like to stress the importance of taking the child's point of view into account during these discussions. The participation of the child in decisions that concern them, including in relation to the digital environment, is a right recalled by the United Nations Convention on the Rights of the Child in 2021. 17 This study found low correlation between children's priorities and those of their parents/physicians: incorporating the child's point of view helps ensure the objectives reflect the needs and preferences of the patient living day-to-day with asthma. Furthermore, research indicates that when pediatric patients feel involved in medical decisions, they demonstrate better medication adherence, quality of life, and health outcomes18–21. Finally, participation in medical decisions helps children develop a sense of autonomy and self-management skills, which will be useful as they take on more and more responsibility for managing their asthma. 22
The strength of this study is that it combined a qualitative approach to identify the objectives and a quantitative approach to a large sample of patients to anticipate how these objectives might be used in future ADMS. The main limitation of this study is that it was conducted in hospital settings in the region of Paris. The objectives identified may not reflect those from other settings, such as in private practice and in other regions or countries. Mothers from different ethnic backgrounds have been shown to have different attitudes to the use of daily inhaled corticosteroids, with some groups rigorously administering them to their children, while others were more reluctant to use them. 23 This could have an impact on their view of the objectives of their child's asthma management. In addition, the study focused on children aged between 8 and 17, and the conclusions cannot be extended to younger children. Finally, the quantitative survey developed was not a validated tool. It was also difficult to complete for some participants. However, we mitigated this last risk by providing an example of a constant-sum questionnaire for each category of participant, and we adapted the constant-sum questionnaire for children by using bags into which they had to put balls (and thus not have to calculate) (supplemental material 3).
Conclusion
This study identified seven objectives related to treatment intake and five objectives related to treatment modalities. These objectives are quantifiable and relevant to the management of asthma in the short and long term. They can therefore be incorporated as parameters for future ADMS. To reach a consensus on the weight to be given to each of the objectives between the preferences of the child, the parent and the physician, shared decision-making currently remains the best option, preserving a child-centered care.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076241227285 for Objectives for algorithmic decision-making systems in childhood asthma: Perspectives of children, parents, and physicians by Omar Masrour, Johan Personnic, Flore Amat, Rola Abou Taam, Blandine Prevost, Guillaume Lezmi, Apolline Gonsard, Nadia Nathan, Alexandra Pirojoc, Christophe Delacourt, Stéphanie Wanin and David Drummond in DIGITAL HEALTH
Acknowledgements
The authors thank the children, parents, and physicians who accepted to participate to the interviews, and Dr M. Le Bourgeois, Dr R. Abou Taam, Dr J. de Blic, Dr L. Giovannini-Chami, Dr G. Lezmi, and Ms. Z. Cintrat for their help in reviewing the semi-structured interview guide.
Footnotes
Contributorship: DD and JP contributed to the study conception and design. DD, JP, and OM analyzed the results and wrote the first draft of the manuscript. All authors participated in data collection, commented on previous versions of the manuscript, and read and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study was approved by the institutional review board CPP Ile de France 2 (n° 21.00315.000005 RIPH 3HPS)
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Guarantor: David Drummond takes full responsibility for the content of this article.
Informed consent: This research was considered by the institutional review board as non-interventional research. In this context, in accordance with the Jardé law in France, children and their legally authorized representatives provided oral consent and we recorded their non-objection to participate in this study in their health record.
ORCID iD: David Drummond https://orcid.org/0000-0002-7324-4992
Supplemental material: Supplemental material for this article is available online.
References
- 1.Zahedivash A, Hanisch D, Dubin AM, et al. Implantable cardioverter defibrillators in infants and toddlers: indications, placement, programming, and outcomes. Circ Arrhythm Electrophysiol 2022; 15: e010557. [DOI] [PubMed] [Google Scholar]
- 2.Breton MD, Kanapka LG, Beck RW, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020; 383: 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gradaus R, Wollmann C, Köbe J, et al. Potential benefit from implantable cardioverter-defibrillator therapy in children and young adolescents. Heart 2004; 90: 328–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham MB, de Bock M, Smith GJ, et al. Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: a randomized clinical trial. JAMA Pediatr 2021; 175: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics 2016; 137: e20152354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson GJ, Loddenkemper R, Lundbäck B, et al. Respiratory health and disease in Europe: the new European lung white book. Eur Respir J 2013; 42: 559–563. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2023. May 2023, www.ginasthma.org (May 2023, accessed 8 May 2023).
- 8.Hammer SC, Robroeks CMHHT, van Rij C, et al. Actual asthma control in a paediatric outpatient clinic population: do patients perceive their actual level of control? Pediatr Allergy Immunol 2008; 19: 626–633. [DOI] [PubMed] [Google Scholar]
- 9.Drummond D, Roukema J, Pijnenburg M. Home monitoring in asthma: towards digital twins. Curr Opin Pulm Med 2023; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 10.Gonsard A, AbouTaam R, Prévost B, et al. Children’s views on artificial intelligence and digital twins for the daily management of their asthma: a mixed-method study. Eur J Pediatr 2022; 182: 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruizinga MD, Essers E, Stuurman FE, et al. Clinical validation of digital biomarkers for paediatric patients with asthma and cystic fibrosis: potential for clinical trials and clinical care. Eur Respir J 2022; 59: 2100208. [DOI] [PubMed] [Google Scholar]
- 12.Gupta RS, Fierstein JL, Boon KL, et al. Sensor-based electronic monitoring for asthma: a randomized controlled trial. Pediatrics 2021; 147: e20201330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toulouse E, Masseguin C, Lafont B, et al. French Legal approach to clinical research. Anaesth Crit Care Pain Med 2018; 37: 607–614. [DOI] [PubMed] [Google Scholar]
- 14.Kallio H, Pietilä AM, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs 2016; 72: 2954–2965. [DOI] [PubMed] [Google Scholar]
- 15.Reinert A. Une méthode de classification descendante hiérarchique: application à l’analyse lexicale par contexte. Cahiers de l’Analyse des Données 1983; 8: 187–198. [Google Scholar]
- 16.Lemanske RF, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 2010; 362: 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.General comment No. 25 (2021) on children’s rights in relation to the digital environment. OHCHR, https://www.ohchr.org/en/documents/general-comments-and-recommendations/general-comment-no-25-2021-childrens-rights-relation (accessed 3 May 2023).
- 18.Liu TL, Taylor YJ, Mahabaleshwarkar R, et al. Shared decision making and time to exacerbation in children with asthma. J Asthma 2018; 55: 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor YJ, Tapp H, Shade LE, et al. Impact of shared decision making on asthma quality of life and asthma control among children. J Asthma 2018; 55: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapp H, Shade L, Mahabaleshwarkar R, et al. Results from a pragmatic prospective cohort study: shared decision making improves outcomes for children with asthma. J Asthma 2017; 54: 392–402. [DOI] [PubMed] [Google Scholar]
- 21.Kew KM, Malik P, Aniruddhan K, et al. Shared decision-making for people with asthma. Cochrane Database Syst Rev 2017; 2017: CD012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond D, Gonsard A, Robinson PD. Digital respiratory medicine for children and young people. In: Pinnock H, Poberezhets V, Drummond D (eds) Digital respiratory healthcare (ERS monograph). Sheffield: European Respiratory Society, 2023, pp.122–131. [Google Scholar]
- 23.van Dellen QM, van Aalderen WMC, Bindels PJE, et al. Asthma beliefs among mothers and children from different ethnic origins living in Amsterdam, The Netherlands. BMC Public Health 2008; 8: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076241227285 for Objectives for algorithmic decision-making systems in childhood asthma: Perspectives of children, parents, and physicians by Omar Masrour, Johan Personnic, Flore Amat, Rola Abou Taam, Blandine Prevost, Guillaume Lezmi, Apolline Gonsard, Nadia Nathan, Alexandra Pirojoc, Christophe Delacourt, Stéphanie Wanin and David Drummond in DIGITAL HEALTH